Abstract
Background
With approximately one-third of their genomes consisting of linear and circular plasmids, the Lyme disease agent cluster of species has the most complex genomes among known bacteria. We report here a comparative analysis of plasmids in eleven Borreliella (also known as Borrelia burgdorferi sensu lato) species.
Results
We sequenced the complete genomes of two B. afzelii, two B. garinii, and individual B. spielmanii, B. bissettiae, B. valaisiana and B. finlandensis isolates. These individual isolates carry between seven and sixteen plasmids, and together harbor 99 plasmids. We report here a comparative analysis of these plasmids, along with 70 additional Borreliella plasmids available in the public sequence databases. We identify only one new putative plasmid compatibility type (the 30th) among these 169 plasmid sequences, suggesting that all or nearly all such types have now been discovered. We find that the linear plasmids in the non-B. burgdorferi species have undergone the same kinds of apparently random, chaotic rearrangements mediated by non-homologous recombination that we previously discovered in B. burgdorferi. These rearrangements occurred independently in the different species lineages, and they, along with an expanded chromosomal phylogeny reported here, allow the identification of several whole plasmid transfer events among these species. Phylogenetic analyses of the plasmid partition genes show that a majority of the plasmid compatibility types arose early, most likely before separation of the Lyme agent Borreliella and relapsing fever Borrelia clades, and this, with occasional cross species plasmid transfers, has resulted in few if any species-specific or geographic region-specific Borreliella plasmid types.
Conclusions
The primordial origin and persistent maintenance of the Borreliella plasmid types support their functional indispensability as well as evolutionary roles in facilitating genome diversity. The improved resolution of Borreliella plasmid phylogeny based on conserved partition-gene clusters will lead to better determination of gene orthology which is essential for prediction of biological function, and it will provide a basis for inferring detailed evolutionary mechanisms of Borreliella genomic variability including homologous gene and plasmid exchanges as well as non-homologous rearrangements.
Electronic supplementary material
The online version of this article (10.1186/s12864-018-4597-x) contains supplementary material, which is available to authorized users.
Keywords: Borreliella, Borrelia, Burgdorferi, Lyme disease, Linear plasmid, Circular plasmid, Plasmid evolution
Background
The growing number of cases of human Lyme and related diseases merits detailed study of the causative pathogens. The bacterial family Borreliaceae fam. Nov. contains two clades that are typified by the causative agents human Lyme disease and human relapsing fever (e. g., [1, 2]). Recent taxonomic analyses have argued that the single original Borrelia genus that contained these bacteria should be split into two genera, Borreliella sp. nov. which contains the Lyme disease agents and close relatives, and Borrelia which contains the relapsing fever agents and close relatives [3]. Although the division of the original single Borrelia genus into these two genera remains somewhat controversial [4, 5], we choose to use the two-genus nomenclature in this report since it is useful and informative in discussing their evolutionary genomics. Each of these genera contains a number of species, and Borreliella currently consists of about twenty species (previously known as “Borrelia burgdorferi sensu lato” species, a term we do not use here) that are carried by Ixodes ticks in the temperate zones of North America and Eurasia and recently found in South America [6]. Lyme disease is the most common vector-borne human disease in the United States and Europe [7, 8], and it has been associated with seven or eight Borreliella species, B. afzelii, B. bavariensis, B. bissettiae, B. burgdorferi, B. garinii, B. lusitaniae, B. mayonii and perhaps B. spielmanii [9–17] (but also see [18]). These species have highly related chromosomes that are about 900 kbp in length, and they carry numerous plasmids that are more variable in sequence than the chromosome and are variably present in individual isolates [1, 15, 19–21]. These bacteria infect a wide variety of mammals and other vertebrates [22, 23] and exhibit different pathogenicities, tropisms and disease manifestations [24–27]. It is likely that many of these phenotypic differences are the result of differences in plasmid-encoded genes.
Electrophoretic separation of whole Borreliella DNAs has invariably shown that these bacteria carry multiple plasmids (e. g., [28, 29]). However, multiple linear plasmids in the same cell often have similar size, and circular plasmids are not resolved, so careful (not draft) sequencing is required to delineate all the plasmids in any isolate. Whole genome sequencing of B. burgdorferi sensu stricto isolates (hereafter referred to as B. burgdorferi) has shown that up to 23 plasmids can be present in a single isolate; the type strain B31’s whole genome sequence contains 21 plasmids [30], and two additional plasmids were shown to have been lost from the sequenced culture [30, 31]. These plasmids carry a large repertoire of genes, many of which encode surface lipoproteins that are important in the detection, prevention and pathogenicity of Borreliella infection [32–34]. In addition, a number of plasmid genes are critical for tick transmission and virulence in mice [35–48].
In order to understand the diversity and evolutionary relationships within the Borreliella lineage, as well as their human pathogenicity and relationship to the relapsing fever clade, we sequenced the complete genomes, including all the plasmids, of twenty-two isolates from seven Borreliella species and a variety of sources [30, 49–53]. These isolates include B. burgdorferi and six other species. The plasmids in the fourteen B. burgdorferi whole genome sequences include linear replicons that range from 5 to 55 kbp in length and circular replicons between 9 and 61 kbp [20]. We used cosmid library scaffolds to assemble the plasmid sequences, and the plasmids in strain B31 were extensively checked by Southern blot restriction mapping to show that our assembly methods generate accurate plasmid sequences [30, 54]. Multiple stretches of very similar sequences in many of the Borreliella plasmids make standard “next generation” short run sequencing methodologies and random library dideoxynucleotide sequencing unable to assemble many of these plasmid sequences. However, recently the plasmids in B. afzelii K78 plasmids have been successfully sequenced using highly accurate next generation sequencing and high stringency sequence assembly methods [21, 55], and B. burgdorferi strains PAbe and PAli and B. mayonii MN14–1420 and MN14–1539 plasmid sequences have been successfully assembled using scaffolds determined by single molecule, real-time (SMRT) long sequence runs (PacBio platform) [15, 21, 55]. The plasmid contents of the above Borreliella closed whole genome sequences range from seven to twenty-three plasmids, and in every case these include both linear and circular plasmids. In addition, apparently complete sequences of individual plasmids have been deposited in the public sequence database from B. garinii Ip21 [56] and 20047 (BioProject PRJNA350560), B. bavariensis PBi [57] and BgVir [58], B. afzelii BO23 (BioProject PNJNA359557) and Tom3107 [59], B. valaisiana Tom4006 [59], B. japonica HO14 (BioProject PRJEB15958) and B. chilensis VA1 [60]. We report here a comparative analysis of all available Borreliella plasmid sequences.
Results
Plasmid types & names
Four hundred and five Borreliella plasmid sequences are compared in this study; 236 are from the genomes of fully sequenced B. burgdorferi isolates, 141 are from non-burgdorferi Borreliella species (hereafter referred to as NBu-Borreliella species, Table 1) with fully sequenced genomes, and 28 are anecdotally sequenced plasmids from other NBu-Borreliella isolates. (We include the five incompletely assembled strain B. finlandensis SV1 lp32–6 and B. spielmanii A14S cp32 and cp9 plasmids in this analysis; see below; plasmids from isolates PAbe and PAli were reported [55] after our analysis was completed and are not included.) Two hundred and twenty-two of these plasmids are linear and 183 are circular. Accession numbers for the Borreliella plasmids in complete genome sequences can be found in references [15, 21, 30, 49–53, 55], and the remaining plasmid accession numbers are listed in Additional file 1: Table S1. All are available at the Borreliabase world-wide web site http://BorreliaBase.org/ [61]. These plasmids are traditionally named according to the “sequence type” of their paralogous protein family 32 (PFam32) ParA partition proteins; the fact that this very likely defines the plasmid compatibility type has been discussed in detail elsewhere ([19, 30, 62–64] and see below). Analysis of the 169 NBu-Borreliella plasmids has not been previously reported, and this study focuses on their relationships.
Table 1.
Number of plasmids in NBu-Borreliella isolates
| Linear plasmids | Circular plasmids | |
|---|---|---|
| B. afzelii | ||
| ACA-1 | 9 | 5 |
| BO23 | 6a | 2a |
| K78 | 8 | 5 |
| PKo | 9 | 8 |
| B. bissettiae | ||
| DN127 | 7 | 9 |
| B. finlandensis | ||
| SV1 | 5 | 5 |
| B. garinii | ||
| Far04 | 6 | 1 |
| PBr | 8 | 3 |
| 20047 | 4a | 1a |
| B. japonica | ||
| HO14 | 3a | 1a |
| B. mayonii | ||
| MN14–1420 | 8 | 7 |
| MN14–1539 | 7 | 7 |
| B. spielmanii | ||
| A14S | 7 | 6b |
| B. valaisiana | ||
| VS116 | 6 | 5 |
aNot all plasmids have been identified and sequenced in this isolate
bBecause the sequences of some circular plasmids in A14S were not closed, it is possible that some cp32s are fused. Thus, the number of different plasmid DNA molecules could be slightly less than this value
Figure 1 lists the plasmids present in each of the fourteen best understood NBu-Borreliella genomes. Nearly all of the sequenced plasmids in these isolates fall into one of the 29 PFam32 types that were previously defined from analysis of the plasmids in fourteen B. burgdorferi genomes [20]. These fourteen isolates contain plasmids from 24 of the previously known 29 PFam32 types; they contain no plasmid types lp21, lp28–1 (but see below), lp28–5, lp28–6 or cp32–8. We searched the available NBu-Borreliella draft genomes for genes encoding the five “missing” PFam32 protein types and found only one cognate match, a 99% amino acid sequence match to lp28–6 PFam32 protein from Colorado, B. bissettiae isolate CO275 (Accession No. OJH14568). It therefore appears that at least lp28–6 is present in some NBu-Borreliella isolates. The other four “missing” PFam32 types were not found, but the number of such genome sequencing projects is still rather small so their absence may not be significant.
Fig. 1.
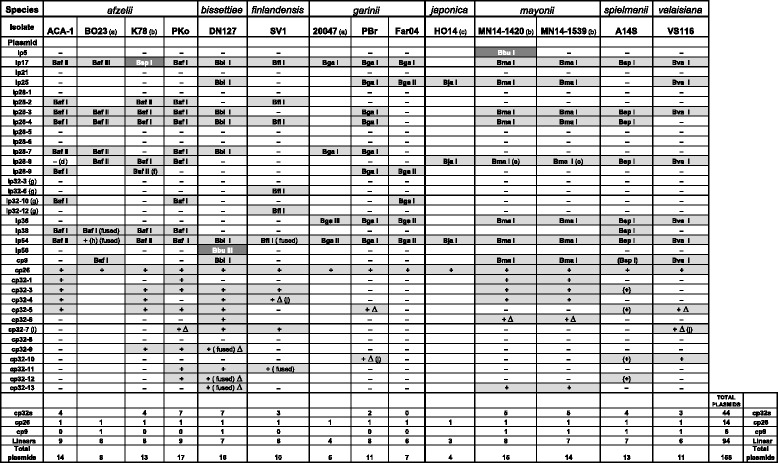
Sequenced plasmids in NBu-Borreliella species. The eleven completely sequenced genomes and three partly sequence genomes are shown as columns where shaded cells indicate the plasmid’s presence, “fused” indicates that the indicated plasmids contain apparently intact PFam32 genes of more than one type (see text); and “∆”, indicates the presence of a substantial deletion relative to other cp32s. Hyphens (−) denote plasmids that are known not to be present, and blank cells indicate that it is not known if that plasmid type is present. For the linear plasmids and cp9, Roman numerals indicate plasmid organizational subtype (see text and [20] for subtype definitions); here subtypes are named by the first letter of the genus name and first two letters of the species name (e.g., B. afzelii subtype I is “Baf I”). Similar subtype numerals in different plasmid types (columns in table) does not imply any relationship, and subtype organizations are always different in the different species except in the three cases that are indicated by dark gray cells (see text). The cp26 plasmids do not exhibit organizational variation and cp32s are too variable, so no subtypes are defined for these plasmids; a “+” indicates that a plasmid of that PFam32 type is present, and parentheses (...) around the subtype name denote the cp9 and four cp32 isolate A14S plasmids whose sequences were not closed. Because the sequences of these plasmids in A14S were not closed, it is possible that some cp32s are fused, so the number of different plasmid DNA molecules is not known precisely in this case. (a) The indicated plasmid sequences from isolates B. afzelii BO23 and B. garinii 20047 have been deposited in GenBank as closed sequences (Additional file 1: Table S1); it is not known what other plasmids these isolates may carry (S. Bontemps-Gallo, Pers. Com.); (b) these plasmids represent the full complement of plasmids of B. afzelii K78 and the two B. mayonii isolates; accession numbers are listed in [15, 21], respectively; (c) the plasmid sequences from isolate B. japonica HO14 (ATCC51557) are “minimal draft” quality sequences; they are included here because they are the right size to be full plasmid sequences but the details of their sequences should be interpreted with caution. (d) plasmid is very likely present in the original ACA-1 isolate, see text; (e) called lp28–10 in reference [15], see text; (f) called lp28–1 in reference [21], see text; (g) the lp32 plasmids very likely have the same compatibility type as cp32 of the same number, see text; (h) the BO23 lp54 sequence does not include the PFam54 gene array that distinguishes the subtypes of this plasmid; (i) previously named cp32–2 and cp32–7 have the same PFam32 protein type; we use cp32–7 to represent this group; and (j) several kbp of typically linear plasmid sequence replaces at least part of the deleted cp32 DNA
Only one new type of plasmid-encoded PFam32 protein, carried by some cp9 plasmids, is present in the NBu-Borreliella genomes. This is shown in the maximum likelihood PFam32 tree in Fig. 2 and in the similar neighbor-joining tree in Additional file 1: Figure S1. Both trees give identical sets of PFam32 types and show that the cp9 PFam32 proteins form a well-separated branch with strong bootstrap support. This new type is encoded by the circular cp9 (8.5–10.5 kbp) plasmids from B. afzelii, B. bissettiae, B. mayonii, B. valaisiana and probably B. spielmanii isolates (see cp9 analysis below). We currently lump the cp9 plasmids together even though the B. burgdorferi and B. garinii versions lack PFam32 genes, because they are otherwise extremely similar. The cp9 PFam32 proteins are 20–25% different in amino acid sequence from their nearest relatives, the cp32–1 PFam32 proteins. The B. mayonii isolates both carry a cp32–1 plasmid in addition to such a cp9, so this level of difference is sufficient to allow them to coexist in the same cell. This is consistent with the observation that the previous most closely related pair of PFam32 protein types that we know must have different compatibilities, B. burgdorferi JD1 cp32–8 and cp32–12 (for example), are also about 25% different in amino acid sequence [20]. We also note that the lp28–9 plasmid group was previously split from the lp28–1 group (Table S4 in [19, 20]), since their PFam32 genes are 17–18% different, so we include B. afzelii K78 “lp28–1” [21] as an lp28–9 in this discussion (Fig. 2 and Additional file 1: Figure S1). In addition, the PFam32 proteins encoded by the two B. mayonii plasmids called “lp28–10” [15] are sufficiently closely related to the canonical lp28–8 plasmids that we include them in the latter group in this discussion (the lp28–8 and lp28–10 PFam32 proteins are only 12–15% different; Fig. 2 and Additional file 1: Figure S1). The precise degree of difference among PFam32 proteins that might allow plasmid incompatibility is not known and could well vary in different specific cases, so lp28–1/lp28–9 compatibility and lp28–8/lp28–10 incompatibility remain speculative since no strains are known that carry both members of these pairs. We also note that Fig. 2 and Additional file 1: Figure S1 indicate that especially substantial PFam32 diversity exists within several of the plasmid types (e.g., lp28–4 and lp38), and it is possible that these could constitute more than one compatibility type. More experimental work will be required to understand Borreliella plasmid compatibility in greater detail. The 169 NBu-Borreliella plasmid sequences analyzed here contribute to the current total of 405 plasmids from 28 isolates that bear out the so far inviolate rule that no Borreliella cell contains two or more plasmids that encode the same PFam32 type; they thus lend additional credence to the idea that these proteins contribute to plasmid compatibility.
Fig. 2.
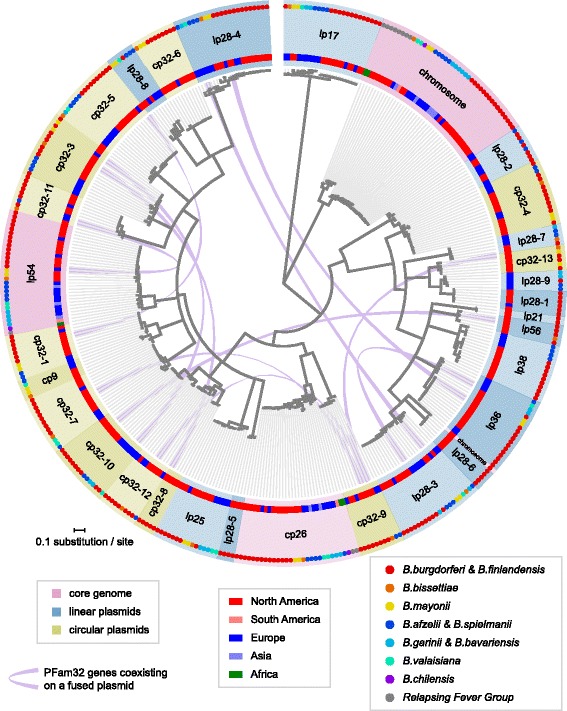
PFam32 gene tree suggests ancestral radiation of plasmid compatibility groups. The midpoint-rooted gene tree (center) is based on a 392-amino acid long alignment of 411 PFam32 homologues from 34 Borreliella genomes and selected sequences from 8 relapsing-fever Borrelia genomes. The rings, from center outward, indicate the (i) continent of origin of each isolate, (ii) plasmid compatibility type names with different shades of pink, blue, and green for the core genome (chromosome, cp26 and lp54), linear, and circular plasmids, respectively, and (iv) species (colored dots). Lavender central ribbons connect multiple PFam32 alleles found on the same replicon, indicating plasmid fusion events. A vertical PFam32 tree is available as Additional file 1: Figure S1, where two PFam32 homologs (one on lp28–9 from BOL26, another on lp56 from DN127) with positions inconsistent with the genome phylogeny (see Fig. 5 below) – indicative of whole-plasmid transfer – are highlighted. Plasmid types lp5 and some cp9s are not shown in the figure as they do not have a PFam32 partition gene
Since only one additional PFam32 type has been found among the 169 NBu-Borreliella plasmid sequences from eight species analyzed here, it is likely that additional Borreliella plasmid types are rare, geographically restricted, or both. However, we have previously noted that the “orphan” (not in a cluster with the other putative partition genes) PFam32 protein BB_F13 encoded by strain B31 lp28–1 suggests the possible existence of a 31st type (see Additional file 1: Figure S1). We also note that the Fritz-Lipmann Institute B. afzelii PKo draft genome sequence (Bioproject PRJNA17057) contains an approximately 13 kbp contig that is not found in our PKo sequence (Bioproject PRJNA68149). It contains a typical cluster of four partition genes that encode PFam49, PFam57, PFam32 and PFam50 proteins (however, the latter is C-terminally truncated), and this PFam32 protein (locus_tag = BAPKO_2556) is about 18% different from its closest relatives, the B. burgdorferi lp21 PFam32 proteins. This PKo protein has one close relative in the current database, a 95% identical homologue (locus_tag = SAMN02983004_01117) in a B. japonica HO14 draft contig. The latter two proteins form a unique and robust branch in the PFam32 tree (Additional file 1: Figure S1) and thus likely represent a relatively rare 32nd Borreliella PFam32 type.
Plasmid frequencies
Among the eleven NBu-Borreliella isolates with completely sequenced genomes, the average number of different plasmids per isolate is 12.8 and the number of different plasmids ranges from seven in B. garinii Far04 to seventeen in B. afzelii PKo (Fig. 1). These plasmid numbers are minimum values since Borreliella plasmids are known to be rather easily lost during laboratory culture [48, 65–68]; indeed, B. afzelii strain ACA-1 was previously shown to harbor a plasmid that carries the vls cassette region [69] that it is missing from our ACA-1 genome sequence (see below), and our B. afzelii PKo genome sequence is missing the novel plasmid mentioned above.
We previously found that among fourteen B. burgdorferi isolates the different plasmids are present at different frequencies. For example, plasmids lp17, lp28–4, lp36, lp54 and cp26 were the most common and universally present in all fourteen isolates, and lp28–3, lp38 and cp32–5 were only missing from two, three and three isolates, respectively [20]. Here we find that all eleven of the completely sequenced NBu-Borreliella isolates carry lp17, lp54 and cp26. Their next most common plasmids are lp28–3 and lp28–4, each of which is present in nine of the genomes, and lp28–5, lp28–8 and lp36 which are each present in six genomes (Fig. 1). The overall ratios of circular to linear plasmids in the completely sequenced genomes are 0.68 in the NBu-Borreliella isolates and 0.92 in the B. burgdorferi isolates. This difference is largely due to fewer cp32 plasmids in the former. Cp32s represent 28% of the plasmids in NBu-Borreliella and 38% in B. burgdorferi isolates, and we note that B. garinii Far04 is the only Borreliella isolate examined to date that contains no cp32 type plasmid (but it does carry a related plasmid lp32–10 that contains about 28 kbp of cp32-like sequences; see below). Since B. garinii PBr has only two cp32s it is possible that the B. garinii species in general carry fewer cp32s than the other Borreliella species, but this sample is currently too small to draw this as a rigorous conclusion.
Linear plasmids
Most linear plasmid PFam32 types in B. burgdorferi are present in different isolates as several different “organizational subtypes” [20]. The present analysis shows that this is also true for the NBu-Borreliella species. Among the 222 Borreliella linear plasmids (99 NBu-Borreliella and 123 B. burgdorferi, respectively), in only a very small number of cases do cognate plasmids have the same gene organization in more than one species (see below). The vast majority have sequence organizations that are unique to their species. Figures 3 and 4 show, by way of example, comparative open reading frame (ORF) maps of the NBu-Borreliella lp17 and lp36 plasmids, respectively. Our previous analysis of B. burgdorferi linear plasmids concluded that during their relatively recent evolution they have undergone many apparently random, duplicative and other types of rearrangements mediated by nonhomologous recombination. Genes were often truncated by these rearrangements or after such events redundant genes have undergone random decay that included bp changes and indel formation [19, 20, 30, 64, 70, 71]. The result of this seemingly chaotic evolution is a strikingly low fraction (for a prokaryote) of protein coding DNA in these plasmids, due to the presence of many apparently non-functional pseudogenes and DNA regions that have no long ORFs. NBu-Borreliella linear plasmid sequence annotation performed by the JCVI Prokaryotic Annotation Pipeline (www.jcvi.org/cms/research/projects/prokaryotic-annotation-pipeline/overview/) shows that the NBu-Borreliella linear plasmids also contain a considerable amount of DNA that appears not to encode proteins. Additional file 1: Figure S2 shows two typical examples, B. afzelii PKo lp28–4 and B. spielmanii A14S lp36. PKo lp28–4 has 42% protein coding DNA and 44% if unique short genes are included, and A14S lp36 has 31% and 34% protein coding DNA calculated by these two methods, respectively.
Fig. 3.
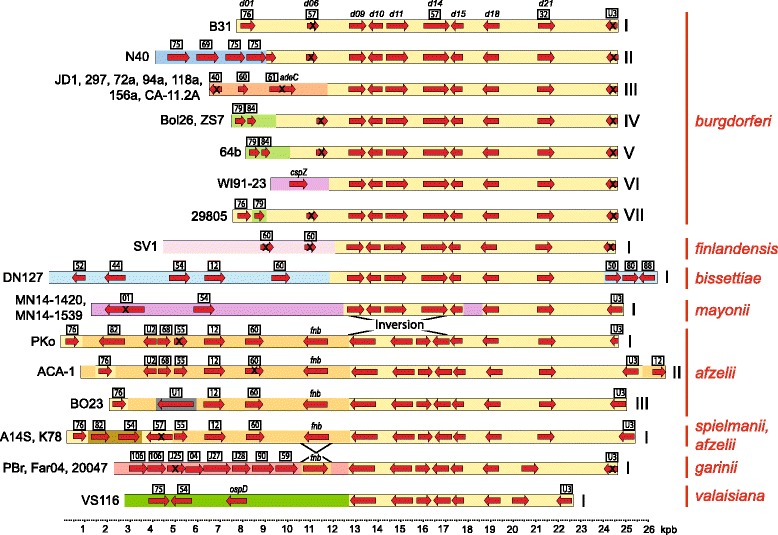
ORF maps of Borreliella lp17 plasmids. The horizontal bars represent all the sequenced lp17 plasmids; identical background colors indicate regions of homologous DNA in the different plasmids. The bacterial species and organizational subtypes (see text) are indicated by Roman numerals and on the right (without their species component, see text), and isolates that carry each subtype are indicated on the left. Selected genes are indicated by red arrows, and black “X”s mark pseudogenes. PFam numbers [19, 30] are indicated in the boxes above; “U”s in boxes are proteins for which no intact gene is known in B. burgdorferi; “J's” in boxes indicate strain B31 homologous proteins for which there is only one gene in strain B31 (i.e., no PFam number exists); adeC, cspZ and fbn refer to adenine deaminase, complement regulator-acquiring surface protein Z and fibronectin binding protein encoding genes, respectively; selected strain B31 gene names are indicated above its map. Black lines between plasmids mark the locations of inversions. See Additional file 1: Figure S3B for locations of indels within generally homologous regions (e.g., deletions within B31-like sections of VS116 lp17 and PBr lp17)
Fig. 4.

ORF maps of Borreliella lp36 plasmids. Plasmids are represented as in Fig. 3, with the same background colors indicating regions of homologous DNA. The organizational subtypes (see text) are indicated by Roman numerals on the right, and isolates that carry each type are indicated at the left
Comparative maps of all the NBu-Borreliella linear plasmid organizational subtypes are displayed in Additional file 1: Figure S3; the different subtypes within each species are defined and named with Roman numerals as in Casjens et al. [20], and to distinguish the different species here we add the first letter of the genus name and first two letters of the species name (e.g., B. afzelii lp17 subtype I is called “lp17 Baf I”. Even though fewer members of each species were analyzed than was done with the fourteen B. burgdorferi isolates [20], in the NBu-Borreliella species where plasmids of the same type have been completely sequenced from multiple isolates, we find substantial organizational variation in those plasmids (subtypes listed in Fig. 1). In the four B. afzelii and B. garinii isolates for which the most information is known, ten plasmids, lp17, lp25, lp28–2, lp28–3, lp28–4, lp28–7, lp28–8, lp28–9, lp36 and lp54, display more than one subtype within at least one of these two species (ignoring the fact that BO23 lp38 type Baf I appears to be joined end-to-end to lp54) (Figs. 3, 4 and Additional file 1: Figure S3). The frequencies of plasmid subtypes in the NBu-Borreliella genomes are similar to those of B. burgdorferi. Interestingly all of the seven B. mayonii linear plasmids are the same subtype in both of the sequenced genomes; either this species harbors less plasmid diversity than other Borreliella species, or the small sample contains two fortuitously closed related B. mayonii genomes.
Thus, the nature of the subtype differences among and within the NBu-Borreliella species are similar to those described for B. burgdorferi, and are characterized by apparently random shuffling of DNA sequence patches among the linear plasmids. Table 2 demonstrates this evolutionary shuffling by listing the varied locations of a number of relatively well-studied genes in the different Borreliella genomes. Clearly, these rearrangements did not happen in a common ancestor, but occurred with the same overall chaotic pattern after the separation of the different species. The common forces that drive this peculiar evolution in the different branches of this bacterial lineage remain mysterious.
Table 2.
Linear plasmid locations of selected NBu-Borreliella genes
| Genea | adeC | arp/erpD | bptA | cpsZ | dbpABc | fnb | plzA | ospABc | ospCc | ospD | pncA | res-mod1 | res-mod2 | sagABC DE | vlsE-liked | vslE & vls cassettes |
|
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PFamb | 61 | 163 | 99 | 92 | 74 | none | 180 | 53 | none | none | none | 1 | 102–167 | none | 170 | 170 | |
| B. burgdorferi | B31 | lp36 | lp28–1 | lp25 | lp28–3 | lp54 | lp36 | – | lp54 | cp26 | lp38 | lp25 | lp25, lp28–3 |
lp56 | – | – | lp28–1 |
| B. bissettiae | DN127 | lp28–7 | – | lp25 | lp28–3 | lp54 | lp28–7 | – | lp54 | cp26 | – | lp25 | lp25, lp28–3 |
– | – | – | – |
| B. finlandensis | SV1 | – | – | lp28–2 | – | lp54h | – | lp32–6f | lp54h | cp26 | – | lp28–2 | lp32–12f | – | – | – | lp32–6 |
| B. mayonii | MN14–1420 | lp36 | – | lp25 | – | lp54 | – | – | lp54 | cp26 | lp28–3 | lp25 | lp25, lp28–4 |
– | – | – | lp28–8e |
| MN14–1539 | lp36 | – | lp25 | – | lp54 | – | – | lp54 | cp26 | lp28–3 | lp25 | lp25, lp28–4 |
– | – | – | lp28–8e | |
| B. afzelii | PKo | lp38 | – | lp28–2 | – | lp54 | lp17 | – | lp54 | cp26 | lp32–10 f | lp28–2 | lp28–3, lp28–4 |
lp32–10 | lp28–8 | – | lp28–8 |
| K78 | lp38 | – | lp28–2 | – | lp54 | lp17 | – | lp54 | cp26 | – | lp28–2 | lp28–3, lp28–4 |
– | lp28–8 | – | lp28–8 | |
| ACA-1 | lp38 | – | lp28–2 | – | lp54 | lp17 | – | lp54 | cp26 | lp32–10f | lp28–2 | lp28–3, lp28–4 |
lp32–10 | lp28–8?j | – | lp28–8?j | |
| BO23l | lp38 i | ND | ND | ND | lp54 | lp17 | ND | lp54h | cp26 | ND | ND | lp28–4 | ND | lp28–8 | ND | lp28–8 | |
| B. garinii | PBr | lp25 | – | lp25 | lp28–4 | lp54 | lp17 | lp28–9 | lp54 | cp26 | cp32–10k | lp25 | lp28–3, lp36 |
lp28–9 f | – | lp28–4, lp36 j |
lp28–3 |
| Far04 | lp25 | – | lp25 | – | lp54 | lp17 | – | lp54 | cp26 | lp32–10 | lp25 | lp36 | lp28–9 f | – | – | lp28–9 | |
| 20047l | ND | ND | ND | ND | lp54 | lp17 | ND | lp54 | cp26 | ND | ND | lp36 | ND | ND | lp36 j | ND | |
| B. spielmanii | A14S | lp36 | – | lp38 | lp28–4, lp28–8 |
lp54 | lp17 | – | lp54 | cp26 | – | lp38 | lp28–3 | – | lp28–8 | – | lp28–8 |
| B. valaisiana | VS116 | right end chrmf |
– | lp25j | lp36 f | lp54 | – | – | lp54 | cp26 | lp17 | lp25 | lp28–3f | cp32–7k | lp28–8 | lp28–3 | lp28–8 |
| B. japonica | HO14l | ND | ND | lp25 | ND | lp54 | ND | NC | lp54 | cp26 | ND | lp25 | ND | ND | lp28–8 | ND | ND |
aThe linear plasmids that carry selected genes are indicated in the table; only apparently intact genes are indicated; hyphens (−) indicate a plasmid is absent from that completely sequenced genome. Gene names are as follows: adeC, adenine deaminase [35]; arp, arthritis related protein [134]; cpsZ, complement regulatory protein Z [86]; dbpAB, decorin binding proteins A and B [135]; fnb, fibronectin protein [136–139]; plzB, cyclic di-GMP binding protein [140, 141]; ospAB, outer surface proteins A and B [142–144]; ospC, outer surface protein C [103, 145], ospD, outer surface protein D [85, 146]; pncA, nicotinamidase [73]; res-mod1, restriction-modification protein PFam01 [147, 148]; res-mod2, restriction-modification protein PFam102/167 [148]; sagABCDE, synthesis of streptolysin S-like toxin [78]; vlsE-like, putative lipoproteins that are related VlsE but are encoded at a location not adjacent to vls cassette array [19]; vlsE and vls, variable outer surface protein cassettes and expression locus [149]
bBorreliella paralogous protein family (see text)
cThe gene contents of lp54 and cp26 gene content is very constant so only a few examples of their many important genes are shown
dvlsE homologue not adjacent to vls cassette region
eNamed lp28–10 in the literature but we include them as lp28–8 type plasmids (see text)
fA relatively large, apparently truncated gene fragment whose functionality is unknown; there is no intact adeC gene in the VS116 genome
gGene is known to have been originally present in this isolate, but its plasmid was lost from the sequenced culture [69]
hlp54 is fused to another plasmid (see text); this gene is in the lp54 portion
ilp38 is fused to lp54; this gene is in the lp38 portion
jTwo genes of this sort are present on this plasmid
kUnusual circular plasmid location; in B31 lp38-like indel in PBr cp32–10 and B31 lp56-like indel in VS116 cp32–7; see Additional file 1: Figure S8
lPlasmid sequences are not complete for this isolate. ND indicates not determined; since all plasmids in this strain have not been sequenced the presence of this gene is not known
Lp5, lp21, lp28–1, lp28–5, and lp28–6
No NBu-Borreliella was found to contain an lp21, lp28–1, lp28–5 or lp28–6 plasmid, all of which are known in B. burgdorferi isolates, and only one other species, B. mayonii, has an lp5. Lp5, the smallest of the known Borreliella plasmids, is a relatively rare plasmid whose function is unknown and which has only been sequenced from two B. burgdorferi isolates and one B. mayonii isolate. Additional file 1: Figure S3A shows aligned reading frame maps of these three plasmids. The relationships among these three very similar plasmids is discussed below in terms of possible horizontal plasmid transfer; however, we note here that all three are extremely similar and the only putative plasmid replication/partition gene on the B. mayonii lp5, which encodes a Pfam57 protein, has a frameshift in codon 116 that likely inactivates its function; perhaps its role can be substituted by a paralogue encoded by another plasmid [63].
Lp17
On the other hand, all the NBu-Borreliella complete genome sequences contain an lp17 plasmid. The fourteen B. burgdorferi lp17s are present as seven different organizational subtypes, and curiously the major differences among these types are all different left end sequences [20]. Figure 3 and Additional file 1: Figure S3B show that the NBu-Borreliella lp17s also have a variety of different sequences at their left ends, and several also have shorter substitutions at their right ends. Within species, the two B. mayonii lp17s are nearly identical, as are the three B. garinii plasmids. The four B. afzelii lp17s, on the other hand are present as four different subtypes (the B. afzelii K78 lp17 is discussed in more detail below). The B. afzelii, B. garinii, B. spielmanii and B. valaisiana lp17s all have different organizations, but they all contain the same inversion of a central gene cluster (homologues of bb_d09 through bb_d14) relative to other lp17s (Fig. 3), supporting the idea derived from chromosome phylogeny ([1] and see Fig. 5 below) that these species have a common ancestor after their separation from the branch that contains B. burgdorferi, B. bissettiae, B. finlandensis and B. mayonii [1, 15, 49, 72].
Fig. 5.

Borreliella chromosome phylogeny. It is based on a concatenated 144,891-long protein sequence alignment of 450 single-copy orthologues universally present in all Borreliella genomes (see Methods) and is rooted with orthologues from relapsing-fever Borrelia genomes (not shown). The four previously designated B. burgdorferi chromosomal “SNP types” A, B, C and D [1] and a new type E are indicated outside the circle in the same color box as the cognate strain names. Isolate names are shown within the outer ring, and species designations of the genomes are indicated by color in the outer ring. Continental origins of the isolates are indicated by colored dots at the tips
Lp25
Several of the NBu-Borreliella species carry lp25 plasmids, but the three B. afzelii and single B. finlandensis SV1 and B. spielmanii A14S complete genomes, do not. The two B. mayonii lp25 plasmids are nearly identical, but the two B. garinii lp25s have substantial differences and represent two different subtypes (Additional file 1: Figure S3C). Interestingly, in spite of major organizational differences, all these lp25s carry a pncA gene ([73, 74] and Additional file 1: Figure S3C), a bptA gene [75] and a bb_e17 homologue. The lack of lp25-borne pncA, bptA and bb_e17 genes in the B. afzelii, B. finlandensis and B. spielmanii genomes is compensated for their presence on lp28–2 in B. afzelii and B. finlandensis and lp38 in B. spielmanii (Table 2). We also note that the B. valaisiana lp25 carries a 10.5 kbp imperfect direct repeat (the two arms of the repeat are about 98% identical) that includes these three genes as well as the partition gene cluster; but one of the pncA reading frames is not intact.
Lp28–2
Only four NBu-Borreliella genomes, three B. afzelii and B. finlandensis SV1 genomes, harbor an lp28–2, all of which have very substantial organizational differences from B. burgdorferi lp28–2s (Additional file 1: Figure S3D). The three B. afzelii plasmids have two very different left and right ends and clearly represent two subtypes. Curiously, the SV1 lp28–2 has a left end sequence that is organizationally the same as the B. afzelii ACA-1 lp28–2 suggesting a possible horizontal exchange of that part of one of these two plasmids. As mentioned above, these B. afzelii and B. finlandensis lp28–2s each carry a pncA, bptA and bb_e17 gene.
Lp28–3
Among the NBu-Borreliella genomes only B. finlandensis lacks an lp28–3 plasmid, and again these are present as a number of different organizational subtypes that have substantial inter-species differences (Additional file 1: Figure S3E). The two B. mayonii lp28–3s are very closely related, and the four B. afzelii plasmids comprise two subtypes. The B. garinii PBr lp28–3 is unique among the NBu-Borreliella genomes in that it carries a set of vls cassettes; the B. burgdorferi Bol26 lp28–3 also carries vls cassettes but, other than similar PFam32 genes it has little else in common with the PBr lp28–3. B. valaisiana lp28–3s large size is unusual at over 82 kbp; this is in part due to 21 kbp of rather highly degraded cp32-like DNA (Additional file 1: Figure S3F).
Lp28–4
Only B. valaisiana VS116 and B. garinii Far04 do not contain an lp28–4. Again, each species contains unique subtypes (Additional file 1: Figure S3G). The two B. mayonii plasmids are very closely related, and the four B. afzelii plasmids comprise two subtypes (curiously the B. afzelii BO23 subtype II is similar to the other cognate B. afzelii plasmids, but appears at the current stage of sequence assembly to be circularly permuted relative to the others; not shown).
Lp28–7
Among the NBu-Borreliella genomes, lp28–7 plasmids are only present in the B. afzelii, B. bissettiae and B. garinii genomes. Again, in every case the different species contain unique subtypes (Additional file 1: Figure S3D). The three B. afzelii plasmids form three subtypes, and the two B. garinii PBr and 20047 lp28–7s form another subtype. The two B. garinii lp28–7s and the B. afzelii ACA-1 and BO23 lp28–7s all contain the 21 kbp cluster of tightly packed genes that are homologous to the section of B. burgdorferi B31 lp28–2 that has features of bacteriophage virion assembly operons [76, 77], but nearly half of the parallel B. afzelii PKo lp28–7 cluster is deleted and partly replaced by other sequences.
Lp28–8
Linear plasmid lp28–8 is not found in the B. bissettiae, B. finlandensis or B. garinii genomes, but each of the other NBu-Borreliella genomes contains a unique lp28–8 subtype (Additional file 1: Figure S3H). Again, the two B. mayonii plasmids are very closely related to one another (we include the B. mayonii “lp28–10s” in the lp28–8 type, see above), and the B. afzelii PKo and K78 lp28–8 s have the same subtype. All nine known lp28–8 s carry a set of vls cassettes, and B. afzelii strain ACA-1 was previously shown to harbor a plasmid that carries the vls cassette region [69], but this plasmid was not present in our ACA-1 sequence. The partial ACA-1 sequence reported by Wang et al. [69] contains 775 bp adjacent to the vls cassette region whose sequence is essentially identical to parallel sequences in the B. afzelii PKo and K78 lp28–8 plasmids, suggesting that the missing ACA-1 plasmid may well be an lp28–8 even though its PFam32 gene was not sequenced. In addition, all the NBu-Borreliella lp28–8 s except those in B. mayonii carry the sagA/B/C/D/E/F gene cluster that is predicted to encode a streptolysin S-like precursor peptide and the enzymes that convert it into a toxin [78]. No other plasmid types have been found to carry these genes. Our previous analysis showed that the sag genes are absent from all of the B. burgdorferi and B. bavariensis isolates tested and are rare if present at all in B. garinii, but they are common in B. afzelii, B. spielmanii and B. valaisiana isolates and may also be present in B. lusitaniae isolates [78].
Lp28–9
Two B. afzelii and two B. garinii genomes contain lp28–9 plasmids, and they constitute four organizational subtypes (Additional file 1: Figure S3D). The B. garinii Far04 lp28–9 carries a set of vls cassettes, and the B. garinii PBr and B. afzelii ACA-1 and K78 plasmids contain the 21 kbp cluster of B. burgdorferi B31 lp28–2 bacteriophage-like genes (above) [76, 77].
Linear cp32-type plasmids
Among the fourteen B. burgdorferi isolates with sequenced genomes, two, 72a and 118a, carry a linear plasmid with a cp32–3 type PFam32 gene. These plasmids were named lp32–3, and they only carry a small number of genes that appear to be derived from cp32 plasmids in addition to the cp32–3-like four-gene partition cluster (Additional file 1: Figure S3I). Five linear plasmids are present in the NBu-Borreliella genomes that have PFam32 genes of the cp32–6, − 10 and − 12 types (Fig. 1). B. finlandensis SV1 carries lp32–6 and lp32–12 plasmids whose PFam32 genes are closely related to those of cp32–6 and − 12, respectively, and one B. garinii isolate (Far04) and two B. afzelii isolates (PKo and ACA-1) carry lp32–10s that have a cp32–10-like PFam32 gene (Fig. 2 and Additional file 1: Figure S1). The B. burgdorferi lp32–3s and SV1 lp32–6 carry a vls cassette region, but have little else in common. The B. afzelii lp32–10s, like the lp32–3s, have little cp32-like DNA beyond their partition gene clusters, but the SV1 lp32–6 and − 12 plasmids and Far04 lp32–10 have much more cp32-like DNA, about 27, 32 and 28 kbp, respectively. The relatively long approximately 49 kbp SV1 lp32–6 plasmid includes a nearly complete cp32 that appears to have integrated by a recombination event in its homologue of the B31 cp32–1 bb_p04 gene. Genes bb_p05-p09 (about 3 kbp) of the expected cp32-like sequence are missing, but some of this sequence could be at the left plasmid terminus that might not have been reached by the sequencing project. This plasmid’s sequence was not closed, so it is not certain that it is linear, but the presence of a vls cassette region at the right end of the contig is strongly suggestive that it is linear because this cassette array only occurs at near ends of linear plasmids in all cases where its location is known. In the 52.5 kbp long SV1 lp32–12, the recombination occurred in the homologue of the B31 cp32–1 bb_P22 gene and sequences from there to bb_P17 are not present. Its BSV1_x50 protein is 73% identical to strain B. burgdorferi CA12 OspE [79, 80] and 69% identical to B31 ErpP [81], and BSV1_x51 protein is 53% identical to N40 Erp27 [82]. The Far04 lp32–10 has apparently incorporated cp32 DNA sequences twice. It contains one approximately 23 kbp section that is homologous to B31 cp32–1 genes bb_p11 through bb_p41 that contains the cp32–10 PFam32 gene and second approximately 6.5 kbp section that is homologous to the B31 cp32–1 region from about bb_p23 through bb_p32. The Far04 lp32–10 contains no intact erp-like gene but does carry homologues of ospD and cspZ, genes that are present on other linear plasmids in other strains [83–86] (Table 2).
Lp36
Lp36 plasmids are present in B. garinii, B. mayonii, B. spielmanii and B. valaisiana, where again each subtype is limited to a single species (Fig. 4 and Additional file 1: Figure S3J). The two B. mayonii lp36s are very closely related, while the three B. garinii plasmids represent three subtypes. The B. garinii 20047 and PBr lp36s are very similar except for a 2.5 kbp replacement at the left end and the different positions of a transposase (PFam82) gene. The adeC (adenine deaminase), bb_k32 (fibronectin binding protein) and bb_k46 genes of B. burgdorferi B31 lp36 are known to be important for B. burgdorferi virulence [35, 45], and in the NBu-Borreliella species adeC is present on lp36 in the B. mayonii and B. spielmanii genomes, but is found on lp25, lp28–7 or lp38 plasmids in other species; only a relatively large adeC fragment is found at the right end of the chromosome in B. valaisiana VS116 (Table 2). Fibronectin binding protein genes are absent from the B. finlandensis and B. mayonii genomes and only a fragment is present on B. valaisiana VS116 lp28–3, but they are present on lp28–7 in B. bissettiae DN127 and lp17 in the B. afzelii, B. garinii and B. spielmanii genomes (Table 2). Curiously, among the NBu-Borreliella genomes, a gene closely related to bb_k46 is only found in B. valaisiana VS116, and it contains a reading frame disruption; more distantly related members of this PFam75 family are found on plasmids in the B. afzelii, B. garinii and B. spielmanii genomes.
Lp38
Among the NBu-Borreliella genomes, lp38 plasmids are only found in the B. afzelii and B. spielmanii genome sequences, and their PFam32 proteins form a separate branch that is 10–12% different from the B. burgdorferi lp38 PFam32s (Fig. 2 and Additional file 1: Figure S1). All four of the B. afzelii plasmids are closely related and belong to the same subtype (Additional file 1: Figure S3K); however, the BO23 lp38 is annotated to be fused end-to-end to lp54 and as such constitutes a second subtype. The B. afzelii and B. spielmanii lp38 plasmids are very different from each other except for the partition gene cluster, and, uniquely, lp38 carries the only pncA, bb_e17 and bptA genes in B. spielmanii A14S and the adeC gene in B. afzelii (above).
Lp54
The Borreliella lp54 plasmid sequences are quite uniform in their gene content and organization with a few differences at their left ends, in the ospA-ospB gene cluster, in the gene bb_a53-bb_a54 region and in the tandem cluster of PFam54 protein encoding genes near the right end. In addition, we find two major rearrangements involving lp54s; the B. finlandensis SV1 lp54 contains an integrated cp32–11 (see below) and the BO23 lp54 is reported to be fused end-to-end with an lp38 (above). Samuels et al. [87] showed previously by Southern analyses that in the strains they analyzed B. afzelii and B. garinii lp54s are several kbp longer than those of B. burgdorferi, and the genome sequences show that B. afzelii, B. garinii and B. spielmanii lp54s have approximately 3 kbp left end extensions relative to B. burgdorferi lp54s (not shown). These extensions are similar in these three species and carry two genes that encode a PFam52 putative lipoprotein (the terminal gene) and a PFam60 putative lipoprotein (penultimate gene). The reported B. japonica HO14 lp54 has a different 2 kbp left end extension that encodes a PFam12 putative lipoprotein and a PFam82 (transposase) protein, and B. chilensis lp54 has a third type (also about 2 kbp) of left end extension that encodes two unique proteins of unknown function. In all three left-end extension types the first gene internal to the extension is a homologue of B. burgdorferi B31 terminal gene bb_a01, a homologue of the PFam48 outer membrane channel forming protein P13 [88, 89]. The leftmost gene in other NBu-Borreliella lp54s is in all cases a B. burgdorferi B31 bb_a01 homologue, but between the telomere and this gene B. bissettiae DN127, B. finlandensis SV1 and the two B. mayonii strain lp54s all have multiple short insertions relative to the B. burgdorferi plasmids that add up to about 100, 200 and 500 bp of “extra” apparently non-protein coding DNA, respectively, compared to B31 lp54 which has 653 bp between the telomere tip and bb_a01 [51, 90]. The B. valaisiana lp54 left end has (different) indels in this region, but the distance from its projected telomere location and the first gene (also a bb_a01 homologue) is about the same as B31 lp54. The ospA-ospB gene region contains one or two genes in different Borreliella isolates. All the completely sequenced NBu-Borreliella lp54s examined except those of B. garinii lp54s contain two genes in this cluster. B. garinii isolates PBr, Far04 and 20047 have only one gene. We also note that close relatives of B. garinii, B. bavariensis isolates PBi and BgVir have two genes here [57], and Yabuki et al. [91] found that a large fraction of Japanese B. garinii isolates have two genes in this cluster. In addition, B. bissettiae DN127 lp54 is missing ~ 760 bp relative to B. burgdorferi lp54s so that it does not have homologues to B. burgdorferi genes bb_a53 and bb_a54; interestingly, B. valaisiana isolates VS116 and Tom4006 are identical to one another in this region and have a slightly different deletion relative to B. burgdorferi lp54 that removes the same two genes. Finally, the B. chilensis lp54 has several organizational (indel) differences from B. burgdorferi lp54 in the 15–17.5 kbp region and an approximately 650 bp insertion at about 21.6 kbp (B31 lp54 coordinates); the latter is predicted to encode a unique 124 amino acid protein (locus_tag OY14_04520). Thus, these lp54 organizational differences correlate with individual species or particular groups of species.
We have previously shown that the PFam54 gene cluster, which encodes proteins that affect host complement function and/or bind host plasminogen [92–95], is the most variable region of the lp54 plasmids, and we have described these variations in in terms of gene losses and gains [96, 97]. These clusters are quite variable in gene content and number both within and between species, and since our previous analysis nine more NBu-Borreliella lp54 sequences have been reported, making any organizational difference correlations (or lack thereof) in these species much more significant. Additional file 1: Figure S4 shows that there are four conserved PFam54 genes make up the constant outside portions of the cluster and that there are 28 different gene types in the variable region (ignoring small, apparently pseudogene fragments that are present in some cases). The number of genes in the variable region ranges from two in B. chilensis to six in some B. afzelii, B. bissettiae, B. garinii and B. valaisiana isolates. We note that the B. afzelii PKo lp54 has been sequenced twice and one version (accession No. CP000396) has two additional genes in the cluster that are identical to B. bavariensis PBi lp54 genes BGA71 and BGA72. If this sequence is accurate, it indicates that these genes have likely been lost from the other sequenced PKo culture and the maximum number of variable genes in the cluster would be eight. The PFam54 cluster differences create two lp54 subtypes in B. afzelii, B. bavariensis and B. garinii and three in B. burgdorferi. In no case is the whole array of PFam54 genes the same in isolates from different species, but the B. afzelii ACA-1 and B. spielmanii A14S arrays are very similar except for a 37% difference in encoded protein sequences between A14S BpsA14S_a0067 and its B. afzelii orthologues (Additional file 1: Figure S4). Essentially identical arrays are often present in different members of the same species. However, similar individual variable region genes occur in different species in several cases. For example, members of this gene type exemplified by B. afzelii PKo gene BafPKo_a0062 also occur in B. spielmanii, B. valaisiana, B. japonica and B. chilensis PFam54 clusters, genes similar to B. afzelii BafPKo_a0066 are present in B. garinii, B. spielmanii and B. bavariensis, and genes of the same type as B. burgdorferi B31 bb_a68 (cspA) are present in B. bissettiae, B. finlandensis and B. mayonii lp54s. The top four species in Additional file 1: Figure S4, B. burgdorferi, B. bissettiae, B. finlandensis and B. mayonii, have no variable gene types in common with the remainder of the Borreliella species in the figure, which agrees with other analyses that suggest that these groups form two separate lineages ([1] and see Fig. 5 below). Single nucleotide polymorphism (SNP) tree analysis showed that the known whole lp54 plasmids have not exchanged between species [1].
Lp56
Lp56 is a relatively uncommon plasmid. Four of the fourteen B. burgdorferi genomes harbor this plasmid type (one of which, the strain B31 lp56 contains an integrated cp32), but B. bissettiae DN127 is the only NBu-Borreliella genome that harbors an lp56, and its lp56 is very similar to the lp56 present in B. burgdorferi CA.11_2A (Additional file 1: Figure S3L). This is discussed in more detail below as a possible recent whole plasmid horizontal transfer.
Linear plasmid-like sequences at chromosome ends
Borreliella chromosomes are very constant except for some differences which have been described previously in the ribosomal RNA gene region [1, 21, 98], in the gene bb_0524 region [1], and at the right end of B. burgdorferi chromosomes [19, 20, 29, 99, 100]. Our previous work showed that most NBu-Borreliella chromosomes terminate at about the same location [29]. Only B. japonica IKA2 left end appeared to be extended with 15–20 kbp of extra plasmid-related DNA, but its sequence is not known. Additional file 1: Figure S displays ORF maps of the sequences at the ends of all the reported NBu-Borreliella chromosomes. Except for the B. mayonii and B. valaisiana VS116 chromosome, the left end chromosome sequences all terminate at about the same place. The mayonii chromosomes have a left end extension about 1100 bp long that carries a gene that encodes a PFam12 protein whose closest relatives are on B. burgdorferi plasmids. The B. valaisiana left end extension contains only small ORFs that include closely related homologues of fragments of VS116 BvaVS116_h0023 and _e0057 proteins (encoded by VS116 plasmids lp28–3 and lp25, respectively). Uniquely, both the known B. valaisiana left ends lack a homologue of the B. burgdorferi B31 left terminal chromosomal gene bb_001, but this gene is present as the terminal gene at these chromosomes’ right ends (Additional file 1: Figure S5B), suggesting a past termini switching event.
Some B. burgdorferi right chromosomal ends have variable lengths (up to about 20 kbp) of “extra” plasmid-like DNA compared to the shortest chromosomes, and restriction mapping suggested that several other Borreliella species chromosomes terminate at about the same location as the minimal B. burgdorferi chromosome (e.g., strain N40) a few hundred bp to the right of the last common chromosomal gene bb_843 ([20] and references therein). Genome sequences show that all the NBu-Borreliella chromosomal right ends do indeed terminate just beyond the right end of their bb_843 gene homologues (Additional file 1: Figure S5B), with the exception of B. valaisiana, which has right end extensions of about 8600 and 8900 bp in the two strains. These extensions are very similar except for a few small indels and an approximately 550 bp inversion. The internal regions these extensions have about 4800 bp that is about 90% identical to B. afzelii plasmid lp38. These are a new type of linear plasmid-like right end chromosome extension that contains PFam26, PFam40 and PFam47 genes as well as the bb_001 homologue and several pseudogenes. Once again, no left or right end extensions are the same in members of different species, so all these rearrangements appear to have occurred after evolutionary separation of these species.
Circular plasmids
Cp9
The NBu-Borreliella species also carry circular plasmids that are similar to B. burgdorferi cp9, cp26 and cp32 plasmids. We noted above that, unlike the B. burgdorferi cp9s, some of the NBu-Borreliella cp9 plasmids encode a unique type PFam32 protein. The cp9s in different species are all of different organizational subtypes (Additional file 1: Figure S6), but all have substantial regions of homology (the functions of the encoded proteins are largely not known). Interestingly the B. burgdorferi cp9s all carry the PFam95 eppA gene [101], but among the NBu-Borreliella complete genomes, only B. bissettiae DN127 cp9 carries a close relative of this gene (more distantly related “BapA” PFam95 proteins are encoded by cp32 plasmids in several of these genomes).
Cp26
The NBu-Borreliella genomes all include a cp26 plasmid, which is the only plasmid that is essential for growth in culture in B. burgdorferi [102]. As has been previously observed, all NBu-Borreliella cp26s gene contents that the same as those of B. burgdorferi [1, 20, 103]. This plasmid encodes the important surface protein OspC, and although the rest of the cp26 sequences have diverged only a few percent, ospC is probably the most variable single-copy gene in the Borreliella genome [1], and it has undergone a substantial number of horizontal exchange events [104–109]. Single nucleotide polymorphism (SNP) tree analysis showed that the known whole cp26 plasmids have not exchanged between species [1].
Cp32s
The Borreliella cp32 plasmids are prophages, and some phage been shown to produce phage virions that can in turn deliver these plasmids to other cells [76, 77, 110, 111]. All B. burgdorferi isolates carry at least five of these homologous and syntenic, but divergent plasmids, and NBu-Borreliella isolates also usually carry multiple cp32 plasmid types (Fig. 1). As mentioned above, the two complete B. garinii genomes carry fewer cp32s than the other Borreliella species that have been examined. B. garinii Far04 has no cp32 plasmid, and PBr has two, both of which have apparent defects. PBr cp32–10 has a 3.8 kbp deletion in the putative virion assembly gene cluster that is replaced by about 10.5 kbp of DNA that is quite similar to sequences found on B. burgdorferi strain B31 lp38, VS116 lp17 and MN14–1420 lp28–4. PBr cp32–5 has two smaller rearrangements, a deletion that truncates BgaPbr_a0033, the PFam49 gene in the partition gene cluster, and an approximately 1.4 kbp deletion that removes two of the three tandem paralogous genes that are homologues of the B31 cp32–1 PFam148 genes bb_p03, _p04 and _p05. MN14–1420 cp32–13, VS116 cp32–7 and SV1 cp32–11 also have apparent reductions of this ancient gene triplication to one or two genes, but such a deletion is not present in any of the B. burgdorferi cp32s. Since the function of these genes is unknown (they are postulated to lie in the phage virion assembly gene operon [76, 77, 111]), it is not known whether such deletions might impair any phage function.
The other NBu-Borreliella genomes have larger cp32 complements than B. garinii. B. burgdorferi isolates average 6.6 cp32s, and the three B. afzelii genomes average five cp32s, B. bissettiae DN127 has seven (that have nine PFam32 genes, see below), B. finlandensis SV1 has three (with four PFam32 genes due to a cp32 fusion event), B. valaisiana VS116 has three, and the two B. mayonii isolates each have five (Fig. 1). B. spielmanii A14S apparently has four cp32s but they have not been completely assembled and remain in draft status (see Additional file 1: Figure S7 for analysis of the incompletely assembled A14S cp32 contigs). Most of the NBu-Borreliella cp32s appear to be intact but 12 (~ 13%) of their 90 completely assembled cp32s have undergone some type of rearrangement (18% of the 120 completely assembled B. burgdorferi cp32s have rearrangements). Additional file 1: Figures S8 and S9 show the locations the various NBu-Borreliella cp32 rearrangements; none of them except the PBr cp32–5 PFam49 gene truncation (above) affect the partition gene cluster, which is expected to be required for long term plasmid maintenance and/or stability (reveiwed by [62, 63]) and none affect the erp/elp/ospEF/Bap gene containing variable regions 3 and 4 (the latter defined in [19]). VS116 cp32–7 and SV1 cp32–4 have substantial insertions of linear plasmid-like DNA that are similar to parts of B. burgdorferi 94a lp56 and B. finlandensis SV1 lp28–4, respectively; the former also has a large approximately 8 kbp inverted duplication that includes the partition genes. DN127 contains a complex 66 kbp cp32-related circular plasmid named “cp32-quad” that contains four different partial cp32 sequences that include three different apparently intact PFam32 genes that correspond to cp32–9, − 12 and − 13 types (see Additional file 1: Figure S9). Like the B. burgdorferi cp32s, NBu-Borreliella cp32s show diversity within species; however, the two B. mayonii genomes each contain six cp32s and each pair of cognate cp32s is virtually identical, once more emphasizing that these isolates are extremely similar. On the other hand, five different cp32s are present in more than one of the three B. afzelii isolates ACA-1, PKo and K78, and in only one case, ACA-1 and K78 cp32–3, do the cognate plasmids from different isolates have similar mlp and/or erp gene regions (see Additional file 1: Figure S8), but even in that case they have an approximately one kbp region of substantial difference that encodes the proteins encoded by genes BafACA1_s16 and BafK78_s015.
Interestingly, in B. finlandensis SV1 cp32–11 is integrated into lp54 to form an 83,377 bp hybrid linear plasmid that is diagrammed in Additional file 1: Figure S10A. The integrated cp32–11 is nearly full-length but contains a deletion in the B. burgdorferi B31 bb_p03, _p04 and _p05 paralogue cluster (above) that fuses the N-terminal end of the B31 cp32–1 bb_p04 homologue to the C-terminal portion of the bb_P05 homologue (annotated as bsv1_a103). Genes were disrupted on both plasmids by the integration event, which occurred within the B31 lp54 gene bb_a65 homologue and the B31 cp32–1 gene bb_p06 homologue with no apparent deletion or insertion of extraneous nucleotides in either plasmid at the site of integration. The deduced fusion sites have no apparent sequence similarity, so integration was achieved by nonhomologous recombination (see Additional file 1: Figure S10B). This cp32 integration location is different from the location at which cp32–10 integrated into lp56 in strain B31 [30] or the cp32 integrations that formed the lp32s above. The facts that the integration sites on these cp32s are all different and that different genes were disrupted in the target plasmids in all cases suggests that these cp32 integration events occurred at random locations by non-homologous recombination.
Discussion
Inter-species whole plasmid mobility
Chromosome tree correlates with species
Horizontal transfer of small regions of DNA has been identified as being fairly common in the Borreliella species [104–109]. However, as described above, linear plasmid organizational types are typically limited to individual species, indicating that whole plasmid exchange between these species is not common. In order to better understand the evolutionary relationships between the chromosome and plasmids, as well as to search for possible whole plasmid exchanges between species, we constructed a maximum likelihood phylogenetic chromosome tree based on 450 proteins that are universally present as single copies in Borreliella chromosomes (Fig. 5). This expanded tree’s major branches correlate with the different Borreliella species, and the tree has the same branching order for the different species as the previously published chromosomal SNP tree for a subset of these species, and it supports the idea that B. finlandensis and B. mayonii are more closely related to B. burgdorferi than to the other Borreliella species. The chromosomal tree also indicates that the newly sequenced isolates BgVir, SZ and NMJW1 are in fact B. bavariensis as has been previously noted by Margos et al. [112] and Gatzmann et al. [113]. This tree agrees perfectly with the B. burgdorferi chromosomal SNP types A, B, C and D that we defined previously [1], and shows that the chromosome of newly sequenced B. burgdorferi isolate CA382 is type A and that of CA8 forms a new type, which we designate type E. We also note that the B. Chilensis chromosome lies on a deep branch that separates it from all the other Borreliella species. Comparison of plasmid sequence relationships with the chromosome tree allowed the discovery of four examples of possible of whole linear plasmid horizontal transfer events between species lineages.
Lp5 exchange
B. mayonii MN14–1420 is the only NBu-Borreliella isolate known to carry the uncommon lp5 plasmid, and only B. burgdorferi isolates B31 and WI91–23 were previously known to carry it (Additional file 1: Figure S3A). In the 5225 bp that have been sequenced in all three lp5s, MN14–1420 lp5 has 16 single bp differences from B31 lp5 but only one bp difference from WI91–23 lp5 (the WI91–23 lp5 sequence is missing 24 bp at the left end and has extra 2 bp at the right end relative to B31 and MN14–1420 lp5s). The remainder of the B. mayonii and B. burgdorferi genomes are substantially more different than this. For example, the B. burgdorferi B31 and WI91–23 chromosomes (99.5% identical to one another) are both 94.8% identical to the B. mayonii chromosomes (which are themselves > 99.99% identical). Thus, this appears to be an example of quite recent cross-species transfer of the whole lp5 plasmid between these two strains from mid-west US. The direction of this putative transfer between the WI91–23 B. burgdorferi chromosomal type B lineage and B. mayonii, cannot be deduced unambiguously, but the alternate hypothesis, lp5 was present in a common ancestor, would require very different mutation rates in the two B. burgdorferi lineages A and B and the less parsimonious losses from the lineages of the twelve other B. burgdorferi isolates, the one B. finlandensis isolate, the one B. bissettiae isolate and one of the B. mayonii isolates.
Lp56 exchange
The ~ 29.5 kbp lp56 plasmids that are present in B. bissettiae DN127 and B. burgdorferi CA.11_2A are 99.97% identical with only eight single bp differences and no indels between the 29,418 bps that have been sequenced in both plasmids (the left and right ends of CA.11_2A sequence are missing ten and twenty terminal bps, respectively, relative to the DN127 sequence). B. burgdorferi WI91–23 lp56 is the only other sequenced plasmid that has a long region of synteny with the CA.11_2A and DN127 lp56 plasmids, and it has a several kbp replacement at its left end relative to them (see Figure S20 in [20]). The homologous DNA between 11 and 25 kbp of DN127 lp56 is only 93.8% identical to the parallel WI91–23 region (with no indels larger than 15 bp) (Additional file 1: Figure S3L). In addition, the CA.11_2A and WI91–23 chromosomes are only 0.48% different while they are 1.73% and 1.74% different from DN127, respectively [1]. This unusually close lp56 similarity very strongly suggest its recent transfer between B. bissettiae and the B. burgdorferi CA.11_2A chromosomal type C lineage. We also note that these lp56s all carry a long region of synteny with B. burgdorferi linear plasmid lp28–2 that includes its genes bb_g10 through bb_g28 which have been proposed by Eggers et al. [110] to constitute a phage virion assembly gene operon, so it is plausible that these lp56 plasmids are prophages and that their horizontal transfer could be mediated by phage virions.
Lp17 exchange
B. spielmanii A14S and B. afzelii K78 lp17s are members of the same unique subtype which we call lp17 Bsp I (Additional file 1: Figure S3B), but their sequences are not as close as the lp5 or lp56 cases discussed above. A14S lp17 has two short indels relative to K78 lp17, both of which are changes in the number of repeats in a short tandem sequence repeat array; one is the deletion in A14S of four of the six and half 21 bp tandem imperfect repeats (centered at about bp 100 in A14S) that are present in K78 lp17, and the other is 11 more copies in A14S than in K78 of the 21 bp repeat in the different imperfect repeat array that begins at bp 20,884 in 14S. If these repeat differences are removed, the entire A14S and K78 lp17 sequences are 99.2% identical (ignoring K78 lp17’s 29 extra bp at its right end and 23 extra bp at its left end due to failure of the A14S sequence to reach the telomeres; we also note that it has not been shown that these differences are not sequence assembly errors). The lp17 subtype Bsp I plasmids are organizationally different from the PKo, ACA-1 and BO23 lp17 subtypes Baf I, II and III, respectively (Fig. 3 and Additional file 1: Figure S3B). The region between 6500 and 19,000 bp of A14S lp17 avoids the above repeat regions and terminal organizational differences and provides 12,500 bp of syntenic DNA for all of these four subtypes of lp17. In this region A14S and K78 lp17s are 0.6% different from one another, but range from 1.2 to 1.8% different from the other three B. afzelii lp17s (lp17 subtypes Baf I, II and III range from 0.4% to 1.8% different from one another). Thus, in addition to the major terminal organizational differences, the large central syntenic regions in the A14S and K78 lp17s are 2- to 3-fold less divergent from one another than they are from the other B. afzelii lp17s. On the other hand, the chromosomes of K78, PKo and ACA-1 are all 0.220–0.254% different from one another and 0.534–0.535% different from the chromosome of A14S. Thus, the K78 chromosome fits perfectly in B. afzelii and not B. spielmanii. These observations indicate that a B. afzelii K78-like predecessor donated its lp17 to the B. spielmanii A14S lineage or vice versa. Light will be shed on this directionality by additional B. spielmanii lp17 sequences - will they be unique or K78-like? The latter would be parsimonious with the B. spielmanii to B. afzelii direction.
Lp28–9 exchange
The European B. burgdorferi isolate Bol26 is the only one of the sixteen sequenced genomes of this species (including recently reported strains PAbe and PAli [55]) that carries an lp28–9, while two of three European B. afzelii genomes and both European B. garinii genomes carry an lp28–9. Its absence from the fifteen other B. burgdorferi genomes suggests that Bol26 might have obtained lp28–9 from one of these European species. The Bol26 lp28–9 organization is most similar to its counterparts in B. afzelii strains ACA-1 and K78 (Additional file 1: Figure S3D), where it is 87% and 84% identical, respectively, to these plasmids over their common 18,000 bp central regions. The ACA-1 and K78 plasmids are 95% identical over this region. There are also organizational differences among the Bbu I, Baf I and Baf II lp28–9 subtypes. Thus, while it is parsimonious to conclude that Bol29 obtained its lp28–9 from a European B. afzelii, if this is true it must have occurred long enough ago to have allowed the observed sequence divergence and terminal organizational changes occur.
Primordial origin of Borreliella plasmids
PFam32 protein phylogeny
To further investigate the origin and evolution of Borreliella plasmids, we first analyzed the phylogenic tree of their PFam32 proteins shown in Fig. 2. These PFam32 proteins are both orthologous and paralogous; the orthologues are similar in amino acid sequence across species due to direct, non-duplicative descent, and the paralogues are divergent within the same genome due to ancestral duplication events. The 30 types of PFam32 orthologues found on characterized Borreliella plasmids (depicted with different shading on the outer ring in Fig. 2) are delineated based on long tree branches as well on the criterion that no two orthologues should coexist within the same genome [19, 20, 64]. Three conclusions can be drawn from this analysis of the PFam32 protein phylogeny. First, the PFam32 tree supports ancestral, primordial radiation of many of the plasmid types. This conclusion is a more parsimonious explanation of the observation that the large majority of plasmid types exist in multiple Borreliella species across the species and continents, than the alternative hypothesis in which the different plasmid types have invaded multiple Borreliella species independently after they arose in one of the species (especially with the observation above that exchange of whole plasmids between species is rare). Most of the plasmid types appear to be present around the world. Only the lp28–9 PFam32 type is unique to Eurasian Borreliella genomes, while PFam32 plasmid types lp21, lp28–1, lp28–5, lp28–6, and cp32–8 (and lp5 which has no PFam32 gene) have to date been found only in North American isolates. However, these findings, especially the Eurasian deficiencies, could be due to the smaller sample sizes of the Eurasian isolates. We also note that although the lp28–1 type is currently unique to North America and the lp28–9 type is unique to Eurasia, the available data do not rule out the possibility that these two groups represent a single compatibility group (above). The ancestral existence of numerous major plasmid groups in the most recent common ancestor of all Borreliella species suggests essential roles of these plasmids in Borreliella as a biological species complex.
The second conclusion drawn from the PFam32 tree in Fig. 2 is that plasmid fusion, apparently mediated by homologous and/or non-homologous recombination, occurs occasionally in Borreliella genomes. The coexistence of two PFam32 paralogues of different type on a single plasmid is indicative of recent plasmid fusion event, since such fusions are not shared by closely related strains. The following plasmids with fusions of this type are present in four Eurasian isolates: lp36/lp28–3 fusion in B. garinii Far04 that has an “orphan” lp28–3 PFam32 gene (not in a partition cluster), lp28–3/lp28–4/lp38 fusion in B. valaisiana VS116 (at this point only the lp28–3 PFam32 gene is intact), cp32–3/cp32–10 fusion in B. burgdorferi ZS7 and B. finlandensis lp54/cp32–11 in SV1. We also note that an lp38/lp54 fusion has been reported for European B. afzelii BO32, but it is not included in this analysis since its genome sequence appears is still in draft status. Six such fusion plasmids are present in North American strains as follows: cp32–9/cp32–12/cp32–13 in the cp32-quad plasmid of B. bissettiae DN127, and the following fusions in B. burgdorferi: lp56/cp32–10 in B31, lp36/lp28–4 in CA11-2A, cp32–7/cp32–9 in 118a, cp32–3/cp32–8 in 64b, cp32–1/cp32–5 in JD1. These plasmid fusions that carry more than one PFam32 gene are indicated by lavender ribbons in Fig. 2. In addition, the lp32–3, − 6, − 10 and − 12 plasmids in which linear plasmids appear to have fused with cp32s and subsequently lost their linear plasmid PFam32 genes are limited to B. burgdorferi chromosomal lineage C, the relatively closely related species B. afzelii and B. garinii, and B. finlandensis, respectively. These findings suggest that these fusion plasmids arose relatively recently in specific lineages.
On the other hand, these fused plasmids are not always shared by closely related isolates suggesting that the fusion events likely occurred after strain divergence. For example, (i) among the B. burgdorferi type A chromosome isolates the lp56/cp32–10 fusion is found in B31, PAli and PAbe, but not in 64b, ZS7 or Bol26, while different cp32 fusions are present in 64b and ZS7 that are not in the other type A isolates, and (ii) the lp36/lp28–4 fusion in CA11-2A and cp32–7/cp32–9 fusion in 118a are not present in other B. burgdorferi type 3 chromosome isolates. Past fusions may or may not resolve rapidly. We postulate that homologous recombination-mediated fusions form frequently and can resolve rapidly. Indeed, recently Margos et al. [55] reported that their culture of B. burgdorferi B31 (B31 NRZ) carries a cp32–1/cp32–5 fusion, while the cp32–5 portion was not present in the MedImmune culture of B31 that was originally sequenced [51, 70]. This suggests that either a resolution of this fused plasmid occurred and the cp32–5 portion was lost in the latter culture, or a fusion of the two plasmids occurred in the former culture. This apparently rapid fusion and resolution of cp32 plasmids likely reflects the fact that homologous recombination can mediate both in the substantial regions of similar sequence that cp32s contain.
Plasmids similar to the fused lp56 present in the New York isolate B31 were identified by Palmer et al. [114] in two (Connecticut) isolates out of eleven New England B. burgdorferi isolates tested. In addition, lp56 plasmids that are essentially identical to B31 lp56 are present in the sequenced genomes of two B. burgdorferi type A isolates PAbe and PAli isolated from two German patients that likely were infected in North America [55, 115]. The fact that these three plasmids are almost certainly all the result of a single past fusion event [20, 30], suggests that reversal of this fusion is not rapid. In the B31 lp56/cp32–10 and SV1 lp54/cp32–11 cases where the parental sequences can be deduced at the fusion site, the fusions are clearly mediated by non-homologous recombination (Additional file 1: Figure S10B and [30, 70]). Such fusions cannot be easily reversed by homologous recombination and so are not expected to resolve rapidly or precisely.
Third, the PFam32 tree supports of the putative horizontal transfer (by lysogeny or transduction) of whole-plasmids discussed above. The donor and recipient genomes of these events can be suggested by comparing the PFam32 tree (Fig. 2 and Additional file 1: Figure S1) and the chromosome tree (Fig. 5). For example, the latter tree indicates that B. spielmanii is an outgroup of the B. afzelii clade; however, the PFam32 orthologue on B. spielmanii A14S lp17 groups with its B. afzelii lp17 orthologues, supporting the displacement (since all current isolates have an lp17) of an lp17 plasmid between an B. spielmanii and B. afzelii (above). Similarly, inconsistent PFam32 groupings indicate an uptake of lp28–9 by B. burgdorferi Bol26 from a B. afzelii strain (above), and an uptake of an lp56 by B. bissettiae DN127 from a B. burgdorferi strain (above).
Early PFam32 divergence before Borreliella-Borrelia divergence
We further examined these ideas by incorporating relapsing fever (RF) PFam32 proteins into our analysis. Less information has been reported about RF plasmids than about Borreliella plasmids, but both branches carry numerous plasmids, and at least some cognate plasmids have mosaic relationships within the RF lineage, similar to those of the Borreliella plasmids ([116, 117] and our unpublished observations). The RF plasmids that have been analyzed have surprisingly little gene content in common with Borreliella plasmids other than partition genes and a small number of other scattered homologous genes [116, 117]. The exception to this that we are aware of is the presence of cp32-like plasmids in RF isolates, some of which are as follows: B. hermsii HS1 plasmid cp28 [118, 119], B. duttonii Ly plasmids lp26, lp27 and lp28 [117], B. miyamotoi CT13–2396 plasmids cp1, cp2, cp3, cp4 and cp5 [120] and B. coriaceae Co53 unnamed plasmids with accession numbers CP005764 and CP005752. Like the Borreliella cp32s they have sizes that are largely in the 25–30 kbp range with the exception of several in the 13–19 kbp range (truncated versions of cp32s are also present in Borreliella isolates ([20] and references therein)). These RF plasmids have overall synteny with the Borreliella cp32s, but their nucleotide and encoded protein sequences are rather distantly related to their Borreliella homologues. In addition, database searches with one of the important phage virion structural genes carried by the cp32 prophage plasmids, the putative head portal proteins of PFam146, identified syntenic but smaller draft sequence contigs in B. hispanica, B. crocidurae, B. persica, and B. parkeri genomes. It thus appears that, as in the Borreliella isolates, multiple cp32-like plasmids can inhabit individual bacteria, and they are quite widely distributed across the RF lineage. When we included 45 plasmid-encoded PFam32 proteins from RF isolates B. hermsii HS1 [119], B. miyamotoi CT13–2396 [120], B. crocidurae Achem (Bioproject PRJNA85135), B. turicatae BET5EL [121], B. duttonii Ly and B. recurrentis A1 [117] in the neighbor-joining PFam32 tree, they represent at least 24 different sequence types, none of which coincide with any of the Borreliella PFam32 types discussed above, yet nearly all of them group robustly inside the Borreliella PFam32 tree (Additional file 1: Figure S11). And as expected none of these RF strains carry more than one plasmid with any given PFam32 type. We conclude that the origin of Borreliella plasmids must predate the divergence of the Borreliella and RF Borrelia clades. The alternate explanations, massive horizontal PFam32 gene transfer or loss, are much less parsimonious.
Partition gene cluster phylogeny
Finally, to further resolve the history of plasmid evolution in Borreliella, we built a phylogeny of the Borreliella plasmids that harbor a complete set of plasmid partition genes [20, 30, 64]. These partition gene clusters consist of four, usually tandemly arranged genes that encode protein members of the PFam57/62, PFam32, PFam50, and PFam49 families. The total length of this seven concatenated alignment is 1327 amino acids (including gaps), more than four times as long as the 392 amino acid alignment of PFam32 sequences alone, so it provides more information for phylogenetic reconstruction. The tree derived from these concatenated sequences is shown in Fig. 6. Trees based on a single locus can be misleading if a locus has undergone rare recombination events, and phylogenetic trees based on sequences of other individual cp32 proteins, including Pfam80, PFam165, PFam144, and PFam96, show large inconsistencies (trees not shown; see also references [19, 84]). Including more partition genes minimizes the effect of such misleading information. Unlike the PFam32 tree (Fig. 2), the partition gene cluster protein tree (Fig. 6) shows monophyly of all cp32 + cp9 plasmids, suggesting a single common origin for them, with the cp32s separated from the cp9s by a relatively shallow branch. Other major monophyletic plasmid groups separated by deep branches from the others include cp26, lp25, lp28–1/lp28–9, lp28–2/lp28–4, lp28–3/lp28–6/lp36/lp38/lp56, lp28–7, lp28–5, lp28–8 and lp54. The unlisted plasmids (lp5, lp17, and lp21), which are the smallest linear plasmids, do not have a full set of all four partition genes and so were not included in this analysis. These plasmid relationships will provide a phylogenetic framework for understanding the history of plasmid rearrangements, between-genome plasmid and gene exchanges, as well as gene gains and losses.
Fig. 6.

Plasmid super-groups revealed by plasmid partition gene clusters. The midpoint-rooted gene tree (center) is based on a 1327 amino acid long concatenated alignment of sequences of four plasmid partition proteins (including PFam57/62, Pfam50, PFam32, and Pfam49). This tree shows a monophyly of all cp32 + cp9 plasmids (left-side, shades of yellow in outer ring). Other monophyletic plasmid super-groups separated by similarly deep branches include (counter-clockwise from bottom) lp28–4/lp28–2, cp26, lp54, lp25, lp28–8, lp28–5, lp28–3/lp28–6/lp36/lp38/lp56, lp28–7, and lp28–1/lp28–9. Plasmids not shown in the figure (lp5, lp17 and lp21) do not have a full set of all four partition genes. A web-interactive version of the tree is available at BorreliaBase website (under the “Replicons” tab)
Conclusions
There are currently 405 Borreliella complete plasmid sequences in the nucleotide sequence database. We previously analyzed and compared the 236 B. burgdorferi plasmids [19, 20, 30], and here we analyzed the remaining 169 plasmid sequences from eight other Borreliella species, B. afzelii, B. bavariensis, B. bissettiae, B. garinii, B. finlandensis, B. spielmanii, B. valaisiana and B. chilensis. This study of complete genomes from seven new species discovered only one new type of PFam32 putative plasmid compatibility type in addition to the 29 types that were previously known, suggesting that all or nearly all of the types have now been identified. However, since there are currently fourteen additional named or proposed Borreliella species whose plasmid sequences have not yet been examined, it remains quite possible that they might harbor new plasmid types. Only a few of the plasmid PFam32 types are apparently species-specific at our current knowledge state; lp21, lp28–5, lp28–6 and cp32–8 are found only in B. burgdorferi strains, and no types are restricted to other Borreliella species. In addition, only a few PFam32 types are currently restricted to the Eastern or Western Hemispheres; the lp28–9 type is unique to Eurasian Borreliella genomes, while plasmid types lp28–1, lp28–5, lp28–6 and cp32–8 have been found only in North American isolates, but these apparent restrictions may be due to the fact numerous Borreliella species from both hemispheres have not yet been analyzed. During their evolution, the linear plasmids in NBu-Borreliella species have undergone the same kinds of chaotic rearrangements mediated by non-homologous recombination that we have previously described in the B. burgdorferi lineage. These rearrangements have occurred independently in each of the different species lineages, and they allowed the identification several rare whole plasmid transfer events between species.
Phylogenetic analyses of the plasmid partition genes showed that a majority of the different plasmid PFam32 types arose early, most likely before separation of the current Borreliella and Borrelia species, and this, perhaps with occasional cross lineage plasmid transfers, has resulted in few if any species-specific PFam32 plasmid types. Indeed, it is possible that the invention, invasion and/or domestication (from prophages in some cases) of these plasmids occurred in evolutionarily rapid succession and is the key evolutionary event that both defined the species and made the Borreliacae successful global parasites. Phylogenetic analysis of the four-gene plasmid partition clusters showed that the cp9 and cp32 plasmid PFam32 genes share a single common origin that is distinct from the other, mostly linear Borreliella plasmids, and it allowed the deduction of an evolutionary hierarchy of related groups among the linear plasmids. The improved resolution of Borreliella chromosome and plasmid phylogeny will lead to better determination of gene orthology, which is essential for prediction of biological function, and it will provide a basis to infer detailed mechanisms of Borreliella genomic variability including homologous gene and plasmid exchanges as well as non-homologous rearrangements.
The persistent maintenance of all major plasmid groups in Borreliella among its global lineages investigated here suggests not only their functional indispensability but also their evolutionary role in enhancing genome diversity and species evolvability. Indeed, being a closed genome with few gains and losses of gene families since their divergence [1], much of the genome variability and dynamism in Borreliella are mediated by plasmids, including whole-plasmid exchanges and structural rearrangements described here and earlier [20], higher sequence diversity as a results of increased homologous recombination and diversifying selection at surface-antigen loci on plasmids [104, 122] and in situ recombination at vls cassettes locus [123]. Disrupting plasmid partition and maintenance may therefore be an effective means to halt the local transmission cycles and geographic expansion of the Borreliella endemics worldwide. Similarly, knowledge of the variability of the Borreliella genome and its many plasmids will be informative in understanding host-range infectivity factors and varying host clinical responses.
Methods
Plasmid types and subtypes
We have previously described the sources of the isolates whose genomes have been sequenced and the methods used for sequence determination [1, 20]. Plasmid types and subtypes were determined and analyzed as described previously [20]. After determination of the encoded PFam32 protein type using ClustalX neighbor-joining tree analysis (Additional file 1: Figure S1), BLASTn [124], as well as DNA Strider [125] (usually initially at a window stringency setting of 17 out of 23 bp identities) and Gephard ([126] dot plot plasmid comparisons were used to identify groups of very similar plasmids (subtypes) that do not have large indels relative to one another. Nucleotide and protein sequence alignments were performed by BLASTn and BLASTp [124], ClustalX [127] and DNA Strider [125]. Neighbor-joining trees were created by ClustalX [127], and the resulting trees were drawn by NJPlot (http://pbil.univ-lyon1.fr/software/njplot.html). Maximum likelihood trees were inferred with RAxML [128]. Determination of percent identity between chromosomes was determined through reference-independent whole-chromosome alignments using MUGSY [129] as described in reference [1].
Chromosome tree
We identified 450 single-copy orthologues on the main chromosome (list available from author WGQ) using homolog identification by pairwise BLASTp and homolog clustering by CD-HIT [130]. Orthologous protein sequences were aligned using MUSCLE [131, 132]. Using the BIOALN utility of the BbWrapper toolkit (https://metacpan.org/release/ROCKY/Bio-BPWrapper-1.02), the protein alignments were concatenated and an approximate maximum likelihood tree was inferred using FastTree [133]. The resulting tree was re-rooted using orthologues from relapsing-fever Borrelia genomes and branches with low bootstrap support (< 0.8, if any) were removed using the BIOTREE utility of the BbWrapper toolkit. The genome tree was custom plotted and annotated using the D3 JavaScript library (version 4, d3js.org).
Par cluster gene tree
The partition gene cluster includes members of paralogous families PFam57/62, PFam50, PFam32, and PFam49 [30, 51]. Members of these gene families in newly sequenced genomes were identified based on clusters generated by CD-HIT. Protein sequences of partition gene clusters from 34 Borreliella and 4 relapsing-fever Borrelia genome sequences were downloaded from BorreliaBase [61]. Orthologous sequences were aligned with MUSCLE [131, 132] and concatenated with BIOALN. Gene trees were inferred using FastTree, mi-point rooted and low-support branches removed by using BIOTREE, and custom plotted and annotated using the D3 JavaScript library (above).
Additional file
Figure S1. Borreliella plasmid PFam32 protein neighbor-joining tree. Figure S2. Two examples of Borreliella linear plasmids with low protein coding potential. Figure S3. Comparative maps of linear plasmids in Borreliella isolates. Maps of the following linear plasmids are shown in the following panels: A, lp5; B, lp17; C, lp25; D, lp28–2, lp28–6, lp28–7 and lp28–9; E, lp28–3; F, VS116 lp28–3; G, lp28–4; H, lp28–8; I, lp32; J, lp36; K, lp38; L, lp56. Figure S4. The PFam54 gene cluster of the Borreliella lp54 plasmids. Figure S5. Ends of the Borreliella linear chromosome sequences. Figure S6. Comparative maps of cp9 plasmids in Borreliella isolates. Figure S7. Orphan cp32-like contigs in the B. spielmanii A14S genome. Figure S8. Rearrangements in cp32-like plasmids in NBu-Borreliella genomes. Figure S9. B. bissettiae DN127 66 kbp circular plasmid cp32-quad. Figure S10. B. finlandensis SV1 integration of cp32 into lp54. Figure S11. Borreliella and relapsing fever Borrelia PFam32 neighbor-joining tree. Table S1. Borreliella and relapsing fever Borrelia PFam32. (PDF 6030 kb)
Acknowledgements
The authors wish to thank S. Bergstrom, I. Livey, R. Marconi, P. Rosa and I. Schwartz, for originally providing the bacterial isolates whose genome sequences we determined and whose plasmids we analyze in this report. We also thank Sébastien Bontemps-Gallo for kind permission to include unpublished strain 20047 and BO23 nucleotide sequences in our analysis.
Funding
This work was supported by grants AI49003 and GM114817 (SRC), AI37256 (BJL), AI07955 (WGQ), N01-AI30071 (CMF with co-investigators SRC, SES, EFM, BJL, WGQ), and MD007599 (Hunter College) from the National Institutes of Health, by a Tami Fund award, and by a Steven and Alexandra Cohen Foundation award.
Availability of data and materials
The plasmid sequences that we determined have been reported and deposited in the GenBank database [30, 49–53]. They are also available through an online browser of Borreliella and Borrelia genomes (http://BorreliaBase.org) [61]). NEXUS files containing sequence alignments and trees for Figs. 2, 5, and 6 have been deposited to TreeBase (Study ID S22200).
Abbreviations
- ORF
open reading frame
- PFam
Borreliella paralogous protein family
- SNP
single nucleotide polymorphism
Authors’ contributions
Conceived and designed the experiments: SRC, WGQ, EFM, BJL, SES and CMF. Performed the study and analyzed the data: SRC, WGQ, LD, and SA and EFM. Wrote the paper: SRC and WGQ. All authors approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Footnotes
Electronic supplementary material
The online version of this article (10.1186/s12864-018-4597-x) contains supplementary material, which is available to authorized users.
Contributor Information
Sherwood R. Casjens, Phone: 801-581-5980, Email: sherwood.casjens@path.utah.edu
Lia Di, Email: dilie66@gmail.com.
Saymon Akther, Email: saymon.akther@gmail.com.
Emmanuel F. Mongodin, Email: emongodin@som.umaryland.edu
Benjamin J. Luft, Email: Benjamin.Luft@stonybrookmedicine.edu
Steven E. Schutzer, Email: schutzer@gmail.com
Claire M. Fraser, Email: cmfraser@som.umaryland.edu
Wei-Gang Qiu, Phone: 212-896-0445, Email: weigang@genectr.hunter.cuny.edu.
References
- 1.Mongodin EF, Casjens SR, Bruno JF, Xu Y, Drabek EF, Riley DR, Cantarel BL, Pagan PE, Hernandez YA, Vargas LC, et al. Inter- and intra-specific pan-genomes of Borrelia burgdorferi sensu lato: genome stability and adaptive radiation. BMC Genomics. 2013;14:693. doi: 10.1186/1471-2164-14-693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gupta RS, Mahmood S, Adeolu M. A phylogenomic and molecular signature based approach for characterization of the phylum Spirochaetes and its major clades: proposal for a taxonomic revision of the phylum. Front Microbiol. 2013;4:217. doi: 10.3389/fmicb.2013.00217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adeolu M, Gupta RS. A phylogenomic and molecular marker based proposal for the division of the genus Borrelia into two genera: the emended genus Borrelia containing only the members of the relapsing fever Borrelia, and the genus Borreliella gen. nov. containing the members of the Lyme disease Borrelia (Borrelia burgdorferi sensu lato complex) Antonie Van Leeuwenhoek. 2014;105:1049–1072. doi: 10.1007/s10482-014-0164-x. [DOI] [PubMed] [Google Scholar]
- 4.Barbour AG, Adeolu M, Gupta RS. Division of the genus Borrelia into two genera (corresponding to Lyme disease and relapsing fever groups) reflects their genetic and phenotypic distinctiveness and will lead to a better understanding of these two groups of microbes. Int J Syst Evol Microbiol. 2017;67:2058–2067. doi: 10.1099/ijsem.0.001815. [DOI] [PubMed] [Google Scholar]
- 5.Margos G, Marosevic D, Cutler S, Derdakova M, Diuk-Wasser M, Emler S, Fish D, Gray J, Hunfeldt KP, Jaulhac B, et al. There is inadequate evidence to support the division of the genus Borrelia. Int J Syst Evol Microbiol. 2017;67:1081–1084. doi: 10.1099/ijsem.0.001717. [DOI] [PubMed] [Google Scholar]
- 6.Ivanova LB, Tomova A, Gonzalez-Acuna D, Murua R, Moreno CX, Hernandez C, Cabello J, Cabello C, Daniels TJ, Godfrey HP, et al. Borrelia chilensis, a new member of the Borrelia burgdorferi sensu lato complex that extends the range of this genospecies in the Southern Hemisphere. Environ Microbiol. 2014;16:1069–1080. doi: 10.1111/1462-2920.12310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Piesman J, Clark KL, Dolan MC, Happ CM, Burkot TR. Geographic survey of vector ticks (Ixodes scapularis and Ixodes pacificus) for infection with the Lyme disease spirochete, Borrelia burgdorferi. J Vector Ecol. 1999;24:91–98. [PubMed] [Google Scholar]
- 8.Piesman J, Gern L. Lyme borreliosis in Europe and North America. Parasitology. 2004;129(Supplement):S191–S220. doi: 10.1017/s0031182003004694. [DOI] [PubMed] [Google Scholar]
- 9.Burgdorfer W, Barbour AG, Hayes SF, Benach JL, Grunwaldt E, Davis JP. Lyme disease-a tick-borne spirochetosis? Science. 1982;216:1317–1319. doi: 10.1126/science.7043737. [DOI] [PubMed] [Google Scholar]
- 10.Assous MV, Postic D, Paul G, Nevot P, Baranton G. Western blot analysis of sera from Lyme borreliosis patients according to the genomic species of the Borrelia strains used as antigens. Eur J Clin Microbiol Infect Dis. 1993;12:261–268. doi: 10.1007/BF01967256. [DOI] [PubMed] [Google Scholar]
- 11.Collares-Pereira M, Couceiro S, Franca I, Kurtenbach K, Schafer SM, Vitorino L, Goncalves L, Baptista S, Vieira ML, Cunha C. First isolation of Borrelia lusitaniae from a human patient. J Clin Microbiol. 2004;42:1316–1318. doi: 10.1128/JCM.42.3.1316-1318.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Carvalho IL, Fonseca JE, Marques JG, Ullmann A, Hojgaard A, Zeidner N, Nuncio MS. Vasculitis-like syndrome associated with Borrelia lusitaniae infection. Clin Rheumatol. 2008;27:1587–1591. doi: 10.1007/s10067-008-1012-z. [DOI] [PubMed] [Google Scholar]
- 13.Diza E, Papa A, Vezyri E, Tsounis S, Milonas I, Antoniadis A. Borrelia valaisiana in cerebrospinal fluid. Emerg Infect Dis. 2004;10:1692–1693. doi: 10.3201/eid1009.030439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fingerle V, Schulte-Spechtel UC, Ruzic-Sabljic E, Leonhard S, Hofmann H, Weber K, Pfister K, Strle F, Wilske B. Epidemiological aspects and molecular characterization of Borrelia burgdorferi s.L. from southern Germany with special respect to the new species Borrelia spielmanii sp. nov. Int J Med Microbiol. 2007;298:279–290. doi: 10.1016/j.ijmm.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 15.Kingry LC, Batra D, Replogle A, Rowe LA, Pritt BS, Petersen JM. Whole genome sequence and comparative genomics of the novel Lyme Borreliosis causing pathogen, Borrelia mayonii. PLoS One. 2016;11:e0168994. doi: 10.1371/journal.pone.0168994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rijpkema SG, Tazelaar DJ, Molkenboer MJ, Noordhoek GT, Plantinga G, Schouls LM, Schellekens JF. Detection of Borrelia afzelii, Borrelia burgdorferi sensu stricto, Borrelia garinii and group VS116 by PCR in skin biopsies of patients with erythema migrans and acrodermatitis chronica atrophicans. Clin Microbiol Infect. 1997;3:109–116. doi: 10.1111/j.1469-0691.1997.tb00259.x. [DOI] [PubMed] [Google Scholar]
- 17.Rudenko N, Golovchenko M, Lin T, Gao L, Grubhoffer L, Oliver JH., Jr Delineation of a new species of the Borrelia burgdorferi Sensu Lato complex, Borrelia americana sp. nov. J Clin Microbiol. 2009;47:3875–3880. doi: 10.1128/JCM.01050-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Margos G, Sing A, Fingerle V. Published data do not support the notion that Borrelia valaisiana is human pathogenic. Infection. 2017;45:567–569. doi: 10.1007/s15010-017-1032-1. [DOI] [PubMed] [Google Scholar]
- 19.Casjens SR, Mongodin EF, Qiu WG, Luft BJ, Schutzer SE, Gilcrease EB, Huang WM, Vujadinovic M, Aron JK, Vargas LC, et al. Genome stability of Lyme disease spirochetes: comparative genomics of Borrelia burgdorferi plasmids. PLoS One. 2012;7:e33280. doi: 10.1371/journal.pone.0033280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Casjens SR, Gilcrease EB, Vujadinovic M, Mongodin EF, Luft BJ, Schutzer SE, Fraser CM, Qiu WG. Plasmid diversity and phylogenetic consistency in the Lyme disease agent Borrelia burgdorferi. BMC Genomics. 2017;18:165. doi: 10.1186/s12864-017-3553-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schuler W, Bunikis I, Weber-Lehman J, Comstedt P, Kutschan-Bunikis S, Stanek G, Huber J, Meinke A, Bergstrom S, Lundberg U. Complete genome sequence of Borrelia afzelii K78 and comparative genome analysis. PLoS One. 2015;10:e0120548. doi: 10.1371/journal.pone.0120548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gern L, Estrada-Pena A, Frandsen F, Gray JS, Jaenson TG, Jongejan F, Kahl O, Korenberg E, Mehl R, Nuttall PA. European reservoir hosts of Borrelia burgdorferi sensu lato. Zentralbl Bakteriol. 1998;287:196–204. doi: 10.1016/s0934-8840(98)80121-7. [DOI] [PubMed] [Google Scholar]
- 23.Norte AC, Alves da Silva A, Alves J, da Silva LP, Nuncio MS, Escudero R, Anda P, Ramos JA, Lopes de Carvalho I. The importance of lizards and small mammals as reservoirs for Borrelia lusitaniae in Portugal. Environ Microbiol Rep. 2015;7:188–193. doi: 10.1111/1758-2229.12218. [DOI] [PubMed] [Google Scholar]
- 24.Steere AC, Strle F, Wormser GP, Hu LT, Branda JA, Hovius JW, Li X, Mead PS. Lyme borreliosis. Nat Rev Dis Primers. 2016;2:16090. doi: 10.1038/nrdp.2016.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ruzic-Sabljic E, Cerar T. Progress in the molecular diagnosis of Lyme disease. Expert Rev Mol Diagn. 2017;17:19–30. doi: 10.1080/14737159.2016.1246959. [DOI] [PubMed] [Google Scholar]
- 26.Smit R, Postma MJ. Lyme borreliosis: reviewing potential vaccines, clinical aspects and health economics. Expert Rev Vaccines. 2015;14:1549–1561. doi: 10.1586/14760584.2015.1091313. [DOI] [PubMed] [Google Scholar]
- 27.Cerar T, Strle F, Stupica D, Ruzic-Sabljic E, McHugh G, Steere AC, Strle K. Differences in genotype, clinical features, and inflammatory potential of Borrelia burgdorferi sensu stricto strains from Europe and the United States. Emerg Infect Dis. 2016;22:818–827. doi: 10.3201/eid2205.151806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barbour AG. Plasmid analysis of Borrelia burgdorferi, the Lyme disease agent. J Clin Microbiol. 1988;26:475–478. doi: 10.1128/jcm.26.3.475-478.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Casjens S, Delange M, Ley HL, Rosa P, Huang WM. Linear chromosomes of Lyme disease agent spirochetes: genetic diversity and conservation of gene order. J Bacteriol. 1995;177:2769–2780. doi: 10.1128/jb.177.10.2769-2780.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Casjens S, Palmer N, van Vugt R, Huang WM, Stevenson B, Rosa P, Lathigra R, Sutton G, Peterson J, Dodson RJ, et al. A bacterial genome in flux: the twelve linear and nine circular extrachromosomal DNAs in an infectious isolate of the Lyme disease spirochete Borrelia burgdorferi. Mol Microbiol. 2000;35:490–516. doi: 10.1046/j.1365-2958.2000.01698.x. [DOI] [PubMed] [Google Scholar]
- 31.Miller J, Bono J, Babb K, El-Hage N, Casjens S, Stevenson B. A second allele of eppA in Borrelia burgdorferi strain B31 is located on the previously undetected circular plasmid cp9-2. J Bacteriol. 2000;182:6254–6258. doi: 10.1128/jb.182.21.6254-6258.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Caine JA, Coburn J. Multifunctional and redundant roles of Borrelia burgdorferi outer surface proteins in tissue adhesion, colonization, and complement evasion. Front Immunol. 2016;7:442. doi: 10.3389/fimmu.2016.00442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kenedy MR, Scott EJ, 2nd, Shrestha B, Anand A, Iqbal H, Radolf JD, Dyer DW, Akins DR. Consensus computational network analysis for identifying candidate outer membrane proteins from Borrelia spirochetes. BMC Microbiol. 2016;16:141. doi: 10.1186/s12866-016-0762-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dowdell AS, Murphy MD, Azodi C, Swanson SK, Florens L, Chen S, Zuckert WR. Comprehensive spatial analysis of the Borrelia burgdorferi Lipoproteome reveals a compartmentalization bias toward the bacterial surface. J Bacteriol. 2017;199:e00658–e00616. doi: 10.1128/JB.00658-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jewett MW, Lawrence K, Bestor AC, Tilly K, Grimm D, Shaw P, VanRaden M, Gherardini F, Rosa PA. The critical role of the linear plasmid lp36 in the infectious cycle of Borrelia burgdorferi. Mol Microbiol. 2007;64:1358–1374. doi: 10.1111/j.1365-2958.2007.05746.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Botkin DJ, Abbott AN, Stewart PE, Rosa PA, Kawabata H, Watanabe H, Norris SJ. Identification of potential virulence determinants by Himar1 transposition of infectious Borrelia burgdorferi B31. Infect Immun. 2006;74:6690–6699. doi: 10.1128/IAI.00993-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Embers ME, Alvarez X, Ooms T, Philipp MT. Immune evasion failure by linear plasmid 28-1-deficient Borrelia burgdorferi is attributable to persistent expression of an outer surface protein. Infect Immun. 2008; [DOI] [PMC free article] [PubMed]
- 38.Grimm D, Eggers CH, Caimano MJ, Tilly K, Stewart PE, Elias AF, Radolf JD, Rosa PA. Experimental assessment of the roles of linear plasmids lp25 and lp28-1 of Borrelia burgdorferi throughout the infectious cycle. Infect Immun. 2004;72:5938–5946. doi: 10.1128/IAI.72.10.5938-5946.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grimm D, Tilly K, Bueschel DM, Fisher MA, Policastro PF, Gherardini FC, Schwan TG, Rosa PA. Defining plasmids required by Borrelia burgdorferi for colonization of tick vector Ixodes scapularis (Acari: Ixodidae) J Med Entomol. 2005;42:676–684. doi: 10.1093/jmedent/42.4.676. [DOI] [PubMed] [Google Scholar]
- 40.Labandeira-Rey M, Seshu J, Skare JT. The absence of linear plasmid 25 or 28-1 of Borrelia burgdorferi dramatically alters the kinetics of experimental infection via distinct mechanisms. Infect Immun. 2003;71:4608–4613. doi: 10.1128/IAI.71.8.4608-4613.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Purser JE, Norris SJ. Correlation between plasmid content and infectivity in Borrelia burgdorferi. Proc Natl Acad Sci U S A. 2000;97:13865–13870. doi: 10.1073/pnas.97.25.13865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Strother KO, de Silva A. Role of Borrelia burgdorferi linear plasmid 25 in infection of Ixodes scapularis ticks. J Bacteriol. 2005;187:5776–5781. doi: 10.1128/JB.187.16.5776-5781.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Strother KO, Broadwater A, De Silva A. Plasmid requirements for infection of ticks by Borrelia burgdorferi. Vector Borne Zoonotic Dis. 2005;5:237–245. doi: 10.1089/vbz.2005.5.237. [DOI] [PubMed] [Google Scholar]
- 44.Dulebohn DP, Bestor A, Rosa PA. Borrelia burgdorferi linear plasmid 28-3 confers a selective advantage in an experimental mouse-tick infection model. Infect Immun. 2013;81:2986–2996. doi: 10.1128/IAI.00219-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ellis TC, Jain S, Linowski AK, Rike K, Bestor A, Rosa PA, Halpern M, Kurhanewicz S, Jewett MW. In vivo expression technology identifies a novel virulence factor critical for Borrelia burgdorferi persistence in mice. PLoS Pathog. 2013;9:e1003567. doi: 10.1371/journal.ppat.1003567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hayes BM, Dulebohn DP, Sarkar A, Tilly K, Bestor A, Ambroggio X, Rosa PA. Regulatory protein BBD18 of the Lyme disease spirochete: essential role during tick acquisition? MBio. 2014;5:e01017–e01014. doi: 10.1128/mBio.01017-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Krupna-Gaylord MA, Liveris D, Love AC, Wormser GP, Schwartz I, Petzke MM. Induction of type I and type III interferons by Borrelia burgdorferi correlates with pathogenesis and requires linear plasmid 36. PLoS One. 2014;9:e100174. doi: 10.1371/journal.pone.0100174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lin T, Gao L, Zhang C, Odeh E, Jacobs MB, Coutte L, Chaconas G, Philipp MT, Norris SJ. Analysis of an ordered, comprehensive STM mutant library in infectious Borrelia burgdorferi: insights into the genes required for mouse infectivity. PLoS One. 2012;7:e47532. doi: 10.1371/journal.pone.0047532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Casjens SR, Fraser-Liggett CM, Mongodin EF, Qiu WG, Dunn JJ, Luft BJ, Schutzer SE. Whole genome sequence of an unusual Borrelia burgdorferi sensu lato isolate. J Bacteriol. 2011;193:1489–1490. doi: 10.1128/JB.01521-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Casjens SR, Mongodin EF, Qiu WG, Dunn JJ, Luft BJ, Fraser-Liggett CM, Schutzer SE. Whole-genome sequences of two Borrelia afzelii and two Borrelia garinii Lyme disease agent isolates. J Bacteriol. 2011;193:6995–6996. doi: 10.1128/JB.05951-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fraser CM, Casjens S, Huang WM, Sutton GG, Clayton R, Lathigra R, White O, Ketchum KA, Dodson R, Hickey EK, et al. Genomic sequence of a Lyme disease spirochaete, Borrelia burgdorferi. Nature. 1997;390:580–586. doi: 10.1038/37551. [DOI] [PubMed] [Google Scholar]
- 52.Schutzer SE, Fraser-Liggett CM, Casjens SR, Qiu WG, Dunn JJ, Mongodin EF, Luft BJ. Whole genome sequences of thirteen isolates of Borrelia burgdorferi. J Bacteriol. 2011;193:1018–1020. doi: 10.1128/JB.01158-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schutzer S, Fraser-Liggett C, Qiu W, Kraiczy P, Mongodin E, Dunn J, Luft B, Casjens S. Whole genome sequences of Borrelia bissettii, Borrelia valaisiana and Borrelia spielmanii. J Bacteriol. 2012;194:545–546. doi: 10.1128/JB.06263-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Casjens S, van Vugt R, Tilly K, Rosa PA, Stevenson B. Homology throughout the multiple 32-kilobase circular plasmids present in Lyme disease spirochetes. J Bacteriol. 1997;179:217–227. doi: 10.1128/jb.179.1.217-227.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Margos G, Hepner S, Mang C, Marosevic D, Reynolds SE, Krebs S, Sing A, Derdakova M, Reiter MA, Fingerle V. Lost in plasmids: next generation sequencing and the complex genome of the tick-borne pathogen Borrelia burgdorferi. BMC Genomics. 2017;18:422. doi: 10.1186/s12864-017-3804-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dunn JJ, Buchstein SR, Butler LL, Fisenne S, Polin DS, Lade BN, Luft BJ. Complete nucleotide sequence of a circular plasmid from the Lyme disease spirochete, Borrelia burgdorferi. J Bacteriol. 1994;176:2706–2717. doi: 10.1128/jb.176.9.2706-2717.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Glöckner G, Lehmann R, Romualdi A, Pradella S, Schulte-Spechtel U, Schilhabel M, Wilske B, Suhnel J, Platzer M. Comparative analysis of the Borrelia garinii genome. Nucleic Acids Res. 2004;32:6038–6046. doi: 10.1093/nar/gkh953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Brenner EV, Kurilshikov AM, Stronin OV, Fomenko NV. Whole-genome sequencing of Borrelia garinii BgVir, isolated from taiga ticks (Ixodes persulcatus) J Bacteriol. 2012;194:5713. doi: 10.1128/JB.01360-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kurilshikov AM, Fomenko NV, Stronin OV, Tikunov AY, Kabilov MR, Tupikin AE, Tikunova NV. Complete genome sequencing of Borrelia valaisiana and Borrelia afzelii isolated from Ixodes persulcatus ticks in western Siberia. Genome Announc. 2014;2:e01315–e01314. doi: 10.1128/genomeA.01315-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Huang W, Ojaimi C, Fallon JT, Travisany D, Maass A, Ivanova L, Tomova A, Gonzalez-Acuna D, Godfrey HP, Cabello FC. Genome sequence of Borrelia chilensis VA1, a south American member of the Lyme Borreliosis group. Genome Announc. 2015;3:e01535–e01514. doi: 10.1128/genomeA.01535-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Di L, Pagan PE, Packer D, Martin CL, Akther S, Ramrattan G, Mongodin EF, Fraser CM, Schutzer SE, Luft BJ, et al. BorreliaBase: a phylogeny-centered browser of Borrelia genomes. BMC Bioinformatics. 2014;15:233. doi: 10.1186/1471-2105-15-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tilly K, Checroun C, Rosa PA. Requirements for Borrelia burgdorferi plasmid maintenance. Plasmid. 2012;68:1–12. doi: 10.1016/j.plasmid.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chaconas G, Norris SJ. Peaceful coexistence amongst Borrelia plasmids: getting by with a little help from their friends? Plasmid. 2013;70:161–167. doi: 10.1016/j.plasmid.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Casjens S, Eggers CH, Schwartz I. Borrelia genomics: chromsosome, plasmids, bacteriophges and genetic variation. In: Samuels DS, Radolf J, editors. Borrelia molecular biology, host interaction and pathogenicity. Norfolk: Caister Academic Press; 2010. pp. 27–53. [Google Scholar]
- 65.Hyde FW, Johnson RC. Characterization of a circular plasmid from Borrelia burgdorferi, etiologic agent of Lyme disease. J Clin Microbiol. 1988;26:2203–2205. doi: 10.1128/jcm.26.10.2203-2205.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schwan TG, Burgdorfer W, Garon CF. Changes in infectivity and plasmid profile of the Lyme disease spirochete, Borrelia burgdorferi, as a result of in vitro cultivation. Infect Immun. 1988;56:1831–1836. doi: 10.1128/iai.56.8.1831-1836.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Xu Y, Kodner C, Coleman L, Johnson RC. Correlation of plasmids with infectivity of Borrelia burgdorferi sensu stricto type strain B31. Infect Immun. 1996;64:3870–3876. doi: 10.1128/iai.64.9.3870-3876.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Norris SJ, Howell JK, Odeh EA, Lin T, Gao L, Edmondson DG. High-throughput plasmid content analysis of Borrelia burgdorferi B31 by using Luminex multiplex technology. Appl Environ Microbiol. 2011;77:1483–1492. doi: 10.1128/AEM.01877-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang D, Botkin DJ, Norris SJ. Characterization of the vls antigenic variation loci of the Lyme disease spirochaetes Borrelia garinii Ip90 and Borrelia afzelii ACAI. Mol Microbiol. 2003;47:1407–1417. doi: 10.1046/j.1365-2958.2003.03386.x. [DOI] [PubMed] [Google Scholar]
- 70.Casjens S. Borrelia genomes in the year 2000. J Mol Microbiol Biotechnol. 2000;2:401–410. [PubMed] [Google Scholar]
- 71.Casjens SR, Huang WM, Gilcrease EB, Qiu WG, McCaig WD, Luft BJ, Schutzer SE. Comparative genomics of Borrelia burgdorferi. In: Cabello FC, Hulinska D, Godfrey HP, editors. Molecular biology of spirochetes. Amsterdam: IOS Press; 2006. pp. 79–95. [Google Scholar]
- 72.Margos G, Lane RS, Fedorova N, Koloczek J, Piesman J, Hojgaard A, Sing A, Fingerle V. Borrelia bissettiae sp. nov. and Borrelia californiensis sp. nov. prevail in diverse enzootic transmission cycles. Int J Syst Evol Microbiol. 2016;66:1447–1452. doi: 10.1099/ijsem.0.000897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Purser JE, Lawrenz MB, Caimano MJ, Howell JK, Radolf JD, Norris SJ. A plasmid-encoded nicotinamidase (PncA) is essential for infectivity of Borrelia burgdorferi in a mammalian host. Mol Microbiol. 2003;48:753–764. doi: 10.1046/j.1365-2958.2003.03452.x. [DOI] [PubMed] [Google Scholar]
- 74.Jewett MW, Jain S, Linowski AK, Sarkar A, Rosa PA. Molecular characterization of the Borrelia burgdorferi in vivo-essential protein PncA. Microbiology. 2011;157:2831–2840. doi: 10.1099/mic.0.051706-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Revel AT, Blevins JS, Almazan C, Neil L, Kocan KM, de la Fuente J, Hagman KE, Norgard MV. bptA (bbe16) is essential for the persistence of the Lyme disease spirochete, Borrelia burgdorferi, in its natural tick vector. Proc Natl Acad Sci U S A. 2005;102:6972–6977. doi: 10.1073/pnas.0502565102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Eggers CH, Casjens S, Hayes SF, Garon CF, Damman CJ, Oliver DB, Samuels DS. Bacteriophages of spirochetes. J Mol Microbiol Biotechnol. 2000;2:365–373. [PubMed] [Google Scholar]
- 77.Eggers CH, Casjens S, Samuels DS. Bacteriophages of Borrelia burgdorferi and other spirochetes. In: Saier M, Garcia-Lara J, editors. The spirochetes Molecilar and celullar biology. Wiltshire: Hozison Scientific Press; 2001. pp. 35–44. [Google Scholar]
- 78.Molloy EM, Casjens SR, Cox CL, Maxson T, Ethridge NA, Margos G, Fingerle V, Mitchell DA. Identification of the minimal cytolytic unit for streptolysin S and an expansion of the toxin family. BMC Microbiol. 2015;15:141. doi: 10.1186/s12866-015-0464-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lam TT, Nguyen TP, Montgomery RR, Kantor FS, Fikrig E, Flavell RA. Outer surface proteins E and F of Borrelia burgdorferi, the agent of Lyme disease. Infect Immun. 1994;62:290–298. doi: 10.1128/iai.62.1.290-298.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Marconi RT, Sung SY, Hughes CA, Carlyon JA. Molecular and evolutionary analyses of a variable series of genes in Borrelia burgdorferi that are related to ospE and ospF, constitute a gene family, and share a common upstream homology box. J Bacteriol. 1996;178:5615–5626. doi: 10.1128/jb.178.19.5615-5626.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Brissette CA, Haupt K, Barthel D, Cooley AE, Bowman A, Skerka C, Wallich R, Zipfel PF, Kraiczy P, Stevenson B. Borrelia burgdorferi infection-associated surface proteins ErpP, ErpA, and ErpC bind human plasminogen. Infect Immun. 2009;77:300–306. doi: 10.1128/IAI.01133-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Stevenson B, Miller JC. Intra- and interbacterial genetic exchange of Lyme disease spirochete erp genes generates sequence identity amidst diversity. J Mol Evol. 2003;57:309–324. doi: 10.1007/s00239-003-2482-x. [DOI] [PubMed] [Google Scholar]
- 83.Stevenson B, Bykowski T, Cooley AE, Babb K, Miller JC, Woodman ME, von Lackum K, Riley SP. The Lyme disease spirochete Erp protein family: structure, function and regulation of expression. In: Cabello FC, Hulinska D, Godfrey HP, editors. Molecular biology of spirochetes. Amsterdam: IOS Press; 2006. pp. 354–372. [Google Scholar]
- 84.Brisson D, Zhou W, Jutras BL, Casjens S, Stevenson B. Distribution of cp32 prophages among Lyme disease-Ccausing spirochetes and natural diversity of their lipoprotein-eEncoding erp loci. Appl Environ Microbiol. 2013;79:4115–4128. doi: 10.1128/AEM.00817-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Norris SJ, Carter CJ, Howell JK, Barbour AG. Low-passage-associated proteins of Borrelia burgdorferi B31: characterization and molecular cloning of OspD, a surface-exposed, plasmid-encoded lipoprotein. Infect Immun. 1992;60:4662–4672. doi: 10.1128/iai.60.11.4662-4672.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kraiczy P, Seling A, Brissette CA, Rossmann E, Hunfeld KP, Bykowski T, Burns LH, Troese MJ, Cooley AE, Miller JC, et al. Borrelia burgdorferi complement regulator-acquiring surface protein 2 (CspZ) as a serological marker of human Lyme disease. Clin Vaccine Immunol. 2008;15:484–491. doi: 10.1128/CVI.00415-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Samuels D, Marconi R, Garon C. Variation in the size of the ospA-containing linear plasmid, but not the linear chromosome, among the three Borrelia species associated with Lyme disease. J Gen Microbiol. 1993;139(Pt 10):2445–2449. doi: 10.1099/00221287-139-10-2445. [DOI] [PubMed] [Google Scholar]
- 88.Pinne M, Ostberg Y, Comstedt P, Bergstrom S. Molecular analysis of the channel-forming protein P13 and its paralogue family 48 from different Lyme disease Borrelia species. Microbiology. 2004;150:549–559. doi: 10.1099/mic.0.26728-0. [DOI] [PubMed] [Google Scholar]
- 89.Pinne M, Denker K, Nilsson E, Benz R, Bergstrom S. The BBA01 protein, a member of paralog family 48 from Borrelia burgdorferi, is potentially interchangeable with the channel-forming protein P13. J Bacteriol. 2006;188:4207–4217. doi: 10.1128/JB.00302-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tourand Y, Deneke J, Moriarty TJ, Chaconas G. Characterization and in vitro reaction properties of 19 unique hairpin telomeres from the linear plasmids of the Lyme disease spirochete. J Biol Chem. 2009;284:7264–7272. doi: 10.1074/jbc.M808918200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yabuki M, Nakao M, Fukunaga M. Genetic diversity and the absence of regional differences of Borrelia garinii as demonstrated by ospA and ospB gene sequence analysis. Microbiol Immunol. 1999;43:1097–1102. doi: 10.1111/j.1348-0421.1999.tb03366.x. [DOI] [PubMed] [Google Scholar]
- 92.Wallich R, Pattathu J, Kitiratschky V, Brenner C, Zipfel PF, Brade V, Simon MM, Kraiczy P. Identification and functional characterization of complement regulator-acquiring surface protein 1 of the Lyme disease spirochetes Borrelia afzelii and Borrelia garinii. Infect Immun. 2005;73:2351–2359. doi: 10.1128/IAI.73.4.2351-2359.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hallstrom T, Siegel C, Morgelin M, Kraiczy P, Skerka C, Zipfel PF. CspA from Borrelia burgdorferi inhibits the terminal complement pathway. MBio. 2013;4 [DOI] [PMC free article] [PubMed]
- 94.Hammerschmidt C, Klevenhaus Y, Koenigs A, Hallstrom T, Fingerle V, Skerka C, Pos KM, Zipfel PF, Wallich R, Kraiczy P. BGA66 and BGA71 facilitate complement resistance of Borrelia bavariensis by inhibiting assembly of the membrane attack complex. Mol Microbiol. 2016;99:407–424. doi: 10.1111/mmi.13239. [DOI] [PubMed] [Google Scholar]
- 95.Koenigs A, Hammerschmidt C, Jutras BL, Pogoryelov D, Barthel D, Skerka C, Kugelstadt D, Wallich R, Stevenson B, Zipfel PF, et al. BBA70 of Borrelia burgdorferi is a novel plasminogen-binding protein. J Biol Chem. 2013;288:25229–25243. doi: 10.1074/jbc.M112.413872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wywial E, Haven J, Casjens SR, Hernandez YA, Singh S, Mongodin EF, Fraser-Liggett CM, Luft BJ, Schutzer SE, Qiu WG. Fast, adaptive evolution at a bacterial host-resistance locus: the PFam54 gene array in Borrelia burgdorferi. Gene. 2009;445:26–37. doi: 10.1016/j.gene.2009.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Qiu WG, Martin CL. Evolutionary genomics of Borrelia burgdorferi sensu lato: findings, hypotheses, and the rise of hybrids. Infect Genet Evol. 2014;27:576–593. doi: 10.1016/j.meegid.2014.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ojaimi C, Davidson BE, Saint Girons I, Old IG. Conservation of gene arrangement and an unusual organization of rRNA genes in the linear chromosomes of the Lyme disease spirochaetes Borrelia burgdorferi, B. garinii and B. afzelii. Microbiology. 1994;140:2931–2940. doi: 10.1099/13500872-140-11-2931. [DOI] [PubMed] [Google Scholar]
- 99.Casjens S, Murphy M, DeLange M, Sampson L, van Vugt R, Huang WM. Telomeres of the linear chromosomes of Lyme disease spirochaetes: nucleotide sequence and possible exchange with linear plasmid telomeres. Mol Microbiol. 1997;26:581–596. doi: 10.1046/j.1365-2958.1997.6051963.x. [DOI] [PubMed] [Google Scholar]
- 100.Huang WM, Robertson M, Aron J, Casjens S. Telomere exchange between linear replicons of Borrelia burgdorferi. J Bacteriol. 2004;186:4134–4141. doi: 10.1128/JB.186.13.4134-4141.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Champion CI, Blanco DR, Skare JT, Haake DA, Giladi M, Foley D, Miller JN, Lovett MA. A 9.0-kilobase-pair circular plasmid of Borrelia burgdorferi encodes an exported protein: evidence for expression only during infection. Infect Immun. 1994;62:2653–2661. doi: 10.1128/iai.62.7.2653-2661.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Jewett MW, Byram R, Bestor A, Tilly K, Lawrence K, Burtnick MN, Gherardini F, Rosa PA. Genetic basis for retention of a critical virulence plasmid of Borrelia burgdorferi. Mol Microbiol. 2007;66:975–990. doi: 10.1111/j.1365-2958.2007.05969.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Tilly K, Casjens S, Stevenson B, Bono JL, Samuels DS, Hogan D, Rosa P. The Borrelia burgdorferi circular plasmid cp26: conservation of plasmid structure and targeted inactivation of the ospC gene. Mol Microbiol. 1997;25:361–373. doi: 10.1046/j.1365-2958.1997.4711838.x. [DOI] [PubMed] [Google Scholar]
- 104.Haven J, Vargas LC, Mongodin EF, Xue V, Hernandez Y, Pagan P, Fraser-Liggett CM, Schutzer SE, Luft BJ, Casjens SR, et al. Pervasive recombination and sympatric genome diversification driven by frequency-dependent selection in Borrelia burgdorferi, the Lyme disease bacterium. Genetics. 2011;189:951–966. doi: 10.1534/genetics.111.130773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Qiu WG, Bosler EM, Campbell JR, Ugine GD, Wang IN, Luft BJ, Dykhuizen DE. A population genetic study of Borrelia burgdorferi sensu stricto from eastern Long Island, New York, suggested frequency-dependent selection, gene flow and host adaptation. Hereditas. 1997;127:203–216. doi: 10.1111/j.1601-5223.1997.00203.x. [DOI] [PubMed] [Google Scholar]
- 106.Barbour AG, Travinsky B. Evolution and distribution of the ospC gene, a transferable serotype determinant of Borrelia burgdorferi. MBio. 2010;1:e00153–e00110. doi: 10.1128/mBio.00153-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Brisson D, Vandermause MF, Meece JK, Reed KD, Dykhuizen DE. Evolution of northeastern and midwestern Borrelia burgdorferi, United States. Emerg Infect Dis. 2010;16:911–917. doi: 10.3201/eid1606.090329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Livey I, Gibbs CP, Schuster R, Dorner F. Evidence for lateral transfer and recombination in OspC variation in Lyme disease Borrelia. Mol Microbiol. 1995;18:257–269. doi: 10.1111/j.1365-2958.1995.mmi_18020257.x. [DOI] [PubMed] [Google Scholar]
- 109.Wang I, Dykhuizen D, Qiu W, Dunn J, Bosler E, Luft B. Genetic diversity of ospC in a local population of Borrelia burgdorferi sensu stricto. Genetics. 1999;151:15–30. doi: 10.1093/genetics/151.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Eggers CH, Gray CM, Preisig AM, Glenn DM, Pereira J, Ayers RW, Alshahrani M, Acabbo C, Becker MR, Bruenn KN, et al. Phage-mediated horizontal gene transfer of both prophage and heterologous DNA by varphiBB-1, a bacteriophage of Borrelia burgdorferi. Pathog Dis. 2016;74 [DOI] [PubMed]
- 111.Zhang H, Marconi RT. Demonstration of cotranscription and 1-methyl-3-nitroso-nitroguanidine induction of a 30-gene operon of Borrelia burgdorferi: evidence that the 32-kilobase circular plasmids are prophages. J Bacteriol. 2005;187:7985–7995. doi: 10.1128/JB.187.23.7985-7995.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Margos G, Wilske B, Sing A, Hizo-Teufel C, Cao WC, Chu C, Scholz H, Straubinger RK, Fingerle V. Borrelia bavariensis sp. nov. is widely distributed in Europe and Asia. Int J Syst Evol Microbiol. 2013;63:4284–4288. doi: 10.1099/ijs.0.052001-0. [DOI] [PubMed] [Google Scholar]
- 113.Gatzmann F, Metzler D, Krebs S, Blum H, Sing A, Takano A, Kawabata H, Fingerle V, Margos G, Becker NS. NGS population genetics analyses reveal divergent evolution of a Lyme Borreliosis agent in Europe and Asia. Ticks Tick Borne Dis. 2015;6:344–351. doi: 10.1016/j.ttbdis.2015.02.008. [DOI] [PubMed] [Google Scholar]
- 114.Palmer N, Fraser C, Casjens S. Distribution of twelve linear extrachromosomal DNAs in natural isolates of the Lyme disease spirochetes. J Bacteriol. 2000;182:2476–2480. doi: 10.1128/jb.182.9.2476-2480.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Castillo-Ramirez S, Fingerle V, Jungnick S, Straubinger RK, Krebs S, Blum H, Meinel DM, Hofmann H, Guertler P, Sing A, et al. Trans-Atlantic exchanges have shaped the population structure of the Lyme disease agent Borrelia burgdorferi sensu stricto. Sci Rep. 2016;6:22794. doi: 10.1038/srep22794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Miller SC, Porcella SF, Raffel SJ, Schwan TG, Barbour AG. Large linear plasmids of Borrelia species that cause relapsing fever. J Bacteriol. 2013;195:3629–3639. doi: 10.1128/JB.00347-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lescot M, Audic S, Robert C, Nguyen TT, Blanc G, Cutler SJ, Wincker P, Couloux A, Claverie JM, Raoult D, et al. The genome of Borrelia recurrentis, the agent of deadly louse-borne relapsing fever, is a degraded subset of tick-borne Borrelia duttonii. PLoS Genet. 2008;4:e1000185. doi: 10.1371/journal.pgen.1000185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Stevenson B, Porcella SF, Oie KL, Fitzpatrick CA, Raffel SJ, Lubke L, Schrumpf ME, Schwan TG. The relapsing fever spirochete Borrelia hermsii contains multiple, antigen-encoding circular plasmids that are homologous to the cp32 plasmids of Lyme disease spirochetes. Infect Immun. 2000;68:3900–3908. doi: 10.1128/iai.68.7.3900-3908.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Barbour AG. Chromosome and plasmids of the tick-borne relapsing fever agent Borrelia hermsii. Genome Announc. 2016;4:e00528–e00516. doi: 10.1128/genomeA.00528-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kingry LC, Replogle A, Batra D, Rowe LA, Sexton C, Dolan M, Connally N, Petersen JM, Schriefer ME. Toward a complete north American Borrelia miyamotoi genome. Genome Announc. 2017;5 [DOI] [PMC free article] [PubMed]
- 121.Kingry LC, Batra D, Replogle A, Sexton C, Rowe L, Stermole BM, Christensen AM, Schriefer ME. Chromosome and linear plasmid sequences of a 2015 human isolate of the tick-borne relapsing fever spirochete, Borrelia turicatae. Genome Announc. 2016;4:e01557–e01516. doi: 10.1128/genomeA.00655-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Qiu WG, Schutzer SE, Bruno JF, Attie O, Xu Y, Dunn JJ, Fraser CM, Casjens SR, Luft BJ. Genetic exchange and plasmid transfers in Borrelia burgdorferi sensu stricto revealed by three-way genome comparisons and multilocus sequence typing. Proc Natl Acad Sci U S A. 2004;101:14150–14155. doi: 10.1073/pnas.0402745101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Graves CJ, Ros VI, Stevenson B, Sniegowski PD, Brisson D. Natural selection promotes antigenic evolvability. PLoS Pathog. 2013;9:e1003766. doi: 10.1371/journal.ppat.1003766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Douglas SE. DNA strider. A Macintosh program for handling protein and nucleic acid sequences. Methods Mol Biol. 1994;25:181–194. doi: 10.1385/0-89603-276-0:181. [DOI] [PubMed] [Google Scholar]
- 126.Krumsiek J, Arnold R, Rattei T. Gepard: a rapid and sensitive tool for creating dotplots on genome scale. Bioinformatics. 2007;23:1026–1028. doi: 10.1093/bioinformatics/btm039. [DOI] [PubMed] [Google Scholar]
- 127.Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, et al. Clustal W and Clustal X version 2.0. Bioinformatics. 2007;23:2947–2948. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- 128.Stamatakis A. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics. 2014;30:1312–1313. doi: 10.1093/bioinformatics/btu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Angiuoli SV, Salzberg SL. Mugsy: fast multiple alignment of closely related whole genomes. Bioinformatics. 2011;27:334–342. doi: 10.1093/bioinformatics/btq665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Fu L, Niu B, Zhu Z, Wu S, Li W. CD-HIT: accelerated for clustering the next-generation sequencing data. Bioinformatics. 2012;28:3150–3152. doi: 10.1093/bioinformatics/bts565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Edgar RC. MUSCLE: a multiple sequence alignment method with reduced time and space complexity. BMC Bioinformatics. 2004;5:113. doi: 10.1186/1471-2105-5-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Edgar RC. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004;32:1792–1797. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Price MN, Dehal PS, Arkin AP. FastTree 2--approximately maximum-likelihood trees for large alignments. PLoS One. 2010;5:e9490. doi: 10.1371/journal.pone.0009490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Feng S, Hodzic E, Freet K, Barthold SW. Immunogenicity of Borrelia burgdorferi arthritis-related protein. Infect Immun. 2003;71:7211–7214. doi: 10.1128/IAI.71.12.7211-7214.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Hagman KE, Lahdenne P, Popova TG, Porcella SF, Akins DR, Radolf JD, Norgard MV. Decorin-binding protein of Borrelia burgdorferi is encoded within a two- gene operon and is protective in the murine model of Lyme borreliosis. Infect Immun. 1998;66:2674–2683. doi: 10.1128/iai.66.6.2674-2683.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Brissette CA, Bykowski T, Cooley AE, Bowman A, Stevenson B. Borrelia burgdorferi RevA antigen binds host fibronectin. Infect Immun. 2009;77:2802–2812. doi: 10.1128/IAI.00227-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Fischer JR, LeBlanc KT, Leong JM. Fibronectin binding protein BBK32 of the Lyme disease spirochete promotes bacterial attachment to glycosaminoglycans. Infect Immun. 2006;74:435–441. doi: 10.1128/IAI.74.1.435-441.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Probert W, Johnson B. Identification of a 47 kd fibronectin-binding protein expressed by Borrelia burgdorferi isolate B31. Mol Microbiol. 1998;30:1003–1015. doi: 10.1046/j.1365-2958.1998.01127.x. [DOI] [PubMed] [Google Scholar]
- 139.Seshu J, Esteve-Gassent MD, Labandeira-Rey M, Kim JH, Trzeciakowski JP, Hook M, Skare JT. Inactivation of the fibronectin-binding adhesin gene bbk32 significantly attenuates the infectivity potential of Borrelia burgdorferi. Mol Microbiol. 2006;59:1591–1601. doi: 10.1111/j.1365-2958.2005.05042.x. [DOI] [PubMed] [Google Scholar]
- 140.Freedman JC, Rogers EA, Kostick JL, Zhang H, Iyer R, Schwartz I, Marconi RT. Identification and molecular characterization of a cyclic-di-GMP effector protein, PlzA (BB0733): additional evidence for the existence of a functional cyclic-di-GMP regulatory network in the Lyme disease spirochete, Borrelia burgdorferi. FEMS Immunol Med Microbiol. 2009;58:285–294. doi: 10.1111/j.1574-695X.2009.00635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Novak EA, Sultan SZ, Motaleb MA. The cyclic-di-GMP signaling pathway in the Lyme disease spirochete, Borrelia burgdorferi. Front Cell Infect Microbiol. 2014;4:56. doi: 10.3389/fcimb.2014.00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Bergstrom S, Bundoc VG, Barbour AG. Molecular analysis of linear plasmid-encoded major surface proteins, OspA and OspB, of the Lyme disease spirochaete Borrelia burgdorferi. Mol Microbiol. 1989;3:479–486. doi: 10.1111/j.1365-2958.1989.tb00194.x. [DOI] [PubMed] [Google Scholar]
- 143.Yang XF, Pal U, Alani SM, Fikrig E, Norgard MV. Essential role for OspA/B in the life cycle of the Lyme disease spirochete. J Exp Med. 2004;199:641–648. doi: 10.1084/jem.20031960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Li H, Dunn JJ, Luft BJ, Lawson CL. Crystal structure of Lyme disease antigen outer surface protein a complexed with an fab. Proc Natl Acad Sci U S A. 1997;94:3584–3589. doi: 10.1073/pnas.94.8.3584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Wilske B, Preac-Mursic V, Jauris S, Hofmann A, Pradel I, Soutschek E, Schwab E, Will G, Wanner G. Immunological and molecular polymorphisms of OspC, an immunodominant major outer surface protein of Borrelia burgdorferi. Infect Immun. 1993;61:2182–2191. doi: 10.1128/iai.61.5.2182-2191.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Marconi RT, Samuels DS, Landry RK, Garon CF. Analysis of the distribution and molecular heterogeneity of the ospD gene among the Lyme disease spirochetes: evidence for lateral gene exchange. J Bacteriol. 1994;176:4572–4582. doi: 10.1128/jb.176.15.4572-4582.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kawabata H, Norris SJ, Watanabe H. BBE02 disruption mutants of Borrelia burgdorferi B31 have a highly transformable, infectious phenotype. Infect Immun. 2004;72:7147–7154. doi: 10.1128/IAI.72.12.7147-7154.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Rego RO, Bestor A, Rosa PA. Defining the plasmid-borne restriction-modification systems of the Lyme disease spirochete Borrelia burgdorferi. J Bacteriol. 2011;193:1161–1171. doi: 10.1128/JB.01176-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Zhang JR, Hardham JM, Barbour AG, Norris SJ. Antigenic variation in Lyme disease borreliae by promiscuous recombination of VMP-like sequence cassettes. Cell. 1997;89:275–285. doi: 10.1016/s0092-8674(00)80206-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Borreliella plasmid PFam32 protein neighbor-joining tree. Figure S2. Two examples of Borreliella linear plasmids with low protein coding potential. Figure S3. Comparative maps of linear plasmids in Borreliella isolates. Maps of the following linear plasmids are shown in the following panels: A, lp5; B, lp17; C, lp25; D, lp28–2, lp28–6, lp28–7 and lp28–9; E, lp28–3; F, VS116 lp28–3; G, lp28–4; H, lp28–8; I, lp32; J, lp36; K, lp38; L, lp56. Figure S4. The PFam54 gene cluster of the Borreliella lp54 plasmids. Figure S5. Ends of the Borreliella linear chromosome sequences. Figure S6. Comparative maps of cp9 plasmids in Borreliella isolates. Figure S7. Orphan cp32-like contigs in the B. spielmanii A14S genome. Figure S8. Rearrangements in cp32-like plasmids in NBu-Borreliella genomes. Figure S9. B. bissettiae DN127 66 kbp circular plasmid cp32-quad. Figure S10. B. finlandensis SV1 integration of cp32 into lp54. Figure S11. Borreliella and relapsing fever Borrelia PFam32 neighbor-joining tree. Table S1. Borreliella and relapsing fever Borrelia PFam32. (PDF 6030 kb)
Data Availability Statement
The plasmid sequences that we determined have been reported and deposited in the GenBank database [30, 49–53]. They are also available through an online browser of Borreliella and Borrelia genomes (http://BorreliaBase.org) [61]). NEXUS files containing sequence alignments and trees for Figs. 2, 5, and 6 have been deposited to TreeBase (Study ID S22200).


