Abstract
Background
In the present study, we aimed to investigate the effects of sinomenine (SIN) on chronic intermittent hypoxia (CIH)- induced lung injury in rats, and to explore the underlying mechanisms.
Material/Methods
To perform the investigation, a CIH rat model was established. ELISA assay was applied to detect the level of inflammatory cytokines. Oxidative stress bio-markers (MDA, SOD, and CAT) were determined in lung tissues. In addition, the expression level of NADPH oxidase 2 (Nox2) was analyzed by Western blotting and qRT-PCR, respectively.
Results
The results showed that compared with other groups, more obvious pulmonary pathological changes were observed in the CIH group. The level of inflammatory cytokines in the CIH group was markedly higher than that in the control and Con-S groups. Compared with the control and Con-S groups, oxidative stress was notably increased in the CIH group. Expression of Nox2 was also increased in the CIH group. The effects caused by CIH in rats were attenuated by SIN treatment.
Conclusions
SIN can reverse chronic intermittent hypoxia-induced lung injury through inhibiting inflammation and oxidative stress.
MeSH Keywords: Inflammation; Lung Injury; Oxidative Stress; Sinomenium; Sleep Apnea, Obstructive
Background
Obstructive sleep apnea syndrome (OSAS), a common disease which can lead to metabolic disorders, is characterized by complete or partial recurrent episodes of upper-respiratory tract obstruction during sleep, causing daytime fatigue, frequent arousals, intermittent hypoxemia, and other problems [1–3]. It has been revealed that OSAS is associated with decreased blood oxygen saturation [4]. The epithelial and endothelial cell injury in lung tissues can be induced by hypoxia. Approximately 2–4% of the adult population suffer from OSAS, and most are older adults [5]. Endothelial dysfunction, systemic inflammation, and oxidative stress are involved in the pathogenesis of OSAS. The typical pathophysiological feature of OSAS is chronic intermittent hypoxia (CIH), which is characterized by repeated alternation of normal oxygen levels and hypoxia. It has been confirmed that the long-term presence of CIH in OSAS patients is the major cause of systemic injury. In recent years, many CIH animal models have been developed to simulate OSAS pathophysiological environments for the study of CIH-induced tissue and organ injury [6–13].
As a pure alkaloid, sinomenine (7,8-didehydro-4-hydroxy3,7-dimethoxy-17-methyl-9α, 13α, 14α-morphinan-6-one; SIN) is extracted from the Chinese medicinal plant Sinomenium acutum, and it has been widely used for inflammatory diseases treatment in China [14,15]. Previous studies have revealed the various pharmacological properties of SIN, such as anti-cancer, anti-inflammation, immunosuppression, cytoprotection, neuroprotection, and protection against acute lung injury [16–23]. Nevertheless, to the best of our knowledge, no study has investigated the effects of SIN on the lung injury induced by CIH or explored the potential underlying molecular mechanisms.
Therefore, in the present study, we aimed to investigate the effects of sinomenine administration on the lung injury induced by CIH in rats, as well as the underlying molecular mechanisms.
Material and Methods
Chronic intermittent hypoxia (CIH) model establishment
A total of 20 SD rats (all males, body weight ~200 g) were supplied by the Vital River Company (Beijing, China). Rats were housed individually under a 12-h light–dark cycle at 25±2°C with 60±5% humidity. All the rats ate and drank freely.
Mice were randomly divided into 4 groups: 1. Control group (rats exposed to normoxia; n=5); 2. Con-S group with 5 rats (normoxia exposure + sinomenine administration 41 μg kg−1·min−1 for 25 days); 3. CIH group with 5 rats (CIH exposure alone); and 4. CIH-S group with 5 rats (CIH exposure+ sinomenine administration at 41 μg kg−1·min−1 for 25 days; n=5). An osmotic mini-pump (Alzet 2004; Alza, USA) was embedded into each rat of the Con-S group, CIH group, and CIH-S group.
The CIH model was established as previously described [24]. In brief, a hypoxic environment was created by using sealed chambers. The compressed air and pure nitrogen were then added into each chamber by means of a solenoid timing valve. The gas was infused into chambers at a cycle of 90 s. At the first 30 s of a cycle, in order to achieve a 5% minimum oxygen concentration, every chamber was infused with pure nitrogen. Chambers were infused with the compressed air for the remaining 60 s to make the concentration of the oxygen gradually return to 20.9%. Air was forced into the chambers of the control and Con-S groups. Finally, all rats were sacrificed by cervical dislocation. Blood was collected and centrifugated, and then the serum was stored at −80°C. One part of the lung tissues was collected and then fixed with 4% phosphate-buffered formaldehyde for histological analysis, and the other part of the lung tissues was immediately frozen in liquid nitrogen and then stored at −80°C. The present study was approved by the Ethics Committee of the Affiliated Hospital of Jiangnan University and the experiments were performed according to the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals.
Histological staining [8]
The lung tissue specimens of the rats were first fixed with 10% formalin, paraffin-embedded, cut into 5-mm-thick slices, and then stained with hematoxylin and eosin (H&E) [8]. Images were obtained using light microscopy (Nikon Instrument Group, USA).
ELISA assay
The expression levels of TNF-α, IL-6, and IL-8 in the plasma of the rats were measured by using ELISA assay following the manufacturer’s instructions for each kit. All experiments were repeated at least 3 times.
Detection of the MDA level and antioxidant enzyme activity
To detect the level of MDA and the activity of the antioxidant enzymes (SOD and CAT), the frozen lung specimens from rats were homogenized using tissue lysis buffer (Beyotime, China) according to the manufacturer’s instructions. Then, the homogenates were centrifuged at 377.3 g (15 min, 4°C). Subsequently, the level of MDA in the supernatants was detected using an MDA detection kit (CST) according to the manufacturer’s instructions. SOD and CAT activity in lung tissues were determined by using commercial assay kits (CST) following the manufacturer’s instructions. The enzyme activity is presented as units/mg of protein [8].
Real-time RT-PCR
Total RNA from the lung tissues was extracted using Trizol reagent (Invitrogen, USA) following the manufacturer’s instructions. cDNAs were generated by performing reverse transcription assay using a reverse transcription kit following the manufacturer’s instructions. Real-time PCR was applied to analyze the cDNAs by using the 2X Maxima SYBR Green/ROX qPCR Master Mix kit (Invitrogen) in line with the manufacturer’s instructions. The amplification conditions were as follows: 94°C for 10 min for denaturation, followed by 38 cycles of 94°C for 30 s, 60°C for 30 s, and 72°C for 60 s. In the present study, GAPDH served as the internal control, and the 2−ΔΔCq method [25] was used to analyze the data. Primer sequences were obtained from Gen Script Co., Ltd. (Nanjing, China) as required.
Western blot analysis
Total proteins from the lung tissues were collected using the lysis buffer. The concentration of proteins was detected by using the bicinchoninic acid (BCA) protein assay (Thermo Scientific, USA) per as the manufacturer’s instructions. Equal amounts (25 μg) of the protein samples were resolved using 12% SDS-PAGE and then transferred onto PVDF membranes (Millipore, USA). Subsequently, the membranes were blocked with non-fat milk, incubated with primary antibodies (anti-β-actin, anti-p-p38, anti-NF-κB (p65), anti-Nox2, anti-Nrf2, anti-HO-1, and anti-NQO-1; with a 1: 1000 dilution for all (Cell Signaling Technology, Inc., Danvers, MA, USA), washed, and then incubated with a secondary antibody (1: 5000 dilution; Cell Signaling Technology, Inc., Danvers, MA, USA) at room temperature for 2 h. The protein blots were finally visualized using the chemiluminescent ECL reagent (Millipore, MA, USA). Densitometry (QuantityOne 4.5.0 software; Bio-Rad Inc., USA) was used to quantify the protein bands.
Statistical analysis
Data are displayed as mean ±SE. SPSS 17.0 software (IBM, USA) was used for statistical analysis. One-way ANOVA or Student’s t-test was applied for the comparisons between groups. A value of p<0.05 was considered as a significant difference.
Results
Histopathological characteristics
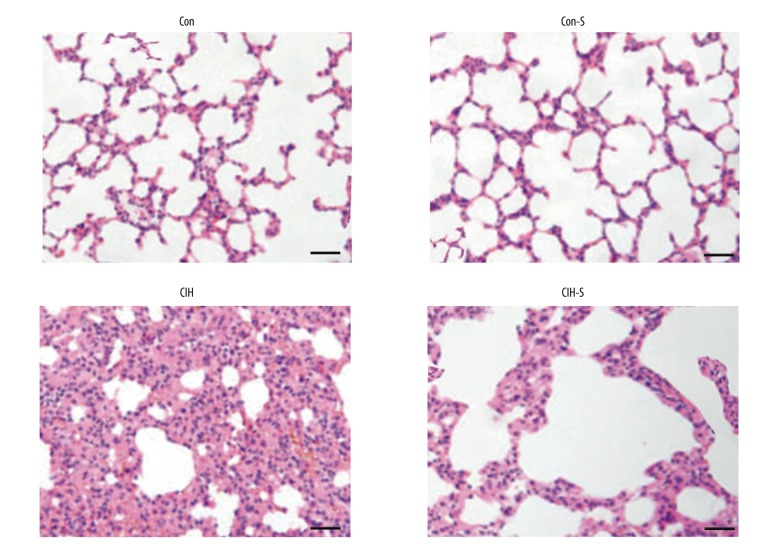
The results of H&E staining indicated that no differences of the histopathological characteristics between the control and Con-S groups were found in the present study. However, compared with control and Con-S groups, the CIH group showed increased infiltration of inflammatory cells, and bleeding, alveolar wall thickness, and edema were markedly increased in the CIH group. Compared with the CIH group, inflammation and pulmonary architecture distortion were markedly decreased in the CIH-S group (Figure 1).
Figure 1.
Histopathological changes in lung tissue. Hematoxylin and eosin staining (200 magnification, bar 100 μm).
SIN down-regulated the release of proinflammatory cytokines
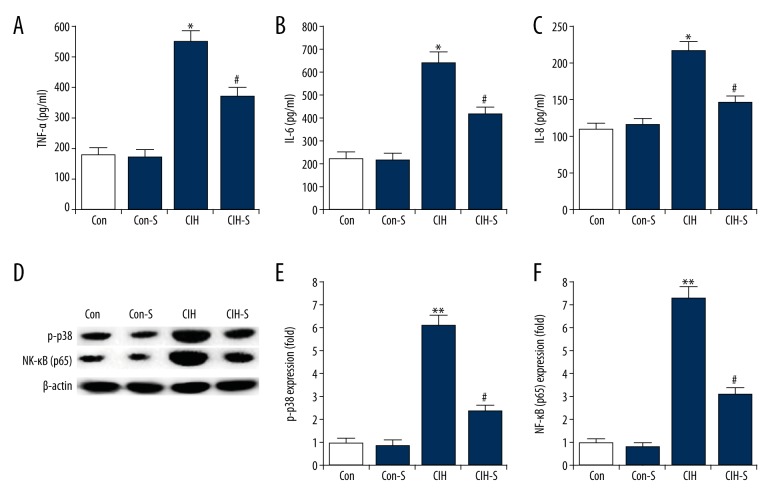
As shown in Figure 2A–2C, no significant differences in the production of TNF-α, IL-6 and IL-8 were found between the control and Con-S groups. Compared with the controls, the level of TNF-α, IL-6, and IL-8 in the plasma was significantly increased in the CIH group, and SIN treatment markedly eliminated this increase.
Figure 2.
Changes in proinflammatory cytokines production. The levels of TNF-α (A), IL-6 (B), and IL-8 (C) in plasma of rats from different groups was determined by ELISA. (D) Protein level of p-p38 and NF-κB (p65) was detected by Western blotting; (E) Expression levels of p38 phosphorylation were expressed as fold of the control; (F) Expression levels of NF-κB (p65) were expressed as fold of the control. Con – Control group (rats exposed to normoxia); Con-S – rats with normoxia exposure + sinomenine administration 41 μg kg−1∙min−1 for 25 days; CIH – rats exposed to CIH alone; CIH-S – rats with CIH exposure + sinomenine administration at 41 μg kg−1∙min−1 for 25 days. Data are reported as means ±SE. *, ** p<0.05, 0.01 vs. control; # p<0.05 vs. CIH.
In addition, p-p38 and NF-κB (p65) were assessed in the present study. We found that the protein levels of p-p38 and NF-κB (p65) were significantly enhanced in the lung tissues by CIH administration, and SIN treatment markedly decreased p-p38 and NF-κB (p65) expression (Figure 2D–2F).
SIN attenuated CIH-induced oxidative stress
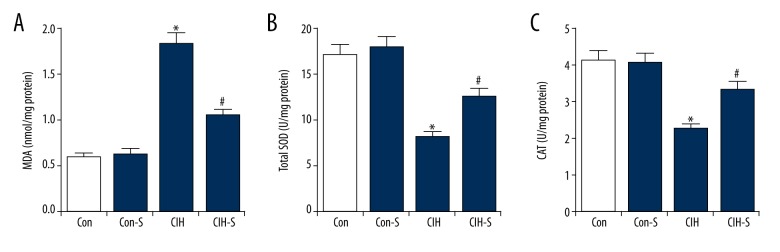
Compared with the controls, the MDA level was significantly increased in the lung tissues of rats from the CIH group, indicating the enhanced oxidative stress in CIH treatment rats. Moreover, the findings suggested that the lung SOD activity and CAT level were both markedly reduced in CIH rats. SIN treatment effectively reduced MDA content and promoted the activity of SOD and the levels of CAT in the lung tissues of the CIH rats (Figure 3).
Figure 3.
SIN reduced oxidative stress in lung tissues. (A) MDA content; (B) Total SOD activity; (C) CAT level. Con – Control group (rats exposed to normoxia); Con-S – rats with normoxia exposure + sinomenine administration 41 μg kg−1∙min−1 for 25 days; CIH – rats exposed to CIH alone; CIH-S – rats with CIH exposure + sinomenine administration at 41 μg kg−1∙min−1 for 25 days. Data are reported as means ±SE. * p<0.05 vs. control; # p<0.05 vs. CIH.
SIN inhibited CIH-induced expression of Nox2
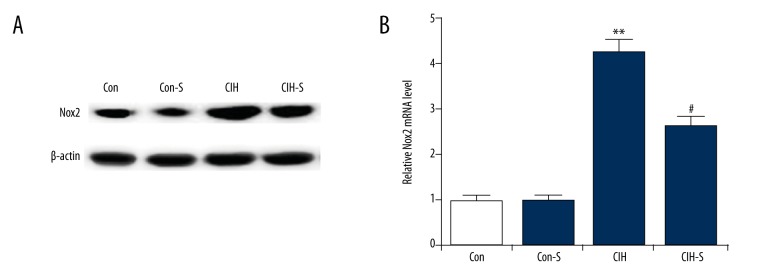
The results indicated that compared with the controls, both protein and mRNA expression of Nox2 were significantly enhanced in the CIH and CIH-S rats, and SIN relieved the CIH-induced increase in Nox2 expression (Figure 4).
Figure 4.
SIN decreased Nox2 expression in rat lung tissues. (A) Protein level of Nox2 in the lung tissues of rats from different groups was measured by Western blot assay; (B) Relative Nox2 mRNA level in the lung tissues of rats from different groups was measured by qRT-PCR. Con – Control group (rats exposed to normoxia); Con-S – rats with normoxia exposure + sinomenine administration 41 μg kg−1∙min−1 for 25 days; CIH – rats exposed to CIH alone; CIH-S – rats with CIH exposure + sinomenine administration at 41 μg kg−1∙min−1 for 25 days. Data are reported as means ±SE. *, ** p<0.05, 0.01 vs. control; # p<0.05 vs. CIH.
SIN activated Nrf2 signaling pathway
The nuclear factor erythroid-derived like 2 (Nrf2) signaling, a key antioxidant mechanism that maintains intracellular redox homeostasis underlying oxidative stress, was also assessed in our study. Compared with the controls, the protein and mRNA levels of Nrf2, HO-1, and NQO-1 was significantly decreased in the lung tissues of rats from the CIH group, and SIN treatment markedly eliminated this decrease (Figure 5).
Figure 5.
SIN activated Nrf2 pathway in rat lung tissues. (A) Protein level of Nrf2, HO-1, and NQO-1 in the lung tissues of rats from different groups was measured by Western blot assay; (B–D) relative Nrf2, HO-1, and NQO-1 mRNA level in the lung tissues of rats from different groups was measured by qRT-PCR. Con – Control group (rats exposed to normoxia); Con-S – rats with normoxia exposure + sinomenine administration 41 μg kg−1∙min−1 for 25 days; CIH – rats exposed to CIH alone; CIH-S – rats with CIH exposure + sinomenine administration at 41 μg kg−1∙min−1 for 25 days. Data are reported as means ±SE. ** p<0.01 vs. control; # p<0.05 vs. CIH.
Discussion
OSAS, a risk factor for refractory hypertension, which is characterized by chronic intermittent hypoxia (CIH), is associated with several cardiovascular diseases. A number of studies have shown that CIH can cause various damage to the body, including myocardial injury, pancreatic injury, renal injury, liver injury, and lung injury [8,24,26–28]. The lungs are the organ most sensitive for hypoxia. Hypoxia can lead to acute physiological changes, including pulmonary artery vasoconstriction and alveolar hypoventilation. Moreover, hypoxia can lead to inflammatory cell infiltration, tissue edema, cytokines elevation, and oxidative stress in the lung. In our present study, we studied the lung injury induced by CIH in rats and explored the protective effect of SIN.
To perform our investigation, a CIH rat model was successfully established. The proinflammatory cytokines, including tumor necrosis factor (TNF-α), interleukin-6 (IL-6), and IL-8, have been revealed to play a critical role in promoting lung injury [29]. The present study showed that the levels of TNF-α, IL-6, and IL-8 were significantly increased in the plasma of CIH rats, and SIN treatment significantly eliminated this increase. To further explore the underlying mechanism, p-p38 and NF-κB (p65) were assessed in the present study. As expected, we found that SIN treatment significantly decreased p-p38 and NF-κB (p65) expression in CIH rats. Thus, SIN can inhibit TNF-α, IL-6, and IL-8 production in CIH rats, partly through repressing p-p38 and NF-κB (p65) activation.
Antioxidant capacity reduction and free radical levels elevation have been reported to be involved in the pathological changes of pulmonary diseases [30]. The effect of SIN on oxidative stress was determined by detecting MDA content, SOD activity, and CAT level in lung tissues. The findings indicated that oxidative stress in the lung tissues of rats was notably enhanced by CIH administration, and SIN treatment significantly inhibited the increased oxidative stress induced by CIH treatment. A previous study revealed that SIN exhibited antioxidant properties mainly through suppressing NADPH oxidase (NOX) [20]. Our results showed that Nox2 was significantly enhanced in the CIH and CIH-S rats, and SIN relieved the CIH-induced increase in Nox2 expression. These results are consistent with the present study. In addition, the Nrf2 signaling, a key antioxidant mechanism, maintains intracellular redox homeostasis underlying oxidative stress, which can be activated by SIN [31], was also assessed in our study, showing that Nrf2 signaling was significantly repressed in the lung tissues of rats from the CIH group, and SIN treatment markedly eliminated this repression.
Taken together, our results indicate that SIN can reverse the lung injury induced by CIH through inhibiting inflammation and oxidative stress. We provide a more complete theoretical basis for the clinical treatment of CIH-induced lung injury.
Conclusions
SIN protects against lung injury induced by CIH via suppressing inflammation and oxidative stress.
Footnotes
Source of support: The study was supported by the National Natural Science Foundation of China (No. 81500071)
Conflicts of interests
None.
References
- 1.Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: A population health perspective. Am J Respir Crit Care Med. 2002;165:1217–39. doi: 10.1164/rccm.2109080. [DOI] [PubMed] [Google Scholar]
- 2.Wang Y, Xu H, Qian Y, et al. Patients with obstructive sleep apnea display decreased flow-mediated dilatation: Evidencefrom a meta-analysis. Med Sci Monit. 2018;24:1069–82. doi: 10.12659/MSM.899716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ekin S, Turan M, Arisoy A, et al. Is there a relationship between obstructive sleep apnea (OSA) and hearing loss? Med Sci Monit. 2016;22:3124–28. doi: 10.12659/MSM.897347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Papandreou C. Levels of TBARS are inversely associated with lowest oxygen saturation in obese patients withOSAS. Sleep Breath. 2013;17:1319–22. doi: 10.1007/s11325-013-0819-2. [DOI] [PubMed] [Google Scholar]
- 5.Epstein LJ, Kristo D, Strollo PJ, Jr, et al. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med. 2009;5:263–76. [PMC free article] [PubMed] [Google Scholar]
- 6.Kalaria RN, Spoors L, Laude EA, et al. Hypoxia of sleep apnoea: Cardiopulmonary and cerebral changes after intermittent hypoxia in rats. Respir Physiol Neurobiol. 2004;140:53–62. doi: 10.1016/j.resp.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 7.Mc Guire M, Mac Dermott M, Bradford A. Effects of chronic intermittent asphyxia on rat diaphragm and limb muscle contractility. Chest. 2003;123:875–81. doi: 10.1378/chest.123.3.875. [DOI] [PubMed] [Google Scholar]
- 8.Lu W, Kang J, Hu K, et al. Angiotensin-(1–7) inhibits inflammation and oxidative stress to relieve lung injury induced by chronic intermittent hypoxia in rats. Braz J Med Biol Res. 2016;49:e5431. doi: 10.1590/1414-431X20165431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ding W, Zhang X, Huang H, et al. Adiponectin protects rat myocardium against chronic intermittent hypoxia-induced injury via inhibition of endoplasmic reticulum stress. PLoS One. 2014;9:e94545. doi: 10.1371/journal.pone.0094545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fu C, Lu H, Wu X, et al. Chronic intermittent hypoxia decreases pulmonary clearance of 99mTc-labelled particulate matter in mice. Am J Transl Res. 2017;9:3060–72. [PMC free article] [PubMed] [Google Scholar]
- 11.Cho HJ, Heo W, Han JW, et al. Chronological change of right ventricle by chronic intermittent hypoxia in mice. Sleep. 2017;40(8) doi: 10.1093/sleep/zsx103. [DOI] [PubMed] [Google Scholar]
- 12.Li G, Jin M, He Y, et al. Fork Head Box Class O1 (FOXO1) activates bim expression to mediate cardiac apoptosis in chronic intermittent hypoxia-induced cardiac hypertrophy. Med Sci Monit. 2018;24:3603–16. doi: 10.12659/MSM.905210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gallego-Martin T, Farré R, Almendros I, et al. Chronic intermittent hypoxia mimicking sleep apnoea increases spontaneous tumorigenesis in mice. Eur Respir J. 2017;49(2) doi: 10.1183/13993003.02111-2016. pii: 1602111. [DOI] [PubMed] [Google Scholar]
- 14.Xu M, Liu L, Qi C, et al. Sinomenine versus NSAIDs for the treatment of rheumatoid arthritis: A systematic review and meta-analysis. Planta Med. 2008;74:1423–29. doi: 10.1055/s-2008-1081346. [DOI] [PubMed] [Google Scholar]
- 15.Cheng Y, Li F, Wang D, et al. Sinomenine inhibits the expression of PDL1 in the peripheral blood mononuclear cells of mesangial proliferative nephritis patients. Mol Med Rep. 2013;7:1223–28. doi: 10.3892/mmr.2013.1302. [DOI] [PubMed] [Google Scholar]
- 16.Cheng Y, Zhang J, Hou W, et al. Immunoregulatory effects of sinomenine on the T-bet/GATA-3 ratio and Th1/Th2 cytokine balance in the treatment of mesangial proliferative nephritis. Int Immunopharmacol. 2009;9:894–99. doi: 10.1016/j.intimp.2009.03.014. [DOI] [PubMed] [Google Scholar]
- 17.Lu XL, Zeng J, Chen YL, et al. Sinomenine hydrochloride inhibits human hepatocellular carcinoma cell growth in vitro and in vivo: Involvement of cell cycle arrest and apoptosis induction. Int J Oncol. 2013;42:229–38. doi: 10.3892/ijo.2012.1704. [DOI] [PubMed] [Google Scholar]
- 18.Li X, Li PP, Liu C, et al. Sinomenine hydrochloride inhibits breast cancer metastasis by attenuating inflammation-related epithelial-mesenchymal transition and cancer stemness. Oncotarget. 2017;8:13560–74. doi: 10.18632/oncotarget.14593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang Q, Li XK. Immunosuppressive and anti-inflammatory activities of sinomenine. Int Immunopharmacol. 2011;11:373–76. doi: 10.1016/j.intimp.2010.11.018. [DOI] [PubMed] [Google Scholar]
- 20.Qian L, Xu Z, Zhang W, et al. Sinomenine, a natural dextrorotatory morphinan analog, is antiinflammatory and neuroprotective through inhibition of microglial NADPH oxidase. J Neuroinflammation. 2007;4:23. doi: 10.1186/1742-2094-4-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu WN, Wu PF, Chen XL, et al. Sinomenine protects against ischaemic brain injury: Involvement of co-inhibition of acid-sensing ion channel 1a and L-type calcium channels. Br J Pharmacol. 2011;164:1445–59. doi: 10.1111/j.1476-5381.2011.01487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang Z, Liu Y, Yuan F, et al. Sinomenine inhibits microglia activation and attenuates brain injury in intracerebral hemorrhage. Mol Immunol. 2014;60:109–14. doi: 10.1016/j.molimm.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 23.Li J, Zhao L, He X, et al. Sinomenine protects against lipopolysaccharide-induced acute lung injury in mice via adenosine A(2A) receptor signaling. PLoS One. 2013;8:e59257. doi: 10.1371/journal.pone.0059257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Feng SZ, Tian JL, Zhang Q, et al. An experimental research on chronic intermittent hypoxia leading to liver injury. Sleep Breath. 2011;15:493–502. doi: 10.1007/s11325-010-0370-3. [DOI] [PubMed] [Google Scholar]
- 25.Zhao L, Lu X, Cao Y. MicroRNA and signal transduction pathways in tumor radiation response. Cell Signal. 2013;25:1625–34. doi: 10.1016/j.cellsig.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yeung HM, Hung MW, Lau CF, Fung ML. Cardioprotective effects of melatonin against myocardial injuries induced by chronicintermittent hypoxia in rats. J Pineal Res. 2015;58:12–25. doi: 10.1111/jpi.12190. [DOI] [PubMed] [Google Scholar]
- 27.Wang Y, Hai B, Niu X, et al. Chronic intermittent hypoxia disturbs insulin secretion and causes pancreatic injury via the MAPK signaling pathway. Biochem Cell Biol. 2017;95:415–20. doi: 10.1139/bcb-2016-0167. [DOI] [PubMed] [Google Scholar]
- 28.Lu W, Kang J, Hu K, et al. Angiotensin-(1–7) relieved renal injury induced by chronic intermittent hypoxia in rats by reducing inflammation, oxidative stress and fibrosis. Braz J Med Biol Res. 2017;50:e5594. doi: 10.1590/1414-431X20165594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bingyang J, Jinping L, Mingzheng L, et al. Effects of urinary protease inhibitor on inflammatory response during on-pump coronary revascularisation. Effect of ulinastatin on inflammatory response. J Cardiovasc Surg (Torino) 2007;48:497–503. [PubMed] [Google Scholar]
- 30.Gao H, Tian Y, Wang W, et al. Levels of interleukin-6, superoxide dismutase and malondialdehyde in the lung tissue of a rat model of hypoxia-induced acute pulmonary edema. Exp Ther Med. 2016;11:993–97. doi: 10.3892/etm.2015.2962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qin T, Du R, Huang F, et al. Sinomenine activation of Nrf2 signaling prevents hyperactive inflammation and kidney injury in a mouse model of obstructive nephropathy. Free Radic Biol Med. 2016;92:90–99. doi: 10.1016/j.freeradbiomed.2016.01.011. [DOI] [PubMed] [Google Scholar]