Abstract
Ireland lost its official freedom from Schmallenberg virus (SBV) in October 2012. The route of introduction is uncertain, with long-distance displacement of infected Culicoides, biting midges, by suitable wind flows considered to be the most likely source. The authors investigated the potential introduction of SBV into Ireland through a Culicoides incursion event in the summer of 2012. They conducted SBV serology on archived bovine sera to identify the prospective dispersal window, then used atmospheric dispersion modelling during periods around this window to identify environmental conditions the authors considered suitable for atmospheric dispersal of Culicoides from potential infected source locations across Southern England. The authors believe that there was one plausible window over the summer of 2012, on August 10–11, based on suitable meteorological conditions. They conclude that a potential long-range transportation event of Culicoides appears to have occurred successfully only once during the 2012 vector competent season. If these incursion events remain at a low frequency, meteorological modelling has the potential to contribute cost-effectively to the alert and response systems for vectorborne diseases in the future.
Keywords: schmallenberg, culicoides, serology, ireland, atmospheric dispersion modelling
Introduction
Schmallenberg virus (SBV), a novel Orthobunyavirus, is a recently emerged viral pathogen of ruminants, new to Europe in 2011.1 2 Like bluetongue virus (BTV), it is a vectorborne disease, transmitted by Culicoides biting midges.3 Vertical transmission is also possible,4 but horizontal animal-to-animal spread has not been reported. It causes a transient disease (diarrhoea, pyrexia, drop in milk yield) in adult animals and can lead to abortion and birth defects in pregnant females. While reproductive and congenital effects are readily clinically apparent, the mildness of the initial acute disease in adult animals often means infection predates detection. Viraemia tends to be short (less than five days) in adult animals, with antibodies appearing after 10 days and eventual herd seroprevalence rates of typically 70–100 per cent for an initial outbreak.5 Natural infection with SBV is presumed to bestow detectable antibodies for at least two years.6
The involvement of vectors in disease spread introduces transmission routes quite independent of direct inter-ruminant contacts or movements. Small infected insects such as Culicoides can be subject to long-distance displacement of several hundred kilometres7 by suitable wind flows. SBV expanded across continental Europe relatively quickly,8 with almost all European countries reporting its circulation. Ireland lost its official freedom from SBV in October 2012,9 one of the last EU countries to do so, nine months after the disease was first identified in the southern UK, its nearest geographical neighbour.10 In the preceding BTV outbreak, there was evidence that wind significantly directed the expansion of the vector-mediated disease on several scales.11 12
Dispersal of vector-driven disease is usually evidenced by a new emergence of pathogen circulation in a previously disease-free region. Culicoides species are estimated to have a flight range of up to 5 km over a few days.13 Longer distance dispersals are thought to remain relatively infrequent events14 due to the particular alignment of atmospheric, ecological and phenological conditions required for successful uplift, atmospheric transportation, deposition and local colonisation by vectors.
Atmospheric dispersion modelling (ADM) has supported windborne theories of various pathogen expansions; however, uncertainty remains around the absolute fine detail of pathways. Various approaches have been used, with most built on existing particle dispersion numerical models, including the System for Integrated Modelling of Atmospheric Composition,15 Population Migration Trajectory model,16 17 Canadian Meteorological Centre,18 Nuclear Accident Model,19 Spatiotemporal Wind-Outbreak Trajectory Simulation14 and the Hybrid Single Particle Lagrangian Integrated Trajectory Model (HYSPLIT). ADM has been used previously to model long-distance dispersal of Culicoides 11 17 20 21 and more specifically outbreaks of SBV.22 23
Using SBV serology on archived bovine sera and utilising existing techniques in ADM, the authors investigated the potential introduction of SBV into Ireland through a Culicoides incursion event in the summer of 2012. This presents a unique opportunity to identify a potential windborne incursion event of a transboundary disease into Ireland.
Materials and methods
SBV serology on archived sera
The archived serum
Following the clinical effects and eventual isolation of SBV in Northern Europe during the autumn of 2011, scanning surveillance in Ireland was reviewed, diagnostics established, and Irish farmers and veterinary practitioners alerted on potential abortions and malformations in ruminants. However, it was in October 2012 before the first confirmed case of SBV malformation was reported,9 significantly later than its most proximate neighbour, the UK. At that stage, it was determined to repurpose any available 2012 serum sample sets for retrospective SBV antibody testing.
In 2012, the Irish cattle population was 6.2 million, with 1.5 million of that being females over 48 months of age.24 The Irish national bovine viral diarrhoea (BVD) eradication programme commenced in January 201225 and generates blood samples for the laboratory confirmation of BVD virus status. The majority of programme samples are from young calves aged less than three months. This age group is the most useful when attempting to determine recent, same-year exposure to pathogens, and samples were representative of geography, production type, breed, birth date and sex.
All available archived samples collected from 12 counties (Carlow, Cork, Dublin, Kildare, Kilkenny, Laois, Limerick, Louth, Tipperary, Waterford, Wexford and Wicklow) in the south, south-east and east of Ireland between March 1 and December 31, 2012 were used in this study. During active and passive surveillance conducted from November 2012 to January 2013, SBV seropositive herds and real-time quantatitive PCR (RT-qPCR)-positive animals were mainly located in these regions of Ireland.26 In addition, herds in these counties were the first with clinical evidence of SBV during late 2012 through to early 2013.27
SBV testing
Samples were tested using ID Screen SBV Competition Multi-species ELISA, according to the manufacturer’s instructions without modification. Absorbances were read in a Tecan Sunrise microplate reader (Tecan Austria, Salzburg, Austria). All results were reported as sample/negative control (S/N%), with samples reporting S/N% ≤40 deemed positive and samples reporting S/N% ≥50 deemed negative. An inconclusive result (40<S/N%<50) was considered positive in subsequent calculations.
Atmospheric dispersion modelling
ADM is the mathematical simulation of how air pollutants, or in this case, Culicoides, disperse or travel in the atmosphere. These models require importation of meteorological data, either observed or forecast, for a domain (area of interest for which there are meteorological data), and apply dispersion or trajectory algorithms to the pollutant being modelled based on the ambient meteorological processes. The source terms of the model refer to the emission type with other corresponding dependencies such as the volume or count of the emission, the time and duration of emission, and whether the emission was from a point source, multiple point sources or over an area. The output capabilities of most models will generally be predicted air concentration plumes, deposition estimates and trajectories. One such model developed by the National Oceanic and Atmospheric Administration, the HYSPLIT_4 ADM (V.4, June 2015 release28), was used in this study to simulate the atmospheric dispersal of Culicoides from potential source locations across Southern England in the Summer of 2012. At that time, this region had been confirmed as diffusely SBV-infected.29 As described, the model must be populated with meteorological data for the appropriate domain and time period. As such, meteorological data were extracted from the European Centre for Medium-Range Weather Forecasts archives at a resolution of 0.125 of a degree. The model was run in particle mode whereby a fixed number of particles are advected about the model domain by the mean wind field and spread by a three-dimensional turbulent component. The model was set to simulate the continuous release of particles from eight source locations from May 1 to September 30, 2012 inclusive to represent the period of most abundance during the vector competent season. A particle model was chosen over a puff model to best represent Culicoides. As Culicoides can sustain flight independent of mechanical transportation by wind, they were assigned zero density in the model. A decay time of 20 hours (after Eagles and others7) was set for the maximum duration that Culicoides could remain airborne after which they exited the model. The eight locations were chosen to give a broad spatial range across which SBV-positive farms were identified. The purpose of running this model was to identify any potential periods with crude suitable meteorological conditions that could have facilitated Culicoides incursion from the source locations into Ireland within the vector competent season. Once these putative dates were identified, additional modelling techniques of calculating backward trajectories and arrival times were used to determine the most likely source location. Backward trajectories were calculated from the approximate farm locations where SBV was first identified in Ireland, starting from the time estimates highlighted by the crude ADM. These models traced backward in time along a trajectory refining the area of the likely putative source. Arrival times calculate the approximate time taken for a plume to extend from a source location through a domain and give a good indication of how long Culicoides would need to remain airborne before reaching Ireland from the putative source locations. Once the putative source area was refined, the local climatic conditions in this area were then explored to better identify which of these dates were theoretically the most suitable for high-level Culicoides activity.
Results
SBV serology on archived sera
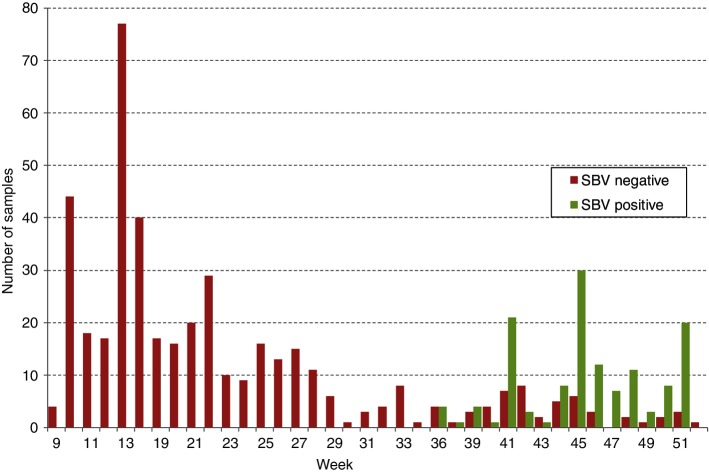
In total, 565 archived sera were tested, from 284 calves, 277 cows and 4 other adult animals. One hundred and thirty one (23.7 per cent) were SBV antibody-positive, including 25.7 per cent of calves and 22.0 per cent of cows. Changes in seropositivity over time are presented in Fig 1. The first SBV antibody-positive sample had been collected in week 36 (on September 5, 2012), from an adult female from Kilkenny. In total, seropositive animals were identified in six counties during this period (Carlow, Cork, Kilkenny, Waterford, Wexford and Wicklow; 39, 3, 9, 44, 31 and 8 seropositive animals, respectively). Fig 2 presents the geographical progression of viral infection in south-east Ireland during the period tested, including the period before and after week 36 of 2012. Assuming a 14-day typical period for detectable seroconversion, week 34±2 was identified as the prospective dispersal window.
FIG 1:
Schmallenberg virus (SBV) serology results in Ireland during 2012; weekly frequencies shown. ELISA testing performed on archived bovine serum samples from counties Carlow, Cork, Kilkenny, Waterford, Wexford and Wicklow.
FIG 2:
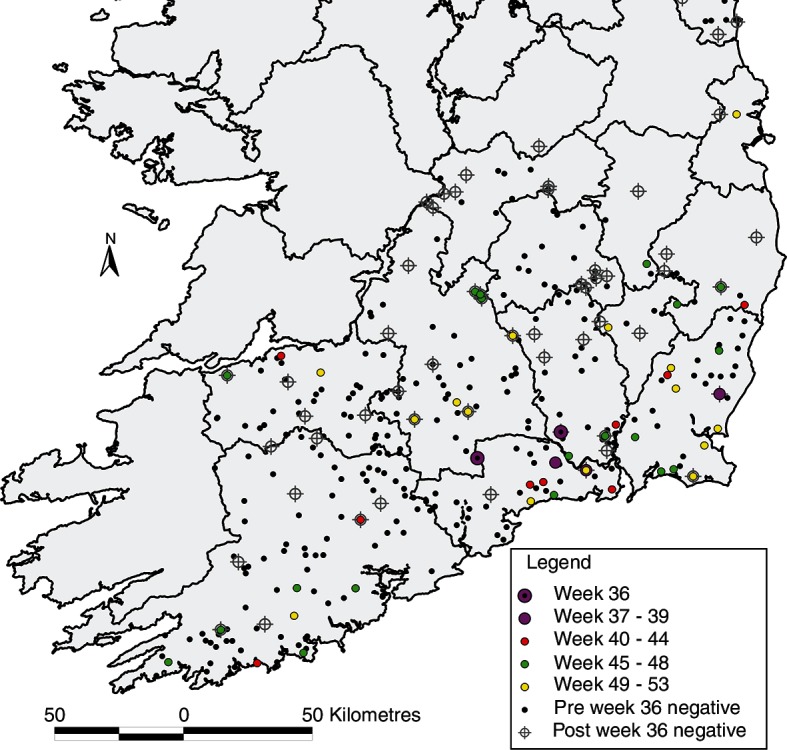
The progression of exposures to Schmallenberg virus in south-east Ireland during March to December 2012, including the period before and after week 36 of 2012.
Atmospheric dispersion modelling
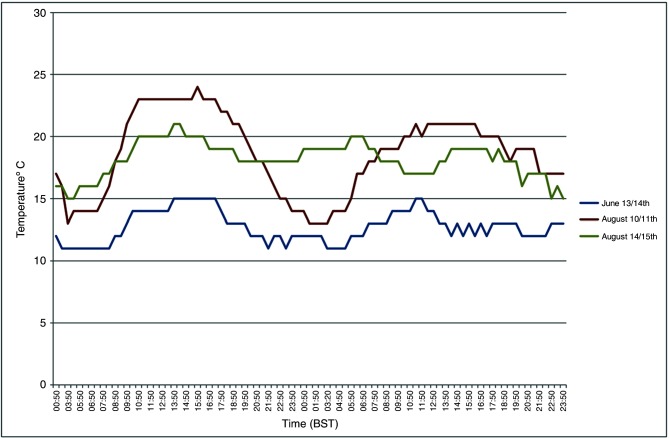
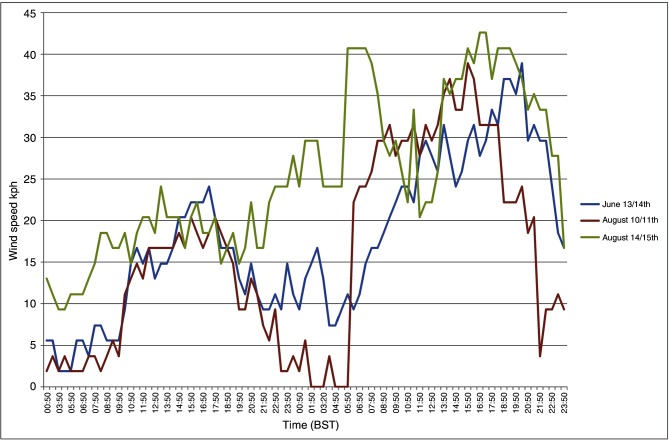
The crude particle concentration model revealed that there were three possible periods which the authors considered to consist of suitable meteorological conditions for basic transportation of Culicoides to Ireland based on wind direction and wind speed over the summer of 2012. These dates were June 13–14, August 10–11 and August 14–15. For each of these time periods, the authors conducted a manual extraction of local weather conditions (temperature, Fig 3; wind speed, Fig 4) from the nearest recording station to the putative source location (Exeter International Airport) based on the results of backward trajectory models from the initial SBV index cases in Ireland. They believe the night of August 10 had the most favourable overall conditions for a Culicoides migration event to occur.
FIG 3:
Temperature data: nearest meteorological recording station (Exeter International Airport) to the putative source locations spanning the identified time periods of interest for potential Culicoides migration events. Time in British Summer Time (BST).
FIG 4:
Wind speed data: nearest meteorological recording station (Exeter International Airport) to the putative source locations spanning the identified time periods of interest for potential Culicoides migration events. Time in British Summer Time (BST).
Arrival times were calculated from the particle concentrations model (Fig 5). The more westerly of the source locations show an arrival time in south-east Ireland of approximately 10 hours. Forward trajectories were calculated from source locations to show the most likely path of a released single particle (Fig 6). The particle concentration model was run to show a release from 21:00 on August 10 to 12:00 on August 11. The legend values are representative of 100,000 Culicoides released, which is an arbitrary representation of a hypothetical value (Fig 7).
FIG 5:
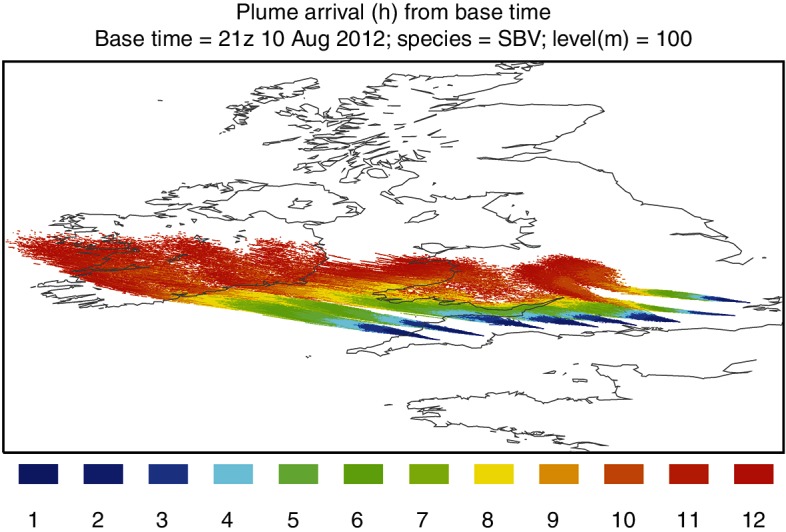
Arrival times calculated from the particle concentration model. Time values are in hours and are based on a release from source locations at 21:00 on August 10, 2012.
FIG 6:
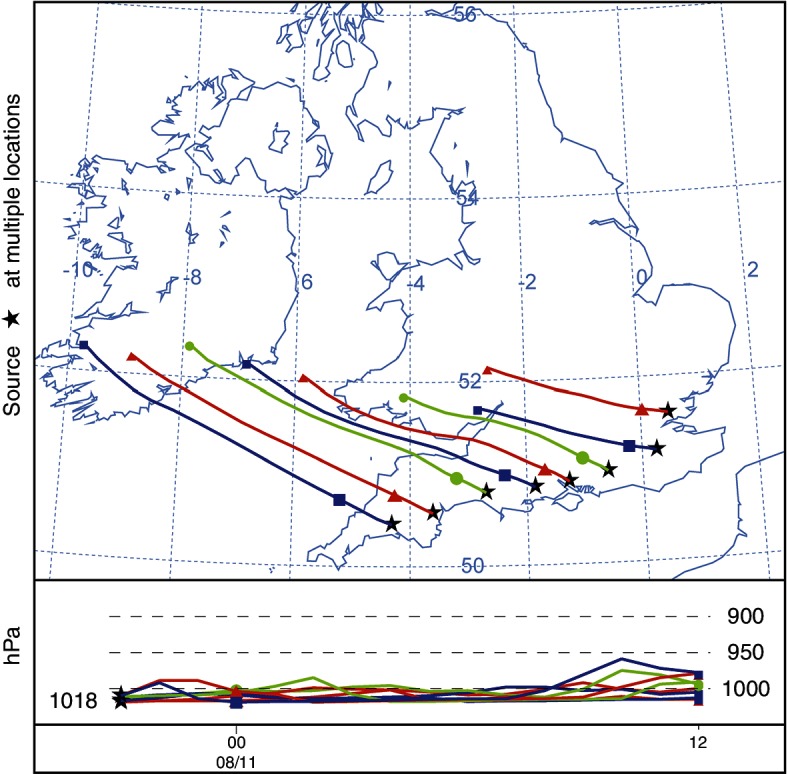
Calculated 15-hour trajectories based on particle release from source locations at 21:00 on August 10, 2012.
FIG 7:
Particle concentration model assuming a continuous release from source locations starting at 21:00 on August 10, 2012 until 12:00 on August 11, 2012. Concentration values calculated from an arbitrary input of 100,000 released particles from each source location over a 15-hour period. Time in Coordinated Universal Time (UTC).
Discussion
The first case of SBV occurring in Ireland was in October 2012. As Ireland is an island separated by water from the rest of Europe, possible sources of infection include wind dispersion of infected Culicoides, importation of infected animals, and introduction via ‘castaway’ infected Culicoides on fomites, in vehicles or on imported fodder, flowers or vegetables. Animal tracing investigations on SBV-positive index farms ruled out importation of infected animals as a potential source. BTV, a virus transmitted in a very similar way, has yet to enter Ireland and infect cattle or sheep via fomites, vehicles or organic imports even though it has been persistent on the continent for many years. While this does not rule out this pathway for the introduction of SBV, the authors believe its most likely method of incursion was windborne. The maximum distance that Culicoides can be transported on winds is uncertain. Case studies provide examples where Culicoides have travelled several hundred kilometres and have remained airborne for 12+ hours.7 11 More specifically, new cases of SBV believed to be a result of wind dispersion have been identified.22 If Ireland fell within the range of wind transportation of SBV-infected Culicoides from England, the estimated flight distance in this case would be approximately 350 km.
To investigate this, two differing but complementary methodologies were used: first, using SBV bovine serology on archived bovine sera from known affected areas, to identify the prospective dispersal window; and secondly, using ADM during periods around this window, to identify environmental conditions suitable for atmospheric dispersal of Culicoides from potential source locations across Southern England. A potential long-range transportation event of infected Culicoides appears to have occurred successfully only once during the 2012 vector competent season.
The initial particle concentration model was run irrespective of local climatic conditions known to favour Culicoides activity at the source locations. The main variables of interest were wind speed and wind direction. At this level of modelling, the only meteorological parameters that the authors assumed to preclude a potential incursion event occurring was continuous wind speeds in excess of 40 km/hour; 8 km/hour has previously been identified as the wind speed above which Culicoides generally do not fly.30 7 The three dates identified, June 13–14, August 10–11 and August 14–15, all spanned dusk to dawn. This is accepted as when Culicoides species are most active and coincides with calmer wind conditions and a lower atmospheric boundary layer.30 Of the three dates, June 13 was the least compatible with Culicoides activity. This was due to lower daytime and evening temperatures (10°C), and relatively high wind speeds with intermittent light precipitation. On August 14, temperatures were favourable, but the wind speeds were relatively high and gusty. The night of August 10 had the most favourable overall conditions. The temperature on August 10 (and previous days) was unseasonably warm (22°C–24°C). There was high humidity with early morning mist. Wind speeds were moderate to light in the afternoon, dropping to completely calm from approximately 23:00 to 01:00. The wind then gradually and steadily increased over several hours to 30+ km/hour by noon. Therefore, August 10 was considered to be the only highly probable date for the dispersion event to have occurred.
Other models31 are specifically designed to include a Culicoides activity score at the point of release. Factors known to influence Culicoides activity include temperature, humidity, rainfall and wind speed.30 32 Differences in activity levels of individual Culicoides species have also been observed based on sex, reproductive status, etc.20 32 Some models include complex population dynamics which contribute heavily to the particle release components of the model source terms.33 This assumes that dispersion is driven by stage in life cycle, appropriate local weather conditions and population density. The modelling in this study ignores population dynamics and presumes presence and abundance of Culicoides species during the entire vector competent season across the whole putative source region.31 As the authors were investigating a rare long-range dispersion event, they had an opportunity to speculate on what the conditions were at the putative source that triggered this event rather than making assumptions from existing studies from other countries, which might not apply to the UK and Ireland. Using the particle concentration model allows the authors to simulate a large release of particles at source and gives an indication of how a migratory Culicoides cloud might disperse. As the authors have no understanding of the number of Culicoides required to successfully introduce the virus into the ruminant population, they cannot perform a sensitivity analysis on their source terms.
Forward trajectories were run from the eight source locations to approximate potential flight paths of Culicoides (Fig 6). Trajectories and concentration models are known to create different results. Trajectories do not include the atmospheric dispersion of a pollutant or particles. In the concentration calculation, many Lagrangian particles are released, transported and dispersed through the domain, whereas trajectory models follow a single path dictated by the meteorological parameters. This passive transit is how the authors would expect a single airborne insect to be carried. Anywhere along the path of the trajectory could be a landing location for transported Culicoides. Unlike typical particles modelled, Culicoides can maintain active flight. This would enable them to remain aloft when passive particles would be deposited by the model. In addition, Culicoides, unlike passive particles, may exhibit choice. Stimuli such as smell, fluctuation in temperature or change in the atmospheric boundary layer might trigger intentional landing. These stimuli would be present where an air flow meets land having crossed a large body of water. The path of the trajectories modelled would suggest that the most promising source locations for a potential incursion occurred between Bournemouth and Exeter.
The involvement of a free-moving, highly mobile vector makes the mapping and control of animal pathogens such as Schmallenberg and bluetongue viruses inherently more complex. Vector ranges can expand locally by active host-seeking behaviours14 and potentially much further by the passive conveyance of vectors due to wind flows34 and inadvertent transfers linked to trade.30 Understanding the spread of vectorborne diseases in farmed animals, such as SBV, is a critical part of the risk assessment process for introductions of such pathogens. It is particularly important for regions such as Ireland with significant agricultural livestock sectors but no previous exposure to these types of diseases.
It can be assumed that the south of England and the arrival areas in Ireland are not beyond the environmental range for the short-term survival and reproduction of the virus because local farm-to-farm transmission continued following the initial incursion. The duration of viraemia is short in cattle and sheep (three to four days). Gubbins and others35 set their farm spread model to have a lower threshold temperature for virus replication of 12.3°C. They estimate that probability of transmission from host to vector is high (14 per cent). In the areas of southern England that experienced cases of SBV, there are no major obstacles such as mountain ranges to impede onward wind transportation of infected Culicoides. The density of ruminant populations is sufficient to make the likelihood of migratory infected vectors finding and infecting a host very high. These areas also have Culicoides species present in very large numbers in the summer months.31 The reasons as to why more dispersion events, such as hypothesised here, do not occur and why dissemination remains largely limited to local incremental expansion are unclear. Many more short-range to medium-range dispersion events should be expected within the British Isles, where local conditions satisfy high Culicoides activity and broader meteorological conditions favour medium-distance to long-distance dispersion. This however is not what is observed. In 2012–2013, SBV cases concentrated in southern parts of England, with only sporadic cases occurring away from these locations. BTV showed a similar lack of long-distance dispersion after initial incursion. Only 2 per cent of cases could be attributed to long-range dispersion of greater than 32 km.14 A possible key to understanding the mechanisms underlying this could be alluded to by Pedgley.36 There is a suggestion that Culicoides dispersion could be triggered by an intentional migratory urge that is distinctly different from their typical behaviour. This would suggest that any long-range or even relatively short-range dispersion events could be more than a chance event of Culicoides being caught unintentionally in an air flow. Local climatic conditions occurring over a period of hours or even days may trigger a migratory response in Culicoides. Currently, models of Culicoides dispersion events are based on conditions at source at a fixed time. These parameters relate to conditions known to favour high levels of Culicoides activity but not to the conditions that may need satisfying to prompt migratory behaviour. There was widespread wind dispersion of SBV and BTV in continental Europe22 that did not occur to the same extent in the UK, and as the authors state in this study it is believed that there was only one SBV incursion event in Ireland. Due to maritime influences, prevailing winds and associated weather patterns, the mean summer temperatures in the UK and Ireland are generally lower than areas of similar latitude in continental Europe, where there was more confirmed wind spread of SBV. The authors speculate that lower mean summer temperatures may inhibit migratory behaviour in native Culicoides species, reducing their range to appetitive or vegetative movements or leading to higher mean attrition rates during transit.37 An additional consideration is the possibility of multiple incursion events of infected Culicoides with no further transmission to livestock. Ireland has a well-established diversity of Culicoides species38; however, species of Culicoides are differentiated by ecology and host,39 and it is possible that imperfect timing and mismatches of these two factors following dispersal events may limit the establishment of newly arrived vectors and related disease. Factors such as the number of vector generations concluded within a season, the length and depth of average winters, and the availability of specialised breeding habitats may dictate the particular viral and insect concurrence required for introduced diseases to persist across years into endemicity.
The findings in this study are based on a number of assumptions. First, the serology captured the initial index cases of SBV. The archived sera were not collected for the purposes of identifying a new disease. Ideally, testing more samples would give more confidence in identifying the true initial index cases. The authors do know that once introduced, there was pronounced local spread of infection (Fig 2). If there had been virus present before week 36, the authors would have expected to see much more local spread of infection as characterised by the pattern of spread from weeks 40 onwards. Secondly, there was no alternative source of SBV introduction via imported infected animals or Culicoides. This has been discussed earlier in this section. Thirdly, the meteorological conditions at the time of the dispersal event were key to triggering a migration rather than it being subject to mere chance. Finally, subsequent incursion events may have occurred following the initial incursion. These would be indistinguishable from cases resulting from local spread. While the authors can find no evidence of this through ADM, it is not possible to entirely rule it out.
Future work should be conducted to compare the predicted spread of SBV within UK and Ireland with that of mainland Europe to identify any differences in the source terms of the models. This would give further weight to a hypothesis of a difference based on the environmental-driven migratory responses of Culicoides. A better understanding of these mechanisms is important for aiding in the development of biologically plausible meteorological early warning systems and the creation of informed disease control strategies.
Conclusion
Using HYSPLIT ADM and contemporary serological testing, a potential once-off incursion event was identified where long-range wind transportation of Culicoides resulted in an outbreak of SBV in Ireland in August 2012.
Acknowledgments
The authors would like to extend their thanks and appreciation to the Department of Agriculture, Food and the Marine, Catherine Boland and Padraig Ross at the CVRL, and to Keith Lambkin at Met Éireann.
Footnotes
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Beer M, Conraths FJ, van der Poel WH. ’Schmallenberg virus'-a novel orthobunyavirus emerging in Europe. Epidemiol Infect 2013;141:1–8. 10.1017/S0950268812002245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hoffmann B, Scheuch M, Höper D, et al. Novel orthobunyavirus in cattle, Europe, 2011. Emerg Infect Dis 2012;18:469–72. 10.3201/eid1803.111905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.De Regge N, Deblauwe I, De Deken R, et al. Detection of Schmallenberg virus in different Culicoides spp. by real-time RT-PCR. Transbound Emerg Dis 2012;59:471–5. 10.1111/tbed.12000 [DOI] [PubMed] [Google Scholar]
- 4.Garigliany MM, Hoffmann B, Dive M, et al. Schmallenberg virus in calf born at term with porencephaly, Belgium. Emerg Infect Dis 2012;18:1005–6. 10.3201/eid1806.120104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Doceul V, Lara E, Sailleau C, et al. Epidemiology, molecular virology and diagnostics of Schmallenberg virus, an emerging orthobunyavirus in Europe. Vet Res 2013;44:31–13. 10.1186/1297-9716-44-31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Elbers AR, Stockhofe-Zurwieden N, van der Poel WH. Schmallenberg virus antibody persistence in adult cattle after natural infection and decay of maternal antibodies in calves. BMC Vet Res 2014;10:103 10.1186/1746-6148-10-103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eagles D, Melville L, Weir R, et al. Long-distance aerial dispersal modelling of Culicoides biting midges: case studies of incursions into Australia. BMC Vet Res 2014;10:135 10.1186/1746-6148-10-135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. European Food Safety Authority (2013). "Schmallenberg" virus: analysis of the epidemiological data: EFSA Supporting Publications, 2012:EN-429. [Google Scholar]
- 9.Bradshaw B, Mooney J, Ross PJ, et al. Schmallenberg virus cases identified in Ireland. Vet Rec 2012;171:540–1. 10.1136/vr.e7928 [DOI] [PubMed] [Google Scholar]
- 10.Steinbach F, Dastjerdi A, Drew T, et al. Continued presentation of cases of Schmallenberg virus in sheep in England. Vet Rec 2012;170:547.1–547. 10.1136/vr.e3640 [DOI] [PubMed] [Google Scholar]
- 11.Burgin LE, Gloster J, Sanders C, et al. Investigating incursions of bluetongue virus using a model of long-distance Culicoides biting midge dispersal. Transbound Emerg Dis 2013;60:263–72. 10.1111/j.1865-1682.2012.01345.x [DOI] [PubMed] [Google Scholar]
- 12.Faes C, van der Stede Y, Guis H, et al. Factors affecting Bluetongue serotype 8 spread in Northern Europe in 2006: the geographical epidemiology. Prev Vet Med 2013;110:149–58. 10.1016/j.prevetmed.2012.11.026 [DOI] [PubMed] [Google Scholar]
- 13.Elbers AR, Meiswinkel R, van Weezep E, et al. Schmallenberg virus in Culicoides biting midges in the Netherlands in 2012. Transbound Emerg Dis 2015;62:339–42. 10.1111/tbed.12128 [DOI] [PubMed] [Google Scholar]
- 14.Sedda L, Brown HE, Purse BV, et al. A new algorithm quantifies the roles of wind and midge flight activity in the bluetongue epizootic in northwest Europe. Proc Biol Sci 2012;279:2354–62. 10.1098/rspb.2011.2555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leskinen M, Markkula I, Koistinen J, et al. Pest insect immigration warning by an atmospheric dispersion model, weather radars and traps. Journal of Applied Entomology 2011;135:55–67. 10.1111/j.1439-0418.2009.01480.x [DOI] [Google Scholar]
- 16.Rochester W. The migration systems of Helicoverpa punctigera (Wallengren) and Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae) in Australia, 1999. The University of Queensland, Brisbane, Australia.. [Google Scholar]
- 17.Eagles D, Deveson T, Walker PJ, et al. Evaluation of long-distance dispersal of Culicoides midges into northern Australia using a migration model. Med Vet Entomol 2012;26:334–40. 10.1111/j.1365-2915.2011.01005.x [DOI] [PubMed] [Google Scholar]
- 18.Hopkinson RF, Soroka JJ. Air trajectory model applied to an in-depth diagnosis of potential diamondback moth infestations on the Canadian Prairies. Agric For Meteorol 2010;150:1–11. 10.1016/j.agrformet.2009.07.015 [DOI] [Google Scholar]
- 19.Agren EC, Burgin L, Lewerin SS, et al. Possible means of introduction of bluetongue virus serotype 8 (BTV-8) to Sweden in August 2008: comparison of results from two models for atmospheric transport of the Culicoides vector. Vet Rec 2010;167:484–8. 10.1136/vr.c3961 [DOI] [PubMed] [Google Scholar]
- 20.Ducheyne E, De Deken R, Bécu S, et al. Quantifying the wind dispersal of Culicoides species in Greece and Bulgaria. Geospat Health 2007;1:177–89. 10.4081/gh.2007.266 [DOI] [PubMed] [Google Scholar]
- 21.García-Lastra R, Leginagoikoa I, Plazaola JM, et al. Bluetongue virus serotype 1 outbreak in the Basque Country (Northern Spain) 2007-2008. Data support a primary vector windborne transport. PLoS One 2012;7:e34421 10.1371/journal.pone.0034421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sedda L, Rogers DJ. The influence of the wind in the Schmallenberg virus outbreak in Europe. Sci Rep 2013;3:3361 10.1038/srep03361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roberts HC, Elbers AR, Conraths FJ, et al. Response to an emerging vector-borne disease: surveillance and preparedness for Schmallenberg virus. Prev Vet Med 2014;116:341–9. 10.1016/j.prevetmed.2014.08.020 [DOI] [PubMed] [Google Scholar]
- 24. Department of Agriculture, Food and the Marine (2012). Animal Identification & Movement (AIM) Bovine Statistics Report. 2012. https://www.agriculture.gov.ie/media/migration/animalhealthwelfare/animalidentificationandmovement/AIMBOVINESTATISTICSREPORT2012220513.pdf
- 25.Graham DA, Lynch M, Coughlan S, et al. Development and review of the voluntary phase of a national BVD eradication programme in Ireland. Vet Rec 2014;174:67 10.1136/vr.101814 [DOI] [PubMed] [Google Scholar]
- 26.Barrett D, More SJ, O’Neill R, et al. Prevalence and distribution of exposure to Schmallenberg virus in Irish cattle during October 2012 to November 2013. BMC Vet Res 2015;11:267 10.1186/s12917-015-0564-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johnson A, Bradshaw B, Boland C, et al. A bulk milk tank study to detect evidence of spread of Schmallenberg virus infection in the south-west of Ireland in 2013. Ir Vet J 2014;67:11 10.1186/2046-0481-67-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Draxler R, Rolph G. HYSPLIT (Hybrid Single-Particle Lagrangian Intergrated Tracjetory) National Oceanic and Atmospheric Administration Air Resource Laboratory, College Park, Maryland. 2015. https://www.arl.noaa.gov/hysplit/hysplit/ (accessed 09 Nov 2017).
- 29. Department for Environment Food and Rural Affairs (2012). Update No.9 on Schmallenberg Virus in Northern Europe. Department for Environment, Food and Rural Affairs, Veterinary & Science Policy Advice, International Disease Monitoring Reference VITT/1200 Schmallenberg virus in North Europe. 2012. http://webarchive.nationalarchives.gov.uk/20130822084033/http://www.defra.gov.uk/animal-diseases/files/poa-schmallenberg-update-120618.pdf (accessed 03 Jan 2017).
- 30.Carpenter S, Szmaragd C, Barber J, et al. An assessment of Culicoides surveillance techniques in Northern Europe: have we underestimated a potential bluetongue virus vector? J Appl Ecol 2008;45:1237–45. [Google Scholar]
- 31.Sanders CJ, Shortall CR, Gubbins S, et al. Influence of season and meteorological parameters on flight activity of Culicoides biting midges. J Appl Ecol 2011;48:1355–64. 10.1111/j.1365-2664.2011.02051.x [DOI] [Google Scholar]
- 32.Blackwell A. Diel flight periodicity of the biting midge Culicoides impunctatus and the effects of meteorological conditions. Med Vet Entomol 1997;11:361–7. 10.1111/j.1365-2915.1997.tb00423.x [DOI] [PubMed] [Google Scholar]
- 33.Kelso JK, Milne GJ. A spatial simulation model for the dispersal of the bluetongue vector Culicoides brevitarsis in Australia. PLoS One 2014;9:e104646 10.1371/journal.pone.0104646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jacquet S, Huber K, Pagès N, et al. Range expansion of the Bluetongue vector, Culicoides imicola, in continental France likely due to rare wind-transport events. Sci Rep 2016;6:27247 10.1038/srep27247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gubbins S, Turner J, Baylis M, et al. Inferences about the transmission of Schmallenberg virus within and between farms. Prev Vet Med 2014;116:380–90. 10.1016/j.prevetmed.2014.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pedgley D. Windborne pests and diseases. Chichester: Ellis Horwood, 1982. [Google Scholar]
- 37.Reynolds DR, Chapman JW, Harrington R. The migration of insect vectors of plant and animal viruses. Adv Virus Res 2006;67:453–517. 10.1016/S0065-3527(06)67012-7 [DOI] [PubMed] [Google Scholar]
- 38.McCARTHY TK, Bateman A, Nowak D, et al. National BTV vector surveillance programme 2007-2009. 2010. http://www.agriculture.gov.ie/media/migration/animalhealthwelfare/diseasecontrols/bluetonguedisease/BTVVectorSurveillance0910FinalReport.pdf (accessed 23 Dec 2016).
- 39.Purse BV, Falconer D, Sullivan MJ, et al. Impacts of climate, host and landscape factors on Culicoides species in Scotland. Med Vet Entomol 2012;26:168–77. 10.1111/j.1365-2915.2011.00991.x [DOI] [PubMed] [Google Scholar]