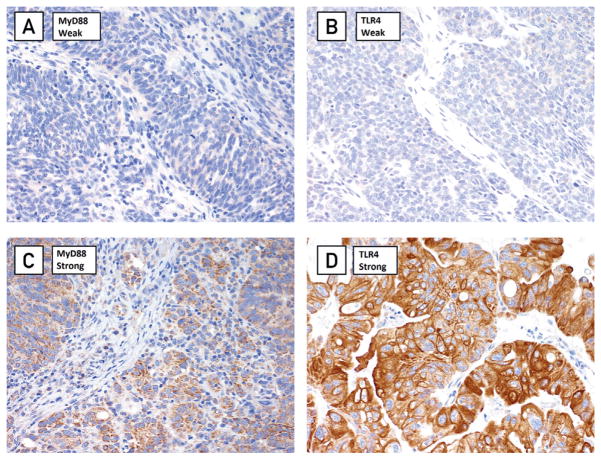
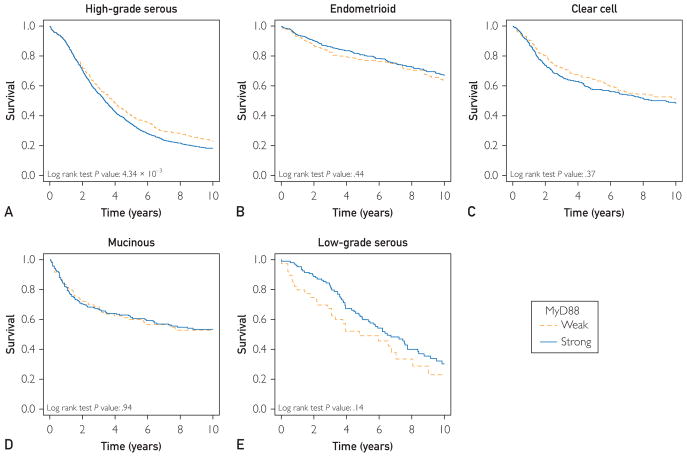
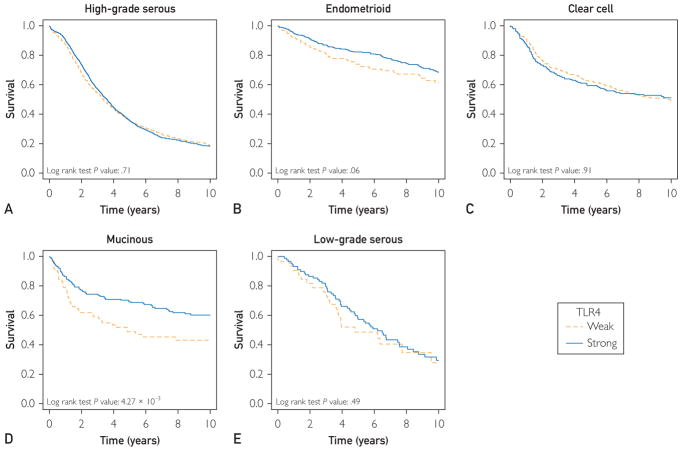
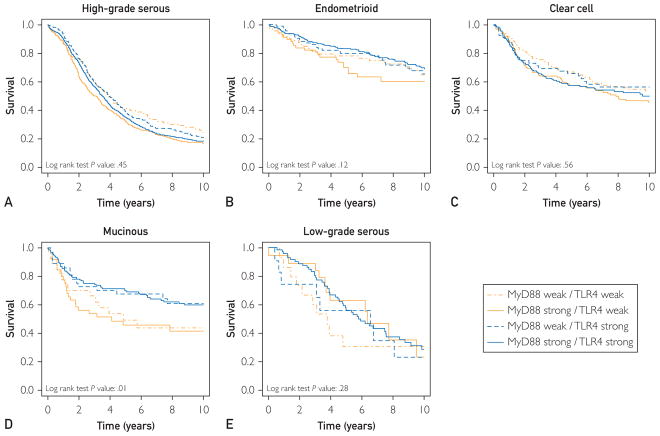
Matthew S Block
Matthew S Block, MD, PhD
1Department of Medical Oncology (M.S.B., K.R.K.) and Department of Health Sciences Research (R.A.V., S.J.W., B.M.M., Z.L., E.L.G.), Mayo Clinic, Rochester, MN; Department of Pathology and Laboratory Medicine, University of Calgary, Calgary, Alberta, Canada (P.F.R., M.K.); Pathology Department, Catholic University of Health and Allied Sciences-Bugando, Mwanza, Tanzania (P.F.R.); Tübingen University Hospital, Department of Women’s Health, Tübingen, Germany (P.W., F.A.T., A.D.H., S.Y.B., S.K.); Sir Peter MacCallum Department of Oncology, the University of Melbourne, Parkville, Victoria, Australia (N.T., D.D.B.); International Hereditary Cancer Center, Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (A.T., O.O., J. Lubiński, J.G.); Department of Gynecology and Obstetrics, Ribeirão Preto Medical School, University of São Paulo, Ribeirão Preto, Brazil (D.G.T., C.B.S., J.M.A., F.J.C.R.); Department of Pathology, Institute of Pathology, Heidelberg University Hospital, Heidelberg, Germany (P.S.); Department of Population Health Science and Policy, Department of Genetics and Genomic Sciences, Icahn School of Medicine at Mount Sinai, New York, NY (W.S., J.H.R.); Pathology West ICPMR Westmead, Westmead Hospital, the University of Sydney, Sydney, Australia (R.S.); University of Western Sydney at Westmead Hospital, Westmead, New South Wales, Australia (R.S.); Medical Oncology Service, Hospital Sant Pau, Barcelona, Spain (T.R.C.); H12O-CNIO Lung Cancer Clinical Research Unit (L.P.-A.), and Oncology Department, Hospital Universitario 12 de Octubre (L.P.-A.), Madrid, Spain; Women’s Cancer Program, Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, CA (S.O., J. Lester, B.Y.K.); the University of Texas School of Public Health, Houston (R.B.N.); Department of Oncology, Division of Gynecologic Oncology, Cumming School of Medicine, University of Calgary, Calgary, Alberta, Canada (G.N., P.G.); Department of Obstetrics, Gynecology and Reproductive Sciences, University of Pittsburgh School of Medicine (F.M., R.P.E.), Department of Epidemiology, University of Pittsburgh Graduate School of Public Health (F.M.), and Womens Cancer Research Program, Magee-Womens Research Institute and University of Pittsburgh Cancer Institute (F.M.), Pittsburgh, PA; Department of Gynecological Surgery and Gynecological Oncology of Adults and Adolescents, Pomeranian Medical University, Szczecin, Poland (J.M., A.C.-G.); Department of Health Research and Policy, Stanford University School of Medicine, Stanford, CA (V.M.); Department of Oncology, University of Cambridge, Strangeways Research Laboratory, Cambridge, UK (M.M., J.A., P.D.P.P.); Department of Pathology, Stanford University, Stanford, CA (T.A.L.); Department of Gynecologic Oncology, the Third Affiliated Hospital of Kunming Medical University (Yunnan Tumor Hospital), Kunming, China (Z.L.); Centre for Cancer Research, the Westmead Institute for Medical Research, the University of Sydney (C.J.K., J.H., P.R.H., B. Gao, A.deF.), and Department of Gynaecological Oncology, Westmead Hospital (C.J.K., J.H., A.deF.), Sydney, New South Wales, Australia; Division of Cancer Epidemiology, German Cancer Research Center (Deutsches Krebsforschungszentrum), Heidelberg, Germany (A.Y.J., U.E., J.C.-C.); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia (S.E.J., G.C.-T.); Department of Histopathology, Addenbrookes Hospital, Cambridge, UK (M.J.-L.); Virus, Lifestyle and Genes, Danish Cancer Society Research Center, Copenhagen, Denmark (A.J., S.K.K.); School of Women’s and Children’s Health, University of New South Wales, Sydney, Australia (M.P.I., S.J.R.); Tissue Bank of the National Center for Tumor Diseases (NCT) Heidelberg (E. Herpel), and Institute of Pathology, University of Heidelberg (E. Herpel), Heidelberg, Germany; Cancer Center, University of Hawaii, Honolulu, HI (B.Y.H.); the Crown Princess Mary Cancer Centre, Westmead Hospital, the University of Sydney, Sydney, Australia (P.R.H., B. Gao); Oncology Department, Hospital General de Albacete, Madrid, Spain (J.M.G.-B.); Department of Cancer Genomics and Genetics, Peter MacCallum Cancer Centre, East Melbourne, Victoria, Australia (S.F.); Pathology Department, Fundación Jiménez Díaz, Madrid, Spain (A.C.); Department of Health Research and Policy and Department of Biomedical Data Science, Stanford University School of Medicine, Stanford, CA (A.S.W.); Department of Obstetrics and Gynecology, Division of Gynecologic Oncology, Royal Alexandra Hospital, Edmonton, Alberta, Canada (H.S.); Tübingen University Hospital, Institute of Pathology, Tübingen, Germany (A.S.); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (K.B.M.); Gynaecological Cancer Research Centre, Department of Women’s Cancer, Institute for Women’s Health, University College London, London, UK (U.M.); Alberta Health Services-Cancer Care, Calgary, Alberta, Canada (J.M.K.); Department of Gynecology, Rigshospitalet, University of Copenhagen, Copenhagen, Denmark (S.K.K.); Department of Public Health Sciences, Medical University of South Carolina and Hollings Cancer Center, Charleston, SC (L.E.K.); Department of Pathology and Laboratory Medicine, University of British Columbia (D.G.H.), and Centre for Translational and Applied Genomics, British Columbia Cancer Agency (D.G.H.), Vancouver, British Columbia, Canada; Molecular Unit, Department of Pathology, Herlev Hospital, University of Copenhagen, Copenhagen, Denmark (E. Høgdall); Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, CA (M.T.G.); Genetic Pathology Evaluation Centre, Vancouver General Hospital and University of British Columbia, Vancouver, British Columbia, Canada (B. Gilks); Human Genetics Group, Spanish National Cancer Research Centre (Centro Nacional de Investigaciones Oncológicas) (M.J.G., J.B.), and Centre for Biomedical Network Research on Rare Diseases (Centro de Investigación Biomédica en Red de Enfermedades Raras), Instituto de Salud Carlos III (M.J.G., J.B.), Madrid, Spain; David Geffen School of Medicine, Department of Medicine, Division of Hematology and Oncology, University of California at Los Angeles (P.A.F.); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Erlangen, Germany (P.A.F.); Department of Histopathology, Queen’s Medical Centre, Nottingham University Hospitals NHS Trust, Nottingham, UK (S.D.); University Cancer Center Hamburg, University Medical Center Hamburg-Eppendorf, Hamburg, Germany (J.C.-C.); Cancer Genetics Laboratory, Research Division, Peter MacCallum Cancer Centre (I.G.C.), Sir Peter MacCallum Department of Oncology, University of Melbourne (I.G.C.), and Department of Pathology, University of Melbourne (I.G.C.), Melbourne, Victoria, Australia; Department of Oncology, University of Cambridge, Hutchison/MRC Research Centre (J.D.B.), Cancer Research UK Cambridge Institute, University of Cambridge, Li Ka Shing Centre (J.D.B.), and Cambridge Experimental Cancer Medicine Centre (J.D.B.), Cambridge, UK; Cancer Genomics Program, Research Department, Peter MacCallum Cancer Centre, East Melbourne, Victoria, Australia (D.D.B.); the Garvan Institute, Sydney, New South Wales, Australia (D.D.B., S.J.R.); and Department of Public Health and Primary Care, University of Cambridge, Strangeways Research Laboratory, Cambridge, UK (P.D.P.P.)
1,
Robert A Vierkant
Robert A Vierkant, MS
1Department of Medical Oncology (M.S.B., K.R.K.) and Department of Health Sciences Research (R.A.V., S.J.W., B.M.M., Z.L., E.L.G.), Mayo Clinic, Rochester, MN; Department of Pathology and Laboratory Medicine, University of Calgary, Calgary, Alberta, Canada (P.F.R., M.K.); Pathology Department, Catholic University of Health and Allied Sciences-Bugando, Mwanza, Tanzania (P.F.R.); Tübingen University Hospital, Department of Women’s Health, Tübingen, Germany (P.W., F.A.T., A.D.H., S.Y.B., S.K.); Sir Peter MacCallum Department of Oncology, the University of Melbourne, Parkville, Victoria, Australia (N.T., D.D.B.); International Hereditary Cancer Center, Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (A.T., O.O., J. Lubiński, J.G.); Department of Gynecology and Obstetrics, Ribeirão Preto Medical School, University of São Paulo, Ribeirão Preto, Brazil (D.G.T., C.B.S., J.M.A., F.J.C.R.); Department of Pathology, Institute of Pathology, Heidelberg University Hospital, Heidelberg, Germany (P.S.); Department of Population Health Science and Policy, Department of Genetics and Genomic Sciences, Icahn School of Medicine at Mount Sinai, New York, NY (W.S., J.H.R.); Pathology West ICPMR Westmead, Westmead Hospital, the University of Sydney, Sydney, Australia (R.S.); University of Western Sydney at Westmead Hospital, Westmead, New South Wales, Australia (R.S.); Medical Oncology Service, Hospital Sant Pau, Barcelona, Spain (T.R.C.); H12O-CNIO Lung Cancer Clinical Research Unit (L.P.-A.), and Oncology Department, Hospital Universitario 12 de Octubre (L.P.-A.), Madrid, Spain; Women’s Cancer Program, Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, CA (S.O., J. Lester, B.Y.K.); the University of Texas School of Public Health, Houston (R.B.N.); Department of Oncology, Division of Gynecologic Oncology, Cumming School of Medicine, University of Calgary, Calgary, Alberta, Canada (G.N., P.G.); Department of Obstetrics, Gynecology and Reproductive Sciences, University of Pittsburgh School of Medicine (F.M., R.P.E.), Department of Epidemiology, University of Pittsburgh Graduate School of Public Health (F.M.), and Womens Cancer Research Program, Magee-Womens Research Institute and University of Pittsburgh Cancer Institute (F.M.), Pittsburgh, PA; Department of Gynecological Surgery and Gynecological Oncology of Adults and Adolescents, Pomeranian Medical University, Szczecin, Poland (J.M., A.C.-G.); Department of Health Research and Policy, Stanford University School of Medicine, Stanford, CA (V.M.); Department of Oncology, University of Cambridge, Strangeways Research Laboratory, Cambridge, UK (M.M., J.A., P.D.P.P.); Department of Pathology, Stanford University, Stanford, CA (T.A.L.); Department of Gynecologic Oncology, the Third Affiliated Hospital of Kunming Medical University (Yunnan Tumor Hospital), Kunming, China (Z.L.); Centre for Cancer Research, the Westmead Institute for Medical Research, the University of Sydney (C.J.K., J.H., P.R.H., B. Gao, A.deF.), and Department of Gynaecological Oncology, Westmead Hospital (C.J.K., J.H., A.deF.), Sydney, New South Wales, Australia; Division of Cancer Epidemiology, German Cancer Research Center (Deutsches Krebsforschungszentrum), Heidelberg, Germany (A.Y.J., U.E., J.C.-C.); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia (S.E.J., G.C.-T.); Department of Histopathology, Addenbrookes Hospital, Cambridge, UK (M.J.-L.); Virus, Lifestyle and Genes, Danish Cancer Society Research Center, Copenhagen, Denmark (A.J., S.K.K.); School of Women’s and Children’s Health, University of New South Wales, Sydney, Australia (M.P.I., S.J.R.); Tissue Bank of the National Center for Tumor Diseases (NCT) Heidelberg (E. Herpel), and Institute of Pathology, University of Heidelberg (E. Herpel), Heidelberg, Germany; Cancer Center, University of Hawaii, Honolulu, HI (B.Y.H.); the Crown Princess Mary Cancer Centre, Westmead Hospital, the University of Sydney, Sydney, Australia (P.R.H., B. Gao); Oncology Department, Hospital General de Albacete, Madrid, Spain (J.M.G.-B.); Department of Cancer Genomics and Genetics, Peter MacCallum Cancer Centre, East Melbourne, Victoria, Australia (S.F.); Pathology Department, Fundación Jiménez Díaz, Madrid, Spain (A.C.); Department of Health Research and Policy and Department of Biomedical Data Science, Stanford University School of Medicine, Stanford, CA (A.S.W.); Department of Obstetrics and Gynecology, Division of Gynecologic Oncology, Royal Alexandra Hospital, Edmonton, Alberta, Canada (H.S.); Tübingen University Hospital, Institute of Pathology, Tübingen, Germany (A.S.); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (K.B.M.); Gynaecological Cancer Research Centre, Department of Women’s Cancer, Institute for Women’s Health, University College London, London, UK (U.M.); Alberta Health Services-Cancer Care, Calgary, Alberta, Canada (J.M.K.); Department of Gynecology, Rigshospitalet, University of Copenhagen, Copenhagen, Denmark (S.K.K.); Department of Public Health Sciences, Medical University of South Carolina and Hollings Cancer Center, Charleston, SC (L.E.K.); Department of Pathology and Laboratory Medicine, University of British Columbia (D.G.H.), and Centre for Translational and Applied Genomics, British Columbia Cancer Agency (D.G.H.), Vancouver, British Columbia, Canada; Molecular Unit, Department of Pathology, Herlev Hospital, University of Copenhagen, Copenhagen, Denmark (E. Høgdall); Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, CA (M.T.G.); Genetic Pathology Evaluation Centre, Vancouver General Hospital and University of British Columbia, Vancouver, British Columbia, Canada (B. Gilks); Human Genetics Group, Spanish National Cancer Research Centre (Centro Nacional de Investigaciones Oncológicas) (M.J.G., J.B.), and Centre for Biomedical Network Research on Rare Diseases (Centro de Investigación Biomédica en Red de Enfermedades Raras), Instituto de Salud Carlos III (M.J.G., J.B.), Madrid, Spain; David Geffen School of Medicine, Department of Medicine, Division of Hematology and Oncology, University of California at Los Angeles (P.A.F.); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Erlangen, Germany (P.A.F.); Department of Histopathology, Queen’s Medical Centre, Nottingham University Hospitals NHS Trust, Nottingham, UK (S.D.); University Cancer Center Hamburg, University Medical Center Hamburg-Eppendorf, Hamburg, Germany (J.C.-C.); Cancer Genetics Laboratory, Research Division, Peter MacCallum Cancer Centre (I.G.C.), Sir Peter MacCallum Department of Oncology, University of Melbourne (I.G.C.), and Department of Pathology, University of Melbourne (I.G.C.), Melbourne, Victoria, Australia; Department of Oncology, University of Cambridge, Hutchison/MRC Research Centre (J.D.B.), Cancer Research UK Cambridge Institute, University of Cambridge, Li Ka Shing Centre (J.D.B.), and Cambridge Experimental Cancer Medicine Centre (J.D.B.), Cambridge, UK; Cancer Genomics Program, Research Department, Peter MacCallum Cancer Centre, East Melbourne, Victoria, Australia (D.D.B.); the Garvan Institute, Sydney, New South Wales, Australia (D.D.B., S.J.R.); and Department of Public Health and Primary Care, University of Cambridge, Strangeways Research Laboratory, Cambridge, UK (P.D.P.P.)
1,
Peter F Rambau
Peter F Rambau, MD
1Department of Medical Oncology (M.S.B., K.R.K.) and Department of Health Sciences Research (R.A.V., S.J.W., B.M.M., Z.L., E.L.G.), Mayo Clinic, Rochester, MN; Department of Pathology and Laboratory Medicine, University of Calgary, Calgary, Alberta, Canada (P.F.R., M.K.); Pathology Department, Catholic University of Health and Allied Sciences-Bugando, Mwanza, Tanzania (P.F.R.); Tübingen University Hospital, Department of Women’s Health, Tübingen, Germany (P.W., F.A.T., A.D.H., S.Y.B., S.K.); Sir Peter MacCallum Department of Oncology, the University of Melbourne, Parkville, Victoria, Australia (N.T., D.D.B.); International Hereditary Cancer Center, Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (A.T., O.O., J. Lubiński, J.G.); Department of Gynecology and Obstetrics, Ribeirão Preto Medical School, University of São Paulo, Ribeirão Preto, Brazil (D.G.T., C.B.S., J.M.A., F.J.C.R.); Department of Pathology, Institute of Pathology, Heidelberg University Hospital, Heidelberg, Germany (P.S.); Department of Population Health Science and Policy, Department of Genetics and Genomic Sciences, Icahn School of Medicine at Mount Sinai, New York, NY (W.S., J.H.R.); Pathology West ICPMR Westmead, Westmead Hospital, the University of Sydney, Sydney, Australia (R.S.); University of Western Sydney at Westmead Hospital, Westmead, New South Wales, Australia (R.S.); Medical Oncology Service, Hospital Sant Pau, Barcelona, Spain (T.R.C.); H12O-CNIO Lung Cancer Clinical Research Unit (L.P.-A.), and Oncology Department, Hospital Universitario 12 de Octubre (L.P.-A.), Madrid, Spain; Women’s Cancer Program, Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, CA (S.O., J. Lester, B.Y.K.); the University of Texas School of Public Health, Houston (R.B.N.); Department of Oncology, Division of Gynecologic Oncology, Cumming School of Medicine, University of Calgary, Calgary, Alberta, Canada (G.N., P.G.); Department of Obstetrics, Gynecology and Reproductive Sciences, University of Pittsburgh School of Medicine (F.M., R.P.E.), Department of Epidemiology, University of Pittsburgh Graduate School of Public Health (F.M.), and Womens Cancer Research Program, Magee-Womens Research Institute and University of Pittsburgh Cancer Institute (F.M.), Pittsburgh, PA; Department of Gynecological Surgery and Gynecological Oncology of Adults and Adolescents, Pomeranian Medical University, Szczecin, Poland (J.M., A.C.-G.); Department of Health Research and Policy, Stanford University School of Medicine, Stanford, CA (V.M.); Department of Oncology, University of Cambridge, Strangeways Research Laboratory, Cambridge, UK (M.M., J.A., P.D.P.P.); Department of Pathology, Stanford University, Stanford, CA (T.A.L.); Department of Gynecologic Oncology, the Third Affiliated Hospital of Kunming Medical University (Yunnan Tumor Hospital), Kunming, China (Z.L.); Centre for Cancer Research, the Westmead Institute for Medical Research, the University of Sydney (C.J.K., J.H., P.R.H., B. Gao, A.deF.), and Department of Gynaecological Oncology, Westmead Hospital (C.J.K., J.H., A.deF.), Sydney, New South Wales, Australia; Division of Cancer Epidemiology, German Cancer Research Center (Deutsches Krebsforschungszentrum), Heidelberg, Germany (A.Y.J., U.E., J.C.-C.); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia (S.E.J., G.C.-T.); Department of Histopathology, Addenbrookes Hospital, Cambridge, UK (M.J.-L.); Virus, Lifestyle and Genes, Danish Cancer Society Research Center, Copenhagen, Denmark (A.J., S.K.K.); School of Women’s and Children’s Health, University of New South Wales, Sydney, Australia (M.P.I., S.J.R.); Tissue Bank of the National Center for Tumor Diseases (NCT) Heidelberg (E. Herpel), and Institute of Pathology, University of Heidelberg (E. Herpel), Heidelberg, Germany; Cancer Center, University of Hawaii, Honolulu, HI (B.Y.H.); the Crown Princess Mary Cancer Centre, Westmead Hospital, the University of Sydney, Sydney, Australia (P.R.H., B. Gao); Oncology Department, Hospital General de Albacete, Madrid, Spain (J.M.G.-B.); Department of Cancer Genomics and Genetics, Peter MacCallum Cancer Centre, East Melbourne, Victoria, Australia (S.F.); Pathology Department, Fundación Jiménez Díaz, Madrid, Spain (A.C.); Department of Health Research and Policy and Department of Biomedical Data Science, Stanford University School of Medicine, Stanford, CA (A.S.W.); Department of Obstetrics and Gynecology, Division of Gynecologic Oncology, Royal Alexandra Hospital, Edmonton, Alberta, Canada (H.S.); Tübingen University Hospital, Institute of Pathology, Tübingen, Germany (A.S.); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (K.B.M.); Gynaecological Cancer Research Centre, Department of Women’s Cancer, Institute for Women’s Health, University College London, London, UK (U.M.); Alberta Health Services-Cancer Care, Calgary, Alberta, Canada (J.M.K.); Department of Gynecology, Rigshospitalet, University of Copenhagen, Copenhagen, Denmark (S.K.K.); Department of Public Health Sciences, Medical University of South Carolina and Hollings Cancer Center, Charleston, SC (L.E.K.); Department of Pathology and Laboratory Medicine, University of British Columbia (D.G.H.), and Centre for Translational and Applied Genomics, British Columbia Cancer Agency (D.G.H.), Vancouver, British Columbia, Canada; Molecular Unit, Department of Pathology, Herlev Hospital, University of Copenhagen, Copenhagen, Denmark (E. Høgdall); Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, CA (M.T.G.); Genetic Pathology Evaluation Centre, Vancouver General Hospital and University of British Columbia, Vancouver, British Columbia, Canada (B. Gilks); Human Genetics Group, Spanish National Cancer Research Centre (Centro Nacional de Investigaciones Oncológicas) (M.J.G., J.B.), and Centre for Biomedical Network Research on Rare Diseases (Centro de Investigación Biomédica en Red de Enfermedades Raras), Instituto de Salud Carlos III (M.J.G., J.B.), Madrid, Spain; David Geffen School of Medicine, Department of Medicine, Division of Hematology and Oncology, University of California at Los Angeles (P.A.F.); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Erlangen, Germany (P.A.F.); Department of Histopathology, Queen’s Medical Centre, Nottingham University Hospitals NHS Trust, Nottingham, UK (S.D.); University Cancer Center Hamburg, University Medical Center Hamburg-Eppendorf, Hamburg, Germany (J.C.-C.); Cancer Genetics Laboratory, Research Division, Peter MacCallum Cancer Centre (I.G.C.), Sir Peter MacCallum Department of Oncology, University of Melbourne (I.G.C.), and Department of Pathology, University of Melbourne (I.G.C.), Melbourne, Victoria, Australia; Department of Oncology, University of Cambridge, Hutchison/MRC Research Centre (J.D.B.), Cancer Research UK Cambridge Institute, University of Cambridge, Li Ka Shing Centre (J.D.B.), and Cambridge Experimental Cancer Medicine Centre (J.D.B.), Cambridge, UK; Cancer Genomics Program, Research Department, Peter MacCallum Cancer Centre, East Melbourne, Victoria, Australia (D.D.B.); the Garvan Institute, Sydney, New South Wales, Australia (D.D.B., S.J.R.); and Department of Public Health and Primary Care, University of Cambridge, Strangeways Research Laboratory, Cambridge, UK (P.D.P.P.)
1,
Stacey J Winham
Stacey J Winham, PhD
1Department of Medical Oncology (M.S.B., K.R.K.) and Department of Health Sciences Research (R.A.V., S.J.W., B.M.M., Z.L., E.L.G.), Mayo Clinic, Rochester, MN; Department of Pathology and Laboratory Medicine, University of Calgary, Calgary, Alberta, Canada (P.F.R., M.K.); Pathology Department, Catholic University of Health and Allied Sciences-Bugando, Mwanza, Tanzania (P.F.R.); Tübingen University Hospital, Department of Women’s Health, Tübingen, Germany (P.W., F.A.T., A.D.H., S.Y.B., S.K.); Sir Peter MacCallum Department of Oncology, the University of Melbourne, Parkville, Victoria, Australia (N.T., D.D.B.); International Hereditary Cancer Center, Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (A.T., O.O., J. Lubiński, J.G.); Department of Gynecology and Obstetrics, Ribeirão Preto Medical School, University of São Paulo, Ribeirão Preto, Brazil (D.G.T., C.B.S., J.M.A., F.J.C.R.); Department of Pathology, Institute of Pathology, Heidelberg University Hospital, Heidelberg, Germany (P.S.); Department of Population Health Science and Policy, Department of Genetics and Genomic Sciences, Icahn School of Medicine at Mount Sinai, New York, NY (W.S., J.H.R.); Pathology West ICPMR Westmead, Westmead Hospital, the University of Sydney, Sydney, Australia (R.S.); University of Western Sydney at Westmead Hospital, Westmead, New South Wales, Australia (R.S.); Medical Oncology Service, Hospital Sant Pau, Barcelona, Spain (T.R.C.); H12O-CNIO Lung Cancer Clinical Research Unit (L.P.-A.), and Oncology Department, Hospital Universitario 12 de Octubre (L.P.-A.), Madrid, Spain; Women’s Cancer Program, Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, CA (S.O., J. Lester, B.Y.K.); the University of Texas School of Public Health, Houston (R.B.N.); Department of Oncology, Division of Gynecologic Oncology, Cumming School of Medicine, University of Calgary, Calgary, Alberta, Canada (G.N., P.G.); Department of Obstetrics, Gynecology and Reproductive Sciences, University of Pittsburgh School of Medicine (F.M., R.P.E.), Department of Epidemiology, University of Pittsburgh Graduate School of Public Health (F.M.), and Womens Cancer Research Program, Magee-Womens Research Institute and University of Pittsburgh Cancer Institute (F.M.), Pittsburgh, PA; Department of Gynecological Surgery and Gynecological Oncology of Adults and Adolescents, Pomeranian Medical University, Szczecin, Poland (J.M., A.C.-G.); Department of Health Research and Policy, Stanford University School of Medicine, Stanford, CA (V.M.); Department of Oncology, University of Cambridge, Strangeways Research Laboratory, Cambridge, UK (M.M., J.A., P.D.P.P.); Department of Pathology, Stanford University, Stanford, CA (T.A.L.); Department of Gynecologic Oncology, the Third Affiliated Hospital of Kunming Medical University (Yunnan Tumor Hospital), Kunming, China (Z.L.); Centre for Cancer Research, the Westmead Institute for Medical Research, the University of Sydney (C.J.K., J.H., P.R.H., B. Gao, A.deF.), and Department of Gynaecological Oncology, Westmead Hospital (C.J.K., J.H., A.deF.), Sydney, New South Wales, Australia; Division of Cancer Epidemiology, German Cancer Research Center (Deutsches Krebsforschungszentrum), Heidelberg, Germany (A.Y.J., U.E., J.C.-C.); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia (S.E.J., G.C.-T.); Department of Histopathology, Addenbrookes Hospital, Cambridge, UK (M.J.-L.); Virus, Lifestyle and Genes, Danish Cancer Society Research Center, Copenhagen, Denmark (A.J., S.K.K.); School of Women’s and Children’s Health, University of New South Wales, Sydney, Australia (M.P.I., S.J.R.); Tissue Bank of the National Center for Tumor Diseases (NCT) Heidelberg (E. Herpel), and Institute of Pathology, University of Heidelberg (E. Herpel), Heidelberg, Germany; Cancer Center, University of Hawaii, Honolulu, HI (B.Y.H.); the Crown Princess Mary Cancer Centre, Westmead Hospital, the University of Sydney, Sydney, Australia (P.R.H., B. Gao); Oncology Department, Hospital General de Albacete, Madrid, Spain (J.M.G.-B.); Department of Cancer Genomics and Genetics, Peter MacCallum Cancer Centre, East Melbourne, Victoria, Australia (S.F.); Pathology Department, Fundación Jiménez Díaz, Madrid, Spain (A.C.); Department of Health Research and Policy and Department of Biomedical Data Science, Stanford University School of Medicine, Stanford, CA (A.S.W.); Department of Obstetrics and Gynecology, Division of Gynecologic Oncology, Royal Alexandra Hospital, Edmonton, Alberta, Canada (H.S.); Tübingen University Hospital, Institute of Pathology, Tübingen, Germany (A.S.); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (K.B.M.); Gynaecological Cancer Research Centre, Department of Women’s Cancer, Institute for Women’s Health, University College London, London, UK (U.M.); Alberta Health Services-Cancer Care, Calgary, Alberta, Canada (J.M.K.); Department of Gynecology, Rigshospitalet, University of Copenhagen, Copenhagen, Denmark (S.K.K.); Department of Public Health Sciences, Medical University of South Carolina and Hollings Cancer Center, Charleston, SC (L.E.K.); Department of Pathology and Laboratory Medicine, University of British Columbia (D.G.H.), and Centre for Translational and Applied Genomics, British Columbia Cancer Agency (D.G.H.), Vancouver, British Columbia, Canada; Molecular Unit, Department of Pathology, Herlev Hospital, University of Copenhagen, Copenhagen, Denmark (E. Høgdall); Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, CA (M.T.G.); Genetic Pathology Evaluation Centre, Vancouver General Hospital and University of British Columbia, Vancouver, British Columbia, Canada (B. Gilks); Human Genetics Group, Spanish National Cancer Research Centre (Centro Nacional de Investigaciones Oncológicas) (M.J.G., J.B.), and Centre for Biomedical Network Research on Rare Diseases (Centro de Investigación Biomédica en Red de Enfermedades Raras), Instituto de Salud Carlos III (M.J.G., J.B.), Madrid, Spain; David Geffen School of Medicine, Department of Medicine, Division of Hematology and Oncology, University of California at Los Angeles (P.A.F.); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Erlangen, Germany (P.A.F.); Department of Histopathology, Queen’s Medical Centre, Nottingham University Hospitals NHS Trust, Nottingham, UK (S.D.); University Cancer Center Hamburg, University Medical Center Hamburg-Eppendorf, Hamburg, Germany (J.C.-C.); Cancer Genetics Laboratory, Research Division, Peter MacCallum Cancer Centre (I.G.C.), Sir Peter MacCallum Department of Oncology, University of Melbourne (I.G.C.), and Department of Pathology, University of Melbourne (I.G.C.), Melbourne, Victoria, Australia; Department of Oncology, University of Cambridge, Hutchison/MRC Research Centre (J.D.B.), Cancer Research UK Cambridge Institute, University of Cambridge, Li Ka Shing Centre (J.D.B.), and Cambridge Experimental Cancer Medicine Centre (J.D.B.), Cambridge, UK; Cancer Genomics Program, Research Department, Peter MacCallum Cancer Centre, East Melbourne, Victoria, Australia (D.D.B.); the Garvan Institute, Sydney, New South Wales, Australia (D.D.B., S.J.R.); and Department of Public Health and Primary Care, University of Cambridge, Strangeways Research Laboratory, Cambridge, UK (P.D.P.P.)
1,
Philipp Wagner
Philipp Wagner, MD
1Department of Medical Oncology (M.S.B., K.R.K.) and Department of Health Sciences Research (R.A.V., S.J.W., B.M.M., Z.L., E.L.G.), Mayo Clinic, Rochester, MN; Department of Pathology and Laboratory Medicine, University of Calgary, Calgary, Alberta, Canada (P.F.R., M.K.); Pathology Department, Catholic University of Health and Allied Sciences-Bugando, Mwanza, Tanzania (P.F.R.); Tübingen University Hospital, Department of Women’s Health, Tübingen, Germany (P.W., F.A.T., A.D.H., S.Y.B., S.K.); Sir Peter MacCallum Department of Oncology, the University of Melbourne, Parkville, Victoria, Australia (N.T., D.D.B.); International Hereditary Cancer Center, Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (A.T., O.O., J. Lubiński, J.G.); Department of Gynecology and Obstetrics, Ribeirão Preto Medical School, University of São Paulo, Ribeirão Preto, Brazil (D.G.T., C.B.S., J.M.A., F.J.C.R.); Department of Pathology, Institute of Pathology, Heidelberg University Hospital, Heidelberg, Germany (P.S.); Department of Population Health Science and Policy, Department of Genetics and Genomic Sciences, Icahn School of Medicine at Mount Sinai, New York, NY (W.S., J.H.R.); Pathology West ICPMR Westmead, Westmead Hospital, the University of Sydney, Sydney, Australia (R.S.); University of Western Sydney at Westmead Hospital, Westmead, New South Wales, Australia (R.S.); Medical Oncology Service, Hospital Sant Pau, Barcelona, Spain (T.R.C.); H12O-CNIO Lung Cancer Clinical Research Unit (L.P.-A.), and Oncology Department, Hospital Universitario 12 de Octubre (L.P.-A.), Madrid, Spain; Women’s Cancer Program, Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, CA (S.O., J. Lester, B.Y.K.); the University of Texas School of Public Health, Houston (R.B.N.); Department of Oncology, Division of Gynecologic Oncology, Cumming School of Medicine, University of Calgary, Calgary, Alberta, Canada (G.N., P.G.); Department of Obstetrics, Gynecology and Reproductive Sciences, University of Pittsburgh School of Medicine (F.M., R.P.E.), Department of Epidemiology, University of Pittsburgh Graduate School of Public Health (F.M.), and Womens Cancer Research Program, Magee-Womens Research Institute and University of Pittsburgh Cancer Institute (F.M.), Pittsburgh, PA; Department of Gynecological Surgery and Gynecological Oncology of Adults and Adolescents, Pomeranian Medical University, Szczecin, Poland (J.M., A.C.-G.); Department of Health Research and Policy, Stanford University School of Medicine, Stanford, CA (V.M.); Department of Oncology, University of Cambridge, Strangeways Research Laboratory, Cambridge, UK (M.M., J.A., P.D.P.P.); Department of Pathology, Stanford University, Stanford, CA (T.A.L.); Department of Gynecologic Oncology, the Third Affiliated Hospital of Kunming Medical University (Yunnan Tumor Hospital), Kunming, China (Z.L.); Centre for Cancer Research, the Westmead Institute for Medical Research, the University of Sydney (C.J.K., J.H., P.R.H., B. Gao, A.deF.), and Department of Gynaecological Oncology, Westmead Hospital (C.J.K., J.H., A.deF.), Sydney, New South Wales, Australia; Division of Cancer Epidemiology, German Cancer Research Center (Deutsches Krebsforschungszentrum), Heidelberg, Germany (A.Y.J., U.E., J.C.-C.); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia (S.E.J., G.C.-T.); Department of Histopathology, Addenbrookes Hospital, Cambridge, UK (M.J.-L.); Virus, Lifestyle and Genes, Danish Cancer Society Research Center, Copenhagen, Denmark (A.J., S.K.K.); School of Women’s and Children’s Health, University of New South Wales, Sydney, Australia (M.P.I., S.J.R.); Tissue Bank of the National Center for Tumor Diseases (NCT) Heidelberg (E. Herpel), and Institute of Pathology, University of Heidelberg (E. Herpel), Heidelberg, Germany; Cancer Center, University of Hawaii, Honolulu, HI (B.Y.H.); the Crown Princess Mary Cancer Centre, Westmead Hospital, the University of Sydney, Sydney, Australia (P.R.H., B. Gao); Oncology Department, Hospital General de Albacete, Madrid, Spain (J.M.G.-B.); Department of Cancer Genomics and Genetics, Peter MacCallum Cancer Centre, East Melbourne, Victoria, Australia (S.F.); Pathology Department, Fundación Jiménez Díaz, Madrid, Spain (A.C.); Department of Health Research and Policy and Department of Biomedical Data Science, Stanford University School of Medicine, Stanford, CA (A.S.W.); Department of Obstetrics and Gynecology, Division of Gynecologic Oncology, Royal Alexandra Hospital, Edmonton, Alberta, Canada (H.S.); Tübingen University Hospital, Institute of Pathology, Tübingen, Germany (A.S.); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (K.B.M.); Gynaecological Cancer Research Centre, Department of Women’s Cancer, Institute for Women’s Health, University College London, London, UK (U.M.); Alberta Health Services-Cancer Care, Calgary, Alberta, Canada (J.M.K.); Department of Gynecology, Rigshospitalet, University of Copenhagen, Copenhagen, Denmark (S.K.K.); Department of Public Health Sciences, Medical University of South Carolina and Hollings Cancer Center, Charleston, SC (L.E.K.); Department of Pathology and Laboratory Medicine, University of British Columbia (D.G.H.), and Centre for Translational and Applied Genomics, British Columbia Cancer Agency (D.G.H.), Vancouver, British Columbia, Canada; Molecular Unit, Department of Pathology, Herlev Hospital, University of Copenhagen, Copenhagen, Denmark (E. Høgdall); Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, CA (M.T.G.); Genetic Pathology Evaluation Centre, Vancouver General Hospital and University of British Columbia, Vancouver, British Columbia, Canada (B. Gilks); Human Genetics Group, Spanish National Cancer Research Centre (Centro Nacional de Investigaciones Oncológicas) (M.J.G., J.B.), and Centre for Biomedical Network Research on Rare Diseases (Centro de Investigación Biomédica en Red de Enfermedades Raras), Instituto de Salud Carlos III (M.J.G., J.B.), Madrid, Spain; David Geffen School of Medicine, Department of Medicine, Division of Hematology and Oncology, University of California at Los Angeles (P.A.F.); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Erlangen, Germany (P.A.F.); Department of Histopathology, Queen’s Medical Centre, Nottingham University Hospitals NHS Trust, Nottingham, UK (S.D.); University Cancer Center Hamburg, University Medical Center Hamburg-Eppendorf, Hamburg, Germany (J.C.-C.); Cancer Genetics Laboratory, Research Division, Peter MacCallum Cancer Centre (I.G.C.), Sir Peter MacCallum Department of Oncology, University of Melbourne (I.G.C.), and Department of Pathology, University of Melbourne (I.G.C.), Melbourne, Victoria, Australia; Department of Oncology, University of Cambridge, Hutchison/MRC Research Centre (J.D.B.), Cancer Research UK Cambridge Institute, University of Cambridge, Li Ka Shing Centre (J.D.B.), and Cambridge Experimental Cancer Medicine Centre (J.D.B.), Cambridge, UK; Cancer Genomics Program, Research Department, Peter MacCallum Cancer Centre, East Melbourne, Victoria, Australia (D.D.B.); the Garvan Institute, Sydney, New South Wales, Australia (D.D.B., S.J.R.); and Department of Public Health and Primary Care, University of Cambridge, Strangeways Research Laboratory, Cambridge, UK (P.D.P.P.)
1,
Nadia Traficante
Nadia Traficante, BSc
1Department of Medical Oncology (M.S.B., K.R.K.) and Department of Health Sciences Research (R.A.V., S.J.W., B.M.M., Z.L., E.L.G.), Mayo Clinic, Rochester, MN; Department of Pathology and Laboratory Medicine, University of Calgary, Calgary, Alberta, Canada (P.F.R., M.K.); Pathology Department, Catholic University of Health and Allied Sciences-Bugando, Mwanza, Tanzania (P.F.R.); Tübingen University Hospital, Department of Women’s Health, Tübingen, Germany (P.W., F.A.T., A.D.H., S.Y.B., S.K.); Sir Peter MacCallum Department of Oncology, the University of Melbourne, Parkville, Victoria, Australia (N.T., D.D.B.); International Hereditary Cancer Center, Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (A.T., O.O., J. Lubiński, J.G.); Department of Gynecology and Obstetrics, Ribeirão Preto Medical School, University of São Paulo, Ribeirão Preto, Brazil (D.G.T., C.B.S., J.M.A., F.J.C.R.); Department of Pathology, Institute of Pathology, Heidelberg University Hospital, Heidelberg, Germany (P.S.); Department of Population Health Science and Policy, Department of Genetics and Genomic Sciences, Icahn School of Medicine at Mount Sinai, New York, NY (W.S., J.H.R.); Pathology West ICPMR Westmead, Westmead Hospital, the University of Sydney, Sydney, Australia (R.S.); University of Western Sydney at Westmead Hospital, Westmead, New South Wales, Australia (R.S.); Medical Oncology Service, Hospital Sant Pau, Barcelona, Spain (T.R.C.); H12O-CNIO Lung Cancer Clinical Research Unit (L.P.-A.), and Oncology Department, Hospital Universitario 12 de Octubre (L.P.-A.), Madrid, Spain; Women’s Cancer Program, Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, CA (S.O., J. Lester, B.Y.K.); the University of Texas School of Public Health, Houston (R.B.N.); Department of Oncology, Division of Gynecologic Oncology, Cumming School of Medicine, University of Calgary, Calgary, Alberta, Canada (G.N., P.G.); Department of Obstetrics, Gynecology and Reproductive Sciences, University of Pittsburgh School of Medicine (F.M., R.P.E.), Department of Epidemiology, University of Pittsburgh Graduate School of Public Health (F.M.), and Womens Cancer Research Program, Magee-Womens Research Institute and University of Pittsburgh Cancer Institute (F.M.), Pittsburgh, PA; Department of Gynecological Surgery and Gynecological Oncology of Adults and Adolescents, Pomeranian Medical University, Szczecin, Poland (J.M., A.C.-G.); Department of Health Research and Policy, Stanford University School of Medicine, Stanford, CA (V.M.); Department of Oncology, University of Cambridge, Strangeways Research Laboratory, Cambridge, UK (M.M., J.A., P.D.P.P.); Department of Pathology, Stanford University, Stanford, CA (T.A.L.); Department of Gynecologic Oncology, the Third Affiliated Hospital of Kunming Medical University (Yunnan Tumor Hospital), Kunming, China (Z.L.); Centre for Cancer Research, the Westmead Institute for Medical Research, the University of Sydney (C.J.K., J.H., P.R.H., B. Gao, A.deF.), and Department of Gynaecological Oncology, Westmead Hospital (C.J.K., J.H., A.deF.), Sydney, New South Wales, Australia; Division of Cancer Epidemiology, German Cancer Research Center (Deutsches Krebsforschungszentrum), Heidelberg, Germany (A.Y.J., U.E., J.C.-C.); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia (S.E.J., G.C.-T.); Department of Histopathology, Addenbrookes Hospital, Cambridge, UK (M.J.-L.); Virus, Lifestyle and Genes, Danish Cancer Society Research Center, Copenhagen, Denmark (A.J., S.K.K.); School of Women’s and Children’s Health, University of New South Wales, Sydney, Australia (M.P.I., S.J.R.); Tissue Bank of the National Center for Tumor Diseases (NCT) Heidelberg (E. Herpel), and Institute of Pathology, University of Heidelberg (E. Herpel), Heidelberg, Germany; Cancer Center, University of Hawaii, Honolulu, HI (B.Y.H.); the Crown Princess Mary Cancer Centre, Westmead Hospital, the University of Sydney, Sydney, Australia (P.R.H., B. Gao); Oncology Department, Hospital General de Albacete, Madrid, Spain (J.M.G.-B.); Department of Cancer Genomics and Genetics, Peter MacCallum Cancer Centre, East Melbourne, Victoria, Australia (S.F.); Pathology Department, Fundación Jiménez Díaz, Madrid, Spain (A.C.); Department of Health Research and Policy and Department of Biomedical Data Science, Stanford University School of Medicine, Stanford, CA (A.S.W.); Department of Obstetrics and Gynecology, Division of Gynecologic Oncology, Royal Alexandra Hospital, Edmonton, Alberta, Canada (H.S.); Tübingen University Hospital, Institute of Pathology, Tübingen, Germany (A.S.); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (K.B.M.); Gynaecological Cancer Research Centre, Department of Women’s Cancer, Institute for Women’s Health, University College London, London, UK (U.M.); Alberta Health Services-Cancer Care, Calgary, Alberta, Canada (J.M.K.); Department of Gynecology, Rigshospitalet, University of Copenhagen, Copenhagen, Denmark (S.K.K.); Department of Public Health Sciences, Medical University of South Carolina and Hollings Cancer Center, Charleston, SC (L.E.K.); Department of Pathology and Laboratory Medicine, University of British Columbia (D.G.H.), and Centre for Translational and Applied Genomics, British Columbia Cancer Agency (D.G.H.), Vancouver, British Columbia, Canada; Molecular Unit, Department of Pathology, Herlev Hospital, University of Copenhagen, Copenhagen, Denmark (E. Høgdall); Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, CA (M.T.G.); Genetic Pathology Evaluation Centre, Vancouver General Hospital and University of British Columbia, Vancouver, British Columbia, Canada (B. Gilks); Human Genetics Group, Spanish National Cancer Research Centre (Centro Nacional de Investigaciones Oncológicas) (M.J.G., J.B.), and Centre for Biomedical Network Research on Rare Diseases (Centro de Investigación Biomédica en Red de Enfermedades Raras), Instituto de Salud Carlos III (M.J.G., J.B.), Madrid, Spain; David Geffen School of Medicine, Department of Medicine, Division of Hematology and Oncology, University of California at Los Angeles (P.A.F.); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Erlangen, Germany (P.A.F.); Department of Histopathology, Queen’s Medical Centre, Nottingham University Hospitals NHS Trust, Nottingham, UK (S.D.); University Cancer Center Hamburg, University Medical Center Hamburg-Eppendorf, Hamburg, Germany (J.C.-C.); Cancer Genetics Laboratory, Research Division, Peter MacCallum Cancer Centre (I.G.C.), Sir Peter MacCallum Department of Oncology, University of Melbourne (I.G.C.), and Department of Pathology, University of Melbourne (I.G.C.), Melbourne, Victoria, Australia; Department of Oncology, University of Cambridge, Hutchison/MRC Research Centre (J.D.B.), Cancer Research UK Cambridge Institute, University of Cambridge, Li Ka Shing Centre (J.D.B.), and Cambridge Experimental Cancer Medicine Centre (J.D.B.), Cambridge, UK; Cancer Genomics Program, Research Department, Peter MacCallum Cancer Centre, East Melbourne, Victoria, Australia (D.D.B.); the Garvan Institute, Sydney, New South Wales, Australia (D.D.B., S.J.R.); and Department of Public Health and Primary Care, University of Cambridge, Strangeways Research Laboratory, Cambridge, UK (P.D.P.P.)
1,
Aleksandra Tołoczko
Aleksandra Tołoczko, MD
1Department of Medical Oncology (M.S.B., K.R.K.) and Department of Health Sciences Research (R.A.V., S.J.W., B.M.M., Z.L., E.L.G.), Mayo Clinic, Rochester, MN; Department of Pathology and Laboratory Medicine, University of Calgary, Calgary, Alberta, Canada (P.F.R., M.K.); Pathology Department, Catholic University of Health and Allied Sciences-Bugando, Mwanza, Tanzania (P.F.R.); Tübingen University Hospital, Department of Women’s Health, Tübingen, Germany (P.W., F.A.T., A.D.H., S.Y.B., S.K.); Sir Peter MacCallum Department of Oncology, the University of Melbourne, Parkville, Victoria, Australia (N.T., D.D.B.); International Hereditary Cancer Center, Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (A.T., O.O., J. Lubiński, J.G.); Department of Gynecology and Obstetrics, Ribeirão Preto Medical School, University of São Paulo, Ribeirão Preto, Brazil (D.G.T., C.B.S., J.M.A., F.J.C.R.); Department of Pathology, Institute of Pathology, Heidelberg University Hospital, Heidelberg, Germany (P.S.); Department of Population Health Science and Policy, Department of Genetics and Genomic Sciences, Icahn School of Medicine at Mount Sinai, New York, NY (W.S., J.H.R.); Pathology West ICPMR Westmead, Westmead Hospital, the University of Sydney, Sydney, Australia (R.S.); University of Western Sydney at Westmead Hospital, Westmead, New South Wales, Australia (R.S.); Medical Oncology Service, Hospital Sant Pau, Barcelona, Spain (T.R.C.); H12O-CNIO Lung Cancer Clinical Research Unit (L.P.-A.), and Oncology Department, Hospital Universitario 12 de Octubre (L.P.-A.), Madrid, Spain; Women’s Cancer Program, Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, CA (S.O., J. Lester, B.Y.K.); the University of Texas School of Public Health, Houston (R.B.N.); Department of Oncology, Division of Gynecologic Oncology, Cumming School of Medicine, University of Calgary, Calgary, Alberta, Canada (G.N., P.G.); Department of Obstetrics, Gynecology and Reproductive Sciences, University of Pittsburgh School of Medicine (F.M., R.P.E.), Department of Epidemiology, University of Pittsburgh Graduate School of Public Health (F.M.), and Womens Cancer Research Program, Magee-Womens Research Institute and University of Pittsburgh Cancer Institute (F.M.), Pittsburgh, PA; Department of Gynecological Surgery and Gynecological Oncology of Adults and Adolescents, Pomeranian Medical University, Szczecin, Poland (J.M., A.C.-G.); Department of Health Research and Policy, Stanford University School of Medicine, Stanford, CA (V.M.); Department of Oncology, University of Cambridge, Strangeways Research Laboratory, Cambridge, UK (M.M., J.A., P.D.P.P.); Department of Pathology, Stanford University, Stanford, CA (T.A.L.); Department of Gynecologic Oncology, the Third Affiliated Hospital of Kunming Medical University (Yunnan Tumor Hospital), Kunming, China (Z.L.); Centre for Cancer Research, the Westmead Institute for Medical Research, the University of Sydney (C.J.K., J.H., P.R.H., B. Gao, A.deF.), and Department of Gynaecological Oncology, Westmead Hospital (C.J.K., J.H., A.deF.), Sydney, New South Wales, Australia; Division of Cancer Epidemiology, German Cancer Research Center (Deutsches Krebsforschungszentrum), Heidelberg, Germany (A.Y.J., U.E., J.C.-C.); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia (S.E.J., G.C.-T.); Department of Histopathology, Addenbrookes Hospital, Cambridge, UK (M.J.-L.); Virus, Lifestyle and Genes, Danish Cancer Society Research Center, Copenhagen, Denmark (A.J., S.K.K.); School of Women’s and Children’s Health, University of New South Wales, Sydney, Australia (M.P.I., S.J.R.); Tissue Bank of the National Center for Tumor Diseases (NCT) Heidelberg (E. Herpel), and Institute of Pathology, University of Heidelberg (E. Herpel), Heidelberg, Germany; Cancer Center, University of Hawaii, Honolulu, HI (B.Y.H.); the Crown Princess Mary Cancer Centre, Westmead Hospital, the University of Sydney, Sydney, Australia (P.R.H., B. Gao); Oncology Department, Hospital General de Albacete, Madrid, Spain (J.M.G.-B.); Department of Cancer Genomics and Genetics, Peter MacCallum Cancer Centre, East Melbourne, Victoria, Australia (S.F.); Pathology Department, Fundación Jiménez Díaz, Madrid, Spain (A.C.); Department of Health Research and Policy and Department of Biomedical Data Science, Stanford University School of Medicine, Stanford, CA (A.S.W.); Department of Obstetrics and Gynecology, Division of Gynecologic Oncology, Royal Alexandra Hospital, Edmonton, Alberta, Canada (H.S.); Tübingen University Hospital, Institute of Pathology, Tübingen, Germany (A.S.); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (K.B.M.); Gynaecological Cancer Research Centre, Department of Women’s Cancer, Institute for Women’s Health, University College London, London, UK (U.M.); Alberta Health Services-Cancer Care, Calgary, Alberta, Canada (J.M.K.); Department of Gynecology, Rigshospitalet, University of Copenhagen, Copenhagen, Denmark (S.K.K.); Department of Public Health Sciences, Medical University of South Carolina and Hollings Cancer Center, Charleston, SC (L.E.K.); Department of Pathology and Laboratory Medicine, University of British Columbia (D.G.H.), and Centre for Translational and Applied Genomics, British Columbia Cancer Agency (D.G.H.), Vancouver, British Columbia, Canada; Molecular Unit, Department of Pathology, Herlev Hospital, University of Copenhagen, Copenhagen, Denmark (E. Høgdall); Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, CA (M.T.G.); Genetic Pathology Evaluation Centre, Vancouver General Hospital and University of British Columbia, Vancouver, British Columbia, Canada (B. Gilks); Human Genetics Group, Spanish National Cancer Research Centre (Centro Nacional de Investigaciones Oncológicas) (M.J.G., J.B.), and Centre for Biomedical Network Research on Rare Diseases (Centro de Investigación Biomédica en Red de Enfermedades Raras), Instituto de Salud Carlos III (M.J.G., J.B.), Madrid, Spain; David Geffen School of Medicine, Department of Medicine, Division of Hematology and Oncology, University of California at Los Angeles (P.A.F.); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Erlangen, Germany (P.A.F.); Department of Histopathology, Queen’s Medical Centre, Nottingham University Hospitals NHS Trust, Nottingham, UK (S.D.); University Cancer Center Hamburg, University Medical Center Hamburg-Eppendorf, Hamburg, Germany (J.C.-C.); Cancer Genetics Laboratory, Research Division, Peter MacCallum Cancer Centre (I.G.C.), Sir Peter MacCallum Department of Oncology, University of Melbourne (I.G.C.), and Department of Pathology, University of Melbourne (I.G.C.), Melbourne, Victoria, Australia; Department of Oncology, University of Cambridge, Hutchison/MRC Research Centre (J.D.B.), Cancer Research UK Cambridge Institute, University of Cambridge, Li Ka Shing Centre (J.D.B.), and Cambridge Experimental Cancer Medicine Centre (J.D.B.), Cambridge, UK; Cancer Genomics Program, Research Department, Peter MacCallum Cancer Centre, East Melbourne, Victoria, Australia (D.D.B.); the Garvan Institute, Sydney, New South Wales, Australia (D.D.B., S.J.R.); and Department of Public Health and Primary Care, University of Cambridge, Strangeways Research Laboratory, Cambridge, UK (P.D.P.P.)
1,
Daniel G Tiezzi
Daniel G Tiezzi, MD, PhD
1Department of Medical Oncology (M.S.B., K.R.K.) and Department of Health Sciences Research (R.A.V., S.J.W., B.M.M., Z.L., E.L.G.), Mayo Clinic, Rochester, MN; Department of Pathology and Laboratory Medicine, University of Calgary, Calgary, Alberta, Canada (P.F.R., M.K.); Pathology Department, Catholic University of Health and Allied Sciences-Bugando, Mwanza, Tanzania (P.F.R.); Tübingen University Hospital, Department of Women’s Health, Tübingen, Germany (P.W., F.A.T., A.D.H., S.Y.B., S.K.); Sir Peter MacCallum Department of Oncology, the University of Melbourne, Parkville, Victoria, Australia (N.T., D.D.B.); International Hereditary Cancer Center, Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (A.T., O.O., J. Lubiński, J.G.); Department of Gynecology and Obstetrics, Ribeirão Preto Medical School, University of São Paulo, Ribeirão Preto, Brazil (D.G.T., C.B.S., J.M.A., F.J.C.R.); Department of Pathology, Institute of Pathology, Heidelberg University Hospital, Heidelberg, Germany (P.S.); Department of Population Health Science and Policy, Department of Genetics and Genomic Sciences, Icahn School of Medicine at Mount Sinai, New York, NY (W.S., J.H.R.); Pathology West ICPMR Westmead, Westmead Hospital, the University of Sydney, Sydney, Australia (R.S.); University of Western Sydney at Westmead Hospital, Westmead, New South Wales, Australia (R.S.); Medical Oncology Service, Hospital Sant Pau, Barcelona, Spain (T.R.C.); H12O-CNIO Lung Cancer Clinical Research Unit (L.P.-A.), and Oncology Department, Hospital Universitario 12 de Octubre (L.P.-A.), Madrid, Spain; Women’s Cancer Program, Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, CA (S.O., J. Lester, B.Y.K.); the University of Texas School of Public Health, Houston (R.B.N.); Department of Oncology, Division of Gynecologic Oncology, Cumming School of Medicine, University of Calgary, Calgary, Alberta, Canada (G.N., P.G.); Department of Obstetrics, Gynecology and Reproductive Sciences, University of Pittsburgh School of Medicine (F.M., R.P.E.), Department of Epidemiology, University of Pittsburgh Graduate School of Public Health (F.M.), and Womens Cancer Research Program, Magee-Womens Research Institute and University of Pittsburgh Cancer Institute (F.M.), Pittsburgh, PA; Department of Gynecological Surgery and Gynecological Oncology of Adults and Adolescents, Pomeranian Medical University, Szczecin, Poland (J.M., A.C.-G.); Department of Health Research and Policy, Stanford University School of Medicine, Stanford, CA (V.M.); Department of Oncology, University of Cambridge, Strangeways Research Laboratory, Cambridge, UK (M.M., J.A., P.D.P.P.); Department of Pathology, Stanford University, Stanford, CA (T.A.L.); Department of Gynecologic Oncology, the Third Affiliated Hospital of Kunming Medical University (Yunnan Tumor Hospital), Kunming, China (Z.L.); Centre for Cancer Research, the Westmead Institute for Medical Research, the University of Sydney (C.J.K., J.H., P.R.H., B. Gao, A.deF.), and Department of Gynaecological Oncology, Westmead Hospital (C.J.K., J.H., A.deF.), Sydney, New South Wales, Australia; Division of Cancer Epidemiology, German Cancer Research Center (Deutsches Krebsforschungszentrum), Heidelberg, Germany (A.Y.J., U.E., J.C.-C.); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia (S.E.J., G.C.-T.); Department of Histopathology, Addenbrookes Hospital, Cambridge, UK (M.J.-L.); Virus, Lifestyle and Genes, Danish Cancer Society Research Center, Copenhagen, Denmark (A.J., S.K.K.); School of Women’s and Children’s Health, University of New South Wales, Sydney, Australia (M.P.I., S.J.R.); Tissue Bank of the National Center for Tumor Diseases (NCT) Heidelberg (E. Herpel), and Institute of Pathology, University of Heidelberg (E. Herpel), Heidelberg, Germany; Cancer Center, University of Hawaii, Honolulu, HI (B.Y.H.); the Crown Princess Mary Cancer Centre, Westmead Hospital, the University of Sydney, Sydney, Australia (P.R.H., B. Gao); Oncology Department, Hospital General de Albacete, Madrid, Spain (J.M.G.-B.); Department of Cancer Genomics and Genetics, Peter MacCallum Cancer Centre, East Melbourne, Victoria, Australia (S.F.); Pathology Department, Fundación Jiménez Díaz, Madrid, Spain (A.C.); Department of Health Research and Policy and Department of Biomedical Data Science, Stanford University School of Medicine, Stanford, CA (A.S.W.); Department of Obstetrics and Gynecology, Division of Gynecologic Oncology, Royal Alexandra Hospital, Edmonton, Alberta, Canada (H.S.); Tübingen University Hospital, Institute of Pathology, Tübingen, Germany (A.S.); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (K.B.M.); Gynaecological Cancer Research Centre, Department of Women’s Cancer, Institute for Women’s Health, University College London, London, UK (U.M.); Alberta Health Services-Cancer Care, Calgary, Alberta, Canada (J.M.K.); Department of Gynecology, Rigshospitalet, University of Copenhagen, Copenhagen, Denmark (S.K.K.); Department of Public Health Sciences, Medical University of South Carolina and Hollings Cancer Center, Charleston, SC (L.E.K.); Department of Pathology and Laboratory Medicine, University of British Columbia (D.G.H.), and Centre for Translational and Applied Genomics, British Columbia Cancer Agency (D.G.H.), Vancouver, British Columbia, Canada; Molecular Unit, Department of Pathology, Herlev Hospital, University of Copenhagen, Copenhagen, Denmark (E. Høgdall); Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, CA (M.T.G.); Genetic Pathology Evaluation Centre, Vancouver General Hospital and University of British Columbia, Vancouver, British Columbia, Canada (B. Gilks); Human Genetics Group, Spanish National Cancer Research Centre (Centro Nacional de Investigaciones Oncológicas) (M.J.G., J.B.), and Centre for Biomedical Network Research on Rare Diseases (Centro de Investigación Biomédica en Red de Enfermedades Raras), Instituto de Salud Carlos III (M.J.G., J.B.), Madrid, Spain; David Geffen School of Medicine, Department of Medicine, Division of Hematology and Oncology, University of California at Los Angeles (P.A.F.); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Erlangen, Germany (P.A.F.); Department of Histopathology, Queen’s Medical Centre, Nottingham University Hospitals NHS Trust, Nottingham, UK (S.D.); University Cancer Center Hamburg, University Medical Center Hamburg-Eppendorf, Hamburg, Germany (J.C.-C.); Cancer Genetics Laboratory, Research Division, Peter MacCallum Cancer Centre (I.G.C.), Sir Peter MacCallum Department of Oncology, University of Melbourne (I.G.C.), and Department of Pathology, University of Melbourne (I.G.C.), Melbourne, Victoria, Australia; Department of Oncology, University of Cambridge, Hutchison/MRC Research Centre (J.D.B.), Cancer Research UK Cambridge Institute, University of Cambridge, Li Ka Shing Centre (J.D.B.), and Cambridge Experimental Cancer Medicine Centre (J.D.B.), Cambridge, UK; Cancer Genomics Program, Research Department, Peter MacCallum Cancer Centre, East Melbourne, Victoria, Australia (D.D.B.); the Garvan Institute, Sydney, New South Wales, Australia (D.D.B., S.J.R.); and Department of Public Health and Primary Care, University of Cambridge, Strangeways Research Laboratory, Cambridge, UK (P.D.P.P.)
1,
Florin Andrei Taran
Florin Andrei Taran, MD
1Department of Medical Oncology (M.S.B., K.R.K.) and Department of Health Sciences Research (R.A.V., S.J.W., B.M.M., Z.L., E.L.G.), Mayo Clinic, Rochester, MN; Department of Pathology and Laboratory Medicine, University of Calgary, Calgary, Alberta, Canada (P.F.R., M.K.); Pathology Department, Catholic University of Health and Allied Sciences-Bugando, Mwanza, Tanzania (P.F.R.); Tübingen University Hospital, Department of Women’s Health, Tübingen, Germany (P.W., F.A.T., A.D.H., S.Y.B., S.K.); Sir Peter MacCallum Department of Oncology, the University of Melbourne, Parkville, Victoria, Australia (N.T., D.D.B.); International Hereditary Cancer Center, Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (A.T., O.O., J. Lubiński, J.G.); Department of Gynecology and Obstetrics, Ribeirão Preto Medical School, University of São Paulo, Ribeirão Preto, Brazil (D.G.T., C.B.S., J.M.A., F.J.C.R.); Department of Pathology, Institute of Pathology, Heidelberg University Hospital, Heidelberg, Germany (P.S.); Department of Population Health Science and Policy, Department of Genetics and Genomic Sciences, Icahn School of Medicine at Mount Sinai, New York, NY (W.S., J.H.R.); Pathology West ICPMR Westmead, Westmead Hospital, the University of Sydney, Sydney, Australia (R.S.); University of Western Sydney at Westmead Hospital, Westmead, New South Wales, Australia (R.S.); Medical Oncology Service, Hospital Sant Pau, Barcelona, Spain (T.R.C.); H12O-CNIO Lung Cancer Clinical Research Unit (L.P.-A.), and Oncology Department, Hospital Universitario 12 de Octubre (L.P.-A.), Madrid, Spain; Women’s Cancer Program, Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, CA (S.O., J. Lester, B.Y.K.); the University of Texas School of Public Health, Houston (R.B.N.); Department of Oncology, Division of Gynecologic Oncology, Cumming School of Medicine, University of Calgary, Calgary, Alberta, Canada (G.N., P.G.); Department of Obstetrics, Gynecology and Reproductive Sciences, University of Pittsburgh School of Medicine (F.M., R.P.E.), Department of Epidemiology, University of Pittsburgh Graduate School of Public Health (F.M.), and Womens Cancer Research Program, Magee-Womens Research Institute and University of Pittsburgh Cancer Institute (F.M.), Pittsburgh, PA; Department of Gynecological Surgery and Gynecological Oncology of Adults and Adolescents, Pomeranian Medical University, Szczecin, Poland (J.M., A.C.-G.); Department of Health Research and Policy, Stanford University School of Medicine, Stanford, CA (V.M.); Department of Oncology, University of Cambridge, Strangeways Research Laboratory, Cambridge, UK (M.M., J.A., P.D.P.P.); Department of Pathology, Stanford University, Stanford, CA (T.A.L.); Department of Gynecologic Oncology, the Third Affiliated Hospital of Kunming Medical University (Yunnan Tumor Hospital), Kunming, China (Z.L.); Centre for Cancer Research, the Westmead Institute for Medical Research, the University of Sydney (C.J.K., J.H., P.R.H., B. Gao, A.deF.), and Department of Gynaecological Oncology, Westmead Hospital (C.J.K., J.H., A.deF.), Sydney, New South Wales, Australia; Division of Cancer Epidemiology, German Cancer Research Center (Deutsches Krebsforschungszentrum), Heidelberg, Germany (A.Y.J., U.E., J.C.-C.); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia (S.E.J., G.C.-T.); Department of Histopathology, Addenbrookes Hospital, Cambridge, UK (M.J.-L.); Virus, Lifestyle and Genes, Danish Cancer Society Research Center, Copenhagen, Denmark (A.J., S.K.K.); School of Women’s and Children’s Health, University of New South Wales, Sydney, Australia (M.P.I., S.J.R.); Tissue Bank of the National Center for Tumor Diseases (NCT) Heidelberg (E. Herpel), and Institute of Pathology, University of Heidelberg (E. Herpel), Heidelberg, Germany; Cancer Center, University of Hawaii, Honolulu, HI (B.Y.H.); the Crown Princess Mary Cancer Centre, Westmead Hospital, the University of Sydney, Sydney, Australia (P.R.H., B. Gao); Oncology Department, Hospital General de Albacete, Madrid, Spain (J.M.G.-B.); Department of Cancer Genomics and Genetics, Peter MacCallum Cancer Centre, East Melbourne, Victoria, Australia (S.F.); Pathology Department, Fundación Jiménez Díaz, Madrid, Spain (A.C.); Department of Health Research and Policy and Department of Biomedical Data Science, Stanford University School of Medicine, Stanford, CA (A.S.W.); Department of Obstetrics and Gynecology, Division of Gynecologic Oncology, Royal Alexandra Hospital, Edmonton, Alberta, Canada (H.S.); Tübingen University Hospital, Institute of Pathology, Tübingen, Germany (A.S.); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (K.B.M.); Gynaecological Cancer Research Centre, Department of Women’s Cancer, Institute for Women’s Health, University College London, London, UK (U.M.); Alberta Health Services-Cancer Care, Calgary, Alberta, Canada (J.M.K.); Department of Gynecology, Rigshospitalet, University of Copenhagen, Copenhagen, Denmark (S.K.K.); Department of Public Health Sciences, Medical University of South Carolina and Hollings Cancer Center, Charleston, SC (L.E.K.); Department of Pathology and Laboratory Medicine, University of British Columbia (D.G.H.), and Centre for Translational and Applied Genomics, British Columbia Cancer Agency (D.G.H.), Vancouver, British Columbia, Canada; Molecular Unit, Department of Pathology, Herlev Hospital, University of Copenhagen, Copenhagen, Denmark (E. Høgdall); Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, CA (M.T.G.); Genetic Pathology Evaluation Centre, Vancouver General Hospital and University of British Columbia, Vancouver, British Columbia, Canada (B. Gilks); Human Genetics Group, Spanish National Cancer Research Centre (Centro Nacional de Investigaciones Oncológicas) (M.J.G., J.B.), and Centre for Biomedical Network Research on Rare Diseases (Centro de Investigación Biomédica en Red de Enfermedades Raras), Instituto de Salud Carlos III (M.J.G., J.B.), Madrid, Spain; David Geffen School of Medicine, Department of Medicine, Division of Hematology and Oncology, University of California at Los Angeles (P.A.F.); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Erlangen, Germany (P.A.F.); Department of Histopathology, Queen’s Medical Centre, Nottingham University Hospitals NHS Trust, Nottingham, UK (S.D.); University Cancer Center Hamburg, University Medical Center Hamburg-Eppendorf, Hamburg, Germany (J.C.-C.); Cancer Genetics Laboratory, Research Division, Peter MacCallum Cancer Centre (I.G.C.), Sir Peter MacCallum Department of Oncology, University of Melbourne (I.G.C.), and Department of Pathology, University of Melbourne (I.G.C.), Melbourne, Victoria, Australia; Department of Oncology, University of Cambridge, Hutchison/MRC Research Centre (J.D.B.), Cancer Research UK Cambridge Institute, University of Cambridge, Li Ka Shing Centre (J.D.B.), and Cambridge Experimental Cancer Medicine Centre (J.D.B.), Cambridge, UK; Cancer Genomics Program, Research Department, Peter MacCallum Cancer Centre, East Melbourne, Victoria, Australia (D.D.B.); the Garvan Institute, Sydney, New South Wales, Australia (D.D.B., S.J.R.); and Department of Public Health and Primary Care, University of Cambridge, Strangeways Research Laboratory, Cambridge, UK (P.D.P.P.)
1,
Peter Sinn
Peter Sinn, MD, PhD
1Department of Medical Oncology (M.S.B., K.R.K.) and Department of Health Sciences Research (R.A.V., S.J.W., B.M.M., Z.L., E.L.G.), Mayo Clinic, Rochester, MN; Department of Pathology and Laboratory Medicine, University of Calgary, Calgary, Alberta, Canada (P.F.R., M.K.); Pathology Department, Catholic University of Health and Allied Sciences-Bugando, Mwanza, Tanzania (P.F.R.); Tübingen University Hospital, Department of Women’s Health, Tübingen, Germany (P.W., F.A.T., A.D.H., S.Y.B., S.K.); Sir Peter MacCallum Department of Oncology, the University of Melbourne, Parkville, Victoria, Australia (N.T., D.D.B.); International Hereditary Cancer Center, Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (A.T., O.O., J. Lubiński, J.G.); Department of Gynecology and Obstetrics, Ribeirão Preto Medical School, University of São Paulo, Ribeirão Preto, Brazil (D.G.T., C.B.S., J.M.A., F.J.C.R.); Department of Pathology, Institute of Pathology, Heidelberg University Hospital, Heidelberg, Germany (P.S.); Department of Population Health Science and Policy, Department of Genetics and Genomic Sciences, Icahn School of Medicine at Mount Sinai, New York, NY (W.S., J.H.R.); Pathology West ICPMR Westmead, Westmead Hospital, the University of Sydney, Sydney, Australia (R.S.); University of Western Sydney at Westmead Hospital, Westmead, New South Wales, Australia (R.S.); Medical Oncology Service, Hospital Sant Pau, Barcelona, Spain (T.R.C.); H12O-CNIO Lung Cancer Clinical Research Unit (L.P.-A.), and Oncology Department, Hospital Universitario 12 de Octubre (L.P.-A.), Madrid, Spain; Women’s Cancer Program, Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, CA (S.O., J. Lester, B.Y.K.); the University of Texas School of Public Health, Houston (R.B.N.); Department of Oncology, Division of Gynecologic Oncology, Cumming School of Medicine, University of Calgary, Calgary, Alberta, Canada (G.N., P.G.); Department of Obstetrics, Gynecology and Reproductive Sciences, University of Pittsburgh School of Medicine (F.M., R.P.E.), Department of Epidemiology, University of Pittsburgh Graduate School of Public Health (F.M.), and Womens Cancer Research Program, Magee-Womens Research Institute and University of Pittsburgh Cancer Institute (F.M.), Pittsburgh, PA; Department of Gynecological Surgery and Gynecological Oncology of Adults and Adolescents, Pomeranian Medical University, Szczecin, Poland (J.M., A.C.-G.); Department of Health Research and Policy, Stanford University School of Medicine, Stanford, CA (V.M.); Department of Oncology, University of Cambridge, Strangeways Research Laboratory, Cambridge, UK (M.M., J.A., P.D.P.P.); Department of Pathology, Stanford University, Stanford, CA (T.A.L.); Department of Gynecologic Oncology, the Third Affiliated Hospital of Kunming Medical University (Yunnan Tumor Hospital), Kunming, China (Z.L.); Centre for Cancer Research, the Westmead Institute for Medical Research, the University of Sydney (C.J.K., J.H., P.R.H., B. Gao, A.deF.), and Department of Gynaecological Oncology, Westmead Hospital (C.J.K., J.H., A.deF.), Sydney, New South Wales, Australia; Division of Cancer Epidemiology, German Cancer Research Center (Deutsches Krebsforschungszentrum), Heidelberg, Germany (A.Y.J., U.E., J.C.-C.); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia (S.E.J., G.C.-T.); Department of Histopathology, Addenbrookes Hospital, Cambridge, UK (M.J.-L.); Virus, Lifestyle and Genes, Danish Cancer Society Research Center, Copenhagen, Denmark (A.J., S.K.K.); School of Women’s and Children’s Health, University of New South Wales, Sydney, Australia (M.P.I., S.J.R.); Tissue Bank of the National Center for Tumor Diseases (NCT) Heidelberg (E. Herpel), and Institute of Pathology, University of Heidelberg (E. Herpel), Heidelberg, Germany; Cancer Center, University of Hawaii, Honolulu, HI (B.Y.H.); the Crown Princess Mary Cancer Centre, Westmead Hospital, the University of Sydney, Sydney, Australia (P.R.H., B. Gao); Oncology Department, Hospital General de Albacete, Madrid, Spain (J.M.G.-B.); Department of Cancer Genomics and Genetics, Peter MacCallum Cancer Centre, East Melbourne, Victoria, Australia (S.F.); Pathology Department, Fundación Jiménez Díaz, Madrid, Spain (A.C.); Department of Health Research and Policy and Department of Biomedical Data Science, Stanford University School of Medicine, Stanford, CA (A.S.W.); Department of Obstetrics and Gynecology, Division of Gynecologic Oncology, Royal Alexandra Hospital, Edmonton, Alberta, Canada (H.S.); Tübingen University Hospital, Institute of Pathology, Tübingen, Germany (A.S.); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (K.B.M.); Gynaecological Cancer Research Centre, Department of Women’s Cancer, Institute for Women’s Health, University College London, London, UK (U.M.); Alberta Health Services-Cancer Care, Calgary, Alberta, Canada (J.M.K.); Department of Gynecology, Rigshospitalet, University of Copenhagen, Copenhagen, Denmark (S.K.K.); Department of Public Health Sciences, Medical University of South Carolina and Hollings Cancer Center, Charleston, SC (L.E.K.); Department of Pathology and Laboratory Medicine, University of British Columbia (D.G.H.), and Centre for Translational and Applied Genomics, British Columbia Cancer Agency (D.G.H.), Vancouver, British Columbia, Canada; Molecular Unit, Department of Pathology, Herlev Hospital, University of Copenhagen, Copenhagen, Denmark (E. Høgdall); Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, CA (M.T.G.); Genetic Pathology Evaluation Centre, Vancouver General Hospital and University of British Columbia, Vancouver, British Columbia, Canada (B. Gilks); Human Genetics Group, Spanish National Cancer Research Centre (Centro Nacional de Investigaciones Oncológicas) (M.J.G., J.B.), and Centre for Biomedical Network Research on Rare Diseases (Centro de Investigación Biomédica en Red de Enfermedades Raras), Instituto de Salud Carlos III (M.J.G., J.B.), Madrid, Spain; David Geffen School of Medicine, Department of Medicine, Division of Hematology and Oncology, University of California at Los Angeles (P.A.F.); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Erlangen, Germany (P.A.F.); Department of Histopathology, Queen’s Medical Centre, Nottingham University Hospitals NHS Trust, Nottingham, UK (S.D.); University Cancer Center Hamburg, University Medical Center Hamburg-Eppendorf, Hamburg, Germany (J.C.-C.); Cancer Genetics Laboratory, Research Division, Peter MacCallum Cancer Centre (I.G.C.), Sir Peter MacCallum Department of Oncology, University of Melbourne (I.G.C.), and Department of Pathology, University of Melbourne (I.G.C.), Melbourne, Victoria, Australia; Department of Oncology, University of Cambridge, Hutchison/MRC Research Centre (J.D.B.), Cancer Research UK Cambridge Institute, University of Cambridge, Li Ka Shing Centre (J.D.B.), and Cambridge Experimental Cancer Medicine Centre (J.D.B.), Cambridge, UK; Cancer Genomics Program, Research Department, Peter MacCallum Cancer Centre, East Melbourne, Victoria, Australia (D.D.B.); the Garvan Institute, Sydney, New South Wales, Australia (D.D.B., S.J.R.); and Department of Public Health and Primary Care, University of Cambridge, Strangeways Research Laboratory, Cambridge, UK (P.D.P.P.)
1,
Weiva Sieh
Weiva Sieh, PhD
1Department of Medical Oncology (M.S.B., K.R.K.) and Department of Health Sciences Research (R.A.V., S.J.W., B.M.M., Z.L., E.L.G.), Mayo Clinic, Rochester, MN; Department of Pathology and Laboratory Medicine, University of Calgary, Calgary, Alberta, Canada (P.F.R., M.K.); Pathology Department, Catholic University of Health and Allied Sciences-Bugando, Mwanza, Tanzania (P.F.R.); Tübingen University Hospital, Department of Women’s Health, Tübingen, Germany (P.W., F.A.T., A.D.H., S.Y.B., S.K.); Sir Peter MacCallum Department of Oncology, the University of Melbourne, Parkville, Victoria, Australia (N.T., D.D.B.); International Hereditary Cancer Center, Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (A.T., O.O., J. Lubiński, J.G.); Department of Gynecology and Obstetrics, Ribeirão Preto Medical School, University of São Paulo, Ribeirão Preto, Brazil (D.G.T., C.B.S., J.M.A., F.J.C.R.); Department of Pathology, Institute of Pathology, Heidelberg University Hospital, Heidelberg, Germany (P.S.); Department of Population Health Science and Policy, Department of Genetics and Genomic Sciences, Icahn School of Medicine at Mount Sinai, New York, NY (W.S., J.H.R.); Pathology West ICPMR Westmead, Westmead Hospital, the University of Sydney, Sydney, Australia (R.S.); University of Western Sydney at Westmead Hospital, Westmead, New South Wales, Australia (R.S.); Medical Oncology Service, Hospital Sant Pau, Barcelona, Spain (T.R.C.); H12O-CNIO Lung Cancer Clinical Research Unit (L.P.-A.), and Oncology Department, Hospital Universitario 12 de Octubre (L.P.-A.), Madrid, Spain; Women’s Cancer Program, Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, CA (S.O., J. Lester, B.Y.K.); the University of Texas School of Public Health, Houston (R.B.N.); Department of Oncology, Division of Gynecologic Oncology, Cumming School of Medicine, University of Calgary, Calgary, Alberta, Canada (G.N., P.G.); Department of Obstetrics, Gynecology and Reproductive Sciences, University of Pittsburgh School of Medicine (F.M., R.P.E.), Department of Epidemiology, University of Pittsburgh Graduate School of Public Health (F.M.), and Womens Cancer Research Program, Magee-Womens Research Institute and University of Pittsburgh Cancer Institute (F.M.), Pittsburgh, PA; Department of Gynecological Surgery and Gynecological Oncology of Adults and Adolescents, Pomeranian Medical University, Szczecin, Poland (J.M., A.C.-G.); Department of Health Research and Policy, Stanford University School of Medicine, Stanford, CA (V.M.); Department of Oncology, University of Cambridge, Strangeways Research Laboratory, Cambridge, UK (M.M., J.A., P.D.P.P.); Department of Pathology, Stanford University, Stanford, CA (T.A.L.); Department of Gynecologic Oncology, the Third Affiliated Hospital of Kunming Medical University (Yunnan Tumor Hospital), Kunming, China (Z.L.); Centre for Cancer Research, the Westmead Institute for Medical Research, the University of Sydney (C.J.K., J.H., P.R.H., B. Gao, A.deF.), and Department of Gynaecological Oncology, Westmead Hospital (C.J.K., J.H., A.deF.), Sydney, New South Wales, Australia; Division of Cancer Epidemiology, German Cancer Research Center (Deutsches Krebsforschungszentrum), Heidelberg, Germany (A.Y.J., U.E., J.C.-C.); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia (S.E.J., G.C.-T.); Department of Histopathology, Addenbrookes Hospital, Cambridge, UK (M.J.-L.); Virus, Lifestyle and Genes, Danish Cancer Society Research Center, Copenhagen, Denmark (A.J., S.K.K.); School of Women’s and Children’s Health, University of New South Wales, Sydney, Australia (M.P.I., S.J.R.); Tissue Bank of the National Center for Tumor Diseases (NCT) Heidelberg (E. Herpel), and Institute of Pathology, University of Heidelberg (E. Herpel), Heidelberg, Germany; Cancer Center, University of Hawaii, Honolulu, HI (B.Y.H.); the Crown Princess Mary Cancer Centre, Westmead Hospital, the University of Sydney, Sydney, Australia (P.R.H., B. Gao); Oncology Department, Hospital General de Albacete, Madrid, Spain (J.M.G.-B.); Department of Cancer Genomics and Genetics, Peter MacCallum Cancer Centre, East Melbourne, Victoria, Australia (S.F.); Pathology Department, Fundación Jiménez Díaz, Madrid, Spain (A.C.); Department of Health Research and Policy and Department of Biomedical Data Science, Stanford University School of Medicine, Stanford, CA (A.S.W.); Department of Obstetrics and Gynecology, Division of Gynecologic Oncology, Royal Alexandra Hospital, Edmonton, Alberta, Canada (H.S.); Tübingen University Hospital, Institute of Pathology, Tübingen, Germany (A.S.); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (K.B.M.); Gynaecological Cancer Research Centre, Department of Women’s Cancer, Institute for Women’s Health, University College London, London, UK (U.M.); Alberta Health Services-Cancer Care, Calgary, Alberta, Canada (J.M.K.); Department of Gynecology, Rigshospitalet, University of Copenhagen, Copenhagen, Denmark (S.K.K.); Department of Public Health Sciences, Medical University of South Carolina and Hollings Cancer Center, Charleston, SC (L.E.K.); Department of Pathology and Laboratory Medicine, University of British Columbia (D.G.H.), and Centre for Translational and Applied Genomics, British Columbia Cancer Agency (D.G.H.), Vancouver, British Columbia, Canada; Molecular Unit, Department of Pathology, Herlev Hospital, University of Copenhagen, Copenhagen, Denmark (E. Høgdall); Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, CA (M.T.G.); Genetic Pathology Evaluation Centre, Vancouver General Hospital and University of British Columbia, Vancouver, British Columbia, Canada (B. Gilks); Human Genetics Group, Spanish National Cancer Research Centre (Centro Nacional de Investigaciones Oncológicas) (M.J.G., J.B.), and Centre for Biomedical Network Research on Rare Diseases (Centro de Investigación Biomédica en Red de Enfermedades Raras), Instituto de Salud Carlos III (M.J.G., J.B.), Madrid, Spain; David Geffen School of Medicine, Department of Medicine, Division of Hematology and Oncology, University of California at Los Angeles (P.A.F.); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Erlangen, Germany (P.A.F.); Department of Histopathology, Queen’s Medical Centre, Nottingham University Hospitals NHS Trust, Nottingham, UK (S.D.); University Cancer Center Hamburg, University Medical Center Hamburg-Eppendorf, Hamburg, Germany (J.C.-C.); Cancer Genetics Laboratory, Research Division, Peter MacCallum Cancer Centre (I.G.C.), Sir Peter MacCallum Department of Oncology, University of Melbourne (I.G.C.), and Department of Pathology, University of Melbourne (I.G.C.), Melbourne, Victoria, Australia; Department of Oncology, University of Cambridge, Hutchison/MRC Research Centre (J.D.B.), Cancer Research UK Cambridge Institute, University of Cambridge, Li Ka Shing Centre (J.D.B.), and Cambridge Experimental Cancer Medicine Centre (J.D.B.), Cambridge, UK; Cancer Genomics Program, Research Department, Peter MacCallum Cancer Centre, East Melbourne, Victoria, Australia (D.D.B.); the Garvan Institute, Sydney, New South Wales, Australia (D.D.B., S.J.R.); and Department of Public Health and Primary Care, University of Cambridge, Strangeways Research Laboratory, Cambridge, UK (P.D.P.P.)
1,
Raghwa Sharma
Raghwa Sharma, MBBS, FRCPA
1Department of Medical Oncology (M.S.B., K.R.K.) and Department of Health Sciences Research (R.A.V., S.J.W., B.M.M., Z.L., E.L.G.), Mayo Clinic, Rochester, MN; Department of Pathology and Laboratory Medicine, University of Calgary, Calgary, Alberta, Canada (P.F.R., M.K.); Pathology Department, Catholic University of Health and Allied Sciences-Bugando, Mwanza, Tanzania (P.F.R.); Tübingen University Hospital, Department of Women’s Health, Tübingen, Germany (P.W., F.A.T., A.D.H., S.Y.B., S.K.); Sir Peter MacCallum Department of Oncology, the University of Melbourne, Parkville, Victoria, Australia (N.T., D.D.B.); International Hereditary Cancer Center, Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (A.T., O.O., J. Lubiński, J.G.); Department of Gynecology and Obstetrics, Ribeirão Preto Medical School, University of São Paulo, Ribeirão Preto, Brazil (D.G.T., C.B.S., J.M.A., F.J.C.R.); Department of Pathology, Institute of Pathology, Heidelberg University Hospital, Heidelberg, Germany (P.S.); Department of Population Health Science and Policy, Department of Genetics and Genomic Sciences, Icahn School of Medicine at Mount Sinai, New York, NY (W.S., J.H.R.); Pathology West ICPMR Westmead, Westmead Hospital, the University of Sydney, Sydney, Australia (R.S.); University of Western Sydney at Westmead Hospital, Westmead, New South Wales, Australia (R.S.); Medical Oncology Service, Hospital Sant Pau, Barcelona, Spain (T.R.C.); H12O-CNIO Lung Cancer Clinical Research Unit (L.P.-A.), and Oncology Department, Hospital Universitario 12 de Octubre (L.P.-A.), Madrid, Spain; Women’s Cancer Program, Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, CA (S.O., J. Lester, B.Y.K.); the University of Texas School of Public Health, Houston (R.B.N.); Department of Oncology, Division of Gynecologic Oncology, Cumming School of Medicine, University of Calgary, Calgary, Alberta, Canada (G.N., P.G.); Department of Obstetrics, Gynecology and Reproductive Sciences, University of Pittsburgh School of Medicine (F.M., R.P.E.), Department of Epidemiology, University of Pittsburgh Graduate School of Public Health (F.M.), and Womens Cancer Research Program, Magee-Womens Research Institute and University of Pittsburgh Cancer Institute (F.M.), Pittsburgh, PA; Department of Gynecological Surgery and Gynecological Oncology of Adults and Adolescents, Pomeranian Medical University, Szczecin, Poland (J.M., A.C.-G.); Department of Health Research and Policy, Stanford University School of Medicine, Stanford, CA (V.M.); Department of Oncology, University of Cambridge, Strangeways Research Laboratory, Cambridge, UK (M.M., J.A., P.D.P.P.); Department of Pathology, Stanford University, Stanford, CA (T.A.L.); Department of Gynecologic Oncology, the Third Affiliated Hospital of Kunming Medical University (Yunnan Tumor Hospital), Kunming, China (Z.L.); Centre for Cancer Research, the Westmead Institute for Medical Research, the University of Sydney (C.J.K., J.H., P.R.H., B. Gao, A.deF.), and Department of Gynaecological Oncology, Westmead Hospital (C.J.K., J.H., A.deF.), Sydney, New South Wales, Australia; Division of Cancer Epidemiology, German Cancer Research Center (Deutsches Krebsforschungszentrum), Heidelberg, Germany (A.Y.J., U.E., J.C.-C.); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia (S.E.J., G.C.-T.); Department of Histopathology, Addenbrookes Hospital, Cambridge, UK (M.J.-L.); Virus, Lifestyle and Genes, Danish Cancer Society Research Center, Copenhagen, Denmark (A.J., S.K.K.); School of Women’s and Children’s Health, University of New South Wales, Sydney, Australia (M.P.I., S.J.R.); Tissue Bank of the National Center for Tumor Diseases (NCT) Heidelberg (E. Herpel), and Institute of Pathology, University of Heidelberg (E. Herpel), Heidelberg, Germany; Cancer Center, University of Hawaii, Honolulu, HI (B.Y.H.); the Crown Princess Mary Cancer Centre, Westmead Hospital, the University of Sydney, Sydney, Australia (P.R.H., B. Gao); Oncology Department, Hospital General de Albacete, Madrid, Spain (J.M.G.-B.); Department of Cancer Genomics and Genetics, Peter MacCallum Cancer Centre, East Melbourne, Victoria, Australia (S.F.); Pathology Department, Fundación Jiménez Díaz, Madrid, Spain (A.C.); Department of Health Research and Policy and Department of Biomedical Data Science, Stanford University School of Medicine, Stanford, CA (A.S.W.); Department of Obstetrics and Gynecology, Division of Gynecologic Oncology, Royal Alexandra Hospital, Edmonton, Alberta, Canada (H.S.); Tübingen University Hospital, Institute of Pathology, Tübingen, Germany (A.S.); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (K.B.M.); Gynaecological Cancer Research Centre, Department of Women’s Cancer, Institute for Women’s Health, University College London, London, UK (U.M.); Alberta Health Services-Cancer Care, Calgary, Alberta, Canada (J.M.K.); Department of Gynecology, Rigshospitalet, University of Copenhagen, Copenhagen, Denmark (S.K.K.); Department of Public Health Sciences, Medical University of South Carolina and Hollings Cancer Center, Charleston, SC (L.E.K.); Department of Pathology and Laboratory Medicine, University of British Columbia (D.G.H.), and Centre for Translational and Applied Genomics, British Columbia Cancer Agency (D.G.H.), Vancouver, British Columbia, Canada; Molecular Unit, Department of Pathology, Herlev Hospital, University of Copenhagen, Copenhagen, Denmark (E. Høgdall); Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, CA (M.T.G.); Genetic Pathology Evaluation Centre, Vancouver General Hospital and University of British Columbia, Vancouver, British Columbia, Canada (B. Gilks); Human Genetics Group, Spanish National Cancer Research Centre (Centro Nacional de Investigaciones Oncológicas) (M.J.G., J.B.), and Centre for Biomedical Network Research on Rare Diseases (Centro de Investigación Biomédica en Red de Enfermedades Raras), Instituto de Salud Carlos III (M.J.G., J.B.), Madrid, Spain; David Geffen School of Medicine, Department of Medicine, Division of Hematology and Oncology, University of California at Los Angeles (P.A.F.); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Erlangen, Germany (P.A.F.); Department of Histopathology, Queen’s Medical Centre, Nottingham University Hospitals NHS Trust, Nottingham, UK (S.D.); University Cancer Center Hamburg, University Medical Center Hamburg-Eppendorf, Hamburg, Germany (J.C.-C.); Cancer Genetics Laboratory, Research Division, Peter MacCallum Cancer Centre (I.G.C.), Sir Peter MacCallum Department of Oncology, University of Melbourne (I.G.C.), and Department of Pathology, University of Melbourne (I.G.C.), Melbourne, Victoria, Australia; Department of Oncology, University of Cambridge, Hutchison/MRC Research Centre (J.D.B.), Cancer Research UK Cambridge Institute, University of Cambridge, Li Ka Shing Centre (J.D.B.), and Cambridge Experimental Cancer Medicine Centre (J.D.B.), Cambridge, UK; Cancer Genomics Program, Research Department, Peter MacCallum Cancer Centre, East Melbourne, Victoria, Australia (D.D.B.); the Garvan Institute, Sydney, New South Wales, Australia (D.D.B., S.J.R.); and Department of Public Health and Primary Care, University of Cambridge, Strangeways Research Laboratory, Cambridge, UK (P.D.P.P.)
1,
Joseph H Rothstein
Joseph H Rothstein, MS
1Department of Medical Oncology (M.S.B., K.R.K.) and Department of Health Sciences Research (R.A.V., S.J.W., B.M.M., Z.L., E.L.G.), Mayo Clinic, Rochester, MN; Department of Pathology and Laboratory Medicine, University of Calgary, Calgary, Alberta, Canada (P.F.R., M.K.); Pathology Department, Catholic University of Health and Allied Sciences-Bugando, Mwanza, Tanzania (P.F.R.); Tübingen University Hospital, Department of Women’s Health, Tübingen, Germany (P.W., F.A.T., A.D.H., S.Y.B., S.K.); Sir Peter MacCallum Department of Oncology, the University of Melbourne, Parkville, Victoria, Australia (N.T., D.D.B.); International Hereditary Cancer Center, Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (A.T., O.O., J. Lubiński, J.G.); Department of Gynecology and Obstetrics, Ribeirão Preto Medical School, University of São Paulo, Ribeirão Preto, Brazil (D.G.T., C.B.S., J.M.A., F.J.C.R.); Department of Pathology, Institute of Pathology, Heidelberg University Hospital, Heidelberg, Germany (P.S.); Department of Population Health Science and Policy, Department of Genetics and Genomic Sciences, Icahn School of Medicine at Mount Sinai, New York, NY (W.S., J.H.R.); Pathology West ICPMR Westmead, Westmead Hospital, the University of Sydney, Sydney, Australia (R.S.); University of Western Sydney at Westmead Hospital, Westmead, New South Wales, Australia (R.S.); Medical Oncology Service, Hospital Sant Pau, Barcelona, Spain (T.R.C.); H12O-CNIO Lung Cancer Clinical Research Unit (L.P.-A.), and Oncology Department, Hospital Universitario 12 de Octubre (L.P.-A.), Madrid, Spain; Women’s Cancer Program, Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, CA (S.O., J. Lester, B.Y.K.); the University of Texas School of Public Health, Houston (R.B.N.); Department of Oncology, Division of Gynecologic Oncology, Cumming School of Medicine, University of Calgary, Calgary, Alberta, Canada (G.N., P.G.); Department of Obstetrics, Gynecology and Reproductive Sciences, University of Pittsburgh School of Medicine (F.M., R.P.E.), Department of Epidemiology, University of Pittsburgh Graduate School of Public Health (F.M.), and Womens Cancer Research Program, Magee-Womens Research Institute and University of Pittsburgh Cancer Institute (F.M.), Pittsburgh, PA; Department of Gynecological Surgery and Gynecological Oncology of Adults and Adolescents, Pomeranian Medical University, Szczecin, Poland (J.M., A.C.-G.); Department of Health Research and Policy, Stanford University School of Medicine, Stanford, CA (V.M.); Department of Oncology, University of Cambridge, Strangeways Research Laboratory, Cambridge, UK (M.M., J.A., P.D.P.P.); Department of Pathology, Stanford University, Stanford, CA (T.A.L.); Department of Gynecologic Oncology, the Third Affiliated Hospital of Kunming Medical University (Yunnan Tumor Hospital), Kunming, China (Z.L.); Centre for Cancer Research, the Westmead Institute for Medical Research, the University of Sydney (C.J.K., J.H., P.R.H., B. Gao, A.deF.), and Department of Gynaecological Oncology, Westmead Hospital (C.J.K., J.H., A.deF.), Sydney, New South Wales, Australia; Division of Cancer Epidemiology, German Cancer Research Center (Deutsches Krebsforschungszentrum), Heidelberg, Germany (A.Y.J., U.E., J.C.-C.); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia (S.E.J., G.C.-T.); Department of Histopathology, Addenbrookes Hospital, Cambridge, UK (M.J.-L.); Virus, Lifestyle and Genes, Danish Cancer Society Research Center, Copenhagen, Denmark (A.J., S.K.K.); School of Women’s and Children’s Health, University of New South Wales, Sydney, Australia (M.P.I., S.J.R.); Tissue Bank of the National Center for Tumor Diseases (NCT) Heidelberg (E. Herpel), and Institute of Pathology, University of Heidelberg (E. Herpel), Heidelberg, Germany; Cancer Center, University of Hawaii, Honolulu, HI (B.Y.H.); the Crown Princess Mary Cancer Centre, Westmead Hospital, the University of Sydney, Sydney, Australia (P.R.H., B. Gao); Oncology Department, Hospital General de Albacete, Madrid, Spain (J.M.G.-B.); Department of Cancer Genomics and Genetics, Peter MacCallum Cancer Centre, East Melbourne, Victoria, Australia (S.F.); Pathology Department, Fundación Jiménez Díaz, Madrid, Spain (A.C.); Department of Health Research and Policy and Department of Biomedical Data Science, Stanford University School of Medicine, Stanford, CA (A.S.W.); Department of Obstetrics and Gynecology, Division of Gynecologic Oncology, Royal Alexandra Hospital, Edmonton, Alberta, Canada (H.S.); Tübingen University Hospital, Institute of Pathology, Tübingen, Germany (A.S.); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (K.B.M.); Gynaecological Cancer Research Centre, Department of Women’s Cancer, Institute for Women’s Health, University College London, London, UK (U.M.); Alberta Health Services-Cancer Care, Calgary, Alberta, Canada (J.M.K.); Department of Gynecology, Rigshospitalet, University of Copenhagen, Copenhagen, Denmark (S.K.K.); Department of Public Health Sciences, Medical University of South Carolina and Hollings Cancer Center, Charleston, SC (L.E.K.); Department of Pathology and Laboratory Medicine, University of British Columbia (D.G.H.), and Centre for Translational and Applied Genomics, British Columbia Cancer Agency (D.G.H.), Vancouver, British Columbia, Canada; Molecular Unit, Department of Pathology, Herlev Hospital, University of Copenhagen, Copenhagen, Denmark (E. Høgdall); Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, CA (M.T.G.); Genetic Pathology Evaluation Centre, Vancouver General Hospital and University of British Columbia, Vancouver, British Columbia, Canada (B. Gilks); Human Genetics Group, Spanish National Cancer Research Centre (Centro Nacional de Investigaciones Oncológicas) (M.J.G., J.B.), and Centre for Biomedical Network Research on Rare Diseases (Centro de Investigación Biomédica en Red de Enfermedades Raras), Instituto de Salud Carlos III (M.J.G., J.B.), Madrid, Spain; David Geffen School of Medicine, Department of Medicine, Division of Hematology and Oncology, University of California at Los Angeles (P.A.F.); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Erlangen, Germany (P.A.F.); Department of Histopathology, Queen’s Medical Centre, Nottingham University Hospitals NHS Trust, Nottingham, UK (S.D.); University Cancer Center Hamburg, University Medical Center Hamburg-Eppendorf, Hamburg, Germany (J.C.-C.); Cancer Genetics Laboratory, Research Division, Peter MacCallum Cancer Centre (I.G.C.), Sir Peter MacCallum Department of Oncology, University of Melbourne (I.G.C.), and Department of Pathology, University of Melbourne (I.G.C.), Melbourne, Victoria, Australia; Department of Oncology, University of Cambridge, Hutchison/MRC Research Centre (J.D.B.), Cancer Research UK Cambridge Institute, University of Cambridge, Li Ka Shing Centre (J.D.B.), and Cambridge Experimental Cancer Medicine Centre (J.D.B.), Cambridge, UK; Cancer Genomics Program, Research Department, Peter MacCallum Cancer Centre, East Melbourne, Victoria, Australia (D.D.B.); the Garvan Institute, Sydney, New South Wales, Australia (D.D.B., S.J.R.); and Department of Public Health and Primary Care, University of Cambridge, Strangeways Research Laboratory, Cambridge, UK (P.D.P.P.)
1,
Teresa Ramón y Cajal
Teresa Ramón y Cajal, MD
1Department of Medical Oncology (M.S.B., K.R.K.) and Department of Health Sciences Research (R.A.V., S.J.W., B.M.M., Z.L., E.L.G.), Mayo Clinic, Rochester, MN; Department of Pathology and Laboratory Medicine, University of Calgary, Calgary, Alberta, Canada (P.F.R., M.K.); Pathology Department, Catholic University of Health and Allied Sciences-Bugando, Mwanza, Tanzania (P.F.R.); Tübingen University Hospital, Department of Women’s Health, Tübingen, Germany (P.W., F.A.T., A.D.H., S.Y.B., S.K.); Sir Peter MacCallum Department of Oncology, the University of Melbourne, Parkville, Victoria, Australia (N.T., D.D.B.); International Hereditary Cancer Center, Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (A.T., O.O., J. Lubiński, J.G.); Department of Gynecology and Obstetrics, Ribeirão Preto Medical School, University of São Paulo, Ribeirão Preto, Brazil (D.G.T., C.B.S., J.M.A., F.J.C.R.); Department of Pathology, Institute of Pathology, Heidelberg University Hospital, Heidelberg, Germany (P.S.); Department of Population Health Science and Policy, Department of Genetics and Genomic Sciences, Icahn School of Medicine at Mount Sinai, New York, NY (W.S., J.H.R.); Pathology West ICPMR Westmead, Westmead Hospital, the University of Sydney, Sydney, Australia (R.S.); University of Western Sydney at Westmead Hospital, Westmead, New South Wales, Australia (R.S.); Medical Oncology Service, Hospital Sant Pau, Barcelona, Spain (T.R.C.); H12O-CNIO Lung Cancer Clinical Research Unit (L.P.-A.), and Oncology Department, Hospital Universitario 12 de Octubre (L.P.-A.), Madrid, Spain; Women’s Cancer Program, Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, CA (S.O., J. Lester, B.Y.K.); the University of Texas School of Public Health, Houston (R.B.N.); Department of Oncology, Division of Gynecologic Oncology, Cumming School of Medicine, University of Calgary, Calgary, Alberta, Canada (G.N., P.G.); Department of Obstetrics, Gynecology and Reproductive Sciences, University of Pittsburgh School of Medicine (F.M., R.P.E.), Department of Epidemiology, University of Pittsburgh Graduate School of Public Health (F.M.), and Womens Cancer Research Program, Magee-Womens Research Institute and University of Pittsburgh Cancer Institute (F.M.), Pittsburgh, PA; Department of Gynecological Surgery and Gynecological Oncology of Adults and Adolescents, Pomeranian Medical University, Szczecin, Poland (J.M., A.C.-G.); Department of Health Research and Policy, Stanford University School of Medicine, Stanford, CA (V.M.); Department of Oncology, University of Cambridge, Strangeways Research Laboratory, Cambridge, UK (M.M., J.A., P.D.P.P.); Department of Pathology, Stanford University, Stanford, CA (T.A.L.); Department of Gynecologic Oncology, the Third Affiliated Hospital of Kunming Medical University (Yunnan Tumor Hospital), Kunming, China (Z.L.); Centre for Cancer Research, the Westmead Institute for Medical Research, the University of Sydney (C.J.K., J.H., P.R.H., B. Gao, A.deF.), and Department of Gynaecological Oncology, Westmead Hospital (C.J.K., J.H., A.deF.), Sydney, New South Wales, Australia; Division of Cancer Epidemiology, German Cancer Research Center (Deutsches Krebsforschungszentrum), Heidelberg, Germany (A.Y.J., U.E., J.C.-C.); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia (S.E.J., G.C.-T.); Department of Histopathology, Addenbrookes Hospital, Cambridge, UK (M.J.-L.); Virus, Lifestyle and Genes, Danish Cancer Society Research Center, Copenhagen, Denmark (A.J., S.K.K.); School of Women’s and Children’s Health, University of New South Wales, Sydney, Australia (M.P.I., S.J.R.); Tissue Bank of the National Center for Tumor Diseases (NCT) Heidelberg (E. Herpel), and Institute of Pathology, University of Heidelberg (E. Herpel), Heidelberg, Germany; Cancer Center, University of Hawaii, Honolulu, HI (B.Y.H.); the Crown Princess Mary Cancer Centre, Westmead Hospital, the University of Sydney, Sydney, Australia (P.R.H., B. Gao); Oncology Department, Hospital General de Albacete, Madrid, Spain (J.M.G.-B.); Department of Cancer Genomics and Genetics, Peter MacCallum Cancer Centre, East Melbourne, Victoria, Australia (S.F.); Pathology Department, Fundación Jiménez Díaz, Madrid, Spain (A.C.); Department of Health Research and Policy and Department of Biomedical Data Science, Stanford University School of Medicine, Stanford, CA (A.S.W.); Department of Obstetrics and Gynecology, Division of Gynecologic Oncology, Royal Alexandra Hospital, Edmonton, Alberta, Canada (H.S.); Tübingen University Hospital, Institute of Pathology, Tübingen, Germany (A.S.); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (K.B.M.); Gynaecological Cancer Research Centre, Department of Women’s Cancer, Institute for Women’s Health, University College London, London, UK (U.M.); Alberta Health Services-Cancer Care, Calgary, Alberta, Canada (J.M.K.); Department of Gynecology, Rigshospitalet, University of Copenhagen, Copenhagen, Denmark (S.K.K.); Department of Public Health Sciences, Medical University of South Carolina and Hollings Cancer Center, Charleston, SC (L.E.K.); Department of Pathology and Laboratory Medicine, University of British Columbia (D.G.H.), and Centre for Translational and Applied Genomics, British Columbia Cancer Agency (D.G.H.), Vancouver, British Columbia, Canada; Molecular Unit, Department of Pathology, Herlev Hospital, University of Copenhagen, Copenhagen, Denmark (E. Høgdall); Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, CA (M.T.G.); Genetic Pathology Evaluation Centre, Vancouver General Hospital and University of British Columbia, Vancouver, British Columbia, Canada (B. Gilks); Human Genetics Group, Spanish National Cancer Research Centre (Centro Nacional de Investigaciones Oncológicas) (M.J.G., J.B.), and Centre for Biomedical Network Research on Rare Diseases (Centro de Investigación Biomédica en Red de Enfermedades Raras), Instituto de Salud Carlos III (M.J.G., J.B.), Madrid, Spain; David Geffen School of Medicine, Department of Medicine, Division of Hematology and Oncology, University of California at Los Angeles (P.A.F.); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Erlangen, Germany (P.A.F.); Department of Histopathology, Queen’s Medical Centre, Nottingham University Hospitals NHS Trust, Nottingham, UK (S.D.); University Cancer Center Hamburg, University Medical Center Hamburg-Eppendorf, Hamburg, Germany (J.C.-C.); Cancer Genetics Laboratory, Research Division, Peter MacCallum Cancer Centre (I.G.C.), Sir Peter MacCallum Department of Oncology, University of Melbourne (I.G.C.), and Department of Pathology, University of Melbourne (I.G.C.), Melbourne, Victoria, Australia; Department of Oncology, University of Cambridge, Hutchison/MRC Research Centre (J.D.B.), Cancer Research UK Cambridge Institute, University of Cambridge, Li Ka Shing Centre (J.D.B.), and Cambridge Experimental Cancer Medicine Centre (J.D.B.), Cambridge, UK; Cancer Genomics Program, Research Department, Peter MacCallum Cancer Centre, East Melbourne, Victoria, Australia (D.D.B.); the Garvan Institute, Sydney, New South Wales, Australia (D.D.B., S.J.R.); and Department of Public Health and Primary Care, University of Cambridge, Strangeways Research Laboratory, Cambridge, UK (P.D.P.P.)
1,
Luis Paz-Ares
Luis Paz-Ares, MD
1Department of Medical Oncology (M.S.B., K.R.K.) and Department of Health Sciences Research (R.A.V., S.J.W., B.M.M., Z.L., E.L.G.), Mayo Clinic, Rochester, MN; Department of Pathology and Laboratory Medicine, University of Calgary, Calgary, Alberta, Canada (P.F.R., M.K.); Pathology Department, Catholic University of Health and Allied Sciences-Bugando, Mwanza, Tanzania (P.F.R.); Tübingen University Hospital, Department of Women’s Health, Tübingen, Germany (P.W., F.A.T., A.D.H., S.Y.B., S.K.); Sir Peter MacCallum Department of Oncology, the University of Melbourne, Parkville, Victoria, Australia (N.T., D.D.B.); International Hereditary Cancer Center, Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (A.T., O.O., J. Lubiński, J.G.); Department of Gynecology and Obstetrics, Ribeirão Preto Medical School, University of São Paulo, Ribeirão Preto, Brazil (D.G.T., C.B.S., J.M.A., F.J.C.R.); Department of Pathology, Institute of Pathology, Heidelberg University Hospital, Heidelberg, Germany (P.S.); Department of Population Health Science and Policy, Department of Genetics and Genomic Sciences, Icahn School of Medicine at Mount Sinai, New York, NY (W.S., J.H.R.); Pathology West ICPMR Westmead, Westmead Hospital, the University of Sydney, Sydney, Australia (R.S.); University of Western Sydney at Westmead Hospital, Westmead, New South Wales, Australia (R.S.); Medical Oncology Service, Hospital Sant Pau, Barcelona, Spain (T.R.C.); H12O-CNIO Lung Cancer Clinical Research Unit (L.P.-A.), and Oncology Department, Hospital Universitario 12 de Octubre (L.P.-A.), Madrid, Spain; Women’s Cancer Program, Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, CA (S.O., J. Lester, B.Y.K.); the University of Texas School of Public Health, Houston (R.B.N.); Department of Oncology, Division of Gynecologic Oncology, Cumming School of Medicine, University of Calgary, Calgary, Alberta, Canada (G.N., P.G.); Department of Obstetrics, Gynecology and Reproductive Sciences, University of Pittsburgh School of Medicine (F.M., R.P.E.), Department of Epidemiology, University of Pittsburgh Graduate School of Public Health (F.M.), and Womens Cancer Research Program, Magee-Womens Research Institute and University of Pittsburgh Cancer Institute (F.M.), Pittsburgh, PA; Department of Gynecological Surgery and Gynecological Oncology of Adults and Adolescents, Pomeranian Medical University, Szczecin, Poland (J.M., A.C.-G.); Department of Health Research and Policy, Stanford University School of Medicine, Stanford, CA (V.M.); Department of Oncology, University of Cambridge, Strangeways Research Laboratory, Cambridge, UK (M.M., J.A., P.D.P.P.); Department of Pathology, Stanford University, Stanford, CA (T.A.L.); Department of Gynecologic Oncology, the Third Affiliated Hospital of Kunming Medical University (Yunnan Tumor Hospital), Kunming, China (Z.L.); Centre for Cancer Research, the Westmead Institute for Medical Research, the University of Sydney (C.J.K., J.H., P.R.H., B. Gao, A.deF.), and Department of Gynaecological Oncology, Westmead Hospital (C.J.K., J.H., A.deF.), Sydney, New South Wales, Australia; Division of Cancer Epidemiology, German Cancer Research Center (Deutsches Krebsforschungszentrum), Heidelberg, Germany (A.Y.J., U.E., J.C.-C.); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia (S.E.J., G.C.-T.); Department of Histopathology, Addenbrookes Hospital, Cambridge, UK (M.J.-L.); Virus, Lifestyle and Genes, Danish Cancer Society Research Center, Copenhagen, Denmark (A.J., S.K.K.); School of Women’s and Children’s Health, University of New South Wales, Sydney, Australia (M.P.I., S.J.R.); Tissue Bank of the National Center for Tumor Diseases (NCT) Heidelberg (E. Herpel), and Institute of Pathology, University of Heidelberg (E. Herpel), Heidelberg, Germany; Cancer Center, University of Hawaii, Honolulu, HI (B.Y.H.); the Crown Princess Mary Cancer Centre, Westmead Hospital, the University of Sydney, Sydney, Australia (P.R.H., B. Gao); Oncology Department, Hospital General de Albacete, Madrid, Spain (J.M.G.-B.); Department of Cancer Genomics and Genetics, Peter MacCallum Cancer Centre, East Melbourne, Victoria, Australia (S.F.); Pathology Department, Fundación Jiménez Díaz, Madrid, Spain (A.C.); Department of Health Research and Policy and Department of Biomedical Data Science, Stanford University School of Medicine, Stanford, CA (A.S.W.); Department of Obstetrics and Gynecology, Division of Gynecologic Oncology, Royal Alexandra Hospital, Edmonton, Alberta, Canada (H.S.); Tübingen University Hospital, Institute of Pathology, Tübingen, Germany (A.S.); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (K.B.M.); Gynaecological Cancer Research Centre, Department of Women’s Cancer, Institute for Women’s Health, University College London, London, UK (U.M.); Alberta Health Services-Cancer Care, Calgary, Alberta, Canada (J.M.K.); Department of Gynecology, Rigshospitalet, University of Copenhagen, Copenhagen, Denmark (S.K.K.); Department of Public Health Sciences, Medical University of South Carolina and Hollings Cancer Center, Charleston, SC (L.E.K.); Department of Pathology and Laboratory Medicine, University of British Columbia (D.G.H.), and Centre for Translational and Applied Genomics, British Columbia Cancer Agency (D.G.H.), Vancouver, British Columbia, Canada; Molecular Unit, Department of Pathology, Herlev Hospital, University of Copenhagen, Copenhagen, Denmark (E. Høgdall); Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, CA (M.T.G.); Genetic Pathology Evaluation Centre, Vancouver General Hospital and University of British Columbia, Vancouver, British Columbia, Canada (B. Gilks); Human Genetics Group, Spanish National Cancer Research Centre (Centro Nacional de Investigaciones Oncológicas) (M.J.G., J.B.), and Centre for Biomedical Network Research on Rare Diseases (Centro de Investigación Biomédica en Red de Enfermedades Raras), Instituto de Salud Carlos III (M.J.G., J.B.), Madrid, Spain; David Geffen School of Medicine, Department of Medicine, Division of Hematology and Oncology, University of California at Los Angeles (P.A.F.); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Erlangen, Germany (P.A.F.); Department of Histopathology, Queen’s Medical Centre, Nottingham University Hospitals NHS Trust, Nottingham, UK (S.D.); University Cancer Center Hamburg, University Medical Center Hamburg-Eppendorf, Hamburg, Germany (J.C.-C.); Cancer Genetics Laboratory, Research Division, Peter MacCallum Cancer Centre (I.G.C.), Sir Peter MacCallum Department of Oncology, University of Melbourne (I.G.C.), and Department of Pathology, University of Melbourne (I.G.C.), Melbourne, Victoria, Australia; Department of Oncology, University of Cambridge, Hutchison/MRC Research Centre (J.D.B.), Cancer Research UK Cambridge Institute, University of Cambridge, Li Ka Shing Centre (J.D.B.), and Cambridge Experimental Cancer Medicine Centre (J.D.B.), Cambridge, UK; Cancer Genomics Program, Research Department, Peter MacCallum Cancer Centre, East Melbourne, Victoria, Australia (D.D.B.); the Garvan Institute, Sydney, New South Wales, Australia (D.D.B., S.J.R.); and Department of Public Health and Primary Care, University of Cambridge, Strangeways Research Laboratory, Cambridge, UK (P.D.P.P.)
1,
Oleg Oszurek
Oleg Oszurek, MD
1Department of Medical Oncology (M.S.B., K.R.K.) and Department of Health Sciences Research (R.A.V., S.J.W., B.M.M., Z.L., E.L.G.), Mayo Clinic, Rochester, MN; Department of Pathology and Laboratory Medicine, University of Calgary, Calgary, Alberta, Canada (P.F.R., M.K.); Pathology Department, Catholic University of Health and Allied Sciences-Bugando, Mwanza, Tanzania (P.F.R.); Tübingen University Hospital, Department of Women’s Health, Tübingen, Germany (P.W., F.A.T., A.D.H., S.Y.B., S.K.); Sir Peter MacCallum Department of Oncology, the University of Melbourne, Parkville, Victoria, Australia (N.T., D.D.B.); International Hereditary Cancer Center, Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (A.T., O.O., J. Lubiński, J.G.); Department of Gynecology and Obstetrics, Ribeirão Preto Medical School, University of São Paulo, Ribeirão Preto, Brazil (D.G.T., C.B.S., J.M.A., F.J.C.R.); Department of Pathology, Institute of Pathology, Heidelberg University Hospital, Heidelberg, Germany (P.S.); Department of Population Health Science and Policy, Department of Genetics and Genomic Sciences, Icahn School of Medicine at Mount Sinai, New York, NY (W.S., J.H.R.); Pathology West ICPMR Westmead, Westmead Hospital, the University of Sydney, Sydney, Australia (R.S.); University of Western Sydney at Westmead Hospital, Westmead, New South Wales, Australia (R.S.); Medical Oncology Service, Hospital Sant Pau, Barcelona, Spain (T.R.C.); H12O-CNIO Lung Cancer Clinical Research Unit (L.P.-A.), and Oncology Department, Hospital Universitario 12 de Octubre (L.P.-A.), Madrid, Spain; Women’s Cancer Program, Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, CA (S.O., J. Lester, B.Y.K.); the University of Texas School of Public Health, Houston (R.B.N.); Department of Oncology, Division of Gynecologic Oncology, Cumming School of Medicine, University of Calgary, Calgary, Alberta, Canada (G.N., P.G.); Department of Obstetrics, Gynecology and Reproductive Sciences, University of Pittsburgh School of Medicine (F.M., R.P.E.), Department of Epidemiology, University of Pittsburgh Graduate School of Public Health (F.M.), and Womens Cancer Research Program, Magee-Womens Research Institute and University of Pittsburgh Cancer Institute (F.M.), Pittsburgh, PA; Department of Gynecological Surgery and Gynecological Oncology of Adults and Adolescents, Pomeranian Medical University, Szczecin, Poland (J.M., A.C.-G.); Department of Health Research and Policy, Stanford University School of Medicine, Stanford, CA (V.M.); Department of Oncology, University of Cambridge, Strangeways Research Laboratory, Cambridge, UK (M.M., J.A., P.D.P.P.); Department of Pathology, Stanford University, Stanford, CA (T.A.L.); Department of Gynecologic Oncology, the Third Affiliated Hospital of Kunming Medical University (Yunnan Tumor Hospital), Kunming, China (Z.L.); Centre for Cancer Research, the Westmead Institute for Medical Research, the University of Sydney (C.J.K., J.H., P.R.H., B. Gao, A.deF.), and Department of Gynaecological Oncology, Westmead Hospital (C.J.K., J.H., A.deF.), Sydney, New South Wales, Australia; Division of Cancer Epidemiology, German Cancer Research Center (Deutsches Krebsforschungszentrum), Heidelberg, Germany (A.Y.J., U.E., J.C.-C.); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia (S.E.J., G.C.-T.); Department of Histopathology, Addenbrookes Hospital, Cambridge, UK (M.J.-L.); Virus, Lifestyle and Genes, Danish Cancer Society Research Center, Copenhagen, Denmark (A.J., S.K.K.); School of Women’s and Children’s Health, University of New South Wales, Sydney, Australia (M.P.I., S.J.R.); Tissue Bank of the National Center for Tumor Diseases (NCT) Heidelberg (E. Herpel), and Institute of Pathology, University of Heidelberg (E. Herpel), Heidelberg, Germany; Cancer Center, University of Hawaii, Honolulu, HI (B.Y.H.); the Crown Princess Mary Cancer Centre, Westmead Hospital, the University of Sydney, Sydney, Australia (P.R.H., B. Gao); Oncology Department, Hospital General de Albacete, Madrid, Spain (J.M.G.-B.); Department of Cancer Genomics and Genetics, Peter MacCallum Cancer Centre, East Melbourne, Victoria, Australia (S.F.); Pathology Department, Fundación Jiménez Díaz, Madrid, Spain (A.C.); Department of Health Research and Policy and Department of Biomedical Data Science, Stanford University School of Medicine, Stanford, CA (A.S.W.); Department of Obstetrics and Gynecology, Division of Gynecologic Oncology, Royal Alexandra Hospital, Edmonton, Alberta, Canada (H.S.); Tübingen University Hospital, Institute of Pathology, Tübingen, Germany (A.S.); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (K.B.M.); Gynaecological Cancer Research Centre, Department of Women’s Cancer, Institute for Women’s Health, University College London, London, UK (U.M.); Alberta Health Services-Cancer Care, Calgary, Alberta, Canada (J.M.K.); Department of Gynecology, Rigshospitalet, University of Copenhagen, Copenhagen, Denmark (S.K.K.); Department of Public Health Sciences, Medical University of South Carolina and Hollings Cancer Center, Charleston, SC (L.E.K.); Department of Pathology and Laboratory Medicine, University of British Columbia (D.G.H.), and Centre for Translational and Applied Genomics, British Columbia Cancer Agency (D.G.H.), Vancouver, British Columbia, Canada; Molecular Unit, Department of Pathology, Herlev Hospital, University of Copenhagen, Copenhagen, Denmark (E. Høgdall); Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, CA (M.T.G.); Genetic Pathology Evaluation Centre, Vancouver General Hospital and University of British Columbia, Vancouver, British Columbia, Canada (B. Gilks); Human Genetics Group, Spanish National Cancer Research Centre (Centro Nacional de Investigaciones Oncológicas) (M.J.G., J.B.), and Centre for Biomedical Network Research on Rare Diseases (Centro de Investigación Biomédica en Red de Enfermedades Raras), Instituto de Salud Carlos III (M.J.G., J.B.), Madrid, Spain; David Geffen School of Medicine, Department of Medicine, Division of Hematology and Oncology, University of California at Los Angeles (P.A.F.); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Erlangen, Germany (P.A.F.); Department of Histopathology, Queen’s Medical Centre, Nottingham University Hospitals NHS Trust, Nottingham, UK (S.D.); University Cancer Center Hamburg, University Medical Center Hamburg-Eppendorf, Hamburg, Germany (J.C.-C.); Cancer Genetics Laboratory, Research Division, Peter MacCallum Cancer Centre (I.G.C.), Sir Peter MacCallum Department of Oncology, University of Melbourne (I.G.C.), and Department of Pathology, University of Melbourne (I.G.C.), Melbourne, Victoria, Australia; Department of Oncology, University of Cambridge, Hutchison/MRC Research Centre (J.D.B.), Cancer Research UK Cambridge Institute, University of Cambridge, Li Ka Shing Centre (J.D.B.), and Cambridge Experimental Cancer Medicine Centre (J.D.B.), Cambridge, UK; Cancer Genomics Program, Research Department, Peter MacCallum Cancer Centre, East Melbourne, Victoria, Australia (D.D.B.); the Garvan Institute, Sydney, New South Wales, Australia (D.D.B., S.J.R.); and Department of Public Health and Primary Care, University of Cambridge, Strangeways Research Laboratory, Cambridge, UK (P.D.P.P.)
1,
Sandra Orsulic
Sandra Orsulic, PhD
1Department of Medical Oncology (M.S.B., K.R.K.) and Department of Health Sciences Research (R.A.V., S.J.W., B.M.M., Z.L., E.L.G.), Mayo Clinic, Rochester, MN; Department of Pathology and Laboratory Medicine, University of Calgary, Calgary, Alberta, Canada (P.F.R., M.K.); Pathology Department, Catholic University of Health and Allied Sciences-Bugando, Mwanza, Tanzania (P.F.R.); Tübingen University Hospital, Department of Women’s Health, Tübingen, Germany (P.W., F.A.T., A.D.H., S.Y.B., S.K.); Sir Peter MacCallum Department of Oncology, the University of Melbourne, Parkville, Victoria, Australia (N.T., D.D.B.); International Hereditary Cancer Center, Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (A.T., O.O., J. Lubiński, J.G.); Department of Gynecology and Obstetrics, Ribeirão Preto Medical School, University of São Paulo, Ribeirão Preto, Brazil (D.G.T., C.B.S., J.M.A., F.J.C.R.); Department of Pathology, Institute of Pathology, Heidelberg University Hospital, Heidelberg, Germany (P.S.); Department of Population Health Science and Policy, Department of Genetics and Genomic Sciences, Icahn School of Medicine at Mount Sinai, New York, NY (W.S., J.H.R.); Pathology West ICPMR Westmead, Westmead Hospital, the University of Sydney, Sydney, Australia (R.S.); University of Western Sydney at Westmead Hospital, Westmead, New South Wales, Australia (R.S.); Medical Oncology Service, Hospital Sant Pau, Barcelona, Spain (T.R.C.); H12O-CNIO Lung Cancer Clinical Research Unit (L.P.-A.), and Oncology Department, Hospital Universitario 12 de Octubre (L.P.-A.), Madrid, Spain; Women’s Cancer Program, Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, CA (S.O., J. Lester, B.Y.K.); the University of Texas School of Public Health, Houston (R.B.N.); Department of Oncology, Division of Gynecologic Oncology, Cumming School of Medicine, University of Calgary, Calgary, Alberta, Canada (G.N., P.G.); Department of Obstetrics, Gynecology and Reproductive Sciences, University of Pittsburgh School of Medicine (F.M., R.P.E.), Department of Epidemiology, University of Pittsburgh Graduate School of Public Health (F.M.), and Womens Cancer Research Program, Magee-Womens Research Institute and University of Pittsburgh Cancer Institute (F.M.), Pittsburgh, PA; Department of Gynecological Surgery and Gynecological Oncology of Adults and Adolescents, Pomeranian Medical University, Szczecin, Poland (J.M., A.C.-G.); Department of Health Research and Policy, Stanford University School of Medicine, Stanford, CA (V.M.); Department of Oncology, University of Cambridge, Strangeways Research Laboratory, Cambridge, UK (M.M., J.A., P.D.P.P.); Department of Pathology, Stanford University, Stanford, CA (T.A.L.); Department of Gynecologic Oncology, the Third Affiliated Hospital of Kunming Medical University (Yunnan Tumor Hospital), Kunming, China (Z.L.); Centre for Cancer Research, the Westmead Institute for Medical Research, the University of Sydney (C.J.K., J.H., P.R.H., B. Gao, A.deF.), and Department of Gynaecological Oncology, Westmead Hospital (C.J.K., J.H., A.deF.), Sydney, New South Wales, Australia; Division of Cancer Epidemiology, German Cancer Research Center (Deutsches Krebsforschungszentrum), Heidelberg, Germany (A.Y.J., U.E., J.C.-C.); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia (S.E.J., G.C.-T.); Department of Histopathology, Addenbrookes Hospital, Cambridge, UK (M.J.-L.); Virus, Lifestyle and Genes, Danish Cancer Society Research Center, Copenhagen, Denmark (A.J., S.K.K.); School of Women’s and Children’s Health, University of New South Wales, Sydney, Australia (M.P.I., S.J.R.); Tissue Bank of the National Center for Tumor Diseases (NCT) Heidelberg (E. Herpel), and Institute of Pathology, University of Heidelberg (E. Herpel), Heidelberg, Germany; Cancer Center, University of Hawaii, Honolulu, HI (B.Y.H.); the Crown Princess Mary Cancer Centre, Westmead Hospital, the University of Sydney, Sydney, Australia (P.R.H., B. Gao); Oncology Department, Hospital General de Albacete, Madrid, Spain (J.M.G.-B.); Department of Cancer Genomics and Genetics, Peter MacCallum Cancer Centre, East Melbourne, Victoria, Australia (S.F.); Pathology Department, Fundación Jiménez Díaz, Madrid, Spain (A.C.); Department of Health Research and Policy and Department of Biomedical Data Science, Stanford University School of Medicine, Stanford, CA (A.S.W.); Department of Obstetrics and Gynecology, Division of Gynecologic Oncology, Royal Alexandra Hospital, Edmonton, Alberta, Canada (H.S.); Tübingen University Hospital, Institute of Pathology, Tübingen, Germany (A.S.); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (K.B.M.); Gynaecological Cancer Research Centre, Department of Women’s Cancer, Institute for Women’s Health, University College London, London, UK (U.M.); Alberta Health Services-Cancer Care, Calgary, Alberta, Canada (J.M.K.); Department of Gynecology, Rigshospitalet, University of Copenhagen, Copenhagen, Denmark (S.K.K.); Department of Public Health Sciences, Medical University of South Carolina and Hollings Cancer Center, Charleston, SC (L.E.K.); Department of Pathology and Laboratory Medicine, University of British Columbia (D.G.H.), and Centre for Translational and Applied Genomics, British Columbia Cancer Agency (D.G.H.), Vancouver, British Columbia, Canada; Molecular Unit, Department of Pathology, Herlev Hospital, University of Copenhagen, Copenhagen, Denmark (E. Høgdall); Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, CA (M.T.G.); Genetic Pathology Evaluation Centre, Vancouver General Hospital and University of British Columbia, Vancouver, British Columbia, Canada (B. Gilks); Human Genetics Group, Spanish National Cancer Research Centre (Centro Nacional de Investigaciones Oncológicas) (M.J.G., J.B.), and Centre for Biomedical Network Research on Rare Diseases (Centro de Investigación Biomédica en Red de Enfermedades Raras), Instituto de Salud Carlos III (M.J.G., J.B.), Madrid, Spain; David Geffen School of Medicine, Department of Medicine, Division of Hematology and Oncology, University of California at Los Angeles (P.A.F.); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Erlangen, Germany (P.A.F.); Department of Histopathology, Queen’s Medical Centre, Nottingham University Hospitals NHS Trust, Nottingham, UK (S.D.); University Cancer Center Hamburg, University Medical Center Hamburg-Eppendorf, Hamburg, Germany (J.C.-C.); Cancer Genetics Laboratory, Research Division, Peter MacCallum Cancer Centre (I.G.C.), Sir Peter MacCallum Department of Oncology, University of Melbourne (I.G.C.), and Department of Pathology, University of Melbourne (I.G.C.), Melbourne, Victoria, Australia; Department of Oncology, University of Cambridge, Hutchison/MRC Research Centre (J.D.B.), Cancer Research UK Cambridge Institute, University of Cambridge, Li Ka Shing Centre (J.D.B.), and Cambridge Experimental Cancer Medicine Centre (J.D.B.), Cambridge, UK; Cancer Genomics Program, Research Department, Peter MacCallum Cancer Centre, East Melbourne, Victoria, Australia (D.D.B.); the Garvan Institute, Sydney, New South Wales, Australia (D.D.B., S.J.R.); and Department of Public Health and Primary Care, University of Cambridge, Strangeways Research Laboratory, Cambridge, UK (P.D.P.P.)
1,
Roberta B Ness
Roberta B Ness, MD, MPH
1Department of Medical Oncology (M.S.B., K.R.K.) and Department of Health Sciences Research (R.A.V., S.J.W., B.M.M., Z.L., E.L.G.), Mayo Clinic, Rochester, MN; Department of Pathology and Laboratory Medicine, University of Calgary, Calgary, Alberta, Canada (P.F.R., M.K.); Pathology Department, Catholic University of Health and Allied Sciences-Bugando, Mwanza, Tanzania (P.F.R.); Tübingen University Hospital, Department of Women’s Health, Tübingen, Germany (P.W., F.A.T., A.D.H., S.Y.B., S.K.); Sir Peter MacCallum Department of Oncology, the University of Melbourne, Parkville, Victoria, Australia (N.T., D.D.B.); International Hereditary Cancer Center, Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (A.T., O.O., J. Lubiński, J.G.); Department of Gynecology and Obstetrics, Ribeirão Preto Medical School, University of São Paulo, Ribeirão Preto, Brazil (D.G.T., C.B.S., J.M.A., F.J.C.R.); Department of Pathology, Institute of Pathology, Heidelberg University Hospital, Heidelberg, Germany (P.S.); Department of Population Health Science and Policy, Department of Genetics and Genomic Sciences, Icahn School of Medicine at Mount Sinai, New York, NY (W.S., J.H.R.); Pathology West ICPMR Westmead, Westmead Hospital, the University of Sydney, Sydney, Australia (R.S.); University of Western Sydney at Westmead Hospital, Westmead, New South Wales, Australia (R.S.); Medical Oncology Service, Hospital Sant Pau, Barcelona, Spain (T.R.C.); H12O-CNIO Lung Cancer Clinical Research Unit (L.P.-A.), and Oncology Department, Hospital Universitario 12 de Octubre (L.P.-A.), Madrid, Spain; Women’s Cancer Program, Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, CA (S.O., J. Lester, B.Y.K.); the University of Texas School of Public Health, Houston (R.B.N.); Department of Oncology, Division of Gynecologic Oncology, Cumming School of Medicine, University of Calgary, Calgary, Alberta, Canada (G.N., P.G.); Department of Obstetrics, Gynecology and Reproductive Sciences, University of Pittsburgh School of Medicine (F.M., R.P.E.), Department of Epidemiology, University of Pittsburgh Graduate School of Public Health (F.M.), and Womens Cancer Research Program, Magee-Womens Research Institute and University of Pittsburgh Cancer Institute (F.M.), Pittsburgh, PA; Department of Gynecological Surgery and Gynecological Oncology of Adults and Adolescents, Pomeranian Medical University, Szczecin, Poland (J.M., A.C.-G.); Department of Health Research and Policy, Stanford University School of Medicine, Stanford, CA (V.M.); Department of Oncology, University of Cambridge, Strangeways Research Laboratory, Cambridge, UK (M.M., J.A., P.D.P.P.); Department of Pathology, Stanford University, Stanford, CA (T.A.L.); Department of Gynecologic Oncology, the Third Affiliated Hospital of Kunming Medical University (Yunnan Tumor Hospital), Kunming, China (Z.L.); Centre for Cancer Research, the Westmead Institute for Medical Research, the University of Sydney (C.J.K., J.H., P.R.H., B. Gao, A.deF.), and Department of Gynaecological Oncology, Westmead Hospital (C.J.K., J.H., A.deF.), Sydney, New South Wales, Australia; Division of Cancer Epidemiology, German Cancer Research Center (Deutsches Krebsforschungszentrum), Heidelberg, Germany (A.Y.J., U.E., J.C.-C.); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia (S.E.J., G.C.-T.); Department of Histopathology, Addenbrookes Hospital, Cambridge, UK (M.J.-L.); Virus, Lifestyle and Genes, Danish Cancer Society Research Center, Copenhagen, Denmark (A.J., S.K.K.); School of Women’s and Children’s Health, University of New South Wales, Sydney, Australia (M.P.I., S.J.R.); Tissue Bank of the National Center for Tumor Diseases (NCT) Heidelberg (E. Herpel), and Institute of Pathology, University of Heidelberg (E. Herpel), Heidelberg, Germany; Cancer Center, University of Hawaii, Honolulu, HI (B.Y.H.); the Crown Princess Mary Cancer Centre, Westmead Hospital, the University of Sydney, Sydney, Australia (P.R.H., B. Gao); Oncology Department, Hospital General de Albacete, Madrid, Spain (J.M.G.-B.); Department of Cancer Genomics and Genetics, Peter MacCallum Cancer Centre, East Melbourne, Victoria, Australia (S.F.); Pathology Department, Fundación Jiménez Díaz, Madrid, Spain (A.C.); Department of Health Research and Policy and Department of Biomedical Data Science, Stanford University School of Medicine, Stanford, CA (A.S.W.); Department of Obstetrics and Gynecology, Division of Gynecologic Oncology, Royal Alexandra Hospital, Edmonton, Alberta, Canada (H.S.); Tübingen University Hospital, Institute of Pathology, Tübingen, Germany (A.S.); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (K.B.M.); Gynaecological Cancer Research Centre, Department of Women’s Cancer, Institute for Women’s Health, University College London, London, UK (U.M.); Alberta Health Services-Cancer Care, Calgary, Alberta, Canada (J.M.K.); Department of Gynecology, Rigshospitalet, University of Copenhagen, Copenhagen, Denmark (S.K.K.); Department of Public Health Sciences, Medical University of South Carolina and Hollings Cancer Center, Charleston, SC (L.E.K.); Department of Pathology and Laboratory Medicine, University of British Columbia (D.G.H.), and Centre for Translational and Applied Genomics, British Columbia Cancer Agency (D.G.H.), Vancouver, British Columbia, Canada; Molecular Unit, Department of Pathology, Herlev Hospital, University of Copenhagen, Copenhagen, Denmark (E. Høgdall); Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, CA (M.T.G.); Genetic Pathology Evaluation Centre, Vancouver General Hospital and University of British Columbia, Vancouver, British Columbia, Canada (B. Gilks); Human Genetics Group, Spanish National Cancer Research Centre (Centro Nacional de Investigaciones Oncológicas) (M.J.G., J.B.), and Centre for Biomedical Network Research on Rare Diseases (Centro de Investigación Biomédica en Red de Enfermedades Raras), Instituto de Salud Carlos III (M.J.G., J.B.), Madrid, Spain; David Geffen School of Medicine, Department of Medicine, Division of Hematology and Oncology, University of California at Los Angeles (P.A.F.); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Erlangen, Germany (P.A.F.); Department of Histopathology, Queen’s Medical Centre, Nottingham University Hospitals NHS Trust, Nottingham, UK (S.D.); University Cancer Center Hamburg, University Medical Center Hamburg-Eppendorf, Hamburg, Germany (J.C.-C.); Cancer Genetics Laboratory, Research Division, Peter MacCallum Cancer Centre (I.G.C.), Sir Peter MacCallum Department of Oncology, University of Melbourne (I.G.C.), and Department of Pathology, University of Melbourne (I.G.C.), Melbourne, Victoria, Australia; Department of Oncology, University of Cambridge, Hutchison/MRC Research Centre (J.D.B.), Cancer Research UK Cambridge Institute, University of Cambridge, Li Ka Shing Centre (J.D.B.), and Cambridge Experimental Cancer Medicine Centre (J.D.B.), Cambridge, UK; Cancer Genomics Program, Research Department, Peter MacCallum Cancer Centre, East Melbourne, Victoria, Australia (D.D.B.); the Garvan Institute, Sydney, New South Wales, Australia (D.D.B., S.J.R.); and Department of Public Health and Primary Care, University of Cambridge, Strangeways Research Laboratory, Cambridge, UK (P.D.P.P.)
1,
Gregg Nelson
Gregg Nelson, MD, PhD
1Department of Medical Oncology (M.S.B., K.R.K.) and Department of Health Sciences Research (R.A.V., S.J.W., B.M.M., Z.L., E.L.G.), Mayo Clinic, Rochester, MN; Department of Pathology and Laboratory Medicine, University of Calgary, Calgary, Alberta, Canada (P.F.R., M.K.); Pathology Department, Catholic University of Health and Allied Sciences-Bugando, Mwanza, Tanzania (P.F.R.); Tübingen University Hospital, Department of Women’s Health, Tübingen, Germany (P.W., F.A.T., A.D.H., S.Y.B., S.K.); Sir Peter MacCallum Department of Oncology, the University of Melbourne, Parkville, Victoria, Australia (N.T., D.D.B.); International Hereditary Cancer Center, Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (A.T., O.O., J. Lubiński, J.G.); Department of Gynecology and Obstetrics, Ribeirão Preto Medical School, University of São Paulo, Ribeirão Preto, Brazil (D.G.T., C.B.S., J.M.A., F.J.C.R.); Department of Pathology, Institute of Pathology, Heidelberg University Hospital, Heidelberg, Germany (P.S.); Department of Population Health Science and Policy, Department of Genetics and Genomic Sciences, Icahn School of Medicine at Mount Sinai, New York, NY (W.S., J.H.R.); Pathology West ICPMR Westmead, Westmead Hospital, the University of Sydney, Sydney, Australia (R.S.); University of Western Sydney at Westmead Hospital, Westmead, New South Wales, Australia (R.S.); Medical Oncology Service, Hospital Sant Pau, Barcelona, Spain (T.R.C.); H12O-CNIO Lung Cancer Clinical Research Unit (L.P.-A.), and Oncology Department, Hospital Universitario 12 de Octubre (L.P.-A.), Madrid, Spain; Women’s Cancer Program, Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, CA (S.O., J. Lester, B.Y.K.); the University of Texas School of Public Health, Houston (R.B.N.); Department of Oncology, Division of Gynecologic Oncology, Cumming School of Medicine, University of Calgary, Calgary, Alberta, Canada (G.N., P.G.); Department of Obstetrics, Gynecology and Reproductive Sciences, University of Pittsburgh School of Medicine (F.M., R.P.E.), Department of Epidemiology, University of Pittsburgh Graduate School of Public Health (F.M.), and Womens Cancer Research Program, Magee-Womens Research Institute and University of Pittsburgh Cancer Institute (F.M.), Pittsburgh, PA; Department of Gynecological Surgery and Gynecological Oncology of Adults and Adolescents, Pomeranian Medical University, Szczecin, Poland (J.M., A.C.-G.); Department of Health Research and Policy, Stanford University School of Medicine, Stanford, CA (V.M.); Department of Oncology, University of Cambridge, Strangeways Research Laboratory, Cambridge, UK (M.M., J.A., P.D.P.P.); Department of Pathology, Stanford University, Stanford, CA (T.A.L.); Department of Gynecologic Oncology, the Third Affiliated Hospital of Kunming Medical University (Yunnan Tumor Hospital), Kunming, China (Z.L.); Centre for Cancer Research, the Westmead Institute for Medical Research, the University of Sydney (C.J.K., J.H., P.R.H., B. Gao, A.deF.), and Department of Gynaecological Oncology, Westmead Hospital (C.J.K., J.H., A.deF.), Sydney, New South Wales, Australia; Division of Cancer Epidemiology, German Cancer Research Center (Deutsches Krebsforschungszentrum), Heidelberg, Germany (A.Y.J., U.E., J.C.-C.); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia (S.E.J., G.C.-T.); Department of Histopathology, Addenbrookes Hospital, Cambridge, UK (M.J.-L.); Virus, Lifestyle and Genes, Danish Cancer Society Research Center, Copenhagen, Denmark (A.J., S.K.K.); School of Women’s and Children’s Health, University of New South Wales, Sydney, Australia (M.P.I., S.J.R.); Tissue Bank of the National Center for Tumor Diseases (NCT) Heidelberg (E. Herpel), and Institute of Pathology, University of Heidelberg (E. Herpel), Heidelberg, Germany; Cancer Center, University of Hawaii, Honolulu, HI (B.Y.H.); the Crown Princess Mary Cancer Centre, Westmead Hospital, the University of Sydney, Sydney, Australia (P.R.H., B. Gao); Oncology Department, Hospital General de Albacete, Madrid, Spain (J.M.G.-B.); Department of Cancer Genomics and Genetics, Peter MacCallum Cancer Centre, East Melbourne, Victoria, Australia (S.F.); Pathology Department, Fundación Jiménez Díaz, Madrid, Spain (A.C.); Department of Health Research and Policy and Department of Biomedical Data Science, Stanford University School of Medicine, Stanford, CA (A.S.W.); Department of Obstetrics and Gynecology, Division of Gynecologic Oncology, Royal Alexandra Hospital, Edmonton, Alberta, Canada (H.S.); Tübingen University Hospital, Institute of Pathology, Tübingen, Germany (A.S.); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (K.B.M.); Gynaecological Cancer Research Centre, Department of Women’s Cancer, Institute for Women’s Health, University College London, London, UK (U.M.); Alberta Health Services-Cancer Care, Calgary, Alberta, Canada (J.M.K.); Department of Gynecology, Rigshospitalet, University of Copenhagen, Copenhagen, Denmark (S.K.K.); Department of Public Health Sciences, Medical University of South Carolina and Hollings Cancer Center, Charleston, SC (L.E.K.); Department of Pathology and Laboratory Medicine, University of British Columbia (D.G.H.), and Centre for Translational and Applied Genomics, British Columbia Cancer Agency (D.G.H.), Vancouver, British Columbia, Canada; Molecular Unit, Department of Pathology, Herlev Hospital, University of Copenhagen, Copenhagen, Denmark (E. Høgdall); Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, CA (M.T.G.); Genetic Pathology Evaluation Centre, Vancouver General Hospital and University of British Columbia, Vancouver, British Columbia, Canada (B. Gilks); Human Genetics Group, Spanish National Cancer Research Centre (Centro Nacional de Investigaciones Oncológicas) (M.J.G., J.B.), and Centre for Biomedical Network Research on Rare Diseases (Centro de Investigación Biomédica en Red de Enfermedades Raras), Instituto de Salud Carlos III (M.J.G., J.B.), Madrid, Spain; David Geffen School of Medicine, Department of Medicine, Division of Hematology and Oncology, University of California at Los Angeles (P.A.F.); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Erlangen, Germany (P.A.F.); Department of Histopathology, Queen’s Medical Centre, Nottingham University Hospitals NHS Trust, Nottingham, UK (S.D.); University Cancer Center Hamburg, University Medical Center Hamburg-Eppendorf, Hamburg, Germany (J.C.-C.); Cancer Genetics Laboratory, Research Division, Peter MacCallum Cancer Centre (I.G.C.), Sir Peter MacCallum Department of Oncology, University of Melbourne (I.G.C.), and Department of Pathology, University of Melbourne (I.G.C.), Melbourne, Victoria, Australia; Department of Oncology, University of Cambridge, Hutchison/MRC Research Centre (J.D.B.), Cancer Research UK Cambridge Institute, University of Cambridge, Li Ka Shing Centre (J.D.B.), and Cambridge Experimental Cancer Medicine Centre (J.D.B.), Cambridge, UK; Cancer Genomics Program, Research Department, Peter MacCallum Cancer Centre, East Melbourne, Victoria, Australia (D.D.B.); the Garvan Institute, Sydney, New South Wales, Australia (D.D.B., S.J.R.); and Department of Public Health and Primary Care, University of Cambridge, Strangeways Research Laboratory, Cambridge, UK (P.D.P.P.)
1,
Francesmary Modugno
Francesmary Modugno, PhD
1Department of Medical Oncology (M.S.B., K.R.K.) and Department of Health Sciences Research (R.A.V., S.J.W., B.M.M., Z.L., E.L.G.), Mayo Clinic, Rochester, MN; Department of Pathology and Laboratory Medicine, University of Calgary, Calgary, Alberta, Canada (P.F.R., M.K.); Pathology Department, Catholic University of Health and Allied Sciences-Bugando, Mwanza, Tanzania (P.F.R.); Tübingen University Hospital, Department of Women’s Health, Tübingen, Germany (P.W., F.A.T., A.D.H., S.Y.B., S.K.); Sir Peter MacCallum Department of Oncology, the University of Melbourne, Parkville, Victoria, Australia (N.T., D.D.B.); International Hereditary Cancer Center, Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (A.T., O.O., J. Lubiński, J.G.); Department of Gynecology and Obstetrics, Ribeirão Preto Medical School, University of São Paulo, Ribeirão Preto, Brazil (D.G.T., C.B.S., J.M.A., F.J.C.R.); Department of Pathology, Institute of Pathology, Heidelberg University Hospital, Heidelberg, Germany (P.S.); Department of Population Health Science and Policy, Department of Genetics and Genomic Sciences, Icahn School of Medicine at Mount Sinai, New York, NY (W.S., J.H.R.); Pathology West ICPMR Westmead, Westmead Hospital, the University of Sydney, Sydney, Australia (R.S.); University of Western Sydney at Westmead Hospital, Westmead, New South Wales, Australia (R.S.); Medical Oncology Service, Hospital Sant Pau, Barcelona, Spain (T.R.C.); H12O-CNIO Lung Cancer Clinical Research Unit (L.P.-A.), and Oncology Department, Hospital Universitario 12 de Octubre (L.P.-A.), Madrid, Spain; Women’s Cancer Program, Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, CA (S.O., J. Lester, B.Y.K.); the University of Texas School of Public Health, Houston (R.B.N.); Department of Oncology, Division of Gynecologic Oncology, Cumming School of Medicine, University of Calgary, Calgary, Alberta, Canada (G.N., P.G.); Department of Obstetrics, Gynecology and Reproductive Sciences, University of Pittsburgh School of Medicine (F.M., R.P.E.), Department of Epidemiology, University of Pittsburgh Graduate School of Public Health (F.M.), and Womens Cancer Research Program, Magee-Womens Research Institute and University of Pittsburgh Cancer Institute (F.M.), Pittsburgh, PA; Department of Gynecological Surgery and Gynecological Oncology of Adults and Adolescents, Pomeranian Medical University, Szczecin, Poland (J.M., A.C.-G.); Department of Health Research and Policy, Stanford University School of Medicine, Stanford, CA (V.M.); Department of Oncology, University of Cambridge, Strangeways Research Laboratory, Cambridge, UK (M.M., J.A., P.D.P.P.); Department of Pathology, Stanford University, Stanford, CA (T.A.L.); Department of Gynecologic Oncology, the Third Affiliated Hospital of Kunming Medical University (Yunnan Tumor Hospital), Kunming, China (Z.L.); Centre for Cancer Research, the Westmead Institute for Medical Research, the University of Sydney (C.J.K., J.H., P.R.H., B. Gao, A.deF.), and Department of Gynaecological Oncology, Westmead Hospital (C.J.K., J.H., A.deF.), Sydney, New South Wales, Australia; Division of Cancer Epidemiology, German Cancer Research Center (Deutsches Krebsforschungszentrum), Heidelberg, Germany (A.Y.J., U.E., J.C.-C.); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia (S.E.J., G.C.-T.); Department of Histopathology, Addenbrookes Hospital, Cambridge, UK (M.J.-L.); Virus, Lifestyle and Genes, Danish Cancer Society Research Center, Copenhagen, Denmark (A.J., S.K.K.); School of Women’s and Children’s Health, University of New South Wales, Sydney, Australia (M.P.I., S.J.R.); Tissue Bank of the National Center for Tumor Diseases (NCT) Heidelberg (E. Herpel), and Institute of Pathology, University of Heidelberg (E. Herpel), Heidelberg, Germany; Cancer Center, University of Hawaii, Honolulu, HI (B.Y.H.); the Crown Princess Mary Cancer Centre, Westmead Hospital, the University of Sydney, Sydney, Australia (P.R.H., B. Gao); Oncology Department, Hospital General de Albacete, Madrid, Spain (J.M.G.-B.); Department of Cancer Genomics and Genetics, Peter MacCallum Cancer Centre, East Melbourne, Victoria, Australia (S.F.); Pathology Department, Fundación Jiménez Díaz, Madrid, Spain (A.C.); Department of Health Research and Policy and Department of Biomedical Data Science, Stanford University School of Medicine, Stanford, CA (A.S.W.); Department of Obstetrics and Gynecology, Division of Gynecologic Oncology, Royal Alexandra Hospital, Edmonton, Alberta, Canada (H.S.); Tübingen University Hospital, Institute of Pathology, Tübingen, Germany (A.S.); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (K.B.M.); Gynaecological Cancer Research Centre, Department of Women’s Cancer, Institute for Women’s Health, University College London, London, UK (U.M.); Alberta Health Services-Cancer Care, Calgary, Alberta, Canada (J.M.K.); Department of Gynecology, Rigshospitalet, University of Copenhagen, Copenhagen, Denmark (S.K.K.); Department of Public Health Sciences, Medical University of South Carolina and Hollings Cancer Center, Charleston, SC (L.E.K.); Department of Pathology and Laboratory Medicine, University of British Columbia (D.G.H.), and Centre for Translational and Applied Genomics, British Columbia Cancer Agency (D.G.H.), Vancouver, British Columbia, Canada; Molecular Unit, Department of Pathology, Herlev Hospital, University of Copenhagen, Copenhagen, Denmark (E. Høgdall); Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, CA (M.T.G.); Genetic Pathology Evaluation Centre, Vancouver General Hospital and University of British Columbia, Vancouver, British Columbia, Canada (B. Gilks); Human Genetics Group, Spanish National Cancer Research Centre (Centro Nacional de Investigaciones Oncológicas) (M.J.G., J.B.), and Centre for Biomedical Network Research on Rare Diseases (Centro de Investigación Biomédica en Red de Enfermedades Raras), Instituto de Salud Carlos III (M.J.G., J.B.), Madrid, Spain; David Geffen School of Medicine, Department of Medicine, Division of Hematology and Oncology, University of California at Los Angeles (P.A.F.); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Erlangen, Germany (P.A.F.); Department of Histopathology, Queen’s Medical Centre, Nottingham University Hospitals NHS Trust, Nottingham, UK (S.D.); University Cancer Center Hamburg, University Medical Center Hamburg-Eppendorf, Hamburg, Germany (J.C.-C.); Cancer Genetics Laboratory, Research Division, Peter MacCallum Cancer Centre (I.G.C.), Sir Peter MacCallum Department of Oncology, University of Melbourne (I.G.C.), and Department of Pathology, University of Melbourne (I.G.C.), Melbourne, Victoria, Australia; Department of Oncology, University of Cambridge, Hutchison/MRC Research Centre (J.D.B.), Cancer Research UK Cambridge Institute, University of Cambridge, Li Ka Shing Centre (J.D.B.), and Cambridge Experimental Cancer Medicine Centre (J.D.B.), Cambridge, UK; Cancer Genomics Program, Research Department, Peter MacCallum Cancer Centre, East Melbourne, Victoria, Australia (D.D.B.); the Garvan Institute, Sydney, New South Wales, Australia (D.D.B., S.J.R.); and Department of Public Health and Primary Care, University of Cambridge, Strangeways Research Laboratory, Cambridge, UK (P.D.P.P.)
1,
Janusz Menkiszak
Janusz Menkiszak, MD, PhD
1Department of Medical Oncology (M.S.B., K.R.K.) and Department of Health Sciences Research (R.A.V., S.J.W., B.M.M., Z.L., E.L.G.), Mayo Clinic, Rochester, MN; Department of Pathology and Laboratory Medicine, University of Calgary, Calgary, Alberta, Canada (P.F.R., M.K.); Pathology Department, Catholic University of Health and Allied Sciences-Bugando, Mwanza, Tanzania (P.F.R.); Tübingen University Hospital, Department of Women’s Health, Tübingen, Germany (P.W., F.A.T., A.D.H., S.Y.B., S.K.); Sir Peter MacCallum Department of Oncology, the University of Melbourne, Parkville, Victoria, Australia (N.T., D.D.B.); International Hereditary Cancer Center, Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (A.T., O.O., J. Lubiński, J.G.); Department of Gynecology and Obstetrics, Ribeirão Preto Medical School, University of São Paulo, Ribeirão Preto, Brazil (D.G.T., C.B.S., J.M.A., F.J.C.R.); Department of Pathology, Institute of Pathology, Heidelberg University Hospital, Heidelberg, Germany (P.S.); Department of Population Health Science and Policy, Department of Genetics and Genomic Sciences, Icahn School of Medicine at Mount Sinai, New York, NY (W.S., J.H.R.); Pathology West ICPMR Westmead, Westmead Hospital, the University of Sydney, Sydney, Australia (R.S.); University of Western Sydney at Westmead Hospital, Westmead, New South Wales, Australia (R.S.); Medical Oncology Service, Hospital Sant Pau, Barcelona, Spain (T.R.C.); H12O-CNIO Lung Cancer Clinical Research Unit (L.P.-A.), and Oncology Department, Hospital Universitario 12 de Octubre (L.P.-A.), Madrid, Spain; Women’s Cancer Program, Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, CA (S.O., J. Lester, B.Y.K.); the University of Texas School of Public Health, Houston (R.B.N.); Department of Oncology, Division of Gynecologic Oncology, Cumming School of Medicine, University of Calgary, Calgary, Alberta, Canada (G.N., P.G.); Department of Obstetrics, Gynecology and Reproductive Sciences, University of Pittsburgh School of Medicine (F.M., R.P.E.), Department of Epidemiology, University of Pittsburgh Graduate School of Public Health (F.M.), and Womens Cancer Research Program, Magee-Womens Research Institute and University of Pittsburgh Cancer Institute (F.M.), Pittsburgh, PA; Department of Gynecological Surgery and Gynecological Oncology of Adults and Adolescents, Pomeranian Medical University, Szczecin, Poland (J.M., A.C.-G.); Department of Health Research and Policy, Stanford University School of Medicine, Stanford, CA (V.M.); Department of Oncology, University of Cambridge, Strangeways Research Laboratory, Cambridge, UK (M.M., J.A., P.D.P.P.); Department of Pathology, Stanford University, Stanford, CA (T.A.L.); Department of Gynecologic Oncology, the Third Affiliated Hospital of Kunming Medical University (Yunnan Tumor Hospital), Kunming, China (Z.L.); Centre for Cancer Research, the Westmead Institute for Medical Research, the University of Sydney (C.J.K., J.H., P.R.H., B. Gao, A.deF.), and Department of Gynaecological Oncology, Westmead Hospital (C.J.K., J.H., A.deF.), Sydney, New South Wales, Australia; Division of Cancer Epidemiology, German Cancer Research Center (Deutsches Krebsforschungszentrum), Heidelberg, Germany (A.Y.J., U.E., J.C.-C.); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia (S.E.J., G.C.-T.); Department of Histopathology, Addenbrookes Hospital, Cambridge, UK (M.J.-L.); Virus, Lifestyle and Genes, Danish Cancer Society Research Center, Copenhagen, Denmark (A.J., S.K.K.); School of Women’s and Children’s Health, University of New South Wales, Sydney, Australia (M.P.I., S.J.R.); Tissue Bank of the National Center for Tumor Diseases (NCT) Heidelberg (E. Herpel), and Institute of Pathology, University of Heidelberg (E. Herpel), Heidelberg, Germany; Cancer Center, University of Hawaii, Honolulu, HI (B.Y.H.); the Crown Princess Mary Cancer Centre, Westmead Hospital, the University of Sydney, Sydney, Australia (P.R.H., B. Gao); Oncology Department, Hospital General de Albacete, Madrid, Spain (J.M.G.-B.); Department of Cancer Genomics and Genetics, Peter MacCallum Cancer Centre, East Melbourne, Victoria, Australia (S.F.); Pathology Department, Fundación Jiménez Díaz, Madrid, Spain (A.C.); Department of Health Research and Policy and Department of Biomedical Data Science, Stanford University School of Medicine, Stanford, CA (A.S.W.); Department of Obstetrics and Gynecology, Division of Gynecologic Oncology, Royal Alexandra Hospital, Edmonton, Alberta, Canada (H.S.); Tübingen University Hospital, Institute of Pathology, Tübingen, Germany (A.S.); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (K.B.M.); Gynaecological Cancer Research Centre, Department of Women’s Cancer, Institute for Women’s Health, University College London, London, UK (U.M.); Alberta Health Services-Cancer Care, Calgary, Alberta, Canada (J.M.K.); Department of Gynecology, Rigshospitalet, University of Copenhagen, Copenhagen, Denmark (S.K.K.); Department of Public Health Sciences, Medical University of South Carolina and Hollings Cancer Center, Charleston, SC (L.E.K.); Department of Pathology and Laboratory Medicine, University of British Columbia (D.G.H.), and Centre for Translational and Applied Genomics, British Columbia Cancer Agency (D.G.H.), Vancouver, British Columbia, Canada; Molecular Unit, Department of Pathology, Herlev Hospital, University of Copenhagen, Copenhagen, Denmark (E. Høgdall); Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, CA (M.T.G.); Genetic Pathology Evaluation Centre, Vancouver General Hospital and University of British Columbia, Vancouver, British Columbia, Canada (B. Gilks); Human Genetics Group, Spanish National Cancer Research Centre (Centro Nacional de Investigaciones Oncológicas) (M.J.G., J.B.), and Centre for Biomedical Network Research on Rare Diseases (Centro de Investigación Biomédica en Red de Enfermedades Raras), Instituto de Salud Carlos III (M.J.G., J.B.), Madrid, Spain; David Geffen School of Medicine, Department of Medicine, Division of Hematology and Oncology, University of California at Los Angeles (P.A.F.); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Erlangen, Germany (P.A.F.); Department of Histopathology, Queen’s Medical Centre, Nottingham University Hospitals NHS Trust, Nottingham, UK (S.D.); University Cancer Center Hamburg, University Medical Center Hamburg-Eppendorf, Hamburg, Germany (J.C.-C.); Cancer Genetics Laboratory, Research Division, Peter MacCallum Cancer Centre (I.G.C.), Sir Peter MacCallum Department of Oncology, University of Melbourne (I.G.C.), and Department of Pathology, University of Melbourne (I.G.C.), Melbourne, Victoria, Australia; Department of Oncology, University of Cambridge, Hutchison/MRC Research Centre (J.D.B.), Cancer Research UK Cambridge Institute, University of Cambridge, Li Ka Shing Centre (J.D.B.), and Cambridge Experimental Cancer Medicine Centre (J.D.B.), Cambridge, UK; Cancer Genomics Program, Research Department, Peter MacCallum Cancer Centre, East Melbourne, Victoria, Australia (D.D.B.); the Garvan Institute, Sydney, New South Wales, Australia (D.D.B., S.J.R.); and Department of Public Health and Primary Care, University of Cambridge, Strangeways Research Laboratory, Cambridge, UK (P.D.P.P.)
1,
Valerie McGuire
Valerie McGuire, PhD
1Department of Medical Oncology (M.S.B., K.R.K.) and Department of Health Sciences Research (R.A.V., S.J.W., B.M.M., Z.L., E.L.G.), Mayo Clinic, Rochester, MN; Department of Pathology and Laboratory Medicine, University of Calgary, Calgary, Alberta, Canada (P.F.R., M.K.); Pathology Department, Catholic University of Health and Allied Sciences-Bugando, Mwanza, Tanzania (P.F.R.); Tübingen University Hospital, Department of Women’s Health, Tübingen, Germany (P.W., F.A.T., A.D.H., S.Y.B., S.K.); Sir Peter MacCallum Department of Oncology, the University of Melbourne, Parkville, Victoria, Australia (N.T., D.D.B.); International Hereditary Cancer Center, Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (A.T., O.O., J. Lubiński, J.G.); Department of Gynecology and Obstetrics, Ribeirão Preto Medical School, University of São Paulo, Ribeirão Preto, Brazil (D.G.T., C.B.S., J.M.A., F.J.C.R.); Department of Pathology, Institute of Pathology, Heidelberg University Hospital, Heidelberg, Germany (P.S.); Department of Population Health Science and Policy, Department of Genetics and Genomic Sciences, Icahn School of Medicine at Mount Sinai, New York, NY (W.S., J.H.R.); Pathology West ICPMR Westmead, Westmead Hospital, the University of Sydney, Sydney, Australia (R.S.); University of Western Sydney at Westmead Hospital, Westmead, New South Wales, Australia (R.S.); Medical Oncology Service, Hospital Sant Pau, Barcelona, Spain (T.R.C.); H12O-CNIO Lung Cancer Clinical Research Unit (L.P.-A.), and Oncology Department, Hospital Universitario 12 de Octubre (L.P.-A.), Madrid, Spain; Women’s Cancer Program, Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, CA (S.O., J. Lester, B.Y.K.); the University of Texas School of Public Health, Houston (R.B.N.); Department of Oncology, Division of Gynecologic Oncology, Cumming School of Medicine, University of Calgary, Calgary, Alberta, Canada (G.N., P.G.); Department of Obstetrics, Gynecology and Reproductive Sciences, University of Pittsburgh School of Medicine (F.M., R.P.E.), Department of Epidemiology, University of Pittsburgh Graduate School of Public Health (F.M.), and Womens Cancer Research Program, Magee-Womens Research Institute and University of Pittsburgh Cancer Institute (F.M.), Pittsburgh, PA; Department of Gynecological Surgery and Gynecological Oncology of Adults and Adolescents, Pomeranian Medical University, Szczecin, Poland (J.M., A.C.-G.); Department of Health Research and Policy, Stanford University School of Medicine, Stanford, CA (V.M.); Department of Oncology, University of Cambridge, Strangeways Research Laboratory, Cambridge, UK (M.M., J.A., P.D.P.P.); Department of Pathology, Stanford University, Stanford, CA (T.A.L.); Department of Gynecologic Oncology, the Third Affiliated Hospital of Kunming Medical University (Yunnan Tumor Hospital), Kunming, China (Z.L.); Centre for Cancer Research, the Westmead Institute for Medical Research, the University of Sydney (C.J.K., J.H., P.R.H., B. Gao, A.deF.), and Department of Gynaecological Oncology, Westmead Hospital (C.J.K., J.H., A.deF.), Sydney, New South Wales, Australia; Division of Cancer Epidemiology, German Cancer Research Center (Deutsches Krebsforschungszentrum), Heidelberg, Germany (A.Y.J., U.E., J.C.-C.); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia (S.E.J., G.C.-T.); Department of Histopathology, Addenbrookes Hospital, Cambridge, UK (M.J.-L.); Virus, Lifestyle and Genes, Danish Cancer Society Research Center, Copenhagen, Denmark (A.J., S.K.K.); School of Women’s and Children’s Health, University of New South Wales, Sydney, Australia (M.P.I., S.J.R.); Tissue Bank of the National Center for Tumor Diseases (NCT) Heidelberg (E. Herpel), and Institute of Pathology, University of Heidelberg (E. Herpel), Heidelberg, Germany; Cancer Center, University of Hawaii, Honolulu, HI (B.Y.H.); the Crown Princess Mary Cancer Centre, Westmead Hospital, the University of Sydney, Sydney, Australia (P.R.H., B. Gao); Oncology Department, Hospital General de Albacete, Madrid, Spain (J.M.G.-B.); Department of Cancer Genomics and Genetics, Peter MacCallum Cancer Centre, East Melbourne, Victoria, Australia (S.F.); Pathology Department, Fundación Jiménez Díaz, Madrid, Spain (A.C.); Department of Health Research and Policy and Department of Biomedical Data Science, Stanford University School of Medicine, Stanford, CA (A.S.W.); Department of Obstetrics and Gynecology, Division of Gynecologic Oncology, Royal Alexandra Hospital, Edmonton, Alberta, Canada (H.S.); Tübingen University Hospital, Institute of Pathology, Tübingen, Germany (A.S.); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (K.B.M.); Gynaecological Cancer Research Centre, Department of Women’s Cancer, Institute for Women’s Health, University College London, London, UK (U.M.); Alberta Health Services-Cancer Care, Calgary, Alberta, Canada (J.M.K.); Department of Gynecology, Rigshospitalet, University of Copenhagen, Copenhagen, Denmark (S.K.K.); Department of Public Health Sciences, Medical University of South Carolina and Hollings Cancer Center, Charleston, SC (L.E.K.); Department of Pathology and Laboratory Medicine, University of British Columbia (D.G.H.), and Centre for Translational and Applied Genomics, British Columbia Cancer Agency (D.G.H.), Vancouver, British Columbia, Canada; Molecular Unit, Department of Pathology, Herlev Hospital, University of Copenhagen, Copenhagen, Denmark (E. Høgdall); Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, CA (M.T.G.); Genetic Pathology Evaluation Centre, Vancouver General Hospital and University of British Columbia, Vancouver, British Columbia, Canada (B. Gilks); Human Genetics Group, Spanish National Cancer Research Centre (Centro Nacional de Investigaciones Oncológicas) (M.J.G., J.B.), and Centre for Biomedical Network Research on Rare Diseases (Centro de Investigación Biomédica en Red de Enfermedades Raras), Instituto de Salud Carlos III (M.J.G., J.B.), Madrid, Spain; David Geffen School of Medicine, Department of Medicine, Division of Hematology and Oncology, University of California at Los Angeles (P.A.F.); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Erlangen, Germany (P.A.F.); Department of Histopathology, Queen’s Medical Centre, Nottingham University Hospitals NHS Trust, Nottingham, UK (S.D.); University Cancer Center Hamburg, University Medical Center Hamburg-Eppendorf, Hamburg, Germany (J.C.-C.); Cancer Genetics Laboratory, Research Division, Peter MacCallum Cancer Centre (I.G.C.), Sir Peter MacCallum Department of Oncology, University of Melbourne (I.G.C.), and Department of Pathology, University of Melbourne (I.G.C.), Melbourne, Victoria, Australia; Department of Oncology, University of Cambridge, Hutchison/MRC Research Centre (J.D.B.), Cancer Research UK Cambridge Institute, University of Cambridge, Li Ka Shing Centre (J.D.B.), and Cambridge Experimental Cancer Medicine Centre (J.D.B.), Cambridge, UK; Cancer Genomics Program, Research Department, Peter MacCallum Cancer Centre, East Melbourne, Victoria, Australia (D.D.B.); the Garvan Institute, Sydney, New South Wales, Australia (D.D.B., S.J.R.); and Department of Public Health and Primary Care, University of Cambridge, Strangeways Research Laboratory, Cambridge, UK (P.D.P.P.)
1,
Bryan M McCauley
Bryan M McCauley, BS
1Department of Medical Oncology (M.S.B., K.R.K.) and Department of Health Sciences Research (R.A.V., S.J.W., B.M.M., Z.L., E.L.G.), Mayo Clinic, Rochester, MN; Department of Pathology and Laboratory Medicine, University of Calgary, Calgary, Alberta, Canada (P.F.R., M.K.); Pathology Department, Catholic University of Health and Allied Sciences-Bugando, Mwanza, Tanzania (P.F.R.); Tübingen University Hospital, Department of Women’s Health, Tübingen, Germany (P.W., F.A.T., A.D.H., S.Y.B., S.K.); Sir Peter MacCallum Department of Oncology, the University of Melbourne, Parkville, Victoria, Australia (N.T., D.D.B.); International Hereditary Cancer Center, Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (A.T., O.O., J. Lubiński, J.G.); Department of Gynecology and Obstetrics, Ribeirão Preto Medical School, University of São Paulo, Ribeirão Preto, Brazil (D.G.T., C.B.S., J.M.A., F.J.C.R.); Department of Pathology, Institute of Pathology, Heidelberg University Hospital, Heidelberg, Germany (P.S.); Department of Population Health Science and Policy, Department of Genetics and Genomic Sciences, Icahn School of Medicine at Mount Sinai, New York, NY (W.S., J.H.R.); Pathology West ICPMR Westmead, Westmead Hospital, the University of Sydney, Sydney, Australia (R.S.); University of Western Sydney at Westmead Hospital, Westmead, New South Wales, Australia (R.S.); Medical Oncology Service, Hospital Sant Pau, Barcelona, Spain (T.R.C.); H12O-CNIO Lung Cancer Clinical Research Unit (L.P.-A.), and Oncology Department, Hospital Universitario 12 de Octubre (L.P.-A.), Madrid, Spain; Women’s Cancer Program, Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, CA (S.O., J. Lester, B.Y.K.); the University of Texas School of Public Health, Houston (R.B.N.); Department of Oncology, Division of Gynecologic Oncology, Cumming School of Medicine, University of Calgary, Calgary, Alberta, Canada (G.N., P.G.); Department of Obstetrics, Gynecology and Reproductive Sciences, University of Pittsburgh School of Medicine (F.M., R.P.E.), Department of Epidemiology, University of Pittsburgh Graduate School of Public Health (F.M.), and Womens Cancer Research Program, Magee-Womens Research Institute and University of Pittsburgh Cancer Institute (F.M.), Pittsburgh, PA; Department of Gynecological Surgery and Gynecological Oncology of Adults and Adolescents, Pomeranian Medical University, Szczecin, Poland (J.M., A.C.-G.); Department of Health Research and Policy, Stanford University School of Medicine, Stanford, CA (V.M.); Department of Oncology, University of Cambridge, Strangeways Research Laboratory, Cambridge, UK (M.M., J.A., P.D.P.P.); Department of Pathology, Stanford University, Stanford, CA (T.A.L.); Department of Gynecologic Oncology, the Third Affiliated Hospital of Kunming Medical University (Yunnan Tumor Hospital), Kunming, China (Z.L.); Centre for Cancer Research, the Westmead Institute for Medical Research, the University of Sydney (C.J.K., J.H., P.R.H., B. Gao, A.deF.), and Department of Gynaecological Oncology, Westmead Hospital (C.J.K., J.H., A.deF.), Sydney, New South Wales, Australia; Division of Cancer Epidemiology, German Cancer Research Center (Deutsches Krebsforschungszentrum), Heidelberg, Germany (A.Y.J., U.E., J.C.-C.); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia (S.E.J., G.C.-T.); Department of Histopathology, Addenbrookes Hospital, Cambridge, UK (M.J.-L.); Virus, Lifestyle and Genes, Danish Cancer Society Research Center, Copenhagen, Denmark (A.J., S.K.K.); School of Women’s and Children’s Health, University of New South Wales, Sydney, Australia (M.P.I., S.J.R.); Tissue Bank of the National Center for Tumor Diseases (NCT) Heidelberg (E. Herpel), and Institute of Pathology, University of Heidelberg (E. Herpel), Heidelberg, Germany; Cancer Center, University of Hawaii, Honolulu, HI (B.Y.H.); the Crown Princess Mary Cancer Centre, Westmead Hospital, the University of Sydney, Sydney, Australia (P.R.H., B. Gao); Oncology Department, Hospital General de Albacete, Madrid, Spain (J.M.G.-B.); Department of Cancer Genomics and Genetics, Peter MacCallum Cancer Centre, East Melbourne, Victoria, Australia (S.F.); Pathology Department, Fundación Jiménez Díaz, Madrid, Spain (A.C.); Department of Health Research and Policy and Department of Biomedical Data Science, Stanford University School of Medicine, Stanford, CA (A.S.W.); Department of Obstetrics and Gynecology, Division of Gynecologic Oncology, Royal Alexandra Hospital, Edmonton, Alberta, Canada (H.S.); Tübingen University Hospital, Institute of Pathology, Tübingen, Germany (A.S.); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (K.B.M.); Gynaecological Cancer Research Centre, Department of Women’s Cancer, Institute for Women’s Health, University College London, London, UK (U.M.); Alberta Health Services-Cancer Care, Calgary, Alberta, Canada (J.M.K.); Department of Gynecology, Rigshospitalet, University of Copenhagen, Copenhagen, Denmark (S.K.K.); Department of Public Health Sciences, Medical University of South Carolina and Hollings Cancer Center, Charleston, SC (L.E.K.); Department of Pathology and Laboratory Medicine, University of British Columbia (D.G.H.), and Centre for Translational and Applied Genomics, British Columbia Cancer Agency (D.G.H.), Vancouver, British Columbia, Canada; Molecular Unit, Department of Pathology, Herlev Hospital, University of Copenhagen, Copenhagen, Denmark (E. Høgdall); Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, CA (M.T.G.); Genetic Pathology Evaluation Centre, Vancouver General Hospital and University of British Columbia, Vancouver, British Columbia, Canada (B. Gilks); Human Genetics Group, Spanish National Cancer Research Centre (Centro Nacional de Investigaciones Oncológicas) (M.J.G., J.B.), and Centre for Biomedical Network Research on Rare Diseases (Centro de Investigación Biomédica en Red de Enfermedades Raras), Instituto de Salud Carlos III (M.J.G., J.B.), Madrid, Spain; David Geffen School of Medicine, Department of Medicine, Division of Hematology and Oncology, University of California at Los Angeles (P.A.F.); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Erlangen, Germany (P.A.F.); Department of Histopathology, Queen’s Medical Centre, Nottingham University Hospitals NHS Trust, Nottingham, UK (S.D.); University Cancer Center Hamburg, University Medical Center Hamburg-Eppendorf, Hamburg, Germany (J.C.-C.); Cancer Genetics Laboratory, Research Division, Peter MacCallum Cancer Centre (I.G.C.), Sir Peter MacCallum Department of Oncology, University of Melbourne (I.G.C.), and Department of Pathology, University of Melbourne (I.G.C.), Melbourne, Victoria, Australia; Department of Oncology, University of Cambridge, Hutchison/MRC Research Centre (J.D.B.), Cancer Research UK Cambridge Institute, University of Cambridge, Li Ka Shing Centre (J.D.B.), and Cambridge Experimental Cancer Medicine Centre (J.D.B.), Cambridge, UK; Cancer Genomics Program, Research Department, Peter MacCallum Cancer Centre, East Melbourne, Victoria, Australia (D.D.B.); the Garvan Institute, Sydney, New South Wales, Australia (D.D.B., S.J.R.); and Department of Public Health and Primary Care, University of Cambridge, Strangeways Research Laboratory, Cambridge, UK (P.D.P.P.)
1,
Marie Mack
Marie Mack, PhD
1Department of Medical Oncology (M.S.B., K.R.K.) and Department of Health Sciences Research (R.A.V., S.J.W., B.M.M., Z.L., E.L.G.), Mayo Clinic, Rochester, MN; Department of Pathology and Laboratory Medicine, University of Calgary, Calgary, Alberta, Canada (P.F.R., M.K.); Pathology Department, Catholic University of Health and Allied Sciences-Bugando, Mwanza, Tanzania (P.F.R.); Tübingen University Hospital, Department of Women’s Health, Tübingen, Germany (P.W., F.A.T., A.D.H., S.Y.B., S.K.); Sir Peter MacCallum Department of Oncology, the University of Melbourne, Parkville, Victoria, Australia (N.T., D.D.B.); International Hereditary Cancer Center, Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (A.T., O.O., J. Lubiński, J.G.); Department of Gynecology and Obstetrics, Ribeirão Preto Medical School, University of São Paulo, Ribeirão Preto, Brazil (D.G.T., C.B.S., J.M.A., F.J.C.R.); Department of Pathology, Institute of Pathology, Heidelberg University Hospital, Heidelberg, Germany (P.S.); Department of Population Health Science and Policy, Department of Genetics and Genomic Sciences, Icahn School of Medicine at Mount Sinai, New York, NY (W.S., J.H.R.); Pathology West ICPMR Westmead, Westmead Hospital, the University of Sydney, Sydney, Australia (R.S.); University of Western Sydney at Westmead Hospital, Westmead, New South Wales, Australia (R.S.); Medical Oncology Service, Hospital Sant Pau, Barcelona, Spain (T.R.C.); H12O-CNIO Lung Cancer Clinical Research Unit (L.P.-A.), and Oncology Department, Hospital Universitario 12 de Octubre (L.P.-A.), Madrid, Spain; Women’s Cancer Program, Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, CA (S.O., J. Lester, B.Y.K.); the University of Texas School of Public Health, Houston (R.B.N.); Department of Oncology, Division of Gynecologic Oncology, Cumming School of Medicine, University of Calgary, Calgary, Alberta, Canada (G.N., P.G.); Department of Obstetrics, Gynecology and Reproductive Sciences, University of Pittsburgh School of Medicine (F.M., R.P.E.), Department of Epidemiology, University of Pittsburgh Graduate School of Public Health (F.M.), and Womens Cancer Research Program, Magee-Womens Research Institute and University of Pittsburgh Cancer Institute (F.M.), Pittsburgh, PA; Department of Gynecological Surgery and Gynecological Oncology of Adults and Adolescents, Pomeranian Medical University, Szczecin, Poland (J.M., A.C.-G.); Department of Health Research and Policy, Stanford University School of Medicine, Stanford, CA (V.M.); Department of Oncology, University of Cambridge, Strangeways Research Laboratory, Cambridge, UK (M.M., J.A., P.D.P.P.); Department of Pathology, Stanford University, Stanford, CA (T.A.L.); Department of Gynecologic Oncology, the Third Affiliated Hospital of Kunming Medical University (Yunnan Tumor Hospital), Kunming, China (Z.L.); Centre for Cancer Research, the Westmead Institute for Medical Research, the University of Sydney (C.J.K., J.H., P.R.H., B. Gao, A.deF.), and Department of Gynaecological Oncology, Westmead Hospital (C.J.K., J.H., A.deF.), Sydney, New South Wales, Australia; Division of Cancer Epidemiology, German Cancer Research Center (Deutsches Krebsforschungszentrum), Heidelberg, Germany (A.Y.J., U.E., J.C.-C.); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia (S.E.J., G.C.-T.); Department of Histopathology, Addenbrookes Hospital, Cambridge, UK (M.J.-L.); Virus, Lifestyle and Genes, Danish Cancer Society Research Center, Copenhagen, Denmark (A.J., S.K.K.); School of Women’s and Children’s Health, University of New South Wales, Sydney, Australia (M.P.I., S.J.R.); Tissue Bank of the National Center for Tumor Diseases (NCT) Heidelberg (E. Herpel), and Institute of Pathology, University of Heidelberg (E. Herpel), Heidelberg, Germany; Cancer Center, University of Hawaii, Honolulu, HI (B.Y.H.); the Crown Princess Mary Cancer Centre, Westmead Hospital, the University of Sydney, Sydney, Australia (P.R.H., B. Gao); Oncology Department, Hospital General de Albacete, Madrid, Spain (J.M.G.-B.); Department of Cancer Genomics and Genetics, Peter MacCallum Cancer Centre, East Melbourne, Victoria, Australia (S.F.); Pathology Department, Fundación Jiménez Díaz, Madrid, Spain (A.C.); Department of Health Research and Policy and Department of Biomedical Data Science, Stanford University School of Medicine, Stanford, CA (A.S.W.); Department of Obstetrics and Gynecology, Division of Gynecologic Oncology, Royal Alexandra Hospital, Edmonton, Alberta, Canada (H.S.); Tübingen University Hospital, Institute of Pathology, Tübingen, Germany (A.S.); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (K.B.M.); Gynaecological Cancer Research Centre, Department of Women’s Cancer, Institute for Women’s Health, University College London, London, UK (U.M.); Alberta Health Services-Cancer Care, Calgary, Alberta, Canada (J.M.K.); Department of Gynecology, Rigshospitalet, University of Copenhagen, Copenhagen, Denmark (S.K.K.); Department of Public Health Sciences, Medical University of South Carolina and Hollings Cancer Center, Charleston, SC (L.E.K.); Department of Pathology and Laboratory Medicine, University of British Columbia (D.G.H.), and Centre for Translational and Applied Genomics, British Columbia Cancer Agency (D.G.H.), Vancouver, British Columbia, Canada; Molecular Unit, Department of Pathology, Herlev Hospital, University of Copenhagen, Copenhagen, Denmark (E. Høgdall); Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, CA (M.T.G.); Genetic Pathology Evaluation Centre, Vancouver General Hospital and University of British Columbia, Vancouver, British Columbia, Canada (B. Gilks); Human Genetics Group, Spanish National Cancer Research Centre (Centro Nacional de Investigaciones Oncológicas) (M.J.G., J.B.), and Centre for Biomedical Network Research on Rare Diseases (Centro de Investigación Biomédica en Red de Enfermedades Raras), Instituto de Salud Carlos III (M.J.G., J.B.), Madrid, Spain; David Geffen School of Medicine, Department of Medicine, Division of Hematology and Oncology, University of California at Los Angeles (P.A.F.); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Erlangen, Germany (P.A.F.); Department of Histopathology, Queen’s Medical Centre, Nottingham University Hospitals NHS Trust, Nottingham, UK (S.D.); University Cancer Center Hamburg, University Medical Center Hamburg-Eppendorf, Hamburg, Germany (J.C.-C.); Cancer Genetics Laboratory, Research Division, Peter MacCallum Cancer Centre (I.G.C.), Sir Peter MacCallum Department of Oncology, University of Melbourne (I.G.C.), and Department of Pathology, University of Melbourne (I.G.C.), Melbourne, Victoria, Australia; Department of Oncology, University of Cambridge, Hutchison/MRC Research Centre (J.D.B.), Cancer Research UK Cambridge Institute, University of Cambridge, Li Ka Shing Centre (J.D.B.), and Cambridge Experimental Cancer Medicine Centre (J.D.B.), Cambridge, UK; Cancer Genomics Program, Research Department, Peter MacCallum Cancer Centre, East Melbourne, Victoria, Australia (D.D.B.); the Garvan Institute, Sydney, New South Wales, Australia (D.D.B., S.J.R.); and Department of Public Health and Primary Care, University of Cambridge, Strangeways Research Laboratory, Cambridge, UK (P.D.P.P.)
1,
Jan Lubiński
Jan Lubiński, MD, PhD
1Department of Medical Oncology (M.S.B., K.R.K.) and Department of Health Sciences Research (R.A.V., S.J.W., B.M.M., Z.L., E.L.G.), Mayo Clinic, Rochester, MN; Department of Pathology and Laboratory Medicine, University of Calgary, Calgary, Alberta, Canada (P.F.R., M.K.); Pathology Department, Catholic University of Health and Allied Sciences-Bugando, Mwanza, Tanzania (P.F.R.); Tübingen University Hospital, Department of Women’s Health, Tübingen, Germany (P.W., F.A.T., A.D.H., S.Y.B., S.K.); Sir Peter MacCallum Department of Oncology, the University of Melbourne, Parkville, Victoria, Australia (N.T., D.D.B.); International Hereditary Cancer Center, Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (A.T., O.O., J. Lubiński, J.G.); Department of Gynecology and Obstetrics, Ribeirão Preto Medical School, University of São Paulo, Ribeirão Preto, Brazil (D.G.T., C.B.S., J.M.A., F.J.C.R.); Department of Pathology, Institute of Pathology, Heidelberg University Hospital, Heidelberg, Germany (P.S.); Department of Population Health Science and Policy, Department of Genetics and Genomic Sciences, Icahn School of Medicine at Mount Sinai, New York, NY (W.S., J.H.R.); Pathology West ICPMR Westmead, Westmead Hospital, the University of Sydney, Sydney, Australia (R.S.); University of Western Sydney at Westmead Hospital, Westmead, New South Wales, Australia (R.S.); Medical Oncology Service, Hospital Sant Pau, Barcelona, Spain (T.R.C.); H12O-CNIO Lung Cancer Clinical Research Unit (L.P.-A.), and Oncology Department, Hospital Universitario 12 de Octubre (L.P.-A.), Madrid, Spain; Women’s Cancer Program, Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, CA (S.O., J. Lester, B.Y.K.); the University of Texas School of Public Health, Houston (R.B.N.); Department of Oncology, Division of Gynecologic Oncology, Cumming School of Medicine, University of Calgary, Calgary, Alberta, Canada (G.N., P.G.); Department of Obstetrics, Gynecology and Reproductive Sciences, University of Pittsburgh School of Medicine (F.M., R.P.E.), Department of Epidemiology, University of Pittsburgh Graduate School of Public Health (F.M.), and Womens Cancer Research Program, Magee-Womens Research Institute and University of Pittsburgh Cancer Institute (F.M.), Pittsburgh, PA; Department of Gynecological Surgery and Gynecological Oncology of Adults and Adolescents, Pomeranian Medical University, Szczecin, Poland (J.M., A.C.-G.); Department of Health Research and Policy, Stanford University School of Medicine, Stanford, CA (V.M.); Department of Oncology, University of Cambridge, Strangeways Research Laboratory, Cambridge, UK (M.M., J.A., P.D.P.P.); Department of Pathology, Stanford University, Stanford, CA (T.A.L.); Department of Gynecologic Oncology, the Third Affiliated Hospital of Kunming Medical University (Yunnan Tumor Hospital), Kunming, China (Z.L.); Centre for Cancer Research, the Westmead Institute for Medical Research, the University of Sydney (C.J.K., J.H., P.R.H., B. Gao, A.deF.), and Department of Gynaecological Oncology, Westmead Hospital (C.J.K., J.H., A.deF.), Sydney, New South Wales, Australia; Division of Cancer Epidemiology, German Cancer Research Center (Deutsches Krebsforschungszentrum), Heidelberg, Germany (A.Y.J., U.E., J.C.-C.); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia (S.E.J., G.C.-T.); Department of Histopathology, Addenbrookes Hospital, Cambridge, UK (M.J.-L.); Virus, Lifestyle and Genes, Danish Cancer Society Research Center, Copenhagen, Denmark (A.J., S.K.K.); School of Women’s and Children’s Health, University of New South Wales, Sydney, Australia (M.P.I., S.J.R.); Tissue Bank of the National Center for Tumor Diseases (NCT) Heidelberg (E. Herpel), and Institute of Pathology, University of Heidelberg (E. Herpel), Heidelberg, Germany; Cancer Center, University of Hawaii, Honolulu, HI (B.Y.H.); the Crown Princess Mary Cancer Centre, Westmead Hospital, the University of Sydney, Sydney, Australia (P.R.H., B. Gao); Oncology Department, Hospital General de Albacete, Madrid, Spain (J.M.G.-B.); Department of Cancer Genomics and Genetics, Peter MacCallum Cancer Centre, East Melbourne, Victoria, Australia (S.F.); Pathology Department, Fundación Jiménez Díaz, Madrid, Spain (A.C.); Department of Health Research and Policy and Department of Biomedical Data Science, Stanford University School of Medicine, Stanford, CA (A.S.W.); Department of Obstetrics and Gynecology, Division of Gynecologic Oncology, Royal Alexandra Hospital, Edmonton, Alberta, Canada (H.S.); Tübingen University Hospital, Institute of Pathology, Tübingen, Germany (A.S.); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (K.B.M.); Gynaecological Cancer Research Centre, Department of Women’s Cancer, Institute for Women’s Health, University College London, London, UK (U.M.); Alberta Health Services-Cancer Care, Calgary, Alberta, Canada (J.M.K.); Department of Gynecology, Rigshospitalet, University of Copenhagen, Copenhagen, Denmark (S.K.K.); Department of Public Health Sciences, Medical University of South Carolina and Hollings Cancer Center, Charleston, SC (L.E.K.); Department of Pathology and Laboratory Medicine, University of British Columbia (D.G.H.), and Centre for Translational and Applied Genomics, British Columbia Cancer Agency (D.G.H.), Vancouver, British Columbia, Canada; Molecular Unit, Department of Pathology, Herlev Hospital, University of Copenhagen, Copenhagen, Denmark (E. Høgdall); Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, CA (M.T.G.); Genetic Pathology Evaluation Centre, Vancouver General Hospital and University of British Columbia, Vancouver, British Columbia, Canada (B. Gilks); Human Genetics Group, Spanish National Cancer Research Centre (Centro Nacional de Investigaciones Oncológicas) (M.J.G., J.B.), and Centre for Biomedical Network Research on Rare Diseases (Centro de Investigación Biomédica en Red de Enfermedades Raras), Instituto de Salud Carlos III (M.J.G., J.B.), Madrid, Spain; David Geffen School of Medicine, Department of Medicine, Division of Hematology and Oncology, University of California at Los Angeles (P.A.F.); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Erlangen, Germany (P.A.F.); Department of Histopathology, Queen’s Medical Centre, Nottingham University Hospitals NHS Trust, Nottingham, UK (S.D.); University Cancer Center Hamburg, University Medical Center Hamburg-Eppendorf, Hamburg, Germany (J.C.-C.); Cancer Genetics Laboratory, Research Division, Peter MacCallum Cancer Centre (I.G.C.), Sir Peter MacCallum Department of Oncology, University of Melbourne (I.G.C.), and Department of Pathology, University of Melbourne (I.G.C.), Melbourne, Victoria, Australia; Department of Oncology, University of Cambridge, Hutchison/MRC Research Centre (J.D.B.), Cancer Research UK Cambridge Institute, University of Cambridge, Li Ka Shing Centre (J.D.B.), and Cambridge Experimental Cancer Medicine Centre (J.D.B.), Cambridge, UK; Cancer Genomics Program, Research Department, Peter MacCallum Cancer Centre, East Melbourne, Victoria, Australia (D.D.B.); the Garvan Institute, Sydney, New South Wales, Australia (D.D.B., S.J.R.); and Department of Public Health and Primary Care, University of Cambridge, Strangeways Research Laboratory, Cambridge, UK (P.D.P.P.)
1,
Teri A Longacre
Teri A Longacre, MD
1Department of Medical Oncology (M.S.B., K.R.K.) and Department of Health Sciences Research (R.A.V., S.J.W., B.M.M., Z.L., E.L.G.), Mayo Clinic, Rochester, MN; Department of Pathology and Laboratory Medicine, University of Calgary, Calgary, Alberta, Canada (P.F.R., M.K.); Pathology Department, Catholic University of Health and Allied Sciences-Bugando, Mwanza, Tanzania (P.F.R.); Tübingen University Hospital, Department of Women’s Health, Tübingen, Germany (P.W., F.A.T., A.D.H., S.Y.B., S.K.); Sir Peter MacCallum Department of Oncology, the University of Melbourne, Parkville, Victoria, Australia (N.T., D.D.B.); International Hereditary Cancer Center, Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (A.T., O.O., J. Lubiński, J.G.); Department of Gynecology and Obstetrics, Ribeirão Preto Medical School, University of São Paulo, Ribeirão Preto, Brazil (D.G.T., C.B.S., J.M.A., F.J.C.R.); Department of Pathology, Institute of Pathology, Heidelberg University Hospital, Heidelberg, Germany (P.S.); Department of Population Health Science and Policy, Department of Genetics and Genomic Sciences, Icahn School of Medicine at Mount Sinai, New York, NY (W.S., J.H.R.); Pathology West ICPMR Westmead, Westmead Hospital, the University of Sydney, Sydney, Australia (R.S.); University of Western Sydney at Westmead Hospital, Westmead, New South Wales, Australia (R.S.); Medical Oncology Service, Hospital Sant Pau, Barcelona, Spain (T.R.C.); H12O-CNIO Lung Cancer Clinical Research Unit (L.P.-A.), and Oncology Department, Hospital Universitario 12 de Octubre (L.P.-A.), Madrid, Spain; Women’s Cancer Program, Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, CA (S.O., J. Lester, B.Y.K.); the University of Texas School of Public Health, Houston (R.B.N.); Department of Oncology, Division of Gynecologic Oncology, Cumming School of Medicine, University of Calgary, Calgary, Alberta, Canada (G.N., P.G.); Department of Obstetrics, Gynecology and Reproductive Sciences, University of Pittsburgh School of Medicine (F.M., R.P.E.), Department of Epidemiology, University of Pittsburgh Graduate School of Public Health (F.M.), and Womens Cancer Research Program, Magee-Womens Research Institute and University of Pittsburgh Cancer Institute (F.M.), Pittsburgh, PA; Department of Gynecological Surgery and Gynecological Oncology of Adults and Adolescents, Pomeranian Medical University, Szczecin, Poland (J.M., A.C.-G.); Department of Health Research and Policy, Stanford University School of Medicine, Stanford, CA (V.M.); Department of Oncology, University of Cambridge, Strangeways Research Laboratory, Cambridge, UK (M.M., J.A., P.D.P.P.); Department of Pathology, Stanford University, Stanford, CA (T.A.L.); Department of Gynecologic Oncology, the Third Affiliated Hospital of Kunming Medical University (Yunnan Tumor Hospital), Kunming, China (Z.L.); Centre for Cancer Research, the Westmead Institute for Medical Research, the University of Sydney (C.J.K., J.H., P.R.H., B. Gao, A.deF.), and Department of Gynaecological Oncology, Westmead Hospital (C.J.K., J.H., A.deF.), Sydney, New South Wales, Australia; Division of Cancer Epidemiology, German Cancer Research Center (Deutsches Krebsforschungszentrum), Heidelberg, Germany (A.Y.J., U.E., J.C.-C.); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia (S.E.J., G.C.-T.); Department of Histopathology, Addenbrookes Hospital, Cambridge, UK (M.J.-L.); Virus, Lifestyle and Genes, Danish Cancer Society Research Center, Copenhagen, Denmark (A.J., S.K.K.); School of Women’s and Children’s Health, University of New South Wales, Sydney, Australia (M.P.I., S.J.R.); Tissue Bank of the National Center for Tumor Diseases (NCT) Heidelberg (E. Herpel), and Institute of Pathology, University of Heidelberg (E. Herpel), Heidelberg, Germany; Cancer Center, University of Hawaii, Honolulu, HI (B.Y.H.); the Crown Princess Mary Cancer Centre, Westmead Hospital, the University of Sydney, Sydney, Australia (P.R.H., B. Gao); Oncology Department, Hospital General de Albacete, Madrid, Spain (J.M.G.-B.); Department of Cancer Genomics and Genetics, Peter MacCallum Cancer Centre, East Melbourne, Victoria, Australia (S.F.); Pathology Department, Fundación Jiménez Díaz, Madrid, Spain (A.C.); Department of Health Research and Policy and Department of Biomedical Data Science, Stanford University School of Medicine, Stanford, CA (A.S.W.); Department of Obstetrics and Gynecology, Division of Gynecologic Oncology, Royal Alexandra Hospital, Edmonton, Alberta, Canada (H.S.); Tübingen University Hospital, Institute of Pathology, Tübingen, Germany (A.S.); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (K.B.M.); Gynaecological Cancer Research Centre, Department of Women’s Cancer, Institute for Women’s Health, University College London, London, UK (U.M.); Alberta Health Services-Cancer Care, Calgary, Alberta, Canada (J.M.K.); Department of Gynecology, Rigshospitalet, University of Copenhagen, Copenhagen, Denmark (S.K.K.); Department of Public Health Sciences, Medical University of South Carolina and Hollings Cancer Center, Charleston, SC (L.E.K.); Department of Pathology and Laboratory Medicine, University of British Columbia (D.G.H.), and Centre for Translational and Applied Genomics, British Columbia Cancer Agency (D.G.H.), Vancouver, British Columbia, Canada; Molecular Unit, Department of Pathology, Herlev Hospital, University of Copenhagen, Copenhagen, Denmark (E. Høgdall); Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, CA (M.T.G.); Genetic Pathology Evaluation Centre, Vancouver General Hospital and University of British Columbia, Vancouver, British Columbia, Canada (B. Gilks); Human Genetics Group, Spanish National Cancer Research Centre (Centro Nacional de Investigaciones Oncológicas) (M.J.G., J.B.), and Centre for Biomedical Network Research on Rare Diseases (Centro de Investigación Biomédica en Red de Enfermedades Raras), Instituto de Salud Carlos III (M.J.G., J.B.), Madrid, Spain; David Geffen School of Medicine, Department of Medicine, Division of Hematology and Oncology, University of California at Los Angeles (P.A.F.); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Erlangen, Germany (P.A.F.); Department of Histopathology, Queen’s Medical Centre, Nottingham University Hospitals NHS Trust, Nottingham, UK (S.D.); University Cancer Center Hamburg, University Medical Center Hamburg-Eppendorf, Hamburg, Germany (J.C.-C.); Cancer Genetics Laboratory, Research Division, Peter MacCallum Cancer Centre (I.G.C.), Sir Peter MacCallum Department of Oncology, University of Melbourne (I.G.C.), and Department of Pathology, University of Melbourne (I.G.C.), Melbourne, Victoria, Australia; Department of Oncology, University of Cambridge, Hutchison/MRC Research Centre (J.D.B.), Cancer Research UK Cambridge Institute, University of Cambridge, Li Ka Shing Centre (J.D.B.), and Cambridge Experimental Cancer Medicine Centre (J.D.B.), Cambridge, UK; Cancer Genomics Program, Research Department, Peter MacCallum Cancer Centre, East Melbourne, Victoria, Australia (D.D.B.); the Garvan Institute, Sydney, New South Wales, Australia (D.D.B., S.J.R.); and Department of Public Health and Primary Care, University of Cambridge, Strangeways Research Laboratory, Cambridge, UK (P.D.P.P.)
1,
Zheng Li
Zheng Li, MD
1Department of Medical Oncology (M.S.B., K.R.K.) and Department of Health Sciences Research (R.A.V., S.J.W., B.M.M., Z.L., E.L.G.), Mayo Clinic, Rochester, MN; Department of Pathology and Laboratory Medicine, University of Calgary, Calgary, Alberta, Canada (P.F.R., M.K.); Pathology Department, Catholic University of Health and Allied Sciences-Bugando, Mwanza, Tanzania (P.F.R.); Tübingen University Hospital, Department of Women’s Health, Tübingen, Germany (P.W., F.A.T., A.D.H., S.Y.B., S.K.); Sir Peter MacCallum Department of Oncology, the University of Melbourne, Parkville, Victoria, Australia (N.T., D.D.B.); International Hereditary Cancer Center, Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (A.T., O.O., J. Lubiński, J.G.); Department of Gynecology and Obstetrics, Ribeirão Preto Medical School, University of São Paulo, Ribeirão Preto, Brazil (D.G.T., C.B.S., J.M.A., F.J.C.R.); Department of Pathology, Institute of Pathology, Heidelberg University Hospital, Heidelberg, Germany (P.S.); Department of Population Health Science and Policy, Department of Genetics and Genomic Sciences, Icahn School of Medicine at Mount Sinai, New York, NY (W.S., J.H.R.); Pathology West ICPMR Westmead, Westmead Hospital, the University of Sydney, Sydney, Australia (R.S.); University of Western Sydney at Westmead Hospital, Westmead, New South Wales, Australia (R.S.); Medical Oncology Service, Hospital Sant Pau, Barcelona, Spain (T.R.C.); H12O-CNIO Lung Cancer Clinical Research Unit (L.P.-A.), and Oncology Department, Hospital Universitario 12 de Octubre (L.P.-A.), Madrid, Spain; Women’s Cancer Program, Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, CA (S.O., J. Lester, B.Y.K.); the University of Texas School of Public Health, Houston (R.B.N.); Department of Oncology, Division of Gynecologic Oncology, Cumming School of Medicine, University of Calgary, Calgary, Alberta, Canada (G.N., P.G.); Department of Obstetrics, Gynecology and Reproductive Sciences, University of Pittsburgh School of Medicine (F.M., R.P.E.), Department of Epidemiology, University of Pittsburgh Graduate School of Public Health (F.M.), and Womens Cancer Research Program, Magee-Womens Research Institute and University of Pittsburgh Cancer Institute (F.M.), Pittsburgh, PA; Department of Gynecological Surgery and Gynecological Oncology of Adults and Adolescents, Pomeranian Medical University, Szczecin, Poland (J.M., A.C.-G.); Department of Health Research and Policy, Stanford University School of Medicine, Stanford, CA (V.M.); Department of Oncology, University of Cambridge, Strangeways Research Laboratory, Cambridge, UK (M.M., J.A., P.D.P.P.); Department of Pathology, Stanford University, Stanford, CA (T.A.L.); Department of Gynecologic Oncology, the Third Affiliated Hospital of Kunming Medical University (Yunnan Tumor Hospital), Kunming, China (Z.L.); Centre for Cancer Research, the Westmead Institute for Medical Research, the University of Sydney (C.J.K., J.H., P.R.H., B. Gao, A.deF.), and Department of Gynaecological Oncology, Westmead Hospital (C.J.K., J.H., A.deF.), Sydney, New South Wales, Australia; Division of Cancer Epidemiology, German Cancer Research Center (Deutsches Krebsforschungszentrum), Heidelberg, Germany (A.Y.J., U.E., J.C.-C.); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia (S.E.J., G.C.-T.); Department of Histopathology, Addenbrookes Hospital, Cambridge, UK (M.J.-L.); Virus, Lifestyle and Genes, Danish Cancer Society Research Center, Copenhagen, Denmark (A.J., S.K.K.); School of Women’s and Children’s Health, University of New South Wales, Sydney, Australia (M.P.I., S.J.R.); Tissue Bank of the National Center for Tumor Diseases (NCT) Heidelberg (E. Herpel), and Institute of Pathology, University of Heidelberg (E. Herpel), Heidelberg, Germany; Cancer Center, University of Hawaii, Honolulu, HI (B.Y.H.); the Crown Princess Mary Cancer Centre, Westmead Hospital, the University of Sydney, Sydney, Australia (P.R.H., B. Gao); Oncology Department, Hospital General de Albacete, Madrid, Spain (J.M.G.-B.); Department of Cancer Genomics and Genetics, Peter MacCallum Cancer Centre, East Melbourne, Victoria, Australia (S.F.); Pathology Department, Fundación Jiménez Díaz, Madrid, Spain (A.C.); Department of Health Research and Policy and Department of Biomedical Data Science, Stanford University School of Medicine, Stanford, CA (A.S.W.); Department of Obstetrics and Gynecology, Division of Gynecologic Oncology, Royal Alexandra Hospital, Edmonton, Alberta, Canada (H.S.); Tübingen University Hospital, Institute of Pathology, Tübingen, Germany (A.S.); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (K.B.M.); Gynaecological Cancer Research Centre, Department of Women’s Cancer, Institute for Women’s Health, University College London, London, UK (U.M.); Alberta Health Services-Cancer Care, Calgary, Alberta, Canada (J.M.K.); Department of Gynecology, Rigshospitalet, University of Copenhagen, Copenhagen, Denmark (S.K.K.); Department of Public Health Sciences, Medical University of South Carolina and Hollings Cancer Center, Charleston, SC (L.E.K.); Department of Pathology and Laboratory Medicine, University of British Columbia (D.G.H.), and Centre for Translational and Applied Genomics, British Columbia Cancer Agency (D.G.H.), Vancouver, British Columbia, Canada; Molecular Unit, Department of Pathology, Herlev Hospital, University of Copenhagen, Copenhagen, Denmark (E. Høgdall); Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, CA (M.T.G.); Genetic Pathology Evaluation Centre, Vancouver General Hospital and University of British Columbia, Vancouver, British Columbia, Canada (B. Gilks); Human Genetics Group, Spanish National Cancer Research Centre (Centro Nacional de Investigaciones Oncológicas) (M.J.G., J.B.), and Centre for Biomedical Network Research on Rare Diseases (Centro de Investigación Biomédica en Red de Enfermedades Raras), Instituto de Salud Carlos III (M.J.G., J.B.), Madrid, Spain; David Geffen School of Medicine, Department of Medicine, Division of Hematology and Oncology, University of California at Los Angeles (P.A.F.); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Erlangen, Germany (P.A.F.); Department of Histopathology, Queen’s Medical Centre, Nottingham University Hospitals NHS Trust, Nottingham, UK (S.D.); University Cancer Center Hamburg, University Medical Center Hamburg-Eppendorf, Hamburg, Germany (J.C.-C.); Cancer Genetics Laboratory, Research Division, Peter MacCallum Cancer Centre (I.G.C.), Sir Peter MacCallum Department of Oncology, University of Melbourne (I.G.C.), and Department of Pathology, University of Melbourne (I.G.C.), Melbourne, Victoria, Australia; Department of Oncology, University of Cambridge, Hutchison/MRC Research Centre (J.D.B.), Cancer Research UK Cambridge Institute, University of Cambridge, Li Ka Shing Centre (J.D.B.), and Cambridge Experimental Cancer Medicine Centre (J.D.B.), Cambridge, UK; Cancer Genomics Program, Research Department, Peter MacCallum Cancer Centre, East Melbourne, Victoria, Australia (D.D.B.); the Garvan Institute, Sydney, New South Wales, Australia (D.D.B., S.J.R.); and Department of Public Health and Primary Care, University of Cambridge, Strangeways Research Laboratory, Cambridge, UK (P.D.P.P.)
1,
Jenny Lester
Jenny Lester, MPH, CCRP
1Department of Medical Oncology (M.S.B., K.R.K.) and Department of Health Sciences Research (R.A.V., S.J.W., B.M.M., Z.L., E.L.G.), Mayo Clinic, Rochester, MN; Department of Pathology and Laboratory Medicine, University of Calgary, Calgary, Alberta, Canada (P.F.R., M.K.); Pathology Department, Catholic University of Health and Allied Sciences-Bugando, Mwanza, Tanzania (P.F.R.); Tübingen University Hospital, Department of Women’s Health, Tübingen, Germany (P.W., F.A.T., A.D.H., S.Y.B., S.K.); Sir Peter MacCallum Department of Oncology, the University of Melbourne, Parkville, Victoria, Australia (N.T., D.D.B.); International Hereditary Cancer Center, Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (A.T., O.O., J. Lubiński, J.G.); Department of Gynecology and Obstetrics, Ribeirão Preto Medical School, University of São Paulo, Ribeirão Preto, Brazil (D.G.T., C.B.S., J.M.A., F.J.C.R.); Department of Pathology, Institute of Pathology, Heidelberg University Hospital, Heidelberg, Germany (P.S.); Department of Population Health Science and Policy, Department of Genetics and Genomic Sciences, Icahn School of Medicine at Mount Sinai, New York, NY (W.S., J.H.R.); Pathology West ICPMR Westmead, Westmead Hospital, the University of Sydney, Sydney, Australia (R.S.); University of Western Sydney at Westmead Hospital, Westmead, New South Wales, Australia (R.S.); Medical Oncology Service, Hospital Sant Pau, Barcelona, Spain (T.R.C.); H12O-CNIO Lung Cancer Clinical Research Unit (L.P.-A.), and Oncology Department, Hospital Universitario 12 de Octubre (L.P.-A.), Madrid, Spain; Women’s Cancer Program, Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, CA (S.O., J. Lester, B.Y.K.); the University of Texas School of Public Health, Houston (R.B.N.); Department of Oncology, Division of Gynecologic Oncology, Cumming School of Medicine, University of Calgary, Calgary, Alberta, Canada (G.N., P.G.); Department of Obstetrics, Gynecology and Reproductive Sciences, University of Pittsburgh School of Medicine (F.M., R.P.E.), Department of Epidemiology, University of Pittsburgh Graduate School of Public Health (F.M.), and Womens Cancer Research Program, Magee-Womens Research Institute and University of Pittsburgh Cancer Institute (F.M.), Pittsburgh, PA; Department of Gynecological Surgery and Gynecological Oncology of Adults and Adolescents, Pomeranian Medical University, Szczecin, Poland (J.M., A.C.-G.); Department of Health Research and Policy, Stanford University School of Medicine, Stanford, CA (V.M.); Department of Oncology, University of Cambridge, Strangeways Research Laboratory, Cambridge, UK (M.M., J.A., P.D.P.P.); Department of Pathology, Stanford University, Stanford, CA (T.A.L.); Department of Gynecologic Oncology, the Third Affiliated Hospital of Kunming Medical University (Yunnan Tumor Hospital), Kunming, China (Z.L.); Centre for Cancer Research, the Westmead Institute for Medical Research, the University of Sydney (C.J.K., J.H., P.R.H., B. Gao, A.deF.), and Department of Gynaecological Oncology, Westmead Hospital (C.J.K., J.H., A.deF.), Sydney, New South Wales, Australia; Division of Cancer Epidemiology, German Cancer Research Center (Deutsches Krebsforschungszentrum), Heidelberg, Germany (A.Y.J., U.E., J.C.-C.); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia (S.E.J., G.C.-T.); Department of Histopathology, Addenbrookes Hospital, Cambridge, UK (M.J.-L.); Virus, Lifestyle and Genes, Danish Cancer Society Research Center, Copenhagen, Denmark (A.J., S.K.K.); School of Women’s and Children’s Health, University of New South Wales, Sydney, Australia (M.P.I., S.J.R.); Tissue Bank of the National Center for Tumor Diseases (NCT) Heidelberg (E. Herpel), and Institute of Pathology, University of Heidelberg (E. Herpel), Heidelberg, Germany; Cancer Center, University of Hawaii, Honolulu, HI (B.Y.H.); the Crown Princess Mary Cancer Centre, Westmead Hospital, the University of Sydney, Sydney, Australia (P.R.H., B. Gao); Oncology Department, Hospital General de Albacete, Madrid, Spain (J.M.G.-B.); Department of Cancer Genomics and Genetics, Peter MacCallum Cancer Centre, East Melbourne, Victoria, Australia (S.F.); Pathology Department, Fundación Jiménez Díaz, Madrid, Spain (A.C.); Department of Health Research and Policy and Department of Biomedical Data Science, Stanford University School of Medicine, Stanford, CA (A.S.W.); Department of Obstetrics and Gynecology, Division of Gynecologic Oncology, Royal Alexandra Hospital, Edmonton, Alberta, Canada (H.S.); Tübingen University Hospital, Institute of Pathology, Tübingen, Germany (A.S.); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (K.B.M.); Gynaecological Cancer Research Centre, Department of Women’s Cancer, Institute for Women’s Health, University College London, London, UK (U.M.); Alberta Health Services-Cancer Care, Calgary, Alberta, Canada (J.M.K.); Department of Gynecology, Rigshospitalet, University of Copenhagen, Copenhagen, Denmark (S.K.K.); Department of Public Health Sciences, Medical University of South Carolina and Hollings Cancer Center, Charleston, SC (L.E.K.); Department of Pathology and Laboratory Medicine, University of British Columbia (D.G.H.), and Centre for Translational and Applied Genomics, British Columbia Cancer Agency (D.G.H.), Vancouver, British Columbia, Canada; Molecular Unit, Department of Pathology, Herlev Hospital, University of Copenhagen, Copenhagen, Denmark (E. Høgdall); Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, CA (M.T.G.); Genetic Pathology Evaluation Centre, Vancouver General Hospital and University of British Columbia, Vancouver, British Columbia, Canada (B. Gilks); Human Genetics Group, Spanish National Cancer Research Centre (Centro Nacional de Investigaciones Oncológicas) (M.J.G., J.B.), and Centre for Biomedical Network Research on Rare Diseases (Centro de Investigación Biomédica en Red de Enfermedades Raras), Instituto de Salud Carlos III (M.J.G., J.B.), Madrid, Spain; David Geffen School of Medicine, Department of Medicine, Division of Hematology and Oncology, University of California at Los Angeles (P.A.F.); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Erlangen, Germany (P.A.F.); Department of Histopathology, Queen’s Medical Centre, Nottingham University Hospitals NHS Trust, Nottingham, UK (S.D.); University Cancer Center Hamburg, University Medical Center Hamburg-Eppendorf, Hamburg, Germany (J.C.-C.); Cancer Genetics Laboratory, Research Division, Peter MacCallum Cancer Centre (I.G.C.), Sir Peter MacCallum Department of Oncology, University of Melbourne (I.G.C.), and Department of Pathology, University of Melbourne (I.G.C.), Melbourne, Victoria, Australia; Department of Oncology, University of Cambridge, Hutchison/MRC Research Centre (J.D.B.), Cancer Research UK Cambridge Institute, University of Cambridge, Li Ka Shing Centre (J.D.B.), and Cambridge Experimental Cancer Medicine Centre (J.D.B.), Cambridge, UK; Cancer Genomics Program, Research Department, Peter MacCallum Cancer Centre, East Melbourne, Victoria, Australia (D.D.B.); the Garvan Institute, Sydney, New South Wales, Australia (D.D.B., S.J.R.); and Department of Public Health and Primary Care, University of Cambridge, Strangeways Research Laboratory, Cambridge, UK (P.D.P.P.)
1,
Catherine J Kennedy
Catherine J Kennedy, BSc (Hons)
1Department of Medical Oncology (M.S.B., K.R.K.) and Department of Health Sciences Research (R.A.V., S.J.W., B.M.M., Z.L., E.L.G.), Mayo Clinic, Rochester, MN; Department of Pathology and Laboratory Medicine, University of Calgary, Calgary, Alberta, Canada (P.F.R., M.K.); Pathology Department, Catholic University of Health and Allied Sciences-Bugando, Mwanza, Tanzania (P.F.R.); Tübingen University Hospital, Department of Women’s Health, Tübingen, Germany (P.W., F.A.T., A.D.H., S.Y.B., S.K.); Sir Peter MacCallum Department of Oncology, the University of Melbourne, Parkville, Victoria, Australia (N.T., D.D.B.); International Hereditary Cancer Center, Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (A.T., O.O., J. Lubiński, J.G.); Department of Gynecology and Obstetrics, Ribeirão Preto Medical School, University of São Paulo, Ribeirão Preto, Brazil (D.G.T., C.B.S., J.M.A., F.J.C.R.); Department of Pathology, Institute of Pathology, Heidelberg University Hospital, Heidelberg, Germany (P.S.); Department of Population Health Science and Policy, Department of Genetics and Genomic Sciences, Icahn School of Medicine at Mount Sinai, New York, NY (W.S., J.H.R.); Pathology West ICPMR Westmead, Westmead Hospital, the University of Sydney, Sydney, Australia (R.S.); University of Western Sydney at Westmead Hospital, Westmead, New South Wales, Australia (R.S.); Medical Oncology Service, Hospital Sant Pau, Barcelona, Spain (T.R.C.); H12O-CNIO Lung Cancer Clinical Research Unit (L.P.-A.), and Oncology Department, Hospital Universitario 12 de Octubre (L.P.-A.), Madrid, Spain; Women’s Cancer Program, Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, CA (S.O., J. Lester, B.Y.K.); the University of Texas School of Public Health, Houston (R.B.N.); Department of Oncology, Division of Gynecologic Oncology, Cumming School of Medicine, University of Calgary, Calgary, Alberta, Canada (G.N., P.G.); Department of Obstetrics, Gynecology and Reproductive Sciences, University of Pittsburgh School of Medicine (F.M., R.P.E.), Department of Epidemiology, University of Pittsburgh Graduate School of Public Health (F.M.), and Womens Cancer Research Program, Magee-Womens Research Institute and University of Pittsburgh Cancer Institute (F.M.), Pittsburgh, PA; Department of Gynecological Surgery and Gynecological Oncology of Adults and Adolescents, Pomeranian Medical University, Szczecin, Poland (J.M., A.C.-G.); Department of Health Research and Policy, Stanford University School of Medicine, Stanford, CA (V.M.); Department of Oncology, University of Cambridge, Strangeways Research Laboratory, Cambridge, UK (M.M., J.A., P.D.P.P.); Department of Pathology, Stanford University, Stanford, CA (T.A.L.); Department of Gynecologic Oncology, the Third Affiliated Hospital of Kunming Medical University (Yunnan Tumor Hospital), Kunming, China (Z.L.); Centre for Cancer Research, the Westmead Institute for Medical Research, the University of Sydney (C.J.K., J.H., P.R.H., B. Gao, A.deF.), and Department of Gynaecological Oncology, Westmead Hospital (C.J.K., J.H., A.deF.), Sydney, New South Wales, Australia; Division of Cancer Epidemiology, German Cancer Research Center (Deutsches Krebsforschungszentrum), Heidelberg, Germany (A.Y.J., U.E., J.C.-C.); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia (S.E.J., G.C.-T.); Department of Histopathology, Addenbrookes Hospital, Cambridge, UK (M.J.-L.); Virus, Lifestyle and Genes, Danish Cancer Society Research Center, Copenhagen, Denmark (A.J., S.K.K.); School of Women’s and Children’s Health, University of New South Wales, Sydney, Australia (M.P.I., S.J.R.); Tissue Bank of the National Center for Tumor Diseases (NCT) Heidelberg (E. Herpel), and Institute of Pathology, University of Heidelberg (E. Herpel), Heidelberg, Germany; Cancer Center, University of Hawaii, Honolulu, HI (B.Y.H.); the Crown Princess Mary Cancer Centre, Westmead Hospital, the University of Sydney, Sydney, Australia (P.R.H., B. Gao); Oncology Department, Hospital General de Albacete, Madrid, Spain (J.M.G.-B.); Department of Cancer Genomics and Genetics, Peter MacCallum Cancer Centre, East Melbourne, Victoria, Australia (S.F.); Pathology Department, Fundación Jiménez Díaz, Madrid, Spain (A.C.); Department of Health Research and Policy and Department of Biomedical Data Science, Stanford University School of Medicine, Stanford, CA (A.S.W.); Department of Obstetrics and Gynecology, Division of Gynecologic Oncology, Royal Alexandra Hospital, Edmonton, Alberta, Canada (H.S.); Tübingen University Hospital, Institute of Pathology, Tübingen, Germany (A.S.); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (K.B.M.); Gynaecological Cancer Research Centre, Department of Women’s Cancer, Institute for Women’s Health, University College London, London, UK (U.M.); Alberta Health Services-Cancer Care, Calgary, Alberta, Canada (J.M.K.); Department of Gynecology, Rigshospitalet, University of Copenhagen, Copenhagen, Denmark (S.K.K.); Department of Public Health Sciences, Medical University of South Carolina and Hollings Cancer Center, Charleston, SC (L.E.K.); Department of Pathology and Laboratory Medicine, University of British Columbia (D.G.H.), and Centre for Translational and Applied Genomics, British Columbia Cancer Agency (D.G.H.), Vancouver, British Columbia, Canada; Molecular Unit, Department of Pathology, Herlev Hospital, University of Copenhagen, Copenhagen, Denmark (E. Høgdall); Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, CA (M.T.G.); Genetic Pathology Evaluation Centre, Vancouver General Hospital and University of British Columbia, Vancouver, British Columbia, Canada (B. Gilks); Human Genetics Group, Spanish National Cancer Research Centre (Centro Nacional de Investigaciones Oncológicas) (M.J.G., J.B.), and Centre for Biomedical Network Research on Rare Diseases (Centro de Investigación Biomédica en Red de Enfermedades Raras), Instituto de Salud Carlos III (M.J.G., J.B.), Madrid, Spain; David Geffen School of Medicine, Department of Medicine, Division of Hematology and Oncology, University of California at Los Angeles (P.A.F.); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Erlangen, Germany (P.A.F.); Department of Histopathology, Queen’s Medical Centre, Nottingham University Hospitals NHS Trust, Nottingham, UK (S.D.); University Cancer Center Hamburg, University Medical Center Hamburg-Eppendorf, Hamburg, Germany (J.C.-C.); Cancer Genetics Laboratory, Research Division, Peter MacCallum Cancer Centre (I.G.C.), Sir Peter MacCallum Department of Oncology, University of Melbourne (I.G.C.), and Department of Pathology, University of Melbourne (I.G.C.), Melbourne, Victoria, Australia; Department of Oncology, University of Cambridge, Hutchison/MRC Research Centre (J.D.B.), Cancer Research UK Cambridge Institute, University of Cambridge, Li Ka Shing Centre (J.D.B.), and Cambridge Experimental Cancer Medicine Centre (J.D.B.), Cambridge, UK; Cancer Genomics Program, Research Department, Peter MacCallum Cancer Centre, East Melbourne, Victoria, Australia (D.D.B.); the Garvan Institute, Sydney, New South Wales, Australia (D.D.B., S.J.R.); and Department of Public Health and Primary Care, University of Cambridge, Strangeways Research Laboratory, Cambridge, UK (P.D.P.P.)
1,
Kimberly R Kalli
Kimberly R Kalli, PhD
1Department of Medical Oncology (M.S.B., K.R.K.) and Department of Health Sciences Research (R.A.V., S.J.W., B.M.M., Z.L., E.L.G.), Mayo Clinic, Rochester, MN; Department of Pathology and Laboratory Medicine, University of Calgary, Calgary, Alberta, Canada (P.F.R., M.K.); Pathology Department, Catholic University of Health and Allied Sciences-Bugando, Mwanza, Tanzania (P.F.R.); Tübingen University Hospital, Department of Women’s Health, Tübingen, Germany (P.W., F.A.T., A.D.H., S.Y.B., S.K.); Sir Peter MacCallum Department of Oncology, the University of Melbourne, Parkville, Victoria, Australia (N.T., D.D.B.); International Hereditary Cancer Center, Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (A.T., O.O., J. Lubiński, J.G.); Department of Gynecology and Obstetrics, Ribeirão Preto Medical School, University of São Paulo, Ribeirão Preto, Brazil (D.G.T., C.B.S., J.M.A., F.J.C.R.); Department of Pathology, Institute of Pathology, Heidelberg University Hospital, Heidelberg, Germany (P.S.); Department of Population Health Science and Policy, Department of Genetics and Genomic Sciences, Icahn School of Medicine at Mount Sinai, New York, NY (W.S., J.H.R.); Pathology West ICPMR Westmead, Westmead Hospital, the University of Sydney, Sydney, Australia (R.S.); University of Western Sydney at Westmead Hospital, Westmead, New South Wales, Australia (R.S.); Medical Oncology Service, Hospital Sant Pau, Barcelona, Spain (T.R.C.); H12O-CNIO Lung Cancer Clinical Research Unit (L.P.-A.), and Oncology Department, Hospital Universitario 12 de Octubre (L.P.-A.), Madrid, Spain; Women’s Cancer Program, Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, CA (S.O., J. Lester, B.Y.K.); the University of Texas School of Public Health, Houston (R.B.N.); Department of Oncology, Division of Gynecologic Oncology, Cumming School of Medicine, University of Calgary, Calgary, Alberta, Canada (G.N., P.G.); Department of Obstetrics, Gynecology and Reproductive Sciences, University of Pittsburgh School of Medicine (F.M., R.P.E.), Department of Epidemiology, University of Pittsburgh Graduate School of Public Health (F.M.), and Womens Cancer Research Program, Magee-Womens Research Institute and University of Pittsburgh Cancer Institute (F.M.), Pittsburgh, PA; Department of Gynecological Surgery and Gynecological Oncology of Adults and Adolescents, Pomeranian Medical University, Szczecin, Poland (J.M., A.C.-G.); Department of Health Research and Policy, Stanford University School of Medicine, Stanford, CA (V.M.); Department of Oncology, University of Cambridge, Strangeways Research Laboratory, Cambridge, UK (M.M., J.A., P.D.P.P.); Department of Pathology, Stanford University, Stanford, CA (T.A.L.); Department of Gynecologic Oncology, the Third Affiliated Hospital of Kunming Medical University (Yunnan Tumor Hospital), Kunming, China (Z.L.); Centre for Cancer Research, the Westmead Institute for Medical Research, the University of Sydney (C.J.K., J.H., P.R.H., B. Gao, A.deF.), and Department of Gynaecological Oncology, Westmead Hospital (C.J.K., J.H., A.deF.), Sydney, New South Wales, Australia; Division of Cancer Epidemiology, German Cancer Research Center (Deutsches Krebsforschungszentrum), Heidelberg, Germany (A.Y.J., U.E., J.C.-C.); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia (S.E.J., G.C.-T.); Department of Histopathology, Addenbrookes Hospital, Cambridge, UK (M.J.-L.); Virus, Lifestyle and Genes, Danish Cancer Society Research Center, Copenhagen, Denmark (A.J., S.K.K.); School of Women’s and Children’s Health, University of New South Wales, Sydney, Australia (M.P.I., S.J.R.); Tissue Bank of the National Center for Tumor Diseases (NCT) Heidelberg (E. Herpel), and Institute of Pathology, University of Heidelberg (E. Herpel), Heidelberg, Germany; Cancer Center, University of Hawaii, Honolulu, HI (B.Y.H.); the Crown Princess Mary Cancer Centre, Westmead Hospital, the University of Sydney, Sydney, Australia (P.R.H., B. Gao); Oncology Department, Hospital General de Albacete, Madrid, Spain (J.M.G.-B.); Department of Cancer Genomics and Genetics, Peter MacCallum Cancer Centre, East Melbourne, Victoria, Australia (S.F.); Pathology Department, Fundación Jiménez Díaz, Madrid, Spain (A.C.); Department of Health Research and Policy and Department of Biomedical Data Science, Stanford University School of Medicine, Stanford, CA (A.S.W.); Department of Obstetrics and Gynecology, Division of Gynecologic Oncology, Royal Alexandra Hospital, Edmonton, Alberta, Canada (H.S.); Tübingen University Hospital, Institute of Pathology, Tübingen, Germany (A.S.); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (K.B.M.); Gynaecological Cancer Research Centre, Department of Women’s Cancer, Institute for Women’s Health, University College London, London, UK (U.M.); Alberta Health Services-Cancer Care, Calgary, Alberta, Canada (J.M.K.); Department of Gynecology, Rigshospitalet, University of Copenhagen, Copenhagen, Denmark (S.K.K.); Department of Public Health Sciences, Medical University of South Carolina and Hollings Cancer Center, Charleston, SC (L.E.K.); Department of Pathology and Laboratory Medicine, University of British Columbia (D.G.H.), and Centre for Translational and Applied Genomics, British Columbia Cancer Agency (D.G.H.), Vancouver, British Columbia, Canada; Molecular Unit, Department of Pathology, Herlev Hospital, University of Copenhagen, Copenhagen, Denmark (E. Høgdall); Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, CA (M.T.G.); Genetic Pathology Evaluation Centre, Vancouver General Hospital and University of British Columbia, Vancouver, British Columbia, Canada (B. Gilks); Human Genetics Group, Spanish National Cancer Research Centre (Centro Nacional de Investigaciones Oncológicas) (M.J.G., J.B.), and Centre for Biomedical Network Research on Rare Diseases (Centro de Investigación Biomédica en Red de Enfermedades Raras), Instituto de Salud Carlos III (M.J.G., J.B.), Madrid, Spain; David Geffen School of Medicine, Department of Medicine, Division of Hematology and Oncology, University of California at Los Angeles (P.A.F.); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Erlangen, Germany (P.A.F.); Department of Histopathology, Queen’s Medical Centre, Nottingham University Hospitals NHS Trust, Nottingham, UK (S.D.); University Cancer Center Hamburg, University Medical Center Hamburg-Eppendorf, Hamburg, Germany (J.C.-C.); Cancer Genetics Laboratory, Research Division, Peter MacCallum Cancer Centre (I.G.C.), Sir Peter MacCallum Department of Oncology, University of Melbourne (I.G.C.), and Department of Pathology, University of Melbourne (I.G.C.), Melbourne, Victoria, Australia; Department of Oncology, University of Cambridge, Hutchison/MRC Research Centre (J.D.B.), Cancer Research UK Cambridge Institute, University of Cambridge, Li Ka Shing Centre (J.D.B.), and Cambridge Experimental Cancer Medicine Centre (J.D.B.), Cambridge, UK; Cancer Genomics Program, Research Department, Peter MacCallum Cancer Centre, East Melbourne, Victoria, Australia (D.D.B.); the Garvan Institute, Sydney, New South Wales, Australia (D.D.B., S.J.R.); and Department of Public Health and Primary Care, University of Cambridge, Strangeways Research Laboratory, Cambridge, UK (P.D.P.P.)
1,
Audrey Y Jung
Audrey Y Jung, PhD
1Department of Medical Oncology (M.S.B., K.R.K.) and Department of Health Sciences Research (R.A.V., S.J.W., B.M.M., Z.L., E.L.G.), Mayo Clinic, Rochester, MN; Department of Pathology and Laboratory Medicine, University of Calgary, Calgary, Alberta, Canada (P.F.R., M.K.); Pathology Department, Catholic University of Health and Allied Sciences-Bugando, Mwanza, Tanzania (P.F.R.); Tübingen University Hospital, Department of Women’s Health, Tübingen, Germany (P.W., F.A.T., A.D.H., S.Y.B., S.K.); Sir Peter MacCallum Department of Oncology, the University of Melbourne, Parkville, Victoria, Australia (N.T., D.D.B.); International Hereditary Cancer Center, Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (A.T., O.O., J. Lubiński, J.G.); Department of Gynecology and Obstetrics, Ribeirão Preto Medical School, University of São Paulo, Ribeirão Preto, Brazil (D.G.T., C.B.S., J.M.A., F.J.C.R.); Department of Pathology, Institute of Pathology, Heidelberg University Hospital, Heidelberg, Germany (P.S.); Department of Population Health Science and Policy, Department of Genetics and Genomic Sciences, Icahn School of Medicine at Mount Sinai, New York, NY (W.S., J.H.R.); Pathology West ICPMR Westmead, Westmead Hospital, the University of Sydney, Sydney, Australia (R.S.); University of Western Sydney at Westmead Hospital, Westmead, New South Wales, Australia (R.S.); Medical Oncology Service, Hospital Sant Pau, Barcelona, Spain (T.R.C.); H12O-CNIO Lung Cancer Clinical Research Unit (L.P.-A.), and Oncology Department, Hospital Universitario 12 de Octubre (L.P.-A.), Madrid, Spain; Women’s Cancer Program, Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, CA (S.O., J. Lester, B.Y.K.); the University of Texas School of Public Health, Houston (R.B.N.); Department of Oncology, Division of Gynecologic Oncology, Cumming School of Medicine, University of Calgary, Calgary, Alberta, Canada (G.N., P.G.); Department of Obstetrics, Gynecology and Reproductive Sciences, University of Pittsburgh School of Medicine (F.M., R.P.E.), Department of Epidemiology, University of Pittsburgh Graduate School of Public Health (F.M.), and Womens Cancer Research Program, Magee-Womens Research Institute and University of Pittsburgh Cancer Institute (F.M.), Pittsburgh, PA; Department of Gynecological Surgery and Gynecological Oncology of Adults and Adolescents, Pomeranian Medical University, Szczecin, Poland (J.M., A.C.-G.); Department of Health Research and Policy, Stanford University School of Medicine, Stanford, CA (V.M.); Department of Oncology, University of Cambridge, Strangeways Research Laboratory, Cambridge, UK (M.M., J.A., P.D.P.P.); Department of Pathology, Stanford University, Stanford, CA (T.A.L.); Department of Gynecologic Oncology, the Third Affiliated Hospital of Kunming Medical University (Yunnan Tumor Hospital), Kunming, China (Z.L.); Centre for Cancer Research, the Westmead Institute for Medical Research, the University of Sydney (C.J.K., J.H., P.R.H., B. Gao, A.deF.), and Department of Gynaecological Oncology, Westmead Hospital (C.J.K., J.H., A.deF.), Sydney, New South Wales, Australia; Division of Cancer Epidemiology, German Cancer Research Center (Deutsches Krebsforschungszentrum), Heidelberg, Germany (A.Y.J., U.E., J.C.-C.); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia (S.E.J., G.C.-T.); Department of Histopathology, Addenbrookes Hospital, Cambridge, UK (M.J.-L.); Virus, Lifestyle and Genes, Danish Cancer Society Research Center, Copenhagen, Denmark (A.J., S.K.K.); School of Women’s and Children’s Health, University of New South Wales, Sydney, Australia (M.P.I., S.J.R.); Tissue Bank of the National Center for Tumor Diseases (NCT) Heidelberg (E. Herpel), and Institute of Pathology, University of Heidelberg (E. Herpel), Heidelberg, Germany; Cancer Center, University of Hawaii, Honolulu, HI (B.Y.H.); the Crown Princess Mary Cancer Centre, Westmead Hospital, the University of Sydney, Sydney, Australia (P.R.H., B. Gao); Oncology Department, Hospital General de Albacete, Madrid, Spain (J.M.G.-B.); Department of Cancer Genomics and Genetics, Peter MacCallum Cancer Centre, East Melbourne, Victoria, Australia (S.F.); Pathology Department, Fundación Jiménez Díaz, Madrid, Spain (A.C.); Department of Health Research and Policy and Department of Biomedical Data Science, Stanford University School of Medicine, Stanford, CA (A.S.W.); Department of Obstetrics and Gynecology, Division of Gynecologic Oncology, Royal Alexandra Hospital, Edmonton, Alberta, Canada (H.S.); Tübingen University Hospital, Institute of Pathology, Tübingen, Germany (A.S.); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (K.B.M.); Gynaecological Cancer Research Centre, Department of Women’s Cancer, Institute for Women’s Health, University College London, London, UK (U.M.); Alberta Health Services-Cancer Care, Calgary, Alberta, Canada (J.M.K.); Department of Gynecology, Rigshospitalet, University of Copenhagen, Copenhagen, Denmark (S.K.K.); Department of Public Health Sciences, Medical University of South Carolina and Hollings Cancer Center, Charleston, SC (L.E.K.); Department of Pathology and Laboratory Medicine, University of British Columbia (D.G.H.), and Centre for Translational and Applied Genomics, British Columbia Cancer Agency (D.G.H.), Vancouver, British Columbia, Canada; Molecular Unit, Department of Pathology, Herlev Hospital, University of Copenhagen, Copenhagen, Denmark (E. Høgdall); Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, CA (M.T.G.); Genetic Pathology Evaluation Centre, Vancouver General Hospital and University of British Columbia, Vancouver, British Columbia, Canada (B. Gilks); Human Genetics Group, Spanish National Cancer Research Centre (Centro Nacional de Investigaciones Oncológicas) (M.J.G., J.B.), and Centre for Biomedical Network Research on Rare Diseases (Centro de Investigación Biomédica en Red de Enfermedades Raras), Instituto de Salud Carlos III (M.J.G., J.B.), Madrid, Spain; David Geffen School of Medicine, Department of Medicine, Division of Hematology and Oncology, University of California at Los Angeles (P.A.F.); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Erlangen, Germany (P.A.F.); Department of Histopathology, Queen’s Medical Centre, Nottingham University Hospitals NHS Trust, Nottingham, UK (S.D.); University Cancer Center Hamburg, University Medical Center Hamburg-Eppendorf, Hamburg, Germany (J.C.-C.); Cancer Genetics Laboratory, Research Division, Peter MacCallum Cancer Centre (I.G.C.), Sir Peter MacCallum Department of Oncology, University of Melbourne (I.G.C.), and Department of Pathology, University of Melbourne (I.G.C.), Melbourne, Victoria, Australia; Department of Oncology, University of Cambridge, Hutchison/MRC Research Centre (J.D.B.), Cancer Research UK Cambridge Institute, University of Cambridge, Li Ka Shing Centre (J.D.B.), and Cambridge Experimental Cancer Medicine Centre (J.D.B.), Cambridge, UK; Cancer Genomics Program, Research Department, Peter MacCallum Cancer Centre, East Melbourne, Victoria, Australia (D.D.B.); the Garvan Institute, Sydney, New South Wales, Australia (D.D.B., S.J.R.); and Department of Public Health and Primary Care, University of Cambridge, Strangeways Research Laboratory, Cambridge, UK (P.D.P.P.)
1,
Sharon E Johnatty
Sharon E Johnatty, PhD
1Department of Medical Oncology (M.S.B., K.R.K.) and Department of Health Sciences Research (R.A.V., S.J.W., B.M.M., Z.L., E.L.G.), Mayo Clinic, Rochester, MN; Department of Pathology and Laboratory Medicine, University of Calgary, Calgary, Alberta, Canada (P.F.R., M.K.); Pathology Department, Catholic University of Health and Allied Sciences-Bugando, Mwanza, Tanzania (P.F.R.); Tübingen University Hospital, Department of Women’s Health, Tübingen, Germany (P.W., F.A.T., A.D.H., S.Y.B., S.K.); Sir Peter MacCallum Department of Oncology, the University of Melbourne, Parkville, Victoria, Australia (N.T., D.D.B.); International Hereditary Cancer Center, Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (A.T., O.O., J. Lubiński, J.G.); Department of Gynecology and Obstetrics, Ribeirão Preto Medical School, University of São Paulo, Ribeirão Preto, Brazil (D.G.T., C.B.S., J.M.A., F.J.C.R.); Department of Pathology, Institute of Pathology, Heidelberg University Hospital, Heidelberg, Germany (P.S.); Department of Population Health Science and Policy, Department of Genetics and Genomic Sciences, Icahn School of Medicine at Mount Sinai, New York, NY (W.S., J.H.R.); Pathology West ICPMR Westmead, Westmead Hospital, the University of Sydney, Sydney, Australia (R.S.); University of Western Sydney at Westmead Hospital, Westmead, New South Wales, Australia (R.S.); Medical Oncology Service, Hospital Sant Pau, Barcelona, Spain (T.R.C.); H12O-CNIO Lung Cancer Clinical Research Unit (L.P.-A.), and Oncology Department, Hospital Universitario 12 de Octubre (L.P.-A.), Madrid, Spain; Women’s Cancer Program, Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, CA (S.O., J. Lester, B.Y.K.); the University of Texas School of Public Health, Houston (R.B.N.); Department of Oncology, Division of Gynecologic Oncology, Cumming School of Medicine, University of Calgary, Calgary, Alberta, Canada (G.N., P.G.); Department of Obstetrics, Gynecology and Reproductive Sciences, University of Pittsburgh School of Medicine (F.M., R.P.E.), Department of Epidemiology, University of Pittsburgh Graduate School of Public Health (F.M.), and Womens Cancer Research Program, Magee-Womens Research Institute and University of Pittsburgh Cancer Institute (F.M.), Pittsburgh, PA; Department of Gynecological Surgery and Gynecological Oncology of Adults and Adolescents, Pomeranian Medical University, Szczecin, Poland (J.M., A.C.-G.); Department of Health Research and Policy, Stanford University School of Medicine, Stanford, CA (V.M.); Department of Oncology, University of Cambridge, Strangeways Research Laboratory, Cambridge, UK (M.M., J.A., P.D.P.P.); Department of Pathology, Stanford University, Stanford, CA (T.A.L.); Department of Gynecologic Oncology, the Third Affiliated Hospital of Kunming Medical University (Yunnan Tumor Hospital), Kunming, China (Z.L.); Centre for Cancer Research, the Westmead Institute for Medical Research, the University of Sydney (C.J.K., J.H., P.R.H., B. Gao, A.deF.), and Department of Gynaecological Oncology, Westmead Hospital (C.J.K., J.H., A.deF.), Sydney, New South Wales, Australia; Division of Cancer Epidemiology, German Cancer Research Center (Deutsches Krebsforschungszentrum), Heidelberg, Germany (A.Y.J., U.E., J.C.-C.); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia (S.E.J., G.C.-T.); Department of Histopathology, Addenbrookes Hospital, Cambridge, UK (M.J.-L.); Virus, Lifestyle and Genes, Danish Cancer Society Research Center, Copenhagen, Denmark (A.J., S.K.K.); School of Women’s and Children’s Health, University of New South Wales, Sydney, Australia (M.P.I., S.J.R.); Tissue Bank of the National Center for Tumor Diseases (NCT) Heidelberg (E. Herpel), and Institute of Pathology, University of Heidelberg (E. Herpel), Heidelberg, Germany; Cancer Center, University of Hawaii, Honolulu, HI (B.Y.H.); the Crown Princess Mary Cancer Centre, Westmead Hospital, the University of Sydney, Sydney, Australia (P.R.H., B. Gao); Oncology Department, Hospital General de Albacete, Madrid, Spain (J.M.G.-B.); Department of Cancer Genomics and Genetics, Peter MacCallum Cancer Centre, East Melbourne, Victoria, Australia (S.F.); Pathology Department, Fundación Jiménez Díaz, Madrid, Spain (A.C.); Department of Health Research and Policy and Department of Biomedical Data Science, Stanford University School of Medicine, Stanford, CA (A.S.W.); Department of Obstetrics and Gynecology, Division of Gynecologic Oncology, Royal Alexandra Hospital, Edmonton, Alberta, Canada (H.S.); Tübingen University Hospital, Institute of Pathology, Tübingen, Germany (A.S.); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (K.B.M.); Gynaecological Cancer Research Centre, Department of Women’s Cancer, Institute for Women’s Health, University College London, London, UK (U.M.); Alberta Health Services-Cancer Care, Calgary, Alberta, Canada (J.M.K.); Department of Gynecology, Rigshospitalet, University of Copenhagen, Copenhagen, Denmark (S.K.K.); Department of Public Health Sciences, Medical University of South Carolina and Hollings Cancer Center, Charleston, SC (L.E.K.); Department of Pathology and Laboratory Medicine, University of British Columbia (D.G.H.), and Centre for Translational and Applied Genomics, British Columbia Cancer Agency (D.G.H.), Vancouver, British Columbia, Canada; Molecular Unit, Department of Pathology, Herlev Hospital, University of Copenhagen, Copenhagen, Denmark (E. Høgdall); Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, CA (M.T.G.); Genetic Pathology Evaluation Centre, Vancouver General Hospital and University of British Columbia, Vancouver, British Columbia, Canada (B. Gilks); Human Genetics Group, Spanish National Cancer Research Centre (Centro Nacional de Investigaciones Oncológicas) (M.J.G., J.B.), and Centre for Biomedical Network Research on Rare Diseases (Centro de Investigación Biomédica en Red de Enfermedades Raras), Instituto de Salud Carlos III (M.J.G., J.B.), Madrid, Spain; David Geffen School of Medicine, Department of Medicine, Division of Hematology and Oncology, University of California at Los Angeles (P.A.F.); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Erlangen, Germany (P.A.F.); Department of Histopathology, Queen’s Medical Centre, Nottingham University Hospitals NHS Trust, Nottingham, UK (S.D.); University Cancer Center Hamburg, University Medical Center Hamburg-Eppendorf, Hamburg, Germany (J.C.-C.); Cancer Genetics Laboratory, Research Division, Peter MacCallum Cancer Centre (I.G.C.), Sir Peter MacCallum Department of Oncology, University of Melbourne (I.G.C.), and Department of Pathology, University of Melbourne (I.G.C.), Melbourne, Victoria, Australia; Department of Oncology, University of Cambridge, Hutchison/MRC Research Centre (J.D.B.), Cancer Research UK Cambridge Institute, University of Cambridge, Li Ka Shing Centre (J.D.B.), and Cambridge Experimental Cancer Medicine Centre (J.D.B.), Cambridge, UK; Cancer Genomics Program, Research Department, Peter MacCallum Cancer Centre, East Melbourne, Victoria, Australia (D.D.B.); the Garvan Institute, Sydney, New South Wales, Australia (D.D.B., S.J.R.); and Department of Public Health and Primary Care, University of Cambridge, Strangeways Research Laboratory, Cambridge, UK (P.D.P.P.)
1,
Mercedes Jimenez-Linan
Mercedes Jimenez-Linan, MD
1Department of Medical Oncology (M.S.B., K.R.K.) and Department of Health Sciences Research (R.A.V., S.J.W., B.M.M., Z.L., E.L.G.), Mayo Clinic, Rochester, MN; Department of Pathology and Laboratory Medicine, University of Calgary, Calgary, Alberta, Canada (P.F.R., M.K.); Pathology Department, Catholic University of Health and Allied Sciences-Bugando, Mwanza, Tanzania (P.F.R.); Tübingen University Hospital, Department of Women’s Health, Tübingen, Germany (P.W., F.A.T., A.D.H., S.Y.B., S.K.); Sir Peter MacCallum Department of Oncology, the University of Melbourne, Parkville, Victoria, Australia (N.T., D.D.B.); International Hereditary Cancer Center, Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (A.T., O.O., J. Lubiński, J.G.); Department of Gynecology and Obstetrics, Ribeirão Preto Medical School, University of São Paulo, Ribeirão Preto, Brazil (D.G.T., C.B.S., J.M.A., F.J.C.R.); Department of Pathology, Institute of Pathology, Heidelberg University Hospital, Heidelberg, Germany (P.S.); Department of Population Health Science and Policy, Department of Genetics and Genomic Sciences, Icahn School of Medicine at Mount Sinai, New York, NY (W.S., J.H.R.); Pathology West ICPMR Westmead, Westmead Hospital, the University of Sydney, Sydney, Australia (R.S.); University of Western Sydney at Westmead Hospital, Westmead, New South Wales, Australia (R.S.); Medical Oncology Service, Hospital Sant Pau, Barcelona, Spain (T.R.C.); H12O-CNIO Lung Cancer Clinical Research Unit (L.P.-A.), and Oncology Department, Hospital Universitario 12 de Octubre (L.P.-A.), Madrid, Spain; Women’s Cancer Program, Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, CA (S.O., J. Lester, B.Y.K.); the University of Texas School of Public Health, Houston (R.B.N.); Department of Oncology, Division of Gynecologic Oncology, Cumming School of Medicine, University of Calgary, Calgary, Alberta, Canada (G.N., P.G.); Department of Obstetrics, Gynecology and Reproductive Sciences, University of Pittsburgh School of Medicine (F.M., R.P.E.), Department of Epidemiology, University of Pittsburgh Graduate School of Public Health (F.M.), and Womens Cancer Research Program, Magee-Womens Research Institute and University of Pittsburgh Cancer Institute (F.M.), Pittsburgh, PA; Department of Gynecological Surgery and Gynecological Oncology of Adults and Adolescents, Pomeranian Medical University, Szczecin, Poland (J.M., A.C.-G.); Department of Health Research and Policy, Stanford University School of Medicine, Stanford, CA (V.M.); Department of Oncology, University of Cambridge, Strangeways Research Laboratory, Cambridge, UK (M.M., J.A., P.D.P.P.); Department of Pathology, Stanford University, Stanford, CA (T.A.L.); Department of Gynecologic Oncology, the Third Affiliated Hospital of Kunming Medical University (Yunnan Tumor Hospital), Kunming, China (Z.L.); Centre for Cancer Research, the Westmead Institute for Medical Research, the University of Sydney (C.J.K., J.H., P.R.H., B. Gao, A.deF.), and Department of Gynaecological Oncology, Westmead Hospital (C.J.K., J.H., A.deF.), Sydney, New South Wales, Australia; Division of Cancer Epidemiology, German Cancer Research Center (Deutsches Krebsforschungszentrum), Heidelberg, Germany (A.Y.J., U.E., J.C.-C.); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia (S.E.J., G.C.-T.); Department of Histopathology, Addenbrookes Hospital, Cambridge, UK (M.J.-L.); Virus, Lifestyle and Genes, Danish Cancer Society Research Center, Copenhagen, Denmark (A.J., S.K.K.); School of Women’s and Children’s Health, University of New South Wales, Sydney, Australia (M.P.I., S.J.R.); Tissue Bank of the National Center for Tumor Diseases (NCT) Heidelberg (E. Herpel), and Institute of Pathology, University of Heidelberg (E. Herpel), Heidelberg, Germany; Cancer Center, University of Hawaii, Honolulu, HI (B.Y.H.); the Crown Princess Mary Cancer Centre, Westmead Hospital, the University of Sydney, Sydney, Australia (P.R.H., B. Gao); Oncology Department, Hospital General de Albacete, Madrid, Spain (J.M.G.-B.); Department of Cancer Genomics and Genetics, Peter MacCallum Cancer Centre, East Melbourne, Victoria, Australia (S.F.); Pathology Department, Fundación Jiménez Díaz, Madrid, Spain (A.C.); Department of Health Research and Policy and Department of Biomedical Data Science, Stanford University School of Medicine, Stanford, CA (A.S.W.); Department of Obstetrics and Gynecology, Division of Gynecologic Oncology, Royal Alexandra Hospital, Edmonton, Alberta, Canada (H.S.); Tübingen University Hospital, Institute of Pathology, Tübingen, Germany (A.S.); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (K.B.M.); Gynaecological Cancer Research Centre, Department of Women’s Cancer, Institute for Women’s Health, University College London, London, UK (U.M.); Alberta Health Services-Cancer Care, Calgary, Alberta, Canada (J.M.K.); Department of Gynecology, Rigshospitalet, University of Copenhagen, Copenhagen, Denmark (S.K.K.); Department of Public Health Sciences, Medical University of South Carolina and Hollings Cancer Center, Charleston, SC (L.E.K.); Department of Pathology and Laboratory Medicine, University of British Columbia (D.G.H.), and Centre for Translational and Applied Genomics, British Columbia Cancer Agency (D.G.H.), Vancouver, British Columbia, Canada; Molecular Unit, Department of Pathology, Herlev Hospital, University of Copenhagen, Copenhagen, Denmark (E. Høgdall); Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, CA (M.T.G.); Genetic Pathology Evaluation Centre, Vancouver General Hospital and University of British Columbia, Vancouver, British Columbia, Canada (B. Gilks); Human Genetics Group, Spanish National Cancer Research Centre (Centro Nacional de Investigaciones Oncológicas) (M.J.G., J.B.), and Centre for Biomedical Network Research on Rare Diseases (Centro de Investigación Biomédica en Red de Enfermedades Raras), Instituto de Salud Carlos III (M.J.G., J.B.), Madrid, Spain; David Geffen School of Medicine, Department of Medicine, Division of Hematology and Oncology, University of California at Los Angeles (P.A.F.); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Erlangen, Germany (P.A.F.); Department of Histopathology, Queen’s Medical Centre, Nottingham University Hospitals NHS Trust, Nottingham, UK (S.D.); University Cancer Center Hamburg, University Medical Center Hamburg-Eppendorf, Hamburg, Germany (J.C.-C.); Cancer Genetics Laboratory, Research Division, Peter MacCallum Cancer Centre (I.G.C.), Sir Peter MacCallum Department of Oncology, University of Melbourne (I.G.C.), and Department of Pathology, University of Melbourne (I.G.C.), Melbourne, Victoria, Australia; Department of Oncology, University of Cambridge, Hutchison/MRC Research Centre (J.D.B.), Cancer Research UK Cambridge Institute, University of Cambridge, Li Ka Shing Centre (J.D.B.), and Cambridge Experimental Cancer Medicine Centre (J.D.B.), Cambridge, UK; Cancer Genomics Program, Research Department, Peter MacCallum Cancer Centre, East Melbourne, Victoria, Australia (D.D.B.); the Garvan Institute, Sydney, New South Wales, Australia (D.D.B., S.J.R.); and Department of Public Health and Primary Care, University of Cambridge, Strangeways Research Laboratory, Cambridge, UK (P.D.P.P.)
1,
Allan Jensen
Allan Jensen, PhD
1Department of Medical Oncology (M.S.B., K.R.K.) and Department of Health Sciences Research (R.A.V., S.J.W., B.M.M., Z.L., E.L.G.), Mayo Clinic, Rochester, MN; Department of Pathology and Laboratory Medicine, University of Calgary, Calgary, Alberta, Canada (P.F.R., M.K.); Pathology Department, Catholic University of Health and Allied Sciences-Bugando, Mwanza, Tanzania (P.F.R.); Tübingen University Hospital, Department of Women’s Health, Tübingen, Germany (P.W., F.A.T., A.D.H., S.Y.B., S.K.); Sir Peter MacCallum Department of Oncology, the University of Melbourne, Parkville, Victoria, Australia (N.T., D.D.B.); International Hereditary Cancer Center, Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (A.T., O.O., J. Lubiński, J.G.); Department of Gynecology and Obstetrics, Ribeirão Preto Medical School, University of São Paulo, Ribeirão Preto, Brazil (D.G.T., C.B.S., J.M.A., F.J.C.R.); Department of Pathology, Institute of Pathology, Heidelberg University Hospital, Heidelberg, Germany (P.S.); Department of Population Health Science and Policy, Department of Genetics and Genomic Sciences, Icahn School of Medicine at Mount Sinai, New York, NY (W.S., J.H.R.); Pathology West ICPMR Westmead, Westmead Hospital, the University of Sydney, Sydney, Australia (R.S.); University of Western Sydney at Westmead Hospital, Westmead, New South Wales, Australia (R.S.); Medical Oncology Service, Hospital Sant Pau, Barcelona, Spain (T.R.C.); H12O-CNIO Lung Cancer Clinical Research Unit (L.P.-A.), and Oncology Department, Hospital Universitario 12 de Octubre (L.P.-A.), Madrid, Spain; Women’s Cancer Program, Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, CA (S.O., J. Lester, B.Y.K.); the University of Texas School of Public Health, Houston (R.B.N.); Department of Oncology, Division of Gynecologic Oncology, Cumming School of Medicine, University of Calgary, Calgary, Alberta, Canada (G.N., P.G.); Department of Obstetrics, Gynecology and Reproductive Sciences, University of Pittsburgh School of Medicine (F.M., R.P.E.), Department of Epidemiology, University of Pittsburgh Graduate School of Public Health (F.M.), and Womens Cancer Research Program, Magee-Womens Research Institute and University of Pittsburgh Cancer Institute (F.M.), Pittsburgh, PA; Department of Gynecological Surgery and Gynecological Oncology of Adults and Adolescents, Pomeranian Medical University, Szczecin, Poland (J.M., A.C.-G.); Department of Health Research and Policy, Stanford University School of Medicine, Stanford, CA (V.M.); Department of Oncology, University of Cambridge, Strangeways Research Laboratory, Cambridge, UK (M.M., J.A., P.D.P.P.); Department of Pathology, Stanford University, Stanford, CA (T.A.L.); Department of Gynecologic Oncology, the Third Affiliated Hospital of Kunming Medical University (Yunnan Tumor Hospital), Kunming, China (Z.L.); Centre for Cancer Research, the Westmead Institute for Medical Research, the University of Sydney (C.J.K., J.H., P.R.H., B. Gao, A.deF.), and Department of Gynaecological Oncology, Westmead Hospital (C.J.K., J.H., A.deF.), Sydney, New South Wales, Australia; Division of Cancer Epidemiology, German Cancer Research Center (Deutsches Krebsforschungszentrum), Heidelberg, Germany (A.Y.J., U.E., J.C.-C.); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia (S.E.J., G.C.-T.); Department of Histopathology, Addenbrookes Hospital, Cambridge, UK (M.J.-L.); Virus, Lifestyle and Genes, Danish Cancer Society Research Center, Copenhagen, Denmark (A.J., S.K.K.); School of Women’s and Children’s Health, University of New South Wales, Sydney, Australia (M.P.I., S.J.R.); Tissue Bank of the National Center for Tumor Diseases (NCT) Heidelberg (E. Herpel), and Institute of Pathology, University of Heidelberg (E. Herpel), Heidelberg, Germany; Cancer Center, University of Hawaii, Honolulu, HI (B.Y.H.); the Crown Princess Mary Cancer Centre, Westmead Hospital, the University of Sydney, Sydney, Australia (P.R.H., B. Gao); Oncology Department, Hospital General de Albacete, Madrid, Spain (J.M.G.-B.); Department of Cancer Genomics and Genetics, Peter MacCallum Cancer Centre, East Melbourne, Victoria, Australia (S.F.); Pathology Department, Fundación Jiménez Díaz, Madrid, Spain (A.C.); Department of Health Research and Policy and Department of Biomedical Data Science, Stanford University School of Medicine, Stanford, CA (A.S.W.); Department of Obstetrics and Gynecology, Division of Gynecologic Oncology, Royal Alexandra Hospital, Edmonton, Alberta, Canada (H.S.); Tübingen University Hospital, Institute of Pathology, Tübingen, Germany (A.S.); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (K.B.M.); Gynaecological Cancer Research Centre, Department of Women’s Cancer, Institute for Women’s Health, University College London, London, UK (U.M.); Alberta Health Services-Cancer Care, Calgary, Alberta, Canada (J.M.K.); Department of Gynecology, Rigshospitalet, University of Copenhagen, Copenhagen, Denmark (S.K.K.); Department of Public Health Sciences, Medical University of South Carolina and Hollings Cancer Center, Charleston, SC (L.E.K.); Department of Pathology and Laboratory Medicine, University of British Columbia (D.G.H.), and Centre for Translational and Applied Genomics, British Columbia Cancer Agency (D.G.H.), Vancouver, British Columbia, Canada; Molecular Unit, Department of Pathology, Herlev Hospital, University of Copenhagen, Copenhagen, Denmark (E. Høgdall); Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, CA (M.T.G.); Genetic Pathology Evaluation Centre, Vancouver General Hospital and University of British Columbia, Vancouver, British Columbia, Canada (B. Gilks); Human Genetics Group, Spanish National Cancer Research Centre (Centro Nacional de Investigaciones Oncológicas) (M.J.G., J.B.), and Centre for Biomedical Network Research on Rare Diseases (Centro de Investigación Biomédica en Red de Enfermedades Raras), Instituto de Salud Carlos III (M.J.G., J.B.), Madrid, Spain; David Geffen School of Medicine, Department of Medicine, Division of Hematology and Oncology, University of California at Los Angeles (P.A.F.); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Erlangen, Germany (P.A.F.); Department of Histopathology, Queen’s Medical Centre, Nottingham University Hospitals NHS Trust, Nottingham, UK (S.D.); University Cancer Center Hamburg, University Medical Center Hamburg-Eppendorf, Hamburg, Germany (J.C.-C.); Cancer Genetics Laboratory, Research Division, Peter MacCallum Cancer Centre (I.G.C.), Sir Peter MacCallum Department of Oncology, University of Melbourne (I.G.C.), and Department of Pathology, University of Melbourne (I.G.C.), Melbourne, Victoria, Australia; Department of Oncology, University of Cambridge, Hutchison/MRC Research Centre (J.D.B.), Cancer Research UK Cambridge Institute, University of Cambridge, Li Ka Shing Centre (J.D.B.), and Cambridge Experimental Cancer Medicine Centre (J.D.B.), Cambridge, UK; Cancer Genomics Program, Research Department, Peter MacCallum Cancer Centre, East Melbourne, Victoria, Australia (D.D.B.); the Garvan Institute, Sydney, New South Wales, Australia (D.D.B., S.J.R.); and Department of Public Health and Primary Care, University of Cambridge, Strangeways Research Laboratory, Cambridge, UK (P.D.P.P.)
1,
Maria P Intermaggio
Maria P Intermaggio, MS
1Department of Medical Oncology (M.S.B., K.R.K.) and Department of Health Sciences Research (R.A.V., S.J.W., B.M.M., Z.L., E.L.G.), Mayo Clinic, Rochester, MN; Department of Pathology and Laboratory Medicine, University of Calgary, Calgary, Alberta, Canada (P.F.R., M.K.); Pathology Department, Catholic University of Health and Allied Sciences-Bugando, Mwanza, Tanzania (P.F.R.); Tübingen University Hospital, Department of Women’s Health, Tübingen, Germany (P.W., F.A.T., A.D.H., S.Y.B., S.K.); Sir Peter MacCallum Department of Oncology, the University of Melbourne, Parkville, Victoria, Australia (N.T., D.D.B.); International Hereditary Cancer Center, Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (A.T., O.O., J. Lubiński, J.G.); Department of Gynecology and Obstetrics, Ribeirão Preto Medical School, University of São Paulo, Ribeirão Preto, Brazil (D.G.T., C.B.S., J.M.A., F.J.C.R.); Department of Pathology, Institute of Pathology, Heidelberg University Hospital, Heidelberg, Germany (P.S.); Department of Population Health Science and Policy, Department of Genetics and Genomic Sciences, Icahn School of Medicine at Mount Sinai, New York, NY (W.S., J.H.R.); Pathology West ICPMR Westmead, Westmead Hospital, the University of Sydney, Sydney, Australia (R.S.); University of Western Sydney at Westmead Hospital, Westmead, New South Wales, Australia (R.S.); Medical Oncology Service, Hospital Sant Pau, Barcelona, Spain (T.R.C.); H12O-CNIO Lung Cancer Clinical Research Unit (L.P.-A.), and Oncology Department, Hospital Universitario 12 de Octubre (L.P.-A.), Madrid, Spain; Women’s Cancer Program, Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, CA (S.O., J. Lester, B.Y.K.); the University of Texas School of Public Health, Houston (R.B.N.); Department of Oncology, Division of Gynecologic Oncology, Cumming School of Medicine, University of Calgary, Calgary, Alberta, Canada (G.N., P.G.); Department of Obstetrics, Gynecology and Reproductive Sciences, University of Pittsburgh School of Medicine (F.M., R.P.E.), Department of Epidemiology, University of Pittsburgh Graduate School of Public Health (F.M.), and Womens Cancer Research Program, Magee-Womens Research Institute and University of Pittsburgh Cancer Institute (F.M.), Pittsburgh, PA; Department of Gynecological Surgery and Gynecological Oncology of Adults and Adolescents, Pomeranian Medical University, Szczecin, Poland (J.M., A.C.-G.); Department of Health Research and Policy, Stanford University School of Medicine, Stanford, CA (V.M.); Department of Oncology, University of Cambridge, Strangeways Research Laboratory, Cambridge, UK (M.M., J.A., P.D.P.P.); Department of Pathology, Stanford University, Stanford, CA (T.A.L.); Department of Gynecologic Oncology, the Third Affiliated Hospital of Kunming Medical University (Yunnan Tumor Hospital), Kunming, China (Z.L.); Centre for Cancer Research, the Westmead Institute for Medical Research, the University of Sydney (C.J.K., J.H., P.R.H., B. Gao, A.deF.), and Department of Gynaecological Oncology, Westmead Hospital (C.J.K., J.H., A.deF.), Sydney, New South Wales, Australia; Division of Cancer Epidemiology, German Cancer Research Center (Deutsches Krebsforschungszentrum), Heidelberg, Germany (A.Y.J., U.E., J.C.-C.); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia (S.E.J., G.C.-T.); Department of Histopathology, Addenbrookes Hospital, Cambridge, UK (M.J.-L.); Virus, Lifestyle and Genes, Danish Cancer Society Research Center, Copenhagen, Denmark (A.J., S.K.K.); School of Women’s and Children’s Health, University of New South Wales, Sydney, Australia (M.P.I., S.J.R.); Tissue Bank of the National Center for Tumor Diseases (NCT) Heidelberg (E. Herpel), and Institute of Pathology, University of Heidelberg (E. Herpel), Heidelberg, Germany; Cancer Center, University of Hawaii, Honolulu, HI (B.Y.H.); the Crown Princess Mary Cancer Centre, Westmead Hospital, the University of Sydney, Sydney, Australia (P.R.H., B. Gao); Oncology Department, Hospital General de Albacete, Madrid, Spain (J.M.G.-B.); Department of Cancer Genomics and Genetics, Peter MacCallum Cancer Centre, East Melbourne, Victoria, Australia (S.F.); Pathology Department, Fundación Jiménez Díaz, Madrid, Spain (A.C.); Department of Health Research and Policy and Department of Biomedical Data Science, Stanford University School of Medicine, Stanford, CA (A.S.W.); Department of Obstetrics and Gynecology, Division of Gynecologic Oncology, Royal Alexandra Hospital, Edmonton, Alberta, Canada (H.S.); Tübingen University Hospital, Institute of Pathology, Tübingen, Germany (A.S.); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (K.B.M.); Gynaecological Cancer Research Centre, Department of Women’s Cancer, Institute for Women’s Health, University College London, London, UK (U.M.); Alberta Health Services-Cancer Care, Calgary, Alberta, Canada (J.M.K.); Department of Gynecology, Rigshospitalet, University of Copenhagen, Copenhagen, Denmark (S.K.K.); Department of Public Health Sciences, Medical University of South Carolina and Hollings Cancer Center, Charleston, SC (L.E.K.); Department of Pathology and Laboratory Medicine, University of British Columbia (D.G.H.), and Centre for Translational and Applied Genomics, British Columbia Cancer Agency (D.G.H.), Vancouver, British Columbia, Canada; Molecular Unit, Department of Pathology, Herlev Hospital, University of Copenhagen, Copenhagen, Denmark (E. Høgdall); Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, CA (M.T.G.); Genetic Pathology Evaluation Centre, Vancouver General Hospital and University of British Columbia, Vancouver, British Columbia, Canada (B. Gilks); Human Genetics Group, Spanish National Cancer Research Centre (Centro Nacional de Investigaciones Oncológicas) (M.J.G., J.B.), and Centre for Biomedical Network Research on Rare Diseases (Centro de Investigación Biomédica en Red de Enfermedades Raras), Instituto de Salud Carlos III (M.J.G., J.B.), Madrid, Spain; David Geffen School of Medicine, Department of Medicine, Division of Hematology and Oncology, University of California at Los Angeles (P.A.F.); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Erlangen, Germany (P.A.F.); Department of Histopathology, Queen’s Medical Centre, Nottingham University Hospitals NHS Trust, Nottingham, UK (S.D.); University Cancer Center Hamburg, University Medical Center Hamburg-Eppendorf, Hamburg, Germany (J.C.-C.); Cancer Genetics Laboratory, Research Division, Peter MacCallum Cancer Centre (I.G.C.), Sir Peter MacCallum Department of Oncology, University of Melbourne (I.G.C.), and Department of Pathology, University of Melbourne (I.G.C.), Melbourne, Victoria, Australia; Department of Oncology, University of Cambridge, Hutchison/MRC Research Centre (J.D.B.), Cancer Research UK Cambridge Institute, University of Cambridge, Li Ka Shing Centre (J.D.B.), and Cambridge Experimental Cancer Medicine Centre (J.D.B.), Cambridge, UK; Cancer Genomics Program, Research Department, Peter MacCallum Cancer Centre, East Melbourne, Victoria, Australia (D.D.B.); the Garvan Institute, Sydney, New South Wales, Australia (D.D.B., S.J.R.); and Department of Public Health and Primary Care, University of Cambridge, Strangeways Research Laboratory, Cambridge, UK (P.D.P.P.)
1,
Jillian Hung
Jillian Hung, MHS
1Department of Medical Oncology (M.S.B., K.R.K.) and Department of Health Sciences Research (R.A.V., S.J.W., B.M.M., Z.L., E.L.G.), Mayo Clinic, Rochester, MN; Department of Pathology and Laboratory Medicine, University of Calgary, Calgary, Alberta, Canada (P.F.R., M.K.); Pathology Department, Catholic University of Health and Allied Sciences-Bugando, Mwanza, Tanzania (P.F.R.); Tübingen University Hospital, Department of Women’s Health, Tübingen, Germany (P.W., F.A.T., A.D.H., S.Y.B., S.K.); Sir Peter MacCallum Department of Oncology, the University of Melbourne, Parkville, Victoria, Australia (N.T., D.D.B.); International Hereditary Cancer Center, Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (A.T., O.O., J. Lubiński, J.G.); Department of Gynecology and Obstetrics, Ribeirão Preto Medical School, University of São Paulo, Ribeirão Preto, Brazil (D.G.T., C.B.S., J.M.A., F.J.C.R.); Department of Pathology, Institute of Pathology, Heidelberg University Hospital, Heidelberg, Germany (P.S.); Department of Population Health Science and Policy, Department of Genetics and Genomic Sciences, Icahn School of Medicine at Mount Sinai, New York, NY (W.S., J.H.R.); Pathology West ICPMR Westmead, Westmead Hospital, the University of Sydney, Sydney, Australia (R.S.); University of Western Sydney at Westmead Hospital, Westmead, New South Wales, Australia (R.S.); Medical Oncology Service, Hospital Sant Pau, Barcelona, Spain (T.R.C.); H12O-CNIO Lung Cancer Clinical Research Unit (L.P.-A.), and Oncology Department, Hospital Universitario 12 de Octubre (L.P.-A.), Madrid, Spain; Women’s Cancer Program, Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, CA (S.O., J. Lester, B.Y.K.); the University of Texas School of Public Health, Houston (R.B.N.); Department of Oncology, Division of Gynecologic Oncology, Cumming School of Medicine, University of Calgary, Calgary, Alberta, Canada (G.N., P.G.); Department of Obstetrics, Gynecology and Reproductive Sciences, University of Pittsburgh School of Medicine (F.M., R.P.E.), Department of Epidemiology, University of Pittsburgh Graduate School of Public Health (F.M.), and Womens Cancer Research Program, Magee-Womens Research Institute and University of Pittsburgh Cancer Institute (F.M.), Pittsburgh, PA; Department of Gynecological Surgery and Gynecological Oncology of Adults and Adolescents, Pomeranian Medical University, Szczecin, Poland (J.M., A.C.-G.); Department of Health Research and Policy, Stanford University School of Medicine, Stanford, CA (V.M.); Department of Oncology, University of Cambridge, Strangeways Research Laboratory, Cambridge, UK (M.M., J.A., P.D.P.P.); Department of Pathology, Stanford University, Stanford, CA (T.A.L.); Department of Gynecologic Oncology, the Third Affiliated Hospital of Kunming Medical University (Yunnan Tumor Hospital), Kunming, China (Z.L.); Centre for Cancer Research, the Westmead Institute for Medical Research, the University of Sydney (C.J.K., J.H., P.R.H., B. Gao, A.deF.), and Department of Gynaecological Oncology, Westmead Hospital (C.J.K., J.H., A.deF.), Sydney, New South Wales, Australia; Division of Cancer Epidemiology, German Cancer Research Center (Deutsches Krebsforschungszentrum), Heidelberg, Germany (A.Y.J., U.E., J.C.-C.); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia (S.E.J., G.C.-T.); Department of Histopathology, Addenbrookes Hospital, Cambridge, UK (M.J.-L.); Virus, Lifestyle and Genes, Danish Cancer Society Research Center, Copenhagen, Denmark (A.J., S.K.K.); School of Women’s and Children’s Health, University of New South Wales, Sydney, Australia (M.P.I., S.J.R.); Tissue Bank of the National Center for Tumor Diseases (NCT) Heidelberg (E. Herpel), and Institute of Pathology, University of Heidelberg (E. Herpel), Heidelberg, Germany; Cancer Center, University of Hawaii, Honolulu, HI (B.Y.H.); the Crown Princess Mary Cancer Centre, Westmead Hospital, the University of Sydney, Sydney, Australia (P.R.H., B. Gao); Oncology Department, Hospital General de Albacete, Madrid, Spain (J.M.G.-B.); Department of Cancer Genomics and Genetics, Peter MacCallum Cancer Centre, East Melbourne, Victoria, Australia (S.F.); Pathology Department, Fundación Jiménez Díaz, Madrid, Spain (A.C.); Department of Health Research and Policy and Department of Biomedical Data Science, Stanford University School of Medicine, Stanford, CA (A.S.W.); Department of Obstetrics and Gynecology, Division of Gynecologic Oncology, Royal Alexandra Hospital, Edmonton, Alberta, Canada (H.S.); Tübingen University Hospital, Institute of Pathology, Tübingen, Germany (A.S.); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (K.B.M.); Gynaecological Cancer Research Centre, Department of Women’s Cancer, Institute for Women’s Health, University College London, London, UK (U.M.); Alberta Health Services-Cancer Care, Calgary, Alberta, Canada (J.M.K.); Department of Gynecology, Rigshospitalet, University of Copenhagen, Copenhagen, Denmark (S.K.K.); Department of Public Health Sciences, Medical University of South Carolina and Hollings Cancer Center, Charleston, SC (L.E.K.); Department of Pathology and Laboratory Medicine, University of British Columbia (D.G.H.), and Centre for Translational and Applied Genomics, British Columbia Cancer Agency (D.G.H.), Vancouver, British Columbia, Canada; Molecular Unit, Department of Pathology, Herlev Hospital, University of Copenhagen, Copenhagen, Denmark (E. Høgdall); Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, CA (M.T.G.); Genetic Pathology Evaluation Centre, Vancouver General Hospital and University of British Columbia, Vancouver, British Columbia, Canada (B. Gilks); Human Genetics Group, Spanish National Cancer Research Centre (Centro Nacional de Investigaciones Oncológicas) (M.J.G., J.B.), and Centre for Biomedical Network Research on Rare Diseases (Centro de Investigación Biomédica en Red de Enfermedades Raras), Instituto de Salud Carlos III (M.J.G., J.B.), Madrid, Spain; David Geffen School of Medicine, Department of Medicine, Division of Hematology and Oncology, University of California at Los Angeles (P.A.F.); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Erlangen, Germany (P.A.F.); Department of Histopathology, Queen’s Medical Centre, Nottingham University Hospitals NHS Trust, Nottingham, UK (S.D.); University Cancer Center Hamburg, University Medical Center Hamburg-Eppendorf, Hamburg, Germany (J.C.-C.); Cancer Genetics Laboratory, Research Division, Peter MacCallum Cancer Centre (I.G.C.), Sir Peter MacCallum Department of Oncology, University of Melbourne (I.G.C.), and Department of Pathology, University of Melbourne (I.G.C.), Melbourne, Victoria, Australia; Department of Oncology, University of Cambridge, Hutchison/MRC Research Centre (J.D.B.), Cancer Research UK Cambridge Institute, University of Cambridge, Li Ka Shing Centre (J.D.B.), and Cambridge Experimental Cancer Medicine Centre (J.D.B.), Cambridge, UK; Cancer Genomics Program, Research Department, Peter MacCallum Cancer Centre, East Melbourne, Victoria, Australia (D.D.B.); the Garvan Institute, Sydney, New South Wales, Australia (D.D.B., S.J.R.); and Department of Public Health and Primary Care, University of Cambridge, Strangeways Research Laboratory, Cambridge, UK (P.D.P.P.)
1,
Esther Herpel
Esther Herpel, MD, PhD
1Department of Medical Oncology (M.S.B., K.R.K.) and Department of Health Sciences Research (R.A.V., S.J.W., B.M.M., Z.L., E.L.G.), Mayo Clinic, Rochester, MN; Department of Pathology and Laboratory Medicine, University of Calgary, Calgary, Alberta, Canada (P.F.R., M.K.); Pathology Department, Catholic University of Health and Allied Sciences-Bugando, Mwanza, Tanzania (P.F.R.); Tübingen University Hospital, Department of Women’s Health, Tübingen, Germany (P.W., F.A.T., A.D.H., S.Y.B., S.K.); Sir Peter MacCallum Department of Oncology, the University of Melbourne, Parkville, Victoria, Australia (N.T., D.D.B.); International Hereditary Cancer Center, Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (A.T., O.O., J. Lubiński, J.G.); Department of Gynecology and Obstetrics, Ribeirão Preto Medical School, University of São Paulo, Ribeirão Preto, Brazil (D.G.T., C.B.S., J.M.A., F.J.C.R.); Department of Pathology, Institute of Pathology, Heidelberg University Hospital, Heidelberg, Germany (P.S.); Department of Population Health Science and Policy, Department of Genetics and Genomic Sciences, Icahn School of Medicine at Mount Sinai, New York, NY (W.S., J.H.R.); Pathology West ICPMR Westmead, Westmead Hospital, the University of Sydney, Sydney, Australia (R.S.); University of Western Sydney at Westmead Hospital, Westmead, New South Wales, Australia (R.S.); Medical Oncology Service, Hospital Sant Pau, Barcelona, Spain (T.R.C.); H12O-CNIO Lung Cancer Clinical Research Unit (L.P.-A.), and Oncology Department, Hospital Universitario 12 de Octubre (L.P.-A.), Madrid, Spain; Women’s Cancer Program, Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, CA (S.O., J. Lester, B.Y.K.); the University of Texas School of Public Health, Houston (R.B.N.); Department of Oncology, Division of Gynecologic Oncology, Cumming School of Medicine, University of Calgary, Calgary, Alberta, Canada (G.N., P.G.); Department of Obstetrics, Gynecology and Reproductive Sciences, University of Pittsburgh School of Medicine (F.M., R.P.E.), Department of Epidemiology, University of Pittsburgh Graduate School of Public Health (F.M.), and Womens Cancer Research Program, Magee-Womens Research Institute and University of Pittsburgh Cancer Institute (F.M.), Pittsburgh, PA; Department of Gynecological Surgery and Gynecological Oncology of Adults and Adolescents, Pomeranian Medical University, Szczecin, Poland (J.M., A.C.-G.); Department of Health Research and Policy, Stanford University School of Medicine, Stanford, CA (V.M.); Department of Oncology, University of Cambridge, Strangeways Research Laboratory, Cambridge, UK (M.M., J.A., P.D.P.P.); Department of Pathology, Stanford University, Stanford, CA (T.A.L.); Department of Gynecologic Oncology, the Third Affiliated Hospital of Kunming Medical University (Yunnan Tumor Hospital), Kunming, China (Z.L.); Centre for Cancer Research, the Westmead Institute for Medical Research, the University of Sydney (C.J.K., J.H., P.R.H., B. Gao, A.deF.), and Department of Gynaecological Oncology, Westmead Hospital (C.J.K., J.H., A.deF.), Sydney, New South Wales, Australia; Division of Cancer Epidemiology, German Cancer Research Center (Deutsches Krebsforschungszentrum), Heidelberg, Germany (A.Y.J., U.E., J.C.-C.); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia (S.E.J., G.C.-T.); Department of Histopathology, Addenbrookes Hospital, Cambridge, UK (M.J.-L.); Virus, Lifestyle and Genes, Danish Cancer Society Research Center, Copenhagen, Denmark (A.J., S.K.K.); School of Women’s and Children’s Health, University of New South Wales, Sydney, Australia (M.P.I., S.J.R.); Tissue Bank of the National Center for Tumor Diseases (NCT) Heidelberg (E. Herpel), and Institute of Pathology, University of Heidelberg (E. Herpel), Heidelberg, Germany; Cancer Center, University of Hawaii, Honolulu, HI (B.Y.H.); the Crown Princess Mary Cancer Centre, Westmead Hospital, the University of Sydney, Sydney, Australia (P.R.H., B. Gao); Oncology Department, Hospital General de Albacete, Madrid, Spain (J.M.G.-B.); Department of Cancer Genomics and Genetics, Peter MacCallum Cancer Centre, East Melbourne, Victoria, Australia (S.F.); Pathology Department, Fundación Jiménez Díaz, Madrid, Spain (A.C.); Department of Health Research and Policy and Department of Biomedical Data Science, Stanford University School of Medicine, Stanford, CA (A.S.W.); Department of Obstetrics and Gynecology, Division of Gynecologic Oncology, Royal Alexandra Hospital, Edmonton, Alberta, Canada (H.S.); Tübingen University Hospital, Institute of Pathology, Tübingen, Germany (A.S.); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (K.B.M.); Gynaecological Cancer Research Centre, Department of Women’s Cancer, Institute for Women’s Health, University College London, London, UK (U.M.); Alberta Health Services-Cancer Care, Calgary, Alberta, Canada (J.M.K.); Department of Gynecology, Rigshospitalet, University of Copenhagen, Copenhagen, Denmark (S.K.K.); Department of Public Health Sciences, Medical University of South Carolina and Hollings Cancer Center, Charleston, SC (L.E.K.); Department of Pathology and Laboratory Medicine, University of British Columbia (D.G.H.), and Centre for Translational and Applied Genomics, British Columbia Cancer Agency (D.G.H.), Vancouver, British Columbia, Canada; Molecular Unit, Department of Pathology, Herlev Hospital, University of Copenhagen, Copenhagen, Denmark (E. Høgdall); Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, CA (M.T.G.); Genetic Pathology Evaluation Centre, Vancouver General Hospital and University of British Columbia, Vancouver, British Columbia, Canada (B. Gilks); Human Genetics Group, Spanish National Cancer Research Centre (Centro Nacional de Investigaciones Oncológicas) (M.J.G., J.B.), and Centre for Biomedical Network Research on Rare Diseases (Centro de Investigación Biomédica en Red de Enfermedades Raras), Instituto de Salud Carlos III (M.J.G., J.B.), Madrid, Spain; David Geffen School of Medicine, Department of Medicine, Division of Hematology and Oncology, University of California at Los Angeles (P.A.F.); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Erlangen, Germany (P.A.F.); Department of Histopathology, Queen’s Medical Centre, Nottingham University Hospitals NHS Trust, Nottingham, UK (S.D.); University Cancer Center Hamburg, University Medical Center Hamburg-Eppendorf, Hamburg, Germany (J.C.-C.); Cancer Genetics Laboratory, Research Division, Peter MacCallum Cancer Centre (I.G.C.), Sir Peter MacCallum Department of Oncology, University of Melbourne (I.G.C.), and Department of Pathology, University of Melbourne (I.G.C.), Melbourne, Victoria, Australia; Department of Oncology, University of Cambridge, Hutchison/MRC Research Centre (J.D.B.), Cancer Research UK Cambridge Institute, University of Cambridge, Li Ka Shing Centre (J.D.B.), and Cambridge Experimental Cancer Medicine Centre (J.D.B.), Cambridge, UK; Cancer Genomics Program, Research Department, Peter MacCallum Cancer Centre, East Melbourne, Victoria, Australia (D.D.B.); the Garvan Institute, Sydney, New South Wales, Australia (D.D.B., S.J.R.); and Department of Public Health and Primary Care, University of Cambridge, Strangeways Research Laboratory, Cambridge, UK (P.D.P.P.)
1,
Brenda Y Hernandez
Brenda Y Hernandez, PhD, MPH
1Department of Medical Oncology (M.S.B., K.R.K.) and Department of Health Sciences Research (R.A.V., S.J.W., B.M.M., Z.L., E.L.G.), Mayo Clinic, Rochester, MN; Department of Pathology and Laboratory Medicine, University of Calgary, Calgary, Alberta, Canada (P.F.R., M.K.); Pathology Department, Catholic University of Health and Allied Sciences-Bugando, Mwanza, Tanzania (P.F.R.); Tübingen University Hospital, Department of Women’s Health, Tübingen, Germany (P.W., F.A.T., A.D.H., S.Y.B., S.K.); Sir Peter MacCallum Department of Oncology, the University of Melbourne, Parkville, Victoria, Australia (N.T., D.D.B.); International Hereditary Cancer Center, Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (A.T., O.O., J. Lubiński, J.G.); Department of Gynecology and Obstetrics, Ribeirão Preto Medical School, University of São Paulo, Ribeirão Preto, Brazil (D.G.T., C.B.S., J.M.A., F.J.C.R.); Department of Pathology, Institute of Pathology, Heidelberg University Hospital, Heidelberg, Germany (P.S.); Department of Population Health Science and Policy, Department of Genetics and Genomic Sciences, Icahn School of Medicine at Mount Sinai, New York, NY (W.S., J.H.R.); Pathology West ICPMR Westmead, Westmead Hospital, the University of Sydney, Sydney, Australia (R.S.); University of Western Sydney at Westmead Hospital, Westmead, New South Wales, Australia (R.S.); Medical Oncology Service, Hospital Sant Pau, Barcelona, Spain (T.R.C.); H12O-CNIO Lung Cancer Clinical Research Unit (L.P.-A.), and Oncology Department, Hospital Universitario 12 de Octubre (L.P.-A.), Madrid, Spain; Women’s Cancer Program, Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, CA (S.O., J. Lester, B.Y.K.); the University of Texas School of Public Health, Houston (R.B.N.); Department of Oncology, Division of Gynecologic Oncology, Cumming School of Medicine, University of Calgary, Calgary, Alberta, Canada (G.N., P.G.); Department of Obstetrics, Gynecology and Reproductive Sciences, University of Pittsburgh School of Medicine (F.M., R.P.E.), Department of Epidemiology, University of Pittsburgh Graduate School of Public Health (F.M.), and Womens Cancer Research Program, Magee-Womens Research Institute and University of Pittsburgh Cancer Institute (F.M.), Pittsburgh, PA; Department of Gynecological Surgery and Gynecological Oncology of Adults and Adolescents, Pomeranian Medical University, Szczecin, Poland (J.M., A.C.-G.); Department of Health Research and Policy, Stanford University School of Medicine, Stanford, CA (V.M.); Department of Oncology, University of Cambridge, Strangeways Research Laboratory, Cambridge, UK (M.M., J.A., P.D.P.P.); Department of Pathology, Stanford University, Stanford, CA (T.A.L.); Department of Gynecologic Oncology, the Third Affiliated Hospital of Kunming Medical University (Yunnan Tumor Hospital), Kunming, China (Z.L.); Centre for Cancer Research, the Westmead Institute for Medical Research, the University of Sydney (C.J.K., J.H., P.R.H., B. Gao, A.deF.), and Department of Gynaecological Oncology, Westmead Hospital (C.J.K., J.H., A.deF.), Sydney, New South Wales, Australia; Division of Cancer Epidemiology, German Cancer Research Center (Deutsches Krebsforschungszentrum), Heidelberg, Germany (A.Y.J., U.E., J.C.-C.); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia (S.E.J., G.C.-T.); Department of Histopathology, Addenbrookes Hospital, Cambridge, UK (M.J.-L.); Virus, Lifestyle and Genes, Danish Cancer Society Research Center, Copenhagen, Denmark (A.J., S.K.K.); School of Women’s and Children’s Health, University of New South Wales, Sydney, Australia (M.P.I., S.J.R.); Tissue Bank of the National Center for Tumor Diseases (NCT) Heidelberg (E. Herpel), and Institute of Pathology, University of Heidelberg (E. Herpel), Heidelberg, Germany; Cancer Center, University of Hawaii, Honolulu, HI (B.Y.H.); the Crown Princess Mary Cancer Centre, Westmead Hospital, the University of Sydney, Sydney, Australia (P.R.H., B. Gao); Oncology Department, Hospital General de Albacete, Madrid, Spain (J.M.G.-B.); Department of Cancer Genomics and Genetics, Peter MacCallum Cancer Centre, East Melbourne, Victoria, Australia (S.F.); Pathology Department, Fundación Jiménez Díaz, Madrid, Spain (A.C.); Department of Health Research and Policy and Department of Biomedical Data Science, Stanford University School of Medicine, Stanford, CA (A.S.W.); Department of Obstetrics and Gynecology, Division of Gynecologic Oncology, Royal Alexandra Hospital, Edmonton, Alberta, Canada (H.S.); Tübingen University Hospital, Institute of Pathology, Tübingen, Germany (A.S.); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (K.B.M.); Gynaecological Cancer Research Centre, Department of Women’s Cancer, Institute for Women’s Health, University College London, London, UK (U.M.); Alberta Health Services-Cancer Care, Calgary, Alberta, Canada (J.M.K.); Department of Gynecology, Rigshospitalet, University of Copenhagen, Copenhagen, Denmark (S.K.K.); Department of Public Health Sciences, Medical University of South Carolina and Hollings Cancer Center, Charleston, SC (L.E.K.); Department of Pathology and Laboratory Medicine, University of British Columbia (D.G.H.), and Centre for Translational and Applied Genomics, British Columbia Cancer Agency (D.G.H.), Vancouver, British Columbia, Canada; Molecular Unit, Department of Pathology, Herlev Hospital, University of Copenhagen, Copenhagen, Denmark (E. Høgdall); Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, CA (M.T.G.); Genetic Pathology Evaluation Centre, Vancouver General Hospital and University of British Columbia, Vancouver, British Columbia, Canada (B. Gilks); Human Genetics Group, Spanish National Cancer Research Centre (Centro Nacional de Investigaciones Oncológicas) (M.J.G., J.B.), and Centre for Biomedical Network Research on Rare Diseases (Centro de Investigación Biomédica en Red de Enfermedades Raras), Instituto de Salud Carlos III (M.J.G., J.B.), Madrid, Spain; David Geffen School of Medicine, Department of Medicine, Division of Hematology and Oncology, University of California at Los Angeles (P.A.F.); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Erlangen, Germany (P.A.F.); Department of Histopathology, Queen’s Medical Centre, Nottingham University Hospitals NHS Trust, Nottingham, UK (S.D.); University Cancer Center Hamburg, University Medical Center Hamburg-Eppendorf, Hamburg, Germany (J.C.-C.); Cancer Genetics Laboratory, Research Division, Peter MacCallum Cancer Centre (I.G.C.), Sir Peter MacCallum Department of Oncology, University of Melbourne (I.G.C.), and Department of Pathology, University of Melbourne (I.G.C.), Melbourne, Victoria, Australia; Department of Oncology, University of Cambridge, Hutchison/MRC Research Centre (J.D.B.), Cancer Research UK Cambridge Institute, University of Cambridge, Li Ka Shing Centre (J.D.B.), and Cambridge Experimental Cancer Medicine Centre (J.D.B.), Cambridge, UK; Cancer Genomics Program, Research Department, Peter MacCallum Cancer Centre, East Melbourne, Victoria, Australia (D.D.B.); the Garvan Institute, Sydney, New South Wales, Australia (D.D.B., S.J.R.); and Department of Public Health and Primary Care, University of Cambridge, Strangeways Research Laboratory, Cambridge, UK (P.D.P.P.)
1,
Andreas D Hartkopf
Andreas D Hartkopf, MD
1Department of Medical Oncology (M.S.B., K.R.K.) and Department of Health Sciences Research (R.A.V., S.J.W., B.M.M., Z.L., E.L.G.), Mayo Clinic, Rochester, MN; Department of Pathology and Laboratory Medicine, University of Calgary, Calgary, Alberta, Canada (P.F.R., M.K.); Pathology Department, Catholic University of Health and Allied Sciences-Bugando, Mwanza, Tanzania (P.F.R.); Tübingen University Hospital, Department of Women’s Health, Tübingen, Germany (P.W., F.A.T., A.D.H., S.Y.B., S.K.); Sir Peter MacCallum Department of Oncology, the University of Melbourne, Parkville, Victoria, Australia (N.T., D.D.B.); International Hereditary Cancer Center, Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (A.T., O.O., J. Lubiński, J.G.); Department of Gynecology and Obstetrics, Ribeirão Preto Medical School, University of São Paulo, Ribeirão Preto, Brazil (D.G.T., C.B.S., J.M.A., F.J.C.R.); Department of Pathology, Institute of Pathology, Heidelberg University Hospital, Heidelberg, Germany (P.S.); Department of Population Health Science and Policy, Department of Genetics and Genomic Sciences, Icahn School of Medicine at Mount Sinai, New York, NY (W.S., J.H.R.); Pathology West ICPMR Westmead, Westmead Hospital, the University of Sydney, Sydney, Australia (R.S.); University of Western Sydney at Westmead Hospital, Westmead, New South Wales, Australia (R.S.); Medical Oncology Service, Hospital Sant Pau, Barcelona, Spain (T.R.C.); H12O-CNIO Lung Cancer Clinical Research Unit (L.P.-A.), and Oncology Department, Hospital Universitario 12 de Octubre (L.P.-A.), Madrid, Spain; Women’s Cancer Program, Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, CA (S.O., J. Lester, B.Y.K.); the University of Texas School of Public Health, Houston (R.B.N.); Department of Oncology, Division of Gynecologic Oncology, Cumming School of Medicine, University of Calgary, Calgary, Alberta, Canada (G.N., P.G.); Department of Obstetrics, Gynecology and Reproductive Sciences, University of Pittsburgh School of Medicine (F.M., R.P.E.), Department of Epidemiology, University of Pittsburgh Graduate School of Public Health (F.M.), and Womens Cancer Research Program, Magee-Womens Research Institute and University of Pittsburgh Cancer Institute (F.M.), Pittsburgh, PA; Department of Gynecological Surgery and Gynecological Oncology of Adults and Adolescents, Pomeranian Medical University, Szczecin, Poland (J.M., A.C.-G.); Department of Health Research and Policy, Stanford University School of Medicine, Stanford, CA (V.M.); Department of Oncology, University of Cambridge, Strangeways Research Laboratory, Cambridge, UK (M.M., J.A., P.D.P.P.); Department of Pathology, Stanford University, Stanford, CA (T.A.L.); Department of Gynecologic Oncology, the Third Affiliated Hospital of Kunming Medical University (Yunnan Tumor Hospital), Kunming, China (Z.L.); Centre for Cancer Research, the Westmead Institute for Medical Research, the University of Sydney (C.J.K., J.H., P.R.H., B. Gao, A.deF.), and Department of Gynaecological Oncology, Westmead Hospital (C.J.K., J.H., A.deF.), Sydney, New South Wales, Australia; Division of Cancer Epidemiology, German Cancer Research Center (Deutsches Krebsforschungszentrum), Heidelberg, Germany (A.Y.J., U.E., J.C.-C.); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia (S.E.J., G.C.-T.); Department of Histopathology, Addenbrookes Hospital, Cambridge, UK (M.J.-L.); Virus, Lifestyle and Genes, Danish Cancer Society Research Center, Copenhagen, Denmark (A.J., S.K.K.); School of Women’s and Children’s Health, University of New South Wales, Sydney, Australia (M.P.I., S.J.R.); Tissue Bank of the National Center for Tumor Diseases (NCT) Heidelberg (E. Herpel), and Institute of Pathology, University of Heidelberg (E. Herpel), Heidelberg, Germany; Cancer Center, University of Hawaii, Honolulu, HI (B.Y.H.); the Crown Princess Mary Cancer Centre, Westmead Hospital, the University of Sydney, Sydney, Australia (P.R.H., B. Gao); Oncology Department, Hospital General de Albacete, Madrid, Spain (J.M.G.-B.); Department of Cancer Genomics and Genetics, Peter MacCallum Cancer Centre, East Melbourne, Victoria, Australia (S.F.); Pathology Department, Fundación Jiménez Díaz, Madrid, Spain (A.C.); Department of Health Research and Policy and Department of Biomedical Data Science, Stanford University School of Medicine, Stanford, CA (A.S.W.); Department of Obstetrics and Gynecology, Division of Gynecologic Oncology, Royal Alexandra Hospital, Edmonton, Alberta, Canada (H.S.); Tübingen University Hospital, Institute of Pathology, Tübingen, Germany (A.S.); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (K.B.M.); Gynaecological Cancer Research Centre, Department of Women’s Cancer, Institute for Women’s Health, University College London, London, UK (U.M.); Alberta Health Services-Cancer Care, Calgary, Alberta, Canada (J.M.K.); Department of Gynecology, Rigshospitalet, University of Copenhagen, Copenhagen, Denmark (S.K.K.); Department of Public Health Sciences, Medical University of South Carolina and Hollings Cancer Center, Charleston, SC (L.E.K.); Department of Pathology and Laboratory Medicine, University of British Columbia (D.G.H.), and Centre for Translational and Applied Genomics, British Columbia Cancer Agency (D.G.H.), Vancouver, British Columbia, Canada; Molecular Unit, Department of Pathology, Herlev Hospital, University of Copenhagen, Copenhagen, Denmark (E. Høgdall); Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, CA (M.T.G.); Genetic Pathology Evaluation Centre, Vancouver General Hospital and University of British Columbia, Vancouver, British Columbia, Canada (B. Gilks); Human Genetics Group, Spanish National Cancer Research Centre (Centro Nacional de Investigaciones Oncológicas) (M.J.G., J.B.), and Centre for Biomedical Network Research on Rare Diseases (Centro de Investigación Biomédica en Red de Enfermedades Raras), Instituto de Salud Carlos III (M.J.G., J.B.), Madrid, Spain; David Geffen School of Medicine, Department of Medicine, Division of Hematology and Oncology, University of California at Los Angeles (P.A.F.); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Erlangen, Germany (P.A.F.); Department of Histopathology, Queen’s Medical Centre, Nottingham University Hospitals NHS Trust, Nottingham, UK (S.D.); University Cancer Center Hamburg, University Medical Center Hamburg-Eppendorf, Hamburg, Germany (J.C.-C.); Cancer Genetics Laboratory, Research Division, Peter MacCallum Cancer Centre (I.G.C.), Sir Peter MacCallum Department of Oncology, University of Melbourne (I.G.C.), and Department of Pathology, University of Melbourne (I.G.C.), Melbourne, Victoria, Australia; Department of Oncology, University of Cambridge, Hutchison/MRC Research Centre (J.D.B.), Cancer Research UK Cambridge Institute, University of Cambridge, Li Ka Shing Centre (J.D.B.), and Cambridge Experimental Cancer Medicine Centre (J.D.B.), Cambridge, UK; Cancer Genomics Program, Research Department, Peter MacCallum Cancer Centre, East Melbourne, Victoria, Australia (D.D.B.); the Garvan Institute, Sydney, New South Wales, Australia (D.D.B., S.J.R.); and Department of Public Health and Primary Care, University of Cambridge, Strangeways Research Laboratory, Cambridge, UK (P.D.P.P.)
1,
Paul R Harnett
Paul R Harnett, MBBS, PhD
1Department of Medical Oncology (M.S.B., K.R.K.) and Department of Health Sciences Research (R.A.V., S.J.W., B.M.M., Z.L., E.L.G.), Mayo Clinic, Rochester, MN; Department of Pathology and Laboratory Medicine, University of Calgary, Calgary, Alberta, Canada (P.F.R., M.K.); Pathology Department, Catholic University of Health and Allied Sciences-Bugando, Mwanza, Tanzania (P.F.R.); Tübingen University Hospital, Department of Women’s Health, Tübingen, Germany (P.W., F.A.T., A.D.H., S.Y.B., S.K.); Sir Peter MacCallum Department of Oncology, the University of Melbourne, Parkville, Victoria, Australia (N.T., D.D.B.); International Hereditary Cancer Center, Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (A.T., O.O., J. Lubiński, J.G.); Department of Gynecology and Obstetrics, Ribeirão Preto Medical School, University of São Paulo, Ribeirão Preto, Brazil (D.G.T., C.B.S., J.M.A., F.J.C.R.); Department of Pathology, Institute of Pathology, Heidelberg University Hospital, Heidelberg, Germany (P.S.); Department of Population Health Science and Policy, Department of Genetics and Genomic Sciences, Icahn School of Medicine at Mount Sinai, New York, NY (W.S., J.H.R.); Pathology West ICPMR Westmead, Westmead Hospital, the University of Sydney, Sydney, Australia (R.S.); University of Western Sydney at Westmead Hospital, Westmead, New South Wales, Australia (R.S.); Medical Oncology Service, Hospital Sant Pau, Barcelona, Spain (T.R.C.); H12O-CNIO Lung Cancer Clinical Research Unit (L.P.-A.), and Oncology Department, Hospital Universitario 12 de Octubre (L.P.-A.), Madrid, Spain; Women’s Cancer Program, Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, CA (S.O., J. Lester, B.Y.K.); the University of Texas School of Public Health, Houston (R.B.N.); Department of Oncology, Division of Gynecologic Oncology, Cumming School of Medicine, University of Calgary, Calgary, Alberta, Canada (G.N., P.G.); Department of Obstetrics, Gynecology and Reproductive Sciences, University of Pittsburgh School of Medicine (F.M., R.P.E.), Department of Epidemiology, University of Pittsburgh Graduate School of Public Health (F.M.), and Womens Cancer Research Program, Magee-Womens Research Institute and University of Pittsburgh Cancer Institute (F.M.), Pittsburgh, PA; Department of Gynecological Surgery and Gynecological Oncology of Adults and Adolescents, Pomeranian Medical University, Szczecin, Poland (J.M., A.C.-G.); Department of Health Research and Policy, Stanford University School of Medicine, Stanford, CA (V.M.); Department of Oncology, University of Cambridge, Strangeways Research Laboratory, Cambridge, UK (M.M., J.A., P.D.P.P.); Department of Pathology, Stanford University, Stanford, CA (T.A.L.); Department of Gynecologic Oncology, the Third Affiliated Hospital of Kunming Medical University (Yunnan Tumor Hospital), Kunming, China (Z.L.); Centre for Cancer Research, the Westmead Institute for Medical Research, the University of Sydney (C.J.K., J.H., P.R.H., B. Gao, A.deF.), and Department of Gynaecological Oncology, Westmead Hospital (C.J.K., J.H., A.deF.), Sydney, New South Wales, Australia; Division of Cancer Epidemiology, German Cancer Research Center (Deutsches Krebsforschungszentrum), Heidelberg, Germany (A.Y.J., U.E., J.C.-C.); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia (S.E.J., G.C.-T.); Department of Histopathology, Addenbrookes Hospital, Cambridge, UK (M.J.-L.); Virus, Lifestyle and Genes, Danish Cancer Society Research Center, Copenhagen, Denmark (A.J., S.K.K.); School of Women’s and Children’s Health, University of New South Wales, Sydney, Australia (M.P.I., S.J.R.); Tissue Bank of the National Center for Tumor Diseases (NCT) Heidelberg (E. Herpel), and Institute of Pathology, University of Heidelberg (E. Herpel), Heidelberg, Germany; Cancer Center, University of Hawaii, Honolulu, HI (B.Y.H.); the Crown Princess Mary Cancer Centre, Westmead Hospital, the University of Sydney, Sydney, Australia (P.R.H., B. Gao); Oncology Department, Hospital General de Albacete, Madrid, Spain (J.M.G.-B.); Department of Cancer Genomics and Genetics, Peter MacCallum Cancer Centre, East Melbourne, Victoria, Australia (S.F.); Pathology Department, Fundación Jiménez Díaz, Madrid, Spain (A.C.); Department of Health Research and Policy and Department of Biomedical Data Science, Stanford University School of Medicine, Stanford, CA (A.S.W.); Department of Obstetrics and Gynecology, Division of Gynecologic Oncology, Royal Alexandra Hospital, Edmonton, Alberta, Canada (H.S.); Tübingen University Hospital, Institute of Pathology, Tübingen, Germany (A.S.); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (K.B.M.); Gynaecological Cancer Research Centre, Department of Women’s Cancer, Institute for Women’s Health, University College London, London, UK (U.M.); Alberta Health Services-Cancer Care, Calgary, Alberta, Canada (J.M.K.); Department of Gynecology, Rigshospitalet, University of Copenhagen, Copenhagen, Denmark (S.K.K.); Department of Public Health Sciences, Medical University of South Carolina and Hollings Cancer Center, Charleston, SC (L.E.K.); Department of Pathology and Laboratory Medicine, University of British Columbia (D.G.H.), and Centre for Translational and Applied Genomics, British Columbia Cancer Agency (D.G.H.), Vancouver, British Columbia, Canada; Molecular Unit, Department of Pathology, Herlev Hospital, University of Copenhagen, Copenhagen, Denmark (E. Høgdall); Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, CA (M.T.G.); Genetic Pathology Evaluation Centre, Vancouver General Hospital and University of British Columbia, Vancouver, British Columbia, Canada (B. Gilks); Human Genetics Group, Spanish National Cancer Research Centre (Centro Nacional de Investigaciones Oncológicas) (M.J.G., J.B.), and Centre for Biomedical Network Research on Rare Diseases (Centro de Investigación Biomédica en Red de Enfermedades Raras), Instituto de Salud Carlos III (M.J.G., J.B.), Madrid, Spain; David Geffen School of Medicine, Department of Medicine, Division of Hematology and Oncology, University of California at Los Angeles (P.A.F.); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Erlangen, Germany (P.A.F.); Department of Histopathology, Queen’s Medical Centre, Nottingham University Hospitals NHS Trust, Nottingham, UK (S.D.); University Cancer Center Hamburg, University Medical Center Hamburg-Eppendorf, Hamburg, Germany (J.C.-C.); Cancer Genetics Laboratory, Research Division, Peter MacCallum Cancer Centre (I.G.C.), Sir Peter MacCallum Department of Oncology, University of Melbourne (I.G.C.), and Department of Pathology, University of Melbourne (I.G.C.), Melbourne, Victoria, Australia; Department of Oncology, University of Cambridge, Hutchison/MRC Research Centre (J.D.B.), Cancer Research UK Cambridge Institute, University of Cambridge, Li Ka Shing Centre (J.D.B.), and Cambridge Experimental Cancer Medicine Centre (J.D.B.), Cambridge, UK; Cancer Genomics Program, Research Department, Peter MacCallum Cancer Centre, East Melbourne, Victoria, Australia (D.D.B.); the Garvan Institute, Sydney, New South Wales, Australia (D.D.B., S.J.R.); and Department of Public Health and Primary Care, University of Cambridge, Strangeways Research Laboratory, Cambridge, UK (P.D.P.P.)
1,
Prafull Ghatage
Prafull Ghatage, MD
1Department of Medical Oncology (M.S.B., K.R.K.) and Department of Health Sciences Research (R.A.V., S.J.W., B.M.M., Z.L., E.L.G.), Mayo Clinic, Rochester, MN; Department of Pathology and Laboratory Medicine, University of Calgary, Calgary, Alberta, Canada (P.F.R., M.K.); Pathology Department, Catholic University of Health and Allied Sciences-Bugando, Mwanza, Tanzania (P.F.R.); Tübingen University Hospital, Department of Women’s Health, Tübingen, Germany (P.W., F.A.T., A.D.H., S.Y.B., S.K.); Sir Peter MacCallum Department of Oncology, the University of Melbourne, Parkville, Victoria, Australia (N.T., D.D.B.); International Hereditary Cancer Center, Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (A.T., O.O., J. Lubiński, J.G.); Department of Gynecology and Obstetrics, Ribeirão Preto Medical School, University of São Paulo, Ribeirão Preto, Brazil (D.G.T., C.B.S., J.M.A., F.J.C.R.); Department of Pathology, Institute of Pathology, Heidelberg University Hospital, Heidelberg, Germany (P.S.); Department of Population Health Science and Policy, Department of Genetics and Genomic Sciences, Icahn School of Medicine at Mount Sinai, New York, NY (W.S., J.H.R.); Pathology West ICPMR Westmead, Westmead Hospital, the University of Sydney, Sydney, Australia (R.S.); University of Western Sydney at Westmead Hospital, Westmead, New South Wales, Australia (R.S.); Medical Oncology Service, Hospital Sant Pau, Barcelona, Spain (T.R.C.); H12O-CNIO Lung Cancer Clinical Research Unit (L.P.-A.), and Oncology Department, Hospital Universitario 12 de Octubre (L.P.-A.), Madrid, Spain; Women’s Cancer Program, Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, CA (S.O., J. Lester, B.Y.K.); the University of Texas School of Public Health, Houston (R.B.N.); Department of Oncology, Division of Gynecologic Oncology, Cumming School of Medicine, University of Calgary, Calgary, Alberta, Canada (G.N., P.G.); Department of Obstetrics, Gynecology and Reproductive Sciences, University of Pittsburgh School of Medicine (F.M., R.P.E.), Department of Epidemiology, University of Pittsburgh Graduate School of Public Health (F.M.), and Womens Cancer Research Program, Magee-Womens Research Institute and University of Pittsburgh Cancer Institute (F.M.), Pittsburgh, PA; Department of Gynecological Surgery and Gynecological Oncology of Adults and Adolescents, Pomeranian Medical University, Szczecin, Poland (J.M., A.C.-G.); Department of Health Research and Policy, Stanford University School of Medicine, Stanford, CA (V.M.); Department of Oncology, University of Cambridge, Strangeways Research Laboratory, Cambridge, UK (M.M., J.A., P.D.P.P.); Department of Pathology, Stanford University, Stanford, CA (T.A.L.); Department of Gynecologic Oncology, the Third Affiliated Hospital of Kunming Medical University (Yunnan Tumor Hospital), Kunming, China (Z.L.); Centre for Cancer Research, the Westmead Institute for Medical Research, the University of Sydney (C.J.K., J.H., P.R.H., B. Gao, A.deF.), and Department of Gynaecological Oncology, Westmead Hospital (C.J.K., J.H., A.deF.), Sydney, New South Wales, Australia; Division of Cancer Epidemiology, German Cancer Research Center (Deutsches Krebsforschungszentrum), Heidelberg, Germany (A.Y.J., U.E., J.C.-C.); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia (S.E.J., G.C.-T.); Department of Histopathology, Addenbrookes Hospital, Cambridge, UK (M.J.-L.); Virus, Lifestyle and Genes, Danish Cancer Society Research Center, Copenhagen, Denmark (A.J., S.K.K.); School of Women’s and Children’s Health, University of New South Wales, Sydney, Australia (M.P.I., S.J.R.); Tissue Bank of the National Center for Tumor Diseases (NCT) Heidelberg (E. Herpel), and Institute of Pathology, University of Heidelberg (E. Herpel), Heidelberg, Germany; Cancer Center, University of Hawaii, Honolulu, HI (B.Y.H.); the Crown Princess Mary Cancer Centre, Westmead Hospital, the University of Sydney, Sydney, Australia (P.R.H., B. Gao); Oncology Department, Hospital General de Albacete, Madrid, Spain (J.M.G.-B.); Department of Cancer Genomics and Genetics, Peter MacCallum Cancer Centre, East Melbourne, Victoria, Australia (S.F.); Pathology Department, Fundación Jiménez Díaz, Madrid, Spain (A.C.); Department of Health Research and Policy and Department of Biomedical Data Science, Stanford University School of Medicine, Stanford, CA (A.S.W.); Department of Obstetrics and Gynecology, Division of Gynecologic Oncology, Royal Alexandra Hospital, Edmonton, Alberta, Canada (H.S.); Tübingen University Hospital, Institute of Pathology, Tübingen, Germany (A.S.); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (K.B.M.); Gynaecological Cancer Research Centre, Department of Women’s Cancer, Institute for Women’s Health, University College London, London, UK (U.M.); Alberta Health Services-Cancer Care, Calgary, Alberta, Canada (J.M.K.); Department of Gynecology, Rigshospitalet, University of Copenhagen, Copenhagen, Denmark (S.K.K.); Department of Public Health Sciences, Medical University of South Carolina and Hollings Cancer Center, Charleston, SC (L.E.K.); Department of Pathology and Laboratory Medicine, University of British Columbia (D.G.H.), and Centre for Translational and Applied Genomics, British Columbia Cancer Agency (D.G.H.), Vancouver, British Columbia, Canada; Molecular Unit, Department of Pathology, Herlev Hospital, University of Copenhagen, Copenhagen, Denmark (E. Høgdall); Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, CA (M.T.G.); Genetic Pathology Evaluation Centre, Vancouver General Hospital and University of British Columbia, Vancouver, British Columbia, Canada (B. Gilks); Human Genetics Group, Spanish National Cancer Research Centre (Centro Nacional de Investigaciones Oncológicas) (M.J.G., J.B.), and Centre for Biomedical Network Research on Rare Diseases (Centro de Investigación Biomédica en Red de Enfermedades Raras), Instituto de Salud Carlos III (M.J.G., J.B.), Madrid, Spain; David Geffen School of Medicine, Department of Medicine, Division of Hematology and Oncology, University of California at Los Angeles (P.A.F.); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Erlangen, Germany (P.A.F.); Department of Histopathology, Queen’s Medical Centre, Nottingham University Hospitals NHS Trust, Nottingham, UK (S.D.); University Cancer Center Hamburg, University Medical Center Hamburg-Eppendorf, Hamburg, Germany (J.C.-C.); Cancer Genetics Laboratory, Research Division, Peter MacCallum Cancer Centre (I.G.C.), Sir Peter MacCallum Department of Oncology, University of Melbourne (I.G.C.), and Department of Pathology, University of Melbourne (I.G.C.), Melbourne, Victoria, Australia; Department of Oncology, University of Cambridge, Hutchison/MRC Research Centre (J.D.B.), Cancer Research UK Cambridge Institute, University of Cambridge, Li Ka Shing Centre (J.D.B.), and Cambridge Experimental Cancer Medicine Centre (J.D.B.), Cambridge, UK; Cancer Genomics Program, Research Department, Peter MacCallum Cancer Centre, East Melbourne, Victoria, Australia (D.D.B.); the Garvan Institute, Sydney, New South Wales, Australia (D.D.B., S.J.R.); and Department of Public Health and Primary Care, University of Cambridge, Strangeways Research Laboratory, Cambridge, UK (P.D.P.P.)
1,
José M García-Bueno
José M García-Bueno, MD
1Department of Medical Oncology (M.S.B., K.R.K.) and Department of Health Sciences Research (R.A.V., S.J.W., B.M.M., Z.L., E.L.G.), Mayo Clinic, Rochester, MN; Department of Pathology and Laboratory Medicine, University of Calgary, Calgary, Alberta, Canada (P.F.R., M.K.); Pathology Department, Catholic University of Health and Allied Sciences-Bugando, Mwanza, Tanzania (P.F.R.); Tübingen University Hospital, Department of Women’s Health, Tübingen, Germany (P.W., F.A.T., A.D.H., S.Y.B., S.K.); Sir Peter MacCallum Department of Oncology, the University of Melbourne, Parkville, Victoria, Australia (N.T., D.D.B.); International Hereditary Cancer Center, Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (A.T., O.O., J. Lubiński, J.G.); Department of Gynecology and Obstetrics, Ribeirão Preto Medical School, University of São Paulo, Ribeirão Preto, Brazil (D.G.T., C.B.S., J.M.A., F.J.C.R.); Department of Pathology, Institute of Pathology, Heidelberg University Hospital, Heidelberg, Germany (P.S.); Department of Population Health Science and Policy, Department of Genetics and Genomic Sciences, Icahn School of Medicine at Mount Sinai, New York, NY (W.S., J.H.R.); Pathology West ICPMR Westmead, Westmead Hospital, the University of Sydney, Sydney, Australia (R.S.); University of Western Sydney at Westmead Hospital, Westmead, New South Wales, Australia (R.S.); Medical Oncology Service, Hospital Sant Pau, Barcelona, Spain (T.R.C.); H12O-CNIO Lung Cancer Clinical Research Unit (L.P.-A.), and Oncology Department, Hospital Universitario 12 de Octubre (L.P.-A.), Madrid, Spain; Women’s Cancer Program, Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, CA (S.O., J. Lester, B.Y.K.); the University of Texas School of Public Health, Houston (R.B.N.); Department of Oncology, Division of Gynecologic Oncology, Cumming School of Medicine, University of Calgary, Calgary, Alberta, Canada (G.N., P.G.); Department of Obstetrics, Gynecology and Reproductive Sciences, University of Pittsburgh School of Medicine (F.M., R.P.E.), Department of Epidemiology, University of Pittsburgh Graduate School of Public Health (F.M.), and Womens Cancer Research Program, Magee-Womens Research Institute and University of Pittsburgh Cancer Institute (F.M.), Pittsburgh, PA; Department of Gynecological Surgery and Gynecological Oncology of Adults and Adolescents, Pomeranian Medical University, Szczecin, Poland (J.M., A.C.-G.); Department of Health Research and Policy, Stanford University School of Medicine, Stanford, CA (V.M.); Department of Oncology, University of Cambridge, Strangeways Research Laboratory, Cambridge, UK (M.M., J.A., P.D.P.P.); Department of Pathology, Stanford University, Stanford, CA (T.A.L.); Department of Gynecologic Oncology, the Third Affiliated Hospital of Kunming Medical University (Yunnan Tumor Hospital), Kunming, China (Z.L.); Centre for Cancer Research, the Westmead Institute for Medical Research, the University of Sydney (C.J.K., J.H., P.R.H., B. Gao, A.deF.), and Department of Gynaecological Oncology, Westmead Hospital (C.J.K., J.H., A.deF.), Sydney, New South Wales, Australia; Division of Cancer Epidemiology, German Cancer Research Center (Deutsches Krebsforschungszentrum), Heidelberg, Germany (A.Y.J., U.E., J.C.-C.); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia (S.E.J., G.C.-T.); Department of Histopathology, Addenbrookes Hospital, Cambridge, UK (M.J.-L.); Virus, Lifestyle and Genes, Danish Cancer Society Research Center, Copenhagen, Denmark (A.J., S.K.K.); School of Women’s and Children’s Health, University of New South Wales, Sydney, Australia (M.P.I., S.J.R.); Tissue Bank of the National Center for Tumor Diseases (NCT) Heidelberg (E. Herpel), and Institute of Pathology, University of Heidelberg (E. Herpel), Heidelberg, Germany; Cancer Center, University of Hawaii, Honolulu, HI (B.Y.H.); the Crown Princess Mary Cancer Centre, Westmead Hospital, the University of Sydney, Sydney, Australia (P.R.H., B. Gao); Oncology Department, Hospital General de Albacete, Madrid, Spain (J.M.G.-B.); Department of Cancer Genomics and Genetics, Peter MacCallum Cancer Centre, East Melbourne, Victoria, Australia (S.F.); Pathology Department, Fundación Jiménez Díaz, Madrid, Spain (A.C.); Department of Health Research and Policy and Department of Biomedical Data Science, Stanford University School of Medicine, Stanford, CA (A.S.W.); Department of Obstetrics and Gynecology, Division of Gynecologic Oncology, Royal Alexandra Hospital, Edmonton, Alberta, Canada (H.S.); Tübingen University Hospital, Institute of Pathology, Tübingen, Germany (A.S.); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (K.B.M.); Gynaecological Cancer Research Centre, Department of Women’s Cancer, Institute for Women’s Health, University College London, London, UK (U.M.); Alberta Health Services-Cancer Care, Calgary, Alberta, Canada (J.M.K.); Department of Gynecology, Rigshospitalet, University of Copenhagen, Copenhagen, Denmark (S.K.K.); Department of Public Health Sciences, Medical University of South Carolina and Hollings Cancer Center, Charleston, SC (L.E.K.); Department of Pathology and Laboratory Medicine, University of British Columbia (D.G.H.), and Centre for Translational and Applied Genomics, British Columbia Cancer Agency (D.G.H.), Vancouver, British Columbia, Canada; Molecular Unit, Department of Pathology, Herlev Hospital, University of Copenhagen, Copenhagen, Denmark (E. Høgdall); Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, CA (M.T.G.); Genetic Pathology Evaluation Centre, Vancouver General Hospital and University of British Columbia, Vancouver, British Columbia, Canada (B. Gilks); Human Genetics Group, Spanish National Cancer Research Centre (Centro Nacional de Investigaciones Oncológicas) (M.J.G., J.B.), and Centre for Biomedical Network Research on Rare Diseases (Centro de Investigación Biomédica en Red de Enfermedades Raras), Instituto de Salud Carlos III (M.J.G., J.B.), Madrid, Spain; David Geffen School of Medicine, Department of Medicine, Division of Hematology and Oncology, University of California at Los Angeles (P.A.F.); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Erlangen, Germany (P.A.F.); Department of Histopathology, Queen’s Medical Centre, Nottingham University Hospitals NHS Trust, Nottingham, UK (S.D.); University Cancer Center Hamburg, University Medical Center Hamburg-Eppendorf, Hamburg, Germany (J.C.-C.); Cancer Genetics Laboratory, Research Division, Peter MacCallum Cancer Centre (I.G.C.), Sir Peter MacCallum Department of Oncology, University of Melbourne (I.G.C.), and Department of Pathology, University of Melbourne (I.G.C.), Melbourne, Victoria, Australia; Department of Oncology, University of Cambridge, Hutchison/MRC Research Centre (J.D.B.), Cancer Research UK Cambridge Institute, University of Cambridge, Li Ka Shing Centre (J.D.B.), and Cambridge Experimental Cancer Medicine Centre (J.D.B.), Cambridge, UK; Cancer Genomics Program, Research Department, Peter MacCallum Cancer Centre, East Melbourne, Victoria, Australia (D.D.B.); the Garvan Institute, Sydney, New South Wales, Australia (D.D.B., S.J.R.); and Department of Public Health and Primary Care, University of Cambridge, Strangeways Research Laboratory, Cambridge, UK (P.D.P.P.)
1,
Bo Gao
Bo Gao, BMed, PhD
1Department of Medical Oncology (M.S.B., K.R.K.) and Department of Health Sciences Research (R.A.V., S.J.W., B.M.M., Z.L., E.L.G.), Mayo Clinic, Rochester, MN; Department of Pathology and Laboratory Medicine, University of Calgary, Calgary, Alberta, Canada (P.F.R., M.K.); Pathology Department, Catholic University of Health and Allied Sciences-Bugando, Mwanza, Tanzania (P.F.R.); Tübingen University Hospital, Department of Women’s Health, Tübingen, Germany (P.W., F.A.T., A.D.H., S.Y.B., S.K.); Sir Peter MacCallum Department of Oncology, the University of Melbourne, Parkville, Victoria, Australia (N.T., D.D.B.); International Hereditary Cancer Center, Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (A.T., O.O., J. Lubiński, J.G.); Department of Gynecology and Obstetrics, Ribeirão Preto Medical School, University of São Paulo, Ribeirão Preto, Brazil (D.G.T., C.B.S., J.M.A., F.J.C.R.); Department of Pathology, Institute of Pathology, Heidelberg University Hospital, Heidelberg, Germany (P.S.); Department of Population Health Science and Policy, Department of Genetics and Genomic Sciences, Icahn School of Medicine at Mount Sinai, New York, NY (W.S., J.H.R.); Pathology West ICPMR Westmead, Westmead Hospital, the University of Sydney, Sydney, Australia (R.S.); University of Western Sydney at Westmead Hospital, Westmead, New South Wales, Australia (R.S.); Medical Oncology Service, Hospital Sant Pau, Barcelona, Spain (T.R.C.); H12O-CNIO Lung Cancer Clinical Research Unit (L.P.-A.), and Oncology Department, Hospital Universitario 12 de Octubre (L.P.-A.), Madrid, Spain; Women’s Cancer Program, Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, CA (S.O., J. Lester, B.Y.K.); the University of Texas School of Public Health, Houston (R.B.N.); Department of Oncology, Division of Gynecologic Oncology, Cumming School of Medicine, University of Calgary, Calgary, Alberta, Canada (G.N., P.G.); Department of Obstetrics, Gynecology and Reproductive Sciences, University of Pittsburgh School of Medicine (F.M., R.P.E.), Department of Epidemiology, University of Pittsburgh Graduate School of Public Health (F.M.), and Womens Cancer Research Program, Magee-Womens Research Institute and University of Pittsburgh Cancer Institute (F.M.), Pittsburgh, PA; Department of Gynecological Surgery and Gynecological Oncology of Adults and Adolescents, Pomeranian Medical University, Szczecin, Poland (J.M., A.C.-G.); Department of Health Research and Policy, Stanford University School of Medicine, Stanford, CA (V.M.); Department of Oncology, University of Cambridge, Strangeways Research Laboratory, Cambridge, UK (M.M., J.A., P.D.P.P.); Department of Pathology, Stanford University, Stanford, CA (T.A.L.); Department of Gynecologic Oncology, the Third Affiliated Hospital of Kunming Medical University (Yunnan Tumor Hospital), Kunming, China (Z.L.); Centre for Cancer Research, the Westmead Institute for Medical Research, the University of Sydney (C.J.K., J.H., P.R.H., B. Gao, A.deF.), and Department of Gynaecological Oncology, Westmead Hospital (C.J.K., J.H., A.deF.), Sydney, New South Wales, Australia; Division of Cancer Epidemiology, German Cancer Research Center (Deutsches Krebsforschungszentrum), Heidelberg, Germany (A.Y.J., U.E., J.C.-C.); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia (S.E.J., G.C.-T.); Department of Histopathology, Addenbrookes Hospital, Cambridge, UK (M.J.-L.); Virus, Lifestyle and Genes, Danish Cancer Society Research Center, Copenhagen, Denmark (A.J., S.K.K.); School of Women’s and Children’s Health, University of New South Wales, Sydney, Australia (M.P.I., S.J.R.); Tissue Bank of the National Center for Tumor Diseases (NCT) Heidelberg (E. Herpel), and Institute of Pathology, University of Heidelberg (E. Herpel), Heidelberg, Germany; Cancer Center, University of Hawaii, Honolulu, HI (B.Y.H.); the Crown Princess Mary Cancer Centre, Westmead Hospital, the University of Sydney, Sydney, Australia (P.R.H., B. Gao); Oncology Department, Hospital General de Albacete, Madrid, Spain (J.M.G.-B.); Department of Cancer Genomics and Genetics, Peter MacCallum Cancer Centre, East Melbourne, Victoria, Australia (S.F.); Pathology Department, Fundación Jiménez Díaz, Madrid, Spain (A.C.); Department of Health Research and Policy and Department of Biomedical Data Science, Stanford University School of Medicine, Stanford, CA (A.S.W.); Department of Obstetrics and Gynecology, Division of Gynecologic Oncology, Royal Alexandra Hospital, Edmonton, Alberta, Canada (H.S.); Tübingen University Hospital, Institute of Pathology, Tübingen, Germany (A.S.); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (K.B.M.); Gynaecological Cancer Research Centre, Department of Women’s Cancer, Institute for Women’s Health, University College London, London, UK (U.M.); Alberta Health Services-Cancer Care, Calgary, Alberta, Canada (J.M.K.); Department of Gynecology, Rigshospitalet, University of Copenhagen, Copenhagen, Denmark (S.K.K.); Department of Public Health Sciences, Medical University of South Carolina and Hollings Cancer Center, Charleston, SC (L.E.K.); Department of Pathology and Laboratory Medicine, University of British Columbia (D.G.H.), and Centre for Translational and Applied Genomics, British Columbia Cancer Agency (D.G.H.), Vancouver, British Columbia, Canada; Molecular Unit, Department of Pathology, Herlev Hospital, University of Copenhagen, Copenhagen, Denmark (E. Høgdall); Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, CA (M.T.G.); Genetic Pathology Evaluation Centre, Vancouver General Hospital and University of British Columbia, Vancouver, British Columbia, Canada (B. Gilks); Human Genetics Group, Spanish National Cancer Research Centre (Centro Nacional de Investigaciones Oncológicas) (M.J.G., J.B.), and Centre for Biomedical Network Research on Rare Diseases (Centro de Investigación Biomédica en Red de Enfermedades Raras), Instituto de Salud Carlos III (M.J.G., J.B.), Madrid, Spain; David Geffen School of Medicine, Department of Medicine, Division of Hematology and Oncology, University of California at Los Angeles (P.A.F.); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Erlangen, Germany (P.A.F.); Department of Histopathology, Queen’s Medical Centre, Nottingham University Hospitals NHS Trust, Nottingham, UK (S.D.); University Cancer Center Hamburg, University Medical Center Hamburg-Eppendorf, Hamburg, Germany (J.C.-C.); Cancer Genetics Laboratory, Research Division, Peter MacCallum Cancer Centre (I.G.C.), Sir Peter MacCallum Department of Oncology, University of Melbourne (I.G.C.), and Department of Pathology, University of Melbourne (I.G.C.), Melbourne, Victoria, Australia; Department of Oncology, University of Cambridge, Hutchison/MRC Research Centre (J.D.B.), Cancer Research UK Cambridge Institute, University of Cambridge, Li Ka Shing Centre (J.D.B.), and Cambridge Experimental Cancer Medicine Centre (J.D.B.), Cambridge, UK; Cancer Genomics Program, Research Department, Peter MacCallum Cancer Centre, East Melbourne, Victoria, Australia (D.D.B.); the Garvan Institute, Sydney, New South Wales, Australia (D.D.B., S.J.R.); and Department of Public Health and Primary Care, University of Cambridge, Strangeways Research Laboratory, Cambridge, UK (P.D.P.P.)
1,
Sian Fereday
Sian Fereday, BSc, BA
1Department of Medical Oncology (M.S.B., K.R.K.) and Department of Health Sciences Research (R.A.V., S.J.W., B.M.M., Z.L., E.L.G.), Mayo Clinic, Rochester, MN; Department of Pathology and Laboratory Medicine, University of Calgary, Calgary, Alberta, Canada (P.F.R., M.K.); Pathology Department, Catholic University of Health and Allied Sciences-Bugando, Mwanza, Tanzania (P.F.R.); Tübingen University Hospital, Department of Women’s Health, Tübingen, Germany (P.W., F.A.T., A.D.H., S.Y.B., S.K.); Sir Peter MacCallum Department of Oncology, the University of Melbourne, Parkville, Victoria, Australia (N.T., D.D.B.); International Hereditary Cancer Center, Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (A.T., O.O., J. Lubiński, J.G.); Department of Gynecology and Obstetrics, Ribeirão Preto Medical School, University of São Paulo, Ribeirão Preto, Brazil (D.G.T., C.B.S., J.M.A., F.J.C.R.); Department of Pathology, Institute of Pathology, Heidelberg University Hospital, Heidelberg, Germany (P.S.); Department of Population Health Science and Policy, Department of Genetics and Genomic Sciences, Icahn School of Medicine at Mount Sinai, New York, NY (W.S., J.H.R.); Pathology West ICPMR Westmead, Westmead Hospital, the University of Sydney, Sydney, Australia (R.S.); University of Western Sydney at Westmead Hospital, Westmead, New South Wales, Australia (R.S.); Medical Oncology Service, Hospital Sant Pau, Barcelona, Spain (T.R.C.); H12O-CNIO Lung Cancer Clinical Research Unit (L.P.-A.), and Oncology Department, Hospital Universitario 12 de Octubre (L.P.-A.), Madrid, Spain; Women’s Cancer Program, Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, CA (S.O., J. Lester, B.Y.K.); the University of Texas School of Public Health, Houston (R.B.N.); Department of Oncology, Division of Gynecologic Oncology, Cumming School of Medicine, University of Calgary, Calgary, Alberta, Canada (G.N., P.G.); Department of Obstetrics, Gynecology and Reproductive Sciences, University of Pittsburgh School of Medicine (F.M., R.P.E.), Department of Epidemiology, University of Pittsburgh Graduate School of Public Health (F.M.), and Womens Cancer Research Program, Magee-Womens Research Institute and University of Pittsburgh Cancer Institute (F.M.), Pittsburgh, PA; Department of Gynecological Surgery and Gynecological Oncology of Adults and Adolescents, Pomeranian Medical University, Szczecin, Poland (J.M., A.C.-G.); Department of Health Research and Policy, Stanford University School of Medicine, Stanford, CA (V.M.); Department of Oncology, University of Cambridge, Strangeways Research Laboratory, Cambridge, UK (M.M., J.A., P.D.P.P.); Department of Pathology, Stanford University, Stanford, CA (T.A.L.); Department of Gynecologic Oncology, the Third Affiliated Hospital of Kunming Medical University (Yunnan Tumor Hospital), Kunming, China (Z.L.); Centre for Cancer Research, the Westmead Institute for Medical Research, the University of Sydney (C.J.K., J.H., P.R.H., B. Gao, A.deF.), and Department of Gynaecological Oncology, Westmead Hospital (C.J.K., J.H., A.deF.), Sydney, New South Wales, Australia; Division of Cancer Epidemiology, German Cancer Research Center (Deutsches Krebsforschungszentrum), Heidelberg, Germany (A.Y.J., U.E., J.C.-C.); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia (S.E.J., G.C.-T.); Department of Histopathology, Addenbrookes Hospital, Cambridge, UK (M.J.-L.); Virus, Lifestyle and Genes, Danish Cancer Society Research Center, Copenhagen, Denmark (A.J., S.K.K.); School of Women’s and Children’s Health, University of New South Wales, Sydney, Australia (M.P.I., S.J.R.); Tissue Bank of the National Center for Tumor Diseases (NCT) Heidelberg (E. Herpel), and Institute of Pathology, University of Heidelberg (E. Herpel), Heidelberg, Germany; Cancer Center, University of Hawaii, Honolulu, HI (B.Y.H.); the Crown Princess Mary Cancer Centre, Westmead Hospital, the University of Sydney, Sydney, Australia (P.R.H., B. Gao); Oncology Department, Hospital General de Albacete, Madrid, Spain (J.M.G.-B.); Department of Cancer Genomics and Genetics, Peter MacCallum Cancer Centre, East Melbourne, Victoria, Australia (S.F.); Pathology Department, Fundación Jiménez Díaz, Madrid, Spain (A.C.); Department of Health Research and Policy and Department of Biomedical Data Science, Stanford University School of Medicine, Stanford, CA (A.S.W.); Department of Obstetrics and Gynecology, Division of Gynecologic Oncology, Royal Alexandra Hospital, Edmonton, Alberta, Canada (H.S.); Tübingen University Hospital, Institute of Pathology, Tübingen, Germany (A.S.); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (K.B.M.); Gynaecological Cancer Research Centre, Department of Women’s Cancer, Institute for Women’s Health, University College London, London, UK (U.M.); Alberta Health Services-Cancer Care, Calgary, Alberta, Canada (J.M.K.); Department of Gynecology, Rigshospitalet, University of Copenhagen, Copenhagen, Denmark (S.K.K.); Department of Public Health Sciences, Medical University of South Carolina and Hollings Cancer Center, Charleston, SC (L.E.K.); Department of Pathology and Laboratory Medicine, University of British Columbia (D.G.H.), and Centre for Translational and Applied Genomics, British Columbia Cancer Agency (D.G.H.), Vancouver, British Columbia, Canada; Molecular Unit, Department of Pathology, Herlev Hospital, University of Copenhagen, Copenhagen, Denmark (E. Høgdall); Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, CA (M.T.G.); Genetic Pathology Evaluation Centre, Vancouver General Hospital and University of British Columbia, Vancouver, British Columbia, Canada (B. Gilks); Human Genetics Group, Spanish National Cancer Research Centre (Centro Nacional de Investigaciones Oncológicas) (M.J.G., J.B.), and Centre for Biomedical Network Research on Rare Diseases (Centro de Investigación Biomédica en Red de Enfermedades Raras), Instituto de Salud Carlos III (M.J.G., J.B.), Madrid, Spain; David Geffen School of Medicine, Department of Medicine, Division of Hematology and Oncology, University of California at Los Angeles (P.A.F.); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Erlangen, Germany (P.A.F.); Department of Histopathology, Queen’s Medical Centre, Nottingham University Hospitals NHS Trust, Nottingham, UK (S.D.); University Cancer Center Hamburg, University Medical Center Hamburg-Eppendorf, Hamburg, Germany (J.C.-C.); Cancer Genetics Laboratory, Research Division, Peter MacCallum Cancer Centre (I.G.C.), Sir Peter MacCallum Department of Oncology, University of Melbourne (I.G.C.), and Department of Pathology, University of Melbourne (I.G.C.), Melbourne, Victoria, Australia; Department of Oncology, University of Cambridge, Hutchison/MRC Research Centre (J.D.B.), Cancer Research UK Cambridge Institute, University of Cambridge, Li Ka Shing Centre (J.D.B.), and Cambridge Experimental Cancer Medicine Centre (J.D.B.), Cambridge, UK; Cancer Genomics Program, Research Department, Peter MacCallum Cancer Centre, East Melbourne, Victoria, Australia (D.D.B.); the Garvan Institute, Sydney, New South Wales, Australia (D.D.B., S.J.R.); and Department of Public Health and Primary Care, University of Cambridge, Strangeways Research Laboratory, Cambridge, UK (P.D.P.P.)
1,
Ursula Eilber
Ursula Eilber, PhD
1Department of Medical Oncology (M.S.B., K.R.K.) and Department of Health Sciences Research (R.A.V., S.J.W., B.M.M., Z.L., E.L.G.), Mayo Clinic, Rochester, MN; Department of Pathology and Laboratory Medicine, University of Calgary, Calgary, Alberta, Canada (P.F.R., M.K.); Pathology Department, Catholic University of Health and Allied Sciences-Bugando, Mwanza, Tanzania (P.F.R.); Tübingen University Hospital, Department of Women’s Health, Tübingen, Germany (P.W., F.A.T., A.D.H., S.Y.B., S.K.); Sir Peter MacCallum Department of Oncology, the University of Melbourne, Parkville, Victoria, Australia (N.T., D.D.B.); International Hereditary Cancer Center, Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (A.T., O.O., J. Lubiński, J.G.); Department of Gynecology and Obstetrics, Ribeirão Preto Medical School, University of São Paulo, Ribeirão Preto, Brazil (D.G.T., C.B.S., J.M.A., F.J.C.R.); Department of Pathology, Institute of Pathology, Heidelberg University Hospital, Heidelberg, Germany (P.S.); Department of Population Health Science and Policy, Department of Genetics and Genomic Sciences, Icahn School of Medicine at Mount Sinai, New York, NY (W.S., J.H.R.); Pathology West ICPMR Westmead, Westmead Hospital, the University of Sydney, Sydney, Australia (R.S.); University of Western Sydney at Westmead Hospital, Westmead, New South Wales, Australia (R.S.); Medical Oncology Service, Hospital Sant Pau, Barcelona, Spain (T.R.C.); H12O-CNIO Lung Cancer Clinical Research Unit (L.P.-A.), and Oncology Department, Hospital Universitario 12 de Octubre (L.P.-A.), Madrid, Spain; Women’s Cancer Program, Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, CA (S.O., J. Lester, B.Y.K.); the University of Texas School of Public Health, Houston (R.B.N.); Department of Oncology, Division of Gynecologic Oncology, Cumming School of Medicine, University of Calgary, Calgary, Alberta, Canada (G.N., P.G.); Department of Obstetrics, Gynecology and Reproductive Sciences, University of Pittsburgh School of Medicine (F.M., R.P.E.), Department of Epidemiology, University of Pittsburgh Graduate School of Public Health (F.M.), and Womens Cancer Research Program, Magee-Womens Research Institute and University of Pittsburgh Cancer Institute (F.M.), Pittsburgh, PA; Department of Gynecological Surgery and Gynecological Oncology of Adults and Adolescents, Pomeranian Medical University, Szczecin, Poland (J.M., A.C.-G.); Department of Health Research and Policy, Stanford University School of Medicine, Stanford, CA (V.M.); Department of Oncology, University of Cambridge, Strangeways Research Laboratory, Cambridge, UK (M.M., J.A., P.D.P.P.); Department of Pathology, Stanford University, Stanford, CA (T.A.L.); Department of Gynecologic Oncology, the Third Affiliated Hospital of Kunming Medical University (Yunnan Tumor Hospital), Kunming, China (Z.L.); Centre for Cancer Research, the Westmead Institute for Medical Research, the University of Sydney (C.J.K., J.H., P.R.H., B. Gao, A.deF.), and Department of Gynaecological Oncology, Westmead Hospital (C.J.K., J.H., A.deF.), Sydney, New South Wales, Australia; Division of Cancer Epidemiology, German Cancer Research Center (Deutsches Krebsforschungszentrum), Heidelberg, Germany (A.Y.J., U.E., J.C.-C.); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia (S.E.J., G.C.-T.); Department of Histopathology, Addenbrookes Hospital, Cambridge, UK (M.J.-L.); Virus, Lifestyle and Genes, Danish Cancer Society Research Center, Copenhagen, Denmark (A.J., S.K.K.); School of Women’s and Children’s Health, University of New South Wales, Sydney, Australia (M.P.I., S.J.R.); Tissue Bank of the National Center for Tumor Diseases (NCT) Heidelberg (E. Herpel), and Institute of Pathology, University of Heidelberg (E. Herpel), Heidelberg, Germany; Cancer Center, University of Hawaii, Honolulu, HI (B.Y.H.); the Crown Princess Mary Cancer Centre, Westmead Hospital, the University of Sydney, Sydney, Australia (P.R.H., B. Gao); Oncology Department, Hospital General de Albacete, Madrid, Spain (J.M.G.-B.); Department of Cancer Genomics and Genetics, Peter MacCallum Cancer Centre, East Melbourne, Victoria, Australia (S.F.); Pathology Department, Fundación Jiménez Díaz, Madrid, Spain (A.C.); Department of Health Research and Policy and Department of Biomedical Data Science, Stanford University School of Medicine, Stanford, CA (A.S.W.); Department of Obstetrics and Gynecology, Division of Gynecologic Oncology, Royal Alexandra Hospital, Edmonton, Alberta, Canada (H.S.); Tübingen University Hospital, Institute of Pathology, Tübingen, Germany (A.S.); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (K.B.M.); Gynaecological Cancer Research Centre, Department of Women’s Cancer, Institute for Women’s Health, University College London, London, UK (U.M.); Alberta Health Services-Cancer Care, Calgary, Alberta, Canada (J.M.K.); Department of Gynecology, Rigshospitalet, University of Copenhagen, Copenhagen, Denmark (S.K.K.); Department of Public Health Sciences, Medical University of South Carolina and Hollings Cancer Center, Charleston, SC (L.E.K.); Department of Pathology and Laboratory Medicine, University of British Columbia (D.G.H.), and Centre for Translational and Applied Genomics, British Columbia Cancer Agency (D.G.H.), Vancouver, British Columbia, Canada; Molecular Unit, Department of Pathology, Herlev Hospital, University of Copenhagen, Copenhagen, Denmark (E. Høgdall); Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, CA (M.T.G.); Genetic Pathology Evaluation Centre, Vancouver General Hospital and University of British Columbia, Vancouver, British Columbia, Canada (B. Gilks); Human Genetics Group, Spanish National Cancer Research Centre (Centro Nacional de Investigaciones Oncológicas) (M.J.G., J.B.), and Centre for Biomedical Network Research on Rare Diseases (Centro de Investigación Biomédica en Red de Enfermedades Raras), Instituto de Salud Carlos III (M.J.G., J.B.), Madrid, Spain; David Geffen School of Medicine, Department of Medicine, Division of Hematology and Oncology, University of California at Los Angeles (P.A.F.); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Erlangen, Germany (P.A.F.); Department of Histopathology, Queen’s Medical Centre, Nottingham University Hospitals NHS Trust, Nottingham, UK (S.D.); University Cancer Center Hamburg, University Medical Center Hamburg-Eppendorf, Hamburg, Germany (J.C.-C.); Cancer Genetics Laboratory, Research Division, Peter MacCallum Cancer Centre (I.G.C.), Sir Peter MacCallum Department of Oncology, University of Melbourne (I.G.C.), and Department of Pathology, University of Melbourne (I.G.C.), Melbourne, Victoria, Australia; Department of Oncology, University of Cambridge, Hutchison/MRC Research Centre (J.D.B.), Cancer Research UK Cambridge Institute, University of Cambridge, Li Ka Shing Centre (J.D.B.), and Cambridge Experimental Cancer Medicine Centre (J.D.B.), Cambridge, UK; Cancer Genomics Program, Research Department, Peter MacCallum Cancer Centre, East Melbourne, Victoria, Australia (D.D.B.); the Garvan Institute, Sydney, New South Wales, Australia (D.D.B., S.J.R.); and Department of Public Health and Primary Care, University of Cambridge, Strangeways Research Laboratory, Cambridge, UK (P.D.P.P.)
1,
Robert P Edwards
Robert P Edwards, MD
1Department of Medical Oncology (M.S.B., K.R.K.) and Department of Health Sciences Research (R.A.V., S.J.W., B.M.M., Z.L., E.L.G.), Mayo Clinic, Rochester, MN; Department of Pathology and Laboratory Medicine, University of Calgary, Calgary, Alberta, Canada (P.F.R., M.K.); Pathology Department, Catholic University of Health and Allied Sciences-Bugando, Mwanza, Tanzania (P.F.R.); Tübingen University Hospital, Department of Women’s Health, Tübingen, Germany (P.W., F.A.T., A.D.H., S.Y.B., S.K.); Sir Peter MacCallum Department of Oncology, the University of Melbourne, Parkville, Victoria, Australia (N.T., D.D.B.); International Hereditary Cancer Center, Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (A.T., O.O., J. Lubiński, J.G.); Department of Gynecology and Obstetrics, Ribeirão Preto Medical School, University of São Paulo, Ribeirão Preto, Brazil (D.G.T., C.B.S., J.M.A., F.J.C.R.); Department of Pathology, Institute of Pathology, Heidelberg University Hospital, Heidelberg, Germany (P.S.); Department of Population Health Science and Policy, Department of Genetics and Genomic Sciences, Icahn School of Medicine at Mount Sinai, New York, NY (W.S., J.H.R.); Pathology West ICPMR Westmead, Westmead Hospital, the University of Sydney, Sydney, Australia (R.S.); University of Western Sydney at Westmead Hospital, Westmead, New South Wales, Australia (R.S.); Medical Oncology Service, Hospital Sant Pau, Barcelona, Spain (T.R.C.); H12O-CNIO Lung Cancer Clinical Research Unit (L.P.-A.), and Oncology Department, Hospital Universitario 12 de Octubre (L.P.-A.), Madrid, Spain; Women’s Cancer Program, Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, CA (S.O., J. Lester, B.Y.K.); the University of Texas School of Public Health, Houston (R.B.N.); Department of Oncology, Division of Gynecologic Oncology, Cumming School of Medicine, University of Calgary, Calgary, Alberta, Canada (G.N., P.G.); Department of Obstetrics, Gynecology and Reproductive Sciences, University of Pittsburgh School of Medicine (F.M., R.P.E.), Department of Epidemiology, University of Pittsburgh Graduate School of Public Health (F.M.), and Womens Cancer Research Program, Magee-Womens Research Institute and University of Pittsburgh Cancer Institute (F.M.), Pittsburgh, PA; Department of Gynecological Surgery and Gynecological Oncology of Adults and Adolescents, Pomeranian Medical University, Szczecin, Poland (J.M., A.C.-G.); Department of Health Research and Policy, Stanford University School of Medicine, Stanford, CA (V.M.); Department of Oncology, University of Cambridge, Strangeways Research Laboratory, Cambridge, UK (M.M., J.A., P.D.P.P.); Department of Pathology, Stanford University, Stanford, CA (T.A.L.); Department of Gynecologic Oncology, the Third Affiliated Hospital of Kunming Medical University (Yunnan Tumor Hospital), Kunming, China (Z.L.); Centre for Cancer Research, the Westmead Institute for Medical Research, the University of Sydney (C.J.K., J.H., P.R.H., B. Gao, A.deF.), and Department of Gynaecological Oncology, Westmead Hospital (C.J.K., J.H., A.deF.), Sydney, New South Wales, Australia; Division of Cancer Epidemiology, German Cancer Research Center (Deutsches Krebsforschungszentrum), Heidelberg, Germany (A.Y.J., U.E., J.C.-C.); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia (S.E.J., G.C.-T.); Department of Histopathology, Addenbrookes Hospital, Cambridge, UK (M.J.-L.); Virus, Lifestyle and Genes, Danish Cancer Society Research Center, Copenhagen, Denmark (A.J., S.K.K.); School of Women’s and Children’s Health, University of New South Wales, Sydney, Australia (M.P.I., S.J.R.); Tissue Bank of the National Center for Tumor Diseases (NCT) Heidelberg (E. Herpel), and Institute of Pathology, University of Heidelberg (E. Herpel), Heidelberg, Germany; Cancer Center, University of Hawaii, Honolulu, HI (B.Y.H.); the Crown Princess Mary Cancer Centre, Westmead Hospital, the University of Sydney, Sydney, Australia (P.R.H., B. Gao); Oncology Department, Hospital General de Albacete, Madrid, Spain (J.M.G.-B.); Department of Cancer Genomics and Genetics, Peter MacCallum Cancer Centre, East Melbourne, Victoria, Australia (S.F.); Pathology Department, Fundación Jiménez Díaz, Madrid, Spain (A.C.); Department of Health Research and Policy and Department of Biomedical Data Science, Stanford University School of Medicine, Stanford, CA (A.S.W.); Department of Obstetrics and Gynecology, Division of Gynecologic Oncology, Royal Alexandra Hospital, Edmonton, Alberta, Canada (H.S.); Tübingen University Hospital, Institute of Pathology, Tübingen, Germany (A.S.); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (K.B.M.); Gynaecological Cancer Research Centre, Department of Women’s Cancer, Institute for Women’s Health, University College London, London, UK (U.M.); Alberta Health Services-Cancer Care, Calgary, Alberta, Canada (J.M.K.); Department of Gynecology, Rigshospitalet, University of Copenhagen, Copenhagen, Denmark (S.K.K.); Department of Public Health Sciences, Medical University of South Carolina and Hollings Cancer Center, Charleston, SC (L.E.K.); Department of Pathology and Laboratory Medicine, University of British Columbia (D.G.H.), and Centre for Translational and Applied Genomics, British Columbia Cancer Agency (D.G.H.), Vancouver, British Columbia, Canada; Molecular Unit, Department of Pathology, Herlev Hospital, University of Copenhagen, Copenhagen, Denmark (E. Høgdall); Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, CA (M.T.G.); Genetic Pathology Evaluation Centre, Vancouver General Hospital and University of British Columbia, Vancouver, British Columbia, Canada (B. Gilks); Human Genetics Group, Spanish National Cancer Research Centre (Centro Nacional de Investigaciones Oncológicas) (M.J.G., J.B.), and Centre for Biomedical Network Research on Rare Diseases (Centro de Investigación Biomédica en Red de Enfermedades Raras), Instituto de Salud Carlos III (M.J.G., J.B.), Madrid, Spain; David Geffen School of Medicine, Department of Medicine, Division of Hematology and Oncology, University of California at Los Angeles (P.A.F.); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Erlangen, Germany (P.A.F.); Department of Histopathology, Queen’s Medical Centre, Nottingham University Hospitals NHS Trust, Nottingham, UK (S.D.); University Cancer Center Hamburg, University Medical Center Hamburg-Eppendorf, Hamburg, Germany (J.C.-C.); Cancer Genetics Laboratory, Research Division, Peter MacCallum Cancer Centre (I.G.C.), Sir Peter MacCallum Department of Oncology, University of Melbourne (I.G.C.), and Department of Pathology, University of Melbourne (I.G.C.), Melbourne, Victoria, Australia; Department of Oncology, University of Cambridge, Hutchison/MRC Research Centre (J.D.B.), Cancer Research UK Cambridge Institute, University of Cambridge, Li Ka Shing Centre (J.D.B.), and Cambridge Experimental Cancer Medicine Centre (J.D.B.), Cambridge, UK; Cancer Genomics Program, Research Department, Peter MacCallum Cancer Centre, East Melbourne, Victoria, Australia (D.D.B.); the Garvan Institute, Sydney, New South Wales, Australia (D.D.B., S.J.R.); and Department of Public Health and Primary Care, University of Cambridge, Strangeways Research Laboratory, Cambridge, UK (P.D.P.P.)
1,
Christiani B de Sousa
Christiani B de Sousa, MD, PhD
1Department of Medical Oncology (M.S.B., K.R.K.) and Department of Health Sciences Research (R.A.V., S.J.W., B.M.M., Z.L., E.L.G.), Mayo Clinic, Rochester, MN; Department of Pathology and Laboratory Medicine, University of Calgary, Calgary, Alberta, Canada (P.F.R., M.K.); Pathology Department, Catholic University of Health and Allied Sciences-Bugando, Mwanza, Tanzania (P.F.R.); Tübingen University Hospital, Department of Women’s Health, Tübingen, Germany (P.W., F.A.T., A.D.H., S.Y.B., S.K.); Sir Peter MacCallum Department of Oncology, the University of Melbourne, Parkville, Victoria, Australia (N.T., D.D.B.); International Hereditary Cancer Center, Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (A.T., O.O., J. Lubiński, J.G.); Department of Gynecology and Obstetrics, Ribeirão Preto Medical School, University of São Paulo, Ribeirão Preto, Brazil (D.G.T., C.B.S., J.M.A., F.J.C.R.); Department of Pathology, Institute of Pathology, Heidelberg University Hospital, Heidelberg, Germany (P.S.); Department of Population Health Science and Policy, Department of Genetics and Genomic Sciences, Icahn School of Medicine at Mount Sinai, New York, NY (W.S., J.H.R.); Pathology West ICPMR Westmead, Westmead Hospital, the University of Sydney, Sydney, Australia (R.S.); University of Western Sydney at Westmead Hospital, Westmead, New South Wales, Australia (R.S.); Medical Oncology Service, Hospital Sant Pau, Barcelona, Spain (T.R.C.); H12O-CNIO Lung Cancer Clinical Research Unit (L.P.-A.), and Oncology Department, Hospital Universitario 12 de Octubre (L.P.-A.), Madrid, Spain; Women’s Cancer Program, Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, CA (S.O., J. Lester, B.Y.K.); the University of Texas School of Public Health, Houston (R.B.N.); Department of Oncology, Division of Gynecologic Oncology, Cumming School of Medicine, University of Calgary, Calgary, Alberta, Canada (G.N., P.G.); Department of Obstetrics, Gynecology and Reproductive Sciences, University of Pittsburgh School of Medicine (F.M., R.P.E.), Department of Epidemiology, University of Pittsburgh Graduate School of Public Health (F.M.), and Womens Cancer Research Program, Magee-Womens Research Institute and University of Pittsburgh Cancer Institute (F.M.), Pittsburgh, PA; Department of Gynecological Surgery and Gynecological Oncology of Adults and Adolescents, Pomeranian Medical University, Szczecin, Poland (J.M., A.C.-G.); Department of Health Research and Policy, Stanford University School of Medicine, Stanford, CA (V.M.); Department of Oncology, University of Cambridge, Strangeways Research Laboratory, Cambridge, UK (M.M., J.A., P.D.P.P.); Department of Pathology, Stanford University, Stanford, CA (T.A.L.); Department of Gynecologic Oncology, the Third Affiliated Hospital of Kunming Medical University (Yunnan Tumor Hospital), Kunming, China (Z.L.); Centre for Cancer Research, the Westmead Institute for Medical Research, the University of Sydney (C.J.K., J.H., P.R.H., B. Gao, A.deF.), and Department of Gynaecological Oncology, Westmead Hospital (C.J.K., J.H., A.deF.), Sydney, New South Wales, Australia; Division of Cancer Epidemiology, German Cancer Research Center (Deutsches Krebsforschungszentrum), Heidelberg, Germany (A.Y.J., U.E., J.C.-C.); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia (S.E.J., G.C.-T.); Department of Histopathology, Addenbrookes Hospital, Cambridge, UK (M.J.-L.); Virus, Lifestyle and Genes, Danish Cancer Society Research Center, Copenhagen, Denmark (A.J., S.K.K.); School of Women’s and Children’s Health, University of New South Wales, Sydney, Australia (M.P.I., S.J.R.); Tissue Bank of the National Center for Tumor Diseases (NCT) Heidelberg (E. Herpel), and Institute of Pathology, University of Heidelberg (E. Herpel), Heidelberg, Germany; Cancer Center, University of Hawaii, Honolulu, HI (B.Y.H.); the Crown Princess Mary Cancer Centre, Westmead Hospital, the University of Sydney, Sydney, Australia (P.R.H., B. Gao); Oncology Department, Hospital General de Albacete, Madrid, Spain (J.M.G.-B.); Department of Cancer Genomics and Genetics, Peter MacCallum Cancer Centre, East Melbourne, Victoria, Australia (S.F.); Pathology Department, Fundación Jiménez Díaz, Madrid, Spain (A.C.); Department of Health Research and Policy and Department of Biomedical Data Science, Stanford University School of Medicine, Stanford, CA (A.S.W.); Department of Obstetrics and Gynecology, Division of Gynecologic Oncology, Royal Alexandra Hospital, Edmonton, Alberta, Canada (H.S.); Tübingen University Hospital, Institute of Pathology, Tübingen, Germany (A.S.); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (K.B.M.); Gynaecological Cancer Research Centre, Department of Women’s Cancer, Institute for Women’s Health, University College London, London, UK (U.M.); Alberta Health Services-Cancer Care, Calgary, Alberta, Canada (J.M.K.); Department of Gynecology, Rigshospitalet, University of Copenhagen, Copenhagen, Denmark (S.K.K.); Department of Public Health Sciences, Medical University of South Carolina and Hollings Cancer Center, Charleston, SC (L.E.K.); Department of Pathology and Laboratory Medicine, University of British Columbia (D.G.H.), and Centre for Translational and Applied Genomics, British Columbia Cancer Agency (D.G.H.), Vancouver, British Columbia, Canada; Molecular Unit, Department of Pathology, Herlev Hospital, University of Copenhagen, Copenhagen, Denmark (E. Høgdall); Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, CA (M.T.G.); Genetic Pathology Evaluation Centre, Vancouver General Hospital and University of British Columbia, Vancouver, British Columbia, Canada (B. Gilks); Human Genetics Group, Spanish National Cancer Research Centre (Centro Nacional de Investigaciones Oncológicas) (M.J.G., J.B.), and Centre for Biomedical Network Research on Rare Diseases (Centro de Investigación Biomédica en Red de Enfermedades Raras), Instituto de Salud Carlos III (M.J.G., J.B.), Madrid, Spain; David Geffen School of Medicine, Department of Medicine, Division of Hematology and Oncology, University of California at Los Angeles (P.A.F.); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Erlangen, Germany (P.A.F.); Department of Histopathology, Queen’s Medical Centre, Nottingham University Hospitals NHS Trust, Nottingham, UK (S.D.); University Cancer Center Hamburg, University Medical Center Hamburg-Eppendorf, Hamburg, Germany (J.C.-C.); Cancer Genetics Laboratory, Research Division, Peter MacCallum Cancer Centre (I.G.C.), Sir Peter MacCallum Department of Oncology, University of Melbourne (I.G.C.), and Department of Pathology, University of Melbourne (I.G.C.), Melbourne, Victoria, Australia; Department of Oncology, University of Cambridge, Hutchison/MRC Research Centre (J.D.B.), Cancer Research UK Cambridge Institute, University of Cambridge, Li Ka Shing Centre (J.D.B.), and Cambridge Experimental Cancer Medicine Centre (J.D.B.), Cambridge, UK; Cancer Genomics Program, Research Department, Peter MacCallum Cancer Centre, East Melbourne, Victoria, Australia (D.D.B.); the Garvan Institute, Sydney, New South Wales, Australia (D.D.B., S.J.R.); and Department of Public Health and Primary Care, University of Cambridge, Strangeways Research Laboratory, Cambridge, UK (P.D.P.P.)
1,
Jurandyr M de Andrade
Jurandyr M de Andrade, MD, PhD
1Department of Medical Oncology (M.S.B., K.R.K.) and Department of Health Sciences Research (R.A.V., S.J.W., B.M.M., Z.L., E.L.G.), Mayo Clinic, Rochester, MN; Department of Pathology and Laboratory Medicine, University of Calgary, Calgary, Alberta, Canada (P.F.R., M.K.); Pathology Department, Catholic University of Health and Allied Sciences-Bugando, Mwanza, Tanzania (P.F.R.); Tübingen University Hospital, Department of Women’s Health, Tübingen, Germany (P.W., F.A.T., A.D.H., S.Y.B., S.K.); Sir Peter MacCallum Department of Oncology, the University of Melbourne, Parkville, Victoria, Australia (N.T., D.D.B.); International Hereditary Cancer Center, Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (A.T., O.O., J. Lubiński, J.G.); Department of Gynecology and Obstetrics, Ribeirão Preto Medical School, University of São Paulo, Ribeirão Preto, Brazil (D.G.T., C.B.S., J.M.A., F.J.C.R.); Department of Pathology, Institute of Pathology, Heidelberg University Hospital, Heidelberg, Germany (P.S.); Department of Population Health Science and Policy, Department of Genetics and Genomic Sciences, Icahn School of Medicine at Mount Sinai, New York, NY (W.S., J.H.R.); Pathology West ICPMR Westmead, Westmead Hospital, the University of Sydney, Sydney, Australia (R.S.); University of Western Sydney at Westmead Hospital, Westmead, New South Wales, Australia (R.S.); Medical Oncology Service, Hospital Sant Pau, Barcelona, Spain (T.R.C.); H12O-CNIO Lung Cancer Clinical Research Unit (L.P.-A.), and Oncology Department, Hospital Universitario 12 de Octubre (L.P.-A.), Madrid, Spain; Women’s Cancer Program, Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, CA (S.O., J. Lester, B.Y.K.); the University of Texas School of Public Health, Houston (R.B.N.); Department of Oncology, Division of Gynecologic Oncology, Cumming School of Medicine, University of Calgary, Calgary, Alberta, Canada (G.N., P.G.); Department of Obstetrics, Gynecology and Reproductive Sciences, University of Pittsburgh School of Medicine (F.M., R.P.E.), Department of Epidemiology, University of Pittsburgh Graduate School of Public Health (F.M.), and Womens Cancer Research Program, Magee-Womens Research Institute and University of Pittsburgh Cancer Institute (F.M.), Pittsburgh, PA; Department of Gynecological Surgery and Gynecological Oncology of Adults and Adolescents, Pomeranian Medical University, Szczecin, Poland (J.M., A.C.-G.); Department of Health Research and Policy, Stanford University School of Medicine, Stanford, CA (V.M.); Department of Oncology, University of Cambridge, Strangeways Research Laboratory, Cambridge, UK (M.M., J.A., P.D.P.P.); Department of Pathology, Stanford University, Stanford, CA (T.A.L.); Department of Gynecologic Oncology, the Third Affiliated Hospital of Kunming Medical University (Yunnan Tumor Hospital), Kunming, China (Z.L.); Centre for Cancer Research, the Westmead Institute for Medical Research, the University of Sydney (C.J.K., J.H., P.R.H., B. Gao, A.deF.), and Department of Gynaecological Oncology, Westmead Hospital (C.J.K., J.H., A.deF.), Sydney, New South Wales, Australia; Division of Cancer Epidemiology, German Cancer Research Center (Deutsches Krebsforschungszentrum), Heidelberg, Germany (A.Y.J., U.E., J.C.-C.); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia (S.E.J., G.C.-T.); Department of Histopathology, Addenbrookes Hospital, Cambridge, UK (M.J.-L.); Virus, Lifestyle and Genes, Danish Cancer Society Research Center, Copenhagen, Denmark (A.J., S.K.K.); School of Women’s and Children’s Health, University of New South Wales, Sydney, Australia (M.P.I., S.J.R.); Tissue Bank of the National Center for Tumor Diseases (NCT) Heidelberg (E. Herpel), and Institute of Pathology, University of Heidelberg (E. Herpel), Heidelberg, Germany; Cancer Center, University of Hawaii, Honolulu, HI (B.Y.H.); the Crown Princess Mary Cancer Centre, Westmead Hospital, the University of Sydney, Sydney, Australia (P.R.H., B. Gao); Oncology Department, Hospital General de Albacete, Madrid, Spain (J.M.G.-B.); Department of Cancer Genomics and Genetics, Peter MacCallum Cancer Centre, East Melbourne, Victoria, Australia (S.F.); Pathology Department, Fundación Jiménez Díaz, Madrid, Spain (A.C.); Department of Health Research and Policy and Department of Biomedical Data Science, Stanford University School of Medicine, Stanford, CA (A.S.W.); Department of Obstetrics and Gynecology, Division of Gynecologic Oncology, Royal Alexandra Hospital, Edmonton, Alberta, Canada (H.S.); Tübingen University Hospital, Institute of Pathology, Tübingen, Germany (A.S.); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (K.B.M.); Gynaecological Cancer Research Centre, Department of Women’s Cancer, Institute for Women’s Health, University College London, London, UK (U.M.); Alberta Health Services-Cancer Care, Calgary, Alberta, Canada (J.M.K.); Department of Gynecology, Rigshospitalet, University of Copenhagen, Copenhagen, Denmark (S.K.K.); Department of Public Health Sciences, Medical University of South Carolina and Hollings Cancer Center, Charleston, SC (L.E.K.); Department of Pathology and Laboratory Medicine, University of British Columbia (D.G.H.), and Centre for Translational and Applied Genomics, British Columbia Cancer Agency (D.G.H.), Vancouver, British Columbia, Canada; Molecular Unit, Department of Pathology, Herlev Hospital, University of Copenhagen, Copenhagen, Denmark (E. Høgdall); Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, CA (M.T.G.); Genetic Pathology Evaluation Centre, Vancouver General Hospital and University of British Columbia, Vancouver, British Columbia, Canada (B. Gilks); Human Genetics Group, Spanish National Cancer Research Centre (Centro Nacional de Investigaciones Oncológicas) (M.J.G., J.B.), and Centre for Biomedical Network Research on Rare Diseases (Centro de Investigación Biomédica en Red de Enfermedades Raras), Instituto de Salud Carlos III (M.J.G., J.B.), Madrid, Spain; David Geffen School of Medicine, Department of Medicine, Division of Hematology and Oncology, University of California at Los Angeles (P.A.F.); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Erlangen, Germany (P.A.F.); Department of Histopathology, Queen’s Medical Centre, Nottingham University Hospitals NHS Trust, Nottingham, UK (S.D.); University Cancer Center Hamburg, University Medical Center Hamburg-Eppendorf, Hamburg, Germany (J.C.-C.); Cancer Genetics Laboratory, Research Division, Peter MacCallum Cancer Centre (I.G.C.), Sir Peter MacCallum Department of Oncology, University of Melbourne (I.G.C.), and Department of Pathology, University of Melbourne (I.G.C.), Melbourne, Victoria, Australia; Department of Oncology, University of Cambridge, Hutchison/MRC Research Centre (J.D.B.), Cancer Research UK Cambridge Institute, University of Cambridge, Li Ka Shing Centre (J.D.B.), and Cambridge Experimental Cancer Medicine Centre (J.D.B.), Cambridge, UK; Cancer Genomics Program, Research Department, Peter MacCallum Cancer Centre, East Melbourne, Victoria, Australia (D.D.B.); the Garvan Institute, Sydney, New South Wales, Australia (D.D.B., S.J.R.); and Department of Public Health and Primary Care, University of Cambridge, Strangeways Research Laboratory, Cambridge, UK (P.D.P.P.)
1,
Anita Chudecka-Głaz
Anita Chudecka-Głaz, MD, PhD
1Department of Medical Oncology (M.S.B., K.R.K.) and Department of Health Sciences Research (R.A.V., S.J.W., B.M.M., Z.L., E.L.G.), Mayo Clinic, Rochester, MN; Department of Pathology and Laboratory Medicine, University of Calgary, Calgary, Alberta, Canada (P.F.R., M.K.); Pathology Department, Catholic University of Health and Allied Sciences-Bugando, Mwanza, Tanzania (P.F.R.); Tübingen University Hospital, Department of Women’s Health, Tübingen, Germany (P.W., F.A.T., A.D.H., S.Y.B., S.K.); Sir Peter MacCallum Department of Oncology, the University of Melbourne, Parkville, Victoria, Australia (N.T., D.D.B.); International Hereditary Cancer Center, Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (A.T., O.O., J. Lubiński, J.G.); Department of Gynecology and Obstetrics, Ribeirão Preto Medical School, University of São Paulo, Ribeirão Preto, Brazil (D.G.T., C.B.S., J.M.A., F.J.C.R.); Department of Pathology, Institute of Pathology, Heidelberg University Hospital, Heidelberg, Germany (P.S.); Department of Population Health Science and Policy, Department of Genetics and Genomic Sciences, Icahn School of Medicine at Mount Sinai, New York, NY (W.S., J.H.R.); Pathology West ICPMR Westmead, Westmead Hospital, the University of Sydney, Sydney, Australia (R.S.); University of Western Sydney at Westmead Hospital, Westmead, New South Wales, Australia (R.S.); Medical Oncology Service, Hospital Sant Pau, Barcelona, Spain (T.R.C.); H12O-CNIO Lung Cancer Clinical Research Unit (L.P.-A.), and Oncology Department, Hospital Universitario 12 de Octubre (L.P.-A.), Madrid, Spain; Women’s Cancer Program, Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, CA (S.O., J. Lester, B.Y.K.); the University of Texas School of Public Health, Houston (R.B.N.); Department of Oncology, Division of Gynecologic Oncology, Cumming School of Medicine, University of Calgary, Calgary, Alberta, Canada (G.N., P.G.); Department of Obstetrics, Gynecology and Reproductive Sciences, University of Pittsburgh School of Medicine (F.M., R.P.E.), Department of Epidemiology, University of Pittsburgh Graduate School of Public Health (F.M.), and Womens Cancer Research Program, Magee-Womens Research Institute and University of Pittsburgh Cancer Institute (F.M.), Pittsburgh, PA; Department of Gynecological Surgery and Gynecological Oncology of Adults and Adolescents, Pomeranian Medical University, Szczecin, Poland (J.M., A.C.-G.); Department of Health Research and Policy, Stanford University School of Medicine, Stanford, CA (V.M.); Department of Oncology, University of Cambridge, Strangeways Research Laboratory, Cambridge, UK (M.M., J.A., P.D.P.P.); Department of Pathology, Stanford University, Stanford, CA (T.A.L.); Department of Gynecologic Oncology, the Third Affiliated Hospital of Kunming Medical University (Yunnan Tumor Hospital), Kunming, China (Z.L.); Centre for Cancer Research, the Westmead Institute for Medical Research, the University of Sydney (C.J.K., J.H., P.R.H., B. Gao, A.deF.), and Department of Gynaecological Oncology, Westmead Hospital (C.J.K., J.H., A.deF.), Sydney, New South Wales, Australia; Division of Cancer Epidemiology, German Cancer Research Center (Deutsches Krebsforschungszentrum), Heidelberg, Germany (A.Y.J., U.E., J.C.-C.); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia (S.E.J., G.C.-T.); Department of Histopathology, Addenbrookes Hospital, Cambridge, UK (M.J.-L.); Virus, Lifestyle and Genes, Danish Cancer Society Research Center, Copenhagen, Denmark (A.J., S.K.K.); School of Women’s and Children’s Health, University of New South Wales, Sydney, Australia (M.P.I., S.J.R.); Tissue Bank of the National Center for Tumor Diseases (NCT) Heidelberg (E. Herpel), and Institute of Pathology, University of Heidelberg (E. Herpel), Heidelberg, Germany; Cancer Center, University of Hawaii, Honolulu, HI (B.Y.H.); the Crown Princess Mary Cancer Centre, Westmead Hospital, the University of Sydney, Sydney, Australia (P.R.H., B. Gao); Oncology Department, Hospital General de Albacete, Madrid, Spain (J.M.G.-B.); Department of Cancer Genomics and Genetics, Peter MacCallum Cancer Centre, East Melbourne, Victoria, Australia (S.F.); Pathology Department, Fundación Jiménez Díaz, Madrid, Spain (A.C.); Department of Health Research and Policy and Department of Biomedical Data Science, Stanford University School of Medicine, Stanford, CA (A.S.W.); Department of Obstetrics and Gynecology, Division of Gynecologic Oncology, Royal Alexandra Hospital, Edmonton, Alberta, Canada (H.S.); Tübingen University Hospital, Institute of Pathology, Tübingen, Germany (A.S.); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (K.B.M.); Gynaecological Cancer Research Centre, Department of Women’s Cancer, Institute for Women’s Health, University College London, London, UK (U.M.); Alberta Health Services-Cancer Care, Calgary, Alberta, Canada (J.M.K.); Department of Gynecology, Rigshospitalet, University of Copenhagen, Copenhagen, Denmark (S.K.K.); Department of Public Health Sciences, Medical University of South Carolina and Hollings Cancer Center, Charleston, SC (L.E.K.); Department of Pathology and Laboratory Medicine, University of British Columbia (D.G.H.), and Centre for Translational and Applied Genomics, British Columbia Cancer Agency (D.G.H.), Vancouver, British Columbia, Canada; Molecular Unit, Department of Pathology, Herlev Hospital, University of Copenhagen, Copenhagen, Denmark (E. Høgdall); Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, CA (M.T.G.); Genetic Pathology Evaluation Centre, Vancouver General Hospital and University of British Columbia, Vancouver, British Columbia, Canada (B. Gilks); Human Genetics Group, Spanish National Cancer Research Centre (Centro Nacional de Investigaciones Oncológicas) (M.J.G., J.B.), and Centre for Biomedical Network Research on Rare Diseases (Centro de Investigación Biomédica en Red de Enfermedades Raras), Instituto de Salud Carlos III (M.J.G., J.B.), Madrid, Spain; David Geffen School of Medicine, Department of Medicine, Division of Hematology and Oncology, University of California at Los Angeles (P.A.F.); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Erlangen, Germany (P.A.F.); Department of Histopathology, Queen’s Medical Centre, Nottingham University Hospitals NHS Trust, Nottingham, UK (S.D.); University Cancer Center Hamburg, University Medical Center Hamburg-Eppendorf, Hamburg, Germany (J.C.-C.); Cancer Genetics Laboratory, Research Division, Peter MacCallum Cancer Centre (I.G.C.), Sir Peter MacCallum Department of Oncology, University of Melbourne (I.G.C.), and Department of Pathology, University of Melbourne (I.G.C.), Melbourne, Victoria, Australia; Department of Oncology, University of Cambridge, Hutchison/MRC Research Centre (J.D.B.), Cancer Research UK Cambridge Institute, University of Cambridge, Li Ka Shing Centre (J.D.B.), and Cambridge Experimental Cancer Medicine Centre (J.D.B.), Cambridge, UK; Cancer Genomics Program, Research Department, Peter MacCallum Cancer Centre, East Melbourne, Victoria, Australia (D.D.B.); the Garvan Institute, Sydney, New South Wales, Australia (D.D.B., S.J.R.); and Department of Public Health and Primary Care, University of Cambridge, Strangeways Research Laboratory, Cambridge, UK (P.D.P.P.)
1,
Georgia Chenevix-Trench
Georgia Chenevix-Trench, PhD
1Department of Medical Oncology (M.S.B., K.R.K.) and Department of Health Sciences Research (R.A.V., S.J.W., B.M.M., Z.L., E.L.G.), Mayo Clinic, Rochester, MN; Department of Pathology and Laboratory Medicine, University of Calgary, Calgary, Alberta, Canada (P.F.R., M.K.); Pathology Department, Catholic University of Health and Allied Sciences-Bugando, Mwanza, Tanzania (P.F.R.); Tübingen University Hospital, Department of Women’s Health, Tübingen, Germany (P.W., F.A.T., A.D.H., S.Y.B., S.K.); Sir Peter MacCallum Department of Oncology, the University of Melbourne, Parkville, Victoria, Australia (N.T., D.D.B.); International Hereditary Cancer Center, Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (A.T., O.O., J. Lubiński, J.G.); Department of Gynecology and Obstetrics, Ribeirão Preto Medical School, University of São Paulo, Ribeirão Preto, Brazil (D.G.T., C.B.S., J.M.A., F.J.C.R.); Department of Pathology, Institute of Pathology, Heidelberg University Hospital, Heidelberg, Germany (P.S.); Department of Population Health Science and Policy, Department of Genetics and Genomic Sciences, Icahn School of Medicine at Mount Sinai, New York, NY (W.S., J.H.R.); Pathology West ICPMR Westmead, Westmead Hospital, the University of Sydney, Sydney, Australia (R.S.); University of Western Sydney at Westmead Hospital, Westmead, New South Wales, Australia (R.S.); Medical Oncology Service, Hospital Sant Pau, Barcelona, Spain (T.R.C.); H12O-CNIO Lung Cancer Clinical Research Unit (L.P.-A.), and Oncology Department, Hospital Universitario 12 de Octubre (L.P.-A.), Madrid, Spain; Women’s Cancer Program, Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, CA (S.O., J. Lester, B.Y.K.); the University of Texas School of Public Health, Houston (R.B.N.); Department of Oncology, Division of Gynecologic Oncology, Cumming School of Medicine, University of Calgary, Calgary, Alberta, Canada (G.N., P.G.); Department of Obstetrics, Gynecology and Reproductive Sciences, University of Pittsburgh School of Medicine (F.M., R.P.E.), Department of Epidemiology, University of Pittsburgh Graduate School of Public Health (F.M.), and Womens Cancer Research Program, Magee-Womens Research Institute and University of Pittsburgh Cancer Institute (F.M.), Pittsburgh, PA; Department of Gynecological Surgery and Gynecological Oncology of Adults and Adolescents, Pomeranian Medical University, Szczecin, Poland (J.M., A.C.-G.); Department of Health Research and Policy, Stanford University School of Medicine, Stanford, CA (V.M.); Department of Oncology, University of Cambridge, Strangeways Research Laboratory, Cambridge, UK (M.M., J.A., P.D.P.P.); Department of Pathology, Stanford University, Stanford, CA (T.A.L.); Department of Gynecologic Oncology, the Third Affiliated Hospital of Kunming Medical University (Yunnan Tumor Hospital), Kunming, China (Z.L.); Centre for Cancer Research, the Westmead Institute for Medical Research, the University of Sydney (C.J.K., J.H., P.R.H., B. Gao, A.deF.), and Department of Gynaecological Oncology, Westmead Hospital (C.J.K., J.H., A.deF.), Sydney, New South Wales, Australia; Division of Cancer Epidemiology, German Cancer Research Center (Deutsches Krebsforschungszentrum), Heidelberg, Germany (A.Y.J., U.E., J.C.-C.); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia (S.E.J., G.C.-T.); Department of Histopathology, Addenbrookes Hospital, Cambridge, UK (M.J.-L.); Virus, Lifestyle and Genes, Danish Cancer Society Research Center, Copenhagen, Denmark (A.J., S.K.K.); School of Women’s and Children’s Health, University of New South Wales, Sydney, Australia (M.P.I., S.J.R.); Tissue Bank of the National Center for Tumor Diseases (NCT) Heidelberg (E. Herpel), and Institute of Pathology, University of Heidelberg (E. Herpel), Heidelberg, Germany; Cancer Center, University of Hawaii, Honolulu, HI (B.Y.H.); the Crown Princess Mary Cancer Centre, Westmead Hospital, the University of Sydney, Sydney, Australia (P.R.H., B. Gao); Oncology Department, Hospital General de Albacete, Madrid, Spain (J.M.G.-B.); Department of Cancer Genomics and Genetics, Peter MacCallum Cancer Centre, East Melbourne, Victoria, Australia (S.F.); Pathology Department, Fundación Jiménez Díaz, Madrid, Spain (A.C.); Department of Health Research and Policy and Department of Biomedical Data Science, Stanford University School of Medicine, Stanford, CA (A.S.W.); Department of Obstetrics and Gynecology, Division of Gynecologic Oncology, Royal Alexandra Hospital, Edmonton, Alberta, Canada (H.S.); Tübingen University Hospital, Institute of Pathology, Tübingen, Germany (A.S.); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (K.B.M.); Gynaecological Cancer Research Centre, Department of Women’s Cancer, Institute for Women’s Health, University College London, London, UK (U.M.); Alberta Health Services-Cancer Care, Calgary, Alberta, Canada (J.M.K.); Department of Gynecology, Rigshospitalet, University of Copenhagen, Copenhagen, Denmark (S.K.K.); Department of Public Health Sciences, Medical University of South Carolina and Hollings Cancer Center, Charleston, SC (L.E.K.); Department of Pathology and Laboratory Medicine, University of British Columbia (D.G.H.), and Centre for Translational and Applied Genomics, British Columbia Cancer Agency (D.G.H.), Vancouver, British Columbia, Canada; Molecular Unit, Department of Pathology, Herlev Hospital, University of Copenhagen, Copenhagen, Denmark (E. Høgdall); Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, CA (M.T.G.); Genetic Pathology Evaluation Centre, Vancouver General Hospital and University of British Columbia, Vancouver, British Columbia, Canada (B. Gilks); Human Genetics Group, Spanish National Cancer Research Centre (Centro Nacional de Investigaciones Oncológicas) (M.J.G., J.B.), and Centre for Biomedical Network Research on Rare Diseases (Centro de Investigación Biomédica en Red de Enfermedades Raras), Instituto de Salud Carlos III (M.J.G., J.B.), Madrid, Spain; David Geffen School of Medicine, Department of Medicine, Division of Hematology and Oncology, University of California at Los Angeles (P.A.F.); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Erlangen, Germany (P.A.F.); Department of Histopathology, Queen’s Medical Centre, Nottingham University Hospitals NHS Trust, Nottingham, UK (S.D.); University Cancer Center Hamburg, University Medical Center Hamburg-Eppendorf, Hamburg, Germany (J.C.-C.); Cancer Genetics Laboratory, Research Division, Peter MacCallum Cancer Centre (I.G.C.), Sir Peter MacCallum Department of Oncology, University of Melbourne (I.G.C.), and Department of Pathology, University of Melbourne (I.G.C.), Melbourne, Victoria, Australia; Department of Oncology, University of Cambridge, Hutchison/MRC Research Centre (J.D.B.), Cancer Research UK Cambridge Institute, University of Cambridge, Li Ka Shing Centre (J.D.B.), and Cambridge Experimental Cancer Medicine Centre (J.D.B.), Cambridge, UK; Cancer Genomics Program, Research Department, Peter MacCallum Cancer Centre, East Melbourne, Victoria, Australia (D.D.B.); the Garvan Institute, Sydney, New South Wales, Australia (D.D.B., S.J.R.); and Department of Public Health and Primary Care, University of Cambridge, Strangeways Research Laboratory, Cambridge, UK (P.D.P.P.)
1,
Alicia Cazorla
Alicia Cazorla, MD
1Department of Medical Oncology (M.S.B., K.R.K.) and Department of Health Sciences Research (R.A.V., S.J.W., B.M.M., Z.L., E.L.G.), Mayo Clinic, Rochester, MN; Department of Pathology and Laboratory Medicine, University of Calgary, Calgary, Alberta, Canada (P.F.R., M.K.); Pathology Department, Catholic University of Health and Allied Sciences-Bugando, Mwanza, Tanzania (P.F.R.); Tübingen University Hospital, Department of Women’s Health, Tübingen, Germany (P.W., F.A.T., A.D.H., S.Y.B., S.K.); Sir Peter MacCallum Department of Oncology, the University of Melbourne, Parkville, Victoria, Australia (N.T., D.D.B.); International Hereditary Cancer Center, Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (A.T., O.O., J. Lubiński, J.G.); Department of Gynecology and Obstetrics, Ribeirão Preto Medical School, University of São Paulo, Ribeirão Preto, Brazil (D.G.T., C.B.S., J.M.A., F.J.C.R.); Department of Pathology, Institute of Pathology, Heidelberg University Hospital, Heidelberg, Germany (P.S.); Department of Population Health Science and Policy, Department of Genetics and Genomic Sciences, Icahn School of Medicine at Mount Sinai, New York, NY (W.S., J.H.R.); Pathology West ICPMR Westmead, Westmead Hospital, the University of Sydney, Sydney, Australia (R.S.); University of Western Sydney at Westmead Hospital, Westmead, New South Wales, Australia (R.S.); Medical Oncology Service, Hospital Sant Pau, Barcelona, Spain (T.R.C.); H12O-CNIO Lung Cancer Clinical Research Unit (L.P.-A.), and Oncology Department, Hospital Universitario 12 de Octubre (L.P.-A.), Madrid, Spain; Women’s Cancer Program, Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, CA (S.O., J. Lester, B.Y.K.); the University of Texas School of Public Health, Houston (R.B.N.); Department of Oncology, Division of Gynecologic Oncology, Cumming School of Medicine, University of Calgary, Calgary, Alberta, Canada (G.N., P.G.); Department of Obstetrics, Gynecology and Reproductive Sciences, University of Pittsburgh School of Medicine (F.M., R.P.E.), Department of Epidemiology, University of Pittsburgh Graduate School of Public Health (F.M.), and Womens Cancer Research Program, Magee-Womens Research Institute and University of Pittsburgh Cancer Institute (F.M.), Pittsburgh, PA; Department of Gynecological Surgery and Gynecological Oncology of Adults and Adolescents, Pomeranian Medical University, Szczecin, Poland (J.M., A.C.-G.); Department of Health Research and Policy, Stanford University School of Medicine, Stanford, CA (V.M.); Department of Oncology, University of Cambridge, Strangeways Research Laboratory, Cambridge, UK (M.M., J.A., P.D.P.P.); Department of Pathology, Stanford University, Stanford, CA (T.A.L.); Department of Gynecologic Oncology, the Third Affiliated Hospital of Kunming Medical University (Yunnan Tumor Hospital), Kunming, China (Z.L.); Centre for Cancer Research, the Westmead Institute for Medical Research, the University of Sydney (C.J.K., J.H., P.R.H., B. Gao, A.deF.), and Department of Gynaecological Oncology, Westmead Hospital (C.J.K., J.H., A.deF.), Sydney, New South Wales, Australia; Division of Cancer Epidemiology, German Cancer Research Center (Deutsches Krebsforschungszentrum), Heidelberg, Germany (A.Y.J., U.E., J.C.-C.); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia (S.E.J., G.C.-T.); Department of Histopathology, Addenbrookes Hospital, Cambridge, UK (M.J.-L.); Virus, Lifestyle and Genes, Danish Cancer Society Research Center, Copenhagen, Denmark (A.J., S.K.K.); School of Women’s and Children’s Health, University of New South Wales, Sydney, Australia (M.P.I., S.J.R.); Tissue Bank of the National Center for Tumor Diseases (NCT) Heidelberg (E. Herpel), and Institute of Pathology, University of Heidelberg (E. Herpel), Heidelberg, Germany; Cancer Center, University of Hawaii, Honolulu, HI (B.Y.H.); the Crown Princess Mary Cancer Centre, Westmead Hospital, the University of Sydney, Sydney, Australia (P.R.H., B. Gao); Oncology Department, Hospital General de Albacete, Madrid, Spain (J.M.G.-B.); Department of Cancer Genomics and Genetics, Peter MacCallum Cancer Centre, East Melbourne, Victoria, Australia (S.F.); Pathology Department, Fundación Jiménez Díaz, Madrid, Spain (A.C.); Department of Health Research and Policy and Department of Biomedical Data Science, Stanford University School of Medicine, Stanford, CA (A.S.W.); Department of Obstetrics and Gynecology, Division of Gynecologic Oncology, Royal Alexandra Hospital, Edmonton, Alberta, Canada (H.S.); Tübingen University Hospital, Institute of Pathology, Tübingen, Germany (A.S.); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (K.B.M.); Gynaecological Cancer Research Centre, Department of Women’s Cancer, Institute for Women’s Health, University College London, London, UK (U.M.); Alberta Health Services-Cancer Care, Calgary, Alberta, Canada (J.M.K.); Department of Gynecology, Rigshospitalet, University of Copenhagen, Copenhagen, Denmark (S.K.K.); Department of Public Health Sciences, Medical University of South Carolina and Hollings Cancer Center, Charleston, SC (L.E.K.); Department of Pathology and Laboratory Medicine, University of British Columbia (D.G.H.), and Centre for Translational and Applied Genomics, British Columbia Cancer Agency (D.G.H.), Vancouver, British Columbia, Canada; Molecular Unit, Department of Pathology, Herlev Hospital, University of Copenhagen, Copenhagen, Denmark (E. Høgdall); Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, CA (M.T.G.); Genetic Pathology Evaluation Centre, Vancouver General Hospital and University of British Columbia, Vancouver, British Columbia, Canada (B. Gilks); Human Genetics Group, Spanish National Cancer Research Centre (Centro Nacional de Investigaciones Oncológicas) (M.J.G., J.B.), and Centre for Biomedical Network Research on Rare Diseases (Centro de Investigación Biomédica en Red de Enfermedades Raras), Instituto de Salud Carlos III (M.J.G., J.B.), Madrid, Spain; David Geffen School of Medicine, Department of Medicine, Division of Hematology and Oncology, University of California at Los Angeles (P.A.F.); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Erlangen, Germany (P.A.F.); Department of Histopathology, Queen’s Medical Centre, Nottingham University Hospitals NHS Trust, Nottingham, UK (S.D.); University Cancer Center Hamburg, University Medical Center Hamburg-Eppendorf, Hamburg, Germany (J.C.-C.); Cancer Genetics Laboratory, Research Division, Peter MacCallum Cancer Centre (I.G.C.), Sir Peter MacCallum Department of Oncology, University of Melbourne (I.G.C.), and Department of Pathology, University of Melbourne (I.G.C.), Melbourne, Victoria, Australia; Department of Oncology, University of Cambridge, Hutchison/MRC Research Centre (J.D.B.), Cancer Research UK Cambridge Institute, University of Cambridge, Li Ka Shing Centre (J.D.B.), and Cambridge Experimental Cancer Medicine Centre (J.D.B.), Cambridge, UK; Cancer Genomics Program, Research Department, Peter MacCallum Cancer Centre, East Melbourne, Victoria, Australia (D.D.B.); the Garvan Institute, Sydney, New South Wales, Australia (D.D.B., S.J.R.); and Department of Public Health and Primary Care, University of Cambridge, Strangeways Research Laboratory, Cambridge, UK (P.D.P.P.)
1,
Sara Y Brucker
Sara Y Brucker, MD, PhD
1Department of Medical Oncology (M.S.B., K.R.K.) and Department of Health Sciences Research (R.A.V., S.J.W., B.M.M., Z.L., E.L.G.), Mayo Clinic, Rochester, MN; Department of Pathology and Laboratory Medicine, University of Calgary, Calgary, Alberta, Canada (P.F.R., M.K.); Pathology Department, Catholic University of Health and Allied Sciences-Bugando, Mwanza, Tanzania (P.F.R.); Tübingen University Hospital, Department of Women’s Health, Tübingen, Germany (P.W., F.A.T., A.D.H., S.Y.B., S.K.); Sir Peter MacCallum Department of Oncology, the University of Melbourne, Parkville, Victoria, Australia (N.T., D.D.B.); International Hereditary Cancer Center, Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (A.T., O.O., J. Lubiński, J.G.); Department of Gynecology and Obstetrics, Ribeirão Preto Medical School, University of São Paulo, Ribeirão Preto, Brazil (D.G.T., C.B.S., J.M.A., F.J.C.R.); Department of Pathology, Institute of Pathology, Heidelberg University Hospital, Heidelberg, Germany (P.S.); Department of Population Health Science and Policy, Department of Genetics and Genomic Sciences, Icahn School of Medicine at Mount Sinai, New York, NY (W.S., J.H.R.); Pathology West ICPMR Westmead, Westmead Hospital, the University of Sydney, Sydney, Australia (R.S.); University of Western Sydney at Westmead Hospital, Westmead, New South Wales, Australia (R.S.); Medical Oncology Service, Hospital Sant Pau, Barcelona, Spain (T.R.C.); H12O-CNIO Lung Cancer Clinical Research Unit (L.P.-A.), and Oncology Department, Hospital Universitario 12 de Octubre (L.P.-A.), Madrid, Spain; Women’s Cancer Program, Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, CA (S.O., J. Lester, B.Y.K.); the University of Texas School of Public Health, Houston (R.B.N.); Department of Oncology, Division of Gynecologic Oncology, Cumming School of Medicine, University of Calgary, Calgary, Alberta, Canada (G.N., P.G.); Department of Obstetrics, Gynecology and Reproductive Sciences, University of Pittsburgh School of Medicine (F.M., R.P.E.), Department of Epidemiology, University of Pittsburgh Graduate School of Public Health (F.M.), and Womens Cancer Research Program, Magee-Womens Research Institute and University of Pittsburgh Cancer Institute (F.M.), Pittsburgh, PA; Department of Gynecological Surgery and Gynecological Oncology of Adults and Adolescents, Pomeranian Medical University, Szczecin, Poland (J.M., A.C.-G.); Department of Health Research and Policy, Stanford University School of Medicine, Stanford, CA (V.M.); Department of Oncology, University of Cambridge, Strangeways Research Laboratory, Cambridge, UK (M.M., J.A., P.D.P.P.); Department of Pathology, Stanford University, Stanford, CA (T.A.L.); Department of Gynecologic Oncology, the Third Affiliated Hospital of Kunming Medical University (Yunnan Tumor Hospital), Kunming, China (Z.L.); Centre for Cancer Research, the Westmead Institute for Medical Research, the University of Sydney (C.J.K., J.H., P.R.H., B. Gao, A.deF.), and Department of Gynaecological Oncology, Westmead Hospital (C.J.K., J.H., A.deF.), Sydney, New South Wales, Australia; Division of Cancer Epidemiology, German Cancer Research Center (Deutsches Krebsforschungszentrum), Heidelberg, Germany (A.Y.J., U.E., J.C.-C.); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia (S.E.J., G.C.-T.); Department of Histopathology, Addenbrookes Hospital, Cambridge, UK (M.J.-L.); Virus, Lifestyle and Genes, Danish Cancer Society Research Center, Copenhagen, Denmark (A.J., S.K.K.); School of Women’s and Children’s Health, University of New South Wales, Sydney, Australia (M.P.I., S.J.R.); Tissue Bank of the National Center for Tumor Diseases (NCT) Heidelberg (E. Herpel), and Institute of Pathology, University of Heidelberg (E. Herpel), Heidelberg, Germany; Cancer Center, University of Hawaii, Honolulu, HI (B.Y.H.); the Crown Princess Mary Cancer Centre, Westmead Hospital, the University of Sydney, Sydney, Australia (P.R.H., B. Gao); Oncology Department, Hospital General de Albacete, Madrid, Spain (J.M.G.-B.); Department of Cancer Genomics and Genetics, Peter MacCallum Cancer Centre, East Melbourne, Victoria, Australia (S.F.); Pathology Department, Fundación Jiménez Díaz, Madrid, Spain (A.C.); Department of Health Research and Policy and Department of Biomedical Data Science, Stanford University School of Medicine, Stanford, CA (A.S.W.); Department of Obstetrics and Gynecology, Division of Gynecologic Oncology, Royal Alexandra Hospital, Edmonton, Alberta, Canada (H.S.); Tübingen University Hospital, Institute of Pathology, Tübingen, Germany (A.S.); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (K.B.M.); Gynaecological Cancer Research Centre, Department of Women’s Cancer, Institute for Women’s Health, University College London, London, UK (U.M.); Alberta Health Services-Cancer Care, Calgary, Alberta, Canada (J.M.K.); Department of Gynecology, Rigshospitalet, University of Copenhagen, Copenhagen, Denmark (S.K.K.); Department of Public Health Sciences, Medical University of South Carolina and Hollings Cancer Center, Charleston, SC (L.E.K.); Department of Pathology and Laboratory Medicine, University of British Columbia (D.G.H.), and Centre for Translational and Applied Genomics, British Columbia Cancer Agency (D.G.H.), Vancouver, British Columbia, Canada; Molecular Unit, Department of Pathology, Herlev Hospital, University of Copenhagen, Copenhagen, Denmark (E. Høgdall); Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, CA (M.T.G.); Genetic Pathology Evaluation Centre, Vancouver General Hospital and University of British Columbia, Vancouver, British Columbia, Canada (B. Gilks); Human Genetics Group, Spanish National Cancer Research Centre (Centro Nacional de Investigaciones Oncológicas) (M.J.G., J.B.), and Centre for Biomedical Network Research on Rare Diseases (Centro de Investigación Biomédica en Red de Enfermedades Raras), Instituto de Salud Carlos III (M.J.G., J.B.), Madrid, Spain; David Geffen School of Medicine, Department of Medicine, Division of Hematology and Oncology, University of California at Los Angeles (P.A.F.); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Erlangen, Germany (P.A.F.); Department of Histopathology, Queen’s Medical Centre, Nottingham University Hospitals NHS Trust, Nottingham, UK (S.D.); University Cancer Center Hamburg, University Medical Center Hamburg-Eppendorf, Hamburg, Germany (J.C.-C.); Cancer Genetics Laboratory, Research Division, Peter MacCallum Cancer Centre (I.G.C.), Sir Peter MacCallum Department of Oncology, University of Melbourne (I.G.C.), and Department of Pathology, University of Melbourne (I.G.C.), Melbourne, Victoria, Australia; Department of Oncology, University of Cambridge, Hutchison/MRC Research Centre (J.D.B.), Cancer Research UK Cambridge Institute, University of Cambridge, Li Ka Shing Centre (J.D.B.), and Cambridge Experimental Cancer Medicine Centre (J.D.B.), Cambridge, UK; Cancer Genomics Program, Research Department, Peter MacCallum Cancer Centre, East Melbourne, Victoria, Australia (D.D.B.); the Garvan Institute, Sydney, New South Wales, Australia (D.D.B., S.J.R.); and Department of Public Health and Primary Care, University of Cambridge, Strangeways Research Laboratory, Cambridge, UK (P.D.P.P.)
1;
Australian Ovarian Cancer Study Group1,*,
Jennifer Alsop
Jennifer Alsop, BA
1Department of Medical Oncology (M.S.B., K.R.K.) and Department of Health Sciences Research (R.A.V., S.J.W., B.M.M., Z.L., E.L.G.), Mayo Clinic, Rochester, MN; Department of Pathology and Laboratory Medicine, University of Calgary, Calgary, Alberta, Canada (P.F.R., M.K.); Pathology Department, Catholic University of Health and Allied Sciences-Bugando, Mwanza, Tanzania (P.F.R.); Tübingen University Hospital, Department of Women’s Health, Tübingen, Germany (P.W., F.A.T., A.D.H., S.Y.B., S.K.); Sir Peter MacCallum Department of Oncology, the University of Melbourne, Parkville, Victoria, Australia (N.T., D.D.B.); International Hereditary Cancer Center, Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (A.T., O.O., J. Lubiński, J.G.); Department of Gynecology and Obstetrics, Ribeirão Preto Medical School, University of São Paulo, Ribeirão Preto, Brazil (D.G.T., C.B.S., J.M.A., F.J.C.R.); Department of Pathology, Institute of Pathology, Heidelberg University Hospital, Heidelberg, Germany (P.S.); Department of Population Health Science and Policy, Department of Genetics and Genomic Sciences, Icahn School of Medicine at Mount Sinai, New York, NY (W.S., J.H.R.); Pathology West ICPMR Westmead, Westmead Hospital, the University of Sydney, Sydney, Australia (R.S.); University of Western Sydney at Westmead Hospital, Westmead, New South Wales, Australia (R.S.); Medical Oncology Service, Hospital Sant Pau, Barcelona, Spain (T.R.C.); H12O-CNIO Lung Cancer Clinical Research Unit (L.P.-A.), and Oncology Department, Hospital Universitario 12 de Octubre (L.P.-A.), Madrid, Spain; Women’s Cancer Program, Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, CA (S.O., J. Lester, B.Y.K.); the University of Texas School of Public Health, Houston (R.B.N.); Department of Oncology, Division of Gynecologic Oncology, Cumming School of Medicine, University of Calgary, Calgary, Alberta, Canada (G.N., P.G.); Department of Obstetrics, Gynecology and Reproductive Sciences, University of Pittsburgh School of Medicine (F.M., R.P.E.), Department of Epidemiology, University of Pittsburgh Graduate School of Public Health (F.M.), and Womens Cancer Research Program, Magee-Womens Research Institute and University of Pittsburgh Cancer Institute (F.M.), Pittsburgh, PA; Department of Gynecological Surgery and Gynecological Oncology of Adults and Adolescents, Pomeranian Medical University, Szczecin, Poland (J.M., A.C.-G.); Department of Health Research and Policy, Stanford University School of Medicine, Stanford, CA (V.M.); Department of Oncology, University of Cambridge, Strangeways Research Laboratory, Cambridge, UK (M.M., J.A., P.D.P.P.); Department of Pathology, Stanford University, Stanford, CA (T.A.L.); Department of Gynecologic Oncology, the Third Affiliated Hospital of Kunming Medical University (Yunnan Tumor Hospital), Kunming, China (Z.L.); Centre for Cancer Research, the Westmead Institute for Medical Research, the University of Sydney (C.J.K., J.H., P.R.H., B. Gao, A.deF.), and Department of Gynaecological Oncology, Westmead Hospital (C.J.K., J.H., A.deF.), Sydney, New South Wales, Australia; Division of Cancer Epidemiology, German Cancer Research Center (Deutsches Krebsforschungszentrum), Heidelberg, Germany (A.Y.J., U.E., J.C.-C.); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia (S.E.J., G.C.-T.); Department of Histopathology, Addenbrookes Hospital, Cambridge, UK (M.J.-L.); Virus, Lifestyle and Genes, Danish Cancer Society Research Center, Copenhagen, Denmark (A.J., S.K.K.); School of Women’s and Children’s Health, University of New South Wales, Sydney, Australia (M.P.I., S.J.R.); Tissue Bank of the National Center for Tumor Diseases (NCT) Heidelberg (E. Herpel), and Institute of Pathology, University of Heidelberg (E. Herpel), Heidelberg, Germany; Cancer Center, University of Hawaii, Honolulu, HI (B.Y.H.); the Crown Princess Mary Cancer Centre, Westmead Hospital, the University of Sydney, Sydney, Australia (P.R.H., B. Gao); Oncology Department, Hospital General de Albacete, Madrid, Spain (J.M.G.-B.); Department of Cancer Genomics and Genetics, Peter MacCallum Cancer Centre, East Melbourne, Victoria, Australia (S.F.); Pathology Department, Fundación Jiménez Díaz, Madrid, Spain (A.C.); Department of Health Research and Policy and Department of Biomedical Data Science, Stanford University School of Medicine, Stanford, CA (A.S.W.); Department of Obstetrics and Gynecology, Division of Gynecologic Oncology, Royal Alexandra Hospital, Edmonton, Alberta, Canada (H.S.); Tübingen University Hospital, Institute of Pathology, Tübingen, Germany (A.S.); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (K.B.M.); Gynaecological Cancer Research Centre, Department of Women’s Cancer, Institute for Women’s Health, University College London, London, UK (U.M.); Alberta Health Services-Cancer Care, Calgary, Alberta, Canada (J.M.K.); Department of Gynecology, Rigshospitalet, University of Copenhagen, Copenhagen, Denmark (S.K.K.); Department of Public Health Sciences, Medical University of South Carolina and Hollings Cancer Center, Charleston, SC (L.E.K.); Department of Pathology and Laboratory Medicine, University of British Columbia (D.G.H.), and Centre for Translational and Applied Genomics, British Columbia Cancer Agency (D.G.H.), Vancouver, British Columbia, Canada; Molecular Unit, Department of Pathology, Herlev Hospital, University of Copenhagen, Copenhagen, Denmark (E. Høgdall); Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, CA (M.T.G.); Genetic Pathology Evaluation Centre, Vancouver General Hospital and University of British Columbia, Vancouver, British Columbia, Canada (B. Gilks); Human Genetics Group, Spanish National Cancer Research Centre (Centro Nacional de Investigaciones Oncológicas) (M.J.G., J.B.), and Centre for Biomedical Network Research on Rare Diseases (Centro de Investigación Biomédica en Red de Enfermedades Raras), Instituto de Salud Carlos III (M.J.G., J.B.), Madrid, Spain; David Geffen School of Medicine, Department of Medicine, Division of Hematology and Oncology, University of California at Los Angeles (P.A.F.); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Erlangen, Germany (P.A.F.); Department of Histopathology, Queen’s Medical Centre, Nottingham University Hospitals NHS Trust, Nottingham, UK (S.D.); University Cancer Center Hamburg, University Medical Center Hamburg-Eppendorf, Hamburg, Germany (J.C.-C.); Cancer Genetics Laboratory, Research Division, Peter MacCallum Cancer Centre (I.G.C.), Sir Peter MacCallum Department of Oncology, University of Melbourne (I.G.C.), and Department of Pathology, University of Melbourne (I.G.C.), Melbourne, Victoria, Australia; Department of Oncology, University of Cambridge, Hutchison/MRC Research Centre (J.D.B.), Cancer Research UK Cambridge Institute, University of Cambridge, Li Ka Shing Centre (J.D.B.), and Cambridge Experimental Cancer Medicine Centre (J.D.B.), Cambridge, UK; Cancer Genomics Program, Research Department, Peter MacCallum Cancer Centre, East Melbourne, Victoria, Australia (D.D.B.); the Garvan Institute, Sydney, New South Wales, Australia (D.D.B., S.J.R.); and Department of Public Health and Primary Care, University of Cambridge, Strangeways Research Laboratory, Cambridge, UK (P.D.P.P.)
1,
Alice S Whittemore
Alice S Whittemore, PhD
1Department of Medical Oncology (M.S.B., K.R.K.) and Department of Health Sciences Research (R.A.V., S.J.W., B.M.M., Z.L., E.L.G.), Mayo Clinic, Rochester, MN; Department of Pathology and Laboratory Medicine, University of Calgary, Calgary, Alberta, Canada (P.F.R., M.K.); Pathology Department, Catholic University of Health and Allied Sciences-Bugando, Mwanza, Tanzania (P.F.R.); Tübingen University Hospital, Department of Women’s Health, Tübingen, Germany (P.W., F.A.T., A.D.H., S.Y.B., S.K.); Sir Peter MacCallum Department of Oncology, the University of Melbourne, Parkville, Victoria, Australia (N.T., D.D.B.); International Hereditary Cancer Center, Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (A.T., O.O., J. Lubiński, J.G.); Department of Gynecology and Obstetrics, Ribeirão Preto Medical School, University of São Paulo, Ribeirão Preto, Brazil (D.G.T., C.B.S., J.M.A., F.J.C.R.); Department of Pathology, Institute of Pathology, Heidelberg University Hospital, Heidelberg, Germany (P.S.); Department of Population Health Science and Policy, Department of Genetics and Genomic Sciences, Icahn School of Medicine at Mount Sinai, New York, NY (W.S., J.H.R.); Pathology West ICPMR Westmead, Westmead Hospital, the University of Sydney, Sydney, Australia (R.S.); University of Western Sydney at Westmead Hospital, Westmead, New South Wales, Australia (R.S.); Medical Oncology Service, Hospital Sant Pau, Barcelona, Spain (T.R.C.); H12O-CNIO Lung Cancer Clinical Research Unit (L.P.-A.), and Oncology Department, Hospital Universitario 12 de Octubre (L.P.-A.), Madrid, Spain; Women’s Cancer Program, Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, CA (S.O., J. Lester, B.Y.K.); the University of Texas School of Public Health, Houston (R.B.N.); Department of Oncology, Division of Gynecologic Oncology, Cumming School of Medicine, University of Calgary, Calgary, Alberta, Canada (G.N., P.G.); Department of Obstetrics, Gynecology and Reproductive Sciences, University of Pittsburgh School of Medicine (F.M., R.P.E.), Department of Epidemiology, University of Pittsburgh Graduate School of Public Health (F.M.), and Womens Cancer Research Program, Magee-Womens Research Institute and University of Pittsburgh Cancer Institute (F.M.), Pittsburgh, PA; Department of Gynecological Surgery and Gynecological Oncology of Adults and Adolescents, Pomeranian Medical University, Szczecin, Poland (J.M., A.C.-G.); Department of Health Research and Policy, Stanford University School of Medicine, Stanford, CA (V.M.); Department of Oncology, University of Cambridge, Strangeways Research Laboratory, Cambridge, UK (M.M., J.A., P.D.P.P.); Department of Pathology, Stanford University, Stanford, CA (T.A.L.); Department of Gynecologic Oncology, the Third Affiliated Hospital of Kunming Medical University (Yunnan Tumor Hospital), Kunming, China (Z.L.); Centre for Cancer Research, the Westmead Institute for Medical Research, the University of Sydney (C.J.K., J.H., P.R.H., B. Gao, A.deF.), and Department of Gynaecological Oncology, Westmead Hospital (C.J.K., J.H., A.deF.), Sydney, New South Wales, Australia; Division of Cancer Epidemiology, German Cancer Research Center (Deutsches Krebsforschungszentrum), Heidelberg, Germany (A.Y.J., U.E., J.C.-C.); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia (S.E.J., G.C.-T.); Department of Histopathology, Addenbrookes Hospital, Cambridge, UK (M.J.-L.); Virus, Lifestyle and Genes, Danish Cancer Society Research Center, Copenhagen, Denmark (A.J., S.K.K.); School of Women’s and Children’s Health, University of New South Wales, Sydney, Australia (M.P.I., S.J.R.); Tissue Bank of the National Center for Tumor Diseases (NCT) Heidelberg (E. Herpel), and Institute of Pathology, University of Heidelberg (E. Herpel), Heidelberg, Germany; Cancer Center, University of Hawaii, Honolulu, HI (B.Y.H.); the Crown Princess Mary Cancer Centre, Westmead Hospital, the University of Sydney, Sydney, Australia (P.R.H., B. Gao); Oncology Department, Hospital General de Albacete, Madrid, Spain (J.M.G.-B.); Department of Cancer Genomics and Genetics, Peter MacCallum Cancer Centre, East Melbourne, Victoria, Australia (S.F.); Pathology Department, Fundación Jiménez Díaz, Madrid, Spain (A.C.); Department of Health Research and Policy and Department of Biomedical Data Science, Stanford University School of Medicine, Stanford, CA (A.S.W.); Department of Obstetrics and Gynecology, Division of Gynecologic Oncology, Royal Alexandra Hospital, Edmonton, Alberta, Canada (H.S.); Tübingen University Hospital, Institute of Pathology, Tübingen, Germany (A.S.); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (K.B.M.); Gynaecological Cancer Research Centre, Department of Women’s Cancer, Institute for Women’s Health, University College London, London, UK (U.M.); Alberta Health Services-Cancer Care, Calgary, Alberta, Canada (J.M.K.); Department of Gynecology, Rigshospitalet, University of Copenhagen, Copenhagen, Denmark (S.K.K.); Department of Public Health Sciences, Medical University of South Carolina and Hollings Cancer Center, Charleston, SC (L.E.K.); Department of Pathology and Laboratory Medicine, University of British Columbia (D.G.H.), and Centre for Translational and Applied Genomics, British Columbia Cancer Agency (D.G.H.), Vancouver, British Columbia, Canada; Molecular Unit, Department of Pathology, Herlev Hospital, University of Copenhagen, Copenhagen, Denmark (E. Høgdall); Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, CA (M.T.G.); Genetic Pathology Evaluation Centre, Vancouver General Hospital and University of British Columbia, Vancouver, British Columbia, Canada (B. Gilks); Human Genetics Group, Spanish National Cancer Research Centre (Centro Nacional de Investigaciones Oncológicas) (M.J.G., J.B.), and Centre for Biomedical Network Research on Rare Diseases (Centro de Investigación Biomédica en Red de Enfermedades Raras), Instituto de Salud Carlos III (M.J.G., J.B.), Madrid, Spain; David Geffen School of Medicine, Department of Medicine, Division of Hematology and Oncology, University of California at Los Angeles (P.A.F.); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Erlangen, Germany (P.A.F.); Department of Histopathology, Queen’s Medical Centre, Nottingham University Hospitals NHS Trust, Nottingham, UK (S.D.); University Cancer Center Hamburg, University Medical Center Hamburg-Eppendorf, Hamburg, Germany (J.C.-C.); Cancer Genetics Laboratory, Research Division, Peter MacCallum Cancer Centre (I.G.C.), Sir Peter MacCallum Department of Oncology, University of Melbourne (I.G.C.), and Department of Pathology, University of Melbourne (I.G.C.), Melbourne, Victoria, Australia; Department of Oncology, University of Cambridge, Hutchison/MRC Research Centre (J.D.B.), Cancer Research UK Cambridge Institute, University of Cambridge, Li Ka Shing Centre (J.D.B.), and Cambridge Experimental Cancer Medicine Centre (J.D.B.), Cambridge, UK; Cancer Genomics Program, Research Department, Peter MacCallum Cancer Centre, East Melbourne, Victoria, Australia (D.D.B.); the Garvan Institute, Sydney, New South Wales, Australia (D.D.B., S.J.R.); and Department of Public Health and Primary Care, University of Cambridge, Strangeways Research Laboratory, Cambridge, UK (P.D.P.P.)
1,
Helen Steed
Helen Steed, MD
1Department of Medical Oncology (M.S.B., K.R.K.) and Department of Health Sciences Research (R.A.V., S.J.W., B.M.M., Z.L., E.L.G.), Mayo Clinic, Rochester, MN; Department of Pathology and Laboratory Medicine, University of Calgary, Calgary, Alberta, Canada (P.F.R., M.K.); Pathology Department, Catholic University of Health and Allied Sciences-Bugando, Mwanza, Tanzania (P.F.R.); Tübingen University Hospital, Department of Women’s Health, Tübingen, Germany (P.W., F.A.T., A.D.H., S.Y.B., S.K.); Sir Peter MacCallum Department of Oncology, the University of Melbourne, Parkville, Victoria, Australia (N.T., D.D.B.); International Hereditary Cancer Center, Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (A.T., O.O., J. Lubiński, J.G.); Department of Gynecology and Obstetrics, Ribeirão Preto Medical School, University of São Paulo, Ribeirão Preto, Brazil (D.G.T., C.B.S., J.M.A., F.J.C.R.); Department of Pathology, Institute of Pathology, Heidelberg University Hospital, Heidelberg, Germany (P.S.); Department of Population Health Science and Policy, Department of Genetics and Genomic Sciences, Icahn School of Medicine at Mount Sinai, New York, NY (W.S., J.H.R.); Pathology West ICPMR Westmead, Westmead Hospital, the University of Sydney, Sydney, Australia (R.S.); University of Western Sydney at Westmead Hospital, Westmead, New South Wales, Australia (R.S.); Medical Oncology Service, Hospital Sant Pau, Barcelona, Spain (T.R.C.); H12O-CNIO Lung Cancer Clinical Research Unit (L.P.-A.), and Oncology Department, Hospital Universitario 12 de Octubre (L.P.-A.), Madrid, Spain; Women’s Cancer Program, Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, CA (S.O., J. Lester, B.Y.K.); the University of Texas School of Public Health, Houston (R.B.N.); Department of Oncology, Division of Gynecologic Oncology, Cumming School of Medicine, University of Calgary, Calgary, Alberta, Canada (G.N., P.G.); Department of Obstetrics, Gynecology and Reproductive Sciences, University of Pittsburgh School of Medicine (F.M., R.P.E.), Department of Epidemiology, University of Pittsburgh Graduate School of Public Health (F.M.), and Womens Cancer Research Program, Magee-Womens Research Institute and University of Pittsburgh Cancer Institute (F.M.), Pittsburgh, PA; Department of Gynecological Surgery and Gynecological Oncology of Adults and Adolescents, Pomeranian Medical University, Szczecin, Poland (J.M., A.C.-G.); Department of Health Research and Policy, Stanford University School of Medicine, Stanford, CA (V.M.); Department of Oncology, University of Cambridge, Strangeways Research Laboratory, Cambridge, UK (M.M., J.A., P.D.P.P.); Department of Pathology, Stanford University, Stanford, CA (T.A.L.); Department of Gynecologic Oncology, the Third Affiliated Hospital of Kunming Medical University (Yunnan Tumor Hospital), Kunming, China (Z.L.); Centre for Cancer Research, the Westmead Institute for Medical Research, the University of Sydney (C.J.K., J.H., P.R.H., B. Gao, A.deF.), and Department of Gynaecological Oncology, Westmead Hospital (C.J.K., J.H., A.deF.), Sydney, New South Wales, Australia; Division of Cancer Epidemiology, German Cancer Research Center (Deutsches Krebsforschungszentrum), Heidelberg, Germany (A.Y.J., U.E., J.C.-C.); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia (S.E.J., G.C.-T.); Department of Histopathology, Addenbrookes Hospital, Cambridge, UK (M.J.-L.); Virus, Lifestyle and Genes, Danish Cancer Society Research Center, Copenhagen, Denmark (A.J., S.K.K.); School of Women’s and Children’s Health, University of New South Wales, Sydney, Australia (M.P.I., S.J.R.); Tissue Bank of the National Center for Tumor Diseases (NCT) Heidelberg (E. Herpel), and Institute of Pathology, University of Heidelberg (E. Herpel), Heidelberg, Germany; Cancer Center, University of Hawaii, Honolulu, HI (B.Y.H.); the Crown Princess Mary Cancer Centre, Westmead Hospital, the University of Sydney, Sydney, Australia (P.R.H., B. Gao); Oncology Department, Hospital General de Albacete, Madrid, Spain (J.M.G.-B.); Department of Cancer Genomics and Genetics, Peter MacCallum Cancer Centre, East Melbourne, Victoria, Australia (S.F.); Pathology Department, Fundación Jiménez Díaz, Madrid, Spain (A.C.); Department of Health Research and Policy and Department of Biomedical Data Science, Stanford University School of Medicine, Stanford, CA (A.S.W.); Department of Obstetrics and Gynecology, Division of Gynecologic Oncology, Royal Alexandra Hospital, Edmonton, Alberta, Canada (H.S.); Tübingen University Hospital, Institute of Pathology, Tübingen, Germany (A.S.); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (K.B.M.); Gynaecological Cancer Research Centre, Department of Women’s Cancer, Institute for Women’s Health, University College London, London, UK (U.M.); Alberta Health Services-Cancer Care, Calgary, Alberta, Canada (J.M.K.); Department of Gynecology, Rigshospitalet, University of Copenhagen, Copenhagen, Denmark (S.K.K.); Department of Public Health Sciences, Medical University of South Carolina and Hollings Cancer Center, Charleston, SC (L.E.K.); Department of Pathology and Laboratory Medicine, University of British Columbia (D.G.H.), and Centre for Translational and Applied Genomics, British Columbia Cancer Agency (D.G.H.), Vancouver, British Columbia, Canada; Molecular Unit, Department of Pathology, Herlev Hospital, University of Copenhagen, Copenhagen, Denmark (E. Høgdall); Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, CA (M.T.G.); Genetic Pathology Evaluation Centre, Vancouver General Hospital and University of British Columbia, Vancouver, British Columbia, Canada (B. Gilks); Human Genetics Group, Spanish National Cancer Research Centre (Centro Nacional de Investigaciones Oncológicas) (M.J.G., J.B.), and Centre for Biomedical Network Research on Rare Diseases (Centro de Investigación Biomédica en Red de Enfermedades Raras), Instituto de Salud Carlos III (M.J.G., J.B.), Madrid, Spain; David Geffen School of Medicine, Department of Medicine, Division of Hematology and Oncology, University of California at Los Angeles (P.A.F.); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Erlangen, Germany (P.A.F.); Department of Histopathology, Queen’s Medical Centre, Nottingham University Hospitals NHS Trust, Nottingham, UK (S.D.); University Cancer Center Hamburg, University Medical Center Hamburg-Eppendorf, Hamburg, Germany (J.C.-C.); Cancer Genetics Laboratory, Research Division, Peter MacCallum Cancer Centre (I.G.C.), Sir Peter MacCallum Department of Oncology, University of Melbourne (I.G.C.), and Department of Pathology, University of Melbourne (I.G.C.), Melbourne, Victoria, Australia; Department of Oncology, University of Cambridge, Hutchison/MRC Research Centre (J.D.B.), Cancer Research UK Cambridge Institute, University of Cambridge, Li Ka Shing Centre (J.D.B.), and Cambridge Experimental Cancer Medicine Centre (J.D.B.), Cambridge, UK; Cancer Genomics Program, Research Department, Peter MacCallum Cancer Centre, East Melbourne, Victoria, Australia (D.D.B.); the Garvan Institute, Sydney, New South Wales, Australia (D.D.B., S.J.R.); and Department of Public Health and Primary Care, University of Cambridge, Strangeways Research Laboratory, Cambridge, UK (P.D.P.P.)
1,
Annette Staebler
Annette Staebler, MD
1Department of Medical Oncology (M.S.B., K.R.K.) and Department of Health Sciences Research (R.A.V., S.J.W., B.M.M., Z.L., E.L.G.), Mayo Clinic, Rochester, MN; Department of Pathology and Laboratory Medicine, University of Calgary, Calgary, Alberta, Canada (P.F.R., M.K.); Pathology Department, Catholic University of Health and Allied Sciences-Bugando, Mwanza, Tanzania (P.F.R.); Tübingen University Hospital, Department of Women’s Health, Tübingen, Germany (P.W., F.A.T., A.D.H., S.Y.B., S.K.); Sir Peter MacCallum Department of Oncology, the University of Melbourne, Parkville, Victoria, Australia (N.T., D.D.B.); International Hereditary Cancer Center, Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (A.T., O.O., J. Lubiński, J.G.); Department of Gynecology and Obstetrics, Ribeirão Preto Medical School, University of São Paulo, Ribeirão Preto, Brazil (D.G.T., C.B.S., J.M.A., F.J.C.R.); Department of Pathology, Institute of Pathology, Heidelberg University Hospital, Heidelberg, Germany (P.S.); Department of Population Health Science and Policy, Department of Genetics and Genomic Sciences, Icahn School of Medicine at Mount Sinai, New York, NY (W.S., J.H.R.); Pathology West ICPMR Westmead, Westmead Hospital, the University of Sydney, Sydney, Australia (R.S.); University of Western Sydney at Westmead Hospital, Westmead, New South Wales, Australia (R.S.); Medical Oncology Service, Hospital Sant Pau, Barcelona, Spain (T.R.C.); H12O-CNIO Lung Cancer Clinical Research Unit (L.P.-A.), and Oncology Department, Hospital Universitario 12 de Octubre (L.P.-A.), Madrid, Spain; Women’s Cancer Program, Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, CA (S.O., J. Lester, B.Y.K.); the University of Texas School of Public Health, Houston (R.B.N.); Department of Oncology, Division of Gynecologic Oncology, Cumming School of Medicine, University of Calgary, Calgary, Alberta, Canada (G.N., P.G.); Department of Obstetrics, Gynecology and Reproductive Sciences, University of Pittsburgh School of Medicine (F.M., R.P.E.), Department of Epidemiology, University of Pittsburgh Graduate School of Public Health (F.M.), and Womens Cancer Research Program, Magee-Womens Research Institute and University of Pittsburgh Cancer Institute (F.M.), Pittsburgh, PA; Department of Gynecological Surgery and Gynecological Oncology of Adults and Adolescents, Pomeranian Medical University, Szczecin, Poland (J.M., A.C.-G.); Department of Health Research and Policy, Stanford University School of Medicine, Stanford, CA (V.M.); Department of Oncology, University of Cambridge, Strangeways Research Laboratory, Cambridge, UK (M.M., J.A., P.D.P.P.); Department of Pathology, Stanford University, Stanford, CA (T.A.L.); Department of Gynecologic Oncology, the Third Affiliated Hospital of Kunming Medical University (Yunnan Tumor Hospital), Kunming, China (Z.L.); Centre for Cancer Research, the Westmead Institute for Medical Research, the University of Sydney (C.J.K., J.H., P.R.H., B. Gao, A.deF.), and Department of Gynaecological Oncology, Westmead Hospital (C.J.K., J.H., A.deF.), Sydney, New South Wales, Australia; Division of Cancer Epidemiology, German Cancer Research Center (Deutsches Krebsforschungszentrum), Heidelberg, Germany (A.Y.J., U.E., J.C.-C.); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia (S.E.J., G.C.-T.); Department of Histopathology, Addenbrookes Hospital, Cambridge, UK (M.J.-L.); Virus, Lifestyle and Genes, Danish Cancer Society Research Center, Copenhagen, Denmark (A.J., S.K.K.); School of Women’s and Children’s Health, University of New South Wales, Sydney, Australia (M.P.I., S.J.R.); Tissue Bank of the National Center for Tumor Diseases (NCT) Heidelberg (E. Herpel), and Institute of Pathology, University of Heidelberg (E. Herpel), Heidelberg, Germany; Cancer Center, University of Hawaii, Honolulu, HI (B.Y.H.); the Crown Princess Mary Cancer Centre, Westmead Hospital, the University of Sydney, Sydney, Australia (P.R.H., B. Gao); Oncology Department, Hospital General de Albacete, Madrid, Spain (J.M.G.-B.); Department of Cancer Genomics and Genetics, Peter MacCallum Cancer Centre, East Melbourne, Victoria, Australia (S.F.); Pathology Department, Fundación Jiménez Díaz, Madrid, Spain (A.C.); Department of Health Research and Policy and Department of Biomedical Data Science, Stanford University School of Medicine, Stanford, CA (A.S.W.); Department of Obstetrics and Gynecology, Division of Gynecologic Oncology, Royal Alexandra Hospital, Edmonton, Alberta, Canada (H.S.); Tübingen University Hospital, Institute of Pathology, Tübingen, Germany (A.S.); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (K.B.M.); Gynaecological Cancer Research Centre, Department of Women’s Cancer, Institute for Women’s Health, University College London, London, UK (U.M.); Alberta Health Services-Cancer Care, Calgary, Alberta, Canada (J.M.K.); Department of Gynecology, Rigshospitalet, University of Copenhagen, Copenhagen, Denmark (S.K.K.); Department of Public Health Sciences, Medical University of South Carolina and Hollings Cancer Center, Charleston, SC (L.E.K.); Department of Pathology and Laboratory Medicine, University of British Columbia (D.G.H.), and Centre for Translational and Applied Genomics, British Columbia Cancer Agency (D.G.H.), Vancouver, British Columbia, Canada; Molecular Unit, Department of Pathology, Herlev Hospital, University of Copenhagen, Copenhagen, Denmark (E. Høgdall); Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, CA (M.T.G.); Genetic Pathology Evaluation Centre, Vancouver General Hospital and University of British Columbia, Vancouver, British Columbia, Canada (B. Gilks); Human Genetics Group, Spanish National Cancer Research Centre (Centro Nacional de Investigaciones Oncológicas) (M.J.G., J.B.), and Centre for Biomedical Network Research on Rare Diseases (Centro de Investigación Biomédica en Red de Enfermedades Raras), Instituto de Salud Carlos III (M.J.G., J.B.), Madrid, Spain; David Geffen School of Medicine, Department of Medicine, Division of Hematology and Oncology, University of California at Los Angeles (P.A.F.); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Erlangen, Germany (P.A.F.); Department of Histopathology, Queen’s Medical Centre, Nottingham University Hospitals NHS Trust, Nottingham, UK (S.D.); University Cancer Center Hamburg, University Medical Center Hamburg-Eppendorf, Hamburg, Germany (J.C.-C.); Cancer Genetics Laboratory, Research Division, Peter MacCallum Cancer Centre (I.G.C.), Sir Peter MacCallum Department of Oncology, University of Melbourne (I.G.C.), and Department of Pathology, University of Melbourne (I.G.C.), Melbourne, Victoria, Australia; Department of Oncology, University of Cambridge, Hutchison/MRC Research Centre (J.D.B.), Cancer Research UK Cambridge Institute, University of Cambridge, Li Ka Shing Centre (J.D.B.), and Cambridge Experimental Cancer Medicine Centre (J.D.B.), Cambridge, UK; Cancer Genomics Program, Research Department, Peter MacCallum Cancer Centre, East Melbourne, Victoria, Australia (D.D.B.); the Garvan Institute, Sydney, New South Wales, Australia (D.D.B., S.J.R.); and Department of Public Health and Primary Care, University of Cambridge, Strangeways Research Laboratory, Cambridge, UK (P.D.P.P.)
1,
Kirsten B Moysich
Kirsten B Moysich, PhD
1Department of Medical Oncology (M.S.B., K.R.K.) and Department of Health Sciences Research (R.A.V., S.J.W., B.M.M., Z.L., E.L.G.), Mayo Clinic, Rochester, MN; Department of Pathology and Laboratory Medicine, University of Calgary, Calgary, Alberta, Canada (P.F.R., M.K.); Pathology Department, Catholic University of Health and Allied Sciences-Bugando, Mwanza, Tanzania (P.F.R.); Tübingen University Hospital, Department of Women’s Health, Tübingen, Germany (P.W., F.A.T., A.D.H., S.Y.B., S.K.); Sir Peter MacCallum Department of Oncology, the University of Melbourne, Parkville, Victoria, Australia (N.T., D.D.B.); International Hereditary Cancer Center, Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (A.T., O.O., J. Lubiński, J.G.); Department of Gynecology and Obstetrics, Ribeirão Preto Medical School, University of São Paulo, Ribeirão Preto, Brazil (D.G.T., C.B.S., J.M.A., F.J.C.R.); Department of Pathology, Institute of Pathology, Heidelberg University Hospital, Heidelberg, Germany (P.S.); Department of Population Health Science and Policy, Department of Genetics and Genomic Sciences, Icahn School of Medicine at Mount Sinai, New York, NY (W.S., J.H.R.); Pathology West ICPMR Westmead, Westmead Hospital, the University of Sydney, Sydney, Australia (R.S.); University of Western Sydney at Westmead Hospital, Westmead, New South Wales, Australia (R.S.); Medical Oncology Service, Hospital Sant Pau, Barcelona, Spain (T.R.C.); H12O-CNIO Lung Cancer Clinical Research Unit (L.P.-A.), and Oncology Department, Hospital Universitario 12 de Octubre (L.P.-A.), Madrid, Spain; Women’s Cancer Program, Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, CA (S.O., J. Lester, B.Y.K.); the University of Texas School of Public Health, Houston (R.B.N.); Department of Oncology, Division of Gynecologic Oncology, Cumming School of Medicine, University of Calgary, Calgary, Alberta, Canada (G.N., P.G.); Department of Obstetrics, Gynecology and Reproductive Sciences, University of Pittsburgh School of Medicine (F.M., R.P.E.), Department of Epidemiology, University of Pittsburgh Graduate School of Public Health (F.M.), and Womens Cancer Research Program, Magee-Womens Research Institute and University of Pittsburgh Cancer Institute (F.M.), Pittsburgh, PA; Department of Gynecological Surgery and Gynecological Oncology of Adults and Adolescents, Pomeranian Medical University, Szczecin, Poland (J.M., A.C.-G.); Department of Health Research and Policy, Stanford University School of Medicine, Stanford, CA (V.M.); Department of Oncology, University of Cambridge, Strangeways Research Laboratory, Cambridge, UK (M.M., J.A., P.D.P.P.); Department of Pathology, Stanford University, Stanford, CA (T.A.L.); Department of Gynecologic Oncology, the Third Affiliated Hospital of Kunming Medical University (Yunnan Tumor Hospital), Kunming, China (Z.L.); Centre for Cancer Research, the Westmead Institute for Medical Research, the University of Sydney (C.J.K., J.H., P.R.H., B. Gao, A.deF.), and Department of Gynaecological Oncology, Westmead Hospital (C.J.K., J.H., A.deF.), Sydney, New South Wales, Australia; Division of Cancer Epidemiology, German Cancer Research Center (Deutsches Krebsforschungszentrum), Heidelberg, Germany (A.Y.J., U.E., J.C.-C.); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia (S.E.J., G.C.-T.); Department of Histopathology, Addenbrookes Hospital, Cambridge, UK (M.J.-L.); Virus, Lifestyle and Genes, Danish Cancer Society Research Center, Copenhagen, Denmark (A.J., S.K.K.); School of Women’s and Children’s Health, University of New South Wales, Sydney, Australia (M.P.I., S.J.R.); Tissue Bank of the National Center for Tumor Diseases (NCT) Heidelberg (E. Herpel), and Institute of Pathology, University of Heidelberg (E. Herpel), Heidelberg, Germany; Cancer Center, University of Hawaii, Honolulu, HI (B.Y.H.); the Crown Princess Mary Cancer Centre, Westmead Hospital, the University of Sydney, Sydney, Australia (P.R.H., B. Gao); Oncology Department, Hospital General de Albacete, Madrid, Spain (J.M.G.-B.); Department of Cancer Genomics and Genetics, Peter MacCallum Cancer Centre, East Melbourne, Victoria, Australia (S.F.); Pathology Department, Fundación Jiménez Díaz, Madrid, Spain (A.C.); Department of Health Research and Policy and Department of Biomedical Data Science, Stanford University School of Medicine, Stanford, CA (A.S.W.); Department of Obstetrics and Gynecology, Division of Gynecologic Oncology, Royal Alexandra Hospital, Edmonton, Alberta, Canada (H.S.); Tübingen University Hospital, Institute of Pathology, Tübingen, Germany (A.S.); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (K.B.M.); Gynaecological Cancer Research Centre, Department of Women’s Cancer, Institute for Women’s Health, University College London, London, UK (U.M.); Alberta Health Services-Cancer Care, Calgary, Alberta, Canada (J.M.K.); Department of Gynecology, Rigshospitalet, University of Copenhagen, Copenhagen, Denmark (S.K.K.); Department of Public Health Sciences, Medical University of South Carolina and Hollings Cancer Center, Charleston, SC (L.E.K.); Department of Pathology and Laboratory Medicine, University of British Columbia (D.G.H.), and Centre for Translational and Applied Genomics, British Columbia Cancer Agency (D.G.H.), Vancouver, British Columbia, Canada; Molecular Unit, Department of Pathology, Herlev Hospital, University of Copenhagen, Copenhagen, Denmark (E. Høgdall); Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, CA (M.T.G.); Genetic Pathology Evaluation Centre, Vancouver General Hospital and University of British Columbia, Vancouver, British Columbia, Canada (B. Gilks); Human Genetics Group, Spanish National Cancer Research Centre (Centro Nacional de Investigaciones Oncológicas) (M.J.G., J.B.), and Centre for Biomedical Network Research on Rare Diseases (Centro de Investigación Biomédica en Red de Enfermedades Raras), Instituto de Salud Carlos III (M.J.G., J.B.), Madrid, Spain; David Geffen School of Medicine, Department of Medicine, Division of Hematology and Oncology, University of California at Los Angeles (P.A.F.); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Erlangen, Germany (P.A.F.); Department of Histopathology, Queen’s Medical Centre, Nottingham University Hospitals NHS Trust, Nottingham, UK (S.D.); University Cancer Center Hamburg, University Medical Center Hamburg-Eppendorf, Hamburg, Germany (J.C.-C.); Cancer Genetics Laboratory, Research Division, Peter MacCallum Cancer Centre (I.G.C.), Sir Peter MacCallum Department of Oncology, University of Melbourne (I.G.C.), and Department of Pathology, University of Melbourne (I.G.C.), Melbourne, Victoria, Australia; Department of Oncology, University of Cambridge, Hutchison/MRC Research Centre (J.D.B.), Cancer Research UK Cambridge Institute, University of Cambridge, Li Ka Shing Centre (J.D.B.), and Cambridge Experimental Cancer Medicine Centre (J.D.B.), Cambridge, UK; Cancer Genomics Program, Research Department, Peter MacCallum Cancer Centre, East Melbourne, Victoria, Australia (D.D.B.); the Garvan Institute, Sydney, New South Wales, Australia (D.D.B., S.J.R.); and Department of Public Health and Primary Care, University of Cambridge, Strangeways Research Laboratory, Cambridge, UK (P.D.P.P.)
1,
Usha Menon
Usha Menon, MD
1Department of Medical Oncology (M.S.B., K.R.K.) and Department of Health Sciences Research (R.A.V., S.J.W., B.M.M., Z.L., E.L.G.), Mayo Clinic, Rochester, MN; Department of Pathology and Laboratory Medicine, University of Calgary, Calgary, Alberta, Canada (P.F.R., M.K.); Pathology Department, Catholic University of Health and Allied Sciences-Bugando, Mwanza, Tanzania (P.F.R.); Tübingen University Hospital, Department of Women’s Health, Tübingen, Germany (P.W., F.A.T., A.D.H., S.Y.B., S.K.); Sir Peter MacCallum Department of Oncology, the University of Melbourne, Parkville, Victoria, Australia (N.T., D.D.B.); International Hereditary Cancer Center, Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (A.T., O.O., J. Lubiński, J.G.); Department of Gynecology and Obstetrics, Ribeirão Preto Medical School, University of São Paulo, Ribeirão Preto, Brazil (D.G.T., C.B.S., J.M.A., F.J.C.R.); Department of Pathology, Institute of Pathology, Heidelberg University Hospital, Heidelberg, Germany (P.S.); Department of Population Health Science and Policy, Department of Genetics and Genomic Sciences, Icahn School of Medicine at Mount Sinai, New York, NY (W.S., J.H.R.); Pathology West ICPMR Westmead, Westmead Hospital, the University of Sydney, Sydney, Australia (R.S.); University of Western Sydney at Westmead Hospital, Westmead, New South Wales, Australia (R.S.); Medical Oncology Service, Hospital Sant Pau, Barcelona, Spain (T.R.C.); H12O-CNIO Lung Cancer Clinical Research Unit (L.P.-A.), and Oncology Department, Hospital Universitario 12 de Octubre (L.P.-A.), Madrid, Spain; Women’s Cancer Program, Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, CA (S.O., J. Lester, B.Y.K.); the University of Texas School of Public Health, Houston (R.B.N.); Department of Oncology, Division of Gynecologic Oncology, Cumming School of Medicine, University of Calgary, Calgary, Alberta, Canada (G.N., P.G.); Department of Obstetrics, Gynecology and Reproductive Sciences, University of Pittsburgh School of Medicine (F.M., R.P.E.), Department of Epidemiology, University of Pittsburgh Graduate School of Public Health (F.M.), and Womens Cancer Research Program, Magee-Womens Research Institute and University of Pittsburgh Cancer Institute (F.M.), Pittsburgh, PA; Department of Gynecological Surgery and Gynecological Oncology of Adults and Adolescents, Pomeranian Medical University, Szczecin, Poland (J.M., A.C.-G.); Department of Health Research and Policy, Stanford University School of Medicine, Stanford, CA (V.M.); Department of Oncology, University of Cambridge, Strangeways Research Laboratory, Cambridge, UK (M.M., J.A., P.D.P.P.); Department of Pathology, Stanford University, Stanford, CA (T.A.L.); Department of Gynecologic Oncology, the Third Affiliated Hospital of Kunming Medical University (Yunnan Tumor Hospital), Kunming, China (Z.L.); Centre for Cancer Research, the Westmead Institute for Medical Research, the University of Sydney (C.J.K., J.H., P.R.H., B. Gao, A.deF.), and Department of Gynaecological Oncology, Westmead Hospital (C.J.K., J.H., A.deF.), Sydney, New South Wales, Australia; Division of Cancer Epidemiology, German Cancer Research Center (Deutsches Krebsforschungszentrum), Heidelberg, Germany (A.Y.J., U.E., J.C.-C.); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia (S.E.J., G.C.-T.); Department of Histopathology, Addenbrookes Hospital, Cambridge, UK (M.J.-L.); Virus, Lifestyle and Genes, Danish Cancer Society Research Center, Copenhagen, Denmark (A.J., S.K.K.); School of Women’s and Children’s Health, University of New South Wales, Sydney, Australia (M.P.I., S.J.R.); Tissue Bank of the National Center for Tumor Diseases (NCT) Heidelberg (E. Herpel), and Institute of Pathology, University of Heidelberg (E. Herpel), Heidelberg, Germany; Cancer Center, University of Hawaii, Honolulu, HI (B.Y.H.); the Crown Princess Mary Cancer Centre, Westmead Hospital, the University of Sydney, Sydney, Australia (P.R.H., B. Gao); Oncology Department, Hospital General de Albacete, Madrid, Spain (J.M.G.-B.); Department of Cancer Genomics and Genetics, Peter MacCallum Cancer Centre, East Melbourne, Victoria, Australia (S.F.); Pathology Department, Fundación Jiménez Díaz, Madrid, Spain (A.C.); Department of Health Research and Policy and Department of Biomedical Data Science, Stanford University School of Medicine, Stanford, CA (A.S.W.); Department of Obstetrics and Gynecology, Division of Gynecologic Oncology, Royal Alexandra Hospital, Edmonton, Alberta, Canada (H.S.); Tübingen University Hospital, Institute of Pathology, Tübingen, Germany (A.S.); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (K.B.M.); Gynaecological Cancer Research Centre, Department of Women’s Cancer, Institute for Women’s Health, University College London, London, UK (U.M.); Alberta Health Services-Cancer Care, Calgary, Alberta, Canada (J.M.K.); Department of Gynecology, Rigshospitalet, University of Copenhagen, Copenhagen, Denmark (S.K.K.); Department of Public Health Sciences, Medical University of South Carolina and Hollings Cancer Center, Charleston, SC (L.E.K.); Department of Pathology and Laboratory Medicine, University of British Columbia (D.G.H.), and Centre for Translational and Applied Genomics, British Columbia Cancer Agency (D.G.H.), Vancouver, British Columbia, Canada; Molecular Unit, Department of Pathology, Herlev Hospital, University of Copenhagen, Copenhagen, Denmark (E. Høgdall); Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, CA (M.T.G.); Genetic Pathology Evaluation Centre, Vancouver General Hospital and University of British Columbia, Vancouver, British Columbia, Canada (B. Gilks); Human Genetics Group, Spanish National Cancer Research Centre (Centro Nacional de Investigaciones Oncológicas) (M.J.G., J.B.), and Centre for Biomedical Network Research on Rare Diseases (Centro de Investigación Biomédica en Red de Enfermedades Raras), Instituto de Salud Carlos III (M.J.G., J.B.), Madrid, Spain; David Geffen School of Medicine, Department of Medicine, Division of Hematology and Oncology, University of California at Los Angeles (P.A.F.); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Erlangen, Germany (P.A.F.); Department of Histopathology, Queen’s Medical Centre, Nottingham University Hospitals NHS Trust, Nottingham, UK (S.D.); University Cancer Center Hamburg, University Medical Center Hamburg-Eppendorf, Hamburg, Germany (J.C.-C.); Cancer Genetics Laboratory, Research Division, Peter MacCallum Cancer Centre (I.G.C.), Sir Peter MacCallum Department of Oncology, University of Melbourne (I.G.C.), and Department of Pathology, University of Melbourne (I.G.C.), Melbourne, Victoria, Australia; Department of Oncology, University of Cambridge, Hutchison/MRC Research Centre (J.D.B.), Cancer Research UK Cambridge Institute, University of Cambridge, Li Ka Shing Centre (J.D.B.), and Cambridge Experimental Cancer Medicine Centre (J.D.B.), Cambridge, UK; Cancer Genomics Program, Research Department, Peter MacCallum Cancer Centre, East Melbourne, Victoria, Australia (D.D.B.); the Garvan Institute, Sydney, New South Wales, Australia (D.D.B., S.J.R.); and Department of Public Health and Primary Care, University of Cambridge, Strangeways Research Laboratory, Cambridge, UK (P.D.P.P.)
1,
Jennifer M Koziak
Jennifer M Koziak, BSc
1Department of Medical Oncology (M.S.B., K.R.K.) and Department of Health Sciences Research (R.A.V., S.J.W., B.M.M., Z.L., E.L.G.), Mayo Clinic, Rochester, MN; Department of Pathology and Laboratory Medicine, University of Calgary, Calgary, Alberta, Canada (P.F.R., M.K.); Pathology Department, Catholic University of Health and Allied Sciences-Bugando, Mwanza, Tanzania (P.F.R.); Tübingen University Hospital, Department of Women’s Health, Tübingen, Germany (P.W., F.A.T., A.D.H., S.Y.B., S.K.); Sir Peter MacCallum Department of Oncology, the University of Melbourne, Parkville, Victoria, Australia (N.T., D.D.B.); International Hereditary Cancer Center, Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (A.T., O.O., J. Lubiński, J.G.); Department of Gynecology and Obstetrics, Ribeirão Preto Medical School, University of São Paulo, Ribeirão Preto, Brazil (D.G.T., C.B.S., J.M.A., F.J.C.R.); Department of Pathology, Institute of Pathology, Heidelberg University Hospital, Heidelberg, Germany (P.S.); Department of Population Health Science and Policy, Department of Genetics and Genomic Sciences, Icahn School of Medicine at Mount Sinai, New York, NY (W.S., J.H.R.); Pathology West ICPMR Westmead, Westmead Hospital, the University of Sydney, Sydney, Australia (R.S.); University of Western Sydney at Westmead Hospital, Westmead, New South Wales, Australia (R.S.); Medical Oncology Service, Hospital Sant Pau, Barcelona, Spain (T.R.C.); H12O-CNIO Lung Cancer Clinical Research Unit (L.P.-A.), and Oncology Department, Hospital Universitario 12 de Octubre (L.P.-A.), Madrid, Spain; Women’s Cancer Program, Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, CA (S.O., J. Lester, B.Y.K.); the University of Texas School of Public Health, Houston (R.B.N.); Department of Oncology, Division of Gynecologic Oncology, Cumming School of Medicine, University of Calgary, Calgary, Alberta, Canada (G.N., P.G.); Department of Obstetrics, Gynecology and Reproductive Sciences, University of Pittsburgh School of Medicine (F.M., R.P.E.), Department of Epidemiology, University of Pittsburgh Graduate School of Public Health (F.M.), and Womens Cancer Research Program, Magee-Womens Research Institute and University of Pittsburgh Cancer Institute (F.M.), Pittsburgh, PA; Department of Gynecological Surgery and Gynecological Oncology of Adults and Adolescents, Pomeranian Medical University, Szczecin, Poland (J.M., A.C.-G.); Department of Health Research and Policy, Stanford University School of Medicine, Stanford, CA (V.M.); Department of Oncology, University of Cambridge, Strangeways Research Laboratory, Cambridge, UK (M.M., J.A., P.D.P.P.); Department of Pathology, Stanford University, Stanford, CA (T.A.L.); Department of Gynecologic Oncology, the Third Affiliated Hospital of Kunming Medical University (Yunnan Tumor Hospital), Kunming, China (Z.L.); Centre for Cancer Research, the Westmead Institute for Medical Research, the University of Sydney (C.J.K., J.H., P.R.H., B. Gao, A.deF.), and Department of Gynaecological Oncology, Westmead Hospital (C.J.K., J.H., A.deF.), Sydney, New South Wales, Australia; Division of Cancer Epidemiology, German Cancer Research Center (Deutsches Krebsforschungszentrum), Heidelberg, Germany (A.Y.J., U.E., J.C.-C.); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia (S.E.J., G.C.-T.); Department of Histopathology, Addenbrookes Hospital, Cambridge, UK (M.J.-L.); Virus, Lifestyle and Genes, Danish Cancer Society Research Center, Copenhagen, Denmark (A.J., S.K.K.); School of Women’s and Children’s Health, University of New South Wales, Sydney, Australia (M.P.I., S.J.R.); Tissue Bank of the National Center for Tumor Diseases (NCT) Heidelberg (E. Herpel), and Institute of Pathology, University of Heidelberg (E. Herpel), Heidelberg, Germany; Cancer Center, University of Hawaii, Honolulu, HI (B.Y.H.); the Crown Princess Mary Cancer Centre, Westmead Hospital, the University of Sydney, Sydney, Australia (P.R.H., B. Gao); Oncology Department, Hospital General de Albacete, Madrid, Spain (J.M.G.-B.); Department of Cancer Genomics and Genetics, Peter MacCallum Cancer Centre, East Melbourne, Victoria, Australia (S.F.); Pathology Department, Fundación Jiménez Díaz, Madrid, Spain (A.C.); Department of Health Research and Policy and Department of Biomedical Data Science, Stanford University School of Medicine, Stanford, CA (A.S.W.); Department of Obstetrics and Gynecology, Division of Gynecologic Oncology, Royal Alexandra Hospital, Edmonton, Alberta, Canada (H.S.); Tübingen University Hospital, Institute of Pathology, Tübingen, Germany (A.S.); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (K.B.M.); Gynaecological Cancer Research Centre, Department of Women’s Cancer, Institute for Women’s Health, University College London, London, UK (U.M.); Alberta Health Services-Cancer Care, Calgary, Alberta, Canada (J.M.K.); Department of Gynecology, Rigshospitalet, University of Copenhagen, Copenhagen, Denmark (S.K.K.); Department of Public Health Sciences, Medical University of South Carolina and Hollings Cancer Center, Charleston, SC (L.E.K.); Department of Pathology and Laboratory Medicine, University of British Columbia (D.G.H.), and Centre for Translational and Applied Genomics, British Columbia Cancer Agency (D.G.H.), Vancouver, British Columbia, Canada; Molecular Unit, Department of Pathology, Herlev Hospital, University of Copenhagen, Copenhagen, Denmark (E. Høgdall); Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, CA (M.T.G.); Genetic Pathology Evaluation Centre, Vancouver General Hospital and University of British Columbia, Vancouver, British Columbia, Canada (B. Gilks); Human Genetics Group, Spanish National Cancer Research Centre (Centro Nacional de Investigaciones Oncológicas) (M.J.G., J.B.), and Centre for Biomedical Network Research on Rare Diseases (Centro de Investigación Biomédica en Red de Enfermedades Raras), Instituto de Salud Carlos III (M.J.G., J.B.), Madrid, Spain; David Geffen School of Medicine, Department of Medicine, Division of Hematology and Oncology, University of California at Los Angeles (P.A.F.); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Erlangen, Germany (P.A.F.); Department of Histopathology, Queen’s Medical Centre, Nottingham University Hospitals NHS Trust, Nottingham, UK (S.D.); University Cancer Center Hamburg, University Medical Center Hamburg-Eppendorf, Hamburg, Germany (J.C.-C.); Cancer Genetics Laboratory, Research Division, Peter MacCallum Cancer Centre (I.G.C.), Sir Peter MacCallum Department of Oncology, University of Melbourne (I.G.C.), and Department of Pathology, University of Melbourne (I.G.C.), Melbourne, Victoria, Australia; Department of Oncology, University of Cambridge, Hutchison/MRC Research Centre (J.D.B.), Cancer Research UK Cambridge Institute, University of Cambridge, Li Ka Shing Centre (J.D.B.), and Cambridge Experimental Cancer Medicine Centre (J.D.B.), Cambridge, UK; Cancer Genomics Program, Research Department, Peter MacCallum Cancer Centre, East Melbourne, Victoria, Australia (D.D.B.); the Garvan Institute, Sydney, New South Wales, Australia (D.D.B., S.J.R.); and Department of Public Health and Primary Care, University of Cambridge, Strangeways Research Laboratory, Cambridge, UK (P.D.P.P.)
1,
Stefan Kommoss
Stefan Kommoss, MD
1Department of Medical Oncology (M.S.B., K.R.K.) and Department of Health Sciences Research (R.A.V., S.J.W., B.M.M., Z.L., E.L.G.), Mayo Clinic, Rochester, MN; Department of Pathology and Laboratory Medicine, University of Calgary, Calgary, Alberta, Canada (P.F.R., M.K.); Pathology Department, Catholic University of Health and Allied Sciences-Bugando, Mwanza, Tanzania (P.F.R.); Tübingen University Hospital, Department of Women’s Health, Tübingen, Germany (P.W., F.A.T., A.D.H., S.Y.B., S.K.); Sir Peter MacCallum Department of Oncology, the University of Melbourne, Parkville, Victoria, Australia (N.T., D.D.B.); International Hereditary Cancer Center, Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (A.T., O.O., J. Lubiński, J.G.); Department of Gynecology and Obstetrics, Ribeirão Preto Medical School, University of São Paulo, Ribeirão Preto, Brazil (D.G.T., C.B.S., J.M.A., F.J.C.R.); Department of Pathology, Institute of Pathology, Heidelberg University Hospital, Heidelberg, Germany (P.S.); Department of Population Health Science and Policy, Department of Genetics and Genomic Sciences, Icahn School of Medicine at Mount Sinai, New York, NY (W.S., J.H.R.); Pathology West ICPMR Westmead, Westmead Hospital, the University of Sydney, Sydney, Australia (R.S.); University of Western Sydney at Westmead Hospital, Westmead, New South Wales, Australia (R.S.); Medical Oncology Service, Hospital Sant Pau, Barcelona, Spain (T.R.C.); H12O-CNIO Lung Cancer Clinical Research Unit (L.P.-A.), and Oncology Department, Hospital Universitario 12 de Octubre (L.P.-A.), Madrid, Spain; Women’s Cancer Program, Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, CA (S.O., J. Lester, B.Y.K.); the University of Texas School of Public Health, Houston (R.B.N.); Department of Oncology, Division of Gynecologic Oncology, Cumming School of Medicine, University of Calgary, Calgary, Alberta, Canada (G.N., P.G.); Department of Obstetrics, Gynecology and Reproductive Sciences, University of Pittsburgh School of Medicine (F.M., R.P.E.), Department of Epidemiology, University of Pittsburgh Graduate School of Public Health (F.M.), and Womens Cancer Research Program, Magee-Womens Research Institute and University of Pittsburgh Cancer Institute (F.M.), Pittsburgh, PA; Department of Gynecological Surgery and Gynecological Oncology of Adults and Adolescents, Pomeranian Medical University, Szczecin, Poland (J.M., A.C.-G.); Department of Health Research and Policy, Stanford University School of Medicine, Stanford, CA (V.M.); Department of Oncology, University of Cambridge, Strangeways Research Laboratory, Cambridge, UK (M.M., J.A., P.D.P.P.); Department of Pathology, Stanford University, Stanford, CA (T.A.L.); Department of Gynecologic Oncology, the Third Affiliated Hospital of Kunming Medical University (Yunnan Tumor Hospital), Kunming, China (Z.L.); Centre for Cancer Research, the Westmead Institute for Medical Research, the University of Sydney (C.J.K., J.H., P.R.H., B. Gao, A.deF.), and Department of Gynaecological Oncology, Westmead Hospital (C.J.K., J.H., A.deF.), Sydney, New South Wales, Australia; Division of Cancer Epidemiology, German Cancer Research Center (Deutsches Krebsforschungszentrum), Heidelberg, Germany (A.Y.J., U.E., J.C.-C.); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia (S.E.J., G.C.-T.); Department of Histopathology, Addenbrookes Hospital, Cambridge, UK (M.J.-L.); Virus, Lifestyle and Genes, Danish Cancer Society Research Center, Copenhagen, Denmark (A.J., S.K.K.); School of Women’s and Children’s Health, University of New South Wales, Sydney, Australia (M.P.I., S.J.R.); Tissue Bank of the National Center for Tumor Diseases (NCT) Heidelberg (E. Herpel), and Institute of Pathology, University of Heidelberg (E. Herpel), Heidelberg, Germany; Cancer Center, University of Hawaii, Honolulu, HI (B.Y.H.); the Crown Princess Mary Cancer Centre, Westmead Hospital, the University of Sydney, Sydney, Australia (P.R.H., B. Gao); Oncology Department, Hospital General de Albacete, Madrid, Spain (J.M.G.-B.); Department of Cancer Genomics and Genetics, Peter MacCallum Cancer Centre, East Melbourne, Victoria, Australia (S.F.); Pathology Department, Fundación Jiménez Díaz, Madrid, Spain (A.C.); Department of Health Research and Policy and Department of Biomedical Data Science, Stanford University School of Medicine, Stanford, CA (A.S.W.); Department of Obstetrics and Gynecology, Division of Gynecologic Oncology, Royal Alexandra Hospital, Edmonton, Alberta, Canada (H.S.); Tübingen University Hospital, Institute of Pathology, Tübingen, Germany (A.S.); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (K.B.M.); Gynaecological Cancer Research Centre, Department of Women’s Cancer, Institute for Women’s Health, University College London, London, UK (U.M.); Alberta Health Services-Cancer Care, Calgary, Alberta, Canada (J.M.K.); Department of Gynecology, Rigshospitalet, University of Copenhagen, Copenhagen, Denmark (S.K.K.); Department of Public Health Sciences, Medical University of South Carolina and Hollings Cancer Center, Charleston, SC (L.E.K.); Department of Pathology and Laboratory Medicine, University of British Columbia (D.G.H.), and Centre for Translational and Applied Genomics, British Columbia Cancer Agency (D.G.H.), Vancouver, British Columbia, Canada; Molecular Unit, Department of Pathology, Herlev Hospital, University of Copenhagen, Copenhagen, Denmark (E. Høgdall); Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, CA (M.T.G.); Genetic Pathology Evaluation Centre, Vancouver General Hospital and University of British Columbia, Vancouver, British Columbia, Canada (B. Gilks); Human Genetics Group, Spanish National Cancer Research Centre (Centro Nacional de Investigaciones Oncológicas) (M.J.G., J.B.), and Centre for Biomedical Network Research on Rare Diseases (Centro de Investigación Biomédica en Red de Enfermedades Raras), Instituto de Salud Carlos III (M.J.G., J.B.), Madrid, Spain; David Geffen School of Medicine, Department of Medicine, Division of Hematology and Oncology, University of California at Los Angeles (P.A.F.); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Erlangen, Germany (P.A.F.); Department of Histopathology, Queen’s Medical Centre, Nottingham University Hospitals NHS Trust, Nottingham, UK (S.D.); University Cancer Center Hamburg, University Medical Center Hamburg-Eppendorf, Hamburg, Germany (J.C.-C.); Cancer Genetics Laboratory, Research Division, Peter MacCallum Cancer Centre (I.G.C.), Sir Peter MacCallum Department of Oncology, University of Melbourne (I.G.C.), and Department of Pathology, University of Melbourne (I.G.C.), Melbourne, Victoria, Australia; Department of Oncology, University of Cambridge, Hutchison/MRC Research Centre (J.D.B.), Cancer Research UK Cambridge Institute, University of Cambridge, Li Ka Shing Centre (J.D.B.), and Cambridge Experimental Cancer Medicine Centre (J.D.B.), Cambridge, UK; Cancer Genomics Program, Research Department, Peter MacCallum Cancer Centre, East Melbourne, Victoria, Australia (D.D.B.); the Garvan Institute, Sydney, New South Wales, Australia (D.D.B., S.J.R.); and Department of Public Health and Primary Care, University of Cambridge, Strangeways Research Laboratory, Cambridge, UK (P.D.P.P.)
1,
Susanne K Kjaer
Susanne K Kjaer, MD
1Department of Medical Oncology (M.S.B., K.R.K.) and Department of Health Sciences Research (R.A.V., S.J.W., B.M.M., Z.L., E.L.G.), Mayo Clinic, Rochester, MN; Department of Pathology and Laboratory Medicine, University of Calgary, Calgary, Alberta, Canada (P.F.R., M.K.); Pathology Department, Catholic University of Health and Allied Sciences-Bugando, Mwanza, Tanzania (P.F.R.); Tübingen University Hospital, Department of Women’s Health, Tübingen, Germany (P.W., F.A.T., A.D.H., S.Y.B., S.K.); Sir Peter MacCallum Department of Oncology, the University of Melbourne, Parkville, Victoria, Australia (N.T., D.D.B.); International Hereditary Cancer Center, Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (A.T., O.O., J. Lubiński, J.G.); Department of Gynecology and Obstetrics, Ribeirão Preto Medical School, University of São Paulo, Ribeirão Preto, Brazil (D.G.T., C.B.S., J.M.A., F.J.C.R.); Department of Pathology, Institute of Pathology, Heidelberg University Hospital, Heidelberg, Germany (P.S.); Department of Population Health Science and Policy, Department of Genetics and Genomic Sciences, Icahn School of Medicine at Mount Sinai, New York, NY (W.S., J.H.R.); Pathology West ICPMR Westmead, Westmead Hospital, the University of Sydney, Sydney, Australia (R.S.); University of Western Sydney at Westmead Hospital, Westmead, New South Wales, Australia (R.S.); Medical Oncology Service, Hospital Sant Pau, Barcelona, Spain (T.R.C.); H12O-CNIO Lung Cancer Clinical Research Unit (L.P.-A.), and Oncology Department, Hospital Universitario 12 de Octubre (L.P.-A.), Madrid, Spain; Women’s Cancer Program, Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, CA (S.O., J. Lester, B.Y.K.); the University of Texas School of Public Health, Houston (R.B.N.); Department of Oncology, Division of Gynecologic Oncology, Cumming School of Medicine, University of Calgary, Calgary, Alberta, Canada (G.N., P.G.); Department of Obstetrics, Gynecology and Reproductive Sciences, University of Pittsburgh School of Medicine (F.M., R.P.E.), Department of Epidemiology, University of Pittsburgh Graduate School of Public Health (F.M.), and Womens Cancer Research Program, Magee-Womens Research Institute and University of Pittsburgh Cancer Institute (F.M.), Pittsburgh, PA; Department of Gynecological Surgery and Gynecological Oncology of Adults and Adolescents, Pomeranian Medical University, Szczecin, Poland (J.M., A.C.-G.); Department of Health Research and Policy, Stanford University School of Medicine, Stanford, CA (V.M.); Department of Oncology, University of Cambridge, Strangeways Research Laboratory, Cambridge, UK (M.M., J.A., P.D.P.P.); Department of Pathology, Stanford University, Stanford, CA (T.A.L.); Department of Gynecologic Oncology, the Third Affiliated Hospital of Kunming Medical University (Yunnan Tumor Hospital), Kunming, China (Z.L.); Centre for Cancer Research, the Westmead Institute for Medical Research, the University of Sydney (C.J.K., J.H., P.R.H., B. Gao, A.deF.), and Department of Gynaecological Oncology, Westmead Hospital (C.J.K., J.H., A.deF.), Sydney, New South Wales, Australia; Division of Cancer Epidemiology, German Cancer Research Center (Deutsches Krebsforschungszentrum), Heidelberg, Germany (A.Y.J., U.E., J.C.-C.); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia (S.E.J., G.C.-T.); Department of Histopathology, Addenbrookes Hospital, Cambridge, UK (M.J.-L.); Virus, Lifestyle and Genes, Danish Cancer Society Research Center, Copenhagen, Denmark (A.J., S.K.K.); School of Women’s and Children’s Health, University of New South Wales, Sydney, Australia (M.P.I., S.J.R.); Tissue Bank of the National Center for Tumor Diseases (NCT) Heidelberg (E. Herpel), and Institute of Pathology, University of Heidelberg (E. Herpel), Heidelberg, Germany; Cancer Center, University of Hawaii, Honolulu, HI (B.Y.H.); the Crown Princess Mary Cancer Centre, Westmead Hospital, the University of Sydney, Sydney, Australia (P.R.H., B. Gao); Oncology Department, Hospital General de Albacete, Madrid, Spain (J.M.G.-B.); Department of Cancer Genomics and Genetics, Peter MacCallum Cancer Centre, East Melbourne, Victoria, Australia (S.F.); Pathology Department, Fundación Jiménez Díaz, Madrid, Spain (A.C.); Department of Health Research and Policy and Department of Biomedical Data Science, Stanford University School of Medicine, Stanford, CA (A.S.W.); Department of Obstetrics and Gynecology, Division of Gynecologic Oncology, Royal Alexandra Hospital, Edmonton, Alberta, Canada (H.S.); Tübingen University Hospital, Institute of Pathology, Tübingen, Germany (A.S.); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (K.B.M.); Gynaecological Cancer Research Centre, Department of Women’s Cancer, Institute for Women’s Health, University College London, London, UK (U.M.); Alberta Health Services-Cancer Care, Calgary, Alberta, Canada (J.M.K.); Department of Gynecology, Rigshospitalet, University of Copenhagen, Copenhagen, Denmark (S.K.K.); Department of Public Health Sciences, Medical University of South Carolina and Hollings Cancer Center, Charleston, SC (L.E.K.); Department of Pathology and Laboratory Medicine, University of British Columbia (D.G.H.), and Centre for Translational and Applied Genomics, British Columbia Cancer Agency (D.G.H.), Vancouver, British Columbia, Canada; Molecular Unit, Department of Pathology, Herlev Hospital, University of Copenhagen, Copenhagen, Denmark (E. Høgdall); Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, CA (M.T.G.); Genetic Pathology Evaluation Centre, Vancouver General Hospital and University of British Columbia, Vancouver, British Columbia, Canada (B. Gilks); Human Genetics Group, Spanish National Cancer Research Centre (Centro Nacional de Investigaciones Oncológicas) (M.J.G., J.B.), and Centre for Biomedical Network Research on Rare Diseases (Centro de Investigación Biomédica en Red de Enfermedades Raras), Instituto de Salud Carlos III (M.J.G., J.B.), Madrid, Spain; David Geffen School of Medicine, Department of Medicine, Division of Hematology and Oncology, University of California at Los Angeles (P.A.F.); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Erlangen, Germany (P.A.F.); Department of Histopathology, Queen’s Medical Centre, Nottingham University Hospitals NHS Trust, Nottingham, UK (S.D.); University Cancer Center Hamburg, University Medical Center Hamburg-Eppendorf, Hamburg, Germany (J.C.-C.); Cancer Genetics Laboratory, Research Division, Peter MacCallum Cancer Centre (I.G.C.), Sir Peter MacCallum Department of Oncology, University of Melbourne (I.G.C.), and Department of Pathology, University of Melbourne (I.G.C.), Melbourne, Victoria, Australia; Department of Oncology, University of Cambridge, Hutchison/MRC Research Centre (J.D.B.), Cancer Research UK Cambridge Institute, University of Cambridge, Li Ka Shing Centre (J.D.B.), and Cambridge Experimental Cancer Medicine Centre (J.D.B.), Cambridge, UK; Cancer Genomics Program, Research Department, Peter MacCallum Cancer Centre, East Melbourne, Victoria, Australia (D.D.B.); the Garvan Institute, Sydney, New South Wales, Australia (D.D.B., S.J.R.); and Department of Public Health and Primary Care, University of Cambridge, Strangeways Research Laboratory, Cambridge, UK (P.D.P.P.)
1,
Linda E Kelemen
Linda E Kelemen, ScD
1Department of Medical Oncology (M.S.B., K.R.K.) and Department of Health Sciences Research (R.A.V., S.J.W., B.M.M., Z.L., E.L.G.), Mayo Clinic, Rochester, MN; Department of Pathology and Laboratory Medicine, University of Calgary, Calgary, Alberta, Canada (P.F.R., M.K.); Pathology Department, Catholic University of Health and Allied Sciences-Bugando, Mwanza, Tanzania (P.F.R.); Tübingen University Hospital, Department of Women’s Health, Tübingen, Germany (P.W., F.A.T., A.D.H., S.Y.B., S.K.); Sir Peter MacCallum Department of Oncology, the University of Melbourne, Parkville, Victoria, Australia (N.T., D.D.B.); International Hereditary Cancer Center, Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (A.T., O.O., J. Lubiński, J.G.); Department of Gynecology and Obstetrics, Ribeirão Preto Medical School, University of São Paulo, Ribeirão Preto, Brazil (D.G.T., C.B.S., J.M.A., F.J.C.R.); Department of Pathology, Institute of Pathology, Heidelberg University Hospital, Heidelberg, Germany (P.S.); Department of Population Health Science and Policy, Department of Genetics and Genomic Sciences, Icahn School of Medicine at Mount Sinai, New York, NY (W.S., J.H.R.); Pathology West ICPMR Westmead, Westmead Hospital, the University of Sydney, Sydney, Australia (R.S.); University of Western Sydney at Westmead Hospital, Westmead, New South Wales, Australia (R.S.); Medical Oncology Service, Hospital Sant Pau, Barcelona, Spain (T.R.C.); H12O-CNIO Lung Cancer Clinical Research Unit (L.P.-A.), and Oncology Department, Hospital Universitario 12 de Octubre (L.P.-A.), Madrid, Spain; Women’s Cancer Program, Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, CA (S.O., J. Lester, B.Y.K.); the University of Texas School of Public Health, Houston (R.B.N.); Department of Oncology, Division of Gynecologic Oncology, Cumming School of Medicine, University of Calgary, Calgary, Alberta, Canada (G.N., P.G.); Department of Obstetrics, Gynecology and Reproductive Sciences, University of Pittsburgh School of Medicine (F.M., R.P.E.), Department of Epidemiology, University of Pittsburgh Graduate School of Public Health (F.M.), and Womens Cancer Research Program, Magee-Womens Research Institute and University of Pittsburgh Cancer Institute (F.M.), Pittsburgh, PA; Department of Gynecological Surgery and Gynecological Oncology of Adults and Adolescents, Pomeranian Medical University, Szczecin, Poland (J.M., A.C.-G.); Department of Health Research and Policy, Stanford University School of Medicine, Stanford, CA (V.M.); Department of Oncology, University of Cambridge, Strangeways Research Laboratory, Cambridge, UK (M.M., J.A., P.D.P.P.); Department of Pathology, Stanford University, Stanford, CA (T.A.L.); Department of Gynecologic Oncology, the Third Affiliated Hospital of Kunming Medical University (Yunnan Tumor Hospital), Kunming, China (Z.L.); Centre for Cancer Research, the Westmead Institute for Medical Research, the University of Sydney (C.J.K., J.H., P.R.H., B. Gao, A.deF.), and Department of Gynaecological Oncology, Westmead Hospital (C.J.K., J.H., A.deF.), Sydney, New South Wales, Australia; Division of Cancer Epidemiology, German Cancer Research Center (Deutsches Krebsforschungszentrum), Heidelberg, Germany (A.Y.J., U.E., J.C.-C.); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia (S.E.J., G.C.-T.); Department of Histopathology, Addenbrookes Hospital, Cambridge, UK (M.J.-L.); Virus, Lifestyle and Genes, Danish Cancer Society Research Center, Copenhagen, Denmark (A.J., S.K.K.); School of Women’s and Children’s Health, University of New South Wales, Sydney, Australia (M.P.I., S.J.R.); Tissue Bank of the National Center for Tumor Diseases (NCT) Heidelberg (E. Herpel), and Institute of Pathology, University of Heidelberg (E. Herpel), Heidelberg, Germany; Cancer Center, University of Hawaii, Honolulu, HI (B.Y.H.); the Crown Princess Mary Cancer Centre, Westmead Hospital, the University of Sydney, Sydney, Australia (P.R.H., B. Gao); Oncology Department, Hospital General de Albacete, Madrid, Spain (J.M.G.-B.); Department of Cancer Genomics and Genetics, Peter MacCallum Cancer Centre, East Melbourne, Victoria, Australia (S.F.); Pathology Department, Fundación Jiménez Díaz, Madrid, Spain (A.C.); Department of Health Research and Policy and Department of Biomedical Data Science, Stanford University School of Medicine, Stanford, CA (A.S.W.); Department of Obstetrics and Gynecology, Division of Gynecologic Oncology, Royal Alexandra Hospital, Edmonton, Alberta, Canada (H.S.); Tübingen University Hospital, Institute of Pathology, Tübingen, Germany (A.S.); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (K.B.M.); Gynaecological Cancer Research Centre, Department of Women’s Cancer, Institute for Women’s Health, University College London, London, UK (U.M.); Alberta Health Services-Cancer Care, Calgary, Alberta, Canada (J.M.K.); Department of Gynecology, Rigshospitalet, University of Copenhagen, Copenhagen, Denmark (S.K.K.); Department of Public Health Sciences, Medical University of South Carolina and Hollings Cancer Center, Charleston, SC (L.E.K.); Department of Pathology and Laboratory Medicine, University of British Columbia (D.G.H.), and Centre for Translational and Applied Genomics, British Columbia Cancer Agency (D.G.H.), Vancouver, British Columbia, Canada; Molecular Unit, Department of Pathology, Herlev Hospital, University of Copenhagen, Copenhagen, Denmark (E. Høgdall); Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, CA (M.T.G.); Genetic Pathology Evaluation Centre, Vancouver General Hospital and University of British Columbia, Vancouver, British Columbia, Canada (B. Gilks); Human Genetics Group, Spanish National Cancer Research Centre (Centro Nacional de Investigaciones Oncológicas) (M.J.G., J.B.), and Centre for Biomedical Network Research on Rare Diseases (Centro de Investigación Biomédica en Red de Enfermedades Raras), Instituto de Salud Carlos III (M.J.G., J.B.), Madrid, Spain; David Geffen School of Medicine, Department of Medicine, Division of Hematology and Oncology, University of California at Los Angeles (P.A.F.); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Erlangen, Germany (P.A.F.); Department of Histopathology, Queen’s Medical Centre, Nottingham University Hospitals NHS Trust, Nottingham, UK (S.D.); University Cancer Center Hamburg, University Medical Center Hamburg-Eppendorf, Hamburg, Germany (J.C.-C.); Cancer Genetics Laboratory, Research Division, Peter MacCallum Cancer Centre (I.G.C.), Sir Peter MacCallum Department of Oncology, University of Melbourne (I.G.C.), and Department of Pathology, University of Melbourne (I.G.C.), Melbourne, Victoria, Australia; Department of Oncology, University of Cambridge, Hutchison/MRC Research Centre (J.D.B.), Cancer Research UK Cambridge Institute, University of Cambridge, Li Ka Shing Centre (J.D.B.), and Cambridge Experimental Cancer Medicine Centre (J.D.B.), Cambridge, UK; Cancer Genomics Program, Research Department, Peter MacCallum Cancer Centre, East Melbourne, Victoria, Australia (D.D.B.); the Garvan Institute, Sydney, New South Wales, Australia (D.D.B., S.J.R.); and Department of Public Health and Primary Care, University of Cambridge, Strangeways Research Laboratory, Cambridge, UK (P.D.P.P.)
1,
Beth Y Karlan
Beth Y Karlan, MD
1Department of Medical Oncology (M.S.B., K.R.K.) and Department of Health Sciences Research (R.A.V., S.J.W., B.M.M., Z.L., E.L.G.), Mayo Clinic, Rochester, MN; Department of Pathology and Laboratory Medicine, University of Calgary, Calgary, Alberta, Canada (P.F.R., M.K.); Pathology Department, Catholic University of Health and Allied Sciences-Bugando, Mwanza, Tanzania (P.F.R.); Tübingen University Hospital, Department of Women’s Health, Tübingen, Germany (P.W., F.A.T., A.D.H., S.Y.B., S.K.); Sir Peter MacCallum Department of Oncology, the University of Melbourne, Parkville, Victoria, Australia (N.T., D.D.B.); International Hereditary Cancer Center, Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (A.T., O.O., J. Lubiński, J.G.); Department of Gynecology and Obstetrics, Ribeirão Preto Medical School, University of São Paulo, Ribeirão Preto, Brazil (D.G.T., C.B.S., J.M.A., F.J.C.R.); Department of Pathology, Institute of Pathology, Heidelberg University Hospital, Heidelberg, Germany (P.S.); Department of Population Health Science and Policy, Department of Genetics and Genomic Sciences, Icahn School of Medicine at Mount Sinai, New York, NY (W.S., J.H.R.); Pathology West ICPMR Westmead, Westmead Hospital, the University of Sydney, Sydney, Australia (R.S.); University of Western Sydney at Westmead Hospital, Westmead, New South Wales, Australia (R.S.); Medical Oncology Service, Hospital Sant Pau, Barcelona, Spain (T.R.C.); H12O-CNIO Lung Cancer Clinical Research Unit (L.P.-A.), and Oncology Department, Hospital Universitario 12 de Octubre (L.P.-A.), Madrid, Spain; Women’s Cancer Program, Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, CA (S.O., J. Lester, B.Y.K.); the University of Texas School of Public Health, Houston (R.B.N.); Department of Oncology, Division of Gynecologic Oncology, Cumming School of Medicine, University of Calgary, Calgary, Alberta, Canada (G.N., P.G.); Department of Obstetrics, Gynecology and Reproductive Sciences, University of Pittsburgh School of Medicine (F.M., R.P.E.), Department of Epidemiology, University of Pittsburgh Graduate School of Public Health (F.M.), and Womens Cancer Research Program, Magee-Womens Research Institute and University of Pittsburgh Cancer Institute (F.M.), Pittsburgh, PA; Department of Gynecological Surgery and Gynecological Oncology of Adults and Adolescents, Pomeranian Medical University, Szczecin, Poland (J.M., A.C.-G.); Department of Health Research and Policy, Stanford University School of Medicine, Stanford, CA (V.M.); Department of Oncology, University of Cambridge, Strangeways Research Laboratory, Cambridge, UK (M.M., J.A., P.D.P.P.); Department of Pathology, Stanford University, Stanford, CA (T.A.L.); Department of Gynecologic Oncology, the Third Affiliated Hospital of Kunming Medical University (Yunnan Tumor Hospital), Kunming, China (Z.L.); Centre for Cancer Research, the Westmead Institute for Medical Research, the University of Sydney (C.J.K., J.H., P.R.H., B. Gao, A.deF.), and Department of Gynaecological Oncology, Westmead Hospital (C.J.K., J.H., A.deF.), Sydney, New South Wales, Australia; Division of Cancer Epidemiology, German Cancer Research Center (Deutsches Krebsforschungszentrum), Heidelberg, Germany (A.Y.J., U.E., J.C.-C.); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia (S.E.J., G.C.-T.); Department of Histopathology, Addenbrookes Hospital, Cambridge, UK (M.J.-L.); Virus, Lifestyle and Genes, Danish Cancer Society Research Center, Copenhagen, Denmark (A.J., S.K.K.); School of Women’s and Children’s Health, University of New South Wales, Sydney, Australia (M.P.I., S.J.R.); Tissue Bank of the National Center for Tumor Diseases (NCT) Heidelberg (E. Herpel), and Institute of Pathology, University of Heidelberg (E. Herpel), Heidelberg, Germany; Cancer Center, University of Hawaii, Honolulu, HI (B.Y.H.); the Crown Princess Mary Cancer Centre, Westmead Hospital, the University of Sydney, Sydney, Australia (P.R.H., B. Gao); Oncology Department, Hospital General de Albacete, Madrid, Spain (J.M.G.-B.); Department of Cancer Genomics and Genetics, Peter MacCallum Cancer Centre, East Melbourne, Victoria, Australia (S.F.); Pathology Department, Fundación Jiménez Díaz, Madrid, Spain (A.C.); Department of Health Research and Policy and Department of Biomedical Data Science, Stanford University School of Medicine, Stanford, CA (A.S.W.); Department of Obstetrics and Gynecology, Division of Gynecologic Oncology, Royal Alexandra Hospital, Edmonton, Alberta, Canada (H.S.); Tübingen University Hospital, Institute of Pathology, Tübingen, Germany (A.S.); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (K.B.M.); Gynaecological Cancer Research Centre, Department of Women’s Cancer, Institute for Women’s Health, University College London, London, UK (U.M.); Alberta Health Services-Cancer Care, Calgary, Alberta, Canada (J.M.K.); Department of Gynecology, Rigshospitalet, University of Copenhagen, Copenhagen, Denmark (S.K.K.); Department of Public Health Sciences, Medical University of South Carolina and Hollings Cancer Center, Charleston, SC (L.E.K.); Department of Pathology and Laboratory Medicine, University of British Columbia (D.G.H.), and Centre for Translational and Applied Genomics, British Columbia Cancer Agency (D.G.H.), Vancouver, British Columbia, Canada; Molecular Unit, Department of Pathology, Herlev Hospital, University of Copenhagen, Copenhagen, Denmark (E. Høgdall); Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, CA (M.T.G.); Genetic Pathology Evaluation Centre, Vancouver General Hospital and University of British Columbia, Vancouver, British Columbia, Canada (B. Gilks); Human Genetics Group, Spanish National Cancer Research Centre (Centro Nacional de Investigaciones Oncológicas) (M.J.G., J.B.), and Centre for Biomedical Network Research on Rare Diseases (Centro de Investigación Biomédica en Red de Enfermedades Raras), Instituto de Salud Carlos III (M.J.G., J.B.), Madrid, Spain; David Geffen School of Medicine, Department of Medicine, Division of Hematology and Oncology, University of California at Los Angeles (P.A.F.); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Erlangen, Germany (P.A.F.); Department of Histopathology, Queen’s Medical Centre, Nottingham University Hospitals NHS Trust, Nottingham, UK (S.D.); University Cancer Center Hamburg, University Medical Center Hamburg-Eppendorf, Hamburg, Germany (J.C.-C.); Cancer Genetics Laboratory, Research Division, Peter MacCallum Cancer Centre (I.G.C.), Sir Peter MacCallum Department of Oncology, University of Melbourne (I.G.C.), and Department of Pathology, University of Melbourne (I.G.C.), Melbourne, Victoria, Australia; Department of Oncology, University of Cambridge, Hutchison/MRC Research Centre (J.D.B.), Cancer Research UK Cambridge Institute, University of Cambridge, Li Ka Shing Centre (J.D.B.), and Cambridge Experimental Cancer Medicine Centre (J.D.B.), Cambridge, UK; Cancer Genomics Program, Research Department, Peter MacCallum Cancer Centre, East Melbourne, Victoria, Australia (D.D.B.); the Garvan Institute, Sydney, New South Wales, Australia (D.D.B., S.J.R.); and Department of Public Health and Primary Care, University of Cambridge, Strangeways Research Laboratory, Cambridge, UK (P.D.P.P.)
1,
David G Huntsman
David G Huntsman, MD
1Department of Medical Oncology (M.S.B., K.R.K.) and Department of Health Sciences Research (R.A.V., S.J.W., B.M.M., Z.L., E.L.G.), Mayo Clinic, Rochester, MN; Department of Pathology and Laboratory Medicine, University of Calgary, Calgary, Alberta, Canada (P.F.R., M.K.); Pathology Department, Catholic University of Health and Allied Sciences-Bugando, Mwanza, Tanzania (P.F.R.); Tübingen University Hospital, Department of Women’s Health, Tübingen, Germany (P.W., F.A.T., A.D.H., S.Y.B., S.K.); Sir Peter MacCallum Department of Oncology, the University of Melbourne, Parkville, Victoria, Australia (N.T., D.D.B.); International Hereditary Cancer Center, Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (A.T., O.O., J. Lubiński, J.G.); Department of Gynecology and Obstetrics, Ribeirão Preto Medical School, University of São Paulo, Ribeirão Preto, Brazil (D.G.T., C.B.S., J.M.A., F.J.C.R.); Department of Pathology, Institute of Pathology, Heidelberg University Hospital, Heidelberg, Germany (P.S.); Department of Population Health Science and Policy, Department of Genetics and Genomic Sciences, Icahn School of Medicine at Mount Sinai, New York, NY (W.S., J.H.R.); Pathology West ICPMR Westmead, Westmead Hospital, the University of Sydney, Sydney, Australia (R.S.); University of Western Sydney at Westmead Hospital, Westmead, New South Wales, Australia (R.S.); Medical Oncology Service, Hospital Sant Pau, Barcelona, Spain (T.R.C.); H12O-CNIO Lung Cancer Clinical Research Unit (L.P.-A.), and Oncology Department, Hospital Universitario 12 de Octubre (L.P.-A.), Madrid, Spain; Women’s Cancer Program, Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, CA (S.O., J. Lester, B.Y.K.); the University of Texas School of Public Health, Houston (R.B.N.); Department of Oncology, Division of Gynecologic Oncology, Cumming School of Medicine, University of Calgary, Calgary, Alberta, Canada (G.N., P.G.); Department of Obstetrics, Gynecology and Reproductive Sciences, University of Pittsburgh School of Medicine (F.M., R.P.E.), Department of Epidemiology, University of Pittsburgh Graduate School of Public Health (F.M.), and Womens Cancer Research Program, Magee-Womens Research Institute and University of Pittsburgh Cancer Institute (F.M.), Pittsburgh, PA; Department of Gynecological Surgery and Gynecological Oncology of Adults and Adolescents, Pomeranian Medical University, Szczecin, Poland (J.M., A.C.-G.); Department of Health Research and Policy, Stanford University School of Medicine, Stanford, CA (V.M.); Department of Oncology, University of Cambridge, Strangeways Research Laboratory, Cambridge, UK (M.M., J.A., P.D.P.P.); Department of Pathology, Stanford University, Stanford, CA (T.A.L.); Department of Gynecologic Oncology, the Third Affiliated Hospital of Kunming Medical University (Yunnan Tumor Hospital), Kunming, China (Z.L.); Centre for Cancer Research, the Westmead Institute for Medical Research, the University of Sydney (C.J.K., J.H., P.R.H., B. Gao, A.deF.), and Department of Gynaecological Oncology, Westmead Hospital (C.J.K., J.H., A.deF.), Sydney, New South Wales, Australia; Division of Cancer Epidemiology, German Cancer Research Center (Deutsches Krebsforschungszentrum), Heidelberg, Germany (A.Y.J., U.E., J.C.-C.); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia (S.E.J., G.C.-T.); Department of Histopathology, Addenbrookes Hospital, Cambridge, UK (M.J.-L.); Virus, Lifestyle and Genes, Danish Cancer Society Research Center, Copenhagen, Denmark (A.J., S.K.K.); School of Women’s and Children’s Health, University of New South Wales, Sydney, Australia (M.P.I., S.J.R.); Tissue Bank of the National Center for Tumor Diseases (NCT) Heidelberg (E. Herpel), and Institute of Pathology, University of Heidelberg (E. Herpel), Heidelberg, Germany; Cancer Center, University of Hawaii, Honolulu, HI (B.Y.H.); the Crown Princess Mary Cancer Centre, Westmead Hospital, the University of Sydney, Sydney, Australia (P.R.H., B. Gao); Oncology Department, Hospital General de Albacete, Madrid, Spain (J.M.G.-B.); Department of Cancer Genomics and Genetics, Peter MacCallum Cancer Centre, East Melbourne, Victoria, Australia (S.F.); Pathology Department, Fundación Jiménez Díaz, Madrid, Spain (A.C.); Department of Health Research and Policy and Department of Biomedical Data Science, Stanford University School of Medicine, Stanford, CA (A.S.W.); Department of Obstetrics and Gynecology, Division of Gynecologic Oncology, Royal Alexandra Hospital, Edmonton, Alberta, Canada (H.S.); Tübingen University Hospital, Institute of Pathology, Tübingen, Germany (A.S.); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (K.B.M.); Gynaecological Cancer Research Centre, Department of Women’s Cancer, Institute for Women’s Health, University College London, London, UK (U.M.); Alberta Health Services-Cancer Care, Calgary, Alberta, Canada (J.M.K.); Department of Gynecology, Rigshospitalet, University of Copenhagen, Copenhagen, Denmark (S.K.K.); Department of Public Health Sciences, Medical University of South Carolina and Hollings Cancer Center, Charleston, SC (L.E.K.); Department of Pathology and Laboratory Medicine, University of British Columbia (D.G.H.), and Centre for Translational and Applied Genomics, British Columbia Cancer Agency (D.G.H.), Vancouver, British Columbia, Canada; Molecular Unit, Department of Pathology, Herlev Hospital, University of Copenhagen, Copenhagen, Denmark (E. Høgdall); Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, CA (M.T.G.); Genetic Pathology Evaluation Centre, Vancouver General Hospital and University of British Columbia, Vancouver, British Columbia, Canada (B. Gilks); Human Genetics Group, Spanish National Cancer Research Centre (Centro Nacional de Investigaciones Oncológicas) (M.J.G., J.B.), and Centre for Biomedical Network Research on Rare Diseases (Centro de Investigación Biomédica en Red de Enfermedades Raras), Instituto de Salud Carlos III (M.J.G., J.B.), Madrid, Spain; David Geffen School of Medicine, Department of Medicine, Division of Hematology and Oncology, University of California at Los Angeles (P.A.F.); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Erlangen, Germany (P.A.F.); Department of Histopathology, Queen’s Medical Centre, Nottingham University Hospitals NHS Trust, Nottingham, UK (S.D.); University Cancer Center Hamburg, University Medical Center Hamburg-Eppendorf, Hamburg, Germany (J.C.-C.); Cancer Genetics Laboratory, Research Division, Peter MacCallum Cancer Centre (I.G.C.), Sir Peter MacCallum Department of Oncology, University of Melbourne (I.G.C.), and Department of Pathology, University of Melbourne (I.G.C.), Melbourne, Victoria, Australia; Department of Oncology, University of Cambridge, Hutchison/MRC Research Centre (J.D.B.), Cancer Research UK Cambridge Institute, University of Cambridge, Li Ka Shing Centre (J.D.B.), and Cambridge Experimental Cancer Medicine Centre (J.D.B.), Cambridge, UK; Cancer Genomics Program, Research Department, Peter MacCallum Cancer Centre, East Melbourne, Victoria, Australia (D.D.B.); the Garvan Institute, Sydney, New South Wales, Australia (D.D.B., S.J.R.); and Department of Public Health and Primary Care, University of Cambridge, Strangeways Research Laboratory, Cambridge, UK (P.D.P.P.)
1,
Estrid Høgdall
Estrid Høgdall, PhD
1Department of Medical Oncology (M.S.B., K.R.K.) and Department of Health Sciences Research (R.A.V., S.J.W., B.M.M., Z.L., E.L.G.), Mayo Clinic, Rochester, MN; Department of Pathology and Laboratory Medicine, University of Calgary, Calgary, Alberta, Canada (P.F.R., M.K.); Pathology Department, Catholic University of Health and Allied Sciences-Bugando, Mwanza, Tanzania (P.F.R.); Tübingen University Hospital, Department of Women’s Health, Tübingen, Germany (P.W., F.A.T., A.D.H., S.Y.B., S.K.); Sir Peter MacCallum Department of Oncology, the University of Melbourne, Parkville, Victoria, Australia (N.T., D.D.B.); International Hereditary Cancer Center, Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (A.T., O.O., J. Lubiński, J.G.); Department of Gynecology and Obstetrics, Ribeirão Preto Medical School, University of São Paulo, Ribeirão Preto, Brazil (D.G.T., C.B.S., J.M.A., F.J.C.R.); Department of Pathology, Institute of Pathology, Heidelberg University Hospital, Heidelberg, Germany (P.S.); Department of Population Health Science and Policy, Department of Genetics and Genomic Sciences, Icahn School of Medicine at Mount Sinai, New York, NY (W.S., J.H.R.); Pathology West ICPMR Westmead, Westmead Hospital, the University of Sydney, Sydney, Australia (R.S.); University of Western Sydney at Westmead Hospital, Westmead, New South Wales, Australia (R.S.); Medical Oncology Service, Hospital Sant Pau, Barcelona, Spain (T.R.C.); H12O-CNIO Lung Cancer Clinical Research Unit (L.P.-A.), and Oncology Department, Hospital Universitario 12 de Octubre (L.P.-A.), Madrid, Spain; Women’s Cancer Program, Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, CA (S.O., J. Lester, B.Y.K.); the University of Texas School of Public Health, Houston (R.B.N.); Department of Oncology, Division of Gynecologic Oncology, Cumming School of Medicine, University of Calgary, Calgary, Alberta, Canada (G.N., P.G.); Department of Obstetrics, Gynecology and Reproductive Sciences, University of Pittsburgh School of Medicine (F.M., R.P.E.), Department of Epidemiology, University of Pittsburgh Graduate School of Public Health (F.M.), and Womens Cancer Research Program, Magee-Womens Research Institute and University of Pittsburgh Cancer Institute (F.M.), Pittsburgh, PA; Department of Gynecological Surgery and Gynecological Oncology of Adults and Adolescents, Pomeranian Medical University, Szczecin, Poland (J.M., A.C.-G.); Department of Health Research and Policy, Stanford University School of Medicine, Stanford, CA (V.M.); Department of Oncology, University of Cambridge, Strangeways Research Laboratory, Cambridge, UK (M.M., J.A., P.D.P.P.); Department of Pathology, Stanford University, Stanford, CA (T.A.L.); Department of Gynecologic Oncology, the Third Affiliated Hospital of Kunming Medical University (Yunnan Tumor Hospital), Kunming, China (Z.L.); Centre for Cancer Research, the Westmead Institute for Medical Research, the University of Sydney (C.J.K., J.H., P.R.H., B. Gao, A.deF.), and Department of Gynaecological Oncology, Westmead Hospital (C.J.K., J.H., A.deF.), Sydney, New South Wales, Australia; Division of Cancer Epidemiology, German Cancer Research Center (Deutsches Krebsforschungszentrum), Heidelberg, Germany (A.Y.J., U.E., J.C.-C.); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia (S.E.J., G.C.-T.); Department of Histopathology, Addenbrookes Hospital, Cambridge, UK (M.J.-L.); Virus, Lifestyle and Genes, Danish Cancer Society Research Center, Copenhagen, Denmark (A.J., S.K.K.); School of Women’s and Children’s Health, University of New South Wales, Sydney, Australia (M.P.I., S.J.R.); Tissue Bank of the National Center for Tumor Diseases (NCT) Heidelberg (E. Herpel), and Institute of Pathology, University of Heidelberg (E. Herpel), Heidelberg, Germany; Cancer Center, University of Hawaii, Honolulu, HI (B.Y.H.); the Crown Princess Mary Cancer Centre, Westmead Hospital, the University of Sydney, Sydney, Australia (P.R.H., B. Gao); Oncology Department, Hospital General de Albacete, Madrid, Spain (J.M.G.-B.); Department of Cancer Genomics and Genetics, Peter MacCallum Cancer Centre, East Melbourne, Victoria, Australia (S.F.); Pathology Department, Fundación Jiménez Díaz, Madrid, Spain (A.C.); Department of Health Research and Policy and Department of Biomedical Data Science, Stanford University School of Medicine, Stanford, CA (A.S.W.); Department of Obstetrics and Gynecology, Division of Gynecologic Oncology, Royal Alexandra Hospital, Edmonton, Alberta, Canada (H.S.); Tübingen University Hospital, Institute of Pathology, Tübingen, Germany (A.S.); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (K.B.M.); Gynaecological Cancer Research Centre, Department of Women’s Cancer, Institute for Women’s Health, University College London, London, UK (U.M.); Alberta Health Services-Cancer Care, Calgary, Alberta, Canada (J.M.K.); Department of Gynecology, Rigshospitalet, University of Copenhagen, Copenhagen, Denmark (S.K.K.); Department of Public Health Sciences, Medical University of South Carolina and Hollings Cancer Center, Charleston, SC (L.E.K.); Department of Pathology and Laboratory Medicine, University of British Columbia (D.G.H.), and Centre for Translational and Applied Genomics, British Columbia Cancer Agency (D.G.H.), Vancouver, British Columbia, Canada; Molecular Unit, Department of Pathology, Herlev Hospital, University of Copenhagen, Copenhagen, Denmark (E. Høgdall); Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, CA (M.T.G.); Genetic Pathology Evaluation Centre, Vancouver General Hospital and University of British Columbia, Vancouver, British Columbia, Canada (B. Gilks); Human Genetics Group, Spanish National Cancer Research Centre (Centro Nacional de Investigaciones Oncológicas) (M.J.G., J.B.), and Centre for Biomedical Network Research on Rare Diseases (Centro de Investigación Biomédica en Red de Enfermedades Raras), Instituto de Salud Carlos III (M.J.G., J.B.), Madrid, Spain; David Geffen School of Medicine, Department of Medicine, Division of Hematology and Oncology, University of California at Los Angeles (P.A.F.); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Erlangen, Germany (P.A.F.); Department of Histopathology, Queen’s Medical Centre, Nottingham University Hospitals NHS Trust, Nottingham, UK (S.D.); University Cancer Center Hamburg, University Medical Center Hamburg-Eppendorf, Hamburg, Germany (J.C.-C.); Cancer Genetics Laboratory, Research Division, Peter MacCallum Cancer Centre (I.G.C.), Sir Peter MacCallum Department of Oncology, University of Melbourne (I.G.C.), and Department of Pathology, University of Melbourne (I.G.C.), Melbourne, Victoria, Australia; Department of Oncology, University of Cambridge, Hutchison/MRC Research Centre (J.D.B.), Cancer Research UK Cambridge Institute, University of Cambridge, Li Ka Shing Centre (J.D.B.), and Cambridge Experimental Cancer Medicine Centre (J.D.B.), Cambridge, UK; Cancer Genomics Program, Research Department, Peter MacCallum Cancer Centre, East Melbourne, Victoria, Australia (D.D.B.); the Garvan Institute, Sydney, New South Wales, Australia (D.D.B., S.J.R.); and Department of Public Health and Primary Care, University of Cambridge, Strangeways Research Laboratory, Cambridge, UK (P.D.P.P.)
1,
Jacek Gronwald
Jacek Gronwald, MD, PhD
1Department of Medical Oncology (M.S.B., K.R.K.) and Department of Health Sciences Research (R.A.V., S.J.W., B.M.M., Z.L., E.L.G.), Mayo Clinic, Rochester, MN; Department of Pathology and Laboratory Medicine, University of Calgary, Calgary, Alberta, Canada (P.F.R., M.K.); Pathology Department, Catholic University of Health and Allied Sciences-Bugando, Mwanza, Tanzania (P.F.R.); Tübingen University Hospital, Department of Women’s Health, Tübingen, Germany (P.W., F.A.T., A.D.H., S.Y.B., S.K.); Sir Peter MacCallum Department of Oncology, the University of Melbourne, Parkville, Victoria, Australia (N.T., D.D.B.); International Hereditary Cancer Center, Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (A.T., O.O., J. Lubiński, J.G.); Department of Gynecology and Obstetrics, Ribeirão Preto Medical School, University of São Paulo, Ribeirão Preto, Brazil (D.G.T., C.B.S., J.M.A., F.J.C.R.); Department of Pathology, Institute of Pathology, Heidelberg University Hospital, Heidelberg, Germany (P.S.); Department of Population Health Science and Policy, Department of Genetics and Genomic Sciences, Icahn School of Medicine at Mount Sinai, New York, NY (W.S., J.H.R.); Pathology West ICPMR Westmead, Westmead Hospital, the University of Sydney, Sydney, Australia (R.S.); University of Western Sydney at Westmead Hospital, Westmead, New South Wales, Australia (R.S.); Medical Oncology Service, Hospital Sant Pau, Barcelona, Spain (T.R.C.); H12O-CNIO Lung Cancer Clinical Research Unit (L.P.-A.), and Oncology Department, Hospital Universitario 12 de Octubre (L.P.-A.), Madrid, Spain; Women’s Cancer Program, Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, CA (S.O., J. Lester, B.Y.K.); the University of Texas School of Public Health, Houston (R.B.N.); Department of Oncology, Division of Gynecologic Oncology, Cumming School of Medicine, University of Calgary, Calgary, Alberta, Canada (G.N., P.G.); Department of Obstetrics, Gynecology and Reproductive Sciences, University of Pittsburgh School of Medicine (F.M., R.P.E.), Department of Epidemiology, University of Pittsburgh Graduate School of Public Health (F.M.), and Womens Cancer Research Program, Magee-Womens Research Institute and University of Pittsburgh Cancer Institute (F.M.), Pittsburgh, PA; Department of Gynecological Surgery and Gynecological Oncology of Adults and Adolescents, Pomeranian Medical University, Szczecin, Poland (J.M., A.C.-G.); Department of Health Research and Policy, Stanford University School of Medicine, Stanford, CA (V.M.); Department of Oncology, University of Cambridge, Strangeways Research Laboratory, Cambridge, UK (M.M., J.A., P.D.P.P.); Department of Pathology, Stanford University, Stanford, CA (T.A.L.); Department of Gynecologic Oncology, the Third Affiliated Hospital of Kunming Medical University (Yunnan Tumor Hospital), Kunming, China (Z.L.); Centre for Cancer Research, the Westmead Institute for Medical Research, the University of Sydney (C.J.K., J.H., P.R.H., B. Gao, A.deF.), and Department of Gynaecological Oncology, Westmead Hospital (C.J.K., J.H., A.deF.), Sydney, New South Wales, Australia; Division of Cancer Epidemiology, German Cancer Research Center (Deutsches Krebsforschungszentrum), Heidelberg, Germany (A.Y.J., U.E., J.C.-C.); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia (S.E.J., G.C.-T.); Department of Histopathology, Addenbrookes Hospital, Cambridge, UK (M.J.-L.); Virus, Lifestyle and Genes, Danish Cancer Society Research Center, Copenhagen, Denmark (A.J., S.K.K.); School of Women’s and Children’s Health, University of New South Wales, Sydney, Australia (M.P.I., S.J.R.); Tissue Bank of the National Center for Tumor Diseases (NCT) Heidelberg (E. Herpel), and Institute of Pathology, University of Heidelberg (E. Herpel), Heidelberg, Germany; Cancer Center, University of Hawaii, Honolulu, HI (B.Y.H.); the Crown Princess Mary Cancer Centre, Westmead Hospital, the University of Sydney, Sydney, Australia (P.R.H., B. Gao); Oncology Department, Hospital General de Albacete, Madrid, Spain (J.M.G.-B.); Department of Cancer Genomics and Genetics, Peter MacCallum Cancer Centre, East Melbourne, Victoria, Australia (S.F.); Pathology Department, Fundación Jiménez Díaz, Madrid, Spain (A.C.); Department of Health Research and Policy and Department of Biomedical Data Science, Stanford University School of Medicine, Stanford, CA (A.S.W.); Department of Obstetrics and Gynecology, Division of Gynecologic Oncology, Royal Alexandra Hospital, Edmonton, Alberta, Canada (H.S.); Tübingen University Hospital, Institute of Pathology, Tübingen, Germany (A.S.); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (K.B.M.); Gynaecological Cancer Research Centre, Department of Women’s Cancer, Institute for Women’s Health, University College London, London, UK (U.M.); Alberta Health Services-Cancer Care, Calgary, Alberta, Canada (J.M.K.); Department of Gynecology, Rigshospitalet, University of Copenhagen, Copenhagen, Denmark (S.K.K.); Department of Public Health Sciences, Medical University of South Carolina and Hollings Cancer Center, Charleston, SC (L.E.K.); Department of Pathology and Laboratory Medicine, University of British Columbia (D.G.H.), and Centre for Translational and Applied Genomics, British Columbia Cancer Agency (D.G.H.), Vancouver, British Columbia, Canada; Molecular Unit, Department of Pathology, Herlev Hospital, University of Copenhagen, Copenhagen, Denmark (E. Høgdall); Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, CA (M.T.G.); Genetic Pathology Evaluation Centre, Vancouver General Hospital and University of British Columbia, Vancouver, British Columbia, Canada (B. Gilks); Human Genetics Group, Spanish National Cancer Research Centre (Centro Nacional de Investigaciones Oncológicas) (M.J.G., J.B.), and Centre for Biomedical Network Research on Rare Diseases (Centro de Investigación Biomédica en Red de Enfermedades Raras), Instituto de Salud Carlos III (M.J.G., J.B.), Madrid, Spain; David Geffen School of Medicine, Department of Medicine, Division of Hematology and Oncology, University of California at Los Angeles (P.A.F.); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Erlangen, Germany (P.A.F.); Department of Histopathology, Queen’s Medical Centre, Nottingham University Hospitals NHS Trust, Nottingham, UK (S.D.); University Cancer Center Hamburg, University Medical Center Hamburg-Eppendorf, Hamburg, Germany (J.C.-C.); Cancer Genetics Laboratory, Research Division, Peter MacCallum Cancer Centre (I.G.C.), Sir Peter MacCallum Department of Oncology, University of Melbourne (I.G.C.), and Department of Pathology, University of Melbourne (I.G.C.), Melbourne, Victoria, Australia; Department of Oncology, University of Cambridge, Hutchison/MRC Research Centre (J.D.B.), Cancer Research UK Cambridge Institute, University of Cambridge, Li Ka Shing Centre (J.D.B.), and Cambridge Experimental Cancer Medicine Centre (J.D.B.), Cambridge, UK; Cancer Genomics Program, Research Department, Peter MacCallum Cancer Centre, East Melbourne, Victoria, Australia (D.D.B.); the Garvan Institute, Sydney, New South Wales, Australia (D.D.B., S.J.R.); and Department of Public Health and Primary Care, University of Cambridge, Strangeways Research Laboratory, Cambridge, UK (P.D.P.P.)
1,
Marc T Goodman
Marc T Goodman, PhD
1Department of Medical Oncology (M.S.B., K.R.K.) and Department of Health Sciences Research (R.A.V., S.J.W., B.M.M., Z.L., E.L.G.), Mayo Clinic, Rochester, MN; Department of Pathology and Laboratory Medicine, University of Calgary, Calgary, Alberta, Canada (P.F.R., M.K.); Pathology Department, Catholic University of Health and Allied Sciences-Bugando, Mwanza, Tanzania (P.F.R.); Tübingen University Hospital, Department of Women’s Health, Tübingen, Germany (P.W., F.A.T., A.D.H., S.Y.B., S.K.); Sir Peter MacCallum Department of Oncology, the University of Melbourne, Parkville, Victoria, Australia (N.T., D.D.B.); International Hereditary Cancer Center, Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (A.T., O.O., J. Lubiński, J.G.); Department of Gynecology and Obstetrics, Ribeirão Preto Medical School, University of São Paulo, Ribeirão Preto, Brazil (D.G.T., C.B.S., J.M.A., F.J.C.R.); Department of Pathology, Institute of Pathology, Heidelberg University Hospital, Heidelberg, Germany (P.S.); Department of Population Health Science and Policy, Department of Genetics and Genomic Sciences, Icahn School of Medicine at Mount Sinai, New York, NY (W.S., J.H.R.); Pathology West ICPMR Westmead, Westmead Hospital, the University of Sydney, Sydney, Australia (R.S.); University of Western Sydney at Westmead Hospital, Westmead, New South Wales, Australia (R.S.); Medical Oncology Service, Hospital Sant Pau, Barcelona, Spain (T.R.C.); H12O-CNIO Lung Cancer Clinical Research Unit (L.P.-A.), and Oncology Department, Hospital Universitario 12 de Octubre (L.P.-A.), Madrid, Spain; Women’s Cancer Program, Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, CA (S.O., J. Lester, B.Y.K.); the University of Texas School of Public Health, Houston (R.B.N.); Department of Oncology, Division of Gynecologic Oncology, Cumming School of Medicine, University of Calgary, Calgary, Alberta, Canada (G.N., P.G.); Department of Obstetrics, Gynecology and Reproductive Sciences, University of Pittsburgh School of Medicine (F.M., R.P.E.), Department of Epidemiology, University of Pittsburgh Graduate School of Public Health (F.M.), and Womens Cancer Research Program, Magee-Womens Research Institute and University of Pittsburgh Cancer Institute (F.M.), Pittsburgh, PA; Department of Gynecological Surgery and Gynecological Oncology of Adults and Adolescents, Pomeranian Medical University, Szczecin, Poland (J.M., A.C.-G.); Department of Health Research and Policy, Stanford University School of Medicine, Stanford, CA (V.M.); Department of Oncology, University of Cambridge, Strangeways Research Laboratory, Cambridge, UK (M.M., J.A., P.D.P.P.); Department of Pathology, Stanford University, Stanford, CA (T.A.L.); Department of Gynecologic Oncology, the Third Affiliated Hospital of Kunming Medical University (Yunnan Tumor Hospital), Kunming, China (Z.L.); Centre for Cancer Research, the Westmead Institute for Medical Research, the University of Sydney (C.J.K., J.H., P.R.H., B. Gao, A.deF.), and Department of Gynaecological Oncology, Westmead Hospital (C.J.K., J.H., A.deF.), Sydney, New South Wales, Australia; Division of Cancer Epidemiology, German Cancer Research Center (Deutsches Krebsforschungszentrum), Heidelberg, Germany (A.Y.J., U.E., J.C.-C.); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia (S.E.J., G.C.-T.); Department of Histopathology, Addenbrookes Hospital, Cambridge, UK (M.J.-L.); Virus, Lifestyle and Genes, Danish Cancer Society Research Center, Copenhagen, Denmark (A.J., S.K.K.); School of Women’s and Children’s Health, University of New South Wales, Sydney, Australia (M.P.I., S.J.R.); Tissue Bank of the National Center for Tumor Diseases (NCT) Heidelberg (E. Herpel), and Institute of Pathology, University of Heidelberg (E. Herpel), Heidelberg, Germany; Cancer Center, University of Hawaii, Honolulu, HI (B.Y.H.); the Crown Princess Mary Cancer Centre, Westmead Hospital, the University of Sydney, Sydney, Australia (P.R.H., B. Gao); Oncology Department, Hospital General de Albacete, Madrid, Spain (J.M.G.-B.); Department of Cancer Genomics and Genetics, Peter MacCallum Cancer Centre, East Melbourne, Victoria, Australia (S.F.); Pathology Department, Fundación Jiménez Díaz, Madrid, Spain (A.C.); Department of Health Research and Policy and Department of Biomedical Data Science, Stanford University School of Medicine, Stanford, CA (A.S.W.); Department of Obstetrics and Gynecology, Division of Gynecologic Oncology, Royal Alexandra Hospital, Edmonton, Alberta, Canada (H.S.); Tübingen University Hospital, Institute of Pathology, Tübingen, Germany (A.S.); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (K.B.M.); Gynaecological Cancer Research Centre, Department of Women’s Cancer, Institute for Women’s Health, University College London, London, UK (U.M.); Alberta Health Services-Cancer Care, Calgary, Alberta, Canada (J.M.K.); Department of Gynecology, Rigshospitalet, University of Copenhagen, Copenhagen, Denmark (S.K.K.); Department of Public Health Sciences, Medical University of South Carolina and Hollings Cancer Center, Charleston, SC (L.E.K.); Department of Pathology and Laboratory Medicine, University of British Columbia (D.G.H.), and Centre for Translational and Applied Genomics, British Columbia Cancer Agency (D.G.H.), Vancouver, British Columbia, Canada; Molecular Unit, Department of Pathology, Herlev Hospital, University of Copenhagen, Copenhagen, Denmark (E. Høgdall); Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, CA (M.T.G.); Genetic Pathology Evaluation Centre, Vancouver General Hospital and University of British Columbia, Vancouver, British Columbia, Canada (B. Gilks); Human Genetics Group, Spanish National Cancer Research Centre (Centro Nacional de Investigaciones Oncológicas) (M.J.G., J.B.), and Centre for Biomedical Network Research on Rare Diseases (Centro de Investigación Biomédica en Red de Enfermedades Raras), Instituto de Salud Carlos III (M.J.G., J.B.), Madrid, Spain; David Geffen School of Medicine, Department of Medicine, Division of Hematology and Oncology, University of California at Los Angeles (P.A.F.); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Erlangen, Germany (P.A.F.); Department of Histopathology, Queen’s Medical Centre, Nottingham University Hospitals NHS Trust, Nottingham, UK (S.D.); University Cancer Center Hamburg, University Medical Center Hamburg-Eppendorf, Hamburg, Germany (J.C.-C.); Cancer Genetics Laboratory, Research Division, Peter MacCallum Cancer Centre (I.G.C.), Sir Peter MacCallum Department of Oncology, University of Melbourne (I.G.C.), and Department of Pathology, University of Melbourne (I.G.C.), Melbourne, Victoria, Australia; Department of Oncology, University of Cambridge, Hutchison/MRC Research Centre (J.D.B.), Cancer Research UK Cambridge Institute, University of Cambridge, Li Ka Shing Centre (J.D.B.), and Cambridge Experimental Cancer Medicine Centre (J.D.B.), Cambridge, UK; Cancer Genomics Program, Research Department, Peter MacCallum Cancer Centre, East Melbourne, Victoria, Australia (D.D.B.); the Garvan Institute, Sydney, New South Wales, Australia (D.D.B., S.J.R.); and Department of Public Health and Primary Care, University of Cambridge, Strangeways Research Laboratory, Cambridge, UK (P.D.P.P.)
1,
Blake Gilks
Blake Gilks, MD
1Department of Medical Oncology (M.S.B., K.R.K.) and Department of Health Sciences Research (R.A.V., S.J.W., B.M.M., Z.L., E.L.G.), Mayo Clinic, Rochester, MN; Department of Pathology and Laboratory Medicine, University of Calgary, Calgary, Alberta, Canada (P.F.R., M.K.); Pathology Department, Catholic University of Health and Allied Sciences-Bugando, Mwanza, Tanzania (P.F.R.); Tübingen University Hospital, Department of Women’s Health, Tübingen, Germany (P.W., F.A.T., A.D.H., S.Y.B., S.K.); Sir Peter MacCallum Department of Oncology, the University of Melbourne, Parkville, Victoria, Australia (N.T., D.D.B.); International Hereditary Cancer Center, Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (A.T., O.O., J. Lubiński, J.G.); Department of Gynecology and Obstetrics, Ribeirão Preto Medical School, University of São Paulo, Ribeirão Preto, Brazil (D.G.T., C.B.S., J.M.A., F.J.C.R.); Department of Pathology, Institute of Pathology, Heidelberg University Hospital, Heidelberg, Germany (P.S.); Department of Population Health Science and Policy, Department of Genetics and Genomic Sciences, Icahn School of Medicine at Mount Sinai, New York, NY (W.S., J.H.R.); Pathology West ICPMR Westmead, Westmead Hospital, the University of Sydney, Sydney, Australia (R.S.); University of Western Sydney at Westmead Hospital, Westmead, New South Wales, Australia (R.S.); Medical Oncology Service, Hospital Sant Pau, Barcelona, Spain (T.R.C.); H12O-CNIO Lung Cancer Clinical Research Unit (L.P.-A.), and Oncology Department, Hospital Universitario 12 de Octubre (L.P.-A.), Madrid, Spain; Women’s Cancer Program, Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, CA (S.O., J. Lester, B.Y.K.); the University of Texas School of Public Health, Houston (R.B.N.); Department of Oncology, Division of Gynecologic Oncology, Cumming School of Medicine, University of Calgary, Calgary, Alberta, Canada (G.N., P.G.); Department of Obstetrics, Gynecology and Reproductive Sciences, University of Pittsburgh School of Medicine (F.M., R.P.E.), Department of Epidemiology, University of Pittsburgh Graduate School of Public Health (F.M.), and Womens Cancer Research Program, Magee-Womens Research Institute and University of Pittsburgh Cancer Institute (F.M.), Pittsburgh, PA; Department of Gynecological Surgery and Gynecological Oncology of Adults and Adolescents, Pomeranian Medical University, Szczecin, Poland (J.M., A.C.-G.); Department of Health Research and Policy, Stanford University School of Medicine, Stanford, CA (V.M.); Department of Oncology, University of Cambridge, Strangeways Research Laboratory, Cambridge, UK (M.M., J.A., P.D.P.P.); Department of Pathology, Stanford University, Stanford, CA (T.A.L.); Department of Gynecologic Oncology, the Third Affiliated Hospital of Kunming Medical University (Yunnan Tumor Hospital), Kunming, China (Z.L.); Centre for Cancer Research, the Westmead Institute for Medical Research, the University of Sydney (C.J.K., J.H., P.R.H., B. Gao, A.deF.), and Department of Gynaecological Oncology, Westmead Hospital (C.J.K., J.H., A.deF.), Sydney, New South Wales, Australia; Division of Cancer Epidemiology, German Cancer Research Center (Deutsches Krebsforschungszentrum), Heidelberg, Germany (A.Y.J., U.E., J.C.-C.); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia (S.E.J., G.C.-T.); Department of Histopathology, Addenbrookes Hospital, Cambridge, UK (M.J.-L.); Virus, Lifestyle and Genes, Danish Cancer Society Research Center, Copenhagen, Denmark (A.J., S.K.K.); School of Women’s and Children’s Health, University of New South Wales, Sydney, Australia (M.P.I., S.J.R.); Tissue Bank of the National Center for Tumor Diseases (NCT) Heidelberg (E. Herpel), and Institute of Pathology, University of Heidelberg (E. Herpel), Heidelberg, Germany; Cancer Center, University of Hawaii, Honolulu, HI (B.Y.H.); the Crown Princess Mary Cancer Centre, Westmead Hospital, the University of Sydney, Sydney, Australia (P.R.H., B. Gao); Oncology Department, Hospital General de Albacete, Madrid, Spain (J.M.G.-B.); Department of Cancer Genomics and Genetics, Peter MacCallum Cancer Centre, East Melbourne, Victoria, Australia (S.F.); Pathology Department, Fundación Jiménez Díaz, Madrid, Spain (A.C.); Department of Health Research and Policy and Department of Biomedical Data Science, Stanford University School of Medicine, Stanford, CA (A.S.W.); Department of Obstetrics and Gynecology, Division of Gynecologic Oncology, Royal Alexandra Hospital, Edmonton, Alberta, Canada (H.S.); Tübingen University Hospital, Institute of Pathology, Tübingen, Germany (A.S.); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (K.B.M.); Gynaecological Cancer Research Centre, Department of Women’s Cancer, Institute for Women’s Health, University College London, London, UK (U.M.); Alberta Health Services-Cancer Care, Calgary, Alberta, Canada (J.M.K.); Department of Gynecology, Rigshospitalet, University of Copenhagen, Copenhagen, Denmark (S.K.K.); Department of Public Health Sciences, Medical University of South Carolina and Hollings Cancer Center, Charleston, SC (L.E.K.); Department of Pathology and Laboratory Medicine, University of British Columbia (D.G.H.), and Centre for Translational and Applied Genomics, British Columbia Cancer Agency (D.G.H.), Vancouver, British Columbia, Canada; Molecular Unit, Department of Pathology, Herlev Hospital, University of Copenhagen, Copenhagen, Denmark (E. Høgdall); Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, CA (M.T.G.); Genetic Pathology Evaluation Centre, Vancouver General Hospital and University of British Columbia, Vancouver, British Columbia, Canada (B. Gilks); Human Genetics Group, Spanish National Cancer Research Centre (Centro Nacional de Investigaciones Oncológicas) (M.J.G., J.B.), and Centre for Biomedical Network Research on Rare Diseases (Centro de Investigación Biomédica en Red de Enfermedades Raras), Instituto de Salud Carlos III (M.J.G., J.B.), Madrid, Spain; David Geffen School of Medicine, Department of Medicine, Division of Hematology and Oncology, University of California at Los Angeles (P.A.F.); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Erlangen, Germany (P.A.F.); Department of Histopathology, Queen’s Medical Centre, Nottingham University Hospitals NHS Trust, Nottingham, UK (S.D.); University Cancer Center Hamburg, University Medical Center Hamburg-Eppendorf, Hamburg, Germany (J.C.-C.); Cancer Genetics Laboratory, Research Division, Peter MacCallum Cancer Centre (I.G.C.), Sir Peter MacCallum Department of Oncology, University of Melbourne (I.G.C.), and Department of Pathology, University of Melbourne (I.G.C.), Melbourne, Victoria, Australia; Department of Oncology, University of Cambridge, Hutchison/MRC Research Centre (J.D.B.), Cancer Research UK Cambridge Institute, University of Cambridge, Li Ka Shing Centre (J.D.B.), and Cambridge Experimental Cancer Medicine Centre (J.D.B.), Cambridge, UK; Cancer Genomics Program, Research Department, Peter MacCallum Cancer Centre, East Melbourne, Victoria, Australia (D.D.B.); the Garvan Institute, Sydney, New South Wales, Australia (D.D.B., S.J.R.); and Department of Public Health and Primary Care, University of Cambridge, Strangeways Research Laboratory, Cambridge, UK (P.D.P.P.)
1,
María José García
María José García, PhD
1Department of Medical Oncology (M.S.B., K.R.K.) and Department of Health Sciences Research (R.A.V., S.J.W., B.M.M., Z.L., E.L.G.), Mayo Clinic, Rochester, MN; Department of Pathology and Laboratory Medicine, University of Calgary, Calgary, Alberta, Canada (P.F.R., M.K.); Pathology Department, Catholic University of Health and Allied Sciences-Bugando, Mwanza, Tanzania (P.F.R.); Tübingen University Hospital, Department of Women’s Health, Tübingen, Germany (P.W., F.A.T., A.D.H., S.Y.B., S.K.); Sir Peter MacCallum Department of Oncology, the University of Melbourne, Parkville, Victoria, Australia (N.T., D.D.B.); International Hereditary Cancer Center, Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (A.T., O.O., J. Lubiński, J.G.); Department of Gynecology and Obstetrics, Ribeirão Preto Medical School, University of São Paulo, Ribeirão Preto, Brazil (D.G.T., C.B.S., J.M.A., F.J.C.R.); Department of Pathology, Institute of Pathology, Heidelberg University Hospital, Heidelberg, Germany (P.S.); Department of Population Health Science and Policy, Department of Genetics and Genomic Sciences, Icahn School of Medicine at Mount Sinai, New York, NY (W.S., J.H.R.); Pathology West ICPMR Westmead, Westmead Hospital, the University of Sydney, Sydney, Australia (R.S.); University of Western Sydney at Westmead Hospital, Westmead, New South Wales, Australia (R.S.); Medical Oncology Service, Hospital Sant Pau, Barcelona, Spain (T.R.C.); H12O-CNIO Lung Cancer Clinical Research Unit (L.P.-A.), and Oncology Department, Hospital Universitario 12 de Octubre (L.P.-A.), Madrid, Spain; Women’s Cancer Program, Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, CA (S.O., J. Lester, B.Y.K.); the University of Texas School of Public Health, Houston (R.B.N.); Department of Oncology, Division of Gynecologic Oncology, Cumming School of Medicine, University of Calgary, Calgary, Alberta, Canada (G.N., P.G.); Department of Obstetrics, Gynecology and Reproductive Sciences, University of Pittsburgh School of Medicine (F.M., R.P.E.), Department of Epidemiology, University of Pittsburgh Graduate School of Public Health (F.M.), and Womens Cancer Research Program, Magee-Womens Research Institute and University of Pittsburgh Cancer Institute (F.M.), Pittsburgh, PA; Department of Gynecological Surgery and Gynecological Oncology of Adults and Adolescents, Pomeranian Medical University, Szczecin, Poland (J.M., A.C.-G.); Department of Health Research and Policy, Stanford University School of Medicine, Stanford, CA (V.M.); Department of Oncology, University of Cambridge, Strangeways Research Laboratory, Cambridge, UK (M.M., J.A., P.D.P.P.); Department of Pathology, Stanford University, Stanford, CA (T.A.L.); Department of Gynecologic Oncology, the Third Affiliated Hospital of Kunming Medical University (Yunnan Tumor Hospital), Kunming, China (Z.L.); Centre for Cancer Research, the Westmead Institute for Medical Research, the University of Sydney (C.J.K., J.H., P.R.H., B. Gao, A.deF.), and Department of Gynaecological Oncology, Westmead Hospital (C.J.K., J.H., A.deF.), Sydney, New South Wales, Australia; Division of Cancer Epidemiology, German Cancer Research Center (Deutsches Krebsforschungszentrum), Heidelberg, Germany (A.Y.J., U.E., J.C.-C.); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia (S.E.J., G.C.-T.); Department of Histopathology, Addenbrookes Hospital, Cambridge, UK (M.J.-L.); Virus, Lifestyle and Genes, Danish Cancer Society Research Center, Copenhagen, Denmark (A.J., S.K.K.); School of Women’s and Children’s Health, University of New South Wales, Sydney, Australia (M.P.I., S.J.R.); Tissue Bank of the National Center for Tumor Diseases (NCT) Heidelberg (E. Herpel), and Institute of Pathology, University of Heidelberg (E. Herpel), Heidelberg, Germany; Cancer Center, University of Hawaii, Honolulu, HI (B.Y.H.); the Crown Princess Mary Cancer Centre, Westmead Hospital, the University of Sydney, Sydney, Australia (P.R.H., B. Gao); Oncology Department, Hospital General de Albacete, Madrid, Spain (J.M.G.-B.); Department of Cancer Genomics and Genetics, Peter MacCallum Cancer Centre, East Melbourne, Victoria, Australia (S.F.); Pathology Department, Fundación Jiménez Díaz, Madrid, Spain (A.C.); Department of Health Research and Policy and Department of Biomedical Data Science, Stanford University School of Medicine, Stanford, CA (A.S.W.); Department of Obstetrics and Gynecology, Division of Gynecologic Oncology, Royal Alexandra Hospital, Edmonton, Alberta, Canada (H.S.); Tübingen University Hospital, Institute of Pathology, Tübingen, Germany (A.S.); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (K.B.M.); Gynaecological Cancer Research Centre, Department of Women’s Cancer, Institute for Women’s Health, University College London, London, UK (U.M.); Alberta Health Services-Cancer Care, Calgary, Alberta, Canada (J.M.K.); Department of Gynecology, Rigshospitalet, University of Copenhagen, Copenhagen, Denmark (S.K.K.); Department of Public Health Sciences, Medical University of South Carolina and Hollings Cancer Center, Charleston, SC (L.E.K.); Department of Pathology and Laboratory Medicine, University of British Columbia (D.G.H.), and Centre for Translational and Applied Genomics, British Columbia Cancer Agency (D.G.H.), Vancouver, British Columbia, Canada; Molecular Unit, Department of Pathology, Herlev Hospital, University of Copenhagen, Copenhagen, Denmark (E. Høgdall); Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, CA (M.T.G.); Genetic Pathology Evaluation Centre, Vancouver General Hospital and University of British Columbia, Vancouver, British Columbia, Canada (B. Gilks); Human Genetics Group, Spanish National Cancer Research Centre (Centro Nacional de Investigaciones Oncológicas) (M.J.G., J.B.), and Centre for Biomedical Network Research on Rare Diseases (Centro de Investigación Biomédica en Red de Enfermedades Raras), Instituto de Salud Carlos III (M.J.G., J.B.), Madrid, Spain; David Geffen School of Medicine, Department of Medicine, Division of Hematology and Oncology, University of California at Los Angeles (P.A.F.); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Erlangen, Germany (P.A.F.); Department of Histopathology, Queen’s Medical Centre, Nottingham University Hospitals NHS Trust, Nottingham, UK (S.D.); University Cancer Center Hamburg, University Medical Center Hamburg-Eppendorf, Hamburg, Germany (J.C.-C.); Cancer Genetics Laboratory, Research Division, Peter MacCallum Cancer Centre (I.G.C.), Sir Peter MacCallum Department of Oncology, University of Melbourne (I.G.C.), and Department of Pathology, University of Melbourne (I.G.C.), Melbourne, Victoria, Australia; Department of Oncology, University of Cambridge, Hutchison/MRC Research Centre (J.D.B.), Cancer Research UK Cambridge Institute, University of Cambridge, Li Ka Shing Centre (J.D.B.), and Cambridge Experimental Cancer Medicine Centre (J.D.B.), Cambridge, UK; Cancer Genomics Program, Research Department, Peter MacCallum Cancer Centre, East Melbourne, Victoria, Australia (D.D.B.); the Garvan Institute, Sydney, New South Wales, Australia (D.D.B., S.J.R.); and Department of Public Health and Primary Care, University of Cambridge, Strangeways Research Laboratory, Cambridge, UK (P.D.P.P.)
1,
Peter A Fasching
Peter A Fasching, MD
1Department of Medical Oncology (M.S.B., K.R.K.) and Department of Health Sciences Research (R.A.V., S.J.W., B.M.M., Z.L., E.L.G.), Mayo Clinic, Rochester, MN; Department of Pathology and Laboratory Medicine, University of Calgary, Calgary, Alberta, Canada (P.F.R., M.K.); Pathology Department, Catholic University of Health and Allied Sciences-Bugando, Mwanza, Tanzania (P.F.R.); Tübingen University Hospital, Department of Women’s Health, Tübingen, Germany (P.W., F.A.T., A.D.H., S.Y.B., S.K.); Sir Peter MacCallum Department of Oncology, the University of Melbourne, Parkville, Victoria, Australia (N.T., D.D.B.); International Hereditary Cancer Center, Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (A.T., O.O., J. Lubiński, J.G.); Department of Gynecology and Obstetrics, Ribeirão Preto Medical School, University of São Paulo, Ribeirão Preto, Brazil (D.G.T., C.B.S., J.M.A., F.J.C.R.); Department of Pathology, Institute of Pathology, Heidelberg University Hospital, Heidelberg, Germany (P.S.); Department of Population Health Science and Policy, Department of Genetics and Genomic Sciences, Icahn School of Medicine at Mount Sinai, New York, NY (W.S., J.H.R.); Pathology West ICPMR Westmead, Westmead Hospital, the University of Sydney, Sydney, Australia (R.S.); University of Western Sydney at Westmead Hospital, Westmead, New South Wales, Australia (R.S.); Medical Oncology Service, Hospital Sant Pau, Barcelona, Spain (T.R.C.); H12O-CNIO Lung Cancer Clinical Research Unit (L.P.-A.), and Oncology Department, Hospital Universitario 12 de Octubre (L.P.-A.), Madrid, Spain; Women’s Cancer Program, Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, CA (S.O., J. Lester, B.Y.K.); the University of Texas School of Public Health, Houston (R.B.N.); Department of Oncology, Division of Gynecologic Oncology, Cumming School of Medicine, University of Calgary, Calgary, Alberta, Canada (G.N., P.G.); Department of Obstetrics, Gynecology and Reproductive Sciences, University of Pittsburgh School of Medicine (F.M., R.P.E.), Department of Epidemiology, University of Pittsburgh Graduate School of Public Health (F.M.), and Womens Cancer Research Program, Magee-Womens Research Institute and University of Pittsburgh Cancer Institute (F.M.), Pittsburgh, PA; Department of Gynecological Surgery and Gynecological Oncology of Adults and Adolescents, Pomeranian Medical University, Szczecin, Poland (J.M., A.C.-G.); Department of Health Research and Policy, Stanford University School of Medicine, Stanford, CA (V.M.); Department of Oncology, University of Cambridge, Strangeways Research Laboratory, Cambridge, UK (M.M., J.A., P.D.P.P.); Department of Pathology, Stanford University, Stanford, CA (T.A.L.); Department of Gynecologic Oncology, the Third Affiliated Hospital of Kunming Medical University (Yunnan Tumor Hospital), Kunming, China (Z.L.); Centre for Cancer Research, the Westmead Institute for Medical Research, the University of Sydney (C.J.K., J.H., P.R.H., B. Gao, A.deF.), and Department of Gynaecological Oncology, Westmead Hospital (C.J.K., J.H., A.deF.), Sydney, New South Wales, Australia; Division of Cancer Epidemiology, German Cancer Research Center (Deutsches Krebsforschungszentrum), Heidelberg, Germany (A.Y.J., U.E., J.C.-C.); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia (S.E.J., G.C.-T.); Department of Histopathology, Addenbrookes Hospital, Cambridge, UK (M.J.-L.); Virus, Lifestyle and Genes, Danish Cancer Society Research Center, Copenhagen, Denmark (A.J., S.K.K.); School of Women’s and Children’s Health, University of New South Wales, Sydney, Australia (M.P.I., S.J.R.); Tissue Bank of the National Center for Tumor Diseases (NCT) Heidelberg (E. Herpel), and Institute of Pathology, University of Heidelberg (E. Herpel), Heidelberg, Germany; Cancer Center, University of Hawaii, Honolulu, HI (B.Y.H.); the Crown Princess Mary Cancer Centre, Westmead Hospital, the University of Sydney, Sydney, Australia (P.R.H., B. Gao); Oncology Department, Hospital General de Albacete, Madrid, Spain (J.M.G.-B.); Department of Cancer Genomics and Genetics, Peter MacCallum Cancer Centre, East Melbourne, Victoria, Australia (S.F.); Pathology Department, Fundación Jiménez Díaz, Madrid, Spain (A.C.); Department of Health Research and Policy and Department of Biomedical Data Science, Stanford University School of Medicine, Stanford, CA (A.S.W.); Department of Obstetrics and Gynecology, Division of Gynecologic Oncology, Royal Alexandra Hospital, Edmonton, Alberta, Canada (H.S.); Tübingen University Hospital, Institute of Pathology, Tübingen, Germany (A.S.); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (K.B.M.); Gynaecological Cancer Research Centre, Department of Women’s Cancer, Institute for Women’s Health, University College London, London, UK (U.M.); Alberta Health Services-Cancer Care, Calgary, Alberta, Canada (J.M.K.); Department of Gynecology, Rigshospitalet, University of Copenhagen, Copenhagen, Denmark (S.K.K.); Department of Public Health Sciences, Medical University of South Carolina and Hollings Cancer Center, Charleston, SC (L.E.K.); Department of Pathology and Laboratory Medicine, University of British Columbia (D.G.H.), and Centre for Translational and Applied Genomics, British Columbia Cancer Agency (D.G.H.), Vancouver, British Columbia, Canada; Molecular Unit, Department of Pathology, Herlev Hospital, University of Copenhagen, Copenhagen, Denmark (E. Høgdall); Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, CA (M.T.G.); Genetic Pathology Evaluation Centre, Vancouver General Hospital and University of British Columbia, Vancouver, British Columbia, Canada (B. Gilks); Human Genetics Group, Spanish National Cancer Research Centre (Centro Nacional de Investigaciones Oncológicas) (M.J.G., J.B.), and Centre for Biomedical Network Research on Rare Diseases (Centro de Investigación Biomédica en Red de Enfermedades Raras), Instituto de Salud Carlos III (M.J.G., J.B.), Madrid, Spain; David Geffen School of Medicine, Department of Medicine, Division of Hematology and Oncology, University of California at Los Angeles (P.A.F.); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Erlangen, Germany (P.A.F.); Department of Histopathology, Queen’s Medical Centre, Nottingham University Hospitals NHS Trust, Nottingham, UK (S.D.); University Cancer Center Hamburg, University Medical Center Hamburg-Eppendorf, Hamburg, Germany (J.C.-C.); Cancer Genetics Laboratory, Research Division, Peter MacCallum Cancer Centre (I.G.C.), Sir Peter MacCallum Department of Oncology, University of Melbourne (I.G.C.), and Department of Pathology, University of Melbourne (I.G.C.), Melbourne, Victoria, Australia; Department of Oncology, University of Cambridge, Hutchison/MRC Research Centre (J.D.B.), Cancer Research UK Cambridge Institute, University of Cambridge, Li Ka Shing Centre (J.D.B.), and Cambridge Experimental Cancer Medicine Centre (J.D.B.), Cambridge, UK; Cancer Genomics Program, Research Department, Peter MacCallum Cancer Centre, East Melbourne, Victoria, Australia (D.D.B.); the Garvan Institute, Sydney, New South Wales, Australia (D.D.B., S.J.R.); and Department of Public Health and Primary Care, University of Cambridge, Strangeways Research Laboratory, Cambridge, UK (P.D.P.P.)
1,
Anna de Fazio
Anna de Fazio, PhD
1Department of Medical Oncology (M.S.B., K.R.K.) and Department of Health Sciences Research (R.A.V., S.J.W., B.M.M., Z.L., E.L.G.), Mayo Clinic, Rochester, MN; Department of Pathology and Laboratory Medicine, University of Calgary, Calgary, Alberta, Canada (P.F.R., M.K.); Pathology Department, Catholic University of Health and Allied Sciences-Bugando, Mwanza, Tanzania (P.F.R.); Tübingen University Hospital, Department of Women’s Health, Tübingen, Germany (P.W., F.A.T., A.D.H., S.Y.B., S.K.); Sir Peter MacCallum Department of Oncology, the University of Melbourne, Parkville, Victoria, Australia (N.T., D.D.B.); International Hereditary Cancer Center, Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (A.T., O.O., J. Lubiński, J.G.); Department of Gynecology and Obstetrics, Ribeirão Preto Medical School, University of São Paulo, Ribeirão Preto, Brazil (D.G.T., C.B.S., J.M.A., F.J.C.R.); Department of Pathology, Institute of Pathology, Heidelberg University Hospital, Heidelberg, Germany (P.S.); Department of Population Health Science and Policy, Department of Genetics and Genomic Sciences, Icahn School of Medicine at Mount Sinai, New York, NY (W.S., J.H.R.); Pathology West ICPMR Westmead, Westmead Hospital, the University of Sydney, Sydney, Australia (R.S.); University of Western Sydney at Westmead Hospital, Westmead, New South Wales, Australia (R.S.); Medical Oncology Service, Hospital Sant Pau, Barcelona, Spain (T.R.C.); H12O-CNIO Lung Cancer Clinical Research Unit (L.P.-A.), and Oncology Department, Hospital Universitario 12 de Octubre (L.P.-A.), Madrid, Spain; Women’s Cancer Program, Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, CA (S.O., J. Lester, B.Y.K.); the University of Texas School of Public Health, Houston (R.B.N.); Department of Oncology, Division of Gynecologic Oncology, Cumming School of Medicine, University of Calgary, Calgary, Alberta, Canada (G.N., P.G.); Department of Obstetrics, Gynecology and Reproductive Sciences, University of Pittsburgh School of Medicine (F.M., R.P.E.), Department of Epidemiology, University of Pittsburgh Graduate School of Public Health (F.M.), and Womens Cancer Research Program, Magee-Womens Research Institute and University of Pittsburgh Cancer Institute (F.M.), Pittsburgh, PA; Department of Gynecological Surgery and Gynecological Oncology of Adults and Adolescents, Pomeranian Medical University, Szczecin, Poland (J.M., A.C.-G.); Department of Health Research and Policy, Stanford University School of Medicine, Stanford, CA (V.M.); Department of Oncology, University of Cambridge, Strangeways Research Laboratory, Cambridge, UK (M.M., J.A., P.D.P.P.); Department of Pathology, Stanford University, Stanford, CA (T.A.L.); Department of Gynecologic Oncology, the Third Affiliated Hospital of Kunming Medical University (Yunnan Tumor Hospital), Kunming, China (Z.L.); Centre for Cancer Research, the Westmead Institute for Medical Research, the University of Sydney (C.J.K., J.H., P.R.H., B. Gao, A.deF.), and Department of Gynaecological Oncology, Westmead Hospital (C.J.K., J.H., A.deF.), Sydney, New South Wales, Australia; Division of Cancer Epidemiology, German Cancer Research Center (Deutsches Krebsforschungszentrum), Heidelberg, Germany (A.Y.J., U.E., J.C.-C.); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia (S.E.J., G.C.-T.); Department of Histopathology, Addenbrookes Hospital, Cambridge, UK (M.J.-L.); Virus, Lifestyle and Genes, Danish Cancer Society Research Center, Copenhagen, Denmark (A.J., S.K.K.); School of Women’s and Children’s Health, University of New South Wales, Sydney, Australia (M.P.I., S.J.R.); Tissue Bank of the National Center for Tumor Diseases (NCT) Heidelberg (E. Herpel), and Institute of Pathology, University of Heidelberg (E. Herpel), Heidelberg, Germany; Cancer Center, University of Hawaii, Honolulu, HI (B.Y.H.); the Crown Princess Mary Cancer Centre, Westmead Hospital, the University of Sydney, Sydney, Australia (P.R.H., B. Gao); Oncology Department, Hospital General de Albacete, Madrid, Spain (J.M.G.-B.); Department of Cancer Genomics and Genetics, Peter MacCallum Cancer Centre, East Melbourne, Victoria, Australia (S.F.); Pathology Department, Fundación Jiménez Díaz, Madrid, Spain (A.C.); Department of Health Research and Policy and Department of Biomedical Data Science, Stanford University School of Medicine, Stanford, CA (A.S.W.); Department of Obstetrics and Gynecology, Division of Gynecologic Oncology, Royal Alexandra Hospital, Edmonton, Alberta, Canada (H.S.); Tübingen University Hospital, Institute of Pathology, Tübingen, Germany (A.S.); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (K.B.M.); Gynaecological Cancer Research Centre, Department of Women’s Cancer, Institute for Women’s Health, University College London, London, UK (U.M.); Alberta Health Services-Cancer Care, Calgary, Alberta, Canada (J.M.K.); Department of Gynecology, Rigshospitalet, University of Copenhagen, Copenhagen, Denmark (S.K.K.); Department of Public Health Sciences, Medical University of South Carolina and Hollings Cancer Center, Charleston, SC (L.E.K.); Department of Pathology and Laboratory Medicine, University of British Columbia (D.G.H.), and Centre for Translational and Applied Genomics, British Columbia Cancer Agency (D.G.H.), Vancouver, British Columbia, Canada; Molecular Unit, Department of Pathology, Herlev Hospital, University of Copenhagen, Copenhagen, Denmark (E. Høgdall); Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, CA (M.T.G.); Genetic Pathology Evaluation Centre, Vancouver General Hospital and University of British Columbia, Vancouver, British Columbia, Canada (B. Gilks); Human Genetics Group, Spanish National Cancer Research Centre (Centro Nacional de Investigaciones Oncológicas) (M.J.G., J.B.), and Centre for Biomedical Network Research on Rare Diseases (Centro de Investigación Biomédica en Red de Enfermedades Raras), Instituto de Salud Carlos III (M.J.G., J.B.), Madrid, Spain; David Geffen School of Medicine, Department of Medicine, Division of Hematology and Oncology, University of California at Los Angeles (P.A.F.); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Erlangen, Germany (P.A.F.); Department of Histopathology, Queen’s Medical Centre, Nottingham University Hospitals NHS Trust, Nottingham, UK (S.D.); University Cancer Center Hamburg, University Medical Center Hamburg-Eppendorf, Hamburg, Germany (J.C.-C.); Cancer Genetics Laboratory, Research Division, Peter MacCallum Cancer Centre (I.G.C.), Sir Peter MacCallum Department of Oncology, University of Melbourne (I.G.C.), and Department of Pathology, University of Melbourne (I.G.C.), Melbourne, Victoria, Australia; Department of Oncology, University of Cambridge, Hutchison/MRC Research Centre (J.D.B.), Cancer Research UK Cambridge Institute, University of Cambridge, Li Ka Shing Centre (J.D.B.), and Cambridge Experimental Cancer Medicine Centre (J.D.B.), Cambridge, UK; Cancer Genomics Program, Research Department, Peter MacCallum Cancer Centre, East Melbourne, Victoria, Australia (D.D.B.); the Garvan Institute, Sydney, New South Wales, Australia (D.D.B., S.J.R.); and Department of Public Health and Primary Care, University of Cambridge, Strangeways Research Laboratory, Cambridge, UK (P.D.P.P.)
1,
Suha Deen
Suha Deen, MBChB
1Department of Medical Oncology (M.S.B., K.R.K.) and Department of Health Sciences Research (R.A.V., S.J.W., B.M.M., Z.L., E.L.G.), Mayo Clinic, Rochester, MN; Department of Pathology and Laboratory Medicine, University of Calgary, Calgary, Alberta, Canada (P.F.R., M.K.); Pathology Department, Catholic University of Health and Allied Sciences-Bugando, Mwanza, Tanzania (P.F.R.); Tübingen University Hospital, Department of Women’s Health, Tübingen, Germany (P.W., F.A.T., A.D.H., S.Y.B., S.K.); Sir Peter MacCallum Department of Oncology, the University of Melbourne, Parkville, Victoria, Australia (N.T., D.D.B.); International Hereditary Cancer Center, Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (A.T., O.O., J. Lubiński, J.G.); Department of Gynecology and Obstetrics, Ribeirão Preto Medical School, University of São Paulo, Ribeirão Preto, Brazil (D.G.T., C.B.S., J.M.A., F.J.C.R.); Department of Pathology, Institute of Pathology, Heidelberg University Hospital, Heidelberg, Germany (P.S.); Department of Population Health Science and Policy, Department of Genetics and Genomic Sciences, Icahn School of Medicine at Mount Sinai, New York, NY (W.S., J.H.R.); Pathology West ICPMR Westmead, Westmead Hospital, the University of Sydney, Sydney, Australia (R.S.); University of Western Sydney at Westmead Hospital, Westmead, New South Wales, Australia (R.S.); Medical Oncology Service, Hospital Sant Pau, Barcelona, Spain (T.R.C.); H12O-CNIO Lung Cancer Clinical Research Unit (L.P.-A.), and Oncology Department, Hospital Universitario 12 de Octubre (L.P.-A.), Madrid, Spain; Women’s Cancer Program, Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, CA (S.O., J. Lester, B.Y.K.); the University of Texas School of Public Health, Houston (R.B.N.); Department of Oncology, Division of Gynecologic Oncology, Cumming School of Medicine, University of Calgary, Calgary, Alberta, Canada (G.N., P.G.); Department of Obstetrics, Gynecology and Reproductive Sciences, University of Pittsburgh School of Medicine (F.M., R.P.E.), Department of Epidemiology, University of Pittsburgh Graduate School of Public Health (F.M.), and Womens Cancer Research Program, Magee-Womens Research Institute and University of Pittsburgh Cancer Institute (F.M.), Pittsburgh, PA; Department of Gynecological Surgery and Gynecological Oncology of Adults and Adolescents, Pomeranian Medical University, Szczecin, Poland (J.M., A.C.-G.); Department of Health Research and Policy, Stanford University School of Medicine, Stanford, CA (V.M.); Department of Oncology, University of Cambridge, Strangeways Research Laboratory, Cambridge, UK (M.M., J.A., P.D.P.P.); Department of Pathology, Stanford University, Stanford, CA (T.A.L.); Department of Gynecologic Oncology, the Third Affiliated Hospital of Kunming Medical University (Yunnan Tumor Hospital), Kunming, China (Z.L.); Centre for Cancer Research, the Westmead Institute for Medical Research, the University of Sydney (C.J.K., J.H., P.R.H., B. Gao, A.deF.), and Department of Gynaecological Oncology, Westmead Hospital (C.J.K., J.H., A.deF.), Sydney, New South Wales, Australia; Division of Cancer Epidemiology, German Cancer Research Center (Deutsches Krebsforschungszentrum), Heidelberg, Germany (A.Y.J., U.E., J.C.-C.); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia (S.E.J., G.C.-T.); Department of Histopathology, Addenbrookes Hospital, Cambridge, UK (M.J.-L.); Virus, Lifestyle and Genes, Danish Cancer Society Research Center, Copenhagen, Denmark (A.J., S.K.K.); School of Women’s and Children’s Health, University of New South Wales, Sydney, Australia (M.P.I., S.J.R.); Tissue Bank of the National Center for Tumor Diseases (NCT) Heidelberg (E. Herpel), and Institute of Pathology, University of Heidelberg (E. Herpel), Heidelberg, Germany; Cancer Center, University of Hawaii, Honolulu, HI (B.Y.H.); the Crown Princess Mary Cancer Centre, Westmead Hospital, the University of Sydney, Sydney, Australia (P.R.H., B. Gao); Oncology Department, Hospital General de Albacete, Madrid, Spain (J.M.G.-B.); Department of Cancer Genomics and Genetics, Peter MacCallum Cancer Centre, East Melbourne, Victoria, Australia (S.F.); Pathology Department, Fundación Jiménez Díaz, Madrid, Spain (A.C.); Department of Health Research and Policy and Department of Biomedical Data Science, Stanford University School of Medicine, Stanford, CA (A.S.W.); Department of Obstetrics and Gynecology, Division of Gynecologic Oncology, Royal Alexandra Hospital, Edmonton, Alberta, Canada (H.S.); Tübingen University Hospital, Institute of Pathology, Tübingen, Germany (A.S.); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (K.B.M.); Gynaecological Cancer Research Centre, Department of Women’s Cancer, Institute for Women’s Health, University College London, London, UK (U.M.); Alberta Health Services-Cancer Care, Calgary, Alberta, Canada (J.M.K.); Department of Gynecology, Rigshospitalet, University of Copenhagen, Copenhagen, Denmark (S.K.K.); Department of Public Health Sciences, Medical University of South Carolina and Hollings Cancer Center, Charleston, SC (L.E.K.); Department of Pathology and Laboratory Medicine, University of British Columbia (D.G.H.), and Centre for Translational and Applied Genomics, British Columbia Cancer Agency (D.G.H.), Vancouver, British Columbia, Canada; Molecular Unit, Department of Pathology, Herlev Hospital, University of Copenhagen, Copenhagen, Denmark (E. Høgdall); Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, CA (M.T.G.); Genetic Pathology Evaluation Centre, Vancouver General Hospital and University of British Columbia, Vancouver, British Columbia, Canada (B. Gilks); Human Genetics Group, Spanish National Cancer Research Centre (Centro Nacional de Investigaciones Oncológicas) (M.J.G., J.B.), and Centre for Biomedical Network Research on Rare Diseases (Centro de Investigación Biomédica en Red de Enfermedades Raras), Instituto de Salud Carlos III (M.J.G., J.B.), Madrid, Spain; David Geffen School of Medicine, Department of Medicine, Division of Hematology and Oncology, University of California at Los Angeles (P.A.F.); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Erlangen, Germany (P.A.F.); Department of Histopathology, Queen’s Medical Centre, Nottingham University Hospitals NHS Trust, Nottingham, UK (S.D.); University Cancer Center Hamburg, University Medical Center Hamburg-Eppendorf, Hamburg, Germany (J.C.-C.); Cancer Genetics Laboratory, Research Division, Peter MacCallum Cancer Centre (I.G.C.), Sir Peter MacCallum Department of Oncology, University of Melbourne (I.G.C.), and Department of Pathology, University of Melbourne (I.G.C.), Melbourne, Victoria, Australia; Department of Oncology, University of Cambridge, Hutchison/MRC Research Centre (J.D.B.), Cancer Research UK Cambridge Institute, University of Cambridge, Li Ka Shing Centre (J.D.B.), and Cambridge Experimental Cancer Medicine Centre (J.D.B.), Cambridge, UK; Cancer Genomics Program, Research Department, Peter MacCallum Cancer Centre, East Melbourne, Victoria, Australia (D.D.B.); the Garvan Institute, Sydney, New South Wales, Australia (D.D.B., S.J.R.); and Department of Public Health and Primary Care, University of Cambridge, Strangeways Research Laboratory, Cambridge, UK (P.D.P.P.)
1,
Jenny Chang-Claude
Jenny Chang-Claude, PhD
1Department of Medical Oncology (M.S.B., K.R.K.) and Department of Health Sciences Research (R.A.V., S.J.W., B.M.M., Z.L., E.L.G.), Mayo Clinic, Rochester, MN; Department of Pathology and Laboratory Medicine, University of Calgary, Calgary, Alberta, Canada (P.F.R., M.K.); Pathology Department, Catholic University of Health and Allied Sciences-Bugando, Mwanza, Tanzania (P.F.R.); Tübingen University Hospital, Department of Women’s Health, Tübingen, Germany (P.W., F.A.T., A.D.H., S.Y.B., S.K.); Sir Peter MacCallum Department of Oncology, the University of Melbourne, Parkville, Victoria, Australia (N.T., D.D.B.); International Hereditary Cancer Center, Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (A.T., O.O., J. Lubiński, J.G.); Department of Gynecology and Obstetrics, Ribeirão Preto Medical School, University of São Paulo, Ribeirão Preto, Brazil (D.G.T., C.B.S., J.M.A., F.J.C.R.); Department of Pathology, Institute of Pathology, Heidelberg University Hospital, Heidelberg, Germany (P.S.); Department of Population Health Science and Policy, Department of Genetics and Genomic Sciences, Icahn School of Medicine at Mount Sinai, New York, NY (W.S., J.H.R.); Pathology West ICPMR Westmead, Westmead Hospital, the University of Sydney, Sydney, Australia (R.S.); University of Western Sydney at Westmead Hospital, Westmead, New South Wales, Australia (R.S.); Medical Oncology Service, Hospital Sant Pau, Barcelona, Spain (T.R.C.); H12O-CNIO Lung Cancer Clinical Research Unit (L.P.-A.), and Oncology Department, Hospital Universitario 12 de Octubre (L.P.-A.), Madrid, Spain; Women’s Cancer Program, Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, CA (S.O., J. Lester, B.Y.K.); the University of Texas School of Public Health, Houston (R.B.N.); Department of Oncology, Division of Gynecologic Oncology, Cumming School of Medicine, University of Calgary, Calgary, Alberta, Canada (G.N., P.G.); Department of Obstetrics, Gynecology and Reproductive Sciences, University of Pittsburgh School of Medicine (F.M., R.P.E.), Department of Epidemiology, University of Pittsburgh Graduate School of Public Health (F.M.), and Womens Cancer Research Program, Magee-Womens Research Institute and University of Pittsburgh Cancer Institute (F.M.), Pittsburgh, PA; Department of Gynecological Surgery and Gynecological Oncology of Adults and Adolescents, Pomeranian Medical University, Szczecin, Poland (J.M., A.C.-G.); Department of Health Research and Policy, Stanford University School of Medicine, Stanford, CA (V.M.); Department of Oncology, University of Cambridge, Strangeways Research Laboratory, Cambridge, UK (M.M., J.A., P.D.P.P.); Department of Pathology, Stanford University, Stanford, CA (T.A.L.); Department of Gynecologic Oncology, the Third Affiliated Hospital of Kunming Medical University (Yunnan Tumor Hospital), Kunming, China (Z.L.); Centre for Cancer Research, the Westmead Institute for Medical Research, the University of Sydney (C.J.K., J.H., P.R.H., B. Gao, A.deF.), and Department of Gynaecological Oncology, Westmead Hospital (C.J.K., J.H., A.deF.), Sydney, New South Wales, Australia; Division of Cancer Epidemiology, German Cancer Research Center (Deutsches Krebsforschungszentrum), Heidelberg, Germany (A.Y.J., U.E., J.C.-C.); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia (S.E.J., G.C.-T.); Department of Histopathology, Addenbrookes Hospital, Cambridge, UK (M.J.-L.); Virus, Lifestyle and Genes, Danish Cancer Society Research Center, Copenhagen, Denmark (A.J., S.K.K.); School of Women’s and Children’s Health, University of New South Wales, Sydney, Australia (M.P.I., S.J.R.); Tissue Bank of the National Center for Tumor Diseases (NCT) Heidelberg (E. Herpel), and Institute of Pathology, University of Heidelberg (E. Herpel), Heidelberg, Germany; Cancer Center, University of Hawaii, Honolulu, HI (B.Y.H.); the Crown Princess Mary Cancer Centre, Westmead Hospital, the University of Sydney, Sydney, Australia (P.R.H., B. Gao); Oncology Department, Hospital General de Albacete, Madrid, Spain (J.M.G.-B.); Department of Cancer Genomics and Genetics, Peter MacCallum Cancer Centre, East Melbourne, Victoria, Australia (S.F.); Pathology Department, Fundación Jiménez Díaz, Madrid, Spain (A.C.); Department of Health Research and Policy and Department of Biomedical Data Science, Stanford University School of Medicine, Stanford, CA (A.S.W.); Department of Obstetrics and Gynecology, Division of Gynecologic Oncology, Royal Alexandra Hospital, Edmonton, Alberta, Canada (H.S.); Tübingen University Hospital, Institute of Pathology, Tübingen, Germany (A.S.); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (K.B.M.); Gynaecological Cancer Research Centre, Department of Women’s Cancer, Institute for Women’s Health, University College London, London, UK (U.M.); Alberta Health Services-Cancer Care, Calgary, Alberta, Canada (J.M.K.); Department of Gynecology, Rigshospitalet, University of Copenhagen, Copenhagen, Denmark (S.K.K.); Department of Public Health Sciences, Medical University of South Carolina and Hollings Cancer Center, Charleston, SC (L.E.K.); Department of Pathology and Laboratory Medicine, University of British Columbia (D.G.H.), and Centre for Translational and Applied Genomics, British Columbia Cancer Agency (D.G.H.), Vancouver, British Columbia, Canada; Molecular Unit, Department of Pathology, Herlev Hospital, University of Copenhagen, Copenhagen, Denmark (E. Høgdall); Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, CA (M.T.G.); Genetic Pathology Evaluation Centre, Vancouver General Hospital and University of British Columbia, Vancouver, British Columbia, Canada (B. Gilks); Human Genetics Group, Spanish National Cancer Research Centre (Centro Nacional de Investigaciones Oncológicas) (M.J.G., J.B.), and Centre for Biomedical Network Research on Rare Diseases (Centro de Investigación Biomédica en Red de Enfermedades Raras), Instituto de Salud Carlos III (M.J.G., J.B.), Madrid, Spain; David Geffen School of Medicine, Department of Medicine, Division of Hematology and Oncology, University of California at Los Angeles (P.A.F.); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Erlangen, Germany (P.A.F.); Department of Histopathology, Queen’s Medical Centre, Nottingham University Hospitals NHS Trust, Nottingham, UK (S.D.); University Cancer Center Hamburg, University Medical Center Hamburg-Eppendorf, Hamburg, Germany (J.C.-C.); Cancer Genetics Laboratory, Research Division, Peter MacCallum Cancer Centre (I.G.C.), Sir Peter MacCallum Department of Oncology, University of Melbourne (I.G.C.), and Department of Pathology, University of Melbourne (I.G.C.), Melbourne, Victoria, Australia; Department of Oncology, University of Cambridge, Hutchison/MRC Research Centre (J.D.B.), Cancer Research UK Cambridge Institute, University of Cambridge, Li Ka Shing Centre (J.D.B.), and Cambridge Experimental Cancer Medicine Centre (J.D.B.), Cambridge, UK; Cancer Genomics Program, Research Department, Peter MacCallum Cancer Centre, East Melbourne, Victoria, Australia (D.D.B.); the Garvan Institute, Sydney, New South Wales, Australia (D.D.B., S.J.R.); and Department of Public Health and Primary Care, University of Cambridge, Strangeways Research Laboratory, Cambridge, UK (P.D.P.P.)
1,
Francisco J Candido dos Reis
Francisco J Candido dos Reis, MD, PhD
1Department of Medical Oncology (M.S.B., K.R.K.) and Department of Health Sciences Research (R.A.V., S.J.W., B.M.M., Z.L., E.L.G.), Mayo Clinic, Rochester, MN; Department of Pathology and Laboratory Medicine, University of Calgary, Calgary, Alberta, Canada (P.F.R., M.K.); Pathology Department, Catholic University of Health and Allied Sciences-Bugando, Mwanza, Tanzania (P.F.R.); Tübingen University Hospital, Department of Women’s Health, Tübingen, Germany (P.W., F.A.T., A.D.H., S.Y.B., S.K.); Sir Peter MacCallum Department of Oncology, the University of Melbourne, Parkville, Victoria, Australia (N.T., D.D.B.); International Hereditary Cancer Center, Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (A.T., O.O., J. Lubiński, J.G.); Department of Gynecology and Obstetrics, Ribeirão Preto Medical School, University of São Paulo, Ribeirão Preto, Brazil (D.G.T., C.B.S., J.M.A., F.J.C.R.); Department of Pathology, Institute of Pathology, Heidelberg University Hospital, Heidelberg, Germany (P.S.); Department of Population Health Science and Policy, Department of Genetics and Genomic Sciences, Icahn School of Medicine at Mount Sinai, New York, NY (W.S., J.H.R.); Pathology West ICPMR Westmead, Westmead Hospital, the University of Sydney, Sydney, Australia (R.S.); University of Western Sydney at Westmead Hospital, Westmead, New South Wales, Australia (R.S.); Medical Oncology Service, Hospital Sant Pau, Barcelona, Spain (T.R.C.); H12O-CNIO Lung Cancer Clinical Research Unit (L.P.-A.), and Oncology Department, Hospital Universitario 12 de Octubre (L.P.-A.), Madrid, Spain; Women’s Cancer Program, Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, CA (S.O., J. Lester, B.Y.K.); the University of Texas School of Public Health, Houston (R.B.N.); Department of Oncology, Division of Gynecologic Oncology, Cumming School of Medicine, University of Calgary, Calgary, Alberta, Canada (G.N., P.G.); Department of Obstetrics, Gynecology and Reproductive Sciences, University of Pittsburgh School of Medicine (F.M., R.P.E.), Department of Epidemiology, University of Pittsburgh Graduate School of Public Health (F.M.), and Womens Cancer Research Program, Magee-Womens Research Institute and University of Pittsburgh Cancer Institute (F.M.), Pittsburgh, PA; Department of Gynecological Surgery and Gynecological Oncology of Adults and Adolescents, Pomeranian Medical University, Szczecin, Poland (J.M., A.C.-G.); Department of Health Research and Policy, Stanford University School of Medicine, Stanford, CA (V.M.); Department of Oncology, University of Cambridge, Strangeways Research Laboratory, Cambridge, UK (M.M., J.A., P.D.P.P.); Department of Pathology, Stanford University, Stanford, CA (T.A.L.); Department of Gynecologic Oncology, the Third Affiliated Hospital of Kunming Medical University (Yunnan Tumor Hospital), Kunming, China (Z.L.); Centre for Cancer Research, the Westmead Institute for Medical Research, the University of Sydney (C.J.K., J.H., P.R.H., B. Gao, A.deF.), and Department of Gynaecological Oncology, Westmead Hospital (C.J.K., J.H., A.deF.), Sydney, New South Wales, Australia; Division of Cancer Epidemiology, German Cancer Research Center (Deutsches Krebsforschungszentrum), Heidelberg, Germany (A.Y.J., U.E., J.C.-C.); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia (S.E.J., G.C.-T.); Department of Histopathology, Addenbrookes Hospital, Cambridge, UK (M.J.-L.); Virus, Lifestyle and Genes, Danish Cancer Society Research Center, Copenhagen, Denmark (A.J., S.K.K.); School of Women’s and Children’s Health, University of New South Wales, Sydney, Australia (M.P.I., S.J.R.); Tissue Bank of the National Center for Tumor Diseases (NCT) Heidelberg (E. Herpel), and Institute of Pathology, University of Heidelberg (E. Herpel), Heidelberg, Germany; Cancer Center, University of Hawaii, Honolulu, HI (B.Y.H.); the Crown Princess Mary Cancer Centre, Westmead Hospital, the University of Sydney, Sydney, Australia (P.R.H., B. Gao); Oncology Department, Hospital General de Albacete, Madrid, Spain (J.M.G.-B.); Department of Cancer Genomics and Genetics, Peter MacCallum Cancer Centre, East Melbourne, Victoria, Australia (S.F.); Pathology Department, Fundación Jiménez Díaz, Madrid, Spain (A.C.); Department of Health Research and Policy and Department of Biomedical Data Science, Stanford University School of Medicine, Stanford, CA (A.S.W.); Department of Obstetrics and Gynecology, Division of Gynecologic Oncology, Royal Alexandra Hospital, Edmonton, Alberta, Canada (H.S.); Tübingen University Hospital, Institute of Pathology, Tübingen, Germany (A.S.); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (K.B.M.); Gynaecological Cancer Research Centre, Department of Women’s Cancer, Institute for Women’s Health, University College London, London, UK (U.M.); Alberta Health Services-Cancer Care, Calgary, Alberta, Canada (J.M.K.); Department of Gynecology, Rigshospitalet, University of Copenhagen, Copenhagen, Denmark (S.K.K.); Department of Public Health Sciences, Medical University of South Carolina and Hollings Cancer Center, Charleston, SC (L.E.K.); Department of Pathology and Laboratory Medicine, University of British Columbia (D.G.H.), and Centre for Translational and Applied Genomics, British Columbia Cancer Agency (D.G.H.), Vancouver, British Columbia, Canada; Molecular Unit, Department of Pathology, Herlev Hospital, University of Copenhagen, Copenhagen, Denmark (E. Høgdall); Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, CA (M.T.G.); Genetic Pathology Evaluation Centre, Vancouver General Hospital and University of British Columbia, Vancouver, British Columbia, Canada (B. Gilks); Human Genetics Group, Spanish National Cancer Research Centre (Centro Nacional de Investigaciones Oncológicas) (M.J.G., J.B.), and Centre for Biomedical Network Research on Rare Diseases (Centro de Investigación Biomédica en Red de Enfermedades Raras), Instituto de Salud Carlos III (M.J.G., J.B.), Madrid, Spain; David Geffen School of Medicine, Department of Medicine, Division of Hematology and Oncology, University of California at Los Angeles (P.A.F.); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Erlangen, Germany (P.A.F.); Department of Histopathology, Queen’s Medical Centre, Nottingham University Hospitals NHS Trust, Nottingham, UK (S.D.); University Cancer Center Hamburg, University Medical Center Hamburg-Eppendorf, Hamburg, Germany (J.C.-C.); Cancer Genetics Laboratory, Research Division, Peter MacCallum Cancer Centre (I.G.C.), Sir Peter MacCallum Department of Oncology, University of Melbourne (I.G.C.), and Department of Pathology, University of Melbourne (I.G.C.), Melbourne, Victoria, Australia; Department of Oncology, University of Cambridge, Hutchison/MRC Research Centre (J.D.B.), Cancer Research UK Cambridge Institute, University of Cambridge, Li Ka Shing Centre (J.D.B.), and Cambridge Experimental Cancer Medicine Centre (J.D.B.), Cambridge, UK; Cancer Genomics Program, Research Department, Peter MacCallum Cancer Centre, East Melbourne, Victoria, Australia (D.D.B.); the Garvan Institute, Sydney, New South Wales, Australia (D.D.B., S.J.R.); and Department of Public Health and Primary Care, University of Cambridge, Strangeways Research Laboratory, Cambridge, UK (P.D.P.P.)
1,
Ian G Campbell
Ian G Campbell, PhD
1Department of Medical Oncology (M.S.B., K.R.K.) and Department of Health Sciences Research (R.A.V., S.J.W., B.M.M., Z.L., E.L.G.), Mayo Clinic, Rochester, MN; Department of Pathology and Laboratory Medicine, University of Calgary, Calgary, Alberta, Canada (P.F.R., M.K.); Pathology Department, Catholic University of Health and Allied Sciences-Bugando, Mwanza, Tanzania (P.F.R.); Tübingen University Hospital, Department of Women’s Health, Tübingen, Germany (P.W., F.A.T., A.D.H., S.Y.B., S.K.); Sir Peter MacCallum Department of Oncology, the University of Melbourne, Parkville, Victoria, Australia (N.T., D.D.B.); International Hereditary Cancer Center, Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (A.T., O.O., J. Lubiński, J.G.); Department of Gynecology and Obstetrics, Ribeirão Preto Medical School, University of São Paulo, Ribeirão Preto, Brazil (D.G.T., C.B.S., J.M.A., F.J.C.R.); Department of Pathology, Institute of Pathology, Heidelberg University Hospital, Heidelberg, Germany (P.S.); Department of Population Health Science and Policy, Department of Genetics and Genomic Sciences, Icahn School of Medicine at Mount Sinai, New York, NY (W.S., J.H.R.); Pathology West ICPMR Westmead, Westmead Hospital, the University of Sydney, Sydney, Australia (R.S.); University of Western Sydney at Westmead Hospital, Westmead, New South Wales, Australia (R.S.); Medical Oncology Service, Hospital Sant Pau, Barcelona, Spain (T.R.C.); H12O-CNIO Lung Cancer Clinical Research Unit (L.P.-A.), and Oncology Department, Hospital Universitario 12 de Octubre (L.P.-A.), Madrid, Spain; Women’s Cancer Program, Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, CA (S.O., J. Lester, B.Y.K.); the University of Texas School of Public Health, Houston (R.B.N.); Department of Oncology, Division of Gynecologic Oncology, Cumming School of Medicine, University of Calgary, Calgary, Alberta, Canada (G.N., P.G.); Department of Obstetrics, Gynecology and Reproductive Sciences, University of Pittsburgh School of Medicine (F.M., R.P.E.), Department of Epidemiology, University of Pittsburgh Graduate School of Public Health (F.M.), and Womens Cancer Research Program, Magee-Womens Research Institute and University of Pittsburgh Cancer Institute (F.M.), Pittsburgh, PA; Department of Gynecological Surgery and Gynecological Oncology of Adults and Adolescents, Pomeranian Medical University, Szczecin, Poland (J.M., A.C.-G.); Department of Health Research and Policy, Stanford University School of Medicine, Stanford, CA (V.M.); Department of Oncology, University of Cambridge, Strangeways Research Laboratory, Cambridge, UK (M.M., J.A., P.D.P.P.); Department of Pathology, Stanford University, Stanford, CA (T.A.L.); Department of Gynecologic Oncology, the Third Affiliated Hospital of Kunming Medical University (Yunnan Tumor Hospital), Kunming, China (Z.L.); Centre for Cancer Research, the Westmead Institute for Medical Research, the University of Sydney (C.J.K., J.H., P.R.H., B. Gao, A.deF.), and Department of Gynaecological Oncology, Westmead Hospital (C.J.K., J.H., A.deF.), Sydney, New South Wales, Australia; Division of Cancer Epidemiology, German Cancer Research Center (Deutsches Krebsforschungszentrum), Heidelberg, Germany (A.Y.J., U.E., J.C.-C.); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia (S.E.J., G.C.-T.); Department of Histopathology, Addenbrookes Hospital, Cambridge, UK (M.J.-L.); Virus, Lifestyle and Genes, Danish Cancer Society Research Center, Copenhagen, Denmark (A.J., S.K.K.); School of Women’s and Children’s Health, University of New South Wales, Sydney, Australia (M.P.I., S.J.R.); Tissue Bank of the National Center for Tumor Diseases (NCT) Heidelberg (E. Herpel), and Institute of Pathology, University of Heidelberg (E. Herpel), Heidelberg, Germany; Cancer Center, University of Hawaii, Honolulu, HI (B.Y.H.); the Crown Princess Mary Cancer Centre, Westmead Hospital, the University of Sydney, Sydney, Australia (P.R.H., B. Gao); Oncology Department, Hospital General de Albacete, Madrid, Spain (J.M.G.-B.); Department of Cancer Genomics and Genetics, Peter MacCallum Cancer Centre, East Melbourne, Victoria, Australia (S.F.); Pathology Department, Fundación Jiménez Díaz, Madrid, Spain (A.C.); Department of Health Research and Policy and Department of Biomedical Data Science, Stanford University School of Medicine, Stanford, CA (A.S.W.); Department of Obstetrics and Gynecology, Division of Gynecologic Oncology, Royal Alexandra Hospital, Edmonton, Alberta, Canada (H.S.); Tübingen University Hospital, Institute of Pathology, Tübingen, Germany (A.S.); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (K.B.M.); Gynaecological Cancer Research Centre, Department of Women’s Cancer, Institute for Women’s Health, University College London, London, UK (U.M.); Alberta Health Services-Cancer Care, Calgary, Alberta, Canada (J.M.K.); Department of Gynecology, Rigshospitalet, University of Copenhagen, Copenhagen, Denmark (S.K.K.); Department of Public Health Sciences, Medical University of South Carolina and Hollings Cancer Center, Charleston, SC (L.E.K.); Department of Pathology and Laboratory Medicine, University of British Columbia (D.G.H.), and Centre for Translational and Applied Genomics, British Columbia Cancer Agency (D.G.H.), Vancouver, British Columbia, Canada; Molecular Unit, Department of Pathology, Herlev Hospital, University of Copenhagen, Copenhagen, Denmark (E. Høgdall); Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, CA (M.T.G.); Genetic Pathology Evaluation Centre, Vancouver General Hospital and University of British Columbia, Vancouver, British Columbia, Canada (B. Gilks); Human Genetics Group, Spanish National Cancer Research Centre (Centro Nacional de Investigaciones Oncológicas) (M.J.G., J.B.), and Centre for Biomedical Network Research on Rare Diseases (Centro de Investigación Biomédica en Red de Enfermedades Raras), Instituto de Salud Carlos III (M.J.G., J.B.), Madrid, Spain; David Geffen School of Medicine, Department of Medicine, Division of Hematology and Oncology, University of California at Los Angeles (P.A.F.); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Erlangen, Germany (P.A.F.); Department of Histopathology, Queen’s Medical Centre, Nottingham University Hospitals NHS Trust, Nottingham, UK (S.D.); University Cancer Center Hamburg, University Medical Center Hamburg-Eppendorf, Hamburg, Germany (J.C.-C.); Cancer Genetics Laboratory, Research Division, Peter MacCallum Cancer Centre (I.G.C.), Sir Peter MacCallum Department of Oncology, University of Melbourne (I.G.C.), and Department of Pathology, University of Melbourne (I.G.C.), Melbourne, Victoria, Australia; Department of Oncology, University of Cambridge, Hutchison/MRC Research Centre (J.D.B.), Cancer Research UK Cambridge Institute, University of Cambridge, Li Ka Shing Centre (J.D.B.), and Cambridge Experimental Cancer Medicine Centre (J.D.B.), Cambridge, UK; Cancer Genomics Program, Research Department, Peter MacCallum Cancer Centre, East Melbourne, Victoria, Australia (D.D.B.); the Garvan Institute, Sydney, New South Wales, Australia (D.D.B., S.J.R.); and Department of Public Health and Primary Care, University of Cambridge, Strangeways Research Laboratory, Cambridge, UK (P.D.P.P.)
1,
James D Brenton
James D Brenton, MD, PhD
1Department of Medical Oncology (M.S.B., K.R.K.) and Department of Health Sciences Research (R.A.V., S.J.W., B.M.M., Z.L., E.L.G.), Mayo Clinic, Rochester, MN; Department of Pathology and Laboratory Medicine, University of Calgary, Calgary, Alberta, Canada (P.F.R., M.K.); Pathology Department, Catholic University of Health and Allied Sciences-Bugando, Mwanza, Tanzania (P.F.R.); Tübingen University Hospital, Department of Women’s Health, Tübingen, Germany (P.W., F.A.T., A.D.H., S.Y.B., S.K.); Sir Peter MacCallum Department of Oncology, the University of Melbourne, Parkville, Victoria, Australia (N.T., D.D.B.); International Hereditary Cancer Center, Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (A.T., O.O., J. Lubiński, J.G.); Department of Gynecology and Obstetrics, Ribeirão Preto Medical School, University of São Paulo, Ribeirão Preto, Brazil (D.G.T., C.B.S., J.M.A., F.J.C.R.); Department of Pathology, Institute of Pathology, Heidelberg University Hospital, Heidelberg, Germany (P.S.); Department of Population Health Science and Policy, Department of Genetics and Genomic Sciences, Icahn School of Medicine at Mount Sinai, New York, NY (W.S., J.H.R.); Pathology West ICPMR Westmead, Westmead Hospital, the University of Sydney, Sydney, Australia (R.S.); University of Western Sydney at Westmead Hospital, Westmead, New South Wales, Australia (R.S.); Medical Oncology Service, Hospital Sant Pau, Barcelona, Spain (T.R.C.); H12O-CNIO Lung Cancer Clinical Research Unit (L.P.-A.), and Oncology Department, Hospital Universitario 12 de Octubre (L.P.-A.), Madrid, Spain; Women’s Cancer Program, Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, CA (S.O., J. Lester, B.Y.K.); the University of Texas School of Public Health, Houston (R.B.N.); Department of Oncology, Division of Gynecologic Oncology, Cumming School of Medicine, University of Calgary, Calgary, Alberta, Canada (G.N., P.G.); Department of Obstetrics, Gynecology and Reproductive Sciences, University of Pittsburgh School of Medicine (F.M., R.P.E.), Department of Epidemiology, University of Pittsburgh Graduate School of Public Health (F.M.), and Womens Cancer Research Program, Magee-Womens Research Institute and University of Pittsburgh Cancer Institute (F.M.), Pittsburgh, PA; Department of Gynecological Surgery and Gynecological Oncology of Adults and Adolescents, Pomeranian Medical University, Szczecin, Poland (J.M., A.C.-G.); Department of Health Research and Policy, Stanford University School of Medicine, Stanford, CA (V.M.); Department of Oncology, University of Cambridge, Strangeways Research Laboratory, Cambridge, UK (M.M., J.A., P.D.P.P.); Department of Pathology, Stanford University, Stanford, CA (T.A.L.); Department of Gynecologic Oncology, the Third Affiliated Hospital of Kunming Medical University (Yunnan Tumor Hospital), Kunming, China (Z.L.); Centre for Cancer Research, the Westmead Institute for Medical Research, the University of Sydney (C.J.K., J.H., P.R.H., B. Gao, A.deF.), and Department of Gynaecological Oncology, Westmead Hospital (C.J.K., J.H., A.deF.), Sydney, New South Wales, Australia; Division of Cancer Epidemiology, German Cancer Research Center (Deutsches Krebsforschungszentrum), Heidelberg, Germany (A.Y.J., U.E., J.C.-C.); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia (S.E.J., G.C.-T.); Department of Histopathology, Addenbrookes Hospital, Cambridge, UK (M.J.-L.); Virus, Lifestyle and Genes, Danish Cancer Society Research Center, Copenhagen, Denmark (A.J., S.K.K.); School of Women’s and Children’s Health, University of New South Wales, Sydney, Australia (M.P.I., S.J.R.); Tissue Bank of the National Center for Tumor Diseases (NCT) Heidelberg (E. Herpel), and Institute of Pathology, University of Heidelberg (E. Herpel), Heidelberg, Germany; Cancer Center, University of Hawaii, Honolulu, HI (B.Y.H.); the Crown Princess Mary Cancer Centre, Westmead Hospital, the University of Sydney, Sydney, Australia (P.R.H., B. Gao); Oncology Department, Hospital General de Albacete, Madrid, Spain (J.M.G.-B.); Department of Cancer Genomics and Genetics, Peter MacCallum Cancer Centre, East Melbourne, Victoria, Australia (S.F.); Pathology Department, Fundación Jiménez Díaz, Madrid, Spain (A.C.); Department of Health Research and Policy and Department of Biomedical Data Science, Stanford University School of Medicine, Stanford, CA (A.S.W.); Department of Obstetrics and Gynecology, Division of Gynecologic Oncology, Royal Alexandra Hospital, Edmonton, Alberta, Canada (H.S.); Tübingen University Hospital, Institute of Pathology, Tübingen, Germany (A.S.); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (K.B.M.); Gynaecological Cancer Research Centre, Department of Women’s Cancer, Institute for Women’s Health, University College London, London, UK (U.M.); Alberta Health Services-Cancer Care, Calgary, Alberta, Canada (J.M.K.); Department of Gynecology, Rigshospitalet, University of Copenhagen, Copenhagen, Denmark (S.K.K.); Department of Public Health Sciences, Medical University of South Carolina and Hollings Cancer Center, Charleston, SC (L.E.K.); Department of Pathology and Laboratory Medicine, University of British Columbia (D.G.H.), and Centre for Translational and Applied Genomics, British Columbia Cancer Agency (D.G.H.), Vancouver, British Columbia, Canada; Molecular Unit, Department of Pathology, Herlev Hospital, University of Copenhagen, Copenhagen, Denmark (E. Høgdall); Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, CA (M.T.G.); Genetic Pathology Evaluation Centre, Vancouver General Hospital and University of British Columbia, Vancouver, British Columbia, Canada (B. Gilks); Human Genetics Group, Spanish National Cancer Research Centre (Centro Nacional de Investigaciones Oncológicas) (M.J.G., J.B.), and Centre for Biomedical Network Research on Rare Diseases (Centro de Investigación Biomédica en Red de Enfermedades Raras), Instituto de Salud Carlos III (M.J.G., J.B.), Madrid, Spain; David Geffen School of Medicine, Department of Medicine, Division of Hematology and Oncology, University of California at Los Angeles (P.A.F.); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Erlangen, Germany (P.A.F.); Department of Histopathology, Queen’s Medical Centre, Nottingham University Hospitals NHS Trust, Nottingham, UK (S.D.); University Cancer Center Hamburg, University Medical Center Hamburg-Eppendorf, Hamburg, Germany (J.C.-C.); Cancer Genetics Laboratory, Research Division, Peter MacCallum Cancer Centre (I.G.C.), Sir Peter MacCallum Department of Oncology, University of Melbourne (I.G.C.), and Department of Pathology, University of Melbourne (I.G.C.), Melbourne, Victoria, Australia; Department of Oncology, University of Cambridge, Hutchison/MRC Research Centre (J.D.B.), Cancer Research UK Cambridge Institute, University of Cambridge, Li Ka Shing Centre (J.D.B.), and Cambridge Experimental Cancer Medicine Centre (J.D.B.), Cambridge, UK; Cancer Genomics Program, Research Department, Peter MacCallum Cancer Centre, East Melbourne, Victoria, Australia (D.D.B.); the Garvan Institute, Sydney, New South Wales, Australia (D.D.B., S.J.R.); and Department of Public Health and Primary Care, University of Cambridge, Strangeways Research Laboratory, Cambridge, UK (P.D.P.P.)
1,
David D Bowtell
David D Bowtell, PhD
1Department of Medical Oncology (M.S.B., K.R.K.) and Department of Health Sciences Research (R.A.V., S.J.W., B.M.M., Z.L., E.L.G.), Mayo Clinic, Rochester, MN; Department of Pathology and Laboratory Medicine, University of Calgary, Calgary, Alberta, Canada (P.F.R., M.K.); Pathology Department, Catholic University of Health and Allied Sciences-Bugando, Mwanza, Tanzania (P.F.R.); Tübingen University Hospital, Department of Women’s Health, Tübingen, Germany (P.W., F.A.T., A.D.H., S.Y.B., S.K.); Sir Peter MacCallum Department of Oncology, the University of Melbourne, Parkville, Victoria, Australia (N.T., D.D.B.); International Hereditary Cancer Center, Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (A.T., O.O., J. Lubiński, J.G.); Department of Gynecology and Obstetrics, Ribeirão Preto Medical School, University of São Paulo, Ribeirão Preto, Brazil (D.G.T., C.B.S., J.M.A., F.J.C.R.); Department of Pathology, Institute of Pathology, Heidelberg University Hospital, Heidelberg, Germany (P.S.); Department of Population Health Science and Policy, Department of Genetics and Genomic Sciences, Icahn School of Medicine at Mount Sinai, New York, NY (W.S., J.H.R.); Pathology West ICPMR Westmead, Westmead Hospital, the University of Sydney, Sydney, Australia (R.S.); University of Western Sydney at Westmead Hospital, Westmead, New South Wales, Australia (R.S.); Medical Oncology Service, Hospital Sant Pau, Barcelona, Spain (T.R.C.); H12O-CNIO Lung Cancer Clinical Research Unit (L.P.-A.), and Oncology Department, Hospital Universitario 12 de Octubre (L.P.-A.), Madrid, Spain; Women’s Cancer Program, Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, CA (S.O., J. Lester, B.Y.K.); the University of Texas School of Public Health, Houston (R.B.N.); Department of Oncology, Division of Gynecologic Oncology, Cumming School of Medicine, University of Calgary, Calgary, Alberta, Canada (G.N., P.G.); Department of Obstetrics, Gynecology and Reproductive Sciences, University of Pittsburgh School of Medicine (F.M., R.P.E.), Department of Epidemiology, University of Pittsburgh Graduate School of Public Health (F.M.), and Womens Cancer Research Program, Magee-Womens Research Institute and University of Pittsburgh Cancer Institute (F.M.), Pittsburgh, PA; Department of Gynecological Surgery and Gynecological Oncology of Adults and Adolescents, Pomeranian Medical University, Szczecin, Poland (J.M., A.C.-G.); Department of Health Research and Policy, Stanford University School of Medicine, Stanford, CA (V.M.); Department of Oncology, University of Cambridge, Strangeways Research Laboratory, Cambridge, UK (M.M., J.A., P.D.P.P.); Department of Pathology, Stanford University, Stanford, CA (T.A.L.); Department of Gynecologic Oncology, the Third Affiliated Hospital of Kunming Medical University (Yunnan Tumor Hospital), Kunming, China (Z.L.); Centre for Cancer Research, the Westmead Institute for Medical Research, the University of Sydney (C.J.K., J.H., P.R.H., B. Gao, A.deF.), and Department of Gynaecological Oncology, Westmead Hospital (C.J.K., J.H., A.deF.), Sydney, New South Wales, Australia; Division of Cancer Epidemiology, German Cancer Research Center (Deutsches Krebsforschungszentrum), Heidelberg, Germany (A.Y.J., U.E., J.C.-C.); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia (S.E.J., G.C.-T.); Department of Histopathology, Addenbrookes Hospital, Cambridge, UK (M.J.-L.); Virus, Lifestyle and Genes, Danish Cancer Society Research Center, Copenhagen, Denmark (A.J., S.K.K.); School of Women’s and Children’s Health, University of New South Wales, Sydney, Australia (M.P.I., S.J.R.); Tissue Bank of the National Center for Tumor Diseases (NCT) Heidelberg (E. Herpel), and Institute of Pathology, University of Heidelberg (E. Herpel), Heidelberg, Germany; Cancer Center, University of Hawaii, Honolulu, HI (B.Y.H.); the Crown Princess Mary Cancer Centre, Westmead Hospital, the University of Sydney, Sydney, Australia (P.R.H., B. Gao); Oncology Department, Hospital General de Albacete, Madrid, Spain (J.M.G.-B.); Department of Cancer Genomics and Genetics, Peter MacCallum Cancer Centre, East Melbourne, Victoria, Australia (S.F.); Pathology Department, Fundación Jiménez Díaz, Madrid, Spain (A.C.); Department of Health Research and Policy and Department of Biomedical Data Science, Stanford University School of Medicine, Stanford, CA (A.S.W.); Department of Obstetrics and Gynecology, Division of Gynecologic Oncology, Royal Alexandra Hospital, Edmonton, Alberta, Canada (H.S.); Tübingen University Hospital, Institute of Pathology, Tübingen, Germany (A.S.); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (K.B.M.); Gynaecological Cancer Research Centre, Department of Women’s Cancer, Institute for Women’s Health, University College London, London, UK (U.M.); Alberta Health Services-Cancer Care, Calgary, Alberta, Canada (J.M.K.); Department of Gynecology, Rigshospitalet, University of Copenhagen, Copenhagen, Denmark (S.K.K.); Department of Public Health Sciences, Medical University of South Carolina and Hollings Cancer Center, Charleston, SC (L.E.K.); Department of Pathology and Laboratory Medicine, University of British Columbia (D.G.H.), and Centre for Translational and Applied Genomics, British Columbia Cancer Agency (D.G.H.), Vancouver, British Columbia, Canada; Molecular Unit, Department of Pathology, Herlev Hospital, University of Copenhagen, Copenhagen, Denmark (E. Høgdall); Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, CA (M.T.G.); Genetic Pathology Evaluation Centre, Vancouver General Hospital and University of British Columbia, Vancouver, British Columbia, Canada (B. Gilks); Human Genetics Group, Spanish National Cancer Research Centre (Centro Nacional de Investigaciones Oncológicas) (M.J.G., J.B.), and Centre for Biomedical Network Research on Rare Diseases (Centro de Investigación Biomédica en Red de Enfermedades Raras), Instituto de Salud Carlos III (M.J.G., J.B.), Madrid, Spain; David Geffen School of Medicine, Department of Medicine, Division of Hematology and Oncology, University of California at Los Angeles (P.A.F.); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Erlangen, Germany (P.A.F.); Department of Histopathology, Queen’s Medical Centre, Nottingham University Hospitals NHS Trust, Nottingham, UK (S.D.); University Cancer Center Hamburg, University Medical Center Hamburg-Eppendorf, Hamburg, Germany (J.C.-C.); Cancer Genetics Laboratory, Research Division, Peter MacCallum Cancer Centre (I.G.C.), Sir Peter MacCallum Department of Oncology, University of Melbourne (I.G.C.), and Department of Pathology, University of Melbourne (I.G.C.), Melbourne, Victoria, Australia; Department of Oncology, University of Cambridge, Hutchison/MRC Research Centre (J.D.B.), Cancer Research UK Cambridge Institute, University of Cambridge, Li Ka Shing Centre (J.D.B.), and Cambridge Experimental Cancer Medicine Centre (J.D.B.), Cambridge, UK; Cancer Genomics Program, Research Department, Peter MacCallum Cancer Centre, East Melbourne, Victoria, Australia (D.D.B.); the Garvan Institute, Sydney, New South Wales, Australia (D.D.B., S.J.R.); and Department of Public Health and Primary Care, University of Cambridge, Strangeways Research Laboratory, Cambridge, UK (P.D.P.P.)
1,
Javier Benítez
Javier Benítez, PhD
1Department of Medical Oncology (M.S.B., K.R.K.) and Department of Health Sciences Research (R.A.V., S.J.W., B.M.M., Z.L., E.L.G.), Mayo Clinic, Rochester, MN; Department of Pathology and Laboratory Medicine, University of Calgary, Calgary, Alberta, Canada (P.F.R., M.K.); Pathology Department, Catholic University of Health and Allied Sciences-Bugando, Mwanza, Tanzania (P.F.R.); Tübingen University Hospital, Department of Women’s Health, Tübingen, Germany (P.W., F.A.T., A.D.H., S.Y.B., S.K.); Sir Peter MacCallum Department of Oncology, the University of Melbourne, Parkville, Victoria, Australia (N.T., D.D.B.); International Hereditary Cancer Center, Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (A.T., O.O., J. Lubiński, J.G.); Department of Gynecology and Obstetrics, Ribeirão Preto Medical School, University of São Paulo, Ribeirão Preto, Brazil (D.G.T., C.B.S., J.M.A., F.J.C.R.); Department of Pathology, Institute of Pathology, Heidelberg University Hospital, Heidelberg, Germany (P.S.); Department of Population Health Science and Policy, Department of Genetics and Genomic Sciences, Icahn School of Medicine at Mount Sinai, New York, NY (W.S., J.H.R.); Pathology West ICPMR Westmead, Westmead Hospital, the University of Sydney, Sydney, Australia (R.S.); University of Western Sydney at Westmead Hospital, Westmead, New South Wales, Australia (R.S.); Medical Oncology Service, Hospital Sant Pau, Barcelona, Spain (T.R.C.); H12O-CNIO Lung Cancer Clinical Research Unit (L.P.-A.), and Oncology Department, Hospital Universitario 12 de Octubre (L.P.-A.), Madrid, Spain; Women’s Cancer Program, Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, CA (S.O., J. Lester, B.Y.K.); the University of Texas School of Public Health, Houston (R.B.N.); Department of Oncology, Division of Gynecologic Oncology, Cumming School of Medicine, University of Calgary, Calgary, Alberta, Canada (G.N., P.G.); Department of Obstetrics, Gynecology and Reproductive Sciences, University of Pittsburgh School of Medicine (F.M., R.P.E.), Department of Epidemiology, University of Pittsburgh Graduate School of Public Health (F.M.), and Womens Cancer Research Program, Magee-Womens Research Institute and University of Pittsburgh Cancer Institute (F.M.), Pittsburgh, PA; Department of Gynecological Surgery and Gynecological Oncology of Adults and Adolescents, Pomeranian Medical University, Szczecin, Poland (J.M., A.C.-G.); Department of Health Research and Policy, Stanford University School of Medicine, Stanford, CA (V.M.); Department of Oncology, University of Cambridge, Strangeways Research Laboratory, Cambridge, UK (M.M., J.A., P.D.P.P.); Department of Pathology, Stanford University, Stanford, CA (T.A.L.); Department of Gynecologic Oncology, the Third Affiliated Hospital of Kunming Medical University (Yunnan Tumor Hospital), Kunming, China (Z.L.); Centre for Cancer Research, the Westmead Institute for Medical Research, the University of Sydney (C.J.K., J.H., P.R.H., B. Gao, A.deF.), and Department of Gynaecological Oncology, Westmead Hospital (C.J.K., J.H., A.deF.), Sydney, New South Wales, Australia; Division of Cancer Epidemiology, German Cancer Research Center (Deutsches Krebsforschungszentrum), Heidelberg, Germany (A.Y.J., U.E., J.C.-C.); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia (S.E.J., G.C.-T.); Department of Histopathology, Addenbrookes Hospital, Cambridge, UK (M.J.-L.); Virus, Lifestyle and Genes, Danish Cancer Society Research Center, Copenhagen, Denmark (A.J., S.K.K.); School of Women’s and Children’s Health, University of New South Wales, Sydney, Australia (M.P.I., S.J.R.); Tissue Bank of the National Center for Tumor Diseases (NCT) Heidelberg (E. Herpel), and Institute of Pathology, University of Heidelberg (E. Herpel), Heidelberg, Germany; Cancer Center, University of Hawaii, Honolulu, HI (B.Y.H.); the Crown Princess Mary Cancer Centre, Westmead Hospital, the University of Sydney, Sydney, Australia (P.R.H., B. Gao); Oncology Department, Hospital General de Albacete, Madrid, Spain (J.M.G.-B.); Department of Cancer Genomics and Genetics, Peter MacCallum Cancer Centre, East Melbourne, Victoria, Australia (S.F.); Pathology Department, Fundación Jiménez Díaz, Madrid, Spain (A.C.); Department of Health Research and Policy and Department of Biomedical Data Science, Stanford University School of Medicine, Stanford, CA (A.S.W.); Department of Obstetrics and Gynecology, Division of Gynecologic Oncology, Royal Alexandra Hospital, Edmonton, Alberta, Canada (H.S.); Tübingen University Hospital, Institute of Pathology, Tübingen, Germany (A.S.); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (K.B.M.); Gynaecological Cancer Research Centre, Department of Women’s Cancer, Institute for Women’s Health, University College London, London, UK (U.M.); Alberta Health Services-Cancer Care, Calgary, Alberta, Canada (J.M.K.); Department of Gynecology, Rigshospitalet, University of Copenhagen, Copenhagen, Denmark (S.K.K.); Department of Public Health Sciences, Medical University of South Carolina and Hollings Cancer Center, Charleston, SC (L.E.K.); Department of Pathology and Laboratory Medicine, University of British Columbia (D.G.H.), and Centre for Translational and Applied Genomics, British Columbia Cancer Agency (D.G.H.), Vancouver, British Columbia, Canada; Molecular Unit, Department of Pathology, Herlev Hospital, University of Copenhagen, Copenhagen, Denmark (E. Høgdall); Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, CA (M.T.G.); Genetic Pathology Evaluation Centre, Vancouver General Hospital and University of British Columbia, Vancouver, British Columbia, Canada (B. Gilks); Human Genetics Group, Spanish National Cancer Research Centre (Centro Nacional de Investigaciones Oncológicas) (M.J.G., J.B.), and Centre for Biomedical Network Research on Rare Diseases (Centro de Investigación Biomédica en Red de Enfermedades Raras), Instituto de Salud Carlos III (M.J.G., J.B.), Madrid, Spain; David Geffen School of Medicine, Department of Medicine, Division of Hematology and Oncology, University of California at Los Angeles (P.A.F.); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Erlangen, Germany (P.A.F.); Department of Histopathology, Queen’s Medical Centre, Nottingham University Hospitals NHS Trust, Nottingham, UK (S.D.); University Cancer Center Hamburg, University Medical Center Hamburg-Eppendorf, Hamburg, Germany (J.C.-C.); Cancer Genetics Laboratory, Research Division, Peter MacCallum Cancer Centre (I.G.C.), Sir Peter MacCallum Department of Oncology, University of Melbourne (I.G.C.), and Department of Pathology, University of Melbourne (I.G.C.), Melbourne, Victoria, Australia; Department of Oncology, University of Cambridge, Hutchison/MRC Research Centre (J.D.B.), Cancer Research UK Cambridge Institute, University of Cambridge, Li Ka Shing Centre (J.D.B.), and Cambridge Experimental Cancer Medicine Centre (J.D.B.), Cambridge, UK; Cancer Genomics Program, Research Department, Peter MacCallum Cancer Centre, East Melbourne, Victoria, Australia (D.D.B.); the Garvan Institute, Sydney, New South Wales, Australia (D.D.B., S.J.R.); and Department of Public Health and Primary Care, University of Cambridge, Strangeways Research Laboratory, Cambridge, UK (P.D.P.P.)
1,
Paul DP Pharoah
Paul DP Pharoah, MD, PhD
1Department of Medical Oncology (M.S.B., K.R.K.) and Department of Health Sciences Research (R.A.V., S.J.W., B.M.M., Z.L., E.L.G.), Mayo Clinic, Rochester, MN; Department of Pathology and Laboratory Medicine, University of Calgary, Calgary, Alberta, Canada (P.F.R., M.K.); Pathology Department, Catholic University of Health and Allied Sciences-Bugando, Mwanza, Tanzania (P.F.R.); Tübingen University Hospital, Department of Women’s Health, Tübingen, Germany (P.W., F.A.T., A.D.H., S.Y.B., S.K.); Sir Peter MacCallum Department of Oncology, the University of Melbourne, Parkville, Victoria, Australia (N.T., D.D.B.); International Hereditary Cancer Center, Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (A.T., O.O., J. Lubiński, J.G.); Department of Gynecology and Obstetrics, Ribeirão Preto Medical School, University of São Paulo, Ribeirão Preto, Brazil (D.G.T., C.B.S., J.M.A., F.J.C.R.); Department of Pathology, Institute of Pathology, Heidelberg University Hospital, Heidelberg, Germany (P.S.); Department of Population Health Science and Policy, Department of Genetics and Genomic Sciences, Icahn School of Medicine at Mount Sinai, New York, NY (W.S., J.H.R.); Pathology West ICPMR Westmead, Westmead Hospital, the University of Sydney, Sydney, Australia (R.S.); University of Western Sydney at Westmead Hospital, Westmead, New South Wales, Australia (R.S.); Medical Oncology Service, Hospital Sant Pau, Barcelona, Spain (T.R.C.); H12O-CNIO Lung Cancer Clinical Research Unit (L.P.-A.), and Oncology Department, Hospital Universitario 12 de Octubre (L.P.-A.), Madrid, Spain; Women’s Cancer Program, Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, CA (S.O., J. Lester, B.Y.K.); the University of Texas School of Public Health, Houston (R.B.N.); Department of Oncology, Division of Gynecologic Oncology, Cumming School of Medicine, University of Calgary, Calgary, Alberta, Canada (G.N., P.G.); Department of Obstetrics, Gynecology and Reproductive Sciences, University of Pittsburgh School of Medicine (F.M., R.P.E.), Department of Epidemiology, University of Pittsburgh Graduate School of Public Health (F.M.), and Womens Cancer Research Program, Magee-Womens Research Institute and University of Pittsburgh Cancer Institute (F.M.), Pittsburgh, PA; Department of Gynecological Surgery and Gynecological Oncology of Adults and Adolescents, Pomeranian Medical University, Szczecin, Poland (J.M., A.C.-G.); Department of Health Research and Policy, Stanford University School of Medicine, Stanford, CA (V.M.); Department of Oncology, University of Cambridge, Strangeways Research Laboratory, Cambridge, UK (M.M., J.A., P.D.P.P.); Department of Pathology, Stanford University, Stanford, CA (T.A.L.); Department of Gynecologic Oncology, the Third Affiliated Hospital of Kunming Medical University (Yunnan Tumor Hospital), Kunming, China (Z.L.); Centre for Cancer Research, the Westmead Institute for Medical Research, the University of Sydney (C.J.K., J.H., P.R.H., B. Gao, A.deF.), and Department of Gynaecological Oncology, Westmead Hospital (C.J.K., J.H., A.deF.), Sydney, New South Wales, Australia; Division of Cancer Epidemiology, German Cancer Research Center (Deutsches Krebsforschungszentrum), Heidelberg, Germany (A.Y.J., U.E., J.C.-C.); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia (S.E.J., G.C.-T.); Department of Histopathology, Addenbrookes Hospital, Cambridge, UK (M.J.-L.); Virus, Lifestyle and Genes, Danish Cancer Society Research Center, Copenhagen, Denmark (A.J., S.K.K.); School of Women’s and Children’s Health, University of New South Wales, Sydney, Australia (M.P.I., S.J.R.); Tissue Bank of the National Center for Tumor Diseases (NCT) Heidelberg (E. Herpel), and Institute of Pathology, University of Heidelberg (E. Herpel), Heidelberg, Germany; Cancer Center, University of Hawaii, Honolulu, HI (B.Y.H.); the Crown Princess Mary Cancer Centre, Westmead Hospital, the University of Sydney, Sydney, Australia (P.R.H., B. Gao); Oncology Department, Hospital General de Albacete, Madrid, Spain (J.M.G.-B.); Department of Cancer Genomics and Genetics, Peter MacCallum Cancer Centre, East Melbourne, Victoria, Australia (S.F.); Pathology Department, Fundación Jiménez Díaz, Madrid, Spain (A.C.); Department of Health Research and Policy and Department of Biomedical Data Science, Stanford University School of Medicine, Stanford, CA (A.S.W.); Department of Obstetrics and Gynecology, Division of Gynecologic Oncology, Royal Alexandra Hospital, Edmonton, Alberta, Canada (H.S.); Tübingen University Hospital, Institute of Pathology, Tübingen, Germany (A.S.); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (K.B.M.); Gynaecological Cancer Research Centre, Department of Women’s Cancer, Institute for Women’s Health, University College London, London, UK (U.M.); Alberta Health Services-Cancer Care, Calgary, Alberta, Canada (J.M.K.); Department of Gynecology, Rigshospitalet, University of Copenhagen, Copenhagen, Denmark (S.K.K.); Department of Public Health Sciences, Medical University of South Carolina and Hollings Cancer Center, Charleston, SC (L.E.K.); Department of Pathology and Laboratory Medicine, University of British Columbia (D.G.H.), and Centre for Translational and Applied Genomics, British Columbia Cancer Agency (D.G.H.), Vancouver, British Columbia, Canada; Molecular Unit, Department of Pathology, Herlev Hospital, University of Copenhagen, Copenhagen, Denmark (E. Høgdall); Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, CA (M.T.G.); Genetic Pathology Evaluation Centre, Vancouver General Hospital and University of British Columbia, Vancouver, British Columbia, Canada (B. Gilks); Human Genetics Group, Spanish National Cancer Research Centre (Centro Nacional de Investigaciones Oncológicas) (M.J.G., J.B.), and Centre for Biomedical Network Research on Rare Diseases (Centro de Investigación Biomédica en Red de Enfermedades Raras), Instituto de Salud Carlos III (M.J.G., J.B.), Madrid, Spain; David Geffen School of Medicine, Department of Medicine, Division of Hematology and Oncology, University of California at Los Angeles (P.A.F.); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Erlangen, Germany (P.A.F.); Department of Histopathology, Queen’s Medical Centre, Nottingham University Hospitals NHS Trust, Nottingham, UK (S.D.); University Cancer Center Hamburg, University Medical Center Hamburg-Eppendorf, Hamburg, Germany (J.C.-C.); Cancer Genetics Laboratory, Research Division, Peter MacCallum Cancer Centre (I.G.C.), Sir Peter MacCallum Department of Oncology, University of Melbourne (I.G.C.), and Department of Pathology, University of Melbourne (I.G.C.), Melbourne, Victoria, Australia; Department of Oncology, University of Cambridge, Hutchison/MRC Research Centre (J.D.B.), Cancer Research UK Cambridge Institute, University of Cambridge, Li Ka Shing Centre (J.D.B.), and Cambridge Experimental Cancer Medicine Centre (J.D.B.), Cambridge, UK; Cancer Genomics Program, Research Department, Peter MacCallum Cancer Centre, East Melbourne, Victoria, Australia (D.D.B.); the Garvan Institute, Sydney, New South Wales, Australia (D.D.B., S.J.R.); and Department of Public Health and Primary Care, University of Cambridge, Strangeways Research Laboratory, Cambridge, UK (P.D.P.P.)
1,
Martin Köbel
Martin Köbel, MD
1Department of Medical Oncology (M.S.B., K.R.K.) and Department of Health Sciences Research (R.A.V., S.J.W., B.M.M., Z.L., E.L.G.), Mayo Clinic, Rochester, MN; Department of Pathology and Laboratory Medicine, University of Calgary, Calgary, Alberta, Canada (P.F.R., M.K.); Pathology Department, Catholic University of Health and Allied Sciences-Bugando, Mwanza, Tanzania (P.F.R.); Tübingen University Hospital, Department of Women’s Health, Tübingen, Germany (P.W., F.A.T., A.D.H., S.Y.B., S.K.); Sir Peter MacCallum Department of Oncology, the University of Melbourne, Parkville, Victoria, Australia (N.T., D.D.B.); International Hereditary Cancer Center, Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (A.T., O.O., J. Lubiński, J.G.); Department of Gynecology and Obstetrics, Ribeirão Preto Medical School, University of São Paulo, Ribeirão Preto, Brazil (D.G.T., C.B.S., J.M.A., F.J.C.R.); Department of Pathology, Institute of Pathology, Heidelberg University Hospital, Heidelberg, Germany (P.S.); Department of Population Health Science and Policy, Department of Genetics and Genomic Sciences, Icahn School of Medicine at Mount Sinai, New York, NY (W.S., J.H.R.); Pathology West ICPMR Westmead, Westmead Hospital, the University of Sydney, Sydney, Australia (R.S.); University of Western Sydney at Westmead Hospital, Westmead, New South Wales, Australia (R.S.); Medical Oncology Service, Hospital Sant Pau, Barcelona, Spain (T.R.C.); H12O-CNIO Lung Cancer Clinical Research Unit (L.P.-A.), and Oncology Department, Hospital Universitario 12 de Octubre (L.P.-A.), Madrid, Spain; Women’s Cancer Program, Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, CA (S.O., J. Lester, B.Y.K.); the University of Texas School of Public Health, Houston (R.B.N.); Department of Oncology, Division of Gynecologic Oncology, Cumming School of Medicine, University of Calgary, Calgary, Alberta, Canada (G.N., P.G.); Department of Obstetrics, Gynecology and Reproductive Sciences, University of Pittsburgh School of Medicine (F.M., R.P.E.), Department of Epidemiology, University of Pittsburgh Graduate School of Public Health (F.M.), and Womens Cancer Research Program, Magee-Womens Research Institute and University of Pittsburgh Cancer Institute (F.M.), Pittsburgh, PA; Department of Gynecological Surgery and Gynecological Oncology of Adults and Adolescents, Pomeranian Medical University, Szczecin, Poland (J.M., A.C.-G.); Department of Health Research and Policy, Stanford University School of Medicine, Stanford, CA (V.M.); Department of Oncology, University of Cambridge, Strangeways Research Laboratory, Cambridge, UK (M.M., J.A., P.D.P.P.); Department of Pathology, Stanford University, Stanford, CA (T.A.L.); Department of Gynecologic Oncology, the Third Affiliated Hospital of Kunming Medical University (Yunnan Tumor Hospital), Kunming, China (Z.L.); Centre for Cancer Research, the Westmead Institute for Medical Research, the University of Sydney (C.J.K., J.H., P.R.H., B. Gao, A.deF.), and Department of Gynaecological Oncology, Westmead Hospital (C.J.K., J.H., A.deF.), Sydney, New South Wales, Australia; Division of Cancer Epidemiology, German Cancer Research Center (Deutsches Krebsforschungszentrum), Heidelberg, Germany (A.Y.J., U.E., J.C.-C.); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia (S.E.J., G.C.-T.); Department of Histopathology, Addenbrookes Hospital, Cambridge, UK (M.J.-L.); Virus, Lifestyle and Genes, Danish Cancer Society Research Center, Copenhagen, Denmark (A.J., S.K.K.); School of Women’s and Children’s Health, University of New South Wales, Sydney, Australia (M.P.I., S.J.R.); Tissue Bank of the National Center for Tumor Diseases (NCT) Heidelberg (E. Herpel), and Institute of Pathology, University of Heidelberg (E. Herpel), Heidelberg, Germany; Cancer Center, University of Hawaii, Honolulu, HI (B.Y.H.); the Crown Princess Mary Cancer Centre, Westmead Hospital, the University of Sydney, Sydney, Australia (P.R.H., B. Gao); Oncology Department, Hospital General de Albacete, Madrid, Spain (J.M.G.-B.); Department of Cancer Genomics and Genetics, Peter MacCallum Cancer Centre, East Melbourne, Victoria, Australia (S.F.); Pathology Department, Fundación Jiménez Díaz, Madrid, Spain (A.C.); Department of Health Research and Policy and Department of Biomedical Data Science, Stanford University School of Medicine, Stanford, CA (A.S.W.); Department of Obstetrics and Gynecology, Division of Gynecologic Oncology, Royal Alexandra Hospital, Edmonton, Alberta, Canada (H.S.); Tübingen University Hospital, Institute of Pathology, Tübingen, Germany (A.S.); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (K.B.M.); Gynaecological Cancer Research Centre, Department of Women’s Cancer, Institute for Women’s Health, University College London, London, UK (U.M.); Alberta Health Services-Cancer Care, Calgary, Alberta, Canada (J.M.K.); Department of Gynecology, Rigshospitalet, University of Copenhagen, Copenhagen, Denmark (S.K.K.); Department of Public Health Sciences, Medical University of South Carolina and Hollings Cancer Center, Charleston, SC (L.E.K.); Department of Pathology and Laboratory Medicine, University of British Columbia (D.G.H.), and Centre for Translational and Applied Genomics, British Columbia Cancer Agency (D.G.H.), Vancouver, British Columbia, Canada; Molecular Unit, Department of Pathology, Herlev Hospital, University of Copenhagen, Copenhagen, Denmark (E. Høgdall); Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, CA (M.T.G.); Genetic Pathology Evaluation Centre, Vancouver General Hospital and University of British Columbia, Vancouver, British Columbia, Canada (B. Gilks); Human Genetics Group, Spanish National Cancer Research Centre (Centro Nacional de Investigaciones Oncológicas) (M.J.G., J.B.), and Centre for Biomedical Network Research on Rare Diseases (Centro de Investigación Biomédica en Red de Enfermedades Raras), Instituto de Salud Carlos III (M.J.G., J.B.), Madrid, Spain; David Geffen School of Medicine, Department of Medicine, Division of Hematology and Oncology, University of California at Los Angeles (P.A.F.); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Erlangen, Germany (P.A.F.); Department of Histopathology, Queen’s Medical Centre, Nottingham University Hospitals NHS Trust, Nottingham, UK (S.D.); University Cancer Center Hamburg, University Medical Center Hamburg-Eppendorf, Hamburg, Germany (J.C.-C.); Cancer Genetics Laboratory, Research Division, Peter MacCallum Cancer Centre (I.G.C.), Sir Peter MacCallum Department of Oncology, University of Melbourne (I.G.C.), and Department of Pathology, University of Melbourne (I.G.C.), Melbourne, Victoria, Australia; Department of Oncology, University of Cambridge, Hutchison/MRC Research Centre (J.D.B.), Cancer Research UK Cambridge Institute, University of Cambridge, Li Ka Shing Centre (J.D.B.), and Cambridge Experimental Cancer Medicine Centre (J.D.B.), Cambridge, UK; Cancer Genomics Program, Research Department, Peter MacCallum Cancer Centre, East Melbourne, Victoria, Australia (D.D.B.); the Garvan Institute, Sydney, New South Wales, Australia (D.D.B., S.J.R.); and Department of Public Health and Primary Care, University of Cambridge, Strangeways Research Laboratory, Cambridge, UK (P.D.P.P.)
1,
Susan J Ramus
Susan J Ramus, PhD
1Department of Medical Oncology (M.S.B., K.R.K.) and Department of Health Sciences Research (R.A.V., S.J.W., B.M.M., Z.L., E.L.G.), Mayo Clinic, Rochester, MN; Department of Pathology and Laboratory Medicine, University of Calgary, Calgary, Alberta, Canada (P.F.R., M.K.); Pathology Department, Catholic University of Health and Allied Sciences-Bugando, Mwanza, Tanzania (P.F.R.); Tübingen University Hospital, Department of Women’s Health, Tübingen, Germany (P.W., F.A.T., A.D.H., S.Y.B., S.K.); Sir Peter MacCallum Department of Oncology, the University of Melbourne, Parkville, Victoria, Australia (N.T., D.D.B.); International Hereditary Cancer Center, Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (A.T., O.O., J. Lubiński, J.G.); Department of Gynecology and Obstetrics, Ribeirão Preto Medical School, University of São Paulo, Ribeirão Preto, Brazil (D.G.T., C.B.S., J.M.A., F.J.C.R.); Department of Pathology, Institute of Pathology, Heidelberg University Hospital, Heidelberg, Germany (P.S.); Department of Population Health Science and Policy, Department of Genetics and Genomic Sciences, Icahn School of Medicine at Mount Sinai, New York, NY (W.S., J.H.R.); Pathology West ICPMR Westmead, Westmead Hospital, the University of Sydney, Sydney, Australia (R.S.); University of Western Sydney at Westmead Hospital, Westmead, New South Wales, Australia (R.S.); Medical Oncology Service, Hospital Sant Pau, Barcelona, Spain (T.R.C.); H12O-CNIO Lung Cancer Clinical Research Unit (L.P.-A.), and Oncology Department, Hospital Universitario 12 de Octubre (L.P.-A.), Madrid, Spain; Women’s Cancer Program, Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, CA (S.O., J. Lester, B.Y.K.); the University of Texas School of Public Health, Houston (R.B.N.); Department of Oncology, Division of Gynecologic Oncology, Cumming School of Medicine, University of Calgary, Calgary, Alberta, Canada (G.N., P.G.); Department of Obstetrics, Gynecology and Reproductive Sciences, University of Pittsburgh School of Medicine (F.M., R.P.E.), Department of Epidemiology, University of Pittsburgh Graduate School of Public Health (F.M.), and Womens Cancer Research Program, Magee-Womens Research Institute and University of Pittsburgh Cancer Institute (F.M.), Pittsburgh, PA; Department of Gynecological Surgery and Gynecological Oncology of Adults and Adolescents, Pomeranian Medical University, Szczecin, Poland (J.M., A.C.-G.); Department of Health Research and Policy, Stanford University School of Medicine, Stanford, CA (V.M.); Department of Oncology, University of Cambridge, Strangeways Research Laboratory, Cambridge, UK (M.M., J.A., P.D.P.P.); Department of Pathology, Stanford University, Stanford, CA (T.A.L.); Department of Gynecologic Oncology, the Third Affiliated Hospital of Kunming Medical University (Yunnan Tumor Hospital), Kunming, China (Z.L.); Centre for Cancer Research, the Westmead Institute for Medical Research, the University of Sydney (C.J.K., J.H., P.R.H., B. Gao, A.deF.), and Department of Gynaecological Oncology, Westmead Hospital (C.J.K., J.H., A.deF.), Sydney, New South Wales, Australia; Division of Cancer Epidemiology, German Cancer Research Center (Deutsches Krebsforschungszentrum), Heidelberg, Germany (A.Y.J., U.E., J.C.-C.); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia (S.E.J., G.C.-T.); Department of Histopathology, Addenbrookes Hospital, Cambridge, UK (M.J.-L.); Virus, Lifestyle and Genes, Danish Cancer Society Research Center, Copenhagen, Denmark (A.J., S.K.K.); School of Women’s and Children’s Health, University of New South Wales, Sydney, Australia (M.P.I., S.J.R.); Tissue Bank of the National Center for Tumor Diseases (NCT) Heidelberg (E. Herpel), and Institute of Pathology, University of Heidelberg (E. Herpel), Heidelberg, Germany; Cancer Center, University of Hawaii, Honolulu, HI (B.Y.H.); the Crown Princess Mary Cancer Centre, Westmead Hospital, the University of Sydney, Sydney, Australia (P.R.H., B. Gao); Oncology Department, Hospital General de Albacete, Madrid, Spain (J.M.G.-B.); Department of Cancer Genomics and Genetics, Peter MacCallum Cancer Centre, East Melbourne, Victoria, Australia (S.F.); Pathology Department, Fundación Jiménez Díaz, Madrid, Spain (A.C.); Department of Health Research and Policy and Department of Biomedical Data Science, Stanford University School of Medicine, Stanford, CA (A.S.W.); Department of Obstetrics and Gynecology, Division of Gynecologic Oncology, Royal Alexandra Hospital, Edmonton, Alberta, Canada (H.S.); Tübingen University Hospital, Institute of Pathology, Tübingen, Germany (A.S.); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (K.B.M.); Gynaecological Cancer Research Centre, Department of Women’s Cancer, Institute for Women’s Health, University College London, London, UK (U.M.); Alberta Health Services-Cancer Care, Calgary, Alberta, Canada (J.M.K.); Department of Gynecology, Rigshospitalet, University of Copenhagen, Copenhagen, Denmark (S.K.K.); Department of Public Health Sciences, Medical University of South Carolina and Hollings Cancer Center, Charleston, SC (L.E.K.); Department of Pathology and Laboratory Medicine, University of British Columbia (D.G.H.), and Centre for Translational and Applied Genomics, British Columbia Cancer Agency (D.G.H.), Vancouver, British Columbia, Canada; Molecular Unit, Department of Pathology, Herlev Hospital, University of Copenhagen, Copenhagen, Denmark (E. Høgdall); Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, CA (M.T.G.); Genetic Pathology Evaluation Centre, Vancouver General Hospital and University of British Columbia, Vancouver, British Columbia, Canada (B. Gilks); Human Genetics Group, Spanish National Cancer Research Centre (Centro Nacional de Investigaciones Oncológicas) (M.J.G., J.B.), and Centre for Biomedical Network Research on Rare Diseases (Centro de Investigación Biomédica en Red de Enfermedades Raras), Instituto de Salud Carlos III (M.J.G., J.B.), Madrid, Spain; David Geffen School of Medicine, Department of Medicine, Division of Hematology and Oncology, University of California at Los Angeles (P.A.F.); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Erlangen, Germany (P.A.F.); Department of Histopathology, Queen’s Medical Centre, Nottingham University Hospitals NHS Trust, Nottingham, UK (S.D.); University Cancer Center Hamburg, University Medical Center Hamburg-Eppendorf, Hamburg, Germany (J.C.-C.); Cancer Genetics Laboratory, Research Division, Peter MacCallum Cancer Centre (I.G.C.), Sir Peter MacCallum Department of Oncology, University of Melbourne (I.G.C.), and Department of Pathology, University of Melbourne (I.G.C.), Melbourne, Victoria, Australia; Department of Oncology, University of Cambridge, Hutchison/MRC Research Centre (J.D.B.), Cancer Research UK Cambridge Institute, University of Cambridge, Li Ka Shing Centre (J.D.B.), and Cambridge Experimental Cancer Medicine Centre (J.D.B.), Cambridge, UK; Cancer Genomics Program, Research Department, Peter MacCallum Cancer Centre, East Melbourne, Victoria, Australia (D.D.B.); the Garvan Institute, Sydney, New South Wales, Australia (D.D.B., S.J.R.); and Department of Public Health and Primary Care, University of Cambridge, Strangeways Research Laboratory, Cambridge, UK (P.D.P.P.)
1,
Ellen L Goode
Ellen L Goode, PhD, MPH
1Department of Medical Oncology (M.S.B., K.R.K.) and Department of Health Sciences Research (R.A.V., S.J.W., B.M.M., Z.L., E.L.G.), Mayo Clinic, Rochester, MN; Department of Pathology and Laboratory Medicine, University of Calgary, Calgary, Alberta, Canada (P.F.R., M.K.); Pathology Department, Catholic University of Health and Allied Sciences-Bugando, Mwanza, Tanzania (P.F.R.); Tübingen University Hospital, Department of Women’s Health, Tübingen, Germany (P.W., F.A.T., A.D.H., S.Y.B., S.K.); Sir Peter MacCallum Department of Oncology, the University of Melbourne, Parkville, Victoria, Australia (N.T., D.D.B.); International Hereditary Cancer Center, Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (A.T., O.O., J. Lubiński, J.G.); Department of Gynecology and Obstetrics, Ribeirão Preto Medical School, University of São Paulo, Ribeirão Preto, Brazil (D.G.T., C.B.S., J.M.A., F.J.C.R.); Department of Pathology, Institute of Pathology, Heidelberg University Hospital, Heidelberg, Germany (P.S.); Department of Population Health Science and Policy, Department of Genetics and Genomic Sciences, Icahn School of Medicine at Mount Sinai, New York, NY (W.S., J.H.R.); Pathology West ICPMR Westmead, Westmead Hospital, the University of Sydney, Sydney, Australia (R.S.); University of Western Sydney at Westmead Hospital, Westmead, New South Wales, Australia (R.S.); Medical Oncology Service, Hospital Sant Pau, Barcelona, Spain (T.R.C.); H12O-CNIO Lung Cancer Clinical Research Unit (L.P.-A.), and Oncology Department, Hospital Universitario 12 de Octubre (L.P.-A.), Madrid, Spain; Women’s Cancer Program, Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, CA (S.O., J. Lester, B.Y.K.); the University of Texas School of Public Health, Houston (R.B.N.); Department of Oncology, Division of Gynecologic Oncology, Cumming School of Medicine, University of Calgary, Calgary, Alberta, Canada (G.N., P.G.); Department of Obstetrics, Gynecology and Reproductive Sciences, University of Pittsburgh School of Medicine (F.M., R.P.E.), Department of Epidemiology, University of Pittsburgh Graduate School of Public Health (F.M.), and Womens Cancer Research Program, Magee-Womens Research Institute and University of Pittsburgh Cancer Institute (F.M.), Pittsburgh, PA; Department of Gynecological Surgery and Gynecological Oncology of Adults and Adolescents, Pomeranian Medical University, Szczecin, Poland (J.M., A.C.-G.); Department of Health Research and Policy, Stanford University School of Medicine, Stanford, CA (V.M.); Department of Oncology, University of Cambridge, Strangeways Research Laboratory, Cambridge, UK (M.M., J.A., P.D.P.P.); Department of Pathology, Stanford University, Stanford, CA (T.A.L.); Department of Gynecologic Oncology, the Third Affiliated Hospital of Kunming Medical University (Yunnan Tumor Hospital), Kunming, China (Z.L.); Centre for Cancer Research, the Westmead Institute for Medical Research, the University of Sydney (C.J.K., J.H., P.R.H., B. Gao, A.deF.), and Department of Gynaecological Oncology, Westmead Hospital (C.J.K., J.H., A.deF.), Sydney, New South Wales, Australia; Division of Cancer Epidemiology, German Cancer Research Center (Deutsches Krebsforschungszentrum), Heidelberg, Germany (A.Y.J., U.E., J.C.-C.); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia (S.E.J., G.C.-T.); Department of Histopathology, Addenbrookes Hospital, Cambridge, UK (M.J.-L.); Virus, Lifestyle and Genes, Danish Cancer Society Research Center, Copenhagen, Denmark (A.J., S.K.K.); School of Women’s and Children’s Health, University of New South Wales, Sydney, Australia (M.P.I., S.J.R.); Tissue Bank of the National Center for Tumor Diseases (NCT) Heidelberg (E. Herpel), and Institute of Pathology, University of Heidelberg (E. Herpel), Heidelberg, Germany; Cancer Center, University of Hawaii, Honolulu, HI (B.Y.H.); the Crown Princess Mary Cancer Centre, Westmead Hospital, the University of Sydney, Sydney, Australia (P.R.H., B. Gao); Oncology Department, Hospital General de Albacete, Madrid, Spain (J.M.G.-B.); Department of Cancer Genomics and Genetics, Peter MacCallum Cancer Centre, East Melbourne, Victoria, Australia (S.F.); Pathology Department, Fundación Jiménez Díaz, Madrid, Spain (A.C.); Department of Health Research and Policy and Department of Biomedical Data Science, Stanford University School of Medicine, Stanford, CA (A.S.W.); Department of Obstetrics and Gynecology, Division of Gynecologic Oncology, Royal Alexandra Hospital, Edmonton, Alberta, Canada (H.S.); Tübingen University Hospital, Institute of Pathology, Tübingen, Germany (A.S.); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (K.B.M.); Gynaecological Cancer Research Centre, Department of Women’s Cancer, Institute for Women’s Health, University College London, London, UK (U.M.); Alberta Health Services-Cancer Care, Calgary, Alberta, Canada (J.M.K.); Department of Gynecology, Rigshospitalet, University of Copenhagen, Copenhagen, Denmark (S.K.K.); Department of Public Health Sciences, Medical University of South Carolina and Hollings Cancer Center, Charleston, SC (L.E.K.); Department of Pathology and Laboratory Medicine, University of British Columbia (D.G.H.), and Centre for Translational and Applied Genomics, British Columbia Cancer Agency (D.G.H.), Vancouver, British Columbia, Canada; Molecular Unit, Department of Pathology, Herlev Hospital, University of Copenhagen, Copenhagen, Denmark (E. Høgdall); Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, CA (M.T.G.); Genetic Pathology Evaluation Centre, Vancouver General Hospital and University of British Columbia, Vancouver, British Columbia, Canada (B. Gilks); Human Genetics Group, Spanish National Cancer Research Centre (Centro Nacional de Investigaciones Oncológicas) (M.J.G., J.B.), and Centre for Biomedical Network Research on Rare Diseases (Centro de Investigación Biomédica en Red de Enfermedades Raras), Instituto de Salud Carlos III (M.J.G., J.B.), Madrid, Spain; David Geffen School of Medicine, Department of Medicine, Division of Hematology and Oncology, University of California at Los Angeles (P.A.F.); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Erlangen, Germany (P.A.F.); Department of Histopathology, Queen’s Medical Centre, Nottingham University Hospitals NHS Trust, Nottingham, UK (S.D.); University Cancer Center Hamburg, University Medical Center Hamburg-Eppendorf, Hamburg, Germany (J.C.-C.); Cancer Genetics Laboratory, Research Division, Peter MacCallum Cancer Centre (I.G.C.), Sir Peter MacCallum Department of Oncology, University of Melbourne (I.G.C.), and Department of Pathology, University of Melbourne (I.G.C.), Melbourne, Victoria, Australia; Department of Oncology, University of Cambridge, Hutchison/MRC Research Centre (J.D.B.), Cancer Research UK Cambridge Institute, University of Cambridge, Li Ka Shing Centre (J.D.B.), and Cambridge Experimental Cancer Medicine Centre (J.D.B.), Cambridge, UK; Cancer Genomics Program, Research Department, Peter MacCallum Cancer Centre, East Melbourne, Victoria, Australia (D.D.B.); the Garvan Institute, Sydney, New South Wales, Australia (D.D.B., S.J.R.); and Department of Public Health and Primary Care, University of Cambridge, Strangeways Research Laboratory, Cambridge, UK (P.D.P.P.)
1