Abstract
Sudan azo dyes are banned for food usage in most countries, but they are illegally used to maintain or enhance the color of food products due to low cost, bright staining, and wide availability of the dyes. In this report, we examined the toxic effects of these azo dyes and their potential reduction metabolites on 11 prevalent human intestinal bacterial strains. Among the tested bacteria, cell growth of 2, 3, 5, 5, and 1 strains was inhibited by Sudan I, II, III, IV, and Para Red, respectively. At the tested concentration of 100 μM, Sudan I and II inhibited growth of Clostridium perfringens and Lactobacillus rhamnosus with decrease of growth rates from 14 to 47%. Sudan II also affected growth of Enterococcus faecalis. Growth of Bifidobacterium catenulatum, C. perfringens, E. faecalis, Escherichia coli, and Peptostreptococcus magnus was affected by Sudan III and IV with decrease in growth rates from 11 to 67%. C. perfringens was the only strain in which growth was affected by Para Red with 47 and 26% growth decreases at 6 and 10 h, respectively. 1-Amino-2-naphthol, a common metabolite of the dyes, was capable of inhibiting growth of most of the tested bacteria with inhibition rates from 8 to 46%. However, the other metabolites of the dyes had no effect on growth of the bacterial strains. The dyes and their metabolites had less effect on cell viability than on cell growth of the tested bacterial strains. Clostridium indolis and Clostridium ramosum were the only two strains with about a 10 % decrease in cell viability in the presence of Sudan azo dyes. The present results suggested that Sudan azo dyes and their metabolites potentially affect the human intestinal bacterial ecology by selectively inhibiting some bacterial species, which may have an adverse effect on human health.
Keywords: Sudan azo dye, Human intestinal bacteria, Metabolite, Cell growth, Cell viability
1. Introduction
Azo dyes are synthetic compounds consisting of a diazotized amine coupled to an amine or a phenol and which are characterized by one or more azo bonds (R1–N═N–R2) [1–3]. They are the largest group of dyes and at least 3,000 of them are extensively used as colorants in food, cosmetics, paper, textile and pharmaceutical industries [4–8]. Through ingestion, inhalation or skin contact, humans are exposed to these compounds. Previous studies have shown that synthetic azo dyes could be metabolized by mammalian and microbial enzyme systems [9–14]. The initial step of the metabolism of azo dyes is reduction of the azo bond catalyzed by azoreductase [15–19]. It has been known that the reduction of the azo bonds is important for toxicity, mutagenicity and carcinogenicity of the azo dyes [20,21]. Azoreductases can be found in many microorganisms and the area has been well reviewed recently [21].
Sudan azo dyes (Sudan I, II, III, IV and Para Red) are fat-soluble azo dyes, which are used for different industrial and scientific applications, such as coloring of plastics, waxes, textiles, and fuel, staining for microscopy [22]. These azo dyes are not permitted for use as food additives due to their potential carcinogenicity. The International Agency for Research on Cancer (IARC) has classified Sudan I–IV as Class 3 carcinogens (not classifiable as to its carcinogenicity to humans). Para Red also could be a genotoxic carcinogen (http://www.food.gov.uk/safereating/chemsafe/parared/). Nevertheless, it has been reported that Sudan azo dyes were found in various foods such as chili powders, eggs, Worcestershire sauce, garlic curry sauce, palm oils, as well as in other food products as contaminants [23,24] which arouse great concern about safety of the food supplies [22,25,26]. Sudan I (C.I. Solvent Yellow 14) is a monoazo dye with the chemical formula of 1-phenylazo-2-naphthol. It can cause tumors in the liver or urinary bladder in mammals and is a potential mutagen and carcinogen for humans [14,27,28]. Recent studies showed that Sudan I induced genotoxic effect in HepG2 cells [29] and increased mutagenic and clastogenic effects in the MCL-5 cell lines compared to the AHH-1 cell line [30]. In addition, Sudan I also gave positive results in Salmonella Typhimurium mutagenicity tests with S9 activation, a post-mitochondrial fraction prepared from the livers of rats [31]. Sudan II (C.I. Solvent Orange 7) is a dimethyl derivative of Sudan I. Early studies showed that Sudan II induced mutation of Salmonella Typhimurium TA 1538 in the presence of a rat liver preparation [32] and gave positive results in both Salmonella assays (the standard plate-incorporation assay and the FMN preincubation modification of the Salmonella assay) [33]. Sudan III (C.I. Solvent Red 23) is a diazo dye and approved for use in drug and cosmetics. In vitro investigation for clastogenic potential of Sudan III using Chinese hamster ovary (CHO) cells without metabolic activation showed that the number of breaks in metaphase was increased following addition of Sudan III [34]. Particular concern regarding safety of Sudan III has been noted due to potential reductive cleavage of the azo bond to produce 4-aminoazobenzene and aniline, which are classified as a category 2 and 3 carcinogens in Annex I of the Directive 67/548/EEC, respectively [35–37]. Sudan IV (C.I. Solvent Red 24) is also a diazo dye. After chemical reduction and microsomal activation, Sudan IV became mutagenic [38]. Additionally, Sudan IV induced Cytochrome P450 1A1 protein and mRNA in Wistar rats, although lower than Sudan I and Sudan III [39]. Para Red is chemically very similar to Sudan I. A recent study demonstrated that Para Red was a genotoxic chemical in AHH-1 cells and oxidative metabolism increased the genotoxic effect of Para Red [30].
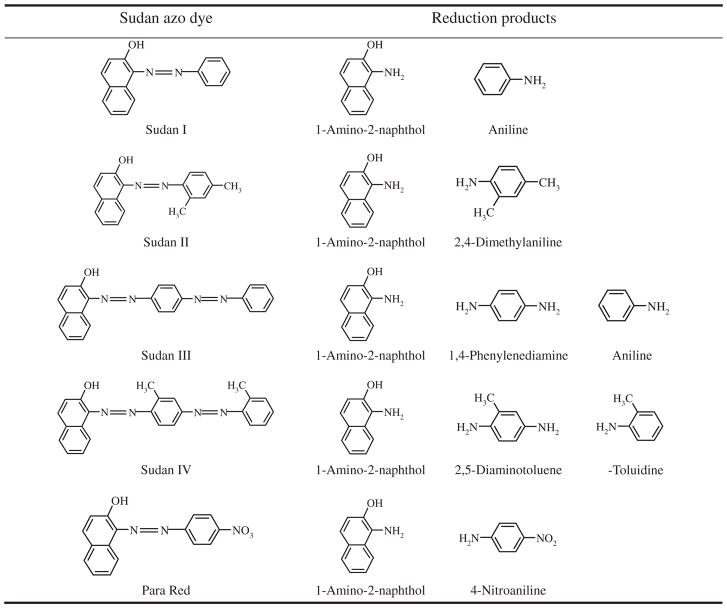
Although azo dyes can be reduced by microsomal and cytosolic reductase of the liver and extra-hepatic tissue, gastrointestinal microbiota play a major role in the metabolism of these dyes [2,21]. The human gastrointestinal tract is colonized by a diverse community of organisms comprising at least thousands of different species of bacteria which have important effects on host health [4,40]. Recent reports elucidated that human intestinal microbiome composition varies and differs between healthy people [41–43] and between obese and lean individuals [44]. There are evidences indicating that human intestinal microbiota anaerobically meta bolize azo dyes to the corresponding carcinogenic aromatic amines [2,3,12,35, 45–47]. Sudan I–IV and Para Red also can be metabolized by human intestinal bacteria [12,35] and their reduction metabolites are shown in Table 1 [35]. Human exposure to these Sudan azo dyes mainly occurs through ingestion of foods contaminated with these dyes [21]. When the foods are ingested, these Sudan azo dyes are initiallycontacted with the gastrointestinal tract, where the dyes can be reduced to corresponding aromatic amines, which are toxic, water-soluble, and easily absorbed by the human intestine [15,48,49]. Very limited information is available related to the toxic effects of these Sudan azo dyes on the human intestinal bacteria. In our previous study, we have demonstrated that human intestinal bacteria are able to metabolize Sudan azo dyes and produce potentially genotoxic metabolites [12,35]. This study investigated effects of Sudan azo dyes and their reduction metabolites on human intestinal bacteria and provides evidence that these compounds are able to selectively inhibit some bacterial species resulting in potential disruption of normal human intestinal ecology.
Table 1.
Chemical structure of Sudan dyes and their theoretical reduction products by human intestinal bacteria.
2. Materials and methods
2.1. Materials
Sudan I (1-phenylazo-2-naphthol), Sudan II (1-(2,4-dimethyl-phenylazo)-2-naphthol), Sudan III (1-[4-(phenylazo)phenylazo]-2-naphthol), Sudan IV (1-[2-Methyl-4-(2-methylphenylazo)phenyl-azo]-2-naphthol), Para Red (1-(4-Nitrophenylazo)naphthalen-2-ol), 1-amino-2-naphthol (hydrochloride), aniline, 2,4-dimeth ylaniline, 1,4-phenylenediamine, 2,5-diaminotoluene (sulfate), o-toluidine, 4-nitroaniline and dimethyl sulfoxide (DMSO) were purchased from Sigma Chemical Co. The LIVE/DEAD BacLight bacterial viability and counting kit containing solutions of 3.34 mM SYTO9 in DMSO (200 μl), 20 mM propidium iodide (PI) in DMSO (200 μl) and a calibrated suspension of microspheres (diameter: 6 μm, 1 ml; concentration: 1.0 × 108 beads/ml) and SYTO9 green fluorescent nucleic acid stain (5 mM solution in DMSO, 100 μl) were purchased from Molecular Probes. Stock solutions of Sudan I, II, III, IV, Para Red,1-amino-2-naphthol, aniline, 2,4-dimethylaniline, 1,4-phenylenedi amine, 2,5-diaminotoluene, o-toluidine and 4-nitroaniline were freshly prepared by dissolving each chemical in DMSO.
2.2. Bacterial strains and culture conditions
Based on our previous research [12,15], 11 prevalent anaerobic human intestinal bacterial species isolates obtained from the American Type Culture Collection (ATCC) were used in the study (Table 2). The bacterial isolates were preserved at −80 °C in 15% glycerol stocks. All strains, except Lactobacillus rhamnosus, which was cultured on deMann–Rogosa–Sharpe (MRS) agar plates (Becton Dickinson & Company), were grown on brain heart infusion (BHI) agar supplemented with vitamin K and hemin (Remel) at 37 °C overnight in an anaerobic chamber (Coy Laboratory Products INC.) [12]. One colony was picked by a loop and inoculated into a 15-ml Falcon centrifuge tubecontaining 10 ml medium(BHI brothsupplemented with vitamin K and hemin or MRS). The culture was incubated in static conditions at 37°C overnight in an anaerobic chamber for use as seed culture.
Table 2.
Effects of Sudan azo dyes on the growth of human intestinal bacteria.
| Strain name | Culture time (h) | Control (cells/ml) | Sudan azo dyes (100 μM) (cells/ml)a | ||||
|---|---|---|---|---|---|---|---|
| Sudan I | Sudan II | Sudan III | Sudan IV | Para Red | |||
| Bacteroides ovatus | 6 | 0.90 × 109 | 1.02 × 109 | 0.88 × 109 | 0.91 × 109 | 1.00 × 109 | 1.02 × 109 |
| ATCC 8483 | 10 | 1.16 × 109 | 1.09 × 109 | 1.09 × 109 | 1.11 × 109 | 1.12 × 109 | 1.20 × 109 |
| Bifidobacterium infantis | 6 | 1.07 × 109 | 0.96 × 109 | 0.97 × 109 | 0.98 × 109 | 0.97 × 109 | 1.04 × 109 |
| ATCC 15697 | 10 | 1.26 × 109 | 1.20 × 109 | 1.19 × 109 | 1.17 × 109 | 1.19 × 109 | 1.29 × 109 |
| Bifidobacterium catenulatum | 6 | 0.74 × 109 | 0.75 × 109 | 0.74 × 109 | 0.66 × 109 | 0.32 × 109 | 0.77 × 109 |
| ATCC 27539 | 10 | 0.87 × 109 | 0.86 × 109 | 0.86 × 109 | 0.72 × 109 | 0.31 × 109 | 0.86 × 109 |
| Clostridium indolis | 6 | 1.12 × 109 | 1.18 × 109 | 1.12 × 109 | 1.13 × 109 | 1.09 × 109 | 1.24 × 109 |
| ATCC 25771 | 10 | 1.30 × 109 | 1.30 × 109 | 1.27 × 109 | 1.27 × 109 | 1.22 × 109 | 1.44 × 109 |
| Clostridium perfringens | 6 | 1.00 × 109 | 0.78 × 109 | 0.65 × 109 | 0.70 × 109 | 0.76 × 109 | 0.53 × 109 |
| ATCC 13124 | 10 | 1.59 × 109 | 1.37 × 109 | 1.32 × 109 | 1.36 × 109 | 1.31 × 109 | 1.18 × 109 |
| Clostridium ramosum | 6 | 1.13 × 109 | 0.99 × 109 | 1.14 × 109 | 1.09 × 109 | 1.06 × 109 | 1.22 × 109 |
| ATCC 25582 | 10 | 1.37 × 109 | 1.22 × 109 | 1.34 × 109 | 1.18 × 109 | 1.19 × 109 | 1.35 × 109 |
| Enterococcus faecalis | 6 | 0.56 × 109 | 0.53 × 109 | 0.50 × 109 | 0.63 × 109 | 0.56 × 109 | 0.72 × 109 |
| ATCC 19433 | 10 | 1.35 × 109 | 1.36 × 109 | 1.35 × 109 | 1.04 × 109 | 0.86 × 109 | 1.28 × 109 |
| Escherichia coli | 6 | 0.71 × 109 | 0.73 × 109 | 0.67 × 109 | 0.30 × 109 | 0.34 × 109 | 0.63 × 109 |
| ATCC 25922 | 10 | 0.81 × 109 | 0.83 × 109 | 0.79 × 109 | 0.57 × 109 | 0.31 × 109 | 0.78 × 109 |
| Lactobacillus rhamnosus | 6 | 0.81 × 108 | 0.55 × 108 | 0.43 × 108 | 0.81 × 108 | 0.70 × 108 | 0.78 × 108 |
| ATCC 53103 | 10 | 5.29 × 108 | 4.54 × 108 | 3.22 × 108 | 4.92 × 108 | 5.24 × 108 | 5.23 × 108 |
| Peptostreptococcus magnus | 6 | 0.67 × 109 | 0.65 × 109 | 0.64 × 109 | 0.35 × 109 | 0.33 × 109 | 0.67 × 109 |
| ATCC 14955 | 10 | 0.85 × 109 | 0.74 × 109 | 0.81 × 109 | 0.60 × 109 | 0.29 × 109 | 0.84 × 109 |
| Ruminococcus obeum | 6 | 0.98 × 109 | 0.98 × 109 | 0.95 × 109 | 0.97 × 109 | 0.94 × 109 | 1.03 × 109 |
| ATCC 29174 | 10 | 1.20 × 109 | 1.21 × 109 | 1.17 × 109 | 1.14 × 109 | 1.09 × 109 | 1.23 × 109 |
Data are presented as mean of triplicate with standard deviations (SD) of <5%. P values < 0.05. Significant inhibition data are highlighted.
2.3. Growth determination of human intestinal bacteria
For growth experiments, the seed culture of the bacteria was inoculated into BHI medium with an inoculation ratio of 0.01% (v/v), except L. rhamnosus, which was inoculated into MRS medium with 1% (v/v) of the seed culture, and then 10 ml aliquots of the cultures were transferred to the centrifuge tubes. Each dye stock solution was added to the cultures at final concentration of 100 μM in triplicate (the concentration of Sudan azo dyes used is in the range of food products contaminated with the dyes and all experiments were triplicate unless otherwise stated). The media containing no dye but an equal volume of DMSO were inoculated with each strain as a control. The cultures were incubated at 37 °C in the anaerobic chamber without agitation. At 6 and 10 h of incubation, an aliquot (10 μL) of each sample was directly diluted for 100-fold into the saline solution. One microliter SYTO9 green fluorescent nucleic acid dye (5 mM) was added and incubated for 15 min in the dark [50]. The bacterial cell number was analyzed on an Accuri C6 flow cytometer (FCM) (Accuri Cytometers), with 488 nm excitation from a blue solid state laser at 50 mW and the standard filter setup. All parameters were collected as logarithmic signals. Green fluorescence was collected in the FL1 channel (530 ± 15 nm). Ten microliter stained sample was quantitatively analyzed on the Accuri C6 FCM.
For metabolites, each stock solution was added to the cultures at final concentration of 100 and 200 μM, respectively. Samples of 200 μl were withdrawn from the cultures at 6 and 10 h and were added to a 96-well plate (Corning incorporated, flat bottom, non-lid) and immediately assayed by measuring the optical density (OD) in a SpectraMax M2 plate reader (Molecular Devices) at 660 nm. Sterile media with each metabolite or DMSO at 6 and 10 h were used as blanks, respectively.
2.4. Bacterial cell viability assay
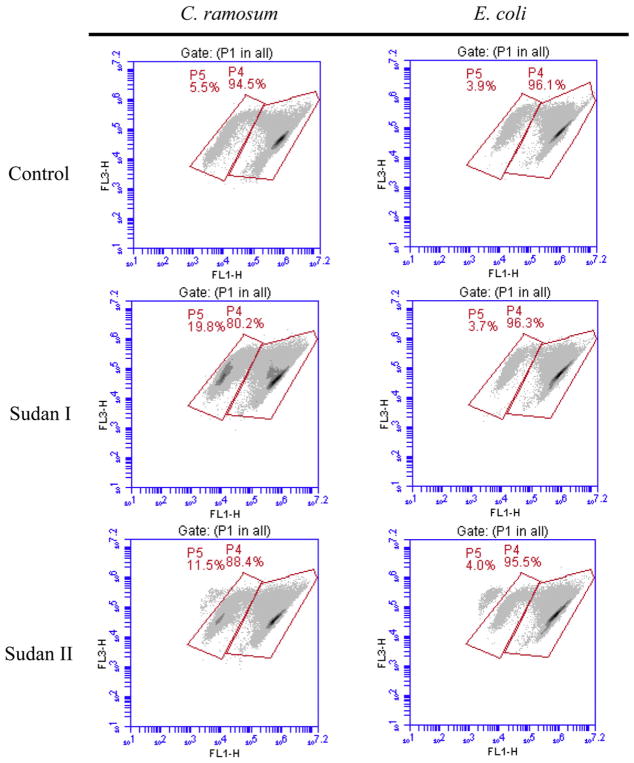
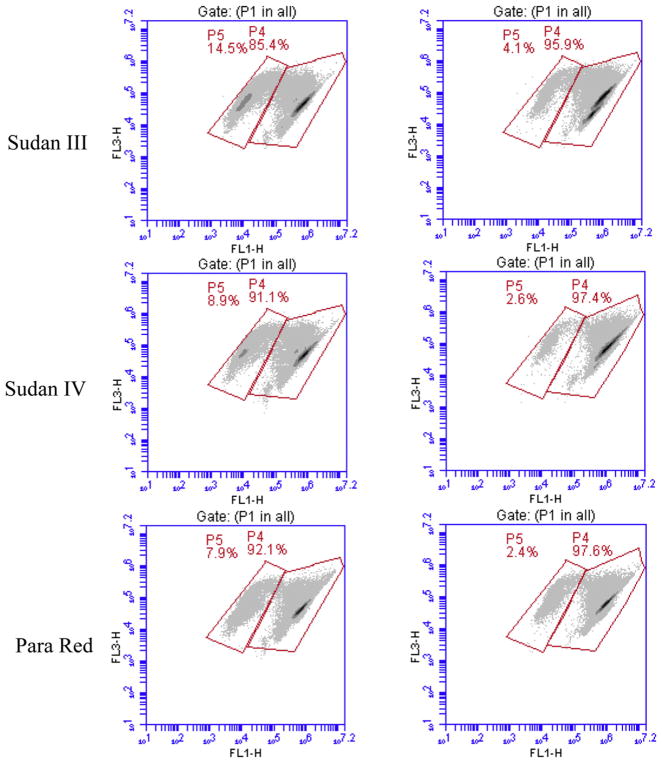
For examining effect of Sudan azo dyes on cell viability of the bacteria, the bacterial seed cultures were inoculated into fresh medium (BHI or MRS) with an inoculation ratio of 1% (v/v) respectively. Then 10 ml medium from each was transferred to the centrifuge tubes. Each Sudan azo dye stock solution was added to the medium at final concentration of 100 μM. The medium containing no dye but an equal volume of DMSO were served as the controls. After 24 h of incubation in an anaerobic chamber at 37 °C without shaking, the samples were collected for cell viability measurement as described below. For evaluating the effects of metabolites on cell viability, the cultures were grown to the stationary phase at 37 °C for 12 h. Then, each metabolite of the dyes was added to the cultures at final concentrations of 100 and 200 μM. The cultures containing no metabolite but an equal volume of DMSO were applied as the controls. After further incubation of the cultures for 12 h under the same conditions, the bacterial cells were harvested and used for cell viability assays. One milliliter samples collected from the cultures were stained according to the manufacture’s instruction using the BacLight LIVE/DEAD bacterial viability and counting kit. The bacterial cell viability assay was performed on the Accuri C6 FCM. Fluorescence filters and detectors were all standardized with green fluorescence collected in the FL1 channel (530 ± 15 nm) and red fluorescence collected in the FL3 channel (>670 nm). All parameters were collected as logarithmic signals. A similar setup of parameters was used as described previously [51]. Data were analyzed using CFlow Plus software. In density plots of light scatter properties, bacterial cells were gated from irrelevant counts for fluorescence analyses. In density plots of fluorescence, the distinct bacterial populations (P4: live cells and P5: dead cells, as shown in Fig. 1) were gated based on the different viability stages. Cell viability (%) = percent of P4 (green cells)/(percent of P4 + percent of P5 (red cells)) × 100.
Fig. 1.
Effects of Sudan azo dyes on the cell viabilities of C. ramosum and E. coli. The samples were stained using SYTO9 plus PI. For all FL1-H/FL3-H density plots, horizontal axes (FL1-H) represent green fluorescence and vertical axes (FL3-H) represent red fluorescence (FL1, 530 ± 15 nm; FL3, >670 nm). Gate P4 and P5 represent those used to define populations designated as live and dead cells, respectively.
2.5. Statistical analysis
Student’s t-test for triplicate samples was used. P values of less than 0.05 were considered statistically significant. All experimental data are shown with P < 0.05.
3. Results and discussion
3.1. Effects of Sudan azo dyes on the cell growth of human intestinal bacteria
Our previous studies demonstrated that a microbial consortium in human fecal suspension and most of the tested prevalent intestinal bacterial species were capable of reducing Sudan azo dyes to produce potentially genotoxic aromatic amines [12,35,52]. As a part of a series of studies concerning Sudan azo dye contamination as a food safety issue, we conducted experiments to evaluate the impact of exposure of Sudan azo dyes and their metabolites on prevalent human intestinal bacteria. We chose 11 intestinal bacterial isolates including obligate and facultative anaerobes which represent several prevalent genera of human intestinal bacteria, which were used in our previous study on Sudan azo dye degradation, for the study. To examine the effects of Sudan azo dyes on the growth of the bacterial strains, the bacterial cell numbers were monitored by FCM at 6 and 10 h incubation times. As shown in Table 2, when bacterial strains were cultured with Sudan I, growth of Clostridium perfringens and L. rhamnosus was inhibited. Cell numbers of the C. perfringens cultures were lower 22 and 14% than the cultures without Sudan I at 6 and 10 h, respectively. For L. rhamnosus, 32 and 14% growth decreases were obtained. The other strains cultured with Sudan I did not display growth inhibition at 6 and 10 h. Sudan II was able to inhibit growth of C. perfringens and L. rhamnosus with decreased cell numbers of 35 and 47% at 6 h and 17 and 39% at 10 h, respectively. Growth of Enterococcus faecalis was inhibited with a decreased cell number value of 11% at 6 h. For each of 11 strains cultured with Sudan III, the cell number values of Bifidobacterium catenulatum, C. perfringens, Escherichia coli, and Peptostreptococcus magnus were reduced by 11, 30, 59, and 48% at 6 h and 17, 14, 30, and 29% at 10 h, respectively. Growth of E. faecalis was inhibited with a decreased cell number value of 23% at 10 h. Sudan IV caused a similar growth inhibition pattern on the tested bacteria to Sudan III. However, the inhibitory effects were stronger when bacteria were grown with Sudan IV compared with Sudan III, except for C. perfringens. Strong inhibitions on the growth of B. catenulatum occurred in the presence of Sudan IV with 57 and 64% decrease of cell number values at 6 and 10 h. Sudan IV also had a significant effect on the growth of E. coli with 52 and 62% of inhibition at 6 and 10 h. Similar growth inhibitions of 51 and 67% were detected when P. magnus was cultured with Sudan IV at 6 and 10 h. Growth of E. faecalis was affected with 36% inhibition cultured with Sudan IV at 10 h. Among the tested bacterial strains, C. perfringens was the only strain to be inhibited by Para Red, with 47 and 26% growth decreases occurring at 6 and 10 h. Di-azo Sudan dyes (Sudan III and IV) had stronger inhibitions on cell growth of the bacteria than those of mono-azo Sudan dyes (Sudan I, II and Para Red). This may result from di-azo dyes binding more strongly to the bacterial outside surface than monoazo dyes, which causes a stronger perturbance of the cell membrane and prevent the transport of essential nutrients from the medium to the cell [53]. On the other hand, the results also showed that cell growth of some bacteria was more sensitive to Sudan azo dyes than the other tested bacteria. Among the tested bacteria, C. perfringens was the most sensitive to Sudan azo dye inhibitions. B. catenulatum, E. coli, and P. magnus were susceptible to diazo Sudan dyes, but not to monoazo Sudan dyes. However, cell growth of Bacteroides ovatus, Bifidobacterium infantis, Clostridium indolis, Clostridium ramosum and Ruminococcus obeum was not significantly affected by Sudan azo dyes. Effect of Sudan dyes on the bacterial cell growth may rely on both structural features of the dyes and physiological characteristics of the bacteria.
3.2. Effects of metabolites of Sudan azo dyes on the cell growth of human intestinal bacteria
ODs660 of 11 bacterial strains incubated with the respective metabolites of Sudan azo dyes at 6 and 10 h are shown in Table 3. At a concentration of 100 μM, the metabolites showed no inhibitory effect on the bacteria; whereas, when the concentration of metabolites were increased to 200 μM, significant inhibitions on the growth of most strains occurred by 1-amino-2-naphthol, with the exception of L. rhamnosus. For example, the OD values of these strains with 1-amino-2-naphthol were about 15–46% lower than that of the control without metabolite at 6 h. With increasing incubation time to10 h, the inhibition of the metabolites on the bacterial growth of the tested strains was partially eliminated. The OD values of C. perfringens and E. faecalis with 1-amino-2-naphthol were similar to those cultures without the metabolite at 10 h. For the other 8 strains, there was still a substantial inhibition of the growth of the bacteria ranging from 8 to 19%. On the contrary, no inhibition on growth of the tested bacteria was observed in the presence of the other metabolites (Table 3). The results showed that 1-amino-2-naphthol, a common metabolite of Sudan azo dyes, was toxic to the most human intestinal bacteria except for L. rhamnosus, which was able to resist the toxic effect by 1-amino-2-naphthol and no significant inhibitory effect was observed at 200 μM. This was similar to our previous study on a skin bacterium, in which 1-amino-2-naphthol was the only metabolite that significantly inhibit cell growth of Staphylococcus aureus [51].
Table 3.
Effects of reduction metabolites of Sudan azo dyes on the growth of human intestinal bacterial strains.a
| Strains No. | Cultured Time (h) |
Control | Metabolites
|
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1-Amino-2-Naphthol
|
Aniline
|
2,4-Dimethylaniline
|
1,4-Phenylenediamine
|
2,5-Diaminotoluene
|
o-Toluidine
|
4-Nitroaniline
|
||||||||||
| 100 μM | 200 μM | 100 μM | 200 μM | 100 μM | 200 μM | 100 μM | 200 μM | 100 μM | 200 μM | 100 μM | 200 μM | 100 μM | 200 μM | |||
| B. ovatus | 6 | 0.85 ± 0.02 | 0.81 ± 0.02 | 0.59 ± 0.00 | 0.82 ± 0.02 | 0.87 ± 0.02 | 0.81 ± 0.01 | 0.86 ± 0.02 | 0.82 ± 0.01 | 0.90 ± 0.01 | 0.83 ± 0.02 | 0.88 ± 0.01 | 0.84 ± 0.01 | 0.88 ± 0.01 | 0.83 ± 0.02 | 0.88 ± 0.01 |
| 10 | 1.00 ± 0.01 | 1.02 ± 0.00 | 0.86 ± 0.03 | 0.97 ± 0.02 | 0.96 ± 0.02 | 1.00 ± 0.02 | 0.99 ± 0.02 | 0.99 ± 0.01 | 0.99 ± 0.01 | 0.95 ± 0.02 | 0.96 ± 0.01 | 0.96 ± 0.01 | 0.97 ± 0.01 | 0.98 ± 0.03 | 0.99 ± 0.03 | |
| B. infantis | 6 | 0.92 ± 0.02 | 0.87 ± 0.00 | 0.78 ± 0.01 | 0.87 ± 0.00 | 0.93 ± 0.02 | 0.88 ± 0.01 | 0.92 ± 0.01 | 0.88 ± 0.02 | 0.95 ± 0.02 | 0.87 ± 0.01 | 0.93 ± 0.01 | 0.88 ± 0.01 | 0.93 ± 0.01 | 0.89 ± 0.01 | 0.92 ± 0.00 |
| 10 | 0.99 ± 0.01 | 0.99 ± 0.01 | 0.91 ± 0.01 | 0.96 ± 0.02 | 0.98 ± 0.01 | 0.96 ± 0.02 | 1.00 ± 0.03 | 0.98 ± 0.01 | 1.01 ± 0.01 | 0.96 ± 0.01 | 1.00 ± 0.02 | 0.96 ± 0.01 | 1.00 ± 0.01 | 0.96 ± 0.02 | 1.02 ± 0.02 | |
| B. catenulatum | 6 | 0.96 ± 0.01 | 0.92 ± 0.01 | 0.69 ± 0.01 | 0.94 ± 0.01 | 0.94 ± 0.02 | 0.95 ± 0.01 | 0.97 ± 0.02 | 0.95 ± 0.02 | 0.97 ± 0.01 | 0.94 ± 0.02 | 0.95 ± 0.01 | 0.95 ± 0.01 | 0.96 ± 0.01 | 0.97 ± 0.02 | 0.97 ± 0.01 |
| 10 | 1.01 ± 0.00 | 0.99 ± 0.01 | 0.85 ± 0.01 | 0.97 ± 0.03 | 0.99 ± 0.02 | 1.00 ± 0.02 | 1.01 ± 0.02 | 1.01 ± 0.01 | 1.01 ± 0.00 | 0.98 ± 0.01 | 1.00 ± 0.01 | 0.97 ± 0.01 | 0.99 ± 0.01 | 1.00 ± 0.02 | 1.02 ± 0.03 | |
| C. indolis | 6 | 0.95 ± 0.02 | 0.93 ± 0.01 | 0.53 ± 0.00 | 0.95 ± 0.02 | 0.91 ± 0.00 | 0.96 ± 0.03 | 0.93 ± 0.01 | 0.95 ± 0.01 | 0.95 ± 0.02 | 0.94 ± 0.01 | 0.95 ± 0.01 | 0.95 ± 0.00 | 0.97 ± 0.04 | 0.96 ± 0.01 | 0.99 ± 0.02 |
| 10 | 0.94 ± 0.01 | 0.94 ± 0.01 | 0.82 ± 0.01 | 0.91 ± 0.01 | 0.93 ± 0.01 | 0.92 ± 0.02 | 0.95 ± 0.03 | 0.92 ± 0.01 | 0.97 ± 0.02 | 0.91 ± 0.00 | 0.97 ± 0.02 | 0.91 ± 0.01 | 0.96 ± 0.03 | 0.92 ± 0.02 | 0.98 ± 0.03 | |
| C. perfringens | 6 | 0.98 ± 0.03 | 0.87 ± 0.01 | 0.76 ± 0.04 | 0.99 ± 0.02 | 0.91 ± 0.03 | 1.00 ± 0.06 | 0.92 ± 0.06 | 1.02 ± 0.01 | 0.96 ± 0.02 | 0.99 ± 0.01 | 0.95 ± 0.04 | 1.00 ± 0.01 | 0.96 ± 0.03 | 1.00 ± 0.02 | 0.82 ± 0.06 |
| 10 | 1.07 ± 0.00 | 1.01 ± 0.01 | 1.03 ± 0.01 | 1.04 ± 0.01 | 1.05 ± 0.02 | 1.07 ± 0.03 | 1.06 ± 0.03 | 1.07 ± 0.01 | 1.07 ± 0.00 | 1.03 ± 0.02 | 1.05 ± 0.02 | 1.04 ± 0.01 | 1.05 ± 0.01 | 1.07 ± 0.02 | 1.04 ± 0.02 | |
| C. ramosum | 6 | 0.90 ± 0.02 | 0.86 ± 0.01 | 0.55 ± 0.02 | 0.89 ± 0.01 | 0.88 ± 0.03 | 0.89 ± 0.05 | 0.90 ± 0.03 | 0.90 ± 0.03 | 0.94 ± 0.02 | 0.88 ± 0.01 | 0.91 ± 0.03 | 0.87 ± 0.03 | 0.93 ± 0.03 | 0.88 ± 0.04 | 0.95 ± 0.02 |
| 10 | 0.97 ± 0.01 | 0.98 ± 0.02 | 0.80 ± 0.03 | 0.96 ± 0.02 | 0.96 ± 0.02 | 0.96 ± 0.02 | 0.99 ± 0.02 | 0.95 ± 0.02 | 1.00 ± 0.02 | 0.93 ± 0.02 | 1.00 ± 0.02 | 0.95 ± 0.02 | 0.01 ± 0.02 | 0.97 ± 0.02 | 1.03 ± 0.02 | |
| E. faecalis | 6 | 0.67 ± 0.00 | 0.61 ± 0.01 | 0.55 ± 0.02 | 0.63 ± 0.01 | 0.63 ± 0.01 | 0.63 ± 0.02 | 0.65 ± 0.02 | 0.67 ± 0.01 | 0.69 ± 0.02 | 0.64 ± 0.02 | 0.67 ± 0.01 | 0.64 ± 0.02 | 0.66 ± 0.01 | 0.64 ± 0.01 | 0.64 ± 0.01 |
| 10 | 0.72 ± 0.01 | 0.72 ± 0.02 | 0.73 ± 0.02 | 0.70 ± 0.02 | 0.71 ± 0.01 | 0.72 ± 0.02 | 0.72 ± 0.02 | 0.71 ± 0.00 | 0.72 ± 0.01 | 0.70 ± 0.01 | 0.70 ± 0.01 | 0.69 ± 0.02 | 0.71 ± 0.01 | 0.70 ± 0.02 | 0.70 ± 0.02 | |
| E. coli | 6 | 0.95 ± 0.01 | 0.90 ± 0.01 | 0.60 ± 0.01 | 0.91 ± 0.02 | 0.91 ± 0.02 | 0.94 ± 0.02 | 0.93 ± 0.02 | 0.94 ± 0.01 | 0.94 ± 0.01 | 0.92 ± 0.00 | 0.92 ± 0.00 | 0.91 ± 0.02 | 0.94 ± 0.01 | 0.92 ± 0.02 | 0.94 ± 0.01 |
| 10 | 1.03 ± 0.01 | 1.01 ± 0.01 | 0.90 ± 0.01 | 1.02 ± 0.02 | 1.03 ± 0.01 | 1.03 ± 0.02 | 1.05 ± 0.02 | 1.01 ± 0.01 | 1.04 ± 0.01 | 1.00 ± 0.02 | 1.00 ± 0.01 | 1.00 ± 0.02 | 1.03 ± 0.01 | 1.01 ± 0.03 | 1.03 ± 0.01 | |
| L. rhamnosus | 6 | 0.41 ± 0.03 | 0.42 ± 0.02 | 0.42 ± 0.03 | 0.40 ± 0.01 | 0.40 ± 0.03 | 0.42 ± 0.04 | 0.38 ± 0.02 | 0.44 ± 0.04 | 0.37 ± 0.05 | 0.44 ± 0.02 | 0.45 ± 0.03 | 0.44 ± 0.01 | 0.47 ± 0.03 | 0.46 ± 0.01 | 0.46 ± 0.07 |
| 10 | 1.34 ± 0.03 | 1.36 ± 0.02 | 1.31 ± 0.01 | 1.33 ± 0.04 | 1.31 ± 0.03 | 1.37 ± 0.03 | 1.31 ± 0.04 | 1.32 ± 0.03 | 1.30 ± 0.02 | 1.32 ± 0.02 | 1.30 ± 0.01 | 1.34 ± 0.00 | 1.30 ± 0.01 | 1.38 ± 0.00 | 1.33 ± 0.02 | |
| P. magnus | 6 | 0.92 ± 0.03 | 0.87 ± 0.02 | 0.63 ± 0.03 | 0.90 ± 0.03 | 0.92 ± 0.03 | 0.90 ± 0.03 | 0.92 ± 0.04 | 0.92 ± 0.02 | 0.95 ± 0.03 | 0.90 ± 0.03 | 0.94 ± 0.03 | 0.91 ± 0.02 | 0.96 ± 0.03 | 0.91 ± 0.03 | 0.96 ± 0.02 |
| 10 | 1.03 ± 0.01 | 1.02 ± 0.00 | 0.89 ± 0.01 | 1.01 ± 0.01 | 1.04 ± 0.01 | 1.03 ± 0.02 | 1.05 ± 0.02 | 1.02 ± 0.01 | 1.07 ± 0.01 | 1.01 ± 0.02 | 1.05 ± 0.02 | 1.01 ± 0.01 | 1.06 ± 0.03 | 1.02 ± 0.02 | 1.05 ± 0.02 | |
| R. obeum | 6 | 1.02 ± 0.02 | 0.97 ± 0.02 | 0.55 ± 0.02 | 1.01 ± 0.02 | 1.02 ± 0.03 | 1.02 ± 0.03 | 1.03 ± 0.03 | 1.01 ± 0.02 | 1.04 ± 0.03 | 0.99 ± 0.01 | 1.03 ± 0.02 | 1.00 ± 0.03 | 1.04 ± 0.02 | 1.03 ± 0.02 | 1.06 ± 0.02 |
| 10 | 1.01 ± 0.01 | 0.98 ± 0.01 | 0.82 ± 0.01 | 1.00 ± 0.03 | 0.98 ± 0.02 | 1.01 ± 0.02 | 1.01 ± 0.01 | 1.00 ± 0.01 | 1.01 ± 0.02 | 0.98 ± 0.01 | 1.00 ± 0.01 | 0.98 ± 0.03 | 1.00 ± 0.02 | 1.00 ± 0.01 | 1.02 ± 0.02 | |
Data shown were mean ± SD. The mean was from triplicate incubations with SDs. P values < 0.05. Significant inhibition data are highlighted.
3.3. Effects of Sudan azo dyes on the human intestinal bacterial cell viability
The cell viabilities of 11 human intestinal strains with Sudan azo dyes were determined by FCM measurements using the BacLight LIVE/DEAD bacterial viability and counting kit. Examples of such effects on C. ramosum and E. coli are shown in Fig. 1. Two distinctive groups can be found. Group P4 is the population of living bacterial cells, while group P5 is the population of dead bacterial cells. The cell viability of each of 11 strains with various dyes is illustrated in Table 4. After 24 h incubation, cell viabilities of 9 out of 11 strains were not affected by the dyes at a concentration of 100 μM. For example, cell viability of E. coli cultured with different dyes was from 93 to 97%, which are similar to those of the cultures without Sudan azo dyes. However the cell viabilities of two Clostridium strains, C. indolis and C. ramosum, were affected by the dyes. Sudan II, III, IV and Para Red caused 5–7% decrease on viability of C. indolis. Sudan I, II, and III caused 6–10% decrease on cell viability of C. ramosum. Since the metabolites of Sudan dyes did not have significant effect on cell viability of the bacteria (described below), decrease of cell viabilities of two Clostridium species may be mainly caused by Sudan azo dyes [12].
Table 4.
Cell viability of 11 intestinal human bacterial strains cultured with Sudan azo dyes at 24 h.a
| Strain name | Control | Sudan azo dye (100 μM)
|
||||
|---|---|---|---|---|---|---|
| Sudan I | Sudan II | Sudan III | Sudan IV | Para red | ||
| B. ovatus | 98.3 ± 0.1 | 99.2 ± 0.0 | 99.2 ± 0.0 | 99.5 ± 0.3 | 99.6 ± 0.2 | 99.2 ± 0.1 |
| B. infantis | 92.7 ± 0.5 | 94.9 ± 0.6 | 95.1 ± 3.0 | 95.9 ± 1.0 | 97.0 ± 1.3 | 96.0 ± 1.2 |
| B. catenulatum | 94.2 ± 1.6 | 93.6 ± 1.3 | 96.4 ± 1.2 | 95.6 ± 1.2 | 94.1 ± 2.0 | 94.8 ± 1.2 |
| C. indolis | 87.7 ± 2.4 | 89.6 ± 2.7 | 81.4 ± 2.5 | 80.8 ± 3.2 | 82.1 ± 2.0 | 80.9 ± 2.5 |
| C. perfringens | 89.5 ± 1.8 | 89.1 ± 1.3 | 89.6 ± 2.5 | 87.4 ± 2.1 | 89.1 ± 1.2 | 89.3 ± 1.2 |
| C. ramosum | 93.9 ± 1.3 | 83.3 ± 4.3 | 87.6 ± 1.3 | 84.5 ± 1.6 | 89.3 ± 2.6 | 92.4 ± 2.7 |
| E. faecalis | 93.4 ± 2.3 | 92.4 ± 0.7 | 92.9 ± 0.3 | 94.4 ± 0.5 | 92.6 ± 0.8 | 93.3 ± 0.2 |
| E. coli | 96.4 ± 0.9 | 93.9 ± 3.0 | 96.1 ± 0.6 | 95.9 ± 0.8 | 96.9 ± 1.8 | 97.4 ± 1.5 |
| L. rhamnosus | 98.7 ± 0.2 | 99.1 ± 0.2 | 98.5 ± 0.3 | 98.3 ± 0.4 | 98.9 ± 0.4 | 98.4 ± 0.4 |
| P. magnus | 98.2 ± 0.1 | 99.0 ± 0.4 | 98.6 ± 0.2 | 96.6 ± 0.5 | 98.3 ± 0.3 | 99.3 ± 0.1 |
| R. obeum | 92.3 ± 1.0 | 93.4 ± 2.0 | 95.7 ± 0.9 | 94.3 ± 1.0 | 96.9 ± 2.7 | 95.3 ± 0.6 |
Data shown were mean ± SD. The mean was from triplicate incubations with SDs. P values < 0.05. Significant inhibition data are highlighted.
3.4. Effects of metabolites Sudan azo dyes on the human intestinal bacterial cells viability
The cell viabilities of 11 strains with individual metabolites of Sudan azo dyes were studied. The data are shown in Table 5. Each metabolite had no significant effect on the cell viabilities of the bacterial strains. For example, at 100 μM of 1-amino-2-naphthol, aniline, 2,4-dimethylaniline, 1,4-phenylenediamine, 2,5-diaminotoluene, o-toluidine, or 4-nitroaniline, cell viabilities of B. ovatus were 97.7, 95.7, 95.9, 95.6, 96.4, 96.3, and 96.7%, respectively. When each metabolite concentration was increased to 200 μM, the cell viabilities of the bacterium were 96.2, 94.9, 96.1, 95.6, 96.5, 95.6, and 96.2%, respectively. Whereas, cell viability of the bacterial control culture without metabolite was 96.6%. Here we have demonstrated that none of the metabolites from Sudan azo dyes had a significant effect on the cell viabilities of the tested strains, which is similar to our previous observation on S. aureus [51].
Table 5.
Effects of reduction metabolites of Sudan azo dyes on the cell viability of 11 human intestinal bacterial species.a
| Species No. | Control | Viability (%)
|
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1-Amino-2-Naphthol
|
Aniline
|
2,4-Dimethylaniline
|
1,4-Phenylenediamine
|
2,5-Diaminotoluene
|
o-Toluidine
|
4-Nitroaniline
|
|||||||||
| 100 μM | 200 μM | 100 μM | 200 μM | 100 μM | 200 μM | 100 μM | 200 μM | 100 μM | 200 μM | 100 μM | 200 μM | 100 μM | 200 μM | ||
| B. ovatus | 96.6 ± 1.8 | 97.7 ± 0.8 | 96.2 ± 0.2 | 95.7 ± 1.6 | 94.9 ± 1.8 | 95.9 ± 2.7 | 96.1 ± 0.8 | 95.6 ± 1.0 | 95.6 ± 0.5 | 96.4 ± 0.9 | 96.5 ± 0.9 | 96.3 ± 0.8 | 95.6 ± 1.7 | 96.7 ± 1.4 | 96.2 ± 0.7 |
| B. infantis | 95.1 ± 1.8 | 96.8 ± 2.0 | 96.0 ± 1.0 | 96.2 ± 0.8 | 93.6 ± 1.4 | 93.9 ± 1.8 | 94.7 ± 2.2 | 95.6 ± 3.1 | 95.4 ± 1.6 | 96.8 ± 2.4 | 95.4 ± 0.3 | 95.4 ± 0.7 | 96.8 ± 0.3 | 95.1 ± 0.8 | 95.7 ± 1.9 |
| B. catenulatum | 92.1 ± 1.5 | 91.5 ± 0.2 | 94.6 ± 2.7 | 91.0 ± 0.9 | 92.8 ± 3.4 | 91.3 ± 1.0 | 94.0 ± 4.2 | 91.2 ± 1.1 | 94.5 ± 1.5 | 91.7 ± 1.3 | 92.7 ± 0.6 | 91.0 ± 0.2 | 91.5 ± 1.4 | 90.6 ± 2.6 | 94.8 ± 1.5 |
| C. indolis | 97.6 ± 1.3 | 97.5 ± 1.3 | 95.8 ± 1.0 | 96.1 ± 4.3 | 95.2 ± 1.7 | 94.3 ± 2.0 | 96.2 ± 1.5 | 92.9 ± 2.6 | 96.9 ± 3.3 | 95.0 ± 2.8 | 97.8 ± 0.6 | 94.7 ± 0.8 | 95.0 ± 1.3 | 96.0 ± 1.2 | 95.3 ± 1.1 |
| C. perfringens | 91.6 ± 0.3 | 92.5 ± 0.7 | 93.6 ± 0.3 | 90.0 ± 3.3 | 92.8 ± 3.2 | 88.3 ± 3.0 | 93.3 ± 2.0 | 91.1 ± 1.6 | 93.5 ± 2.2 | 94.4 ± 1.0 | 94.5 ± 1.5 | 96.5 ± 0.8 | 93.3 ± 1.8 | 96.2 ± 0.3 | 92.9 ± 0.1 |
| C. ramosum | 97.2 ± 1.0 | 97.0 ± 1.2 | 95.7 ± 0.5 | 97.3 ± 3.4 | 94.3 ± 2.6 | 98.5 ± 0.6 | 94.9 ± 1.6 | 96.1 ± 0.9 | 95.1 ± 0.3 | 94.8 ± 0.7 | 95.9 ± 0.7 | 94.5 ± 1.6 | 96.3 ± 1.6 | 94.9 ± 1.2 | 95.1 ± 2.3 |
| E. faecalis | 90.8 ± 2.3 | 89.4 ± 2.3 | 89.7 ± 3.7 | 87.3 ± 2.6 | 88.5 ± 1.6 | 89.8 ± 3.6 | 90.7 ± 0.8 | 90.0 ± 2.6 | 90.1 ± 4.4 | 90.6 ± 1.2 | 90.5 ± 1.5 | 89.7 ± 1.4 | 89.9 ± 3.4 | 89.8 ± 0.8 | 88.9 ± 1.5 |
| E. coli | 98.7 ± 0.7 | 98.9 ± 0.5 | 97.4 ± 1.2 | 98.4 ± 0.8 | 98.0 ± 0.4 | 99.0 ± 0.2 | 97.9 ± 0.2 | 98.4 ± 0.3 | 99.1 ± 0.5 | 97.6 ± 0.3 | 98.2 ± 0.4 | 98.3 ± 0.5 | 99.0 ± 0.7 | 98.0 ± 0.2 | 98.3 ± 0.4 |
| L. rhamnosus | 98.8 ± 0.1 | 98.5 ± 0.5 | 98.4 ± 0.1 | 98.3 ± 0.5 | 97.8 ± 1.2 | 98.6 ± 0.2 | 97.4 ± 0.4 | 97.6 ± 1.9 | 98.5 ± 0.2 | 97.9 ± 1.3 | 98.3 ± 0.1 | 98.7 ± 0.2 | 97.3 ± 0.9 | 98.3 ± 0.1 | 97.6 ± 0.9 |
| P. magnus | 94.6 ± 0.7 | 95.3 ± 0.3 | 92.3 ± 1.9 | 95.0 ± 0.2 | 96.3 ± 2.5 | 94.6 ± 1.0 | 93.9 ± 0.6 | 96.1 ± 0.5 | 95.9 ± 0.9 | 94.6 ± 0.1 | 96.9 ± 1.4 | 95.1 ± 0.7 | 96.7 ± 0.5 | 94.8 ± 0.7 | 96.5 ± 1.8 |
| R. obeum | 92.6 ± 0.6 | 92.2 ± 1.9 | 89.8 ± 2.4 | 91.3 ± 1.4 | 90.7 ± 1.0 | 90.6 ± 0.4 | 92.4 ± 1.6 | 92.9 ± 2.1 | 90.0 ± 2.9 | 93.1 ± 4.1 | 90.2 ± 1.8 | 92.7 ± 1.2 | 92.4 ± 0.1 | 93.7 ± 1.7 | 93.7 ± 1.0 |
Each metabolite of the dyes was added after incubating the cultures in anaerobic chamber at 37 °C for 12 h. After exposure of the bacterial cells to the metabolites for 12 h, the bacterial cultures were collected for cell viability measurement. Data were presented in mean ± SD. The mean was from triplicate incubations with SD. P values < 0.05.
4. Conclusions
Sudan azo dyes are banned for food usage in most countries, but they are illegally used to maintain or enhance the color of food products due to low cost, bright staining, and wide availability of the dyes. In this study, we demonstrated that di-azo Sudan dyes (Sudan III and IV) had stronger inhibition than mono-azo Sudan dyes (Sudan I, II and Para Red) on growth of human intestinal bacteria. Sudan azo dyes can selectively inhibit cell viabilities of two Clostridium species. Among the metabolites of Sudan azo dyes reduction, the common metabolite 1-amino-2-naphthol can inhibit the cell growth of the bacteria, whereas, none of the metabolites from the dyes reduced by the bacteria had effects on the cell viabilities of the bacteria. The study suggested that Sudan azo dyes and their metabolites have potential effect on human intestinal bacterial ecology by selectively inhibiting some bacterial species and long-term exposure to the dyes may have adverse effect on human health. This investigation provides data examining the potential adverse effects of Sudan azo dyes, as food contaminants, on human intestinal bacteria. The data provided here will be useful in the risk assessment process to evaluate public exposure to food products contaminated with these azo dyes. On the basis of the current results, further studies on the toxicological effects of Sudan azo dyes on the intestinal microbiota in vivo are warranted.
Acknowledgments
This study was funded by National Center for Toxicological Research, United States Food and Drug Administration, and supported in part by appointments (H.P and J. F) to the Postgraduate Research Fellowship Program by the Oak Ridge Institute for Science and Education through an interagency agreement between the U.S. Department of Energy and the U.S. Food and Drug Administration. The views presented in this article do not necessarily reflect those of the Food and Drug Administration.
References
- 1.Collier SW, Storm JE, Bronaugh RL. Reduction of azo dyes during in vitro percutaneous absorption. Toxicol Appl Pharmacol. 1993;118:73–9. doi: 10.1006/taap.1993.1011. [DOI] [PubMed] [Google Scholar]
- 2.Chung KT, Stevens SE, Jr, Cerniglia CE. The reduction of azo dyes by the intestinal microflora. Crit Rev Microbiol. 1992;18:175–90. doi: 10.3109/10408419209114557. [DOI] [PubMed] [Google Scholar]
- 3.Rafii F, Hall JD, Cerniglia CE. Mutagenicity of azo dyes used in foods, drugs and cosmetics before and after reduction by Clostridium species from the human intestinal tract. Food Chem Toxicol. 1997;35:897–901. doi: 10.1016/s0278-6915(97)00060-4. [DOI] [PubMed] [Google Scholar]
- 4.Rowland I. The influence of the gut microflora on food toxicity. Proc Nutr Soc. 1981;40:67–74. doi: 10.1079/pns19810011. [DOI] [PubMed] [Google Scholar]
- 5.Stingley RL, Zou W, Heinze TM, Chen H, Cerniglia CE. Metabolism of azo dyes by human skin microbiota. J Med Microbiol. 2010;59:108–14. doi: 10.1099/jmm.0.012617-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feng J, Cerniglia CE, Chen H. Toxicological significance of azo dye metabolism by human intestinal microbiota. Front Biosci (Elite Ed) 2012;4:568–86. doi: 10.2741/400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen KC, Wu JY, Liou DJ, Hwang SC. Decolorization of the textile dyes by newly isolated bacterial strains. J Biotechnol. 2003;101:57–68. doi: 10.1016/s0168-1656(02)00303-6. [DOI] [PubMed] [Google Scholar]
- 8.Stolz A. Basic and applied aspects in the microbial degradation of azo dyes. Appl Microbiol Biotechnol. 2001;56:69–80. doi: 10.1007/s002530100686. [DOI] [PubMed] [Google Scholar]
- 9.Medvedev ZA, Crowne HM, Medvedeva MN. Age related variations of hepatocarcinogenic effect of azo dye (3′-MDAB) as linked to the level of hepatocyte polyploidization. Mech Ageing Dev. 1988;46:159–74. doi: 10.1016/0047-6374(88)90123-6. [DOI] [PubMed] [Google Scholar]
- 10.Cerniglia CE, Freeman JP, Franklin W, Pack LD. Metabolism of azo dyes derived from benzidine, 3,3′-dimethyl-benzidine and 3,3′-dimethoxybenzidine to potentially carcinogenic aromatic amines by intestinal bacteria. Carcinogenesis. 1982;3:1255–60. doi: 10.1093/carcin/3.11.1255. [DOI] [PubMed] [Google Scholar]
- 11.Cerniglia CE, Zhuo Z, Manning BW, Federle TW, Heflich RH. Mutagenic activation of the benzidine-based dye direct black 38 by human intestinal microflora. Mutat Res. 1986;175:11–6. doi: 10.1016/0165-7992(86)90138-7. [DOI] [PubMed] [Google Scholar]
- 12.Xu H, Heinze TM, Paine DD, Cerniglia CE, Chen H. Sudan azo dyes and para red degradation by prevalent bacteria of the human gastrointestinal tract. Anaerobe. 2010;16:114–9. doi: 10.1016/j.anaerobe.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen H, Xu H, Kweon O, Chen S, Cerniglia CE. Functional role of Trp-105 of Enterococcus faecalis azoreductase (AzoA) as resolved by structural and mutational analysis. Microbiology. 2008;154:2659–67. doi: 10.1099/mic.0.2008/019877-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stiborova M, Martinek V, Rydlova H, Hodek P, Frei E. Sudan I is a potential carcinogen for humans: evidence for its metabolic activation and detoxication by human recombinant cytochrome P450 1A1 and liver microsomes. Cancer Res. 2002;62:5678–84. [PubMed] [Google Scholar]
- 15.Chen H, Wang RF, Cerniglia CE. Molecular cloning, overexpression, purification, and characterization of an aerobic FMN-dependent azoreductase from Enterococcus faecalis. Protein Expr Purif. 2004;34:302–10. doi: 10.1016/j.pep.2003.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feng J, Heinze TM, Xu H, Cerniglia CE, Chen H. Evidence for significantly enhancing reduction of Azo dyes in Escherichia coli by expressed cytoplasmic Azoreductase (AzoA) of Enterococcus faecalis. Protein Pept Lett. 2010;17:578–84. doi: 10.2174/092986610791112701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen H, Hopper SL, Cerniglia CE. Biochemical and molecular characterization of an azoreductase from Staphylococcus aureus, a tetrameric NADPH-dependent flavoprotein. Microbiology. 2005;151:1433–41. doi: 10.1099/mic.0.27805-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen H, Feng J, Kweon O, Xu H, Cerniglia CE. Identification and molecular characterization of a novel flavin-free NADPH preferred azoreductase encoded by azoB in Pigmentiphaga kullae K24. BMC Biochem. 2010;11:13. doi: 10.1186/1471-2091-11-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu ZJ, Chen H, Shaw N, Hopper SL, Chen L, Chen S, et al. Crystal structure of an aerobic FMN-dependent azoreductase (AzoA) from Enterococcus faecalis. Arch Biochem Biophys. 2007;463:68–77. doi: 10.1016/j.abb.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 20.Parkinson TM, Brown JP. Metabolic fate of food colorants. Annu Rev Nutr. 1981;1:175–205. doi: 10.1146/annurev.nu.01.070181.001135. [DOI] [PubMed] [Google Scholar]
- 21.Chen H. Recent advances in azo dye degrading enzyme research. Curr Protein Pept Sci. 2006;7:101–11. doi: 10.2174/138920306776359786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rebane R, Leito I, Yurchenko S, Herodes K. A review of analytical techniques for determination of Sudan I–IV dyes in food matrixes. J Chromatogr A. 2010;1217:2747–57. doi: 10.1016/j.chroma.2010.02.038. [DOI] [PubMed] [Google Scholar]
- 23.Yan H, Wang H, Qiao J, Yang G. Molecularly imprinted matrix solid-phase dispersion combined with dispersive liquid–liquid microextraction for the determination of four Sudan dyes in egg yolk. J Chromatogr A. 2011;1218:2182–8. doi: 10.1016/j.chroma.2011.02.042. [DOI] [PubMed] [Google Scholar]
- 24.Calbiani F, Careri M, Elviri L, Mangia A, Pistara L, Zagnoni I. Development and in-house validation of a liquid chromatography-electrospray-tandem mass spectrometry method for the simultaneous determination of Sudan I, Sudan II, Sudan III and Sudan IV in hot chilli products. J Chromatogr A. 2004;1042:123–30. doi: 10.1016/j.chroma.2004.05.027. [DOI] [PubMed] [Google Scholar]
- 25.Chailapakul O, Wonsawat W, Siangproh W, Grudpan K, Zhao Y, Zhu Z. Analysis of Sudan I, Sudan II, Sudan III, and Sudan IV in food by HPLC with electrochemical detection: comparison of glassy carbon electrode with carbon nanotube-ionic liquid gel modified electrode. Food Chem. 2008;109:876–82. doi: 10.1016/j.foodchem.2008.01.018. [DOI] [PubMed] [Google Scholar]
- 26.Ertas E, Özer H, Alasalvar C. A rapid HPLC method for determination of Sudan dyes and para red in red chilli pepper. Food Chem. 2007;105:756–60. [Google Scholar]
- 27.Moller P, Wallin H. Genotoxic hazards of azo pigments and other colorants related to 1-phenylazo-2-hydroxynaphthalene. Mutat Res. 2000;462:13–30. doi: 10.1016/s1383-5742(99)00090-3. [DOI] [PubMed] [Google Scholar]
- 28.Westmoreland C, Gatehouse DG. The differential clastogenicity of solvent yellow 14 and FD & C yellow no. 6 in vivo in the rodent micronucleus test (observations on species and tissue specificity) Carcinogenesis. 1991;12:1403–7. doi: 10.1093/carcin/12.8.1403. [DOI] [PubMed] [Google Scholar]
- 29.An Y, Jiang L, Cao J, Geng C, Zhong L. Sudan I induces genotoxic effects and oxidative DNA damage in HepG2 cells. Mutat Res. 2007;627:164–70. doi: 10.1016/j.mrgentox.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 30.Johnson GE, Quick EL, Parry EM, Parry JM. Metabolic influences for mutation induction curves after exposure to Sudan-1 and para red. Mutagenesis. 2010;25:327–33. doi: 10.1093/mutage/geq009. [DOI] [PubMed] [Google Scholar]
- 31.Zeiger E, Anderson B, Haworth S, Lawlor T, Mortelmans K. Salmonella mutagenicity tests: IV. Results from the testing of 300 chemicals. Environ Mol Mutagen. 1988;11(Suppl 12):1–157. [PubMed] [Google Scholar]
- 32.Garner RC, Nutman CA. Testing of some azo dyes and their reduction products for mutagenicity using Salmonella typhimurium TA 1538. Mutat Res. 1977;44:9–19. doi: 10.1016/0027-5107(77)90110-5. [DOI] [PubMed] [Google Scholar]
- 33.Cameron TP, Hughes TJ, Kirby PE, Fung VA, Dunkel VC. Mutagenic activity of 27 dyes and related chemicals in the salmonella/microsome and mouse lymphoma TK+/− assays. Mutat Res. 1987;189:223–61. doi: 10.1016/0165-1218(87)90056-5. [DOI] [PubMed] [Google Scholar]
- 34.Au W, Hsu TC. Studies on the clastogenic effects of biologic stains and dyes. Environ Mutagen. 1979;1:27–35. doi: 10.1002/em.2860010109. [DOI] [PubMed] [Google Scholar]
- 35.Xu H, Heinze TM, Chen S, Cerniglia CE, Chen H. Anaerobic metabolism of 1-amino-2-naphthol-based azo dyes (Sudan dyes) by human intestinal micro-flora. Appl Environ Microbiol. 2007;73:7759–62. doi: 10.1128/AEM.01410-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fare G. Rat skin carcinogenesis by topical applications of some azo dyes. Cancer Res. 1966;26:2406–8. [PubMed] [Google Scholar]
- 37.Federal Institute for Risk Assessment. Dyes Sudan I to IV in food. 2003 http://www.bfr.bund.de/cm/245/dyes_sudan_I_IV.pdf.
- 38.Brown JP, Roehm GW, Brown RJ. Mutagenicity testing of certified food colors and related azo, xanthene and triphenylmethane dyes with the Salmonella/microsome system. Mutat Res. 1978;56:249–71. doi: 10.1016/0027-5107(78)90192-6. [DOI] [PubMed] [Google Scholar]
- 39.Refat NA, Ibrahim ZS, Moustafa GG, Sakamoto KQ, Ishizuka M, Fujita S. The induction of cytochrome P450 1A1 by Sudan dyes. J Biochem Mol Toxicol. 2008;22:77–84. doi: 10.1002/jbt.20220. [DOI] [PubMed] [Google Scholar]
- 40.Simon GL, Gorbach SL. The human intestinal microflora. Dig Dis Sci. 1986;31:147S–62S. doi: 10.1007/BF01295996. [DOI] [PubMed] [Google Scholar]
- 41.Li M, Wang B, Zhang M, Rantalainen M, Wang S, Zhou H, et al. Symbiotic gut microbes modulate human metabolic phenotypes. Proc Natl Acad Sci U S A. 2008;105:2117–22. doi: 10.1073/pnas.0712038105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nicholson JK, Holmes E, Wilson ID. Gut microorganisms, mammalian metabolism and personalized health care. Nat Rev Microbiol. 2005;3:431–8. doi: 10.1038/nrmicro1152. [DOI] [PubMed] [Google Scholar]
- 43.Eckburg PB, Bik EM, Bernstein CN, Purdom E, Dethlefsen L, Sargent M, et al. Diversity of the human intestinal microbial flora. Science. 2005;308:1635–8. doi: 10.1126/science.1110591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: human gut microbes associated with obesity. Nature. 2006;444:1022–3. doi: 10.1038/4441022a. [DOI] [PubMed] [Google Scholar]
- 45.Manning BW, Cerniglia CE, Federle TW. Metabolism of the benzidine-based azo dye direct black 38 by human intestinal microbiota. Appl Environ Microbiol. 1985;50:10–5. doi: 10.1128/aem.50.1.10-15.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rafii F, Franklin W, Cerniglia CE. Azoreductase activity of anaerobic bacteria isolated from human intestinal microflora. Appl Environ Microbiol. 1990;56:2146–51. doi: 10.1128/aem.56.7.2146-2151.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bras R, Gomes A, Ferra MI, Pinheiro HM, Goncalves IC. Monoazo and diazo dye decolourisation studies in a methanogenic UASB reactor. J Biotechnol. 2005;115:57–66. doi: 10.1016/j.jbiotec.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 48.Wu X, Kannan S, Ramanujam VM, Khan MF. Iron release and oxidative DNA damage in splenic toxicity of aniline. J Toxicol Environ Health A. 2005;68:657–66. doi: 10.1080/15287390590921757. [DOI] [PubMed] [Google Scholar]
- 49.Bomhard EM, Herbold BA. Genotoxic activities of aniline and its metabolites and their relationship to the carcinogenicity of aniline in the spleen of rats. Crit Rev Toxicol. 2005;35:783–835. doi: 10.1080/10408440500442384. [DOI] [PubMed] [Google Scholar]
- 50.Lebaron P, Parthuisot N, Catala P. Comparison of blue nucleic acid dyes for flow cytometric enumeration of bacteria in aquatic systems. Appl Environ Microbiol. 1998;64:1725–30. doi: 10.1128/aem.64.5.1725-1730.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pan H, Feng J, Cerniglia CE, Chen H. Effects of Orange II and Sudan III azo dyes and their metabolites on Staphylococcus aureus. J Ind Microbiol Biotechnol. 2011;38:1729–38. doi: 10.1007/s10295-011-0962-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen H, Xu H, Heinze TM, Cerniglia CE. Decolorization of water and oil-soluble azo dyes by Lactobacillus acidophilus and Lactobacillus fermentum. J Ind Microbiol Biotechnol. 2009;36:1459–66. doi: 10.1007/s10295-009-0633-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ren JR, Zhao HP, Song C, Wang SL, Li L, Xu YT, et al. Comparative trans-membrane transports of four typical lipophilic organic chemicals. Bioresour Technol. 2010;101:8632–8. doi: 10.1016/j.biortech.2010.06.121. [DOI] [PubMed] [Google Scholar]