Abstract
Background
Polysaccharides from bivalves have multiple bioactivities in various aspects of biology. However, the role of a polysaccharide derived from Amusium pleuronectes on potential hepatoprotective effects remains unclear.
Material/Methods
A water-soluble polysaccharide was isolated from Amusium pleuronectes (APS-1) using ultrasound-assisted hot-water extraction. The molecular weight of APS-1 was approximately 11.7 kDa and was determined by calibration with dextran. APS-1 was analyzed by high-performance liquid chromatography (HPLC), and mainly consisted of a uniform glucose polymer. The protective effect of APS-1 on Schistosoma japonicum-induced liver fibrosis was investigated in a mouse model.
Results
Treatment with APS-1 increased serum levels of interleukin (IL)-12 and interferon (IFN)-γ, increased superoxide dismutase (SOD) activity, and decreased levels of IL-13 and IL-5, and hyaluronidase activity. Moreover, immunohistochemical analysis revealed that the collagen content of hepatic tissue of APS-1-treated mice, including that of collagen I, II, and IV, was dramatically decreased. Furthermore, our data showed that combined treatment of APS-1 with praziquantel had more pronounced effects than treatment with either APS-1 or praziquantel alone.
Conclusions
Our findings suggest that the treatment using APS-1 in combination with praziquantel attenuated S. japonicum egg-induced hepatic fibrosis, and possessed potent hepatoprotective activity.
MeSH Keywords: Liver Cirrhosis, Polysaccharides, Schistosoma Japonicum
Background
Schistosomiasis is a serious endemic disease caused by infection with Schistosoma spp that affect more than 200 million people worldwide [1,2]. Schistosoma japonicum (S. japonicum), one of the 3 species that affects humans, is predominantly prevalent in the southern part of China and creates an important public health problem [3,4]. When trapped in the liver and gut, the eggs laid by adult worms of S. japonicum induce a vigorous granulomatous response [5]. Egg-induced progressive hepatic fibrosis is one of the major consequences of S. japonicum-related infections and leads to further development of portal hypertension, hepatosplenomegaly, ascites, and hepatic cirrhosis [6].
After Schistosoma infection, when progressing to the chronic phase, soluble egg antigen (SEA) that is locally secreted by Schistosoma eggs suppresses T helper type 1 (Th1)-mediated responses and facilitates Th2-mediated inflammatory responses [6]. In previous studies, it was demonstrated that Th2-related cytokines such as interleukin (IL)-5 and IL-13 actively participate in hepatic fibrogenesis in S. japonicum-infected mice [7–9].
Bivalves are a type of shelled mollusk found in China and other parts of the world. Since ancient times, bivalves have been used by humans not only as an excellent source of high-quality nutrition, but also as a traditional Chinese medicinal drug [10]. Increasing evidence has demonstrated that the polysaccharides present in bivalves have various bioactivities. For example, a mytilan isolated from the mantle of the mussel Crenomytilus grayanus has high immunomodulating activity [11]. In addition, a polysaccharide derived from Corbicula fluminea was found to have antioxidant and anti-tumor activity [12,13]. A polysaccharide derived from Cyclina sinensis was investigated and had antioxidant, anti-tumor, and hepatoprotective activities [14,15]. Moreover, polysaccharides from Crassostrea gigas have multifunctional activities, including hepatoprotective activities [10], and antihypertensive activities [16]. Unfortunately, data regarding the hepatoprotective effects of polysaccharides derived from Amusium pleuronectes (A. pleuronectes), one of various types of bivalves, are limited.
In the present study, we isolated and characterized a water-soluble polysaccharide from A. pleuronectes (APS-1) and evaluated the potential hepatoprotective effects of APS-1 in mice. Preliminary characterization of APS-1 was assessed by high-performance liquid chromatography (HPLC). Moreover, hepatoprotective effects of APS-1 were investigated in a S. japonicum egg-induced hepatic granuloma and fibrosis model in mice by measuring serum levels of IL-12, interferon (IFN)-γ, IL-5, and IL-13, as well as the activities of hyaluronidase (HA) and superoxide dismutase (SOD), and tissue levels of collagen type I, type II, and type IV, as well as by evaluating histopathological changes.
Material and Methods
Materials
A. pleuronectes were collected along the coast of Shanghai on the East China Sea in May of 2015. After manually removing the shells, the bodies were cleaned and freeze-dried prior to extraction.
S. japonicum (Chinese mainland strain)-infected Oncomelania hupensis snails were obtained from the Jiangsu Provincial Institute of Parasitic Diseases (Wuxi, China).
PMP (1-Phenyl-3-methyl-5-pyrazolone) was purchased from Sigma-Aldrich (Shanghai, China). The TSK gel G4000PWXL column was obtained from TOSOH (Shanghai, China). Hypersil BDS C18 HPLC columns were purchased from Thermo Fisher Scientific (Shanghai, China). Pepsin was purchased from Sigma Chemicals (St. Louis, MO, USA). Assay kits for IL-12, IFN-γ, IL-5, IL-13, HA, and SOD were purchased from Cusabio Biotech Co. Ltd. (Wuhan, China). Primary antibodies, including rabbit anti-COL2A1 polyclonal antibody (Cat no. D120453), rabbit anti-COL4A1 polyclonal antibody (Cat no. D160202), and horseradish peroxidase immunohistochemistry kit (Rabbit) (Cat no. E670016) were purchased from Sangon Biotech Co., Ltd (Shanghai, China). Primary rabbit anti-COL1A1 monoclonal antibody (Cat no. AF1840) was obtained from Beyotime (Shanghai, China), and praziquantel (PZQ) was purchased from Jiangsu Kanglong Medicine Co., Ltd. (Nanjing, China).
Separation of a water-soluble polysaccharides of A. pleuronectes
After being degreased with acetone for 24 h, ground freeze-dried samples of A. pleuronectes (100 g) were resuspended in water at 1: 40 (w/v) and successively extracted using ultrasound-assisted hot water (4000 mL, 90°C) for 4 h (power=160 w). The solution was adjusted to pH 5.0 for 12 h and centrifuged for 30 min at 4000×g. The resulting supernatant was hydrolyzed by pepsin (Sigma-Aldrich, Shanghai, China) (pH 2.0) for 2 h and centrifuged at 4000×g for 30 min. The supernatant obtained was adjusted to pH 7.0 and subjected to a hollow-fiber ultrafiltration module (Hangzhou Kaijie Membrane Separation Technology Co., Ltd., Hangzhou, China) with a 10-kDa cut-off polysulfone hollow-fiber membrane (effective membrane area: 0.25 m2). After being pumped by a peristaltic pump, the retentate solution was freeze-dried. The yield of A. pleuronectes-derived polysaccharide APS-1 was calculated relative to the original dry weight of A. pleuronectes.
Measurement of the molecular weight of APS-1
The molecular weight (MW) of APS-1 was determined by passing the samples through a TSK gel GMPWXL column (7.5×300 mm, Shanghai, China) in H2O. Standard water-soluble polysaccharides included dextrans with MWs of 2700, 5250, 9450, 13050, 36800, and 64650 Da.
Monosaccharide composition analysis of APS-1
The monosaccharide composition of APS-1 was determined using a previously described method with minor modifications [17]. APS-1 (5 mg) was dissolved in 6 mL of 0.1 mol/L sulfuric acid and incubated at 121°C for 2 h. Then, each reaction mixture was evaporated to dryness under reduced pressure. A set of monosaccharide standards, containing mannose, glucosamine, rhamnose, glucuronic acid, galacturonic acid, galactosamine, glucose, galactose, arabinose, and fucose (10 nM each), was treated in the same manner as the polysaccharide. The dried hydrolyzed APS-1 and monosaccharide standards were labeled by the addition of 20 μL of PMP solution (0.5 mol/L in methanol) and 20 μL of NaOH (0.3 mol/L) and incubated at 70°C for 2 h for derivatization. Subsequently, the mixture was neutralized by the addition of 20 μL of HCl (0.3 mol/L). Next, trichloromethane (0.5 mL) was added to the mixture, and vortexed for 10s. The organic phase was carefully removed and discarded. The resulting aqueous phase (20 μL) of the PMP-labeled monosaccharides was analyzed on a Thermo C18 column (4.6×250 mm) connected to a Waters HPLC system (2695 binary HPLC pump and 2487 dual λ absorbance detector, Shanghai, China). PMP-labeled monosaccharides were separated using a buffer consisting of 0.1 mol/L PBS (pH 6.7) with 17% acetonitrile on a HPLC system. The flow rate was set to 0.8 mL/min and the wavelength for UV detection was 245 nm [17].
Animals and treatment protocol
Fifty female BALB/c mice (6–8 weeks old) were purchased from the Comparative Medical Center of Yangzhou University (Yangzhou, Jiangsu, China) and housed at a temperature of 22±2°C, 60–70% relative humidity, and a 12-h light-dark cycle. All experimental protocols were approved by the Animal Ethics Committee of Wannan Medical College (Wuhu, Anhui, China).
BALB/c mice were randomly divided into 5 groups (n=10 per group) and treated as follows: (1) Group A, saline (1 mL daily) for 8 weeks, and served as a negative control; the remaining animals were infected using 30±2 freshly shed cercaria percutaneously after they had been anesthetized with isoflurane. After 8 weeks, 40 mice (except mice in Group A) were randomly divided into 4 groups and treated as follows: (2) Group B, saline (1 mL daily) for 8 weeks as a negative control; (3) Group C, APS-1 [200 mg/(kg·d) body weight (b.w.) oral dose (p.o.)] on day 57 for 2 days in a row; (4) Group D, PZQ (500 mg/(kg·d) b.w. p.o.) on day 57 for 2 days; (4) Group E, PZQ (500 mg/(kg·d) b.w. p.o.) day 57 for 2 days + APS-1 (200 mg/(kg·d) b.w. p.o.) on day 59 for 8 weeks. Mice were euthanized 24 h after administration of the last treatment, and non-anticoagulated blood and liver were collected.
Assays of IL-12, IFN-γ, IL-5, IL-13, HA, and SOD in mouse serum
Blood was kept at 4°C for 12 h, then centrifuged at 1500×g for 30 min to obtain serum. Levels of IL-12, IFN-γ, IL-5, and IL-13, as well as HA and SOD activities, were measured using kits according to the manufacturer’s guidelines.
Histopathology and immunohistochemistry of hepatic tissue
Hepatic tissues were fixed overnight in 10% phosphate-buffered neutral formalin and embedded in paraffin. The serial sections (5 μm) of hepatic tissues were stained with hematoxylin and eosin to calculate the number of eggs and nodules, and the degree of fibrosis was evaluated using a light microscope (IX51, Olympus, Tokyo, Japan). For each specimen, at least 5 random fields were evaluated.
Tissue was processed for immunohistochemical staining with anti-collagen I, II, or IV antibodies. At least 5 non-contiguous tissue sections were evaluated, and the mean values from 6–8 mice were calculated using ImageJ 1.45s freeware (National Institutes of Health, Rockville, MD, USA) and used for statistical analysis.
Statistical analysis
Experiments were performed in triplicate. Data are presented as the mean ± standard error of the mean (SEM). The data were analyzed by one-way analysis of variance (ANOVA) using GraphPad Prism 7.0 for Windows (GraphPad Software, San Diego, CA, USA). P<0.05 was considered statistically significant.
Results
Preliminary characterization of APS-1
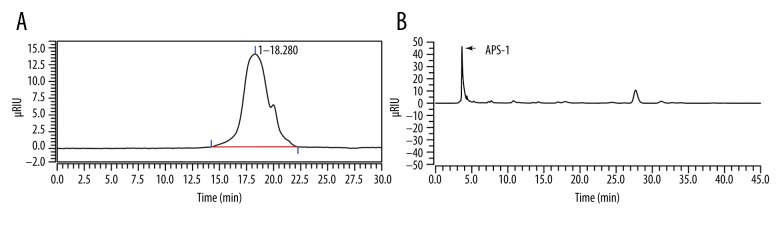
In the absence of proteins, isolated APS-1 appeared as a white powder. The average MW of APS-1 was calculated as 11.7 kDa by a GMPWXL column using dextrans with different MWs as standard water-soluble polysaccharides (Figure 1A).
Figure 1.
(A) Estimation of the molecular weight of APS-1 using high-performance liquid chromatography (HPLC) with a TSK GMPWXL column. (B) HPLC analysis of PMP-labeled monosaccharides derived from APS-1. RIU – refractive index unit.
APS-1 was mainly composed of glucose as determined by HPLC analysis of a PMP-derivatized sample (Figure 1B). However, additional Fourier-transform infrared (FT-IR) spectroscopy and 13C nuclear magnetic resonance (NMR) spectral analysis of APS-1 could not be well-established for glucose configuration (data not shown).
APS-1 treatment suppresses S. japonicum egg-induced hepatic fibrosis
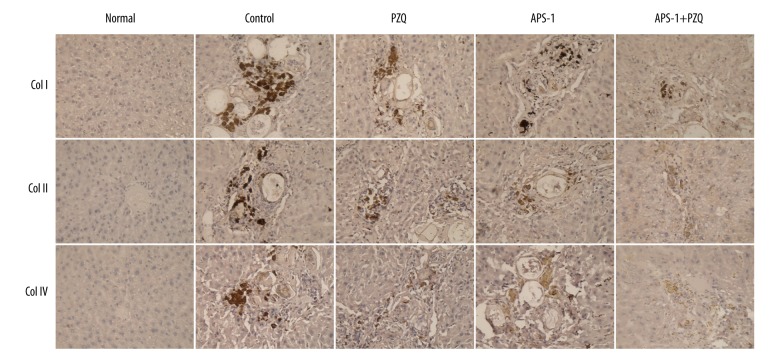
To investigate the effect of APS-1 on S. japonicum egg-induced hepatic fibrosis, after being infected with S. japonicum cercaria, mice were treated with APS-1 at a dose of 200 mg/(kg·d) b.w. p.o. daily for 8 consecutive weeks. At 24 h after the last APS-1 treatment, mice were euthanized and the expression of collagen I, II, and IV was determined for monitoring the formation of hepatic fibrosis using immunohistochemical analysis. As shown in Figure 2, hepatic fibrosis was suppressed in mice treated with PZQ, APS-1, or APS-1+PZQ when compared with mice in the control group (Figure 2).
Figure 2.
APS-1 treatment ameliorates hepatic fibrosis in S. japonicum-infected mice. Magnification, 400×.
APS-1 treatment affects egg burden
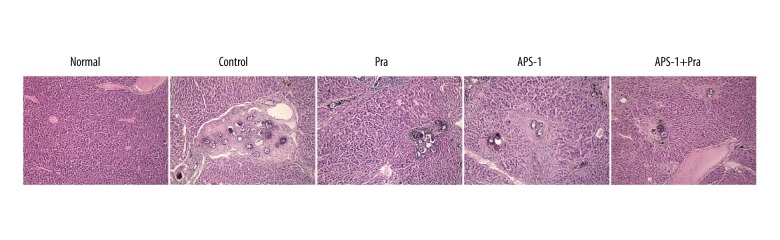
We assessed whether the above-mentioned differences in hepatic fibrosis are due to dissimilar total numbers of parasite eggs in the liver. We found that the total number of parasite eggs in the livers was similar between APS-1- and PZQ-treated mice and obviously decreased when compared with mice in the control group. These findings demonstrate that the reduction in hepatic fibrosis was due to differences in the intensity of egg burden (Figure 3). Moreover, reduced hepatic fibrosis was most significant in the APS-1+PZQ group compared to other groups, except for the normal group.
Figure 3.
Effect of APS-1 treatment on S. japonicum egg burden in mice by hematoxylin and eosin staining. Magnification, 100×.
Correlation of IL-13 with hepatic fibrosis after S. japonicum infection
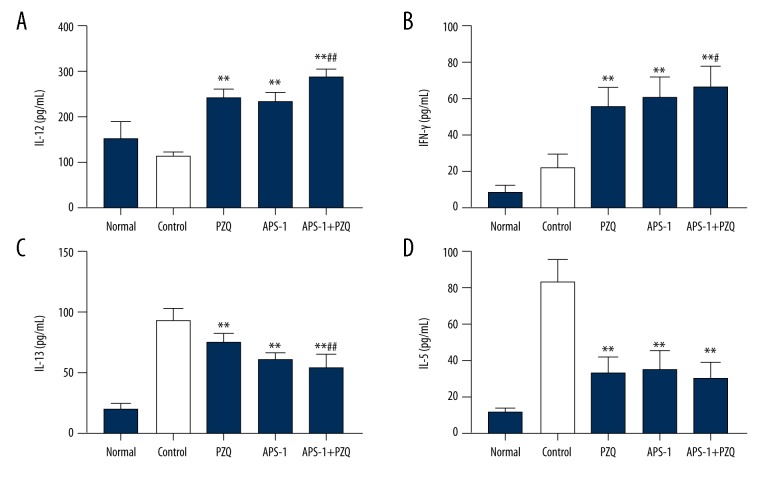
To evaluate the effect of APS-1 on cytokines, ELISA was performed to assess IL-12, IFN-γ, IL-13, and IL-5 in serum. We found that the production of IL-12 and IFN-γ was significantly increased in mice treated with PZQ, APS-1, and APS-1+PZQ compared with the control group (P<0.01; Figure 4). Moreover, mice treated with a combination of APS-1+PZQ produced higher levels of IL-12 and IFN-γ compared to those in the APS-1 and PZQ groups (P<0.01). Conversely, mice treated with APS-1, PZQ, or APS-1+PZQ produced lower amounts of IL-13 and IL-5 compared to mice in the control group (P<0.01).
Figure 4.
Changes of serum cytokines in APS-1-treated mice after S. japonicum infection by quantitative ELISA. Data are shown as the mean ±SD of 10 mice per group. ** P<0.01, compared with the control group; ## P<0.01, compared with the PZQ group and/or APS-1 group.
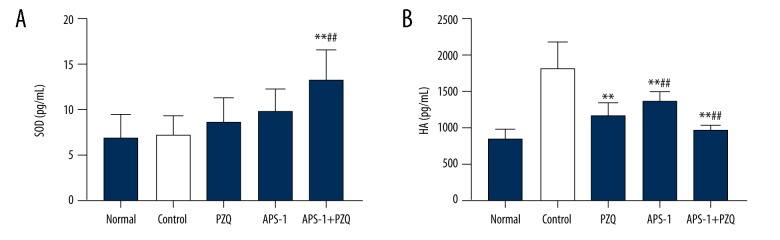
APS-1 treatment causes increased SOD activity and decreased HA activity after S. japonicum infection
In this study, we examined the activities of serum SOD and HA to determine possible mechanisms of the effects of APS-1 administration on S. japonicum-infected mice. The serum SOD and HA activities indicated that SOD activities in the PZQ group, the APS-1 group, and the APS-1+PZQ group were increased when compared to the control group (P<0.01; Figure 5). Moreover, the results showed that APS-1 significantly decreased HA levels.
Figure 5.
The effect of APS-1 treatment on serum SOD and HA activities in S. japonicum-infected mice. (A) SOD activity, (B) HA activity. Data are presented as the mean ±SD from 10 mice per group. ** P<0.01, compared with control group; # P<0.05, compared with PZQ group; ## P<0.01, compared with APS-1 group.
Discussion
In the present study, we isolated the APS-1 polysaccharides from A. pleuronectes and, after characterization, found that it has a MW of 11.7 kDa and represents a glucose-type polymer. Subsequent bioactivity analysis showed that APS-1 treatment attenuated hepatic fibrosis in S. japonicum-infected mice by reducing collagen levels that contributed to decreased egg burden. The mechanism involved may involve increased levels of classic Th1 cytokines (IL-12 and IFNγ) and SOD activity, as well as by decreased levels of the classic Th2 cytokine IL-13 and HA activity.
Liver fibrosis caused by S. japonicum is one of the most serious pathological changes that induce hepatic disorders and hepatic cancer [18]. Due to the continuous stimulation of egg SEA of S. japonicum, hepatic fibrosis symptoms caused by S. japonicum are the most grievous among those of the prevalent schistosomiasis in China [9]. Therefore, identifying effective strategies to prevent or even reverse hepatic fibrosis is critical to overcome the battle against S. japonicum infection. However, safe drug intervention has been impossible because of the poorly understood immunopathogenesis and mechanism of action of hepatic fibrosis in this disease [19,20].
In schistosomiasis, eggs are laid by adult worms inside the mesenteric and portal vein system of the human body. Eggs are mainly trapped in the liver and gut, where they induce a vigorous granulomatous response [5], and subsequently contribute to hepatic fibrosis. Hepatic fibrosis is a serious consequence of S. japonicum infection that involves excessive deposition of collagen, primarily by hepatic stellate cells [19,21–23]. In the present study, we found that after S. japonicum infection, the livers of mice in the control group showed an increased number of eggs and high levels of collagen. However, APS-1 and/or PZQ treatment significantly attenuated egg burden and high collagen expression. These findings suggest that APS-1 has a hepatoprotective activity in S. japonicum infection.
Acute infection by S. japonicum is characterized by a shift from the Th1 immune response toward a predominantly Th2 response during chronic stages [24]. As a profibrogenic factor, IL-13 is involved in a protective mechanism that is characterized by formation of CD4+ T cell-driven liver granulomas, leading to liver fibrosis [25–27]. IL-13 mediates the secretion of collagen, and contributes to the progression of hepatic fibrosis in S. japonicum in human and animal models [27–29]. Our data showed that the level of IL-13 expression was upregulated during hepatic fibrosis. Moreover, after treatment with APS-1 and/or PZQ, IL-13 levels decreased and the classic Th1 cytokines (IL-12 and IFNγ) were increased. These results indicate that APS-1 corrected the imbalance of the Th1/Th2 ratio induced by S. japonicum infection.
Oxidative stress plays a pivotal role in the process of hepatic fibrosis [30]. In schistosome-induced liver damage, abnormal SOD expression was found to promote liver fibrosis [31,32]. In a previous study, it was shown that serum HA levels were significantly increased in rat liver fibrosis [33,34]. In the present study, we showed that S. japonicum infection induced serum HA levels and reduced SOD activities in the liver. However, ASP-1 and/or PZQ treatment reversed these levels. Thus, our data indicate that ASP-1 has an antioxidative role in schistosome-induced liver fibrosis.
Conclusions
In this study, the preliminary characterization and in vivo hepatoprotective activity of the APS-1 polysaccharide derived from A. pleuronectes were investigated. Preliminary structural composition analysis indicated that ASP-1 primarily consisted of a glucose polymer with an average MW of 11.7 kDa. In addition, in vivo studies confirmed that ASP-1 plays a hepatoprotective role in schistosomiasis liver fibrosis and possibly abrogates liver fibrogenesis by rectifying the imbalance of the Th1/Th2 ratio and decreasing the collagen expression, as well as having an antioxidative effect on the liver. The results of this study suggest that APS-1 in combination with praziquantel may be beneficial in treatment of hepatic fibrosis. However, additional studies that focus on elucidating the exact mechanisms of APS-1 regulation of the imbalance of Th1/Th2 ratio and mediating collagen secretion during S. japonicum infection are required.
Footnotes
Source of support: This work was supported by the National Natural Science Foundation of China (81172790 and 81671586), the Key Project of Natural Science Foundation of Anhui Provincial Educational Department (KJ2014A270), and the Academic and Technical Leader Project of Wannan Medical College (010202041703)
Conflict of Interest
None.
References
- 1.Molyneux DH, Malecela MN. Neglected tropical diseases and the millennium development goals: Why the “other diseases” matter: Reality versus rhetoric. Parasit Vectors. 2011;4:234. doi: 10.1186/1756-3305-4-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Steinmann P, Keiser J, Bos R, et al. Schistosomiasis and water resources development: Systematic review, meta-analysis, and estimates of people at risk. Lancet Infect Dis. 2006;6(7):411–25. doi: 10.1016/S1473-3099(06)70521-7. [DOI] [PubMed] [Google Scholar]
- 3.Lei ZL, Zhang LJ, Xu ZM, et al. [Endemic status of schistosomiasis in People’s Republic of China in 2014]. Zhongguo Xue Xi Chong Bing Fang Zhi Za Zhi. 2015;27(6):563–69. [PubMed] [Google Scholar]
- 4.Zhou XN, Wang LY, Chen MG, et al. The public health significance and control of schistosomiasis in China – then and now. Acta Trop. 2005;96(2–3):97–105. doi: 10.1016/j.actatropica.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 5.Liu M, Wu Q, Chen P, et al. A boswellic acid-containing extract ameliorates schistosomiasis liver granuloma and fibrosis through regulating NF-kappaB signaling in mice. PLoS One. 2014;9(6):e100129. doi: 10.1371/journal.pone.0100129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wilson MS, Mentink-Kane MM, Pesce JT, et al. Immunopathology of schistosomiasis. Immunol Cell Biol. 2007;85(2):148–54. doi: 10.1038/sj.icb.7100014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu X, Wen X, Chi Y, et al. Activation-induced T helper cell death contributes to Th1/Th2 polarization following murine Schistosoma japonicum infection. J Biomed Biotechnol. 2010;2010:202397. doi: 10.1155/2010/202397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wynn TA. IL-13 effector functions. Annu Rev Immunol. 2003;21:425–56. doi: 10.1146/annurev.immunol.21.120601.141142. [DOI] [PubMed] [Google Scholar]
- 9.Tang J, Huang H, Ji X, et al. Involvement of IL-13 and tissue transglutaminase in liver granuloma and fibrosis after Schistosoma japonicum infection. Mediators Inflamm. 2014;2014:753483. doi: 10.1155/2014/753483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shi X, Ma H, Tong C, et al. Hepatoprotective effect of a polysaccharide from Crassostrea gigas on acute and chronic models of liver injury. Int J Biol Macromol. 2015;78:142–48. doi: 10.1016/j.ijbiomac.2015.03.056. [DOI] [PubMed] [Google Scholar]
- 11.Ovodova RG, Glazkova VE, Mikheyskaya LV, et al. The structure of mytilan, a bioglycan-immunomodulator isolated from the mussel Crenomytilus grayanus. Carbohydre Res. 1992;223:221–26. doi: 10.1016/0008-6215(92)80018-v. [DOI] [PubMed] [Google Scholar]
- 12.Liao N, Chen S, Ye X, et al. Antioxidant and anti-tumor activity of a polysaccharide from freshwater clam, Corbicula fluminea. Food Funct. 2013;4(4):539–48. doi: 10.1039/c2fo30178d. [DOI] [PubMed] [Google Scholar]
- 13.Liao N, Zhong J, Zhang R, et al. Protein-bound polysaccharide from corbicula fluminea inhibits cell growth in MCF-7 and MDA-MB-231 human breast cancer cells. PLoS One. 2016;11(12):e0167889. doi: 10.1371/journal.pone.0167889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jiang C, Xiong Q, Gan D, et al. Antioxidant activity and potential hepatoprotective effect of polysaccharides from Cyclina sinensis. Carbohydre Polym. 2013;91(1):262–68. doi: 10.1016/j.carbpol.2012.08.029. [DOI] [PubMed] [Google Scholar]
- 15.Jiang C, Xiong Q, Li S, et al. Structural characterization, sulfation and antitumor activity of a polysaccharide fraction from Cyclina sinensis. Carbohydr Polym. 2015;115:200–6. doi: 10.1016/j.carbpol.2014.08.095. [DOI] [PubMed] [Google Scholar]
- 16.Wang T, Ding J, Li H, et al. Antihypertensive activity of polysaccharide from Crassostrea gigas. Int J Biol Macromol. 2016;83:195–97. doi: 10.1016/j.ijbiomac.2015.11.078. [DOI] [PubMed] [Google Scholar]
- 17.Fu D, O’Neill RA. Monosaccharide composition analysis of oligosaccharides and glycoproteins by high-performance liquid chromatography. Anal Biochem. 1995;227(2):377–84. doi: 10.1006/abio.1995.1294. [DOI] [PubMed] [Google Scholar]
- 18.Qiu DC, Hubbard AE, Zhong B, et al. A matched, case-control study of the association between Schistosoma japonicum and liver and colon cancers, in rural China. Ann Trop Med Parasitol. 2005;99(1):47–52. doi: 10.1179/136485905X19883. [DOI] [PubMed] [Google Scholar]
- 19.Wen Z, Ji X, Tang J, et al. Positive feedback regulation between transglutaminase 2 and toll-like receptor 4 signaling in hepatic stellate cells correlates with liver fibrosis post Schistosoma japonicum infection. Front Immunol. 2017;8:1808. doi: 10.3389/fimmu.2017.01808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou CL, Kong DL, Liu JF, et al. MHC II(−), but not MHC II(+), hepatic Stellate cells contribute to liver fibrosis of mice in infection with Schistosoma japonicum. Biochim Biophys Acta. 2017;1863(7):1848–57. doi: 10.1016/j.bbadis.2017.05.002. [DOI] [PubMed] [Google Scholar]
- 21.Mohamed-Ali Q, Elwali NE, Abdelhameed AA, et al. Susceptibility to periportal (Symmers) fibrosis in human schistosoma mansoni infections: Evidence that intensity and duration of infection, gender, and inherited factors are critical in disease progression. J Infect Dis. 1999;180(4):1298–306. doi: 10.1086/314999. [DOI] [PubMed] [Google Scholar]
- 22.Wu Y, Liang Y, Zhu Y, et al. Protective effect of the omega-3 polyunsaturated fatty acids on the schistosomiasis liver fibrosis in mice. Int J Clin Exp Med. 2015;8(6):9470–76. [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou S, Jin X, Li Y, et al. Blockade of PD-1 signaling enhances Th2 Cell responses and aggravates liver immunopathology in mice with Schistosomiasis japonica. PLoS Negl Trop Dis. 2016;10(10):e0005094. doi: 10.1371/journal.pntd.0005094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fairfax K, Nascimento M, Huang SC, et al. Th2 responses in schistosomiasis. Semin Immunopathol. 2012;34(6):863–71. doi: 10.1007/s00281-012-0354-4. [DOI] [PubMed] [Google Scholar]
- 25.Andrade ZA. Schistosomiasis and liver fibrosis. Parasite Immunol. 2009;31(11):656–63. doi: 10.1111/j.1365-3024.2009.01157.x. [DOI] [PubMed] [Google Scholar]
- 26.Co DO, Hogan LH, Il-Kim S, et al. T cell contributions to the different phases of granuloma formation. Immunol Lett. 2004;92(1–2):135–42. doi: 10.1016/j.imlet.2003.11.023. [DOI] [PubMed] [Google Scholar]
- 27.Long X, Chen Q, Zhao J, et al. An IL-13 promoter polymorphism associated with liver fibrosis in patients with Schistosoma japonicum. PloS One. 2015;10(8):e0135360. doi: 10.1371/journal.pone.0135360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mata-Santos HA, Dutra FF, Rocha CC, et al. Silymarin reduces profibrogenic cytokines and reverses hepatic fibrosis in chronic murine schistosomiasis. Antimicrob Agents Chemother. 2014;58(4):2076–83. doi: 10.1128/AAC.01936-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chiaramonte MG, Cheever AW, Malley JD, et al. Studies of murine schistosomiasis reveal interleukin-13 blockade as a treatment for established and progressive liver fibrosis. Hepatology. 2001;34(2):273–82. doi: 10.1053/jhep.2001.26376. [DOI] [PubMed] [Google Scholar]
- 30.Wang X, Zhang R, Du J, et al. Vitamin E reduces hepatic fibrosis in mice with Schistosoma japonicum infection. Mol Med Rep. 2012;5(2):465–68. doi: 10.3892/mmr.2011.654. [DOI] [PubMed] [Google Scholar]
- 31.Chen Y, Xiao Z. [Therapeutic effect of resveratrol as well as resveratrol combined with praziquantel on the liver fibrosis due to Schistosoma japonicum infection in mice]. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi. 2013;31(5):337–41. [in Chinese] [PubMed] [Google Scholar]
- 32.Abdallahi OM, Hanna S, De Reggi M, et al. Visualization of oxygen radical production in mouse liver in response to infection with Schistosoma mansoni. Liver. 1999;19(6):495–500. doi: 10.1111/j.1478-3231.1999.tb00082.x. [DOI] [PubMed] [Google Scholar]
- 33.Tan Y, Lv ZP, Bai XC, et al. Traditional Chinese medicine Bao Gan Ning increase phosphorylation of CREB in liver fibrosis in vivo and in vitro. J Ethnopharmacol. 2006;105(1–2):69–75. doi: 10.1016/j.jep.2005.09.040. [DOI] [PubMed] [Google Scholar]
- 34.Wu T, Chen JM, Xiao TG, et al. Qinggan Huoxue Recipe suppresses epithelial-to-mesenchymal transition in alcoholic liver fibrosis through TGF-beta1/Smad signaling pathway. World J Gastroenterol. 2016;22(19):4695–706. doi: 10.3748/wjg.v22.i19.4695. [DOI] [PMC free article] [PubMed] [Google Scholar]