Abstract
Objective
Single-cell RNA sequencing studies have revealed that the type-2 diabetes associated two-pore domain K+ (K2P) channel TALK-1 is abundantly expressed in somatostatin-secreting δ-cells. However, a physiological role for TALK-1 in δ-cells remains unknown. We previously determined that in β-cells, K+ flux through endoplasmic reticulum (ER)-localized TALK-1 channels enhances ER Ca2+ leak, modulating Ca2+ handling and insulin secretion. As glucose amplification of islet somatostatin release relies on Ca2+-induced Ca2+ release (CICR) from the δ-cell ER, we investigated whether TALK-1 modulates δ-cell Ca2+ handling and somatostatin secretion.
Methods
To define the functions of islet δ-cell TALK-1 channels, we generated control and TALK-1 channel-deficient (TALK-1 KO) mice expressing fluorescent reporters specifically in δ- and α-cells to facilitate cell type identification. Using immunofluorescence, patch clamp electrophysiology, Ca2+ imaging, and hormone secretion assays, we assessed how TALK-1 channel activity impacts δ- and α-cell function.
Results
TALK-1 channels are expressed in both mouse and human δ-cells, where they modulate glucose-stimulated changes in cytosolic Ca2+ and somatostatin secretion. Measurement of cytosolic Ca2+ levels in response to membrane potential depolarization revealed enhanced CICR in TALK-1 KO δ-cells that could be abolished by depleting ER Ca2+ with sarco/endoplasmic reticulum Ca2+ ATPase (SERCA) inhibitors. Consistent with elevated somatostatin inhibitory tone, we observed significantly reduced glucagon secretion and α-cell Ca2+ oscillations in TALK-1 KO islets, and found that blockade of α-cell somatostatin signaling with a somatostatin receptor 2 (SSTR2) antagonist restored glucagon secretion in TALK-1 KO islets.
Conclusions
These data indicate that TALK-1 reduces δ-cell cytosolic Ca2+ elevations and somatostatin release by limiting δ-cell CICR, modulating the intraislet paracrine signaling mechanisms that control glucagon secretion.
Keywords: Two-pore domain K+ channel, KCNK16, Paracrine, Islet, Endoplasmic reticulum, Hormone secretion
Highlights
-
•
The two-pore domain K+ channel TALK-1 is expressed in islet and gastric δ-cells.
-
•
TALK-1 channels set δ-cell ER Ca2+ stores, regulating CICR and somatostatin release.
-
•
TALK-1 control of δ-cell function tunes α-cell Ca2+ handling and glucagon secretion.
-
•
Glucose enhances δ-cell Ca2+ signals and coordinates δ-cell function with β-cells.
1. Introduction
Somatostatin is a potent inhibitory peptide, which regulates many physiological processes, such as hormone secretion, neurotransmission, gastric function, and cell proliferation. Somatostatin signals through plasma membrane Gαi-coupled receptors, suppressing cellular function through a number of mechanisms including inhibition of cAMP-dependent pathways and activation of inward-rectifier K+ channels. In most cases, secreted somatostatin acts locally to regulate the activity of surrounding cells [1]. This is exemplified by intraislet somatostatin release, which inhibits glucagon and insulin secretion [2], [3], [4]. Islet somatostatin secretion is increased by elevated blood glucose levels [5], providing a paracrine feedback mechanism to curtail excessive insulin and glucagon secretion and minimize large fluctuations in glycemia. Although the necessity of proper islet somatostatin secretion for glucose homeostasis is increasingly appreciated, the molecular mechanisms underlying δ-cell function remain poorly understood. Emerging data suggest that dysregulated islet somatostatin secretion contributes to hyperglycemia in type 1 and type 2 diabetes mellitus (T2DM), highlighting the importance of determining the molecular physiology of δ-cells [2], [6], [7], [8], [9].
Somatostatin secretion requires extracellular Ca2+ influx through voltage-dependent Ca2+ channels (VDCCs) [10], [11], [12]. VDCC opening is determined by the plasma membrane potential (Vm), which is largely controlled by the activity of hyperpolarizing K+ channels such as ATP-sensitive K+ (KATP) channels. Pharmacological or metabolic inhibition of KATP channels stimulates Ca2+ entry and somatostatin secretion [3], [9], [13]. However, δ-cells lacking functional KATP channels still exhibit glucose-stimulated somatostatin secretion (GSSS), indicating that other mechanisms in addition to KATP channels contribute to GSSS [11]. Previous studies demonstrate that GSSS also relies upon paracrine stimulation by β-cells and Ca2+-induced Ca2+ release (CICR) from the δ-cell ER [9], [11]. CICR is sensitive to both cytoplasmic Ca2+ and total ER Ca2+ content [14], [15], [16]. Glucose metabolism accelerates δ-cell sarco/endoplasmic reticulum Ca2+-ATPase (SERCA) activity, resulting in elevated ER Ca2+ content, which enhances CICR [11]. Pharmacological inhibition of SERCA activity and subsequent depletion of ER Ca2+ significantly reduces somatostatin secretion, indicating that δ-cell ER Ca2+ stores serve a critical role in GSSS [11], [17]. However, the mechanisms which govern δ-cell Ca2+ influx, ER Ca2+ stores, and CICR under different glucose conditions are largely unknown.
Recent studies demonstrate that the two-pore domain K+ (K2P) channel TALK-1 (encoded by the KCNK16 gene) is abundantly expressed in δ-cells of the islet and gastric epithelium [18], [19], [20]. Although the function of δ-cell TALK-1 channels remains unknown, β-cell TALK-1 channels control Ca2+ influx and insulin secretion by modulating electrical activity and ER Ca2+ homeostasis [21]. TALK-1 regulates β-cell ER Ca2+ handling by conducting K+ countercurrents across the ER membrane which promote ER Ca2+ leak; inhibiting TALK-1 channel activity augments ER Ca2+ stores [22]. TALK-1 channels are also implicated in T2DM pathogenesis through a non-synonymous polymorphism (rs1535500, encoding TALK-1 A277E) which causes a gain-of-function in TALK-1 channel activity [21]. The rs1535500 polymorphism is associated with impaired insulin secretion in T2DM patients and increased T2DM susceptibility [23], [24], [25], [26], [27]. These data, along with the prominent expression of TALK-1 channels in the islet, suggest that defects in δ-cell function induced by TALK-1 A277E may contribute to islet dysfunction and exacerbate hyperglycemia in patients with T2DM.
Given the role TALK-1 channels serve in regulating ER Ca2+ stores, prominent expression of TALK-1 mRNA in δ-cells, and sensitivity of CICR to ER Ca2+ levels, we investigated whether TALK-1 channels modulate δ-cell Ca2+ handling and somatostatin secretion. We found that TALK-1 forms functional channels in mouse and human δ-cells, where it limits CICR and somatostatin secretion. CICR and ER Ca2+ stores are enhanced in δ-cells lacking TALK-1 channels, leading to increased somatostatin secretion and reduced glucagon secretion. These findings highlight the physiological importance of TALK-1 channel modulation of δ-cell homeostasis in regulating islet somatostatin signaling, and contribute to an improved understanding of the molecular mechanisms underlying GSSS.
2. Materials and methods
2.1. Chemicals
All chemicals were purchased from Sigma–Aldrich (St. Louis, MO) unless specified otherwise.
2.2. Biological materials and study approval
The mice used in this study were 10–15 week-old males on a C57Bl6/J background. Mice were housed in a 12-hour light/dark cycle with access to standard chow (Lab Diets, 5L0D) ad libitum. Wild-type (WT) and TALK-1 KO transgenic mice expressing tdRFP specifically in somatostatin-positive cells (RFP δ-cells) were generated by crossing mice expressing Sst-IRES-Cre [28] with mice expressing a tdRFP fluorescent reporter preceded by a loxP-flanked STOP cassette [29]. Transgenic mice expressing GCaMP6s specifically in somatostatin-positive cells were generated by crossing WT and TALK-1 KO mice expressing Sst-IRES-Cre with those possessing the genetically encoded Ca2+ indicator GCaMP6s preceded by a loxP-flanked STOP cassette [36]. Mouse islets were isolated by digesting the pancreas with collagenase P (Roche) and performing density gradient centrifugation as previously described [30]. We obtained human islets from adult non-diabetic donors from multiple isolation centers organized by the Integrated Islet Distribution Program (human islet donor characteristics are provided in Table S1). Mouse and human islets were cultured in RPMI 1640 (Gibco) containing 15% fetal bovine serum (FBS), 100 IU∙ml−1 penicillin, and 100 mg ml−1 streptomycin, in an incubator maintained at 37 °C, 5% CO2. Although the concentration of glucose in RPMI 1640 (11 mM) is higher than typical resting glucose in humans (5.6 mM), human cells are more amenable to patch clamp recording when cultured under these conditions. Moreover, we do not observe significant differences in δ-cell electrical excitability when cultured with high (11 mM) and low (1 mM) glucose. Islets and cells were seeded to poly-d-lysine-coated 35 mm glass-bottom dishes (CellVis). In experiments using single islet cells, islets were triturated in 0.0075% trypsin-EDTA prior to plating. Dispersed cells were cultured for approximately 6 h in 100 μL of medium prior to being refed with 2 mL of fresh medium. In human δ-cell experiments, cells were transfected with TALK-1 DN- or mCherry-expressing plasmids and identified by post-staining for somatostatin as previously described [21]. Briefly, cells were transfected for 18 h using 1 μL Lipofectamine 3000 and 1 μL P3000 (Thermo Fisher) with 400 ng DNA in a total volume of 100 μL of the transfection mixture. After 18 h the cells were re-fed with fresh media. For Ca2+ imaging experiments using primary human δ-cells, cells were infected 48 prior to imaging with Psst-mCherry adenovirus to facilitate identification of δ-cells [31]. All mouse procedures performed in this study were done in compliance with protocols reviewed and approved by the Vanderbilt University Institutional Animal Care and Use Committee, according to guidelines set forth by the National Institutes of Health.
2.3. Immunofluorescence
Paraffin-embedded mouse and human pancreas sections were processed and stained as previously described (human donor information is provided in Table S2) [21]. Sections were stained using primary antibodies against somatostatin (Santa Cruz Biotechnology sc-7819: 1:250), TALK-1 (Novus Biologicals #NBP1-83071; 1:175) or TALK-1a (Antibody Verify AAS72353C; 1:250), glucagon (Proteintech #15954-I-AP: 1:500), and the endoplasmic reticulum marker GRP94 (Novus Biologicals #NB300-619; 1:100); secondary antibodies used were Alexa Fluor 488-conjugated donkey anti-rabbit (Jackson Immunoresearch #711-546-152; 1:300), DyLight 650-conjugated donkey anti-goat (Thermo Fisher #SA5-10089; 1:250), and Cy3-conjugated donkey anti-mouse (Jackson Immunoresearch #715-166-150; 1:500).
2.4. Calcium imaging
Ca2+ imaging was performed as previously described using epifluorescent or confocal miscroscopy with Ca2+ dyes (Fura-2 AM, Cal520-AM, or Cal590-AM) or genetic indicators (GCaMP3 or GCaMP6s) [22]. Further details on the protocols used to measure can be found in the supplemental data.
2.5. Cross-correlation analysis
The glucose-mediated synchronization of islet β- and δ-cell Ca2+ oscillations was calculated using the cross-correlation function of Clampfit 10. Changes in the GCaMP6s signal of individual δ-cells within an islet were correlated to Cal590-AM signal in the same islet at 1 mM glucose, 11 mM glucose, and 11 mM glucose with 50 μM CPA. As Cal590-AM was utilized as an indicator of β-cell , areas displaying GCaMP6s fluorescence were excluded from analysis. Correlation coefficients for β-cell/δ-cell synchronization were determined as a function of lag time between Cal590-AM and δ-cell GCaMP6s signals [32].
2.6. Patch clamp electrophysiology
An Axopatch 200B amplifier (Molecular Devices) was used to measure whole-cell K+ channel currents in the voltage-clamp mode; currents were digitized using a Digidata 1440, lowpass filtered at 1 kHz and sampled at 10 kHz. During recording, samples were perfused with KRB without CaCl2 and supplemented with (in mM): 0.2 tolbutamide (MP Biomedicals), 10 tetraethylammonium chloride hydrate (TEA, Thermo Fisher Scientific), 1 EGTA, pH 7.35 with NaOH, and 11 mM glucose. Patch electrodes were pulled to a resistance of 3–4 MΩ and filled with an intracellular solution containing (in mM): 140 KCl, 1 MgCl2, 10 EGTA, 10 HEPES, and 3 Mg-ATP, pH 7.22 with KOH. For Vm recordings, pipettes were filled with an intracellular solution containing (in mM) 28.4 K2SO4, 63.7 KCl, 11.8 NaCl, 1 MgCl2, 20.8 HEPES, 0.5 EGTA (pH 7.22 with KOH) and ∼0.05 mg ml−1 amphotericin B. δ-cells were continuously perfused with KRB solutions at 35–36 °C. VDCC currents were recorded from dispersed mouse δ-cells as previously described [33]. Patch electrodes were pulled to a resistance of 3–4 MΩ when filled with an intracellular solution containing (in mM): 120 CsCl, 10 TEA, 1 MgCl2, 3 EGTA, 10 HEPES, 3 MgATP, pH 7.22 with CsOH. Cells were patched in KRB solution supplemented with 11 mM glucose; upon obtaining the whole-cell configuration with a seal resistance >1 GΩ, the bath solution was exchanged for a buffer containing (in mM): 82 N-methyl-d-glucamine, 20 TEA, 30 CaCl2, 1 MgCl2, 5 CsCl, 10 HEPES, 11 glucose, 0.2 tolbutamide, pH 7.35 with HCl, osmolarity adjusted with sucrose. δ-cells were perfused for 3 min with this solution prior to initiating the VDCC recording protocol. Voltage steps of 10 mV were applied from a holding potential of −80 mV; linear leak currents were subtracted online using a P/4 protocol. Data were analyzed using Clampfit 10 and Microsoft Excel.
2.7. Hormone secretion
For all experiments, islets were allowed to recover for 24 h following isolation in RPMI 1640 supplemented with 15% FBS and 11 mM glucose. Glucagon secretion was then determined by radioimmunoassay from perifused islets stimulated with 1 and 11 mM glucose (Vanderbilt Islet Procurement & Analysis Core; supported by the Vanderbilt Diabetes Research and Training Center; P60 DK020593) [21]. Glucagon and somatostatin secretion measurements from static incubations were performed as previously described [34]. Somatostatin was measured using a fluorescent EIA kit according to the manufacturer's instructions (Phoenix Pharmaceuticals #FEK-060-03).
2.8. Statistical analysis
The data are shown as recordings that are averaged or representative of results obtained from at least three independent cultures. The values presented are the mean ± SE. Statistical differences between means were assessed using two-tailed unpaired Student's t-test unless stated otherwise. A P < 0.05 was considered as significant.
3. Results
3.1. TALK-1 channels are expressed in δ-cells
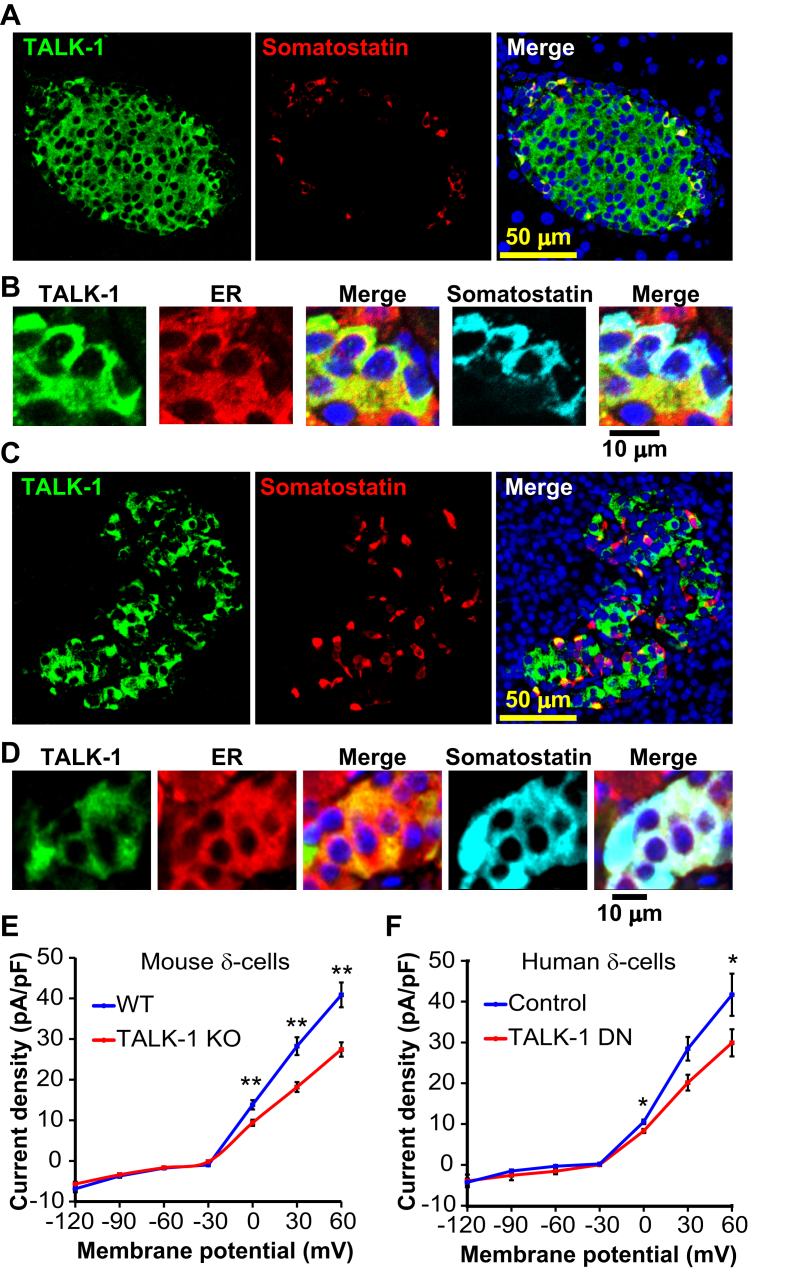
We first sought to determine whether functional TALK-1 channels are expressed in the ER of δ-cells. Somatostatin-positive cells from mouse and human pancreas sections displayed strong intracellular localization of TALK-1, which resembled the staining pattern for an ER marker (GRP94). This suggests that, as with β-cells, TALK-1 may serve a role in the ER of islet δ-cells (Figure 1A–D) [22]. As TALK-1 expression has also been reported in gastric δ-cells [19], we stained paraffin embedded sections of human duodenum and stomach for TALK-1 and somatostatin. In agreement with transcriptome analyses, TALK-1 was detectable in gastric somatostatin-positive cells (Figs. S1A,B). We next went on to test whether TALK-1 forms functional K+ channels in mouse islet δ-cells using TALK-1 deficient mice (TALK-1 KO) [21]. We dispersed these islet cell preparations and recorded whole-cell K2P currents from single RFP δ-cells, which were significantly reduced in δ-cells from TALK-1 KO mice when compared to WT (Figure 1E). To assess whether TALK-1 also forms a functional channel in human δ-cells, we expressed a TALK-1 dominant-negative (DN) mutant to inhibit endogenous TALK-1 channel activity [21]. The TALK-1 DN relies on a mutation (G110E) in the K+ selectivity filter that abolishes K+ conductance upon interaction with wild-type TALK-1; the TALK-1 DN construct also possesses an mCherry fluorescent reporter separated from TALK-1 DN by a P2A sequence, facilitating identification of transfected cells [21]. Expression of the TALK-1 DN in single human δ-cells (confirmed by post-staining for somatostatin) resulted in inhibition of whole-cell K2P currents (Figure 1F). These observations indicate that TALK-1 forms K+ channels in δ-cells.
Figure 1.
TALK-1 channels are expressed in mouse and human δ-cells. (A) Mouse pancreas section stained for TALK-1 (green) and somatostatin (red) (representative of N = 3 mice). (B) Mouse pancreas section stained for TALK-1 (green), ER (GRP94, red), and SST (cyan). (C) Human pancreas section stained for TALK-1 (green) and somatostatin (red) (representative of N = 3 pancreata). (D) Human pancreas section stained for TALK-1 (green), ER (GRP94, red), and somatostatin (E) K2P currents recorded from WT and TALK-1 KO δ-cells (N = 3 mice per genotype). (F) K2P currents recorded from human δ-cells expressing TALK-1 DN or control mCherry. (N = 3 islet preparations); *P < 0.05, **P < 0.005.
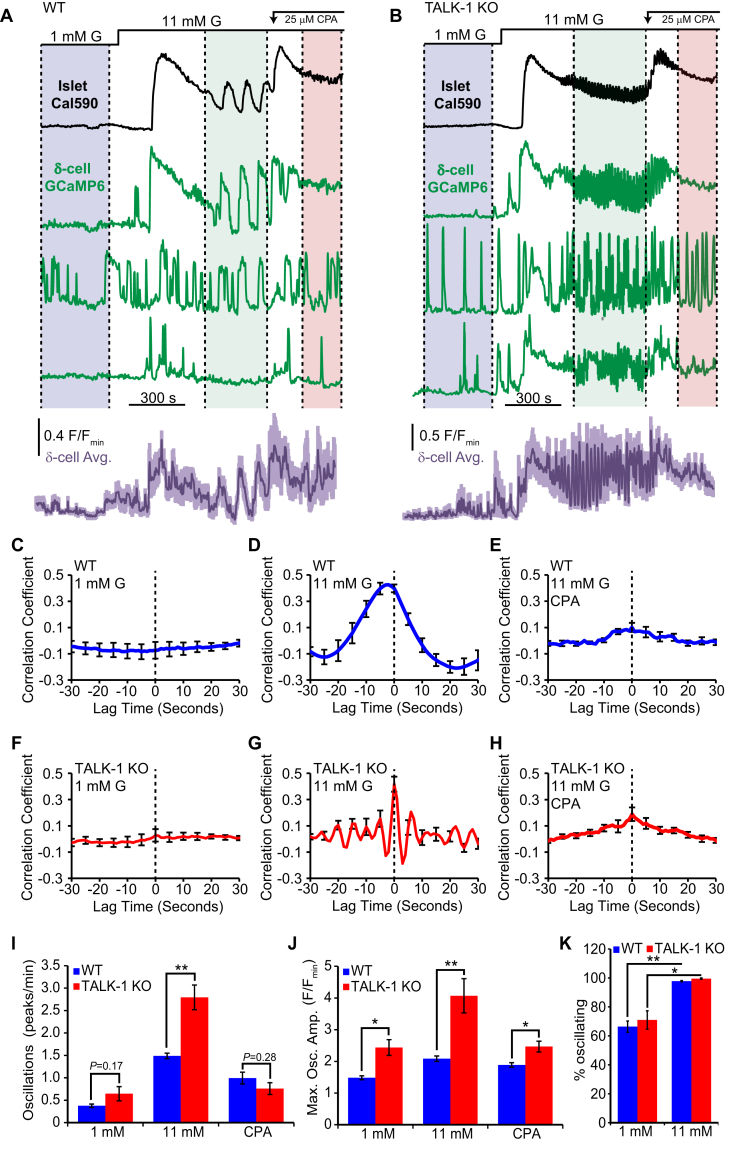
Physiological δ-cell function depends upon the islet microenvironment which enables paracrine feedback mechanisms between β-, α-, and δ-cells [9], [35], [36]. Therefore, we also examined how TALK-1 channel activity impacts δ-cell cytosolic Ca2+ () homeostasis in intact islets. This was accomplished by crossing our TALK-1 KO/Sst-IRES-Cre mice with those possessing the genetically encoded Ca2+ indicator GCaMP6s preceded by a loxP-flanked STOP cassette [37], generating islets with GCaMP6s expressed specifically in δ-cells. As δ-cell has been reported to oscillate in synchrony with β-cells [31], we loaded GCaMP6s-expressing islets with the red synthetic Ca2+ indicator Cal590-AM to examine the relationship between β- and δ-cell in whole islets. As approximately 85% of islet cells are β-cells, total islet Cal590-AM signal excluding GCaMP6s positive δ-cells was monitored as an indicator of β-cell response [38]. In low (1 mM) glucose there was no coordination between β- and δ-cell oscillations as 66.4 ± 3.9% WT and 70.0 ± 6.4% TALK-1 KO δ-cells exhibited oscillations (Figure 2A–C,F, and K), whereas β-cells remained quiescent. Under high (11 mM) glucose, nearly all δ-cells responded with increased that correlated with β-cell oscillations (Figure 2D,G). In TALK-1 KO islets, islet oscillation frequency is significantly accelerated [21], and this phenotype was also observed in TALK-1 KO δ-cells in intact islets (Figure 2I). We also found that intact islet TALK-1 KO δ-cells produced larger amplitude oscillations compared to WT δ-cells under both low and high glucose conditions (Figure 2J). In WT and TALK-1 KO δ-cells, glucose increased the amplitude of oscillations, consistent with observations that glucose metabolism enhances δ-cell CICR [11]. The height of the oscillations in elevated glucose was reduced to the amplitude observed in low glucose by depleting ER Ca2+ with the SERCA inhibitor CPA (Figure 2J) [22], [39], [40]. Interestingly, following the addition of CPA oscillations ceased in β-cells but continued in a sub-population of δ-cells indicating a decrease in glucose-induced coupling between β- and δ-cells (Figure 2E,H). This suggests that ER Ca2+ handling may be involved in this relationship.
Figure 2.
Glucose synchronizes WT and TALK-1 KO δ-cellwith β-cells. (A) Recording of from a WT islet expressing GCaMP6s in δ-cells and loaded with the red Ca2+ dye Cal590-AM (black: representative WT islet Cal590-AM signal excluding GCaMP6s-positive δ-cells, green: representative WT δ-cell GCaMP6s signals, purple: average WT δ-cell GCaMP6s signal; lines above indicate glucose concentrations; colored regions delineate periods used for cross-correlation analysis). (B) Recording of from a TALK-1 KO islet expressing GCaMP6s in δ-cells and loaded with the red Ca2+ dye Cal590-AM (black: representative TALK-1 KO islet Cal590-AM signal excluding GCaMP6s-positive δ-cells, green: representative TALK-1 KO δ-cell GCaMP6s signals, and purple: average TALK-1 KO δ-cell GCaMP6s signal). (C) Average correlation between WT β- and δ-cells at 1 mM glucose (N = 13 islets). (D) Average correlation between WT β- and δ-cells at 11 mM glucose (N = 13 islets). (E) Average correlation between WT β- and δ-cells at 11 mM glucose + 50 μM CPA (N = 13 islets). (F) Average correlation between TALK-1 KO β- and δ-cells at 1 mM glucose (N = 12 islets). (G) Average correlation between TALK-1 KO β- and δ-cells at 11 mM glucose (N = 12 islets). (H) Average correlation between TALK-1 KO β- and δ-cells at 11 mM glucose + 50 μM CPA (N = 12 islets). (I). Average δ-cell Ca2+ oscillation frequency in WT and TALK-1 KO δ-cells measured under the indicated conditions (N = 3 mice per genotype). (J) Average maximum GCaMP6s fluorescence amplitude in WT and TALK-1 KO δ-cells measured under the indicated conditions (N = 3 mice per genotype). (K) Comparison of percent of δ-cells exhibiting Ca2+ oscillations under indicated glucose conditions (N = 3 mice per genotype); *P < 0.05; **P < 0.005.
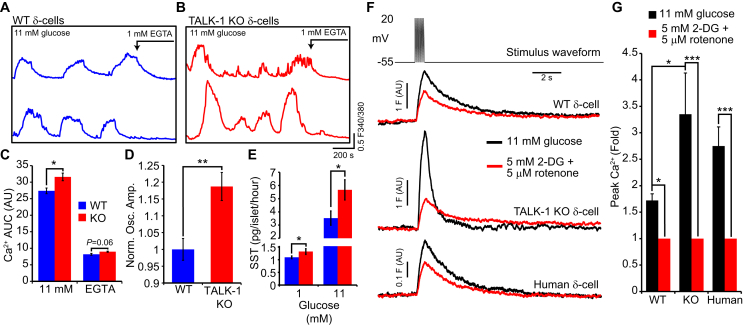
We next investigated whether the absence of TALK-1 channels altered handling in single δ-cells. Using dispersed islet-cells, we monitored single δ-cell under stimulatory (11 mM) glucose conditions in WT and TALK-1 KO δ-cells (Figure 3A,B). Under these conditions, both WT and TALK-1 KO δ-cells exhibited oscillations in . Area under the Ca2+ curve (AUC) analysis between 0 and 800 s revealed that under these conditions the change in was significantly greater in TALK-1 KO δ-cells as compared to WT δ-cells (Figure 3C); a difference which was abolished by removal of extracellular Ca2+ (AUC was analyzed between 1300 and 1500 s). The amplitude of δ-cell Ca2+ transients was also significantly greater in TALK-1 KO islets (Figure 2J) and in single TALK-1 KO δ-cells (Figure 3D) than in corresponding WT islets and WT δ-cells. As an elevation is critical for somatostatin secretion, we went on to measure somatostatin release from WT and TALK-1 KO islets. Compared to WT islets, somatostatin secretion was increased by approximately 20% under basal (1 mM glucose) conditions and enhanced by over 60% under stimulatory (11 mM glucose) conditions from islets lacking TALK-1 (Figure 3E).
Figure 3.
TALK-1 limits changes in δ-celland somatostatin secretion. (A) recorded in single Fura-2-loaded WT δ-cells perfused with the indicated treatments (representative of N = 3 mice per genotype). (B) recorded in single Fura-2-loaded TALK-1 KO δ-cells perfused with the indicated treatments (representative of N = 3 mice per genotype). (C) Increase in (area under the curve, AUC) in WT and TALK-1 KO δ-cells (AUC with 11 mM glucose was quantified between 0 and 800 s and AUC with EGTA and no extracellular Ca2+ was quantified between 1300 and 1500 s; N = 3 mice per genotype). (D) Maximum Ca2+c oscillation amplitude (F/Fmin) measured in WT and TALK-1 KO δ-cells (N = 6 mice per genotype). (E) Somatostatin secretion from WT and TALK-1 KO islets at 1 and 11 mM glucose (N = 4–7 islet preparations per genotype). (F) Representative transients in WT, TALK-1 KO, and human δ-cells subjected to depolarizing pulses in the presence of 11 mM glucose or 5 mM 2-deoxyglucose and 5 μM rotenone. The fold increase in δ-cell Ca2+ in response to increasingly is quantified in (G) (N = 12 cells (WT); 12 cells (TALK-1 KO); 7 cells (human)). *P < 0.05, **P < 0.005, ***P < 0.0005; ANOVA followed by Tukey's multiple comparison test.
GSSS relies on glucose metabolism, and inhibition of glucose metabolism significantly reduces depolarization-induced somatostatin secretion [11]. To assess how glucose metabolism contributes to the amplification of δ-cell transients, we measured depolarization-induced changes in patch-clamped single δ-cells. Application of 2-deoxyglucose, which inhibits glycolysis, and rotenone, which limits mitochondrial ATP synthesis, reduced the increase in following depolarization in control, TALK-1 KO, and human δ-cells (Figure 3F,G). Additionally, the reduction in transient amplitude caused by inhibition of glucose metabolism was significantly greater in TALK-1 KO δ-cells when compared to controls, suggesting that TALK-1 channels limit elevations in δ-cell . Interestingly, δ-cell returned to baseline more rapidly following depolarization in TALK-1 KO compared to WT δ-cells. This may be due to enhanced CICR in TALK-1 KO δ-cells, which would be expected to lead to a greater drop in ER Ca2+ in TALK-1 KO compared to WT δ-cells. As SERCAs are activated by depletion of ER Ca2+ [41], [42], SERCA activity would presumably be higher in TALK-1 KO δ-cells resulting in faster removal of Ca2+ from the cytoplasm. Taken together, these observations indicate that δ-cell TALK-1 channels regulate δ-cell , demonstrate that there is significant crosstalk between β-cells and δ-cells under glucose conditions which stimulate β-cell Ca2+ influx, and confirm that glucose metabolism exerts an amplifying effect on δ-cell .
3.2. TALK-1 KO δ-cells are modestly depolarized
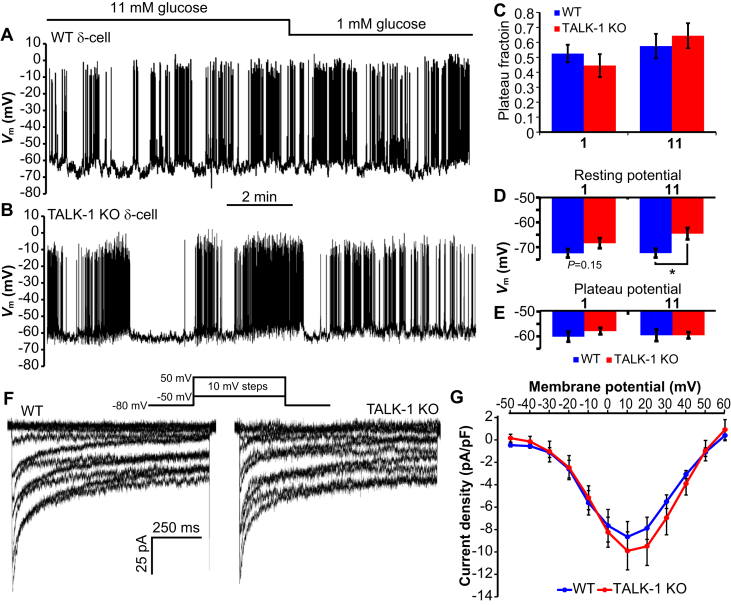
As TALK-1 produces detectable K+ currents at the plasma membrane in δ-cells, we next investigated electrical activity in TALK-1 KO δ-cells. Islets were dispersed into clusters of cells to ensure accurate positioning of the recording pipette on RFP δ-cells. In both WT and TALK-1 KO δ-cells, we found that the Vm was largely insensitive to changes in extracellular glucose (Figure 4A–E), with action potential firing in low (1 mM) glucose. Similarly, we found no significant difference in the plateau fraction (the ratio of time spent in electrically excitable periods divided by the total time examined) comparing either the effects of glucose or genotype (Figure 4C). TALK-1 KO δ-cells were slightly depolarized in high glucose during electrically silent periods (Figure 4D), but we detected no difference in the plateau Vm from which action potentials fire (Figure 4E). There were also no appreciable differences in the pattern or frequency of action potentials in TALK-1 KO δ-cells when compared to WT δ-cells; however, action potential upstroke and afterhyperpolarization (AHP) had larger amplitudes in KO δ-cells (Table 1). We also measured VDCC currents to assess whether changes in Ca2+ channel function could account for the greater change in in TALK-1 KO δ-cells. However, we found no difference in VDCC currents (Figure 4F,G), indicating that alterations in VDCCs per se are unlikely a major source of increased in TALK-1 KO δ-cells. Thus, we went on to assess whether the increased observed in TALK-1 KO δ-cells was due to altered intracellular Ca2+ handling.
Figure 4.
Electrical activity and VDCC currents in WT and TALK-1 KO δ-cells. (A) Vm recording from a WT δ-cell treated with indicated glucose concentrations (representative of recordings obtained from N = 12 cells/4 mice per genotype). (B) Vm recording from a TALK-1 KO δ-cell treated with indicated glucose concentrations (representative of recordings obtained from N = 12 cells/4 mice per genotype). (C) Quantification of plateau fraction in WT and TALK-1 KO δ-cells (N = 12 cells/4 mice per genotype). (D and E) Average Vm in WT and TALK-1 δ-cells at 1 and 11 mM glucose. (N = 12 cells/4 mice per genotype). (F) VDCC currents recorded from WT (representative of N = 9 cells/3 mice per genotype) and TALK-1 KO (representative of N = 8 cells/3 mice per genotype) δ-cells. VDCC current densities are quantified in (G); *P < 0.05.
Table 1.
Action potential characteristics in WT and TALK-1 KO δ-cells. Action potential parameters were determined over a period of 60 s in the second oscillation of electrical activity in δ-cells treated with 11 mM glucose and are reported relative to the plateau Vm (N = 12 cells/3 mice per genotype).
| Parameter | WT | TALK-1 KO | P value |
|---|---|---|---|
| Peak amplitude (mV) | 40.8 ± 1.3 | 51.0 ± 2.7 | 0.002 |
| AHP amplitude (mV) | −5.3 ± 0.7 | −6.9 ± 0.4 | 0.05 |
| Event frequency (Hz) | 1.5 ± 0.2 | 1.7 ± 0.2 | 0.36 |
3.3. CICR is enhanced in TALK-1 KO δ-cells
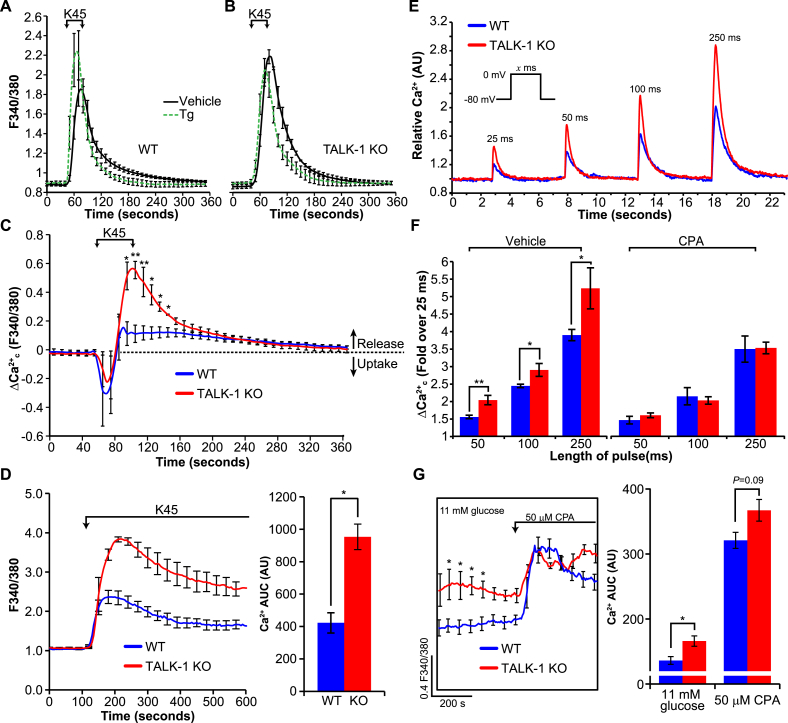
One of the mechanisms proposed to underlie the glucose-induced increase in somatostatin secretion is enhanced CICR [11]. As TALK-1 channels modulate ER Ca2+ content, we examined whether elevated in TALK-1 KO δ-cells was a consequence of increased CICR. First, we determined whether ER Ca2+ stores were increased in δ-cells lacking TALK-1 channels by measuring CPA-induced ER Ca2+ release. Based on δ-cell current clamp Vm recordings, the impact of TALK-1 at the plasma membrane on ER Ca2+ levels was predicted to be minimal; however, to eliminate this possibility Ca2+ influx was clamped with high K+ and diazoxide prior to removal of Ca2+ from the recording solution. Similar to our observations in TALK-1 KO β-cells [22], ER Ca2+ stores were higher in TALK-1 KO δ-cells (WT: 22.9 ± 4.5 AUC and TALK-1 KO: 35.7 ± 4.4 AUC, P < 0.005, Figs. S2A,B). The CPA-induced rate of ER Ca2+ release was also accelerated in TALK-1 KO compared to WT δ-cells (Fig. S2C). To further examine δ-cell Ca2+ handling independent of glucose-induced changes in the Vm, we treated single WT and TALK-1 KO δ-cells with a solution containing 11 mM glucose to energize SERCAs and the KATP channel activator diazoxide to suppress electrical excitability. Under these conditions oscillations were mostly abolished, but a 40-second depolarization with high (45 mM) K+ elicited a rapid increase in that slowly returned to basal levels upon removal of the depolarizing K+ stimulus (Figure 5A,B). To investigate the contribution of the ER to changes in under these conditions, we repeated this experiment in δ-cells pre-treated with the SERCA inhibitor thapsigargin (Figure 5A,B). Subtraction of the signal obtained from thapsigargin-treated cells from the signal of vehicle-treated cells revealed that depolarization induces a transient uptake of by the ER followed by ER Ca2+ release (Figure 5C), similar to observations in β-cells [43]. In δ-cells, ER Ca2+ release preceded the end of the high K+ pulse and was substantially greater in TALK-1 KO compared to WT δ-cells (Figure 5C). Similarly, we observed a larger rise in in TALK-1 KO δ-cells compared to WT δ-cells when KATP was activated with diazoxide and Ca2+ was clamped with high K+ for several minutes (WT:422.8 ± 62.6 AUC and TALK-1 KO: 953.3 ± 78.9 AUC, P < 0.01, Figure 5D). This is consistent with past reports that documented sustained CICR over a period of several minutes in the presence of a stimulus [44], [45]. To determine whether increased ER Ca2+ release in TALK-1 KO δ-cells is a consequence of enhanced CICR, we next used fast imaging to measure changes in patch-clamped δ-cells. 25–250 ms step depolarizations (−80 mV–0 mV) induced prompt increases in which decayed to basal levels upon stepping back to the pre-pulse potential (Figure 5E). The depolarization-induced increases in were significantly greater in TALK-1 KO δ-cells, a difference which could be eliminated by depleting ER Ca2+ with CPA to indirectly preclude CICR (Figure 5F). As these data suggested that enhanced CICR contributes to elevated in TALK-1 KO δ-cells, we assessed whether depleting ER Ca2+ with CPA would return glucose-stimulated in KO δ-cells to a level comparable to WT δ-cells (Figure 5G). Indeed, whereas levels were significantly higher in TALK-1 KO δ-cells in 11 mM glucose (Figure 5G [see also Figures 2J, 3C, and 3D]), CPA treatment resulted in comparable levels in WT and TALK-1 KO δ-cells. Interestingly, although ER Ca2+ depletion with a SERCA inhibitor suppressed glucose-stimulated somatostatin release [11], [17] and oscillation amplitude (Figure 2F), CPA treatment paradoxically elevated bulk δ-cell levels (Figure 5G). The CPA-induced elevation in average δ-cell may be due to Vm depolarization caused by activation of store-operated currents (SOCs). Indeed, in the presence of Vm-hyperpolarizing diazoxide, basal in thapsigargin-treated δ-cells was not elevated (Figure 5A,B). These observations suggest that SOCs regulate δ-cell by modulating the Vm and VDCCs, and highlight that somatostatin secretion is very sensitive to ER Ca2+ release.
Figure 5.
CICR is increased in TALK-1 KO δ-cells. (A, B) Average increase in following high K+ (45 mM)-induced depolarization in WT and TALK-1 KO δ-cells, treated with vehicle or with 5 μM thapsigargin (Tg). SERCAs were energized with 11 mM glucose and KATP was activated with 125 μM diazoxide. Subtraction of the signal obtained from Tg treated cells from vehicle treated cells reveals the contribution of the ER to the signal (C) (N = 3 mice per genotype). (D) Average change in in response to treatment with diazoxide (125 μM) and depolarization with 45 mM K+ in WT and TALK-1 KO δ-cells (N = 3 mice per genotype); *P < 0.05. (E) Representative transients in WT and TALK-1 KO δ-cells subjected to short depolarizing pulses. The fold increase in δ-cell Ca2+ in response to increasingly long depolarizations in the presence or absence of CPA (25 μM) is quantified in (F) (N = 9 cells (WT); 7 cells (TALK-1 KO); 3 mice per genotype). (G) in WT and TALK-1 KO δ-cells was assessed before and after treatment with CPA. (N = 3 mice per genotype); *P < 0.05, **P < 0.005.
3.4. Glucagon secretion is reduced from TALK-1 KO islets
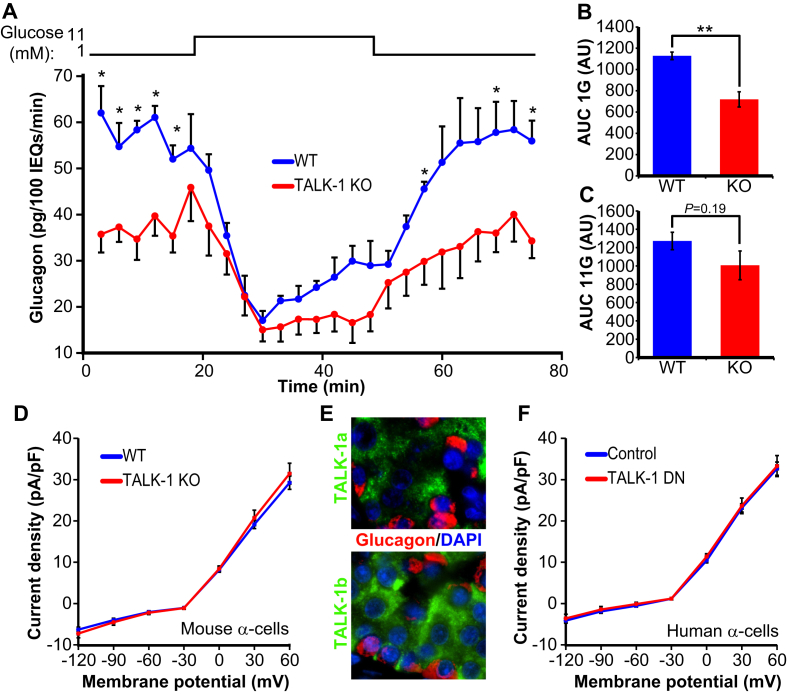
The acute sensitivity of α-cells to somatostatin under low glucose conditions is highlighted by the several-fold increase in glucagon secretion when somatostatin signaling is blocked [3], [46], [47]. Thus, somatostatin exerts an inhibitory tone on α-cells in low glucose, and increased basal somatostatin secretion leads to reduced glucagon secretion [3]. In TALK-1 KO islets, we found that glucagon release is significantly impaired under low glucose conditions (Figure 6A–C). While RNA sequencing of sorted α-cells has revealed high levels of KCNK16 mRNA (gene encoding TALK-1), TALK-1 protein is not detected in mouse or human α-cells [8], [21], [48], [49]. It is not clear why KCNK16 mRNA is present in α-cells but not TALK-1 protein; however, Blodgett and colleagues also observed high levels of insulin mRNA but not protein in α-cells [48]. These observations underscore the importance of functional experimentation in support of transcriptome analysis and that the molecular mechanisms regulating islet-cell hormone mRNA expression are still incompletely understood. To further verify the absence of TALK-1 channels in α-cells, we recorded α-cell K2P currents, but we did not detect a difference between WT and TALK-1 KO α-cells (Figure 6D). Immunofluorescent analysis of human pancreas sections using two different TALK-1 antibodies failed to demonstrate TALK-1 in α-cells (Figure 6E), and expression of the TALK-1 DN mutant in human α-cells (confirmed by post-staining) had no effect on K2P currents (Figure 6F). As TALK-1 channels are not expressed in α-cells, it is likely that reduced glucagon secretion from TALK-1 KO islets was due to paracrine effects. While insulin is a paracrine inhibitor of glucagon secretion [50], insulin secretion from TALK-1 KO islets was indistinguishable from WT islets in 1 mM glucose [21]. This suggests that the reduced glucagon secretion observed in TALK-1 KO islets is a consequence of increased somatostatin secretion.
Figure 6.
Reduced glucagon secretion from TALK-1 KO islets. (A) Isolated islets from WT and TALK-1 KO mice were perifused with the indicated glucose concentrations (N = 4 mice per genotype). (B) Glucagon AUC for the period corresponding to glucagon secretion in 1 mM glucose (0–18 min). (C) Glucagon AUC for the period corresponding to glucagon secretion in 11 mM glucose (18–45 min). (D) K2P current density in WT and TALK-1 KO α-cells (N = 3 mice per genotype). (E) Human pancreas sections stained for TALK-1 using two different antibodies and glucagon. (F) K2P current density in human α-cells expressing either TALK-1 DN mutant or mCherry control. Cells were post-stained for glucagon; only glucagon-positive cells were analyzed (N = 10 α-cells per condition/five donors); *P < 0.05; **P < 0.005.
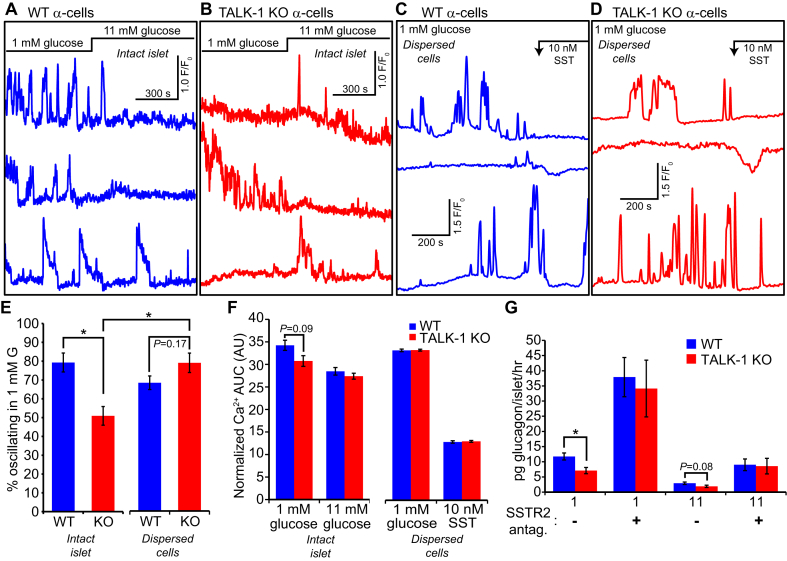
Somatostatin receptor signaling inhibits α-cell function through multiple mechanisms, including reduction of cellular cAMP levels [50], suppression of glucagon granule exocytosis [51], and activation of Vm-hyperpolarizing K+ currents [52], [53], [54]. As Vm hyperpolarization limits α-cell Ca2+ influx and glucagon secretion [55], we hypothesized that increased basal somatostatin release in TALK-1 KO islets would impact α-cell dynamics. We tested this possibility by measuring α-cell oscillations in WT and TALK-1 KO islets, using mice which express the genetically encoded Ca2+ indicator GCaMP3 specifically in α-cells [34]. In agreement with findings that somatostatin suppresses α-cell electrical activity and increases in [54], [56], we found that significantly fewer TALK-1 KO α-cells exhibited oscillations in low (1 mM) glucose when compared to controls (Figure 7A,B, and E). However, disrupting intraislet somatostatin paracrine signaling by dispersing islets into single cells normalized the oscillation frequency of WT and TALK-1 KO α-cells (Figure 7C,D, and E). Similarly, we found a tendency towards lower in intact islet TALK-1 KO α-cells, a difference which was not found in single TALK-1 KO α-cells (Figure 7F). Single α-cells were still capable of responding to somatostatin, as addition of 10 nM somatostatin caused a transient suppression of Ca2+ oscillations (Figure 7C,D), consistent with its effects on α-cell electrical activity [54]. To ascertain the relationship between somatostatin signaling and TALK-1 KO α-cell function, we measured glucagon secretion from WT and TALK-1 KO islets in the presence of the SSTR2 antagonist CYN154806. Blockade of SSTR2, which is the dominant α-cell SSTR [56], [57], potently increased glucagon secretion under both low and high glucose conditions and abrogated the reduced glucagon secretion of TALK-1 KO islets (Figure 7G). Together, these findings indicate that TALK-1 modulation of δ-cell somatostatin secretion impacts α-cell Ca2+ handling and glucagon secretion.
Figure 7.
TALK-1 KO α-cells exhibit altered Ca2+dynamics only in intact islets. (A) Recordings of intracellular Ca2+ responses in WT α-cells, in islets expressing the genetically encoded Ca2+ indicator GCaMP3 specifically in α-cells (representative of islet α-cells from N = 3 mice). (B) Recordings of intracellular Ca2+ responses in TALK-1 KO α-cells, in islets expressing the genetically encoded Ca2+ indicator GCaMP3 specifically in α-cells (representative of islet α-cells from N = 3 mice). (C) Recordings of intracellular Ca2+ responses in single WT α-cells expressing the genetically encoded Ca2+ indicator GCaMP3 (representative of α-cells from N = 3 mice). (D) Recordings of intracellular Ca2+ responses in single TALK-1 KO α-cells expressing the genetically encoded Ca2+ indicator GCaMP3 (representative of α-cells from N = 3 mice). (E and F) Comparison of percent oscillating α-cells and Ca2+ AUC determined from WT and TALK-1 KO α-cells under the indicated conditions (N = 3 mice per genotype). (G) Islets were incubated for 1 h with the indicated treatments (SSTR2 antagonist: 500 nM CYN154806); N = 10 mice per genotype (glucose only); 3 mice per genotype (+CYN154806); *P < 0.05.
4. Discussion
Although it is increasingly clear that islet δ-cells serve an important role in glucose homeostasis, our understanding of the mechanisms underlying δ-cell stimulus-secretion coupling has lagged behind that of β- and α-cells. CICR contributes to GSSS [11], but the molecular components which tune δ-cell CICR have remained largely unknown. Recent observations identified TALK-1 channels as important regulators of β-cell ER Ca2+ homeostasis and demonstrated prominent expression of TALK-1 in δ-cells [18], [19], [22]. Here, we assessed the functional roles of δ-cell TALK-1 channels and examined how TALK-1 modulation of δ-cell somatostatin secretion impacts paracrine regulation of α-cells. Our data suggest that δ-cell TALK-1 channels limit CICR, reducing somatostatin secretion and increasing glucagon release. These observations highlight the importance of CICR during GSSS and demonstrate that TALK-1 channel participate in the regulation of this distinct mode of Ca2+ handling.
Ca2+ influx through VDCCs is critical for δ-cell exocytosis. Indeed, removal of extracellular Ca2+, Vm hyperpolarization, and pharmacological VDCC blockade all reduce or inhibit δ-cell somatostatin secretion [9], [10], [11], [58]. While VDCC-mediated Ca2+ influx provides the trigger for exocytosis in δ-cells, GSSS is augmented by metabolically enhanced ER Ca2+ release [11]. Accordingly, depletion of ER Ca2+ (by inhibiting SERCAs) strongly inhibits somatostatin secretion [11], [17], [58]. The results presented here demonstrate that glucose metabolism augments δ-cell through increased CICR, as hypothesized by Zhang and colleagues [11]. We also found that depletion of ER Ca2+ stores significantly blunted depolarization-induced transients, strongly supporting the hypothesis that Ca2+ release from the δ-cell ER makes a substantial contribution to GSSS [11]. These data emphasize that metabolic enhancement of δ-cell ER Ca2+ stores and subsequent CICR is critical for somatostatin secretion.
Our findings indicate that TALK-1 channels provide a control mechanism for δ-cell ER Ca2+ handling and somatostatin secretion. In β-cells TALK-1 channels act as a countercurrent for ER Ca2+ leak [22]. The data suggest that TALK-1 may also modulate δ-cell ER Ca2+ handling and thereby reduce ER Ca2+ content. Similar to β-cells lacking TALK-1, ER Ca2+ stores were increased in TALK-1 KO δ-cells, presumably due to reduced ER Ca2+ leak [22]. However, despite increased ER Ca2+ storage in TALK-1 KO δ-cells, the rate of ER Ca2+ release following SERCA inhibition was faster. One possible explanation is that the driving force for ER Ca2+ extrusion is increased in response to higher ER Ca2+ stores. ER Ca2+ stores are also strongly correlated with CICR [14], [15], [16], therefore, greater CICR occurs with elevated ER Ca2+ storage. The importance of countercurrents in this process is exemplified in muscle cells lacking ER K+ countercurrents mediated by TRIC-A channels, which results in augmented ER Ca2+ stores and enhanced ligand mediated ER Ca2+ release (e.g. CICR) [59], [60]. An analogous phenotype was found in TALK-1 KO δ-cells, which exhibited significantly higher ER Ca2+ stores compared to WT δ-cells and larger glucose- and depolarization-induced transients. Elevated fluctuations in δ-cells lacking TALK-1 channels were normalized by ER Ca2+ depletion, demonstrating that increased ER Ca2+ release was responsible for augmented TALK-1 KO δ-cell transients. The observation that TALK-1 channels modulate δ-cell ER Ca2+ handling is consistent with the role of TALK-1 in limiting ER Ca2+ leak in β-cells and underscores the importance of ER Ca2+ homeostasis in tuning δ-cell CICR [22].
CICR provides a glucose-dependent mechanism to amplify δ-cell levels and somatostatin secretion, and is conceivably tuned by ATP-sensitive proteins such as SERCAs and ER Ca2+ release channels [11]. The findings presented here suggest that TALK-1 could have multiple effects on δ-cell ER Ca2+ homeostasis which influence CICR and somatostatin secretion. As discussed above, TALK-1 channel activity modulates ER Ca2+ handling, controlling ER Ca2+ levels which determines the driving force for Ca2+ to exit the ER. Under conditions of increased ER Ca2+ stores, such as in TALK-1 KO δ-cells or in high glucose when SERCAs are energized, elevated ER Ca2+ content would be predicted to enhance Ca2+ currents through ER Ca2+ release channels. In addition, changes in ER Ca2+ levels may also shift the sensitivity of δ-cell ER Ca2+ handling proteins. For example, the open probability of both ryanodine receptors and inositol trisphosphate receptors are influenced by the free Ca2+ concentration in the ER, modulating the sensitivity and magnitude of CICR [61], [62]. Therefore, up- or downregulation of TALK-1 channel activity may provide a mechanism to control the dynamic range of δ-cell CICR, scaling Ca2+ release and somatostatin secretion to maintain glycemia. It remains to be determined whether gastric δ-cells share the stimulus-secretion mechanisms of islet δ-cells, although TALK-1 is highly expressed in both. Adriaenssens and colleagues found that several incretin hormones elicit Ca2+ release from intracellular stores in gastric δ-cells [19], and the findings of this study suggest that TALK-1 channels may serve a role in regulating these responses. Interestingly, one of the few known physiological regulators of TALK-1 currents is extracellular pH [63]. While pH fluctuations around the islet are not expected to be of sufficient magnitude to impact islet cell TALK-1 currents, pH changes in the gastrointestinal tract could regulate gastric δ-cell Ca2+ handling and somatostatin secretion. Future studies will define the mechanisms which control the expression and activity of both islet and gastric δ-cell TALK-1 channels to better understand the mechanisms which regulate somatostatin secretion.
TALK-1 modulation of ER Ca2+ handling regulates distinct cellular responses in β-cells (e.g., modulation of Ca2+-activated K+ currents and electrical activity [21], [22]) and δ-cells (e.g., control of CICR). While the role of δ-cell TALK-1 channels in CICR-dependent modulation of the Vm is unclear, we found minimal defects in TALK-1 KO δ-cell electrical activity. It is important to note that our Vm recordings of TALK-1 KO δ-cells were performed in clusters of islet cells, whereas our β-cell Vm recordings [21] were performed in intact islets. Under these conditions, paracrine interactions between β-, δ-, and α-cells which affect δ-cell ER Ca2+ handling may be perturbed. It is increasingly clear that signaling between β- and δ-cells serves an important function in synchronizing islet hormone secretion, and our findings indicate that islet TALK-1 channels participate in this process. The functional coupling of β- and δ-cells is demonstrated by observations of simultaneous oscillations of insulin and somatostatin release from perfused pancreas [64] as well as synchronized islet β- and δ-cell oscillations [31]. “Chemical coupling” of β- and δ-cells is mediated by the peptide hormone urocortin3 (Ucn3), which is co-released with insulin to stimulate δ-cell type 2 corticotropin releasing hormone receptors (Crhr2) [9]. The effects of Ucn3 on δ-cell Ca2+ handling have not been determined, but Crhr2 activation can trigger IP3R-dependent ER Ca2+ release in heterologous expression systems and neurons [65], [66]. This suggests the possibility that β- to δ-cell signaling incorporates δ-cell ER Ca2+ release, the magnitude of which is controlled by TALK-1 channels. In agreement with such a role for ER Ca2+ release, we found that depletion of ER Ca2+ (via SERCA inhibition) disrupted the coordination of β- and δ-cell oscillations. We also observed an increased frequency of δ-cell oscillations in TALK-1 KO islets, similar to TALK-1 KO β-cells. This phenotype is likely due to increased β-cell electrical and oscillations [21], as we found no difference between WT and TALK-1 KO δ-cell oscillation frequency in low glucose when β-cells are quiescent. In addition to a possible alteration in chemical coupling between TALK-1 KO β- and δ-cells, TALK-1 may also influence electrical coupling between β- and δ-cells; indeed, gap junctions have been detected between β- and δ-cells [67], [68], [69]. Although the mechanisms and hierarchy of β- and δ-cell functional coupling remain to be determined, TALK-1 control of the integration of inputs from β- and δ-cells likely serves an important role in modulating glucagon secretion.
Inhibition of islet TALK-1 channel activity inhibits glucagon secretion while stimulating insulin and somatostatin secretion. Therefore, inhibition of TALK-1 channels could be beneficial in patients with T2DM. Indeed, TALK-1 KO mice fed a high-fat diet exhibit reduced fasting glycemia [21]. The findings described here suggest that the lower fasting glycemia in TALK-1 KO mice may arise due to elevated somatostatin secretion and reduced glucagon secretion, in addition to increased insulin secretion. Increased glucagon secretion at 1 mM glucose in the presence of an SSTR2 inhibitor highlights the importance of δ-cell paracrine signaling to the inhibitory action of TALK-1 on glucagon secretion. While the basis for this effect remains to be determined, it is likely related to decreased α-cell and cAMP levels. SSTR2 signaling in α-cells inhibits adenylyl cyclase synthesis of cAMP and also activates G protein-coupled inwardly-rectifying K+ channels, which hyperpolarize Vm and inhibit VDCCs [70]. Interestingly, SSTR2 signaling has been shown to reduce α-cell cAMP only at low glucose [71]. Our observations together with these previously published data highlight that α-cells are very sensitive to somatostatin at low glucose, but that alternate mechanisms predominate at high glucose. Taken together, these data also indicate that defects that lead to increases in TALK-1 channel activity may contribute to fasting hyperglycemia under metabolically stressful conditions. In agreement with this hypothesis, a gain-of-function polymorphism in TALK-1 (rs1535500, encoding TALK-1 A277E) is associated with an increased risk for T2DM [24], [25], [27]. This polymorphism would be predicted to reduce ER Ca2+ levels [22]. In δ-cells, increased TALK-1 channel activity would diminish CICR and lower somatostatin secretion. In turn, decreased somatostatin secretion would be expected to increase glucagon secretion and contribute to hyperglycemia. In addition, rs1535500-induced defects in β-cell function may impair β-cell to δ-cell signaling, further contributing to hyperglycemia by allowing inappropriately elevated glucagon secretion. Further studies are needed to understand TALK-1 regulation of δ-cells and the intraislet feedback mechanisms which contribute to glucose control in health and disease.
In conclusion, this study reveals that TALK-1 channels serve a key role in shaping δ-cell and controlling somatostatin secretion by modulating δ-cell ER Ca2+ handling. We find that TALK-1 channel activity regulates δ-cell CICR, limiting elevations in and somatostatin secretion. Our data also suggest that defects which lead to increases in TALK-1 channel activity (such as those induced by rs1535500) may participate in the pathogenesis of T2DM by negatively impacting δ-cell function, contributing to elevated glucagon release. These observations improve our understanding of the molecular mechanisms controlling δ-cell stimulus-secretion coupling and highlight the potential clinical utility of TALK-1 channels as a therapeutic target to reduce elevated glucagon secretion in patients with T2DM.
Acknowledgements
We thank Professor Anders Tengholm (Department of Medical Cell Biology, Uppsala University, Sweden) for generously providing the Psst-mCherry adenovirus. This project was funded by National Institutes of Health grants K01DK081666, R01DK097392, Vanderbilt Diabetes Research and Training Center Pilot and Feasibility Grant P60DK20593, and American Diabetes Association grant 1-17-IBS-024 (DAJ); Vanderbilt Molecular Endocrinology Training Program (METP) grant 5T32DK07563 and National Institutes of Health grant 1F31DK109625 (NCV); and Vanderbilt METP grant 5T32DK007563-28 (MKA). The Vanderbilt Translational Pathology Shared Resource assisted in the preparation and processing of paraffin-embedded pancreata (2P30 CA068485-14; 5U24DK059637-13). Confocal microscopy was performed using the Vanderbilt Cell Imaging Shared Resource (DK020593).
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.molmet.2018.01.016.
Author contributions
NCV and DAJ conceived the project; NCV, MTD, KLJ, PKD, KAK, MKA, SCM, and DAJ performed experiments and analyzed the data; NCV, MTD, and DAJ wrote and edited the manuscript.
Conflict of interest
The authors declare no conflict of interest.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Patel Y.C. Somatostatin and its receptor family. Frontiers in Neuroendocrinology. 1999;20(3):157–198. doi: 10.1006/frne.1999.0183. [DOI] [PubMed] [Google Scholar]
- 2.Hauge-Evans A.C., King A.J., Carmignac D., Richardson C.C., Robinson I.C., Low M.J. Somatostatin secreted by islet δ-cells fulfills multiple roles as a paracrine regulator of islet function. Diabetes. 2009;58(2):403–411. doi: 10.2337/db08-0792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cheng-Xue R., Gomez-Ruiz A., Antoine N., Noel L.A., Chae H.Y., Ravier M.A. Tolbutamide controls glucagon release from mouse islets differently than glucose: involvement of K(ATP) channels from both alpha-cells and delta-cells. Diabetes. 2013;62(5):1612–1622. doi: 10.2337/db12-0347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nilsson T., Arkhammar P., Rorsman P., Berggren P.O. Suppression of insulin release by galanin and somatostatin is mediated by a G-protein. An effect involving repolarization and reduction in cytoplasmic free Ca2+ concentration. Journal of Biological Chemistry. 1989;264(2):973–980. [PubMed] [Google Scholar]
- 5.Efendic S., Nylen A., Roovete A., Uvnas-Wallenstein K. Effects of glucose and arginine on the release of immunoreactive somatostatin from the isolated perfused rat pancreas. FEBS Letters. 1978;92(1):33–35. doi: 10.1016/0014-5793(78)80715-7. [DOI] [PubMed] [Google Scholar]
- 6.Brereton M.F., Vergari E., Zhang Q., Clark A. Alpha-, delta-and PP-cells: are they the architectural cornerstones of islet structure and co-ordination. Journal of Histochemistry and Cytochemistry. 2015;63(8):575–591. doi: 10.1369/0022155415583535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Girard J. Glucagon, a key factor in the pathophysiology of type 2 diabetes. Biochimie. 2017 doi: 10.1016/j.biochi.2017.10.004. [DOI] [PubMed] [Google Scholar]
- 8.Segerstolpe Å., Palasantza A., Eliasson P., Andersson E.-M., Andréasson A.-C., Sun X. Single-cell transcriptome profiling of human pancreatic islets in health and type 2 diabetes. Cell Metabolism. 2016;24(4):593–607. doi: 10.1016/j.cmet.2016.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van der Meulen T., Donaldson C.J., Caceres E., Hunter A.E., Cowing-Zitron C., Pound L.D. Urocortin3 mediates somatostatin-dependent negative feedback control of insulin secretion. Nature Medicine. 2015;21(7):769–776. doi: 10.1038/nm.3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Efendic S., Grill V., Nylen A., Ostensson C.G. Difference in calcium dependency of insulin, glucagon and somatostatin secretion in response to glibenclamide in perfused rat pancreas. Diabetologia. 1982;22(6):475–479. doi: 10.1007/BF00282593. [DOI] [PubMed] [Google Scholar]
- 11.Zhang Q., Bengtsson M., Partridge C., Salehi A., Braun M., Cox R. R-type Ca(2+)-channel-evoked CICR regulates glucose-induced somatostatin secretion. Nature Cell Biology. 2007;9(4):453–460. doi: 10.1038/ncb1563. [DOI] [PubMed] [Google Scholar]
- 12.Braun M., Ramracheya R., Amisten S., Bengtsson M., Moritoh Y., Zhang Q. Somatostatin release, electrical activity, membrane currents and exocytosis in human pancreatic delta cells. Diabetologia. 2009;52(8):1566–1578. doi: 10.1007/s00125-009-1382-z. [DOI] [PubMed] [Google Scholar]
- 13.Berts A., Ball A., Dryselius G., Gylfe E., Hellman B. Glucose stimulation of somatostatin-producing islet cells involves oscillatory Ca2+ signaling. Endocrinology. 1996;137(2):693–697. doi: 10.1210/endo.137.2.8593819. [DOI] [PubMed] [Google Scholar]
- 14.Nelson T.E., Nelson K.E. Intra- and extraluminal sarcoplasmic reticulum membrane regulatory sites for Ca2(+)-induced Ca2+ release. FEBS Letters. 1990;263(2):292–294. doi: 10.1016/0014-5793(90)81396-6. [DOI] [PubMed] [Google Scholar]
- 15.Gyorke S., Terentyev D. Modulation of ryanodine receptor by luminal calcium and accessory proteins in health and cardiac disease. Cardiovascular Research. 2008;77(2):245–255. doi: 10.1093/cvr/cvm038. [DOI] [PubMed] [Google Scholar]
- 16.Missiaen L., De Smedt H., Droogmans G., Casteels R. Ca2+ release induced by inositol 1,4,5-trisphosphate is a steady-state phenomenon controlled by luminal Ca2+ in permeabilized cells. Nature. 1992;357(6379):599–602. doi: 10.1038/357599a0. [DOI] [PubMed] [Google Scholar]
- 17.Vieira E., Salehi A., Gylfe E. Glucose inhibits glucagon secretion by a direct effect on mouse pancreatic alpha cells. Diabetologia. 2007;50(2):370–379. doi: 10.1007/s00125-006-0511-1. [DOI] [PubMed] [Google Scholar]
- 18.Li J., Klughammer J., Farlik M., Penz T., Spittler A., Barbieux C. Single-cell transcriptomes reveal characteristic features of human pancreatic islet cell types. EMBO Reports. 2016;17(2):178–187. doi: 10.15252/embr.201540946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Adriaenssens A., Lam B.Y., Billing L., Skeffington K., Sewing S., Reimann F. A transcriptome-led exploration of molecular mechanisms regulating somatostatin-producing D-cells in the gastric epithelium. Endocrinology. 2015;156(11):3924–3936. doi: 10.1210/en.2015-1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Adriaenssens A.E., Svendsen B., Lam B.Y., Yeo G.S., Holst J.J., Reimann F. Transcriptomic profiling of pancreatic alpha, beta and delta cell populations identifies delta cells as a principal target for ghrelin in mouse islets. Diabetologia. 2016;59(10):2156–2165. doi: 10.1007/s00125-016-4033-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vierra N.C., Dadi P.K., Jeong I., Dickerson M., Powell D.R., Jacobson D.A. Type 2 diabetes-associated K+ channel TALK-1 modulates beta-cell electrical excitability, second-phase insulin secretion, and glucose homeostasis. Diabetes. 2015;64(11):3818–3828. doi: 10.2337/db15-0280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vierra N.C., Dadi P.K., Milian S.C., Dickerson M., Jordan K.L., Gilon P. 2017. TALK-1 channels control β-cell endoplasmic reticulum Ca2+ homeostasis Sci Signal. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dadi P.K., Vierra N.C., Ustione A., Piston D.W., Colbran R.J., Jacobson D.A. Inhibition of pancreatic beta-cell Ca2+/calmodulin-dependent protein kinase II reduces glucose-stimulated calcium influx and insulin secretion, impairing glucose tolerance. Journal of Biological Chemistry. 2014;289(18):12435–12445. doi: 10.1074/jbc.M114.562587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cho Y.S., Chen C.H., Hu C., Long J., Ong R.T., Sim X. Meta-analysis of genome-wide association studies identifies eight new loci for type 2 diabetes in east Asians. Nature Genetics. 2012;44(1):67–72. doi: 10.1038/ng.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Replication, D.I.G, Meta-analysis, C, Asian Genetic Epidemiology Network Type 2 Diabetes, C, South Asian Type 2 Diabetes, C, Mexican American Type 2 Diabetes, C, Type 2 Diabetes Genetic Exploration by Next-generation sequencing in muylti-Ethnic Samples, C Genome-wide trans-ancestry meta-analysis provides insight into the genetic architecture of type 2 diabetes susceptibility. Nature Genetics. 2014;46(3):234–244. doi: 10.1038/ng.2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wood A.R., Jonsson A., Jackson A.U., Wang N., van Leewen N., Palmer N.D. A genome-wide association study of IVGTT-based measures of first phase insulin secretion refines the underlying physiology of type 2 diabetes variants. Diabetes. 2017 doi: 10.2337/db16-1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Muller Y.L., Piaggi P., Chen P., Wiessner G., Okani C., Kobes S. Assessing variation across eight established east asian loci for type 2 diabetes in american indians: suggestive evidence for new sex-specific diabetes signals in GLIS3 and ZFAND3. Diabetes Metabolism Research Review. 2016 doi: 10.1002/dmrr.2869. [DOI] [PubMed] [Google Scholar]
- 28.Taniguchi H., He M., Wu P., Kim S., Paik R., Sugino K. A resource of Cre driver lines for genetic targeting of GABAergic neurons in cerebral cortex. Neuron. 2011;71(6):995–1013. doi: 10.1016/j.neuron.2011.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Luche H., Weber O., Nageswara Rao T., Blum C., Fehling H.J. Faithful activation of an extra-bright red fluorescent protein in "knock-in" Cre-reporter mice ideally suited for lineage tracing studies. European Journal of Immunology. 2007;37(1):43–53. doi: 10.1002/eji.200636745. [DOI] [PubMed] [Google Scholar]
- 30.Roe M.W., Philipson L.H., Frangakis C.J., Kuznetsov A., Mertz R.J., Lancaster M.E. Defective glucose-dependent endoplasmic reticulum Ca2+ sequestration in diabetic mouse islets of Langerhans. Journal of Biological Chemistry. 1994;269(28):18279–18282. [PubMed] [Google Scholar]
- 31.Shuai H., Xu Y., Yu Q., Gylfe E., Tengholm A. Fluorescent protein vectors for pancreatic islet cell identification in live-cell imaging. Pflugers Archiv. 2016;468(10):1765–1777. doi: 10.1007/s00424-016-1864-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li J., Shuai H., Gylfe E., Tengholm A. Oscillations of sub-membrane ATP in glucose-stimulated beta cells depend on negative feedback from Ca2+ Diabetologia. 2013;56(7):1577–1586. doi: 10.1007/s00125-013-2894-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhu L., Almaca J., Dadi P.K., Hong H., Sakamoto W., Rossi M. beta-arrestin-2 is an essential regulator of pancreatic beta-cell function under physiological and pathophysiological conditions. Nature Communications. 2017;8:14295. doi: 10.1038/ncomms14295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dadi P.K., Luo B., Vierra N.C., Jacobson D.A. TASK-1 potassium channels limit pancreatic alpha-cell calcium influx and glucagon secretion. Molecular Endocrinology. 2015;29(5):777–787. doi: 10.1210/me.2014-1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.DiGruccio M.R., Mawla A.M., Donaldson C.J., Noguchi G.M., Vaughan J., Cowing-Zitron C. Comprehensive alpha, beta and delta cell transcriptomes reveal that ghrelin selectively activates delta cells and promotes somatostatin release from pancreatic islets. Molecular Metabolism. 2016;5(7):449–458. doi: 10.1016/j.molmet.2016.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Molina J., Rodriguez-Diaz R., Fachado A., Jacques-Silva M.C., Berggren P.O., Caicedo A. Control of insulin secretion by cholinergic signaling in the human pancreatic islet. Diabetes. 2014;63(8):2714–2726. doi: 10.2337/db13-1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Madisen L., Garner A.R., Shimaoka D., Chuong A.S., Klapoetke N.C., Li L. Transgenic mice for intersectional targeting of neural sensors and effectors with high specificity and performance. Neuron. 2015;85(5):942–958. doi: 10.1016/j.neuron.2015.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kilimnik G., Jo J., Periwal V., Zielinski M.C., Hara M. Quantification of islet size and architecture. Islets. 2012;4(2):167–172. doi: 10.4161/isl.19256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Beck A., Zur Nieden R., Schneider H.-P., Deitmer J.W. Calcium release from intracellular stores in rodent astrocytes and neurons in situ. Cell Calcium. 2004;35(1):47–58. doi: 10.1016/s0143-4160(03)00171-4. [DOI] [PubMed] [Google Scholar]
- 40.Dyachok O., Gylfe E. Store-operated influx of Ca2+ in pancreatic β-cells exhibits graded dependence on the filling of the endoplasmic reticulum. Journal of Cell Science. 2001;114(11):2179–2186. doi: 10.1242/jcs.114.11.2179. [DOI] [PubMed] [Google Scholar]
- 41.Brini M., Bano D., Manni S., Rizzuto R., Carafoli E. Effects of PMCA and SERCA pump overexpression on the kinetics of cell Ca 2+ signalling. The EMBO Journal. 2000;19(18):4926–4935. doi: 10.1093/emboj/19.18.4926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Solovyova N., Veselovsky N., Toescu E., Verkhratsky A. Ca2+ dynamics in the lumen of the endoplasmic reticulum in sensory neurons: direct visualization of Ca2+-induced Ca2+ release triggered by physiological Ca2+ entry. The EMBO Journal. 2002;21(4):622–630. doi: 10.1093/emboj/21.4.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gilon P., Arredouani A., Gailly P., Gromada J., Henquin J.C. Uptake and release of Ca2+ by the endoplasmic reticulum contribute to the oscillations of the cytosolic Ca2+ concentration triggered by Ca2+ influx in the electrically excitable pancreatic B-cell. Journal of Biological Chemistry. 1999;274(29):20197–20205. doi: 10.1074/jbc.274.29.20197. [DOI] [PubMed] [Google Scholar]
- 44.Beauvois M.C., Arredouani A., Jonas J.C., Rolland J.F., Schuit F., Henquin J.C. Atypical Ca2+-induced Ca2+ release from a sarco-endoplasmic reticulum Ca2+-ATPase 3-dependent Ca2+ pool in mouse pancreatic β-cells. The Journal of Physiology. 2004;559(1):141–156. doi: 10.1113/jphysiol.2004.067454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dyachok O., Gylfe E. Ca2+-induced Ca2+ release via inositol 1, 4, 5-trisphosphate receptors is amplified by protein kinase A and triggers exocytosis in pancreatic β-cells. Journal of Biological Chemistry. 2004;279(44):45455–45461. doi: 10.1074/jbc.M407673200. [DOI] [PubMed] [Google Scholar]
- 46.Li J., Yu Q., Ahooghalandari P., Gribble F.M., Reimann F., Tengholm A. Submembrane ATP and Ca2+ kinetics in alpha-cells: unexpected signaling for glucagon secretion. The FASEB Journal. 2015;29(8):3379–3388. doi: 10.1096/fj.14-265918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang Q., Ramracheya R., Lahmann C., Tarasov A., Bengtsson M., Braha O. Role of KATP channels in glucose-regulated glucagon secretion and impaired counterregulation in type 2 diabetes. Cell Metabolism. 2013;18(6):871–882. doi: 10.1016/j.cmet.2013.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Blodgett D.M., Nowosielska A., Afik S., Pechhold S., Cura A.J., Kennedy N.J. Novel observations from next generation RNA sequencing of highly purified human adult and fetal islet cell subsets. Diabetes. 2015 doi: 10.2337/db15-0039. db150039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xin Y., Kim J., Okamoto H., Ni M., Wei Y., Adler C. RNA sequencing of single human islet cells reveals type 2 diabetes genes. Cell Metabolism. 2016;24(4):608–615. doi: 10.1016/j.cmet.2016.08.018. [DOI] [PubMed] [Google Scholar]
- 50.Elliott A.D., Ustione A., Piston D.W. Somatostatin and insulin mediate glucose-inhibited glucagon secretion in the pancreatic alpha-cell by lowering cAMP. American Journal of Physiology. Endocrinology and Metabolism. 2015;308(2):E130–E143. doi: 10.1152/ajpendo.00344.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gromada J., Hoy M., Buschard K., Salehi A., Rorsman P. Somatostatin inhibits exocytosis in rat pancreatic alpha-cells by G(i2)-dependent activation of calcineurin and depriming of secretory granules. The Journal of Physiology. 2001;535(Pt 2):519–532. doi: 10.1111/j.1469-7793.2001.00519.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yoshimoto Y., Fukuyama Y., Horio Y., Inanobe A., Gotoh M., Kurachi Y. Somatostatin induces hyperpolarization in pancreatic islet alpha cells by activating a G protein-gated K+ channel. FEBS Letters. 1999;444(2–3):265–269. doi: 10.1016/s0014-5793(99)00076-9. [DOI] [PubMed] [Google Scholar]
- 53.Gromada J., Hoy M., Olsen H.L., Gotfredsen C.F., Buschard K., Rorsman P. Gi2 proteins couple somatostatin receptors to low-conductance K+ channels in rat pancreatic alpha-cells. Pflugers Archiv. 2001;442(1):19–26. doi: 10.1007/s004240000474. [DOI] [PubMed] [Google Scholar]
- 54.Ramracheya R., Ward C., Shigeto M., Walker J.N., Amisten S., Zhang Q. Membrane potential-dependent inactivation of voltage-gated ion channels in alpha-cells inhibits glucagon secretion from human islets. Diabetes. 2010;59(9):2198–2208. doi: 10.2337/db09-1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Le Marchand S.J., Piston D.W. Glucose decouples intracellular Ca2+ activity from glucagon secretion in mouse pancreatic islet alpha-cells. PLoS One. 2012;7(10) doi: 10.1371/journal.pone.0047084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kailey B., van de Bunt M., Cheley S., Johnson P.R., MacDonald P.E., Gloyn A.L. SSTR2 is the functionally dominant somatostatin receptor in human pancreatic beta- and alpha-cells. American Journal of Physiology. Endocrinology and Metabolism. 2012;303(9):E1107–E1116. doi: 10.1152/ajpendo.00207.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Strowski M.Z., Parmar R.M., Blake A.D., Schaeffer J.M. Somatostatin inhibits insulin and glucagon secretion via two receptors subtypes: an in vitro study of pancreatic islets from somatostatin receptor 2 knockout mice. Endocrinology. 2000;141(1):111–117. doi: 10.1210/endo.141.1.7263. [DOI] [PubMed] [Google Scholar]
- 58.Li Q., Cui M., Yang F., Li N., Jiang B., Yu Z. A cullin 4B-RING E3 ligase complex fine-tunes pancreatic delta cell paracrine interactions. Journal of Clinical Investigation. 2017 doi: 10.1172/JCI91348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhao X., Yamazaki D., Park K.H., Komazaki S., Tjondrokoesoemo A., Nishi M. Ca2+ overload and sarcoplasmic reticulum instability in tric-a null skeletal muscle. Journal of Biological Chemistry. 2010;285(48):37370–37376. doi: 10.1074/jbc.M110.170084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yamazaki D., Tabara Y., Kita S., Hanada H., Komazaki S., Naitou D. TRIC-A channels in vascular smooth muscle contribute to blood pressure maintenance. Cell Metabolism. 2011;14(2):231–241. doi: 10.1016/j.cmet.2011.05.011. [DOI] [PubMed] [Google Scholar]
- 61.Laver D.R. Ca2+ stores regulate ryanodine receptor Ca2+ release channels via luminal and cytosolic Ca2+ sites. Biophysical Journal. 2007;92(10):3541–3555. doi: 10.1529/biophysj.106.099028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vais H., Foskett J.K., Ullah G., Pearson J.E., Mak D.O. Permeant calcium ion feed-through regulation of single inositol 1,4,5-trisphosphate receptor channel gating. The Journal of General Physiology. 2012;140(6):697–716. doi: 10.1085/jgp.201210804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Girard C., Duprat F., Terrenoire C., Tinel N., Fosset M., Romey G. Genomic and functional characteristics of novel human pancreatic 2P domain K(+) channels. Biochemical and Biophysical Research Communications. 2001;282(1):249–256. doi: 10.1006/bbrc.2001.4562. [DOI] [PubMed] [Google Scholar]
- 64.Stagner J.I., Samols E., Weir G.C. Sustained oscillations of insulin, glucagon, and somatostatin from the isolated canine pancreas during exposure to a constant glucose concentration. Journal of Clinical Investigation. 1980;65(4):939–942. doi: 10.1172/JCI109750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gutknecht E., Van der Linden I., Van Kolen K., Verhoeven K.F., Vauquelin G., Dautzenberg F.M. Molecular mechanisms of corticotropin-releasing factor receptor-induced calcium signaling. Molecular Pharmacology. 2009;75(3):648–657. doi: 10.1124/mol.108.050427. [DOI] [PubMed] [Google Scholar]
- 66.Brailoiu G.C., Deliu E., Tica A.A., Chitravanshi V.C., Brailoiu E. Urocortin 3 elevates cytosolic calcium in nucleus ambiguus neurons. Journal of Neurochemistry. 2012;122(6):1129–1136. doi: 10.1111/j.1471-4159.2012.07869.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Briant L., Reinbothe T., Spiliotis I., Miranda C., Rodriguez B., Rorsman P. δ-cells and β-cells are electrically coupled and regulate α-cell activity via somatostatin. The Journal of Physiology. 2017 doi: 10.1113/JP274581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Meda P., Kohen E., Kohen C., Rabinovitch A., Orci L. Direct communication of homologous and heterologous endocrine islet cells in culture. The Journal of Cell Biology. 1982;92(1):221–226. doi: 10.1083/jcb.92.1.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Michaels R.L., Sheridan J.D. Islets of Langerhans: dye coupling among immunocytochemically distinct cell types. Science. 1981;214(4522):801–803. doi: 10.1126/science.6117129. [DOI] [PubMed] [Google Scholar]
- 70.Briant L., Salehi A., Vergari E., Zhang Q., Rorsman P. Glucagon secretion from pancreatic α-cells. Upsala Journal of Medical Sciences. 2016;121(2):113–119. doi: 10.3109/03009734.2016.1156789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tengholm A., Gylfe E. cAMP signaling in insulin and glucagon secretion. Diabetes, Obesity and Metabolism. 2017;19:42–53. doi: 10.1111/dom.12993. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.