SUMMARY
Cardiac stem/progenitor cells hold great potential for regenerative therapies; however, the mechanisms regulating their expansion and differentiation remain insufficiently defined. Here we show that Ldb1 is a central regulator of genome organization in cardiac progenitor cells, which is crucial for cardiac lineage differentiation and heart development. We demonstrate that Ldb1 binds to the key regulator of cardiac progenitors, Isl1, and protects it from degradation. Furthermore, the Isl1/Ldb1 complex promotes long-range enhancer-promoter interactions at the loci of the core cardiac transcription factors Mef2c and Hand2. Chromosome conformation capture followed by sequencing identified specific Ldb1-mediated interactions of the Isl1/Ldb1 responsive Mef2c anterior heart field enhancer with genes that play key roles in cardiac progenitor cell function and cardiovascular development. Importantly, the expression of these genes was downregulated upon Ldb1 depletion and Isl1/Ldb1 haplodeficiency. In conclusion, the Isl1/Ldb1 complex orchestrates a network for heart-specific transcriptional regulation and coordination in three-dimensional space during cardiogenesis.
In Brief
Caputo et al. identify Ldb1 as a crucial regulator of cardiogenesis. Ldb1 binds the key cardiac transcription factor Isl1 and protects it from degradation. The stabilized Isl1/Ldb1 complex orchestrates a network for transcriptional regulation and coordination in three-dimensional space, driving cardiac progenitor cell differentiation and heart development.
INTRODUCTION
Heart failure is the leading cause of morbidity and mortality worldwide. A number of cardiac regenerative strategies have been proposed, among which stem/progenitor cells hold great promise for heart repair (Aguirre et al., 2013; Hansson et al., 2009). Knowledge accumulated from developmental studies has significantly improved the methods for in vitro cardiac differentiation of embryonic stem cells (ESCs) and studies utilizing ESC differentiation have brought further insights into the regulatory networks that integrate multiple transcription factors and signaling molecules and strictly control the distinct steps of cardiogenesis. However, the current limitations for the use of stem/progenitor cells in regenerative medicine, e.g., those linked to their expansion, differentiation efficiency, and functional integration, call for a more complete understanding of the mechanisms driving cardiovascular lineage commitment and differentiation.
During embryogenesis the heart is generated by a common progenitor at gastrulation that segregates into two distinct populations, termed first and second heart fields. The first heart field (FHF) fuses at the midline and differentiates into the myocardium of the heart tube. After the initial heart tube formation, the heart tube grows by the addition of Isl1-positive second heart field (SHF) progenitor cells to its anterior and venous poles (Cai et al., 2003; Evans et al., 2010; Vincent and Buckingham, 2010). Studies in different model systems revealed the crucial function of the LIM-homeodomain (LIM-HD) transcription factor Isl1 in heart morphogenesis (Cai et al., 2003; de Pater et al., 2009; Witzel et al., 2012). Isl1-deficient mouse embryos lack the right ventricle and the outflow tract, both structures derived from the SHF, as Isl1 is required for the proliferation, survival, and migration of these cells into the forming heart (Cai et al., 2003). Importantly, the Isl1-positive cardiovascular progenitors are multipotent and can differentiate into all three cardiovascular lineages: cardiomyocytes, smooth muscle cells, and endothelial cells (Moretti et al., 2006). Moreover, Isl1 is required for the differentiation of these cells into the cardiomyocyte and smooth muscle lineage (Kwon et al., 2009), but the mechanisms underlying its function are poorly understood.
The acquisition of cellular identity involves genome reorganization and a coordinated series of large-scale transcriptional changes (Dixon et al., 2015; Gorkin et al., 2014; Peric-Hupkes et al., 2010). Studies using chromosome conformation capture (3C) assays and 3C-based technologies (de Wit and de Laat, 2012; Dekker et al., 2002) have suggested that CTCF together with Cohesin might be involved in general formation of chromatin structures by promoting constitutive long-range DNA interactions, whereas specific transcription factors and their cofactors together with CTCF, Cohesin, and the Mediator complex might be involved in controlling locus-specific looping interactions and lineage-specific transcription (Kagey et al., 2010; Phillips-Cremins et al., 2013; Shen et al., 2012). In ESCs for example, the binding of the key pluripotency transcription factors Oct4, Sox2, and Nanog was shown to be a key determinant of genome organization, as regions with a high density of binding sites for these factors tend to colocalize in nuclear space (de Wit et al., 2013; Denholtz et al., 2013). Another example for a cofactor involved in promoting locus-specific and cell-type-specific interactions is LIM-domain-binding protein 1 (Ldb1) (Deng et al., 2012; Morcillo et al., 1997; Soler et al., 2010; Song et al., 2007). Ldb1 directly binds to LIM domain proteins and Otx proteins and is found in large complexes with bHLH and GATA transcription factors (Bach et al., 1997; Meier et al., 2006; Tripic et al., 2009). By bringing together distinct transcription factors and their co-regulators that are associated with different transcriptional control elements, Ldb1 facilitates long-range enhancer-promoter interactions and regulates cell-type-specific expression patterns (Deng et al., 2012; Soler et al., 2010; Song et al., 2007).
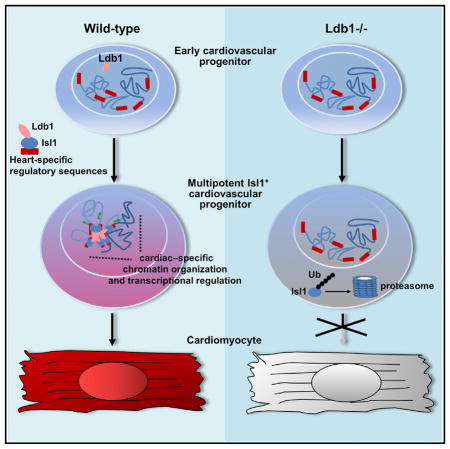
Here we show that Ldb1 is a multifunctional regulator of cardiac progenitor cell differentiation and SHF development. On the one hand, Ldb1 binds to Isl1 and protects it from proteasomal degradation, which leads to an almost complete loss of Isl1+ cardiovascular progenitor cells upon Ldb1 deficiency. On the other hand, the Isl1/Ldb1 complex plays a central role in transcriptional regulation and chromatin organization in three-dimensional space, driving cardiac progenitor cell differentiation and SHF development.
RESULTS
Ldb1 Regulates Cardiac Progenitor Cell Differentiation and SHF Development
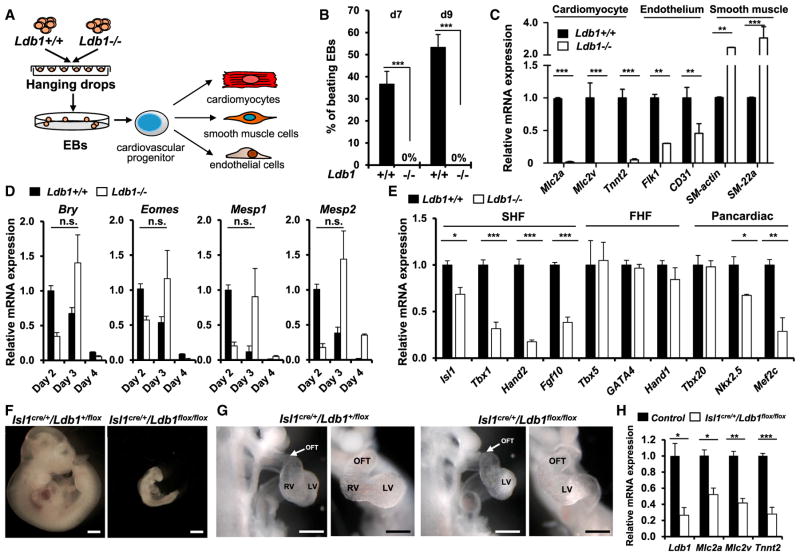
The LIM domain transcription factor Isl1 is required for proliferation, survival, and differentiation of SHF cardiac progenitor cells (Cai et al., 2003). The cofactor Ldb1 regulates spatial chromatin organization and Ldb1 deficiency results in early embryonic lethality with a series of developmental defects, including lack of heart formation (Mukhopadhyay et al., 2003; Mylona et al., 2013). Since Ldb1 is broadly expressed, it is attractive to speculate that via its diverse DNA binding partners, it mediates distinct long-range interactions, leading to cell-type-specific transcriptional programs. Therefore, we reasoned that Isl1 and Ldb1 might work in concert to regulate cardiac progenitor cell function. To elucidate the role of Ldb1 in cardiogenesis, we first utilized WT and Ldb1 knockout (Ldb1−/−) ESCs and differentiated them in embryoid bodies (EBs) to facilitate the generation and differentiation of cardiac progenitors (Figure 1A). Importantly, in contrast to control EBs, which started beating already at day 6 (d6), the EBs differentiated from Ldb1−/− cells showed no beating (Figure 1B). Consistent with this, the expression of cardiomyocyte marker genes was dramatically reduced (Figure 1C). Additionally, the expression of endothelial markers was also downregulated, whereas the expression of smooth muscle genes was upregulated (Figure 1C), suggesting that Ldb1 is important for proper differentiation into cardiomyocyte and endothelial cell lineages. Next, we analyzed whether Ldb1 deficiency affects early developmental decisions, e.g., mesoderm induction, which could subsequently affect cardiac progenitor cell differentiation. Real-time PCR analysis for mesoderm markers (Eomes and Bry) and cardiac mesoderm markers (Mesp1 and Mesp2) showed no significant difference in the peak expression level between control and Ldb1-deficient EBs; however, the peak expression of these markers was delayed by one day (Figure 1D). Next, we analyzed the expression of pancardiac genes and genes specifically expressed in the FHF and the SHF (Figure 1E). Interestingly, while the expression of Isl1, Tbx1, Hand2, and Fgf10, which play important roles in the SHF, and the pancardiac genes Nkx2-5 and Mef2c was significantly downregulated at d4, d5, and d6, genes expressed in the FHF (Tbx5, Hand1, and Gata4) showed no significant change (Figure 1E, data not shown), suggesting a role of Ldb1 in the regulation of SHF progenitor cells. To further investigate the role of Ldb1 in SHF development, we ablated Ldb1 using an Isl1-Cre driver line that results in Cre recombination in SHF progenitor cells (Cai et al., 2003). Isl1-Cre:Ldb1flox/flox embryos died by E10.5 with shortened outflow tract and small right ventricle (Figures 1F–1G, Figure S1A). Furthermore, we observed significant downregulation of cardiomyocyte marker genes in the right ventricle and the outflow tract, both SHF-derived structures, whereas no change in these genes was observed in the left ventricle, derived mainly from the FHF (Figure 1H, Figure S1B). Taken together, these results confirm that Ldb1 is essential for cardiac progenitor cell differentiation and SHF development.
Figure 1. Ablation of Ldb1 Results in Defects in Cardiac Progenitor Cell Differentiation and SHF Development.
(A) Schematic diagram of the experimental setup.
(B) Percentage of beating EBs in WT and Ldb1−/− ESCs.
(C) Relative mRNA expression of cardiomyocyte (Mlc2a, Mlc2v, and Tnnt2), endothelial (Flk-1 and CD31), and smooth muscle (SM-actin, SM-22α) genes in d9 EBs differentiated from WT and Ldb1−/− ESCs.
(D) Relative mRNA expression of mesodermal markers (Eomes, Bry, Mesp1, and Mesp2) in EBs differentiated from control and Ldb1−/− ESCs at different days.
(E) Relative mRNA expression of cardiac progenitor marker genes in d4 EBs differentiated from Ldb1+/+ and Ldb1−/− ESCs.
(F) Gross appearance of control (Isl1cre/+/Ldb1+/flox) and Isl1cre/+/Ldb1flox/flox embryos at E10.5. Scale bars, 500 μm.
(G) Higher magnification of E9.5 embryos viewed from the right (left panels) and the front (right panels). Scale bars, 200 μm. OFT, outflow tract; RV, right ventricle; LV, left ventricle.
(H) Relative mRNA expression analysis of Ldb1 and cardiomyocyte genes in dissected outflow tract and right ventricle of E9.25 WT and Isl1cre/+/Ldb1flox/flox embryos. Data are mean ± SEMs, n = 3 for each genotype.
Data in (B)–(E) are mean ± SEMs, n = 3. *p < 0.05, **p < 0.01, ***p < 0.005. See also Figure S1.
Ldb1 Loss Does Not Affect Early Cardiovascular Progenitor Cell Commitment but Leads to a Compete Loss of Isl1+ Cells
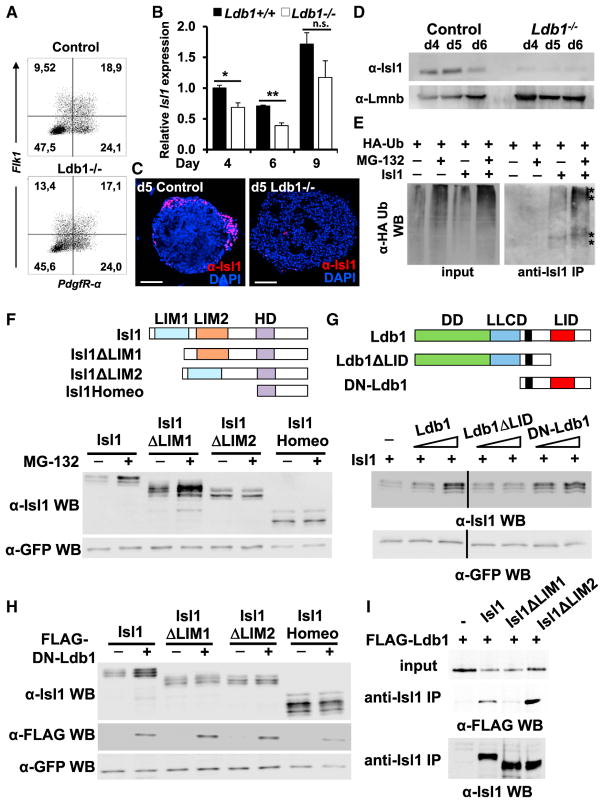
To gain a better understanding of Ldb1 function in cardiogenesis, we analyzed cardiovascular progenitors in more detail. FACS analysis for Flk-1 and PdgfR-α demonstrated no significant differences in early cardiovascular progenitor numbers (Figure 2A). Interestingly, although Isl1 mRNA levels were only slightly reduced in EBs differentiated from Ldb1−/− ESCs (Figures 1E and 2B), Isl1+ cells were virtually absent in Ldb1−/− EBs (Figure 2C). Western blot analysis confirmed the dramatically reduced levels of Isl1 in Ldb1-deficient EBs (Figure 2D). The absence of Isl1 protein, without pronounced changes of Isl1 mRNA levels, suggested that Isl1 is regulated at the protein level. Pulldown of Isl1 detected slower-migrating ubiquitinated forms of Isl1 that were increased in the presence of the proteasomal inhibitor MG-132, indicating that Isl1 is targeted for proteasomal degradation (Figure 2E). To analyze this in more detail, we transfected HEK293T cells with Isl1 deletion constructs lacking the LIM1 and/or LIM2 domain and treated them with MG-132. The levels of the truncated proteins lacking LIM2 or containing only the homeodomain were relatively unchanged, but the levels of the Isl1 protein lacking LIM1 were significantly increased upon MG-132 treatment, suggesting that LIM2 is subjected to ubiquitination (Figure 2F). Next, we analyzed whether binding of Ldb1 to Isl1 might protect it from proteasomal degradation, similarly to Lhx3 (Güngör et al., 2007). To assess this possibility we transfected HEK293T cells with an Isl1 plasmid alone or together with increasing amounts of Ldb1 or Ldb1 deletion constructs and constant amounts of GFP, which served as a control for transfection efficiency (Figure 2G). Importantly, increasing levels of Ldb1 and truncated Ldb1 protein lacking the dimerization domain (DN-Ldb1) led to a significant increase of Isl1 protein levels, but did not change the levels of truncated Isl1 proteins lacking either the LIM1 or LIM2 domain or harboring only the Isl1 homeodomain (Figures 2G and 2H). Furthermore, a truncated Ldb1 protein lacking the LIM-interaction domain (LID) did not have an effect on Isl1 protein levels (Figure 2G). Co-immunoprecipitations (co-IPs) using Isl1 deletion constructs revealed a critical role of the LIM1 domain of Isl1 in mediating the interaction with Ldb1 (Figure 2I), corroborating previous findings (Jurata et al., 1996). Together, these data suggest that the binding of Ldb1 to the LIM1 domain of Isl1 protects it from ubiquitination at the LIM2 domain and from subsequent proteasomal degradation (Figure 7E).
Figure 2. Ldb1 Binds to Isl1 and Protects It from Proteasomal Degradation.
(A) FACS analysis of Flk-1 and PdgfR-α expression in d3.75 EBs differentiated from control and Ldb1−/− ESCs.
(B) Relative mRNA expression of Isl1 in EBs differentiated from control and Ldb1−/− ESCs at different days. Data are mean ± SEMs, n = 3. *p < 0.05, **p < 0.01.
(C) Isl1 immunostaining on vibratome sections from d5 EBs differentiated from control and Ldb1−/− ESCs. Scale bars, 100 μm.
(D) Western blot analysis of total protein extracts of day 4, 5, and 6 EBs differentiated from control and Ldb1−/− ESCs. Lamin B1 served as loading control.
(E) HA-tagged ubiquitin and Isl1 were transiently expressed in HEK293T cells. Cells were either treated with DMSO or with MG-132 6 hr before being harvested. Equivalent amounts of total cellular protein were immunoprecipitated with an anti-Isl1 antibody and detected with an anti-HA antibody.
(F) Schematic representation of Isl1 and Isl1 deletion constructs lacking either LIM1 or LIM2 or harboring only the homeodomain (top). Western blot analysis of whole-cell lysates of HEK293T cells transfected with Isl1, Isl1ΔLIM1, Isl1ΔLIM2, and Isl1Homeo expression plasmids, treated with DMSO or MG-132 is shown.
(G) Schematic representation of Ldb1 and Ldb1 deletion constructs. Ldb1 harbors three domains important for its function: dimerization domain (DD), the Ldb1/Chip conserved domain (LLCD), and the LIM-interaction domain (LID) (top panel). HEK293T cells were transfected with constant amounts of Isl1 (10 μg) and GFP (1 μg, used as a control of transfection efficiency) expression plasmids and increasing amounts of Ldb1, Ldb1ΔLID, and DN-Ldb1 (5 and 9 μg). Immunoblot analysis of equal amounts of total protein extracts was performed using either anti-Isl1 or anti-GFP antibodies.
(H) Western blot analysis of whole-cell lysates from HEK293T cells transfected with Isl1, Isl1ΔLIM1, Isl1ΔLIM2 and Isl1Homeo alone or together with DN-Ldb1 and GFP using anti-Isl1, anti-FLAG, and anti-GFP antibodies.
(I) FLAG-HA-Ldb1 and Isl1 or Isl1 deletion constructs were transiently expressed in HEK293T cells and immunoprecipitation with an anti-Isl1 antibody was followed by immunoblot analysis with an anti-FLAG antibody.
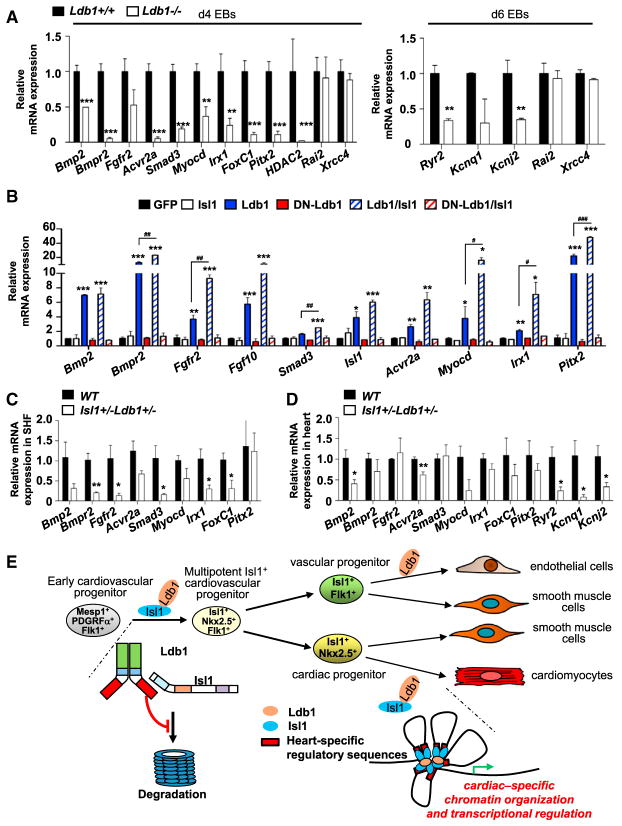
Figure 7. The Isl1/Ldb1 Complex Orchestrates a Network for Transcriptional Regulation and Coordination in Three-Dimensional Space, Driving Cardiogenesis.
(A) Relative mRNA expression analysis in d4 and d6 WT and Ldb1−/− EBs of selected genes identified in the 3C-seq analysis to specifically interact with the Mef2c-AHF in WT, but not in Ldb1−/− EBs.
(B) Relative mRNA expression analysis of selected genes in d5 Ldb1−/−EBs overexpressing either GFP alone or GFP together with Isl1, Ldb1, DN-Ldb1, or these in different combinations.
(C and D) Relative mRNA expression analysis of selected genes in SHF (C) or heart (D) of WT or Isl1+/− Ldb1+/− E9.25 embryos. Data are shown as mean ± SEMs, n = 4. *p < 0.05, **p < 0.01, ***p < 0.005.
(E) Model of the role of Ldb1 in cardiac lineage differentiation. Ldb1 binds to Isl1 and protects it from proteasomal degradation. The stabilized Isl1/Ldb1 complex orchestrates a network for transcriptional regulation and coordination in three-dimensional space, driving cardiac progenitor cell differentiation and heart development.
Ldb1 and Isl1 Physically, Functionally, and Genetically Interact to Regulate Cardiac Progenitor Cell Differentiation and Heart Development
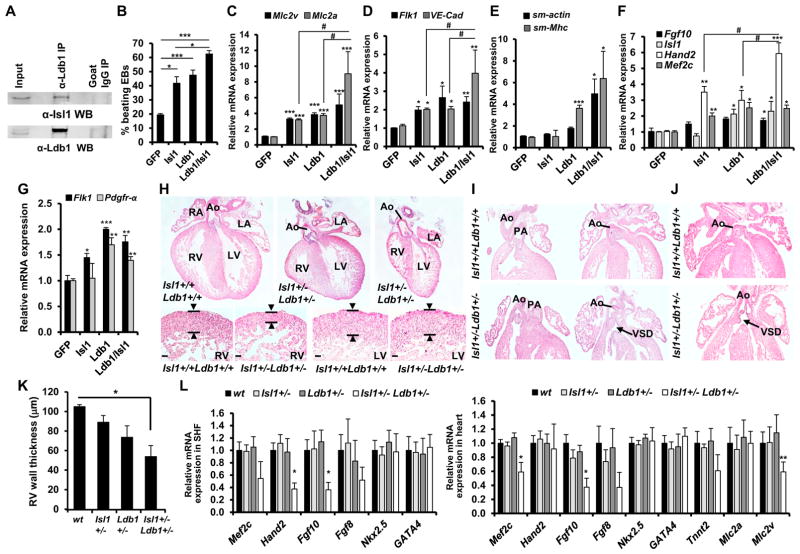
Ldb1 plays fundamental roles in development and cell differentiation as a cofactor for LIM-domain proteins, suggesting that Ldb1 and Isl1 might functionally interact with each other during cardiogenesis. To test this, we first confirmed that Ldb1 and Isl1 interact in cardiac progenitor cells expressing endogenous levels of each protein by performing immunoprecipitation of Ldb1 from nuclear extracts of d5 EBs, a stage enriched in cardiac progenitors (Figure 3A). Next, we transfected mouse ESCs with an expression plasmid carrying GFP alone or together with Isl1, Ldb1, or both Isl1 and Ldb1 (Figures S2A and S2B). GFP-expressing cells, isolated by FACS, were subjected to differentiation in EBs. Overexpression of Isl1 or Ldb1 alone significantly increased the percentage of beating EBs. Importantly, EBs differentiated from Isl1/Ldb1-overexpressing cells showed a higher number of beating foci compared to control EBs or EBs overexpressing Isl1 or Ldb1 alone, which had similar levels of Isl1 and Ldb1 compared to Isl1/Ldb1-overexpressing cells (Figure 3B, Figures S2A and S2B). Consistently, the expression of cardiomyocyte marker genes was markedly increased and we observed a significant synergistic effect of Isl1 and Ldb1 on Mlc2a expression (Figure 3C). Furthermore, the expression of endothelial and smooth muscle markers was also increased (Figures 3D and 3E). Moreover, cardiac progenitor genes (endogenous Isl1, Mef2c, Hand2, and Fgf10) were significantly upregulated (Figure 3F). Interestingly, overexpression of Ldb1 significantly increased Flk-1+PdgfR-α+ cardiovascular progenitor numbers and the expression of Flk-1 and PDGFR-α (Figure 3G, Figure S2C), which may account for the increased expression of markers of all major cardiovascular lineages in Ldb1 and Isl1/Ldb1-overexpressing cells. Finally, we tested whether Isl1 and Ldb1 genetically interact during heart development. Crossing of Isl1 heterozygous and Ldb1 heterozygous mice revealed that only 5% of pups were compound heterozygotes at the weaning stage, whereas an expected ratio of 25% was observed during mid- to late gestation (Figure S2D), suggesting that the compound heterozygotes die after birth. Histological analysis revealed that Isl1/Ldb1 double haplodeficient embryos had various heart abnormalities, including a small and thin right ventricle, ventricular septal defect (VSD), overriding aorta (OA), and double outlet right ventricle (DORV) (Figures 3H–3K). To analyze the primary cause of the observed heart defects we microdissected the heart and the SHF from WT, Isl1+/−, Ldb1+/−, and Isl1+/−Ldb1+/− E9.25 embryos. Importantly, real-time PCR analysis revealed significant downregulation of Hand2, Mef2c, Fgf10, and Mlc2v expression in Isl1+/−Ldb1+/−, supporting a key role of the Isl1/Ldb1 complex in cardiomyocyte differentiation and Mef2c, Hand2, and Fgf10 expression (Figure 3L).
Figure 3. Ldb1 and Isl1 Interact to Regulate Cardiogenesis.
(A) Co-immunoprecipitation of nuclear extracts from d5 EBs using anti-Ldb1 antibody and detected with anti-Isl1 antibody.
(B) Percentage of beating d7 EBs derived from ESCs overexpressing either GFP alone (control) or GFP together with Isl1, Ldb1, or both.
(C–E) Relative mRNA expression of cardiomyocyte (C), endothelial (D), and smooth muscle marker genes (E) in d7 EBs differentiated from ESCs overexpressing either GFP alone or GFP together with Isl1, Ldb1, or Ldb1+Isl1.
(F and G) Relative mRNA expression of cardiac progenitor marker genes in d4 EBs (F) and Flk-1 and PdgfR-α in d3.75 EBs (G) differentiated from ESCs over-expressing either GFP alone or GFP together with Isl1, Ldb1, or Ldb1+Isl1. For qPCR analysis of endogenous Isl1 levels, primers located in the 5′ UTR were utilized.
(H–J) H&E staining of representative paraffin sections of E16.5 hearts of WT and Isl1+/−Ldb1+/− embryos (H, top panels), and higher magnification of right and left ventricles showing thinner compact myocardium of the right ventricle in Isl1+/−Ldb1+/− embryos (H, bottom panels), DORV (I), or OA in E18.5 hearts (J) with VSD (I and J). Ao, Aorta; PA, pulmonary artery; LA, left atrium; LV, left ventricle; RA, right atrium; RV, right ventricle; DORV, double outlet right ventricle; OA, overriding aorta; VSD, ventricular septal defect.
(K) Morphometric analysis of right ventricle compact myocardial thickness. Data are mean ± SEMs, n = 4.
(L) Relative mRNA expression analysis of cardiac progenitor and cardiomyocyte genes in dissected hearts and SHF of E9.25 WT, Isl1+/−, Ldb1+/− (controls), and Isl1+/−Ldb1+/− embryos. Data are mean ± SEMs, n = 4 for each genotype. *p < 0.05, **p < 0.01, ***p < 0.005.
Data in (B)–(G) are mean ± SEMs, n = 3. *p < 0.05, **p < 0.01, ***p < 0.005. See also Figure S2.
The Dimerization Domain of Ldb1 Is Required for SHF Development and Cardiac Progenitor Cell Differentiation
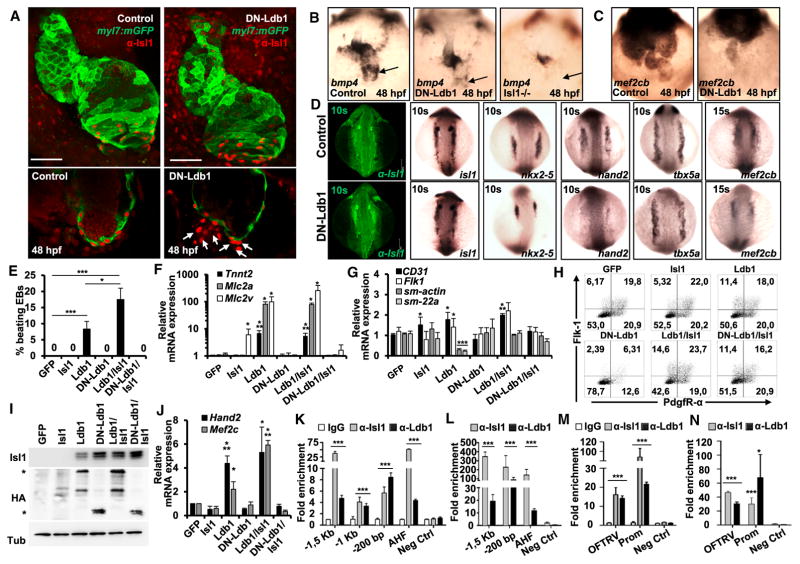
Next, we ectopically overexpressed a truncated form of Ldb1 lacking the dimerization domain (DN-Ldb1, Figure 2G, Figure S3A) in embryos of the zebrafish transgenic line Tg(myl7:EGFP-HsHRAS)s883, which allows the easy monitoring of cardiac morphology (D’Amico et al., 2007). DN-Ldb1 contains the highly conserved LID and can stabilize Isl1 protein levels (Figures 2G and 2H), but lacks the dimerization domain necessary to promote long-range enhancer-promoter interactions. Overexpression of this mutant DN-Ldb1 protein results in a competition with WT Ldb-s for binding to LIM-HD proteins as it acts in a dominant-negative manner (Bach et al., 1999; Becker et al., 2002). Consistent with previous studies, overexpression of DN-Ldb1 led to defects in eye and brain development (Figure S3B; (Becker et al., 2002)). Additionally, we observed that the heart contracted irregularly and with a reduced frequency (Figures S3C and S3D) in the embryos expressing DN-Ldb1. Furthermore the atrium of DN-Ldb1-expressing embryos was significantly shorter (Figure S3E). A similar phenotype was observed in isl1 mutant zebra-fish embryos as a result of a failure of cardiomyocyte differentiation at the venous pole (de Pater et al., 2009). Confocal images of control and DN-Ldb1-expressing embryos stained with anti-Isl1 antibodies at 48 hours post-fertilization (hpf) revealed dramatically fewer Isl1+ cardiomyocytes at the venous pole of the heart. In mutants, Isl1+ cells were found outside of the heart, but did not express the cardiomyocyte marker myl7, supporting a key role of Ldb1 dimerization in Isl1+ cardiomyocyte differentiation (Figure 4A). Further, bmp4 expression at the sinus venosus was strongly reduced in DN-Ldb1-expressing embryos, in striking parallel to isl1 mutant embryos (Figure 4B). Additionally, the expression of mef2cb was also downregulated (Figure 4C). Next, we addressed whether this phenotype can be attributed to defects at early stages of cardiogenesis by performing in situ hybridization and whole-mount anti-Isl1 immunostaining of control and DN-Ldb1-overexpressing embryos at the 10 and 15 somites stage. No significant change of isl1 mRNA expression was detected (Figure 4D). However, Isl1 staining appeared to be stronger in the injected embryos, consistent with the stabilizing effect of DN-Ldb1 on Isl1 protein levels (Figure 4D, Figure 2). Importantly, we observed strong downregulation of hand2, mef2cb, and nkx2-5, which play important roles in the SHF development (Figure 4D), whereas no change was observed in tbx5a expression. These findings suggest that not only the stabilization of Isl1 protein levels but also the formation of higher-order complexes mediated by the Ldb1 dimerization domain might be important for proper cardiac progenitor cell differentiation during SHF development. To gain further support of this hypothesis, we generated stable Ldb1-deficient ESC lines overexpressing GFP alone, Isl1, Ldb1, DN-Ldb1, or these in combinations. Consistent with an important role of Ldb1 in regulating Isl1 stability, Isl1-overexpressing Ldb1−/− ESCs showed dramatically lower Isl1 protein levels compared to Ldb1−/− ESCs overexpressing Isl1 in combination with Ldb1 or the DN-Ldb1 (Figure S4A), although Isl1 mRNA levels were similar (Figure S4B). Importantly, Ldb1 overexpression led to a rescue of cardiac differentiation, as measured by the increased percentage of beating EBs and mRNA levels of cardiomyocyte marker genes (Figures 4E and 4F). The functional rescue was further potentiated by overexpression of Isl1, confirming a synergistic role of these proteins in cardiomyocyte differentiation. By contrast, overexpression of DN-Ldb1 alone or in combination with Isl1 did not rescue the complete loss of cardiac differentiation of Ldb1-deficient EBs (Figures 4E and 4F). Furthermore, the expression of endothelial marker genes was increased in Ldb1−/− EBs overexpressing Ldb1, whereas smooth muscle genes were decreased by Ldb1 overexpression (Figure 4G), supporting a role of Ldb1 in the differentiation of cardiovascular progenitors into the cardiomyocyte and endothelial cell lineages and suppression of the smooth muscle lineage. FACS analysis for Flk-1 and PdgfR-α showed no significant differences in the Flk-1+PdgfR-α+ cardiovascular progenitor numbers upon Ldb1 and Isl1/Ldb1 overexpression in Ldb1-deficient EBs (Figure 4H), demonstrating that the dramatically increased cardiac differentiation is not due to an increased induction of cardiovascular progenitor cells. However, we observed a pronounced decrease of the Flk-1+/PdgfR-α+ population upon DN-Ldb1 expression (Figure 4H). Interestingly this decrease was rescued by Isl1 overexpression in combination with DN-Ldb1 (Figure 4H), suggesting that the decrease in cardiovascular progenitor cells upon DN-Ldb1 overexpression might be due to interference with the function of LIM only or LIM-HD proteins. Furthermore, we saw rescue of Isl1+ cells and Isl1 expression upon Ldb1 and DN-Ldb1 expression (Figure 4I, Figure S4C), consistent with the role of Ldb1 and DN-Ldb1 in stabilizing Isl1 protein levels, suggesting that the inability of DN-Ldb1 to rescue the cardiac differentiation defects of the Ldb1-deficient cells is not due to lack of Isl1+ cells. Importantly, the expression of Mef2c and Hand2, factors crucial for SHF development and differentiation, was significantly upregulated upon Ldb1 overexpression, but not upon overexpression of DN-Ldb1 (Figure 4J). Isl1 has been shown to directly bind to the anterior heart field (AHF) enhancer, which directs the expression of Mef2c in the AHF (Dodou et al., 2004, Witzel et al., 2012). Additionally, we found several conserved Isl1 consensus motifs (YTAATGR; TAAKKR; Mazzoni et al., 2013) between −1.5 kb and the Mef2c transcription start site (Figure S5). Chromatin immunoprecipitation (ChIP) analysis of nuclear extracts from d5 EBs and pools of E8–9 embryos demonstrated specific binding of Isl1 and Ldb1 to the conserved Isl1 binding sites (Figures 4K and 4L). Additionally, we found Hand2 expression to be significantly altered upon loss and gain of function of Ldb1. Hand2 plays a key role in SHF development (Srivastava et al., 1997), and its expression in the heart is specifically driven by a cardiac-specific enhancer located between −4.5 kb and −2.7 kb of the Hand2 transcription start site (Figure S6; OFTRV enhancer) (McFadden et al., 2000). In silico analysis revealed several Isl1 consensus binding sites in the proximal promoter and the OFTRV enhancer of Hand2 (Figure S6). ChIP analysis of d5 EBs and E8–9 embryos showed strong binding of both Isl1 and Ldb1 at these sites (Figures 4M and 4N). Taken together, our data indicate that Mef2c and Hand2 are direct targets of the Isl1/Ldb1 transcriptional complex.
Figure 4. The Dimerization Domain of Ldb1 Is Required for Cardiomyocyte Differentiation.
(A) Confocal images of control and DN-Ldb1-overexpressing Tg(myl7:EGFP-HsHRAS)s883 zebrafish embryos at 48 hr post fertilization (hpf) stained with anti-GFP and anti-Isl1 (red) antibodies. Arrows indicate Isl1+ cells outside of the heart, which do not express the cardiomyocyte marker myl7. Scale bars, 50 μm.
(B and C) In situ hybridization of control, DN-Ldb1-overexpressing, and isl1 mutant embryos at 48 hpf with bmp4 (B) and mef2cb (C) probes. The arrows indicate bmp4 expression at the sinus venosus.
(D) Confocal images of control and DN-Ldb1-overexpressing embryos stained with anti-Isl1 antibody (leftmost panels) and in situ hybridization of control and DN-Ldb1-overexpressing embryos at 10–15 somite stages with isl1, nkx2.5, hand2, tbx5a, and mef2cb probes.
(E) Percentage of beating d7 EBs derived from Ldb1−/− ESCs overexpressing either GFP alone (control) or GFP together with Isl1, Ldb1, DN-Ldb1, or these in different combinations.
(F and G) Relative mRNA expression analysis of cardiomyocyte (F), smooth muscle, and endothelial marker (G) genes in d7 EBs.
(H) FACS analysis of Flk-1 and PdgfR-α expression in d3.75 Ldb1−/− EBs overexpressing either GFP alone or GFP together with Isl1, Ldb1, DN-Ldb1, or these in different combinations.
(I) Western blot analysis of total protein extracts of d5 EBs.
(J) Relative mRNA expression of Mef2c and Hand2 in d4 EBs.
(K and L) ChIP of nuclear extracts from d5 EBs (K) and pools of dissected SHF from E8–9 embryos (L) using anti-Isl1 and anti-Ldb1 antibodies or IgG as control. PCRs were performed using primers flanking conserved Isl1 binding sites in the Mef2c promoter and the AHF enhancer. Fold enrichment values for EBs were calculated relative to IgG control and for embryos relative to a genomic region that does not contain conserved Isl1 binding sites. Data are mean ± SEMs, n = 3.
(M and N) ChIP of nuclear extracts from d5 EBs (M) and pools of dissected SHF from E8–9 embryos (N) using anti-Isl1 and anti-Ldb1 antibodies or IgG as a control. Data are mean ± SEMs, n = 3.
Data in (E)–(G) and (J) are mean ± SEMs, n = 3. *p < 0.05, **p < 0.01, ***p < 0.005. See also Figures S3–S7.
Ldb1 Facilitates Enhancer-Promoter Interactions within the Hand2 and Mef2c Loci
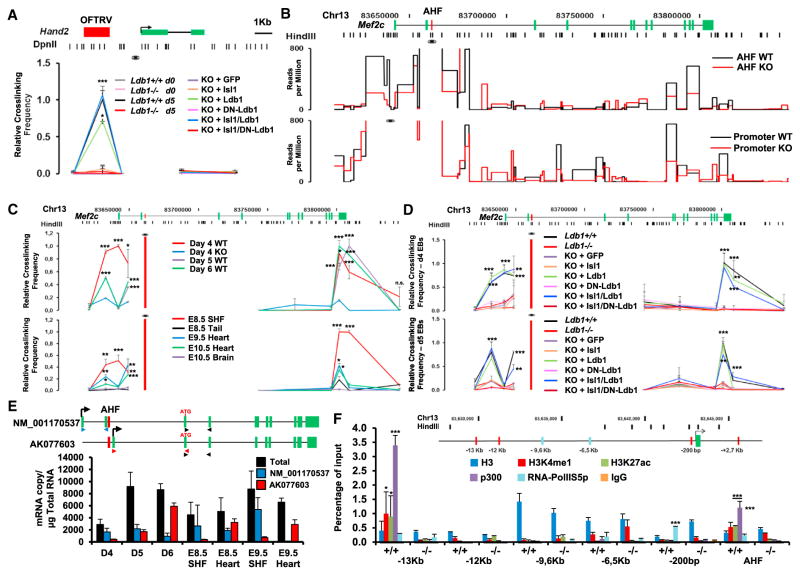
Ldb1 promotes chromatin looping events that bring distal enhancers into close proximity to promoters, thereby regulating gene expression (Deng et al., 2012; Soler et al., 2010; Song et al., 2007). To assess whether a similar mechanism controls cardiac progenitor cell gene expression, we analyzed whether binding of the Isl1/Ldb1 complex promotes chromatin loop formation at the relatively small Hand2 locus. Using 3C-qPCR analysis with the Hand2 promoter as a viewpoint, we observed a specific close proximity of the promoter with the OFTRV enhancer in d5 EBs from WT ESCs, but not in d5 EBs from Ldb1-deficient cells (Figure 5A). A similar interaction pattern was observed in Ldb1−/− EBs overexpressing Ldb1 and Isl1/Ldb1, but not in EBs overexpressing DN-Ldb1 or Isl1/DN-Ldb1 (Figure 5A, Figure S7A). No interaction was observed between the promoter and negative regions for Isl1 and Ldb1 binding. Further, no interaction was detected in WT and Ldb1-deficient ESCs, which do not express Isl1 (Figure 5A, Figure S7A).
Figure 5. Ldb1 Facilitates Enhancer-Promoter Interactions within the Hand2 and Mef2c Loci.
(A) Schematic representation of the Hand2 genomic locus and the position of the DpnII restriction sites used in the 3C assay (top). 3C-qPCR relative crosslinking frequency in WT, Ldb1−/− ESCs, WT and Ldb1−/− ESCs, or d5 EBs derived from Ldb1−/− ESCs overexpressing either GFP alone or GFP together with Isl1, Ldb1, DN-Ldb1, or these in different combinations is shown. The Hand2 promoter was used as the viewpoint. Values were normalized to the β-actin locus and the value for the OFTRV enhancer in d5 WT EBs was set as one. Data are mean ± SEMs, n = 3.
(B) Schematic representation of the Mef2c genomic locus and the position of the restriction sites of HindIII, used in the 3C assay (top). 3C-seq analysis of Mef2c AHF- (middle panel) and Mef2c promoter-associated regions (bottom panel) in d5 WT and Ldb1−/− EBs is shown. The viewpoint is indicated with an eye symbol. Only statistically significant interactions identified by the r3Cseq package (Thongjuea et al., 2013) are presented (p < 0.05).
(C) 3C-qPCR relative crosslinking frequency in EBs at different days (top panel) and in microdissected SHF region, heart, brain, or tail from embryos at different developmental stages (bottom panel). Data are mean ± SEMs, n = 3.
(D) 3C-qPCR relative crosslinking frequency observed in d4 (top) and d5 (bottom) EBs derived from WT and Ldb1−/− ESCs or Ldb1−/− ESCs overexpressing either GFP alone or GFP together with Isl1, Ldb1, and DN-Ldb1 in different combinations. Data are mean ± SEMs, n = 3. For the 3C-qPCR in (C) and (D) the HindIII fragment containing the Mef2c-AHF was used as the viewpoint (red bar, eye symbol).
(E) Schematic representation of alternative Mef2c transcripts (top) and absolute quantification of these transcripts using primers indicated in the scheme (bottom).
(F) ChIP of d4 EBs derived from WT and Ldb1−/− ESCs using antibodies against H3, H3K4me1, H3K27Ac, p300, RNA-PolIIS5p, and IgG as a control. Data are mean ± SEMs, n = 3. *p < 0.05, **p < 0.01, ***p < 0.005. See also Figure S7.
We next performed 3C-seq (Stadhouders et al., 2013) to characterize the spatial interactions within the Mef2c locus using two viewpoints: the Mef2c AHF enhancer, which drives Mef2c expression specifically in the AHF, and the Mef2c promoter, which drives Mef2c expression in different cell types. Multiple promoter-interacting elements were detected in the Mef2c locus, of which some showed a lower signal in Ldb1-deficient EBs (Figure 5B). Importantly, we observed interactions of the AHF enhancer with the promoter area and with the 3′ end of the Mef2c gene, which were decreased in Ldb1-deficient EBs (Figure 5B). 3C-qPCR experiments revealed a similar long-range interaction pattern in WT versus Ldb1−/− EBs during the course of cardiac differentiation and in dissected SHF and hearts of E8–10.5 embryos, confirming the 3C-seq results (Figure 5C). Interestingly, we observed interactions of the AHF enhancer with the Mef2c proximal promoter co-occupied by Isl1 and Ldb1 in d4 EBs at the onset of Mef2c expression and in dissected SHF of E8.5 embryos, whereas in d5 EBs and hearts from E9.5–10.5 embryos, we observed strong interactions with regions more distal to the transcription start site (Figures 5C and 5D). Additionally, no interactions of the AHF enhancer with the promoter and with the 3′ end of the Mef2c gene were observed in tail and brain, suggesting that these chromatin loops are specifically formed in cardiac progenitor cells. Importantly, the 3C results correlated with the binding of Isl1 and Ldb1 at the Mef2c locus (Figure S7B). Next, we analyzed whether Ldb1 dimerization is required for chromatin loop formation by performing 3C-qPCR in d4 and d5 EBs from Ldb1−/− ESCs overexpressing Isl1, Ldb1, and DN-Ldb1 alone or in combinations. Using the AHF enhancer as a viewpoint, we observed a specific interaction of the AHF enhancer with the Mef2c promoter and the 3′end of the Mef2c gene in Ldb1−/− EBs overexpressing Ldb1 and Isl1/Ldb1, but we observed no interaction of these genomic regions in Ldb1−/− EBs overexpressing DN-Ldb1 and Isl1/DN-Ldb1 (Figure 5D). To analyze whether the inability of DN-Ldb1 to promote loop formation was due to its inability to bind to the Isl1/Ldb1 binding sites, we performed ChIP of Ldb1−/− ESCs overexpressing Isl1 alone or in combinations with HA-Ldb1 or HA-DN-Ldb1 using anti-HA antibody. Importantly, we observed similar enrichment of Ldb1 and DN-Ldb1 at the Mef2c promoter and AHF enhancer (Figure S7C). Thus, the DN-Ldb1/Isl1 complex binds at its target sites, but it cannot promote long-range enhancer-promoter interactions necessary for gene expression. Finally, luciferase assays revealed a significant synergistic effect of Isl1 and Ldb1 on a luciferase construct containing the Mef2c promoter upstream and the Mef2c AHF enhancer downstream of a luciferase gene, compared to a reporter construct harboring the Mef2c promoter alone (Figure S7D), suggesting that Isl1/Ldb1 complexes are required for enhancer-promoter interactions to ensure high levels of Mef2c expression.
To understand the functional significance of the dynamic chromatin looping at the Mef2c locus, we analyzed the expression of annotated alternative Mef2c transcripts. Interestingly, while the longer reference sequence transcript (NM_001170537) was highly expressed in d4 EBs and in dissected SHF, the transcript with the alternative transcriptional start site (TSS) 1.5 kb downstream of the AHF enhancer was more abundant later during EB differentiation and in dissected hearts (Figure 5E), implying that the observed dynamic chromatin looping correlates with the expression of alternative transcripts for Mef2c. To better characterize the −14 kb to −5.5 kb genomic region found in close proximity to the AHF enhancer, we screened this region for known enhancer-associated chromatin marks H3K27ac, H3K4me1, p300, and Pol II. We found a marked enrichment of these marks −13 kb upstream of the TSS, which correlated with strong binding of Isl1 and Ldb1 at these sites (Figure 5F, Figure S7B). Sites within the −14 kb to −5.5 kb genomic region that were not bound by Isl1 and Ldb1 did not show significant enrichment of enhancer-associated chromatin marks (Figure 5F).
Ldb1 Orchestrates a Network for Transcriptional Regulation and Coordination in Three-Dimensional Space during Cardiogenesis
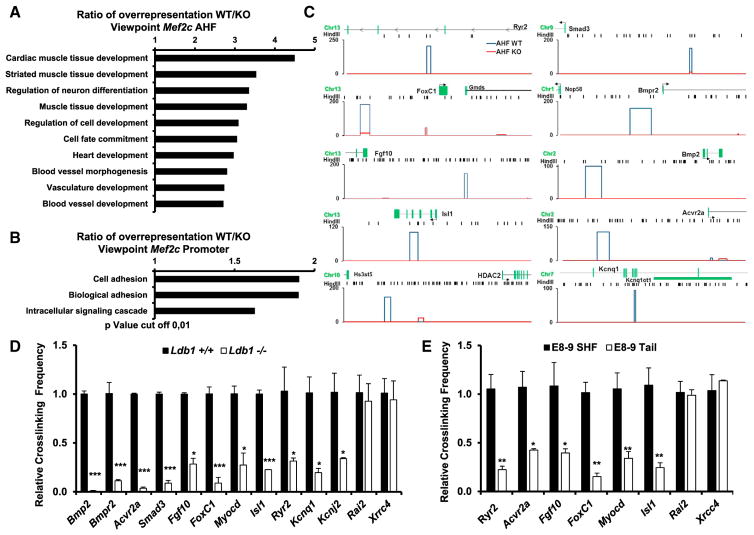
Gene Ontology (GO) analysis of sequences found by the 3C-seq in close proximity to the AHF enhancer over-represented in WT versus Ldb1−/− EBs showed an enrichment of genes involved in heart and cardiac muscle development, cell fate commitment, and vasculature development within the first ten most enriched GO terms (Figure 6A, p < 0.01). By contrast, a similar analysis using the sequences found in proximity to the Mef2c promoter showed no overrepresentation of GO terms involved in heart development (Figure 6B). Importantly, we found that the AHF enhancer is involved in contacts with multiple genes that play key roles during cardiac progenitor cell differentiation and heart development (Figure 6C, Table S1). 3C-qPCR analysis of WT versus Ldb1−/− EBs, as well as of dissected SHF or tails of E8–9 embryos, confirmed the specificity and the Ldb1 dependence of these interactions (Figures 6D and 6E). Importantly, in Ldb1-deficient EBs we observed significant downregulation of selected genes that show higher association with the AHF enhancer in WT versus Ldb1−/− EBs (Figure 7A). By contrast, genes showing similar association with the AHF enhancer in WT and Ldb1−/− EBs (Rai2 and Xrcc4) were not altered. Furthermore, overexpression of Ldb1 or Ldb1 with Isl1, but not DN-Ldb1, strongly promoted the expression of the genes that were highly associated with the AHF enhancer in wild-type EBs (Figure 7B). Moreover, we observed significant downregulation of these genes in hearts and dissected SHF regions of Isl1+/− Ldb1+/− E9.25 embryos compared to WT embryos (Figures 7C and 7D). Taken together, these data provide strong evidence that the Isl1/Ldb1-containing transcription complexes orchestrate a network of transcriptional regulation and coordination in three-dimensional space to regulate cardiac progenitor cell differentiation and heart development (Figure 7E).
Figure 6. Ldb1 Promotes Chromatin Looping between the AHF Enhancer and Genes That Play Key Roles in Cardiovascular Development.
(A and B) Gene ontology analysis of genes interacting with the Mef2c AHF (A) or the Mef2c promoter (B), overrepresented in WT versus Ldb1−/− cells.
(C) Schematic representation of 3C-seq results showing specific interactions of the Mef2c AHF with genes that play key roles in cardiovascular development. The HindIII restriction sites are shown as black bars. y axes: reads per million.
(D and E) 3C-qPCR validation of the interactions observed using the 3C-seq approach in WT or Ldb1−/−d5 EBs (D) or in microdissected SHF regions and tails of E8–9 embryos (E) using the Mef2c-AHF as viewpoint. Data are mean ± SEMs, n = 3. *p < 0.05, **p < 0.01, ***p < 0.005.
DISCUSSION
The differentiation of cardiac progenitor cells into distinct lineages involves a coordinated series of large-scale transcriptional changes, but how these events are coordinated at the molecular level has remained poorly understood. Our study shows that a complex between the central SHF transcription factor Isl1 and the multi-adaptor protein Ldb1 plays a crucial role in directing chromatin organization and coordinating a cardiac-lineage-specific gene expression program.
We show that Ldb1-deficiency results in a markedly decreased expression of SHF marker genes and subsequently impaired differentiation into the cardiomyocyte and endothelial lineages, while differentiation into the smooth muscle lineage was increased. Ldb1 gain-of-function experiments confirmed the requirement of Ldb1 in the differentiation of cardiovascular progenitors in cardiomyocytes and endothelial cells and in the suppression of the smooth muscle lineage. FACS analysis for Flk-1 and PdgfR-α revealed no significant differences in cardiovascular progenitor numbers upon Ldb1 deficiency, showing that Ldb1 loss affects the differentiation of cardiovascular progenitor cells, but not cardiogenic lineage commitment. Interestingly, overexpression of Ldb1 in WT EBs significantly increased the number of Flk-1+PdgfR-α+ cardiovascular progenitors, suggesting that modulation of Ldb1 levels might affect cardiac lineage commitment. Consistent with this, competition of full-length Ldb-s for binding to LIM-domain proteins by DN-Ldb1 led to a significant decrease in Flk-1+PdgfR-α+ cardiovascular progenitor cells, implying functional redundancy between Ldb1 and Ldb2 in cardiac lineage commitment. We show that the requirement of Ldb1 for cardiac progenitor cell differentiation and SHF development is two-fold (Figure 7E): (1) Ldb1 binds to Isl1 and protects it from proteasomal degradation, as a consequence of which Ldb1 deficiency leads to an almost complete loss of Isl1+ cardiovascular progenitor cells. We show that Isl1 is ubiquitinated at its LIM2 domain and that Ldb1 protects Isl1 from degradation mainly via binding to the Isl1 LIM1 domain. This is consistent with previous studies showing that LIM-HD protein levels are regulated by the proteasome and that binding of Ldb1 to LIM domain proteins protects them from degradation (Güngör et al., 2007). (2) The Isl1/Ldb1 complex orchestrates a network for transcriptional regulation and coordination in three-dimensional space in cardiac progenitors, driving cardiac progenitor cell differentiation and heart development. Consistent with this, a truncated Ldb1 protein (DN-Ldb1), which protects Isl1 protein from degradation, but cannot promote long-range enhancer-promoter interactions (Krivega et al., 2014), did not collaborate with Isl1 to regulate the expression of their common targets and failed to rescue the cardiac differentiation defects of Ldb1-deficient cells (Figures 4E and 4F). Importantly, overexpression of DN-Ldb1 in zebrafish embryos leads to defects in the differentiation of Isl1+ cardiac progenitors in striking parallel with isl1 mutant fishes (de Pater et al., 2009), presumably by competing with full-length Ldb1 for binding to Isl1 (Bach et al., 1999). Further, overexpression of DN-Ldb1 caused significant downregulation of hand2 and mef2cb, transcription factors that play important roles in cardiomyocyte differentiation and heart development (Lin et al., 1997; Srivastava et al., 1997). Similarly, we observed decreased expression of Hand2 and Mef2c in Ldb1-deficient EBs, which showed complete blockade of cardiomyocyte differentiation, and in Isl1/Ldb1 haplodeficient embryos, which developed various cardiac anomalies. Our results also show that the Isl1/Ldb1 complex directly regulates Mef2c and Hand2 expression by binding to their heart-specific enhancers and stimulating enhancer-promoter interactions. Similarly, Ldb1 is required for the looping of the β-globin locus control region (LCR) to the β-globin promoter and transcriptional activation (Deng et al., 2012; Krivega et al., 2014; Soler et al., 2010; Song et al., 2007). 3C-seq and 3C-qPCR using the Isl1- and GATA-responsive AHF enhancer of Mef2c (Dodou et al., 2004) as a viewpoint showed close proximity of the promoter region, the AHF enhancer, and the 3′ end of the gene. Gene looping has been shown to bring promoter and terminator regions in close proximity in the early stages of transcriptional activation, facilitating RNA Pol II re-initiation and high-level expression of the long genes FMP27 and SEN1 in Saccharomyces cerevisiae (O’Sullivan et al., 2004). Similarly, the observed loop between the promoter and the 3′end of the Mef2c gene could generate a functional re-initiation complex for subsequent rounds of Mef2c transcription. In addition we observed dynamic chromatin looping at the Mef2c locus during heart development and in the course of cardiac progenitor cell differentiation. The AHF enhancer contacts the proximal promoter during the onset of Mef2c gene activation in the AHF, whereas later this contact is lost, correlating with the expression of an alternative transcript with a TSS 1.5 kb downstream of the AHF enhancer. A similar developmental switch in chromatin looping was observed during erythroid differentiation at the β-globin locus, from favoring the expression of embryonic globin genes in erythroid progenitors to favoring the expression of the adult globin genes in definitive erythrocytes (Palstra et al., 2003).
Remarkably, our 3C analysis identified specific, Ldb1-mediated interactions of the AHF enhancer with multiple genes that play key roles in cardiovascular development as well as cell fate commitment. This could be due to these genes being in close proximity in the same transcription factory, or Ldb1 may be regulating a cardiac-specific interaction network around the Mef2c AHF enhancer. The interactions are cell type specific, as they are only observed in cardiac cells and they are lost upon Ldb1 deficiency. Recent studies analyzing enhancer contacts during Drosophila development revealed that a large number of enhancer interactions are unchanged between different tissues and developmental stages and only few show significant changes (Ghavi-Helm et al., 2014). Notably, Ldb1 appears to mediate cell-type-specific chromatin loops and transcriptional programs via its diverse DNA binding partners (Figure 5, Figure 6, and Figure 7; Soler et al., 2010; Song et al., 2007). Importantly, Ldb1 deficiency led to dramatically decreased expression of the genes associated with the AHF enhancer and overexpression of Isl1 and Ldb1 strongly promoted their expression. The dosage-sensitive interdependence between Isl1 and Ldb1 in the expression of these key factors in cardiogenesis further supports a key role of the Isl1/Ldb1 complex in coordinating genome organization and transcriptional regulation upstream of a regulatory network driving cardiac differentiation and heart development.
In conclusion, our study highlights a central role for Ldb1 in regulating cardiac progenitor cell differentiation and SHF development and provides exciting novel insights into the molecular machinery that orchestrates chromatin organization and coordinated gene expression driving cardiogenesis.
EXPERIMENTAL PROCEDURES
A detailed description of the experimental procedures is provided in the Supplemental Experimental Procedures.
Antibodies
The following primary antibodies were used: rabbit anti-HA (Y-11, Santa Cruz Biotechnology), mouse anti-FLAG (M2, Sigma), goat anti-Ldb1 (N-18, Santa Cruz Biotechnology), mouse anti-Ldb1 (C-9, Santa Cruz Biotechnology), mouse anti-Islet1 39.4D5 (Developmental Studies Hybridoma Bank); goat anti-Lamin B (C-20, Santa Cruz Biotechnology), mouse anti-tubulin (T5168, Sigma), anti-GFP (ab6556, Abcam), APC-conjugated anti-Flk-1 (e-Bioscience 17-5821-81), and PE-conjugated anti-PdgfR-α (e-Bioscience 12-1401-81).
Immunoprecipitation and ChIP
Co-IPs and ChIP were performed as described in Witzel et al. (2012).
Chromosome Conformation Capture Assays: 3C-seq and 3C-qPCR
3C-seq and 3C-qPCR were performed as described in Stadhouders et al. (2013). In brief, 107 cells or dissected SHF regions or tails of 20 E8–9 embryos were crosslinked with 2% formaldehyde at room temperature for 10 min, which was followed by glycine quenching; cell lysis; HindIII, NlaIII, or DpnII digestion; and T4 ligation. Primer sequences used in the 3C assays are listed in the Supplemental Experimental Procedures. r3Cseq, an R/Bioconductor package, was used for the discovery of long-range genomic interactions in the 3C-seq datasets (Thongjuea et al., 2013).
Zebrafish Strains
The following mutant and transgenic lines were used: Tg(myl7:EGFP-HsHRAS)s883 (D’Amico et al., 2007) and isl1sa0029 (Sanger Institute, Zebrafish Mutation Resource).
Mouse Lines
The Ldb1tm1a(EUCOMM)Hmgu line was generated from the microinjection of Ldb1tm1a(EUCOMM)Hmgu ESCs, obtained from the European Conditional Mouse Mutagenesis Program, into blastocysts. All animal experiments were done in accordance with the regulations issued by the Committee for Animal Rights Protection of the State of Hessen (Regierungspraesidium Darmstadt).
mRNA Injection
mRNA synthesis was performed using mMESSAGE mMACHINE SP6 Transcription Kit (Ambion). mRNA was injected in the one-cell stage of fertilized zebrafish eggs (volume, 2 nl; mRNA concentration, 200 ng/μl).
In Situ Hybridization, Whole-Mount Immunostaining, and Confocal Microscopy
In situ hybridization and whole-mount staining were performed as described (Witzel et al., 2012). Confocal images were acquired by a Zeiss LSM 710 system and the Z-stacks were projected by Zeiss LSM 710 software.
Supplementary Material
Highlights.
Ldb1 is required for differentiation of multipotent cardiac progenitor cells
Ldb1 regulates second heart field development
Ldb1 protects the key regulator of cardiac progenitors, Isl1, from degradation
The Isl1/Ldb1 complex orchestrates cardiac-specific chromatin organization
Acknowledgments
We thank Ingrid Konrad, Susanne Kreutzer, Kerstin Richter, and Monika Müller-Boche for technical assistance. We are grateful to Boyan Garvalov for careful reading of the manuscript and the constructive comments. This work was supported by the LOEWE Center for Cell and Gene Therapy (CGT), financed by the Hessian Ministry of Higher Education, Research and Arts (III L 4-518/17.004 [2013]), an Emmy-Noether Program grant DO 1323/1-1, the SFB TRR 81, and the EXC 147 of the DFG (Germany).
Footnotes
ACCESSION NUMBERS
The accession number for the 3C-seq data reported in this paper is SRA: SRP055800.
Supplemental Information for this article includes Supplemental Experimental Procedures, seven figures, and one table and can be found with this article online at http://dx.doi.org/10.1016/j.stem.2015.08.007.
References
- Aguirre A, Sancho-Martinez I, Izpisua Belmonte JC. Reprogramming toward heart regeneration: stem cells and beyond. Cell Stem Cell. 2013;12:275–284. doi: 10.1016/j.stem.2013.02.008. [DOI] [PubMed] [Google Scholar]
- Bach I, Carrière C, Ostendorff HP, Andersen B, Rosenfeld MG. A family of LIM domain-associated cofactors confer transcriptional synergism between LIM and Otx homeodomain proteins. Genes Dev. 1997;11:1370–1380. doi: 10.1101/gad.11.11.1370. [DOI] [PubMed] [Google Scholar]
- Bach I, Rodriguez-Esteban C, Carrière C, Bhushan A, Krones A, Rose DW, Glass CK, Andersen B, Izpisúa Belmonte JC, Rosenfeld MG. RLIM inhibits functional activity of LIM homeodomain transcription factors via recruitment of the histone deacetylase complex. Nat Genet. 1999;22:394–399. doi: 10.1038/11970. [DOI] [PubMed] [Google Scholar]
- Becker T, Ostendorff HP, Bossenz M, Schlüter A, Becker CG, Peirano RI, Bach I. Multiple functions of LIM domain-binding CLIM/NLI/ Ldb cofactors during zebrafish development. Mech Dev. 2002;117:75–85. doi: 10.1016/s0925-4773(02)00178-8. [DOI] [PubMed] [Google Scholar]
- Cai CL, Liang X, Shi Y, Chu PH, Pfaff SL, Chen J, Evans S. Isl1 identifies a cardiac progenitor population that proliferates prior to differentiation and contributes a majority of cells to the heart. Dev Cell. 2003;5:877–889. doi: 10.1016/s1534-5807(03)00363-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Amico L, Scott IC, Jungblut B, Stainier DY. A mutation in zebrafish hmgcr1b reveals a role for isoprenoids in vertebrate heart-tube formation. Curr Biol. 2007;17:252–259. doi: 10.1016/j.cub.2006.12.023. [DOI] [PubMed] [Google Scholar]
- de Pater E, Clijsters L, Marques SR, Lin YF, Garavito-Aguilar ZV, Yelon D, Bakkers J. Distinct phases of cardiomyocyte differentiation regulate growth of the zebrafish heart. Development. 2009;136:1633–1641. doi: 10.1242/dev.030924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit E, de Laat W. A decade of 3C technologies: insights into nuclear organization. Genes Dev. 2012;26:11–24. doi: 10.1101/gad.179804.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit E, Bouwman BAM, Zhu Y, Klous P, Splinter E, Verstegen MJAM, Krijger PHL, Festuccia N, Nora EP, Welling M, et al. The pluripotent genome in three dimensions is shaped around pluripotency factors. Nature. 2013;501:227–231. doi: 10.1038/nature12420. [DOI] [PubMed] [Google Scholar]
- Dekker J, Rippe K, Dekker M, Kleckner N. Capturing chromosome conformation. Science. 2002;295:1306–1311. doi: 10.1126/science.1067799. [DOI] [PubMed] [Google Scholar]
- Deng W, Lee J, Wang H, Miller J, Reik A, Gregory PD, Dean A, Blobel GA. Controlling long-range genomic interactions at a native locus by targeted tethering of a looping factor. Cell. 2012;149:1233–1244. doi: 10.1016/j.cell.2012.03.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denholtz M, Bonora G, Chronis C, Splinter E, de Laat W, Ernst J, Pellegrini M, Plath K. Long-range chromatin contacts in embryonic stem cells reveal a role for pluripotency factors and polycomb proteins in genome organization. Cell Stem Cell. 2013;13:602–616. doi: 10.1016/j.stem.2013.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon JR, Jung I, Selvaraj S, Shen Y, Antosiewicz-Bourget JE, Lee AY, Ye Z, Kim A, Rajagopal N, Xie W, et al. Chromatin architecture reorganization during stem cell differentiation. Nature. 2015;518:331–336. doi: 10.1038/nature14222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodou E, Verzi MP, Anderson JP, Xu SM, Black BL. Mef2c is a direct transcriptional target of ISL1 and GATA factors in the anterior heart field during mouse embryonic development. Development. 2004;131:3931–3942. doi: 10.1242/dev.01256. [DOI] [PubMed] [Google Scholar]
- Evans SM, Yelon D, Conlon FL, Kirby ML. Myocardial lineage development. Circ Res. 2010;107:1428–1444. doi: 10.1161/CIRCRESAHA.110.227405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghavi-Helm Y, Klein FA, Pakozdi T, Ciglar L, Noordermeer D, Huber W, Furlong EE. Enhancer loops appear stable during development and are associated with paused polymerase. Nature. 2014;512:96–100. doi: 10.1038/nature13417. [DOI] [PubMed] [Google Scholar]
- Gorkin DU, Leung D, Ren B. The 3D genome in transcriptional regulation and pluripotency. Cell Stem Cell. 2014;14:762–775. doi: 10.1016/j.stem.2014.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Güngör C, Taniguchi-Ishigaki N, Ma H, Drung A, Tursun B, Ostendorff HP, Bossenz M, Becker CG, Becker T, Bach I. Proteasomal selection of multiprotein complexes recruited by LIM homeodomain transcription factors. Proc Natl Acad Sci USA. 2007;104:15000–15005. doi: 10.1073/pnas.0703738104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson EM, Lindsay ME, Chien KR. Regeneration next: toward heart stem cell therapeutics. Cell Stem Cell. 2009;5:364–377. doi: 10.1016/j.stem.2009.09.004. [DOI] [PubMed] [Google Scholar]
- Jurata LW, Kenny DA, Gill GN. Nuclear LIM interactor, a rhombotin and LIM homeodomain interacting protein, is expressed early in neuronal development. Proc Natl Acad Sci USA. 1996;93:11693–11698. doi: 10.1073/pnas.93.21.11693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagey MH, Newman JJ, Bilodeau S, Zhan Y, Orlando DA, van Berkum NL, Ebmeier CC, Goossens J, Rahl PB, Levine SS, et al. Mediator and cohesin connect gene expression and chromatin architecture. Nature. 2010;467:430–435. doi: 10.1038/nature09380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krivega I, Dale RK, Dean A. Role of LDB1 in the transition from chromatin looping to transcription activation. Genes Dev. 2014;28:1278–1290. doi: 10.1101/gad.239749.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon C, Qian L, Cheng P, Nigam V, Arnold J, Srivastava D. A regulatory pathway involving Notch1/beta-catenin/Isl1 determines cardiac progenitor cell fate. Nat Cell Biol. 2009;11:951–957. doi: 10.1038/ncb1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Q, Schwarz J, Bucana C, Olson EN. Control of mouse cardiac morphogenesis and myogenesis by transcription factor MEF2C. Science. 1997;276:1404–1407. doi: 10.1126/science.276.5317.1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzoni EO, Mahony S, Closser M, Morrison CA, Nedelec S, Williams DJ, An D, Gifford DK, Wichterle H. Synergistic binding of transcription factors to cell-specific enhancers programs motor neuron identity. Nat Neurosci. 2013;16:1219–1227. doi: 10.1038/nn.3467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFadden DG, Charité J, Richardson JA, Srivastava D, Firulli AB, Olson EN. A GATA-dependent right ventricular enhancer controls dHAND transcription in the developing heart. Development. 2000;127:5331–5341. doi: 10.1242/dev.127.24.5331. [DOI] [PubMed] [Google Scholar]
- Meier N, Krpic S, Rodriguez P, Strouboulis J, Monti M, Krijgsveld J, Gering M, Patient R, Hostert A, Grosveld F. Novel binding partners of Ldb1 are required for haematopoietic development. Development. 2006;133:4913–4923. doi: 10.1242/dev.02656. [DOI] [PubMed] [Google Scholar]
- Morcillo P, Rosen C, Baylies MK, Dorsett D. Chip, a widely expressed chromosomal protein required for segmentation and activity of a remote wing margin enhancer in Drosophila. Genes Dev. 1997;11:2729–2740. doi: 10.1101/gad.11.20.2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moretti A, Caron L, Nakano A, Lam JT, Bernshausen A, Chen Y, Qyang Y, Bu L, Sasaki M, Martin-Puig S, et al. Multipotent embryonic isl1+ progenitor cells lead to cardiac, smooth muscle, and endothelial cell diversification. Cell. 2006;127:1151–1165. doi: 10.1016/j.cell.2006.10.029. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay M, Teufel A, Yamashita T, Agulnick AD, Chen L, Downs KM, Schindler A, Grinberg A, Huang SP, Dorward D, Westphal H. Functional ablation of the mouse Ldb1 gene results in severe patterning defects during gastrulation. Development. 2003;130:495–505. doi: 10.1242/dev.00225. [DOI] [PubMed] [Google Scholar]
- Mylona A, Andrieu-Soler C, Thongjuea S, Martella A, Soler E, Jorna R, Hou J, Kockx C, van Ijcken W, Lenhard B, Grosveld F. Genome-wide analysis shows that Ldb1 controls essential hematopoietic genes/pathways in mouse early development and reveals novel players in hematopoiesis. Blood. 2013;121:2902–2913. doi: 10.1182/blood-2012-11-467654. [DOI] [PubMed] [Google Scholar]
- O’Sullivan JM, Tan-Wong SM, Morillon A, Lee B, Coles J, Mellor J, Proudfoot NJ. Gene loops juxtapose promoters and terminators in yeast. Nat Genet. 2004;36:1014–1018. doi: 10.1038/ng1411. [DOI] [PubMed] [Google Scholar]
- Palstra RJ, Tolhuis B, Splinter E, Nijmeijer R, Grosveld F, de Laat W. The beta-globin nuclear compartment in development and erythroid differentiation. Nat Genet. 2003;35:190–194. doi: 10.1038/ng1244. [DOI] [PubMed] [Google Scholar]
- Peric-Hupkes D, Meuleman W, Pagie L, Bruggeman SW, Solovei I, Brugman W, Gräf S, Flicek P, Kerkhoven RM, van Lohuizen M, et al. Molecular maps of the reorganization of genome-nuclear lamina interactions during differentiation. Mol Cell. 2010;38:603–613. doi: 10.1016/j.molcel.2010.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips-Cremins JE, Sauria ME, Sanyal A, Gerasimova TI, Lajoie BR, Bell JS, Ong CT, Hookway TA, Guo C, Sun Y, et al. Architectural protein subclasses shape 3D organization of genomes during lineage commitment. Cell. 2013;153:1281–1295. doi: 10.1016/j.cell.2013.04.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y, Yue F, McCleary DF, Ye Z, Edsall L, Kuan S, Wagner U, Dixon J, Lee L, Lobanenkov VV, Ren B. A map of the cis-regulatory sequences in the mouse genome. Nature. 2012;488:116–120. doi: 10.1038/nature11243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soler E, Andrieu-Soler C, de Boer E, Bryne JC, Thongjuea S, Stadhouders R, Palstra RJ, Stevens M, Kockx C, van Ijcken W, et al. The genome-wide dynamics of the binding of Ldb1 complexes during erythroid differentiation. Genes Dev. 2010;24:277–289. doi: 10.1101/gad.551810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song SH, Hou C, Dean A. A positive role for NLI/Ldb1 in long-range beta-globin locus control region function. Mol Cell. 2007;28:810–822. doi: 10.1016/j.molcel.2007.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava D, Thomas T, Lin Q, Kirby ML, Brown D, Olson EN. Regulation of cardiac mesodermal and neural crest development by the bHLH transcription factor, dHAND. Nat Genet. 1997;16:154–160. doi: 10.1038/ng0697-154. [DOI] [PubMed] [Google Scholar]
- Stadhouders R, Kolovos P, Brouwer R, Zuin J, van den Heuvel A, Kockx C, Palstra RJ, Wendt KS, Grosveld F, van Ijcken W, Soler E. Multiplexed chromosome conformation capture sequencing for rapid genome-scale high-resolution detection of long-range chromatin interactions. Nat Protoc. 2013;8:509–524. doi: 10.1038/nprot.2013.018. [DOI] [PubMed] [Google Scholar]
- Thongjuea S, Stadhouders R, Grosveld FG, Soler E, Lenhard B. r3Cseq: an R/Bioconductor package for the discovery of long-range genomic interactions from chromosome conformation capture and next-generation sequencing data. Nucleic Acids Res. 2013;41:e132. doi: 10.1093/nar/gkt373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripic T, Deng W, Cheng Y, Zhang Y, Vakoc CR, Gregory GD, Hardison RC, Blobel GA. SCL and associated proteins distinguish active from repressive GATA transcription factor complexes. Blood. 2009;113:2191–2201. doi: 10.1182/blood-2008-07-169417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent SD, Buckingham ME. How to make a heart: the origin and regulation of cardiac progenitor cells. Curr Top Dev Biol. 2010;90:1–41. doi: 10.1016/S0070-2153(10)90001-X. [DOI] [PubMed] [Google Scholar]
- Witzel HR, Jungblut B, Choe CP, Crump JG, Braun T, Dobreva G. The LIM protein Ajuba restricts the second heart field progenitor pool by regulating Isl1 activity. Dev Cell. 2012;23:58–70. doi: 10.1016/j.devcel.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.