Abstract
Background:
Hypovitaminosis D is highly prevalent and has several adverse health effects. This study aims to assess the relationship of serum concentrations of 25-hydroxyvitamin D (25[OH] D) and liver enzymes in adolescents.
Methods:
This population-based cross-sectional survey was conducted among a nationally representative multistage sample of 1095 adolescents (52% boys), aged 10–18 years, living in different provinces of Iran. Serum 25(OH)D concentration <30 ng/mL was considered as hypovitaminosis D, and liver enzymes (alanine aminotransaminase [ALT] and aspartate aminotransaminase [AST]) of >40 U/L was considered as high level. To determine the association between serum 25(OH)D categories and elevated levels of liver enzymes, multiple regression models and linear regression analysis were applied, after adjustment for potential confounders. Odds ratios (95% confidence interval) of serum 25(OH)D and elevated liver enzymes were assessed by logistic regression analysis.
Results:
Higher rates of Vitamin D deficiency were documented among individuals with increased levels of liver enzymes. Compared to boys, median of 25(OH)D was lower in girls with elevated levels of liver function tests (12.75 vs. 25.60 ng/mL for ALT and 13 vs. 14.10 ng/mL for AST), with marginally significant gender differences regarding AST.
Conclusions:
We found a relatively high frequency of hypovitaminosis D among adolescents with abnormal liver function. Further prospective studies are needed to examine these associations from early life.
Keywords: Adolescent, liver function tests, Vitamin D
Introduction
Vitamin D, as a fat-soluble vitamin, is produced in the human body through sunlight exposure and could also be obtained from dietary sources and supplements. Vitamin D insufficiency is recognized as a major public health problem and one of the most common nutritional deficiencies worldwide.[1,2,3] It has been estimated that the prevalence of hypovitaminosis D varies from 5% to 30% in adult population.[4] High prevalence of Vitamin D deficiency has been also documented in pediatric age groups,[5] for example, 61.6% of Qatari adolescents,[2] 24% of Mexican preschool children,[6] and 20% of healthy adolescents in the United Arab Emirates.[7] The prevalence of Vitamin D deficiency in normal weight, overweight, obese, and severely obese children in the US is reported as 21, 29, 34, and 49%, respectively.[8] Vitamin D deficiency is also highly prevalent in Iran among children and adolescents.[9,10,11]
It is well documented that lower levels of 25-hydroxyvitamin D (25[OH]D) contribute to growth retardation, skeletal deformation,[12] as well as the wide range of nonskeletal adverse effects including increased prevalence of certain cancers,[13,14] cardiovascular disease,[15] obesity,[16] metabolic syndrome,[17] insulin resistance,[18] infections,[19] allergy,[20,21] and autoimmune disorders.[22] Vitamin D deficiency is also prevalent among patients with chronic liver disease.[23,24,25] It is not well defined whether Vitamin D deficiency could contribute to liver dysfunction or is just result from the reduced liver function; however, a previous longitudinal study revealed an inverse association between Vitamin D status and incidence of liver disease.[26]
Current research about the relationship between 25(OH)D levels and liver function tests is mostly conducted among the adult population,[27] and limited evidence is available in the pediatric age group.[28] The aim of this study is to determine the association of serum 25(OH)D with liver enzymes among adolescents.
Methods
Participants
This cross-sectional population-based survey was performed as a substudy of the third survey of a national surveillance program entitled Childhood and Adolescence Surveillance and Prevention of Adult Noncommunicable disease (CASPIAN) study. The study protocol has been described before.[29] Briefly, the CASPIAN-III survey was conducted on a stratified multistage probability sample of Iranian children and adolescents from the urban and rural area of 27 provinces. The current study was approved by Ethical Committees of relevant national organizations. Voluntary informed consent form and verbal assent were obtained from parents and participants, respectively.
Anthropometric data collection
In total, 5528 school students aged 10–18 years participated in the current nationwide study. Trained health-care providers documented the sociodemographic characteristics and conducted physical examination including anthropometric measurements such as height, weight, and waist circumference (WC) under standard protocols and using calibrated equipment. Body mass index (BMI) was then calculated using the ratio of weight in kilograms to height in square meters. WC was measured to the nearest 0.5 cm, using a nonelastic tape.
Blood sample analysis
Blood samples used for these analyses included liver enzymes and serum 25(OH)D levels after a 12 h overnight fasting state. Serum concentration of 25(OH)D was analyzed quantitatively by direct competitive immunoassay chemiluminescence method using LIASON® 25(OH)D assay TOTAL (DiaSorin, Inc.), with a coefficient of variation of 9.8%. The fresh sera were then tested for alanine aminotransaminase (ALT) and aspartate aminotransaminase (AST) levels using Pars Azmoon reagents kit (Tehran, Iran).
Serum 25(OH)D concentration of <30 ng/mL was considered as Vitamin D deficiency.[30] The cutoff for elevated liver enzymes was considered as >40 U/L mg/dL.[31]
Statistical analysis
Statistical analyses were performed with SPSS (version 16.0, SPSS, Chicago, IL, USA). Mean, standard deviations (SDs), and percentages were calculated for continuous and categorical variables, respectively. We expressed serum 25(OH)D concentration categories as a median and interquartile range (IQR). Baseline characteristics of the students were compared using t-test/Mann–Whitney test for continuous variables and Chi-square test for qualitative variables. To determine the association of serum 25(OH)D concentration categories and elevated levels of liver enzymes, multiple regression models were applied, adjusted for potential confounders including age, gender, living area, family history of chronic disease, type of consumed milk during infancy (formula vs. breast milk), breastfeeding duration, parental education, physical activity, socioeconomic status, BMI, and WC. Adjusted B-coefficient and standard error of serum 25(OH)D concentration categories and ALT to AST ratio resulted from the linear regression analysis are presented. Odds ratios (ORs) (95% confidence interval) of serum 25(OH)D concentrations and elevated levels of liver enzymes were computed. The significance level was set at P < 0.05.
Results
This cross-sectional survey was performed among 1095 Iranian adolescents (52% boys). The mean (SD) age of participants was 14.74 (2.61) years. More than 90% of students studied at public schools. Mean (SD) for BMI and WC was 19.37 (4.58) kg/m2 and 67.82 (12.23) cm, respectively. Elevated ALT was documented among 1.4% and 2.7% of boys and girls, respectively. Moreover, 4.4% of boys and 3.8% of girls had elevated levels of AST. Seventy-seven percent of boys and 80.6% of girls were Vitamin D deficient.
The demographic, anthropometric, and social characteristics of the study participants are summarized in Table 1. The median and IQR of serum 25(OH)D was 16.10 (4.40, 29.62) ng/mL among those who had elevated ALT levels and 15.5 (8.55, 29.75) ng/mL for those with elevated AST levels. Compared to boys, median (IQR) of Vitamin D was lower among girls with abnormal levels of liver function tests (12.75 [4.37, 25.77] ng/mL vs. 25.60 [7.68, 40.85] ng/mL for ALT and 13 [7.29, 25.95] ng/mL vs. 14.10 [7.59, 23.40] ng/mL for AST), with marginally significant gender differences regarding AST (P = 0.08). Among Vitamin D-deficient individuals, respectively, 77.3%and 75% had elevated ALT and AST levels. The corresponding figure for hypovitaminosis D regarding elevated values of ALT was 62.5% and 85.7% for boys and girls, respectively. Of boys with raised AST levels, 83.3% were Vitamin D deficient, whereas 65% of girls with elevated AST had Vitamin D deficiency. The differences were not statistically significant according to gender, except for the marginally significant prevalence of hypovitaminosis D among girls with elevated values of AST (P = 0.08).
Table 1.
Characteristics of participants according to the gender: The Childhood and Adolescence Surveillance and PreventIon of Adult Noncommunicable disease-III study
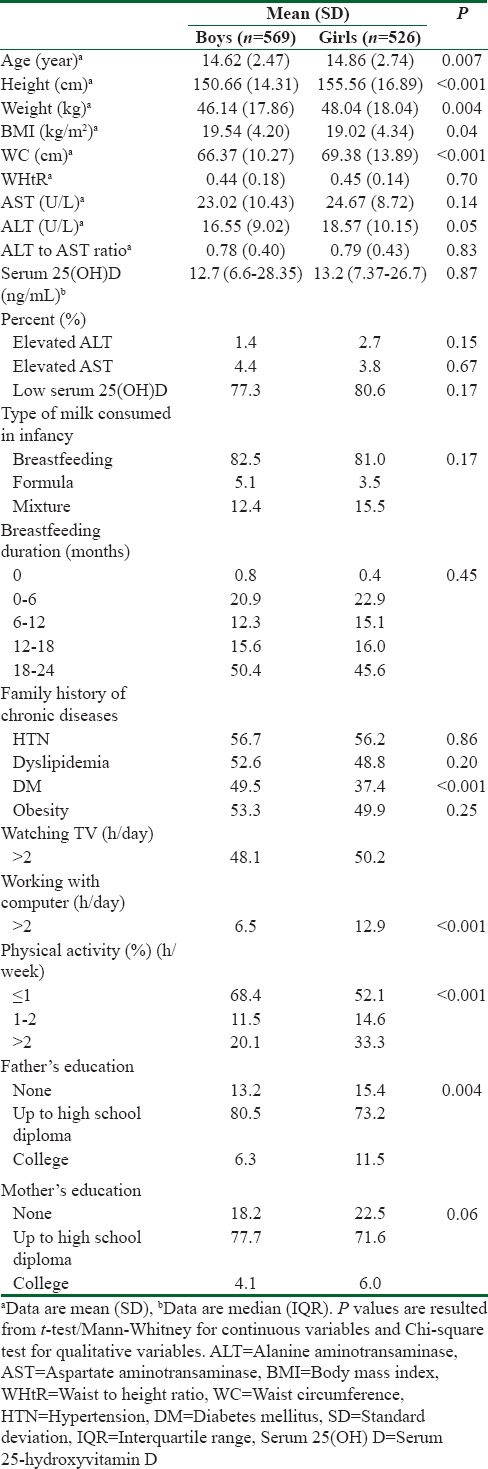
Among Vitamin D deficient individuals, the mean (SD) for ALT, AST, and ALT: AST ratio was 17.43 (9.75) mg/dL, 23.62 (9.66) mg/dL, and 0.79 (0.43), respectively. Mean (SD) of ALT among Vitamin D-deficient boys and girls was 16.61 (9.14) mg/dL and 18.28 (10.29) mg/dL, respectively. Among Vitamin D-deficient individuals, the mean (SD) of AST was not significantly different among boys and girls (23 [10.84] mg/dL vs. 24.26 [8.27] mg/dL, respectively, P > 0.05).
Adjusted logistic regression models [Table 2] showed that Vitamin D deficiency did not increase the OR of elevated liver enzymes, before and after adjustments for potential confounders. Linear regression analysis revealed no significant relationship between serum 25(OH)D concentration categories and ALT: AST ratio, before and after applying adjusted models for confounders [Table 3].
Table 2.
Odds ratios (95% confidence interval) for serum 25-hydroxyvitamin D concentration categories and elevated liver enzymes: The Childhood and Adolescence Surveillance and PreventIon of Adult Noncommunicable disease-III study
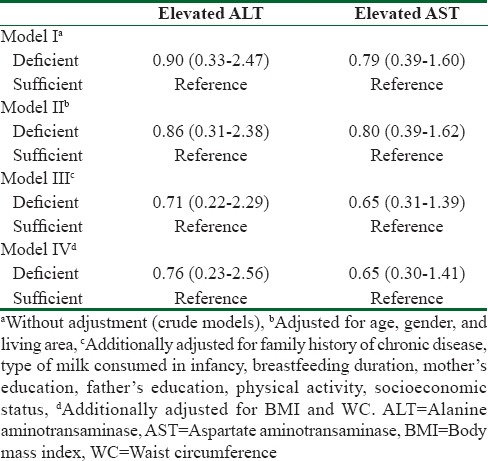
Table 3.
Association of serum 25-hydroxyvitamin D concentration categories and alanine aminotransaminase to aspartate aminotransaminase ratio in the linear regression analysis: The Childhood and Adolescence Surveillance and PreventIon of Adult Noncommunicable disease-III study

Discussion
The present study examined the association between serum 25(OH)D levels and liver function tests among adolescents. We found a higher frequency of Vitamin D deficiency among adolescents with elevated levels of liver enzymes compared to those with normal values. Vitamin D deficiency did not increase the risk of elevated liver enzymes, even after adjustments for potential confounders. The possible association of Vitamin D deficiency and hepatic disorders is of great interest and under the study. Previous studies have revealed a high prevalence of Vitamin D deficiency and higher levels of liver enzymes; however, the relationship is not clearly understood in the pediatric age group.
In the current study, higher rates of Vitamin D deficiency were found among subjects with abnormal levels of liver enzymes, compared to those with normal values. This finding is in line with previous findings of a high frequency of Vitamin D deficiency in patients with liver disease.[23,24] A growing body of evidence suggests that Vitamin D could possibly play a key role in the pathogenesis of liver disease.[32] Liver damages caused by Vitamin D deficiency include increased inflammation, fibrosis, and reduced antiviral response.[33,34,35]
In our study, sufficient levels of Vitamin D reduced the risk of elevated liver enzymes; however, it was not statistically significant. It is worth noting that no previous studies have explored the association between Vitamin D status and liver enzymes in the adolescents’ population, except for only one study that has investigated this association with the development of liver disease in a general adult population.[26]
Our findings are in line with a previous study that revealed that the risk of having a greater value of liver enzymes tended to be more for lower serum 25(OH)D status although not statistically significant. They also found an inverse association between Vitamin D status and the prevalence of hepatic disorders.[26]
In our study, we found a relatively high frequency of Vitamin D deficiency among adolescents (77.3% boys vs. 80.6% girls). In line with these findings, increasing rate of Vitamin D deficiency has been reported among children and adolescents as well as adult population from other parts of the country.[9,10,11,36] Despite the differences in the methods used for measuring serum 25(OH)D levels, various cutoff points to define Vitamin D deficiency and/or insufficiency, wide fluctuations in serum Vitamin D status due to seasonal variations and sunlight exposure, and the way of reporting 25(OH)D between studies, the findings of the present study indicate that more than half of Iranian adolescents are Vitamin D deficient. Iranian girls have less outdoor physical activities and different clothing styles than boys. These might partly explain the higher prevalence of Vitamin D deficiency among girl students. Lower levels of serum Vitamin D among girls have been repeatedly reported by previous studies.[2,9,37,38]
Liver function test abnormalities were, however, prevalent among adolescents consistent with previous reports from Iran and some other countries.[31,39] The relationship between Vitamin D status and features of metabolic syndrome or cardiometabolic risk factors has been analyzed in adolescents[30,40] but has been little explored with liver enzymes in pediatric age group. A study conducted among a sample of school adolescents demonstrated a higher frequency of abnormal ALT in boys (44%) than girls (7%), using a cut point of 40 IU/L for both genders.[41] We found that the prevalence of elevated ALT was lower in boys (1.4%) than girls (2.7%). In our study, consistent with previous evidence,[31,42,43,44,45] greater WC and WHtR, as indicators of central adiposity, might possibly cause elevated ALT levels in girls compared to boys. It has recently been suggested that the role of body fat mass as a “metabolic well” could reduce the bioavailability of Vitamin D and its transformation to 25(OH)D.[46]
Higher rates of Vitamin D deficiency documented among individuals with increased levels of liver enzymes might simply represent unfavorable lifestyle habits such as inadequate dietary intakes of Vitamin D, as well as poor sunlight exposure, air pollution, and engaging in sedentary behaviors. These all might contribute to the development and progression of fatty liver disease and the adverse subsequent metabolic abnormalities as metabolic syndrome and insulin resistance which have been found to associate with the severity of hypovitaminosis D.[47]
Study limitation and strengths
First, because of its cross-sectional design, we cannot conclude cause and effect relationships. Second, we could not examine the pubertal status to explore the effect of pubertal stage on liver enzymes. Third, the single measurement of 25(OH)D does not necessarily indicate Vitamin D status of adolescents for the entire year. It would also be recommended to assess other biomarkers of liver disease as serum albumin or international normalized ratio indicating liver function rather than hepatic damages as evaluated by ALT and AST.[48] Worth mentioning, chronic viral hepatitis was not included because of routine vaccination for hepatitis B and a very low incidence of hepatitis C virus at that age.[49] The strengths of the study are its novelty in adolescent's age group, its nationwide coverage, and including a large nationally representative sample of participants.
Conclusions
We found a considerably high frequency of hypovitaminosis D among adolescents with elevated liver function tests. The extraskeletal consequences of hypovitaminosis D should be highlighted in the pediatric age group. Given that Vitamin D deficiency might possibly act as a contributor to liver disease initiation and progression, prevention and control of hypovitaminosis D is necessary. Further longitudinal studies are needed to examine these associations from early life.
Financial support and sponsorship
Financial supports of Iranian Ministry of Health and Medical Education, cooperation of Ministry of Education and Training, Child Growth and Development Research Center, and Isfahan University of Medical Sciences are sincerely appreciated.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The authors would like to thank the large team working with this project, as well as the students, their parents, and school principals who willingly participated in the study.
References
- 1.Holick MF. Vitamin D: Extraskeletal health. Rheum Dis Clin North Am. 2012;38:141–60. doi: 10.1016/j.rdc.2012.03.013. [DOI] [PubMed] [Google Scholar]
- 2.Bener A, Al-Ali M, Hoffmann GF. High prevalence of Vitamin D deficiency in young children in a highly sunny Humid country: A global health problem. Minerva Pediatr. 2009;61:15–22. [PubMed] [Google Scholar]
- 3.Shin YH, Shin HJ, Lee YJ. Vitamin D status and childhood health. Korean J Pediatr. 2013;56:417–23. doi: 10.3345/kjp.2013.56.10.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bruyère O, Malaise O, Neuprez A, Collette J, Reginster JY. Prevalence of Vitamin D inadequacy in European postmenopausal women. Curr Med Res Opin. 2007;23:1939–44. doi: 10.1185/030079907X219562. [DOI] [PubMed] [Google Scholar]
- 5.Rovner AJ, O'Brien KO. Hypovitaminosis D among healthy children in the United States: A review of the current evidence. Arch Pediatr Adolesc Med. 2008;162:513–9. doi: 10.1001/archpedi.162.6.513. [DOI] [PubMed] [Google Scholar]
- 6.Flores M, Macias N, Lozada A, Sánchez LM, Díaz E, Barquera S, et al. Serum 25-hydroxyvitamin D levels among mexican children ages 2 y to 12 y: A national survey. Nutrition. 2013;29:802–4. doi: 10.1016/j.nut.2012.12.024. [DOI] [PubMed] [Google Scholar]
- 7.Muhairi SJ, Mehairi AE, Khouri AA, Naqbi MM, Maskari FA, Al Kaabi J, et al. Vitamin D deficiency among healthy adolescents in Al Ain, United Arab Emirates. BMC Public Health. 2013;13:33. doi: 10.1186/1471-2458-13-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Turer CB, Lin H, Flores G. Prevalence of Vitamin D deficiency among overweight and obese US children. Pediatrics. 2013;131:e152–61. doi: 10.1542/peds.2012-1711. [DOI] [PubMed] [Google Scholar]
- 9.Neyestani TR, Hajifaraji M, Omidvar N, Eshraghian MR, Shariatzadeh N, Kalayi A, et al. High prevalence of Vitamin D deficiency in school-age children in Tehran, 2008: A red alert. Public Health Nutr. 2012;15:324–30. doi: 10.1017/S1368980011000188. [DOI] [PubMed] [Google Scholar]
- 10.Ardestani PM, Salek M, Keshteli AH, Nejadnik H, Amini M, Hosseini SM, et al. Vitamin D status of 6- to 7-year-old children living in Isfahan, Iran. Endokrynol Pol. 2010;61:377–82. [PubMed] [Google Scholar]
- 11.Kaykhaei MA, Hashemi M, Narouie B, Shikhzadeh A, Rashidi H, Moulaei N, et al. High prevalence of Vitamin D deficiency in Zahedan, Southeast Iran. Ann Nutr Metab. 2011;58:37–41. doi: 10.1159/000323749. [DOI] [PubMed] [Google Scholar]
- 12.Boot AM, Krenning EP, de Muinck Keizer-Schrama SM. The relation between 25-hydroxyvitamin D with peak bone mineral density and body composition in healthy young adults. J Pediatr Endocrinol Metab. 2011;24:355–60. doi: 10.1515/jpem.2011.052. [DOI] [PubMed] [Google Scholar]
- 13.Jenab M, Bueno-de-Mesquita HB, Ferrari P, van Duijnhoven FJ, Norat T, Pischon T, et al. Association between pre-diagnostic circulating Vitamin D concentration and risk of colorectal cancer in European populations: A nested case-control study. BMJ. 2010;340:b5500. doi: 10.1136/bmj.b5500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holick MF. Vitamin D: Importance in the prevention of cancers, type 1 diabetes, heart disease, and osteoporosis. Am J Clin Nutr. 2004;79:362–71. doi: 10.1093/ajcn/79.3.362. [DOI] [PubMed] [Google Scholar]
- 15.Temmerman JC. Vitamin D and cardiovascular disease. J Am Coll Nutr. 2011;30:167–70. doi: 10.1080/07315724.2011.10719956. [DOI] [PubMed] [Google Scholar]
- 16.Harel Z, Flanagan P, Forcier M, Harel D. Low Vitamin D status among obese adolescents: Prevalence and response to treatment. J Adolesc Health. 2011;48:448–52. doi: 10.1016/j.jadohealth.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 17.Ganji V, Zhang X, Shaikh N, Tangpricha V. Serum 25-hydroxyvitamin D concentrations are associated with prevalence of metabolic syndrome and various cardiometabolic risk factors in US children and adolescents based on assay-adjusted serum 25-hydroxyvitamin D data from NHANES 2001-2006. Am J Clin Nutr. 2011;94:225–33. doi: 10.3945/ajcn.111.013516. [DOI] [PubMed] [Google Scholar]
- 18.Shin YH, Kim KE, Lee C, Shin HJ, Kang MS, Lee HR, et al. High prevalence of Vitamin D insufficiency or deficiency in young adolescents in Korea. Eur J Pediatr. 2012;171:1475–80. doi: 10.1007/s00431-012-1746-0. [DOI] [PubMed] [Google Scholar]
- 19.Madden K, Feldman HA, Smith EM, Gordon CM, Keisling SM, Sullivan RM, et al. Vitamin D deficiency in critically ill children. Pediatrics. 2012;130:421–8. doi: 10.1542/peds.2011-3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sharief S, Jariwala S, Kumar J, Muntner P, Melamed ML. Vitamin D levels and food and environmental allergies in the United States: Results from the National Health and Nutrition Examination Survey 2005-2006. J Allergy Clin Immunol. 2011;127:1195–202. doi: 10.1016/j.jaci.2011.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brehm JM, Acosta-Perez E, Klei L, Roeder K, Barmada M, Boutaoui N, et al. Vitamin D insufficiency and severe asthma exacerbations in Puerto Rican children. Am J Respir Crit Care Med. 2012;186:140–6. doi: 10.1164/rccm.201203-0431OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Camurdan OM, Döǧer E, Bideci A, Celik N, Cinaz P. Vitamin D status in children with hashimoto thyroiditis. J Pediatr Endocrinol Metab. 2012;25:467–70. [PubMed] [Google Scholar]
- 23.Arteh J, Narra S, Nair S. Prevalence of Vitamin D deficiency in chronic liver disease. Dig Dis Sci. 2010;55:2624–8. doi: 10.1007/s10620-009-1069-9. [DOI] [PubMed] [Google Scholar]
- 24.Malham M, Jørgensen SP, Ott P, Agnholt J, Vilstrup H, Borre M, et al. Vitamin D deficiency in cirrhosis relates to liver dysfunction rather than aetiology. World J Gastroenterol. 2011;17:922–5. doi: 10.3748/wjg.v17.i7.922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Putz-Bankuti C, Pilz S, Stojakovic T, Scharnagl H, Pieber TR, Trauner M, et al. Association of 25-hydroxyvitamin D levels with liver dysfunction and mortality in chronic liver disease. Liver Int. 2012;32:845–51. doi: 10.1111/j.1478-3231.2011.02735.x. [DOI] [PubMed] [Google Scholar]
- 26.Skaaby T, Husemoen LL, Borglykke A, Jørgensen T, Thuesen BH, Pisinger C, et al. Vitamin D status, liver enzymes, and incident liver disease and mortality: A general population study. Endocrine. 2014;47:213–20. doi: 10.1007/s12020-013-0107-8. [DOI] [PubMed] [Google Scholar]
- 27.Stokes CS, Krawczyk M, Reichel C, Lammert F, Grünhage F. Vitamin D deficiency is associated with mortality in patients with advanced liver cirrhosis. Eur J Clin Invest. 2014;44:176–83. doi: 10.1111/eci.12205. [DOI] [PubMed] [Google Scholar]
- 28.Mohammadi B, Najafi M, Farahmand F, Motamed F, Ghajarzadeh M, Mohammadi J, et al. Prevalence of vitamin D deficiency and rickets in children with cholestasis in Iran. Acta Med Iran. 2012;50:482–5. [PubMed] [Google Scholar]
- 29.Kelishadi R, Heshmat R, Motlagh ME, Majdzadeh R, Keramatian K, Qorbani M, et al. Methodology and early findings of the third survey of CASPIAN study: A national school-based surveillance of students’ high risk behaviors. Int J Prev Med. 2012;3:394–401. [PMC free article] [PubMed] [Google Scholar]
- 30.Kelishadi R, Ardalan G, Motlagh ME, Shariatinejad K, Heshmat R, Poursafa P, et al. National report on the association of serum Vitamin D with cardiometabolic risk factors in the pediatric population of the Middle East and North Africa (MENA): The CASPIAN-III study. Nutrition. 2014;30:33–8. doi: 10.1016/j.nut.2013.05.018. [DOI] [PubMed] [Google Scholar]
- 31.Kelishadi R, Abtahi SH, Qorbani M, Heshmat R, Esmaeil Motlagh M, Taslimi M, et al. First national report on aminotransaminases’ percentiles in children of the Middle East and North Africa (MENA): The CASPIAN-III study. Hepat Mon. 2012;12:e7711. doi: 10.5812/hepatmon.7711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zúñiga S, Firrincieli D, Housset C, Chignard N. Vitamin D and the Vitamin D receptor in liver pathophysiology. Clin Res Hepatol Gastroenterol. 2011;35:295–302. doi: 10.1016/j.clinre.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 33.Pappa HM, Bern E, Kamin D, Grand RJ. Vitamin D status in gastrointestinal and liver disease. Curr Opin Gastroenterol. 2008;24:176–83. doi: 10.1097/MOG.0b013e3282f4d2f3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bouillon R, Carmeliet G, Verlinden L, van Etten E, Verstuyf A, Luderer HF, et al. Vitamin D and human health: Lessons from Vitamin D receptor null mice. Endocr Rev. 2008;29:726–76. doi: 10.1210/er.2008-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bikle DD. Vitamin D insufficiency/deficiency in gastrointestinal disorders. J Bone Miner Res. 2007;22 Suppl 2:V50–4. doi: 10.1359/jbmr.07s208. [DOI] [PubMed] [Google Scholar]
- 36.Hovsepian S, Amini M, Aminorroaya A, Amini P, Iraj B. Prevalence of Vitamin D deficiency among adult population of Isfahan city, Iran. J Health Popul Nutr. 2011;29:149–55. doi: 10.3329/jhpn.v29i2.7857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moussavi M, Heidarpour R, Aminorroaya A, Pournaghshband Z, Amini M. Prevalence of Vitamin D deficiency in Isfahani high school students in 2004. Horm Res. 2005;64:144–8. doi: 10.1159/000088588. [DOI] [PubMed] [Google Scholar]
- 38.Sahu M, Bhatia V, Aggarwal A, Rawat V, Saxena P, Pandey A, et al. Vitamin D deficiency in rural girls and pregnant women despite abundant sunshine in Northern India. Clin Endocrinol (Oxf) 2009;70:680–4. doi: 10.1111/j.1365-2265.2008.03360.x. [DOI] [PubMed] [Google Scholar]
- 39.Di Bonito P, Sanguigno E, Di Fraia T, Forziato C, Boccia G, Saitta F, et al. Association of elevated serum alanine aminotransferase with metabolic factors in obese children: Sex-related analysis. Metabolism. 2009;58:368–72. doi: 10.1016/j.metabol.2008.10.010. [DOI] [PubMed] [Google Scholar]
- 40.Kelishadi R, Cook SR, Adibi A, Faghihimani Z, Ghatrehsamani S, Beihaghi A, et al. Association of the components of the metabolic syndrome with non-alcoholic fatty liver disease among normal-weight, overweight and obese children and adolescents. Diabetol Metab Syndr. 2009;1:29. doi: 10.1186/1758-5996-1-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schwimmer JB, McGreal N, Deutsch R, Finegold MJ, Lavine JE. Influence of gender, race, and ethnicity on suspected fatty liver in obese adolescents. Pediatrics. 2005;115:e561–5. doi: 10.1542/peds.2004-1832. [DOI] [PubMed] [Google Scholar]
- 42.Jamali R, Pourshams A, Amini S, Deyhim MR, Rezvan H, Malekzadeh R, et al. The upper normal limit of serum alanine aminotransferase in Golestan Province, Northeast Iran. Arch Iran Med. 2008;11:602–7. [PubMed] [Google Scholar]
- 43.Poustchi H, George J, Esmaili S, Esna-Ashari F, Ardalan G, Sepanlou SG, et al. Gender differences in healthy ranges for serum alanine aminotransferase levels in adolescence. PLoS One. 2011;6:e21178. doi: 10.1371/journal.pone.0021178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kariv R, Leshno M, Beth-Or A, Strul H, Blendis L, Kokia E, et al. Re-evaluation of serum alanine aminotransferase upper normal limit and its modulating factors in a large-scale population study. Liver Int. 2006;26:445–50. doi: 10.1111/j.1478-3231.2006.01197.x. [DOI] [PubMed] [Google Scholar]
- 45.Mohamadnejad M, Pourshams A, Malekzadeh R, Mohamadkhani A, Rajabiani A, Asgari AA, et al. Healthy ranges of serum alanine aminotransferase levels in Iranian blood donors. World J Gastroenterol. 2003;9:2322–4. doi: 10.3748/wjg.v9.i10.2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wortsman J, Matsuoka LY, Chen TC, Lu Z, Holick MF. Decreased bioavailability of Vitamin D in obesity. Am J Clin Nutr. 2000;72:690–3. doi: 10.1093/ajcn/72.3.690. [DOI] [PubMed] [Google Scholar]
- 47.Targher G, Bertolini L, Scala L, Cigolini M, Zenari L, Falezza G, et al. Associations between serum 25-hydroxyvitamin D3 concentrations and liver histology in patients with non-alcoholic fatty liver disease. Nutr Metab Cardiovasc Dis. 2007;17:517–24. doi: 10.1016/j.numecd.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 48.Rochling FA. Evaluation of abnormal liver tests. Clin Cornerstone. 2001;3:1–2. doi: 10.1016/s1098-3597(01)90074-2. [DOI] [PubMed] [Google Scholar]
- 49.Kangin M, Turhanoglu M, Gulsun S, Cakabay B. Seroprevalence of hepatitis B and C among children in endemic areas of Turkey. Hepat Mon. 2010;10:36–41. [PMC free article] [PubMed] [Google Scholar]


