Abstract
Background
Arsenic trioxide (As2O3) has a dramatic therapeutic effect on acute promyelocytic leukemia (APL) patients. It can also cause apoptosis in various tumor cells. This study investigated whether As2O3 has an antitumor effect on glioma and explored the underlying mechanism.
Results
MTT and trypan blue assays showed that As2O3 remarkably inhibited growth of C6 and 9 L glioma cells. Cell viability decreased in glioma cells to a greater extent than in normal glia cells. The annexin V-FITC/PI and Hoechest/PI staining assays revealed a significant increase in apoptosis that correlated with the duration of As2O3 treatment and occurred in glioma cells to a greater extent than in normal glial cells. As2O3 treatment induced reactive oxygen species (ROS) production in C6 and 9 L cells in a time-dependent manner. Cells pretreated with the antioxidant N-acetylcysteine (NAC) showed significantly lower As2O3-induced ROS generation. As2O3 significantly inhibited the expression of the anti-apoptotic gene Bcl-2, and upregulated the proapoptotic gene Bax in both C6 and 9 L glioma cells in a time-dependent manner.
Conclusions
As2O3 can significantly inhibit the growth of glioma cells and it can induce cell apoptosis in a time- and concentration-dependent manner. ROS were found to be responsible for apoptosis in glioma cells induced by As2O3. These results suggest As2O3 is a promising agent for the treatment of glioma.
Keywords: Arsenic trioxide (As2O3), Reactive oxygen species (ROS), Glioma, Apoptosis
Background
Despite commonly being known as a toxic metalloid, arsenic trioxide (As2O3) has applications in traditional medicine in China. As early as the 1970s, a research group at the First Affiliated Hospital of Harbin Medical University discovered that As2O3 can induce remissions in up to 70% of acute promyelocytic leukemia (APL) patients [1, 2]. The dramatic therapeutic effect of As2O3 on APL was achieved primarily through the induction of cell differentiation and apoptosis [2, 3]. At low concentrations, As2O3 promoted cell differentiation, while at concentrations above 0.5 μmol/l, it induced cell apoptosis [4, 5].
As2O3 induced apoptosis not only in NB4 cells (an APL cell line) but also in various other tumor cell lines [6, 7]. The underlying mechanism remained unclear, but inhibition of cell differentiation and growth and induction of apoptosis are speculated to be the general mechanisms for tumor treatment [8] and As2O3 action [9, 10]. Further research on As2O3 in APL showed that reactive oxygen species (ROS) play an important role in the induction of apoptosis, and that APL cells are sensitive to the intracellular ROS levels [11]. However, there is still some discussion about whether ROS are involved in As2O3 inhibition of the growth of tumor cells [11–14].
Due to the existence of the blood–brain barrier, it is hard for therapeutics drugs to affect glioma cells. New therapeutics are required to overcome this challenge. Although it is still unclear how As2O3 could cross the blood–brain barrier, several studies of As2O3 in glioma indicate that it is a potential therapeutic agent for this type of cancer [9, 15].
The effective concentrations of As2O3 applied in those studies were extremely high, ranging from 4.0 μM to 5.0 mM [16, 17]. High concentrations of As2O3 carry a major health risk. Side effects include mild gastrointestinal discomfort, transient elevation of liver enzymes, reversible neuropathy, hypokalemia, hyperglycemia and cardiac toxicity. Prolongation of the life quality has been detected in as many as 38% of patients treated with As2O3 [18, 19]. In this study, we investigated the anti-tumor effect of a low concentration range (0–8 μmol/l) of As2O3 in the glioma cell lines C6 and 9 L, assessed changes to non-tumor (glial) cells, and explored the underlying mechanism by studying ROS.
Methods
Cell culture
As2O3 was obtained from Yida. Stock solutions were prepared in phosphate buffered saline (PBS) to exclude any unknown influence from other solvents. Working solutions were diluted in RPMI-1640 medium (Gibco) and Dulbecco’s modified Eagle’s medium (DMEM; Gibco), supplemented with 10% heat-inactivated fetal calf serum (FCS).
Rat C6 and 9 L glioma cells were obtained from Harbin Medical Neurosurgical Institute and were respectively cultured in 10% RPMI-1640 medium and 10% DMEM, in both cases supplemented with 10% FCS. Primary glial cells were isolated from new suckling Wistar mice within 24 h of birth using the method of McCarthy and de Vellis [20]. The cell concentration was adjusted to 5 × 105 cells/ml in 15% DMEM. The fourth generation (after about 20 days of culture) was used. The cells were maintained at 37 °C, 95% air and 5% CO2 in a humidified incubator (Heraeus).
Determination of cell viability
To test cell viability, cell suspensions of 2 × 105 cells/ml were mixed with 0.4% trypan blue. After 5–10 min, dye exclusion was examined for viable cells under a light microscope. The 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) bromide assay was also used to determine the number of viable cells after exposure to As2O3. 200 μl cell suspensions (4 × 104 cells/ml) were seeded in 96-well plates. Serially diluted As2O3 was added at final concentrations of 0 (control), 0.5, 1.0, 3.0, 5.0, 6.0, 7.0 and 8.0 μmol/l. Each experiment was performed in quadruplicate and repeated at least three times. After 24, 48 and 72 h, the MTT products were quantified and the results were presented as the percentage of viable cells and normalized to the level of controls. The optimal concentration was determined as 5.0 μmol/l and used to treat the rat C6 and 9 L cells.
Measurement of apoptosis
After cultured for 24, 48 and 72 h, cell apoptosis was assessed using propidium iodide (PI) and annexin-V conjugated to fluorescein isothiocyanate (FITC) according to the manufacturer’s instructions (BD Biosciences). Briefly, cells with or without As2O3 were incubated with FITC-conjugated annexin-V. Then, the cells were collected, washed and centrifuged at 200 g for 10 min. The cell pellet was gently resuspended in 200 μl PI and incubated in the dark for 30 min at room temperature. Apoptosis was then assessed using flow cytometry.
Cell apoptosis and necrosis were further examined by staining with Hoechst 33,342 (HOE) and PI, respectively. Cells were plated into 96-well plates and treated with 5.0 μmol/l As2O3 for 24, 48 and 72 h. Cells (5 × 106 cells/ml) were incubated for 15 min at 37 °C with HOE (10 μg/ml in PBS), centrifuged, washed in PBS, and resuspended at density of 1 × 107 cells/ml. PI (50 μg/ml in PBS) was added before observation. Cells were examined using a light microscope (Olympus) equipped with a fluorescent light source and a UV-2A filter cube with an excitation wavelength of 330–380 nm and a barrier filter of 420 nm. All experiments were repeated at least three times.
Measurement of ROS levels
The generation of ROS was measured as previously described [21]. Briefly, cell suspensions (2 × 106 cells/ml) were exposed to As2O3 at 5.0 μmol/l for 24, 48 and 72 h. To evaluate the major organelles that governed the ROS-mediated stress in glioma cells, C6 and 9 L cells were pretreated with 5 nM antioxidant N-acetylcysteine (NAC) for 2 h, and were exposed to As2O3 at 5.0 μmol/l for 24 h [22]. After exposure, cells were incubated in 10 μM of 2′,7′-dichlorofluorescein diacetate (DCFH-DA; Molecular Probes) at 37 °C for 30 min. The cells were harvested and washed with cold PBS three times. Then, ROS levels were determined through fluorescence-activated cell sorting.
Measurement of apoptotic proteins
Levels of apoptosis-related proteins (Bcl-2, Bax and Fas) were analyzed using Western blot as previously described [23]. Briefly, cells were lysed at 4 °C via RIPA. Proteins were separated using 10% SDS-PAGE, transferred to nitrocellulose membranes and incubated with primary antibodies against Bcl-2, Bax, Fas and actin (1:100, Santa Cruz Biotechnology). Then, the membranes were incubated with horseradish peroxidase-conjugated secondary antibodies, and detected using an enhanced chemiluminescence (ECL) kit (Beyotime).
Statistical analysis
All quantitative data measurements were performed in triplicate and the results are presented as means ± standard deviation. One-way analysis of variance (ANOVA) was performed. The post hoc tests were Dunnett’s tests. Probability values (p) less than 0.05 were considered statistically significant.
Results
As2O3 decreased cell viability in C6 and 9 L glioma cells
The cytotoxicity of As2O3 in C6 and 9 L cells was assessed using the MTT and trypan blue assays. As2O3 was applied at 0.5, 1.0, 3.0, 5.0, 6.0, 7.0 and 8.0 μmol/l, and the inhibitory rates were determined after 24, 48 and 72 h (Fig. 1). The MTT assay showed that the As2O3-induced inhibitory rates for C6 and 9 L cells were dose and time dependent (Fig. 1a). The inhibitory effects of As2O3 on C6 and 9 L cells were significantly stronger than on normal glial cells. For example, the inhibition rate for normal glial cells exposed to 5.0 μmol/l As2O3 was less than 10% of that for glioma cells, suggesting that As2O3 inhibited the growth of glioma cells but not normal glial cells in range of 0–8 μmol/l. The calculated IC50 values for C6 and 9 L cells were respectively 5.0 and 5.6 μmol/l As2O3, so 5.0 μmol/l As2O3 was used in the following experiments.
Fig. 1.
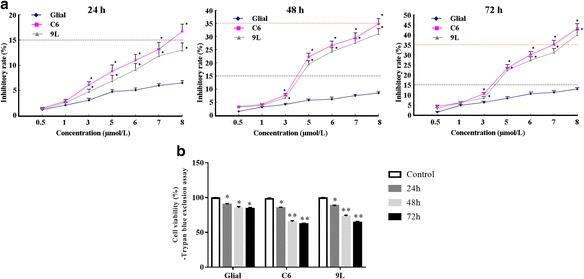
The inhibitory effects of As2O3 on the cell viability of C6 glioma cells, 9 L sarcoma cells and normal non-tumor (glial) cells (control). a The inhibitory rate was determined using the MTT assay 24, 48 and 72 h after exposure to As2O3 and it is expressed as % of control exposure at 24 h. b Cell viability was determined using the Trypan blue exclusion assay. *p < 0.05, **p < 0.01 vs. glial cells
The trypan blue assay showed that 5.0 μmol/l As2O3 significantly decreased cell viabilities in C6 and 9 L in a time-dependent manner (Fig. 1b). Although the cell viability in normal glial cells was also significantly decreased, the change was smaller than for glioma cells, suggesting a greater inhibitory role in glioma than in glial cells.
As2O3 induced apoptosis in C6 and 9 L glioma cells
An annexin V-FITC/PI assay was used to assess cell apoptosis of glioma cells after exposure to 5 μM As2O3. The numbers of early (annexin V+/PI–) and late (annexin V+/PI+) apoptotic cells were calculated. In both C6 and 9 L glioma cells, apoptosis (seen as both early and late apoptotic cells) was significantly induced by 5 μM As2O3 in a time-dependent manner (Fig. 2a and b). The maximal percentages of apoptotic cells in both cell lines were reached at 72 h (14.35% of C6 cells, and 13.13% of 9 L cells; Fig. 2). However, the apoptotic rate for glial cells was only 3.59% (Fig. 2c and d). For cells without As2O3 treatment, the apoptosis rate was close to 0 (data not shown).
Fig. 2.
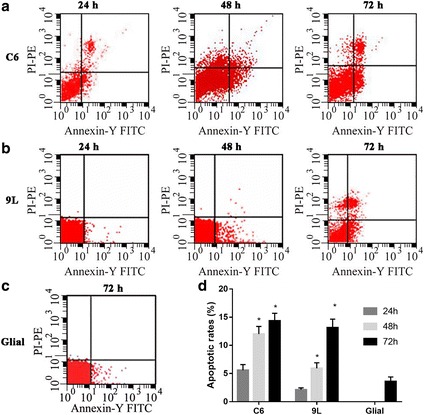
The annexin V-FITC/PI assay for apoptotic and necrotic cells. Cells were stained with annexin V-FITC and PI, and analyzed using a FACSScan flow cytometer. a C6 cells were treated with 5 μmol/l As2O3 for 24, 48 and 72 h. The apoptotic rate was 5.57, 12.01 and 14.35%, respectively. b 9 L cells were treated with 5 μmol/l As2O3 for 24, 48 and 72 h. The apoptotic rate was 2.12, 5.92 and 13.13%, respectively. c Glial cells were treated with 5 μmol/l As2O3 for 72 h. The apoptotic rate was 3.59%. d The quantification data. *p < 0.05 vs. glial cells
The apoptosis in glial and glioma cells after exposure to 5 μM of As2O3 for 72 h was further confirmed with HOE/PI double staining (Fig. 3). Cell uptake of PI indicated necrosis. Cells with clear nuclear condensation but no PI uptake indicated apoptosis. After exposure to As2O3 for 72 h, C6 and 9 L cells showed increases in both necrosis and apoptosis. The level of apoptosis and necrosis induced by As2O3 was higher in C6 and 9 L cells than in glial cells.
Fig. 3.
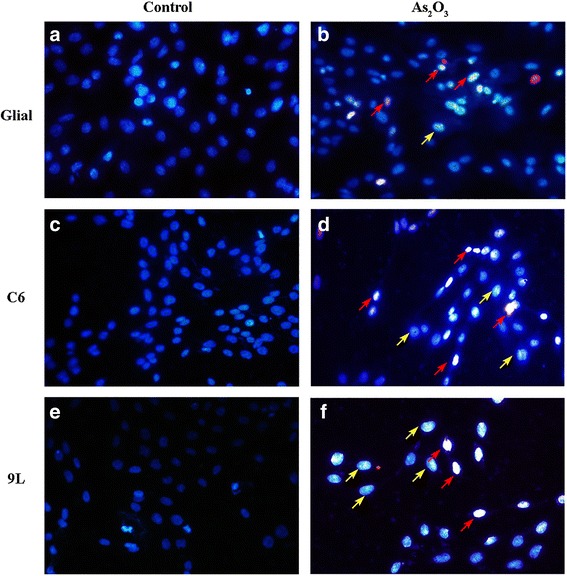
HOE/PI staining assay in glial (a, b), C6 glioma (c, d) and 9 L sarcoma (e, f) cells before (a, c, e) or after (b, d, f) treatment with 5 μmol/l As2O3 for 72 h. Magnification: 60×. Red arrows indicate necrosis. Yellow arrows indicate apoptosis. As2O3 might change the cell morphology of 9 L cells
Production of ROS in C6 and 9 L glioma cells exposed to As2O3
The extent of cellular oxidative stress in living cells was estimated by monitoring ROS generation using the fluorescent dye DCFH-DA (Fig. 4). The mean fluorescence intensity in C6 cells was 7.58, 200.37, 344.80 and 501.74 at 0, 24, 48 and 72 h, respectively. The mean fluorescence intensity in 9 L cells was 3.01, 180.27, 248.32 and 485.90 at 0, 24, 48 and 72 h, respectively. Thus, the level of ROS level positively correlates with DCF intensity. In both C6 (Fig. 4a and b) and 9 L (Fig. 4b and c) cells, intracellular ROS increased significantly with increasing incubation time with 5 μmol/l As2O3 (p < 0.01). Cells pretreated with NAC significantly inhibited the increase in ROS in response to 24 h exposure to As2O3 (Fig. 4a and b).
Fig. 4.
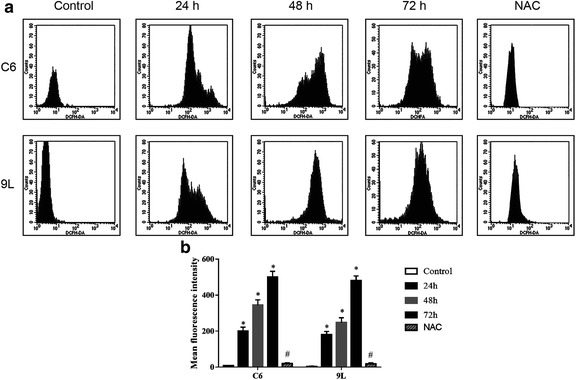
Effects of As2O3 on ROS generation in C6 and 9 L cells as measured using DCFH-DA. a C6 cells without exposure to 5 μmol/l As2O3 showed a mean fluorescence intensity of 7.58, while those with exposure for 24, 48 and 72 h showed 200.37, 344.80 and 501.74, respectively. 9 L cells without exposure to 5 μmol/l As2O3 showed a mean fluorescence intensity of 3.01, while those with exposure for 24, 48 and 72 h showed 180.27, 248.32 and 485.90, respectively. b Quantification data of ROS. Cells pretreated with NAC significantly inhibited the 24 h As2O3 increase in ROS levels. *p < 0.05 vs. control, #p < 0.05 vs. 24 h
Effects of As2O3 on the expression of apoptotic proteins Bcl-2, Bax and Fas
To validate the apoptosis process, the expression levels of apoptosis markers, including Bcl-2, Bax and Fas, were examined in C6 and 9 L glioma cells using western blotting. As2O3 significantly inhibited expression of the anti-apoptotic gene Bcl-2 and upregulated the pro-apoptotic gene Bax in both C6 and 9 L glioma cells in a time-dependent manner (Fig. 5a and b). The expression of Fas did not significantly change after exposure to As2O3 (Fig. 5a and b).
Fig. 5.
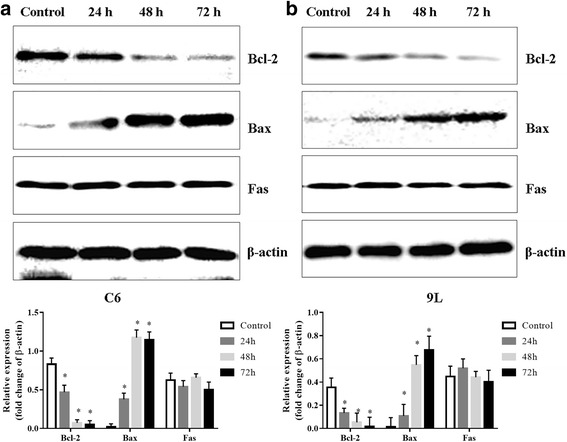
Western blot of Bcl-2, Bax and Fas in C6 glioma and 9 L sarcoma cells with or without treatment with As2O3 (5 μmol/l) for 24, 48 and 72 h. Bcl-2 expression reduced in a time dependent manner, while Bax expression increased in a time dependent manner. a Blots and quantification data for C6 cells. b Blots and quantification data for 9 L cells. *p < 0.05 vs. control
Discussion
Because of its ability to induce apoptosis in various malignant tumor cells, As2O3 has potential as a treatment agent for malignant tumors [24, 25]. Gliomas are highly aggressive tumors that respond poorly to existing clinical therapeutic agents. In previous studies, it was shown that As2O3 treatment could inhibit cell growth of glioma cells, but the studies did not yield guidance on the effective doses [26–28].
Here, we investigated the effective doses of As2O3 using rat glioma cells and comparing them with non-tumor glial cells. Our results showed that As2O3 inhibited the growth of glioma cells in time- and concentration-dependent manners, and that 5.0 μmol/l As2O3 is the optimum concentration for inhibiting cell viability in both C6 and 9 L glioma cells. The inhibitory rate for non-tumor cells was less than 10% of that for the glioma cells, indicating that As2O3 is a promising drug. Due to the exist of the blood–brain barrier, it remains unclear how the 5 μmol/l concentration can be obtained in human blood such that it would be useful for treating glioma cells. Further studies using in vivo animal models are needed.
Both the HOE/PI and annexin-V/PI assays showed that 5.0 μM As2O3 induced apoptosis. However, the mechanism of apoptosis in solid tumor cells is far from clear. In glioma cells treated with As2O3, one of the most likely mechanisms for triggering an antitumor effect is the induction of ROS [29, 30]. Like other heavy metals, including iron, copper, chromium, cadmium, lead and mercury, arsenic affects cells by causing oxidative damage, primarily through disruption of the endogenous cellular antioxidant–redox balance [29, 30]. Cysteine thiol is the functional site for most redox proteins. Arsenic can directly bind to this site and destroy protein function, thereby affecting ROS production and clearance [29, 30]. Cell viability, ROS levels, apoptosis and autophagy in human glioblastoma cell line have been shown to be regulated by As2O3 [31, 32] and/or As2O3 in combination with other agents [33]. As2O3 induces ROS production and apoptosis in glioma cells through the upregulation of the mitoferrin-2 gene [34]. Consistently with the results of those studies, we also found that intracellular ROS levels increased significantly after As2O3 treatment.
The brain appears to be especially sensitive to ROS stress when compared to other organs. Although comprising only 2% of human body weight, the human brain consumes up to 20% of the oxygen supply. Such a high level of oxygen consumption indicates that large quantities of ROS are generated during oxidative phosphorylation in brain tissue. In addition, iron content has been shown to increase brain sites in which ROS production may be greater [35]. Tumor cells are vulnerable to ROS stress. Thus, therapeutic approaches directed at ROS intervention may have an antitumor effect, and As2O3 is a promising antitumor reagent for gliomas.
As2O3 downregulated the expression of Bcl-2, an anti-apoptotic protein, and upregulated the expression of Bax, a pro-apoptotic protein, thus shifting the Bax/Bcl-2 ratio in favor of apoptosis. Fas protein expression remained unchanged. These findings indicate that Bcl-2 and Bax play an important role in As2O3-induced apoptosis in C6 and 9 L glioma cells.
Our results hinted at the possible involvement of mitochondrial dysfunction in As2O3-induced apoptosis. The Bcl-2 family of proteins appear to control cell death by regulating mitochondrial physiology [36]. A change in the mitochondrial electrochemical potential results in the release of apoptotic proteins, such as cytochrome c, Smac/DIABLO, pro-caspases 2, 3 and 9, and apoptosis-inducing factor.
Under physiological and pathophysiological conditions, ROS contributes to trigger and mediate apoptosis [37]. The mitochondria are highly susceptible to oxidative damage, and Bcl-2 exerts its anti-apoptotic function by reducing intracellular ROS. As2O3 downregulated Bcl-2 and rendered C6 and 9 L glioma cells vulnerable to apoptotic cell death. In cells pretreated with NAC, As2O3-induced apoptosis was inhibited, suggesting that a mitochondrial death pathway plays an important role in As2O3-induced apoptosis.
Conclusion
As2O3 strongly inhibits cell viability and induces apoptosis of rat C6 and 9 L glioma cells in vitro when used at an optimal concentration of 5 μmol/l. This action is related to the induction of ROS generation. Moreover, As2O3 showed lower cytotoxicity to normal glial cells than glioma cells, indicating that As2O3 may be a potentially potent chemotherapeutic agent for treating brain tumors.
Acknowledgments
Funding
This study was supported by the National Natural Science Foundation of China (Grant No. 30600641) and the Administration of Education, Heilongjiang Province (Grant No. 11511209).
Availability of data and materials
Please contact the author with data requests.
Abbreviations
- APL
Promyelocytic leukemia
- As2O3
Arsenic trioxide
- DMEM
Dulbecco’s modified Eagle’s medium
- ECL
Enhanced chemiluminescence
- FCS
Fetal calf serum
- FITC
Fluorescein isothiocyanate
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- PBS
Phosphate buffered saline
- PI
Propidium iodide
- ROS
Reaction oxygen species
Authors’ contributions
YS, CW, LW and ZD performed the experiments. All the authors contributed to the data analysis and manuscript preparation. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Yuanyuan Sun, Email: sululansi@sohu.com.
Chen Wang, Email: ciyseq@sina.com.
Ligang Wang, Email: lbnjjz@sina.com.
Zhibo Dai, Email: dzjjrx@sina.com.
Kongbin Yang, Email: dk5732@163.com, Email: ykbnewrosurgery@sina.com.
References
- 1.Mervis J. Ancient remedy performs new tricks. Science. 1996;273:578. doi: 10.1126/science.273.5275.578. [DOI] [PubMed] [Google Scholar]
- 2.Nellessen CM, Janzen V, Mayer K, Giovannini G, Gembruch U, Brossart P, Merz WM. Successful treatment of acute promyelocytic leukemia in pregnancy with single-agent all-trans retinoic acid. Arch Gynecol Obstet. 2017; 10.1007/s00404-017-4583-6. [DOI] [PubMed]
- 3.Hassani S, Khaleghian A, Ahmadian S, Alizadeh S, Alimoghaddam K, Ghavamzadeh A, Ghaffari SH. Redistribution of cell cycle by arsenic trioxide is associated with demethylation and expression changes of cell cycle related genes in acute promyelocytic leukemia cell line (NB4). Ann Hematol. 2017; 10.1007/s00277-017-3163-y. [DOI] [PubMed]
- 4.Chen GQ, Zhu J, Shi XG, Ni JH, Zhong HJ, Si GY, Jin XL, Tang W, Li XS, Xong SM, Shen ZX, Sun GL, Ma J, Zhang P, Zhang TD, Gazin C, Naoe T, Chen SJ, Wang ZY, Chen Z. In vitro studies on cellular and molecular mechanisms of arsenic trioxide (As2O3) in the treatment of acute promyelocytic leukemia: As2O3 induces NB4 cell apoptosis with downregulation of Bcl-2 expression and modulation of PML-RAR alpha/PML proteins. Blood. 1996;88:1052–1061. [PubMed] [Google Scholar]
- 5.Chen GQ, Shi XG, Tang W, Xiong SM, Zhu J, Cai X, Han ZG, Ni JH, Shi GY, Jia PM, Liu MM, He KL, Niu C, Ma J, Zhang P, Zhang TD, Paul P, Naoe T, Kitamura K, Miller W, Waxman S, Wang ZY, De The H, Chen SJ, Chen Z. Use of arsenic trioxide (As2O3) in the treatment of acute promyelocytic leukemia (APL): I. As2O3 exerts dose-dependent dual effects on APL cells. Blood. 1997;89:3345–3353. [PubMed] [Google Scholar]
- 6.Zhang P, Zhao X, Zhang W, He A, Lei B, Zhang W, Chen Y. Leukemia-associated gene MLAA-34 reduces arsenic trioxide-induced apoptosis in HeLa cells via activation of the Wnt/beta-catenin signaling pathway. PLoS One. 2017;12:e0186868. doi: 10.1371/journal.pone.0186868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang F, Zhang CM, Li S, Wang KK, Guo BB, Fu Y, Liu LY, Zhang Y, Jiang HY, Wu CJ. Low dosage of arsenic trioxide inhibits vasculogenic mimicry in hepatoblastoma without cell apoptosis. Mol Med Rep. 2018;17:1573–1582. doi: 10.3892/mmr.2017.8046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zeng Y, Liu X, Yan Z, Xie L. Sphingosine 1-phosphate regulates proliferation, cell cycle and apoptosis of hepatocellular carcinoma cells via syndecan-1. Prog Biophys Mol Biol. 2017; 10.1016/j.pbiomolbio.2017.11.006. [DOI] [PubMed]
- 9.Wang GB, Liu JH, Hu J, Xue K. Mechanism of As2O3 induces apoptosis of glioma U87 cells. Eur Rev Med Pharmacol Sci. 2017;21:4875–4881. [PubMed] [Google Scholar]
- 10.Pan L, Li Y, Zhang HY, Zheng Y, Liu XL, Hu Z, Wang Y, Wang J, Cai YH, Liu Q, Chen WL, Guo Y, Huang YM, Qian F, Jin L, Wang J, Wang SY. DHX15 is associated with poor prognosis in acute myeloid leukemia (AML) and regulates cell apoptosis via the NF-kB signaling pathway. Oncotarget. 2017;8:89643–89654. doi: 10.18632/oncotarget.20288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jing Y, Dai J, Chalmers-Redman RM, Tatton WG, Waxman Arsenic S. Trioxide selectively induces acute promyelocytic leukemia cell apoptosis via a hydrogen peroxide-dependent pathway. Blood. 1999;94:2102–2111. [PubMed] [Google Scholar]
- 12.Yi J, Yang J, He R, Gao F, Sang H, Tang X, Ye RD. Emodin enhances arsenic trioxide-induced apoptosis via generation of reactive oxygen species and inhibition of survival signaling. Cancer Res. 2004;64:108–116. doi: 10.1158/0008-5472.CAN-2820-2. [DOI] [PubMed] [Google Scholar]
- 13.Maeda H, Hori S, Nishitoh H, Ichijo H, Ogawa O, Kakehi Y, Kakizuka Tumor A. Growth inhibition by arsenic trioxide (As2O3) in the orthotopic metastasis model of androgen-independent prostate cancer. Cancer Res. 2001;61:5432–5440. [PubMed] [Google Scholar]
- 14.Kajiguchi T, Yamamoto K, Hossain K, Akhand AA, Nakashima I, Naoe T, Saito H, Emi Sustained N. Activation of c-jun-terminal kinase (JNK) is closely related to arsenic trioxide-induced apoptosis in an acute myeloid leukemia (M2)-derived cell line, NKM-1. Leukemia. 2003;17:2189–2195. doi: 10.1038/sj.leu.2403120. [DOI] [PubMed] [Google Scholar]
- 15.Cheng Y, Li Y, Ma C, Song Y, Xu H, Yu H, Xu S, Mu Q, Li H, Chen Y, Zhao G. Arsenic trioxide inhibits glioma cell growth through induction of telomerase displacement and telomere dysfunction. Oncotarget. 2016;7:12682–12692. doi: 10.18632/oncotarget.7259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xia ZB, Wu XJ, Qi TW, Huang ZS. Study on inhibitory effect of arsenic trioxide on growth of rat C6 glioma cells. Zhongguo Zhong Yao Za Zhi. 2008;33:2150–2153. [PubMed] [Google Scholar]
- 17.Xu Y, Zhang Y, Liu X, Wang Z, Ma J, Wang J, Yue The W. Effects of ultrasound and arsenic trioxide on neurogliocytoma cells and secondary activation of macrophages. Tumori. 2009;95:780–788. doi: 10.1177/030089160909500622. [DOI] [PubMed] [Google Scholar]
- 18.Barbey JT, Pezzullo JC, Soignet SL. Effect of arsenic trioxide on QT interval in patients with advanced malignancies. J Clin Oncol. 2003;21:3609–3615. doi: 10.1200/JCO.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 19.Huang SY, Chang CS, Tang JL, Tien HF, Kuo TL, Huang SF, Yao YT, Chou WC, Chung CY, Wang CH, Shen MC, Chen YC. Acute and chronic arsenic poisoning associated with treatment of acute promyelocytic leukaemia. Br J Haematol. 1998;103:1092–1095. doi: 10.1046/j.1365-2141.1998.01079.x. [DOI] [PubMed] [Google Scholar]
- 20.McCarthy KD, de Vellis J. Preparation of separate astroglial and oligodendroglial cell cultures from rat cerebral tissue. J Cell Biol. 1980;85:890–902. doi: 10.1083/jcb.85.3.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tan Z, Jia X, Ma F, Feng Y, Lu H, Jin JO, Wu D, Yin L, Liu L, Zhang L. Increased MMAB level in mitochondria as a novel biomarker of hepatotoxicity induced by Efavirenz. PLoS One. 2017;12:e0188366. doi: 10.1371/journal.pone.0188366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yi D, Hou Y, Xiao H, Wang L, Zhang Y, Chen H, Wu T, Ding B, Hu CA, Wu G. N-acetylcysteine improves intestinal function in lipopolysaccharides-challenged piglets through multiple signaling pathways. Amino Acids. 2017;49:1915–1929. doi: 10.1007/s00726-017-2389-2. [DOI] [PubMed] [Google Scholar]
- 23.Zeng Y, Yao X, Chen L, Yan Z, Liu J, Zhang Y, Feng T, Wu J, Liu X. Sphingosine-1-phosphate induced epithelial-mesenchymal transition of hepatocellular carcinoma via an MMP-7/ syndecan-1/TGF-beta autocrine loop. Oncotarget. 2016;7:63324–63337. doi: 10.18632/oncotarget.11450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klinger PH, Andrade AF, Delsin LE, Queiroz RG, Scrideli CA, Tone LG, Valera ET. Inhibition of SHH pathway mechanisms by arsenic trioxide in pediatric medulloblastomas: a comprehensive literature review. Genet Mol Res. 2017;16 10.4238/gmr16019412. [DOI] [PubMed]
- 25.Chang HR, Munkhjargal A, Kim MJ, Park SY, Jung E, Ryu JH, Yang Y, Lim JS, Kim Y. The functional roles of PML nuclear bodies in genome maintenance. Mutat Res. 2017; 10.1016/j.mrfmmm.2017.05.002. [DOI] [PubMed]
- 26.Zhao S, Tsuchida T, Kawakami K, Shi C, Kawamoto K. Effect of As2O3 on cell cycle progression and cyclins D1 and B1 expression in two glioblastoma cell lines differing in p53 status. Int J Oncol. 2002;21:49–55. [PubMed] [Google Scholar]
- 27.Akao Y, Nakagawa Y, Akiyama K. Arsenic trioxide induces apoptosis in neuroblastoma cell lines through the activation of caspase 3 in vitro. FEBS Lett. 1999;455:59–62. doi: 10.1016/S0014-5793(99)00841-8. [DOI] [PubMed] [Google Scholar]
- 28.Kanzawa T, Kondo Y, Ito H, Kondo S, Germano I. Induction Of autophagic cell death in malignant glioma cells by arsenic trioxide. Cancer Res. 2003;63:2103–2108. [PubMed] [Google Scholar]
- 29.Chakraborti T, Das S, Mondal M, Roychoudhury S, Chakraborti S. Oxidant Mitochondria and calcium: an overview. Cell Signal. 1999;11:77–85. doi: 10.1016/S0898-6568(98)00025-4. [DOI] [PubMed] [Google Scholar]
- 30.Forman HJ, Torres Reactive M. Oxygen species and cell signaling: respiratory burst in macrophage signaling. Am J Respir Crit Care Med. 2002;166:S4–S8. doi: 10.1164/rccm.2206007. [DOI] [PubMed] [Google Scholar]
- 31.Pucer A, Castino R, Mirkovic B, Falnoga I, Slejkovec Z, Isidoro C, Lah TT. Differential Role of cathepsins B and L in autophagy-associated cell death induced by arsenic trioxide in U87 human glioblastoma cells. Biol Chem. 2010;391:519–531. doi: 10.1515/bc.2010.050. [DOI] [PubMed] [Google Scholar]
- 32.Primon M, Huszthy PC, Motaln H, Talasila KM, Torkar A, Bjerkvig R, Lah Turnsek T. Cathepsin L silencing enhances arsenic trioxide mediated in vitro cytotoxicity and apoptosis in glioblastoma U87MG spheroids. Exp Cell Res. 2013;319:2637–2648. doi: 10.1016/j.yexcr.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 33.Klauser E, Gulden M, Maser E, Seibert S, Seibert H. Additivity, antagonism, and synergy in arsenic trioxide-induced growth inhibition of C6 glioma cells: effects of genistein, quercetin and buthionine-sulfoximine. Food Chem Toxicol. 2014;67:212–221. doi: 10.1016/j.fct.2014.02.039. [DOI] [PubMed] [Google Scholar]
- 34.Wang C, Chen X, Zou H, Chen X, Liu Y, Zhao S. The roles of mitoferrin-2 in the process of arsenic trioxide-induced cell damage in human gliomas. Eur J Med Res. 2014;19:49. doi: 10.1186/s40001-014-0049-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gerlach M, Ben-Shachar D, Riederer P, Youdim MB. Altered Brain metabolism of iron as a cause of neurodegenerative diseases? J Neurochem. 1994;63:793–807. doi: 10.1046/j.1471-4159.1994.63030793.x. [DOI] [PubMed] [Google Scholar]
- 36.Kitazawa H, Numakawa T, Adachi N, Kumamaru E, Tuerxun T, Kudo M, Kunugi H. Cyclophosphamide promotes cell survival via activation of intracellular signaling in cultured cortical neurons. Neurosci Lett. 2010;470:139–144. doi: 10.1016/j.neulet.2009.12.073. [DOI] [PubMed] [Google Scholar]
- 37.Bauer G. Reactive oxygen and nitrogen species: efficient, selective, and interactive signals during intercellular induction of apoptosis. Anticancer Res. 2000;20:4115–4139. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Please contact the author with data requests.


