Abstract
Objective
The growth differentiation factor 15 (GDF15) is a stress-sensitive circulating factor that regulates systemic energy balance. Since exercise is a transient physiological stress that has pleiotropic effects on whole-body energy metabolism, we herein explored the effect of exercise on a) circulating GDF15 levels and b) GDF15 release from skeletal muscle in humans.
Methods
Seven healthy males either rested or exercised at 67% of their VO2max for 1 h and blood was sampled from the femoral artery and femoral vein before, during, and after exercise. Plasma GDF15 concentrations were determined in these samples.
Results
Plasma GDF15 levels increased 34% with exercise (p < 0.001) and further increased to 64% above resting values at 120 min (p < 0.001) after the cessation of exercise. There was no difference between the arterial and venous GDF15 concentration before, during, and after exercise. During a resting control trial, GDF15 levels measured in the same subjects were unaltered.
Conclusions
Vigorous submaximal exercise increases circulating GDF15 levels in humans, but skeletal muscle tissue does not appear to be the source.
Keywords: Skeletal muscle, Growth differentiation factor 15, Recovery, Physical activity
Highlights
-
•
Circulating GDF15 increases during exercise and during recovery from exercise in humans.
-
•
Skeletal muscle tissue appears not to be the source for this exercise-induced increase in GDF15 levels.
-
•
Exercise history should be considered when evaluating GDF15 as a clinical biomarker.
1. Introduction
Obesity and associated health complications afflict millions of people worldwide. Growth differentiation factor 15 (GDF15) has emerged as a potential anti-obesity agent. It circulates as a 25-kDa homodimer and is a member of the transforming growth factor- β (TGF-β) super family. Originally identified in 1997 as a factor that inhibits macrophage activation [1], a role for GDF15 in the regulation of energy balance was established in 2007, when it was demonstrated that GDF15 suppresses food intake [2]. Subsequent pharmacological and genetic studies confirmed that GDF15 administration lowers body weight, largely by decreasing appetite [3], [4], [5], [6], [7]. Some studies have also suggested that GDF15 can directly increase thermogenesis and improve insulin sensitivity [8], [9], [10]. Recently, the GDNF family receptor α-like (GFRAL), located in the area postrema of the hindbrain, was identified as the receptor that mediates the anorexic effects of GDF15 [4], [5], [6], [7].
GDF15 is expressed in most tissues [11] including skeletal muscle [12], and its abundance generally increases in response to cellular stress or injury. For example, circulating GDF15 levels are elevated in patients with cancer or following myocardial injury (reviewed in [13], [14]). In mouse skeletal muscle, GDF15 expression markedly increases in response to mitochondrial stress [8], [15], which can lead to increased plasma GDF15 levels [8]. In humans, circulating GDF15 levels are elevated in patients with muscle atrophy [12] and in patients with mitochondrial myopathy [16]. These data suggest that in response to a stress stimulus, skeletal muscle could release GDF15 into the circulation in humans.
A bout of exercise represents a transient physiological stress [17] that affects overall energy metabolism by increasing energy expenditure [17], improving insulin sensitivity [18], and altering food preference and food intake patterns [19]. During exercise, the contracting skeletal muscle is challenged by energy (e.g. ADP to ATP ratio), mechanical (e.g. stretch) and chemical (e.g. reactive oxygen species) stress. In addition, metformin has been shown to increase serum GDF15 levels in humans [20]. Both metformin and exercise activate the energy sensor AMP-activated protein kinase (AMPK) in skeletal muscle [21], [22]. We therefore tested the hypothesis that exercise increases circulating GDF15 level in humans, by triggering GDF15 release from skeletal muscle.
2. Material and methods
2.1. Subjects and diet
Seven men were recruited for the study, which was approved by the Copenhagen Ethics Committee (Reg. number H-16040740) and performed in accordance with the Declaration of Helsinki. Informed written consent was received from each participant prior to study inclusion. All subjects were healthy, moderately physically active, and with no family history of diabetes. The subjects were 27 ± 1.0 (means ± SEM) years old, with a body weight of 85 ± 3.6 kg, body mass index (BMI) of 24 ± 0.7 kg/m2 and maximal oxygen uptake (VO2 peak) of 50 ± 1.2 ml/kg/min.
Maximal oxygen uptake was measured by an incremental exercise test on a Monark Ergomedic 839E bicycle ergometer (Monark, Sweden).
Subjects were studied twice, at least two weeks apart, in a randomized order. One trial was an exercise trial and the other a rest trial. Before each trial subjects consumed a eucaloric controlled diet for 3 days to ensure the same conditions before each trial. The daily energy requirements were individually determined from weighed dietary registrations and predicted equations from WHO/FAO/UNU. All food items were weighed and prepared in the metabolic kitchen and menus were delivered to the subjects and ingested at home. The diet consisted of 60 energy-percent (E%) carbohydrate, 25E% fat, and 15E% protein.
2.2. Experimental protocol
After three days on the control diet, subjects ingested a light standardized breakfast (1.6 MJ) at 6 A.M at home. Subjects arrived at the institute at 8 A.M, and after 10–15 min of supine rest, teflon catheters were inserted into the femoral artery and vein under local anesthesia. After continued supine rest, basal blood samples were obtained from the femoral artery and vein at 10 A.M (0 min). For the exercise trial, subjects then initiated a 60 min exercise bout on the Monark Ergomedic 839E bicycle ergometer at 67 ± 1% of maximal oxygen uptake (VO2max). Femoral arterial and femoral venous samples were collected simultaneously during exercise at 20, 40, and 60 min and again after 10 and 120 min of resting supine recovery without food intake. For the rest trial, subjects remained in the supine position for 4 h. Arterial blood was collected at 10 A.M. (0 min) and again after 60 and 180 min of rest matching the time schedule of the exercise trial.
2.3. Analysis
Blood was sampled in heparinized syringes and transferred to EDTA-containing tubes and centrifuged at 4 °C at 3000g for 5 min. Plasma was aspirated, frozen, and stored at −80 °C until analysis. GDF15 was measured in plasma using the Quantikine ELISA Human GDF-15 Immunoassay (ELISA, R&D systems, Inc., Minneapolis, USA, catalog number: DGD150). Arterial plasma glucose and blood lactate concentrations and blood hematocrit were measured on an ABL 800 flex (Radiometer Medical, Denmark).
2.4. Statistics
Statistical analyses were performed in SigmaPlot 13.0 (Systat Software, Inc., Germany) or in GraphPad Prism 7 (GraphPad Software, Inc., USA). For the exercise trial, a two-way ANOVA with repeated measurements (RM) was used. The 20 and 40 min time points were excluded in this analysis, because four blood samples could not be analyzed (Table S1). Therefore, for the a–v difference data (Figure 1B) a regular one-way ANOVA was performed to compare differences among the time points. A two-way ANOVA with RM was used for the data from the rest trial. When ANOVA analyses revealed significant differences, the Tukey post hoc test was used for multiple comparisons. p < 0.05 was considered statistically significant.
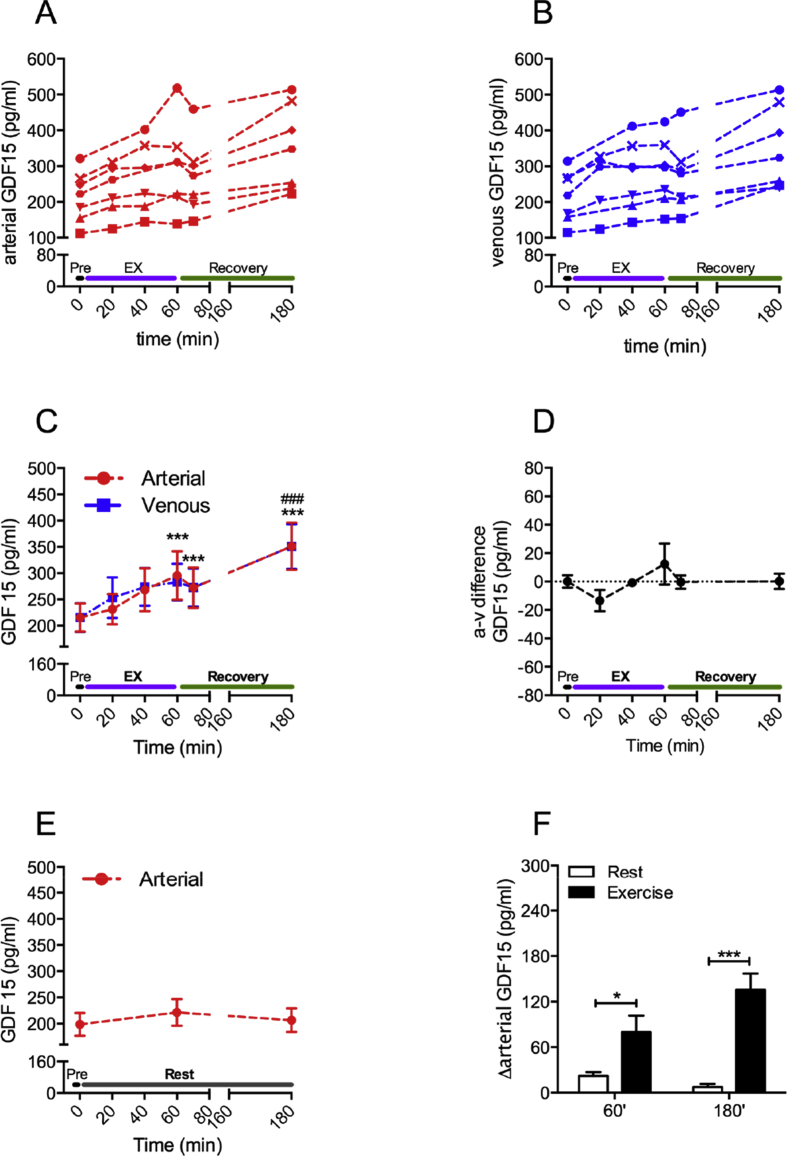
Figure 1.
Vigorous submaximal exercise increases circulating GDF15 in humans. Seven healthy males exercised at 67% of their VO2max for 1 h. Blood, sampled from the femoral artery and femoral vein, was drawn before (Pre), during (EX) and after (Recovery) exercise at indicated time points. Plasma GDF15 concentrations were determined in these samples and are shown for each individual subject (A,B) and as means ± SEM (C). The matching symbol in A and B represents the same subject. The arteriovenous (a–v) GDF15 difference was calculated (D). In the same subjects, plasma GDF15 levels were determined in blood from the femoral artery, collected during a separate rest study (E). Delta (Δ) GDF15 levels were calculated for the exercise and rest trial for the indicated time points (F). Data for D–F are also means ± SEM. *p < 0.05, ***p < 0.001 compared to Pre (in C) or as indicated (in F); ###p < 0.001 compared to 60 min.
3. Results
During the exercise trial, plasma GDF15 levels increased from ∼215 pg/ml at rest to ∼295 pg/ml (p < 0.001) at the end of the exercise bout (Figure 1A). GDF15 further increased to ∼350 pg/ml (p < 0.001) at the end of the recovery (Figure 1A). This exercise-induced increase in circulating GDF15 was remarkably consistent, with GDF15 increasing in every subject. At every time-point, GDF15 concentrations between the femoral artery and femoral vein were similar (Figure 1A–C). In agreement, the arteriovenous (a–v) GDF15 difference was unaffected by exercise and by recovery from exercise (Figure 1D). Arterial GDF15 plasma levels, measured in the same subjects during the time- and diet-matched rest study, remained unchanged (Figure 1E). Absolute changes in arterial GDF15 levels were 2.6-fold (p < 0.05) and 16.7-fold (p < 0.001) higher during the exercise trial compared to the rest trial at 60 min (exercise stop) and 180 min (recovery), respectively (Figure 1F).
Baseline (0 min) respiratory exchange ratio (RER), oxygen uptake, plasma glucose, blood lactate, and hematocrit concentrations were similar in the two trials (Table 1). Throughout the rest trial, these parameters remained unchanged. During the 60 min-long exercise bout, oxygen uptake, blood lactate, and hematocrit concentrations increased significantly and returned to baseline levels after 2 h of recovery (Table 1). Heart rate was 167 ± 5 bpm at the end of exercise.
Table 1.
Respiratory exchange ratio (RER) and oxygen uptake (VO2), as well as plasma glucose, blood lactate and hematocrit (Hct) concentrations determined in femoral arterial blood sampled at baseline, during 60 min of exercise and subsequent recovery, or during 60 and 180 min of rest.
| Baseline |
Exercise/rest |
Recovery |
|||
|---|---|---|---|---|---|
| 0 min | 20 min | 40 min | 60 min | 180 min | |
| RER | |||||
| EX | 0.87 ± 0.02 | 0.98 ± 0.01 | 0.95 ± 0.01 | 0.96 ± 0.01††∗∗ | 0.85 ± 0.04 |
| REST | 0.88 ± 0.03 | – | – | 0.88 ± 0.01 | 0.89 ± 0.02 |
| VO2(L⋅min−1) | |||||
| EX | 0.3 ± 0.0 | 2.7 ± 0.1 | 2.9 ± 0.1 | 2.9 ± 0.2†††∗∗∗ | 0.3 ± 0.0 |
| REST | 0.3 ± 0.0 | – | – | 0.3 ± 0.0 | 0.3 ± 0.0 |
| Arterial glucose (mmol∙L−1) | |||||
| EX | 5.4 ± 0.1 | 5.2 ± 0.2 | 5.1 ± 0.2 | 5.7 ± 0.5 | 5.4 ± 0.1 |
| REST | 5.5 ± 0.1 | – | – | 5.4 ± 0.1 | 5.3 ± 0.1 |
| Arterial lactate (mmol∙L−1) | |||||
| EX | 0.7 ± 0.0 | 3.9 ± 0.5 | 3.4 ± 0.4 | 4.1 ± 0.5†††∗∗∗ | 0.6 ± 0.0 |
| REST | 0.7 ± 0.1 | – | – | 0.7 ± 0.1 | 0.7 ± 0.1 |
| Hct (%) | |||||
| EX | 45.1 ± 1.0 | 49.3 ± 0.9 | 49.5 ± 0.8 | 48.8 ± 0.8 †††∗∗∗ | 45.0 ± 1.0 |
| REST | 44.6 ± 1.3 | – | – | 45.0 ± 1.3 | 45.0 ± 1.4 |
Data are expressed as means ± SEM. ††p < 0.01, †††p < 0.001 different from baseline and recovery values. **p < 0.01, ***p < 0.001 different from REST at the same time point.
20 and 40 min values were excluded from statistical analysis because values at these time points were not obtained in the rest trial.
4. Discussion
Our data demonstrate that circulating GDF15 levels increase with an acute bout of vigorous submaximal exercise in humans. Furthermore, we show that this increase does not appear to be mediated by an exercise-induced release of GDF15 from the exercising muscle, because venous and arterial concentrations of GDF15 across the exercised leg were similar. This is the first well-controlled study to measure GDF15 levels during a bout of exercise and in the subsequent recovery period from exercise. A previous study reported higher GDF15 levels in subjects after a 246 km-long ultramarathon race [23]. However, this is an extreme form of exercise that lasts for ∼24–36 h; also, the study lacked crucial time- and diet-matched control groups, which are imperative for interpretation, especially since GDF15 levels exhibit considerable diurnal variation in humans [24]. In addition, the role of skeletal muscle as a potential source of GDF15 has not been previously assessed in humans.
Our finding of an exercise-induced increase in GDF15 levels gives rise to several hypotheses and implications. First, future studies should clarify whether GDF15 is necessary for some of the exercise-associated metabolic benefits. Second, given the anorexic nature of GDF15, it is tempting to speculate that GDF15 is involved in the transient exercise-induced suppression of appetite in humans [25]. Third, GDF15 is being actively considered as a clinical biomarker, for example, to predict cardiac complications [14]. Exercise history should now be accounted for in such applications. Fourth, since skeletal muscle is not the source of the rise in plasma GDF15 during vigorous submaximal exercise, the responsible organ(s) as well as the mechanisms remain to be identified in humans. It remains possible that GDF15 is secreted from muscle to act locally in an autocrine or paracrine manner, but, notably, Gfral expression was not detected in peripheral tissues, including skeletal muscle, in the mouse [4].
Our findings also have limitations. For now, they apply to young, relatively fit, male subjects. Factors such as sex, age, exercise intensity, the type of exercise, and initial fitness level will have to be considered in future studies. In addition, a minor part of the increase in plasma GDF15 during exercise can likely be attributed to an exercise-induced hemoconcentration, since we detected a rise in hematocrit concentration of 8–9% during exercise. During recovery, however, hematocrit levels returned to baseline and therefore, hemoconcentration does not contribute to the marked increase in circulating GDF15 2 h after the exercise bout.
5. Conclusion
We have identified exercise as a disease-unrelated, physiological stimulus that increases endogenous circulating GDF15 levels in humans. This exercise effect appears to occur without direct contribution from skeletal muscle.
Author contribution
MK analyzed the data and wrote the manuscript. CC assisted in the interpretation of the data and with the preparation of the manuscript. KAS and CSC recruited the subjects and participated in conducting the studies and in the data analysis. JJ assisted in the study design and data interpretation. JFPW, BK, and EAR designed the study, executed the experiments and helped write the manuscript. All authors commented on and approved the final version of the manuscript. EAR is the guarantor of this work and, as such, has full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analyses.
Acknowledgments
We acknowledge the skilled technical assistance of Irene Bech Nielsen and Betina Bolmgren (University of Copenhagen). We also acknowledge the volunteers that participated in the study. The study was supported by Novo-Nordisk A/S. MK is supported by postdoctoral research grant from the Danish Council for Independent Research/Medicine (grant: 4004-00233). CC receives funding from the Lundbeck Foundation (Fellowship) (R238-2016-2859) and the Novo Nordisk Foundation (grant: NNF17OC0026114). Novo Nordisk Foundation Center for Basic Metabolic Research is an independent Research Center, based at the University of Copenhagen, Denmark and partially funded by an unconditional donation from the Novo Nordisk Foundation (www.metabol.ku.dk). KAS was supported by a postdoctoral research grant from the Danish Council for Independent Research/Medicine (grant: 4092-00309).
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.molmet.2017.12.016.
Conflicts of interest
None.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Bootcov M.R., Bauskin A.R., Valenzuela S.M., Moore A.G., Bansal M., He X.Y. MIC-1, a novel macrophage inhibitory cytokine, is a divergent member of the TGF-β superfamily. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:11514–11519. doi: 10.1073/pnas.94.21.11514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johnen H., Lin S., Kuffner T., Brown D.A., Tsai V.W.-W., Bauskin A.R. Tumor-induced anorexia and weight loss are mediated by the TGF-β superfamily cytokine MIC-1. Nature Medicine. 2007;13:1333. doi: 10.1038/nm1677. [DOI] [PubMed] [Google Scholar]
- 3.Macia L., Tsai V.W.-W., Nguyen A.D., Johnen H., Kuffner T., Shi Y.C. Macrophage inhibitory cytokine 1 (MIC-1/GDF15) decreases food intake, body weight and improves glucose tolerance in mice on normal & obesogenic diets. PLoS One. 2012;7 doi: 10.1371/journal.pone.0034868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mullican S.E., Lin-Schmidt X., Chin C.N., Chavez J.A., Furman J.L., Armstrong A.A. GFRAL is the receptor for GDF15 and the ligand promotes weight loss in mice and nonhuman primates. Nature Medicine. 2017;23:1150. doi: 10.1038/nm.4392. [DOI] [PubMed] [Google Scholar]
- 5.Emmerson P.J., Wang F., Du Y., Liu Q., Pickard R.T., Gonciarz M.D. The metabolic effects of GDF15 are mediated by the orphan receptor GFRAL. Nature Medicine. 2017;23:1215. doi: 10.1038/nm.4393. [DOI] [PubMed] [Google Scholar]
- 6.Yang L., Chang C.C., Sun Z., Madsen D., Zhu H., Padkjær S.B. GFRAL is the receptor for GDF15 and is required for the anti-obesity effects of the ligand. Nature Medicine. 2017;23:1158. doi: 10.1038/nm.4394. [DOI] [PubMed] [Google Scholar]
- 7.Hsu J.Y., Crawley S., Chen M., Ayupova D.A., Lindhout D.A., Higbee J. Non-homeostatic body weight regulation through a brainstem-restricted receptor for GDF15. Nature. 2017;550:255. doi: 10.1038/nature24042. [DOI] [PubMed] [Google Scholar]
- 8.Chung H.K., Ryu D., Kim K.S., Chang J.Y., Kim Y.K., Yi H.S. Growth differentiation factor 15 is a myomitokine governing systemic energy homeostasis. The Journal of Cell Biology. 2017;216:149–165. doi: 10.1083/jcb.201607110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chrysovergis K., Wang X., Kosak J., Lee S.H., Kim J.S., Foley J.F. NAG-1/GDF15 prevents obesity by increasing thermogenesis, lipolysis and oxidative metabolism. International Journal of Obesity (London) 2014;38:1555–1564. doi: 10.1038/ijo.2014.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee S.E., Kang S.G., Choi M.J., Jung S.B., Ryu M.J., Chung H.K. Growth differentiation factor 15 mediates systemic glucose regulatory action of t-helper type 2 cytokines. Diabetes. 2017;66:2774. doi: 10.2337/db17-0333. [DOI] [PubMed] [Google Scholar]
- 11.Ding Q., Mracek T., Gonzalez-Muniesa P., Kos K., Wilding J., Trayhurn P. Identification of macrophage inhibitory cytokine-1 in adipose tissue and its secretion as an adipokine by human adipocytes. Endocrinology. 2009;150:1688–1696. doi: 10.1210/en.2008-0952. [DOI] [PubMed] [Google Scholar]
- 12.Bloch S.A.A., Lee J.Y., Syburra T., Rosendahl U., Griffiths M.J.D., Kemp P.R. Increased expression of GDF-15 may mediate ICU-acquired weakness by down-regulating muscle microRNAs. Thorax. 2015;70:219–228. doi: 10.1136/thoraxjnl-2014-206225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bauskin A.R., Brown D.A., Kuffner T., Johnen H., Luo X.W., Hunter M. Role of macrophage inhibitory cytokine-1 in tumorigenesis and diagnosis of cancer. Cancer Research. 2006;66:4983. doi: 10.1158/0008-5472.CAN-05-4067. [DOI] [PubMed] [Google Scholar]
- 14.Wollert K.C., Kempf T., Wallentin L. Growth differentiation factor 15 as a biomarker in cardiovascular disease. Clinical Chemistry. 2017;63:140. doi: 10.1373/clinchem.2016.255174. [DOI] [PubMed] [Google Scholar]
- 15.Ost M., Keipert S., van Schothorst E.M., Donner V., van der Stelt I., Kipp A.P. Muscle mitohormesis promotes cellular survival via serine/glycine pathway flux. The FASEB Journal. 2015;29:1314–1328. doi: 10.1096/fj.14-261503. [DOI] [PubMed] [Google Scholar]
- 16.Kalko S.G., Paco S., Jou C., Rodríguez M.A., Meznaric M., Rogac M. Transcriptomic profiling of TK2 deficient human skeletal muscle suggests a role for the p53 signalling pathway and identifies growth and differentiation factor-15 as a potential novel biomarker for mitochondrial myopathies. BMC Genomics. 2014;15:91. doi: 10.1186/1471-2164-15-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hawley J.A., Hargreaves M., Joyner M.J., Zierath J.R. Integrative biology of exercise. Cell. 2014;159:738–749. doi: 10.1016/j.cell.2014.10.029. [DOI] [PubMed] [Google Scholar]
- 18.Sylow L., Kleinert M., Richter E.A., Jensen T.E. Exercise-stimulated glucose uptake – regulation and implications for glycaemic control. Nature Reviews Endocrinology. 2016;13:133. doi: 10.1038/nrendo.2016.162. [DOI] [PubMed] [Google Scholar]
- 19.Bellisle, F. 1999. Food choice, appetite and physical activity. 2007/01/02: 357–361. [DOI] [PubMed]
- 20.Gerstein H.C., Pare G., Hess S., Ford R.J., Sjaarda J., Raman K. Growth differentiation factor 15 as a novel biomarker for metformin. Diabetes Care. 2016 doi: 10.2337/dc16-1682. [DOI] [PubMed] [Google Scholar]
- 21.Musi N., Hirshman M.F., Nygren J., Svanfeldt M., Bavenholm P., Rooyackers O. Metformin increases AMP-activated protein kinase activity in skeletal muscle of subjects with type 2 diabetes. Diabetes. 2002;51:2074. doi: 10.2337/diabetes.51.7.2074. [DOI] [PubMed] [Google Scholar]
- 22.Winder W.W., Hardie D.G. Inactivation of acetyl-CoA carboxylase and activation of AMP-activated protein kinase in muscle during exercise. American Journal of Physiology, Endocrinology and Metabolism. 1996;270:E299. doi: 10.1152/ajpendo.1996.270.2.E299. [DOI] [PubMed] [Google Scholar]
- 23.Tchou I., Margeli A., Tsironi M., Skenderi K., Barnet M., Kanaka-Gantenbein C. Growth-differentiation factor-15, endoglin and N-terminal pro-brain natriuretic peptide induction in athletes participating in an ultramarathon foot race. Biomarkers. 2009;14:418–422. doi: 10.1080/13547500903062976. [DOI] [PubMed] [Google Scholar]
- 24.Tsai V.W.-W., Macia L., Feinle-Bisset C., Manandhar R., Astrup A., Raben A. Serum levels of human MIC-1/GDF15 vary in a diurnal pattern, do not display a profile suggestive of a satiety factor and are related to BMI. PLoS One. 2015;10:e0133362. doi: 10.1371/journal.pone.0133362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.King N.A., Burley V.J., Blundell J.E. Exercise-induced suppression of appetite: effects on food intake and implications for energy balance. European Journal of Clinical Nutrition. 1994;48:715–724. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.