Abstract
Hematopoietic cell transplantation (HCT) has been used as a part of cancer therapy for over half a decade. Beyond the necessity for donor-derived cells to reconstitute hematopoiesis after radiation and chemotherapy, immunologic reconstitution from allogeneic cells is important for the elimination of residual tumor cells. Natural killer (NK) cells are first among lymphocytes to reconstitute post-transplant and protect against cancer relapse. In this review, we provide a historical perspective on the role of NK cells in cancer control in the transplant setting and focus on current research aimed at improving NK cell responses for therapeutic benefit.
1 The Dawn of Hematopoietic Cell Transplantation
The modern era of hematopoietic cell transplantation (HCT) began with a series of experiments by Jacobson, Lorenz and colleagues, who demonstrated that mice could be rescued from otherwise lethal doses of irradiation by shielding the spleen with lead (Jacobson et al. 1950) or by intravenous marrow infusion (Lorenz et al. 1951). Initially, it was postulated that this protective effect was mediated by an as yet undefined humoral factor produced within hematopoietic tissue and that this factor promotes the functional reconstitution of many cell types in multiple organs (Jacobson 1952). By the mid-1950s, however, genetic markers were used by several groups to show that reconstitution of recipient marrow by donor cells was responsible for the protective effect against lethal irradiation (Lindsley et al. 1955; Nowell et al. 1956; Ford et al. 1956). Successful bone marrow transplant (BMT) studies in rodents, canines, and primates led physicians to speculate that bone marrow grafts from healthy donors could be applied to victims of radiation accidents and patients with immune disorders or leukemia that are treated with total body irradiation (TBI) (Thomas et al. 1957).
Despite the therapeutic potential of BMT, the next decade was disappointing as clinicians learned that allogeneic transplantation in humans is a complicated and difficult endeavor. Most allogeneic grafts were given to terminally ill patients who did not survive long enough for the treatment to be sufficiently evaluated, and there was a high incidence of complete failure of engraftment. The few successful allogeneic grafts were followed by lethal immune reactions of the graft against the host (Mathe et al. 1967). Because of the immunosuppression and leukopenia associated with BMT, high incidences of viral infections were observed during the first 100 days post-transplant. One of the most common and serious complications observed was interstitial pneumonia primarily caused by cytomegalovirus (CMV). Before the advent of effective anti-viral therapies, the incidence of CMV pneumonia was estimated to be close to 50 %, and mortality among this group of patients due to pulmonary infiltration was between 50 and 60 % (Neiman et al. 1973).
Subsequent advances in histocompatibility typing and the prevention and treatment of graft-versus-host disease (GvHD), a multi-organ system disease caused by immune reaction of donor cells against histocompatibility antigen disparities between the donor and host, significantly improved outcomes. The folic acid analog methotrexate and immunosuppressive drug cyclosporine were shown to reduce the incidence of GvHD in BMT recipients (Deeg et al. 1985). Ganciclovir, a drug that acts as a potent inhibitor of CMV DNA polymerase (Field et al. 1983), proved to be effective in the treatment of CMV disease in BMT recipients (Selby et al. 1986). By the mid-1980s, intensive chemotherapy and TBI followed by transplantation of allogeneic bone marrow became established as an effective and potentially curative therapy for patients with various hematological malignancies (Johnson et al. 1981; Dinsmore et al. 1984; Appelbaum et al. 1984).
2 NK Cells Enter the Transplant Picture
As chemotherapy and TBI followed by BMT gained popularity as a means to treat hematological malignancies, questions arose as to which aspects of this combinatorial approach contributed to the antileukemic effect. Some clinicians and researchers argued that high-dose chemotherapy and TBI was solely responsible for eradication of the leukemia and that transplantation of allogeneic marrow simply acts in a supporting role to reconstitute hematopoiesis. Others postulated that immunologic reconstitution from allogeneic cells contributed significantly to leukemia control. Immune control of relapse was supported by studies in rodents (Mathe et al. 1977), and later in humans (Weiden et al. 1979, 1981), showing that the occurrence of GvHD was associated with a decreased risk of leukemia relapse.
In 1986, Ritz and colleagues sought to determine whether donor cells with direct cytotoxicity against leukemic cells arise following BMT and whether this activity could be distinguished from T cell-mediated GvHD. To this end, they cryopre-served leukemic blasts from a single patient with T cell acute lymphoblastic leukemia (T-ALL) at the time of relapse 5 months prior to transplantation with T cell-depleted allogeneic marrow. The authors found that the predominant population of reconstituting donor cells within the first 3 weeks after transplant had an NK cell phenotype, and only after 1 month did significant numbers of T cells develop. NK cells still constituted 24 % of total peripheral blood mononuclear cells (PBMCs) 3 months after transplant and exhibited cytotoxic activity against thawed patient leukemia blasts in vitro. Thus, these results provided evidence for an NK cell-mediated graft-versus-leukemia (GvL) effect that is independent of T cell-mediated GvHD (Hercend et al. 1986).
In addition to their antileukemic effect, early studies in mice showed that NK cells become activated and proliferate in response to viral infections (Welsh 1978; Biron and Welsh 1982; Biron et al. 1983). Adoptive transfer experiments definitively demonstrated that NK cells are necessary and sufficient to provide resistance to mouse cytomegalovirus (MCMV) in vivo (Bukowski et al. 1985). Given these results, there was reason to believe that NK cells could mediate direct activity against CMV following BMT. Meyers and colleagues analyzed 45 patients at high risk for CMV infection during the first 100 days after allogeneic marrow transplant to determine whether there was a relationship between immune cell cytotoxicity and CMV infection. In vitro cytotoxicity assays using peripheral blood lymphocytes from these patients against CMV-infected target cells showed that NK cells were the primary lymphocyte population responsible for lysis of CMV-infected targets. Importantly, superior survival after CMV infection was observed in BMT recipients whose NK cells exhibited greater than or equal to 15 % lysis of CMV-infected targets (Bowden et al. 1987). Hokland and colleagues used a different but complementary approach to demonstrate an association between CMV and NK cell function following allogeneic BMT. This group tracked NK cell cytotoxicity and NK cell numbers early after transplant and found that both NK cell function and total NK cell numbers steadily increased throughout the first 28 days post-transplant. The increase in NK cell-mediated target lysis was more pronounced in patients with CMV infections (either primary or reactivated) (Hokland et al. 1988). Adoptive transfer and cell depletion experiments in mice also demonstrated that both CD8+ and CD4+ T cells play important roles in the resolution of cytomegalovirus (Reddehase et al. 1987; Jonjic et al. 1989). Together, these studies pointed toward the conclusion that both endogenous NK cell and T cell expansion and activation are important correlates for the resolution of CMV infections in the BMT setting.
3 Using Cytokines to Kick NK Cells into Gear
Although accumulating evidence pointed toward an anti-tumor role for NK cells in transplant recipients, there was a realization that donor NK cell function early post-transplant is not optimal. In studies where reconstituting donor NK cells were cloned by limiting dilution, some clones were cytotoxic while others were completely unresponsive (Hercend et al. 1986). Additionally, depressed functional responses of NK cells from transplant recipients relative to healthy individuals had been described (Dupont et al. 1984). Thus, there was interest in approaches both to expand NK cells and to amplify their functional responses to improve clinical outcomes. The first such strategy involved use of interleukin (IL)-2. Rosenberg and colleagues found that incubation of murine splenocytes or human PBMCs with IL-2 led to the generation of lymphokine-activated killer (Milone et al. 2009) cells capable of potent lysis of syngeneic or allogeneic tumors (Yron et al. 1980; Lotze et al. 1981; Grimm et al. 1982). Serial intraperitoneal injections of high doses of IL-2 into mice with established pulmonary tumors induced LAK activity and led to significant tumor regression (Rosenberg et al. 1985).
While the anti-tumor effects of LAK cells and high-dose IL-2 were impressive in mice, significant obstacles were encountered when attempting to translate this therapy to humans. Limited clinical responses were observed in cancer patients treated with IL-2 alone. Combination therapy of LAK cells and IL-2 did lead to objective cancer remissions in 22 % of 106 patients with a variety of advanced metastatic cancer, but therapeutic efficacy was limited by toxicity associated with higher doses IL-2 (Rosenberg et al. 1987). In particular, dose escalations of IL-2 with or without the addition of LAK cells led to the development of life-threatening vascular leak syndrome and severe intrahepatic cholestasis (Ettinghausen et al. 1988; Hoffman et al. 1989).
Because both LAK cells represent a mixture of T cells and NK cells, questions remained with respect to their relative contributions to anti-tumor responses. To directly test the anti-tumor effects of NK cells stimulated with IL-2, our group evaluated lower doses of IL-2 in combination with adoptively transferred NK cells derived from haploidentical donors to treat patients with poor-prognosis acute myeloid leukemia (AML). We found that the intensity of the conditioning regimen that recipients received had a significant impact upon subsequent adoptive NK cell expansion. Donor NK cells transferred into patients who received low-dose chemotherapy persisted transiently but did not expand in vivo. In contrast, adoptively transferred donor NK cells expanded in patients who received high-dose chemotherapy, and complete hematologic remission was observed in 5 of 19 patients. The expansion of adoptively transferred NK cells was associated with high endogenous IL-15 concentrations in plasma in these patients, which was presumably a consequence of lymphopenia and reduced competition for IL-15 from residual recipient lymphocytes. These results suggested that IL-15 might enhance NK cell survival in vivo relative to IL-2. Alternatively, IL-15 may synergize with IL-2 to promote NK cell expansion and survival (Miller et al. 2005). Our group is currently conducting a phase I trial to test the use of recombinant human IL-15 in NK cell adoptive transfer to treat AML patients.
An important issue to consider with the use of IL-2 is the ability of this cytokine to drive the expansion of regulatory T cells (Tregs), which express the high-affinity IL-2 receptor α chain (CD25). Tregs can indirectly inhibit NK cell function by limiting the bioavailability of IL-2 (Gasteiger et al. 2013; Sitrin et al. 2013). Tregs can also directly inhibit NK cell functions in a transforming growth factor (TGF)-β-dependent manner (Ghiringhelli et al. 2005). In applications of donor NK cell infusions to treat ovarian cancer, breast cancer, and refractory lymphoma, we found that host Tregs persist after conditioning and expand rapidly when IL-2 is administered after adoptive NK cell transfer (Bachanova et al. 2010; Geller et al. 2011). To overcome the inhibitory effects of Tregs, we conducted a trial using recombinant IL-2 diphtheria toxin (IL2DT; Ontak) for Treg depletion after adoptive NK cell transfer. We treated 57 refractory AML patients with high-dose chemotherapy followed by IL-2 administration. Donor NK cell expansion was observed in 10 % (4 of 42) of patients that received haploidentical NK cell infusions and IL-2 alone, whereas NK cell expansion occurred in 27 % (4 of 15) of patients that were also given IL2DT. Importantly, the addition of IL2DT was associated with higher complete remission rates at day 28 and improved disease-free survival at 6 months post-adoptive transfer (Bachanova et al. 2014). These results demonstrate that Treg expansion as a result of IL-2 administration limits NK cell expansion in vivo and negate their therapeutic effect.
One potential way to get around this issue is by the clinical use of IL-15 in place of IL-2. IL-15 was originally identified as a T cell stimulatory factor that binds components of the IL-2 receptor (Grabstein et al. 1994). Subsequently, it was shown that IL-15 also stimulates NK cell proliferation and function in a pattern that is similar, but not identical, to that of IL-2 (Carson et al. 1994). In a series of co-culture experiments, we found that Tregs potently suppressed the proliferation of NK cells cultured with IL-2 but not IL-15 in a concentration-dependent manner (Bachanova et al. 2014). This is likely due to the fact that while high concentrations of IL-15 can induce signal transducer and activator of transcription (STAT) 5 phosphorylation and forkhead box P3 (FOXP3) induction through low-affinity binding to the β and γ subunits of the IL-2 receptor in Tregs, only in the presence of IL-2 do Tregs acquire potent suppressor function (Wuest et al. 2008).
Another cytokine-based approach that has recently shown promising results in mouse models of cancer is preconditioning of NK cells with a combination of IL-15, IL-12, and IL-18 prior to adoptive transfer. IL-12 synergizes with IL-18 to epigenetically prime NK cells for enhanced interferon (IFN)-γ production (Chan et al. 1991; Okamura et al. 1995; Luetke-Eversloh et al. 2014). Cerwenka and colleagues tested the in vivo anti-tumor activity of cytokine preconditioning by incubating syngeneic NK cells with either IL-15 alone or IL-12/15/18 for 16 h ex vivo before adoptive transfer into irradiated MHC class I-deficient RMA-S tumor-bearing mice. Sustained tumor clearance and NK cell persistence was observed in 22 % of the mice that received IL-12/15/18-preactivated NK cells. No beneficial effect was observed in mice that received IL-15- or IL-2-preactivated NK cells. Similar results were observed in a RAE-1ε melanoma model (Ni et al. 2012).
Raulet and colleagues also tested the effect of IL-12 and IL-18 in mice with established RMA-S tumors. They observed that injection of IL-12 and IL-18 every other day for 4 weeks significantly prolonged average survival time, and a higher percentage of mice survived long term. In this model, IL-12 and IL-18 appeared to be particularly effective in reversing NK cell anergy induced by tumor cells lacking the expression of class I MHC molecules (Ardolino et al. 2014). Administering potent inflammatory cytokines such as IL-12 and/or IL-18 to transplant recipients or as part of an adoptive NK cell transfer strategy may not be desirable given the toxicity observed in patients given IL-2 (Miller et al. 2005). However, ex vivo priming of allogeneic or autologous NK cells with IL-12/15/18 prior to infusion is an approach that warrants further testing.
4 NK Cell Alloreactivity and Beyond
Tumor cells frequently show quantitative or qualitative alterations in their expression of class I major histocompatibility complex (MHC) molecules relative to cells in corresponding normal tissue. Reduced expression of one or more MHC molecules allows tumor cells to escape recognition by cytotoxic T cells (Doherty et al. 1984). However, there are examples where tumor progression is closely associated with the increased expression of MHC molecules (De Baetselier et al. 1980; Katzav et al. 1983; Eisenbach et al. 1983), suggesting that loss of T cell recognition of MHC is not the only way by which tumors escape immune control. As an explanation for these paradoxical findings, Ljunggren and Kärre hypothesized that a T cell-independent system exists for the recognition and elimination of cells with reduced or absent expression of MHC molecules. To test this hypothesis, they selected MHC class I-deficient lymphoma cells and compared these to MHC class I-sufficient cells for the ability to establish tumors in mice. After inoculation into syngeneic mice, MHC class I-sufficient cells showed progressive growth, while MHC class I-deficient cells failed to grow out. Similar results were observed in nude mice lacking T cells. However, in mice receiving injections of an NK cell-depleting antibody, MHC class I-deficient tumor cells grew progressively (Ljunggren and Karre 1985). In chromium-release assays, NK cells but not cytotoxic T cells directly mediated specific lysis against MHC class I-negative tumor cells (Karre et al. 1986). Together, these studies demonstrated that recognition of and response to missing-self (i.e., MHC class I antigen) is a fundamental aspect of NK cell biology. These studies also provided a definitive mechanistic explanation for the phenomenon of hybrid resistance, whereby normal and neoplastic hematopoietic cells fail to graft in certain lethally irradiated mice, including F1 hybrid recipients of parental cells (Kiessling et al. 1977; Daley and Nakamura 1984; Bordignon et al. 1985).
Once the MHC-dependent discrimination of normal and transformed tissue by NK cells had been described, the race was on to identify the receptors that mediate this effect. Immunization of mice with human NK cells led to the discovery of monoclonal antibodies that could be used to reverse the inhibition of NK cell-mediated lysis in functional assays. These antibodies immunoprecipitated 58-Kd and 70-Kd surface glycoproteins corresponding to the human leukocyte antigen (HLA)-C and HLA-Bw4 specificity of NK cell clones, respectively (Moretta et al. 1993; Litwin et al. 1994). Cloning and sequencing of cDNAs by several laboratories revealed that these receptors, known as killer immunoglobulin-like receptors (KIR), are type I glycoproteins with homology to proteins within the immunoglobulin superfamily (Colonna and Samaridis 1995; D’Andrea et al. 1995; Wagtmann et al. 1995; Dohring et al. 1996). These receptors are encoded by a highly polymorphic family of related genes on chromosome 19q13.4 (Suto et al. 1996) and share a common structure of either two or three extracellular immunoglobulin-like domains, a transmembrane region, and a cytoplasmic tail. Inhibitory KIR possess long cytoplasmic tails with tandem immunoreceptor tyrosine-based inhibitory motifs (ITIMs) that recruit tyrosine phosphatases and inhibit NK cell function (Olcese et al. 1996). Certain isoforms of KIR lack ITIM sequences and activate, rather than inhibit, NK cells. Activating KIR non-covalently associates with the DAP12 signaling molecule. DAP12 contains an immunoreceptor tyrosine-based activation motif (ITAM) in its cytoplasmic domain and recruits the protein tyrosine kinases SYK and ZAP-70 upon phosphorylation (Lanier et al. 1998).
The identification of KIR as mediators of missing-self responses by NK cells led to the hypothesis that mismatches between donor KIR and recipient HLA class I could potentiate GvL activity by increasing NK function. Velardi and colleagues tested this hypothesis by investigating the role of NK cell alloreactivity in HLA haplotype-mismatched HCT recipients. NK cells of donor origin were isolated from HCT recipients and tested ex vivo for lysis of cryopreserved pretransplant recipient lymphocytes. In these assays, donor alloreactive NK cells were highly effective in killing recipient leukemia cells of myeloid but not lymphoid origin (Ruggeri et al. 1999).
In a follow-up study, this group reported clinical data and outcomes in HLA haplotype-mismatched transplants with and without KIR ligand incompatibility, which was defined as the absence in recipients of donor HLA class I allele groups recognized by KIRs. They found that hematopoietic cell grafts with KIR ligand incompatibility was associated with a profound GvL effect and lower probability of relapse for AML patients at 5 years (0 % with KIR ligand incompatibility versus 75 % without KIR ligand incompatibility) (Ruggeri et al. 2002) without evidence of GvHD. We retrospectively analyzed data from 2062 patients undergoing unrelated donor hematopoietic cell transplantation (HCT) for AML, chronic myeloid leukemia (CML), and myelodysplastic syndrome (MDS). Missing one or more KIR ligands was associated with significant protection against relapse in patients with early myeloid leukemia (Miller et al. 2007), supporting a beneficial role for KIR ligand mismatch in the treatment of myeloid leukemia.
While KIR ligand mismatch appears to be an effective strategy for the treatment of AML, it is worth keeping in mind the complexity of HCT and the impacts of transplant-related variables. In the studies reported by Velardi and colleagues, all patients received myeloablative conditioning and were transplanted with T cell-depleted granulocyte-colony stimulating factor-mobilized hematopoietic cell grafts. In a later trial that used less T cell depletion, KIR ligand mismatch patients developed more acute GvHD and had poorer overall survival (Bishara et al. 2004). In another study, the use of minimally T cell depleted, KIR ligand mismatched donor grafts did not improve clinical outcomes. In fact, the expansion of alloreactive T cells overwhelmed the beneficial effect of alloreactive NK cells and was associated with an increased risk of both acute and chronic GvHD and death (Lowe et al. 2003).
We examined the clinical impact of KIR ligand mismatch in 257 recipients of umbilical cord blood (UCB) grafts after either myeloablative or reduced intensity conditioning regimens. In our analysis, KIR ligand mismatch had no effect on GvHD, relapse, transplantation-related mortality (TRM), or survival after my-eloablative conditioning. After reduced intensity conditioning, KIR ligand mismatch was associated with significantly higher rates of acute GvHD and inferior survival (Brunstein et al. 2009). Although UCB units are relatively T cell depleted, the composition of T cells may influence outcomes. Compared with adult grafts, which contain a mixture of naïve, effector, and memory T cells, the T cells in UCB grafts are essentially all naïve and may outcompete NK cells in some transplant scenarios. Thus, HCT is a balancing act, and many challenges still remain in selecting the conditioning regimen, hematopoietic cell source, and degree of mismatch that will lead to optimal relapse prevention and overall survival.
Diverse KIR haplotypes can be simplified into two biologically distinct groups: A and B. Group A haplotypes contain a fixed number of genes that exclusively encode inhibitory receptors, with the exception of KIR2DS4. Group B haplotypes, on the other hand, have variable KIR gene content and include genes encoding the activating receptors KIR2DS1, KIR2DS2, KIR2DS3, KIR2DS5, and KIR3DS17 (Uhrberg et al. 1997). We genotyped donors and recipients from 209 HLA-matched and 239 HLA-mismatched, T cell replete unrelated donor transplantations for AML. In our analysis, three-year overall survival was significantly higher after transplantation from a donor with at least one KIR B haplotype allele (KIR B/x). Multivariable analysis demonstrated a 30 % improvement in the relative risk of relapse-free survival with B/x donors relative to A/A donors (Cooley et al. 2009). In a follow-up study of an expanded cohort of 1532 unrelated donor transplants without T cell depletion, we found that the relapse protection associated with donor KIR B haplotypes is enhanced in recipients who have one or two HLA-C1 alleles compared to C2 homozygous recipients (Cooley et al. 2014). In support of a favorable association between the KIR B haplotype and transplant outcomes, a recent study by Oevermann et al. (2014) found that acute lymphocytic leukemia (ALL) patients transplanted with cells from KIR B haplotype donors had significantly better event-free survival and lower relapse risk relative to KIR A haplotype donors.
It is tempting to speculate that one or more activating KIR present in the KIR B haplotype is directly responsible for prevention of leukemia relapse. Venstrom et al. (2012) evaluated the effect of activating KIR2DS1 from donors on the outcome of allogeneic HCT in 1277 patients with AML and reported a considerable KIR2DS1-dependent GvL effect restricted to donors with at least one copy of HLA-C1. However, Cooley et al. (2014) found that all KIR B genes were equally protective and KIR2DS1 was not dominant. Whether a unique direct interaction between KIR2DS1 and HLA-C2 is responsible for the observed antileukemic effects is still unclear. Notably, it has been shown that HLA-C2 is a general risk factor in HCT for patients with myeloid leukemia independent of genotypic differences in KIR genotypes (Fischer et al. 2007). One possible explanation for the beneficial effect of donor KIR2DS1 on overall survival post-transplant is that it predominantly protects against infection-related mortality. Clinical studies have shown that the presence of activating KIRs correlates with protection against acquired immunodeficiency syndrome (AIDS) progression and hepatitis C virus infection (Martin et al. 2002; Khakoo et al. 2004). In an analysis of 69 transplants with donor-versus-recipient alloreactivity, Velardi and colleagues recently reported that transplantation from donors with KIR2DS1 and/or KIR3DS1 was associated with reduced risk of non-relapse mortality that was largely related to a 50 % reduction in infection rate. The protective effect was mainly associated with viral infections and, to a lesser extent, fungal infections (Mancusi et al. 2015).
Thus, donor KIR haplotypes and individual KIR appear to be relevant for transplant outcomes. However, variability in KIR binding affinities and extensive linkage disequilibrium between KIR genes makes it difficult to pinpoint the exact underlying molecular mechanism of these effects. Future studies that incorporate high-resolution KIR typing and functional binding assays may shed more light on the precise role of activating KIR in the transplant setting.
5 Cytomegalovirus: Driving NK Cells to Adapt Post-HCT
CMV is a β-herpesvirus that is generally acquired early in life. Seroprevalence is ~50–80 % in the USA and Europe depending on socioeconomic status and approaches 100 % in Africa and Asia (Cannon et al. 2010). Infections are generally mild or asymptomatic in healthy individuals, as they are well controlled by T cell and NK cell responses (Quinnan et al. 1982; Biron et al. 1989; Sylwester et al. 2005). Despite robust host responses, CMV efficiently adapts to the immune system and is never completely eliminated from an infected individual. Multiple immune evasion genes have been identified within the CMV genome, allowing the virus to subvert both innate and adaptive immune responses and prevent viral clearance. Thus, the immune system and the virus are at a standoff in infected individuals, and lifelong latency is eventually established primarily in cells of the myeloid lineage (Poole et al. 2011).
Reactivation of CMV is a frequent occurrence in immunodeficient transplant recipients and was previously associated with high rates of morbidity and mortality (Neiman et al. 1973). The widespread use of drugs such as ganciclovir and foscarnet that can be used as preemptive therapy to limit CMV replication has brought these rates down considerably, and lethal CMV pneumonia is now uncommon. Because CMV is able to elicit such strong innate and adaptive immune responses, several groups have explored the possibility that CMV reactivation early after transplant protects against cancer relapse. Lönnqvist et al. published one of the first reports describing an association between CMV infection and relapse protection nearly 30 years ago. They analyzed one-year relapse rates in 72 BMT recipients with various hematological malignancies and found that the probability of relapse was lower in recipients that exhibited the evidence of CMV infection (Lonnqvist et al. 1986).
More recently, several reports have confirmed an association between CMV and reduced leukemia relapse following HCT. Elmaagacli et al. evaluated the impact of early CMV reactivation on relapse in 266 allogeneic HCT recipients with AML. In 77 patients, CMV reactivation, as detected by pp65 antigenemia, occurred within the first 100 days post-transplant. The cumulative relapse incidence was 42 % in patients without early pp65 antigenemia compared to 9 % in patients with early pp65 antigenemia (Elmaagacli et al. 2011). An obvious confounding factor is that CMV reactivation is a known trigger for acute GvHD (Ljungman 1998). However, within the group of 187 individuals with grade II–IV acute GvHD in this study, CMV reactivation still had an independent impact on relapse (Elmaagacli et al. 2011). An association between CMV lower relapse risk was also reported in analyses of a cohort of 110 CML patients (Ito et al. 2013), a cohort of 140 pediatric patients with acute leukemia (Behrendt et al. 2009) and a cohort of 103 patients with various hematological malignancies (Nachbaur et al. 2001). However, some of these findings were not entirely reproduced in larger observational cohorts (Remberger and Ringden 2002; Ljungman et al. 2003; Kollman et al. 2001; Beck et al. 2010).
Given the somewhat conflicting data on CMV and transplant outcomes, Green et al. undertook an analysis of a cohort of 2566 patients with AML, ALL, CML, MDS, and lymphoma undergoing allogeneic HCT at a single center. After adjusting for underlying variables, CMV reactivation was significantly associated with a decreased risk of relapse independent of acute GvHD in patients with AML by day 100 and approached significance at one year. CMV reactivation trended toward an association with relapse protection in multivariable models in patients with ALL, CML, MDS, and lymphoma. When all disease groups were combined, the authors observed a 53 % decreased risk of relapse by day 100 and a 32 % decreased risk of relapse by one year (Green et al. 2013). While results are variable between cohorts and transplant centers, there is a growing consensus that an association exists between CMV reactivation and relapse protection.
The obvious question posed by these findings is what biological mechanism(s) underlies the association between CMV reactivation and relapse observed in HCT recipients with different hematological malignancies. A possible explanation is that the effect is mediated through CMV-specific donor T cells. However, this was not supported by Green et al. (2013) who found that donor CMV serological status did not impact relapse rates. Furthermore, adoptive transfer of CMV-specific T cells does not appear to have a protective effect on relapse (Thomson et al. 2012). One promising hypothesis is that CMV reactivation drives the differentiation and expansion of unique subsets of NK cells that have activity against residual tumor cells. Groundbreaking work by López-Botet and colleagues has revealed a specific association between the DAP12-coupled activating receptor NKG2C and NK cell responses to CMV infection (Guma et al. 2004, 2006).
We have shown that CMV reactivation early after transplant is associated with the expansion and persistence of NK cells that express NKG2C, lack the inhibitory receptor NKG2A, express self-KIR, and preferentially acquire the maturation marker CD57. NKG2C+ NK cells produced significantly more IFN-γ in response to the K562 myeloid leukemia cell line relative to NKG2C− NK cells (Foley et al. 2012a, b). NKG2C+ NK cells also expanded in the absence of detectable CMV viremia when both the donor and recipient were CMV seropositive, implying that latent CMV antigen can drive the expansion of NK cells expressing NKG2C. Furthermore, NKG2C+ NK cells transplanted from seropositive donors exhibited heightened function in response to secondary CMV events compared with NKG2C+ NK cells from CMV seronegative donors (Foley et al. 2012a, b).
NKG2C is expressed on the surface of NK cells as a heterodimer with CD94 and binds the non-classical class I HLA molecule, HLA-E (Braud et al. 1998). While CMV-encoded proteins stabilize HLA-E expression (Tomasec et al. 2000; Ulbrecht et al. 2000) and influence NK cell recognition, NKG2C+ NK cells do not appear to have strict specificity for CMV antigens as evidenced by their heightened responses to K562 cells (Foley et al. 2012a, b). Like virally infected cells, cancer cells can downregulate classical class I HLA molecules while retaining expression of HLA-E (Nguyen et al. 2005; Marin et al. 2003; Lo Monaco et al. 2011). Thus, it is possible that the switch predominant in receptor usage from inhibitory NKG2A to activating NKG2C could be a key mechanism by which these cells mediate GvL effects.
In many respects, CD56dimCD57+NKG2C+ NK cells appear to represent a human analog of Ly49H+ memory-like NK cells that recognize the MCMV-encoded m157 protein (Arase et al. 2002) and participate in the clearance of CMV infections in mice (Brown et al. 2001). Lanier and colleagues have shown that Ly49H+ NK cells expand dramatically in the spleen and liver of mice infected with MCMV and produce elevated levels of IFN-γ. Ly49H+ cells then undergo a contraction phase and expand rapidly upon secondary viral challenge (Sun et al. 2009). The specificity and kinetics of the NK cell response to MCMV infection mirrors that of cytotoxic T cells and provides compelling evidence for the capacity of NK cells to generate memory-like or adaptive responses, at least to CMV infection.
We have recently shown that the adaptive NK cell response to CMV infection goes well beyond NKG2C expression. We identified subsets of human NK cells stochastically lacking expression of the B cell- and myeloid cell-related signaling proteins FcεR1γ, EWS/FLI1-activated transcript (EAT)-2, and spleen tyrosine kinase (SYK) along with reduced expression of the transcription factor promyelocytic leukemia zinc finger protein (PLZF) in healthy CMV seropositive individuals and in response to CMV reactivation post-HCT. These NK cell subsets displayed an adaptive surface receptor phenotype, as they were enriched for the expression of DAP12-coupled activating receptors, preferentially expressed CD57 and expressed low levels of NKG2A. A comparative analysis of NK cell and CD8+ T cell subsets revealed striking genome-wide epigenetic differences among NK cell subsets, with adaptive NK cell differentiation paralleling that of effector CD8+ T cells.
Functionally, CD56dim FcεR1γ− adaptive NK cells produced significantly less IFN-γ in response to IL-12 and IL-18 co-stimulation relative to other NK cell subsets, and this lack of responsiveness was associated with the reduced transcription of IL-12 (IL12RB2) and IL-18 (IL18RAP) receptor subunits. Despite their lack of responsiveness to innate cytokines, regulatory regions within IFNG and TNF were hypomethylated in adaptive NK cells, and these cells more frequently produced IFN-γ and TNF in response to stimulation through the low-affinity Fc-binding receptor CD16. Thus, adaptive NK cells appear to be primed for enhanced antibody-dependent cellular cytotoxicity (ADCC). Interestingly, adaptive NK cells lacking FcεR1γ and/or EAT-2 displayed significantly diminished degranulation in response to autologous, activated T cells. Thus, there appears to exist a functional dichotomy between canonical and adaptive NK cells in terms of survival, regulatory function, and responses to ITAM-dependent activating receptor signaling. We also examined the expansion of adaptive NK cells lacking FcεR1γ in response to autologous monocytes infected with CMV. The frequency of the FcεR1γ− subset increased to a modest extent in response to CMV-infected monocytes, and the addition of anti-CMV antibody accentuated FcεR1γ− NK cell expansion, driving robust proliferation (Schlums et al. 2015). CMV antibody-driven expansion of adaptive FcεR1γ− NK cells has also been reported by Kim and colleagues (Zhang et al. 2013; Lee et al. 2015) and by Muntasell and colleagues (Costa-Garcia et al. 2015).
Further analyses of HCT recipients that incorporate these novel adaptive NK cell markers may lead to a more clear definition of the association between CMV-induced adaptive NK cells and relapse protection and the mechanisms underlying this effect. Our data suggest that adaptive NK cells may have direct anti-tumor function through enhanced functional responses triggered by activating receptors or may indirectly contribute to relapse protection through reduced killing of activated T cells. Studies addressing these hypotheses are currently in progress.
6 Conclusion
The dedicated efforts of countless research groups have advanced our understanding of basic NK cell biology over the past several decades (Fig. 1). We have gone from a view of NK cells as spontaneous killers to one where NK cell function is carefully tuned by the environment and triggered by subtle changes in MHC expression. While we once viewed NK cells as short-lived innate lymphocytes, we are now exploring their adaptive traits. The next frontier will be to use what we have learned to design new, effective strategies for NK cell immunotherapy to treat patients with cancer.
Fig. 1. Timeline of NK cells in HCT.
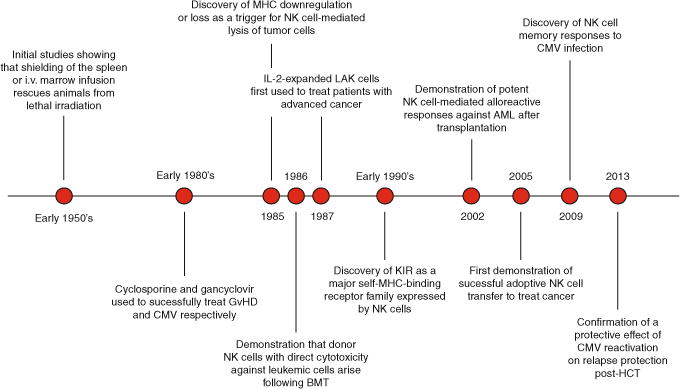
This timeline illustrates milestones in the history of hematopoietic cell transplantation and the therapeutic benefit of NK cells from the early 1950’s to the present
Acknowledgments
This work was supported by P01 CA111412 (FC, MRV, SC, YTB, DJW, JSM), P01 CA65493 (FC, MRV SC, CGB, BRB, JW, JSM) and R01CA72669 (BRB).
Contributor Information
Frank Cichocki, Department of Medicine, University of Minnesota, Minneapolis, MN, USA.
Michael R. Verneris, Department of Pediatrics, University of Minnesota, Minneapolis, MN, USA
Sarah Cooley, Department of Medicine, University of Minnesota, Minneapolis, MN, USA.
Veronika Bachanova, Department of Medicine, University of Minnesota, Minneapolis, MN, USA.
Claudio G. Brunstein, Department of Medicine, University of Minnesota, Minneapolis, MN, USA
Bruce R. Blazar, Department of Pediatrics, University of Minnesota, Minneapolis, MN, USA
John Wagner, Department of Pediatrics, University of Minnesota, Minneapolis, MN, USA.
Heinrich Schlums, Centre for Infectious Medicine, Department of Medicine, Karolinska Institutet, Karolinska University Hospital Huddinge, Stockholm, Sweden.
Yenan T. Bryceson, Centre for Infectious Medicine, Department of Medicine, Karolinska Institutet, Karolinska University Hospital Huddinge, Stockholm, Sweden Broeglmann Research Laboratory, Clinical Institute, University of Bergen, Bergen, Norway.
Daniel J. Weisdorf, Department of Medicine, University of Minnesota, Minneapolis, MN, USA
Jeffrey S. Miller, Department of Medicine, University of Minnesota, Minneapolis, MN, USA MMC 806, Division of Hematology, Oncology and Transplantation, University of Minnesota Cancer Center, Harvard Street at East River Road, Minneapolis, MN 55455, USA.
References
- Appelbaum FR, Dahlberg S, Thomas ED, et al. Bone marrow transplantation or chemotherapy after remission induction for adults with acute nonlymphoblastic leukemia. A prospective comparison. Ann Intern Med. 1984;101(5):581–588. doi: 10.7326/0003-4819-101-5-581. [DOI] [PubMed] [Google Scholar]
- Arase H, Mocarski ES, Campbell AE, Hill AB, Lanier LL. Direct recognition of cytomegalovirus by activating and inhibitory NK cell receptors. Science. 2002;296(5571):1323–1326. doi: 10.1126/science.1070884. [DOI] [PubMed] [Google Scholar]
- Ardolino M, Azimi CS, Iannello A, et al. Cytokine therapy reverses NK cell anergy in MHC-deficient tumors. J Clin Invest. 2014;124(11):4781–4794. doi: 10.1172/JCI74337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachanova V, Burns LJ, McKenna DH, et al. Allogeneic natural killer cells for refractory lymphoma. Cancer Immunol Immunother. 2010;59(11):1739–1744. doi: 10.1007/s00262-010-0896-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachanova V, Cooley S, Defor TE, et al. Clearance of acute myeloid leukemia by haploidentical natural killer cells is improved using IL-2 diphtheria toxin fusion protein. Blood. 2014;123(25):3855–3863. doi: 10.1182/blood-2013-10-532531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck JC, Wagner JE, DeFor TE, et al. Impact of cytomegalovirus (CMV) reactivation after umbilical cord blood transplantation. Biol Blood Marrow Transplant. 2010;16(2):215–222. doi: 10.1016/j.bbmt.2009.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrendt CE, Rosenthal J, Bolotin E, Nakamura R, Zaia J, Forman SJ. Donor and recipient CMV serostatus and outcome of pediatric allogeneic HSCT for acute leukemia in the era of CMV-preemptive therapy. Biol Blood Marrow Transplant. 2009;15(1):54–60. doi: 10.1016/j.bbmt.2008.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biron CA, Welsh RM. Blastogenesis of natural killer cells during viral infection in vivo. J Immunol. 1982;129(6):2788–2795. [PubMed] [Google Scholar]
- Biron CA, Turgiss LR, Welsh RM. Increase in NK cell number and turnover rate during acute viral infection. J Immunol. 1983;131(3):1539–1545. [PubMed] [Google Scholar]
- Biron CA, Byron KS, Sullivan JL. Severe herpesvirus infections in an adolescent without natural killer cells. N Engl J Med. 1989;320(26):1731–1735. doi: 10.1056/NEJM198906293202605. [DOI] [PubMed] [Google Scholar]
- Bishara A, De Santis D, Witt CC, et al. The beneficial role of inhibitory KIR genes of HLA class I NK epitopes in haploidentically mismatched stem cell allografts may be masked by residual donor-alloreactive T cells causing GVHD. Tissue Antigens. 2004;63(3):204–211. doi: 10.1111/j.0001-2815.2004.00182.x. [DOI] [PubMed] [Google Scholar]
- Bordignon C, Daley JP, Nakamura I. Hematopoietic histoincompatibility reactions by NK cells in vitro: model for genetic resistance to marrow grafts. Science. 1985;230(4732):1398–1401. doi: 10.1126/science.3906897. [DOI] [PubMed] [Google Scholar]
- Bowden RA, Day LM, Amos DE, Meyers JD. Natural cytotoxic activity against cytomegalovirus-infected target cells following marrow transplantation. Transplantation. 1987;44(4):504–508. doi: 10.1097/00007890-198710000-00009. [DOI] [PubMed] [Google Scholar]
- Braud VM, Allan DS, O’Callaghan CA, et al. HLA-E binds to natural killer cell receptors CD94/NKG2A, B and C. Nature. 1998;391(6669):795–799. doi: 10.1038/35869. [DOI] [PubMed] [Google Scholar]
- Brown MG, Dokun AO, Heusel JW, et al. Vital involvement of a natural killer cell activation receptor in resistance to viral infection. Science. 2001;292(5518):934–937. doi: 10.1126/science.1060042. [DOI] [PubMed] [Google Scholar]
- Brunstein CG, Wagner JE, Weisdorf DJ, et al. Negative effect of KIR alloreactivity in recipients of umbilical cord blood transplant depends on transplantation conditioning intensity. Blood. 2009;113(22):5628–5634. doi: 10.1182/blood-2008-12-197467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukowski JF, Warner JF, Dennert G, Welsh RM. Adoptive transfer studies demonstrating the antiviral effect of natural killer cells in vivo. J Exp Med. 1985;161(1):40–52. doi: 10.1084/jem.161.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon MJ, Schmid DS, Hyde TB. Review of cytomegalovirus seroprevalence and demographic characteristics associated with infection. Rev Med Virol. 2010;20(4):202–213. doi: 10.1002/rmv.655. [DOI] [PubMed] [Google Scholar]
- Carson WE, Giri JG, Lindemann MJ, et al. Interleukin (IL) 15 is a novel cytokine that activates human natural killer cells via components of the IL-2 receptor. J Exp Med. 1994;180(4):1395–1403. doi: 10.1084/jem.180.4.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan SH, Perussia B, Gupta JW, et al. Induction of interferon gamma production by natural killer cell stimulatory factor: characterization of the responder cells and synergy with other inducers. J Exp Med. 1991;173(4):869–879. doi: 10.1084/jem.173.4.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colonna M, Samaridis J. Cloning of immunoglobulin-superfamily members associated with HLA-C and HLA-B recognition by human natural killer cells. Science. 1995;268(5209):405–408. doi: 10.1126/science.7716543. [DOI] [PubMed] [Google Scholar]
- Cooley S, Trachtenberg E, Bergemann TL, et al. Donors with group B KIR haplotypes improve relapse-free survival after unrelated hematopoietic cell transplantation for acute myelogenous leukemia. Blood. 2009;113(3):726–732. doi: 10.1182/blood-2008-07-171926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooley S, Weisdorf DJ, Guethlein LA, et al. Donor killer cell Ig-like receptor B haplotypes, recipient HLA-C1, and HLA-C mismatch enhance the clinical benefit of unrelated transplantation for acute myelogenous leukemia. J Immunol. 2014;192(10):4592–4600. doi: 10.4049/jimmunol.1302517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa-Garcia M, Vera A, Moraru M, Vilches C, Lopez-Botet M, Muntasell A. Antibody-Mediated Response of NKG2Cbright NK Cells against Human Cytomegalovirus. J Immunol. 2015;194(6):2715–2724. doi: 10.4049/jimmunol.1402281. [DOI] [PubMed] [Google Scholar]
- Daley JP, Nakamura I. Natural resistance of lethally irradiated F1 hybrid mice to parental marrow grafts is a function of H-2/Hh-restricted effectors. J Exp Med. 1984;159(4):1132–1148. doi: 10.1084/jem.159.4.1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Andrea A, Chang C, Franz-Bacon K, McClanahan T, Phillips JH, Lanier LL. Molecular cloning of NKB1. A natural killer cell receptor for HLA-B allotypes. J Immunol. 1995;155(5):2306–2310. [PubMed] [Google Scholar]
- De Baetselier P, Katzav S, Gorelik E, Feldman M, Segal S. Differential expression of H-2 gene products in tumour cells in associated with their metastatogenic properties. Nature. 1980;288(5787):179–181. doi: 10.1038/288179a0. [DOI] [PubMed] [Google Scholar]
- Deeg HJ, Storb R, Thomas ED, et al. Cyclosporine as prophylaxis for graft-versus-host disease: a randomized study in patients undergoing marrow transplantation for acute nonlymphoblastic leukemia. Blood. 1985;65(6):1325–1334. [PubMed] [Google Scholar]
- Dinsmore R, Kirkpatrick D, Flomenberg N, et al. Allogeneic bone marrow transplantation for patients with acute nonlymphocytic leukemia. Blood. 1984;63(3):649–656. [PubMed] [Google Scholar]
- Doherty PC, Knowles BB, Wettstein PJ. Immunological surveillance of tumors in the context of major histocompatibility complex restriction of T cell function. Adv Cancer Res. 1984;42:1–65. doi: 10.1016/s0065-230x(08)60455-8. [DOI] [PubMed] [Google Scholar]
- Dohring C, Scheidegger D, Samaridis J, Cella M, Colonna M. A human killer inhibitory receptor specific for HLA-A1,2. J Immunol. 1996;156(9):3098–3101. [PubMed] [Google Scholar]
- Dupont E, Huygen K, Schandene L, Vandercruys M, Palfliet K, Wybran J. Depressed natural killer function in transplant recipients: an analysis. Transplant Proc. 1984;16(6):1506–1508. [PubMed] [Google Scholar]
- Eisenbach L, Segal S, Feldman M. MHC imbalance and metastatic spread in Lewis lung carcinoma clones. Int J Cancer. 1983;32(1):113–120. doi: 10.1002/ijc.2910320118. [DOI] [PubMed] [Google Scholar]
- Elmaagacli AH, Steckel NK, Koldehoff M, et al. Early human cytomegalovirus replication after transplantation is associated with a decreased relapse risk: evidence for a putative virus-versus-leukemia effect in acute myeloid leukemia patients. Blood. 2011;118(5):1402–1412. doi: 10.1182/blood-2010-08-304121. [DOI] [PubMed] [Google Scholar]
- Ettinghausen SE, Puri RK, Rosenberg SA. Increased vascular permeability in organs mediated by the systemic administration of lymphokine-activated killer cells and recombinant interleukin-2 in mice. J Natl Cancer Inst. 1988;80(3):177–188. doi: 10.1093/jnci/80.3.177. [DOI] [PubMed] [Google Scholar]
- Field AK, Davies ME, DeWitt C, et al. 9-([2-hydroxy-1-(hydroxymethyl)ethoxy]methyl) guanine: a selective inhibitor of herpes group virus replication. Proc Natl Acad Sci U S A. 1983;80(13):4139–4143. doi: 10.1073/pnas.80.13.4139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer JC, Ottinger H, Ferencik S, et al. Relevance of C1 and C2 epitopes for hemopoietic stem cell transplantation: role for sequential acquisition of HLA-C-specific inhibitory killer Ig-like receptor. J Immunol. 2007;178(6):3918–3923. doi: 10.4049/jimmunol.178.6.3918. [DOI] [PubMed] [Google Scholar]
- Foley B, Cooley S, Verneris MR, et al. Cytomegalovirus reactivation after allogeneic transplantation promotes a lasting increase in educated NKG2C+ natural killer cells with potent function. Blood. 2012a;119(11):2665–2674. doi: 10.1182/blood-2011-10-386995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley B, Cooley S, Verneris MR, et al. Human cytomegalovirus (CMV)-induced memory-like NKG2C(+) NK cells are transplantable and expand in vivo in response to recipient CMV antigen. J Immunol. 2012b;189(10):5082–5088. doi: 10.4049/jimmunol.1201964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford CE, Hamerton JL, Barnes DW, Loutit JF. Cytological identification of radiation-chimaeras. Nature. 1956;177(4506):452–454. doi: 10.1038/177452a0. [DOI] [PubMed] [Google Scholar]
- Gasteiger G, Hemmers S, Firth MA, et al. IL-2-dependent tuning of NK cell sensitivity for target cells is controlled by regulatory T cells. J Exp Med. 2013;210(6):1167–1178. doi: 10.1084/jem.20122462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geller MA, Cooley S, Judson PL, et al. A phase II study of allogeneic natural killer cell therapy to treat patients with recurrent ovarian and breast cancer. Cytotherapy. 2011;13(1):98–107. doi: 10.3109/14653249.2010.515582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghiringhelli F, Menard C, Terme M, et al. CD4+ CD25+ regulatory T cells inhibit natural killer cell functions in a transforming growth factor-beta-dependent manner. J Exp Med. 2005;202(8):1075–1085. doi: 10.1084/jem.20051511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabstein KH, Eisenman J, Shanebeck K, et al. Cloning of a T cell growth factor that interacts with the beta chain of the interleukin-2 receptor. Science. 1994;264(5161):965–968. doi: 10.1126/science.8178155. [DOI] [PubMed] [Google Scholar]
- Green ML, Leisenring WM, Xie H, et al. CMV reactivation after allogeneic HCT and relapse risk: evidence for early protection in acute myeloid leukemia. Blood. 2013;122(7):1316–1324. doi: 10.1182/blood-2013-02-487074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm EA, Mazumder A, Zhang HZ, Rosenberg SA. Lymphokine-activated killer cell phenomenon. Lysis of natural killer-resistant fresh solid tumor cells by interleukin 2-activated autologous human peripheral blood lymphocytes. J Exp Med. 1982;155(6):1823–1841. doi: 10.1084/jem.155.6.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guma M, Angulo A, Vilches C, Gomez-Lozano N, Malats N, Lopez-Botet M. Imprint of human cytomegalovirus infection on the NK cell receptor repertoire. Blood. 2004;104(12):3664–3671. doi: 10.1182/blood-2004-05-2058. [DOI] [PubMed] [Google Scholar]
- Guma M, Budt M, Saez A, et al. Expansion of CD94/NKG2C+ NK cells in response to human cytomegalovirus-infected fibroblasts. Blood. 2006;107(9):3624–3631. doi: 10.1182/blood-2005-09-3682. [DOI] [PubMed] [Google Scholar]
- Hercend T, Takvorian T, Nowill A, et al. Characterization of natural killer cells with antileukemia activity following allogeneic bone marrow transplantation. Blood. 1986;67(3):722–728. [PubMed] [Google Scholar]
- Hoffman M, Mittelman A, Dworkin B, et al. Severe intrahepatic cholestasis in patients treated with recombinant interleukin-2 and lymphokine-activated killer cells. J Cancer Res Clin Oncol. 1989;115(2):175–178. doi: 10.1007/BF00397920. [DOI] [PubMed] [Google Scholar]
- Hokland M, Jacobsen N, Ellegaard J, Hokland P. Natural killer function following allogeneic bone marrow transplantation. Very early reemergence but strong dependence of cytomegalovirus infection. Transplantation. 1988;45(6):1080–1084. doi: 10.1097/00007890-198806000-00016. [DOI] [PubMed] [Google Scholar]
- Ito S, Pophali P, Co W, et al. CMV reactivation is associated with a lower incidence of relapse after allo-SCT for CML. Bone Marrow Transplant. 2013;48(10):1313–1316. doi: 10.1038/bmt.2013.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson LO. Evidence for a humoral factor (or factors) concerned in recovery from radiation injury: a review. Cancer Res. 1952;12(5):315–325. [PubMed] [Google Scholar]
- Jacobson LO, Simmons EL, Marks EK, Robson MJ, Bethard WF, Gaston EO. The role of the spleen in radiation injury and recovery. J Lab Clin Med. 1950;35(5):746–770. [PubMed] [Google Scholar]
- Johnson FL, Thomas ED, Clark BS, Chard RL, Hartmann JR, Storb R. A comparison of marrow transplantation with chemotherapy for children with acute lymphoblastic leukemia in second or subsequent remission. N Engl J Med. 1981;305(15):846–851. doi: 10.1056/NEJM198110083051502. [DOI] [PubMed] [Google Scholar]
- Jonjic S, Mutter W, Weiland F, Reddehase MJ, Koszinowski UH. Site-restricted persistent cytomegalovirus infection after selective long-term depletion of CD4+ T lymphocytes. J Exp Med. 1989;169(4):1199–1212. doi: 10.1084/jem.169.4.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karre K, Ljunggren HG, Piontek G, Kiessling R. Selective rejection of H-2-deficient lymphoma variants suggests alternative immune defence strategy. Nature. 1986;319(6055):675–678. doi: 10.1038/319675a0. [DOI] [PubMed] [Google Scholar]
- Katzav S, De Baetselier P, Tartakovsky B, Feldman M, Segal S. Alterations in major histocompatibility complex phenotypes of mouse cloned T10 sarcoma cells: association with shifts from nonmetastatic to metastatic cells. J Natl Cancer Inst. 1983;71(2):317–324. [PubMed] [Google Scholar]
- Khakoo SI, Thio CL, Martin MP, et al. HLA and NK cell inhibitory receptor genes in resolving hepatitis C virus infection. Science. 2004;305(5685):872–874. doi: 10.1126/science.1097670. [DOI] [PubMed] [Google Scholar]
- Kiessling R, Hochman PS, Haller O, Shearer GM, Wigzell H, Cudkowicz G. Evidence for a similar or common mechanism for natural killer cell activity and resistance to hemopoietic grafts. Eur J Immunol. 1977;7(9):655–663. doi: 10.1002/eji.1830070915. [DOI] [PubMed] [Google Scholar]
- Kollman C, Howe CW, Anasetti C, et al. Donor characteristics as risk factors in recipients after transplantation of bone marrow from unrelated donors: the effect of donor age. Blood. 2001;98(7):2043–2051. doi: 10.1182/blood.v98.7.2043. [DOI] [PubMed] [Google Scholar]
- Lanier LL, Corliss BC, Wu J, Leong C, Phillips JH. Immunoreceptor DAP12 bearing a tyrosine-based activation motif is involved in activating NK cells. Nature. 1998;391(6668):703–707. doi: 10.1038/35642. [DOI] [PubMed] [Google Scholar]
- Lee J, Zhang T, Hwang I, et al. Epigenetic Modification and Antibody-Dependent Expansion of Memory-like NK Cells in Human Cytomegalovirus-Infected Individuals. Immunity. 2015;42(3):431–442. doi: 10.1016/j.immuni.2015.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsley DL, Odell TT, Jr, Tausche FG. Implantation of functional erythropoietic elements following total-body irradiation. Proc Soc Exp Biol Med. 1955;90(2):512–515. doi: 10.3181/00379727-90-22082. [DOI] [PubMed] [Google Scholar]
- Litwin V, Gumperz J, Parham P, Phillips JH, Lanier LL. NKB1: a natural killer cell receptor involved in the recognition of polymorphic HLA-B molecules. J Exp Med. 1994;180(2):537–543. doi: 10.1084/jem.180.2.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ljunggren HG, Karre K. Host resistance directed selectively against H-2-deficient lymphoma variants. Analysis of the mechanism. J Exp Med. 1985;162(6):1745–1759. doi: 10.1084/jem.162.6.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ljungman P. Immune reconstitution and viral infections after stem cell transplantation. Bone Marrow Transplant. 1998;21(Suppl 2):S72–S74. [PubMed] [Google Scholar]
- Ljungman P, Brand R, Einsele H, Frassoni F, Niederwieser D, Cordonnier C. Donor CMV serologic status and outcome of CMV-seropositive recipients after unrelated donor stem cell transplantation: an EBMT megafile analysis. Blood. 2003;102(13):4255–4260. doi: 10.1182/blood-2002-10-3263. [DOI] [PubMed] [Google Scholar]
- Lo Monaco E, Tremante E, Cerboni C, et al. Human leukocyte antigen E contributes to protect tumor cells from lysis by natural killer cells. Neoplasia. 2011;13(9):822–830. doi: 10.1593/neo.101684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonnqvist B, Ringden O, Ljungman P, Wahren B, Gahrton G. Reduced risk of recurrent leukaemia in bone marrow transplant recipients after cytomegalovirus infection. Br J Haematol. 1986;63(4):671–679. doi: 10.1111/j.1365-2141.1986.tb07551.x. [DOI] [PubMed] [Google Scholar]
- Lorenz E, Uphoff D, Reid TR, Shelton E. Modification of irradiation injury in mice and guinea pigs by bone marrow injections. J Natl Cancer Inst. 1951;12(1):197–201. [PubMed] [Google Scholar]
- Lotze MT, Grimm EA, Mazumder A, Strausser JL, Rosenberg SA. Lysis of fresh and cultured autologous tumor by human lymphocytes cultured in T-cell growth factor. Cancer Res. 1981;41(11 Pt 1):4420–4425. [PubMed] [Google Scholar]
- Lowe EJ, Turner V, Handgretinger R, et al. T-cell alloreactivity dominates natural killer cell alloreactivity in minimally T-cell-depleted HLA-non-identical paediatric bone marrow transplantation. Br J Haematol. 2003;123(2):323–326. doi: 10.1046/j.1365-2141.2003.04604.x. [DOI] [PubMed] [Google Scholar]
- Luetke-Eversloh M, Hammer Q, Durek P, et al. Human cytomegalovirus drives epigenetic imprinting of the IFNG locus in NKG2Chi natural killer cells. PLoS Pathog. 2014;10(10):e1004441. doi: 10.1371/journal.ppat.1004441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancusi A, Ruggeri L, Urbani E, et al. Haploidentical hematopoietic transplantation from KIR ligand-mismatched donors with activating KIRs reduces non-relapse mortality. Blood. 2015;125(20):3173–3182. doi: 10.1182/blood-2014-09-599993. [DOI] [PubMed] [Google Scholar]
- Marin R, Ruiz-Cabello F, Pedrinaci S, et al. Analysis of HLA-E expression in human tumors. Immunogenetics. 2003;54(11):767–775. doi: 10.1007/s00251-002-0526-9. [DOI] [PubMed] [Google Scholar]
- Martin MP, Gao X, Lee JH, et al. Epistatic interaction between KIR3DS1 and HLA-B delays the progression to AIDS. Nat Genet. 2002;31(4):429–434. doi: 10.1038/ng934. [DOI] [PubMed] [Google Scholar]
- Mathe G, Schwarzenberg L, Amiel JL, et al. Immunogenetic and immunological problems of allogeneic haemopoietic radio-chimaeras in man. Scand J Haematol. 1967;4(3):193–216. doi: 10.1111/j.1600-0609.1967.tb01621.x. [DOI] [PubMed] [Google Scholar]
- Mathe G, Schwarzenberg L, Halle-Pannenko O, Abuaf N, Pouillart P. Of mice and men in bone marrow transplantation. Transplant Proc. 1977;9(1):155–168. [PubMed] [Google Scholar]
- Miller JS, Soignier Y, Panoskaltsis-Mortari A, et al. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood. 2005;105(8):3051–3057. doi: 10.1182/blood-2004-07-2974. [DOI] [PubMed] [Google Scholar]
- Miller JS, Cooley S, Parham P, et al. Missing KIR ligands are associated with less relapse and increased graft-versus-host disease (GVHD) following unrelated donor allogeneic HCT. Blood. 2007;109(11):5058–5061. doi: 10.1182/blood-2007-01-065383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milone MC, Fish JD, Carpenito C, et al. Chimeric receptors containing CD137 signal transduction domains mediate enhanced survival of T cells and increased antileukemic efficacy in vivo. Mol Ther. 2009;17(8):1453–1464. doi: 10.1038/mt.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moretta A, Vitale M, Bottino C, et al. P58 molecules as putative receptors for major histocompatibility complex (MHC) class I molecules in human natural killer (NK) cells. Anti-p58 antibodies reconstitute lysis of MHC class I-protected cells in NK clones displaying different specificities. J Exp Med. 1993;178(2):597–604. doi: 10.1084/jem.178.2.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachbaur D, Bonatti H, Oberaigner W, et al. Survival after bone marrow transplantation from cytomegalovirus seropositive sibling donors. Lancet. 2001;358(9288):1157–1159. doi: 10.1016/S0140-6736(01)06275-4. [DOI] [PubMed] [Google Scholar]
- Neiman P, Wasserman PB, Wentworth BB, et al. Interstitial pneumonia and cytomegalo-virus infection as complications of human marrow transplantation. Transplantation. 1973;15(5):478–485. [PubMed] [Google Scholar]
- Nguyen S, Dhedin N, Vernant JP, et al. NK-cell reconstitution after haploidentical hematopoietic stem-cell transplantations: immaturity of NK cells and inhibitory effect of NKG2A override GvL effect. Blood. 2005;105(10):4135–4142. doi: 10.1182/blood-2004-10-4113. [DOI] [PubMed] [Google Scholar]
- Ni J, Miller M, Stojanovic A, Garbi N, Cerwenka A. Sustained effector function of IL-12/15/18-preactivated NK cells against established tumors. J Exp Med. 2012;209(13):2351–2365. doi: 10.1084/jem.20120944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowell PC, Cole LJ, Habermeyer JG, Roan PL. Growth and continued function of rat marrow cells in x-radiated mice. Cancer Res. 1956;16(3):258–261. [PubMed] [Google Scholar]
- Oevermann L, Michaelis SU, Mezger M, et al. KIR B haplotype donors confer a reduced risk for relapse after haploidentical transplantation in children with ALL. Blood. 2014;124(17):2744–2747. doi: 10.1182/blood-2014-03-565069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamura H, Tsutsi H, Komatsu T, et al. Cloning of a new cytokine that induces IFN-gamma production by T cells. Nature. 1995;378(6552):88–91. doi: 10.1038/378088a0. [DOI] [PubMed] [Google Scholar]
- Olcese L, Lang P, Vely F, et al. Human and mouse killer-cell inhibitory receptors recruit PTP1C and PTP1D protein tyrosine phosphatases. J Immunol. 1996;156(12):4531–4534. [PubMed] [Google Scholar]
- Poole E, McGregor Dallas SR, Colston J, Joseph RS, Sinclair J. Virally induced changes in cellular microRNAs maintain latency of human cytomegalovirus in CD34(+) progenitors. J Gen Virol. 2011;92(Pt 7):1539–1549. doi: 10.1099/vir.0.031377-0. [DOI] [PubMed] [Google Scholar]
- Quinnan GV, Jr, Kirmani N, Rook AH, et al. Cytotoxic t cells in cytomegalovirus infection: HLA-restricted T-lymphocyte and non-T-lymphocyte cytotoxic responses correlate with recovery from cytomegalovirus infection in bone-marrow-transplant recipients. N Engl J Med. 1982;307(1):7–13. doi: 10.1056/NEJM198207013070102. [DOI] [PubMed] [Google Scholar]
- Reddehase MJ, Mutter W, Munch K, Buhring HJ, Koszinowski UH. CD8-positive T lymphocytes specific for murine cytomegalovirus immediate-early antigens mediate protective immunity. J Virol. 1987;61(10):3102–3108. doi: 10.1128/jvi.61.10.3102-3108.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remberger M, Ringden O. Survival after bone-marrow transplantation. Lancet. 2002;359(9309):888. doi: 10.1016/S0140-6736(02)07926-6. [DOI] [PubMed] [Google Scholar]
- Rosenberg SA, Mule JJ, Spiess PJ, Reichert CM, Schwarz SL. Regression of established pulmonary metastases and subcutaneous tumor mediated by the systemic administration of high-dose recombinant interleukin 2. J Exp Med. 1985;161(5):1169–1188. doi: 10.1084/jem.161.5.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg SA, Lotze MT, Muul LM, et al. A progress report on the treatment of 157 patients with advanced cancer using lymphokine-activated killer cells and interleukin-2 or high-dose interleukin-2 alone. N Engl J Med. 1987;316(15):889–897. doi: 10.1056/NEJM198704093161501. [DOI] [PubMed] [Google Scholar]
- Ruggeri L, Capanni M, Casucci M, et al. Role of natural killer cell alloreactivity in HLA-mismatched hematopoietic stem cell transplantation. Blood. 1999;94(1):333–339. [PubMed] [Google Scholar]
- Ruggeri L, Capanni M, Urbani E, et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science. 2002;295(5562):2097–2100. doi: 10.1126/science.1068440. [DOI] [PubMed] [Google Scholar]
- Schlums H, Cichocki F, Tesi B, et al. Cytomegalovirus Infection Drives Adaptive Epigenetic Diversification of NK Cells with Altered Signaling and Effector Function. Immunity. 2015;42(3):443–456. doi: 10.1016/j.immuni.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selby P, Powles RL, Jameson B, Stolle K, Tryhorn Y, Stern H. Treatment of cytomegalovirus pneumonitis after bone marrow transplantation with 9-[2-hydroxy-1-(hydroxymethyl) ethoxymethyl] guanine. Lancet. 1986;1(8494):1377–1378. doi: 10.1016/s0140-6736(86)91682-x. [DOI] [PubMed] [Google Scholar]
- Sitrin J, Ring A, Garcia KC, Benoist C, Mathis D. Regulatory T cells control NK cells in an insulitic lesion by depriving them of IL-2. J Exp Med. 2013;210(6):1153–1165. doi: 10.1084/jem.20122248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun JC, Beilke JN, Lanier LL. Adaptive immune features of natural killer cells. Nature. 2009;457(7229):557–561. doi: 10.1038/nature07665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suto Y, Maenaka K, Yabe T, et al. Chromosomal localization of the human natural killer cell class I receptor family genes to 19q13.4 by fluorescence in situ hybridization. Genomics. 1996;35(1):270–272. doi: 10.1006/geno.1996.0355. [DOI] [PubMed] [Google Scholar]
- Sylwester AW, Mitchell BL, Edgar JB, et al. Broadly targeted human cytomegalovirus-specific CD4+ and CD8+ T cells dominate the memory compartments of exposed subjects. J Exp Med. 2005;202(5):673–685. doi: 10.1084/jem.20050882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas ED, Lochte HL, Jr, Lu WC, Ferrebee JW. Intravenous infusion of bone marrow in patients receiving radiation and chemotherapy. N Engl J Med. 1957;257(11):491–496. doi: 10.1056/NEJM195709122571102. [DOI] [PubMed] [Google Scholar]
- Thomson KJ, Mackinnon S, Peggs KS. CMV-specific cellular therapy for acute myeloid leukemia? Blood. 2012;119(4):1088–1090. doi: 10.1182/blood-2011-10-383943. author reply 1090–1081. [DOI] [PubMed] [Google Scholar]
- Tomasec P, Braud VM, Rickards C, et al. Surface expression of HLA-E, an inhibitor of natural killer cells, enhanced by human cytomegalovirus gpUL40. Science. 2000;287(5455):1031. doi: 10.1126/science.287.5455.1031. [DOI] [PubMed] [Google Scholar]
- Uhrberg M, Valiante NM, Shum BP, et al. Human diversity in killer cell inhibitory receptor genes. Immunity. 1997;7(6):753–763. doi: 10.1016/s1074-7613(00)80394-5. [DOI] [PubMed] [Google Scholar]
- Ulbrecht M, Martinozzi S, Grzeschik M, et al. Cutting edge: the human cytomegalovirus UL40 gene product contains a ligand for HLA-E and prevents NK cell-mediated lysis. J Immunol. 2000;164(10):5019–5022. doi: 10.4049/jimmunol.164.10.5019. [DOI] [PubMed] [Google Scholar]
- Venstrom JM, Pittari G, Gooley TA, et al. HLA-C-dependent prevention of leukemia relapse by donor activating KIR2DS1. N Engl J Med. 2012;367(9):805–816. doi: 10.1056/NEJMoa1200503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagtmann N, Biassoni R, Cantoni C, et al. Molecular clones of the p58 NK cell receptor reveal immunoglobulin-related molecules with diversity in both the extra- and intracellular domains. Immunity. 1995;2(5):439–449. doi: 10.1016/1074-7613(95)90025-x. [DOI] [PubMed] [Google Scholar]
- Weiden PL, Flournoy N, Thomas ED, et al. Antileukemic effect of graft-versus-host disease in human recipients of allogeneic-marrow grafts. N Engl J Med. 1979;300(19):1068–1073. doi: 10.1056/NEJM197905103001902. [DOI] [PubMed] [Google Scholar]
- Weiden PL, Sullivan KM, Flournoy N, Storb R, Thomas ED. Antileukemic effect of chronic graft-versus-host disease: contribution to improved survival after allogeneic marrow transplantation. N Engl J Med. 1981;304(25):1529–1533. doi: 10.1056/NEJM198106183042507. [DOI] [PubMed] [Google Scholar]
- Welsh RM., Jr Mouse natural killer cells: induction specificity, and function. J Immunol. 1978;121(5):1631–1635. [PubMed] [Google Scholar]
- Wuest TY, Willette-Brown J, Durum SK, Hurwitz AA. The influence of IL-2 family cytokines on activation and function of naturally occurring regulatory T cells. J Leukoc Biol. 2008;84(4):973–980. doi: 10.1189/jlb.1107778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yron I, Wood TA, Jr, Spiess PJ, Rosenberg SA. In vitro growth of murine T cells. V. The isolation and growth of lymphoid cells infiltrating syngeneic solid tumors. J Immunol. 1980;125(1):238–245. [PubMed] [Google Scholar]
- Zhang T, Scott JM, Hwang I, Kim S. Cutting edge: antibody-dependent memory-like NK cells distinguished by FcRgamma deficiency. J Immunol. 2013;190(4):1402–1406. doi: 10.4049/jimmunol.1203034. [DOI] [PMC free article] [PubMed] [Google Scholar]


