Abstract
Background
Circulating tumor cells (CTCs) are tumor cells that have shed from a primary tumor and circulate in the peripheral blood. Recent experimental and clinical studies show that CTCs can be detected in early-stage disease.
Case presentation
We report three cases of pituitary adenoma (PA) in which tumor cells with particles were detected in the interstitial vascular compartment by transmission electron microscopy. Tumors were completely resected. Immunohistochemical analysis showed a β-catenin score of 10.5 ± 1.5 in the three cases with CTCs compared with 2.4 ± 0.5 in 24 control adenomas. The Ki-67 labeling index was 2.1 ± 0.7 in CTCs vs. 0.2 ± 0.3 in control cases (p = 0.043), and the p53 score was 4.33 ± 1.3 vs. 0.31 ± 0.17 (p = 0.000). The E-cadherin score did not differ significantly between the two groups.
Conclusions
CTCs can be detected in benign tumors such as PAs and not only in late-stage malignant tumors with apparent distant metastases. The present findings suggest that pituitary carcinomas develop from adenomas.
Keywords: Pituitary adenomas, Pituitary carcinomas, Circulating tumor cells, β-catenin, Ki-67, p53
Background
Pituitary adenomas (PAs) account for 15% of intracranial neoplasms and are generally considered benign, although they exhibit a wide range of behaviors [1]. Many factors affect the proliferation of pituitary adenomas, such as angiogenesis [2], apoptosis [3], growth factors [4], oncogenes [5], tumor suppressor genes, and hormone receptors [6]. The most important predictor of recurrence in functioning adenomas is the basal postoperative hormone level, whereas in nonfunctioning adenomas, no single convincing factor could be identified. Age, gender, tumor size, and invasion are not related to recurrence [7]. The ability to predict tumor recurrence at the initial surgery would be helpful for decision-making regarding adjunctive therapy and to decrease morbidity. Pituitary carcinomas are very rare tumors and are defined as primary neoplasms of the adenohypophysis that undergo craniospinal and/or systemic spread [8]. Whether pituitary carcinomas develop from adenomas or regrowth remains unclear.
Circulating tumor cells (CTCs) are tumor cells that have shed from a primary tumor and circulate in the peripheral blood. Recent studies show that CTCs can be detected not only in late-stage malignant tumors with apparent distant metastases, but also in early-stage disease. In addition, CTCs are potentially useful clinical markers for the diagnosis of malignant tumors and for decision-making regarding treatment [9]. The enumeration of CTCs is correlated with clinical outcome in prostate cancer and has been tested and used in clinical practice [10]. CTCs have been detected after chemotherapy in inflammatory breast cancer patients at high risk for relapse [11]. In the present study, we reported three cases of PA in which tumor cells with secretory granules were detected in tumor blood vessels. The expression of β-catenin, E-Cadherin, Ki-67, and p53 was analyzed in cases and in 24 control PAs to explore the mechanisms underlying recurrence and metastasis.
Case presentation
Case 1
A 44-year-old man was admitted to the hospital with altered visual acuity for 6 months. Magnetic resonance imaging (MRI) revealed a mass measuring 32 × 33 × 21 mm in the sellar region with cystic components. Microsurgical resection was performed by transnasal endoscopic excision of the PA. The tumor was soft and gray red with medium blood supply. On pathological examination, the tumor was negative for prolactin (PRL), growth hormone (GH), adrenocorticotrophic hormone (ACTH), follicle-stimulating hormone (FSH), luteinizing hormone (LH), and thyroid-stimulating hormone (TSH). Tumor cells with particles were detected in the interstitial vascular compartment with thickening of the basement membrane (Fig. 1a). Transmission electron microscopy (TEM): The amount of mitochondria was significantly increased in the cytoplasm of tumor cells, with small round and large irregular shaped particles (Fig. 1b).
Fig. 1.
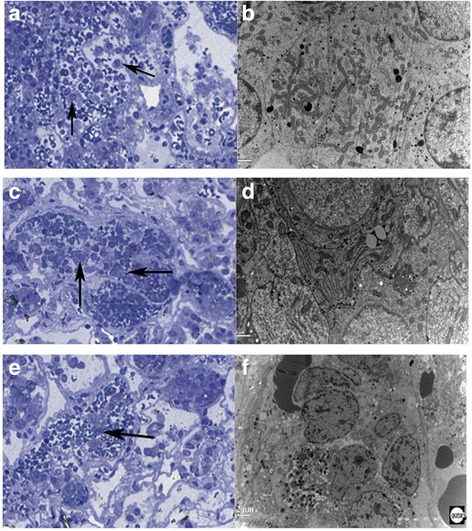
CTCs in three cases were shown. Case 1: ab, Case 2:cd, Case 3: ef. ACE: Tumor cells with particles in the interstitial vascular (Arrow shown) by Azure methylene blue staining.100×. BDF: TEM
Case 2
A 25-year-old man was admitted to the hospital with intermittent headache, dizziness for 5 years, and decreased left eye vision for 1 year. Defects in the left temporal retina and partial nasal visual field were observed. MRI revealed a mass measuring 3 × 22 × 22 mm in the sellar region. Microsurgical resection was performed by transnasal endoscopic excision of the PA. The tumor was soft and gray red with medium blood supply. In the pathological examination, the tumor was positive for GH and PRL (scattered). Tumor cells with particles were detected in the interstitial vascular compartment with thickening of the basement membrane (Fig. 1c). TEM: Large particles and circular particles were observed in the cytoplasm of tumor cells. The rough endoplasmic reticulum was rich in lamellar assemblies and swelling of mitochondria was observed (Fig. 1d).
Case 3
A 40-year-old woman was admitted to the hospital with headache and amenorrhea for 6 months. MRI revealed a mass measuring 20 × 20 × 15 mm in the sellar region. Microsurgical resection was performed by transnasal endoscopic excision of the PA. The tumor was soft and gray red with medium blood supply. In the pathological examination, the tumor was positive for GH and PRL (scattered). Tumor cells with particles were detected in the interstitial vascular compartment with thickening of the basement membrane (Fig. 1e). TEM: There were round or irregular particles in the cytoplasm of tumor cells. Nuclei were varied in size, with irregular shape and increased heterochromatin (Fig. 1f).
The CARE guidelines were followed when reporting these cases.
Expression of the mesenchymal transition biomarkers β-catenin, E-cadherin, Ki-67, and p53 in PA specimens
The expression of β-catenin, E-cadherin, Ki-67, and p53 was analyzed in three cases with CTC and 24 PA specimens. The specimens were fixed in 4% buffered formalin, embedded in paraffin, and incubated with anti-β-catenin (ZSGB-BIO, China), anti-E-cadherin (Abcam, UK), anti-Ki-67 (Leica, Germany), and anti-p53 (Leica, Germany) antibodies followed by immunodetection using the two-step polymer detection system (Polink-2 plus Kit; GBI Labs, England) and visualization with 3,3′-diaminobenzidine. Five fields at 100× magnification were randomly selected.
Staining scores were determined by a semi-quantitative system. The proportion of positive cells was scored as follows: 0: < 10%, 1: 10–25%, 2: 26–50%, 3: 51–75%, and 4: > 75%. Staining intensity was evaluated as follows: 0: no staining, 1: weak staining, light yellow, 2: moderate staining, yellowish brown, and 3: strong staining, brown. The sum score was determined by multiplying the positive proportion score by the intensity score. Immunohistochemical analysis showed that the β-catenin score in the three cases with CTCs was 10.5 ± 1.5 compared with 2.4 ± 0.5 in the control adenomas (Fig. 2a and b). The difference was statistically significant according to Fisher’s exact test (p = 0.020). The Ki-67 labeling index (Fig. 2c and d) was 2.1 ± 0.7 in the three cases with CTCs compared with 0.2 ± 0.3 in control cases (p = 0.043). The p53 score (Fig. 2e and f) was 4.33 ± 1.3 vs. 0.31 ± 0.17 in the controls (p = 0.000). The E-cadherin score did not differ significantly between the two groups (data not shown).
Fig. 2.
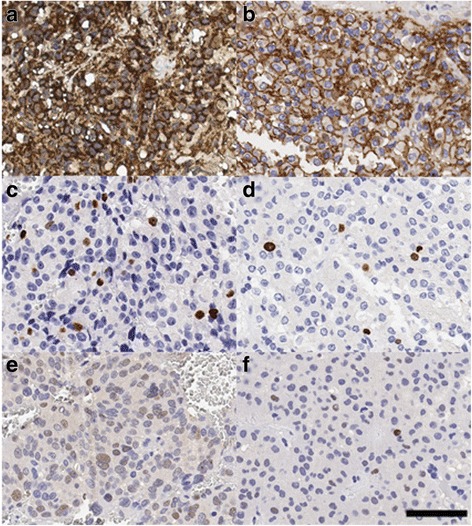
IHC analysis of the β-catenin and Ki67 expression. a β-catenin expression in PAs with CTCs. b β-catenin expression in control PAs. c Ki67 expression in PAs with CTCs. d Ki67 expression in control PAs. e p53 expression in PAs with CTCs. f p53 expression in control PAs. Bar = 60 μm
Molecular information obtained from the blood of cancer patients is an emerging and powerful research tool with great potential as a companion diagnostic factor for patient stratification and monitoring [12]. We detected CTCs with secretory granules in the vessels of patients with PAs. Recent experimental and clinical studies show that CTCs can be detected not only in late-stage malignant tumors with apparent distant metastases, but also in early-stage disease [13]. Furthermore, CTCs were identified as prognostic factors in several solid tumors such as colorectal, pancreatic, gastric, breast, and genitourinary cancers [14].
Pituitary carcinomas are classically defined as pituitary tumors with subcranial, brain, or systemic metastases [15]. In general, pituitary carcinomas are associated with poor prognosis as therapeutic options are limited. Whether pituitary carcinomas develop from adenomas or if they occur de novo remains unclear; in addition, the effect of hormonal subtypes on tumor aggressiveness, treatment outcomes, and prognosis has not been elucidated [16]. The present findings provide indirect evidence supporting that pituitary carcinomas develop from adenomas.
Data from a retrospective case-control study of 410 patients led to the classification of PAs according to tumor size and immunohistochemical type, and the assessment of invasion and proliferation provides prognostic information for predicting postoperative disease-free outcome or recurrence/progression status [17]. Epithelial-mesenchymal transition (EMT) is widely recognized as a key process associated with malignant transformation, and the Wnt/β-catenin pathway plays a regulatory role in EMT because of its involvement in maintaining epithelial integrity and tight adhesion junctions [18]. β-catenin, an important effector in the Wnt signaling pathway, is important for cell-cell adhesion. The E-cadherin/ β-catenin complex functions in intercellular junctions that promote cell adhesion [19]. We found that the Ki-67 LI was > 10%, and the β-catenin positivity rate was > 90% (score: 10.5 ± 1.5) in cases with CTCs. However, there was no statistically significant difference in the level of E-cadherin between the two groups. Abnormal β-catenin expression was correlated with more advanced disease stage, and as a consequence, with poor outcome in gastric cancer [20]. Nuclear E-cadherin is common in nonfunctioning PAs and a subset of growth hormone-secreting adenomas, in which it is associated with tumor size and invasion [21].
Ki-67, a marker of cellular proliferation, has been studied extensively in pituitary neoplasias [22]. It is of relevance to various clinicopathological parameters, including tumor subtype, size, invasiveness, and recurrence, as well as patient age and sex [23]. In malignant prolactinoma, the Ki-67 index of the tumor was 24.8%, and 60% of tumor cells were positive for p53 [24]. In another study, pathology revealed a high Ki-67 index in metastatic tumor tissues in two cases (24.2% and 10%) with positive p53 staining in one pituitary carcinoma [25]. In the current tumor classification of the World Health Organization, the definition of an atypical PA includes a Ki-67 labeling LI > 3% and extensive p53 positivity. In fact, PAs with Ki-67 expression ≥1.5% showed a higher recurrence risk and a worse disease-free survival compared with those with Ki-67 expression < 1.5% [26].
Conclusion
Tumor recurrence is a significant clinical problem in PAs in which patients are asymptomatic because disseminated cells that become dormant are undetectable by clinical tools. Patients with CTCs may benefit from aggressive treatment such as radiotherapy and close surveillance.
Acknowledgments
Funding
This work was supported by Beijing Natural Science Foundation of China (7162035) and Beijing high-level talent plan (2015–3-040).
Availability of data and materials
We agree that all datasets on which the conclusions of the manuscript rely to be either deposited in publicly available repositories (where available and appropriate) or presented in the main paper or additional supporting files, in machine-readable format (such as spreadsheets rather than PDFs) whenever possible. Beijing Natural Science Foundation of China: collection and interpretation of data; Beijing high-level talent plan: writing and revising the manuscript.
Abbreviations
- ACTH
Adrenocorti cotrophic hormone
- CTCs
Circulating tumor cells
- EMT
Epithelial-mesenchymal transition
- FSH
Follicle-Stimulating Hormone
- GH
Growth hormone
- Ki67 LI
Ki67 labelling index
- LH
Luteinizing hormone
- MRI
Magnetic resonance imaging
- PAs
Pituitary adenomas
- PRL
Prolactin
- TEM
Transmission electron microscope
- TSH
Thyroid-stimulating hormone
Authors’ contributions
GH design and writing, HYJ the experiment of transmission electron microscope, LQ staining; WJC staining and operation, ZYZ scanning the slides and operation. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Informed consent was obtained from all individuals and ethical approval was obtained from the Institutional Review Board of Beijing Tiantan Hospital Affiliated to Capital Medical University (KY2013–015-02).
Consent for publication
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor of this journal.
Competing interests
The authors declare that they have no competing of interest.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Yu R, Melmed S. Pathogenesis of pituitary tumors. Prog Brain Res. 2010;182(3):207–227. doi: 10.1016/S0079-6123(10)82009-6. [DOI] [PubMed] [Google Scholar]
- 2.Turner HE, Nagy Z, Gatter KC, Esiri MM, Harris AL, Wass JA. Angiogenesis in pituitary adenomas - relationship to endocrine function, treatment and outcome. J Endocrinol. 2000;165(2):475–481. doi: 10.1677/joe.0.1650475. [DOI] [PubMed] [Google Scholar]
- 3.Sambaziotis D, Kapranos N, Kontogeorgos G. Correlation of Bcl-2 and Bax with apoptosis in human pituitary adenomas. Pituitary. 2003;6(3):127–133. doi: 10.1023/B:PITU.0000011173.04191.37. [DOI] [PubMed] [Google Scholar]
- 4.Spada A, Lania A. Growth factors and human pituitary adenomas. Molec Cell Endocrinol. 2002;197(3):63–68. doi: 10.1016/S0303-7207(02)00279-4. [DOI] [PubMed] [Google Scholar]
- 5.Jiang X, Zhang X. The molecular pathogenesis of pituitary adenomas: an update. Endocrinol Metab (Seoul) 2013;28(4):245–254. doi: 10.3803/EnM.2013.28.4.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Antonio S, Giuseppe M, Andrea R, Esposito V, Jaffrain-Rea ML, Delfini R. Biochemical remission and recurrence rate of secreting pituitary adenomas after transsphenoidal adenomectomy: long-term endocrinologic follow-up results. Surg Neurol. 2007;68(5):513–518. doi: 10.1016/j.surneu.2007.05.057. [DOI] [PubMed] [Google Scholar]
- 7.Roelfsema F, Biermasz NR, Pereira AM. Clinical factors involved in the recurrence of pituitary adenomas after surgical remission: a structured review and meta-analysis. Pituitary. 2012;15(1):71–83. doi: 10.1007/s11102-011-0347-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pernicone PJ, Scheithauer BW, Sebo TJ, Kovacs KT, Horvath E, Young WF, Jr, et al. Pituitary carcinoma: a clinicopathologic study of 15 cases. Cancer. 1997;79(4):804–812. doi: 10.1002/(SICI)1097-0142(19970215)79:4<804::AID-CNCR18>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 9.Andergassen U, Zebisch M, Kölbl AC, König A, Heublein S, Schröder L, et al. Real-Time qPCRbased detection of circulating tumor cells from blood samples of adjuvant breast cancer patient: a preliminary study. Breast Care (Basel). 2016;11(3):194–8. [DOI] [PMC free article] [PubMed]
- 10.Galletti G, Portella L, Tagawa ST, Kirby BJ, Giannakakou P, Nanus DM. Circulating tumor cells in prostate cancer diagnosis and monitoring: an appraisal of clinical potential. Molec Diagnos Ther. 2014;18(4):389–402. doi: 10.1007/s40291-014-0101-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hall CS, Karhade M, Laubacher BA, Kuerer HM, Krishnamurthy S, DeSnyder S, et al. Circulating tumor cells and recurrence after primary systemic therapy in stage III inflammatory breast cancer. Jnci J N Cancer Ins. 2015;107(11). [DOI] [PMC free article] [PubMed]
- 12.Pantel K, Alix-Panabières C. Functional studies on viable circulating tumor cells. Clin Chem. 2016;62(2):328–334. doi: 10.1373/clinchem.2015.242537. [DOI] [PubMed] [Google Scholar]
- 13.Allard WJ, Matera J, Miller MC, Repollet M, Connelly MC, Rao C, et al. Tumor cells circulate in the peripheral blood of all major carcinomas but not in healthy subjects or patients with nonmalignant diseases. Clin Cancer Res. 2004;10:6897–6904. doi: 10.1158/1078-0432.CCR-04-0378. [DOI] [PubMed] [Google Scholar]
- 14.Alix-Panabières C. EPISPOT assay: detection of viable DTCs/CTCs in solid tumor patients. Recent Results Cancer Res. 2012;195:69–76. doi: 10.1007/978-3-642-28160-0_6. [DOI] [PubMed] [Google Scholar]
- 15.Raverot G, Burman P, McCormack A, Heaney A, Petersenn S, Popovic V, et al. European Society of Endocrinology. European Society of Endocrinology Clinical Practice Guidelines for the management of aggressive pituitary tumours and carcinomas. Eur J Endocrinol. 2018;178(1):G1–G24. doi: 10.1530/EJE-17-0796. [DOI] [PubMed] [Google Scholar]
- 16.Colao A, Ochoa AS, Auriemma RS, Faggiano A, Pivonello R, Lombardi G. Pituitary carcinomas. Front Horm Res. 2010;38(38):94–108. doi: 10.1159/000318499. [DOI] [PubMed] [Google Scholar]
- 17.Raverot G, Vasiljevic A, Jouanneau E, Trouillas JA. Prognostic clinicopathologic classification of pituitary endocrine tumors. Endocrinol Metab Clin N Am. 2015;44(1):11–18. doi: 10.1016/j.ecl.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 18.Zhao S, Bellone S, Lopez S, Thakral D, Schwab C, English DP, et al. Mutational landscape of uterine and ovarian carcinosarcomas implicates histone genes in epithelial–mesenchymal transition. PNAS. 2016;113(43):12238–43. doi: 10.1073/pnas.1614120113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chaw SY, Majeed AA, Dalley AJ, Chan A, Stein S, Farah CS. Epithelial to mesenchymal transition (EMT) biomarkers-E-cadherin, beta-catenin, APC and vimentin-in oral squamous cell carcinogenesis and transformation. Oral Oncol. 2012;48(10):997–1006. doi: 10.1016/j.oraloncology.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 20.Bartolomeo MD, Pietrantonio F, Pellegrinelli A, Martinetti A, Mariani L, Daidone MG, et al. Osteopontin, E-cadherin, and β-catenin expression as prognostic biomarkers in patients with radically resected gastric cancer. Gastric Cancer. 2016;19(2):412–420. doi: 10.1007/s10120-015-0495-y. [DOI] [PubMed] [Google Scholar]
- 21.Elston MS, Gill AJ, Conaglen JV, Clarkson A, Cook RJ, Little NS, Robinson BG, Clifton-Bligh RJ, McDonald KL. Nuclear accumulation of e-cadherin correlates with loss of cytoplasmic membrane staining and invasion in pituitary adenomas. J Clin Endocrinol Metab. 2009;94(4):1436–1442. doi: 10.1210/jc.2008-2075. [DOI] [PubMed] [Google Scholar]
- 22.Vasiljevic A, Jouanneau E, Trouillas J, Raverot G. Clinicopathological prognostic and theranostic markers in pituitary tumors. Minerva Endocrinol. 2016;41(3):377–389. [PubMed] [Google Scholar]
- 23.Salehi F, Agur A, Scheithauer BW, Kovacs K, Lloyd RV, Cusimano M. Ki-67 in pituitary neoplasms: a review--part I. Neurosurgery. 2009;65(3):429–437. doi: 10.1227/01.NEU.0000349930.66434.82. [DOI] [PubMed] [Google Scholar]
- 24.Phillips J, East HE, French SE, Melcescu E, Hamilton RD, Nicholas WC, et al. What causes a prolactinoma to be aggressive or to become a pituitary carcinoma? Hormones (Athens) 2012;11(4):477–482. doi: 10.14310/horm.2002.1380. [DOI] [PubMed] [Google Scholar]
- 25.Carlos KM, David C, Steven GW, Christopher HC, Anita M, Paul DB, et al. Radiotherapy with concurrent temozolomide for the management of extraneural metastases in pituitary carcinoma. Pituitary. 2016;19(4):415–421. doi: 10.1007/s11102-016-0721-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chiloiro S, Doglietto F, Trapasso B, Iacovazzo D, Giampietro A, Di Nardo F, et al. Typical and atypical pituitary adenomas: a single-center analysis of outcome and prognosis. Neuroendocrinology. 2015;101(2):143–150. doi: 10.1159/000375448. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
We agree that all datasets on which the conclusions of the manuscript rely to be either deposited in publicly available repositories (where available and appropriate) or presented in the main paper or additional supporting files, in machine-readable format (such as spreadsheets rather than PDFs) whenever possible. Beijing Natural Science Foundation of China: collection and interpretation of data; Beijing high-level talent plan: writing and revising the manuscript.


