Abstract
Patient: Male, 31
Final Diagnosis: Pneumonia from Human Metapneumovirus pulmonary infection
Symptoms: Cough • fatigue
Medication: —
Clinical Procedure: Hernia repair
Specialty: Anesthesiology
Objective:
Unknown ethiology
Background:
Providing anesthesia to immunocompromised patients introduces unique challenges, including difficulty in detecting respiratory infections. Detailed preoperative evaluation and preparation for perioperative complications is crucial. Human metapneumovirus is a common but lesser known respiratory virus that can lead to pneumonia and respiratory compromise and is challenging to detect in the immunocompromised patient.
Case Report:
We present a case of an immunocompromised individual scheduled for umbilical hernia repair who developed severe bronchospasm and intraoperative respiratory failure after induction of general anesthesia. Preoperative evaluation of this patient revealed only minor respiratory symptoms and minimal rhonchi on lung auscultation. This patient did not meet extubation criteria in the operating room and was transferred to the medical intensive care unit. Human metapneumovirus was detected in his lower respiratory tract as the cause of the pneumonia and respiratory failure.
Conclusions:
This case illustrates the difficulty in predicting pulmonary complications in immunocompromised patients and the potential severity of a respiratory infection with Human metapneumovirus. Detecting respiratory infections preoperatively in the immunocompromised patient is important for considering preoperative treatment or postponing elective surgery and potentially avoiding intraoperative respiratory failure.
MeSH Keywords: Intraoperative Care, Intraoperative Complications, Metapneumovirus, Respiratory System Abnormalities
Background
Pulmonary complications are important causes of perioperative morbidity. The incidence has been reported to be as high as 1% to 2% in even minor surgeries and upwards of 10% to 20% in upper abdominal or thoracic surgeries [1]. In addition, pneumonia is the most common invasive infection in immunocompromised patients and carries a high morbidity and mortality rate. Evaluation of immunocompromised patients can be difficult as signs and symptoms of infection can be subtle; missing a pulmonary infection during the perioperative period in this population can lead to devastating consequences.
Human metapneumovirus (hMPV) is a single negative-stranded RNA-enveloped virus in the Pneumovirinae subfamily of viruses and results in both upper and lower respiratory tract infections. Symptoms are wide ranging: from a mild cough to life-threatening pneumonia and bronchiolitis. It is especially common and severe in immunocompromised patients [2,3].
This case report is an illustration of how important it is to perform a thorough preoperative evaluation in immunocompromised patients and highlights the importance picking up on subtle signs of infection in this population. Our patient suffered from an intractable bronchospasm from just manipulating of the airway, illustrating the vulnerability of his respiratory status. Our case brings up the question of how much preoperative evaluation should be done in this sensitive population especially for minor elective surgeries.
Case Report
The patient was a 31-year-old male with end stage renal disease status post kidney transplant, immunosuppression, hypertension, obesity, and sleep apnea who presented for elective umbilical hernia repair. He had no history of pulmonary disease. His daily medications included myfortic, tacrolimus, and prednisone. His preoperative evaluation was unremarkable except for a minor cough and fatigue during the preceding week. The patient’s lungs were clear bilaterally and his vital signs were normal. Induction in the operating room was performed uneventfully with propofol, fentanyl, succinylcho-line, and ketamine. Shortly after induction, stress dose hydro-cortisone and rocuronium were given. He was maintained on 1 MAC of sevoflurane. Prior to surgical incision, the patient began coughing. His peak airway pressures increased to greater than 30 cm H2O and his saturation acutely decreased to less than 85%. He was immediately given 100% oxygen, additional neuromuscular blockade, and the sevoflurane concentration was increased while he was hand-ventilated. His lung compliance was very low, and he was difficult to ventilate. Albuterol was delivered, and an emergent bronchoscopy was undertaken which revealed diffuse erythema and copious white mucous throughout the airway bilaterally, which was suctioned. An arterial blood gas was sent and showed a Pa02 <80 mm Hg despite being on 100% oxygen. Following discussion with the surgeon, the decision was made to cancel surgery. The patient continued to be difficult to ventilate and oxygenate and therefore was transferred to the medical intensive care unit intubated. Chest x-ray upon arrival illustrated bilateral opacities, pulmonary congestion, and atelectasis (Figure 1). In the medical intensive care unit, a bronchoalveolar lavage was performed which revealed respiratory hMPV. The patient continued to have intermittent bronchospasms over the next 12 hours while being treated in the intensive care unit. The patient’s respiratory function normalized with resolution of the bronchospasm on hospital day 2 and he was extubated uneventfully. He fully recovered and was discharged to home on hospital day 3. The patient currently has decided not to have his umbilical hernia repaired.
Figure 1.
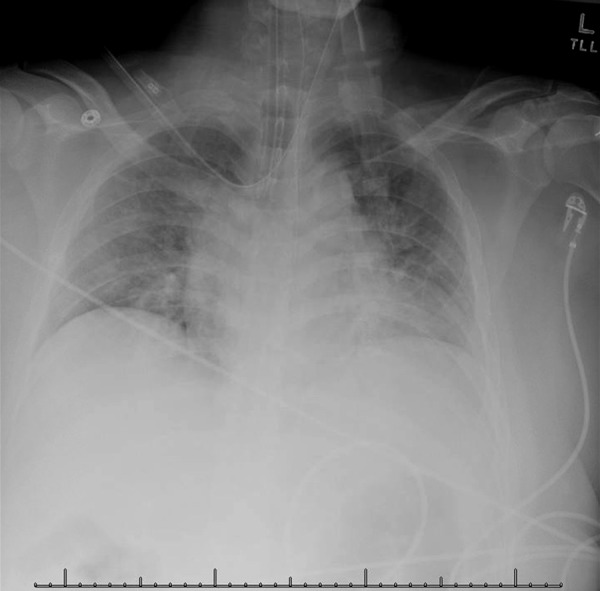
Chest x-ray upon arrival to the medical intensive care unit.
Discussion
Immunocompromised patients can pose perioperative challenges, and in this case, it was an initial subclinical presentation of a respiratory viral infection. Data regarding perioperative evaluation and management of immunocompromised adult patients is not significantly divergent from our normal standard [4,5]. With just a cough and fatigue in the setting of a minor elective surgery, we felt that no further workup was indicated. Indeed, it would have been difficult to predict his respiratory infection preoperatively and his severe bronchospasm following induction of general anesthesia and airway manipulation.
Perioperative pulmonary complications occur in 6% of patients who undergo major abdominal surgery. Etiologies include infection, hypoxia, respiratory failure, bronchospasm, and obstructive disease exacerbation. It leads to increased hospital stay and a higher in hospital mortality rate [6,7]. Additionally, the estimated annual cost of perioperative pulmonary complications in the United States is 3.42 billion dollars. The ARISCAT study, a prospective multicenter study attempting to create a risk index for these complications, found that age >80 years, preoperative Sp02 <90%, preoperative hemoglobin <10 g/dL, upper abdominal or thoracic surgical incision, and surgery duration greater than 2 hours were all associated with increased pulmonary risk [8]. Other studies have also noted smoking, pulmonary hypertension, congestive heart failure, obstructive sleep apnea, and combined metabolic and nutritional factors to be important risk factors [8]. Although our patient did not have these factors, he was immunosuppressed, which put him at increased risk for infection.
Common viruses associated with respiratory tract infections include respiratory syncytial virus, parainfluenza, and adenovirus [9]. As stated previously, a less known, but common virus discovered in 2001 is hMPV [10]. It is a single stranded, negative sense, nonsegmented RNA virus that is a member of the Paramyxoviridae family, and one of the leading causes of upper and lower respiratory tract infections in children, elderly, and immunocompromised individuals [2,11]. Signs and symptoms range from the common cold or upper respiratory tract infection to bronchiolitis, croup, pneumonia, exacerbation of reactive airway disease, and sepsis [6,12]. In a retrospective study of 769 patients who underwent stem cell transplantation and presented with upper or lower respiratory tract infection, 2.5% of the patients had hMPV. Of those patients found to have hMPV, 72% presented with upper respiratory tract infection (cough, fever, headache, wheezing) or influenza-like symptoms. The remaining 28% had symptoms consistent with a lower respiratory tract infection (pneumonia) [13]. Risk factors for transmission and increased severity of symptoms include extremes of age, immunocompromised status, underlying pulmonary disease, and seasonality. Immune status is an important factor that can determine the illness severity, and in addition, the majority of hMPV case mortalities were in immunocompromised patients. Transmission is via large particle respiratory secretions. Treatment is largely supportive respiratory care and antivirals. Vaccines are currently being explored [2,3,6,11,14–16]. Interestingly, in immunocompromised patients who may have subtle symptomatology and an equivocal chest x-ray, a CT scan may be necessary to diagnosis involvement in the lower respiratory tract.
Conclusions
This case illustrates the difficulty in predicting pulmonary complications in immunocompromised patients and the potential severity of hMPV pulmonary infection in these individuals. Our patient with hMPV initially presented with severe bronchospasm and intraoperative respiratory failure. Intubation with airway manipulation was the instigating factor that made this first evident. Having a high index of suspicion for pulmonary infection, even with minor respiratory symptoms in this patient population is prudent. In retrospect, preoperative treatment or postponement of surgery may have avoided the severity of his pulmonary complications and may have allowed for a safe repair of his umbilical hernia.
Footnotes
Conflict of interest
None.
References:
- 1.Degani-Costa LH, Faresin SM, dos Reis Falcao LF. Preoperative evaluation of the patient with pulmonary disease. Braz J Anesthesiol. 2014;64(1):22–34. doi: 10.1016/j.bjane.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 2.Falsey AR. Human metapneumovirus infection in adults. Pediatr Infect Dis J. 2008;27(10 Suppl.):S80–83. doi: 10.1097/INF.0b013e3181684dac. [DOI] [PubMed] [Google Scholar]
- 3.Samuel S, Nanjappa S, Cooper CD, Greene JN. Human metapneumovirus infection in immunocompromised patients. Cancer Control. 2016;23(4):442–45. doi: 10.1177/107327481602300416. [DOI] [PubMed] [Google Scholar]
- 4.Littlewood KE. The immunocompromised adult patient and surgery. Best Pract Res Clin Anaesthesiol. 2008;22(3):585–609. doi: 10.1016/j.bpa.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 5.Neskovic V. Preoperative assesment of the immunocompromised patient. Acta Chir Iugosl. 2011;58(2):185–92. doi: 10.2298/aci1102185n. [DOI] [PubMed] [Google Scholar]
- 6.Crowe JE., Jr Human metapneumovirus as a major cause of human respiratory tract disease. Pediatr Infect Dis J. 2004;23(11 Suppl.):S215–21. doi: 10.1097/01.inf.0000144668.81573.6d. [DOI] [PubMed] [Google Scholar]
- 7.Smetana GW, Lawrence VA, Cornell JE, American College of Physicians Preoperative pulmonary risk stratification for noncardiothoracic surgery:Ssystematic review for the American College of Physicians. Ann Intern Med. 2006;144(8):581–95. doi: 10.7326/0003-4819-144-8-200604180-00009. [DOI] [PubMed] [Google Scholar]
- 8.Canet J, Gallart L, Gomar C, et al. Prediction of postoperative pulmonary complications in a population-based surgical cohort. Anesthesiology. 2010;113(6):1338–50. doi: 10.1097/ALN.0b013e3181fc6e0a. [DOI] [PubMed] [Google Scholar]
- 9.Jain S, Self WH, Wunderink RG, et al. Community-acquired pneumonia requiring hospitalization among U.S. adults. N Engl J Med. 2015;373(5):415–27. doi: 10.1056/NEJMoa1500245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Amer HM. Molecular epidemiology of human metapneumovirus in Riyadh Province, Saudi Arabia. J Mol Microbiol Biotechnol. 2016;26(6):414–21. doi: 10.1159/000448374. [DOI] [PubMed] [Google Scholar]
- 11.Kroll JL, Weinberg A. Human metapneumovirus. Semin Respir Crit Care Med. 2011;32(4):447–53. doi: 10.1055/s-0031-1283284. [DOI] [PubMed] [Google Scholar]
- 12.Mazzoncini JP, Jr, Crowell CB, Kang CS. Human metapneumovirus: An emerging respiratory pathogen. J Emerg Med. 2010;38(4):456–59. doi: 10.1016/j.jemermed.2007.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Egli A, Bucher C, Dumoulin A, et al. Human metapneumovirus infection after allogeneic hematopoietic stem cell transplantation. Infection. 2012;40(6):677–84. doi: 10.1007/s15010-012-0279-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Debur MC, Vidal LR, Stroparo E, et al. Human metapneumovirus infection in hematopoietic stem cell transplant recipients. Transpl Infect Dis. 2010;12(2):173–79. doi: 10.1111/j.1399-3062.2009.00465.x. [DOI] [PubMed] [Google Scholar]
- 15.Falsey AR, Hennessey PA, Formica MA, et al. Humoral immunity to human metapneumovirus infection in adults. Vaccine. 2010;28(6):1477–80. doi: 10.1016/j.vaccine.2009.11.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maitre NL, Williams JV. Human metapneumovirus in the preterm neonate: Current perspectives. Res Rep Neonatol. 2016;6:41–49. doi: 10.2147/RRN.S76270. [DOI] [PMC free article] [PubMed] [Google Scholar]


