Abstract
Background
A vaccine against all four dengue virus (DENV) serotypes includes the formulation of one genotype of each serotype. Although genetic similarities among genotypes within a serotype are higher as compared to those among serotypes, differences in the immunogenicity of the included genotypes would be a critical issue in maximizing successful dengue vaccine development. Thus, we determined the neutralizing antibody responses against three genotypes of dengue virus serotype 2 (DENV-2), namely Cosmopolitan, Asian I, and Asian/American, after primary and secondary inoculation with DENV-2 in a dengue animal model, the common marmoset (Callithrix jacchus).
Methods
A total of fifty-four plasma samples were obtained from thirty-four marmosets that were inoculated with clinically-isolated DENV strains or DENV candidate vaccines, were used in this study. Plasma samples were obtained from marmosets after primary inoculation with DENV-2 infection, secondary inoculation with homologous or heterologous genotypes, and tertiary inoculation with heterologous DENV. Neutralizing antibody titers against DENV-2 (Cosmopolitan, Asian I, and Asian/American genotypes) and DENV-1 were determined using a conventional plaque reduction neutralization assay.
Results
In marmosets that were inoculated with the Cosmopolitan genotype in primary infection, neutralizing antibody neutralized 3 genotypes, and the titers to Asian I genotype were significantly higher than those to homologous Cosmopolitan genotype. After secondary DENV-2 infection with heterologous genotype (Asian I in primary and Asian/American in secondary), neutralizing antibody titers to Asian/American genotype was significantly higher than those against Cosmopolitan and Asian I genotypes. Following tertiary infection with DENV-1 following DENV-2 Asian I and Cosmopolitan genotypes, neutralizing antibody titers to Asian/American were also significantly higher than those against Cosmopolitan and Asian I genotypes.
Conclusion
The present study demonstrated that different levels of neutralizing antibodies were induced against variable DENV-2 genotypes after primary, secondary and tertiary infections, and that neutralizing antibody titers to some heterologous genotypes were higher than those to homologous genotypes within a serotype. The results indicate that heterogeneity and homogeneity of infecting genotypes influence the levels and cross-reactivity of neutralizing antibodies induced in following infections. The results also suggest that certain genotypes may possess advantage in terms of breakthrough infections against vaccination.
Electronic supplementary material
The online version of this article (10.1186/s12985-018-0967-x) contains supplementary material, which is available to authorized users.
Keywords: Dengue, Antibody response, Genotype, Animal model
Background
Dengue (DEN) is a major public health threat in tropical and subtropical regions, including Southeast Asia, the South Pacific, the Eastern Mediterranean, and the Americas [1, 2]. It is estimated that 50 to 100 million cases of dengue virus (DENV) infection occur annually worldwide. DENV is transmitted mainly by the Aedes aegypti and Aedes albopictus mosquitoes. The symptoms of DENV infection may range from undifferentiated viral fever, dengue without warning signs, and dengue with warning signs to life-threatening severe dengue [3]. There are four antigenically distinct serotypes of DENV, referred to as DENV1–4. There remains an urgent need for a high-efficacy dengue vaccine that offers long-term protection against all four DENV serotypes. All four DENV serotypes have similar clinical presentations and co-circulates in endemic areas. Among four serotypes, DENV-2 is the most prevalent serotype in the current worldwide dengue epidemics [4]. There are six genotypes of DENV-2, based on E-protein gene analysis: American, Asian/American, Asian I, Asian II, Cosmopolitan and Sylvatic [5, 6]. It is hypothesized that each DENV-2 genotype differs in terms of virulence and incidence [7].
Neutralizing antibodies play a central role in protection against DENV infection and disease pathogenesis [8]. Infection with one serotype of DENV confers life-long protection against the same serotype, but protection against infection with other serotypes is short-lived [9, 10]. DENV structural proteins, such as the E protein and the precursor membrane (pre-M), are the principal targets of antibody responses [11]. Because DENV serotypes are antigenically related, a majority of the antibody responses against the E-protein can cross-react with more than one DENV serotype [11, 12]. While serotype-specific neutralizing antibodies are induced after infection, these antibodies are highly genotype-specific, and strain variation within each serotype affects the ability of neutralizing antibodies to offer protection [6]. DENV- immunized mice have been shown to possess variable levels of neutralizing antibodies for different strains [13]. Currently, in DENV vaccine formulation, a single genotype is used for each serotype. Although genetic similarities among genotypes within a serotype are higher as compared to those among serotypes, the issue of differences in antigenicity among genotypes is likely to be critical in maximizing successful dengue vaccine development.
The objective of this research was to define genotype-specific and cross-reactive neutralizing antibody responses within a serotype. The common marmoset (Callithrix jacchus) model has been proved useful in primary and secondary DENV infection studies [14, 15]. The model demonstrates antibody response patterns consistent with those of human DENV infection and in candidate vaccines recipients [14–16]. In the present study, using this non-human primate model, we characterized in detail the neutralizing antibody responses to three DENV-2 genotypes in marmosets after primary, secondary and tertiary infections.
Methods
Viruses
Three strains comprised of DENV serotype-2 (DENV-2) genotypes were used in the study. DENV-2 DHF0663 strain (GenBank accession no. AB189122) was isolated from a DHF patient from Indonesia, and belonged to the Cosmopolitan genotype. DENV-2 00–43 strain (GenBank accession no. AB111452) was isolated from an imported dengue fever case from Indonesia, and belonged to the Asian I genotype. DENV-2 08–77 strain (GenBank accession no. AB545874) was isolated from an imported dengue fever case from the Maldives, and belonged to the Asian/American genotype (Additional file 1). These virus strains were first isolated using C6/36 cells, and passaged in baby hamster kidney (BHK) cells. Virus stocks were used within four cell culture passages. Culture supernatants collected from infected BHK cells were centrifuged at 3000 rpm for 5 min to remove cell debris. Virus stocks were stored at − 80 °C before use.
DENV-2 16,881 PDK-53 was a monovalent live attenuated DENV-2 vaccine candidate that were attenuated by serial passages of PDK cells (GenBank accession no. M84728). DENV-1 16,007 PDK-13 was a monovalent live attenuated DENV-1 vaccine candidate that were attenuated by serial passage of PDK cells (GenBank accession no. AF180818). All these vaccine strains were prepared at the Institute of Molecular Biosciences, Mahidol University, Nakhon Pathom, Thailand, shipped in liquid form on dry ice to National Institute of Infectious Diseases, Tokyo, Japan, and stored at − 80 °C before use. The DENV-1 and DENV-2 candidate vaccine and parent strains were kindly provided by Dr. Sutee Yoksan of Institute of Molecular Biosciences, Mahidol University, Thailand.
Samples collected from marmosets
Common marmosets (Callithrix jacchus) were obtained from CLEA Japan, Inc. (Tokyo, Japan) and maintained in specific pathogen-free conditions at the National Institute of Infectious Diseases (Tokyo, Japan). A total of 54 blood samples were collected from 34 marmosets (21 females and 13 males) in the study. All marmosets used in this study were adult marmosets, with an age range of 4–8 years and weight range of 230–451 g. In groups 1–10, 38 plasma samples were collected from 26 marmosets with primary (N = 23), secondary (N = 12), and tertiary (N = 3) dengue virus infections (Table 1). In these group, each of the marmosets were inoculated subcutaneously on the back with 106 plaque forming unit (PFU)/dose of DENV. The intervals between DENV inoculations were between 6 and 12 months. In groups 11–15, sixteen blood samples were collected from eight marmosets that were inoculated subcutaneously on the back with a monovalent live-attenuated dengue vaccine candidates: DENV-1 16,007 PDK-13 strain and DENV-2 16,881 PDK-53 strain (Table 1). In groups 11–15, marmosets received 104 PFU/dose of DENV-1 and DENV-2 vaccine strains. About 6–9 months after the first administration, the marmosets were inoculated subcutaneously on the back with 105 PFU/dose of DENV-1 or DENV-2. A total of 1 mL of whole blood was collected in EDTA tubes from each marmoset on day 0 (before the virus inoculation), and on days 4, 7, and 14 post-inoculation (p.i). Next, the blood samples were centrifuged at 2000 rpm for 10 min at 4 °C. The plasma samples were stored at − 80 °C before use. After primary inoculation, all marmosets were confirmed positive for DENV-specific IgM antibodies (Additional file 2).
Table 1.
List of the marmosets that were used in this study inoculated with clinically isolated dengue virus
| Type of infection | Primary infection | Secondary infection | Tertiary infection | Intervals between DENV infection | Marmosets ID |
|---|---|---|---|---|---|
| Serotype (Genotype) | Serotype (Genotype) | Serotype (Genotype) | |||
| Primary infection | |||||
| Group 1 | D1 (GI) | M1, M2 | |||
| Group 2 | D1 (GII) | M3–1, M4–1, M5–1, M6, | |||
| Group 3 | D2 (Cosmopolitan) | M7–1, M8–1, M9–1, M10, M11, M12, M13, M14 | |||
| Group 4 | D2 (Asian I) | M15–1, M16–1, M17–1, M18, M19, M20 | |||
| Group 5 | D2 (Asian/American) | M21, M22, M23 | |||
| Secondary infection | |||||
| Homologous genotype | |||||
| Group 6 | D2 (Cosmopolitan) | D2 (Cosmopolitan) | 6 months | M24, M25 | |
| Heterologous genotype | |||||
| Group 7 | D2 (Asian I) | D2 (Cosmopolitan) | 6 months | M15–2, M16–2, M17–2, M26 | |
| Homologous serotype | |||||
| Group 8 | D1 (GII) | D1 (GI) | 7 months | M3–2, M4–2, M5–2 | |
| Heterologous serotype | |||||
| Group 9 | D2 (Cosmopolitan) | D1 (GI) | 12 months | M7–2, M8–2, M9–2 | |
| Tertiary infection | |||||
| Group 10 | D2 (Asian I) | D2 (Cosmopolitan) | D1 (GI) | 12 months | M15–3, M16–3, M17–3 |
| Primary infection with monovalent live-attenuated DEN vaccine candidate | |||||
| Group 11 | D1 16007 PDK-13 (GII) | V1–1, V2–1, V3–1, V4–1 | |||
| Group 12 | D2 16881 PDK-53 (Asian I) | V5–1, V6–1 | |||
| Secondary infection | |||||
| Group 13 | D1 16007 PDK-13 (GII) | D1 (GI) | 7 months | V1–2, V2–2, V3–2, V4–2 | |
| Group 14 | D2 16881 PDK-53 (Asian I) | D2 (Cosmopolitan) | 6 months | V5–2, V6–2, V7–1, V8 | |
| Tertiary infection | |||||
| Group 15 | D2 16881 PDK-53 (Asian I) | D2 (Cosmopolitan) | D1 (GI) | 12 months | V5–3, V7–2 |
Determination of neutralizing titer of the antibodies using a plaque reduction neutralization test assay
The neutralizing titers of the antibodies against the DENV-2 genotypes Cosmopolitan, Asian I, and Asian/American were determined using a plaque reduction neutralization test assay (PRNT). BHK cells were seeded in 12-wells plate in Minimum Essential Medium (MEM) (Sigma Aldrich, USA) supplemented with 10% of heat-inactivated fetal bovine serum (Hi-FBS) (Thermo Fisher Scientific, USA)) and were incubated at 37 °C overnight until the cells monolayer reached approximately 70% confluency. Plasma samples were heat-inactivated at 56 °C for 30 min before use. The heat-inactivated plasma samples were serially diluted two-fold starting from 1:10 to 1:20,480 in MEM supplemented with 10% Hi-FBS. The virus-antibody complexes were prepared by mixing 25 μL of DENV at titers of 5000 PFU/ml with 25 μL serially diluted plasma samples to make a final dilution series ranging from 1:20 to 1:40, 960. The control samples were prepared by mixing 25 μL DENV at titers of 5000 PFU/ml with 25 μL MEM supplemented with 10% Hi-FBS. Next, the virus-antibody complexes were incubated at 37 °C for 60 min. A total of 50 μL of the mixture was then inoculated onto BHK cell monolayers in 12-wells plates. After incubation for 60 min, 1 mL of maintenance medium containing MEM and 1% of methyl cellulose supplemented with 2% of Hi-FBS was added. The plates were incubated at 37 °C in 5% CO2 until visible plaques were observed (5–7 days of incubation). Cells were fixed with 10% formaldehyde and stained with methylene blue, washed with water, and then the number of plaques was counted. All tests were conducted in duplicate. The neutralizing antibody titer was expressed as the maximum dilution of plasma sample that yielded a ≥ 50% plaque reduction in the virus inoculum compared with control virus samples. Results are shown as 50% PRNT50 values, expressed as the reciprocal of the highest plasma dilution (end-point titer) that results in ≤50% of the input plaque count.
Data analysis
Data were analyzed using the statistical analysis toolpack in Microsoft Excel 2016 (Microsoft Corporation, USA) and GraphPad Prism 7 (GraphPad Software Inc., USA). Student’s test was performed to compare the mean titers of neutralizing antibodies. p values less than 0.05 were considered statistically significant.
Ethics statement
The animal studies were conducted in accordance with the guidelines of the Institutional Animal Care and Use Committee of the National Institute of Infectious Diseases (NIID), Tokyo, Japan. The study was approved by the Institutional Animal Care and Use Committee of NIID (approval no. 613006 and 516,010). All animal and infection experiments were performed according to the NIID Institutional Guidelines, in additions to the guidelines of the Science Council, and local rules and regulations.
Results
Levels of neutralizing antibodies to different DENV-2 genotypes following primary infection
Marmosets were inoculated with one of 3 genotypes of DENV-2, and neutralizing antibody titers were examined to homologous and heterologous genotypes. Neutralizing antibodies were not detected to any of the 3 genotypes (Cosmopolitan, Asian I, and Asian/America genotypes) from days 0 to 7 post-inoculation (p.i) (Fig. 1a-c). However, the marmosets inoculated with Cosmopolitan, Asian I, and Asian/American genotypes (N = 23) presented neutralizing antibodies to the Cosmopolitan, Asian I, and Asian/American genotypes by day 14 p.i (Fig. 1a-c). In eight marmosets that were inoculated with the Cosmopolitan genotype (Group 3), titers of neutralizing antibody to the Asian I genotype were significantly higher as compared to those for the Cosmopolitan genotype (p = 0.007) on the day 14 p.i (Fig. 1a). In marmosets inoculated with the Asian I (group 4) or Asian/American (group 5) genotypes, neutralizing antibody was also detected to the 3 genotypes, but the titers were found to be at similar levels among these genotypes (Fig. 1b-c). In contrast, in the primary DENV-1 infection (group 1 and group 2), neutralizing antibodies to Cosmopolitan, Asian I, and Asian/American genotypes of DENV-2 were absent (< 20) on days 0, as well as on days 4, 7, and 14 p.i. (Fig. 1d-e). The results confirm that neutralizing antibodies induced by primary infection with DENV-2 do not neutralize heterologous infection with DENV-1.
Fig. 1.
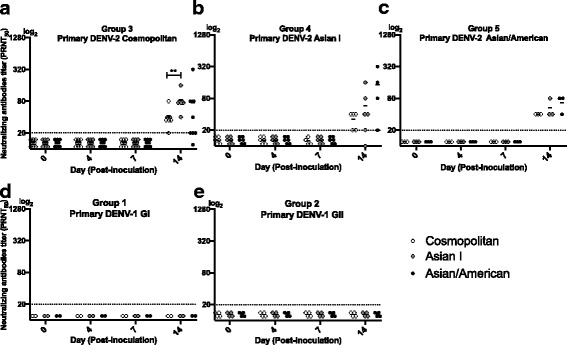
Neutralizing antibodies titers to DENV-2 genotypes (Cosmopolitan, Asian I and Asian/American) during primary DENV inoculation. Neutralizing antibody titers in marmosets inoculated with: a DENV-2 genotype Cosmopolitan, b DENV-2 genotype Asian I, c DENV-2 genotype Asian/American, d DENV-1 genotype I, e DENV-1 genotype II. Double asterisks (**) indicate that there were statistically significant differences (p < 0.05) in the titers of neutralizing antibodies between genotypes. Student’s test analysis was used to determine the differences in mean titers of neutralizing antibody between CM vs AI, CM vs AA, and AI vs AA. CM indicates Cosmopolitan genotype. A1 indicates Asian I genotype. AA indicates Asian/American genotype
Neutralizing antibody responses after secondary DENV-2 infection in marmosets which were previously infected with homologous or heterologous DENV-2 genotype
Marmosets which had been previously infected with DENV-2 were infected with homologous and heterologous genotypes (Fig. 2a and b). Prior to secondary infection with homologous genotype (group 6), neutralizing antibody titers to Asian/American genotypes were detected at day 0, indicating that neutralizing antibody induced during primary infection cross-neutralize different genotypes within the same infecting serotype. Additionally, neutralization antibody levels to Asian/American genotype was higher as compared to the infecting genotype (Cosmopolitan genotype), after primary infection. The results suggest that while antibodies neutralize different genotypes within a serotype, the levels varies among genotypes. The result indicate that after secondary infection with homologous genotype (group 6), neutralizing antibody titers rapidly increased from day 7, but titers did not differ significantly among 3 genotypes (Cosmopolitan, Asian I and Asian/American) at day 14 p.i. (Fig. 2a).
Fig. 2.
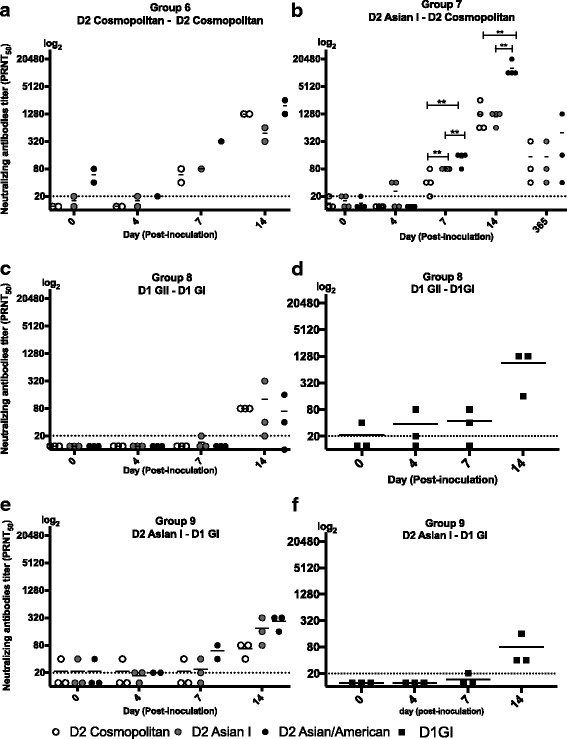
Neutralizing antibodies titers to DENV-2 genotypes (Cosmopolitan, Asian I and Asian/American) after secondary DENV inoculation. Neutralizing antibody titers in marmosets inoculated with: a Homologous DENV-2 genotype(Cosmopolitan), b Heterologous DENV-2 genotypes (Asian1 in primary, Cosmopolitan in secondary), c two DENV-1 genotypes (Genotype 1 in primary, Genotype II in secondary), d Neutralizing antibody titers to DENV-1 genotype I during two DENV-1 genotypes (Genotype 1 in primary, Genotype II in secondary), e Heterologous serotypes (DENV-2 Asian1 genotype in primary, DENV-1 Genotype 1 in secondary), f Neutralizing antibody titers to DENV-1 genotype I after inoculation with heterologous serotypes (DENV-2 Asian1 genotype in primary, DENV-1 Genotype 1 in secondary). Double asterisks (**) indicates that there were statistically significant differences (p < 0.05) in the titers of neutralizing antibodies between genotypes. Student’s test analysis was used to determine the differences in mean titers of neutralizing antibody between CM vs AI, CM vs AA, and AI vs AA. Neutralizing antibody data at day 365 post-inoculation of (b) are the same data as day0 of Fig. 4b. CM indicates Cosmopolitan genotype, A1 indicates Asian I genotype and AA indicates Asian/American genotype
After secondary DENV-2 infection with heterologous genotype (group 7), levels of neutralizing antibody rapidly increased from day 7. The titers of neutralizing antibody to Asian/American genotype were significantly higher as compared to those against the Cosmopolitan (p = 0.029) and Asian I (p = 0.020) genotypes at day 7 p.i. Titers of neutralizing antibody to the Asian I genotype were also significantly higher than those against Cosmopolitan (p = 0.034) at day 7. Similarly, at day 14 p.i, the neutralizing antibody titers against the Asian/American genotype were significantly higher as compared to titers against the Cosmopolitan (p = 0.010) and Asian I (p = 0.009) genotypes. It is of interest that the antibodies possessed higher neutralization activities towards the Asian/American genotype, which were not uses in primary and secondary infection, than towards the Cosmopolitan and Asian I genotypes. Neutralizing antibody titers against the Asian I and Asian/American genotypes after secondary infection with heterologous genotype (Asian I-Cosmopolitan) (Fig. 2b) were significantly higher as compared to those against homologous genotype (Cosmopolitan-Cosmopolitan) secondary infection (Fig. 2a); (p = 0.030) and (p = 0.023), respectively. These results suggest that higher neutralizing activity was induced after secondary infection with heterologous genotype. At day 365 p.i (day 0 prior to third challenge; Figs. 2b and 4c), the levels of neutralizing antibodies to Cosmopolitan, Asian I, and Asian/American were still detected but there were no significant differences between neutralizing activity amongst the genotypes.
Fig. 4.
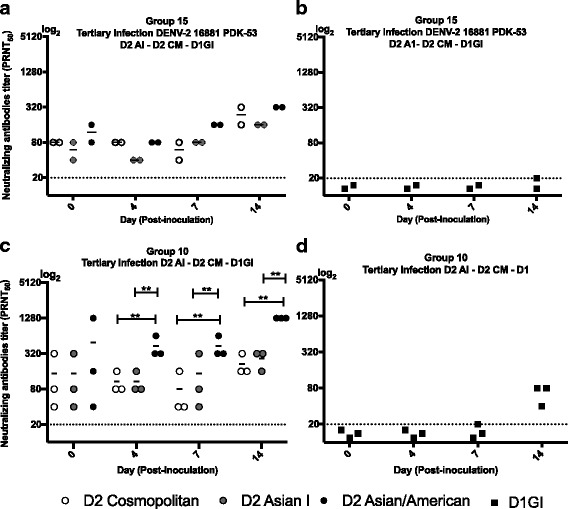
Neutralizing antibodies titers to DENV-2 genotypes (Cosmopolitan, Asian I and Asian/American) after tertiary inoculation. a Marmosets which had been first inoculated with DENV-2 16,881 PDK-53 (Asian I genotype) and then challenged with DENV-2 genotype Cosmopolitan were infected with DENV-1 in tertiary inoculation. b Neutralizing antibody titers to DENV-1, genotype 1 in the same marmosets in (a). c Marmosets that were first inoculated with the DENV-2 Asian I genotype and then challenged with the DENV-2 genotype Cosmopolitan were infected with DENV-1 genotype I in tertiary inoculation. d Neutralizing antibody titers to DENV-1, genotype 1 in the same marmosets in (c). Double asterisks (**) indicate that there were statistically significant differences (p < 0.05) in the titers of neutralizing antibodies between genotypes. Student’s test analysis was used to determine the differences in mean titers of neutralizing antibody between CM vs AI, CM vs AA, and AI vs AA. CM indicates Cosmopolitan genotype. A1 indicates Asian I genotype. AA indicates Asian/American genotype
Neutralizing antibody responses to DENV-2 after secondary infection with DENV-1 in marmosets which were previously infected with DENV-1
Marmosets in group 8 which had been previously infected with DENV-1 genotype II were infected with heterologous genotype I of DENV-1, and neutralizing antibody titers to 3 genotypes of DENV-2 were examined (Fig. 2c and d).
Neutralizing antibodies titers against 3 DENV-2 genotypes (Cosmopolitan, Asian I, and Asian/American genotypes) were below the detection levels at days 0, 4, and 7 p.i (Fig. 2c). However, the marmosets demonstrated neutralizing activities to three DENV-2 genotypes at day 14 p.i, and the titers were at similar levels. Neutralizing antibody to DENV-1 were detected in all the marmosets on day 14 (Fig. 2d). The results indicate that two inoculations with DENV-1 induced antibodies which neutralized 3 genotypes (Cosmopolitan, Asian I and Asian/American) of heterologous serotype (DENV-2) (Fig. 2c), as well as homologous serotype (DENV-1) (Fig. 2d).
Neutralizing antibody responses after secondary DENV-1 infection in marmosets which were previously infected with DENV-2
Marmosets in group 9 which had been previously infected with DENV-2 Asian I genotype were infected with DENV-1, and neutralizing antibody titers to 3 genotypes of DENV-2 were examined (Fig. 2e and f).
One marmoset demonstrated neutralizing antibodies to Cosmopolitan, Asian I, and Asian/American genotypes prior to dengue infection at day 0 (Fig. 2e). Neutralizing antibodies titers to Cosmopolitan, Asian I, and Asian/American genotypes increased in marmosets from days 7 p.i, and there were no significant differences in neutralizing antibody titers for these different genotypes (Fig. 2e). Neutralizing antibodies to the DENV-1 serotype were detected by day 7 p.i (Fig. 2f). Levels of neutralizing antibodies to the Asian/American genotype after two inoculations with homologous (DENV-2) and heterologous (DENV-1) serotypes were significantly higher than those after the two inoculations with DENV-1 (p = 0.020) (Fig. 2c).
The neutralizing antibody response to DENV-2 genotypes in marmosets inoculated with DENV-1 and DENV-2 candidate vaccine
In this series of experiments, we used live-attenuated vaccine candidate to determine whether similar pattern of genotype cross-reactivity is induced by vaccine candidates. Marmosets were immunized with DENV-2 16,881 PDK-53 (Asian I genotype) and challenged with DENV-2 Cosmopolitan genotype. Neutralizing antibody titers to Cosmopolitan, Asian I and Asian/American genotypes were examined. One of two marmoset that was inoculated with the DENV-2 16,881 PDK-53 candidate vaccine (Asian I genotype), however, presented neutralizing antibodies to DENV-2 Cosmopolitan, Asian I, and Asian/American at the day 14 p.i (Fig. 3a). After challenge (secondary infection) with DENV-2 Cosmopolitan genotype, neutralizing antibodies were detected to 3 genotypes in all the 4 marmosets on day 7 and day 14 p.i (Fig. 3b). Neutralizing antibody titers against the Asian/American genotype were also significantly higher compared to those against the Cosmopolitan and Asian I genotypes at the day 14 p.i (p = 0.005 and p = 0.003, respectively).
Fig. 3.
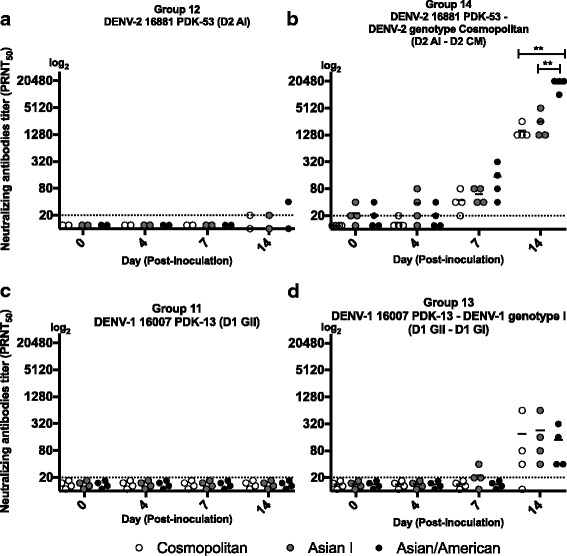
Neutralizing antibodies titers to DENV-2 genotypes (Cosmopolitan, Asian I and Asian/American) in marmosets inoculated with candidate dengue vaccines and challenged with isolated DENV. Neutralizing antibody titers in marmosets inoculated with: a Candidate vaccine DENV-2 16,881 PDK-53, b Candidate vaccine DENV-2 16,881 PDK-53 in primary and with DENV-2 genotype Cosmopolitan in secondary, c Vaccine candidate DENV-1 16,007 PDK-13, d Vaccine candidate DENV-1 16,007 PDK-13 in primary and DENV-1 genotype I in secondary. Double asterisks (**) indicate that there were statistically significant differences (p < 0.05) in the titers of neutralizing antibodies between genotypes. Student’s test analysis was used to determine the differences in mean titers of neutralizing antibody between CM vs AI, CM vs AA, and AI vs AA. CM indicates Cosmopolitan genotype. A1 indicates Asian I genotype. AA indicates Asian/American genotype
Other group of marmosets were immunized with DENV-1 16,007 PDK-13 (genotype II) and challenged with DENV-1 genotype I. Neutralizing antibody activity was not detected on days 0–14 after inoculation with DENV-1 16,007 PDK-13 (Fig. 3c). After challenge with DENV-1 genotype I (secondary infection), serotype cross-reactive antibodies to three genotypes of DENV-2 (Cosmopolitan, Asian I, and Asian/American genotype) were detected at day 14 p.i (Fig. 3d). The results are consistent with shown in Fig. 2c, and indicate that two inoculations with DENV-1 induced antibodies which neutralized 3 genotypes of heterologous serotype (DENV-2) as well as homologous serotype.
Neutralizing antibody responses after tertiary DENV infection
The neutralizing antibody responses to 3 genotypes of DENV-1 were also examined after tertiary DENV inoculation (group 10 and group 15). Marmosets which had been first inoculated with DENV-2 16,881 PDK-53 (Asian I genotype) and then challenged with DENV-2 genotype Cosmopolitan were infected with DENV-1 in tertiary inoculation (Fig. 4a). Neutralizing antibody to Cosmopolitan, Asian I and Asian/American genotypes were detected from day 0 p.i. The levels increased by day 14, but the titers were at similar levels amongst 3 genotypes of DENV-2 (40–320, p = 0.250). Neutralizing activity to DENV-1 genotype 1 was not detected except for one marmosets on day 14 (Fig. 4b).
Three marmosets that were first inoculated with the DENV-2 Asian I genotype and then challenged with the DENV-2 genotype Cosmopolitan were infected with DENV-1 genotype I in tertiary inoculation (Fig. 4c). Neutralizing antibody to Cosmopolitan, Asian I and Asian/American genotypes were detected prior to tertiary virus inoculation. The levels of neutralizing antibody to the Asian/American genotype increased and the titers were higher compared to those against the Cosmopolitan and Asian I genotypes at days 4, 7, and 14 p.i (pday4 = 0.029, pday7 = 0.017, and pday14 = 0.001 for the Cosmopolitan genotype, and pday4 = 0.029, pday7 = 0.034, and pday14 = 0.001 for the Asian I genotype). These marmosets (n = 3) demonstrated DENV-1 neutralizing antibodies at PRNT50 titers between 40 and 80 at day 14 p.i (Fig. 4d).
Discussion
It has been reported that after primary infection, serotype cross-reactive anti-E antibodies were induced, and neutralizing antibody levels were the highest for the infecting homologous serotype [17–20]. In the present study, we found that neutralizing antibodies induced during primary infection with a single genotype neutralized multiple genotypes within DENV-2. The levels of neutralizing antibody to the 3 genotypes of DENV-2 (Cosmopolitan, Asian I, and Asian/American genotypes) (group 4, Fig. 1b) were decreased 6-months after the primary infection (Fig. 2b, day 0) and was consistent with other studies using samples from non-human primates and human [21].
We found that marmosets that were inoculated with heterologous genotype/serotype infection had variable levels of neutralizing antibody in response to 3 different genotype of DENV-2 (Cosmopolitan, Asian I, and Asian/American genotypes) as compared to those marmosets with homologous genotype infection. In marmosets that were inoculated with secondary homologous genotype infection, neutralizing antibodies neutralized Cosmopolitan, Asian I and Asian/American genotypes at similar levels. Interestingly, in marmosets with the secondary heterologous genotype infection, levels of neutralizing antibodies to heterologous Asian/American genotype were significantly higher as compared to those against the Cosmopolitan and Asian I genotypes. Similar results were also observed during the tertiary heterologous serotype infection indicating that cross-neutralizing antibody responses consistently recognized similar antigens even though the marmosets were not inoculated with Asian/American genotypes [21]. In marmosets inoculated with DENV-2 vaccine candidate (Asian I genotype) and infected with Cosmopolitan genotype, antibody neutralized three genotypes of DENV-2 (Cosmopolitan, Asian I, and Asian/American), but neutralizing titers to Asian/American genotype was significantly higher than those to the Cosmopolitan and Asian I genotypes (Fig. 3b). These results are consistent with those obtained using isolated strains shown in Fig. 2b. In our study, the differences in the levels of neutralizing antibodies against 3 genotypes were most prominent when antibody titers were at high levels (PRNT50 = 1:640–20,480). Of note, although the differences were not significant in the primary infection with Asian 1 genotype, inoculation with heterologous genotype (Cosmopolitan) in the secondary infection induced high levels of antibodies that significantly better neutralized the Asian/American genotype than Asian I and Cosmopolitan genotypes. This pattern was not observed after two infections with homologous genotype (Cosmopolitan genotype) (Fig. 2a) and after two infections with heterologous serotypes (DENV-2 Asian 1 and DENV-1 genotype 1) (Fig. 2e). These results suggest that heterogeneity in antigenic molecules between genotypes may lead to the induction of higher levels of antibody to the epitope of the third genotype (Asian/American in the experiment), or synergize in neutralization of the third virus. However, elevated levels of neutralizing antibodies to the Asian/American genotype were not observed one year after secondary infection, when the levels of neutralizing antibodies decreased. These data suggest the presence of a subset of antibodies that better neutralize the Asian/American genotype, and that the subset and neutralizing effects were only observed when the overall neutralizing antibody activities were at high levels (Figs. 2b and 4c).
The marmosets presented neutralizing antibodies to DENV-2 genotypes after two infections with DENV-1 (DENV-1 GII in primary and DENV-1 GI in secondary infections) (Fig. 2c). These data suggest that serotype cross-reactive neutralizing antibodies are induced to some degrees, and that a robust secondary homologous immune response (mean secondary homologous DENV-1 PRNT50 = 1:907; primary DENV-1 PRNT50 = 1:60) may make serotype cross-reactive neutralizing antibody response more apparent. While infection with a single serotype typically leads to high levels of serotype-specific antibodies, it is likely that by two infections with homologous serotype, serotype cross-reactive antibodies may be boosted over threshold levels and are able to cross-neutralize other serotypes. In this regard, secondary homologous infection may partially contribute to serotype cross-protection in residents of DENV hyperendemic areas, due to a higher risk of repeated exposure to the same serotype. The next step would be to further expand our understanding of the spectrum of the neutralizing antibody patterns after heterologous and homologous infection, using different genotype and serotype combinations.
Cross-reactive neutralizing antibodies induced during sequential infection with different serotypes generally possess broad cross-reactivity and neutralizes multiple serotypes including non-infecting serotypes [19, 22, 23]. Previous studies from other groups on DEN cohort patients demonstrated that after heterologous secondary infection, neutralizing antibodies cross-react and neutralize multiple serotypes [24, 25] Notably, marmosets with heterologous serotype infection (primary DENV-2, secondary DENV-1) presented higher levels of neutralizing antibodies to previously infecting serotypes, compared to those against the secondary serotype. Studies examining maternal DENV antibodies demonstrated that the antibody decay could lead to partial protection and virus infection-enhancement [26, 27]. Similarly, although high levels of neutralizing antibodies to the previous infecting serotypes persisted for up to nine months after sequential infection, the antibody levels in marmosets decreased with time. Furthermore, the subset of antibodies that better neutralize the Asian/American genotype were diluted over time. Because this study was conducted based on one single technique and an animal model, further studies would be needed to address the correlation between levels of neutralizing antibody and protection against DENV infection. Previous studies have reported that during secondary homologous serotype infection, the marmosets were protected against re-infection with same serotype of DENV [14, 28]. In secondary heterologous serotype infection and tertiary heterologous infection, levels of viremia (4 × 102 – 2 × 105 pfu/ml) were still detected in the marmosets in groups 9, 10, and 15, at day 4–7 p.i when infected with different serotype of DENV (unpublished results). Thus, further studies using a larger set of data is expected to address the levels of neutralizing antibodies and protection against infection with heterologous serotypes.
Overall, this study highlighted that antibodies neutralize different genotypes within infecting serotypes, and that the levels were significantly different among different genotypes. While clinical trials of candidate vaccines demonstrated that vaccines are immunogenic in both humans and animal models, there are limited data available on the variability of neutralizing activities against different genotypes within serotype. Our study demonstrate that neutralizing antibodies induced by the Asian I and Cosmopolitan genotypes possessed higher neutralizing activities against the Asian/American genotype than against the homologous genotypes: Cosmopolitan and Asian I. The variability in the neutralizing antibodies titers between genotypes suggest that sequence heterogeneity between genotypes could result in complex genotype cross-reactive immune responses; leading to distinct neutralizing antibody patterns among the different genotypes [23, 29–32]. Similarly, marmosets inoculated with a candidate vaccine demonstrated variability in their neutralizing antibody responses comparable to those induced by the parental strain; suggesting that while attenuation leads to decreased pathogenicity, immunogenicity patterns between genotypes are retained. Thus, determination of the spectrum of neutralizing activity in marmosets in relation to the ability to offer protection to multiple serotypes and genotypes offers deep insights into dengue vaccine efficacy issues and disease control.
Conclusion
The present study demonstrated that different levels of neutralizing antibodies were induced against various DENV-2 genotypes after primary and secondary infections and, that neutralizing antibody titers to certain heterologous genotype were higher than those to homologous genotypes within a serotype. The result indicates that heterogeneity and homogeneity of genotypes in primary and secondary infections influence the levels and cross-reactivity of neutralizing antibodies induced after secondary infections. The results also suggest that certain genotypes may possess advantage in terms of breakthrough infections against dengue vaccination.
Additional files
Phylogenetic tree of the dengue virus serotype 2 strains that were used in this study. The phylogenetic tree was constructed by using the nucleotide sequence of the complete envelope protein region. Closed arrows indicates virus strains that were used in this study, DENV-2 strain DHF0663 belongs to genotype Cosmopolitan, DENV-2 strain 00–43 belongs to genotype Asian I, and DENV-2 strain 08–77 belongs to genotype Asian/American. (PDF 48 kb)
Levels of IgM antibody in plasma samples of the marmosets according to the group and type of infection. The levels of IgM antibody were determined using IgM ELISA. The positive detection of IgM is determined when the positive/negative ratio ≥ 2.0. Dash lines indicate the baseline of the positive detection of IgM antibody. (PDF 57 kb)
Acknowledgements
DENV-2 16881 PDK-53 and DENV-1 16007 PDK strains vaccine and parent strains were kindly provided by Dr. Sutee Yoksan, Institute of Molecular Biosciences, Mahidol University, Salaya, Nakhon Pathom, Thailand.
Funding
This work was supported by grants from the Research Program on Emerging and Re-emerging Infectious Diseases of the Japan Agency for Medical Research and Development (AMED), grant-in-aid for Scientific Research (Kiban B no.25293112) from the Ministry of Education, Culture, Sports, Science and Technology of Japan and MSD Life Science Foundation International. The marmosets of groups 4–6 were generously donated by CLEA, Japan. The funding bodies had no role in the study design, data collection, interpretation, or the decision to submit the work for publication.
Availability of data and materials
The datasets used and analyzed during the current study are available from the corresponding author upon reasonable request.
Abbreviations
- AA
Dengue virus 2 genotype Asian/American
- AI
Dengue virus 2 genotype Asian I
- CM
Dengue virus 2 genotype Cosmopolitan
- DENV
Dengue virus
- p.i
Post-inoculation
- PRNT
Plaque reduction neutralization test
Authors’ contributions
NAMA performed the immunoassay experiment, analysed data and drafted the manuscript. MLM provided reagents and materials, conceived the study and designed the experiment, performed experiments, analysed data and drafted the manuscript. YA provided reagents and materials, performed the experiments, analysed data and provided insights into the marmoset experiment. YS provided reagents and materials, performed experiments. CKL provided reagents and materials, performed experiment and provided insights for the data analysis. ST performed experiments and assisted in data analysis. MS provided insights for the data analysis and data interpretation. TT provided reagents and materials, performed and designed experiment, provided insight on data analysis. IK provided study materials, conceived the study, participated in the study design and coordination, and drafted the manuscript. All authors have read and approved the final version of the manuscript.
Ethics approval and consent to participate
The animal studies were conducted in accordance with the guidelines of the Institutional Animal Care and Use Committee of the National Institute of Infectious Diseases (NIID), Tokyo, Japan. The study was approved by the Institutional Animal Care and Use Committee of NIID (approval no. 613006 and 516,010). All animal and infection experiments were performed according to the NIID Institutional Guidelines, in additions to the guidelines of the Science Council, and local rules and regulations.
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
The online version of this article (10.1186/s12985-018-0967-x) contains supplementary material, which is available to authorized users.
Contributor Information
Nor Azila Muhammad Azami, Email: azila@nih.go.jp.
Meng Ling Moi, Email: sherry@nagasaki-u.ac.jp.
Yasushi Ami, Email: yami@nih.go.jp.
Yuriko Suzaki, Email: ysuzaki@nih.go.jp.
Chang-Kweng Lim, Email: ck@nih.go.jp.
Satoshi Taniguchi, Email: rei-tani@nih.go.jp.
Masayuki Saijo, Email: msaijo@nih.go.jp.
Tomohiko Takasaki, Email: takasaki.jp58@pref.kanagawa.jp.
Ichiro Kurane, Phone: +81-03-5285-1111, Email: kurane@nih.go.jp.
References
- 1.Halstead S. Dengue. 2008. [Google Scholar]
- 2.Guzman MC, Halstead SB, Artsob H, Buchy P, Jeremy F, Gubler DJ, et al. Dengue: a continuing global threat. Nat Rev Microbiol. 2010;8:S7–16. doi: 10.1038/nrmicro2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.WHO . Dengue guidelines for diagnosis, treatment, prevention and control: new edition. Geneva: World Health organization; 2009. [PubMed] [Google Scholar]
- 4.Waman VP, Kolekar P, Ramtirthkar MR, Kale MM, Kulkarni-Kale U. Analysis of genotype diversity and evolution of dengue virus serotype 2 using complete genomes. PeerJ. 2016;4:e2326. doi: 10.7717/peerj.2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Usme-Ciro JA, Méndez JA, Laiton KD, Páez A. The relevance of dengue virus genotypes surveillance at country level before vaccine approval. Hum Vaccin Immunother. 2014;10:2674–2678. doi: 10.4161/hv.29563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brien JD, Austin SK, Sukupolvi-Petty S, O’Brien KM, Johnson S, Fremont DH, et al. Genotype-specific neutralization and protection by antibodies against dengue virus type 3. J Virol. 2010;84:10630–10643. doi: 10.1128/JVI.01190-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Twiddy SS, Farrar JJ, Vinh Chau N, Wills B, Gould EA, Gritsun T, et al. Phylogenetic relationships and differential selection pressures among genotypes of dengue-2 virus. Virology. 2002;298:63–72. doi: 10.1006/viro.2002.1447. [DOI] [PubMed] [Google Scholar]
- 8.Moi ML, Takasaki T, Kurane I. Human antibody response to dengue virus: implications for dengue vaccine design. Trop Med Health. 2016;44:1. doi: 10.1186/s41182-016-0004-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morrison AC, Minnick SL, Rocha C, Forshey BM, Stoddard ST, Getis A, et al. Epidemiology of dengue virus in Iquitos, Peru 1999 to 2005: Interepidemic and epidemic patterns of transmission. PLoS Negl Trop Dis. 2010;4:e670. doi: 10.1371/journal.pntd.0000670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moi ML, Lim CK, Kotaki A, Takasaki T, Kurane I. Discrepancy in dengue virus neutralizing antibody titers between plaque reduction neutralizing tests with fc gamma receptor (fc gamma R)-negative and fc gamma R-expressing BHK-21 cells. Clin Vaccine Immunol. 2010;17:402–407. doi: 10.1128/CVI.00396-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rothman AL. Immunity to dengue virus: a tale of original antigenic sin and tropical cytokine storms. Nat Rev Immunol. 2011;11:532–543. doi: 10.1038/nri3014. [DOI] [PubMed] [Google Scholar]
- 12.Schieffelin JS, Costin JM, Nicholson CO, Orgeron NM, Fontaine KA, Isern S, et al. Neutralizing and non-neutralizing monoclonal antibodies against dengue virus E protein derived from a naturally infected patient. Virol J. 2010;7:28. doi: 10.1186/1743-422X-7-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bernardo L, Yndart A, Vázquez S, Morier L, Guzmán MG. Antibody responses to Asian and American genotypes of dengue 2 virus in immunized mice. Clin Diagn Lab Immunol. 2005;12:361–362. doi: 10.1128/CDLI.12.2.361-362.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Omatsu T, Moi ML, Hirayama T, Takasaki T, Nakamura S, Tajima S, et al. Common marmoset (Callithrix jacchus) as a primate model of dengue virus infection: development of high levels of viraemia and demonstration of protective immunity. J Gen Virol. 2011;92:2272–2280. doi: 10.1099/vir.0.031229-0. [DOI] [PubMed] [Google Scholar]
- 15.Moi MLL, Takasaki T, Omatsu T, Nakamura S, Katakai Y, Ami Y, et al. Demonstration of marmosets (Callithrix jacchus) as a non-human primate model for secondary dengue virus infection: high levels of viraemia and serotype cross-reactive antibody responses consistent with secondary infection of humans. J Gen Virol. 2014;95:591–600. doi: 10.1099/vir.0.060384-0. [DOI] [PubMed] [Google Scholar]
- 16.Omatsu T, Moi ML, Takasaki T, Nakamura S, Katakai Y, Tajima S, et al. Changes in hematological and serum biochemical parameters in common marmosets (Callithrix jacchus) after inoculation with dengue virus. J Med Primatol. 2012;41:289–296. doi: 10.1111/j.1600-0684.2012.00552.x. [DOI] [PubMed] [Google Scholar]
- 17.de Alwis R, Beltramello M, Messer WB, Sukupolvi-Petty S, Wahala WMPB, Kraus A, et al. In-depth analysis of the antibody response of individuals exposed to primary dengue virus infection. PLoS Negl Trop Dis. 2011;5:e1188. doi: 10.1371/journal.pntd.0001188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rothman AL, Innis B, Houghten R, Henchal L, Muller-Eberhard H. Dengue: defining protective versus pathologic immunity. J Clin Invest. 2004;113:946–951. doi: 10.1172/JCI21512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mathew A, West K, Kalayanarooj S, Gibbons RV, Srikiatkhachorn A, Green S, et al. B-cell responses during primary and secondary dengue virus infections in humans. J Infect Dis. 2011;204:1514–1522. doi: 10.1093/infdis/jir607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lai C-Y, Tsai W-Y, Lin S-R, Kao C-L, Hu H-P, King C-C, et al. Antibodies to envelope glycoprotein of dengue virus during the natural course of infection are predominantly cross-reactive and recognize epitopes containing highly conserved residues at the fusion loop of domain II. J Virol. 2008;82:6631–6643. doi: 10.1128/JVI.00316-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Katzelnick LC, Fonville JM, Gromowski GD, Arriaga JB, Green A, James SL, et al. Dengue viruses cluster antigenically but not as discrete serotypes. Science (80- ) 2015;349:1338–1343. doi: 10.1126/science.aac5017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mathew A, Kurane I, Green S, Stephens HA, Vaughn DW, Kalayanarooj S, et al. Predominance of HLA-restricted cytotoxic T-lymphocyte responses to serotype-cross-reactive epitopes on nonstructural proteins following natural secondary dengue virus infection. J Virol. 1998;72:3999–4004. doi: 10.1128/jvi.72.5.3999-4004.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.WMPB W, De Silva AM. The human antibody response to dengue virus infection. Viruses. 2011;3:2374–95. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3280510. Cited 22 May 2017. [DOI] [PMC free article] [PubMed]
- 24.Kosasih H, Yusuf H, Sudjana P, Alisjahbana B, Wuryadi S, Irsiana Tan R, et al. Report of four volunteers with primary, secondary and tertiary dengue infections during a prospective cohort study. Dengue Bull. 2006;30 Available from: http://apps.who.int/iris/bitstream/10665/170253/1/db2006v30p87.pdf. Cited 23 May 2017.
- 25.Corbett KS, Katzelnick L, Tissera H, Amerasinghe A, de Silva AD, de Silva AM. Preexisting neutralizing antibody responses distinguish clinically Inapparent and apparent dengue virus infections in a Sri Lankan pediatric cohort. J Infect Dis. 2015;211:590–599. doi: 10.1093/infdis/jiu481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guzman MG, Vazquez S. The complexity of antibody-dependent enhancement of dengue virus infection. Viruses. 2010;2:2649–2662. doi: 10.3390/v2122649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nimmanitya S, Kliks SC, Burke DS, Nisalak A. Evidence that maternal dengue antibodies are important in the development of dengue hemorrhagic fever in infants. Am J Trop Med Hyg. 1988;38:411–419. doi: 10.4269/ajtmh.1988.38.411. [DOI] [PubMed] [Google Scholar]
- 28.Moi ML, Ami Y, Muhammad Azami NA, Shirai K, Yoksan S, Suzaki Y, et al. Marmosets (Callithrix jacchus) as a non-human primate model for evaluation of candidate dengue vaccines: induction and maintenance of specific protective immunity against challenges with clinical isolates. J Gen Virol. 2017; Available from: http://jgv.microbiologyresearch.org/content/journal/jgv/10.1099/jgv.0.000913.v1. Cited 22 May 2017. [DOI] [PubMed]
- 29.Leitmeyer KC, Vaughn DW, Watts DM, Salas R, Villalobos I, Chacon d, et al. Dengue virus structural differences that correlate with pathogenesis. J Virol. 1999;73:4738–4747. doi: 10.1128/jvi.73.6.4738-4747.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen Y, Maguire T, Marks RM. Demonstration of binding of dengue virus envelope protein to target cells. J Virol. 1996;70:8765–8772. doi: 10.1128/jvi.70.12.8765-8772.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zulueta A, Martín J, Hermida L, Alvarez M, Valdés I, Prado I, et al. Amino acid changes in the recombinant dengue 3 envelope domain III determine its antigenicity and immunogenicity in mice. Virus Res. 2006;121:65–73. doi: 10.1016/j.virusres.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 32.Sánchez MD, Pierson TC, McAllister D, Hanna SL, Puffer BA, Valentine LE, et al. Characterization of neutralizing antibodies to West Nile virus. Virology. 2005;336:70–82. doi: 10.1016/j.virol.2005.02.020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Phylogenetic tree of the dengue virus serotype 2 strains that were used in this study. The phylogenetic tree was constructed by using the nucleotide sequence of the complete envelope protein region. Closed arrows indicates virus strains that were used in this study, DENV-2 strain DHF0663 belongs to genotype Cosmopolitan, DENV-2 strain 00–43 belongs to genotype Asian I, and DENV-2 strain 08–77 belongs to genotype Asian/American. (PDF 48 kb)
Levels of IgM antibody in plasma samples of the marmosets according to the group and type of infection. The levels of IgM antibody were determined using IgM ELISA. The positive detection of IgM is determined when the positive/negative ratio ≥ 2.0. Dash lines indicate the baseline of the positive detection of IgM antibody. (PDF 57 kb)
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author upon reasonable request.


