Abstract
This review covers the literature published by chemists from China during the 2015–2016 on natural products (NPs), with 1,985 citations referring to 6,944 new compounds isolated from marine or terrestrial microorganisms, plants, and animals. The emphasis is on 730 new compounds with a novel skeleton or/and significant bioactivity, together with their source organism and country of origin.
Keywords: natural products, China, structure, bioactivity, novel skeleton
Introduction
Natural products (NPs) play an indispensable role in the drug development process and have provided various molecules for research over the years. Among the 1,211 small-molecule drugs approved from 1981 to 2014, 33% were based on NPs or their derivatives (Newman and Cragg, 2016).
Using natural resources to treat disease was the wisdom of the ancient Chinese and there is a long history of Chinese people using traditional Chinese medicines (TCMs) to treat a variety of diseases. NPs research in China originated in the 1920s and began with separation and identification of the main components of TCMs, such as Panax notoginsen (Zhao, 1937), Brucea javanica (Xu and Pan, 1955) as well as Aconitum carmichaeli (Zhao, 1936). The improvement of science and technology after the founding of the People's Republic of China in 1949, along with the establishment of new methods (Colegate and Molyneux, 2007) involved in detection and analysis of compounds led to the rapid development and great achievements of China's NPs research. In particular, huperzine A (Wang et al., 2006), a novel acetylcholinesterase (AChE) inhibitor derived from the Chinese medicinal herb Huperzia serrata, has been used to treat Alzheimer's disease and Youyou Tu was awarded the Nobel Prize in Physiology or Medicine in 2015 for her major contribution to the discovery of artemisinin (Tu, 2011).
By the 1980s, chemists from China not only focused on NPs isolated from TCMs or other terrestrial sources, but also expanded the research to marine natural products (MNPs). The number of new MNPs discovered in China increased exponentially since the 1990s, making China the second most country involved in MNPs discovery behind Japan (Blunt et al., 2016). Taken together, these breakthroughs led to the renewal of NPs research in China.
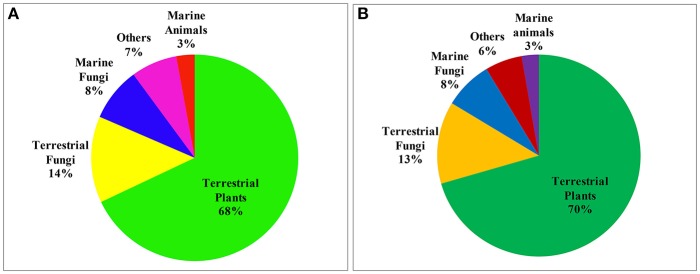
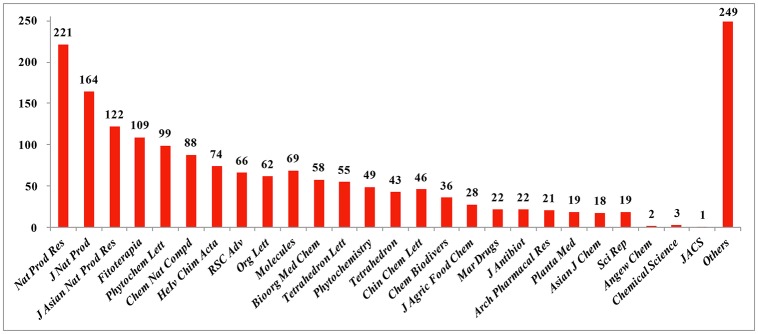
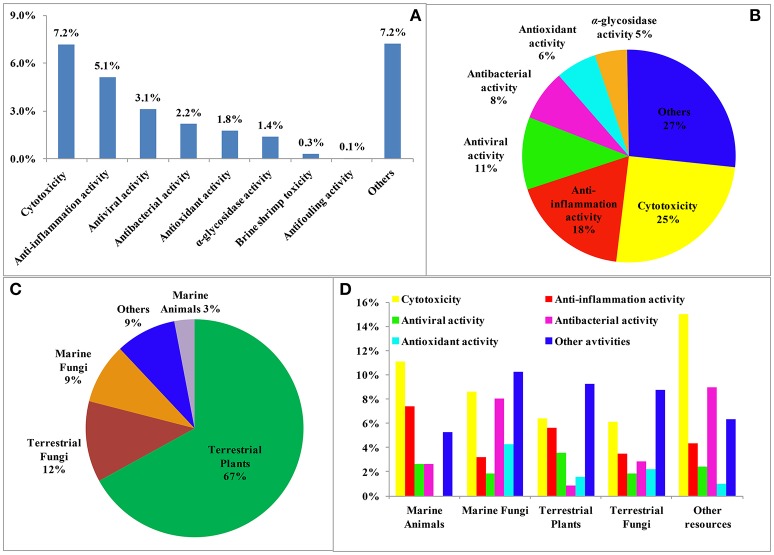
Reviews of NPs are generally based on their producers [mangrove (Wu et al., 2008), Paeonia (Zhao et al., 2016), fungi (Wang et al., 2011), etc.], chemical structures [sesquiterpenoids (Wang et al., 2014), triterpenoids (Xiao et al., 2008), lignans (Zhang et al., 2014), alkaloids (Ma et al., 2016e), etc.] and bioactivities [analgesic activity (Xiao et al., 2016a), antiviral activity (Jiang et al., 2010), cytotoxicity (Wang et al., 2014), etc.] while few reviews are based on the country where the authors come from. According to our current research, more than 6,500 papers have been published by chemists from China in the past 2 years covering all aspects related to NPs, and of these, 1,985 were related to new NPs, a total of about 30%. This review covers the literature published from 2015 to 2016 with 1,985 citations (1,103 for 2015 and 882 for 2016) referring to new NPs isolated from terrestrial- or marine-sourced animals, plants, and microorganisms. In total, 6,944 new small-molecule compounds are summarized (3,891 for 2015 and 3,053 for 2016). The emphasis is on new compounds with a novel skeleton or/and significant biological activity. Pharmaceutical data are directly cited from the original paper, and only comparable or more potent activity relative to the positive control is defined as significant activity. For the cytotoxicity values, significant activity means a half maximal inhibitory concentration (IC50) value below 1 μM or 0.5 μg/mL. Chemical structures, together with classifications, taxonomic origins, locations of collections, and biological activities are described in detail. The numbers for all structures in this review are shown in non-italicized bold font.
Marine microorganisms
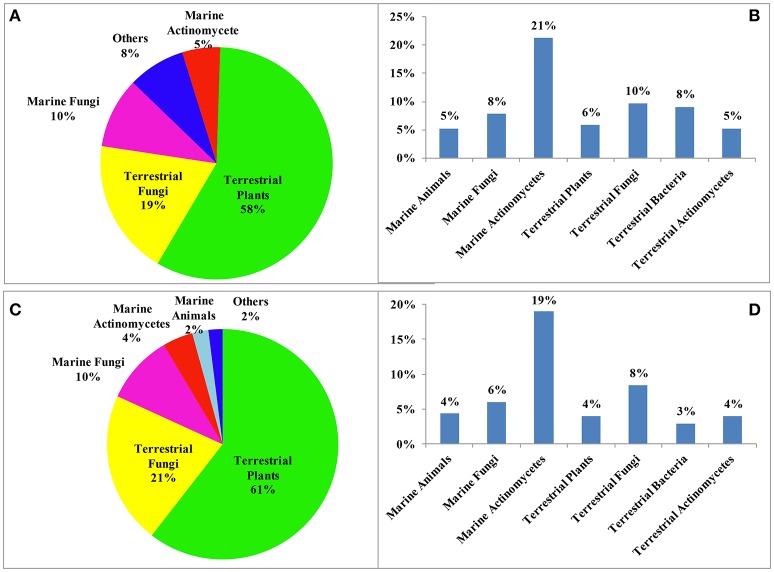
A total of 612 novel NPs was isolated from marine microorganisms in the last 2 years. The percentage of compounds with new skeletons from marine bacteria (19.5%) in this review is much higher than average (5.1%), the percentage of bioactive compounds from marine fungi (36.3%) is also higher than the average (28.4%). A total of 46 references related to 101 NPs with a novel skeleton or/and significant bioactivity are listed below.
Marine-derived bacteria (including mangrove-derived bacteria)
Marine-derived bacteria (except mangrove-derived bacteria)
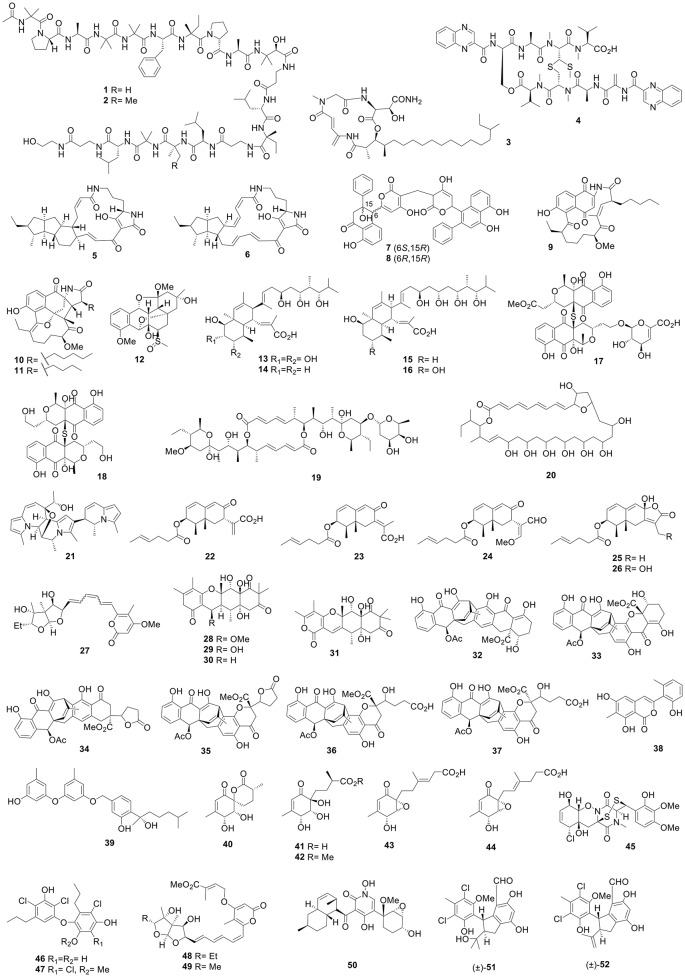
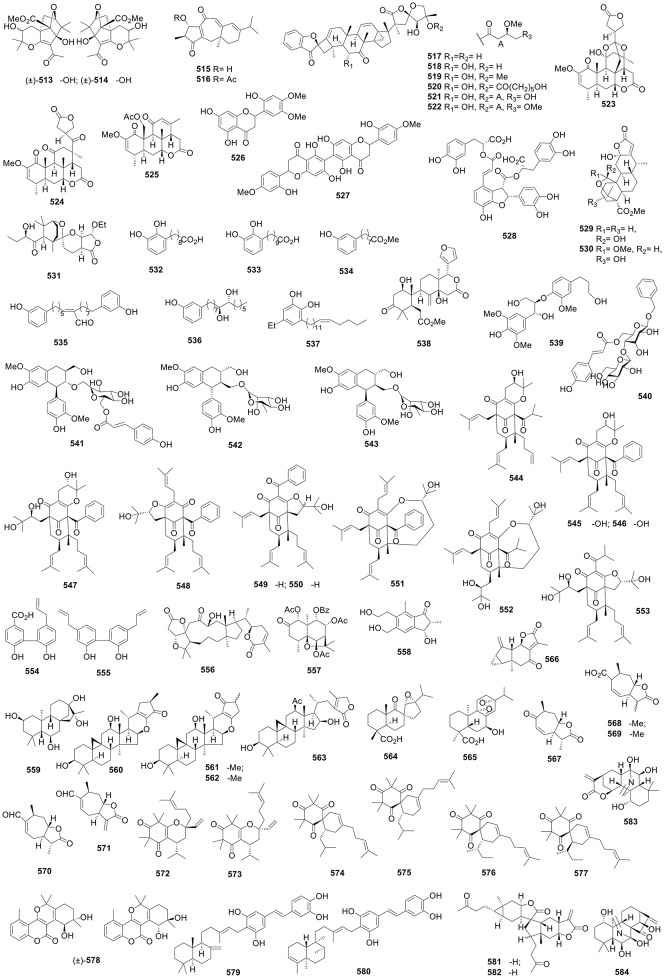
Microbacterins A (1) and B (2) (Figure 1), two new peptaibols, were produced by the deep-sea actinomycete Microbacterium sediminis sp. nov. YLB-01(T). Microbacterin B (2) displayed potent inhibitory activity against BGC-823 cells with an IC50 value of 1.03 μM (Liu et al., 2015b). The marine sediment-derived Micromonospora sp. FIM02-523 (Fujian, China) produced a new depsipeptide, rakicidin B1 (3), which possessed inhibitory activity against HCT-8, MGC803, A549, and A375 cells with IC50 range of 0.11–0.64 μM (Lin et al., 2016b). The marine sponge-derived Streptomyces sp. LS298 (Gelliodes carnosa, Lingshui Bay, Hainan, China) produced a new analog of echinomycin, quinomycin G (4), which exhibited remarkable cytotoxic activity against the Jurkat cells with the IC50 value of 0.414 μM (Zhen et al., 2015). Six new polycyclic tetramate macrolactams (PTMs), pactamides A–F, were isolated from the marine-derived Streptomyces pactum SCSIO 02999 by activating a PTM gene cluster, among which pactamides A (5) and C (6) displayed potent cytotoxicities against SF-268, MCF-7, NCI-H460, and Hep-G2 cell lines with IC50 values of 0.24–0.51 and 0.71–2.42 μM, respectively (Saha et al., 2017). Cultivation of Streptomyces sp. OUCMDZ-3434 (Enteromorpha prolifera, Zhanqiao Beach, Qingdao, China) produced two new polyketides bearing duble 6-(2-phenylnaphthalene-1-yl)pyrane-2-one nuclei and a methylene linkage, wailupemycins H (7) and I (8), both of which showed α-glucosidase inhibitority activities with Ki/IC50 values of 16.8/19.7 and 6.0/8.3 μM, respectively (Chen et al., 2016g). Neoansamycins A–C (9-11), three novel naphthalenic octaketide ansamycins with unprecedented n-pentylmalonyl-CoA or n-butylmalonyl-CoA extender units, were produced by Streptomyces sp. LZ35 and resulted from activation of a cryptic ansamycin biosynthetic gene cluster (nam) (Li et al., 2015f). The first sulfur angucyclinone with an unusual ether-bridged system, grisemycin (12), which exhibited a novel cage-like structure, was obtained from the marine sediment-derived Streptomyces griseus M268 (Kiaochow Bay, China) (Xie et al., 2016b). Nahuoic acids B–E (13-16), polyol polyketides with a decalin ring, were produced by the Streptomyces sp. SCSGAA 0027 (Melitodes squamata, South China Sea) (Nong et al., 2016). Naquihexcins A (17) and B (18), s-bridged pyranonaphthoquinone dimers with a rare unsaturated hexuronic acid moiety, were isolated from a culture of the sponge-derived Streptomyces sp. HDN-10-293 (Che et al., 2016).
Figure 1.
Structures of compounds 1-52.
Mangrove-derived bacteria
The culture of mangrove soil-derived Streptomyces sp. 219807 (Hainan, China) afforded eight elaiophylin derivatives including halichoblelide D (19), which showed significant cytotoxic activity toward MCF-7 and HeLa cells with the IC50 values of 0.33 and 0.30 μM, respectively (Han et al., 2016b). Six new polyene-polyol macrolides, reedsmycins A–F, were afforded by the reed rhizosphere soil-derived Streptomyces sp. CHQ-64 (Guangdong, China), among which reedsmycin F (20) possessed a rare 31-membered macrocyclic system containing a tetrahydrofuran motif (Che et al., 2015).
Marine-derived fungi (including mangrove-derived fungi)
Marine-derived fungi (except mangrove-derived fungi)
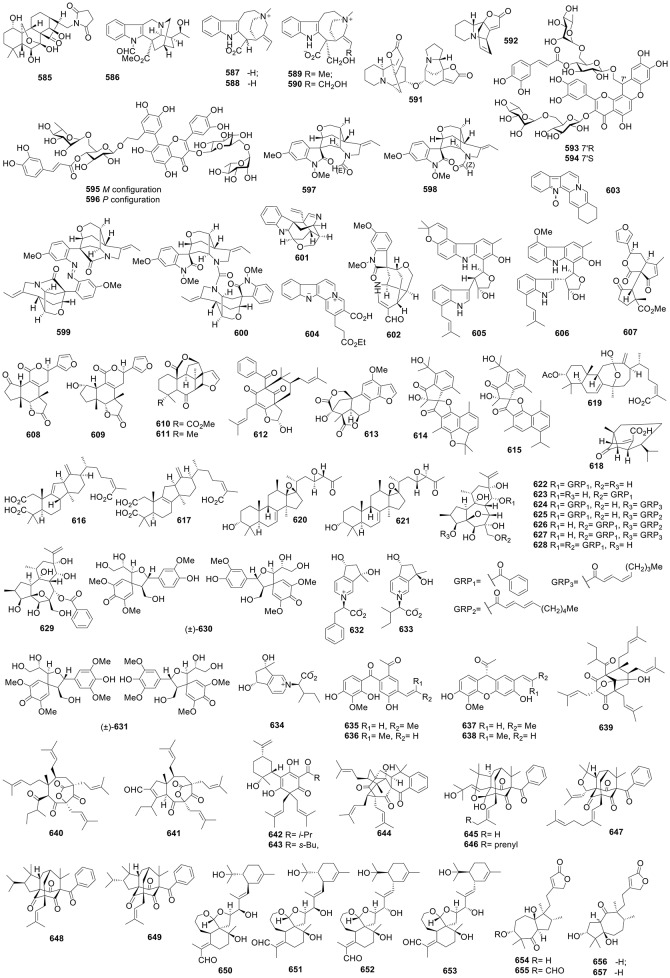
Curindolizine (21), an indolizine alkaloid with an unprecedented skeleton, was obtained from the white croaker-associated fungus Curvularia sp. IFB-Z10 and displayed anti-inflammatory action in lipopolysaccharide (LPS)-induced RAW 264.7 macrophages with an IC50 value of 5.31 μM (positive control dexamethasone, 2.17 μM) (Han et al., 2016c). Acremeremophilanes A–O, 15 new eremophilane-type sesquiterpenoids, were isolated from a deep-sea sediment-derived Acremonium sp. (South Atlantic Ocean), among which acremeremophilanes B–F (22-26) contained a novel 4-hexenoic acid unit. Acremeremophilane B (22) showed the inhibition on the LPS-induced NO production in RAW 264.7 macrophage cell lines with an IC50 value of 8 μM (reference quercetin, 15 μM) (Cheng et al., 2016). The fungus Aspergillus ochraceopetaliformis SCSIO 05702 (sediment, Chinese Antarctic station) produced five highly oxygenated α-pyrone merosesquiterpenoids, ochraceopones A–E, along with a new isomer of asteltoxin, isoasteltoxin (27). Ochraceopones A–D (28-31) were new α-pyrone merosesquiterpenoids with a linear tetracyclic carbon skeleton that had not been described previously. Compound 27 inhibited H1N1 and H3N2 influenza viruses with IC50 values of 0.23 and 0.66 μM (tamiflu, 16.9 and 18.5 nM), respectively (Wang et al., 2016b). Six new polyketides (32-37) with an anthraquinone-xanthone basic structure were produced by Cacospongia scalaris-derived fungus, Engyodontium album LF069 (Limski Fjord, Croatia). Compounds 33–37 represented the first examples of a 23,28 seco-beticolin carbon skeleton. Compounds 32 and 33 showed inhibitory activities against the methicillin resistant Staphylococcus aureus (MRSA) with IC50 at 0.17 and 0.24 μM, respectively, 10-fold stronger than chloramphenicol (Wu et al., 2016). Pleosporalone A (38), the first azaphilone derivative with an aromatic A-ring, were produced by the sediment-derived fungus Pleosporales sp. CF09-01 (Bohai Sea, China). Compound 38 showed antifungal activity to three plant pathogenic fungi, Rhizopus oryzae, Botrytis cinerea, and Phytophthora capsici, with minimal inhibitory concentration (MIC) values of 0.78, 0.39, and 0.78 μM, more potent than carbendazim (MIC 1.56, 0.78, and 1.56 μM), respectively (Cao et al., 2016a). Deep sea sediment-derived Penicillium aculeatum SD-321 (South China sea) produced three novel phenolic bisabolane sesquiterpenes, peniciaculins A (39) and B, and 1-hydroxyboivinianin A. Peniciaculin A (39) displayed antibacteria activity to Micrococcus luteus and Vibrio alginolyticus with MIC values of 1.0 and 2.0 μg/mL, respectively, comparable to chloramphenicol (8.0 and 0.5 μg/mL). Peniciaculin A (39) also showed inhibitory activity against Alternaria brassicae with an MIC value at 0.5 μg/mL, much better than amphotericin B (32 μg/mL) (Li et al., 2015h).
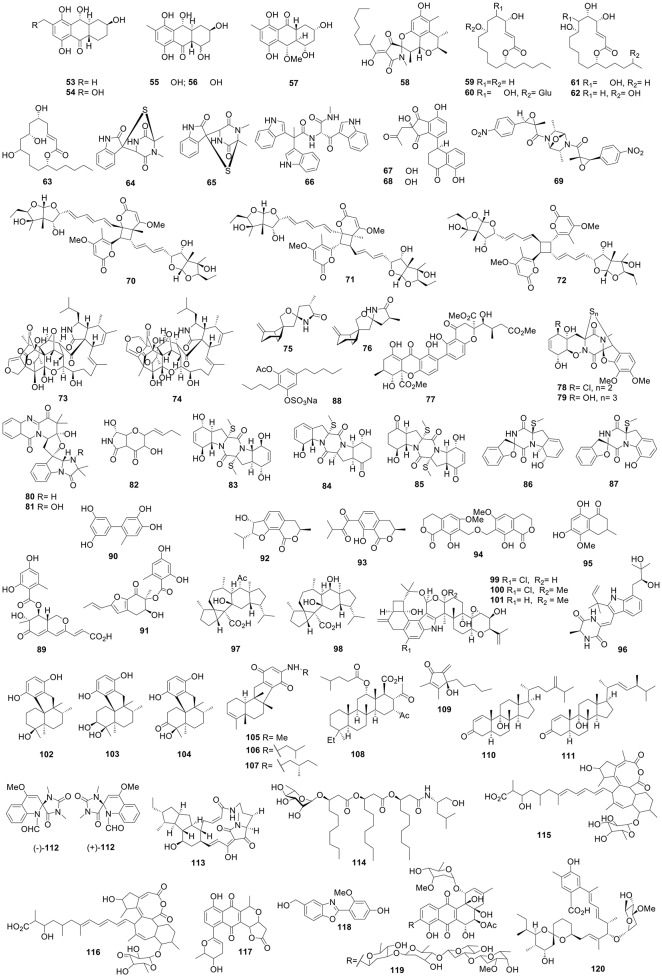
New ambuic acid analogs, penicyclones A–E (40-44), were produced by the deep sea-derived fungus Penicillium sp. F23-2 and were found to show antimicrobial activities against S. aureus with MICs of 0.3–1.0 μg/mL (Guo et al., 2015a). Adametizines A and B, two novel bisthiodiketopiperazine derivatives, were afforded by the sponge-derived fungus Penicillium adametzioides AS-53 (Hainan, China). Adametizine A (45) exhibited brine shrimp lethality with a median lethal dose (LD50) value of 4.8 μM (colchicine, 8.1 μM) and antimicrobial activity against S. aureus, Aeromonas hydrophilia, and V. parahaemolyticus with a same MIC value of 8 μg/mL (Liu et al., 2015i). A culture broth of the deep-sea sediment-derived fungus Spiromastix sp. MCCC 3A00308 (2869 m, South Atlantic Ocean) contained 11 new polyphenols, spiromastols A–K, among which spiromastols A (46) and C (47) showed antimicrobial activities against Agrobacterium tumefaciens ATCC11158, Bacillus thuringensis ATCC10792, B. subtilis CMCC63501, Pseudomonas lachrymans ATCC11921, Ralstonia solanacearum ATCC11696, S. aureus ATCC25923, and Xanthomanes vesicatoria ATCC11633 with MICs of 0.25–0.5 μg/mL. The MIC range of chloroamphenicol toward the same bacteria was 1.0–2.0 μg/mL (Niu et al., 2015). The marine sponge-derived Aspergillus sp. SCSIO XWS02F40 (Callyspongia sp, Guangdong, China) yielded asteltoxins E (48) and F (49), which exhibited anti- virus activities against H3N2 virus with IC50 of 6.2 and 8.9 μM, respectively. Asteltoxin E (48) also active to H1N1 cirus with the IC50 value of 3.5 μM (Tian et al., 2015c). Research into the sponge-derived fungus Arthrinium arundinis ZSDS1-F3 (Xisha Islands, China) led to the identification of three novel 4-hydroxy-2-pyridone alkaloids, arthpyrones A–C. Arthpyrone C (50) significantly inhibited AchE with an IC50 value of 0.81 μM (tacrine, 0.48 μM) (Wang et al., 2015e). The fungus Pestalotiopsis ZJ-2009-7-6 associated with Sarcophyton sp. (Yongxing Island, China) afforded two new chlorinated enantiomeric diphenylmethanes, (±)-pestalachlorides E (51) and F (52), both of which showed significant antifouling activities with half maximal effective concentration (EC50) values of 1.65 and 0.55 μg/mL, respectively, while SeaNine 211 acted as the positive control with an EC50 value of 1.23 μg/mL (Xing et al., 2016). The algicolous fungus Talaromyces islandicus EN-501 from Laurencia okamurai (Shandong, China) afforded five new polyhydroxylated hydroanthraquinone derivatives (53-57) (Figure 2), which displayed better antioxidant activities against 2,2-diphenyl-1-picryl-hydrazyl (DPPH) radicals than butylated hydroxytoluene (BHT, 61 μM) with an IC50 range of 12–52 μM (Li et al., 2017).
Figure 2.
Structures of compounds 53-120.
The marine sediment-derived fungus Penicillium citrinum (Langqi Island, Fujian, China) afforded penicitrinine A (58), an alkaloid with a unique spiro skeleton (Liu et al., 2015d). Five 14-membered macrolides, gliomasolides A–E (59-63), were isolated from a culture broth of the sponge-associated fungus Gliomastix sp. ZSDS1-F7-2 (Phakellia fusca, South China Sea) (Zhang et al., 2015h). Fermentation of the fungus Pseudallescheria ellipsoidea F42-3 associated with the coral Lobophytum crassum (Sanya, Hainan, China) afforded three new compounds (64-66) with a unique skeleton. Pseudellones A (64) and B (65) were irregularly bridged epimonothiodiketopiperazine diastereomers with an unusual 3-indolylglycine residue (Liu et al., 2015e). Chrysamides A–C, three dimeric nitrophenyl trans-epoxyamides, were produced by the sediment fungus Penicillium chrysogenum SCSIO41001 (Indian Ocean). Chrysamide A (67) represented a centrosymmetric dimer skeleton with an novel 7-oxa-2,5-diazabicyclo[2.2.1]heptane nucleus (Chen et al., 2016f). Four new tetralone derivatives, clindanones A (68) and B (69) along with cladosporols F and G, were afforded by the deep-sea sediment-derived fungus Cladosporium cladosporioides HDN14-342 (Indian Ocean), among which compounds 68 and 69 featured new dimeric skeleton coupling indanone and 1-tetralone units (Zhang et al., 2016m). Three novel asteltoxin-bearing dimers, diasteltoxins A–C (70-72), were produced by a mutated sponge fungus, Emericella variecolor XSA-07-2 (Long et al., 2016). Epicochalasines A (73) and B (74), two new cytochalasans featured a hendecacyclic 5/6/11/5/6/5/6/5/6/6/5 ring system containing fused aspochalasin and epicoccine dimer moieties, were produced by Aspergillus flavipes (Zhu et al., 2016). A pair of unusual epimeric spiroaminal derivatives bearing a 6/4/5/5 tetracyclic ring system, sporulaminals A (75) and B (76), were afforded by the marine sediment-derived fungus Paraconiothyrium sporulosum YK-03 (Bohai Bay, China) (Zhang et al., 2016d).
Mangrove-derived fungi
Versixanthones A–F, six unusual xanthone-chromanone dimers, were obtained from the mangrove-derived fungus Aspergillus versicolor HDN1009 (Guangdong Province, China); versixanthone F (77) exhibited cytotoxic activity against HCT-116 with the IC50 value of 0.7 μM (Wu et al., 2015a). The mangrove endophytic fungus Penicillium janthinellum HDN13-309 (Hainan Province, China) produced six new epipolythiodioxopiperazine (ETP) alkaloids, penicisulfuranols A–F. Penicisulfuranols A (78) and C (79) displayed cytotoxicities against HeLa and HL-60 cells with IC50 values of 0.5/0.1 (HeLa/HL-60) and 0.3/1.2 μM, respectively (Zhu et al., 2017). Endophytic fungus Neosartorya udagawae HDN13-313 (Aricennia marina, Hainan, China) produced neosartoryadins A (80) and B (81), which possessed a unique 6/6/6/5 quinazoline ring system directly connected to the 6/5/5 imidazoindolone ring. Neosartoryadins A (80) and B (81) inhibited H1N1 influenza viruses with IC50 values of 66 and 58 μM, respectively, better than ribavirin of 94 μM (Yu et al., 2016a). The mangrove fungus Penicillium brocae MA-231 (Avicennia marina, Hainan, China) produced one novel derivative of polyoxygenated dihydropyrano[2,3-c]pyrrole-4,5-dione, pyranonigrin F (82), five new sulfide diketopiperazine derivatives (penicibrocazines A–E), as well as four diketopiperazines (spirobrocazines A–C along with brocazine G). Pyranonigrin F (82) displayed potent antimicrobial activity against S. aureus, Vibrio. harveyi, and Vibrio. parahaemolyticus with the same MIC value of 0.5 μg/mL, more potent than chloromycetin with MICs of 8.0, 2.0, and 128.0 μg/mL, respectively (Meng et al., 2015b). Penicibrocazine C (83) inhibited S. aureus and M. luteus with the same MIC value of 0.25 μg/mL (chloromycetin, 4.0 and 2.0 μg/mL), while both penicibrocazines B (84) and E (85) inhibited Gaeumannomyces graminis with the same MIC value of 0.25 μg/mL (amphotericin B, 16 μg/mL) (Meng et al., 2015c). Spirobrocazines A (86) and B (87) showed a rare 6/5/6/5/6 cyclic system which had a spirocyclic center at C-2 (Meng et al., 2016a).
A fermentation broth of the mangrove fungus Stemphylium sp. 33231 endophytic with Brguiera sexangula var. rhynchopetala (South China Sea) afforded two novel stemphol sulfates, stemphols A and B. Stemphol B (88) showed antimicrobial activities against Escherichia coli and B. cereus with the same MIC value of 0.6 μg/mL (ciprofloxacin, 0.3 μg/mL) (Zhou et al., 2015d). Endophytic fungus Penicillium sp. HN29-3B1 (Cerbera manghas, Dongzhaigang, Hainan, China) produced pinazaphilones A and B, penicidone D, and two phenolic compounds. Pinazaphilone B (89) and phenolic compound 90 showed more effective α-glucosidase inhibition than acarbose (446.7 μM) with IC50 values of 28.0 and 2.2 μM, respectively (Liu et al., 2015j). Aspergifuranone (91) was identified from the algicolous fungus Aspergillus sp. 16-5B with Sonneratia apetala (Dongzhaigang, Hainan, China) and exhibited significant α-glucosidase inhibition with an IC50 value of 9.05 μM (acarbose, 553.7 μM) (Liu et al., 2015h). Eight isocoumarin derivatives and one isoquinoline were afforded by the mangrove fungus Aspergillus sp. 085242 endophytic with Acanthus ilicifolius (Shankou, Guangxi, China). Asperisocoumarins B (92), E (93), and F (94) exhibited α-glucosidase inhibition with IC50 values of 87.8, 52.3, and 95.6 μM, respectively (acarbose, 628.3 μM) (Xiao et al., 2016b). Six new isocoumarins and two new benzofurans were produced by the mangrove fungus Talaromyces amestolkiae YX1 endophytic with Kandelia obovate (Zhanjiang, Guangdong, China), among which new isocoumarin 95 showed better α-glucosidase inhibition than acarbose with IC50 value of 89.4 and 958.3 μM, respectively (Chen et al., 2016e). Mangrove rhizospheric soil-derived Eurotium rubrum MA-150 (Andaman Sea coastline, Thailand) yielded three new indolediketopiperazine alkaloids, rubrumazines A–C. Among these compounds, rubrumazine B (96) exhibited the best brine shrimp lethality with an LD50 value of 2.4 μM (colchicine, 19.4 μM) (Meng et al., 2015a).
Two new sesterterpenoids, aspterpenacids A (97) and B (98), from Aspergillus terreus H010, the endophytic fungus of Kandelia obovata, represented an unusual 5/3/7/6/5 carbon ring skeleton (Liu et al., 2016e). Twenty indole-diterpenes including three new ones were obtained from a culture of the mangrove fungus Mucor irregularis QEN-189 associated with Rhizophora stylosa, (Hainan, China). Compounds 99–101 possessed a rare 4,6,6,8,5,6,6,6,6-fused indole-diterpene ring system (Gao et al., 2016b).
Marine animals
A total of 189 new NPs was identified from marine animals in 2015–2016 including eight (4.2%) with novel skeletons and 55 (29.1%) with various bioactivities. Herein, we list six references that report 11 NPs with a novel skeleton or/and significant bioactivity.
Four new 6/6/5/6-fused tetracyclic meroterpenes, dysiherbols A–C (102-104) and dysideanone E, were obtained from a marine sponge Dysidea sp. (South China Sea, at a depth of 10 m). Dysiherbol A (102) showed potent nuclear factor-kappaB (NF-κB) inhibition and cytotoxicity with IC50 values of 0.49 and 0.58 μM, respectively (Jiao et al., 2016). Dysidea fragilis (South China Sea) was also the source of three new sesquiterpene aminoquinones ferturing the rearranged avarone skeleton, dysifragilones A–C (105-107), which inhibited the production of NO stimulated by LPS in mouse RAW 264.7 macrophages with IC50 values of 6.61, 9.83, and 17.22 μM, respectively (positive control hydrocortisone, 45.72 μM) (Jiao et al., 2015a). Eight novel scalarane sesterterpenoids, carteriofenones D–K, were discovered from the marine sponge Carteriospongia foliascens (Dongluoxigu Island, China). Carteriofenone D (108) was cytotoxic to a mouse lymphocytic leukemia cell line (P388) with an IC50 value of 0.96 μM (Cao et al., 2015a). Five novel metabolites were isolated from the soft coral Sinularia verruca (Ximao Island, Hainan, China), among which compound 109 showed anti-human immunodeficiency virus (HIV)-1 activity with an EC50 of 5.8 μM (Yuan et al., 2016). Investigation of the gorgonian coral Subergorgia rubra (South China sea) led to the isolation of three new Δ1-9-hydroxy-3-ketosteroids, subergosterones A–C. Subergosterones B (110) and C (111) exhibited antibacterial activities to B. cereus with an MIC value of 1.56 μM, similar to that of ciprofloxacin (1.25 μM) (Sun et al., 2015c). A pair of novel bisheterocyclic quinolineimidazole alkaloids, (±)-spiroreticulatine ((±)-112), obtained from sponge Fascaplysinopsis reticulata (South China Sea), were the first example of spiro quinoline-imidazole alkaloids from sponge (Wang et al., 2015i).
Terrestrial microorganisms
A total of 1,081 new NPs was produced by terrestrial microorganisms, of which 970 were produced by fungi. Among the new compounds isolated from fungi, more than 7.9% possessed an unprecedented skeleton. All 158 NPs with novel skeletons or/and significant bioactivities are listed.
Terrestrial-sourced bacteria
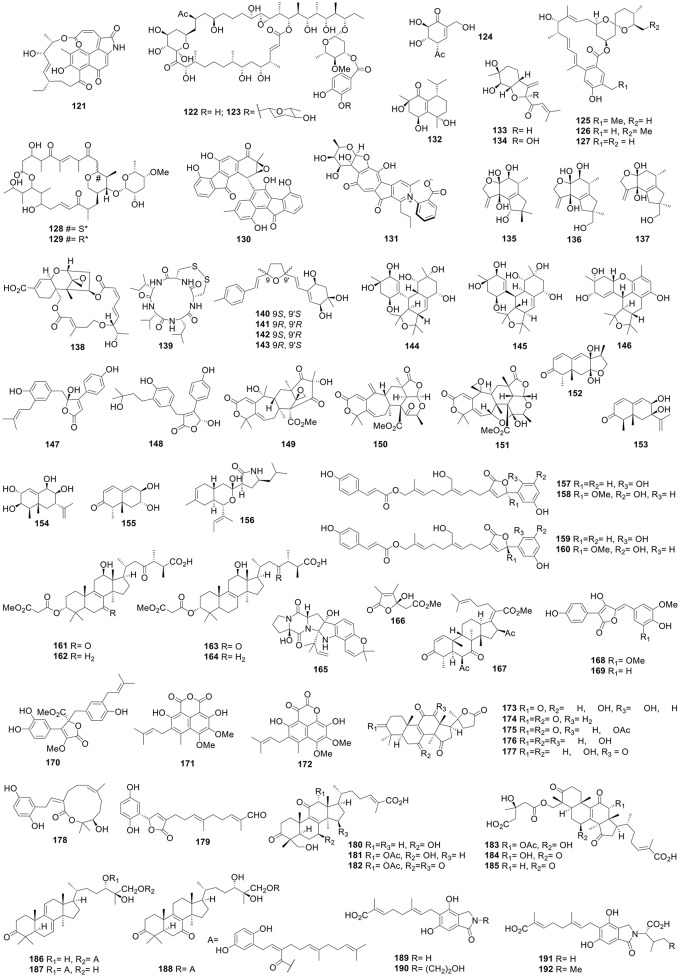
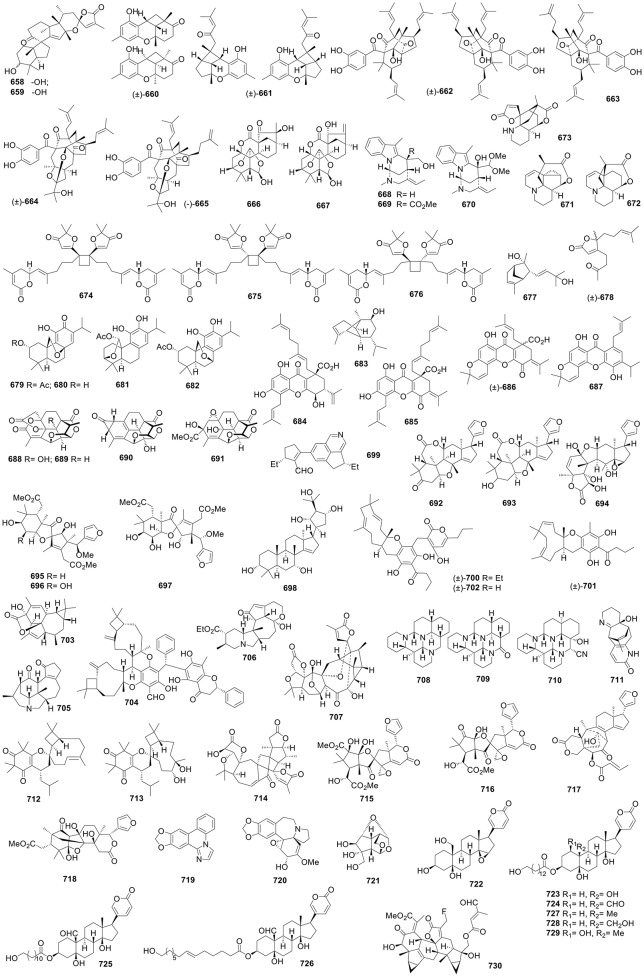
A culture of Lysobacter enzymogenes (University of Nebraska Lincoln, U.S.) afforded two novel PTMs, lysobacteramides A and B. Lysobacteramide B (113) was cytotoxic to A549 cells with an IC50 of 0.8 μM (Xu et al., 2015b). Rhizoleucinoside (114) with a trimeric 3-hydroxy heptylate nucleus, was obtained from a culture of the rhizobial Bradyrhizobium sp. BTAi1 (ATCC BAA-1182) (Chen et al., 2016c). Three polyene antibiotics, aurantinins B–D, were obtained from the fermentation of Bacillus subtilis fmb60 (Jiangsu, China). Aurantinins C (115) and D (116) showed antibacterial activity against Clostridium sporogenes with a MIC of 0.78 μg/mL (erythromycin gluceptate, 3.12 μg/mL) (Yang et al., 2016f). The karst cave soil-derived Streptomyces sp. CC8-201 (Chongqing, China) produced a new pyranonaphthoquinone (PNQ) antibiotic, xiakemycin A (117), which possessed cytotoxicity against HeLa, HCT-116, SH-SY5Y, and PC-3 cells with IC50 values of 0.43–0.98 μM (Jiang et al., 2015b). The saltmarsh soil-derived Nocardiopsis lucentensis DSM 44048 produced seven new benzoxazole derivatives, nocarbenzoxazoles A–G. Nocarbenzoxazole G (118) displayed selective cytotoxicity against HeLa cells with an IC50 of 1 μM (Sun et al., 2015a). Chattamycins A and B, two novel angucycline antibiotics, were produced by Streptomyces chattanoogensis L10 (CGMCC 2644). Chattamycin B (119) showed stronger cytotoxicity against MCF-7 cells with an IC50 of 1.08 μM when compared with the positive control (Zhou et al., 2015e). A culture of the ΔaveCDE mutant strain Streptomyces avermectinius afforded three novel 1,19-seco-avermectin (AVE) analogs, among which compound 120 (Figure 3) was cytotoxic against the Saos-2 cells with an IC50 value of 0.7 μM (Sun et al., 2015b). The hgc1-overexpressed mutant strain Streptomyces sp. LZ35 produced three new hygrocins, among which hygrocin H (121) displayed significant cytotoxic activity against HeLa cells with an IC50 of 0.8 μM (Li et al., 2015g).
Figure 3.
Structures of compounds 121-192.
Soil-derived Streptomyces phytohabitans HBERC-20821 from Wawushan Hill (Sichuan Province, China) afforded two novel 32-membered macrolides, novonestmycins A (122) and B (123), both of which were mycostatic against the phytophathogenic fungi Septoria nodorum, Corynespora cassiicola, and Rhizoctonia solani with MIC values of 0.78, 0.78, and 0.39 μg/mL, respectively. Compounds 122 and 123 were also cytotoxic against HepG2, MCF-7, HeLa, BGC-823, and BEL-7402/5-FU cells with IC50 values of 0.15–0.48 and 0.24–1.34 μg/mL, respectively (Wan et al., 2015). Two new polyoxygenated cyclohexenone, gabosines P (124) and Q, together with two known cyclic dipeptides were obtained from a culture of Streptomycetes strain no. 8 (Qinling Mountains, Shaanxi, China). Gabosine P (124) showed α-glucosidase inhibition with an IC50 value of 9.07 μM, more potent than acarbose (663.28 μM) (Wei et al., 2016a). 13α-Hydroxy-4-ethyl milbemycin β3 (125), 13α-hydroxy-25-ethylmilbemycin β3 (126), and 13α-hydroxymilbemycin β3 (127), three novel β-class milbemycins, were obtained from a fermentation broth of the genetically engineered strain Streptomyces avermitilis MHJ1011. The aveA1 gene was seamlessly replaced by the milA1 gene, and compounds 125-127 showed significant acaricidal activity with LC50 values of 0.0210, 0.1023, and 0.1090 mg/L, respectively (milbemycins A3/A4, 0.0324 mg/L; tenvermectins A/B, 0.0050 mg/L) (Pan et al., 2016a). Two new macrolides with a tetrahydropyran moiety, FXJ15321 (128) and FXJ15322 (129), were obtained from a fermentation broth of the red soil-derived Streptomyces sp. FXJ1.532 (Jiangxi, China) (Guo et al., 2015b). Culture of Streptomyces coelicolor YF11 with a heterologous expression of the intact fls-gene cluster in a 3% sea salt medium led to the isolation of an unusual heterodimer, difluostatin A (130) (Yang et al., 2015a). Rubrolone B (131), a new class of rubrolone analog possessing a rare benzoic acid-pyridine inner salt moiety, was afforded by Camellia sinensis-derived Streptomyces sp. KIB-H033 (Yan et al., 2016).
Terrestrial-sourced fungi
The endophytic fungus Aspergillus clavatus from the leaves of Tripterygium hypoglaucum yielded a new cadinane-type sesquiterpenoid, aspergillusone D (132), which exhibited cytotoxicity against the A549 cells with an IC50 value of 0.2 μM (Wang et al., 2015b). Two new bisabolane-type sesquiterpenoids, pleurotons A (133) and B (134), along with three clitocybulol derivatives, clitocybulols D–F (135-137) were produced by the edible fungus Pleurotus cystidiosus (Qingyun mountains, Fujian, China). These compounds possessed significant inhibitory activities against two human prostate cancer cells, DU-145 and C42B, with IC50 values of 28–233 and 52–163 nM, respectively (Zheng et al., 2015b). Epiroridin acid (138), a roridin-type trichothecene macrolide, was obtained from the liquid culture of Myrothecium roridum A553 associated with Pogostemon cablin (Guangdong, China), and showed significant cytotoxicity against MCF-7, SF-268, NCI-H460, and HepG-2 cells with IC50 values of 0.170, 0.751, 0.360, and 0.380 μM, respectively (Liu et al., 2016c).
Research into the fungus FR02 endophytic with roots of Ficus carica (Qinling Mountain, Shaanxi, China) led to the discovery of 14-membered cyclic dipeptides, among which malformin E (139) showed cytotoxicity against MCF-7 and A549 cell lines with IC50 values of 0.65 and 2.42 μM, respectively. Meanwhile, malformin E (139) also displayed potent antibacterial activity against B. subtilis with an MIC value of 0.91 μM (gentamicin, 4.06 μM) (Ma et al., 2016d). Seven dimeric acremines, bisacremines A–D (140-143) (Wu et al., 2015d) and bisacremines E–G (144-146) (Wu et al., 2015e), were produced by the soil-derived Acremonium persicinum SC0105 (Dinghu Mountain, Guangdong, China). Bisacremine G (146) inhibited the LPS-stimulated production of TNF-α, IL-6, and NO in macrophage and NO in macrophages with 80.1, 89.4, and 55.7% inhibitions, respectively at 50 μM (dexamethasone with 78.0, 92.6, and 62.6% inhibition, respectively). The endophytic fungus Aspergillus terreus PR-P-2 from Camellia sinensis var. assamica (Yunnan, China) yielded three new butenolides, asperteretals A–C. Asperteretals A (147) and C (148) inhibited the NO production induced by LPS in RAW 264.7 macrophages with IC50 values of 26.64 and 17.21 μM, respectively (hydrocortisone, 48.66 μM) (Guo et al., 2016). Five new meroterpenoids, purpurogenolides A–E, were obtained from a solid culture of the soil-derived Penicillium purpurogenum MHz 111 (Heilongjiang, China). Among them, purpurogenolides B–D (149-151) showed inhibitory activities against NO production in LPS-activated BV-2 microglial cells with IC50 values of 15.5, 8.8, and 0.8 μM, respectively (indomethacin, 34.5 μM) (Sun et al., 2016b).
The endophytic fungus Periconia sp. F-31 with Annona muricata (Hainan, China) yielded nine new polyoxygenated eremophilane sesquiterpenes, periconianones C–K and other four new polyketide synthase-nonribosomal peptide synthetase (PKS-NRPS) hybrid metabolites, pericoannosins A–D. Periconianone C (152) was the first furan-type isoeremophilane containing a C-8/C-11 linkage and a 7,12-epoxy moiety (Liu et al., 2016d), while pericoannosin A (156) was characterized by a novel hexahydro-1H-isochromen-5-isobutylpyrrolidin-2-one skeleton (Zhang et al., 2015b). Periconianones D (153), G (154), and K (155) inhibited NO production in LPS-activated BV-2 microglial cells by 10.2, 18.3, and 16.1% inhibition, respectively at 1.0 μM (curcumin 12.9%) (Liu et al., 2016d). Ganosinensols A–D (157-160), four new farnesyl phenolic compounds, were obtained from the fruiting bodies of Ganoderma sinense, and inhibited LPS-induced NO production in RAW 264.7 macrophages with IC50 values of 1.15–2.26 μM (hydrocortisone, 58.79 μM) (Wang et al., 2016d). Eight 24-methyllanostane triterpenes, officimalonic acids A–H together with one known lanostane triterpene were obtained from the fruiting bodies of Fomes officinalis (Xinjiang, China). Officimalonic acids D (161), E (162), G (163), and H (164) were active against NO production in LPS-stimulated RAW 264.7 cells with IC50 values of 5.1–8.9 μM (dexamethasone 22.2 μM) (Han et al., 2016a).
Prenylated indole alkaloid speramides A and B, were produced by the fresh water-derived fungus Aspergillus ochraceus KM007 (Lake Fuxian, Yunnan, China). Speramide A (165) displayed antibacterial activity against P. aeruginosa with an MIC value of 0.8 μM (Chang et al., 2016). A new furanone (166) was obtained from the edible mushroom Grifola frondosa (Wuyi Mountain, Fujian, China), and exhibited antifungal activity against the human pathogen Pseudallescheria boydii (amphotericin B, 0.31 μg/mL) as well as the plant pathogens Piricularia oryzae, Fusarium oxysporum, and Gibberella zeae (carbendazim, 5, 10, and 2.5 μg/ mL) with MIC values of 0.15, 1.25, 2.5, and 2.5 μg/mL, respectively (He et al., 2016). A culture of the endophytic fungus Fusarium sp. from the leaves of Ficus carica (Qinling Mountain, Shaanxi, China) afforded a new helvolic acid derivative 167, which displayed activity against some Gram positive and negative (G+/G−) bacteria and plant pathogenic fungi with MIC values of 3.13–25 μg/mL, more potent than streptomycin sulfate (MIC 7.8 μg/mL for bacteria) and penicillin and carbendazim (MIC 31.2 and 62.5 μg/mL for fungi, respectively) (Liang et al., 2016).
The wetland mud-derived fungus Aspergillus flavipes PJ03-11 (Red Beach National Nature Reserve, Liaoning, China) produced three novel butenolides (Zhang et al., 2016f) and three new phenalenone derivatives, flaviphenalenones A–C (Zhang et al., 2016e). Aspulvinones P (168), Q (169), and methybutyrolactone III (170), as well as flaviphenalenones B (171) and C (172) showed more potent α-glucosidase inhibitory activities than acarbose (0.685 mM) with IC50 values of 0.079, 0022, 0.016, 0.095, and 0.079 mM, respectively. The fruiting bodies of Ganoderma lucidum (Kingsci Biotechnology Co. Ltd., China) afforded 12 highly oxygenated lanostane triterpenoids, among which compounds 173-177 were nor-lanostanoids containing a 17β-pentatomic lactone ring. Compounds 173, 177 and verapamil could increase the adriamycin (ADM) accumulation in MCF-7/ADR cells ~3-fold at a concentration of 20 μM when compared with the negative control. Furthermore, compounds 174, 176, and 177 showed α-glucosidase inhibition with IC50 values of 81.8, 41.7, and 91.3 μM (acarbose, 669.7 μM) (Zhao et al., 2015c).
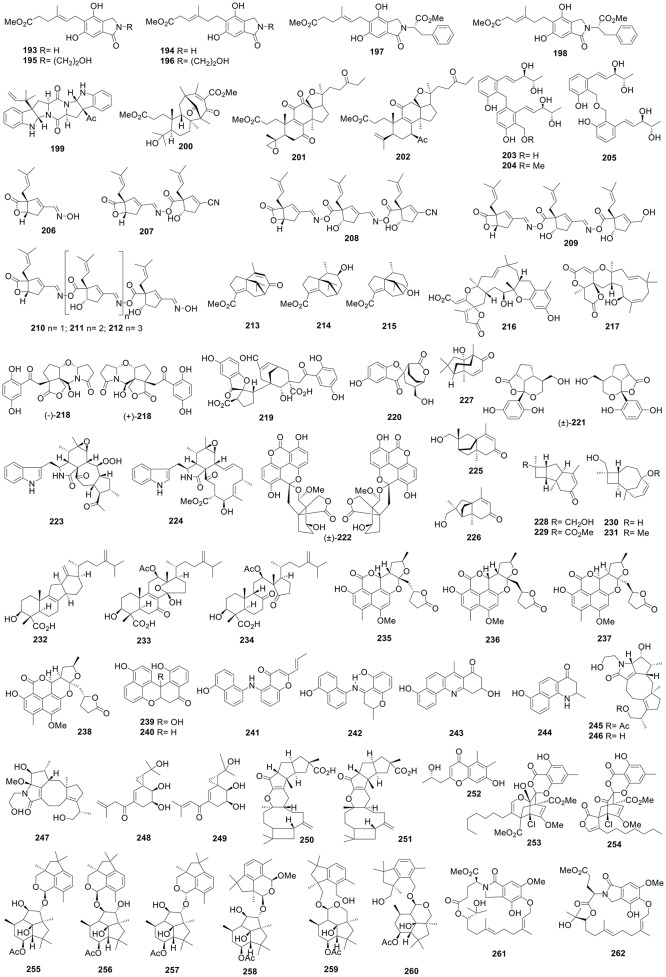
Meroterpenoids ganoleucins A (178) and C (179) were afforded by the fruiting bodies of Ganoderma leucocontextum (Nyingchi, Tibet, China) and exhibited stronger α-glucuronidase inhibition than acarbose with IC50 values of 6.3, 12.7, and 273 μM (Wang et al., 2016c). Sixteen new lanostane triterpenes, ganoleucoins A–P, were also produced by a Ganoderma leucocontextum. Ganoleucoins A (180), C (181), F (182), and J–N (183-187) showed stronger HMG-CoA reductase inhibition than atorvastatin (IC50, 32.1 μM) with IC50 values of 10.7–26.6 μM. Ganoleucoins M (186), N (187), and P (188) showed potent α-glucosidase inhibition with IC50 values of 13.6, 2.5, and 5.9 μM in contrast with acarbose (273.1 μM) (Wang et al., 2015g). Solid-state fermentation the mushroom Hericium erinaceus (Tibet, China) produced 10 new isoindolin-1-ones, erinacerins C–L (189-198) (Figure 4), most of which possessed α-glucosidase inhibition with IC50 values of 5.3–145.1 μM (acarbose, 382.7 μM) (Wang et al., 2015f). Aspergillus terreus CGMCC 3.05358 produced amauromine B (199), a novel diketopiperazine alkaloid which displayed more potent α-glucosidase inhibition than reference acarbose with IC50 values of 0.30 and 0.66 mM, respectively (Shan et al., 2015). Asperterpene B (200) possessing a noval 1,2,5-trimethyl-4,9-dioxobicyclo[3.3.1]non-2-ene-3-carboxylic acid moiety, was produced by soil-derived A. terreus (Yangzi River, Hubei, China), and was a growth inhibitor of BACE1 with an IC50 of 59 nM (positive control LY2811376, 260 nM) (Qi et al., 2016).
Figure 4.
Structures of compounds 193-262.
The fruiting bodies of Ganoderma boninense (Hainan, China) contained ganoboninones A–F, six new 3,4-seco-27-norlanostane triterpenes. Ganoboninones B (201) and F (202) showed in vitro antimalarial activities against Plasmodium falciparum with IC50 values of 15.68 and 2.03 μM, more potent than artemisinin (18.61 μM) (Ma et al., 2015c). Three novel sordariol dimmers 203–205 were afforded by plant fungus Sordaria macrospora from Ilex cornuta and showed antioxidation against 2,2-azino-bis-3-ethylbenzothiazoline-6-sulfonic acid (ABTS) radical with EC50 values of 12.2–14.5 μM (reference trolox, 26.7 μM) (Li et al., 2016h). Research into Boreostereum vibrans (Kunming Botanical Garden, China) led to the isolation of 16 new oximes and oxime esters. Vibralactoximes A (206), D (207), E (208), G (209), and I–K (210-212) displayed significant pancreatic lipase inhibitory activities with IC50 values of 11.1–28.6 μM (vibralactone, 48.7 μM) (Chen et al., 2016b). Microbial transformation of methyl cyperenoate by Cunninghamella elegans CGMCC 3.2028 led to the isolation of eight new derivatives, among which compounds 213-215 displayed in vitro antiplatelet aggregation activities with 93.01, 96.37, and 82.68% inhibition, respectively at 400 μg/mL, equivalent to aspirin (86.32%) (Tian et al., 2016b). Phomanolides A (216) and B (217), unique meroterpenoids with new pentacyclic and tetracyclic skeletons, respectively, were isolated from the solid cultures of the soil-derived Phoma sp. (Qinghai-Tibetan plateau, China) (Zhang et al., 2015i). The novel hybrid metabolite (±)-sinensilactam A ((±)-218) was isolated from the fruit bodies of Ganoderma sinensis, and possessed a unique 2H-pyrrolo[2,1-b][1,3]oxazin-6(7H)-one ring system (Luo et al., 2015b).
Culture of Ganoderma applanatum produced applanatumin A (219) with a new hexacyclic skeleton bearing a spiro[benzo furan-2,1′-cyclopentane] motif (Luo et al., 2015a). applanatumol A (220) with a new spiro[benzofuran-2,2′-bicyclo[3.2.2]nonane] ring system and (±)-applanatumol B ((±)-221) with an unusual dioxacyclopenta[cd]inden motif (Luo et al., 2016a), as well as (±)-ganoapplanin ((±)-222) with a dioxaspirocyclic skeleton constructed from a 6/6/6/6 tetracyclic system and a tricyclo[4.3.3.03′, 7′]dodecane motif (Li et al., 2016f). Armochaeglobines A (223) and B (224), two cytochalasan alkaloids, were produced by the insect-derived Chaetomium globosum TW1-1 associated with Armadillidium vulgare. Armochaeglobine A (223) possessed an unprecedented tetracyclic 5/6/7/5 system, while armochaeglobine B (224) possessed a rare 12-membered cyclic carbon scaffold (Chen et al., 2015a). Polycyclic compounds with unusual skeletons (225-231) were produced by the endophytic Aspergillus tubingensis KJ-9 through remodeling of the β-caryophyllene skeleton, among which compound 225 featured a novel 5/5/6 sesquiterpene skeleton (Tang et al., 2015). Ten new ergosteroids, gloeophyllins A–J, were isolated from the solid cultures of Gloeophyllum abietinum. Gloeophyllin A (232) had a rare C-nor-D-homosteroid skeleton, while gloeophyllin I (233) possessed an unprecedented ergostane skeleton with the 6/5 fused C/D rings replacing by a 10-oxabicyclo[4.3.1]decane moiety, and gloeophyllin C (234) represented the first example of ergosteroid that featured the cleavage of a C8–C14 bond (Han et al., 2015). Four new oxaphenalenone ketals, neonectrolides B–E (235-238), incorporating the furo[2,3-b]isochromeno [3,4,5-def] chromen-11(6aH)-one skeleton, were produced by the soil-derived Neonectria sp. (Qinghai-Tibetan plateau, China) (Ren et al., 2015b). Mutadalesols A–F (239-244), new naphthalene-based molecules, were mycosynthesized by using the ΔpksTL mutant strain of insect-derived Daldinia eschscholzii from Tenodora aridifolia (Tian et al., 2015b). Diterpenoid alkaloids, pericolactines A–C (245-247) with an unusual 5/5/8/5 tetracyclic system, were afforded by Periconia sp. (Changbai Mountain, Jilin, China) (Wu et al., 2015c). Pestalotriols A (248) and B (249), featuring an unprecedented spiro[2.5]octane skeleton, were afforded by Pestalotiopsis fici endophytic with C. sinensis (Zhejiang, China) (Liu et al., 2015c).
Heterodimeric sesquiterpenes, sterhirsutins C (250) and D (251), together with sesquiterpenoids, sterhirsutins E–L, were produced by Stereum hirsutum. Sterhirsutins C (250) and D (251) possessed an unusual 5/5/5/6/9/4 fused ring system (Qi et al., 2015). Four new chromones, chaetosemins B–E, along with chaetosemin A and S(+)-chaetoquadrin J, were isolated from a solid culture of Chaetomium seminudum (Shaanxi, China), among which chaetosemin D (252) possessed a new skeleton (Li et al., 2015b). Two new spiroketals with an unique [4,7]methanochromene and dispirotrione skeleton, chlorotheolides A (253) and B (254), were isolated from a solid culture of Pestalotiopsis theae N635 associated with C. sinensis (Zhejiang, China) (Liu et al., 2016f). Six new heterodimeric botryane ethers, hypocriols A–F (255-260), were produced by the insect-derived Hypocrea sp. EC1-35 (Septobasidium-infected Serrataspis sp.) (Ren et al., 2016). The endophytic Emericella nidulans HDN12-249 (Laizhou Bay, China) produced six isoindolones, emericellolides A–C (261-263) (Figure 5) and emeriphenolicins E–G (264-266) (Zhou et al., 2016a). Three dimeric spiciferones with an acyclobutane ring, lecanicillones A–C (267-269), were afforded by the entomopathogenic Lecanicillium sp. PR-M-3 (Wang et al., 2016h). Culture of the insect-associated Daldinia eschscholzii IFB-TL01 from Tenodera aridifolia led to the isolation of selesconol ((±)-270) that could induce the differentiation of rat bone marrow mesenchymal stem cells into neural cells (Zhang et al., 2016a).
Figure 5.
Structures of compounds 263-322.
Terrestrial plants
Terrestrial plants afforded 4,875 new NPs in the past 2 years and undoubtedly, are the most irreplaceable source of novel NPs in China. Compounds with unique skeletons and bioactivities are also mainly from terrestrial plants, although the numbers of both are lower than average. A total of 451 new NPs with novel skeletons or/and significant bioactivities are listed here.
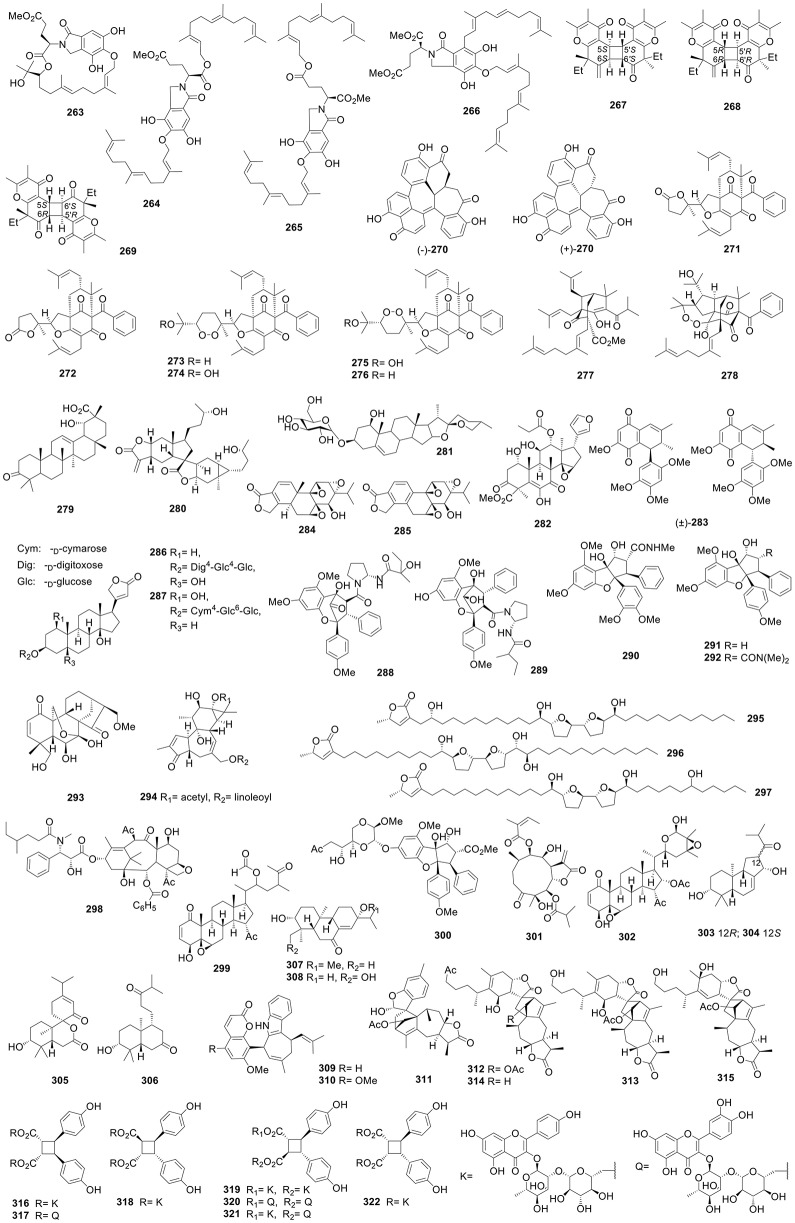
Hyperisampsins H–M (271-276) were new polycyclic polyprenylated acylphloroglucinols (PPAPs) obtained from the aerial parts of Hypericum sampsonii (Dabie Mountain, Hubei, China). Hyperisampsins H (271) and I (272) represented the first examples of PPAPs featuring an unusual γ-lactone ring at C-23, while hyperisampsins J–M (273-276) had an 1,2-dioxane ring. Hyperisampsin J (273) exhibited significant cytotoxic activity to HL-60, SMMC-7721, A-549, MCF-7, and SW480 cell lines with the IC50 range of 0.56–2.49 μM (reference DDP, 1.17–15.86 μM) (Zhu et al., 2015a). Another two PPAPs, hypersubones A (277) and B (278), were also obtained by culture of Hypericum subsessile. Hypersubone A (277) had a new seco-adamantane skeleton, while hypersubone B (278) had a tetracyclo-[6.3.1.1.0]-tridecane core fused in a peroxide ring. Hypersubone B (278) was cytotoxic to HepG2 and Eca109 cells with IC50 values of 1.58 and 0.07 μM (Liao et al., 2015b). A new triterpenoid (279) was isolated from Maytenus austroyunnanensis (Yunnan, China) and displayed inhibitory activity against HeLa cells with an IC50 of 1.48 μM (Tan et al., 2016). Isolation of whole plants of Carpesium abrotanoides gave a novel dimeric sesquiterpene with a cyclopentane linker, dicarabrol (280). Dicarabrol (280) was active against SGC-7901, U937, HeLa, DU145, and HL-60 cells with the IC50 range of 0.10–0.71 μM. Dicarabrol (280) was also antimycobacterial with an MIC of 3.7 μM, close to controlisoniazid (2.0 μM) (Wang et al., 2015d). Isolation of fresh rhizomes of Tupistra chinensis (Shennongjia, Hubei, China) afforded three new spirostanol saponins, tupistrosides G–I, and one new flavane-O-glucoside, tupichiside A. Tupistroside H (281) showed significant cytotoxicity against LoVo and BGC-823 cell lines with IC50 values of 0.267 and 0.327 μM, respectively, in contrast to that of 5.92 and 4.59 μM for cisplatin (Xiao et al., 2015). The leaves of Trichilia americana (Mengla, Yunnan, China) contained 10 novel cedrelone limonoids, among which compound 282 exhibited cytotoxic activity against MCF-7, SMMC-7721, HL-60, A-549, and SW480 cells with IC50 values ranging from 1.0 to 3.1 μM (Ji et al., 2015). A pair of novel quinone enantiomers, (±)-merrilliaquinone ((±)-283), was obtained from the branches and leaves of Illicium merrillianum (Gongshan, Yunnan, China). Compound (+)-283 selectively inhibited SMMC7721 and HuH7 cells with IC50 values of 0.91 and 1.29 μM in contrast with the CC50 of QSG7701 and L02 cells at 47.79 and 36.71 μM, respectively (Tian et al., 2015a).
The leaves of Tripterygium wilfordii (Taining, Fujian, China) contained six new abietane diterpenoids, tripterlides A–F. Tripterlides E (284) and F (285) displayed potent cytotoxicities against HCT-116, HepG2, BGC-823, and H460 cells with IC50 ranges of 0.93–3.16 and 0.17–0.90 μM, respectively (Wang et al., 2015a). Six new cardenolide glycosides were discovered from the roots of Streptocaulon juventas (Yunnan, China). Compounds 286 and 287 showed significant activities to A549 cells with IC50 values of 0.016 and 0.38 μM, respectively (Ye et al., 2015). The leaves of Aglaia odorata (Xishuangbanna, Yunnan, China) afforded nine novel flavaglines, aglaodoratins A–I. Aglaodoratin C (288) exhibited cytotoxicity against MG-63 and HT-29 cells with IC50 values of 1.2 and 0.097 μM, and aglaodoratin D (289) was cytotoxic against the MG-63 cells with an IC50 of 0.75 μM (An et al., 2015). The twigs of Aglaia odorata (Longzhou, Guangxi, China) afforded compound 290, which was active against SGC-7901, HeLa, and A-549 cells with IC50 values of 0.12, 0.32, and 0.25 μM, respectively (Peng et al., 2016b). The roots of Aglaia odorata contained rocaglaol (291) and rocaglamide (292), both were active against MCF-7, SMMC-7721, HL-60, A-549, and SW480 cells with IC50 ranges of 0.007–0.095 μM (Liu and Xu, 2016). Neolaxiflorins I–Y, 17 novel ent-kaurane diterpenoids, were obtained from the Isodon eriocalyx var. laxiflora leaves (Yunnan, China), among which neolaxiflorin P (293) displayed the best activity against A-549, HL-60, MCF-7, SMMC-7721, and SW-480 cells with IC50 ranges of 0.45–1.12 μM (Wang et al., 2015j).
Research into the seeds of Croton tiglium (Sichuan, China) afforded four new 4-deoxy-4β-phorbol diesters. Compound 294 was cytotoxic toward SNU387 cell line with an IC50 value of 0.71 μM (Zhang et al., 2016j). The seeds of Annona squamosa (Guangdong, China) afforded four new annonaceous acetogenins (ACGs), squamocins I–III (295-297), and squamoxinone D. Compound 297 were most active against H460 cell line with an IC50 value of 0.0492 μg/mL (Miao et al., 2016). Four new taxane derivatives were obtained from the whole plants of Taxus wallichiana. var. mairer (Jiangsu, China); compound 298 was cytotoxic against the MCF-7 cell line with an IC50 of 0.077 μM (Wang et al., 2016f). Six new withanolides and four new withanolide glucosides were obtained from Physalis pubescens (Liaoning, China). Compounds 299 and 300 were active against C4-2B, CWR22Rvl, 786-O, A-498, Caki-2, ACHN, A375, and L02 cell lines with IC50 values ranging from 0.17 to 1.22 μM (Xia et al., 2016). Aglapervirisin A (301) was isolated from the leaves of Aglaia perviridis (Xishuangbanna, Yunnan, China), and showed cytotoxicity against HepG2, HL-60, MCF-7, and HT-29 cell lines with IC50 values from 0.008 to 0.014 μM (An et al., 2016b). Whole plants of Carpesium cernuum (Guizhou, China) contained 10 novel highly oxygenated germacranolides, cernuumolides A–J, amomg which cernuumolide H (302) displayed the best cytotoxicity against HCT-116 cells with an IC50 value of 0.87 μM (Liu et al., 2016g).
Sessilifols A–N, 14 new ent-abietane-type diterpenoids, together with three related new norditerpenoids, were obtained from Chloranthus sessilifolius (Fengqi Mountains, Sichuan, China). Sessilifols A (303) and B (304) possessed a rearranged skeleton. Sessilifol C (305) was a rare 7,8-seco-9-spiro-fused ent-abietane, while sessilifol O (306) represented the naturally-occurring 14-norabietane diterpenoid. Sessilifols F (307) and I (308) showed anti-neuroinflammatory activities against the NO production in LPS-stimulated murine BV-2 microglial cells with IC50 values of 8.3 and 7.4 μM (N-monomethyl-L-arginine (L-NMMA), 14.4 μM) (Wang et al., 2015h). Two heterodimers of an isopentenyl indole and a coumarin unit by a new fused cycloheptene linker, exotines A (309) and B (310), were obtained Murraya exotica roots. Compounds 309 and 310 inhibited the NO production in LPS-induced BV-2 microglial cells with IC50 values of 9.2 and 39.9 μM (positive control quercetin, 17.4 μM) (Liu et al., 2015a). One unusual sesterterpenoid (311) and four new sesquiterpene dimers (312-315) were afforded by Inula britannica. Compounds 311-315 showed inhibition on the NO production induced by LPS in RAW 264.7 macrophages with IC50 values of 10.86–49.44 μM (aminoguanidine, 7.90 μM) (Zhang et al., 2015l). Biginkgosides A–I (316-324) (Figure 6), new flavonol glycoside dimers possing a cyclobutane moiety, were isolated from the leaves of Ginkgo biloba (Chongming Island, China). Biginkgosides E (320) and H (323) showed inhibitory avtivities on NO production induced by LPS in BV-2 microglial cells with IC50 values of 2.91 and 17.23 μM (L-NMMA, 14.40 μM) (Ma et al., 2016a).
Figure 6.
Structures of compounds 323-391.
(±)-Hendersine A ((±)-325), a pair of novel isoquinoline alkaloids coupled by an isoquinoline and a succinic acid derivative, along with a new isoquinoline hendersine B (326), were isolated from Corydalis hendersonii (Tibet, China). (±)-Hendersine A ((±)-325) and hendersine B (326) exhibited a protective effect against the LPS-stimulated H9c2 myocyte injuries with 30–40% protection at 10 μM (quercetin, 45%) (Yin et al., 2016). An aqueous extract of Isatis indigotica leaves (Hebei, China) contained isatidifoliumindolinones A (327) and B (328), two enantiomers with the novel 2-[1′-(4″-hydroxy-3″,5″-dimethoxyphenyl)ethyl]-2-methoxyindolin-3-one carbon skeleton, which showed stereochemical-dependent inhibition on the LPS-induced NO production in BV2 cells with 1.2 and 21.4% inhibition at 10 μM (curcumin, 41.2%) (Li et al., 2016c). Interestingly, an aqueous extract of Isatis indigotica roots contained insatindibisindolamides A (329) and B (330), a pair of indole alkaloid enantiomers with a new bisindolylacetamide skeleton, which displayed antiviral activities against the Coxsackie virus B3 (CVB3) with the same IC50 value of 33.3 μM and selection index (SI) value of 5.3 (ribavirin, 292.5/6.8) (Liu et al., 2015g).
Rhizomes of Curcuma phaeocaulis (Chengdou, Sichuan, China) contained three new guaiane-type sesquiterpenes, phaeocaulisins K–M, along with phagermadiol. Phaeocaulisins L (331) and M (332) inhibited NO production induced by LPS in RAW 264.7 macrophages with IC50 values of 54.27 and 6.05 μM, respectively (hydrocortisone, 58.66 μM) (Ma et al., 2015b). Research into the flowers of Hypericum monogynum (Jiangsu, China) led to the identification of 10 rare methylated polycyclic polyprenylated acylphloroglucinol derivatives, hypermongones A–J. Hypermongones A–C (333–335) and hypermongones E-H (336-339) were found to be active against NO production induced by LPS in macrophages with IC50 values from 9.5 to 27.3 μM, better than that of L-NMMA (39.2 μM) (Xu et al., 2015c). Twigs of Tricalysia fruticosa (Xishuangbanna, Yunnan, China) afforded eight new cafestol-type diterpenoids, tricalysins A–H, among which tricalysin H (340) inhibited NO production in LPS-activated RAW 264.7 macrophages with an IC50 value of 6.6 μM (L-NMMA, 40.5 μM). Further investigation indicated tricalysin H (340) inhibited the expression of iNOS and production of the pro-inflammatory cytokines IL-6 and TNF-α through activation of NF-κB and phosphorylation of MAPKs (ERK, JNK and p38) (Shen et al., 2015). The leaves and stems of Murraya tetramera (Wuming, Guangxi, China) gave two unusual trimeric carbazole alkaloids, murratrines A and B, as well as 11 new carbazole dimers, murradines A–K. Murradine B (341) showed better inhibition on LPS-stimulated NO production in BV-2 microglial cells activity than quercetin (17.4 μM) with an IC50 of 11.4 μM (Lv et al., 2015). Three unique cyclolanostane triterpenoids 342-344 and six new isopimarane diterpenoids were isolated from the leaves and twigs of Dysoxylum gotadhora (Hainan, China). Compound 342 represented a naturally occurring 21,24-epoxy cyclolanostane-type triterpenoids, while compounds 343 and 344 were the first examples of 21,25-epoxy cyclolanostanetype triterpenoids. Triterpenoid 342 as well as isopimarane diterpenoids 345 and 346 were active against NO production induced by LPS in RAW 264.7 cells with IC50 values of 25.5, 27.4, and 14.5 μM (indomethacin, 21.5 μM) (Jiang et al., 2015a). Isolation of the root bark of Aphanamixis grandifolia (Yunnan, China) afforded 14 new diterpene dimers, aphanamenes C–P. Aphanamenes G (347) and I (348) exhibited potent inhibitory activities on NO production in RAW 264.7 macrophages with IC50 values of 7.75 and 8.86 μM (L-NMMA, 40.45 μM) (Zhang et al., 2015e).
A phytochemical study into the trunks of Jatropha integerrima (Guangdong, China) led to the isolation of two pairs of novel sesquineolignan enantiomers, (±)-jatrointelignans A and B, as well as one pair of new neolignan enantiomers, (±)-jatrointelignan D, and two new neolignans, (+)-jatrointelignan C and (+)-schisphenlignan I. (–)-Jatrointelignan D ((–)-349) and (+)-schisphenlignan I ((+)-350) inhibited the LPS-induced NO production in BV-2 microglial cells with IC50 values of 8.9 and 5.9 μM (quercetin,17.0 μM) (Zhu et al., 2015b). Sixteen new withanolides, physangulatins A–N, together with withaphysalins Y and Z were obtained from the leaves and stems of Physalis angulata (Guangxi, China). Physangulatins 351-362 as well as withaphysalins Y (363) and Z (364) were moderate active against the NO production with IC50 values of 3.51–71.69 μM (hydrocortisone, 58.79 μM) (Sun et al., 2016a). Research into the bulbs of Fritillaria pallidiflora (Xinjiang, China) gave four new isosteroidal alkaloids, yibeinones A–D. Yibeinones C (365) and D (366) showed potent inhibition on the Ach-induced contraction of rat isolated tracheas with EC50 values of 0.65 and 3.00 μM, better than nifedipine (6.50 μM) (Li et al., 2016j). Four new spirostanol saponins (Xiang et al., 2016a) and 10 new furostanol saponins (Xiang et al., 2016b) were identified from the rhizomes of Tupistra chinensis (Shennongjia, Hubei, China). Spirostanol saponins 367-370 and furostanol saponins 371-373 displayed inhibition on the LPS-stimulated NO production in RAW 264.7 macrophage cells with IC50 values of 3.1–46.2 μM (indomethacin, 47.4 μM). The stem bark of Entandrophragma angolense (Brong Ahafo Region, Ghana) afforded 16 new structurally-diverse limonoids, entangolensins A–P, among which entangolensins E (374) and K (375) inhibited the NO production induced by LPS in RAW 264.7 macrophages with IC50 values of 1.75 and 7.94 μM (L-NMMA, 32.55 μM) (Zhang et al., 2016h).
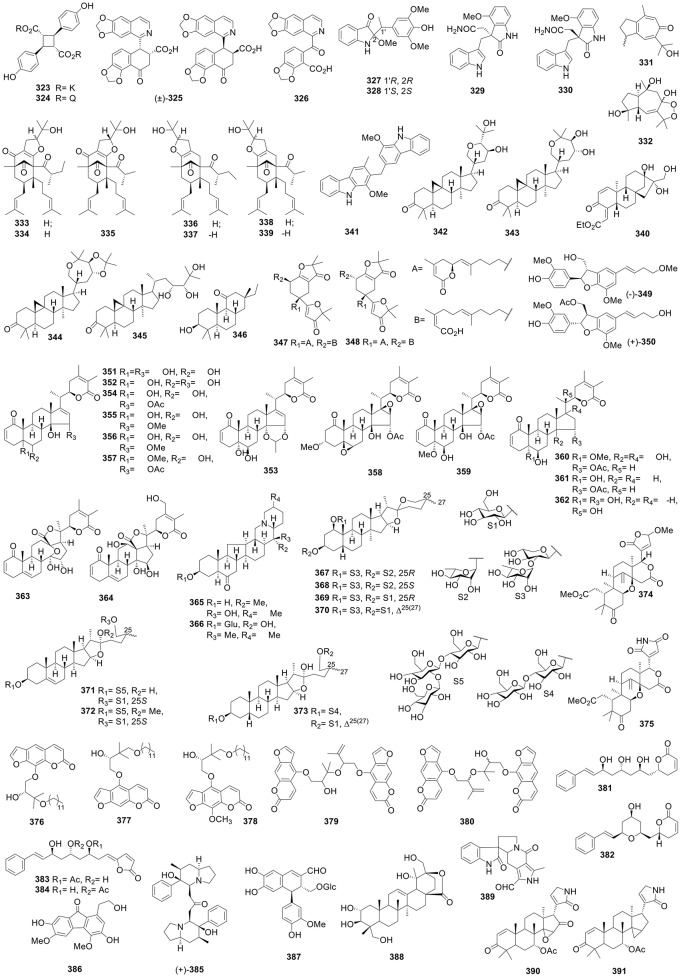
Two coumarins together with 10 coumarins with hydrophobic groups, andafocoumarins A–J, were afforded by Angelica dahurica cv. roots. (Hangbaizhi, Zhejiang, China). Andafocoumarins A–C (376–378) moderately inhibited NO production induced by LPS in mouse RAW 264.7 macrophage cells with IC50 values of 19.7, 13.9, and 25.9 μM, respectively (L-N6-(1-iminoethyl)-lysine, 23.7 μM) (Wei et al., 2016b). Three new dimeric furanocoumarins, dahuribiethrins H–J, were also isolated from roots of Angelica dahurica (Anhui, China). Dahuribiethrins H (379) and I (380) inhibited LPS-stimulated NO production in RAW 264.7 macrophage cells with IC50 values of 8.7 and 27.3 μM (indometacin, 38.6 μM) (Yang et al., 2017). Arylalkenyl α,β-unsaturated δ-lactone cryptoconcatones A–H, along with arylalkenyl α,β-unsaturated γ-lactone cryptoconcatones I and J, were isolated from the leaves and twigs of Cryptocarya concinna (Guangdong, China). Cryptoconcatones D (381), H (382), I (383), and J (384) moderately inhibited NO production in LPS-stimulated mouse RAW 264.7 macrophage cells with IC50 values of 3.2, 4.2, 3.4, and 7.5 μM respectively, better than L-NMMA (IC50 45 μM) (Yang et al., 2016a). A pair of racemic indolizidine enantiomers, (±)-homocrepidine A, and a piperidine derivative, homocrepidine B, were obtained from stems of Dendrobium crepidatum (Yunnan, China). (+)-Homocrepidine A ((+)-385) showed inhibition on the LPS-induced NO production in RAW 264.7 macrophages with an IC50 value of 3.6 μM (indomethacin, IC50 42.2 μM) (Hu et al., 2016c). 1-Ethoxy-3,7-dihydroxy-4,6-dimethoxy-9-fluorenone (386) was obtained from the roots of Litsea cubeba (Anhui, China), and showed a TNF-α inhibition with an IC50 value of 28.2 μM (Lin et al., 2016a). Vitexnegheteroins E–G, three new phenylnaphthalene-type lignans, and vitexnegheteroin H, a new polyoxygenated ursane-type triterpene, were identified from the seeds of Vitex negundo var. heterophylla (Huludao, Liaoning, China). Vitexnegheteroins E (387) and H (388) displayed activities against LPS-induced NO production with IC50 values of 17.27 and 17.23 μM (positive control SMT, 1.83 μM) (Hu et al., 2016b). The stems of Nauclea officinalis (Hainan, China) contained two novel indole alkaloids, namely nauclealises A and B. Nauclealise A (389) showed activity against the LPS-induced NO production in RAW 264.7 macrophages with an IC50 value of 0.82 μM, more active than aminoguanidine (1.80 μM) (Chen et al., 2016a). Investigation into the root bark of Toona sinensis (Sichuan, China) led to the isolation of 12 new azadirone-type and gedunin-type limonoids, toonasinemines A–K. Toonasinemines A (390), B (391), F (392), H (393), and I (394) (Figure 7) displayed inhibitory activity against the LPS-induced NO production in RAW 264.7 macrophages with IC50 values of 10.21–20.68 μM (L-NMMA, 32.55 μM) (Li et al., 2016e).
Figure 7.
Structures of compounds 392-447.
Three new pentacyclic triterpenes 395-397 were discovered from the acorns of Quercus serrata var. brevipetiolata (Dabie Mountains, Anhui, China) and showed activity against the LPS-induced NO production in RAW 264.7 macrophages with IC50 values of 8.2, 12.8, and 19.1 μM, respectively, more effective than indometacin (47.4 μM) (Huang et al., 2016). 13,14-Seco-withanolide minisecolides A–D were identified from the whole plants of Physalis minima (Anhui Province, China). Minisecolides B (398) and C (399) inhibited LPS-induced NO production in RAW 264.7 macrophages with IC50 values of 25.34 and 20.81 μM, respectively (L-NMMA, 30.11 μM) (Lin et al., 2016c). One new diphenol and two new isocoumarin carbonates were obtained from the aerial parts of Lawsonia inermis (Taiwan, China), among which compound 400 exhibited inhibition on the LPS-induced NO production in RAW 264.7 cells with an IC50 value of 5.63 μg/mL, more effective than indomethacin (IC50, 78.56 μg/mL) (Yang et al., 2016b). Two novel bischromones, fistulains A (401) and B (402), were isolated from the bark of Cassia fistula. Fistulain A (401) derived from a chromone and a tricyclic alkaloid through a unique C-14–N linkage, while istulain B (402) had an unusual C-14–C-5′ linkage. Fistulain A (401) displayed anti-TMV (tobacco mosaic virus) activity with an IC50 value of 43.8 μM (ningnamycin, 52.4 μM) (Zhou et al., 2015c).
Myrifamines A–C (403-405), three new myrioneuron alkaloids with unusual carbon skeletons, were obtained from Myrioneuron faberi (Sichuan, China). Myrifamine C (405) was the first example of symmetric dimers of the myrioneuron alkaloids (Cao et al., 2015b). Research into the whole plants of Munronia henryi (Wenshan, Yunnan, China) afforded 14 new limonoids, munronins A–N. Munronins B (406) and H–L (407-411) showed anti-TMV activities with IC50 values ranging from 19.6 to 44.4 μg/mL (ningnanmycin, 44.6μg/mL). Munronin A (412) exhibited cytotoxic effects on HL-60 and SW480 cells with IC50 values of 0.44 and 0.86 μM, respectively (Yan et al., 2015b). Seven novel daphnane diterpenoids, stelleralides D–J, were obtained from the roots of Stellera chamaejasme (Baotou, Inner Mongolia, China). Stelleralides F (413), G (414), and H (415) exhibited anti-HIV activities with EC50 values of 0.93, 0.73, and 0.98 nM and SI values of >10,000, much more potent than zidovudine (EC50 32 nM, SI ≥ 116) (Yan et al., 2015a). Seven filicinic acid-based meroterpenoids comprised 6/6/11, 6/6/7/5, or 6/6/10 ring systems were obtained from Hypericum japonicum. (+)-Hyperjaponols B ((+)-416) and D (417) exhibited anti-Epstein-Barr virus activities with EC50 values of 0.57 and 0.49 μM, respectively, better than ganciclovir (IC50, 2.86 μM) (Hu et al., 2016a).
Flavesines A–F (418-423), six unusual matrine-type alkaloid dimers, were obtained from the roots of Sophora flavescens (Shaanxi, China) and displayed inhibitoriy activities against hepatitis B virus with IC50 values of 17.16–86.60 μM (foscarnet, 105 μM) (Zhang et al., 2016l). The roots of the Illicium oligandrum (Guangxi, China) contained 10 novel prenylated C6-C3 compounds, namely illioliganpyranones B–G, illioliganones J–K, illioliganpyranol A, and illioliganfuranol A. Illioliganpyranones B (424), C (425), E (426), and F (427) as well as illioliganfuranol A (428) showed significant activities against CVB3 virus with IC50 and SI value ranges of 3.70–11.11 μM and 5.2–21.1, respectively (ribavirin, 2.12 mM/3.87). Illioliganpyranone D (429) exhibited potent activity against H3N2 influenza virus A with an IC50 value of 5.55 μM and SI value of 18.0 (oseltamivir, 4.39 μM/700) (Ma et al., 2016c). Whole plants of Spiraea japonica var. acuminata (Yunnan, China) contained five new diterpenes, among which spirimine B (430) and spiramilactone F (431) showed anti-TMV virus activities with protective/curative rates of 92.91%/41.30% and < 20%/69.40% at 100 μg/mL, respectively (ningnanmycin at 48.20%/50.72%) (Ma et al., 2016f). Research into whole plants of Lavandula angustifolia (Yunnan, China) afforded three novel phenylpropanoids 432-434 with 33.9–38.4% inhibition on the TMV infection at 20 μM (ningnanmycin, 33.6%) (Tang et al., 2016a).
Tabasesquiterpene B (435) (Shang et al., 2016), nicosesquiterpenes A (436) and B (437) (Shen et al., 2016) were obtained from leaves of Nicotiana tabacum (Yunnan, China), and showed anti-TMV activities with 35.2, 36.7, and 45.6% inhibition at 20 μM, respectively, better than ningnanmycin. Ananasin A (438), a flavonolignan, was discovered from Ananas comosus (Hainan, China), and was active against S. aureus and E. coli with the same MIC value of 0.156 μg/mL (ciprofloxacin, MIC 0.156 μg/mL) (Huang et al., 2015). Callistrilones A (439) and B (440) with an unprecedented [1]benzofuro-[2,3-a] xanthene or [1]benzofuro [3,2-b]xanthene pentacyclic ring system was obtained from the leaves of Callistemon rigidus (Guangdong, China). Callistrilone A (439) showed antibacterial activity against multiresistant S. aureus ATCC33591, S. aureus Mu50, and Enterococcus faecium 13-01 with IC50 values of 16–32 μg/mL, more potent than oxacillin (IC50, 256–512 μg/mL) (Cao et al., 2016b). Tetracyclic triterpenoids, ricinodols A–G, were isolated from the stems and leaves of Ricinodendron heudelotii (Hainan, China). Ricinodols A (441) and B (442) possessed a novel concurrent rearrangement of Me-19 (10→9) and Me-30 (14→8). Ricinodol E (443) inhibited both human and mouse 11β-hydroxysteroid dehydrogenase type 1 (11β-HSD1) with IC50 values of 0.36 and 0.84 μM, respectively (Yu et al., 2015).
Ten new phenylpropanoid glucosides, tadehaginosides A–J (444-453) (Figure 8), were obtained from Tadehagi triquetrum (Hainan, China). Tadehaginosides A (444) and B (445) had an unusual bicyclo[2.2.2]octene skeleton, while tadehaginosides C (446) and D (447) contained a unique cyclobutane basic core in their carbon scaffolds. Tadehaginosides C–J (446-453), particularly tadehaginoside D (447), significantly increased the basal and insulin-elicited glucose uptake, with an efficacy comparable to 100 nM of insulin (Zhang et al., 2016i). New depside derivatives were obtained from the flowers of Impatiens balsamina (Nanjing, Jiangsu, China), among which compound 454 showed α-glucosidase inhibition with an IC50 value of 0.72 μg/mL (acarbose, 3.36 μg/mL) (Li et al., 2015e). The root bark of Morus alba var. tatarica (Xinjiang, China) afforded four new flavonoids, mortatarins A–D, among which mortatarin D (455) showed potent α-glucosidase inhibition with an IC50 value of 5.0 μM (genistein, 17.8 μM) (Zhang et al., 2015m).
Figure 8.
Structures of compounds 448-512.
Hupehenols A–E, new 20,21,22,23,24,25,26,27-octanordam marane triterpenoids, were isolated from Viburnum latifolia (Caojian, Yunnan, China). Interestingly, all the triterpenoids selectively inhibited human 11β-HSD1 with IC50 values of 15.3–194.5 nM, while the IC50 range for 11β-HSD2 was 11.3–299.6 μM. The SI (the IC50 ratio of HSD2/HSD1) for hupehenols A–E ranged from 131.0 to 3,967.0, much higher than that of glycyrrhetinic acid (GA, 0.1), indicating these compounds were highly selective inhibitors of human 11β-HSD1. Hupehenol B (456) even showed better activity against human 11β-HSD1 with an IC50 of 15.3 nM than GA (29.1 nM) (Chen et al., 2015b). A new ursane triterpene acid (457), together with a new oleanane triterpene acid, was isolated from the whole plants of Spermacoce latifolia (South China Botanical Garden, Guangdong, China). Compound 457 showed α-glucosidase inhibition with an IC50 value of 0.42 mM, equivalent to acarbose (0.409 mM) (Luo et al., 2015c). Research into the seeds of Vaccaria hispanica (Anguo, Hebei, China) led to the discovery of new diantheramide (458) and segelin I (459), both which showed significant α-glucosidase inhibition with IC50 values of 0.08 and 0.28 mM, respectively (acarbose, 0.41 mM) (Zheng et al., 2015a). Roots of Phlomis tuberose (Shangdu Town, Inner Mongolia, China) afforded new diterpenoids 460-463 that exhibited better α-glucosidase inhibitory activities than acarbose (3.72 mM) with IC50 of 0.067–0.379 mM (Yang et al., 2015d). The twigs of Dysoxylum mollissimum (Ledong, Hainan, China) contained new limonoids, dysoxylumosins A–M. Dysoxylumosin F (464) displayed an human 11β-HSD1 with an IC50 value of 9.6 nM (glycyrrhetinic acid, 8.8 nM) (Zhou et al., 2015a).
Flavone derivatives, falandiosides A (465) and B (466), and glucuronide (467), were isolated from the fruits of strawberry (Guangdong, China). Compounds 465–467 showed modest antioxidation against ABTS radical with IC50 values of 5.74, 8.80, and 4.42 μM, respectively (L-ascorbic acid, 14.21 μM). Compounds 466 and 467 also showed α-glucosidase inhibition with IC50 values of 107.52 and 65.22 μM, respectively (acarbose, 619.94 μM) (Yang et al., 2016d). Two new phragmalin-type limonoids were obtained from the stems of Chukrasia tabularis A (Hainan, China). Compound 468 showed α-glucosidase inhibitory activity with an IC50 value of 0.96 mM (acarbose, 0.95 mM) (Peng et al., 2016a). Philippin C (469) was from the root bark of Flemingia philippinensis and showed potent inhibitory activity of protein tyrosine phosphatase 1B (PTP1B) with an IC50 value of 6.5 μM (ersolic acid, 15.5 μM) (Wang et al., 2016g). New conjugates of sesquiterpenoids and acylphloroglucinols were isolated from the leaves of Eucalyptus robusta (Guangxi, China), among which eucarobustols A (470) and B (471) were the first examples of conjugates with aristolane and acylphloroglucinol units. Eucarobustols A (470), C (472), and D (473) displayed PTP1B inhibition with IC50 values of 1.3, 1.8, and 1.6 μM (oleanolic acid, 2.3 μM), respectively (Yu et al., 2016c).
Roots of the rare chloranthaceae plant Chloranthus oldhamii (Jinggang Mountains, Jiangxi, China) contained chlorabietols A–C (474-476), three abietane-type diterpenoids linked with different alkenyl phloroglucinol units through forming a 2,3-dihydrofuran ring. Compounds 474-476 showed PTP1B inhibition with IC50 values of 12.6, 5.3, and 4.9 μM, respectively (oleanolic acid, 3.2 μM) (Xiong et al., 2015a). The stems of Artocarpus nanchuanensis (Jinfoshan Mountain, Chongqing, China) contained four new stilbene derivatives, hypargystilbenes B–E, among which hypargystilbenes B (477), D (478), and E (479) were inhibitory toward PTP1B with IC50 values of 3.23, 37.31, and 2.53 nM, respectively (oleanolic acid, 1.60 nM) (Zhang et al., 2015j). Portulacatone (480) (Yue et al., 2015), oleraceins K (481) and L (482) (Jiao et al., 2015b), isolated from Portulaca oleracea (Shandong, China) displayed dose-dependent DPPH radical scavenging activities with EC50 values of 14.36, 15.30, and 16.13 μM, better than vitamin C. Portulaca oleracea was also the source of the new alkaloids, oleracimine (483), oleracimine A (484), and oleracone A (485), as well as azulene compound, oleracone B (486) (Li et al., 2016b).
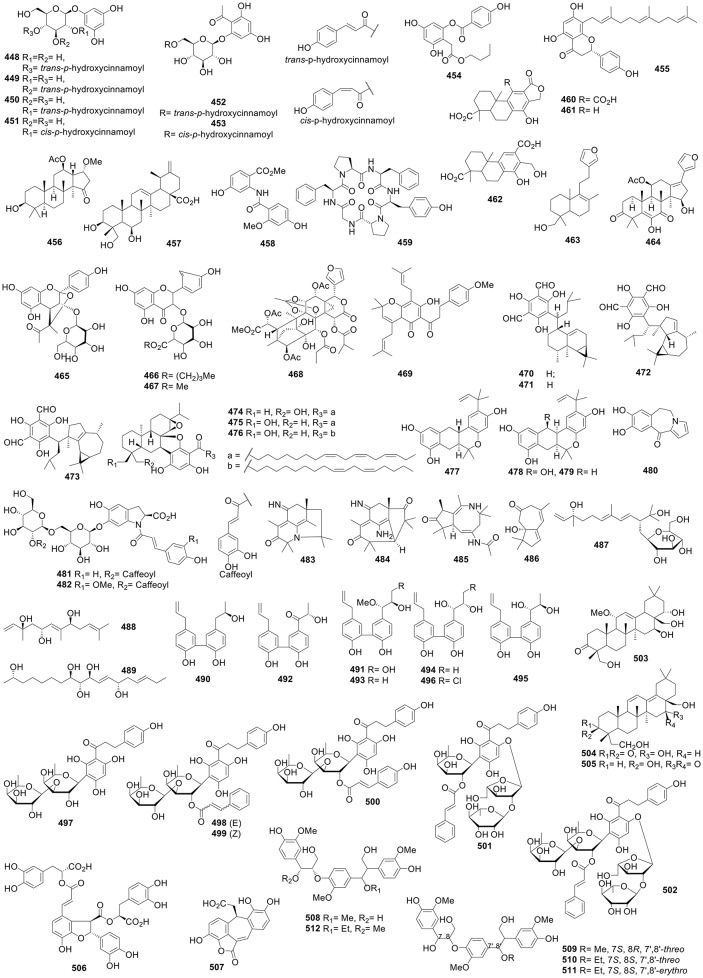
Murraya koenigii (Xishuangbanna, Yunnan, China) afforded four new alkenes, three of which (487-489) showed antioxidative activities against DPPH radical with IC50 values of 38.4, 23.5, and 25.4 μM, respectively (chlorogenic acid, 56.4 μM) (Ma et al., 2016b). Clypearianins A–G (490-496), seven new 3,3′-neolignans, were discovered from the twigs and leaves of Portulaca clypearia (Guangxi, China), and exhibited significant ABTS radical scavenging activity with IC50 values ranging from 4.3 to 14.9 μg/mL (trolox, 14.1 μg/mL) (Lou et al., 2016). Six new dihydrochalcone C-glycosides, carambolasides E–J (497–502), were obtained from the fruits of Averrhoa carambola (Guangdong, China) and displayed potent ABTS radical scavenging activity with IC50 values ranging from 2.54 to 4.52 μM, more effective than L-ascorbic acid (14.21 μM) (Yang et al., 2016c). Research into the roots of Bupleurum chinense afforded 17 triterpenoids, three of which oleanane triterpenes (503–505) exhibited neuroprotective effects against H2O2-induced SH-SY5Y cell death (Li et al., 2016d). Salvia miltiorrhiza afforded three new minor phenolic acids and six known compounds, and new compound 506 showed 84.3% scavenging rate of DPPH radical at 2 mM, better than vitamin C (74.9%) (Si et al., 2016). Two new norditerpenoids, miltiolactones A and B, and seven new neolignans, miltiolignanolides A–G, were obtained from the root of Salvia miltiorrhiza (Shandong, China). Miltiolignanolide C (507) displayed ABTS radical scavenging activity with an IC50 value of 3.73 μM (trolox, 5.10 μM) (Li et al., 2016g). Seven new neolignans were isolated from the seeds of hawthorn, among which compounds 508-512 acted as modest ABTS-radical scavengers with IC50 values ranging from 4.4 to 7.9 μM (trolox, 18.2 μM) (Peng et al., 2016c). (±)-Melicolone A ((±)-513) (Figure 9) and (±)-melicolone B ((±)-514), a pair of rearranged prenylated acetophenone epimers with an unprecedented 9-oxatricyclo-[3.2.1.13, 8]nonane core, were afforded by Melicope ptelefolia leaves (Southeast Asia) and displayed cell protecting activities against oxidative stress in human vein endothelial cells induced by high glucose, equivalent to resveratrol at 5 μM (Xu et al., 2014). Jatrocurcadiones A (515) and B (516) possessing a novel 10,11-seco-premyrsinane diterpenoid skeleton, were obtained from the twigs of Jatropha curcas. Jatrocurcadione A (515) showed inhibition of thioredoxin reductase (TrxR) with an IC50 of 10.0 μM (curcumin, 25.0 μM) (Bao et al., 2015).
Figure 9.
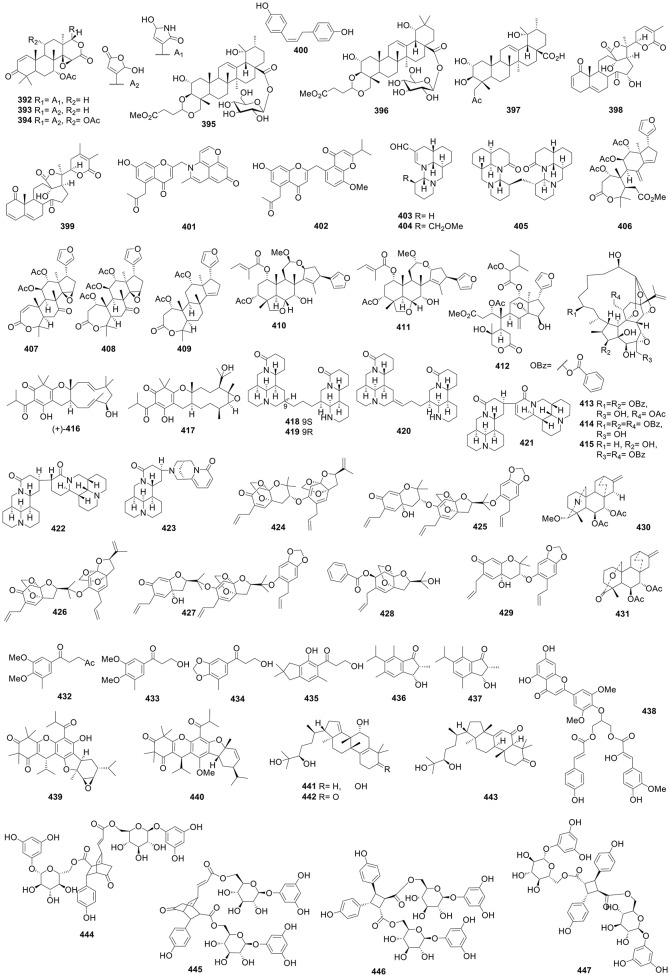
Structures of compounds 513-584.
Phainnanoids A–F (517-522) incorporating 4,5- and 5,5-spirocyclic unique motifs, were isolated from Phyllanthu hainanensis (Hainan, China), among which phainanoid F (522) showed the best immunosuppressive activities in vitro against the proliferation of T and B lymphocytes with IC50 values of 2.04 and 1.60 nM, respectively (CsA, IC50 14.21 and 352.87 nM) (Fan et al., 2015b). A new 20S- quassinoid with an unusual cagelike 2,4-dioxaadamantane nucleus and a migrated side chain, perforalactone A (523), together with perforalactones B (524) and C (525), were produced by twigs and stems of Harrisonia perforata. Compounds 523 and 524 showed inhibitory activites against nicotinic acetylcholine receptor (nAChR) with IC50 values of 15.8 and 1.26 nM, respectively (imidacloprid, 0.79 nM) (Fang et al., 2015).
The roots of Artocarpus heterophyllus (Nanning, Guangxi, China) contained two new flavanones, artoheterones A (526) and B (527), which inhibited the respiratory burst of rat PMNs with IC50 values of 1.67 and 0.19 μM, respectively (quercetin, 4.68 μM) (Ren et al., 2015a). Salvianolic acid Y (528) from the dried roots of Salvia officinalis protected PC12 cells from H2O2-induced injury with 54.2% protection rate at 10 μM (salvianolic acid B, 35.2%) (Gong et al., 2015). Caesalsappanins A–L, 12 new cassane-type diterpenes, were isolated from the seeds of Caesalpinia sappan (Nanning, Guangxi, China), among which caesalsappanins G (529) and H (530) exhibited antimalarial activities against the chloroquine-resistant P. falciparum K1 with IC50/SI values of 0.78/17.6 and 0.52/16.4 μM, respectively (Chloroquine, IC50/SI 0.37/129.5 μM) (Ma et al., 2015a). Leonuketal (531) possessing an unprecedented tetracyclic diterpenoid skeleton within a spiroketal moiety, was isolated from the aerial parts of Leonurus japonicas and displayed significant vasorelaxant activity against contraction of rat aorta induced by KCl with an EC50 value of 2.32 μM (methoxyverapamil, 0.58 μM) (Xiong et al., 2015b).
Eight new urushiol-type compounds were isolated from the resins of Toxicodendron vernicifluum (Bozhou, Anhui, China), among which compounds 532-537 inhibited AA-induced platelet aggregation with IC50 values of 3.09–11.83 μM (aspirin, 25.59 μM) (Xie et al., 2016a). Research into the seeds of Khaya senegalensis (Guangdonge, China) afforded 12 new limonoids, khasenegasins O–Z. Khasenegasin Z (538) exhibited protection for the injury induced by glutamate in primary rat cerebellar granule neuronal cells by increasing 83.3% and 80.3% viability at 10 μM and 1 μM, respectively (edaravone, 86.7% at 50 μM) (Tian et al., 2016c). Three new phenolic compounds were obtained from the roots of Alangium chinense (Guangxi, China). Compound 539 showed moderate inhibition against rat liver microsomal lipid peroxidation induced by Fe2+-cysteine with an IC50 value of 8.18 μM (vitamin E, 54.2 μM) (Zhang et al., 2016k). Compounds 540-543 were isolated from the aerial parts of Lespedeza cuneata (Henan, China) and active toward the transcription of XBP1 with EC50 values from 0.18 to 0.64 μM (Zhou et al., 2016b). Eleven new PPAPs, uraliones A–K, were afforded by whole plants of Hypericum uralum (Yunnan, China). Almost all compounds (544-553) were active against corticosterone-induced PC12 cell injury except uralione I (Zhou et al., 2016e). Tzumins A (554) and B (555), two novel lignan derivatives, were obtained from the bark of Sassafras tzumu (Guangxi, China), and displayed potent AChE inhibition with IC50 values of 2.00 and 1.81 μM, respectively (galanthamine, 2.99 μM) (Lu et al., 2017).
Stems of Schisandra pubescens (Jinfo mountain, Chongqing, China) contained a new triterpenoid 556 with hepatoprotective activity against D-GalN-induced cell injury in QSG7701 cells with 60.5% survival rates at 10 μM (silybin, 66.2%) (Wang et al., 2016a). The seeds of Celastrus monospermus (Guangdong, China) afforded 15 novel β-dihydroagarofuran-type sesquiterpenes, among which celaspermin E (557) showed lifespan extending effects of C. elegans with an extention rate of 37% at 50 μM, similar to the positive control rapamycin (38%) (Gao et al., 2016a). The aerial parts of Pteris cretica (Guizhou, China) afforded four new pterosin sesquiterpenoids and a new ent-kaurane diterpenoid. Compounds 558 and 559 showed more potent lipid-lowering activity than the positive control berberine in 3T3-L1 adipocyte (Luo et al., 2016b). Cimyunnins A–D (560-563) and cimyunnin D (563), characterized a fused cyclopentenone ring G and a rearranged γ-lactone ring F, respectively, were identified from the fruit of Cimicifuga yunnanensis (Daocheng, Sichuan, China). Cimyunnin A (560) displayed a similar anti-angiogenic activity as sunitinib both in vitro and ex vivo (Nian et al., 2015). Macrophypenes A–E, five new diterpenoids, were isolated from leaves of Callicarpa macrophylla (Guangxi, China). Macrophypene A (564) was a novel spiroditerpenoid, while macrophypene E (565) was a rare ent-abietane diterpenoid with a peroxide bridge (Xu et al., 2015a).
Eight new sesquiterpenes and two new lignans, were isolated from the fruits of Xanthium sibiricum (Helen City, Heilongjiang, China). Sibirolide A (566) was the first example of a 3/5/6/5 tetracyclic eudesmane sesquiterpene lactone formed at C-6 and C-7, and norxanthantolide B (567) was the first example of the naturally-occuring xanthane tetranorsesquiterpene, while norxanthantolides C–F (568-571) were the first xanthane trinorsesquiterpenes to date (Shi et al., 2015). Six novel Diels-Alder adducts of a polymethylated phloroglucinol derivative with myrcene, calliviminones C–H (572–577), were produced by fruits of Callistemon viminalis (Guangdong, China) (Wu et al., 2015f). A pair of coumarin enantiomers ((±)-578) with a rare polycyclic pyrano[3-2c] skeleton were isolated from the whole plants of Ainsliaea fragrans (Shiyany, Hubei, China) (Xue et al., 2015). Denticulatains A (579) and B (580), two novel heterodimers formed from a diterpene and a stilbene, were isolated from Macaranga denticulate (Yang et al., 2015b). Dicarabrones A (581) and B (582), a pair of epimers of two sesquiterpene lactone units linked by a cyclopentane ring, were isolated from the whole plants of Carpesium abrotanoides (Wu et al., 2015b).
Three new diterpene alkaloids, kaurines A–C (583-585) (Figure 10), were obtained from Isodon rubescens (Jianshi County, Hubei, China). Kaurines A (583) and B (584) had a unique 7,20-aza-ent- kaurane skeleton, while kaurine C (585) contained a rare succinimide moiety (Liu et al., 2015f). Monoterpenoid indole alkaloid ervatamines A–I, were isolated from Ervatamia hainanensis (Tunchang, Hainan, China). Ervatamine A (586) was a ring-C-contracted ibogan-type monoterpenoid indole alkaloid with an unusual 6/5/6/6/6 pentacyclic system. Ervatamines B–E (587-590) displayed a rare aza- 9/6 ring system (Zhang et al., 2015c). Flueggether A (591) and virosinine A (592) were obtained from a Chinese medicinal plant Flueggea virosa. Flueggether A (591) represented the first example of a securinega alkaloid oligomer with an ether bridge and virosinine A (592) had a new heterocyclic backbone (Zhang et al., 2015f). Forsythoneosides A–D (593-596), four unusual adducts of a flavonoid unit fused to a phenylethanoid glycoside through a pyran ring or carbon-carbon bond, together with four new phenylethanoid glycosides, were isolated from the fruits of Forsythia suspense (Yuncheng, Shanxi, China) (Zhang et al., 2015d). The first rotameric monoterpenoid indole alkaloids (MIAs) 597 and 598, along with two dimeric MIAs 599 and 600 linked by a azo- and an urea unit, respectively, were isolated from roots of Gelsemium elegans (Conghua, Guangdong, China) (Zhang et al., 2015k). Leaves and vine stems of Gelsemium elegans (Xishuangbanna, Yunnan, China) afforded nine new koumine-, humantenine-, and yohimbine- type alkaloids as well as 12 known analogs. Compound 601 was the first example of N-4-demethyl koumine type alkaloid, orhumantenine A (602) was the first norhumantenine alkaloid, and compounds 603 and 604 were the first N-1-oxide and seco-E-ring yohimbane type alkaloids, respectively (Xu et al., 2015d).
Figure 10.
Structures of compounds 585-657.
Stems of Glycosmis pentaphylla afforded glycosmisines A (605) and B (606), two unique alkaloids dimeric from a carbazole unit and a indole unit (Chen et al., 2015c). Three rearranged labdane-type diterpenoids, hapmnioides A–C (607–609), were obtained from Haplomitrium mnioides (Guizhou, China) (Zhou et al., 2016c), Two unprecedented labdane-type diterpenoids with a six-rings' system, haplomintrins A (610) and B (611), were also obtained from Haplomitrium mnioides (Zhou et al., 2015b). Nine new PPAPs, hyperattenins A–I, were obtained from the aerial parts of Hypericum attenuatum (Qichun, Hubei, China). Hyperattenin A (612) was characterized as a bicycle bicyclo[3.3.1]nonane derivative containing a rare hemiacetal functionality (Li et al., 2015a). A new rearranged 17-norpimarane, icacintrichantholide 613, was isolated from Icacina trichantha (Zhao et al., 2015a). Three dimeric cadinane sesquiterpenoids, involucratustones A–C, were produced by the rhizomes of Stahlianthus involucratus. Involucratustones A (614) and B (615) were two rearranged homodimers fused with a unique 1-oxaspiro[4.4]nonane core, and involucratustone C was a novel 3′,4′-seco-cadinane-dimer (Li et al., 2015d). Ten new triterpene acids, kadcoccinic acids A–J, were afforded by stems of Kadsura coccinea (Ziyuan, Guangxi, China). Kadcoccinic acids A (616) and B (617) were the first examples of 2,3-seco-6/6/5/6-fused tetracyclic triterpenoids (Liang et al., 2015).
The stems of Kadsura coccinea afforded kadcoccinin A (618), a sesquiterpenoid within a tricyclo[4.4.0.03, 10]decane scaffold (Hu et al., 2016d). Kadsura coccinea was also the source of six new lanostane-related triterpenoids, kadcoccinones A–F. Kadcoccinone C (619) possessed an unprecedented 6/6/9-fused carbocyclic core containing a rare oxabicyclo[4.3.1]decane system, while kadcoccinones D (620) and E (621) were two novel 18(13→12)-abeo-26-norlanostane triterpenoids (Hu et al., 2015). Neogenkwanines A–H (622-629), daphnane-type diterpenes with a 4,7- or 4,6-oxo bridge, were isolated from Daphne genkwa (Mianyang, Sichuan, China) (Li et al., 2015c). (±)-Subaveniumins A ((±)-630) and B ((±)-631), two pairs of racemic neolignans with a rare 2-oxaspiro[4.5] deca-6,9-dien-8-one motif, were isolated from the bark of Cinnamomum subavenium (Laifeng, Hubei, China) (Lai et al., 2015). Three new zwitterionic alkaloids, ningpoensines A–C (632-634), were obtained from the roots of Scrophularia ningpoensis (Zhang et al., 2015g). Two pairs of rearranged cis/trans neolignane isomers, penchinones A–D (635–638), were identified from Penthorum chinense (Guling, Sichuan, China), among which penchinones C (637) and D (638) featured an unprecedented 7,30-neolignane carbon skeleton (He et al., 2015). Eleven new PPAP-type derivatives, hyphenrones G–Q (639-649), were obtained from Hypericum henryi (Dongchuan, Yunnan, China) (Yang et al., 2015c).
Four new iridals with an α-terpineol moiety resulting from cyclization of the homofarnesylside chain, polycycloiridals A–D (650-653), were from the rhizomes of Iris tectorum (Zhang et al., 2015a). Diterpenoid randainins A–D (654-657) with a trans-fused 7/5 or 5/7 ring system were isolated from leaves and twigs of Callicarpa randaiensis (Nantou, Taiwan, China) (Cheng et al., 2015). Research into Abies chensiensis led to the discovery of triterpenoid spirochensilides A (658) (Figure 11) and B (659) possessing a 8,10-cyclo-9,10-seco skeleton (Zhao et al., 2015b). Two pairs of enantiomeric meroterpenoids, (±)-rhodonoids A ((±)-660) and B ((±)-661) with a unique 6/6/6/4 ring system, were isolated from Rhododendron capitatum (Liao et al., 2015a). Garcinia multiflora was the source of (±)-garcimulins A ((±)-662) and B (663) as well as (±)-garmultin A ((±)-664) and (–)-garmultin B ((–)-665). (±)-Garcimulins A ((±)-662) and B (663) included a tetracyclo[5.4.11, 5.1.09, 13]tridecane skeleton (Fan et al., 2015a), while (±)-garmultin A ((±)-664) and (–)-garmultin B ((–)-665) coupled a 2,11-dioxatricyclo[4.4.1.03, 9] undecane and a tricyclo [4.3.1.03, 7]decane (Tian et al., 2016a). Thirteen enmein-type ent-kaurane diterpenoids were obtained from aerial parts of Isodon phyllostachys (Sichuane, China), among which phyllostacins J (666) and K (667) were the first examples of 3,20:6,20-diepoxyenmein-type ent-kauranoids (Yang et al., 2016e). Alstoscholarisines H–J (668–670), three new monoterpenoid indole alkaloids with a new skeleton created by forming a C-3/N-1 bond, were obtained from the leaves Alstonia scholaris that was registered as an investigational new botanical drug (No. 2011L01436) and approved for clinical trials (phases I and II) by the China Food and Drug Administration (CFDA) (Pan et al., 2016b).
Figure 11.
Structures of compounds 658-730.
Annotinolides A–C (671-673), three novel 7,8-seco-lycopodanoid 8,5-lactones, were afforded by Lycopodium annotinum (Tang et al., 2016b). Aphanamixis grandifolia (Hainan, China) produced three novel diterpenoids dimers, aphadilactones E–G (674-676) (Zhang et al., 2016b). Artaboterpenoid A (677) and (±)-artaboterpenoid B ((±)-678), two new bisabolene-derived sesquiterpenoids, were obtained from the roots of Artabotrys hexapetalus (Xi et al., 2016). Six new compounds were identified from Salvia plebeian, among which compounds 679–682 were diterpenoids bearing an oxygen bridge while compound 683 was a copane-type sesquiterpenoid with a bridged tricyclic framework (Xu et al., 2016b). Oliganthic acids A–D (684-687), four prenylated isopropyl xanthone derivatives, were isolated from the leaves of Garcinia oligantha (Tang et al., 2016c). Cephalotanins A–D (688–691), four polycyclic norditerpenoids with highly rigid ring systems, were obtained from Cephalotaxus sinensis (Guangxi, China) (Xu et al., 2016a).
Three new ring B-seco limonoids, ciliatonoids A–C (692-694), were produced by Toona ciliate. Ciliatonoids A (692) and B (693) featured an unprecedented limonoid architecture (Liu et al., 2016a). Cipadessa cinerascens was the source of four limonoids with spirocyclic skeletons, cipacinoids A–D. Cipacinoids A–C (695-697) were the first examples of 17S- limonoids (Yu et al., 2016b). Phytochemical investigation of the stems of Picrasma quassioides (Jiangxi, China) led to the isolation of picraquassin A (698) featuring an unprecedented 21,24-cycloapotirucallane skeleton (Xu et al., 2016c). Delavatine A (699), a cyclopenta[de]isoquinoline alkaloid, was afforded by Incarvillea delavayi (Yunnan, China) (Zhang et al., 2016n). Dryopteris championii (Hainan, China) produced (±)-drychampones A–C ((±)-700, (±)-701, and (±)-702), three novel meroterpenoids constructed by a 11/6/6 ring system and a pyronone moiety (Chen et al., 2016d). Euphorikanin A (703), characterized by an unusual 5/6/7/3-fused tetracyclic ring skeleton, was obtained from the roots of Euphorbia kansui (Fei et al., 2016). Guajavadimer A (704), a dimeric meroterpenoid featuring two caryophyllenes, one benzylphlorogulcinol, as well as one flavonone-fused complicated stereochemical skeleton, was isolated from the leaves of Psidium guajava (Li et al., 2016a). Daphniphyllum himalense produced himalensines A (705) and B (706) with unprecedented carbon skeletons (Zhang et al., 2016c).
Schisandra lancifolia was the source of lancolide E (707) that possessed a complex tetracyclo [5.4.0.02, 4.03, 7]undecane-bridged system (Shi et al., 2016). Three new alkaloids with the heterohexacyclic skeleton, myritonines A–C (708-710), were afforded by Myrioneuron tonkinensis (Guangxi, China) (Li et al., 2016i). A new C16N2 lycopodium alkaloid, affordedphlefargesiine A (711), was isolated from Phlegmariurus fargesii (Meng et al., 2016b). Rhodomentones A (712) and B (713), two new meroterpenoids bearing a caryophyllene-conjugated oxa-spiro teradecadiene skeleton, were afforded by Rhodomyrtus tomentosa (Jiangxi, China) (Liu et al., 2016b). A schinortriterpenoid possessing a tricyclo[5.2.1.01, 6]decane-bridged system, schincalide A (714), was obtained from the stems and leaves of Schisandra incarnate (Hubei, China) (Zhou et al., 2016d). Fruits of Trichilia connaroides contained spirotrichilins A (715) and B (716), two novel limonoids with a spiro (cyclopenta[b]furan-2,1′-cyclopentane) system (An et al., 2016a). Another two novel limonoids, trichiconlides A (717) and B (718), were also isolated from fruits of T. connaroides (An et al., 2016c). The first naturally occurring imidazo[1,2-f]phenanthridine alkaloid, zephycandidine A (719), was produced by Zephyranthes candida (Hubei,China) (Zhan et al., 2016). A new cephalotaxus alkaloid, cephalolancine A (720), was isolated from Cephalotaxus lanceolata (Ni et al., 2016). Valeriridoid P (721), an unusual iridoid containing a two oxo-bridge fragment, was discovered from the roots of Valeriana officinalis var. latiofolia (Guizhou, China) (Wang et al., 2015c).
Terrestrial animals
A total of 90 new NPs was isolated from terrestrial insects and other animals. Of these, 37.8% compounds exhibited various bioactivities, much higher than the average of 28.4%. Nine NPs reported in two references with significant bioactivities are listed in this section.
The eggs of toad Bufo bufo gargarizans afforded two novel 19-norbufadienolides, a new 14,15-epoxy bufadienolide as well as eight rare bufadienolide-fatty acid conjugates, among which compounds 722-729 displayed cytotoxic activities against MCF-7 and MDA-MB-231 cells with IC50 ranges of 0.027–0.315 and 0.05–1.96 μM, respectively (Zhang et al., 2016g). Twelve new sesquiterpenoid dimmers, fortunilides A–L, were isolated from the malaria parasite Plasmodium falciparum (Guangxi, China). Fortunilide A (730) exhibited P. falciparum growth inhibition with an IC50 of 5.2 nM (artemisinin, 4.0 nM) (Zhou et al., 2017).
Conclusion
This review covers the NPs literatures from 2015 to 2016 by chemists from China and describes 6,944 new NPs reported from 1,985 papers. The average number of new compounds per paper is 3.5. Compared with 2015, the number of new NPs decreased by 22% in 2016 from 3,891 to 3,053. Elucidation of new compounds and assessment of their bioactivities along with methods for their syntheses, corrections of stereochemistry, and mechanistic as well as biosynthetic studies were two major areas of NPs research. However, the discovery of new compounds was undoubtedly the basis of all studies. In recent years, based on the large numbers of identified compounds, chemists increasingly began to focus on the other aspects described above, resulting in the decline of the identification of new compounds in 2016.
According to the survey of the literature from 2015 to 2016 (Supplementary Table 1), 730 new NPs out of 6,944 identified compounds possessed significant bioactivity and/or a novel skeleton. Their detailed information is compiled in this review.
According to our review, terrestrial plants (number of publications/compounds, 1,348/4,895), terrestrial fungi (269/910), and marine fungi (167/535) were the main sources of new NPs at 68%,14%, and 8% in terms of total papers referenced (Figure 12A) and 70%, 13%, 8% in terms of total compounds (Figure 12B), respectively.
Figure 12.
Biological sources of publications (A) and new NPs (B) in China (2015–2016).
A total of 1,765 papers were published in over 110 international journals in the past two years. Figure 13 shows the total number of NP articles from China sorted by journal. Natural Product Research (publications/percentage in total publications of China, 221/12%) was the first choice for chemists from China to publish their newly discovered NPs followed by Journal of Natural Products (164/9%), Journal of Asian Natural Products Research (122/7%), and Fitoterapia (109/6%). Notably, more than a quarter of articles (457 articles) were published in 38 journals with impact factors ≥3.0. The Journal of Natural Products, as a recognized journal in the field of NPs, undoubtedly attracted the most contributions (164 articles) and was followed by RSC Advances (66 articles), Organic Letters (62 articles), and Marine Drugs (22 articles); among all articles related to new NPs published in these journals, 34, 47, 50, and 35% were contributed by Chinese NPs chemists, respectively (Figure 14).
Figure 13.
Numbers of NPs articles from China published in each journal (2015–2016).
Figure 14.
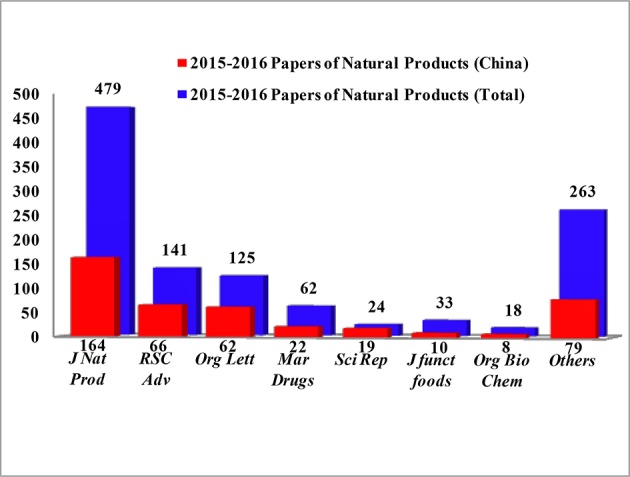
Numbers of new NPs papers with an impact factor of greater than 3.0 (2015–2016).
These results indicate that chemists from China play an irreplaceable role in NPs research and have made great contributions to the discovery of new compounds. It is also worth noting that there were only a few articles relevant to new NPs published in top chemistry journals with significant worldwide influence, such as Angewandte Chemie International Edition (China/total, 2/27), Chemical Science (3/4), and Journal of the American Chemical Society (2/5) (Figure 13). This review of the current progress of NPs chemistry shows that a multidisciplinary approach is henceforth an inevitable trend. Strengthening interdisciplinary cooperation is essential and biosynthesis, bioinformatics, and pharmacology as well as computer-aided technologies should be integrated to advance the discovery of more lead compounds with the potential to be developed into drugs. Currently, chemists from China face the challenging task of value mining of identified NPs rather than simply elucidating the structures of novel compounds. Molecules with unprecedented skeletons always attract attention and interest in the NPs research field. In 2015–2016, 134 papers (7% in total) were published and reported 352 NPs (5% in total) with novel skeletons. The dominant biological sources of new skeletal NPs were terrestrial plants (papers/percentage in 134 papers, 79/58%), terrestrial fungi (26/19%), and marine fungi (13/10%) (Figure 15A), from which 213 (percentage in all novel skeletons, 61%), 75 (21%), and 34 (10%) new skeletal NPs were isolated, respectively (Figure 15C). However, the percent occurrence of novel skeletons out of each biological source was slightly different. Marine actinomyctes possessed the highest occurrence of novel skeletons in 21% of papers (Figure 15B) and 19% of NPs (Figure 15D) followed by terrestrial fungi (10%, 8%), terrestrial bacteria (8%, 3%), and marine fungi (8%, 6%). This indicated that the microorganism, especially marine actinomyctes, was the major biological source of new skeletal compounds in China with great potential for research, and deserved to be extensively studied.
Figure 15.
Occurrence of papers describing novel skeletal NPs (A); papers related to novel skeletons out of the same biological source (B); biological sources of novel skeletal NPs (C); occurrence of novel skeletal compounds out of the same biological source (D).
The results showed that 28% of new NPs displayed a variety of bioactivities. The dominant bioactivities were cytotoxicity, anti-inflammatory activity, and antiviral activity with 7%, 5%, and 3% ratios in all NPs (Figure 16A) and 25%, 18%, and 11% ratios in all bioactive NPs (Figure 16B), respectively. Biological sources were also taken into consideration when compiling the data. A total of 68% of the bioactive compounds were isolated from terrestrial plants followed by terrestrial and marine fungi with 12% and 9% ratios, respectively (Figure 16C). The distribution between biological sources and bioactivities was also analyzed (Figure 16D). Cytotoxicity and anti-inflammatory activity were the major bioactivities of NPs isolated from marine animals and terrestrial plants, whereas cytotoxic and antibacterial activities were dominant for those from marine fungi. This analysis may serve as a guideline for purposeful investigation of bioactive NPs.
Figure 16.
The percent of each type bioactive NPs out of all NPs (A) and all bioactive NPs (B); biological sources of bioactive NPs (C) and distribution between biological sources and bioactivities (D).
As reviewed in this article, China had made enormous strides in NPs chemistry development and contributed substantial numbers of promising bioactive molecules to drug research in the past two years. We believe that there will continue to be a steady development of NPs research in China, and large numbers of more extensive studies as well as innovative approaches will be available in the near future.
Author contributions
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (Nos. 81561148012, U1501221, and 21172204). We thank Renee Mosi, PhD, from Liwen Bianji, Edanz Group China (www.liwenbianji.cn/ac), for editing the English text of a draft of this manuscript.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fchem.2018.00045/full#supplementary-material
References
- An F., Luo J., Li R. J., Luo J., Wang X. B., Yang M. H., et al. (2016a). Spirotrichilins A and B: two rearranged spirocyclic limonoids from Trichilia connaroides. Org. Lett. 18, 1924–1927. 10.1021/acs.orglett.6b00738 [DOI] [PubMed] [Google Scholar]
- An F. L., Luo J., Wang X. B., Yang M. H., Kong L. Y. (2016c). Trichiconlides A and B two novel limonoids from the fruits of Trichilia connaroides. Org. Biomol. Chem. 14, 1231–1235. 10.1039/C5OB02300A [DOI] [PubMed] [Google Scholar]
- An F., Wang J., Wang H., Wang X., Yang M., Guo Q., et al. (2015). Cytotoxic flavonol-diamide [3+2] adducts from the leaves of Aglaia odorata. Tetrahedron 71, 2450–2457. 10.1016/j.tet.2015.02.028 [DOI] [Google Scholar]
- An F. L., Wang X. B., Wang H., Li Z. R., Yang M. H., Luo J., et al. (2016b). Cytotoxic rocaglate derivatives from leaves of Aglaia perviridis. Sci. Rep. 6, 20045–20056. 10.1038/srep20045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao J., Su Z., Lou L., Zhu J., Tang G., Gan L., et al. (2015). Jatrocurcadiones A and B: two novel diterpenoids with an unusual 10,11-seco-premyrsinane skeleton from Jatropha curcas. RSC Adv. 5, 62921–62925. 10.1039/C5RA11380F [DOI] [Google Scholar]
- Blunt J. W., Copp B. R., Keyzers R. A., Munro M. H., Prinsep M. R. (2016). Marine natural products. Nat. Prod. Rep. 33, 382–431. 10.1039/c5np00156k [DOI] [PubMed] [Google Scholar]
- Cao F., Wu Z. H., Shao C. L., Pang S., Liang X. Y., de Voogd N. J., et al. (2015a). Cytotoxic scalarane sesterterpenoids from the South China Sea sponge Carteriospongia foliascens. Org. Biomol. Chem. 13, 4016–4024. 10.1039/C4OB02532F [DOI] [PubMed] [Google Scholar]
- Cao F., Yang J. K., Liu Y. F., Zhu H. J., Wang C. Y. (2016a). Pleosporalone A, the first azaphilone characterized with aromatic A-ring from a marine-derived Pleosporales sp. fungus. Nat. Prod. Res. 30, 2448–2452. 10.1080/14786419.2016.1198352 [DOI] [PubMed] [Google Scholar]
- Cao J. Q., Huang X. J., Li Y. T., Wang Y., Wang L., Jiang R. W., et al. (2016b). Callistrilones A and B, triketone-phloroglucinol-monoterpene hybrids with a new skeleton from Callistemon rigidus. Org. Lett. 18, 120–123. 10.1021/acs.orglett.5b03360 [DOI] [PubMed] [Google Scholar]
- Cao M. M., Zhang Y., Huang S. D., Di Y. T., Peng Z. G., Jiang J. D., et al. (2015b). Alkaloids with different carbon units from Myrioneuron faberi. J. Nat. Prod. 78, 2609–2616. 10.1021/acs.jnatprod.5b00543 [DOI] [PubMed] [Google Scholar]
- Chang Y., Yuan C., Zhang J., Liu S., Cao P., Hua H., et al. (2016). Speramides A–B, two new prenylated indole alkaloids from the freshwater-derived fungus Aspergillus ochraceus KM007. Tetrahedron Lett. 57, 4952–4955. 10.1016/j.tetlet.2016.09.071 [DOI] [Google Scholar]
- Che Q., Li T., Liu X., Yao T., Li J., Gu Q., et al. (2015). Genome scanning inspired isolation of reedsmycins A–F, polyene-polyol macrolides from Streptomyces sp. CHQ-64. RSC Adv. 5, 22777–22782. 10.1039/c4ra15415k [DOI] [Google Scholar]
- Che Q., Tan H., Han X., Zhang X., Gu Q., Zhu T., et al. (2016). Naquihexcin A, a S-bridged pyranonaphthoquinone dimer bearing an unsaturated hexuronic acid moiety from a sponge-derived Streptomyces sp. HDN-10-293. Org. Lett. 18, 3358–3361. 10.1021/acs.orglett.6b01485 [DOI] [PubMed] [Google Scholar]
- Chen C., Zhu H., Li X. N., Yang J., Wang J., Li G., et al. (2015a). Armochaeglobines A and B, two new indole-based alkaloids from the arthropod-derived fungus Chaetomium globosum. Org. Lett. 17, 644–647. 10.1021/ol503666b [DOI] [PubMed] [Google Scholar]
- Chen D., Ma G., He M., Liu Y., Wang X., Yang X. (2016a). Anti-inflammatory activity on two new indole alkaloids from the stems of Nauclea officinalis. Helv. Chim. Acta. 99, 742–746. 10.1002/hlca.201600159 [DOI] [Google Scholar]
- Chen H. P., Zhao Z. Z., Li Z. H., Dong Z. J., Wei K., Bai X., et al. (2016b). Novel natural oximes and oxime esters with a vibralactone backbone from the Basidiomycete Boreostereum vibrans. Chemistryopen 5, 142–149. 10.1002/open.201500198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Sun J., Deering R. W., DaSilva N., Seeram N. P., Wang H., et al. (2016c). Rhizoleucinoside, a rhamnolipid-amino alcohol hybrid from the rhizobial symbiont Bradyrhizobium sp. BTAi1. Org. Lett. 18, 1490–1493. 10.1021/acs.orglett.6b00461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen N. H., Zhang Y. B., Huang X. J., Jiang L., Jiang S. Q., Li G. Q., et al. (2016d). Drychampones A–C: three meroterpenoids from Dryopteris championii. J. Org. Chem. 81, 9443–9448. 10.1021/acs.joc.6b01720 [DOI] [PubMed] [Google Scholar]
- Chen S., Liu Y., Liu Z., Cai R., Lu Y., Huang X., et al. (2016e). Isocoumarins and benzofurans from the mangrove endophytic fungus Talaromyces amestolkiae possess a-glucosidase inhibitory and antibacterial activities. RSC Adv. 6, 26412–26420. 10.1039/C6RA02566H [DOI] [Google Scholar]
- Chen S., Wang J., Lin X., Zhao B., Wei X., Li G., et al. (2016f). Chrysamides A–C, three dimeric nitrophenyltrans-epoxyamides produced by the deep-sea-derived fungus penicillium chrysogenum SCSIO41001. Org. Lett. 18, 3650–3653. 10.1021/acs.orglett.6b01699 [DOI] [PubMed] [Google Scholar]
- Chen X. Q., Shao L., Pal M., Shen Y., Cheng X., Xu G., et al. (2015b). Hupehenols A–E, selective 11β-hydroxysteroid dehydrogenase type 1 (11β-HSD1) inhibitors from Viburnum hupehense. J. Nat. Prod. 78, 330–334. 10.1021/np500896n [DOI] [PubMed] [Google Scholar]
- Chen Y., Tang C., Wu Y., Mo S., Wang S., Yang G., et al. (2015c). Glycosmisines A and B: isolation of two new carbazole-indole–type dimeric alkaloids from Glycosmis pentaphylla and an evaluation of their antiproliferative activities. Org. Biomol. Chem. 13, 6773–6781. 10.1002/chin.201544218 [DOI] [PubMed] [Google Scholar]
- Chen Z., Hao J., Wang L., Wang Y., Kong F., Zhu W. (2016g). New α-glucosidase inhibitors from marine alga-derived Streptomyces sp. OUCMDZ-3434. Sci. Rep. 6, 20004–20012. 10.1038/srep20004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H. H., Cheng Y. B., Hwang T. L., Kuo Y. H., Chen C. H., Shen Y. C. (2015). Randainins A–D, based on unique diterpenoid architectures, from Callicarpa randaiensis. J. Nat. Prod. 78, 1823–1828. 10.1021/acs.jnatprod.5b00012 [DOI] [PubMed] [Google Scholar]
- Cheng Z., Zhao J., Liu D., Proksch P., Zhao Z., Lin W. (2016). Eremophilane-type sesquiterpenoids from an Acremonium sp. fungus isolated from deep-sea sediments. J. Nat. Prod. 79, 1035–1047. 10.1021/acs.jnatprod.5b01103 [DOI] [PubMed] [Google Scholar]
- Colegate S. M., Molyneux R. J. (2007). Bioactive Natural Products: Detection, Isolation, and Structural Determination. Boca Raton, FL: CRC Press. [Google Scholar]
- Fan Y. M., Yi P., Li Y., Yan C., Huang T., Gu W., et al. (2015a). Two unusual polycyclic polyprenylated acylphloroglucinols, Iincluding a pair of enantiomers from Garcinia multiflora. Org. Lett. 17, 2066–2069. 10.1021/acs.orglett.5b00588 [DOI] [PubMed] [Google Scholar]
- Fan Y. Y., Zhang H., Zhou Y., Liu H., Tang W., Zhou B., et al. (2015b). Phainanoids A–F, a new class of potent immunosuppressive triterpenoids with an unprecedented carbon skeleton from Phyllanthus hainanensis. J. Am. Chem. Soc. 137, 138–141. 10.1021/ja511813g [DOI] [PubMed] [Google Scholar]
- Fang X., Di Y. T., Zhang Y., Xu Z. P., Lu Y., Chen Q. Q., et al. (2015). Unprecedented quassinoids with promising biological activity from Harrisonia perforata. Angew. Chem. 127, 5684–5687. 10.1002/ange.201412126 [DOI] [PubMed] [Google Scholar]
- Fei D. Q., Dong L. L., Qi F. M., Fan G. X., Li H. H., Li Z. Y., et al. (2016). Euphorikanin A, a diterpenoid lactone with a fused 5/6/7/3 ring system from Euphorbia kansui. Org. Lett. 18, 2844–2847. 10.1021/acs.orglett.6b01093 [DOI] [PubMed] [Google Scholar]
- Gao L., Zhang R., Lan J., Ning R., Wu D., Chen D., et al. (2016a). β-Dihydroagarofuran-type sesquiterpenes from the seeds of Celastrus monospermus and their lifespan extending effects on the Nematode Caenorhabditis elegans. J. Nat. Prod. 79, 3039–3046. 10.1021/acs.jnatprod.6b00648 [DOI] [PubMed] [Google Scholar]
- Gao S. S., Li X. M., Williams K., Proksch P., Ji N. Y., Wang B. G. (2016b). Rhizovarins A–F, indole-diterpenes from the mangrove-derived endophytic fungus Mucor irregularis QEN-189. J. Nat. Prod. 79, 2066–2074. 10.1021/acs.jnatprod.6b00403 [DOI] [PubMed] [Google Scholar]
- Gong J., Ju A., Zhou D., Li D., Zhou W., Geng W., et al. (2015). Salvianolic acid Y: a new protector of PC12 cells against hydrogen peroxide-induced injury from Salvia officinalis. Molecules 20, 683–692. 10.3390/molecules20010683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo F., Li Z., Xu X., Wang K., Shao M., Zhao F., et al. (2016). Butenolide derivatives from the plant endophytic fungus Aspergillus terreus. Fitoterapia 113, 44–50. 10.1016/j.fitote.2016.06.014 [DOI] [PubMed] [Google Scholar]
- Guo W., Zhang Z., Zhu T., Gu Q., Li D. (2015a). Penicyclones A–E, antibacterial polyketides from the deep-sea-derived fungus Penicillium sp. F23-2. J. Nat. Prod. 78, 2699–2703. 10.1021/acs.jnatprod.5b00655 [DOI] [PubMed] [Google Scholar]
- Guo X., Liu N., Li X., Ding Y., Shang F., Gao Y., et al. (2015b). Red soils harbor diverse culturable actinomycetes that are promising sources of novel secondary metabolites. Appl. Environ. Microbiol. 81, 3086–3103. 10.1128/AEM.03859-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J. J., Bao L. L., Tao Q. Q., Yao Y. J., Liu X. Z., Yin W. B., et al. (2015). Gloeophyllins A–J, cytotoxic ergosteroids with various skeletons from a Chinese Tibet fungus Gloeophyllum abietinum. Org. Lett. 17, 2538–2541. 10.1021/acs.orglett.5b01080 [DOI] [PubMed] [Google Scholar]
- Han J., Li L., Zhong J., Tohtaton Z., Ren Q., Han L., et al. (2016a). Officimalonic acids A–H, lanostane triterpenes from the fruiting bodies of Fomes officinalis. Phytochemistry 130, 193–200. 10.1016/j.phytochem.2016.05.004 [DOI] [PubMed] [Google Scholar]
- Han W. B., Zhang A. H., Deng X. Z., Lei X., Tan R. X. (2016b). Curindolizine, an anti-inflammatory agent assembled via Michael addition of pyrrole alkaloids inside fungal cells. Org. Lett. 18, 1816–1819. 10.1021/acs.orglett.6b00549 [DOI] [PubMed] [Google Scholar]
- Han Y., Tian E., Xu D., Ma M., Deng Z., Hong K. (2016c). Halichoblelide D, a new elaiophylin derivative with potent cytotoxic activity from mangrove-derived Streptomyces sp. 21980. Molecules 21, 970–976. 10.3390/molecules21080970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X., Du X., Zang X., Dong L., Gu Z., Cao L., et al. (2016). Extraction, identification and antimicrobial activity of a new furanone, grifolaone A, from Grifola frondosa. Nat. Prod. Res. 30, 941–947. 10.1080/14786419.2015.1081197 [DOI] [PubMed] [Google Scholar]
- He Y., Peng C., Xie X., Chen M., Li X., Li M., et al. (2015). Penchinones A–D, two pairs of cis-trans isomers with rearranged neolignane carbon skeletons from Penthorum chinense. RSC Adv. 5, 76788–76794. 10.1039/c5ra15982b [DOI] [Google Scholar]
- Hu L., Zhang Y., Zhu H., Liu J., Li H., Li X., et al. (2016a). Filicinic acid based meroterpenoids with anti-epstein-barr virus activities from Hypericum japonicum. Org. Lett. 18, 2272–2275. 10.1021/acs.orglett.6b00906 [DOI] [PubMed] [Google Scholar]
- Hu P., Li D., Hu X., Li S., Sai C., Sun X., et al. (2016b). Lignans and triterpenoids from Vitex negundo var. heterophylla and their biological evaluation. Fitoterapia 111, 147–153. 10.1016/j.fitote.2016.04.020 [DOI] [PubMed] [Google Scholar]
- Hu Y., Zhang C., Zhao X., Wang Y., Feng D., Zhang M., et al. (2016c). (±)-Homocrepidine A, a pair of anti-inflammatory enantiomeric octahydroindolizine alkaloid dimers from Dendrobium crepidatum. J. Nat. Prod. 79, 252–256. 10.1021/acs.jnatprod.5b00801 [DOI] [PubMed] [Google Scholar]
- Hu Z. X., Shi Y. M., Wang W. N., Li X., Du X., Liu M., et al. (2015). Kadcoccinones A–F, new biogenetically related lanostane-type triterpenoids with diverse skeletons from Kadsura coccinea. Org. Lett. 17, 4616–4619. 10.1021/acs.orglett.5b02360 [DOI] [PubMed] [Google Scholar]
- Hu Z., Shi Y., Wang W., Tang J., Zhou M., Du X., et al. (2016d). Structural characterization of kadcoccinin A, a sesquiterpenoid with a tricyclo[4.4.0.03,10]decane scaffold from Kadsura coccinea. Org. Lett. 18, 2284–2287. 10.1021/acs.orglett.6b00919 [DOI] [PubMed] [Google Scholar]
- Huang J., Wang Y., Li C., Wang X., He X. (2016). Triterpenes isolated from acorns of Quercus serrata var. brevipetiolata exert anti-inflammatory activity. Ind. Crops Prod. 91, 302–309. 10.1016/j.indcrop.2016.07.033 [DOI] [Google Scholar]
- Huang X., Cheng W., Ji M., Guo F., Shu M., Zheng C. (2015). Chemical constituents from leaves of Ananas comosusand their biological activities. Chin. Tradit. Herbal Drugs 46, 949–954. 10.7501/j.issn.0253-2670.2015.07.002 [DOI] [Google Scholar]
- Ji K. L., Zhang P., Li X. N., Guo J., Hu H. B., Xiao C. F., et al. (2015). Cytotoxic limonoids from Trichilia americana leaves. Phytochemistry 118, 61–67. 10.1016/j.phytochem.2015.08.014 [DOI] [PubMed] [Google Scholar]
- Jiang K., Chen L. L., Wang S. F., Wang Y., Li Y., Gao K. (2015a). Anti-inflammatory terpenoids from the leaves and twigs of Dysoxylum gotadhora. J. Nat. Prod. 78, 1037–1044. 10.1021/np5010196 [DOI] [PubMed] [Google Scholar]
- Jiang Y., Ng T. B., Wang C. R., Zhang D., Cheng Z. H., Liu Z. K., et al. (2010). Inhibitors from natural products to HIV-1 reverse transcriptase, protease and integrase. Mini Rev. Med. Chem. 10, 1331–1344. 10.2174/138955710793564133 [DOI] [PubMed] [Google Scholar]
- Jiang Z. K., Guo L., Chen C., Liu S. W., Zhang L., Dai S. J., et al. (2015b). Xiakemycin A, a novel pyranonaphthoquinone antibiotic, produced by the Streptomyces sp. CC8-201 from the soil of a karst cave. J. Antibiot. 68, 771–774. 10.1038/ja.2015.70 [DOI] [PubMed] [Google Scholar]
- Jiao W. H., Shi G. H., Xu T. T., Chen G. D., Gu B. B., Wang Z., et al. (2016). Dysiherbols A–C and dysideanone E, cytotoxic and NF-κB inhibitory tetracyclic meroterpenes from a Dysidea sp. marine sponge. J. Nat. Prod. 79, 406–411. 10.1021/acs.jnatprod.5b01079 [DOI] [PubMed] [Google Scholar]
- Jiao W., Xu T., Zhao F., Gao H., Shi G., Wang J., et al. (2015a). Dysifragilones A–C, unusual sesquiterpene aminoquinones and inhibitors of NO production from the South China Sea sponge Dysidea fragilis. Eur. J. Org. Chem. 2015, 960–966. 10.1002/ejoc.201403487 [DOI] [Google Scholar]
- Jiao Z. Z., Yue S., Sun H. X., Jin T. Y., Wang H. N., Zhu R. X., et al. (2015b). Indoline amide glucosides from Portulaca oleracea: isolation, structure, and DPPH radical scavenging activity. J. Nat. Prod. 78, 2588–2597. 10.1021/acs.jnatprod.5b00524 [DOI] [PubMed] [Google Scholar]
- Lai Y., Liu T., Sa R., Wei X., Xue Y., Wu Z., et al. (2015). Neolignans with a rare 2-oxaspiro[4.5]deca-6,9-dien-8-one motif from the stem bark of Cinnamomum subavenium. J. Nat. Prod. 78, 1740–1744. 10.1021/np5010533 [DOI] [PubMed] [Google Scholar]
- Li C. J., Ma J., Sun H., Zhang D., Zhang D. M. (2016a). Guajavadimer A, a dimeric caryophyllene-derived meroterpenoid with a new carbon skeleton from the leaves of Psidium guajava. Org. Lett. 18, 168–171. 10.1021/acs.orglett.5b03117 [DOI] [PubMed] [Google Scholar]
- Li C., Meng Y., Ying Z., Xu N., Hao D., Gao M., et al. (2016b). Three novel alkaloids from Portulaca oleracea L. and their antiinflammatory effects. J. Agric. Food Chem. 64, 5837–5844. 10.1021/acs.jafc.6b02673 [DOI] [PubMed] [Google Scholar]
- Li D., Guo Q., Meng X., Zhu C., Xu C., Shi J. (2016c). Two pairs of unusual scalemic enantiomers from Isatis indigotica leaves. Chin. Chem. Lett. 12, 1745–1750. 10.1016/j.cclet.2016.08.006 [DOI] [Google Scholar]
- Li D., Xue Y., Zhu H., Li Y., Sun B., Liu J., et al. (2015a). Hyperattenins A–I, bioactive polyprenylated acylphloroglucinols from Hypericum attenuatum choisy. RSC Adv. 5, 5277–5287. 10.1039/c4ra11675e [DOI] [Google Scholar]
- Li D. Q., Zhou L., Wang D., Wu J., Li L. Z., Huang X. X., et al. (2016d). Neuroprotective oleanane triterpenes from the roots of Bupleurum chinense. Bioorg. Med. Chem. Lett. 26, 1594–1598. 10.1016/j.bmcl.2016.02.004 [DOI] [PubMed] [Google Scholar]
- Li H. L., Li X. M., Li X., Wang C. Y., Liu H., Kassack M. U., et al. (2017). Antioxidant hydroanthraquinones from the marine algal-derived endophytic fungus Talaromyces islandicus EN-501. J. Nat. Prod. 80, 162–168. 10.1021/acs.jnatprod.6b00797 [DOI] [PubMed] [Google Scholar]
- Li H., Tian J., Tang H., Pan S., Zhang A., Gao J. (2015b). Chaetosemins A–E, new chromones isolated from an ascomycete Chaetomium seminudum and their biological activities. RSC Adv. 5, 29185–29192. 10.1039/c5ra00525f [DOI] [Google Scholar]
- Li J., Li Y., An F., Zhou M., Luo J., Jian K., et al. (2016e). Limonoids with modified furan rings from root barks of Toona sinensis. Tetrahedron. 72, 7481–7487. 10.1016/j.tet.2016.09.061 [DOI] [Google Scholar]
- Li L., Li H., Peng X., Hou B., Yu M., Dong J., et al. (2016f). (±)-Ganoapplanin, a pair of polycyclic meroterpenoid enantiomers from Ganoderma applanatum. Org. Lett. 18, 6078–6081. 10.1021/acs.orglett.6b03064 [DOI] [PubMed] [Google Scholar]
- Li L. Z., Liang X., Sun X., Qi X. L., Wang J., Zhao Q. C., et al. (2016g). Bioactive norditerpenoids and neolignans from the roots of salvia miltiorrhiza. Org. Biomol. Chem. 14, 10050–10057. 10.1039/c6ob01784c [DOI] [PubMed] [Google Scholar]
- Li L., Song S., Gao P., Li F., Wang L., Liu Q., et al. (2015c). Neogenkwanines A–H: daphnane-type diterpenes containing 4,7 or 4,6-ether groups from the flower bud of Daphne genkwa. RSC Adv. 5, 4143–4152. 10.1039/c4ra13167c [DOI] [Google Scholar]
- Li Q. M., Luo J. G., Zhang Y. M., Li Z. R., Wang X. B., Yang M. H., et al. (2015d). Involucratustones A–C: unprecedented sesquiterpene dimers containing multiple contiguous quaternary carbons from Stahlianthus involucratus. Chemistry 21, 13206–13209. 10.1002/chem.201502631 [DOI] [PubMed] [Google Scholar]
- Li Q., Zhang X., Cao J., Guo Z., Lou Y., Ding M., et al. (2015e). Depside derivatives with anti-hepatic fibrosis and anti-diabetic activities from Impatiens balsamina L. flowers. Fitoterapia 105, 234–239. 10.1016/j.fitote.2015.07.007 [DOI] [PubMed] [Google Scholar]
- Li S., Li Y., Lu C., Zhang J., Zhu J., Wang H., et al. (2015f). Activating a cryptic ansamycin biosynthetic gene cluster to produce three new naphthalenic octaketide ansamycins with n-pentyl and n-butyl side chains. Org. Lett. 17, 3706–3709. 10.1021/acs.orglett.5b01686 [DOI] [PubMed] [Google Scholar]
- Li S., Lu C., Ou J., Deng J., Shen Y. (2015g). Overexpression of hgc1 increases the production and diversity of hygrocins in Streptomyces sp. LZ35. RSC Adv. 5, 83843–83846. 10.1039/C5RA12623A [DOI] [Google Scholar]
- Li T., Wang X., Luo J., Yang M., Kong L. (2016h). Antioxidant sordariol dimers from Sordaria macrospora and the absolute configuration determinations of their two simultaneous linear 1,2-diols. Tetrahedron Lett. 57, 2754–2757. 10.1016/j.tetlet.2016.05.014 [DOI] [Google Scholar]
- Li X. H., Zhang Y., Zhang J. H., Li X. N., Cao M. M., Di Y. T., et al. (2016i). Myritonines A–C, alkaloids from myrioneuron tonkinensis based on a novel hexacyclic skeleton. J. Nat. Prod. 79, 1203–1207. 10.1021/acs.jnatprod.5b01130 [DOI] [PubMed] [Google Scholar]
- Li X. D., Li X. M., Xu G. M., Zhang P., Wang B. G. (2015h). Antimicrobial phenolic bisabolanes and related derivatives from Penicillium aculeatum SD-321, a deep sea sediment-derived fungus. J. Nat. Prod. 78, 844–849. 10.1021/acs.jnatprod.5b00004 [DOI] [PubMed] [Google Scholar]
- Li Y., Yili A., Li J., Muhamat A., Aisa H. A. (2016j). New isosteroidal alkaloids with tracheal relaxant effect from the bulbs of Fritillaria pallidiflora schrenk. Bioorg. Med. Chem. Lett. 26, 1983–1987. 10.1016/j.bmcl.2016.03.001 [DOI] [PubMed] [Google Scholar]
- Liang C. Q., Shi Y. M., Wang W. G., Hu Z. X., Li Y., Zheng Y. T., et al. (2015). Kadcoccinic acids A–J, triterpene acids from Kadsura coccinea. J. Nat. Prod. 78, 2067–2073. 10.1021/acs.jnatprod.5b00392 [DOI] [PubMed] [Google Scholar]
- Liang X. A., Ma Y. M., Zhang H. C., Liu R. (2016). A new helvolic acid derivative from an endophytic Fusarium sp. of Ficus carica. Nat. Prod. Res. 30, 2407–2412. 10.1080/14786419.2016.1190722 [DOI] [PubMed] [Google Scholar]
- Liao H. B., Lei C., Gao L. X., Li J. Y., Li J., Hou A. J. (2015a). Two enantiomeric pairs of meroterpenoids from Rhododendron capitatum. Org. Lett. 17, 5040–5043. 10.1021/acs.orglett.5b02515 [DOI] [PubMed] [Google Scholar]
- Liao Y., Liu X., Yang J., Lao Y. Z., Yang X. W., Li X. N., et al. (2015b). Hypersubones A and B, new polycyclic acylphloroglucinols with intriguing adamantane type cores from Hypericum subsessile. Org. Lett. 17, 1172–1175. 10.1021/acs.orglett.5b00100 [DOI] [PubMed] [Google Scholar]
- Lin B., Sun L. N., Xin H. L., Nian H., Song H. T., Jiang Y. P., et al. (2016a). Anti inflammatory constituents from the root of Litseacubeba in LPS induced RAW 264 7 macrophages. Pharm. Biol. 54, 1–7. 10.3109/13880209.2015.1126619 [DOI] [PubMed] [Google Scholar]
- Lin F., Wang C., Jiang H., Zhou J., Zhao W., Chen X. (2016b). Rakicidin B1, a new rakicidin analogue with antitumor activities from marine Micromonosporasp. Chin. J. Antibiot. 41, 510–515. 10.13461/j.cnki.cja.005776 [DOI] [Google Scholar]
- Lin R., Guan Y. Z., Li R. J., Xu X. M., Luo J. G., Kong L. Y. (2016c). 13,14-seco-Withanolides from Physalis minima with potential anti-inflammatory activity. Chem. Biodivers. 13, 884–890. 10.1002/cbdv.201500282 [DOI] [PubMed] [Google Scholar]
- Liu B., Xu Y. (2016). Cytotoxicity and synergistic effect of the constituents from roots of Aglaia odorata (Meliaceae). Nat. Prod. Res. 30, 433–437. 10.1080/01480545.2016.1193866 [DOI] [PubMed] [Google Scholar]
- Liu B. Y., Zhang C., Zeng K. W., Li J., Guo X. Y., Zhao M. B., et al. (2015a). Exotines A and B, two heterodimers of isopentenyl-substituted indole and coumarin derivatives from Murraya exotica. Org. Lett. 17, 4380–4383. 10.1021/acs.orglett.5b02230 [DOI] [PubMed] [Google Scholar]
- Liu C. P., Wang G. C., Gan L. S., Xu C. H., Liu Q. F., Ding J., et al. (2016a). Ciliatonoids A and B, two limonoids from Toona ciliata. Org. Lett. 18, 2894–2897. 10.1021/acs.orglett.6b01213 [DOI] [PubMed] [Google Scholar]
- Liu D., Lin H., Proksch P., Tang X., Shao Z., Lin W. (2015b). Microbacterins A and B, new peptaibols from the deep sea actinomycete Microbacterium sediminis sp. nov. YLB-01(T). Org. Lett. 17, 1220–1223. 10.1021/acs.orglett.5b00172 [DOI] [PubMed] [Google Scholar]
- Liu H. X., Chen K., Yuan Y., Xu Z. F., Tan H. B., Qiu S. X. (2016b). Rhodomentones A and B, novel meroterpenoids with unique NMR characteristics from Rhodomyrtus tomentosa. Org. Biomol. Chem. 14, 7354–7360. 10.1039/c6ob01215a [DOI] [PubMed] [Google Scholar]
- Liu H. X., Liu W. Z., Chen Y. C., Sun Z. H., Tan Y. Z., Li H. H., et al. (2016c). Cytotoxic trichothecene macrolides from the endophyte fungus Myrothecium roridum. J. Asian Nat. Prod. Res. 18, 684–689. 10.1080/10286020.2015.1134505 [DOI] [PubMed] [Google Scholar]
- Liu J., Zhang D., Zhang M., Zhao J., Chen R., Wang N., et al. (2016d). Eremophilane sesquiterpenes from an endophytic fungus Periconia species. J. Nat. Prod.. 79, 2229–2235. 10.1021/acs.jnatprod.6b00299 [DOI] [PubMed] [Google Scholar]
- Liu L., Han Y., Xiao J., Li L., Guo L., Jiang X., et al. (2016f). Chlorotheolides A and B, spiroketals generated via Diels-Alder reactions in the endophytic fungus Pestalotiopsis theae. J. Nat. Prod. 79, 2616–2623. 10.1021/acs.jnatprod.6b00550 [DOI] [PubMed] [Google Scholar]
- Liu L., Zhao C., Li L., Guo L., Che Y. (2015c). Pestalotriols A and B, new spiro[2.5]octane derivatives from the endophytic fungus Pestalotiopsis fici. RSC Adv. 5, 78708–78711. 10.1039/c5ra14009a [DOI] [Google Scholar]
- Liu Q. X., Yang Y. X., Zhang J. P., Chen L. P., Shen Y. H., Li H. L., et al. (2016g). Isolation, structure elucidation, and absolute configuration of highly oxygenated germacranolides from Carpesium cernuum. J. Nat. Prod. 79, 2479–2486. 10.1021/acs.jnatprod.6b00315 [DOI] [PubMed] [Google Scholar]
- Liu Q. Y., Zhou T., Zhao Y. Y., Chen L., Gong M. W., Xia Q. W., et al. (2015d). Antitumor effects and related mechanisms of penicitrinine A, a novel alkaloid with a unique spiro skeleton from the marine fungus Penicillium citrinum. Mar. Drugs 13, 4733–4753. 10.3390/md13084733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W., Li H. J., Xu M. Y., Ju Y. C., Wang L. Y., Xu J., et al. (2015e). Pseudellones A–C, three alkaloids from the marine-derived fungus Pseudallescheria ellipsoidea F42-3. Org. Lett. 17, 5156–5159. 10.1021/acs.orglett.5b02311 [DOI] [PubMed] [Google Scholar]
- Liu X., Yang J., Wang W., Li Y., Wu J. Z., Pu J. X., et al. (2015f). Diterpene alkaloids with an aza-ent-kaurane skeleton from Isodon rubescens. J. Nat. Prod. 78, 196–201. 10.1021/np5006136 [DOI] [PubMed] [Google Scholar]
- Liu Y., Chen M., Wang X., Guo Q., Zhu C., Lin S., et al. (2015g). Antiviral enantiomers of a bisindole alkaloid with a new carbon skeleton from the roots of Isatis indigotica. Chin. Chem. Lett. 26, 931–936. 10.1016/j.cclet.2015.05.052 [DOI] [Google Scholar]
- Liu Y., Chen S., Liu Z., Lu Y., Xia G., Liu H., et al. (2015h). Bioactive metabolites from mangrove endophytic fungus Aspergillus sp. 16-5B. Mar. Drugs 13, 3091–3102. 10.3390/md13053091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Li X. M., Meng L. H., Jiang W. L., Xu G. M., Huang C. G., et al. (2015i). Bisthiodiketopiperazines and acorane sesquiterpenes produced by the marine-derived fungus Penicillium adametzioides AS-53 on different culture media. J. Nat. Prod. 78, 1294–1299. 10.1021/acs.jnatprod.5b00102 [DOI] [PubMed] [Google Scholar]
- Liu Y., Yang Q., Xia G., Huang H., Li H., Ma L., et al. (2015j). Polyketides with alpha-glucosidase inhibitory activity from a mangrove endophytic fungus, Penicillium sp. HN29-3B1. J. Nat. Prod. 78, 1816–1822. 10.1021/np500885f [DOI] [PubMed] [Google Scholar]
- Liu Z., Chen Y., Chen S., Liu Y., Lu Y., Chen D., et al. (2016e). Aspterpenacids A and B, two sesterterpenoids from a mangrove endophytic fungus Aspergillus terreus H010. Org. Lett. 18, 1406–1409. 10.1021/acs.orglett.6b00336 [DOI] [PubMed] [Google Scholar]
- Long H., Cheng Z., Huang W., Wu Q., Li X., Cui J., et al. (2016). Diasteltoxins A–C, asteltoxin-based dimers from a mutant of the sponge-associated Emericella variecolor fungus. Org. Lett. 18, 4678–4681. 10.1021/acs.orglett.6b02313 [DOI] [PubMed] [Google Scholar]
- Lou L. L., Li L. G., Liu Q. B., Li D. Q., Liu Z. X., Huang X. X., et al. (2016). 3, 3'-Neolignans from Pithecellobium clypearia benth and their anti-inflammatory activity. Fitoterapia 112, 16–21. 10.1016/j.fitote.2016.04.021 [DOI] [PubMed] [Google Scholar]
- Lu H., Wu F., Jiang M., Liang W. (2017). Tzumin A and B, two new lignan derivatives from the barks of Sassafras tzumu. Nat. Prod. Res. 31, 829–834. 10.1080/14786419.2016.1250085 [DOI] [PubMed] [Google Scholar]
- Luo Q., Di L., Dai W. F., Lu Q., Yan Y. M., Yang Z. L., et al. (2015a). Applanatumin A, a new dimeric meroterpenoid from Ganoderma applanatum that displays potent antifibrotic activity. Org. Lett. 17, 1110–1113. 10.1021/ol503610b [DOI] [PubMed] [Google Scholar]
- Luo Q., Di L., Yang X., Cheng Y. (2016a). Applanatumols A and B, meroterpenoids with unprecedented skeletons from Ganoderma applanatum. RSC Adv. 6, 45963–45967. 10.1039/C6RA05148K [DOI] [Google Scholar]
- Luo Q., Tian L., Di L., Yan Y. M., Wei X. Y., Wang X. F., et al. (2015b). (+/−)-Sinensilactam A, a pair of rare hybrid metabolites with Smad3 phosphorylation inhibition from Ganoderma sinensis. Org. Lett. 17, 1565–1568. 10.1021/acs.orglett.5b00448 [DOI] [PubMed] [Google Scholar]
- Luo X., Li C., Luo P., Lin X., Ma H., Seeram N. P., et al. (2016b). Pterosin sesquiterpenoids from Pteris cretica as hypolipidemic agents via activating liver X receptors. J. Nat. Prod. 79, 3014–3021. 10.1021/acs.jnatprod.6b00558 [DOI] [PubMed] [Google Scholar]
- Luo Y., Xu Q., Dong L., Zhou Z., Chen Y., Zhang W., et al. (2015c). A new ursane and a new oleanane triterpene acids from the whole plant of Spermacoce latifolia. Phytochem Lett. 11, 127–131. 10.1016/j.phytol.2014.12.005 [DOI] [Google Scholar]
- Lv H. N., Wen R., Zhou Y., Zeng K. W., Li J., Guo X. Y., et al. (2015). Nitrogen oxide inhibitory trimeric and dimeric carbazole alkaloids from Murraya tetramera. J. Nat. Prod. 78, 2432–2439. 10.1021/acs.jnatprod.5b00527 [DOI] [PubMed] [Google Scholar]
- Ma G., Wu H., Chen D., Zhu N., Zhu Y., Sun Z., et al. (2015a). Antimalarial and antiproliferative cassane diterpenes of Caesalpinia sappan. J. Nat. Prod. 78, 2364–2371. 10.1021/acs.jnatprod.5b00317 [DOI] [PubMed] [Google Scholar]
- Ma G. L., Xiong J., Yang G. X., Pan L. L., Hu C. L., Wang W., et al. (2016a). Biginkgosides A–I, unexpected minor dimeric flavonol diglycosidic truxinate and truxillate esters from Ginkgo biloba leaves and their antineuroinflammatory and neuroprotective activities. J. Nat. Prod. 79, 1354–1364. 10.1021/acs.jnatprod.6b00061 [DOI] [PubMed] [Google Scholar]
- Ma J. H., Wang Y., Liu Y., Gao S. Y., Ding L. Q., Zhao F., et al. (2015b). Four new sesquiterpenes from the rhizomes of Curcuma phaeocaulis and their iNOS inhibitory activities. J. Asian Nat. Prod. Res. 17, 532–540. 10.1080/10286020.2015.1046449 [DOI] [PubMed] [Google Scholar]
- Ma K., Li L., Bao L., He L., Sun C., Zhou B., et al. (2015c). Six new 3,4-seco-27-norlanostane triterpenes from the medicinal mushroom Ganoderma boninense and their antiplasmodial activity and agonistic activity to LXRβ. Tetrahedron 71, 1808–1814. 10.1016/j.tet.2015.02.002 [DOI] [Google Scholar]
- Ma Q., Xu K., Sang Z., Wei R., Liu W., Su Y., et al. (2016b). Alkenes with antioxidative activities from Murraya koenigii (L.) Spreng. Bioorg. Med. Chem. Lett. 26, 799–803. 10.1016/j.bmcl.2015.12.091 [DOI] [PubMed] [Google Scholar]
- Ma S., Gao Y., Wang H., Li L., Liu Y., Qu J., et al. (2016c). Antiviral mono- and bis-prenylated C6-C3 derivatives from the roots of Illicium oligandrum. Tetrahedron 72, 3003–3013. 10.1016/j.tet.2016.04.016 [DOI] [Google Scholar]
- Ma Y. M., Liang X. A., Kong Y., Jia B. (2016d). Structural diversity and biological activities of indole diketopiperazine alkaloids from fungi. J. Agric. Food Chem. 64, 6659–6671. 10.1021/acs.jafc.6b01772 [DOI] [PubMed] [Google Scholar]
- Ma Y. M., Liang X. A., Zhang H. C., Liu R. (2016e). Cytotoxic and antibiotic cyclic pentapeptide from an endophytic Aspergillus tamarii of Ficus carica. J. Agric. Food Chem. 64, 3789–3793. 10.1021/acs.jafc.6b01051 [DOI] [PubMed] [Google Scholar]
- Ma Y., Mao X., Huang L., Fan Y., Gu W., Yan C., et al. (2016f). Diterpene alkaloids and diterpenes from Spiraea japonica and their antitobacco mosaic virus activity. Fitoterapia 109, 8–13. 10.1016/j.fitote.2015.11.019 [DOI] [PubMed] [Google Scholar]
- Meng L. H., Du F. Y., Li X. M., Pedpradab P., Xu G. M., Wang B. G. (2015a). Rubrumazines A–C, indolediketopiperazines of the isoechinulin class from Eurotium rubrum MA-150, a fungus obtained from marine mangrove-derived rhizospheric soil. J. Nat. Prod. 78, 909–913. 10.1021/np5007839 [DOI] [PubMed] [Google Scholar]
- Meng L., Li X., Liu Y., Wang B. (2015b). Polyoxygenated dihydropyrano[2,3-c]pyrrole-4,5-dione derivatives from the marine mangrove-derived endophytic fungus Penicillium brocae MA-231 and their antimicrobial activity. Chin. Chem. Lett. 26, 610–612. 10.1016/j.cclet.2015.01.024 [DOI] [Google Scholar]
- Meng L., Wang C., Mándi A., Li X., Hu X., Kassack M., et al. (2016a). Three diketopiperazine alkaloids with spirocyclic skeletons and one bisthiodiketopiperazine derivative from the mangrove-derived endophytic fungus Penicillium brocae MA-231. Org. Lett. 18, 5304–5307. 10.1021/acs.orglett.6b02620 [DOI] [PubMed] [Google Scholar]
- Meng L. H., Zhang P., Li X. M., Wang B. G. (2015c). Penicibrocazines A–E, five new sulfide diketopiperazines from the marine-derived endophytic fungus Penicillium brocae. Mar. Drugs 13, 276–287. 10.3390/md13010276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng W., Xiong J., Wang W., Zhang H., Zeng H., Hu J. (2016b). Phlefargesiine A, a C16N2 Lycopodium alkaloid with an unprecedented [6/7/6/6] -tetracyclic skeleton from Phlegmariurus fargesii. Tetrahedron Lett. 57, 3218–3221. 10.1016/j.tetlet.2016.06.050 [DOI] [Google Scholar]
- Miao Y., Xu X., Yuan F., Shi Y., Chen Y., Chen J., et al. (2016). Four cytotoxic annonaceous acetogenins from the seeds of Annona squamosa. Nat. Prod. Res. 30, 1273–1279. 10.1080/14786419.2015.1055490 [DOI] [PubMed] [Google Scholar]
- Newman D. J., Cragg G. M. (2016). Natural products as sources of new drugs from 1981 to 2014. J. Nat. Prod. 79, 629–661. 10.1021/acs.jnatprod.5b01055 [DOI] [PubMed] [Google Scholar]
- Ni L., Schinnerl J., Bao M., Zhang B., Wu J., Cai X. (2016). Two key biogenetic intermediates of cephalotaxus alkaloids from Cephalotaxus oliveri and C. lanceolata. Tetrahedron Lett. 57, 5201–5204. 10.1016/j.tetlet.2016.10.026 [DOI] [Google Scholar]
- Nian Y., Yang J., Liu T. Y., Luo Y., Zhang J. H., Qiu M. H. (2015). New anti-angiogenic leading structure discovered in the fruit of Cimicifuga yunnanensis. Sci. Rep. 5, 9026–9032. 10.1038/srep09026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu S., Liu D., Proksch P., Shao Z., Lin W. (2015). New polyphenols from a deep sea Spiromastix sp. Fungus, and their antibacterial activities. Mar. Drugs 13, 2526–2540. 10.3390/md13042526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nong X. H., Zhang X. Y., Xu X. Y., Wang J., Qi S. H. (2016). Nahuoic acids B–E, polyhydroxy polyketides from the marine-derived Streptomyces sp. SCSGAA 0027. J. Nat. Prod. 79, 141–148. 10.1021/acs.jnatprod.5b00805 [DOI] [PubMed] [Google Scholar]
- Pan J. J., Wan X., Zhang H., Chen Z., Huang J., Yang B., et al. (2016a). Three new milbemycins from a genetically engineered strain S. avermitilis MHJ1011. J. Antibiot. 69, 104–107. 10.1038/ja.2015.90 [DOI] [PubMed] [Google Scholar]
- Pan Z., Qin X. J., Liu Y. P., Wu T., Luo X. D., Xia C. (2016b). Alstoscholarisines H–J, indole alkaloids from Alstonia scholaris: structural evaluation and bioinspired synthesis of alstoscholarisine H. Org. Lett. 18, 654–657. 10.1021/acs.orglett.5b03583 [DOI] [PubMed] [Google Scholar]
- Peng J., Kong J. F., Liu Z., Wang P., Gai C., Jiang B., et al. (2016a). Two new phragmalin-type limonoids from stems of Chukrasia tabularis. Phytochem Lett. 15, 230–233. 10.1016/j.phytol.2016.01.003 [DOI] [Google Scholar]
- Peng L., Fu W. X., Zeng C. X., Zhou L., Bao M. F., Cai X. H. (2016b). Two new lignans from twigs of Aglaia odorata. J. Asian Nat. Prod. Res. 18, 147–152. 10.1080/10286020.2015.1057575 [DOI] [PubMed] [Google Scholar]
- Peng Y., Lou L. F., Liu S. L., Zhou L., Huang X. X., Song S. J. (2016c). Antioxidant and anti-inflammatory neolignans from the seeds of hawthorn. Bioorg. Med. Chem. Lett. 26, 5501–5506. 10.1016/j.bmcl.2016.10.012 [DOI] [PubMed] [Google Scholar]
- Qi C., Bao J., Wang J., Zhu H., Xue Y., Wang X., et al. (2016). Asperterpenes A and B, two unprecedented meroterpenoids from Aspergillus terreus with BACE1 inhibitory activities. Chem. Sci. 7, 6563–6572. 10.1039/c6sc02464e [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi Q. Y., Ren J. W., Sun L. W., He L. W., Bao L., Yue W., et al. (2015). Stucturally diverse sesquiterpenes produced by a Chinese Tibet fungus Stereum hirsutum and their cytotoxic and immunosuppressant activities. Org. Lett. 17, 3098–3101. 10.1021/acs.orglett.5b01356 [DOI] [PubMed] [Google Scholar]
- Ren F., Zhu S., Wang B., Li L., Liu X., Su R., et al. (2016). Hypocriols A–F, heterodimeric botryane ethers from Hypocrea sp., an insect-associated fungus. J. Nat. Prod. 79, 1848–1856. 10.1021/acs.jnatprod.6b00394 [DOI] [PubMed] [Google Scholar]
- Ren G., Peng J., Liu A., Liang J., Yuan W., Wang H., et al. (2015a). Structure elucidation and NMR assignments of two new flavanones from the roots of Artocarpus heterophyllus. Magn. Reson. Chem. 53, 872–874. 10.1002/mrc.4285 [DOI] [PubMed] [Google Scholar]
- Ren J., Niu S., Li L., Geng Z., Liu X., Che Y. (2015b). Identification of oxaphenalenone ketals from the ascomycete Fungus Neonectria sp. J. Nat. Prod. 78, 1316–1321. 10.1021/acs.jnatprod.5b00159 [DOI] [PubMed] [Google Scholar]
- Saha S., Zhang W., Zhang G., Zhu Y., Chen Y., Liu W., et al. (2017). Activation and characterization of a cryptic gene cluster reveals a cyclization cascade for polycyclic tetramate macrolactams. Chem. Sci. 8, 1607–1612. 10.1039/c6sc03875a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan W., Wu Z., Pang W., Ma L., Ying Y., Zhan Z. (2015). α-Glucosidase inhibitors from the fungus Aspergillus terreus 3.05358. Chem. Biodivers. 12, 1718–1724. 10.1002/cbdv.201500027 [DOI] [PubMed] [Google Scholar]
- Shang S. Z., Zhao W., Tang J. G., Xu X. M., Sun H. D., Pu J. X., et al. (2016). Antiviral sesquiterpenes from leaves of Nicotiana tabacum. Fitoterapia 108, 1–4. 10.1016/j.fitote.2015.11.004 [DOI] [PubMed] [Google Scholar]
- Shen C. P., Luo J. G., Yang M. H., Kong L. Y. (2015). Cafestol-type diterpenoids from the twigs of Tricalysia fruticosa with potential anti-inflammatory activity. J. Nat. Prod. 78, 1322–1329. 10.1021/acs.jnatprod.5b00165 [DOI] [PubMed] [Google Scholar]
- Shen Q., Xu X., Li L., Zhao W., Xiang N., Yang G., et al. (2016). Sesquiterpenes from the leaves of Nicotiana tabacum and their anti-tobacco mosaic virus activity. Chin. Chem. Lett. 27, 753–756. 10.1016/j.cclet.2016.01.048 [DOI] [PubMed] [Google Scholar]
- Shi Y. M., Cai S. L., Li X. N., Liu M., Shang S. Z., Du X., et al. (2016). LC-UV-guided isolation and structure determination of lancolide E. Org. Lett. 18, 100–103. 10.1021/acs.orglett.5b03334 [DOI] [PubMed] [Google Scholar]
- Shi Y. S., Liu Y. B., Ma S. G., Li Y., Qu J., Li L., et al. (2015). Bioactive sesquiterpenes and lignans from the fruits of Xanthium sibiricum. J. Nat. Prod. 78, 1526–1535. 10.1021/np500951s [DOI] [PubMed] [Google Scholar]
- Si Y., Li N., Tong L., Lin B., Wang W., Xing Y., et al. (2016). Bioactive minor components of the total salvianolic acids injection prepared from Salvia miltiorrhiza Bge. Bioorg. Med. Chem. Lett. 26, 82–86. 10.1016/j.bmcl.2015.11.028 [DOI] [PubMed] [Google Scholar]
- Sun C. P., Qiu C. Y., Yuan T., Nie X. F., Sun H. X., Zhang Q., et al. (2016a). Antiproliferative and anti-inflammatory withanolides from Physalis angulata. J. Nat. Prod. 79, 1586–1597. 10.1021/acs.jnatprod.6b00094 [DOI] [PubMed] [Google Scholar]
- Sun J., Zhu Z. X., Song Y. L., Dong D., Zheng J., Liu T., et al. (2016b). Nitric oxide inhibitory meroterpenoids from the fungus Penicillium purpurogenum MHZ 111. J. Nat. Prod. 79, 1415–1422. 10.1021/acs.jnatprod.6b00160 [DOI] [PubMed] [Google Scholar]
- Sun M., Zhang X., Hao H., Li W., Lu C. (2015a). Nocarbenzoxazoles A–G, benzoxazoles produced by halophilic Nocardiopsis lucentensis DSM 44048. J. Nat. Prod. 78, 2123–2127. 10.1021/acs.jnatprod.5b00031 [DOI] [PubMed] [Google Scholar]
- Sun P., Zhao Q., Wu Z., Zhang W., Liu W. (2015b). 1,19-seco-Avermectin analogues from a DeltaaveCDE mutant Streptomyces avermectinius strain. J. Nat. Prod. 78, 301–305. 10.1021/np500468f [DOI] [PubMed] [Google Scholar]
- Sun X. P., Cao F., Shao C. L., Wang M., Zhang X. L., Wang C. Y. (2015c). Antibacterial Δ1-3-Ketosteroids from the South China Sea gorgonian coral Subergorgia rubra. Chem. Biodivers. 12, 1068–1074. 10.1002/cbdv.201400217 [DOI] [PubMed] [Google Scholar]
- Tan W., Pu D., Feng Y., Li H. (2016). A new triterpenoid from Maytenus austroyunnanensis. Nat. Prod. Res. Dev. 28, 173–178. 10.16333/j.1001-6880.2016.2.001 [DOI] [Google Scholar]
- Tang H., Gao J., Zhang Q. (2015). Endophyte inspired chemical diversity from beta-caryophyllene. RSC Adv. 5, 72433–72436. 10.1039/c5ra14243a [DOI] [Google Scholar]
- Tang S., Shi J., Liu C., Jiang R., Zhao W., Liu X., et al. (2016a). Three new phenylpropanoids from Lavandula angustifolia and their bioactivities. Nat. Prod. Res. 31, 1351–1357. 10.1080/14786419.2016.1247081 [DOI] [PubMed] [Google Scholar]
- Tang Y. X., Fu W. W., Wu R., Tan H. S., Shen Z. W., Xu H. X. (2016b). Bioassay-guided isolation of prenylated xanthone derivatives from the leaves of Garcinia oligantha. J. Nat. Prod. 79, 1752–1761. 10.1021/acs.jnatprod.6b00137 [DOI] [PubMed] [Google Scholar]
- Tang Y., Xiong J., Zhang J. J., Wang W., Zhang H. Y., Hu J. F. (2016c). Annotinolides A–C, three lycopodane-derived 8,5-Lactones with polycyclic skeletons from Lycopodium annotinum. Org. Lett. 18, 4376–4379. 10.1021/acs.orglett.6b02132 [DOI] [PubMed] [Google Scholar]
- Tian D. S., Yi P., Xia L., Xiao X., Fan Y. M., Gu W., et al. (2016a). Garmultins A–G, biogenetically related polycyclic acylphloroglucinols from Garcinia multiflora. Org. Lett. 18, 5904–5907. 10.1021/acs.orglett.6b03004 [DOI] [PubMed] [Google Scholar]
- Tian J., Chen Y., Wang Y., Huang X., Sun X., Liu K., et al. (2016b). Microbial transformation of methyl cyperenoate by Cunninghamella elegans AS 3.2028 and the antithrombotic activities of its metabolites. RSC Adv. 6, 112712–112720. 10.1039/c6ra24332k [DOI] [Google Scholar]
- Tian X., Li H., An F., Li R., Zhou M., Yang M., et al. (2016c). New structurally diverse limonoids from the seeds of Khaya senegalensis. Planta Med. 83, 341–350. 10.1055/s-0042-117114 [DOI] [PubMed] [Google Scholar]
- Tian X., Li L., Pei J., Yue R., Fang X., Zhang J., et al. (2015a). (−) and (+)-Merrilliaquinone, a pair of new quinone enantiomers from Illicium merrillianum and their distinctive effect on human hepatoma and hepatic cells. RSC Adv. 5, 75857–75862. 10.1039/c5ra15074d [DOI] [Google Scholar]
- Tian Y., Jiang N., Zhang A. H., Chen C. J., Deng X. Z., Zhang W. J., et al. (2015b). Muta-mycosynthesis of naphthalene analogs. Org. Lett. 17, 1457–1460. 10.1021/acs.orglett.5b00335 [DOI] [PubMed] [Google Scholar]
- Tian Y. Q., Lin X. P., Wang Z., Zhou X. F., Qin X. C., Kaliyaperumal K., et al. (2015c). Asteltoxins with antiviral activities from the marine sponge-derived fungus Aspergillus sp. SCSIO XWS02F40. Molecules 21, 34–43. 10.3390/molecules21010034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu Y. (2011). The discovery of artemisinin (qinghaosu) and gifts from Chinese medicine. Nat. Med. 17, 1217–1220. 10.1038/nm.2471 [DOI] [PubMed] [Google Scholar]
- Wan Z., Fang W., Shi L., Wang K., Zhang Y., Zhang Z., et al. (2015). Novonestmycins A and B, two new 32-membered bioactive macrolides from Streptomyces phytohabitans HBERC-20821. J. Antibiot. 68, 185–190. 10.1038/ja.2014.123 [DOI] [PubMed] [Google Scholar]
- Wang C., Li C., Ma J., Yang J., Chen X., Li L., et al. (2015a). Bioactive 18(4→3)-abeo-abietanoid derivatives from the leaves of Tripterygium wilfordii. RSC Adv. 5, 30046–30052. 10.1039/c5ra02174j [DOI] [Google Scholar]
- Wang G. W., Deng L. Q., Luo Y. P., Liao Z. H., Chen M. (2016a). Hepatoprotective triterpenoids and lignans from the stems of Schisandra pubescens. Nat. Prod. Res. 31, 1855–1860. 10.1080/14786419.2016.1261348 [DOI] [PubMed] [Google Scholar]
- Wang G. W., Qin J. J., Cheng X. R., Shen Y. H., Shan L., Jin H. Z., et al. (2014). Inula sesquiterpenoids: structural diversity, cytotoxicity and anti-tumor activity. Expert Opin. Investig. Drugs 23, 317–345. 10.1517/13543784.2014.868882 [DOI] [PubMed] [Google Scholar]
- Wang J., Bai G., Liu Y., Wang H., Li Y., Yin W., et al. (2015b). Cytotoxic metabolites produced by the endophytic fungus Aspergillus clavatus. Chem. Lett. 44, 1148–1149. 10.1246/cl.150417 [DOI] [Google Scholar]
- Wang J., Han Z., Li H., Zhang W. (2015c). A new iridoid from Valeriana officinalis var. latiofolia. Chin. Tradit. Herbal Drugs 46, 11–14. 10.7501/j.issn.0253-2670.2015.01.003 [DOI] [Google Scholar]
- Wang J. F., He W. J., Zhang X. X., Zhao B. Q., Liu Y. H., Zhou X. J. (2015d). Dicarabrol, a new dimeric sesquiterpene from Carpesium abrotanoides L. Bioorg. Med. Chem. Lett. 25, 4082–4084. 10.1016/j.bmcl.2015.08.034 [DOI] [PubMed] [Google Scholar]
- Wang J., Wei X., Qin X., Lin X., Zhou X., Liao S., et al. (2015e). Arthpyrones A–C, pyridone alkaloids from a sponge-derived fungus Arthrinium arundinis ZSDS1-F3. Org. Lett.. 17, 656–659. 10.1021/ol503646c [DOI] [PubMed] [Google Scholar]
- Wang J., Wei X., Qin X., Tian X., Liao L., Li K., et al. (2016b). Antiviral merosesquiterpenoids produced by the antarctic Fungus Aspergillus ochraceopetaliformis SCSIO 05702. J. Nat. Prod. 79, 59–65. 10.1021/acs.jnatprod.5b00650 [DOI] [PubMed] [Google Scholar]
- Wang K., Bao L., Ma K., Zhang J., Chen B., Han J., et al. (2016c). A novel class of α-glucosidase and HMG-CoA reductase inhibitors from Ganoderma leucocontextum and the anti-diabetic properties of ganomycin I in KK-Ay mice. Eur. J. Med. Chem. 127, 1035–1046. 10.1016/j.ejmech.2016.11.015 [DOI] [PubMed] [Google Scholar]
- Wang K., Bao L., Qi Q., Zhao F., Ma K., Pei Y., et al. (2015f). Erinacerins C–L, isoindolin-1-ones with α-glucosidase inhibitory activity from cultures of the medicinal mushroom Hericium erinaceus. J. Nat. Prod. 78, 146–154. 10.1021/np5004388 [DOI] [PubMed] [Google Scholar]
- Wang K., Bao L., Xiong W., Ma K., Han J., Wang W., et al. (2015g). Lanostane triterpenes from the Tibetan medicinal mushroom Ganoderma leucocontextum and their inhibitory effects on HMG-CoA reductase and α-glucosidase. J. Nat. Prod. 78, 1977–1989. 10.1021/acs.jnatprod.5b00331 [DOI] [PubMed] [Google Scholar]
- Wang L. W., Zhang Y. L., Lin F. C., Hu Y. Z., Zhang C. L. (2011). Natural products with antitumor activity from endophytic fungi. Mini. Rev. Med. Chem. 11, 1056–1074. 10.2174/138955711797247716 [DOI] [PubMed] [Google Scholar]
- Wang L. J., Xiong J., Liu S. T., Pan L. L., Yang G. X., Hu J. F. (2015h). Ent-abietane-type and related seco-/nor-diterpenoids from the rare chloranthaceae plant Chloranthus sessilifolius and their antineuroinflammatory activities. J. Nat. Prod. 78, 1635–1646. 10.1021/acs.jnatprod.5b00195 [DOI] [PubMed] [Google Scholar]
- Wang M., Wang F., Xu F., Ding L. Q., Zhang Q., Li H. X., et al. (2016d). Two pairs of farnesyl phenolic enantiomers as natural nitric oxide inhibitors from Ganoderma sinense. Bioorg. Med. Chem. Lett. 26, 3342–3345. 10.1016/j.bmcl.2016.05.037 [DOI] [PubMed] [Google Scholar]
- Wang Q., Tang X., Luo X., de Voogd N. J., Li P., Li G. (2015i). (+)− and (-)-Spiroreticulatine, a pair of unusual spiro bisheterocyclic quinoline-imidazole alkaloids from the South China Sea sponge Fascaplysinopsis reticulata. Org. Lett. 17, 3458–3461. 10.1021/acs.orglett.5b01503 [DOI] [PubMed] [Google Scholar]
- Wang R., Yan H., Tang X. (2006). Progress in studies of huperzine A, a natural cholinesterase inhibitor from Chinese herbal medicine. Acta Pharmacol. Sin. 27, 1–26. 10.1111/j.1745-7254.2006.00255.x [DOI] [PubMed] [Google Scholar]
- Wang W., Yang J., Wu H., Kong L., Su J., Li X., et al. (2015j). Ent-kauranoids isolated from Isodon eriocalyx var. laxiflora and their structure activity relationship analyses. Tetrahedron 71, 9161–9171. 10.1016/j.tet.2015.09.066 [DOI] [Google Scholar]
- Wang Y., Wang J., Wang H., Ye W. (2016f). Novel taxane derivatives from Taxus wallichiana with highanticancer potency on tumor cells. Chem. Biol. Drug Des. 88, 556–561. 10.1111/cbdd.12782 [DOI] [PubMed] [Google Scholar]
- Wang Y., Yuk H. J., Kim J. Y., Kim D. W., Song Y. H., Tan X. F., et al. (2016g). Novel chromenedione derivatives displaying inhibition of protein tyrosine phosphatase 1B (PTP1B) from Flemingia philippinensis. Bioorg. Med. Chem. Lett. 26, 318–321. 10.1016/j.bmcl.2015.12.021 [DOI] [PubMed] [Google Scholar]
- Wang Z., Sang X., Sun K., Huang S., Chen S., Xue C., et al. (2016h). Lecanicillones A–C, three dimeric isomers of spiciferone A with a cyclobutane ring from an entomopathogenic fungus Lecanicillium sp. PR-M-3. RSC Adv. 6, 82348–82351. 10.1039/C6RA11422A [DOI] [Google Scholar]
- Wei J., Liu L., Dong S., Li H., Tang D., Zhang Q., et al. (2016a). Gabosines P and Q, new carbasugars from Streptomyces sp. and their α-glucosidase inhibitory activity. Bioorg. Med. Chem. Lett. 26, 4903–4906. 10.1016/j.bmcl.2016.09.021 [DOI] [PubMed] [Google Scholar]
- Wei W., Wu X. W., Deng G. G., Yang X. W. (2016b). Anti-inflammatory coumarins with short- and long-chain hydrophobic groups from roots of Angelica dahurica cv. Hangbaizhi. Phytochemistry 123, 58–68. 10.1016/j.phytochem.2016.01.006 [DOI] [PubMed] [Google Scholar]
- Wu B., Wiese J., Wenzel-Storjohann A., Malien S., Schmaljohann R., Imhoff J. F. (2016). Engyodontochones, antibiotic polyketides from the marine fungus Engyodontium album Strain LF069. Chemistry 22, 7452–7462. 10.1002/chem.201600430 [DOI] [PubMed] [Google Scholar]
- Wu G., Yu G., Kurtán T., Mándi A., Peng J., Mo X., et al. (2015a). Versixanthones A–F, cytotoxic xanthone-chromanone dimers from the marine-derived fungus Aspergillus versicolor HDN1009. J. Nat. Prod. 78, 2691–2698. 10.1021/acs.jnatprod.5b00636 [DOI] [PubMed] [Google Scholar]
- Wu J., Tang C., Chen L., Qiao Y., Geng M., Ye Y. (2015b). Dicarabrones A and B, a pair of new epimers dimerized from sesquiterpene lactones via a [3 + 2] cycloaddition from Carpesium abrotanoides. Org. Lett. 17, 1656–1659. 10.1021/acs.orglett.5b00371 [DOI] [PubMed] [Google Scholar]
- Wu J., Xiao Q., Xu J., Li M. Y., Pan J. Y., Yang M. H. (2008). Natural products from true mangrove flora: source, chemistry and bioactivities. Nat. Prod. Rep. 25, 955–981. 10.1039/b807365a [DOI] [PubMed] [Google Scholar]
- Wu L., Luo J., Wang X., Li R., Zhang Y., Kong L. (2015c). Calliviminones C–H six new hetero- and carbon-Diels-Alder adducts with unusual skeletons from the fruits of Callistemon viminalis. RSC Adv. 5, 93900–93906. 10.1039/c5ra19651e [DOI] [Google Scholar]
- Wu P., Xue J., Yao L., Xu L., Li H., Wei X. (2015d). Bisacremines E–G, three polycyclic dimeric acremines produced by Acremonium persicinum SC0105. Org. Lett. 17, 4922–4925. 10.1021/acs.orglett.5b02536 [DOI] [PubMed] [Google Scholar]
- Wu P., Yao L., Xu L., Xue J., Wei X. (2015e). Bisacremines A–D, dimeric acremines produced by a soil-derived Acremonium persicinum strain. J. Nat. Prod. 78, 2161–2166. 10.1021/np501037x [DOI] [PubMed] [Google Scholar]
- Wu Y. H., Chen G. D., He R. R., Wang C. X., Hu D., Wang G. Q., et al. (2015f). Pericolactines A–C, a new class of diterpenoid alkaloids with unusual tetracyclic skeleton. Sci. Rep. 5, 17082–17089. 10.1038/srep17082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi F. M., Ma S. G., Liu Y. B., Li L., Yu S. S. (2016). Artaboterpenoids A and B, bisabolene-derived sesquiterpenoids from Artabotrys hexapetalus. Org. Lett. 18, 3374–3377. 10.1021/acs.orglett.6b01519 [DOI] [PubMed] [Google Scholar]
- Xia G., Li Y., Sun J., Wang L., Tang X., Lin B., et al. (2016). Withanolides from the stems and leaves of Physalis pubescens and their cytotoxic activity. Steroids 115, 136–146. 10.1016/j.steroids.2016.09.002 [DOI] [PubMed] [Google Scholar]
- Xiang L., Wang Y., Yi X., He X. (2016a). Antiproliferative and anti-inflammatory furostanol saponins from the rhizomes of Tupistra chinensis. Steroids 116, 28–37. 10.1016/j.steroids.2016.10.005 [DOI] [PubMed] [Google Scholar]
- Xiang L., Wang Y., Yi X., Feng J., He X. (2016b). Furospirostanol and spirostanol saponins from the rhizome of Tupistra chinensis and their cytotoxic and anti-inflammatory activities. Tetrahedron. 72, 134–141. 10.1016/j.tet.2015.11.012 [DOI] [Google Scholar]
- Xiao W. L., Li R. T., Huang S. X., Pu J. X., Sun H. D. (2008). Triterpenoids from the Schisandraceae family. Nat. Prod. Rep.. 25, 871–891. 10.1039/b719905h [DOI] [PubMed] [Google Scholar]
- Xiao X., Wang X., Gui X., Chen L., Huang B. (2016a). Natural flavonoids as promising analgesic candidates: a aystematic review. Chem. Biodivers. 13, 1427–1440. 10.1002/cbdv.201600060 [DOI] [PubMed] [Google Scholar]
- Xiao Y. H., Yin H. L., Chen L., Tian Y., Liu S. J., Zhang G. J., et al. (2015). Three spirostanol saponins and a flavane-O-glucoside from the fresh rhizomes of Tupistra chinensis. Fitoterapia 102, 102–108. 10.1016/j.fitote.2015.02.008 [DOI] [PubMed] [Google Scholar]
- Xiao Z., Chen S., Cai R., Lin S., Hong K., She Z. (2016b). New furoisocoumarins and isocoumarins from the mangrove endophytic fungus Aspergillus sp. 085242. Beilstein J. Org. Chem.. 12, 2077–2085. 10.3762/bjoc.12.196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y., Zhang J., Liu W., Xie N., Feng F., Qu W. (2016a). New urushiols with platelet aggregation inhibitory activities from resin of Toxicodendron vernicifluum. Fitoterapia 112, 38–44. 10.1016/j.fitote.2016.05.001 [DOI] [PubMed] [Google Scholar]
- Xie Z., Zhou L., Guo L., Yang X., Qu G., Wu C., et al. (2016b). Grisemycin, a bridged angucyclinone with a methylsulfinyl moiety from a marine-derived Streptomyces sp. Org. Lett.. 18, 1402–1405. 10.1021/acs.orglett.6b00332 [DOI] [PubMed] [Google Scholar]
- Xing Q., Gan L., Mou X., Wang W., Wang C., Wei M., et al. (2016). Isolation, resolution and biological evaluation of pestalachlorides E and F containing both point and axial chirality. RSC Adv. 6, 22653–22658. 10.1039/c6ra00374e [DOI] [Google Scholar]
- Xiong J., Hong Z. L., Gao L. X., Shen J., Liu S. T., Yang G. X., et al. (2015a). Chlorabietols A–C, phloroglucinol-diterpene adducts from the chloranthaceae plant Chloranthus oldhamii. J. Org. Chem. 80, 11080–11085. 10.1021/acs.joc.5b01658 [DOI] [PubMed] [Google Scholar]
- Xiong L., Zhou Q. M., Zou Y., Chen M. H., Guo L., Hu G. Y., et al. (2015b). Leonuketal, a spiroketal diterpenoid from Leonurus japonicus. Org. Lett. 17, 6238–6241. 10.1021/acs.orglett.5b03227 [DOI] [PubMed] [Google Scholar]
- Xu J. B., Fan Y. Y., Gan L. S., Zhou Y. B., Li J., Yue J. M. (2016a). Cephalotanins A–D, four norditerpenoids represent three highly rigid carbon skeletons from Cephalotaxus sinensis. Chemistry 22, 14648–14654. 10.1002/chem.201603373 [DOI] [PubMed] [Google Scholar]
- Xu J., Sun Y., Wang M., Ren Q., Li S., Wang H., et al. (2015a). Bioactive diterpenoids from the leaves of Callicarpa macrophylla. J. Nat. Prod. 78, 1563–1569. 10.1021/acs.jnatprod.5b00018 [DOI] [PubMed] [Google Scholar]
- Xu J., Wang M., Sun X., Ren Q., Cao X., Li S., et al. (2016b). Bioactive terpenoids from Salvia plebeia: structures, NO inhibitory activities, and interactions with iNOS. J. Nat. Prod. 79, 2924–2932. 10.1021/acs.jnatprod.6b00733 [DOI] [PubMed] [Google Scholar]
- Xu J., Xiao D., Lin Q. H., He J. F., Liu W. Y., Xie N., et al. (2016c). Cytotoxic tirucallane and apotirucallane triterpenoids from the stems of Picrasma quassioides. J. Nat. Prod. 79, 1899–1910. 10.1021/acs.jnatprod.5b01137 [DOI] [PubMed] [Google Scholar]
- Xu J., Zhao H., Wang X., Li Z., Luo J., Yang M., et al. (2014). (±)-Melicolones A and B, rearranged prenylated acetophenone stereoisomers with an unusual 9 oxatricyclo[3.2.1.13,8]nonane core from the leaves of Melicope ptelefolia. Org. Lett. 17, 146–149. 10.1021/ol5033738 [DOI] [PubMed] [Google Scholar]
- Xu L., Wu P., Wright S. J., Du L., Wei X. (2015b). Bioactive polycyclic tetramate macrolactams from Lysobacter enzymogenes and their absolute configurations by theoretical ECD calculations. J. Nat. Prod.. 78, 1841–1847. 10.1021/acs.jnatprod.5b00099 [DOI] [PubMed] [Google Scholar]
- Xu W. J., Zhu M. D., Wang X. B., Yang M. H., Luo J., Kong L. Y. (2015c). Hypermongones A–J, rare methylated polycyclic polyprenylated acylphloroglucinols from the flowers of Hypericum monogynum. J. Nat. Prod. 78, 1093–1100. 10.1021/acs.jnatprod.5b00066 [DOI] [PubMed] [Google Scholar]
- Xu Y. K., Yang L., Liao S. G., Cao P., Wu B., Hu H. B., et al. (2015d). Koumine, humantenine, and yohimbane alkaloids from Gelsemium elegans. J. Nat. Prod. 78, 1511–1517. 10.1021/np5009619 [DOI] [PubMed] [Google Scholar]
- Xu Z., Pan J. (1955). Study on the chemical constituents of Chinese herbs Brucea Javanica. Acta Pharmaceut. Sin. 3, 211–221. [Google Scholar]
- Xue Y. B., Liu Z., Peng S. S., He Y., Zhang Y., Fang R., et al. (2015). Coumarin derivatives from Ainsliaea fragrans and their anticoagulant activity. Sci. Rep. 5, 13544–13552. 10.1007/s40262-016-0481-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan M., Lu Y., Chen C. H., Zhao Y., Lee K. H., Chen D. F. (2015a). Stelleralides D–J and anti-HIV daphnane diterpenes from Stellera chamaejasme. J. Nat. Prod. 78, 2712–2718. 10.1021/acs.jnatprod.5b00660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Y., Ma Y. T., Yang J., Horsman G. P., Luo D., Ji X., et al. (2016). Tropolone ring construction in the biosynthesis of rubrolone B, a cationic tropolone alkaloid from endophytic Streptomyces. Org. Lett. 18, 1254–1257. 10.1021/acs.orglett.6b00074 [DOI] [PubMed] [Google Scholar]
- Yan Y., Zhang J. X., Huang T., Mao X. Y., Gu W., He H. P., et al. (2015b). Bioactive limonoid constituents of Munronia henryi. J. Nat. Prod. 78, 811–821. 10.1021/np501057f [DOI] [PubMed] [Google Scholar]
- Yang B., Kong L., Wang X., Zhang Y., Li R., Yang M., et al. (2016a). Nitric oxide inhibitory activity and absolute configurations of arylalkenyl α,β-unsaturated δ/γ-lactones from Cryptocarya concinna. J. Nat. Prod.. 79, 196–203. 10.1021/acs.jnatprod.5b00839 [DOI] [PubMed] [Google Scholar]
- Yang C., Huang C., Zhang W., Zhu Y., Zhang C. (2015a). Heterologous expression of fluostatin gene cluster leads to a bioactive heterodimer. Org. Lett. 17, 5324–5327. 10.1021/acs.orglett.5b02683 [DOI] [PubMed] [Google Scholar]
- Yang C. S., Huang H. C., Wang S. Y., Sung P. J., Huang G. J., Chen J. J., et al. (2016b). New diphenol and isocoumarins from the aerial part of Lawsonia inermis and their inhibitory activities against NO production. Molecules 21, 1299–1307. 10.3390/molecules21101299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D., Jia X., Xie H., Wei X. (2016c). Further dihydrochalcone C-glycosides from the fruit of Averrhoa carambola. LWT Food Sci. Technol. 26, 604–609. 10.1016/j.lwt.2015.08.061 [DOI] [Google Scholar]
- Yang D., Li Z., Wang X., Yan H., Yang Y., Luo H., et al. (2015b). Denticulatains A and B unique stilbene-diterpene heterodimers from Macaranga denticulata. RSC Adv. 5, 13886–13890. 10.1039/c4ra14805c [DOI] [Google Scholar]
- Yang D., Xie H., Jiang Y., Wei X. (2016d). Phenolics from strawberry cv. Falandi and their antioxidant and α-glucosidase inhibitory activities. Food Chem. 194, 857–863. 10.1016/j.foodchem.2015.08.091 [DOI] [PubMed] [Google Scholar]
- Yang J., Wang W., Wu H., Du X., Li X., Li Y., et al. (2016e). Bioactive enmein-type ent-kaurane diterpenoids from Isodon phyllostachys. J. Nat. Prod. 79, 132–140. 10.1021/acs.jnatprod.5b00802 [DOI] [PubMed] [Google Scholar]
- Yang J., Zhu X., Cao M., Wang C., Zhang C., Lu Z., et al. (2016f). Genomics-inspired discovery of three antibacterial active metabolites, aurantinins B, C, and D from compost-associated Bacillus subtilis fmb60. J. Agric. Food Chem. 64, 8811–8820. 10.1021/acs.jafc.6b04455 [DOI] [PubMed] [Google Scholar]
- Yang W. Q., Zhu Z. X., Song Y. L., Qi B. W., Wang J., Su C., et al. (2017). Dimeric furanocoumarins from the roots of Angelica dahurica. Nat. Prod. Res. 31, 870–877. 10.1080/14786419.2016.1250090 [DOI] [PubMed] [Google Scholar]
- Yang X. W., Li M. M., Liu X., Ferreira D., Ding Y., Zhang J. J., et al. (2015c). Polycyclic polyprenylated acylphloroglucinol congeners possessing diverse structures from Hypericum henryi. J. Nat. Prod. 78, 885–895. 10.1021/acs.jnatprod.5b00057 [DOI] [PubMed] [Google Scholar]
- Yang Y., Gu L., Xiao Y., Liu Q., Hu H., Chen K., et al. (2015d). Rapid identification of alpha-glucosidase inhibitors from Phlomis tuberosa by sepbox chromatography and thin-layer chromatography bioautography. PLoS ONE 10:e0116922. 10.1371/journal.pone.0116922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye C., Wang H., Xue R., Han N., Wang L., Yang J., et al. (2015). Minor cytotoxic cardenolide glycosides from the root of Streptocaulon juventas. Steroids 93, 39–46. 10.1016/j.steroids.2014.10.005 [DOI] [PubMed] [Google Scholar]
- Yin X., Bai R., Guo Q., Su G., Wang J., Yang X., et al. (2016). Hendersine A, a novel isoquinoline alkaloid from Corydalis hendersonii. Tetrahedron Lett. 57, 4858–4862. 10.1016/j.tetlet.2016.09.064 [DOI] [Google Scholar]
- Yu G., Zhou G., Zhu M., Wang W., Zhu T., Gu Q., et al. (2016a). Neosartoryadins A and B, fumiquinazoline alkaloids from a mangrove-derived fungus, Neosartorya udagawae HDN13-313. Org. Lett. 18, 244–247. 10.1021/acs.orglett.5b02964 [DOI] [PubMed] [Google Scholar]
- Yu J. H., Liu Q. F., Sheng L., Wang G. C., Li J., Yue J. M. (2016b). Cipacinoids A–D, four limonoids with spirocyclic skeletons from Cipadessa cinerascens. Org. Lett. 18, 444–447. 10.1021/acs.orglett.5b03487 [DOI] [PubMed] [Google Scholar]
- Yu J., Shen Y., Wu Y., Leng Y., Zhang H., Yue J. (2015). Ricinodols A–G: new tetracyclic triterpenoids as 11β-HSD1 inhibitors from Ricinodendron heudelotii. RSC Adv. 5, 26777–26784. 10.1039/c5ra01857a [DOI] [Google Scholar]
- Yu Y., Gan L. S., Yang S. P., Sheng L., Liu Q. F., Chen S. N., et al. (2016c). Eucarobustols A–I, conjugates of sesquiterpenoids and acylphloroglucinols from Eucalyptus robusta. J. Nat. Prod. 79, 1365–1372. 10.1021/acs.jnatprod.6b00090 [DOI] [PubMed] [Google Scholar]
- Yuan W., Cheng S., Fu W., Zhao M., Li X., Cai Y., et al. (2016). Structurally diverse metabolites from the soft coral Sinularia verruca collected in the South China Sea. J. Nat. Prod. 79, 1124–1131. 10.1021/acs.jnatprod.6b00031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue S., Jiao Z., Sun H., Jin T., Xiang L. (2015). A new tricyclic alkaloid from Portulaca oleracea L. Helv. Chim. Acta 98, 961–966. 10.1002/hlca.201400374 [DOI] [Google Scholar]
- Zhan G., Qu X., Liu J., Tong Q., Zhou J., Sun B., et al. (2016). Zephycandidine A, the first naturally occurring imidazo[1,2-f]phenanthridine alkaloid from Zephyranthes candida, exhibits significant anti-tumor and anti-acetylcholinesteraseaActivities. Sci. Rep. 6, 33990–33998. 10.1038/srep33990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang A. H., Tan R., Jiang N., Yusupu K., Wang G., Wang X. L., et al. (2016a). Selesconol, a fungal polyketide that induces stem cell differentiation. Org. Lett. 18, 5488–5491. 10.1021/acs.orglett.6b02688 [DOI] [PubMed] [Google Scholar]
- Zhang C. L., Liu Y. F., Wang Y., Liang D., Jiang Z. B., Li L., et al. (2015a). Polycycloiridals A–D, four iridal-type triterpenoids with an alpha-terpineol moiety from Iris tectorum. Org. Lett. 17, 5686–5689. 10.1021/acs.orglett.5b02982 [DOI] [PubMed] [Google Scholar]
- Zhang D., Tao X., Chen R., Liu J., Li L., Fang X., et al. (2015b). Pericoannosin A, a polyketide synthase-nonribosomal peptide synthetase hybrid metabolite with new carbon skeleton from the endophytic fungus Periconia sp. Org. Lett. 17, 4304–4307. 10.1021/acs.orglett.5b02123 [DOI] [PubMed] [Google Scholar]
- Zhang D. B., Yu D. G., Sun M., Zhu X. X., Yao X. J., Zhou S. Y., et al. (2015c). Ervatamines A–I, anti-inflammatory monoterpenoid indole alkaloids with diverse skeletons from Ervatamia hainanensis. J. Nat. Prod. 78, 1253–1261. 10.1021/acs.jnatprod.5b00051 [DOI] [PubMed] [Google Scholar]
- Zhang F., Yang Y. N., Song X. Y., Shao S. Y., Feng Z. M., Jiang J. S., et al. (2015d). Forsythoneosides A–D, neuroprotective phenethanoid and flavone glycoside heterodimers from the fruits of Forsythia suspensa. J. Nat. Prod. 78, 2390–2397. 10.1021/acs.jnatprod.5b00372 [DOI] [PubMed] [Google Scholar]
- Zhang H., Liu J., Gan L. S., Dalal S., Cassera M. B., Yue J. M. (2016b). Antimalarial diterpenoid dimers of a new carbon skeleton from Aphanamixis grandifolia. Org. Biomol. Chem. 14, 957–962. 10.1039/c5ob02296g [DOI] [PubMed] [Google Scholar]
- Zhang H., Shyaula S. L., Li J. Y., Li J., Yue J. M. (2016c). Himalensines A and B, alkaloids from Daphniphyllum himalense. Org. Lett. 18, 1202–1205. 10.1021/acs.orglett.6b00362 [DOI] [PubMed] [Google Scholar]
- Zhang H. J., Zhang Y. M., Luo J. G., Luo J., Kong L. Y. (2015e). Anti–inflammatory diterpene dimers from the root barks of Aphanamixis grandifolia. Org. Biomol. Chem. 13, 7452–7458. 10.1039/c5ob00674k [DOI] [PubMed] [Google Scholar]
- Zhang H., Zhu K. K., Han Y. S., Luo C., Wainberg M. A., Yue J. M. (2015f). Flueggether A and virosinine A, anti-HIV alkaloids from Flueggea virosa. Org. Lett. 17, 6274–6277. 10.1021/acs.orglett.5b03320 [DOI] [PubMed] [Google Scholar]
- Zhang J., Chen J., Liang Z., Zhao C. (2014). New lignans and their biological activities. 11, 1–54. 10.1002/cbdv.201100433 [DOI] [PubMed] [Google Scholar]
- Zhang J., Ip F. C. F., Tong E. P. S., Chan K. W., Li L., Ng Y. P., et al. (2015g). Ningpoensines A–C: unusual zwitterionic alkaloids from Scrophularia ningpoensis. Tetrahedron Lett. 56, 5453–5456. 10.1016/j.tetlet.2015.08.021 [DOI] [Google Scholar]
- Zhang J., Lin X., Li L., Zhong B., Liao X., Liu Y., et al. (2015h). Gliomasolides A–E, unusual macrolides from a sponge-derived fungus Gliomastix sp. ZSDS1-F7-2. RSC Adv. 5, 54645–54648. 10.1039/c5ra08559d [DOI] [Google Scholar]
- Zhang J., Liu L., Wang B., Zhang Y., Wang L., Liu X., et al. (2015i). Phomanolides A and B from the fungus Phoma sp.: meroterpenoids derived from a putative tropolonic sesquiterpene via Hetero-Diels-Alder reactions. J. Nat. Prod. 78, 3058–3066. 10.1021/acs.jnatprod.5b00969 [DOI] [PubMed] [Google Scholar]
- Zhang L., Feng B., Chen G., Li S., Sun Y., Wu H., et al. (2016d). Sporulaminals A and B a pair of unusual epimeric spiroaminal derivatives from a marine-derived fungus Paraconiothyrium sporulosum YK-03. RSC Adv. 6, 42361–42366. 10.1039/C6RA01401A [DOI] [Google Scholar]
- Zhang L., Feng B., Sun Y., Wu H., Li S., Liu B., et al. (2016e). Flaviphenalenones A–C, three new phenalenone derivatives from the fungus Aspergillus flavipes PJ03-11. Tetrahedron Lett. 57, 645–649. 10.1016/j.tetlet.2015.12.099 [DOI] [Google Scholar]
- Zhang L. H., Feng B. M., Zhao Y. Q., Sun Y., Liu B., Liu F., et al. (2016f). Polyketide butenolide, diphenyl ether, and benzophenone derivatives from the fungus Aspergillus flavipes PJ03-11. Bioorg. Med. Chem. Lett. 26, 346–350. 10.1016/j.bmcl.2015.12.009 [DOI] [PubMed] [Google Scholar]
- Zhang P. Z., Gu J., Zhang G. L. (2015j). Novel stilbenes from Artocarpus nanchuanensis. J. Asian Nat. Prod. Res. 17, 217–223. 10.1080/10286020.2015.1006202 [DOI] [PubMed] [Google Scholar]
- Zhang P., Tian H., Nie Q., Wang L., Zhou S., Ye W., et al. (2016g). Structures and inhibitory activity against breast cancer cells of new bufadienolides from the eggs of toad Bufo bufo gargarizans. RSC Adv. 6, 93832–93841. 10.1039/c6ra18676a [DOI] [Google Scholar]
- Zhang W., An F., Zhou M., Chen M., Jian K., Quasie O., et al. (2016h). Limonoids with diverse frameworks from the stem bark of Entandrophragma angolense and their bioactivities. RSC Adv. 6, 97160–97171. 10.1039/C6RA19532F [DOI] [Google Scholar]
- Zhang W., Huang X. J., Zhang S. Y., Zhang D. M., Jiang R. W., Hu J. Y., et al. (2015k). Geleganidines A–C, unusual monoterpenoid indole alkaloids from Gelsemium elegans. J. Nat. Prod. 78, 2036–2044. 10.1021/acs.jnatprod.5b00351 [DOI] [PubMed] [Google Scholar]
- Zhang X., Chen C., Li Y., Chen D., Dong L., Na W., et al. (2016i). Tadehaginosides A–J, phenylpropanoid glucosides from Tadehagi triquetrum, enhance glucose uptake via the upregulation of PPARγ and GLUT-4 in C2C12 myotubes. J. Nat. Prod. 79, 1249–1258. 10.1021/acs.jnatprod.5b00820 [DOI] [PubMed] [Google Scholar]
- Zhang X., Khan A., Wang L., Yu K., Li F., Wang M. (2016j). Four new phorbol diesters from Croton tiglium and their cytotoxic activities. Phytochem. Lett. 16, 82–86. 10.1016/j.phytol.2016.03.008 [DOI] [Google Scholar]
- Zhang X., Ren J., Cheng X., Jin H., Zhang W. (2015l). One new unusual sesterterpenoid and four new sesquiterpene dimers from Inula britannica. RSC Adv. 5, 1979–1982. 10.1039/c4ra11171k [DOI] [Google Scholar]
- Zhang Y., Liu Y., Li Y., Ma S., Li L., Qu J., et al. (2016k). Phenolic constituents from the roots of Alangium chinense. Chin. Chem. Lett. 28, 32–36. 10.1016/j.cclet.2016.05.012 [DOI] [Google Scholar]
- Zhang Y., Luo J., Wan C., Zhou Z., Kong L. (2015m). Four new flavonoids with α-glucosidase inhibitory activities from Morus alba var. tatarica. Chem. Biodivers. 12, 1768–1776. 10.1002/cbdv.201500005 [DOI] [PubMed] [Google Scholar]
- Zhang Y. B., Zhan L. Q., Li G. Q., Wang F., Wang Y., Li Y. L., et al. (2016l). Dimeric matrine-type alkaloids from the roots of Sophora flavescens and their anti-hepatitis B virus activities. J. Org. Chem. 81, 6273–6280. 10.1021/acs.joc.6b00804 [DOI] [PubMed] [Google Scholar]
- Zhang Z., He X., Liu C., Che Q., Zhu T., Gu Q., et al. (2016m). Clindanones A and B and cladosporols F and G, polyketides from the deep-sea derived fungus Cladosporium cladosporioides HDN14-342. RSC Adv. 6, 76498–76504. 10.1039/C6RA14640F [DOI] [Google Scholar]
- Zhang Z., Yang F., Fu J., Shen Y., He W., Zhang W. (2016n). Delavatine A, a structurally unusual cyclopenta[de]isoquinoline alkaloid from Incarvillea delavayi. RSC Adv. 6, 65885–65888. 10.1039/C6RA11915H [DOI] [Google Scholar]
- Zhao C. (1936). Research of Chinese tripterygium. Chin. J. Physiol. 10:529. [Google Scholar]
- Zhao C. (1937). Two saponins from Panax notoginseng. Chin. J. Physiol. 12:59. [Google Scholar]
- Zhao D. D., Jiang L. L., Li H. Y., Yan P. F., Zhang Y. L. (2016). Chemical components and pharmacological activities of terpene natural products from the Genus Paeonia. Molecules 21, 1361–1374. 10.3390/molecules21101362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao M., Onakpa M. M., Santarsiero B. D., Huang X. J., Zhang X. Q., Chen J., et al. (2015a). Icacinlactone H and icacintrichantholide from the tuber of Icacina trichantha. Org. Lett. 17, 3834–3837. 10.1021/acs.orglett.5b01806 [DOI] [PubMed] [Google Scholar]
- Zhao Q., Song Q., Jiang K., Li G., Wei W., Li Y., et al. (2015b). Spirochensilides A and B, two new rearranged triterpenoids from Abies chensiensis. Org. Lett. 17, 2760–2763. 10.1021/acs.orglett.5b01166 [DOI] [PubMed] [Google Scholar]
- Zhao X. R., Huo X. K., Dong P. P., Wang C., Huang S. S., Zhang B. J., et al. (2015c). Inhibitory effects of highly oxygenated lanostane derivatives from the fungus Ganoderma lucidum on P-glycoprotein and alpha-glucosidase. J. Nat. Prod. 78, 1868–1876. 10.1021/acs.jnatprod.5b00132 [DOI] [PubMed] [Google Scholar]
- Zhen X., Gong T., Liu F., Zhang P. C., Zhou W. Q., Li Y., et al. (2015). A new analogue of echinomycin and a new cyclic dipeptide from a marine-derived Streptomyces sp. LS298. Mar. Drugs 13, 6947–6961. 10.3390/md13116947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng M., Ren H., Luo Y., Dong L., Wang L., Zhou Z., et al. (2015a). A new diantheramide and a new cyclic peptide from the seeds of Vaccaria hispanica. Phytochem. Lett. 11, 240–244. 10.1016/j.phytol.2015.01.007 [DOI] [Google Scholar]
- Zheng Y., Pang H., Wang J., Shi G., Huang J. (2015b). New apoptosis-inducing sesquiterpenoids from the mycelial culture of Chinese edible fungus Pleurotus cystidiosus. J. Agric. Food Chem. 63, 545–551. 10.1021/jf504931n [DOI] [PubMed] [Google Scholar]
- Zhou B., Shen Y., Wu Y., Leng Y., Yue J. (2015a). Limonoids with 11beta-hydroxysteroid dehydrogenase type 1 inhibitory activities from Dysoxylum mollissimum. J. Nat. Prod. 78, 2116–2122. 10.1021/acs.jnatprod.5b00442 [DOI] [PubMed] [Google Scholar]
- Zhou B., Wu Y., Dalal S., Merino E. F., Liu Q. F., Xu C. H., et al. (2017). Nanomolar antimalarial agents against chloroquine-resistant Plasmodium falciparum from medicinal plants and their structure-activity relationships. J. Nat. Prod. 80, 96–107. 10.1021/acs.jnatprod.6b00744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H., Sun X., Li N., Che Q., Zhu T., Gu Q., et al. (2016a). Isoindolone-containing meroperpenoids from the endophytic fungus Emericella nidulans HDN12-249. Org. Lett. 18, 4670–4673. 10.1021/acs.orglett.6b02297 [DOI] [PubMed] [Google Scholar]
- Zhou J., Li C. J., Yang J. Z., Ma J., Wu L. Q., Wang W. J., et al. (2016b). Phenylpropanoid and lignan glycosides from the aerial parts of Lespedeza cuneata. Phytochemistry 121, 58–64. 10.1016/j.phytochem.2015.10.002 [DOI] [PubMed] [Google Scholar]
- Zhou J., Zhang J., Cheng A., Xiong Y., Liu L., Lou H. (2015b). Highly rigid labdane-type diterpenoids from a Chinese liverwort and light-driven structure diversification. Org. Lett. 17, 3560–3563. 10.1021/acs.orglett.5b01664 [DOI] [PubMed] [Google Scholar]
- Zhou J., Zhang J., Li R., Liu J., Fan P., Li Y., et al. (2016c). Hapmnioides A–C, rearranged labdane-type diterpenoids from the Chinese liverwort Haplomitrium mnioides. Org. Lett. 18, 4274–4276. 10.1021/acs.orglett.6b01854 [DOI] [PubMed] [Google Scholar]
- Zhou M., Liu Y., Song J., Peng X. G., Cheng Q., Cao H., et al. (2016d). Schincalide A, a schinortriterpenoid with a tricyclo[5.2.1.0(1,6)]decane-bridged system from the stems and leaves of Schisandra incarnate. Org. Lett. 18, 4558–4561. 10.1021/acs.orglett.6b02197 [DOI] [PubMed] [Google Scholar]
- Zhou M., Zhou K., Gao X. M., Jiang Z. Y., Lv J. J., Liu Z. H., et al. (2015c). Fistulains A and B, new bischromones from the bark of Cassia fistula, and their activities. Org. Lett. 17, 2638–2641. 10.1021/acs.orglett.5b01007 [DOI] [PubMed] [Google Scholar]
- Zhou X. M., Zheng C. J., Chen G. Y., Song X. P., Han C. R., Tang X. Z., et al. (2015d). Two new stemphol sulfates from the mangrove endophytic fungus Stemphylium sp. 33231. J. Antibiot. 68, 501–503. 10.1038/ja.2015.16 [DOI] [PubMed] [Google Scholar]
- Zhou Z. B., Li Z. R., Wang X. B., Luo J. G., Kong L. Y. (2016e). Polycyclic polyprenylated derivatives from Hypericum uralum: neuroprotective effects and antidepressant-like activity of uralodin A. J. Nat. Prod. 79, 1231–1240. 10.1021/acs.jnatprod.5b00667 [DOI] [PubMed] [Google Scholar]
- Zhou Z., Xu Q., Bu Q., Guo Y., Liu S., Liu Y., et al. (2015e). Genome mining-directed activation of a silent angucycline biosynthetic gene cluster in Streptomyces chattanoogensis. Chem. Bio. Chem. 16, 496–502. 10.1002/cbic.201402577 [DOI] [PubMed] [Google Scholar]
- Zhu H., Chen C., Tong Q., Chen X., Yang J., Liu J., et al. (2015a). Hyperisampsins H–M, cytotoxic polycyclic polyprenylated acylphloroglucinols from Hypericum sampsonii. Sci. Rep. 5, 14772–14782. 10.1038/srep14772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H., Chen C., Tong Q., Li X., Yang J., Xue Y., et al. (2016). Epicochalasines A and B two bioactive merocytochalasans bearing caged epicoccine dimer units from Aspergillus flavipes. Angw. Chem. 128, 3547–3551. 10.1002/anie.201511315 [DOI] [PubMed] [Google Scholar]
- Zhu J., Cheng B., Zheng Y., Dong Z., Lin S., Tang G., et al. (2015b). Enantiomeric neolignans and sesquineolignans from Jatropha integerrima and their absolute configurations. RSC Adv. 5, 12202–12208. 10.1039/c4ra15966g [DOI] [Google Scholar]
- Zhu M., Zhang X., Feng H., Dai J., Li J., Che Q., et al. (2017). Penicisulfuranols A–F, alkaloids from the mangrove endophytic fungus Penicillium janthinellum HDN13-309. J. Nat. Prod. 80, 71–75. 10.1021/acs.jnatprod.6b00483 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.