Abstract
Alitretinoin is a new oral retinoid authorized for use in grownups that have severe chronic hand eczema (CHE). A comprehensive search to solicit all studies of alitretinoin for the treatment of CHE. A comprehensive search to solicit all studies of alitretinoin for the treatment of CHE including randomized controlled trials (RCTs) or uncontrolled trials, re-treatment studies, open-label studies, or observational studies, along with case series of 10 or more participants. Physician global assessment (PGA), patient global assessment (PaGA) and modified total lesion symptom score (mTLSS) are the methods and outcomes criteria. Generated effect size and 95% confidence intervals were calculated for the outcomes. Heterogeneity and publication bias were also tested for all selected trials. When a noteworthy Q statistic (P < 0.1) demonstrates the heterogeneity crosswise over studies, an arbitrary impact model is used. On the other hand, a settled effect model is when heterogeneity is not shown. The initial search yielded 408 records of which 15 articles were selected. The 15 clinical trials included 3734 patients with CHE. Among alitretinoin-treated patients, the PGA effect size was directly proportional to the drug dosage, ranging from 40% to 69%, while the PaGA score ranged from 28.8% to 62.4%, and mTLSS ranged from 60.4% to 76.9%, much higher than placebo. A higher drug dose was about twice as effective as lower dose. The odds ratio for a better outcome with drug treatment taking duration into account was about 3–4 times that versus placebo. In conclusions, alitretinoin cleared lesions in about 50% of cases, particularly using a higher dose for a longer duration.
Keywords: Alitretinoin, chronic hand eczema, meta-analysis
Introduction
Hand eczema is the most widely recognized dermatitis influencing the hands. Chronic hand eczema (CHE) is ordinarily observed in clinical practice. In the etiology of CHE, exposures to irritants or allergens, and endogenous factors, such as atopic dermatitis have interconnected roles. Subsequently, it is seldom potential, to recognize every single causative part and remove all of them.[1]
Typical characteristics of skin inflammation limited to the hands including erythema, edema, vesiculation, blistering, thickening, fissures, pruritus, and pain represent the clinical marks and symptoms of CHE. The reliable use of emollients and topical steroids and attempting to keep away from aggravations can cure mild cases of CHE, however, severe CHE is wrecking on the off-chance that it is unmanageable to this standard regimen. Severe CHE is accompanied with continuous and distorting changes in the hand appearance[2] and causes much practical, social, workable, and psychological disability.[3]
Nowadays, patients with severe CHE insusceptible to topical steroids have restricted treatment alternatives appropriate for chronic use, and few controlled clinical researches have explored new treatments in this framework.[4]
Alitretinoin (an endogenous retinoid) is mainly a group of vitamin A derivatives with an extensive variety of pharmacological properties and therapeutic applications in dermatology. They impact cell proliferation and differentiation, apoptosis, angiogenesis, keratinization, and sebum excretion, and have immunomodulatory features.[5] Retinoid is subject to binding with two sub-sorts of nuclear receptors, retinoic acid receptors (RARs) and retinoid X receptors (RXRs). The natural ligand for RARs is tretinoin (all-trans retinoic corrosive); ligand connected to RARs is tweaked by RXRs, for which the regular ligand is alitretinoin (9-cis-retinoic corrosive). RXRs likewise adjust the legend bounded by thyroid hormones.[6] Retinoids have built up roles as topical or systemic medicines for severe acne vulgaris, psoriasis and certain malignancy, though little has been written about their use of the therapy for CHE. Alitretinoin (Toctino, Stiefel Laboratories) is an oral retinoid authorized for use in an adult who has serious CHE inert to intense topical steroids. The prescribed dosage is 30 mg/day, lessening to 10 mg/day if unfavorable impacts are unsatisfactory. A course of medication is between 12 and 24 weeks.[7]
In various clinical attempts, the physician’s global assessment (PGA)[8] is the essential, effective factor for the therapeutic response of alitretinoin in CHE. PGA gives a general estimation of the disease seriousness by the doctor, using a 5-point, predefined scale to rate CHE from “clear” to “severe.” Concerning the secondary effective factors, the patient’s global assessment (PaGA),[8] modified total lesion symptom score (mTLSS),[8] degree of disease and time for reaction have been incorporated. The PaGA includes patients’ self-evaluating of their general illness which changes from baseline to the end of treatment by choosing one of six portrayals, running from “clear” or “almost clear” disease to “worsening” of disease. The seven individual CHE symptoms; (erythema, scaling, lichenification or hyperkeratosis, vesiculation, edema, fissures, and pruritus/pain) form the composite scale of mTLSS’ strength and each one of them scores from 0 to 3. The scores are summed, extending from a base estimation of 0 (no signs or symptoms) to the most extreme of 21 (more serious disease). The rate of hand area (palm and dorsum) influenced by eczema represents the degree of the disease.[8,9] The data on the effectiveness of oral alitretinoin in the treatment of CHE has not been predictable until now, and understanding of the after effects of various clinical experiments has been disputable. Hence, this meta-analysis is conducted to separate and break down all accessible data to have an appropriate measure of the curative impact of this medication.
Methods
Research strategy
The criteria of preferred reporting items for systematic reviews and meta-analyses are followed in this meta-analysis.[10] A comprehensive search strategy is conducted to identify all relevant studies of oral alitretinoin use for the treatment of CHE. Articles in all languages were considered potentially eligible; searches covered all the time from January 1998 to January 2016. An electronically published search was performed using EBSCO/Medline, ProQuest/CENTRAL, and PUBMED databases. The researchers use the following keywords in this study “(hand eczema or CHE or eczema) or dermatitis,” for the treatment “(study, trial or comparison), (treatment, drug or therapy), and (alitretinoin or 9-cis-retinoic acid).” The search of publicizing work is limited to, the studies on humans, whereas the reviews and case reports have been abandoned. Duplicate citations were removed. We tried our best also to search all abstracts of presentations pertinent to the subject.
Selection criteria
The criteria used in this study were developed in advance and included studies of oral alitretinoin use for CHE, with dose regimens of 10, 20, 30, or 40 mg daily, duration of treatment of 12–24 weeks, and treatment of adults (aged 16–85 years). Eligible designs were RCTs or uncontrolled trials or re-treatment studies or open-label studies or observational studies, as well as case series of 10 or more participants. The non-original data, such as the studies which are not carried out on humans, in-vitro studies studies, studies with no clinical endpoint, case reports or case series for <5 patients, and incomplete article (e.g., letter) as well as reviews and abstracts were excluded. The articles with the largest size or the most recently published were incorporated when more than one article was published by similar writers using similar sample series.
Study selection
Title and abstract were examined, and full articles of all references that met the prior criteria were chosen for reviewers and appraisal. Thus, only full articles were checked for incorporation or avoidance and information extraction. One reviewer (AMS) independently reviewed all studies that reported cure rates of different interventions. Where there was a discrepancy in the views, the second author (SAA) determined whether the trial should be included. Disagreements were resolved by discussion. A standardized eligibility form was used for the review.
Data extraction and quality assessment
The data were extracted according to research features (country, research design), study population, information about alitretinoin treatment (duration, the different type of dosage, follow-up), study endpoints, response measurement, and tolerability. In all studies (RCTs, uncontrolled studies, double-blind, and extended studies), the main relative changes in serious clinical cases beginning from the baseline represent the outcomes of the study. Using of observational studies in this meta-analysis was done according to the recommendations of the MOOSE group.[11]
Exclusively, the dynamic treatment groups of the RCTs were considered keeping in mind the end goal to have the capacity to compare their outcomes with uncontrolled researches. Furthermore, the researchers abstracted the related data about the comparative effectiveness of alitretinoin (against placebo) from reports on RCTs. With respect to surrogate factors for the medication safety, frequencies of typical adverse events of alitretinoin (headache, flushing, upper respiratory tract infection [URTI], mucocutaneous change, erythema, cheilitis, and increase in serum cholesterol and serum triglyceride) were abstracted. Adequate case definition, the definition of eligible criteria, description of the population study, randomization and blinding, the use of approved results, satisfactory follow-up rate, and the conduct of intention-to-treat analysis were the bases for the study quality evaluation. The abstracted information was entered directly into a Microsoft Excel spreadsheet and later imported to Comprehensive Meta-analysis software 3.0 for Windows (Biostat, Englewood, NJ, United States).
Statistical analysis
The effect size was measured by the event rate for PGA, PaGA and mTLSS (frequency of improvement) that was calculated for each trial within each study in addition to the pooled sample of all studies in both fixed and random models. To evaluate the difference in effect sizes for different drug doses or drug versus placebo, the pooled odds ratio (ORs) and their 95% confidence intervals (CIs) are identified for each research and all pooled studies. Besides, the within- and between-study heterogeneity is assessed using Cochran’s Q statistic. The possibility of the null hypothesis that all studies are assessing a similar impact is evaluated by the heterogeneity test. An arbitrary effect model was used for meta-analysis when a noteworthy Q statistic (P < 0.1) demonstrates heterogeneity crosswise over researches. On the other hand, a settled effect model was used when heterogeneity is not indicated.[12] The arbitrary effect model displaying the approval of the studies indicates significantly different qualities and evaluates both the within-study sampling errors and between-study variances.[13]
When study groups are homogenous, the two models are similar, but if this is not the case, a random effects model usually provides wider CIs than a fixed effects model. A random effects model is best used in the presence of significant between-study heterogeneity.[13] We quantified the effect of heterogeneity using the recently developed I2 measure, where I2=100% × (Q–df)/Q.[14] The I2 measure ranges between 0 and 100%, and represents the proportion of inter-study variability attributable to heterogeneity rather than chance. I2 values of 25%, 50%, and 75% were defined as low, moderate, and high estimates, respectively.
Evaluation of publication bias
Due to the limitations of the funnel plot, which requires a range of studies of varying sizes involving subjective judgments, we evaluated publication bias through Egger’s linear regression test,[15,16] which measures funnel plot asymmetry using a natural logarithmic scale for ORs.
Results
From 28 reports that matched inclusion criteria, we selected 15 involving 3734 patients with CHE [Figure 1 and Table 1] [1,5,8,9,17-27] Six were blinded, randomized controlled clinical trials with placebo; the other 9 reports were of non-controlled trials. These 15 clinical trials have included 30 study groups, 24 involved 3010 cases treated with the drug alitretinoin using doses ranging between 10 and 40 mg for a duration of 12–36 weeks, while in the other 6 study groups, 724 patients were treated with a placebo for the same duration. Patient mean age was 50.5 years with a male-to-female ratio of 1968/1478. All these patients were recruited from the European and American population.
Figure 1.
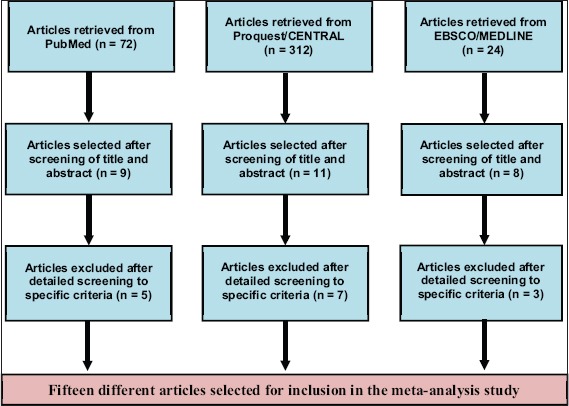
Articles evaluated for inclusion in the meta-analysis
Table 1.
Data collected from 15 clinical trials of alitretinoin for treatment of chronic hand eczema

The forest plot presented in Figure 2 shows the PGA results in patients with CHE either treated with the drug alitretinoin or placebo considering the dose and duration in a random model. Patients receiving alitretinoin showed an effect size PGA of 40% (30–51%) for the dose of 10 mg/day from 7 study groups; 52% (28–75%) for 20 mg/day from 2 study groups; 58% (50–66%) for the dose 30 mg/day from 13 study groups; and 69% (47–85%) for the dose 40 mg/day from 2 study groups while those receiving placebo showed a PGA rate of 23% (15–32%) from 6 study groups. Thus, among alitretinoin-treated patients, the PGA effect size was directly proportional to the drug dosage ranging from 40% to 69% way better than that for placebo.
Figure 2.
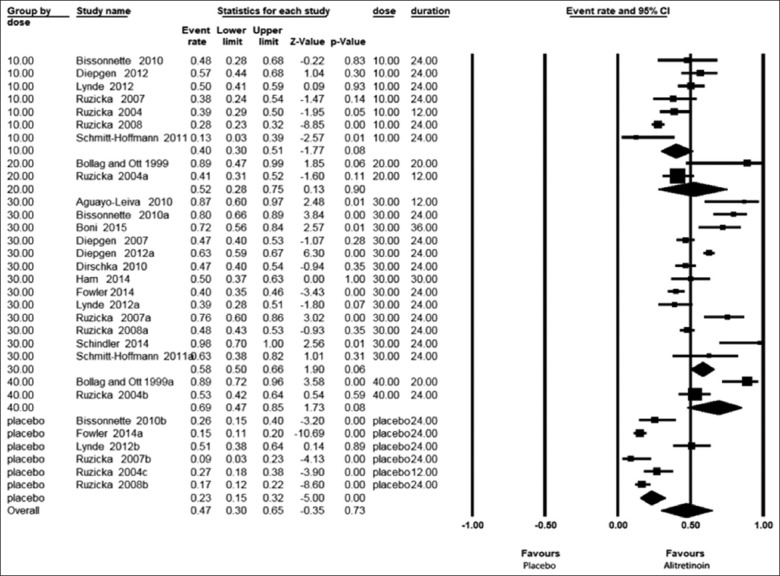
Forest plots of physician global assessment in all patients with chronic hand eczema either treated with alitretinoin or placebo taking duration into consideration in a random model
The forest plot presented in Figure 3 shows the PaGA results in all patients with CHE either treated with the drug alitretinoin or placebo considering also the duration of treatment in a random model. Patients receiving alitretinoin showed an effect size PaGA of 28.8% (17.8–42.9%) for the dose of 10 mg/day from three study groups; 43.4% (22.5–66.9%) for the 20 mg/day from 2 study groups; 49% (37.8–60.3%) for the dose 30 mg/day from 5 study groups; and 62.4% (41–79.8%) for the dose 40 mg/day from 2 study groups while those received placebo showed a PaGA rate of 18.9% (12.1–28.4%) from 4 study groups. From this plot, we could deduce that patient’s perception (PaGA) was less than that of the physician (PGA) ranging from 28.8% to 62.4% but still far better than that of placebo.
Figure 3.
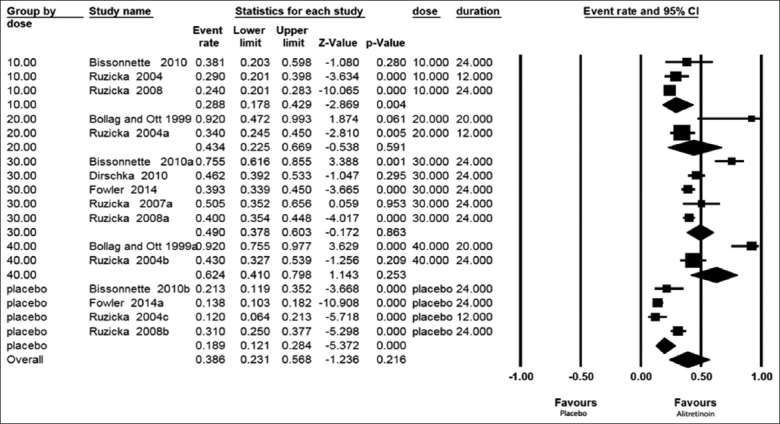
Forest plots of a patient of patient global assessment in all patients with chronic hand eczema either treated with alitretinoin or placebo taking duration into consideration in a random model
The forest plot presented in Figure 4 shows the mTLSS results in all patients with CHE either treated with the drug alitretinoin or placebo considering also the duration of treatment in the random model.
Figure 4.
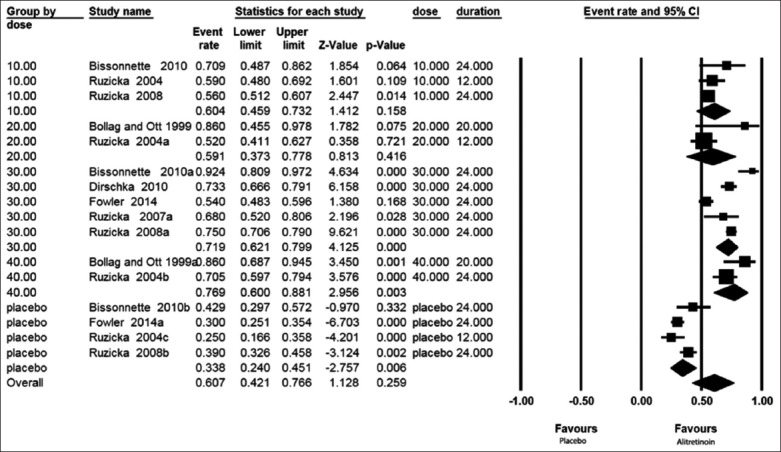
Forest plots of the modified total lesion symptom score in all patients with chronic hand eczema either treated with alitretinoin or placebo taking duration into consideration in a random model
Patients receiving alitretinoin showed an effect size mTLSS of −60.4% (−45.9% –−73.2%) for the dose of 10 mg/day from 3 study groups; −59.1% (−37.3%–−77.8%) for the 20 mg/day from 2 study groups; −71.9% (−62.1%–−79.9%) for the dose 30 mg/day from 5 study groups; and 76.9% (−60%–−88.1%) for the dose 40 mg/day from 2 study groups while those received placebo showed a mTLSS rate of −33.8% (−24%–−45.1%) from 4 study groups.
Testing heterogeneity using the Q test and I2 values showed that these studies were as heterogeneous (P = 0.01) as has been expected, mainly due to the difference in patient’s ethnicity, age and gender in addition to disease characteristics in various studies. The heterogeneity of PGA was I2 = 92.76 (P = 0.01), PaGA I2 = 91.30 (P = 0.01), and mTLSS I2 = 93.90 (P = 0.01). Therefore, a random model was used in the analysis. Publication bias was not significant (Egger regression test, P > 0.05) as being present in the funnel plots, in which the Egger regression test for PGA was 0.74, for PaGA was 0.41, and for mTLSS was 0.45.
We compared the PGA score in patients with CHE receiving high doses of the drug alitretinoin (30-40 mg/day) versus those receiving a lower dose of the drug (10-20 mg/day). Figure 5 shows that the effect size PGA at the high dose, 56% (49–63%), is much higher than the smaller dose, 40% (32–49%). The OR (95% CI) for PGA was 2 (1.3–3.1) [Figure 6], indicating that the effect of high-dose treatment was about twice that of the low-dose.
Figure 5.
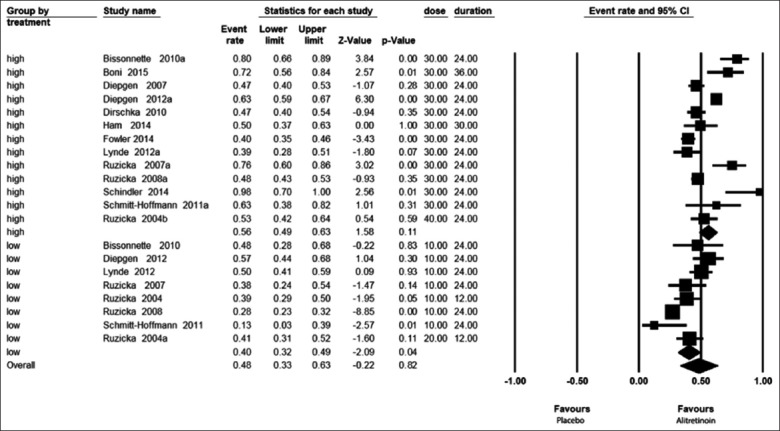
Forest plots showing effect size by physician global assessment with high- and low-dose alitretinoin taking duration into consideration in a random model
Figure 6.
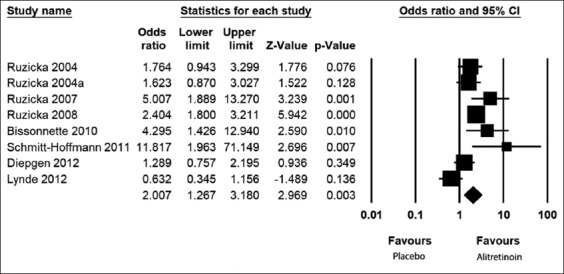
Forest plots showing odds ratios by physician global assessment with high-dose alitretinoin versus low-dose taking duration into consideration in a random model
Comparative meta-analysis of the effect size PGA, PaGA, and mTLSS scores among cases with CHE treated with alitretinoin versus placebo considered both the dose and duration in the final calculations. Due to the significant heterogeneity of outcomes, a random model was selected for analysis. The OR (95% CI), when taking duration into consideration, was 2.89 (1.83–4.56) for PGA, 2.88 (1.52–5.46) for PaGA, and 3.88 (2.68–5.64) for mTLSS, indicating that the effect of treatment with alitretinoin was about 3 to 4 times that of the placebo.
The most common complication reported was headache, which averaged about 15% of treated cases followed by a high cholesterol level in 10% of cases. Relapse was reported in 2 trials in about 44% of patients after stopping the drug in a follow-up period of 48 weeks [Table 2].
Table 2.
Alitretinoin side effects and relapse rate

Discussion
Hand eczema, as often as possible, forms into a chronic state, with disease staying dynamic even if of allergens and/or aggravations thought to be causative are avoided.[28] It is assessed that severe CHE is reported for 5–7% of hand eczema patients and 2–4% are inert to standard treatment.[1] The National Institute for Health and Care Excellence has authorized and suggested alitretinoin as the first and only evidence-based therapy for healing the severe CHE unresponsive to topical corticosteroids.[7] Half of the patients cured for up to 24 weeks have “clear” or “almost clear” status. For further reaction, 39% and 50% of the patients can be cured within another 12–24 weeks of treatment.[7] The researchers have endeavored to reveal insight into the effectiveness of alitretinoin treatment for CHE noticing that - as far as the authors know - this is the first meta-analysis on this subject. Different scores were utilized to survey the change of patients’ health state with CHE; the essential endpoint was the PGA, and the reaction was characterized as “clear” or “almost clear” toward the end of treatment. Secondary endpoints included PaGA, mTLSS, extent of disease and time to respond.[9]
Various outcomes of treatment for CHE using alitretinoin have been reported by dermatological researchers. For instance, a very low PGA outcome (13%) was reported by Schmitt-Hoffmann et al. (2011)[21] at a dose of 10 mg/day, while the very high PGA outcome (98%) was reported by Schindler et al. (2014)[26] at dose 30 mg/day. Placebo therapy in some reports showed a degree of improvement in PGA ranging between 9% as reported by Ruzicka et al. (2007)[17] and 51% reported by Lynde et al. (2012).[22] In our meta-analysis, the effect size of PGA amounted to approximately 50% of treated cases. This signifies that this drug gave potentially promising results in these patients from the viewpoint of the treating physicians. On the other hand, the PaGA, which reflects the viewpoint of patients, also varied between studies. Examples of a very high PaGA outcome were reported by Bollag and Ott (1999)[5] (92%), while a very low PaGA outcome was reported by Ruzicka et al. (2004)[8] (24%) at dose 10 mg/day. Furthermore, placebo therapy in some reports showed a degree of improvement in PGA ranging between 14% reported by Fowler et al. (2014)[25] and 31% reported by Ruzicka et al. (2008).[9] Interestingly, the patient viewpoint assessment reflected by the PaGA score was lower than the physician assessment. This meta-analysis showed a PaGA effect size of approximately 40%, which is lower than the PGA. Similarly, the outcomes for mTLSS varied by dermatologists, ranging between 52% and 92%, according to Ruzicka et al. (2004)[8] and Bissonnette et al. (2010),[19] respectively. The same was true with placebo therapy. In fact, some researchers reported a far better result versus placebo, such as Ruzicka et al. (2007)[17] at a dose of 30 mg/day (76% vs. 9%, OR = 31.16). On the contrary, Lynde et al. (2012)[22] at an alitretinoin dose of 30 mg/day reported lower PGA results of the drug versus placebo (39% vs. 51%, OR = 0.98). Furthermore, in PaGA and mTLSS, far better results were reported for alitretinoin versus placebo. As an example, a very high PaGA was shown by Bissonnette et al. (2010)[19] at a dose 30 mg/day (75% vs. 21%, OR = 11.4), while Ruzicka et al. (2008)[9] reported worse results at a dose 10 mg/day (24% vs. 31%, OR = 0.7). In this meta-analysis, we compared the results of alitretinoin-treated patients with those treated with placebo. Among pooled studies, the OR for effect size for the PGA, PaGA, and mTLSS scores was 3–4 times better with alitretinoin versus placebo.
Some researchers reported a better result with alitretinoin at a high dose (30–40 mg/day) as reported by Schindler et al. (2014)[26] (98%) versus that reported by Fowler et al. (2014) (40%).[25] Schmitt-Hoffmann et al. (2011)[21] reported 13% with a low-dose (10–20 mg/day) versus 89% reported by Bollag and Ott (1999).[5] Interestingly, some authors classified the etiology of CHE as hyperkeratotic, pompholyx, fingertip dermatitis, or not specified.[5,9,20-22,24,26] Others described the etiology of CHE as irritant, atopic, allergic, vesicular/pompholyx, hyperkeratotic-rhagadiform, and fingertip chronic irritation.[23] It was apparent that no matter the type of eczema, alitretinoin proved effective. The majority of the side effects of alitretinoin therapy are dose-related and generally well tolerated; all side effects were considered mild to moderate. Headaches and flushing have been the most frequently reported side effects.[9] In this meta-analysis, the major side effects were in the form of a headache (15%), flushing (7%), upper respiratory tract infection (5%), mucocutaneous (3%), erythema (5%), and cheilitis (6%), while the laboratory findings were elevated cholesterol (10%) and elevation in triglycerides (7%). After a dose reduction, side effects decreased or disappeared[5] therefore, a low-dose reduces the risk of side effects.
Two incidents of relapse after stopping the drug were reported by Ruzicka et al. (2008)[9] (33%) and Aguayo-Leiva et al. (2011)[18] (54%); the relapses occurred within the 24-week follow-up period.
The students included in the meta-analysis did not state clearly whether CHE was also associated with atopic dermatitis, or not except for one study attributing causation to genetic factors (early with atopic dermatitis) in 41% of cases.[29] Similarly, there was no clear statement on the response rate in cases of chronic eczema associated with atopic dermatitis versus others.
Top topical potency steroids with or without occlusion for 4–8 weeks, followed by alitretinoin 30 mg/day for at least 3–6 months represent the reference treatment for CHE. The researchers prefer alternative treatments such as phototherapy, methotrexate, cyclosporine, and mycophenolate mofetil and others if there is attrition for clearing followed by topical medications.[30] Another study by Politiek et al. (2016)[31] comparing alitretinoin and acitretin in severe CHE found that both treatments were effective in hyperkeratotic hand eczema. Fewer patients stopped using alitretinoin compared with acitretin due to side effects.
For treatment other than alitretinoin, there is no strong evidence of efficacy (RCTs), and no other systemic treatments are licensed. Alitretinoin was effective in a large non-interventional study; however, the clinical experience with alitretinoin is still limited. The cost is significantly higher than other retinoid products,[32] and the traditionally used systemic drugs have not compared with “head-to-head” trials yet. In Switzerland, alitretinoin is a cost-effective alternative for the therapy of CHE patients.[33] In addition; an incremental cost-effectiveness ratio ICER of £12,931 per quality-adjusted life-year QALY gained versus supportive care (placebo) was recorded for alitretinoin.[34]
Based on this meta-analysis, we conclude that when considering tolerability, efficacy and patient satisfaction, alitretinoin therapy for treatment of CHE resulted in a good percentage of clearance of the lesion in about 50% of cases, particularly using a higher dose of the drug for the duration of 24 weeks.
References
- 1.Diepgen TL, Agner T, Aberer W, Berth-Jones J, Cambazard F, Elsner P, et al. Management of chronic hand eczema. Contact Dermatitis. 2007;57:203–10. doi: 10.1111/j.1600-0536.2007.01179.x. [DOI] [PubMed] [Google Scholar]
- 2.Held E, Skoet R, Johansen JD, Agner T. The hand eczema severity index (HECSI):A scoring system for clinical assessment of hand eczema. A study of inter- and intraobserver reliability. Br J Dermatol. 2005;152:302–7. doi: 10.1111/j.1365-2133.2004.06305.x. [DOI] [PubMed] [Google Scholar]
- 3.Cvetkovski RS, Zachariae R, Jensen H, Olsen J, Johansen JD, Agner T, et al. Quality of life and depression in a population of occupational hand eczema patients. Contact Dermatitis. 2006;54:106–11. doi: 10.1111/j.0105-1873.2006.00783.x. [DOI] [PubMed] [Google Scholar]
- 4.Van Coevorden AM, Coenraads PJ, Svensson A, Bavinck JN, Diepgen TL, Naldi L, et al. Overview of studies of treatments for hand eczema-the EDEN hand eczema survey. Br J Dermatol. 2004;151:446–51. doi: 10.1111/j.1365-2133.2004.06040.x. [DOI] [PubMed] [Google Scholar]
- 5.Bollag W, Ott F. Successful treatment of chronic hand eczema with oral 9-cis retinoic acid. Dermatology. 1999;199:308–12. doi: 10.1159/000018280. [DOI] [PubMed] [Google Scholar]
- 6.Geiger JM, Hommel L, Harms M, Saurat JH. Oral 13-cis retinoic acid is superior to 9-cis retinoic acid in sebosuppression in human beings. J Am Acad Dermatol. 1996;34:513–5. doi: 10.1016/s0190-9622(96)90462-4. [DOI] [PubMed] [Google Scholar]
- 7.King T, McKenna J, Alexandroff AB. Alitretinoin for the treatment of severe chronic hand eczema. Patient Prefer Adherence. 2014;8:1629–34. doi: 10.2147/PPA.S38830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ruzicka T, Larsen FG, Galewicz D, Horva'th A, Coenraads PJ, Thestrup-Pedersen K, et al. Oral alitretinoin (9-cisretinoic acid) therapy for chronic hand dermatitis in patients refractory to standard therapy:Results of a randomized, double-blind, placebocontrolled, multicenter trial. Arch Dermatol. 2004;140:1453–9. doi: 10.1001/archderm.140.12.1453. [DOI] [PubMed] [Google Scholar]
- 9.Ruzicka T, Lynde CW, Jemec GB, Diepgen T, Berth-Jones J, Coenraads PJ, et al. Efficacy and safety of oral alitretinoin (9- cis retinoic acid) in patients with severe chronic hand eczema refractory to topical corticosteroids:Results of a randomized, double-blind, placebo-controlled, multicentre trial. Br J Dermatol. 2008;158:808–17. doi: 10.1111/j.1365-2133.2008.08487.x. [DOI] [PubMed] [Google Scholar]
- 10.Moher D, Liberati A, Tetzlaff J, Altman DG PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses:The PRISMA statement. Int J Surg. 2010;8:336–41. doi: 10.1016/j.ijsu.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 11.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology:A proposal for reporting. meta-analysis of observational studies in epidemiology (MOOSE) group. JAMA. 2000;283:2008–12. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 12.Smith GD, Egger M. Meta-analyses of randomised controlled trials. Lancet. 1997;350:1182. doi: 10.1016/s0140-6736(05)63833-0. [DOI] [PubMed] [Google Scholar]
- 13.Der Simonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 14.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–58. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 15.Duval S, Tweedie R. Trim and fill:A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56:455–63. doi: 10.1111/j.0006-341x.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- 16.Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ruzicka T, Gupta A, Jemec G, Gerlach B, Maares J, Wevelsiep L. Efficacy and safety of alitretinoin (9-cis retinoic acid) in severe chronic hand eczema refractory to topical treatment (Benefit of Alitretinoin in Chronic Hand eczema;BACH Study) Poster Present Eur Acad Dermatol Venereol (EADV) 2007:275. [Google Scholar]
- 18.Aguayo-Leiva IR, Urrutia S, Jaén-Olasolo P. Response to treatment with oral alitretinoin in patients with chronic hand eczema that is refractory to treatment with potent topical corticosteroids:Experience in 15 patients. Actas Dermosifiliogr. 2011;102:616–22. doi: 10.1016/j.ad.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 19.Bissonnette R, Diepgen TL, Elsner P, English J, Graham-Brown R, Homey BL, et al. Redefining treatment options in chronic hand eczema (CHE) J Eur Acad Dermatol Venereol. 2010;24(suppl 3):1–20. doi: 10.1111/j.1468-3083.2010.03615.x. [DOI] [PubMed] [Google Scholar]
- 20.Dirschka T, Reich K, Bissonnette R, Maares J, Brown T, Diepgen TL. An open-label study assessing the safety and efficacy of alitretinoin in patients with severe chronic hand eczema unresponsive to topical corticosteroids. Clin Exp Dermatol. 2010;36:149–54. doi: 10.1111/j.1365-2230.2010.03955.x. [DOI] [PubMed] [Google Scholar]
- 21.Schmitt-Hoffmann AH, Roos B, Sauer J, Spickermann J, Stoeckel K, Edwards D. Pharmacokinetics, efficacy and safety of alitretinoin in moderate or severe chronic hand eczema. Clin Exp Dermatol. 2011;36(Suppl 2):29–34. doi: 10.1111/j.1365-2230.2011.04035.x. [DOI] [PubMed] [Google Scholar]
- 22.Lynde C, Cambazard F, Ruzicka T, Sebastian M, Brown TC, Maares J. Extended treatment with oral alitretinoin for patients with chronic hand eczema not fully responding to initial treatment. Clin Exp Dermatol. 2012;37:712–7. doi: 10.1111/j.1365-2230.2012.04396.x. [DOI] [PubMed] [Google Scholar]
- 23.Diepgen TL, Pfarr E, Zimmermann T. Efficacy and tolerability of alitretinoin for chronic hand eczema under daily practice conditions:Results of the TOCCATA open study comprising 680 patients. Acta Derm Venereol. 2012;92:251–5. doi: 10.2340/00015555-1256. [DOI] [PubMed] [Google Scholar]
- 24.Ham K, Maini P, Gooderham MJ. Real-world experience with alitretinoin in a community dermatology practice setting in patients with chronic hand dermatitis. J Cutan Med Surg. 2014;18:332–6. doi: 10.2310/7750.2014.13195. [DOI] [PubMed] [Google Scholar]
- 25.Fowler JF, Graff O, Hamedani AG. A phase 3, randomized, double-blind, placebo-controlled study evaluating the efficacy and safety of alitretinoin (BAL4079) in the treatment of severe chronic hand eczema refractory to potent topical corticosteroid therapy. J Drugs Dermatol. 2014;13:1198–204. [PubMed] [Google Scholar]
- 26.Schindler M, Drozdenko G, Kühl AA, Worm M. Immunomodulation in patients with chronic hand eczema treated with oral alitretinoin. Int Arch Allergy Immunol. 2014;165:18–26. doi: 10.1159/000365659. [DOI] [PubMed] [Google Scholar]
- 27.Boni E, Pattini S, Guanti M, Giusti F, Pellacani G, Pepe1 P. Hand eczema and other inflammatory skin diseases:Efficacy of oral alitretinoin. Clin Transl Allergy. 2015;5(Suppl 1):O23. [Google Scholar]
- 28.Meding B, Wrangsjo K, Jarvholm B. Fifteen-year follow-up of hand eczema:Persistence and consequences. Br J Dermatol. 2005;152:975–80. doi: 10.1111/j.1365-2133.2005.06494.x. [DOI] [PubMed] [Google Scholar]
- 29.Lerbaeck A, Kyvik KO, Mortensen J, Bryld LE, Menné T, Agner T. Heritability of hand eczema is not explained by comorbidity with atopic dermatitis. J Invest Dermatol. 2007;127:1632–40. doi: 10.1038/sj.jid.5700750. [DOI] [PubMed] [Google Scholar]
- 30.Lahfa M. Management of chronic hand eczema. Ann Dermatol Venereol. 2014;141(Suppl 1):S143–50. doi: 10.1016/S0151-9638(14)70151-6. [DOI] [PubMed] [Google Scholar]
- 31.Politiek K, Christoffers WA, Coenraads PJ, Schuttelaar MA. Alitretinoin and acitretin in severe chronic hand eczema;results from a retrospective daily practice study. Dermatol Ther. 2016;29:364–71. doi: 10.1111/dth.12362. [DOI] [PubMed] [Google Scholar]
- 32.Diepgen TL, Andersen KE, Chosidow O, Coenraads PJ, Elsner P, English J, et al. Guidelines for diagnosis, prevention and treatment of hand eczema. J Dtsch Dermatol Ges. 2015;13:e1–22. doi: 10.1111/ddg.12510_1. [DOI] [PubMed] [Google Scholar]
- 33.Blank PR, Blank AA, Szucs TD. Cost-effectiveness of oral alitretinoin in patients with severe chronic hand eczema--a long-term analysis from a Swiss perspective. BMC Dermatol. 2010;10:4. doi: 10.1186/1471-5945-10-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Paulden M, Rodgers M, Griffin S, Slack R, Duffy S, Ingram JR, et al. Alitretinoin for the treatment of severe chronic hand eczema. Health Technol Assess. 2010;14(Suppl 1):39–46. doi: 10.3310/hta14Suppl1/06. [DOI] [PubMed] [Google Scholar]


