Abstract
Introduction:
This study aims to study the changing trends and outcomes associated with the adoption of minimally invasive distal pancreatectomy (MIDP) at a single centre.
Materials and Methods:
Retrospective review of sixty consecutive patients who underwent MIDP from September 2006 to November 2016 at a single institution. To study the evolution of MIDP, the study population was divided into three groups consisting of twenty patients (Group I, Group II and Group III).
Results:
Sixty patients underwent MIDP with 11 (18.3%) requiring open conversions. The median operation time was 305 (range: 85–775) min and the median post-operative stay was 6 (range: 3–73) days. Fifteen procedures were spleen-saving pancreatectomies. Major post-operative morbidity (>Grade 2) occurred in 12 (20.0%) patients and there was no mortality or reoperations. There were 33 (55.0%) pancreatic fistulas, of which 15 (25.0%) were Grade B fistulas of which 12 (20.0%) required percutaneous drainage. Comparison between the three groups demonstrated a statistically significant increase in the frequency of procedures performed, increase in robotic-assisted procedures and proportion of asymptomatic tumours resected. There also tended to be non-significant decrease in open conversion rates from 25% to 5% between the three groups and increase in tumour size resected from 24 to 40 mm.
Conclusion:
Comparison between the three groups demonstrated that MIDP was performed with increased frequency. There was a statistically significant increase in the frequency of resections performed for asymptomatic tumours and resections performed through robotic assistance. There was also a non-significant trend towards a decrease in open conversions and increase in the size of tumours resected.
Keywords: Laparoscopic distal pancreatectomy, minimally invasive distal pancreatectomy, robotic pancreatectomy
INTRODUCTION
The first laparoscopic distal pancreatectomy (LDP) was reported by Cushieri et al. in 1996.[1] Subsequently, studies reporting on LDP have been increasingly reported worldwide.[2,3,4] To date, numerous retrospective studies have confirmed the superiority of LDP over the open approach in terms of shorter hospital stay, decreased blood loss and decreased use of analgesia.[2,3,4] However, although the advantages of LDP have been confirmed by several systematic reviews and meta-analyses, level 1 evidence in terms of a prospective randomised control trial supporting its use is still lacking.[5] Furthermore, it is important to note that, although LDP is not as technically demanding as proximal pancreatic resections, it still remains a challenging procedure as evidenced by the high open conversion rates of 16%–31% reported even from high volume specialised centres, especially by early adopters during the learning phase.[6,7,8] Due to these challenges, some centres have adopted robotic-assisted laparoscopic or hand-assisted laparoscopic approaches to shorten the learning curve and improve operative outcomes.[6,9,10]
In the present study, we report our experience with minimally invasive distal pancreatectomy (MIDP) and its evolution since it was first introduced in 2006. It aims to describe the changing trends and outcomes associated with the adoption of MIDP at a single centre. To the best of our knowledge, this series represents the largest reported surgical series of MIDP in Southeast Asia to date.
MATERIALS AND METHODS
All consecutive patients who underwent MIDP at a single institution from September 2006 to November 2016 were identified from a prospectively maintained surgical database. All patients’ data were subsequently obtained retrospectively from the patients’ clinical, radiological and pathological records. Clinical data were collected from a prospective computerised clinical database (Sunrise Clinical Manager version 5.8, Eclipsys Corporation, Atlanta, Georgia) and patient's clinical charts whereas operative data were obtained from another prospective computerised database (OTM 10, IBM, Armonk, New York, USA). Sixty consecutive patients were identified. The data of forty of these patients have been reported in a previous study with a different hypothesis.[11] We divided the sixty patients into three equal groups of twenty consecutive patients (Groups 1–3) to study the evolution of MIDP at our institution over time.
Clinicopathological data including relevant pre-, intra- and post-operative outcomes such as patient demographics, presence of symptoms, history of abdominal surgery, American Society of Anaesthesiology score, tumour size, tumour pathology, operation time, blood loss, blood transfusion, post-operative morbidity and post-operative hospitalisation were recorded. All post-operative morbidities and mortalities were recorded up to 30 days postoperatively. The post-operative complications were classified according to the Clavien-Dindo grading system.[12]
Surgical technique
Various operative techniques were adopted depending on the individual surgeon preference. Our operative technique for laparoscopic or robotic pancreatectomies has been described previously.[11,13,14] In general, the patient was placed in the reverse Trendelenburg position with the left shoulder elevated with or without the legs apart. Various laparoscopic energy devices were utilised such as the Harmonic Scalpel (Ethicon Endo-Surgery, Cincinnati, OH, USA), ENSEAL (Ethicon Endo-Surgery, Cincinnati, OH, USA), LigaSure (Covidien, Boulder, CO, USA) or Thunderbeat (Olympus, Tokyo, Japan). Dissection of the pancreas proceeded from the medial to lateral position in most cases except for distal lesions in the pancreatic tail. Endoscopic staplers were used to transect the pancreas and were reinforced with sutures in selected cases.
Definitions
Subtotal pancreatectomy was defined as when the transection of the pancreatic parenchyma was located at or to the right of the portal vein/splenic vein junction. Extended pancreatectomy was defined according to the recent international study group definition which included any pancreatectomy with adjacent organ resection such as the stomach, colon or vascular resection due to local tumour involvement.[15]
Post-operative pancreatic fistula was defined and graded according to the grading system proposed by the International Study Group of Pancreatic Fistula.[16,17] This was defined as any amount of drain fluid with an amylase content >3 times the upper normal limit of serum amylase or >300 IU/L on or after post-operative day 3. In this study, asymptomatic purely biochemical Grade A pancreatic fistulas were reported but were not considered a morbidity according to the Clavien-Dindo grading system.
All statistical analyses were performed using the computer program Statistical Package for the Social Sciences for Windows, version 21.0 (SPSS Inc., Chicago, IL, USA). Univariate analyses were performed using Kruskal–Wallis test or Chi-squared tests as appropriate. All statistical tests were two-sided and P < 0.05 was considered statistically significant.
RESULTS
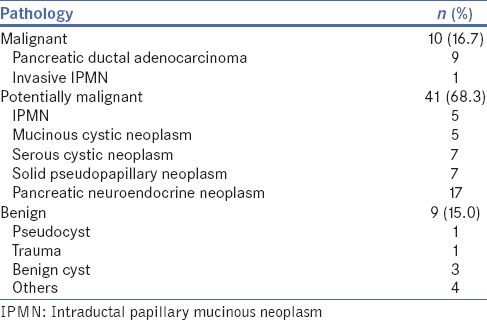
During the study period, sixty consecutive patients underwent MIDP at our institution. The clinicopathological features and perioperative outcomes of these patients are summarised in Tables 1 and 2. The patients had a median age of 56 (range: 21–79) years and there were 27 males (45.0%). Table 3 summarises the pathology of tumours resected. The most common pathology was pancreatic neuroendocrine neoplasms in 17 patients and 10 MIDP were performed for invasive malignancies. There was one emergency resection for pancreatic trauma. The type of MIDP attempted was conventional laparoscopic in 45 (75%), robotic-assisted in 14 (23.3) and hand-assisted in 1 (1.7%).
Table 1.
Comparison between the baseline demographic and perioperative data of patients who underwent minimally invasive distal pancreatectomy
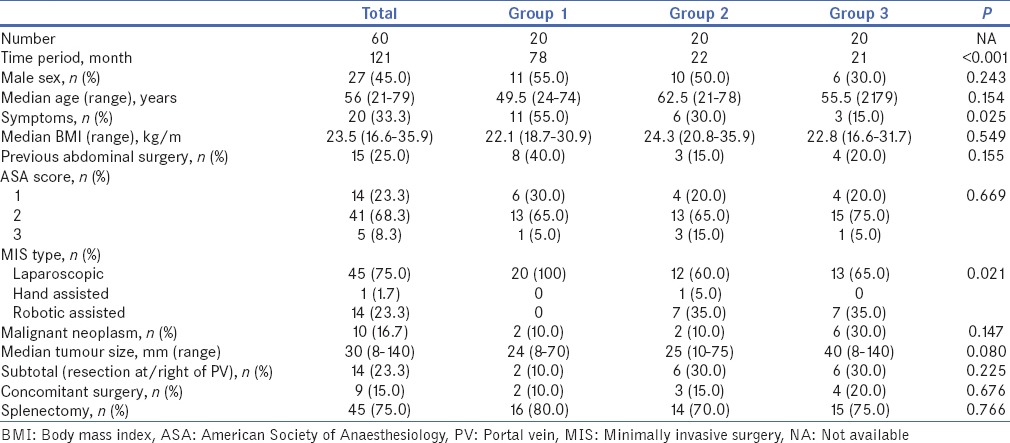
Table 2.
Comparison between the perioperative and oncologic outcomes of patients who underwent minimally invasive distal pancreatectomy across the three groups
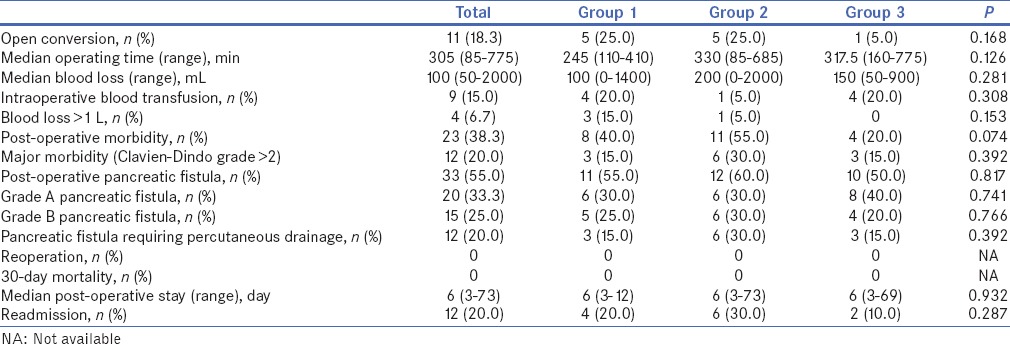
Table 3.
Pathology of tumours resected
Nine (15%) patients had synchronous operations. These included cholecystectomies (n = 5), gastric resection (n = 3), extended right hemicolectomy (n = 1) and bilateral salpingo-oophorectomy (n = 1). One patient had an extended pancreatectomy with gastric resection for locally advanced pancreatic malignancy. The median operating time was 305 (range: 85–775) min and 9 (15.0%) patients required intra-operative blood transfusions. The median post-operative stay was 6 (3–73) days. Eleven (18.3%) operations were converted to open to complete the procedure. This was due to local tumour extension (n = 5), dense adhesions (n = 4) and bleeding (n = 2).
Morbidity
Overall, 23 patients (38.3%) experienced post-operative complications. Twelve patients (20%) experienced major (> Grade 2) complications. All 12 major complications were Grade B pancreatic fistulas requiring post-operative percutaneous drainage (Grade 3a). There were no reoperations or 30-day mortalities. In this series, there were twenty (33.3%) pancreatic fistulas, of which 15 were Grade B and 12 required percutaneous drainage. There were no Grade C fistulas. The median post-operative stay was 6 (range: 3–73) days and 12 (20.0%) patients required readmission for the treatment of post-operative complications.
Evolution of minimally invasive distal pancreatectomy over the study period
Tables 1 and 2 summarise the baseline demographic, clinicopathologic and perioperative data of the patients across the three groups. Comparison between the baseline demographic and pre-operative outcomes of patients who underwent MIDP across the three groups demonstrated that there was a statistically significant increase in the frequency of MIDP performed, increase in robotic-assisted procedures and increase in the proportion of asymptomatic tumours resected. There also tended to be non-significant decrease in open conversion rates from 25% to 5% between the three groups and a non-significant increase in the size of tumours resected from 24 to 40 mm. There was no significant difference in other perioperative outcomes such as operation time, intra-operative blood transfusion rate, median blood loss, post-operative morbidity, readmission rate and post-operative stay.
DISCUSSION
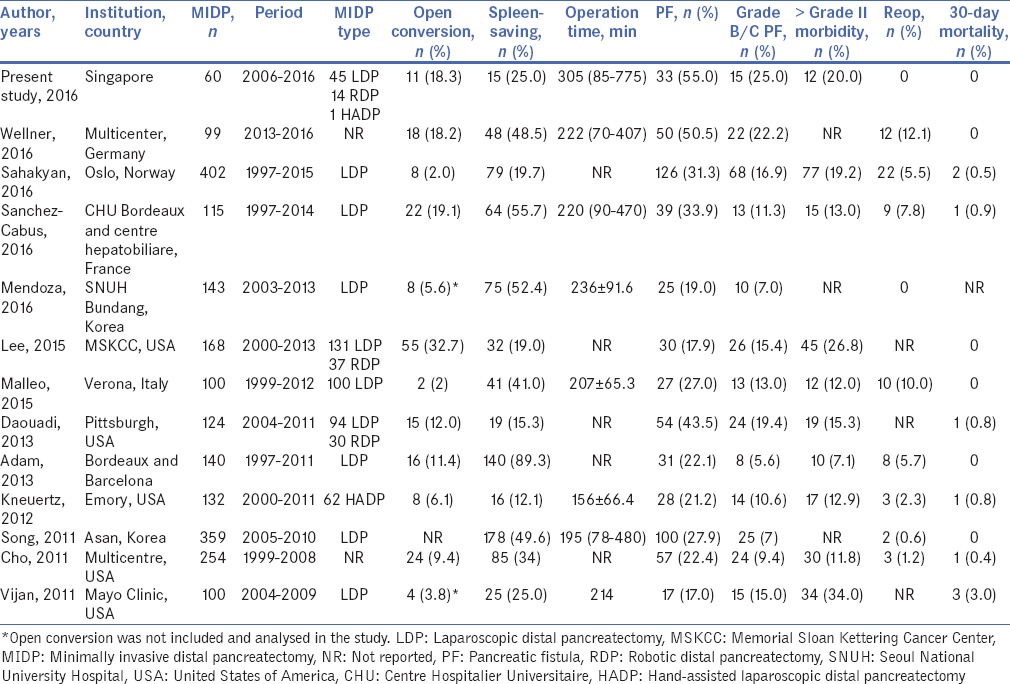
The first MIDP was reported by Cuschieri et al. in 1996[1] 2 years after its feasibility was reported in a pig model in 1994.[18] However, although minimally invasive surgery has grown rapidly in the field of abdominal surgery with many procedures such as cholecystectomies and appendicectomies now routinely performed through laparoscopy,[19,20] the development of minimally invasive surgery for pancreatic surgery has been relatively slow.[9] This is likely due to the deep anatomical location of the pancreas, its propensity for bleeding and high risk of pancreatic leakage which makes minimally invasive surgery of the pancreas highly complex and challenging.[9] However, with the rapid advancements in laparoscopic technology and devices in the past decade, MIDP has increasingly been adopted in recent times with the number of surgical series published in the literature increasing exponentially.[2,3,4,5] Table 4 summarises several recent large case series reporting on MIDP published in the literature.[6,7,21,22,23,24,25,26,27,28,29,30]
Table 4.
Summary of selected recently published large surgical series reporting on minimally invasive distal pancreatectomy
In the present study, we noted that the frequency of MIDP performed at our institution increased over time. The first twenty cases (Group 1) were performed over a period of 78 months whereas the more recent twenty cases (Group 3) were performed over a period of 21 months. There was also a significant increase in the number of asymptomatic tumours resected. This is consistent with several studies which have reported that, with the increased use of cross-sectional imaging today, the incidence of incidental tumours such as pancreatic cystic neoplasms being detected is rapidly increasing.[31,32] We also noted a non-significant trend of larger tumours being resected across the three groups. These findings suggest that the indications for MIDP at our institution expanded with increasing case volume and experience. Kneuertz et al. also noted a similar trend of an increasing proportion of larger tumours being resected as their institution experience increased.[24]
Although MIDP is increasingly adopted today, it remains a technically challenging procedure associated with relatively high open conversion rates reported even from high volume tertiary referral centres, especially during the learning curve.[11] Hence, robotic-assisted laparoscopic surgery[7,13,33] and hand-assisted laparoscopic surgery have been proposed by some authors[6,24] to overcome some of the limitations associated with conventional laparoscopy. In the recent study of 168 MIDP from the Memorial Sloan Kettering Cancer Center, the overall conversion rate was 33%.[6] In another large study from Pittsburgh, the conversion rate for 94 conventional LDP was 16% whereas there was no conversion for the 30 robotic distal pancreatectomies (RDP).[7] In this study, the overall conversion rate was 18.3%. However, we observed a non-significant trend towards decreasing open conversion rates from 25% in Group 1 to 5% in Group 2 with increasing institution experience. It has been previously reported that both institution experience and individual surgeon experience were important factors associated with open conversion rate after MIDP.[11,34,35] There was also an increasing trend towards the adoption of robotic-assisted laparoscopic surgery in the present study although comparison between our more recent experience of RDP with conventional LDP did not demonstrate a significant difference in open conversion rate (unpublished data).
Despite advances in surgical technique and perioperative care, DP is still reportedly associated with a high morbidity rate. The most common complication after is the post-operative pancreatic fistula.[17] Although MIDP has been reported to be associated with superior short-term outcomes compared to open DP, the pancreatic fistula rate remains unchanged with a similar incidence of 10%–50% reported regardless of whether the open or laparoscopic approach is adopted.[11] In this study, the pancreatic fistula rate was 55% and the clinically significant fistula rate was 25% which was consistent with that report by others [Table 4]. Our pancreatic fistula rate remained constant even with increasing experience. This was consistent with previous reports in the literature whereby the reported pancreatic fistula rates after MIDP remained relatively constant even in large series from high volume centres whereby the learning curve had presumably been overcome.[6,7,21,22] Major (> Grade 2) morbidity is not uncommon after MIDP with 20% of patients in the present study experiencing a major morbidity. This was consistent with the 7% to 34% major morbidity rate reported in the literature [Table 4]. Although there were no reoperations in our series, early reoperation after MIDP for bleeding or Grade C pancreatic fistula is also not infrequent with reoperation rates of up to 12% reported in the literature [Table 4].
CONCLUSION
This study demonstrated that MIDP was performed with increased frequency at our institution. There was a statistically significant increase in the frequency of resections performed for asymptomatic tumours and resections performed through robotic assistance over time. There also was a non-significant trend towards a decrease in open conversions and increase in the size of tumours resected.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Cuschieri A, Jakimowicz J, van Spreeuwel J. Laparoscopic distal 70% pancreatectomy and splenectomy for chronic pancreatitis. Ann Surg. 1996;223:280–5. doi: 10.1097/00000658-199603000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Venkat R, Edil BH, Schulick RD, Lidor AO, Makary MA, Wolfgang CL. Laparoscopic distal pancreatectomy is associated with significantly less overall morbidity compared to the open technique: A systematic review and meta-analysis. Ann Surg. 2012;255:1048–59. doi: 10.1097/SLA.0b013e318251ee09. [DOI] [PubMed] [Google Scholar]
- 3.Mehrabi A, Hafezi M, Arvin J, Esmaeilzadeh M, Garoussi C, Emami G, et al. Asystematic review and meta-analysis of laparoscopic versus open distal pancreatectomy for benign and malignant lesions of the pancreas: It's time to randomize. Surgery. 2015;157:45–55. doi: 10.1016/j.surg.2014.06.081. [DOI] [PubMed] [Google Scholar]
- 4.Nigri GR, Rosman AS, Petrucciani N, Fancellu A, Pisano M, Zorcolo L, et al. Metaanalysis of trials comparing minimally invasive and open distal pancreatectomies. Surg Endosc. 2011;25:1642–51. doi: 10.1007/s00464-010-1456-5. [DOI] [PubMed] [Google Scholar]
- 5.Ricci C, Casadei R, Taffurelli G, Pacilio CA, Minni F. Laparoscopic distal pancreatectomy: Many meta-analyses, few certainties. Updates Surg. 2016;68:225–34. doi: 10.1007/s13304-016-0389-5. [DOI] [PubMed] [Google Scholar]
- 6.Lee SY, Allen PJ, Sadot E, D'Angelica MI, DeMatteo RP, Fong Y, et al. Distal pancreatectomy: A single institution's experience in open, laparoscopic, and robotic approaches. J Am Coll Surg. 2015;220:18–27. doi: 10.1016/j.jamcollsurg.2014.10.004. [DOI] [PubMed] [Google Scholar]
- 7.Daouadi M, Zureikat AH, Zenati MS, Choudry H, Tsung A, Bartlett DL, et al. Robot-assisted minimally invasive distal pancreatectomy is superior to the laparoscopic technique. Ann Surg. 2013;257:128–32. doi: 10.1097/SLA.0b013e31825fff08. [DOI] [PubMed] [Google Scholar]
- 8.Taylor C, O'Rourke N, Nathanson L, Martin I, Hopkins G, Layani L, et al. Laparoscopic distal pancreatectomy: The Brisbane experience of forty-six cases. HPB (Oxford) 2008;10:38–42. doi: 10.1080/13651820701802312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang K, Fan Y. Minimally invasive distal pancreatectomy: Review of the english literature. J Laparoendosc Adv Surg Tech A. 2017;27:134–40. doi: 10.1089/lap.2016.0132. [DOI] [PubMed] [Google Scholar]
- 10.Soh YF, Kow AW, Wong KY, Wang B, Chan CY, Liau KH, et al. Perioperative outcomes of laparoscopic and open distal pancreatectomy: Our institution's 5-year experience. Asian J Surg. 2012;35:29–36. doi: 10.1016/j.asjsur.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 11.Goh BK, Chan CY, Lee SY, Chan WH, Cheow PC, Chow PK et al In press. Factors associated with and consequences of open conversion after laparoscopic distal pancreatectomy: Initial experience at a single institution. ANZ J Surg. 2016 doi: 10.1111/ans.13661. [DOI] [PubMed] [Google Scholar]
- 12.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–13. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goh BK, Chan CY, Soh HL, Lee SY, Cheow PC, Chow PK, et al. Acomparison between robotic-assisted laparoscopic distal pancreatectomy versus laparoscopic distal pancreatectomy. Int J Med Robot. 2017;13 doi: 10.1002/rcs.1733. doi:101002/rcs.1733. [DOI] [PubMed] [Google Scholar]
- 14.Goh BK, Wong JS, Chan CY, Cheow PC, Ooi LL, Chung AY, et al. First experience with robotic spleen-saving, vessel-preserving distal pancreatectomy in Singapore: A report of three consecutive cases. Singapore Med J. 2016;57:464–9. doi: 10.11622/smedj.2016020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hartwig W, Vollmer CM, Fingerhut A, Yeo CJ, Neoptolemos JP, Adham M, et al. Extended pancreatectomy in pancreatic ductal adenocarcinoma: Definition and consensus of the International Study Group for Pancreatic Surgery (ISGPS) Surgery. 2014;156:1–14. doi: 10.1016/j.surg.2014.02.009. [DOI] [PubMed] [Google Scholar]
- 16.Bassi C, Dervenis C, Butturini G, Fingerhut A, Yeo C, Izbicki J, et al. Postoperative pancreatic fistula: An International Study Group (ISGPF) definition. Surgery. 2005;138:8–13. doi: 10.1016/j.surg.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 17.Goh BK, Tan YM, Chung YF, Cheow PC, Ong HS, Chan WH, et al. Critical appraisal of 232 consecutive distal pancreatectomies with emphasis on risk factors, outcome, and management of the postoperative pancreatic fistula: A 21-year experience at a single institution. Arch Surg. 2008;143:956–65. doi: 10.1001/archsurg.143.10.956. [DOI] [PubMed] [Google Scholar]
- 18.Soper NJ, Brunt LM, Dunnegan DL, Meininger TA. Laparoscopic distal pancreatectomy in the porcine model. Surg Endosc. 1994;8:57–60. doi: 10.1007/BF02909495. [DOI] [PubMed] [Google Scholar]
- 19.Keus F, de Jong JA, Gooszen HG, van Laarhoven CJ. Laparoscopic versus open cholecystectomy for patients with symptomatic cholecystolithiasis. Cochrane Database Syst Rev. 2006;4:CD006231. doi: 10.1002/14651858.CD006231. [DOI] [PubMed] [Google Scholar]
- 20.Goh BK, Chui CH, Yap TL, Low Y, Lama TK, Alkouder G, et al. Is early laparoscopic appendectomy feasible in children with acute appendicitis presenting with an appendiceal mass? A prospective study. J Pediatr Surg. 2005;40:1134–7. doi: 10.1016/j.jpedsurg.2005.03.046. [DOI] [PubMed] [Google Scholar]
- 21.Vijan SS, Ahmed KA, Harmsen WS, Que FG, Reid-Lombardo KM, Nagorney DM, et al. Laparoscopic vs. open distal pancreatectomy: A single-institution comparative study. Arch Surg. 2010;145:616–21. doi: 10.1001/archsurg.2010.120. [DOI] [PubMed] [Google Scholar]
- 22.Cho CS, Kooby DA, Schmidt CM, Nakeeb A, Bentrem DJ, Merchant NB, et al. Laparoscopic versus open left pancreatectomy: Can preoperative factors indicate the safer technique? Ann Surg. 2011;253:975–80. doi: 10.1097/SLA.0b013e3182128869. [DOI] [PubMed] [Google Scholar]
- 23.Song KB, Kim SC, Park JB, Kim YH, Jung YS, Kim MH, et al. Single-center experience of laparoscopic left pancreatic resection in 359 consecutive patients: Changing the surgical paradigm of left pancreatic resection. Surg Endosc. 2011;25:3364–72. doi: 10.1007/s00464-011-1727-9. [DOI] [PubMed] [Google Scholar]
- 24.Kneuertz PJ, Patel SH, Chu CK, Fisher SB, Maithel SK, Sarmiento JM, et al. Laparoscopic distal pancreatectomy: Trends and lessons learned through an 11-year experience. J Am Coll Surg. 2012;215:167–76. doi: 10.1016/j.jamcollsurg.2012.03.023. [DOI] [PubMed] [Google Scholar]
- 25.Adam JP, Jacquin A, Laurent C, Collet D, Masson B, Fernández-Cruz L, et al. Laparoscopic spleen-preserving distal pancreatectomy: Splenic vessel preservation compared with the Warshaw technique. JAMA Surg. 2013;148:246–52. doi: 10.1001/jamasurg.2013.768. [DOI] [PubMed] [Google Scholar]
- 26.Malleo G, Damoli I, Marchegiani G, Esposito A, Marchese T, Salvia R, et al. Laparoscopic distal pancreatectomy: Analysis of trends in surgical techniques, patient selection, and outcomes. Surg Endosc. 2015;29:1952–62. doi: 10.1007/s00464-014-3890-2. [DOI] [PubMed] [Google Scholar]
- 27.Mendoza AS 3rd, Han HS, Ahn S, Yoon YS, Cho JY, Choi Y. Predictive factors associated with postoperative pancreatic fistula after laparoscopic distal pancreatectomy: A 10-year single-institution experience. Surg Endosc. 2016;30:649–56. doi: 10.1007/s00464-015-4255-1. [DOI] [PubMed] [Google Scholar]
- 28.Sánchez-Cabús S, Adam JP, Pittau G, Gelli M, Cunha AS. Laparoscopic left pancreatectomy: Early results after 115 consecutive patients. Surg Endosc. 2016;30:4480–8. doi: 10.1007/s00464-016-4780-6. [DOI] [PubMed] [Google Scholar]
- 29.Sahakyan MA, Edwin B, Kazaryan AM, Barkhatov L, Buanes T, Ignjatovic D, et al. Perioperative outcomes and survival in elderly patients undergoing laparoscopic distal pancreatectomy. J Hepatobiliary Pancreat Sci. 2017;24:42–8. doi: 10.1002/jhbp.409. [DOI] [PubMed] [Google Scholar]
- 30.Wellner UF, Lapshyn H, Bartsch DK, Mintziras I, Hopt UT, Wittel U, et al. Laparoscopic versus open distal pancreatectomy-a propensity score-matched analysis from the German StuDoQ|Pancreas registry. Int J Colorectal Dis. 2017;32:273–80. doi: 10.1007/s00384-016-2693-4. [DOI] [PubMed] [Google Scholar]
- 31.Goh BK, Tan YM, Cheow PC, Chung YF, Chow PK, Wong WK, et al. Cystic lesions of the pancreas: An appraisal of an aggressive resectional policy adopted at a single institution during 15 years. Am J Surg. 2006;192:148–54. doi: 10.1016/j.amjsurg.2006.02.020. [DOI] [PubMed] [Google Scholar]
- 32.Brugge WR, Lauwers GY, Sahani D, Fernandez-del Castillo C, Warshaw AL. Cystic neoplasms of the pancreas. N Engl J Med. 2004;351:1218–26. doi: 10.1056/NEJMra031623. [DOI] [PubMed] [Google Scholar]
- 33.Goh BK, Lee SY. Robotic pancreatectomy.What is the current evidence? Adv Robot Autom. 2015;S2:004. [Google Scholar]
- 34.Braga M, Ridolfi C, Balzano G, Castoldi R, Pecorelli N, Di Carlo V. Learning curve for laparoscopic distal pancreatectomy in a high-volume hospital. Updates Surg. 2012;64:179–83. doi: 10.1007/s13304-012-0163-2. [DOI] [PubMed] [Google Scholar]
- 35.Ricci C, Casadei R, Buscemi S, Taffurelli G, D'Ambra M, Pacilio CA, et al. Laparoscopic distal pancreatectomy: What factors are related to the learning curve? Surg Today. 2015;45:50–6. doi: 10.1007/s00595-014-0872-x. [DOI] [PubMed] [Google Scholar]


