Abstract
Background
Aspergillus fumigatus is frequently encountered in sputum samples of patients with cystic fibrosis (CF), which traditionally has been interpreted as saprophytic airway colonization. However, this mere bystander role has been challenged by recent data. There is now evidence that Aspergillus fumigatus accelerates the decline of pulmonary function.
(1→3)-β-D-glucan (BDG) and galactomannan (GM) are highly sensitive fungal biomarkers that are used to diagnose invasive fungal disease. However, their diagnostic value in CF patients is largely unknown.
Methods
We conducted a retrospective cohort study on 104 CF patients to determine whether serum BDG and GM levels correlate with parameters such as Aspergillus-positive sputum cultures and lung function.
Results
Aspergillus fumigatus was persistently detected in 22 of the 104 CF patients (21%). Mean serum BDG and GM levels in the Aspergillus-positive patients were significantly higher than in those without persistent Aspergillus detection (89 versus 40 pg/ml [p = 0.022] and 0.30 versus 0.15 ODI [p = 0.013], respectively). 27 and 7 patients had elevated BDG (≥ 60 pg/ml) or GM levels (> 0.5 ODI), respectivly. BDG and GM levels showed a significant correlation (p = 0.004). Patients with increased serum concentrations of BDG were more frequently Aspergillus-positive (40.7 versus 14.3%, p = 0.004) and had a significantly lower forced expiratory volume in one second (FEV1) than patients with a normal BDG (61.6 versus 77.1%, p = 0.007). In the multivariate analysis, BDG but not GM or the growth of A. fumigatus, proved to be an independent predictor for the FEV1.
Conclusions
CF patients with persistent Aspergillus detection have elevated BDG and GM levels which ranged between healthy and invasively infected patients. Serum BDG may be superior to GM and fungal culture in predicting an impaired lung function in CF patients.
Keywords: (1→3)-β-D-glucan, Galactomannan, Biomarker, Cystic fibrosis, Aspergillus, Allergic bronchopulmonary aspergillosis, ABPA, Aspergillosis, Lung function, FEV1
Background
Cystic fibrosis (CF) is the most frequent lethal autosomal recessive disorder in Caucasians. It is caused by mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) gene leading to impaired ion transport across epithelial cells. The disease affects primarily the exocrine glands, resulting in pancreatic insufficiency and progressive pulmonary disease; the latter is the major cause of morbidity and mortality in CF patients. Within the lungs, highly viscous sputum facilitates the colonization and infection of the lower respiratory tract with various microorganisms [1].
While the role of bacterial pathogens, like Staphylococcus aureus, Pseudomonas aeruginosa or Burkholderia cepacia complex, for the progression of the disease is well established, far less is known about the impact of chronic airway colonization with fungi. Aspergillus fumigatus, Scedosporium species, Candida albicans and Exophiala dermatitidis are frequently encountered in sputum samples of CF patients [2]. In particular, the traditional view on A. fumigatus, which has been regarded as saprophytic microorganism with doubtful clinical significance, is challenged by recent data. There is evidence that chronic A. fumigatus colonization is associated with an increased risk of hospitalization for pulmonary exacerbation and lower lung function and that this effect is aggravated by P. aeruginosa co-infection [3, 4]. Furthermore, it seems that antifungal therapy is of benefit for patients who are non-responsive to antibiotics and that the administration of itraconazole to A. fumigatus-sensitized CF patients can improve lung function [5, 6].
Galactomannan (GM) is a major component of the fungal cell wall. It is primarily detected in the serum or bronchoalveolar lavage fluid specimens of patients with invasive aspergillosis [7]. Another fungal antigen used for this purpose is (1→3)-β-D-glucan (BDG). BDG differs in several aspects from GM. Firstly, it is produced by all medically relevant fungi (e.g. Candida spp., Aspergillus spp., Pneumocystis jirovecii) apart from zygomycetes and Cryptococcus neoformans which contain no or only low amounts of BDG in their cell walls [8]. Secondly, BDG has superior sensitivity for diagnosis of invasive aspergillosis compared to serum GM [9].
Only little is known about serum BDG and GM levels in patients with CF. Because of their high sensitivity, BDG and/or GM measurements might help to identify patients with fungus-associated morbidity. Therefore, we initiated a retrospective study in order to analyze causal relationships between clinical and microbiological parameters and BDG- as well as GM-antigenemia.
Methods
Study population
We conducted a retrospective cohort study at the University Hospital Erlangen, Germany, a 1400-bed tertiary care hospital. All patients with CF (children, adolescents and adults), who presented to the CF outpatient clinic between September 2015 and October 2016, were enrolled. Patients with a history of lung transplantation were excluded. As part of the routine diagnostic work-up paired respiratory and serum samples were taken.
Microbiological analyses
Respiratory samples were analyzed for the growth of bacteria (e.g. S. aureus, P. aeruginosa) and fungi (e.g. A. fumigatus) using Columbia blood agar plates, chocolate agar plates, endo agar plates and Sabouraud dextrose agar plates with 0.5 mg/ml chloramphenicol. If necessary, the samples were pretreated with dithiothreitol at a final concentration of 50 μg/ml. The agar plates were then incubated for at least seven days at 37 °C in ambient air with 5% CO2 for the culture of bacteria and at 28 °C in ambient air for the culture of fungi. Relevant microorganisms were differentiated to the species level by Matrix Assisted Laser Desorption Ionization - Time of Flight Mass Spectrometry (MALDI-TOF-MS; Bruker Daltonik GmbH, Germany). Filamentous fungi were differentiated by microscopy of a lactophenol cotton blue wet mount preparation.
Serum samples were tested for Aspergillus-specific antibodies (Aspergillose Fumouze, Fumouze Diagnostics, France), Aspergillus-specific IgE, recombinant Aspergillus antigens f4 and f6 [rAsp f4 IgE, rAsp f6 IgE] (ImmunoCAP 250, Thermo Fisher Scientific, Sweden) and IgG against alkaline protease, elastase and exotoxin A of P. aeruginosa (Mediagnost GmbH, Germany). The sera were then stored at − 20 °C. In October 2016, the stored samples were tested in batch for their content of BDG (Fungitell® assay; Associates of Cape Cod, USA) and GM (Platelia™ Aspergillus Ag assay; Bio-Rad, France). Both assays were performed according to the manufacturer’s instructions. BDG levels above the upper validation limit were diluted and retested. BDG levels below the lower validation limit were calculated by extrapolation. In July 2017, the same serum samples were tested for human fatty acid binding protein 2 (FABP2) using the Quantikine ELISA human FABP2/I-FABP kit (R&D Systems Europe, UK) according to the manufacturer’s instructions. The use of these sera was approved by the local ethics committee (application number 7-17B).
Clinical data and classification of patients
Demographic data and clinical information (body mass index [BMI], cough frequency, sputum production, annual number of pulmonary exacerbations, anti-infective therapy, corticosteroid use, exocrine pancreatic insufficiency, CF-related Diabetes mellitus [CFRD], CF-related liver disease [CFLD], forced expiratory volume in 1 s [FEV1predicted] at serum sampling, and immunological results (C-reactive protein [CRP], leucocyte count [WBC], total serum IgG and IgE) were obtained from all patients analyzed. Following the Global Initiative for Chronic Obstructive Lung Disease (GOLD) classification of severity of airflow obstruction, the study population was divided into patients with mild or no airflow limitation (FEV1predicted ≥ 80%) and patients with moderate to very severe airflow limitation (FEV1predicted < 80%) at the time of serum sampling. Furthermore, on the basis of the Leed’s criteria for chronic P. aeruginosa infection [10], patients were classified according to their respiratory culture results during the past two years in persistently colonized (growth of A. fumigatus in > 50% of total samples or at least in all of the last three samples before serum sampling), intermittently colonized (detection of A. fumigatus that does not fulfil the criteria for persistently colonized patients) and not colonized (no detection of A. fumigatus).
Statistical analysis
Statistical analysis was performed using SPSS-V24 (SPSS Inc., USA) and MedCalc Statistical Software-V16.8 (MedCalc Software bvba, Belgium). Data are given as mean +/− standard deviation (SD) or median with interquartile range (IQR). Receiver-operating-characteristic (ROC)-analysis was used to evaluate the ability of BDG and GM to distinguish between Aspergillus-positive and Aspergillus-negative patients. The highest Youden index indicated the optimal cut-off. As BDG and GM values were not normally distributed, the chi-square test, the Mann-Whitney-U test and the Spearman coefficient of correlation were used for comparison of variables. Differences were considered significant when p values were < 0.05.
Results
Study population
The local cystic fibrosis cohort consisted of 137 patients. Archived serum samples were available from 107 patients. Three patients had to be excluded, because BDG-testing repeatedly produced discrepant results (n = 2) or a respiratory tract specimen for microbiological analysis was not taken at the day of serum sampling (n = 1). Thus, 104 patients were included in the study.
Demographic data, clinical and microbiological results for all patients as well as for subgroups (stratified according to FEV1predicted values at the time of serum sampling, the growth of A. fumigatus and the concentration of serum BDG and GM) are shown in Tables 1 and 2. Correlations of FEV1predicted values, BDG and GM concentrations with continuous clinical and microbiological parameters are given in Table 3 and Fig. 1.
Table 1.
Patient demographics, microbiological and clinical results stratified after FEV1 at serum sampling and persistent A. fumigatus detection
| All patients | FEV1 at serum sampling | Persistent A. fumigatus detection | |||||
|---|---|---|---|---|---|---|---|
| (n = 104) | (< 80% pred.) (n = 55) | (≥80% pred.) (n = 42) | P value | No (n = 82) | Yes (n = 22) | P value | |
| Age [years (min-max)] | 19.3 (4–51) | 24.0 (6–51) | 15.7 (7–31) | < 0.001 | 17.9 (4–51) | 24.8 (10–51) | 0.001 |
| Sex [female/male] | 43/61 | 22/33 | 17/25 | 0.962 | 32/50 | 11/11 | 0.353 |
| BMI [kg/m2 (± STD)] | 19.7 (± 3.9) | 20.4 (± 3.6) | 19.2 (± 3.6) | 0.114 | 19.2 (± 3.7) | 21.6 (± 3.8) | 0.006 |
| Mean BDG [pg/ml (± STD)] | 50 (± 84) | 68 (± 100) | 32 (± 57) | 0.003 | 40 (± 63) | 89 (± 131) | 0.022 |
| Median BDG [pg/ml (IQR)] | 22 (3–62) | 37 (12–82) | 11 (0–42) | 18 (2–48) | 50 (14–115) | ||
| BDG [normal/elevated, (% elevated)] | 77/27 (26.0%) | 36/19 (34.5%) | 36/6 (14.3%) | 0.024 | 66/16 (19.5%) | 11/11 (50.0%) | 0.004 |
| Mean GM [ODI (± STD)] | 0.18 (± 0.22) | 0.22 (± 0.28) | 0.14 (± 0.10) | 0.150 | 0.15 (± 0.12) | 0.30 (± 0.40) | 0.013 |
| Median GM [ODI (IQR)] | 0.10 (0.1–0.2) | 0.10 (0.1–0.2) | 0.10 (0.1–0.1) | 0.10 (0.1–0.1) | 0.10 (0.1–0.3) | ||
| GM [normal/elevated, (% elevated)] | 97/7 (6.7%) | 49/6 (10.9%) | 41/1 (2.4%) | 0.108 | 78/4 (4.9%) | 19/3 (13.6%) | 0.145 |
| Persistent A. fumigatus detection [no. of patients (%)] | 22 (21.2%) | 16 (29.1%) | 6 (14.3%) | 0.084 | – | – | – |
| A. fumigatus-specific antibodies [normal/elevated, (% elevated)] | 100/4 (3.8%) | 52/3 (5.5%) | 41/1 (2.4%) | 0.451 | 78/4 (4.9%) | 22/0 (0%) | 0.291 |
| A. fumigatus-specific IgE level [kU/l, (± STD)] | 3.8 (± 9.9) | 5.2 (± 11.3) | 2.3 (± 8.2) | 0.004 | 4.5 (± 10.9) | 1.1 (± 2.0) | 0.687 |
| rAsp f4-IgE level [kUA/l, (± STD)] | 0.5 (± 2.5) | 0.7 (± 3.1) | 0.3 (± 1.5) | 0.073 | 0.6 (± 2.7) | 0.1 (± 0.2) | 0.879 |
| rAsp f6-IgE level [kUA/l (± STD)] | 0.5 (± 2.3) | 0.7 (± 3.0) | 0.1 (± 0.7) | 0.245 | 0.6 (± 2.5) | 0.0 (± 0.0) | 0.118 |
| S. aureus detection [no. of patients (%)] | 11 (10.6%) | 2 (3.6%) | 7 (16.7%) | 0.028 | 10 (12.2%) | 1 (4.5%) | 0.300 |
| P. aeruginosa detection [no. of patients (%)] | 35 (33.7%) | 31 (56.4%) | 4 (9.5%) | < 0.001 | 22 (26.8%) | 13 (59.1%) | 0.004 |
| P. aeruginosa-specific antibodies [normal/elevated, (% elevated)] | |||||||
| against alkaline protease | 70/14 (16.7%) | 26/12 (31.6%) | 38/1 (2.6%) | 0.001 | 62/10 (13.9%) | 8/4 (33.3%) | 0.094 |
| against elastase | 69/15 (17.9%) | 25/13 (34.2%) | 38/1 (2.6%) | < 0.001 | 60/12 (16.7%) | 9/3 (25.0%) | 0.485 |
| against exotoxin A | 65/19 (22.6%) | 24/14 (36.8%) | 34/5 (12.8%) | 0.015 | 58/14 (19.4%) | 7/5 (41.7%) | 0.088 |
| WBC [× 103/μl (± STD)] | 9.1 (± 4.3) | 10.1 (± 5.2) | 7.5 (± 2.4) | 0.002 | 8.8 (± 3.3) | 10.1 (± 7.0) | 0.891 |
| CRP [mg/l (± STD)] | 6.5 (± 19.1) | 11.6 (± 25.1) | 0.7 (± 1.0) | < 0.001 | 4.6 (± 12.2) | 13.6 (± 33.6) | 0.114 |
| Total IgG [lowered/normal/elevated] | 12/62/25 | 3/26/21 | 7/31/4 | 0.002 | 12/49/19 | 0/13/6 | 0.187 |
| Total IgE level [normal/elevated, (% elevated)] | 65/37 (36.3%) | 27/26 (49.1%) | 33/9 (21.4%) | 0.006 | 54/27 | 11/10 | 0.225 |
| Fatty acid-binding protein 2 [pg/ml (± STD)] | 2407 (± 1471) | 2279 (± 1429) | 2565 (± 1524) | 0.248 | 2540 (± 1574) | 1889 (± 871) | 0.115 |
| FEV1 at serum sampling [% predicted (± STD)] | 73.1 (± 24.1) | 55.8 (± 15.9) | 95.8 (± 9.9) | – | 75.7 (± 23.9) | 64.2 (± 23.1) | 0.057 |
| Cough frequency [none/temporarily/permanently/at night] | 11/64/19/6 | 1/33/14/4 | 8/28/4/1 | 0.007 | 11/50/16/4 | 0/14/3/2 | 0.278 |
| Number of pulmonary exacerbations within the last 12 months | 0.56 (± 0.77) | 0.76 (± 0.90) | 0.40 (± 0.55) | 0.044 | 0.52 (± 0.72) | 0.74 (± 0.93) | 0.293 |
| Systemic antibiotic therapy at serum sampling [no. of patients (%)] | 30 (28.8%) | 19 (34.5%) | 10 (23.8%) | 0.252 | 23 (28.0%) | 7 (31.8%) | 0.729 |
| Systemic antibiotic therapy courses (no. (± STD) | |||||||
| 12 month before serum sampling | 1.6 (± 2.0) | 2.2 (± 2.3) | 0.9 (± 1.1) | < 0.001 | 1.4 (± 1.6) | 2.2 (± 3.0) | 0.477 |
| 6 month after serum sampling | 1.0 (± 1.4) | 1.3 (± 1.7) | 0.5 (± 0.8) | 0.008 | 0.8 (± 1.1) | 1.5 (± 2.4) | 0.314 |
| Nebulized antibiotic [none/temporarily/permanently] | |||||||
| 12 month before serum sampling | 58/16/30 | 18/9/28 | 33/7/2 | < 0.001 | 54/11/17 | 4/5/13 | < 0.001 |
| at serum sampling | 51/38 | 14/34 | 30/4 | < 0.001 | 48/23 | 3/15 | < 0.001 |
| 6 month after serum sampling | 57/21/26 | 17/14/24 | 33/7/2 | < 0.001 | 52/16/14 | 5/5/15 | 0.001 |
| Inhaled corticosteroids [no. of patients (%)] | 14 (14.6%) | 9 (18.4%) | 5 (12.5%) | 0.449 | 9 (11.7%) | 5 (26.3%) | 0.106 |
| Exocrine pancreatic insufficiency [no. of patients (%)] | 93 (89.4%) | 50 (90.9%) | 39 (92.9%) | 0.730 | 72 (87.8%) | 21 (95.5%) | 0.300 |
| CF-related diabetes mellitus [no. of patients (%)] | 11 (10.6%) | 8 (14.5%) | 3 (7.1%) | 0.255 | 9 (11.0%) | 2 (9.1%) | 0.799 |
| CF-related liver disease [no. of patients (%)] | 30 (28.8%) | 16 (29.1%) | 14 (33.3%) | 0.654 | 22 (27.8%) | 8 (32.0%) | 0.729 |
FEV1 forced expiratory volume in 1 s, BMI body mass index, STD standard deviation, BDG (1→3)-β-D-glucan, IQR interquartile range, GM galactomannan, ODI optical density index, WBC white blood cell count, CRP C-reactive protein, no number, CF cystic fibrosis
Table 2.
Patient demographics, clinical and microbiological results stratified after serum BDG and GM positivity
| All patients | Serum BDG level | Serum GM level | |||||
|---|---|---|---|---|---|---|---|
| (n = 104) | Normal (< 60) (n = 77) | Elevated (≥60) (n = 27) | P value | Normal (< 0.5) (n = 97) | Elevated (≥0.5) (n = 7) | P value | |
| Age [years (min-max)] | 19.3 (4–51) | 18.1 (4–48) | 22.7 (4–51) | 0.079 | 18.6 (4–51) | 29.6 (14–51) | 0.047 |
| Sex [female/male] | 43/61 | 31/46 | 12/15 | 0.704 | 40/57 | 3/4 | 0.933 |
| BMI [kg/m2 (± STD)] | 19.7 (± 3.9) | 19.8 (± 3.9) | 19.4 (± 3.9) | 0.739 | 19.8 (± 3.9) | 19.3 (± 2.7) | 0.897 |
| Mean BDG [pg/ml (± STD)] | 50 (± 84) | 16 (± 17) | 148 (± 116) | – | 50 (± 86) | 57 (± 26) | 0.044 |
| Median BDG [pg/ml (IQR)] | 22 (3–62) | 11 (0–28) | 108 (69–179) | 20 (3–56) | 59 (48–68) | ||
| BDG [normal/elevated, (% elevated)] | 77/27 (26.0%) | – | – | – | 73/24 (24.7%) | 4/3 (42.9%) | 0.291 |
| Mean GM [ODI (± STD)] | 0.18 (± 0.22) | 0.15 (± 0.17) | 0.24 (± 0.32) | 0.013 | 0.13 (± 0.1) | 0.89 (± 0.4) | – |
| Median GM [ODI (IQR)] | 0.10 (0.1–0.2) | 0.10 (0.10) | 0.10 (0.10) | 0.10 (0.10) | 0.70 (0.6–1.2) | ||
| GM [normal/elevated, (% elevated)] | 97/7 (6.7%) | 73/4 (5.2%) | 24/3 (11.1%) | 0.291 | – | – | – |
| Persistent A. fumigatus detection [no. of patients (%)] | 22 (21.2%) | 11 (14.3%) | 11 (40.7%) | 0.004 | 19 (19.6%) | 3 (42.9%) | 0.145 |
| S. aureus colonization/infection [no. of patients (%)] | 11 (10.6%) | 10 (13.0%) | 1 (3.7%) | 0.177 | 11 (11.3%) | 0 (0.0%) | 0.346 |
| P. aeruginosa colonization/infection [no. of patients (%)] | 35 (33.7%) | 23 (29.9%) | 12 (44.4%) | 0.168 | 30 (30.9%) | 5 (71.4%) | 0.029 |
| P. aeruginosa-specific antibodies [normal/elevated, (% elevated)] | |||||||
| against alkaline protease | 70/14 (16.7%) | 52/11 (17.5%) | 18/3 (14.3%) | 0.735 | 67/12 (15.2%) | 3/2 (40.0%) | 0.149 |
| against elastase | 69/15 (17.9%) | 52/11 (17.5%) | 17/4 (19.0%) | 0.869 | 67/12 (15.2%) | 2/3 (60.0%) | 0.011 |
| against exotoxin A | 65/19 (22.6%) | 48/15 (23.8%) | 17/4 (19.0%) | 0.651 | 65/14 (17.7%) | 0/5 (100.0%) | < 0.001 |
| A. fumigatus-specific antibodies [normal/elevated, (% elevated)] | 100/4 (3.8%) | 74/3 (3.9%) | 26/1 (3.7%) | 0.964 | 93/4 (4.1%) | 7/0 (0.0%) | 0.584 |
| A. fumigatus-specific IgE level [kU/l, (± STD)] | 3.8 (± 9.9) | 3.6 (± 9.5) | 4.4 (± 11.2) | 0.777 | 4.0 (± 10.2) | 1.8 (± 1.7) | 0.051 |
| rAsp f4-IgE level | 0.5 (± 2.5) | 0.2 (± 1.1) | 1.3 (± 4.5) | 0.768 | 0.5 (± 2.5) | 0.3 (± 0.4) | 0.048 |
| rAsp f6-IgE level | 0.5 (± 2.3) | 0.2 (± 0.8) | 1.2 (± 4.3) | 0.930 | 0.5 (± 2.4) | 0.0 (± 0.0) | 0.391 |
| WBC [×103/μl (± STD)] | 9.1 (± 4.3) | 8.6 (± 3.3) | 10.3 (± 6.3) | 0.183 | 9.0 (± 4.5) | 9.2 (± 2.3) | 0.529 |
| CRP [mg/l (± STD)] | 6.5 (± 19.1) | 4.5 (± 12.2) | 12.1 (± 30.9) | 0.125 | 6.5 (± 19.6) | 6.6 (± 9.0) | 0.506 |
| Total IgG [lowered/normal/elevated] | 12/62/25 | 9/49/17 | 3/13/8 | 0.553 | 12/58/22 | 0/4/3 | 0.392 |
| Total IgE level [normal/elevated] | 65/37 | 53/24 | 12/13 | 0.060 | 61/34 | 4/3 | 0.707 |
| Fatty acid-binding protein 2 [pg/ml (± STD)] | 2411 (± 1481) | 2468 (± 1588) | 2236 (± 1105) | 0.712 | 2373 (± 1469) | 2917 (± 1673) | 0.392 |
| FEV1 at serum sampling [% predicted (± STD)] | 73.1 (± 24.1) | 77.1 (±22.8) | 61.6 (±25.5) | 0.007 | 73.6 (± 24.7) | 66.1 (± 14.1) | 0.407 |
| Cough frequency [none/temporarily/permanently/at night] | 11/64/19/6 | 11/49/11/3 | 0/15/8/3 | 0.038 | 11/58/18/6 | 0/6/1/0 | 0.589 |
| Number of pulmonary exacerbations within the last 12 months | 0.56 (± 0.77) | 0.46 (± 0.72) | 0.85 (± 0.83) | 0.008 | 0.57 (± 0.78) | 0.43 (± 0.54) | 0.762 |
| Systemic antibiotic therapy at serum sampling [no. of patients (%)] | 30 (28.8%) | 18 (23.4%) | 12 (44.4%) | 0.038 | 28 (28.9%) | 2 (28.6%) | 0.987 |
| Systemic antibiotic therapy courses (no. (± STD) | |||||||
| 12 month before serum sampling | 1.6 (± 2.0) | 1.4 (± 1.8) | 2.0 (± 2.4) | 0.415 | 1.5 (± 2.0) | 2.1 (± 1.6) | 0.120 |
| 6 month after serum sampling | 1.0 (± 1.4) | 0.8 (± 1.1) | 1.5 (± 2.1) | 0.047 | 1.0 (± 1.5) | 1.0 (± 1.0) | 0.619 |
| Nebulized antibiotic [none/temporarily/permanently] | |||||||
| 12 month before serum sampling | 58/16/30 | 49/10/18 | 9/6/12 | 0.024 | 56/16/25 | 2/0/5 | 0.032 |
| at serum sampling | 51/38 | 43/25 | 8/13 | 0.042 | 49/33 | 2/5 | 0.109 |
| 6 month after serum sampling | 57/21/26 | 46/15/16 | 11/6/10 | 0.173 | 55/20/22 | 2/1/4 | 0.124 |
| Inhaled corticosteroids [no. of patients (%)] | 14 (14.6%) | 10 (13.0%) | 4 (14.8%) | 0.662 | 13 (13.4%) | 1 (14.3%) | 0.982 |
| Exocrine pancreatic insufficiency [no. of patients (%)] | 93 (89.4%) | 67 (87.0%) | 26 (96.3%) | 0.177 | 86 (88.7%) | 7 (100%) | 0.346 |
| CF-related diabetes mellitus [no. of patients (%)] | 11 (10.6%) | 7 (9.1%) | 4 (14.8%) | 0.405 | 9 (9.3%) | 2 (28.6%) | 0.109 |
| CF-related liver disease [no. of patients (%)] | 30 (28.8%) | 25 (32.5%) | 5 (18.5%) | 0.169 | 28 (28.9%) | 2 (28.6%) | 0.987 |
BDG (1→3)-β-D-glucan, GM galactomannan, BMI body mass index, STD standard deviation, IQR interquartile range, ODI optical density index, WBC white blood cell count, CRP C-reactive protein, no number, FEV1 forced expiratory volume in 1 s, CF cystic fibrosis
Table 3.
Correlation of FEV1 at serum sampling, BDG and GM with continuous clinical and microbiological parameters (Spearman-Rho test)
| FEV1 at serum sampling | BDG | GM | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Correlation Coefficient r | Significance level p-value | Coefficient of determination r2 | Correlation Coefficient r | Significance level p-value | Coefficient of determination r2 | Correlation Coefficient r | Significance level p-value | Coefficient of determination r2 | |
| Age | −0.382 | < 0.001 | 0.146 | 0.187 | 0.057 | 0.035 | 0.186 | 0.058 | 0.035 |
| BMI | −0.028 | 0.787 | 0.001 | - 0.075 | 0.452 | 0.006 | −0.041 | 0.682 | 0.002 |
| FEV1 at serum sampling | – | – | – | - 0.460 | < 0.001 | 0.212 | −0.213 | 0.037 | 0.045 |
| No. of pulmonary exacerbations (last 12 month) | −0.301 | 0.004 | 0.091 | 0.266 | 0.009 | 0.071 | 0.024 | 0.818 | 0.001 |
| Systemic antibiotic therapy courses | |||||||||
| 12 month before serum sampling | −0.438 | < 0.001 | 0.192 | 0.281 | 0.004 | 0.079 | 0.186 | 0.059 | 0.035 |
| 6 month after serum sampling | −0.338 | 0.001 | 0.114 | 0.277 | 0.005 | 0.077 | 0.232 | 0.019 | 0.054 |
| WBC | −0.450 | < 0.001 | 0.203 | 0.246 | 0.012 | 0.061 | 0.168 | 0.091 | 0.028 |
| CRP | −0.621 | < 0.001 | 0.386 | 0.267 | 0.006 | 0.071 | 0.131 | 0.188 | 0.017 |
| BDG | −0.460 | < 0.001 | 0.212 | – | – | – | 0.281 | 0.004 | 0.079 |
| GM | − 0.213 | 0.037 | 0.045 | 0.281 | 0.004 | 0.079 | – | – | – |
| A. fumigatus-specific IgE level | −0.311 | 0.002 | 0.097 | 0.181 | 0.070 | 0.033 | 0.062 | 0.154 | 0.004 |
| rAsp f4-IgE level | −0.203 | 0.051 | 0.041 | 0.151 | 0.134 | 0.023 | 0.041 | 0.688 | 0.002 |
| rAsp f6-IgE level | −0.066 | 0.532 | 0.004 | 0.051 | 0.613 | 0.003 | −0.179 | 0.074 | 0.032 |
| Fatty acid-binding protein 2 | 0.159 | 0.125 | 0.025 | 0.044 | 0.666 | 0.002 | 0.072 | 0.475 | 0.005 |
FEV1 forced expiratory volume in 1 s, BDG (1→3)-β-D-glucan, GM galactomannan, BMI body mass index, no number, WBC white blood cell count, CRP C-reactive protein
Fig. 1.
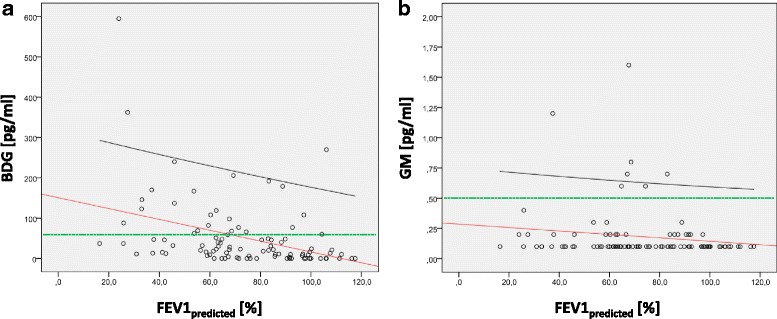
Correlation of the FEV1 at serum sampling with BDG and GM. BDG (a.) and GM (b.) show a significant correlation with the FEV1 (p < 0.001 and 0.037, respectively). The effect size is 0.5 for BDG and 0.2 for GM, respectively. This corresponds to a medium effect for BDG and a small effect for GM according to Cohen [11]. The regression line is depicted in red, the 95%-confidence interval in black and the manufacturer’s cut-off value for invasive fungal disease in green. FEV1: forced expiratory volume in 1 s; BDG: (1→3)-β-D-glucan; GM: galactomannan
Microbiological predictors of an impaired FEV1predicted value
Fifty-five patients (56.7%) of our CF cohort suffered from moderate to very severe airflow limitation (FEV1predicted < 80%), whereas 42 patients (43.3%) had only mild or no airflow limitation (FEV1predicted ≥ 80%). In the univariate analysis, the group of patients with a FEV1predicted < 80% was significantly older (24.0 versus 15.7 years, p < 0.001), had a significantly higher serum concentration of BDG (68 versus 32 pg/ml, p = 0.003), but not of GM (0.22 versus 0.14 optical density index [ODI], p = 0.150), and contained a higher percentage of patients with an elevated BDG (34.5% versus 14.3%, p = 0.024). In addition, this group had higher levels of A. fumigatus-specific IgE and there was a trend towards a higher percentage of patients with persistent A. fumigatus detection (29.1 versus 14.3%, p = 0.084) (Table 1).
Patients with a FEV1predicted < 80% were more often colonized by P. aeruginosa and less likely colonized by S. aureus. Pseudomonas aeruginosa-specific antibodies, WBC, CRP, total IgG and IgE, cough frequency, number of pulmonary exacerbations within the last 12 months and consumption of systemic and nebulized antibiotics before and after serum sampling were also significantly elevated in this group (Table 1).
To examine, which parameters are independent predictors of the lung function, we performed a multivariate analysis with FEV1predicted as dependent variable and all parameters with a significant difference in the univariate analysis as independent variables. The results are shown in Table 4. BDG, WBC, S. aureus- and P. aeruginosa-colonization turned out to be independent predictors of the FEV1predicted in the multivariate analysis. These parameters were able to explain 52.6% of the variation of FEV1predicted which corresponds to a strong effect according to Cohen [11]. An increase of the BDG concentration of 10 pg/ml was associated with an average loss in FEV1predicted of 1%.
Table 4.
Multivariate analysis with FEV1predicted at serum sampling as dependent variable
| Independent variables | Regression coefficient | p-value | R2 corr. | Effect size f |
|---|---|---|---|---|
| Age | −0.073 | 0.772 | 52.6% | 1.05 (strong effect) |
| BDG | −0.098 | 0.002 | ||
| Persistent A. fumigatus detection | −2.951 | 0.562 | ||
| A. fumigatus-specific IgE level | 0.045 | 0.810 | ||
| S. aureus detection | 16.337 | 0.011 | ||
| P. aeruginosa detection | −13.628 | 0.023 | ||
| WBC | −2.911 | < 0.001 | ||
| CRP | −0.015 | 0.933 | ||
| Systemic antibiotic therapy within the last 12 month | − 1.939 | 0.115 | ||
| Nebulized antibiotic within the last 12 month | 0.890 | 0.832 |
FEV1 forced expiratory volume in 1 s, BDG (1→3)-β-D-glucan, WBC white blood cell count, CRP C-reactive protein, R2 coefficient of determination
Characteristics of patients positive for A. fumigatus
Analysis of fungal cultures revealed that 22 patients (21.2%) were persistently colonized/infected with A. fumigatus, whereas one patient was only transiently colonized and 81 patients (77.9%) were negative for A. fumigatus (Table1). In addition, Candida spp. were detected in 55 patients (52.9%), Penicillium spp. in three patients (2.9%), Scedosporium sp. in two patients (1.9%) and Lomentospora prolificans, Exophiala dermatitidis and Trichosporon asahii in one patient each.
The mean serum concentrations of BDG and GM in patients that were persistently positive for A. fumigatus were significantly higher (89 pg/ml and 0.30 ODI, respectively) than in patients without persistent growth of A. fumigatus (40 pg/ml [p = 0.022] and 0.15 ODI [p = 0.013], respectively) (Fig. 2). Although the difference in BDG concentrations between the two subgroups was small, there were significantly more patients with elevated BDG levels (≥ 60 pg/ml) in the A. fumigatus-positive subgroup (50.0 versus 19.5%, p = 0.004) (Table 1). ROC-analysis for discrimination between A. fumigatus-positive and -negative patients gave an area under the ROC curve (AUC) of 0.658 (95%-CI: 0.559–0.748) for BDG and 0.635 (0.535–0.727) for GM (Fig. 3). The AUC of BDG and GM was not significantly different (p = 0.760). The cut-off with the highest Youden index was ≥60 pg/ml for BDG (sensitivity 50.0%, specificity 80.5%) and > 0.1 ODI for GM (sensitivity 45.5%, specificity 78.0%). In addition, patients with persistent A. fumigatus detection were significantly older (24.8 versus 17.9 years, p = 0.001), had a higher BMI (21.6 versus 19.2 kg/m2, p = 0.006) and were more often colonized with P. aeruginosa (59.1 versus 26.8%, 0.004) (Table 1). The FEV1predicted was lower in patients with a positive culture for A. fumigatus (64.2 versus 75.7%, p = 0.057) and they were treated more frequently with nebulized antibiotics in the year before, at and during the 6 months after serum sampling. Although the frequency of systemic antibiotic therapy was also higher in the A. fumigatus-positive subgroup, these differences were not statistically significant. Interestingly, none of the Aspergillus-specific antibodies was significantly elevated in A. fumigatus-positive patients (Table 1).
Fig. 2.
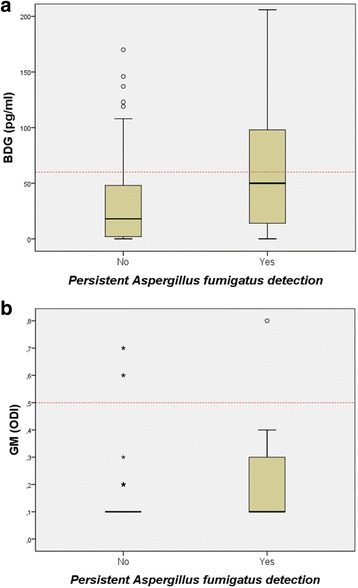
Box-Plots of BDG (a) and GM (b) results stratified by persistent A. fumigatus detection. The red dotted line indicates the manufacturer’s cut-off value for the biomarker. The small circles depict outliers and the stars extreme outliers. For better display not all extreme outliers are shown. BDG: (1→3)-β-D-glucan; GM: galactomannan
Fig. 3.
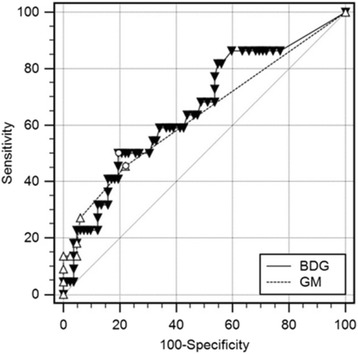
Receiver-operating-characteristic (ROC)-analysis for discrimination between A. fumigatus-positive and -negative patients. The cut-off with the highest Youden index (black circles) was ≥60 pg/ml for BDG (sensitivity 50.0%, specificity 80.5%) and > 0.1 ODI for GM (sensitivity 45.5%, specificity 78.0%). The area under the ROC-curve was not significantly different for BDG and GM (0.658 versus 0.635, p = 0.760). BDG: (1→3)-β-D-glucan; GM: galactomannan
Characteristics of patients with elevated BDG and GM serum concentrations
To further investigate possible differences in the diagnostic value of fungal cultures versus biomarker measurements, we also analyzed the association of BDG and GM with clinical parameters. Of our CF cohort, 27 patients (26.0%) had elevated BDG levels (median 108 pg/ml, IQR 69–179) and 7 patients (6.7%) elevated GM levels (median ODI 0.7, IQR 0.6–1.2). Patients with an elevated serum BDG (≥ 60 pg/ml) had a significantly higher serum GM (0.24 versus 0.15, p = 0.013) than patients with a normal BDG and vice versa (p = 0.044) (Table 2). BDG and GM levels showed a significant correlation but with a very weak effect size (Table 3).
Patients with an elevated serum BDG were more frequently A. fumigatus-positive (40.7 versus 14.3%, p = 0.004). They had a significantly lower FEV1predicted than patients with a normal BDG (61.6 versus 77.1%, p = 0.007) and BDG levels correlated inversely with the paired FEV1predicted (Table 4). Furthermore, patients with an elevated BDG tended to be older (22.7 versus 18.1 years, p = 0.079), had a significantly higher cough rate and more pulmonary exacerbations during the last year. They had received more frequently a nebulized antibiotic, had a higher probability of systemic and nebulized antibiotic therapy at serum sampling and were treated more often with systemic antibiotics during the 6 months follow-up (Table 2).
Patients with elevated GM levels (> 0.5 ODI) were also older and received more often nebulized antibiotics during the last year. However, in contrast to BDG, patients with an elevated serum concentration of GM showed higher levels of A. fumigatus-specific IgE and significantly lower levels of rAsp f4-IgE. Interestingly, patients with an elevated GM were more frequently colonized/infected with P. aeruginosa and showed more often elevated antibody levels against P. aeruginosa elastase and exotoxin A (Table 2).
Most importantly, patients with elevated GM levels did not have significantly reduced FEV1predicted values (66.1% versus 73.6%, p = 0.407). However, this was only the case if the cut-off value was used that is recommended for the diagnosis of invasive fungal disease (ODI ≥ 0.5). After applying the cut-off calculated by our ROC-analysis (ODI > 0.1), patients with elevated GM levels (n = 18) showed significantly reduced FEV1predicted values (65.4% versus 76.1%, p = 0.042).
Discussion
Aspergillus fumigatus is cultured from respiratory samples of CF patients with a prevalence of 6–57% [2]. There is an ongoing debate whether its presence is contributing to the decline of pulmonary function in these patients [12]. If this were the case, it would have far-reaching implications because CF patients might profit from antifungal therapy.
In our cohort, 21.2% of patients were persistently colonized or infected with A. fumigatus. Univariate analysis revealed that these patients were in a more advanced stage of disease, i.e. they were older, had a higher BMI, were more often colonized or infected with P. aeruginosa, were treated more frequently with nebulized but not systemic antibiotics and, most importantly, had a lower FEV1predicted at serum sampling. Similar findings concerning the association between culture positivity for Aspergillus and increasing age [13–15], lower BMI [13], detection of P. aeruginosa [13, 16], exposure to nebulized antibiotics [13, 14, 17–19] and reduced FEV1 [3, 4, 13] were reported in the past. However, there are also studies that could not find such an association between the detection of Aspergillus spp. and lung function [18, 20]. So far, the reasons for these conflicting results are unclear.
In the present study, we performed for the first time a detailed correlative analysis of BDG and GM antigenemia, colonization/infection with A. fumigatus and pulmonary function in CF patients. Our data shows that BDG and GM were significantly higher in Aspergillus-positive compared to Aspergillus-negative patients (BDG 89 versus 40 pg/ml and GM 0.3 versus 0.15 ODI, respectively). These concentrations are lower than the BDG and GM levels that have been observed in patients with invasive pulmonary aspergillosis (186 pg/ml and 0.70 ODI, respectively) [21]. Accordingly, most of our patients with Aspergillus detected in their airways would not fall into the category “serum antigen positive” if the cut-off values for invasive disease were applied (≥ 80 pg/ml and ≥ 0.5 ODI, respectively). However, it seems plausible that in CF patients with chronic Aspergillus colonization/infection less BDG or GM is released into the blood as compared to severely immunocompromised patients with angioinvasive disease. This is supported by our ROC analysis which gave optimal BDG and GM cut-off values for discrimination between Aspergillus-positive and Aspergillus-negative patients of ≥60 pg/ml and > 0.1 ODI, respectively. After applying these cut-offs to our study population, there were significantly more patients with elevated BDG and GM levels in the Aspergillus-positive than in the Aspergillus-negative subgroup. Interestingly, in case of BDG there is an “indeterminate” range between 60 and 80 pg/ml defined by the manufacturer and patients without invasive fungal disease have BDG-levels below 60 pg/ml. Thus, it seems that CF-patients with persistent Aspergillus detection have BDG and GM levels that range between healthy and invasively infected patients.
The diagnostic performance of BDG and GM to distinguish between Aspergillus-positive and Aspergillus-negative patients was, despite the optimized cut-offs, rather low. The sensitivity and specificity were around 50% or 80%, respectively, for both biomarkers. This means that half the patients with respiratory cultures positive for Aspergillus did not have elevated biomarker levels. However, it also means that 80% of patients with elevated biomarker levels were Aspergillus-positive. This constellation of low sensitivity and high specificity could be explained by the existence of two subgroups within the Aspergillus-positive patients. One subgroup consists of patients that are merely colonized with A. fumigatus (exhibiting normal biomarker levels) and the other of patients with true A. fumigatus infection (exhibiting elevated biomarker levels). If this is the case, BDG and GM could potentially differentiate between these two subgroups. If one further assumes that only infected but not colonized patients will develop an Aspergillus-associated impairment of their lung function, it would imply that biomarkers are superior to culture in predicting the FEV1 evolution.
The analysis of the FEV1 in our CF population supports this hypothesis. In the univariate analysis BDG and GM levels are correlating inversely with the FEV1 and patients with elevated BDG or GM levels have significantly lower FEV1 values. Moreover, the multivariate analysis revealed that BDG, but not the culture of A. fumigatus, proved to be an independent predictor of the FEV1. These data also offer an explanation why previous studies reported inconsistent results concerning the correlation between Aspergillus detection and lung function decline.
Data on fungal biomarker levels in sera of CF patients with proven colonization/infection with Aspergillus are scarce. Recently, Rautemaa et al. [22] reported on the only study examining BDG levels in CF patients. They analyzed serum samples from an adult CF cohort (n = 46) at the time of stable disease and during pulmonary exacerbation. There was no significant difference in the BDG levels of patients with stable versus exacerbated disease (40.2 versus 48.7 pg/ml, p = 0.544) or between patients with versus without positive fungal culture (76 versus 37 pg/ml, p = 0.227). Unfortunately, the authors did not specify the fungi they detected. According to a personal communication by V. Rauteema (University of Manchester, UK), the cultured fungi were predominantly Candida spp., whereas only few Aspergillus spp. were isolated. Separate statistical analysis of both genera also did not show significant differences in BDG levels. However, the low number of patients with cultures positive for Aspergillus could explain why the BDG levels between Aspergillus-positive and -negative patients were not significantly different in their study.
Furthermore, Rautemaa et al. [22] reported that multiple serum samples collected over a period of 13 months from 6 patients showed that BDG-positive patients stayed positive and BDG-negative patients remained negative. This would support our hypothesis that BDG-antigenemia in CF patients is not a transient phenomenon but rather the consequence of a persistent condition like chronic Aspergillus infection.
Interestingly, Rautemaa et al. [22] found significantly elevated BDG-levels in patients with exocrine pancreatic insufficiency (PI) and CF-related diabetes (CFRD) and hypothesized that the chronically inflamed intestinal epithelium in CF could allow a translocation of BDG from the gut into the blood. However, we were not able to confirm this data. BDG levels were independent of PI and CFRD (data not shown). To further examine the gut as possible source of serum BDG, we measured intestinal fatty acid binding protein 2 (FABP2), a specific marker for gut mucosal injury [23–25], in the serum of our CF population. FABP2 was not elevated in patients with positive BDG levels arguing against an intestinal source of BDG.
So far, the only study on GM and the detection of Aspergillus in CF patients was performed by Warren et al. [26]. They analyzed GM levels from 138 CF patients. 43% of patients were persistently and 23% transiently colonized/infected with Aspergillus, whereas 34% of the patients were negative for Aspergillus. The GM levels of these three groups were identical and none of the patients had GM levels above an ODI of 0.5. These results are contradictory to our findings. Comparing the findings by Warren et al. [26] with our data, it is striking that 43% and 23% of their patients were persistently or intermittently culture-positive for Aspergillus, whereas in our cohort only 21% of patients were persistently and 1% were transiently Aspergillus-positive. This suggests that there were either profound differences in the study populations or in the diagnostic methodology. Patient recruitment and the utilized GM assay were identical. Unfortunately, the authors did not specify their fungal culture methodology. Also, the definitions for persistent and transient detection of Aspergillus were different. Warren et al. [26] defined ≥2 positive Aspergillus cultures in the last year as persistent colonization/infection, whereas we required > 50% positive cultures in the past 2 years. Essentially, this could mean that our patients were more heavily colonized/infected and therefore had higher GM levels. Interestingly, in the study by Warren et al. [26], patients colonized with Aspergillus were significantly younger than patients without. Again, this finding contrasts with our result (colonized patients were older), which is in accordance with other data showing that higher age is as a risk factor for Aspergillus colonization [13, 15].
Our study has some limitations. The retrospective and cross-sectional design leads to difficulties in distinguishing between cause and effect. It is currently unclear, whether colonization/infection with Aspergillus leads to a more rapid decline in lung function or whether patients with more severe disease exhibit an increased susceptibility to Aspergillus. Furthermore, we did not use culture media selective for Scedosporium spp.. Consequently, the number of patients with Scedosporium spp. present in respiratory samples might be underestimated. Since Scedosporium spp. also produce BDG, we cannot exclude the possibility that part of the BDG detected in the patients´ sera was derived from fungi other than A. fumigatus. Finally, it has been shown that the overall sensitivity of fungal culture is rather poor and that sputum GM or nucleic acid amplification techniques (NAT) are able to detect Aspergillus DNA in a considerable number of samples that were culture-negative [27]. In this context it seems plausible that patients with a negative Aspergillus culture but a positive sputum GM or NAT test are less heavily colonized than culture-positive patients. This would implicate that most Aspergillus-positive patients of our study were heavily colonized and that we might have missed the low-level colonized patients. Further research has to investigate whether these patients are serum BDG and GM positive and if low-level colonization will have an effect on pulmonary function. Therefore, prospective and longitudinal studies using sputum GM and NAT-assisted Aspergillus detection together with serum biomarker measurement are necessary to confirm and expand our data.
Conclusions
We have shown that serum BDG and GM levels are significantly elevated in CF patients with persistent detection of A. fumigatus in respiratory specimens. BDG-, GM- and Aspergillus-positive patients were in a more advanced stage of disease. However, only BDG proved to be an independent predictor of the FEV1. Our data suggest that the serum level of BDG rather than the level of GM or the detection of Aspergillus by culture, might allow to predict the decline of lung function in CF and to identify patients that would profit from antifungal therapy.
Acknowledgments
We would like to thank Sebastian Meyer from the Department of Medical Informatics, Biometry & Epidemiology of the Friedrich-Alexander-Universität (FAU) Erlangen-Nürnberg for his assistance with the statistical analysis and the team of the cystic fibrosis outpatient clinic for the diagnostic work-up and the treatment of the patients.
Funding
No specific funding.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- AUC
Area under the ROC curve
- BDG
(1→3)-β-D-glucan
- BMI
Body mass index
- CF
Cystic fibrosis
- CFLD
CF-related liver disease
- CFRD
CF-related Diabetes mellitus
- CFTR
Cystic fibrosis transmembrane conductance regulator
- CRP
C-reactive protein
- FABP2
Fatty acid binding protein 2
- FEV1
Forced expiratory volume in 1 s
- GM
Galactomannan
- GOLD
Global Initiative for Chronic Obstructive Lung Disease
- Ig
Immunoglobulin
- IQR
Interquartile range
- MALDI-TOF-MS
Matrix Assisted Laser Desorption Ionization-Time of Flight Mass Spectrometry
- NAT
Nucleic acid amplification techniques
- ODI
Optical density index
- PI
Exocrine pancreatic insufficiency
- ROC
Receiver-operating-characteristic
- SD
Standard deviation
- WBC
Leucocyte count
Authors’ contributions
JT collected clinical data, performed BDG and FABP2 tests and analyzed the data. VOM aquired clinical data and critically revised the manuscript, RM and MR performed diagnostic tests, CB critically revised the manuscript, JH planned the study, analyzed the data and drafted the manuscript. All authors read and approved the final manuscript. All authors agreed to be accountable for all aspects of the work.
Ethics approval and consent to participate
The study protocol was approved by the ethics committee of the Medical Faculty of the Friedrich-Alexander-Universität (FAU) Erlangen-Nürnberg, Germany (application number 7-17B). The need for informed consent was waived.
Consent for publication
Not applicable.
Competing interests
JH received royalties for lectures from MSD as well as lecture royalties and a research grant from Pfizer. The remaining authors declare no conflict of interest.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Johannes Träger, Email: johannes.traeger@fau.de.
Volker Otto Melichar, Email: volker.melichar@gmx.net.
Renate Meyer, Email: renate.meyer@uk-erlangen.de.
Manfred Rauh, Email: manfred.rauh@uk-erlangen.de.
Christian Bogdan, Email: christian.bogdan@uk-erlangen.de.
Jürgen Held, Phone: +49-9131 85 46903, Email: juergen.held@uk-erlangen.de.
References
- 1.Rowe SM, Miller S, Sorscher EJ. Cystic fibrosis. N Engl J Med. 2005;352:1992–2001. doi: 10.1056/NEJMra043184. [DOI] [PubMed] [Google Scholar]
- 2.Pihet M, Carrere J, Cimon B, Chabasse D, Delhaes L, Symoens F, Bouchara JP. Occurrence and relevance of filamentous fungi in respiratory secretions of patients with cystic fibrosis--a review. Med Mycol. 2009;47:387–397. doi: 10.1080/13693780802609604. [DOI] [PubMed] [Google Scholar]
- 3.Amin R, Dupuis A, Aaron SD, Ratjen F. The effect of chronic infection with aspergillus fumigatus on lung function and hospitalization in patients with cystic fibrosis. Chest. 2010;137:171–176. doi: 10.1378/chest.09-1103. [DOI] [PubMed] [Google Scholar]
- 4.Fillaux J, Bremont F, Murris M, Cassaing S, Rittie JL, Tetu L, Segonds C, Abbal M, Bieth E, Berry A, et al. Assessment of aspergillus sensitization or persistent carriage as a factor in lung function impairment in cystic fibrosis patients. Scand J Infect Dis. 2012;44:842–847. doi: 10.3109/00365548.2012.695454. [DOI] [PubMed] [Google Scholar]
- 5.Shoseyov D, Brownlee KG, Conway SP, Kerem E. Aspergillus bronchitis in cystic fibrosis. Chest. 2006;130:222–226. doi: 10.1378/chest.130.1.222. [DOI] [PubMed] [Google Scholar]
- 6.Kanthan SK, Bush A, Kemp M, Buchdahl R. Factors effecting impact of aspergillus fumigatus sensitization in cystic fibrosis. Pediatr Pulmonol. 2007;42:785–793. doi: 10.1002/ppul.20656. [DOI] [PubMed] [Google Scholar]
- 7.Mennink-Kersten MA, Donnelly JP, Verweij PE. Detection of circulating galactomannan for the diagnosis and management of invasive aspergillosis. Lancet Infect Dis. 2004;4:349–357. doi: 10.1016/S1473-3099(04)01045-X. [DOI] [PubMed] [Google Scholar]
- 8.Finkelman MA. Pneumocystis jirovecii infection: cell wall (1-3)-ß-D-glucan biology and diagnostic utility. Crit Rev Microbiol. 2010;36:271–281. doi: 10.3109/1040841X.2010.484001. [DOI] [PubMed] [Google Scholar]
- 9.Sulahian A, Porcher R, Bergeron A, Touratier S, Raffoux E, Menotti J, Derouin F, Ribaud P. Use and limits of (1-3)-beta-d-glucan assay (Fungitell), compared to galactomannan determination (Platelia aspergillus), for diagnosis of invasive aspergillosis. J Clin Microbiol. 2014;52:2328–2333. doi: 10.1128/JCM.03567-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee TW, Brownlee KG, Conway SP, Denton M, Littlewood JM. Evaluation of a new definition for chronic Pseudomonas aeruginosa infection in cystic fibrosis patients. J Cyst Fibros. 2003;2:29–34. doi: 10.1016/S1569-1993(02)00141-8. [DOI] [PubMed] [Google Scholar]
- 11.Cohen J. A power primer. Psychol Bull. 1992;112:155–159. doi: 10.1037/0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- 12.Chotirmall SH, McElvaney NG. Fungi in the cystic fibrosis lung: bystanders or pathogens? Int J Biochem Cell Biol. 2014;52:161–173. doi: 10.1016/j.biocel.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 13.de Vrankrijker AM, van der Ent CK, van Berkhout FT, Stellato RK, Willems RJ, Bonten MJ, Wolfs TF. Aspergillus fumigatus colonization in cystic fibrosis: implications for lung function? Clin Microbiol Infect. 2011;17:1381–1386. doi: 10.1111/j.1469-0691.2010.03429.x. [DOI] [PubMed] [Google Scholar]
- 14.Cheer SM, Waugh J, Noble S. Inhaled tobramycin (TOBI): a review of its use in the management of Pseudomonas aeruginosa infections in patients with cystic fibrosis. Drugs. 2003;63:2501–2520. doi: 10.2165/00003495-200363220-00015. [DOI] [PubMed] [Google Scholar]
- 15.Milla CE, Wielinski CL, Regelmann WE. Clinical significance of the recovery of aspergillus species from the respiratory secretions of cystic fibrosis patients. Pediatr Pulmonol. 1996;21:6–10. doi: 10.1002/(SICI)1099-0496(199601)21:1<6::AID-PPUL1>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 16.Baxter CG, Rautemaa R, Jones AM, Webb AK, Bull M, Mahenthiralingam E, Denning DW. Intravenous antibiotics reduce the presence of aspergillus in adult cystic fibrosis sputum. Thorax. 2013;68:652–657. doi: 10.1136/thoraxjnl-2012-202412. [DOI] [PubMed] [Google Scholar]
- 17.Burns JL, Emerson J, Stapp JR, Yim DL, Krzewinski J, Louden L, Ramsey BW, Clausen CR. Microbiology of sputum from patients at cystic fibrosis centers in the United States. Clin Infect Dis. 1998;27:158–163. doi: 10.1086/514631. [DOI] [PubMed] [Google Scholar]
- 18.Bargon J, Dauletbaev N, Kohler B, Wolf M, Posselt HG, Wagner TO Prophylactic antibiotic therapy is associated with an increased prevalence of aspergillus colonization in adult cystic fibrosis patients. Respir Med. 1999;93:835–838. doi: 10.1016/S0954-6111(99)90270-6. [DOI] [PubMed] [Google Scholar]
- 19.Hodson ME, Gallagher CG, Govan JR. A randomised clinical trial of nebulised tobramycin or colistin in cystic fibrosis. Eur Respir J. 2002;20:658–664. doi: 10.1183/09031936.02.00248102. [DOI] [PubMed] [Google Scholar]
- 20.Jubin V, Ranque S, Stremler Le Bel N, Sarles J, Dubus JC. Risk factors for aspergillus colonization and allergic bronchopulmonary aspergillosis in children with cystic fibrosis. Pediatr Pulmonol. 2010;45:764–771. doi: 10.1002/ppul.21240. [DOI] [PubMed] [Google Scholar]
- 21.Cai X, Ni W, Wei C, Cui J. Diagnostic value of the serum galactomannan and (1, 3)-beta-D-glucan assays for invasive pulmonary aspergillosis in non-neutropenic patients. Intern Med. 2014;53:2433–2437. doi: 10.2169/internalmedicine.53.2381. [DOI] [PubMed] [Google Scholar]
- 22.Rautemaa V, Green HD, Jones AM, Rautemaa-Richardson R. High level of beta-(1,3)-d-glucan antigenaemia in cystic fibrosis in the absence of invasive fungal disease. Diagn Microbiol Infect Dis. 2017; [DOI] [PubMed]
- 23.Schurink M, Kooi EM, Hulzebos CV, Kox RG, Groen H, Heineman E, Bos AF, Hulscher JB. Intestinal fatty acid-binding protein as a diagnostic marker for complicated and uncomplicated necrotizing enterocolitis: a prospective cohort study. PLoS One. 2015;10:e0121336. doi: 10.1371/journal.pone.0121336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schellekens DH, Grootjans J, Dello SA, van Bijnen AA, van Dam RM, Dejong CH, Derikx JP, Buurman WA. Plasma intestinal fatty acid-binding protein levels correlate with morphologic epithelial intestinal damage in a human translational ischemia-reperfusion model. J Clin Gastroenterol. 2014;48:253–260. doi: 10.1097/MCG.0b013e3182a87e3e. [DOI] [PubMed] [Google Scholar]
- 25.Kanda T, Fujii H, Tani T, Murakami H, Suda T, Sakai Y, Ono T, Hatakeyama K. Intestinal fatty acid-binding protein is a useful diagnostic marker for mesenteric infarction in humans. Gastroenterology. 1996;110:339–343. doi: 10.1053/gast.1996.v110.pm8566578. [DOI] [PubMed] [Google Scholar]
- 26.Warren TA, Yau Y, Ratjen F, Tullis E, Waters V. Serum galactomannan in cystic fibrosis patients colonized with aspergillus species. Med Mycol. 2012;50:658–660. doi: 10.3109/13693786.2012.676739. [DOI] [PubMed] [Google Scholar]
- 27.Nagano Y, Elborn JS, Millar BC, Walker JM, Goldsmith CE, Rendall J, Moore JE. Comparison of techniques to examine the diversity of fungi in adult patients with cystic fibrosis. Med Mycol. 2010;48:166–176. doi: 10.3109/13693780903127506. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.


