Heart failure (HF) with preserved ejection fraction (HFPEF) is a common syndrome of increasing prevalence. The observation of the co-occurrence of fluid overload and overt HF in the presence of a normal left ventricular ejection fraction was made over three decades ago; yet, the pathophysiology behind HFPEF is incompletely understood. Currently, the incidence and the hospitalization rates of HF with reduced ejection fraction (HFREF) are declining in the Western world, partly due to better management of coronary artery disease (CAD) and its risk factors.1 In contrast, the prevalence of HFPEF is increasing, but it is unknown if a changing presentation of CAD may contribute to this trend.1 Indeed, individuals with HFPEF have a high burden of CAD (as assessed by autopsy and angiographic studies), and CAD may adversely affect the clinical course of HFPEF, but a causal role of CAD in HFPEF development is uncertain.2 We used the community-based sample of the Framingham Heart Study to test whether a genetic risk score (GRS) associated with CAD was also associated with HFREF versus HFPEF risk. We further investigated the prognostic importance of a CAD-related GRS in survival after HFPEF and HFREF.
We followed attendees from the Original and Offspring cohorts, who had blood drawn for DNA extraction, from the date of blood draw until the occurrence of HF, death or the end of follow-up (December 31, 2014). All participants gave their written informed consent before participating and the study was approved by the Boston University Medical Center Institutional Review Board. Genotyping was done on the Affymetrix Gene Chip 500K Array Set & 50K Human Gene Focused Panel (Affymetrix, Santa Clara, CA, USA) and only variants with call rate ≥97% and in Hardy–Weinberg equilibrium (p-value>10−6) were included. Genetic variants were imputed to the 1000 Genomes Project phase I release 3 panel by MACH version 1.0 and variants with imputation quality <0.3 were removed. CAD-related variants were identified from prior genome-wide and exome-wide association studies and we compiled a GRS for CAD based on 58 variants using a weighted additive allele risk score model (beta estimates were retrieved from a recent review).3
HF was defined as clinical HF requiring hospitalization. A systolic left ventricular ejection fraction ≥50% was used to distinguish HFPEF from HFREF. Cox regression models (adjusting for 10-year estimated risk of cardiovascular disease based on the Framingham risk score4 and familial relatedness) were used to estimate the hazards ratio associated with GRS for the risk of developing HF. Separate models were run for any HF, HFPEF, and HFREF, respectively. Tests for differences in hazards ratio estimates associated with GRS for HFPEF vs. HFREF were done using the Lunn-McNeil test.5 A two-sided p-value of <0.05 was considered statistically significant.
A total of 4390 participants (mean age 65 years, 55% women) were included. Mean values and distribution of GRS by heart failure development are presented in Figure 1A. We confirmed a statistically significant association of GRS with incident CAD in our sample (sex and age-adjusted hazards ratio 1.97 [95% confidence interval 1.51–2.58] per 1 unit GRS, p<0.0001). During follow-up (mean 12 years; limits 0.1–27 years), 472 participants (11%) developed HF (196 HFPEF and 212 HFREF; 64 HF cases were unclassified due to unknown ejection fraction). The GRS was associated with any HF and HFREF, but not with HFPEF (Figure 1B); p for equal hazards ratios HFREF vs. HFPEF = 0.0045. Additional adjustments for baseline and interim myocardial infarction and for the 10-year estimated risk of cardiovascular disease did not alter these associations (Figure 1B). Also, the differences in hazards ratios associated with GRS (for HFREF vs. HFPEF) remained statistically significant (p=0.04). The risk of developing HF associated with a high GRS burden was not modified by occurrence of either a baseline or an interim myocardial infarction or by a high 10-year estimated Framingham Risk Score (p for interactions >0.5).
Figure 1. Box plots of GRS values in individuals who did not and did develop heart failure and hazards ratios associated with GRS for heart failure development.
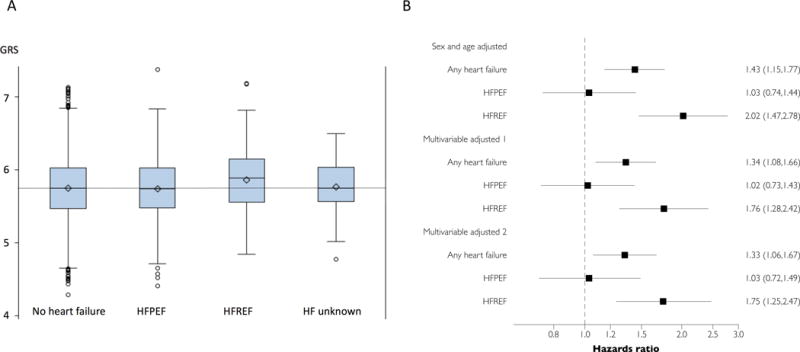
A: Box presents the median value (middle line), mean value (diamond) and first and third quartiles (lower and upper bounds of the box) of genetic risk score (GRS) values. The two error bars present the maximum and minimum values within the upper/lower fence (fences are placed 1.5 interquartile ranges [IQR] away from Q1 and Q3; IQR = Q3 - Q1), and the circles represent more extreme data points. B: Hazards ratios associated with 1 unit increase in GRS based on Cox regression models. Model 1 adjusted for age, sex and myocardial infarction (prevalent at baseline and interim); model 2 additionally adjusted for estimated 10-year risk of developing cardiovascular disease (Framingham Risk score). ‘HF unknown’ refers to heart failure with unknown ejection fraction.
The GRS was not significantly associated with post-HF mortality among any of the groups: hazards ratios 0.90 [0.71–1.14] for any HF [371 deaths], 0.79 [0.54–1.14] for HFPEF [175 deaths], and 0.87 [0.62–1.21] for HFREF [156 deaths].
In conclusion, a GRS for CAD was strongly associated with risk for incident HFREF but not HFPEF in our sample, suggesting that CAD may contribute differentially to the propensity for these two conditions. Because our GRS captured only a modest part of the variability in CAD risk, more studies are warranted to establish if CAD may be a causal factor for the development of HFPEF, or if it is simply a frequent concomitant in elderly people presenting with the condition. Additional investigations are also needed to understand if competing risks could, in part, explain the lack of association between the GRS and the risk of developing HFPEF (i.e., that people with several risk factors for coronary artery disease tend to develop HFREF before they can develop HFPEF), although we did not observe any effect modification by myocardial infarction or a high 10-year risk of developing cardiovascular disease. Other limitations to bear in mind when interpreting the data include the small study sample of predominantly white people of European ancestry and the definition of HF, which was based on the Framingham Study criteria (and only included hospitalized events).
Acknowledgments
We want to thank all the participants and staff of the Framingham Heart Study for their valuable contributions.
Sources of funding
The present work was founded by the National Heart, Lung and Blood Institute’s Framingham Heart Study (contracts N01HC25195 and HHSN268201500001I), and the National Institutes of Health grants R01HL093328 (RSV), R01HL107385 (RSV), and R01HL126136 (RSV). The funding sources had no influence on the present work.
Footnotes
Data sharing: Data can be accessed through dbGAP: https://www.ncbi.nlm.nih.gov/gap/ddb
Conflict of Interest Disclosures
None.
References
- 1.Vasan RS, Xanthakis V, Lyass A, Andersson C, Tsao C, Cheng S, Aragam J, Benjamin EJ, Larson MG. Epidemiology of Left Ventricular Systolic Dysfunction and Heart Failure in the Framingham Study: An Echocardiographic Study Over 3 Decades. JACC Cardiovasc Imaging. 2017 doi: 10.1016/j.jcmg.2017.08.007. online published ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hwang SJ, Melenovsky V, Borlaug BA. Implications of coronary artery disease in heart failure with preserved ejection fraction. J Am Coll Cardiol. 2014;63:2817–2827. doi: 10.1016/j.jacc.2014.03.034. [DOI] [PubMed] [Google Scholar]
- 3.Kessler T, Vilne B, Schunkert H. The impact of genome-wide association studies on the pathophysiology and therapy of cardiovascular disease. EMBO Mol Med. 2016;8:688–701. doi: 10.15252/emmm.201506174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.D’Agostino RB, Sr, Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM, Kannel WB. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117:743–753. doi: 10.1161/CIRCULATIONAHA.107.699579. [DOI] [PubMed] [Google Scholar]
- 5.Lunn M, McNeil D. Applying Cox regression to competing risks. Biometrics. 1995;51:524–532. [PubMed] [Google Scholar]


