Abstract
Background
This study sought to specify (1) the position of nonmedical prescription opioids (NMPO) in drug initiation sequences among Millennials (1979–96), Generation X (1964–79), and Baby Boomers (1949–64) and (2) gender and racial/ethnic differences in sequences among Millennials.
Methods
Data are from the 2013–2014 National Surveys on Drug Use and Health (n = 73,026). We identified statistically significant drug initiation sequences involving alcohol/cigarettes, marijuana, NMPO, cocaine, and heroin using a novel method distinguishing significant sequences from patterns expected only due to correlations induced by common liability among drugs.
Results
Alcohol/cigarettes followed by marijuana was the most common sequence. NMPO or cocaine use after marijuana, and heroin use after NMPO or cocaine, differed by generation. Among successively younger generations, NMPO after marijuana and heroin after NMPO increased. Millennials were more likely to initiate NMPO than cocaine after marijuana; Generation X and Baby Boomers were less likely (odds ratios = 1.4;0.3;0.2). Millennials were more likely than Generation X and Baby Boomers to use heroin after NMPO (hazards ratios = 7.1;3.4;2.5). In each generation, heroin users were far more likely to start heroin after both NMPO and cocaine than either alone. Sequences were similar by gender. Fewer paths were significant among African-Americans.
Conclusions
NMPOs play a more prominent role in drug initiation sequences among Millennials than prior generations. Among Millennials, NMPO use is more likely than cocaine to follow marijuana use. In all generations, transition to heroin from NMPO significantly occurs only when both NMPO and cocaine have been used. Delineation of drug sequences suggests optimal points in development for prevention and treatment efforts.
Keywords: Drug initiation sequences, Nonmedical prescription opioids, Heroin, Cocaine, Generations
1. Introduction
Heroin use increased sharply over the last several years among nonmedical users of prescription opioids (NMPO), especially heavy and dependent users (Banerjee et al., 2016; Cerdá et al., 2015; Jones, 2013; Jones et al., 2015; Martins et al., 2016; Muhuri et al., 2013). NMPO users are also more likely than non-users to use marijuana, stimulants or cocaine (Banerjee et al., 2016; Boyd et al., 2009; Catalano et al., 2011; Han et al., 2015; McCabe et al., 2011; Wu et al., 2008; Young et al., 2012). The prevalence of legal and illegal drug use, including NMPO, has been examined across birth cohorts (Bluthenthal et al., 2017; Degenhardt et al., 2007; Golub and Johnson, 2001; Johnson and Gerstein, 1998). Use of heroin and marijuana peaked in the early 1970’s, cocaine in the 1980’s, and NMPO in the mid-2000’s. Progression through stages of drug use from nonuse to alcohol/tobacco, marijuana, and hard drugs (cocaine, heroin) observed from 1979 to 1997 for 1910–1971 birth cohorts was examined by Golub and Johnson (2001). Progression to each stage peaked with the 1960 birth cohort. To the best of our knowledge, except for that study, generational changes in developmental patterns of drug initiation in the population, in particular, pathways involving NMPO and NMPO in relation to cocaine and heroin, have not been examined.
Drug usage starts with alcohol or cigarettes and proceeds to illegal drugs, even in recent periods when rates of marijuana use surpass those of cigarette use (Keyes et al., 2016). Marijuana, in turn, precedes use of cocaine and other illicit substances (Cleveland and Wiebe, 2008; Degenhardt et al., 2010; Fergusson et al., 2006; Kandel, 2002; Kandel et al., 2006; Lynskey et al., 2012; Rebellon and Van Gundy, 2006; Wagner and Anthony, 2002). This progression, observed in the US and internationally, led to the notion of the Gateway Hypothesis: alcohol, tobacco or marijuana are gateways to using other substances. The notion of developmental stages in drug behavior does not imply that these stages are obligatory nor that entry into a lower stage drug inexorably leads to higher stage drugs. Translational research in rodents supports a causal mechanism through which the gateway sequence arises between two drugs. Nicotine pretreatment (in mice) and alcohol (in rats) enhances responses to later cocaine exposure but not vice versa (Griffin et al., 2017; Kandel and Kandel, 2014; Levine et al., 2011). Nicotine acts as a gateway drug and exerts a priming effect on cocaine through increased global histone acetylation in the nucleus accumbens, and this creates an environment primed for induction of gene expression.
Another perspective, the Common Liability Model, posits that use of multiple drugs reflects a common liability for drug use, with no specific influence of one particular drug leading to use of another (Palmer et al., 2009; Vanyukov et al., 2012). Generalized risks include not only common genetic predispositions but psychosocial and environmental factors including drug availability (Bailey et al., 2011; Cleveland and Wiebe, 2008; Rebellon and Van Gundy, 2006; Wagner and Anthony, 2002).
The position of NMPO use in the sequence of drug involvement remains to be established, especially nationally, and may vary between birth cohorts who differ in their drug experiences. Catalano et al. (2011) inferred an order between NMPO and other drugs from rates of use of different drugs from first grade to age 21. While Harrell and Broman (2009) concluded that alcohol and marijuana use in adolescence predicted any nonmedical prescription drug (NMPD) use six years later, including NMPO, the sequence of initiation among these drugs could not be identified because neither NMPD use at the initial interview nor onset age were ascertained. Others reported that marijuana use prior to age 18 was associated with NMPO use by age 25 (Fiellin et al., 2013), and cigarette and marijuana use by 12th grade predicted NMPO use by age 23 (Miech et al., 2015). Several studies documented that initiation of NMPO use occurred before heroin (Banerjee et al., 2016; Cerdá et al., 2015; Jones, 2013; Muhuri et al., 2013; Novak et al., 2016). This sequence became more prevalent in the population between 2002–04 and 2008–10 (Jones, 2013) and among those born after 1980 (Novak et al., 2016). Except for these studies, changes in patterns of heroin initiation in relation to NMPO and other drugs in different historical periods and at different points in the lifespan have not been examined.
Drug use is an age-graded behavior, and historical differences in prevalence of drug use experienced by different birth cohorts at ages at highest risk for drug initiation may influence the drug use careers of different generations. We consider developmental patterns of use across three generational cohorts spanning ages 18–64 in 2013–2014: Millennials (born in 1979–96), Generation X (born in 1964–79), and Baby Boomers (born in 1949–64). The birth years defining generations vary slightly across investigations (Fry, 2016; Pew Research Center, 2015), but generations provide a useful way of considering the behaviors of individuals born in different time periods. Trend data as of 1982 among 18–34 years olds illustrate that, while prevalence of lifetime use of different drug classes varied greatly over the last 40 years, the relative ranking of these drugs remained the same, except for NMPO (Supplementary Fig. 1). At ages 18–34, Baby Boomers lived through a period of increased drug experimentation, while Generation X lived through a period of decreasing prevalence of use of different drugs. Millennials experienced increases in use, especially for NMPO and heroin. Hence, we would expect sequences of drug initiation to vary across generations, especially regarding the position of NMPO.
We analyze developmental patterns of involvement in legal and illegal drugs with a focus on sequences between NMPO, cocaine, and heroin. Using a novel simulation method that distinguishes significant drug initiation sequences from patterns expected to occur by chance due to correlations induced by common liability between use of different drugs, we address the following questions: (1) what is the position of NMPO use in drug initiation sequences in three generations (Millennials, Generation X, and Baby Boomers?) and (2) what are gender and racial/ethnic differences in these patterns among Millennials, the youngest generation?
2. Methods
2.1. Sample
Data are from two aggregated surveys (2013–2014) from the National Survey on Drug Use and Health (NSDUH), annual cross-sectional surveys of drug use in multistage representative probability samples of the US population aged 12 and older (CBHSQ, 2015a). All states are represented. The target civilian non-instituionalized population represents over 98% of the population. Persons in non-institutional group quarters (homeless shelters, rooming houses, college dormitories) and civilians on military bases are included; individuals on active military duty, in jail, drug treatment programs, hospitals, and homeless not in shelters are excluded. Age groups at highest risk for drug use (12–17 and 18–25) are oversampled. Overall response rates were 60.2% in 2013 and 58.3% in 2014. Public use data were used for ages 18–64 (n = 73,026).
The study was granted expedited approval by the New York State Psychiatric Institute – Columbia University Department of Psychiatry Institutional Review Board.
2.2. Data
Data were collected by CBHSQ with computer-assisted personal interviews (CAPI) by an interviewer, and audio-computer assisted self-interviewing (ACASI) for substance use. Respondents were asked about use of prescription pain relievers (opioids) without a prescription or for the experience or feeling they caused; 21 pain relievers were listed.
2.2.1. Selected constructed variables
Lifetime use and onset age of cigarettes and/or alcohol, marijuana, cocaine, heroin, nonmedical prescription opioids were included. If both cigarettes and alcohol were used, onset age was set to the earlier age.
Three generations were defined: birth cohorts matched as closely as possible the generations defined by Pew Research Center (2015). Deviations were due to age groupings in NSDUH: Millennials, born in 1979–96 (vs. 1981–97 in Pew), Generation X, born in 1964–79 (vs. 1965–80), Baby Boomers, born in 1949–64 (vs. 1946–64), aged 18–34, 35–49, and 50–64, respectively, in 2013–2014. Since NSDUH groups ages and two surveys were aggregated, two birth-year cohorts (1964 (1979) were included in two generations.
The generations cover different ranges of the lifespan, ages 18–34 for Millennials, 35–49 for Generation X, and 50–64 for Baby Boomers.
2.3. Analytical strategy
Three analyses were implemented: (1) estimation of prevalence of lifetime use of the five drugs; (2) identification of significant drug initiation sequences using a novel simulation-based method; (3) estimation through survival analysis of risk of (a) NMPO or cocaine initiation next after marijuana (discrete-time survival model) and (b) heroin initiation next after NMPO or cocaine initiation (Cox proportional hazards model for dichotomous outcome). Analyses were implemented by generation, and by gender and race/ethnicity among Millennials.
2.3.1. Identification of significant drug initiation sequences
Drug initiation sequences were identified based on ordered, reported ages of first use of up to five drugs. There were 326 possible sequences: none, 5 one-drug sequences, and 20, 60, and 120 sequences of 2, 3, 4, and 5 drugs, respectively. Subjects reporting initiating illicit drugs before age 5 were excluded (n = 64). Ties in age of onset were randomly assigned an order based on the prevalence of initiation orders observed in the total sample. Less than 1% of ties involved more than two drugs, the most common (8.5%) being between alcohol/cigarettes and marijuana.
Statistically significant drug sequences compared to those expected by chance under a common liability model were identified with a simulation-based approach. The method simulates a population with characteristics (ages at survey, prevalence of lifetime use, and distributions of onset age for each drug) identical to the observed NSDUH sample but with sequential patterns of drug use onset generated randomly: (1) a multivariate probit model simulated correlated dichotomous lifetime use of all 5 drugs and (2) a multinomial distribution simulated onset age for each drug conditional on lifetime use. Population values for correlations for lifetime use in (1) and age of onset in (2) were fixed at the observed values from the NSDUH sample for each age at time of survey.
The method calculated the expected prevalence of the 326 possible drug initiation sequences in the simulated population data and compared these to the observed prevalence of each drug sequence in NSDUH using a standard test of proportions. A statistically significant drug sequence implied that it occurred more (or less) often than by chance. Hence, the order of drugs (i.e., gateway), not just the combination of drugs (i.e., common liability), was significant. The p-value was based on the standard normal distribution, with level of significance Bonferroni adjusted (Holland and Copenhaver, 1988) for the total number of sequences tested. Censoring of drug initiation, particularly at younger survey ages, was controlled within the method by simulating drug sequences and comparing them conditional upon age at time of survey. The simulation-based analyses were performed within each generation, and by gender and race/ethnicity within Millennials. Sequences were excluded when observed counts were < 25 in each generation and < 10 in each demographic subgroup.
The simulation-based method was implemented in SAS 9.4 (SAS Institute Inc., 2013) with code available from authors; other analyses were implemented in SUDAAN 11.0.1 (RTI, 2012). Analyses used sample weights reflecting selection probabilities at various stages of the sampling design.
3. Results
3.1. Lifetime use of five drug classes among generations
While the absolute prevalence of lifetime use of the drugs varied across generations, relative rankings were similar except for NMPO and cocaine (Table 1). Alcohol or cigarette use was most prevalent. Marijuana use was next in prevalence, with rates higher among Millennials and Baby Boomers than Generation X. NMPO use increased among each successively younger generation while cocaine use decreased. Among Millennials, NMPO use was more prevalent [21.5% (95% CI = 20.9%–22.1%) than cocaine use [15.0% (95% CI = 14.6%–15.5%)]; among Generation X, and especially Baby Boomers, cocaine use was more prevalent than NMPO use: Generation X cocaine = 18.6% (95% CI = 17.9%–19.3%) versus NMPO = 15.3% (95% CI = 14.7%–16.1%); Baby Boomers = 22.9% (95% CI = 21.8%–24.2%) versus 12.0% (95% CI = 11.1%–12.8%). Lifetime heroin use was the least prevalent. The rates were higher among Millennials (2.6%, 95% CI = 2.4%–2.8%) and Baby Boomers (2.5%, 95% CI = 2.1%–3.0%) than Generation X (1.9%, 95% CI = 1.6%–2.1%).
Table 1.
Prevalence of lifetime use of five drug classesa in three generations: NSDUH 2013–2014, ages 18–64b.
| Lifetime Use | Millennial 1979–96 Birth Cohorts (Ages 18–34)
|
Generation X 1964–79 Birth Cohorts (Ages 35–49)
|
Baby Boomer 1949–64 Birth Cohorts (Ages 50–64)
|
|||
|---|---|---|---|---|---|---|
| % | 95% CI | % | 95% CI | % | 95% CI | |
| Alcohol/cigarettes | 89.2 | (88.8–89.6)c | 91.8 | (91.1–92.5)d | 92.4 | (91.6–93.1)d |
| Marijuana | 54.5 | (53.8–55.2)c | 49.9 | (48.9–50.9)d | 54.0 | (52.5–55.5)c |
| NMPO | 21.5 | (20.9–22.1)c | 15.3 | (14.7–16.1)d | 12.0 | (11.1–12.8)e |
| Cocaine | 15.0 | (14.6–15.5)c | 18.6 | (17.9–19.3)d | 22.9 | (21.8–24.2)e |
| Heroin | 2.6 | (2.4–2.8)c | 1.9 | (1.6–2.1)d | 2.5 | (2.1–3.0)c |
| n | (45,010) | (18,727) | (9289) | |||
Weighed percentage, unweighted n.
Alcohol/cigarettes = alcohol or cigarettes; NMPO = nonmedical prescription opioids.
Excludes 64 cases missing onset ages of marijuana, cocaine, nonmedical prescription opioids, or heroin.
Different superscriptes indicate statistically significant (P < 0.05) differences by generation for each drug.
Based on self-reported ages of onset, only very small percentages of Generation X or Baby Boomers report initiating any of the drugs after age 34, ranging from 0.3% for heroin in both generations, 0.5% and 0.9% for alcohol/cigarettes, 0.7% and 1.0% for marijuana, and 0.5% and 1.3% for cocaine among Generation X and Baby Boomers, respectively. The largest percentages of initiates occur for nonmedical prescription opioids, at 2.8% for Generation X and 4.0% Baby Boomers.
3.2. Drug initiation sequences for five drug classes among generations
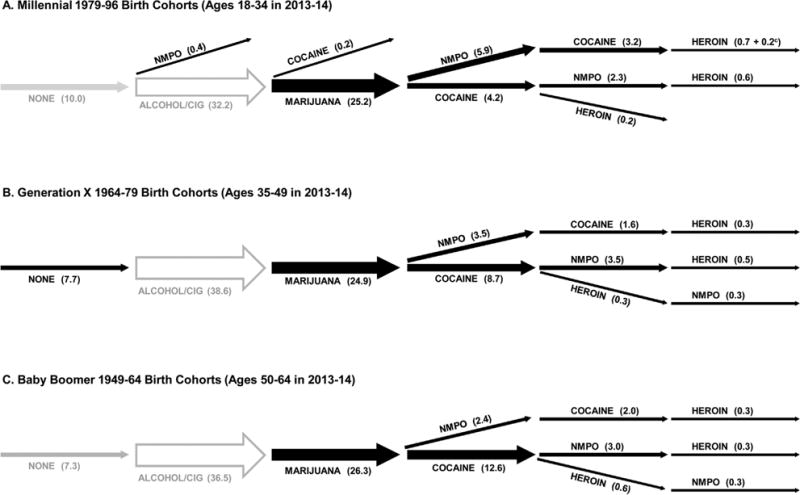
Of 326 possible drug initiation sequences among the five drugs, 13 sequences, including no drug use, occurred significantly more frequently than expected by chance (z > 3.7, P’s < 0.0002) in at least one generation. Fig. 1 displays proportions of individuals in each generation who followed unique significant sequences according to the last drug they used (see also Supplementary Table 1).
Fig. 1.
Significant drug initiation sequencesa among five drug classesb by three generations: NSDUH 2013–2014, ages 18–64 (n = 73,026).
Ten percent of Millennials, 7.7% of Generation X, and 7.3% of Baby Boomers had never used any drugs, a pattern which occurred significantly more frequently than expected by chance among Generation X. Exclusive lifetime use of alcohol or cigarettes occurred as expected by chance and ranged from 32.2% among Millennials to 38.6% among Generation X (Fig. 1). The most common statistically significant sequence observed at the same rate (≈25%) across the three generations was alcohol/cigarettes followed by marijuana.
The sequences of alcohol/cigarettes, then marijuana followed by NMPO or by cocaine were statistically significant in each generation, although each generation initiated NMPO or cocaine use after marijuana at different rates. Marijuana followed by NMPO occurred more frequently among Millennials (5.9%) than Generation X (3.5%) or Baby Boomers (2.4%); marijuana followed by cocaine occurred less frequently among Millennials (4.2%) than Generation X (8.7%) and especially Baby Boomers (12.6%). The proportion initiating NMPO first, followed by cocaine, increased from Baby Boomers and Generation X to Millennials (Supplementary Table 1).
The sequences of alcohol/cigarettes, marijuana, NMPO and/or cocaine followed by heroin were statistically significant for all generations. Patterns were similar for Generation X and Baby Boomers but differed for Millennials. Among Millennials, NMPO followed by cocaine more frequently preceded heroin use (0.9%) than did cocaine followed by NMPO (0.6%); among Generation X and Baby Boomers these two paths were equally prevalent (0.3%). Among all generations, a direct path from cocaine to heroin was also observed, but among Millennials it was much weaker than the path from cocaine to heroin through NMPO. Generation X and Baby Boomers included a path from cocaine to heroin followed by NMPO (Fig. 1; Supplementary Table 1).
A statistically significant single drug sequence, use of NMPO prior to any other drugs, emerged only among Millennials (0.4%).
3.2.1. NMPO or cocaine initiation next after marijuana
To highlight generational differences in drug sequences following marijuana use, combined patterns of NMPO or cocaine initiation following marijuana use, without considering subsequent sequences, were examined. The overall proportions who started using cocaine or NMPO after marijuana varied little across generations (approximately 40% of marijuana users) (Fig. 2). However, as noted above, Millennials were more likely to start using NMPO than cocaine next after marijuana, while the pattern was reversed for Generation X and Baby Boomers (Fig. 2). Among Millennials, 24.0% started using NMPO next after marijuana and 16.9% started cocaine next after marijuana. By contrast, 12.6% of Generation X and 10.1% of Baby Boomers started using NMPO next after marijuana compared with 31.0% and 35.3%, respectively, who started cocaine after marijuana (P’s < 0.001). Thus, the risk of first starting NMPO versus cocaine was positive among Millennials (odds ratio [OR] = 1.4 [95% CI = 1.3–1.5]) and negative among Generation X (OR = 0.29 [95% CI = 0.26–0.32]) and Baby Boomers (OR = 0.18 [95% CI = 0.15–0.22]) (Table 2-A).
Fig. 2.
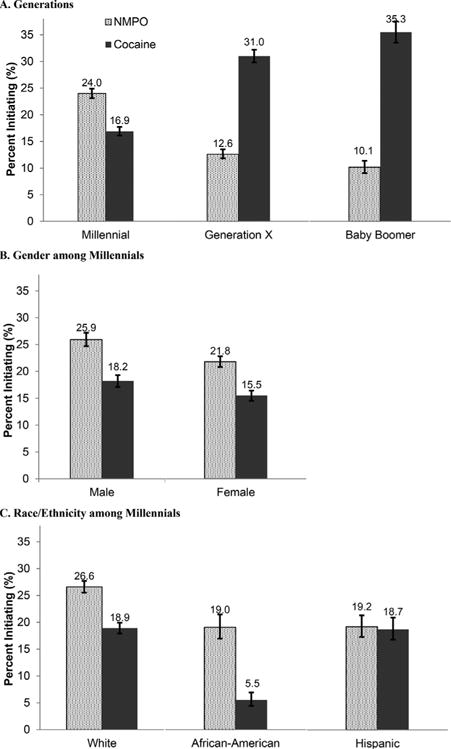
Rates of nonmedical prescription opioid (NMPO) or cocaine initiation next after marijuana among marijuana usersa in three generations and among Millennials by gender and race/ethnicity: NSDUH 2013–2014, ages 18–64 (n = 37,459). aExcludes those who initiated NMPO, cocaine or heroin before marijuana (n = 1,450); includes those who initiated alcohol/cigarettes after marijuana. See also Supplementary Table 2.
Table 2.
(A) Odds of initiating nonmedical prescription opioids (NMPO) or cocaine after marijuana in discrete-time survival analyses, and (B) hazards ratios of initiating heroin after NMPO or cocaine among marijuana users in Cox proportional hazards models, by three generations and among Millennials by gender and race/ethnicity: NSDUH 2013–2014, ages 18–64.
| Sample | A. NMPO vs Cocaine Next Initiation after Marijuana | B. Heroin Initiation Next after NMPO or Cocaine among Marijuana Users | |||||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| NMPO vs. Cocaine | NMPO vs. No NMPO | Cocaine vs. No Cocaine | NMPO and Cocaine vs. NMPO only | NMPO and Cocaine vs. Cocaine only | NMPO and Cocaine vs. Neither Cocaine/NMPO | Total (n) | |
| ORa (95% CI) | HRa (95% CI) | HRa (95% CI) | HRa (95% CI) | HRa (95% CI) | HRa (95% CI) | ||
| Generation | |||||||
| Millennial | 1.4 (1.3–1.5)***,b | 7.1 (5.6–9.1)***,b | 16.6 (13.2–20.9)***,b | 7.8 (5.8–10.5)***,b,c | 2.6 (1.9–3.4)***,b | 39.6 (27.3–57.3)***,b | (23,183) |
| Generation X | 0.3 (0.3–0.3)***,c | 3.4 (2.6–4.3)***,c | 17.5 (11.4–27.8)***,b | 11.1 (5.4–22.8)***,b | 1.6 (1.3–2.1)***,c | 27.4 (15.4–48.8)***,b | (9401) |
| Baby Boomer | 0.2 (0.2–0.2)***,d | 2.5 (1.6–4.0)***,c | 4.1 (2.7−6.2)***,c | 3.6 (1.6–8.2)***,c | 1.7 (1.0–3.0)*,bc | 6.2 (3.5–10.8)***,c | (4875) |
| Millennial | |||||||
| Gender | |||||||
| Male | 1.5 (1.4–1.6)*** | 6.8 (4.9–9.5)*** | 15.4 (11.1–21.2)*** | 7.8 (5.4–11.4)*** | 2.8 (1.8–4.0)*** | 34.9 (21.1–57.8)*** | (11,573) |
| Female | 1.4 (1.2–1.5)*** | 7.3 (5.2–10.1)*** | 17.7 (12.3–25.5)*** | 7.5 (4.7–12.0)*** | 2.4 (1.7–3.4)*** | 44.7 (25.1–79.7)*** | (11,610) |
| Race/Ethnicity | |||||||
| White | 1.5 (1.4–1.6)***,b | 6.5 (5.0–8.5)*** | 15.0 (11.4–19.6)*** | 6.1 (4.5–8.4)*** | 2.2 (1.6–3.0)*** | 41.3 (26.2–65.0)***,b | (14,373) |
| African-American | 3.5 (2.5–4.7)***,c | –***,e | –e | –e | –e | –e | (2960) |
| Hispanic | 1.1 (0.9–1.3)d | 5.7 (2.9–11.1)*** | 8.7 (4.3–17.7)*** | 13.2 (5.0–35.4)*** | 4.3 (1.8–10.4)*** | 14.7 (6.4–33.9)***,c | (3647) |
OR = Odds ratio; HR = hazards ratio.
Different superscripts indicate statistically significant (P < 0.05) differences by generation and by race/ethnicity among Millennials.
Insufficient cases for analysis.
P < 0.05.
P < 0.01.
P < 0.001.
Compared to other generations, Millennials experienced an increase in those who, after marijuana, used NMPO before cocaine or only NMPO, and a sharp decrease of those who used only cocaine (Supplementary Table 2).
3.2.2. Heroin initiation after NMPO and cocaine
Marijuana users in every generation, especially Millennials, were much more likely to start using heroin after using both NMPO and cocaine (=18.8% vs. 12.1% Generation X, 11.5% Baby Boomers, P’s < 0.001) than cocaine alone (7.7%, 7.4%, 6.7%, respectively, P’s < 0.01) or especially NMPO alone (=2.5% versus 1.1% and 3.3%, respectively, P’s < 0.05) (Fig. 3, STable 3). The risk of heroin initiation after NMPO, irrespective of cocaine initiation, was higher among Millennials (hazards ratio [HR] = 7.1 [95% CI = 5.6–9.1]) than Generation X (HR = 3.4 [95% CI = 2.6–4.3]) and Baby Boomers (HR = 2.5 [95% CI = 1.6–4.0]) (Table 2-B). In all generations, the risk of heroin initiation after cocaine was much higher than the risk of heroin initiation after NMPO. However, the risk was similar among Millennials (HR = 16.6 [95% CI = 13.2–20.9]) and Generation X (HR = 17.5 [95% CI = 11.4–27.8]) but much lower among Baby Boomers (HR = 4.1 [95% CI = 2.7–6.2]) (Table 2-B; Supplementary Table 3).
Fig. 3.
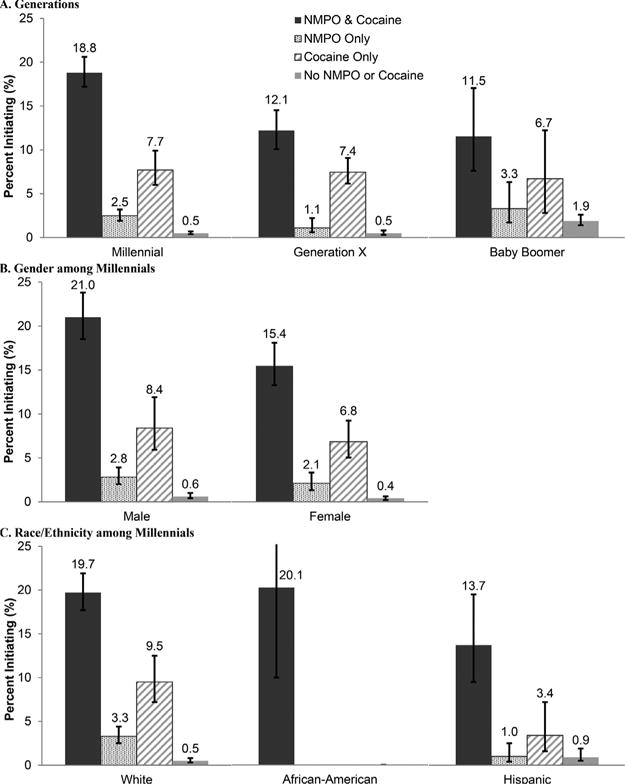
Rates of heroin initiation by four patterns of nonmedical prescription opioid (NMPO) or cocaine initiation after marijuana among marijuana usersa in three generations and among Millennials by gender and race/ethnicity: NSDUH 2013–2014, ages 18–64 (n = 37,459). aExcludes those who initiated NMPO, cocaine or heroin before marijuana (n = 1,450); includes those who initiated alcohol/cigarettes after marijuana. See also Supplementary Table 3.
3.3. Gender and racial/ethnic differences among Millennials
3.3.1. Lifetime use of five drug classes
Among Millennials, males had higher rates of lifetime use of each drug than females, as did whites compared to minorities (Supplementary Table 4). Marijuana use was higher among African-Americans than Hispanics; cocaine and heroin use was higher among Hispanics than African-Americans. Cocaine and heroin use was very low among African-Americans, precluding the identification of sequences involving these drugs.
3.3.2. Drug initiation sequences for five drug classes
Sequences were the same among males and females (Supplementary Table 5). More paths were statistically significant among whites than minorities (Fig. 4; Supplementary Table 5). Among all racial/ethnic groups, the most frequent sequence was alcohol/cigarettes to marijuana. The sequences from marijuana to NMPO or cocaine were more frequent among whites than minorities (NMPO: 7.3% whites, 5.4% African-Americans, 3.4% Hispanics; cocaine: 5.1%, 1.2%, 4.0%). Whites, and especially African-Americans, were more likely to use NMPO than cocaine after marijuana, while Hispanics were equally like to use either drug after marijuana (Table 2-A, Fig. 2-C). Whites and Hispanics progressed to heroin after both NMPO and cocaine in any order; whites also progressed to heroin directly after only cocaine (Fig. 4).
Fig. 4.
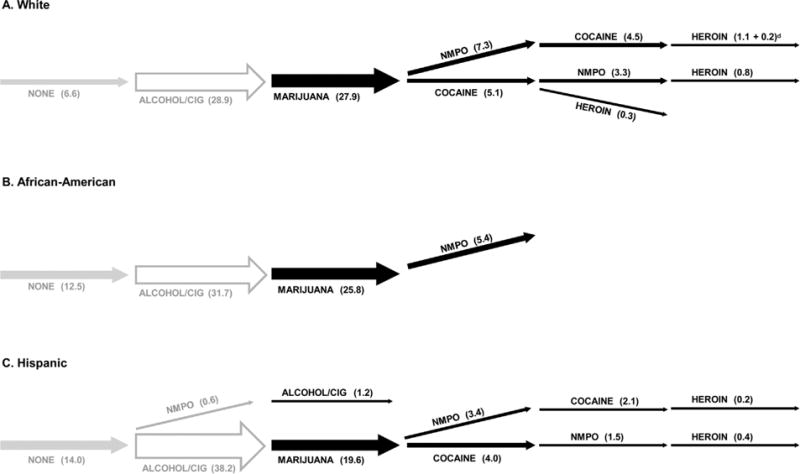
Significant drug initiation sequencesa among five drug classesb among Millennials by race/ethnicityc: NSDUH 2013-14, ages 18–34 (n = 40,134).
4. Discussion
Across three generations in the US, NMPO use fits within a developmental sequence of drug involvement that starts with alcohol or cigarettes, proceeds to marijuana, then to NMPO and/or cocaine, and finally to heroin. The combined rates of initiating either NMPO or cocaine among marijuana users do not vary across generations, whereas the sequential patterns between the two drugs do. Among Millennials, NMPO is more likely than cocaine to follow directly after marijuana, and the reverse occurs among Generation X and, especially, Baby Boomers. Heroin almost never follows directly marijuana; either NMPO or cocaine are initiated after marijuana. In each generation, the highest rates of heroin initiation follow use of both NMPO and cocaine. Both NMPO and cocaine use need to be taken into account in the progression to heroin use. A decreasing proportion initiate heroin next after cocaine, without NMPO, from Baby Boomers to Millennials. The mechanisms underlying the stronger impact of NMPO on subsequent heroin initiation when NMPO precedes rather than follows cocaine among Millennials remain to be elucidated. While increasing use of NMPO prior to heroin in more recent cohorts was previously noted (Jones, 2013; Novak et al., 2016; Martins et al., 2016), to the best of our knowledge, the importance of cocaine either prior to or next after NMPO in the progression to heroin has not previously been noted.
The increasingly higher risk of progression from NMPO to heroin from Baby Boomers and Generation X to Millennials may be partially accounted for by changing individual and environmental factors. Contributing factors may be recent increases in high-intensity NMPO use (Jones, 2013), abuse, and need for opioids combined with the greater availability and affordability of heroin (Cicero et al., 2014; Compton et al., 2016; NIDA, 2015). In additional analyses, we found that prevalence of high-intensity NMPO use among lifetime NMPO users, i.e., those using NMPO at least 200 times or meeting DSM-IV criteria for prescription opioid disorder within the past year (Han et al., 2015), was higher among Millennials (7.5%) than Generation X (5.2%) and Baby Boomers (4.3%). In every generation, high-intensity NMPO use was associated with the highest rates of heroin use. In 2013–14, among Millennials aged 18–34, 21.1% of past year high-intensity NMPO users used heroin compared with 4.6% of non-heavy, 1.0% of former, and 0.1% of never users. While the direction of the association between intensive NMPO use and heroin cannot be determined from cross-sectional data, Muhuri et al. (2013) and Compton et al. (2016), who analyzed restricted NSDUH data where new heroin users could be identified, found that NMPO dependence/abuse preceded heroin uptake. The increase in the progression from prescription opioids to heroin among Millennials compared to older generations may be due not only to increases in high intensity prescription opioid use but also to the greater availability and affordability of heroin. Baby Boomers, having easier access to medically prescribed opioids, would have less need to shift to heroin. However, for all generations, the progression from prescription opioids to heroin needs to take cocaine into account, since cocaine is an integral part of the progression from NMPO to heroin.
The racial/ethnic patterns reproduce differences that have historically been consistently observed. African-Americans are less likely than other groups to follow statistically significant progression patterns (Dean et al., 2014; Golub and Johnson, 2002; Sartor et al., 2013). The absence of significant progression to heroin among African-American Millennials is striking.
In parallel to increases in intensive NMPO use, medical practices and policy changes over the last twenty years, including the introduction of long-acting opioids such as OxyContin in 1995, changes in pain management, increases in opioid prescribing, and overprescribing have led to wider availability and abuse of prescription opioids and a resulting increase in heroin use (Kolodny et al., 2015; Novak et al., 2016; Paulozzi, 2012). Drug consumption is also influenced by the availability of different substances, drug exposure opportunities, and geographical residence (Anthony, 2012; CDC, 2017).
A limitation of the analyses is that they are based on retrospectively reported onset ages. Despite recall bias, analyses of sequential order depend on relative ages, which may be less prone to error than exact onset ages. We compared rates of self-reported lifetime use across the five drugs among the same Baby Boomer birth cohorts in 2013–14, at ages 50–65, and 2002–03, at ages 35–49. Recalled rates of drug initiation were very similar. Most importantly, the order between marijuana, NMPO, cocaine, and heroin were the same at both periods; percentages following specific pathways among marijuana users were also similar, although slightly lower in 2013–14 than in 2002–03 (Supplementary Table 6).
The generations concept is useful for summarizing broad differences across birth cohorts, although the delineation of boundaries between generations is imprecise. Such boundaries imply that generations are homogeneous. However, a linear trend among sequences within cohorts, which could test for homogeneity, could not be estimated because single birth cohorts cannot be identified due to age aggregation in the NSDUH public use data. Furthermore, we analyze and describe generations in the population at the same point in time and recognize that the different generations cover different ranges of the lifespan. While older generations have had longer periods of time in which to start experimenting with different drugs, only a small proportion of Generation X and Baby Boomers report initiating new drugs after age 34, the upper age of the Millennials. Hence, differences in prevalence between generations are not substantially driven by different lifespans, but instead by different exposures during periods of risk for drug initiation in adolescence and young adulthood.
The present findings add to the debate regarding the appropriateness of the Gateway and Common Liability perspectives regarding developmental pathways of involvement in drugs. We implemented a novel method that simulated drug initiation sequences that would occur by chance simply based on correlations between drugs, the common liability, and overall ages of onset without specific information about ordered relationships between drugs. A significant sequence from this method implies that it occurs more (or less) often than expected under the Common Liability Model and therefore reflects a gateway effect. Drugs at a lower stage increase the risk of using drugs at a higher stage. However, rather than being viewed as two opposed explanations of drug behaviors, the Gateway Hypothesis and Common Liability Model complement each other. The Common Liability Model explains the use of drugs in general due to individual psychosocial characteristics, family context, social conditions, and genetic factors, while the Gateway Hypothesis, as tested here, describes how individuals use specific drugs in a particular sequence and how use of one drug increases the risk of using another drug in the sequence, taking the common liability into account.
The delineation of drug sequences and, specifically, the position of NMPO and cocaine in these sequences in different generations, highlights the points in development at onset of different drugs when prevention and treatment efforts would be optimal. Reducing the prescribing of opioids is one strategy that has been advocated for reducing harms associated with NMPO use and its link to heroin (Dowell et al., 2016; Kolodny et al., 2015). In addition to NMPO, marijuana and especially cocaine should be particular targets of clinical and public health interventions and policy. Without antecedent or subsequent use of cocaine, NMPO use does not significantly lead to heroin, especially among Millennials. The finding is important, as the role of cocaine in addition to NMPO in accounting for the use of heroin has not been considered in discussions of the relationship between NMPO and heroin use (Compton et al., 2016; Jones, 2013). Furthermore, use of other drugs concomitant with NMPO explains a significant portion of the increase in prescription opioid-related overdose deaths in the US over the last decade; concomitant cocaine use accounts specifically for increases in non-methadone synthetic opioid deaths (e.g., illicit fentanyl) (Kandel et al., 2017). This finding parallels a recent report on the contribution of opioids, both heroin and non-methadone synthetic opioids, to recent increases in cocaine-related overdose deaths (Jones et al., 2017).
Because marijuana precedes NMPO, cocaine and heroin, it is also important for future research to examine how marijuana legalization in the US will impact the pathways that were identified.
Supplementary Material
Acknowledgments
We thank Mr. Benjamin Jenkins for his assistance in the preparation of the manuscript. We would also like to thank the reviewers of the manuscript for their constructive critiques and suggestions.
Role of funding
This research was supported by grant R01 DA036748 from the National Institute on Drug Abuse (D. Kandel, PI). Support was also provided by the New York State Psychiatric Institute (P. Griesler). The funding agency had no part in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.drugalcdep.2017.10.013.
Footnotes
Contributors
All the authors contributed to the design of the analysis, reviewed the analysis, and participated in the writing of the manuscript. All authors approved of the final version of the manuscript before submission.
Conflict of interest disclosures
The authors report no conflicts of interest.
References
- Anthony JC. Steppingstone and gateway ideas: a discussion of origins, research challenges, and promising lines of research for the future. Drug Alcohol Depend. 2012:S99–S104. doi: 10.1016/j.drugalcdep.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey JA, Hill KG, Meacham MC, Young SE, Hawkins JD. Strategies for characterizing complex phenotypes and environments: general and specific family environmental predictors of young adult tobacco dependence, alcohol use disorder, and co-occurring problems. Drug Alcohol Depend. 2011;118:444–451. doi: 10.1016/j.drugalcdep.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee G, Edelman EJ, Barry DT, Becker WC, Cerdá M, Crystal S, Gaither JR, Gordon AJ, Gordon KS, Kerns RD, Martins SS, Fiellin DA, Marshall BDL. Non-medical use of prescription opioids is associated with heroin initiation among US veterans: a prospective cohort study. Addiction. 2016;111:2021–2031. doi: 10.1111/add.13491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bluthenthal RN, Wenger L, Chu D, Bourgois P, Kral AH. Drug use generations and patterns of injection drug use Birth cohort differences among people who inject drugs in Los Angeles and San Francisco, California. Drug Alcohol Depend. 2017;175:210–218. doi: 10.1016/j.drugalcdep.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd CJ, Teter CJ, West BT, Morales M, McCabe SE. Non-medical use of prescription analgesics: a three-year national longitudinal study. J Addict Dis. 2009;28:232–242. doi: 10.1080/10550880903028452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Center for Behavioral Health Statistics and Quality [CBHSQ] Substance Abuse and Mental Services Administration (SAMHSA) Rockville, MD: 2015a. Results from the 2014 National Survey on Drug Use and Health: Detailed Tables. [Google Scholar]
- Center for Behavioral Health Statistics, Quality [CBHSQ] Substance Abuse and Mental Health Services Administration (SAMHSA) Center for Behavioral Health Statistics and Quality; Rockville, MD: 2015b. National Survey on Drug Use and Health: Methodological Summary and Definitions. [Google Scholar]
- Centers for Disease Control and Prevention (CDC) Annual Surveillance Report of Drug-Related Risks and Outcomes—United States. Department of Health and Human Services; Atlanta, GA: 2017. [Google Scholar]
- Catalano RF, White HR, Fleming CB, Haggerty KP. Is nonmedical prescription opiate use a unique form of illicit drug use? Addict Behav. 2011;36:79–86. doi: 10.1016/j.addbeh.2010.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerdá M, Santaella J, Marshall BDL, Kim JH, Martins SS. Nonmedical prescription opioid use in childhood and early adolescence predicts transitions to heroin use in young adulthood: a national study. J Pediatr. 2015;167(12):e1–2. doi: 10.1016/j.jpeds.2015.04.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicero TJ, Ellis MS, Surratt HL, Kurtz SP. The changing face of heroin use in the United States: a retrospective analysis of the past 50 years. JAMA Psychiatry. 2014;71:821–826. doi: 10.1001/jamapsychiatry.2014.366. [DOI] [PubMed] [Google Scholar]
- Cleveland HH, Wiebe RP. Understanding the association between adolescent marijuana use and later serious drug use: gateway effect or developmental trajectory. Dev Psychopathol. 2008;20:615–632. doi: 10.1017/S0954579408000308. [DOI] [PubMed] [Google Scholar]
- Compton WM, Jones CM, Baldwin GT. Relationship between nonmedical prescription-opioid use and heroin use. N Engl J Med. 2016;374:154–163. doi: 10.1056/NEJMra1508490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean DO, Cole V, Bauer DJ. Delineating prototypical patterns of substance use initiations over time. Addiction. 2014;110:585–594. doi: 10.1111/add.12816. [DOI] [PubMed] [Google Scholar]
- Degenhardt L, Chiu WT, Sampson N, Kessler RC, Anthony JC. Epidemiological patterns of drug use in the United States: evidence from the National Comorbidity Survey Replication, 2001–2003. Drug Alcohol Depend. 2007;90:210–223. doi: 10.1016/j.drugalcdep.2007.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degenhardt L, Dierker L, Chiu WT, Medina-Mora ME, Neymark Y, Sampson N, Alonso J, Angermeyer M, Anthony JC, Bruffaerts R, de Girolamo G, De Graaf R, Gureje O, Karam AN, Kostyuchenko S, Lee S, Lépine JP, Levinson D, Nakamura Y, Posada-Villa J, Stein D, Wells JE, Kessler RC. Evaluating the drug use gateway theory using cross-national data: consistency and associations of the order of initiation of drug use among participants in the WHO World Mental Health surveys. Drug Alcohol Depend. 2010;108:84–97. doi: 10.1016/j.drugalcdep.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain—United States, 2016. MMWR Recomm Rep. 2016;65:1–52. doi: 10.15585/mmwr.rr6501e1. [DOI] [PubMed] [Google Scholar]
- Fergusson DM, Boden JM, Horwood LJ. Cannabis use and other illicit drug use: testing the cannabis gateway hypothesis. Addiction. 2006;101:556–569. doi: 10.1111/j.1360-0443.2005.01322.x. [DOI] [PubMed] [Google Scholar]
- Fiellin LE, Tetrault JM, Becker WC, Fiellin DA, Hoff RA. Previous use of alcohol, cigarettes, and marijuana and subsequent abuse of prescription opioids in young adults. J Adolesc Health. 2013;52:158–163. doi: 10.1016/j.jadohealth.2012.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry R. Millennials Overtake Baby Boomers as America’s Largest Generation. Fact Tank: News in the Numbers. 2016 (Accessed 25.04.16) [Google Scholar]
- Golub A, Johnson BD. Variation in youthful risks of progression from alcohol and tobacco to marijuana and to hard drugs across generations. Am J Public Health. 2001;91:225–232. doi: 10.2105/ajph.91.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golub A, Johnson BD. In: Substance Use Progression and Hard Drug Use in Inner-city New York, in Stages and Pathways of Drug Involvement: Examining the Gateway Hypothesis. Kandel DB, editor. Cambridge University Press; Cambridge, UK: 2002. pp. 90–112. [Google Scholar]
- Griffin EA, Jr, Melas PA, Zhou R, Li Y, Mercado P, Kempadoo KA, Colnaghi L, Taylor K, Hu MC, Kandel ER, Kandel DB. Prior alcohol use enhances vulnerability to compulsive cocaine self-administration by promoting degradation of HDAC4 and HDAC5. Sci Adv. 2017 Nov;3(11):e1701682. doi: 10.1126/sciadv.1701682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han B, Compton WM, Jones CM, Cai R. Nonmedical prescription opioid use and use disorders among adults aged 18 through 64 years in the United States, 2003–2013. J Am Med Assoc. 2015;314:1468–1478. doi: 10.1001/jama.2015.11859. [DOI] [PubMed] [Google Scholar]
- Harrell ZAT, Broman CL. Racial/ethnic differences in correlates of prescription drug misuse among young adults. Drug Alcohol Depend. 2009;104:268–271. doi: 10.1016/j.drugalcdep.2009.05.017. [DOI] [PubMed] [Google Scholar]
- Holland BS, Copenhaver MD. Improved Bonferroni-type multiple testing procedures. Psychol Bull. 1988;104:145–149. [Google Scholar]
- Johnson RA, Gerstein DR. Initiation of use of alcohol, cigarettes, marijuana, cocaine, and other substances in US birth cohorts since 1919. Am J Public Health. 1998;88:27–33. doi: 10.2105/ajph.88.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones CM, Logan J, Gladden M, Bohm MK. Vital signs: demographic and substance use trends among heroin users –United States, 2002–2013. MMWR Morb Mortal Wkly Rep. 2015;64:719–725. [PMC free article] [PubMed] [Google Scholar]
- Jones CM, Baldwin GT, Compton WM. Recent increases in cocaine-related overdose deaths and the role of opioids. Am J Public Health. 2017;107:430–432. doi: 10.2105/AJPH.2016.303627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones CM. Heroin use and heroin use risk behaviors among nonmedical users of prescription opioid pain relievers–United States, 2002–2004 and 2008–2010. Drug Alcohol Depend. 2013;132:95–100. doi: 10.1016/j.drugalcdep.2013.01.007. [DOI] [PubMed] [Google Scholar]
- Kandel D, Kandel E. The Gateway Hypothesis of substance abuse: developmental, biological and societal perspectives. Acta Paediatr. 2014;104:130–137. doi: 10.1111/apa.12851. [DOI] [PubMed] [Google Scholar]
- Kandel DB, Yamaguchi K, Klein LC. Testing the gateway hypothesis. Addiction. 2006;101:470–472. doi: 10.1111/j.1360-0443.2006.01426.x. discussion 474–476. [DOI] [PubMed] [Google Scholar]
- Kandel D, Hu MC, Griesler PC, Wall MM. Increases from 2002 to 2015 in prescription opioid overdose deaths in combination with other substances. Drug Alcohol Depend. 2017;178:501–511. doi: 10.1016/j.drugalcdep.2017.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel DB. Stages and Pathways of Drug Involvement: Examining the Gateway Hypothesis. Cambridge University Press; Cambridge U.K: 2002. [Google Scholar]
- Keyes KM, Hamilton A, Kandel D. Smoking and subsequent marijuana and cocaine use. Am J Public Health. 2016;106:1143–1149. doi: 10.2105/AJPH.2016.303128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolodny A, Courtwright DT, Hwang CS, Kreiner P, Eadie JL, Clark TW, Alexander GC. The prescription opioid and heroin crisis: a public health approach to an epidemic of addiction. Annu Rev Public Health. 2015;36:25. doi: 10.1146/annurev-publhealth-031914-122957. (1–25.16) [DOI] [PubMed] [Google Scholar]
- Levine A, Huang Y, Drisaldi B, Griffin EA, Jr, Pollak DD, Xu S, Yin D, Schaffran C, Kandel DB, Kandel ER. Molecular mechanism for a gateway drug: epigenetic changes initiated by nicotine prime gene expression by cocaine. Sci Transl Med. 2011;3:107–109. doi: 10.1126/scitranslmed.3003062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynskey MT, Agrawal A, Henders A, Nelson EC, Madden PA, Martin NG. An Australian twin study of cannabis and other illicit drug use and misuse: and other psychopathology. Twin Res Hum Genet. 2012;15:631–641. doi: 10.1017/thg.2012.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins SS, Segura LE, Santaella-Tenorio J, Perimutter A, Fenton MC, Cerdá M, Keyes KM, Ghandour LA, Storr CL, Hasin DS. Prescription opioid use disorder and heroin use among 12–34 year-olds in the United States from 2002 to 2014. Addict Behav. 2016:236–241. doi: 10.1016/j.addbeh.2016.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe SE, West BT, Cranford JA, Ross-Durow P, Young AM, Teter CJ, Boyd CJ. Medical misuse of controlled medications among adolescents. Arch Pediatr Adolesc Med. 2011;165:729–735. doi: 10.1001/archpediatrics.2011.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miech R, Johnston L, O’Malley PM, Keyes KM, Heard K. Prescription opioids in adolescence and future opioid misuse. Pediatrics. 2015;136:e1169–1177. doi: 10.1542/peds.2015-1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhuri PK, Gfroerer JC, Davies C. Associations of nonmedical pain reliever use and initiation of heroin use in the United States. CBHSQ Data Rev. 2013 Aug;2013:1–17. [Google Scholar]
- National Institute on Drug Abuse (NIDA) Prescription Opioids and Heroin Research Report Series. NIH, U.S. Department of Health and Human Services (HHS); 2015. pp. 1–8. [Google Scholar]
- Novak SP, Bluthenthal R, Wenger L, Chu D, Kral AH. Initiation of heroin and prescription opioid pain relievers by birth cohort. Am J Public Health. 2016;106:298–300. doi: 10.2105/AJPH.2015.302972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer RH, Young SE, Hopfer CJ, Corley RP, Stallings MC, Crowley TJ, Hewitt JK. Developmental epidemiology of drug use and abuse in adolescence and young adulthood: evidence of generalized risk. Drug Alcohol Depend. 2009;102:78–87. doi: 10.1016/j.drugalcdep.2009.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulozzi LJ. Prescription drug overdoses: a review. J Saf Res. 2012;43:283–289. doi: 10.1016/j.jsr.2012.08.009. [DOI] [PubMed] [Google Scholar]
- Pew Research Center. The Whys and Hows of Generations Research. :2015. Accessed at: http://www.people-press.org/2015/09/03/the-whys-and-hows-of-generations-research.
- Research Triangle Institute (RTI) SUDAAN Language Manual, Version 11.0.1, 2012. 1 and 2. Research Triangle Institute; Research Triangle Park, NC.: 2012. [Google Scholar]
- Rebellon CJ, Van Gundy K. Can social psychological delinquency theory explain the link between marijuana and other illicit drug use? A longitudinal analysis of the gateway hypothesis. J Drug Issues. 2006;36:515–540. [Google Scholar]
- SAS Institute Inc. Statistical Procedures. Second. S.I Inc.; Cary, NC: 2013. Base SAS® 9.4 Procedures Guide. [Google Scholar]
- Sartor CE, Agrawal A, Lynskey MT, Duncan AE, Grant JD, Nelson EC, Madden PA, Heath AC, Bucholz KK. Cannabis or alcohol first? Differences by ethnicity and in risk for rapid progression to cannabis-related problems in women. Psychol Med. 2013;43:813–823. doi: 10.1017/S0033291712001493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanyukov MM, Tarter RE, Kirillova GP, Kirisci L, Reynolds MD, Kreek MJ, Conway KP, Maher BS, Iacono WG, Bierut L, Neale MC, Clark DB, Ridenour TA. Common liability to addiction and Gateway Hypothesis: theoretical, empirical and evolutionary perspective. Drug Alcohol Depend. 2012;123(Suppl. 1):S3–17. doi: 10.1016/j.drugalcdep.2011.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner FA, Anthony JC. Into the world of illegal drug use Exposure opportunity and other mechanisms linking the use of alcohol, tobacco, marijuana, and cocaine. Am J Epidemiol. 2002;155:918–925. doi: 10.1093/aje/155.10.918. [DOI] [PubMed] [Google Scholar]
- Wu LT, Pilowsky DJ, Patkar AA. Non-prescribed use of pain relievers among adolescents in the United States. Drug Alcohol Depend. 2008;94:1–11. doi: 10.1016/j.drugalcdep.2007.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young AM, Glover N, Havens JR. Nonmedical use of prescription medications among adolescents in the United States: a systematic review. J Adolesc Health. 2012;51:6–17. doi: 10.1016/j.jadohealth.2012.01.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


