Abstract
Background
Avian pathogenic Escherichia coli (APEC) are causative agent of extraintestinal infections, collectively known as colibacillosis, which results significant losses in poultry industries. The extraintestinal survival of E. coli is facilitated by numerous virulence factors which are coded by virulence genes. This study was conducted to find out the pattern of antibiotic resistance and virulence genes content in the APEC strains isolated from broiler chickens at National Avian Disease Investigation Laboratory and Veterinary Teaching Hospital, Rampur, Chitwan, Nepal.
Results
A total of 50 E. coli strains were isolated from 50 colibacillosis suspected broiler chickens. Out of 50 isolates of E. coli, 47 (94%) showed resistant to three or more antimicrobials. The highest levels (22%) of multidrug-resistant E. coli were observed for five different types of antimicrobials. Antibiogram profiles of 50 E. coli strains showed the maximum resistance to ampicillin (98%), followed by co-trimoxazole (90%), and doxycycline (62%). The highest intermediate resistance was shown by colistin (50%) and the highest sensitivity was against amikacin (84%), followed by nitrofurantoin (55%). Based on the genetic criteria, 45 (90%) E. coli isolates were considered as pathogenic (APEC) which contained more than five virulence genes. Out of total APEC genes detected, we found the combination of iss, iucD, hlyF, ompT, iroN, and iutA genes were mostly associated with the APEC and additionally, to some lesser extent irp2, papC, Cva/cvi, and tsh genes showed the critical role for virulent traits of APEC strains.
Conclusion
In this study, high prevalent of antimicrobial resistant pattern was found with avian pathogenic E. coli strains isolated from broiler chickens. To our knowledge, this is the first molecular analysis which confirmed the prevalence of APEC strains in poultry sector in Nepal. These finding suggest the need of surveillance and intervention system to control misuse of antibiotics and APEC outbreak in the poultry farm.
Electronic supplementary material
The online version of this article (10.1186/s12917-018-1442-z) contains supplementary material, which is available to authorized users.
Keywords: Avian pathogenic Escherichia coli (APEC), Antimicrobial resistance, Virulence gene, Broiler chicken, PCR
Background
Escherichia coli are considered as normal inhabitant of gastrointestinal tract of man and animals. It is also a part of normal intestinal microflora in bird [1]. But, certain pathogenic strains of E. coli invade different organs of bird and causes pericarditis, air sacculitis, perihepatitis, peritonitis, and other extraintestinal diseases, collectively termed as colibacillosis [1, 2]. Colibacillosis may be localized or systemic and caused by avian pathogenic Escherichia coli (APEC) [3]. The pathogenic ability of E. coli strain is facilitated by broad range of virulence factors which are coded by virulence-associated genes (iutA, iss, papC, iucD, tsh, irp-2, ompT, hlyF, iron, cva/cvi, and astA). According to the genetic criteria, the pathogenicity of APEC strain is determined by presence of at least five virulence genes [1]. Some human and avian extraintestinal pathogenic E.coli (ExPEC) has similar phylogenic backgrounds and shares similar virulence genes possessing zoonotic risk [4]. The sequencing of the genome of the APEC strain O1:K1:H7 revealed a high similarity to the genome of human uropathogenic E. coli (UPEC) and neonatal meningitis E. coli (NMEC) [5].
Antibiotics are commonly used in the poultry farm to circumvent the challenges arise due to APEC strains. In many countries including Nepal, there is excessive use of antibiotics in poultry industries. Studies have reported that antibiotics have been used in chicken broilers as growth promoter and disease preventive measures [6, 7]. However, utilization of antimicrobials in food producing animals have created several adverse effects, such as changes in intestinal micro flora, presence of residual antibiotics in food products, impact on public environment, and emergence of antimicrobial resistance in microorganisms [8, 9]. Multiple antibiotic resistant microbes have challenged in the treatment of zoonotic diseases and its transmission from animal to human have led to the threatening situation in health sectors [10].
Poultry industries are emerging rapidly in the developing countries like Nepal. Chitwan, Kathmandu, and Kaski districts are the major areas of poultry farms in Nepal [6]. Chitwan district solely produces 68% and 10% of total eggs and chicken meats, respectively in the country [11]. But, outbreaks of different types of diseases in the poultry farms cause significant economic losses. Among the diseases reported, the outbreak of colibacillosis is one of the major problems of poultry industries [12]. Colibacillosis among broiler chicken is endemic in Nepal. Studies have reported that the prevalence of colibacillosis ranges from 10 to 60% in Chitwan district. The frequent incidence of avian diseases has been increased tremendously [11, 13]. In the context of Nepal, only few literatures are available regarding assessment and investigation of APEC disease. In addition, the trend of pathological investigations of colibacillosis in Nepal is based on clinical symptoms and isolation of E. coli from fecal samples [11]. These conventional approaches of investigations always pose the risk of reporting non-pathogenic E. coli. Furthermore, studies on virulence genes and molecular detection of APEC strains from broiler chicken of Nepal have not been reported yet. Therefore, this study was conducted to find the pattern of antibiotic resistance and virulence genes content among APEC strains isolated from broiler chickens.
Methods
Sample collections, bacterial isolation and identification
Fifty liver samples were collected from 50 colibacillosis suspected broiler chickens which were attended from May 2016 to March 2017 for routine diagnosis at National Avian Disease Investigation Laboratory (NADIL) and Veterinary Teaching Hospital, Rampur, Nepal. For isolation of E. coli strains, swab from the liver sample was aseptically streaked directly on the MacConkey agar (HiMedia, M081) and incubated aerobically at 37 °C for 24 h. The pure colonies were further streaked in the eosine methylene blue (EMB) agar (HiMedia, M317) and incubated overnight at 37 °C. Colonies with the green metallic sheen on EMB agar were suspected as E. coli strains and the further confirmation was done by following the standard microbiological techniques which include studies of colony morphology, Gram staining, and biochemical tests (indole, methyl red, Voges-Proskauer, citrate, catalase, oxidase, and motility indole ornithinase test) [14, 15].
Antibiotic susceptibility testing
Antibiotic susceptibility test of the isolates was performed following modified Kirby-bauer disk diffusion method as recommended by Clinical and Laboratory Standards Institute (CLSI) [16]. The antibiotics used in this study were amikacin (AK), nitrofurantoin (NIT), ciprofloxacin (CIP), levofloxacin (LE), gentamicin (GEN), ampicillin (AMP), co-trimoxazole (COT), doxycycline hydrochloride (DO), and colisitin (CL). These antibiotics were selected due to their extensive consumption in the poultry feed and treatment of colibacillosis and other avian diseases [7, 11, 13]. All the antibiotic discs used in this study were purchased from Himedia, India. For quality control, E coli ATCC 25922 and Pseudomonas aeruginosa ATCC 27853 were used as reference strains.
Detection of virulence genes
Isolated E. coli strains were investigated for the presence of eleven virulence genes (iutA, iss, papC, iucD, tsh, irp-2, ompT, hlyF, iron, cva/cvi, and astA) which are associated with colibacillosis. For the detection of virulence genes, genomic DNA was extracted from pure cultures of E. coli grown overnight in the MacConkey agar at 37 °C by using the DNeasy Blood and Tissue Kit (Qiagen, catalogue no. 69506). The quality of genomic DNA was checked by gel electrophoresis and measuring absorbance at A260/A280 and A260/A230 ratios using the MaestroNano spectrophotometer (MaestroGen; Model name: MN-913). The conventional PCR was used to amplify the virulence genes. The primers used for amplification were those described previously (Table 1) [17, 18]. The PCR was performed in 25 μL volume containing 12.5 μL Hot start Taq 2X master mix (BioLab Inc., New England), 1 μL each primer, 2 μL DNA template, and 8.5 μL nuclease free water. The PCR amplifications were conducted in MultiGene OptiMax Thermal Cycler (Labnet International, Inc., North America) and the cycling conditions were identical for all the samples as follows: 94 °C for 4 min; 35 cycles of 30 s at 94 °C, 1 min at 60 °C, and 2 min at 68 °C; and 72 °C for 7 min. The amplicons were analyzed by agarose gel electrophoresis with 1.5% agarose gel (Sigma-Aldrich, A4718) prepared in 1× TBE buffer (ThermoFisher Scientific, B52). All the PCR products were stained with ethidium bromide. After electrophoresis, the bands were visualized and photographed under UV light. The amplified product was considered to contain virulence gene if it produced band of the expected size.
Table 1.
Primer sets for detection of target virulence genes from avian pathogenic Escherichia coli (APEC) isolates
| Genes | Primer Sequence (5′–3′) | Amplicon size (bp) |
|---|---|---|
| iutA | F: GGCTGGACATCATGGGAACTGG R: CGTCGGGAACGGGTAGAATCG |
302 |
| iss | F: CAGCAACCCGAACCACTTGATG R: AGCATTGCCAGAGCGGCAGAA |
323 |
| papC | F: TGATATCACGCAGTCAGTAGC R: CCGGCCATATTCACATAA |
501 |
| iucD | F: ACAAAAAGTTCTATCGCTTCC R: CCTGATCCAGATGATGCTC |
714 |
| tsh | F: ACTATTCTCTGCAGGAAGTC R: CTTCCGATGTTCTGAACGT |
824 |
| irp-2 | F: AAGGATTCGCTGTTACCGGAC R: AACTCCTGATACAGGTGGC |
413 |
| ompT | F: TCATCCCGGAAGCCTCCCTCACTACTAT R: TAGCGTTTGCTGCACTGGCTTCTGATAC |
496 |
| hlyF | F: GGCCACAGTCGTTTAGGGTGCTTACC R: GGCGGTTTAGGCATTCCGATACTCAG |
450 |
| iroN | F: AATCCGGCAAAGAGACGAACCGCCT R:GTTCGGGCAACCCCTGCTTTGACTTT |
553 |
| cva/cvi | F: TGGTAGAATGTGCCAGAGCAAG R: GAGCTGTTTGTAGCGAAGCC |
1181 |
| astA | F: TGCCATCAACACAGTATATCC R: TCAGGTCGCGAGTGACGGC |
116 |
Statistical analysis
Data entry and analysis were done using the program Microsoft Office Excel 2010 and Chi-square test was performed. The p-value was calculated and considered significant only when it was less than 0.05. The Multiple Antibiotic Resistance (MAR) index was calculated as a/b [19], where ‘a’ is the number of resistance antibiotics and ‘b’ is the number of antibiotics used.
Results
A total of 50 E. coli strains were isolated from 50 liver swab samples of colibacillosis suspected broiler chickens. The antibiogram profile of E. coli isolates showed highest resistance to ampicillin (98%) and least resistance to amikacin (16%) (Fig. 1). Out of 50 E. coli isolates, 47 (94%) isolates were resistant to three or more antibiotics. The MAR index analysis showed 94% of E. coli isolates had MAR index value of > 0.2 and 6% had MAR index value of ≤0.2. The proportions of isolates with the MAR index values of 0.3, 0.4, 0.5, and 0.6 were 31%, 21%, 22%, and 20%, respectively. There was no significant association of prevalence of antibiotic resistant strains with the type of E. coli strains (P > 0.05).
Fig. 1.
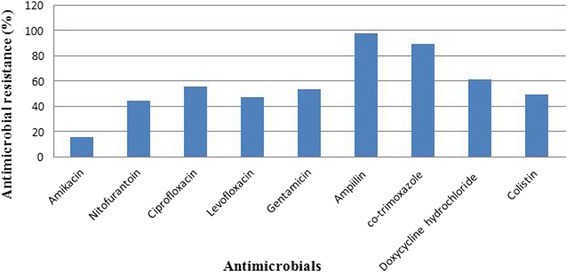
Antimicrobial resistance pattern of tested antibiotics among E. coli strains isolated from colibacillosis suspected broiler chickens
Based on the genetic criteria for the pathogenicity, isolates containing at least five virulence genes were considered as the APEC strains and isolates containing less than five virulence genes were considered as the avian non-pathogenic Escherichia coli (non-APEC) strains. Out of 50 E. coli isolates, 45 (90%) isolates were found to be APEC strains and 5 (10%) isolates were found to be non-APEC strains (Table 2). Among 50 E. coli strains, 7 strains contained all the eleven virulence genes, 14 strains contained ten virulence genes, 15 strains contained nine virulence genes, 5 strains contained eight virulence genes, 2 strains contained seven virulence genes, 2 strains contained five virulence genes, 4 strains contained 4 virulence genes, and 1 strain contained 3 virulence genes. The virulence genes iss, ompT, hlyF, and iroN were detected from all the 45 APEC strains. The frequencies of iucD, astA, iutA, irp-2, and tsh genes in the APEC strains were 97.8%, 95.6%, 82.2%, 73.3%, and 62.2%, respectively. The presence of virulence genes cva/cvi and papC showed the lowest frequency among APEC strains (Table 2; Additional file 1: Figure S1). High frequency of the five virulence genes (iss, ompT, hlyF, iroN, and iucD) was observed among the APEC isolates compared to the non-APEC. Statistical analysis showed that the distribution of virulence genes was significantly associated with the APEC and the non-APEC strains of E. coli (P < 0.05).
Table 2.
Virulence gene frequency in avian pathogenic Escherichia coli (APEC) and avian non-pathogenic Escherichia coli (Non-APEC) isolates
| Genes | APEC isolates (n = 45) n(%) | Non-APEC isolates (n = 5) n(%) | Total (n = 50) n(%) |
|---|---|---|---|
| iutA | 37(82.2) | 1 (20) | 38 (76) |
| iss | 45 (100) | 0 (0) | 45 (90) |
| papC | 25 (55.6) | 0 (0) | 25 (50 |
| iucD | 44 (97.8) | 3 (60) | 47 (94) |
| tsh | 28 (62.2) | 0 (0) | 28 (56) |
| irp-2 | 33 (73.3) | 3 (60) | 36 (72) |
| ompT | 45 (100) | 4 (80) | 49 (98) |
| hlyF | 45 (100) | 3 (60) | 48 (96) |
| iroN | 45 (100) | 1 (20) | 46 (92) |
| cva/cvi | 26 (57.8) | 2 (40) | 28 (56) |
| astA | 43 (95.6) | 2 (40) | 45 (90) |
Discussion
Commercialized poultry industries consume wide range of antibiotics for disease prevention and growth promotion [20]. Globally, the consumption of antimicrobials in food animal production is projected to rise by 67%, by 2030. The antimicrobial use in the chicken is expected to rise by 129%, by 2030 in the Asia-Pacific region [21]. Avian colibacillosis is the major disease in chicken which has been reported by several previous studies [3, 12, 22]. The therapeutic use of antimicrobials in poultry industries is considered as efficient control measure for colibacillosis. However, evolution of multidrug resistant strains along with the transmission of resistance genes has created challenges in reducing risk of APEC infections. In Nepal, the accurate data of prevalence of multi-antibiotic resistant APEC strains is hardly documented. Next, molecular based studies for the detection of virulence genes associated with colibacillosis are lacking. Therefore, this study has attempted to find the multiple antibiotic resistant patterns and detects eleven virulence genes using conventional PCR technology among the APEC strains isolated from diseased broiler chicken.
Out of nine antibiotics tested, none of the antibiotic showed 100% effectiveness against the E coli strains. We found the highest 98% of E. coli isolates were resistant to ampicillin and the lowest 16% of E. coli isolates were resistant to amikacin. Cotrimoxazole, doxycycline hydrochloride, and ciprofloxacin account more than 60% resistivity among the tested E. coli isolates. These antibiotic resistivity patterns of E. coli strains are comparable with the previous studies [3, 7, 13, 23]. Conversely, aminoglycoside amikacin was the most effective against 84% of E. coli strains, which is close to the study published by Bist et al. [24]. Inappropriate utilization of different types of antibiotics in the poultry feed and for disease prevention is the common practice in Chitwan districts and other regions of Nepal. Previous data have showed the marked increase in veterinary antibiotic sales in Nepal [13, 25]. Indiscriminate use of the antibiotics exerts a selection pressure leading to the development of drugs resistance strain of bacteria. The antibiotic resistant patterns found in this study suggest a threatening situation of prevalence of the antibiotic resistant E. coli strains among broiler chickens in Chitwan district. Multiple antibiotic resistant patterns showed 94% of the isolates were resistant to the three or more antimicrobials. The high prevalent of multidrug resistance in E. coli have been reported in Bangladesh [3], China [26], and Korea [27]. The proportion of the isolates with MAR index greater than 0.2 is 94%, and less than or equal to 0.2 is 6%. MAR index value greater than 0.2 indicates high-risk sources of contamination, where several antibiotics may often use for the control of diseases [28]. This suggests the strong indication about an indiscriminative and abusive administration of multiple antibiotics for prophylaxis or infection. Such multi-drug resistances ultimately replace the drug sensitive microorganisms from the antibiotic saturated environment [29].
In this study, the frequency of eleven virulence genes and their role in the pathogenicity was evaluated among APEC and non-APEC strains. APEC strains are characterized by the possession of at least five virulence genes, which enable them to survive an extraintestinal life [1]. To the best of our knowledge, this is the first report in Nepal regarding the study of APEC virulence associated genes by PCR technique. We found the virulence associated genes were more frequently appeared among APEC strains compared to non-APEC strains. The detection rates of iss, iucD, ompT, hlyF, iroN, astA, and iutA among APEC isolates were relatively higher than the detection rates of corresponding genes in the non-APEC strains. High frequency of virulence genes ompT, hlyF, iucD, and irp-2 were found in both the APEC and non-APEC isolates. In contrast, the virulence genes iss, papC, and tsh were not detected from the non-APEC isolates. The detection rate of irp-2 and cva/cvi genes revealed that these genes were frequently distributed in both the APEC and non-APEC strains. The frequency of four genes iss, ompT, hlyF, and iroN were 100% among the APEC isolates (Table 2). The virulence genes irp2 and iucD, both are related to iron acquisition system which demonstrated different detection frequency between the APEC and non-APEC isolates. Among the 45 APEC isolates, 97.8% showed presence of iucD genes, whereas, presence of irp-2 gene was lower (73.3%). Due to the comparable detection rate, this study indicates the possession of iucD gene is important characteristics of both APEC and non-APEC isolates. The frequency of virulence genes detected in this study is comparable with other studies. Kwon et al. studied the presence of eight genes among 18 APEC strains and found the frequency of virulence genes as: iss (100%), tsh (94%), vat (89%), iucD (83%), irp-2 (67%), astA (56%), cva/cvi (16%), and papC (11%) [30]. In another study, De Carli et al. has reported high frequency of virulence genes (hlyF, 100%; iroN, 98.8%; ompT, 100%; iss, 96.3%; and iutA, 81.5%) among the APEC strains [1]. The detection rate of papC is low (55.6%) among the APEC isolates and not detected among the non-APEC isolates. Due to the higher detection rates (80–90%), virulence genes astA, iucD, and iutA are considered as essential genes for pathogenicity. This study also revealed that there was no uniform and absolute combination of the virulence genes which can differentiate APEC and non-APEC strains of E. coli. Additionally, the detection of iss, papC, and tsh genes exclusively only among the APEC strains could be considered as important virulent factors for colibacillosis. The high prevalence of APEC strains of E. coli found in this study based on molecular investigation is the first report to reveal the severity of APEC strains in Chitwan district.
Conclusions
This study showed high prevalence of multiple antimicrobials resistant E. coli and high frequency of virulence genes in APEC strains isolated from the colibacillosis suspected broiler chickens in Chitwan, Nepal. Regular screening and monitoring of the virulence genes associated with the antibiotic resistant APEC strains is essential for implementing intervention program to reduce risk of colibacillosis. A holistic approach is required for the prevention and the control of avian colibacillosis in Nepal and other regions of the country. This can be achieved with the active involvement and cooperation of farmers, hatchery operators, drug importers and marketers, veterinary and allied professionals, and government regulatory agencies.
Additional file
Figure S1. is provided as supplementary material in a separate additional file. (PDF 965 kb)
Acknowledgements
We express sincere gratitude to all the members of the advisory committee, National Avian Disease Investigation Laboratory (NADIL) and faculty members of Agriculture and Forestry University.
Funding
This work was supported financially by the National Avian Disease Investigation Laboratory (NADIL) and National Agricultural Research Development Fund (NARDEF), Nepal.
Availability of data and materials
All data obtained during this study are available within the article.
Abbreviations
- APEC
Avian pathogenic Escherichia coli
- ATCC
American Type Culture Collection
- CLSI
Clinical and Laboratory Standards Institute
- EMB
Eosine methylene blue
- ExPEC
Avian extraintestinal pathogenic E.coli
- MAR
Multiple Antibiotic Resistance
- NADIL
National Avian Disease Investigation Laboratory
- NMEC
Neonatal meningitis E. coli
- UPEC
Human uropathogenic E. coli
Authors’ contributions
HL, BD and RKB conceived the concept, design, and supervised this study. MS and SP performed experimental work. MS, SP, AS, PP, and DKC analyzed data and prepared the final draft of the manuscript. All authors read and approved the final manuscript.
Ethics approval
Ethical approval was obtained from Research Ethics Committee of Agriculture and Forestry University, Rampur, Chitwan, Nepal. No human sample was involved in this study. The animal samples were processed according to the animal research ethical guidelines approved by Research Ethics Committee of Agriculture and Forestry University, Rampur, Chitwan, Nepal.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Footnotes
Electronic supplementary material
The online version of this article (10.1186/s12917-018-1442-z) contains supplementary material, which is available to authorized users.
Contributor Information
Manita Subedi, Email: ms.manitasubedi@gmail.com.
Himal Luitel, Email: drhimal@gmail.com.
Bhuminanda Devkota, Email: bhuminand@gmail.com.
Rebanta Kumar Bhattarai, Email: rkbhattarai@afu.edu.
Sarita Phuyal, Email: sarita.phuyal85@gmail.com.
Prabhat Panthi, Email: panthi.prabhat@gmail.com.
Anil Shrestha, Email: neal.shr2010@gmail.com.
Dhiraj Kumar Chaudhary, Phone: +977-9841441236, Email: dhirajchaudhary2042@gmail.com.
References
- 1.De Carli S, Ikuta N, Lehmann FK, da Silveira VP, de Melo Predebon G, Fonseca AS, et al. Virulence gene content in Escherichia coli isolates from poultry flocks with clinical signs of colibacillosis in Brazil. Poult Sci. 2015;94:2635–2640. doi: 10.3382/ps/pev256. [DOI] [PubMed] [Google Scholar]
- 2.Matter LB, Barbieri NL, Nordhoff M, Ewers C, Horn F. Avian pathogenic Escherichia coli MT78 invades chicken fibroblasts. Vet Microbiol. 2011;148:51–59. doi: 10.1016/j.vetmic.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 3.Matin MA, Islam MA, Khatun MM. Prevalence of colibacillosis in chickens in greater Mymensingh district of Bangladesh. Vet World. 2017;10:29–33. doi: 10.14202/vetworld.2017.29-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Manges AR, Johnson JR. Food-borne origins of Escherichia coli causing extraintestinal infections. Clin Infect Dis. 2012;55:712–719. doi: 10.1093/cid/cis502. [DOI] [PubMed] [Google Scholar]
- 5.Johnson TJ, Kariyawasam S, Wannemuehler Y, Mangiamele P, Johnson SJ, Doetkott C, et al. The genome sequence of avian pathogenic Escherichia coli strain O1:K1:H7 shares strong similarities with human extraintestinal pathogenic E. coli genomes. J Bacteriol. 2007:3228–36. [DOI] [PMC free article] [PubMed]
- 6.Osti R, Bhattarai D, Chaudhary H, Singh V. Poultry production in Nepal: characteristics, productivity and constraints. Int J Appl Sci Biotechnol. 2017;5:222–226. doi: 10.3126/ijasbt.v5i2.17616. [DOI] [Google Scholar]
- 7.Shrestha A, Bajracharya AM, Subedi H, Turha RS, Kafle S, Sharma S, et al. Multi-drug resistance and extended spectrum beta lactamase producing gram negative bacteria from chicken meat in Bharatpur metropolitan, Nepal. BMC Res Notes. 2017;10:574. doi: 10.1186/s13104-017-2917-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miles TD, McLaughlin W, Brown PD. Antimicrobial resistance of Escherichia coli isolates from broiler chickens and humans. BMC Vet Res. 2006;2:7. doi: 10.1186/1746-6148-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Umolu PI, Ohenhen ER, Okwu IG, Ogiehor IS. Multiple antibiotic resistant index and plasmid of Escherichia coli in beef in Ekpoma. J Am Sci. 2006;2:16–18. [Google Scholar]
- 10.Centers for Disease Control and Prevention (CDC). Antibiotic resistance threats in the United States . U. S. CDC: Department of Health and Human Services; 2013. p. 2013. [Google Scholar]
- 11.Gautam G, Devkota B, Thapaliya S. Recent case flow pattern in veterinary teaching Hospital of Agriculture and Forestry University, Chitwan, Nepal. Journal of Agriculture and Forestry University. 2017;1:119–128. [Google Scholar]
- 12.Mellata M. Human and avian extraintestinal pathogenic Escherichia coli: infections, zoonotic risks, and antibiotic resistance trends. Foodborne Pathog Dis. 2013;10:916–932. doi: 10.1089/fpd.2013.1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shrestha EK, Dhakal IP, Sapkota M, Manandhar P, Rijal TB. Antimicrobial resistance pattern of Eshcerichia coli isolates from chicken and human samples in Chitwan. Nepalese. Vet J. 2011;30:38–44. [Google Scholar]
- 14.da Silva N, Taniwaki MH, Junqueira VC, Silveira N, do Nascimento MS, RAR G. Microbiological examination methods of food and water: a laboratory manual. London, UK: Taylor & Francis Group; 2013. [Google Scholar]
- 15.Overdevest I, Willemsen I, Rijnsburger M, Eustace A, Xu L, Hawkey P, et al. Extended-spectrum β-lactamase genes of Escherichia coli in chicken meat and humans, the Netherlands. Emerg Infect Dis. 2011;17:1216–1222. doi: 10.3201/eid1707.110209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clinical Laboratory Standards Institute (CLSI) Performance standards for antimicrobial susceptibility testing; 24th informational supplement (M100-S23) Wayne PA, USA: CLSI; 2014. [Google Scholar]
- 17.Ewers C, Janssen T, Kiessling S, Philipp HC, Wieler LH. Rapid detection of virulence-associated genes in avian pathogenic Escherichia coli by multiplex polymerase chain reaction. Avian Dis. 2005;49:269–273. doi: 10.1637/7293-102604R. [DOI] [PubMed] [Google Scholar]
- 18.Radwan IAE, Salam HSH, Abd-Alwanis AA, Al-Sayed MAY. Frequency of some virulence associated genes among multidrug-resistant Escherischia coli isolated from septicemic broiler chicken. Int. J Adv Res. 2014;2:867–874. [Google Scholar]
- 19.Krumperman PH. Multiple antibiotic resistance indexing of Escherichia coli to identify high-risk sources of fecal contamination of foods. Appl Environ Microbiol. 1983;46:165–170. doi: 10.1128/aem.46.1.165-170.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bhandari S, Singh S. Annual consumption of veterinary medicines and feed supplement in Nepal. Nepalese Vet J. 2004;28:25–32. [Google Scholar]
- 21.Van Boeckel TP, Brower C, Gilbert M, Grenfell BT, Levin SA, Robinson TP, et al. Global trends in antimicrobial use in foods animals. Proc Natl Acad Sci U S A. 2015;112:5649–5654. doi: 10.1073/pnas.1503141112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tonu NS, Sufian MA, Sarkar S, Kamal MM, Rahman MH, Hossain MM. Pathological study on colibacillosis in chickens and detection of Escherichia coli by PCR. Bangladesh. J Vet Med. 2001;9:17–25. [Google Scholar]
- 23.Bakhshi M, Bafghi MF, Astani A, Ranjbar VR, Zandi H, Vakili M. Antimicrobial resistence pattern of Escherichia coli isolated from chickens with colibacillosis in Yazd, Iran. J Food Qual Hazards Control. 2017;4:74–78.
- 24.Bist B, Sharma B, Jain U. Virulence associated factors and antibiotic sensitivity pattern of Escherichia coli isolated from cattle and soil. Vet World. 2014;3:69–72.
- 25.Basnyat B, Pokharel P, Dixit S, Giri S. Antibiotic use, its resistance in Nepal and recommendations for action: a situation analysis. J Nepal Health Res Counc. 2015;13:102–111. [PubMed] [Google Scholar]
- 26.Yang H, Chen S, White DG, Zhao S, Mc Dermott P, Walker R, et al. Characterization of multiple-antimicrobial-resistant Eshcerichia coli isolates from diseased chickens and swine in China. J Clin Microbiol. 2004;42:3483–3489. doi: 10.1128/JCM.42.8.3483-3489.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim TE, Jeong YW, Cho SH, Kim SJ, Kwon HJ. Chronological study of antibiotic resistances and their relevant genes in Korean avian pathogenic Eshcerichia coli isolates. J Clin Microbiol. 2007;45:3309–3315. doi: 10.1128/JCM.01922-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chitanand MP, Kadam TA, Gyananath G, Totewad ND, Balhal DK. Multiple antibiotic resistance indexing of coliforms to identify high risk contamination sites in aquatic environment. Indian J Microbiol. 2010;50:216–220. doi: 10.1007/s12088-010-0042-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Van den Bogaard AE, London N, Driessen C, Stobberingh EE. Antibiotic resistance of faecal Escherichia coli in poultry, poultry farmers and poultry slaughterers. J Antimicrob Chemother. 2001;47:763–771. doi: 10.1093/jac/47.6.763. [DOI] [PubMed] [Google Scholar]
- 30.Kwon SG, Cha SY, Choi EJ, Kim B, Song HJ, Jang HK. Epidemiological prevalence of avian pathogenic Escherichia coli differentiated multiplex PCR from commercial chickens and hatchery in Korea. J Bacteriol Virol. 2008;38:179–188. doi: 10.4167/jbv.2008.38.4.179. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. is provided as supplementary material in a separate additional file. (PDF 965 kb)
Data Availability Statement
All data obtained during this study are available within the article.


