Abstract
Background
It has been reported that D1 receptor (D1R) activation reduces GABAA receptor (GABAAR) current, and baicalin (BAI) displays therapeutic efficacy in diseases involving cognitive impairment.
Methods
We investigated the mechanisms by which BAI could improve DA-induced minimal hepatic encephalopathy (MHE) using immunoblotting, immunofluorescence, and co-immunoprecipitation.
Results
BAI did not induce toxicity on the primary cultured neurons. And no obvious toxicity of BAI to the brain was found in rats. DA activated D1R/dopamine and adenosine 3′5′-monophosphate-regulated phospho-protein (DARPP32) to reduce the GABAAR current; BAI treatment did not change the D1R/DARPP32 levels but blocked DA-induced reduction of GABAAR levels in primary cultured neurons. DA decreased the interaction of GABAAR with TrkB and the expression of downstream AKT, which was blocked by BAI treatment. Moreover, BAI reversed the decrease in the expression of GABAAR/TrkB/AKT and prevented the impairment of synaptogenesis and memory deficits in MHE rats.
Conclusions
These results suggest that BAI has neuroprotective and synaptoprotective effects on MHE which are not related to upstream D1R/DARPP32 signaling, but to the targeting of downstream GABAAR signaling to TrkB.
Keywords: Minimal hepatic encephalopathy, Dopamine, Baicalin, GABAAR, DARPP32
Introduction
Patients with minimal hepatic encephalopathy (MHE) show a complex neuropsychiatric syndrome including mild cognitive impairment, attention deficits, psychomotor slowing, and impaired bimanual and visuo-motor coordination, which can be unveiled using psychometric tests (Amodio et al. 2004; Montoliu et al. 2007). Our several previous studies showed that intracranial dopamine (DA) levels are abnormally elevated in MHE rats, and the elevation of intracranial DA plays an essential role in learning and memory impairment in MHE rats (Ding et al. 2013, 2014a, b, c). The pathogenesis of MHE has been confirmed to be caused by the elevation of DA (Ding et al. 2013). Although DA is known to modulate voltage-dependent ion channels in neostriatal neurons (Surmeier et al. 1995), elevated DA levels have been implicated in several neurological and psychiatric disorders, including schizophrenia, Huntington’s disease, Alzheimer’s disease, attention deficit disorder, and addictive behavior (Blum et al. 2012; Ungless and Grace 2012). However, the deeper mechanism underlying the effects of DA on the pathogenesis of MHE remains unclear, and therapeutic drugs for MHE are lacking.
The toxic actions of DA appear to involve several distinct mechanisms, including autoxidation, oxidative stress, and D1 receptor activation (Chen et al. 2004). Importantly, DA released by midbrain dopaminergic neurons in both the striatum and the substantia nigra (Cheramy et al. 1981; Geffen et al. 1976) controls g-aminobutyric acid (GABA) release through the activation of D1 receptors (D1Rs) located at the somato-dendrites and terminals of striato-nigral medium spiny neurons (Acosta-Garcia et al. 2009). A variety of biochemical studies as well as targeted deletion and mutation of dopamine and adenosine 3′5′-monophosphate-regulated phospho-protein (DARPP32) in mice have shown that DARPP32 plays a critical role in the actions of DA (Svenningsson et al. 2003). Mice lacking DARPP32 exhibit profound deficits in their molecular, electrophysiological, and behavioral responses to DA (Greengard et al. 1999). D1R stimulation in neostriatal medium spiny neurons reduces postsynaptic GABAA receptor currents by activating a PKA/dopamine- and adenosine 3′5′-monophosphate-regulated DARPP32/protein phosphatase 1 signaling cascade targeting GABAA receptor β1 subunits (Flores-Hernandez et al. 2000). However, we hypothesized that the mechanisms of cognitive loss are related to the action of D1R/DARPP32/GABAAR signaling in MHE.
Baicalin (7-d-glucuronic acid-5,6-dihydroxyflavone; BAI) is a flavone isolated from the Chinese medicinal herb Scutellaria baicalensis Georgi that has been used to treat inflammatory diseases and ischemic stroke for thousands of years in China (Zhang et al. 2006). BAI has been identified as a novel and promising therapeutic agent for human central nervous system diseases, because it can pass through the blood–brain barrier into the central nervous system and distribute within the brain tissue, specifically in the hippocampus, striatum, cortex, and thalamus (Tarragó et al. 2008). In order to establish the optimal therapeutic usage of BAI, its physiological effects need to be thoroughly elucidated. The neuroprotective effects of BAI have been described in many experimental models, such as cerebral ischemia (Cheng et al. 2013; Zhou et al. 2014), spinal cord injury (Cao et al. 2010), epilepsy (Liu et al. 2012), learning and memory deficits (Lee et al. 2014), and cultured neurons (Yin et al. 2011). We hypothesized that BAI protects against memory decline in MHE.
Baicalin, along with its aglycone baicalein, is a positive allosteric modulator of the benzodiazepine site and/or non-benzodiazepine site of the GABAA receptor (Hui et al. 2000). Recent studies further demonstrated the anxiolytic-like effect of BAI in a Vogel conflict test and an elevated plus maze test and suggested that its pharmacological action occurred through GABAA receptors (Xu et al. 2006). BAI may inhibit D1R-stimulated GABAAR suppression. However, the neuroprotective effects of BAI in the MHE rat brain may be related to the suppression of DA-induced GABAAR inhibition. However, there is little information about the therapeutic effects of BAI on DA-induced toxicity in vivo. Here, we tested the efficacy of BAI in MHE rats and in a DA injection rat model and investigated the potential mechanisms of its function.
The present study investigated the hypothesis that BAI protects against MHE and that the neuroprotective effects are associated with the activation of GABAAR, the modulation of downstream tropomyosin receptor kinase B (TrkB) signaling, and the expression of synapse-related proteins. The study focused on the evaluation of the effects of BAI on MHE and explored the potential molecular mechanisms using MHE and DA-injected rats.
Materials and methods
Primary hippocampal and cortical neuron cultures and treatments
Primary hippocampal neurons (PHNs) or primary hippocampal neurons (PCNs) were prepared from 1-day-old Sprague–Dawley rat pups. PHNs or PCNs were dissociated from the freshly dissected hippocampus or cerebral cortex by mechanical disruption in the presence of trypsin and DNase and then plated in poly-l-lysine-precoated six-well plates. Cells were seeded at a density of 2 × 106 cells per well in a Neurobasal Medium (1X) supplemented with 0.5 mM GlutaMAX™-I, B-27® incubated at 37 °C, 5% CO2. The medium was changed after 4 days. Neurons remained untreated or were stimulated with DA (10 μM) in the presence or absence of BAI (1, 2.5, 5, 10, or 30 μM). PHNs transfected with TrkB siRNA were treated with DA (10 μM) in the presence or absence of BAI.
Gene and siRNA transfection to PCNs
PCNs were transfected with 0.25 μg of TrkB siRNA (Santa Cruz, CA, USA) using Lipofectamine 2000 (Invitrogen) according to the protocol suggested by the manufacturer. The final concentration of siRNA was 10 nM. The Silencer Negative Control No. 1 siRNA (scrambled siRNA) was used as a control (Santa Cruz, CA, USA). After 48 h, cells were washed with Hank’s Balanced Salt Solution and treated with DA in the presence or absence of BAI.
MHE models
Sprague–Dawley rats (Experimental Animal Center of the Chinese Academy of Sciences in Shanghai) weighing 220–250 g were used. Rats were housed under controlled temperature (24 ± 1 °C) and light (12 h light starting at 07:00 a.m.) conditions, and all experiments were carried out in accordance with the guidelines laid down by the Ethics Committees of the Affiliated Hospital of Wenzhou Medical University regarding the care and use of animals for experimental procedures (Albrecht et al. 2000).
Before experimenting, all animals underwent two behavioral tests: the Y-maze (YM) and the water-finding task (WFT). The normal values of these behavioral tests were obtained. Rats (n = 55) were then randomly divided into two groups: the control group (n = 10) and the thioacetamid (TAA) group (n = 45). Liver cirrhosis was induced by intraperitoneal (IP) injection of TAA (200 mg/kg in normal saline, Sigma-Aldrich) twice per week for 8 weeks. TAA-treated rats with symptoms were diagnosed with HE according to the following criteria: delayed development of decreased motor activity, lethargy, and eventual progression to coma. TAA-treated rats with no HE symptoms were then again subjected to behavioral tests to confirm whether or not they had MHE. If TAA-treated rats met either of the following criteria, they were included in the MHE group: (a) YM value lower than mean ± 1.96·SD, (b) WFT value greater than mean ± 1.96·SD.
WT and MHE rats were injected intraperitoneally with BAI (20, 50, or 100 mg/kg) three times per week for 1 week. At 24 h after the last injection, rats were subjected to YM and WFT tests. Then, all rats were sacrificed to collect the blood, liver tissues, and cerebral cortex tissues.
DA-treated rat models and treatments
Rats (n = 40) were randomly divided into two groups: a DA-treated group (n = 30) and a control group (n = 10). Rats were anesthetized with an intraperitoneal injection of 10% chloralhydrate (0.4 g/kg). The rats were then placed on a stereotaxic apparatus. An intracerebroventricular injection of dopamine hydrochloride (10 μg/3 μl in saline) was stereotaxically injected in the left lateral ventricles of rats three times at 7-day intervals (anterior–posterior, 0.3 mm; lateral, 1.0 mm; horizontal, 3.0 mm from the bregma; n = 30). Then, DA-treated rats were intraperitoneally injected with BAI (20, 50, or 100 mg/kg) three times in 1 week. At 24 h after the last injection, rats underwent YM and WFT tests and were then killed to collect the blood, liver tissues, and cerebral cortex tissues.
Y-maze test
Rats were individually placed at the end of an arm and allowed to explore the maze freely for 8 min. Spontaneous alternation percentage (SA%) was defined as the ratio of the arm choices that differed from the previous two choices (“successful choices”) to the total choices during the run (“total entry minus 2” because the first two entries could not be evaluated) (Yamada et al. 2005).
Water-finding task
A rat was placed at the near-right corner of the apparatus and allowed to explore it freely for 3 min. The elapsed times until the entry into the alcove (entry latency (EL)), until the touching/sniffing/licking of the water tube (contacting latency (CL)), and until the initiation of drinking from the water tube (drinking latency (DL)) were measured (Kawasumi et al. 2004).
Co-immunoprecipitations
Lysates were centrifuged, and supernatants were incubated with antibodies overnight (4 °C) and subsequently incubated with protein G agarose beads (Millipore) for 5 h (4 °C). Beads were washed three times with lysis buffer, and immunoprecipitated proteins were resolved by SDS-PAGE. Following transfer, proteins were probed using primary antibodies and secondary antibodies. Data are expressed as fold change relative to control for three or four independent experiments.
MTT assay
Cells were incubated with medium containing 0.5 mg/ml MTT at 37 °C for 3 h and then treated with dimethylsulfoxide. The absorbance of each aliquot at 490 nm was determined using a microplate reader (Tecan). Cell viability was expressed as the ratio of the signals obtained from the treated and control cultures.
Semi-quantitative PCR
Total RNA was extracted from cerebral cortex tissues or PCAs using TRIzol Reagent (Invitrogen). First-strand cDNA was synthesized from 1 μg total RNA using Omniscript Reverse Transcriptase (QIAGEN). PCR amplification with Taq DNA polymerase (Sigma-Aldrich) was performed under denaturation at 94 °C for 30 s, annealing at 60 °C for 30 s, and elongation at 72 °C for 90 s, repeating the indicated cycles. The sequences for the forward and reverse primers are as follows: GABAAR β1, forward 5’-CCGGCAAGGGGCGCA-3’ and reverse 5’-TCAGTCAAGTCGGGGATCTTCACT-3’; GABAAR β3, forward 5’-AGAGCATGCCCAAGGAAGG-3’ and reverse 5’-AGGTGGGTCTTCTTGTGCG-3’; TrkB, forward 5’-CGGAATTCATGTCGCCCTGGCCGAGGTG-3’ and reverse 5’-CGGGATCCCAGCCTTGTCTTTCCTTTATCT-3’; PSD95, forward 5’-CAAAGACCGTGCCAACGAT-3’ and reverse 5’-GGGACACAGGATCCAAACTTGT-3’; synapsin I, forward 5’-TTCAGCATGGCACGTAATGG-3’ and reverse 5’-CCAGCATACTGCAGCCCAAT-3’; GAPDH, forward 5’-ACCCAGAAGACTGTGGATGG-3’ and reverse 5’-ACACATTGGGGGTAGGAACA-3’.
Immunoblotting
The total amount of protein in the lysates was determined by BCA protein assay (AMRESCO). Samples (50 μg protein) were separated by 10% SDS-PAGE and electroblotted to PVDF membranes, which were blocked by incubation in 5% non-fat dry milk dissolved in TBS-T (150 mM NaCl, 50 mM Tris, 0.05% Tween 20). Following transfer, proteins were probed using the following primary antibodies: D1R, DARPP32, GABAARβ1, GABAARβ3, TrkB, pAKT, AKT, synaptotagmin, synapsin I, PSD95, spinophilin, and β-actin (Abcam). Then, horseradish peroxidase-conjugated secondary antibody was used. After extensive washing, protein bands detected by antibodies were visualized by ECL reagent (Thermo) after exposure on Kodak BioMax film (Kodak).
Double-labeled fluorescent staining
Four-micron frozen cerebral cortex sections or PCAs cultured on glass coverslips were fixed with 4% paraformaldehyde for 30 min and then treated with 0.1% Triton X-100 for 10 min at room temperature. Blocking was achieved with PBS containing 5% normal goat serum for 1 h at room temperature. Sections were then incubated overnight at 4 °C with the following primary antibodies: GABAARβ1, GABAARβ3, TrkB, pAKT, synapsin I, PSD95, GAP43, VGAT, bassoon, and MAP2 (Abcam). Binding of primary antibodies was detected by incubating the sections for 30 min with FITC (green)/Alexa Fluor 594 (red)-conjugated secondary antibody. Imaging was performed with a Leica TCS SP2 confocal laser scanning microscope. For the analysis of synaptogenesis in PHNs, the neurons were co-stained with anti-VGAT and anti-bassoon antibodies. The number and size of the synapses were analyzed with the ImageJ software.
Electrophysiological analysis
Rats were anesthetized with isoflurane and decapitated, and the hippocampi were cut into 400-mm thick transverse slices using a vibratome. After incubation in artificial cerebrospinal fluid (aCSF) at room temperature for 60–90 min, slices were placed in a recording chamber on the stage of an upright microscope (Olympus CX-31) and perfused with aCSF (containing 1 mM MgCl2) at a rate of 3 ml per min at 23–24 °C. A 0.1 MΩ tungsten monopolar electrode was used to stimulate the Schaffer collaterals. The field excitatory postsynaptic potentials (fEPSPs) were recorded in CA1 stratum radiatum by a glass microelectrode filled with aCSF, with resistance of 3–4 MΩ. Field potential input–output curves were constructed by measuring the fEPSP slope corresponding to the stimulus intensity increase from 1 to 7 V in 0.5 V increments. A long-term potentiation (LTP) of fEPSPs was induced by three theta-burst stimulation (TBS), consisting of four pulses at 100 Hz repeated three times at 200-ms intervals. Paired-pulse facilitation (PPF) was examined by applying pairs of pulses at 20–500 ms intervals. The magnitude of LTP is expressed as the mean percentage of the initial baseline fEPSP slope.
Statistical analysis
Data are presented as mean ± SD. The statistical significance of between-group comparisons was determined by one-way analysis of variance (ANOVA). Values of P < 0.05 or P < 0.01 were considered to be statistically significant.
Results
BAI has no effect on DA-mediated DARPP32 signaling in vitro
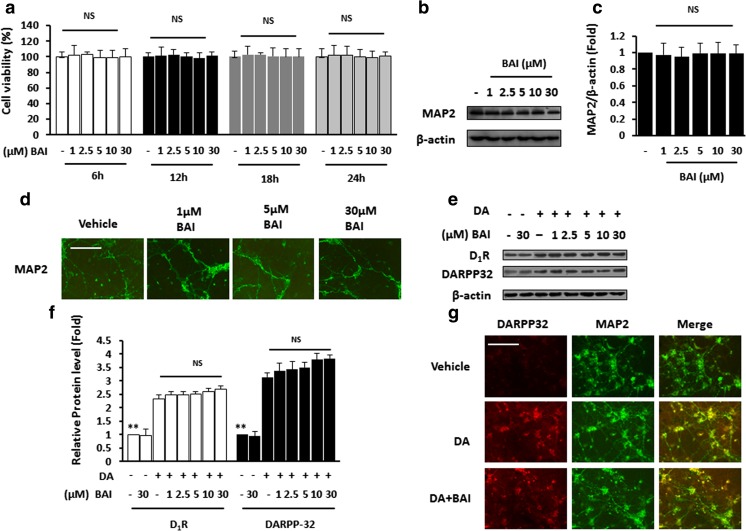
The potential toxicity of BAI to neurons had never been evaluated prior to this study. We first examined the effect of BAI on cell viability. The MTT assay showed that 0–30 μM BAI did not result in any cell death or significant effects on the cell viability of the PHNs (Fig. 1a). No obvious differences in the expression of MAP2 were found between the vehicle- and BAI-treated PHNs (Fig. 1b, c). MAP2 immunostaining revealed no obvious differences in the distribution or intensity of the immunofluorescence between the vehicle- and the BAI-treated PHNs (Fig. 1d). These results suggest that BAI has no toxic effect on neurons in vitro.
Fig. 1.
BAI has no effect on DA-mediated DARPP32 signaling in vitro. a MTT results for PHNs stimulated with 1–30 μM BAI at different time points. b Immunoblot analysis of lysates of PHNs exposed to various doses of BAI (1–30 μM) using anti-MAP2 and anti-β-actin antibodies and c subsequent densitometry. d Immunofluorescence staining of PHNs in the presence of various doses of BAI (1–30 μM) using antibodies against MAP2 (green). e Immunoblot analysis of lysates of PHNs stimulated with 10 μM DA in the presence of various doses of BAI (1–30 μM) using anti-D1R/DARPP32 and anti-β-actin antibodies and f subsequent densitometry. g Double immunofluorescence staining of PHNs stimulated with 10 μM DA in the presence of various doses of BAI (1–30 μM) using antibodies against DARPP32 (red), MAP2 (green). NS not significant. **P < 0.01 vs. DA-treated PCNs. Scale bar, 25 μm. MRGD, merged image
It has been reported that D1R stimulation in neostriatal medium spiny neurons reduces postsynaptic GABAA receptor currents, and GABAAR activation triggers synaptic scaling (Wenner 2014). Since BAI is known as a modulator of GABAAR activation (Flores-Hernandez et al. 2000), we aimed to examine whether BAI enhanced the effect of DA on DARPP32 signaling. Immunoblotting from cell lysates showed that increasing concentrations of DA significantly increased the expression of D1R and DARPP32, but GABAAR activation by BAI had no effect on D1R or DARPP32 levels (Fig. 1e, f). Immunostaining showed increases in cell-bound DARPP32 in rat PCNs exposed to DA. The addition of BAI did not impact DA-induced increases in cell-bound DARPP32 (Fig. 1g).
BAI inhibits the DA-induced inactivation of GABAAR in vitro
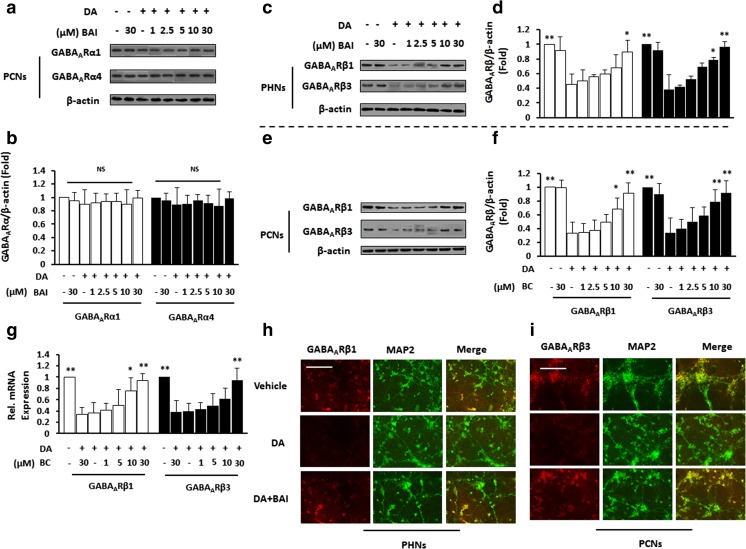
TrkB signaling has a direct role in the formation and maintenance of synapses (Hiester et al. 2013). We therefore examined whether BAI triggered GABAAR to interact with TrkB. IB analysis showed no obvious differences in the expression of GABAARα between the vehicle- and the DA-treated PHNs. The addition of 0–30 μM BAI also had no significant effect on the expression of GABAARα (Fig. 2a, b).
Fig. 2.
BAI reverses DA-induced inactivation of GABAAR. a Immunoblot analysis of lysates of PHNs stimulated with 10 μM DA in the presence of various doses of BAI (1–30 μM) using anti-GABAARα1/GABAARα2 and anti-β-actin antibodies and b subsequent densitometry. c–f Immunoblot analysis and densitometry of PHNs (c, d) and PCNs (e, f) stimulated with 10 μM DA in the presence of various doses of BAI (1–30 μM) using anti-GABAARβ1/GABAARβ3 and anti-β-actin antibodies. g GABAARβ1/GABAARβ3 mRNA in PCNs exposed to 10 μM DA in the presence of various doses of BAI (1–30 μM) were monitored by qPCR. h Double immunofluorescence staining of PHNs stimulated with 10 μM DA in the presence of BAI (30 μM) using antibodies against GABAARβ1 (red), MAP2 (green). Scale bar, 25 μm. MRGD, merged image. i Double immunofluorescence staining of PCNs stimulated with 10 μM DA in the presence of BAI (30 μM) using antibodies against GABAARβ 3 (red), MAP2 (green). *P < 0.05, **P < 0.01 vs. DA-treated PCNs. Scale bar, 25 μm. MRGD, merged image
IB analysis showed that DA significantly decreased GABAARβ1 and GABAARβ3 expression in PHNs compared with unstimulated cells. Treatment with BAI abolished the effect of DA on the expression of GABAARβ in a dose-dependent manner (Fig. 2c, d). In PCNs, DA treatment decreased the expression of GABAARβ1 and GABAARβ3, and the addition of BAI abrogated the effect of DA on GABAARβ1 and GABAARβ3 expression in a dose-dependent manner (Fig. 2e, f). QPCR showed that DA decreased GABAARβ1 and GABAARβ3 mRNA, and BAI treatment enhanced this effect in a dose-dependent manner in PHNs (Fig. 2g). Immunostaining further confirmed that DA significantly increased the expression of GABAARβ1 in PHNs (Fig. 2h) and GABAARβ3 in PCNs (Fig. 2i). BAI abolished the DA-stimulated reduction of GABAARβ levels. These results suggest that both DA and BAI do not affect the activation of GABAARα but induce the activation of GABAARβ.
BAI improves the interaction of GABAAR with TrkB and downstream signaling in DA-treated neurons
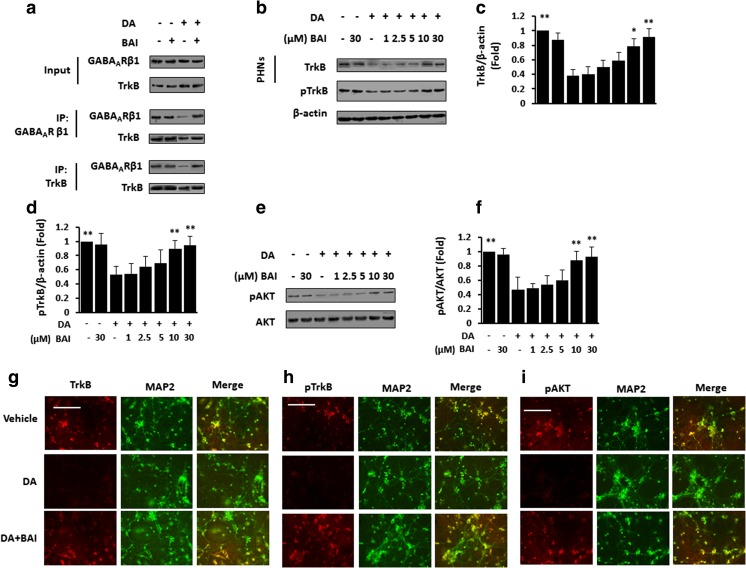
We examined the effect of BAI on the DA-mediated interaction of GABAARβ and TrkB by co-immunoprecipitation. Then, lysates of PHNs were immunoprecipitated with a GABAAARβ antibody; as predicted, DA induced a decrease in GABAARβ and also decreased the amount of TrkB that co-immunoprecipitated with GABAARβ, which was blocked by BAI. When lysates of PHNs were immunoprecipitated with TrkB antibody, as predicted, DA also decreased the amount of GABAARβ that co-immunoprecipitated with TrkB and increased the level of TrkB, which was also blocked by BAI (Fig. 3a). Immunoblotting confirmed that DA decreased TrkB expression and BAI treatment enhanced this effect in a dose-dependent manner in PHNs (Fig. 3b, c); additionally, DA decreased the phosphorylation of TrkB, and BAI treatment enhanced the DA-induced decrease in phosphorylated TrkB (pTrkB) in a dose-dependent manner in PHNs (Fig. 3b, d). Moreover, DA decreased the phosphorylation of AKT, and BAI enhanced this effect in a dose-dependent manner in PCNs (Fig. 3e, f). IF analysis confirmed that TrkB (Fig. 3g), pTrkB (Fig. 3h), and pAKT (Fig. 3i) were strongly expressed in the DA-treated PHNs, while BAI reduced the expression of TrkB/pTrkB/pAKT. These data suggest that BAI inhibits the DA-induced inactivation of the GABAARβ/TrkB signaling pathway in neurons.
Fig. 3.
BAI reverses DA-induced disruption of the interaction of GABAAR with TrkB and downstream signaling inactivation in vitro. a Immunoprecipitation of lysates from PHNs stimulated with 10 μM DA in the presence of BAI (30 μM) with control IgG, anti-TrkB, or anti-GABAARβ1 antibodies. Complexes were immunoblotted with anti-TrkB, or anti-GABAARβ1 antibodies. b Immunoblot analysis and densitometry of PHNs stimulated with 10 μM DA in the presence of various doses of BAI (1–30 μM) using anti-TrkB/pTrkB and anti-β-actin antibodies and subsequent densitometry (c, d). e Immunoblot analysis of PHNs stimulated with 10 μM DA in the presence of various doses of BAI (1–30 μM) using anti-pAKT and anti-AKT antibodies and subsequent densitometry (f). g–i Double immunofluorescence staining of PHNs stimulated with 10 μM DA in the presence of BAI (30 μM) using antibodies against TrkB (g)/pTrkB (h)/pAKT (i) (red), MAP2 (green). *P < 0.05, **P < 0.01 vs. DA-treated PCNs. Scale bar, 25 μm. MRGD, merged image
BAI prevents DA-induced impairment of synaptogenesis in vitro
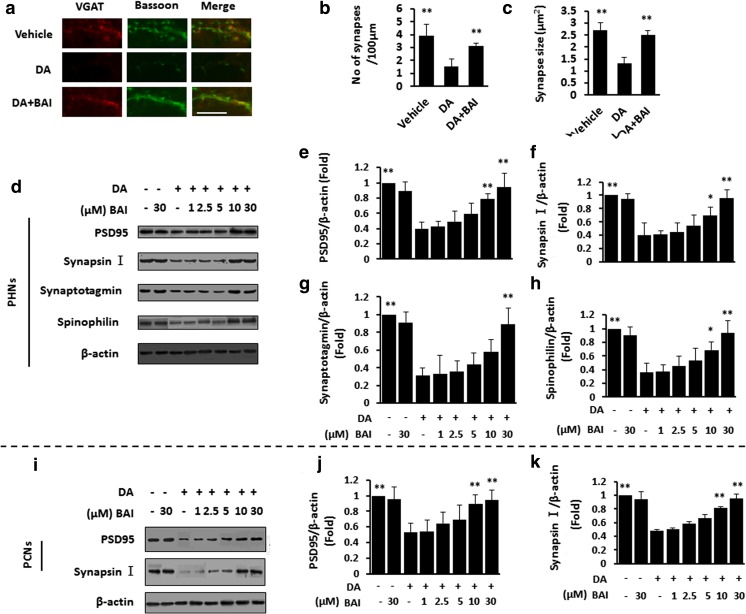
Next, we examined the effect of BAI on the DA-induced impairment of synaptic formation. For the analysis of synaptogenesis in primary hippocampal neurons, the neurons were co-stained with anti-VGAT and anti-bassoon antibodies. The results showed that DA treatment decreased the number of presynaptic structures expressing VGAT and bassoon, and the addition of BAI reversed this effect (Fig. 4a–c).
Fig. 4.
BAI prevents DA-induced impairment of synaptogenesis in vitro. a Double immunofluorescence staining of PHNs stimulated with 10 μM DA in the presence of BAI (30 μM) using the presynaptic markers VGAT antibody (green) and bassoon antibody (red). The number of synapses (b) and synapse size (c) were quantified. (d) Immunoblot analysis of PHNs stimulated with 10 μM DA in the presence of various doses of BAI (1–30 μM) using anti-PSD95/synapsin/synaptotagmin/spinophilin and anti-β-actin antibodies and subsequent densitometry (e–h). *P < 0.05, **P < 0.01 vs. vehicle-treated PCNs. i Immunoblot analysis of PCNs stimulated with 10 μM DA in the presence of various doses of BAI (1–30 μM) using anti-PSD95/synapsin I and anti-β-actin antibodies and subsequent densitometry (j). k PSD95/synapsin I mRNA in PHNs exposed to 10 μM DA in the presence of various doses of BAI (1–30 μM) were monitored by qPCR. * treated with DA in the presence or absence of BAI. *P < 0.05, **P < 0.01 vs. DA-treated PCNs. Scale bar, 25 μm. MRGD, merged image
IB analysis showed that DA treatment decreased the expression of PSD95/synapsin I/synaptotagmin/spinophilin, and the addition of BAI to the DA mixture nearly restored the four proteins to the basal level in PHNs (Fig. 4d–h). We confirmed that PSD95 and synapsin I were weakly expressed in response to DA and strongly expressed in response to BAI in PCNs (Fig. 4i, j). QPCR showed decreased PSD95/synapsin I mRNA in PHNs in response to DA, and the addition of BAI abrogated this effect (Fig. 4k).
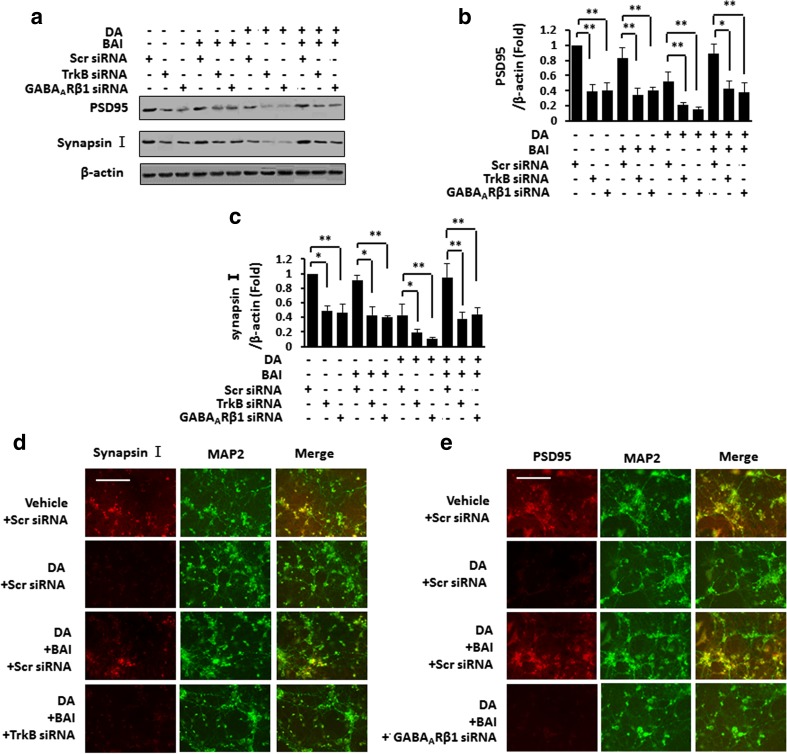
We then examined whether BAI reversed the DA-induced synaptic loss via TrkB signaling. IB analysis indicated that PSD and synapsin I levels were decreased by DA treatment, which was reversed by the addition of BAI in PHNs. Additionally, the effect of BAI was inhibited by TrkB or GABAARβ1 silencing (Fig. 5a–c). Double IF staining shows that both PSD95 (Fig. 5d) and synapsin I (Fig. 5e) levels were significantly decreased in DA-treated PHNs and were recovered to normal levels by the administration of BAI, while the effect of BAI was blocked by either TrkB siRNA or GABAARβ1 siRNA. These data suggest that BAI affects DA.
Fig. 5.
BAI prevents DA-induced impairment of synaptogenesis in vitro. a Immunoblot analysis of PHNs after TrkB/GABAARβ1 siRNA transfection stimulated with 10 μM DA in the presence of BAI (30 μM) using anti-PSD95/synapsin I and anti-β-actin antibodies and subsequent densitometry (b, c). d, e Double immunofluorescence staining of PHNs after TrkB/GABAARβ1 siRNA transfection stimulated with 10 μM DA in the presence of BAI (30 μM) using antibodies against PSD95 (d)/synapsin I (e) (red), MAP2 (green). *P < 0.05, **P < 0.01 vs. DA-treated PCNs. Scale bar, 25 μm. MRGD, merged image
BAI does not induce toxicity in the hippocampus and cerebral cortex
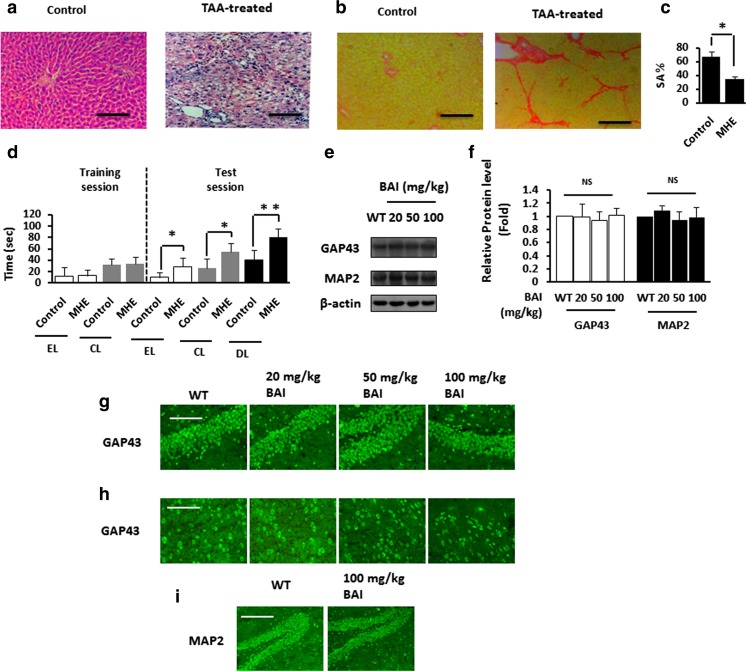
First, we successfully established the MHE rat model (Fig. 6a, b) and DA-injected rat model (Fig. 6c, d). Then, we evaluated the potential toxicity of orally administered BAI to the brain in vivo. Immunoblotting showed no significant difference in the expression levels of 43-kD growth-associated protein (GAP-43) and MAP2 between the control and the BAI groups (Fig. 6e, f). Furthermore, GAP-43 immunostaining revealed no obvious differences between the vehicle- and the BAI-treated rats in the distribution and intensity of immunofluorescence in the hippocampus (Fig. 6g) and cerebral cortex (Fig. 6h). Likewise, there were no significant differences between the vehicle- and the BAI-treated rats in the distribution and intensity of MAP2 immunofluorescence in the hippocampus (Fig. 6i). These findings indicate that BAI causes no obvious toxicity to the rat brain.
Fig. 6.
BAI does not induce toxicity in hippocampus and cerebral cortex. a HE staining of liver sections from TAA-treated rats. Scale bar, 50 μm. b Sirius red staining of liver sections from TAA-treated rats. Scale bar, 50 μm. c Spontaneous alternation percentages (SA%) in YM of normal rats and MHE rats. Data are shown as mean ± SD. *P < 0.05 vs. controls. d WFT results (EL entry latency, CL contacting latency, DL drinking latency) of normal rats and MHE rats. Data are shown as mean ± SD. *P < 0.05, **P < 0.01 vs. controls. e Immunoblot analysis of hippocampal lysates from WT rats treated with various concentrations of BAI (20, 50, 100 mg/kg) using anti-GAP43/MAP2 and anti-β-actin antibodies and subsequent densitometry (f). g Immunofluorescence staining of the hippocampus sections from WT rats treated with various concentrations of BAI (20, 50, 100 mg/kg) using antibodies against GAP43 (green). Scale bar, 50 μm. h Immunofluorescence staining of cortical sections from WT rats treated with various concentrations of BAI (20, 50, 100 mg/kg) using antibodies against GAP43 (green). Scale bar, 25 μm. i Immunofluorescence staining of hippocampal sections from WT rats treated with various concentrations of BAI (20, 50, 100 mg/kg) using antibodies against MAP2 (green). Scale bar, 100 μm. NS not significant
BAI reverses the inactivation of the GABAARβ/TrkB signaling pathway in MHE rats and DA-treated rats
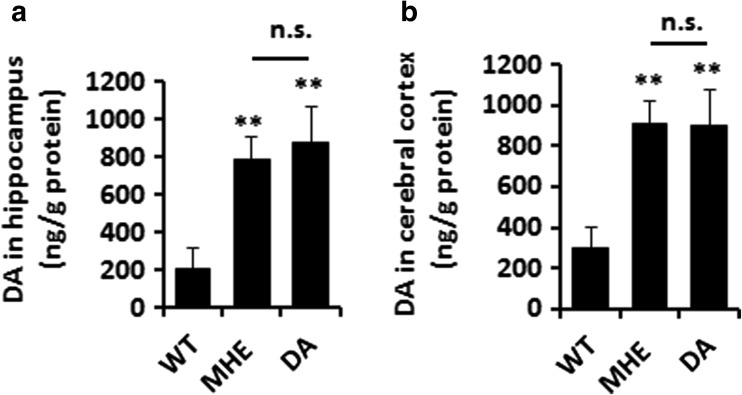
We examined whether the concentration of dopamine in the hippocampus and cortex of MHE rats was as high as that in DA-treated rats. We confirmed increased levels of DA in the hippocampus (Fig. 7a) and cerebral cortex (Fig. 7b) in both MHE rats and DA-treated rats compared with control rats, and the DA levels were as high in MHE rats as in DA-treated rats.
Fig. 7.
Concentration of intracranial DA in MHE and DA-treated rats. a Hippocampal homogenates from MHE and DA-treated rats were analyzed for DA concentration by high-performance liquid chromatography. b Cerebral cortex homogenates from MHE and DA-treated rats were analyzed for DA concentration by high-performance liquid chromatography
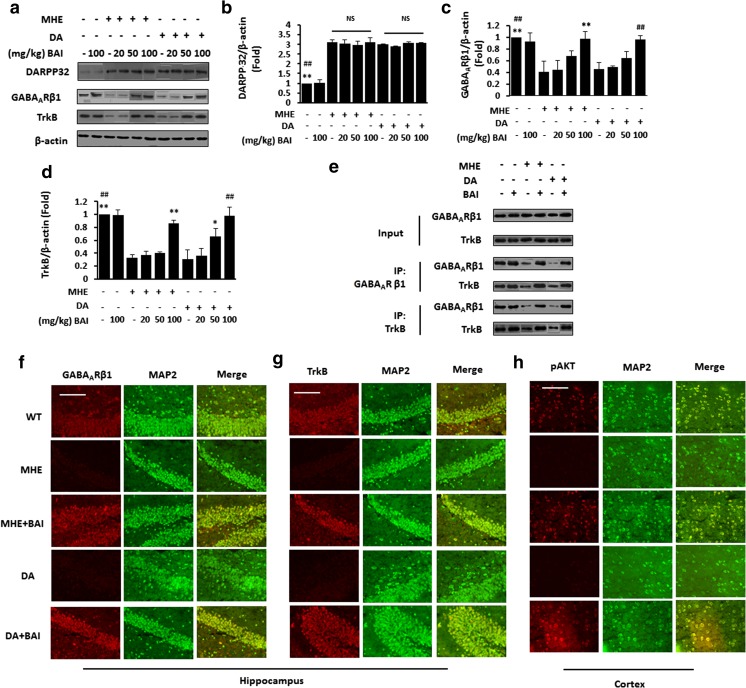
Based on the previous result, we examined the effect of BAI on DARPP32 levels in vivo. IB analysis showed increased expression of DARPP32 in the hippocampus in MHE and DA-treated rats, which was unaffected by BAI administration (Fig. 8a, b).
Fig. 8.
Effect of BAI on the DARPP32/GABAAR/TrkB signaling pathway in MHE rats and DA-treated rats. a Immunoblot analysis of hippocampal lysates from MHE and DA-treated rats administered various concentrations of BAI (20, 50, or 100 mg/kg) using anti-DARPP 32/GABAARβ1/TrkB and anti-β-actin antibodies and (b–d) subsequent densitometry. e Immunoprecipitation of hippocampal lysates from MHE and DA-treated rats administered BAI (100 mg/kg) with control IgG, anti-TrkB, or anti-GABAARβ1 antibodies. Complexes were immunoblotted with anti-TrkB or anti-GABAARβ1 antibodies. f Double immunofluorescence staining of hippocampal sections from MHE or DA-treated rats administered BAI (100 mg/kg) using antibodies against GABAARβ1 (red), MAP2 (green). Scale bar, 25 μm. g Double immunofluorescence staining of hippocampus sections from MHE or DA-treated rats administered BAI (100 mg/kg) using antibodies against TrkB (red), MAP2 (green). Scale bar, 100 μm. h Double immunofluorescence staining of cortical sections from MHE or DA-treated rats administered BAI (100 mg/kg) using antibodies against pAKT (red), MAP2 (green). Scale bar, 50 μm. *P < 0.05, **P < 0.01 vs. MHE rats; #P < 0.05, ##P < 0.01 vs. DA-treated rats. NS not significant. Scale bar, 25 μm. MRGD, merged image
Then, we examined the effect of BAI on the interaction of GABAARβ with TrkB in vivo. Decreased hippocampal GABAARβ (Fig. 8a, c) and TrkB (Fig. 8a, d) expression was found in MHE and DA-treated rats, and the administration of BAI increased the expression of GABAARβ and TrkB. Further co-immunoprecipitation experiments confirmed decreased interaction between GABAARβ and TrkB in MHE and DA-treated rats when GABAARβ and TrkB were immunoprecipitated in the cerebral cortex and hippocampus, and BAI administration improved the interaction (Fig. 8e), consistent with the Western blot results. Immunofluorescence staining showed that GABAARβ (Fig. 8f) and TrkB (Fig. 8g) were weakly expressed in the hippocampal neurons of MHE and DA-treated rats but strongly expressed after the administration of BAI. In the cerebral cortex, immunofluorescence revealed decreased expression of pAKT (Fig. 8h) in both MHE rats and DA-treated rats, and BAI ameliorated the decrease in pAKT expression. These results suggest that BAI does not affect the DA-induced activation of DARPP32 signaling and reverses the DA-mediated inactivation of the downstream GABAARβ/TrkB signaling pathway in MHE rats.
BAI prevents the impairment of synaptogenesis in MHE rats and DA-treated rats
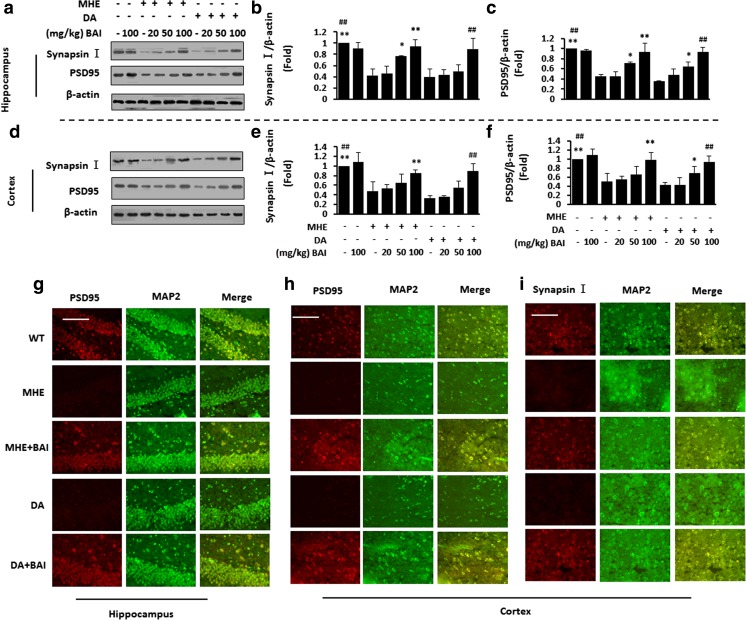
We further examined the effect of BAI on presynaptic markers (synapsin I) and postsynaptic markers (PSD95) in vivo. Hippocampal fractions from MHE and DA-treated rats showed significant decreases in PSD95/synapsin I expression, and BAI treatment improved the expression of synaptic markers (Fig. 9a–c). Significant decreases in the expression of PSD95/synapsin I were also found in the cortices of MHE and DA-treated rats, and BAI treatment reversed this decrease (Fig. 9d–f). IF staining of the hippocampus (Fig. 9g) and cortex (Fig. 9h) in MHE and DA-treated rats showed decreased expression of PSD95, and BAI treatment decreased the levels of both proteins. IF staining also showed decreased levels of synapsin I in the cortices of MHE and DA-treated rats, and BAI treatment increased the protein levels (Fig. 9i). These results suggest that the induction of synaptogenesis by BAI inhibits the DA-induced loss of synaptogenesis via the activation of GABAARβ/TrkB in MHE rats.
Fig. 9.
BAI prevents synaptic loss in MHE rats and DA-treated rats. a–f Immunoblot analysis and subsequent densitometry of hippocampal (a–c) and cortical (d–f) lysates from MHE or DA-treated rats administered various concentrations of BAI (20, 50, 100 mg/kg) using anti-PSD95/synapsin I and anti-β-actin antibodies. g Double immunofluorescence staining of hippocampal sections from MHE or DA-treated rats administered BAI (100 mg/kg) using antibodies against PSD95 (red), MAP2 (green). (h, i) Double immunofluorescence staining of cortical sections from MHE or DA-treated rats administered BAI (100 mg/kg) using antibodies against PSD95 (h)/synapsin I (i) (red), MAP2 (green). *P < 0.05, **P < 0.01 vs. MHE rats; #P < 0.05, ##P < 0.01 vs. DA-treated rats. Scale bar, 25 μm. MRGD, merged image
BAI reverses the impairment of memory function in MHE rats and DA-treated rats
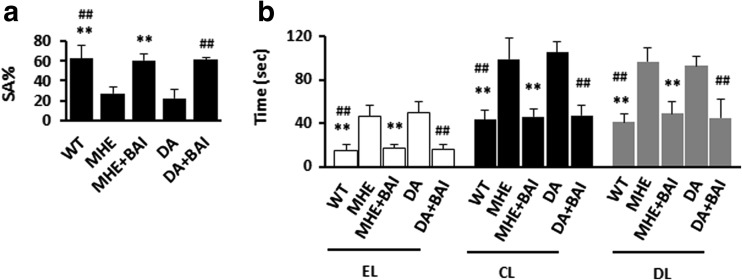
Finally, we assessed whether BAI improved memory impairment in vivo. TAA-treated rats with no HE symptoms were subjected to behavioral tests (a YM and a WFT). In the YM, the decreases in SA% in MHE rats and DA-treated rats were reversed by the administration of BAI (Fig. 10a). In the WFT, the significant delays in EL, CL, and DL in MHE rats and DA-treated rats were recovered to the normal levels by BAI treatment (Fig. 10b). These findings indicate that BAI reverses DA-induced cognitive decline in MHE rats.
Fig. 10.
Effects of BAI on cognitive function in MHE rats and DA-treated rats. a Spontaneous alternation percentage (SA%) in YM of MHE rats or DA-treated rats administered 100 mg/kg BAI. b WFT results (EL entry latency, CL contacting latency, DL drinking latency) of MHE rats or DA-treated rats administered 100 mg/kg BAI. *P < 0.05, **P < 0.01 vs. MHE rats; #P < 0.05, ##P < 0.01 vs. DA-treated rats
Discussion
The GABAA receptor, which belongs to the fast-acting ligand-gated ion channel superfamily, mediates most of the inhibitory synaptic transmissions in the central nervous system and mediates GABAergic inhibition in cortical network assemblies (Macdonald and Olsen 1994). The ionotropic GABAA receptors are heteromeric pentamers composed of different subunits from seven classes (α1–α6, β1–β3, γ1–γ3, δ, ϵ, π, and ρ). The majority of native receptors contain two α subunits, two β subunits, and one γ subunit (McKernan and Whiting 1996). The role of GABAA receptors (GABAARs) in conjunctive object-location learning remains to be established. In this respect, it is interesting to note a potential effect of several subunits of the GABAA receptor in object recognition behavior (Pirker et al. 2000). Alterations in the GABAergic system, including changes in tonic inhibition, are likely to be involved in the cognitive deficits associated with schizophrenia (Damgaard et al. 2011). Many pharmacological aspects of the GABAergic neurotransmitter system have been elucidated, including its anxiolytic, anti-convulsive, and anesthetic activities (Brailowsky and García 1999). In addition, the involvement of the GABAergic neurotransmitter system in learning and memory is supported by a substantial body of evidence (Makkar et al. 2010). Only a handful of studies have attempted to determine how DA influences this intrastriatal GABAergic pathway. Most of these studies have focused on the DA’s actions at D1R. For example, D1 dopamine receptor stimulation was found to increase GABA release (Harsing and Zigmond 1997). However, D1 dopamine receptor agonists appear to be ineffective in modulating GABAergic synaptic potentials in dorsal neostriatal neurons, despite the fact that they inhibit GABAergic signaling in the nucleus accumbens (Nicola and Malenka 1998). At face value, the inability of D1R agonists to modulate GABAergic synaptic potentials in medium spiny neurons is surprising (Surmeier et al. 1995). Excess DA has been shown to modulate GABAA receptors in the hippocampus and cerebral cortex. D1R receptors positively couple to DARPP32, resulting in the inactivation of GABAAR in MHE. The results of the present study suggest that the overstimulation of D1R by DA overload heightens the efficacy of DARPP-32. The occurrence of tonic GABAA receptor inhibition is irrelevant to the expression of α1 and α4 subunits. These results suggest that at high levels of D1R stimulation, DARPP32 effectively blocks the expression of GABAA receptor β1 and β3 subunits. The upregulation of D1R and downregulation of the β1 and β3 subunits of GABAA receptors in MHE rats imply that both D1R and GABAARβ are involved in the underlying pathology of MHE.
The family of compounds with a flavonoid structure and binding affinity to GABAA receptors, which includes BAI, has been shown to exert benzodiazepine (BZ)-like pharmacological actions with a wide spectrum of efficacies (Wang et al. 2005). A characteristic of GABAA receptors is their capacity for modulation by classical BZs, such as diazepam (Rabow et al. 1995). Zhang et al. (2006) demonstrated that BAI passed through the blood–brain barrier and distributed within brain tissue, specifically in the hippocampus, striatum, cortex, and thalamus, although the exact mechanism was not reported. Several studies have shown that BAI prevents cognitive dysfunction and reduces the apoptosis of hippocampal pyramidal cells in global cerebral ischemia/reperfusion injury in rats and humans (Cheng et al. 2012). Moreover, BAI protects the central nervous system activity following seizure-induced brain injury (Cao et al. 2010; Liu et al. 2012), promotes neuronal differentiation (Li et al. 2011), and guards against degeneration and neuronal dysfunction (Tu et al. 2011). It is worth noting that BAI activates GABAergic signaling in global ischemia, which may be a mechanism underlying its neuroprotective effect (Dai et al. 2013). In this study, we observed a decrease in the expression of GABAARβs in the hippocampi of MHE and DA-treated rats and found that BAI exerted a beneficial effect on the expression of GABAARβ. Our results demonstrate that BAI mimics GABA and exerts neurotrophic actions by protecting cortical and hippocampal neurons from DA-induced toxicity and dendritic and synaptic loss via the dopaminergic suppression of GABAAR currents in vitro. In addition, our data showed that intranasal administration of BAI did not significantly alter the structures or expression levels of GAP-43 and MAP2 in hippocampal or primary cortical neurons, suggesting that oral administration of BAI may represent a safe and noninvasive therapeutic strategy for the treatment of MHE.
All of these findings suggest that BAI exerts its neurotrophic activity through GABAARβ. TrkB signaling plays a direct role in the formation and maintenance of synapses (Hiester et al. 2013), and TrkB stimulation plays critical roles in neuronal plasticity, survival, and neurogenesis (Diniz and Teixeira 2011; Zuccato and Cattaneo 2009). TrkB activation is required for multiple aspects of neuronal function, including neuronal survival, morphological change, and synaptic plasticity (Diniz and Teixeira 2011; Zuccato and Cattaneo 2009). In our study, the expression of synaptic markers was increased by BAI treatment, and the silencing of TrkB blocked the effect of BAI, indicating that BAI exerts a profound protective effect on synapses by activating TrkB in vitro. Rat models of MHE showed deficits in hippocampal LTP, which correlated with the impairment in the hippocampus-dependent memory. LTP is regarded as a cellular mechanism for learning and memory (Wen et al. 2013). Furthermore, synaptic loss appears to be the best pathologic correlate of dementia in HE (Schroeter et al. 2015).
Given the key roles that GABAARβ–TrkB signaling plays in learning and memory, we proposed that BAI could prevent memory decline in MHE rats. Tyrosine receptor kinase B (TrkB) signaling in neurons promotes the formation and postsynaptic localization of GABAAR clusters (Binder 2007; Kafitz et al. 1999) and regulates GABAAR functions (Elmariah et al. 2005; Lindholm et al. 1994) and responses (Pearce 1993; Pearce et al. 1995). Meanwhile, TrkB signaling cascade is functionally coupled with GABAAR (Squinto et al. 1991; Tanaka et al. 1997). We previously reported that BAI binds to GABAARβ and quickly induces TrkB dimerization, phosphorylation, and the activation of downstream AKT/synapsin I signaling pathways (Andero et al. 2011; Jang et al. 2010). The results of the present study demonstrate that the activation of GABAARβ is coupled with the upregulation of TrkB, and BAI displays neurotrophic actions by protecting PHNs and PCNs from DA-induced synaptic loss in vitro. These findings suggest that BAI simulates the physiological actions of the GABAARβ/TrkB signaling pathway and prevents synaptic dysfunction and cognitive deficits in a rodent model of MHE.
Based on these findings, we hypothesized that synaptic dysfunction is a major pathophysiological hallmark of MHE. It has been suggested that “synaptoprotective” therapy may have more clinical relevance than neuroprotective therapy for MHE (Zhang et al. 2006). One finding suggested that GABAergic transmission may be a critical step in the process of homeostatic plasticity, such that activity blockage reduces GABA release and therefore GABAARβ activation, which then triggers synaptic scaling (Wenner 2014). If this is true, we would predict that activity blockage and GABAARβ blockage would induce scaling via a common mechanism: a shift in the chloride reversal potential. Our study identified an essential role for BAI in the synaptic integration of hippocampal and cortical neurons and suggested an activity-dependent mechanism for synaptic contact. Like GABA, BAI promotes the expression of presynaptic markers (synaptotagmin and synapsin) and postsynaptic markers (PSD95 and spinophilin), leading to synaptogenesis. Furthermore, chronic exposure of the brain to a high concentration of BAI is sufficient to exert a protective effect in vivo.
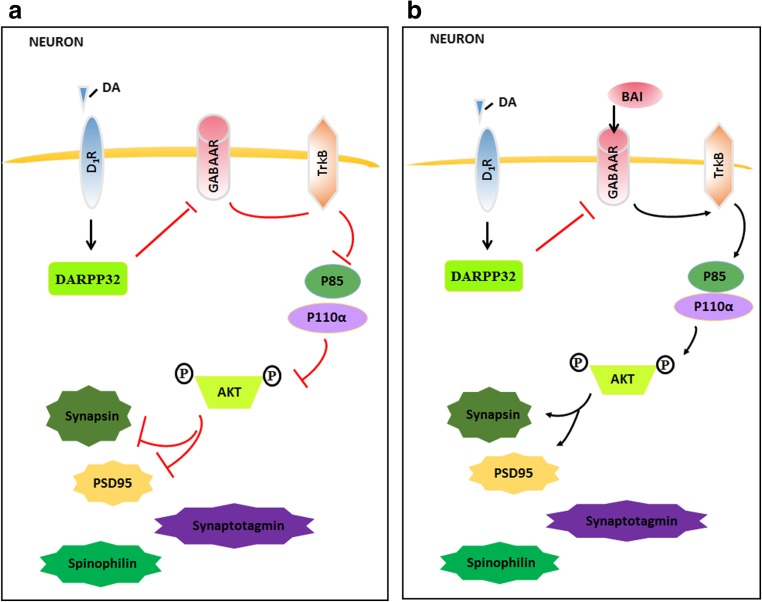
In summary, this study demonstrated that the administration of BAI exerts a therapeutic effect in MHE rats. This effect can largely be attributed to the protective effect of BAI on synapses. The neuroprotective effects of BAI may be linked to the modulation of the interaction of GABAARβ and TrkB and the elevation of synaptic protein expression. Our study identified BAI as a novel synaptoprotective strategy for the treatment of MHE. Our data also showed that BAI regulates GABAARβ to activate the TrkB/AKT/synapse-related protein pathway. BAI can also promote the activation of GABAARβ, a likely mechanism by which BAI reverses DA-induced LTP impairments in MHE rats. Based on the results of the present study, BAI may serve as a useful drug for the treatment of patients with MHE; however, it is essential to further explore whether other modulators are involved in the neuroprotective effects of BAI against MHE (Fig. 11).
Fig. 11.
DA mediated the destruction of the synaptogenesis signaling pathway. a DA initiates its effects by binding to a dopamine receptor. Once activated, the DR recruits DARPP32, which inhibits the activation of GABAARβ, then destroys the TrkB signaling, and subsequently dephosphorylates AKT. Inactivated AKT leads to the downregulation of presynaptic markers (synaptotagmin and synapsin I) and postsynaptic markers (PSD95 and spinophilin), which induces synaptic loss. b Conversely, BAI is recruited to bind to GABAARβ1, which activates TrkB signaling and subsequently phosphorylates AKT. Activated AKT leads to the upregulation of presynaptic markers (synaptotagmin and synapsin I) and postsynaptic markers (PSD95 and spinophilin), which potentiates synaptic loss
Funding information
This study was funded by the National Natural Science Foundation of China (81671042, 81300308, 81171088, 81371396).
References
- Acosta-Garcia J, Hernandez-Chan N, Paz-Bermudez F, Sierra A, Erlij D, Aceves J, Floran B. D4 and D1 dopamine receptors modulate [3H] GABA release in the substantia nigra pars reticulata of the rat. Neuropharmacology. 2009;57:725–730. doi: 10.1016/j.neuropharm.2009.08.010. [DOI] [PubMed] [Google Scholar]
- Albrecht J, Hilgier W, Zielinska M, Januszewski S, Hesselink M, Quack G. Extracellular concentrations of taurine, glutamate, and aspartate in the cerebral cortex of rats at the asymptomatic stage of thioacetamide-induced hepatic failure: modulation by ketamine anesthesia. Neurochem Res. 2000;25(11):1497–1502. doi: 10.1023/A:1007680210114. [DOI] [PubMed] [Google Scholar]
- Amodio P, Montagnese S, Gatta A, Morgan MY. Characteristics of minimal hepatic encephalopathy. Metab Brain Dis. 2004;19(3/4):253–267. doi: 10.1023/B:MEBR.0000043975.01841.de. [DOI] [PubMed] [Google Scholar]
- Andero R, Heldt SA, Ye K, Liu X, Armario A, Ressler KJ. Effect of 7,8-dihydroxyflavone, a small-molecule TrkB agonist, on emotional learning. Am J Psychiatry. 2011;168(2):163–172. doi: 10.1176/appi.ajp.2010.10030326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder DK (2007) Neurotrophins in the dentate gyrus. Prog Brain Res 163:371–397 [DOI] [PubMed]
- Blum K, Chen ALC, Giordano J, Borsten J, Chen TJH, Hauser M, Simpatico T, Femino J, Braverman ER, Barh D. The addictive brain: all roads lead to dopamine. J Psychoactive Drugs. 2012;44(2):134–143. doi: 10.1080/02791072.2012.685407. [DOI] [PubMed] [Google Scholar]
- Brailowsky S, García O. Ethanol, GABA and epilepsy. Arch Med Res. 1999;30(1):3–9. doi: 10.1016/S0188-0128(98)00013-X. [DOI] [PubMed] [Google Scholar]
- Cao Y, Li G, Wang YF, Fan ZK, Yu DS, Wang ZD, Bi YL. Neuroprotective effect of baicalin on compression spinal cord injury in rats. Brain Res. 2010;1357:115–123. doi: 10.1016/j.brainres.2010.07.108. [DOI] [PubMed] [Google Scholar]
- Chen J, Rusnak M, Luedtke RR, Sidhu A. D1 dopamine receptor mediates dopamine-induced cytotoxicity via the ERK signal cascade. J Biol Chem. 2004;279(38):39317–39330. doi: 10.1074/jbc.M403891200. [DOI] [PubMed] [Google Scholar]
- Cheng O, Li Z, Han Y, Jiang Q, Yan Y, Cheng K. Baicalin improved the spatial learning ability of global ischemia/reperfusion rats by reducing hippocampal apoptosis. Brain Res. 2012;1470:111–118. doi: 10.1016/j.brainres.2012.06.026. [DOI] [PubMed] [Google Scholar]
- Cheng FF, Lu Y, Zhong XG, Song WT, Wang XQ, Sun XG, Qin JG, Guo SY, Wang QG (2013) Baicalin’s therapeutic time window of neuroprotection during transient focal cerebral ischemia and its antioxidative effects in vitro and in vivo. Evid Based Complement Alternat Med [DOI] [PMC free article] [PubMed]
- Cheramy A, Leviel V, Glowinski J. Dendritic release of dopamine in the substantia nigra. Nature. 1981;289:537–542. doi: 10.1038/289537a0. [DOI] [PubMed] [Google Scholar]
- Dai J, Chen L, Qiu YM, Li SQ, Xiong WH, Yin YH, Jia F, Jiang JY. Activations of GABAergic signaling, HSP70 and MAPK cascades are involved in baicalin’s neuroprotection against gerbil global ischemia/reperfusion injury. Brain Res Bull. 2013;90:1–9. doi: 10.1016/j.brainresbull.2012.09.014. [DOI] [PubMed] [Google Scholar]
- Damgaard T, Plath N, Neill JC, Hansen SL. Extrasynaptic GABAA receptor activation reverses recognition memory deficits in an animal model of schizophrenia. Psychopharmacology. 2011;214(2):403–413. doi: 10.1007/s00213-010-2039-9. [DOI] [PubMed] [Google Scholar]
- Ding S, Liu L, Jing H, Xie J, Wang X, Mao J, Zhang X, Chen B, Zhuge QC. Dopamine from cirrhotic liver contributes to the impaired learning and memory ability of hippocampus in minimal hepatic encephalopathy. Hepatol Int. 2013;7:923–936. doi: 10.1007/s12072-013-9431-6. [DOI] [PubMed] [Google Scholar]
- Ding S, Hu J, Yang J, Liu L, Huang W, Gu X, Ye Y, Huang L, Liang Y, Chen B, Zhuge Q. The inactivation of JAK2/STAT3 signaling and desensitization of M1 mAChR in minimal hepatic encephalopathy (MHE) and the protection of naringin against MHE. Cell Physiol Biochem. 2014;34(6):1933–1950. doi: 10.1159/000366391. [DOI] [PubMed] [Google Scholar]
- Ding S, Huang W, Ye Y, Yang J, Hu J, Wang X, Liu L, Lu Q, Lin Y. Elevated intracranial dopamine impairs the glutamatenitric oxidecyclic guanosine monophosphate pathway in cortical astrocytes in rats with minimal hepatic encephalopathy. Mol Med Rep. 2014;10(3):1215–1224. doi: 10.3892/mmr.2014.2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding S, Yang J, Liu L, Ye Y, Wang X, Hu J, Chen B, Zhuge Q. Elevated dopamine induces minimal hepatic encephalopathy by activation of astrocytic NADPH oxidase and astrocytic protein tyrosine nitration. Int J Biochem Cell Biol. 2014;55:252–263. doi: 10.1016/j.biocel.2014.09.003. [DOI] [PubMed] [Google Scholar]
- Diniz BS, Teixeira AL. Brain-derived neurotrophic factor and Alzheimer’s disease: physiopathology and beyond. NeuroMolecular Med. 2011;13(4):217–222. doi: 10.1007/s12017-011-8154-x. [DOI] [PubMed] [Google Scholar]
- Elmariah SB, Oh EJ, Hughes EG, Balice-Gordon RJ (2005) Astrocytes regulate inhibitory synapse formation via Trk-mediated modulation of postsynaptic GABAA receptors. J Neurosci Off J Soc Neurosci 25:3638–3650 [DOI] [PMC free article] [PubMed]
- Flores-Hernandez J, Hernandez S, Snyder GL, Yan Z, Fienberg AA, Moss SJ, Greengard P, Surmeier DJ. D(1) dopamine receptor activation reduces GABA(A) receptor currents in neostriatal neurons through a PKA/DARPP-32/PP1 signaling cascade. J Neurophysiol. 2000;83(5):2996–3004. doi: 10.1152/jn.2000.83.5.2996. [DOI] [PubMed] [Google Scholar]
- Geffen LB, Jessell TM, Cuello AC, Iversen LL. Release of dopamine from dendrites in rat substantia nigra. Nature. 1976;260(5548):258–260. doi: 10.1038/260258a0. [DOI] [PubMed] [Google Scholar]
- Greengard P, Allen PB, Nairn AC. Beyond the dopamine receptor: the DARPP-32/protein phosphatase-1 cascade. Neuron. 1999;23(3):435–447. doi: 10.1016/S0896-6273(00)80798-9. [DOI] [PubMed] [Google Scholar]
- Harsing LG, Jr, Zigmond MJ. Influence of dopamine on GABA release in striatum: evidence for D1–D2 interactions and non-synaptic influences. Neuroscience. 1997;77(2):419–429. doi: 10.1016/S0306-4522(96)00475-7. [DOI] [PubMed] [Google Scholar]
- Hiester BG, Galati DF, Salinas PC, Jones KR. Neurotrophin and Wnt signaling cooperatively regulate dendritic spine formation. Mol Cell Neurosci. 2013;56:115–127. doi: 10.1016/j.mcn.2013.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui KM, Wang XH, Xue H. Interaction of flavones from the roots of Scutellaria baicalensis with the benzodiazepine site. Planta Med. 2000;66(1):91–93. doi: 10.1055/s-0029-1243121. [DOI] [PubMed] [Google Scholar]
- Jang SW, Liu X, Yepes M, Shepherd KR, Miller GW, Liu Y, Wilson WD, Xiao G, Blanchi B, Sun YE, Ye K. A selective TrkB agonist with potent neurotrophic activities by 7,8-dihydroxyflavone. Proc Natl Acad Sci U S A. 2010;107(6):2687–2692. doi: 10.1073/pnas.0913572107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kafitz KW, Rose CR, Thoenen H, Konnerth A (1999) Neurotrophin-evoked rapid excitation through TrkB receptors. Nature 401:918–921 [DOI] [PubMed]
- Kawasumi M, Chiba T, Yamada M, Miyamae-Kaneko M, Matsuoka M, Nakahara J, Tomita T, Iwatsubo T, Kato S, Aiso S, Nishimoto I, Kouyama K. Targeted introduction of V642I mutation in amyloid precursor protein gene causes functional abnormality resembling early stage of Alzheimer’s disease in aged mice. Eur J Neurosci. 2004;19(10):2826–2838. doi: 10.1111/j.0953-816X.2004.03397.x. [DOI] [PubMed] [Google Scholar]
- Lee B, Sur B, Shim I, Lee H, Hahm DH. Baicalin improves chronic corticosterone-induced learning and memory deficits via the enhancement of impaired hippocampal brain-derived neurotrophic factor and cAMP response element-binding protein expression in the rat. J Nat Med. 2014;68:132–143. doi: 10.1007/s11418-013-0782-z. [DOI] [PubMed] [Google Scholar]
- Li M, Tsang KS, Choi ST, Li K, Shaw PC, Lau KF. Neuronal differentiation of C17.2 neural stem cells induced by a natural flavonoid, baicalin. Chembiochem. 2011;12(3):449–456. doi: 10.1002/cbic.201000570. [DOI] [PubMed] [Google Scholar]
- Lindholm D, Castrén E, Berzaghi M, Blöchl A, Thoenen H (1994) Activity-dependent and hormonal regulation of neurotrophin mRNA levels in the brain--implications for neuronal plasticity. J Neurobiol 25:1362–1372 [DOI] [PubMed]
- Liu YF, Gao F, Li XW, Jia RH, Meng XD, Zhao R, Jing YY, Wang Y, Jiang W. The anticonvulsant and neuroprotective effects of baicalin on pilocarpine-induced epileptic model in rats. Neurochem Res. 2012;37(8):1670–1680. doi: 10.1007/s11064-012-0771-8. [DOI] [PubMed] [Google Scholar]
- Macdonald RL, Olsen RW. GABAA receptor channels. Annu Rev Neurosci. 1994;17(1):569–602. doi: 10.1146/annurev.ne.17.030194.003033. [DOI] [PubMed] [Google Scholar]
- Makkar SR, Zhang SQ, Cranney J. Behavioral and neural analysis of GABA in the acquisition, consolidation, reconsolidation, and extinction of fear memory. Neuropsychopharmacology. 2010;35(8):1625–1652. doi: 10.1038/npp.2010.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKernan RM, Whiting PJ. Which GABAA-receptor subtypes really occur in the brain? Trends Neurosci. 1996;19(4):139–143. doi: 10.1016/S0166-2236(96)80023-3. [DOI] [PubMed] [Google Scholar]
- Montoliu C, Piedrafita B, Serra MA, del Olmo JA, Ferrandez A, Rodrigo JM, Felipo V. Activation of soluble guanylate cyclase by nitric oxide in lymphocytes correlates with minimal hepatic encephalopathy in cirrhotic patients. J Mol Med. 2007;85(3):237–245. doi: 10.1007/s00109-006-0149-y. [DOI] [PubMed] [Google Scholar]
- Nicola SM, Malenka RC. Modulation of synaptic transmission by dopamine and norepinephrine in ventral but not dorsal striatum. J Neurophysiol. 1998;79(4):1768–1776. doi: 10.1152/jn.1998.79.4.1768. [DOI] [PubMed] [Google Scholar]
- Pearce RA (1993) Physiological evidence for two distinct GABA A responses in rat hippocampus. Neuron 10:189 [DOI] [PubMed]
- Pearce RA, Grunder SD, Faucher LD (1995) Different mechanisms for use-dependent depression of two GABAA-mediated IPSCs in rat hippocampus. J Physiol 484( Pt 2):425–435 [DOI] [PMC free article] [PubMed]
- Pirker S, Schwarzer C, Wieselthaler A, Sieghart W, Sperk G. GABA(A) receptors: immunocytochemical distribution of 13 subunits in the adult rat brain. Neuroscience. 2000;101:815–850. doi: 10.1016/S0306-4522(00)00442-5. [DOI] [PubMed] [Google Scholar]
- Rabow LE, Russek SJ, Farb DH. From ion currents to genomic analysis: recent advances in GABAA receptor research. Synapse. 1995;21(3):189–274. doi: 10.1002/syn.890210302. [DOI] [PubMed] [Google Scholar]
- Schroeter A, Wen S, Molders A, Erlenhardt N, Stein V, Klocker N. Depletion of the AMPAR reserve pool impairs synaptic plasticity in a model of hepatic encephalopathy. Mol Cell Neurosci. 2015;68:331–339. doi: 10.1016/j.mcn.2015.09.001. [DOI] [PubMed] [Google Scholar]
- Squinto SP, Stitt TN, Aldrich TH, Davis S, Blanco SM, Radziejewski C, Glass DJ, Masiakowski P, Furth ME, Valenzuela DM (1991) trk B encodes a functional receptor for brain-derived neurotrophic factor and neurotrophin-3 but not nerve growth factor. Cell 65:885–893 [DOI] [PubMed]
- Surmeier DJ, Bargas J, Hemmings HC, Jr, Nairn AC, Greengard P. Modulation of calcium currents by a D1 dopaminergic protein kinase/phosphatase cascade in rat neostriatal neurons. Neuron. 1995;14(2):385–397. doi: 10.1016/0896-6273(95)90294-5. [DOI] [PubMed] [Google Scholar]
- Svenningsson P, Tzavara ET, Carruthers R, Rachleff I, Wattler S, Nehls M, McKinzie DL, Fienberg AA, Nomikos GG, Greengard P. Diverse psychotomimetics act through a common signaling pathway. Science. 2003;302(5649):1412–1415. doi: 10.1126/science.1089681. [DOI] [PubMed] [Google Scholar]
- Tanaka T, Saito H, Matsuki N (1997) Inhibition of GABAA synaptic responses by brain-derived neurotrophic factor (BDNF) in rat hippocampus. J Neurosci 17:2959–2966 [DOI] [PMC free article] [PubMed]
- Tarragó T, Kichik N, Claasen B, Prades R, Teixidó M, Giralt E. Baicalin, a prodrug able to reach the CNS, is a prolyl oligopeptidase inhibitor. Bioorg Med Chem. 2008;16(15):7516–7524. doi: 10.1016/j.bmc.2008.04.067. [DOI] [PubMed] [Google Scholar]
- Tu XK, Yang WZ, Liang RS, Shi SS, Chen JP, Chen CM, Wang CH, Xie HS, Chen Y, Ouyang LQ. Effect of baicalin on matrix metalloproteinase-9 expression and blood-brain barrier permeability following focal cerebral ischemia in rats. Neurochem Res. 2011;36(11):2022–2028. doi: 10.1007/s11064-011-0526-y. [DOI] [PubMed] [Google Scholar]
- Ungless MA, Grace AA. Are you or aren’t you? Challenges associated with physiologically identifying dopamine neurons. Trends Neurosci. 2012;35(7):422–430. doi: 10.1016/j.tins.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Shing M, Huen Y, Tsang SY, Xue H. Neuroactive flavonoids interacting with GABAA receptor complex. Curr Drug Targets CNS Neurol Disord. 2005;4(5):575–585. doi: 10.2174/156800705774322030. [DOI] [PubMed] [Google Scholar]
- Wen S, Schroeter A, Klocker N. Synaptic plasticity in hepatic encephalopathy—a molecular perspective. Arch Biochem Biophys. 2013;536:183–188. doi: 10.1016/j.abb.2013.04.008. [DOI] [PubMed] [Google Scholar]
- Wenner P. Homeostatic synaptic plasticity in developing spinal networks driven by excitatory GABAergic currents. Neuropharmacology. 2014;78:55–62. doi: 10.1016/j.neuropharm.2013.04.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z, Wang F, Tsang SY, Ho KH, Zheng H, Yuen CT, Chow CY, Xue H. Anxiolytic-like effect of baicalin and its additivity with other anxiolytics. Planta Med. 2006;72(02):189–192. doi: 10.1055/s-2005-873193. [DOI] [PubMed] [Google Scholar]
- Yamada M, Chiba T, Sasabe J, Nawa M, Tajima H, Niikura T, Terashita K, Aiso S, Kita Y, Matsuoka M, Nishimoto I. Implanted cannula-mediated repetitive administration of Abeta25–35 into the mouse cerebral ventricle effectively impairs spatial working memory. Behav Brain Res. 2005;164:139–146. doi: 10.1016/j.bbr.2005.03.026. [DOI] [PubMed] [Google Scholar]
- Yin F, Liu J, Ji X, Wang Y, Zidichouski J, Zhang J. Baicalin prevents the production of hydrogen peroxide and oxidative stress induced by Aβ aggregation in SH-SY5Y cells. Neurosci Lett. 2011;492(2):76–79. doi: 10.1016/j.neulet.2011.01.055. [DOI] [PubMed] [Google Scholar]
- Zhang L, Xing D, Wang W, Wang R, Du L. Kinetic difference of baicalin in rat blood and cerebral nuclei after intravenous administration of Scutellariae Radix extract. J Ethnopharmacol. 2006;103(1):120–125. doi: 10.1016/j.jep.2005.07.013. [DOI] [PubMed] [Google Scholar]
- Zhou QB, Duan CZ, Jia Q, Liu P, Li LY. Baicalin attenuates focal cerebral ischemic reperfusion injury by inhibition of protease-activated receptor-1 and apoptosis. Chin J Integr Med. 2014;20(2):116–122. doi: 10.1007/s11655-013-1441-7. [DOI] [PubMed] [Google Scholar]
- Zuccato C, Cattaneo E. Brain-derived neurotrophic factor in neurodegenerative diseases. Nat Rev Neurol. 2009;5(6):311–322. doi: 10.1038/nrneurol.2009.54. [DOI] [PubMed] [Google Scholar]