Abstract
Despite its well-described safety and efficacy in the treatment of sickle cell anemia (SCA) in high-income settings, hydroxyurea remains largely unavailable in sub-Saharan Africa, where more than 75% of annual SCA births occur and many comorbidities exist. Realizing Effectiveness Across Continents with Hydroxyurea (REACH, ClinicalTrials.gov NCT01966731) is a prospective, Phase I/II open-label trial of hydroxyurea designed to evaluate the feasibility, safety, and benefits of hydroxyurea treatment for children with SCA in four sub-Saharan African countries. Following comprehensive training of local research teams, REACH was approved by local Ethics Committees and achieved full enrollment ahead of projections with 635 participants enrolled over a 30-month period, despite half of families living >12km from their clinical site. At enrollment, study participants (age 5.4±2.4 years) had substantial morbidity, including a history of vaso-occlusive pain (98%), transfusion (68%), malaria (85%), and stroke (6%). Significant differences in laboratory characteristics were noted across sites, with lower hemoglobin concentrations (p<0.01) in Angola (7.2±1.0 g/dL) and the DRC (7.0±0.9 g/dL) compared to Kenya (7.4±1.1 g/dL) and Uganda (7.5±1.1 g/dL). Analysis of known genetic modifiers of SCA demonstrated a high frequency of α-thalassemia (58.4% with at least a single α-globin gene deletion) and G6PD deficiency (19.7% of males and 2.4% of females) across sites. The CAR β-globin haplotype was present in 99% of participants. The full enrollment to REACH confirms the feasibility of conducting high-quality SCA research in Africa; this study will provide vital information to guide safe and effective dosing of hydroxyurea for children with SCA living in Africa.
Keywords: Sickle cell anemia, children, feasibility, hydroxyurea, Africa
INTRODUCTION
Sickle cell anemia (SCA) is an inherited hematological disorder that is associated with severe morbidity and early mortality,1 and represents a common yet overlooked global health problem with over 300,000 affected infants born each year.2 Approximately 75% of these infants are born in sub-Saharan Africa, where early diagnosis and life-saving treatments are essentially unavailable.3,4 Awareness of SCA appears to be slowly improving with screening programs demonstrating the burden and benefits of early diagnosis.6–10 As more children with SCA survive infancy following early and accurate diagnosis with better access to preventive care, their medical needs resulting from untreated SCA will further strain the limited healthcare resources of most African countries.
Hydroxyurea represents the only currently practical disease-modifying therapy for children with SCA living in Africa and is included on the WHO List of Essential Medicines for Children12 and for Adults,13 but remains largely unavailable for most persons with SCA living in the region. Although the benefits of hydroxyurea are well proven in high-income settings,14–21 there are virtually no data regarding the feasibility, dosing, or safety of hydroxyurea treatment in sub-Saharan Africa.3 Despite experiences demonstrating that the benefits of hydroxyurea are optimized when the dose is escalated to maximum tolerated dose (MTD),15,22 it is unclear if MTD is practical in low and middle income (LMIC) settings or whether lower doses may be the best option in clinical settings where resources for monitoring dosing and toxicity are limited.23–25
Realizing Effectiveness Across Continents with Hydroxyurea (REACH, ClinicalTrials.gov NCT01966731) is a prospective, phase I/II open-label dose escalation trial of hydroxyurea in four sub-Saharan African countries designed to evaluate the safety, dosing, feasibility, and benefits of hydroxyurea for children with SCA.26 An important objective of the study is to gather local evidence in African settings, such that dosing and laboratory monitoring guidelines can be developed to increase availability of and access to hydroxyurea for the hundreds of thousands of children who desperately need it. In addition to investigating practical clinical outcomes, REACH also aims to develop local research capacity within a strong collaborative international research network.3 We now report the successful full enrollment of the REACH trial, and provide descriptions of the baseline characteristics of this large and multi-national study population.
METHODS
Study Overview
REACH is a prospective phase I/II open label trial of hydroxyurea therapy for young children (age 1–10 years at enrollment) at four sub-Saharan African clinical sites: Luanda, Angola (Site 1); Kinshasa, Democratic Republic of Congo (Site 2); Kilifi, Kenya (Site 3); and Mbale, Uganda (Site 4). The trial is approved by the Cincinnati Children’s Hospital Medical Center (CCHMC) Institutional Review Board and by the Ethics Committees, along with appropriate local and national oversight bodies, at all four clinical sites. The rationale and details of the study design have been previously published.26 REACH aimed to enroll 150 participants per site, which gives 90% power in a Simon two-stage design to show the frequency of dose-limiting cytopenias (the primary safety endpoint) is less than 30% if the true rate is 20%. Patients were recruited locally and selected from the large clinical population at each site, at the discretion of the local lead investigators. Each site had substantially more eligible patients than the study could enroll, and therefore chose which patients to recruit. Inclusion/exclusion criteria were intentionally broad requiring only a diagnosis of sickle cell anemia and a lack of concurrent chronic diseases or severe malnutrition (z-score <−3). There was purposefully no specification in regard to the frequency or severity of SCA complications, allowing local site investigators the ability to select participants with the most need for hydroxyurea, including patients with the highest clinical severity or the highest socioeconomic need (i.e., those who could not afford hydroxyurea if purchased outside of the study). Three sites provided group education early in the study recruitment period about hydroxyurea treatment and the REACH study to garner interest among their patient population and subsequently, approached families on an individual basis as they were seen at the clinic or hospital with acute complications of SCA.
Upon enrollment, participants entered a 2-month screening period to confirm study treatment eligibility, document adherence to clinic visits, and provide pre-treatment laboratory data and clinical events. Three of the four sites agreed that nominal reimbursement for travel was beneficial, while one site (Site 1) determined that enrollment in the study and the receipt of hydroxyurea was sufficient reimbursement and that travel reimbursement might create additional advantages from the rest of their large patient population. Study participants then entered a hydroxyurea treatment phase at a fixed dose of 20 mg/kg/day, which was maintained for six months before escalating to maximum tolerated dose (MTD) based on pre-specified laboratory criteria.26 The study, which is still ongoing, will continues to a common termination date, currently scheduled for four years from the time the first child initiated hydroxyurea treatment.26
Data Collection and Analysis
Data were entered locally at each site into the internet-based Research Electronic Data Capture (REDCapTM) system, using web-based case report forms and instructions in three languages (English for Kenya and Uganda, French for the DRC, and Portuguese for Angola). Baseline data collection for all enrolled participants included demographic characteristics, comprehensive clinical history and physical examination, immunization history, concomitant medications, and steady-state laboratory results. The clinical history details were provided as self-report by the parent or from the available medical records at each clinical site. For each study participant, z-scores were calculated for height-for-age (HAZ), weight-for-height (WHZ), and weight-for-age (WAZ) using the WHO growth metrics.27 HAZ is a measure of chronic malnutrition with values <−2 defined as stunting; WHZ is a measure of acute malnutrition with values <−2 defined as wasting; and WAZ is a less specific measure with values <−2 defined as underweight. Z-scores <−3 identify children with severe malnutrition and HAZ or WHZ<−3 was an exclusion criteria for REACH. Most study data were captured on paper, with primary source documents uploaded into the REDCap system to allow ongoing remote study monitoring, which helped ensure high quality and accurate study data.
Standardized Laboratory Methods
Each REACH site used local laboratory equipment to perform basic study measurements. Automated complete (full) blood counts were performed using a Sysmex XT-2000i Hematology Analyser (Sites 1 and 3), Sysmex XN-1000 (Site 2) and Sysmex XS-1000i (Site 4). Absolute reticulocyte counts were measured using Sysmex equipment at Sites 1–3 but calculated by microscopy with vital staining at Site 4. Quantitative measurement of fetal hemoglobin (%HbF) was performed locally or as a send-out test, using either capillary zone electrophoresis (Sebia Minicap Flex Piercing for Sites 1 and 4; Sebia Capillarys 2 for Site 2) or HPLC (BioRad D-10 at Site 3). Serum chemistries were measured using Biosystems A15 Random Access Analyzer (Site 1), Roche Cobas C111 (Site 2), ILab Aries Chemistry Analyser (Site 3), or Humastar 200 (Site 4). At baseline, serum was collected and whole blood was spotted onto dried blood spots (FTA cards, GE Healthcare Bio-Sciences, Pittsburgh, PA) for future extraction of DNA. FTA cards and serum samples were stored at −20°C or −80°C until further analysis.
Genetic Modifiers
FTA cards were kept frozen until transport to CCHMC, where DNA-based studies were performed to (1) confirm the diagnosis of SCA; (2) identify concomitant G6PD deficiency; (3) document the presence of α-thalassemia trait; and (4) determine the β-globin haplotype. DNA was prepared from FTA cards using the InstaGene Matrix protocol (Bio-Rad, Hercules, CA) as previously described.28 Briefly, the diagnosis of SCA was determined using a customized TaqMan probe designed for the HbS (sickle, rs334) polymorphism. For the small number of samples that were not homozygous for βS, β-globin gene sequencing was performed to identify additional thalassemic mutations. The G6PD A− variant was identified using three commercially available TaqMan probe sets (Applied Biosystems Foster City, CA); G202A (rs1050829) distinguished A and B isoforms, A376G (rs1050828) identified the A− variant, and sex was determined using SRY_VIC, and ABCD1_CCHS0H-FAM for Y- and X-chromosomes, respectively. For the detection of alpha-thalassemia trait, the 3.7 kb α-globin gene deletion (−α 3.7) was scored using a copy-number variant TaqMan assay with custom TaqMan probes as described.29 This assay allows the quantification of α-globin genes with the following results: no gene deletions (αα/αα), 1 gene deletion (−α3.7/αα), or 2 gene deletion (−α3.7/−α3.7). The β-globin haplotype was determined using a combination of TaqMan probes and restriction digestion patterns to identify the classical polymorphisms that distinguish the five common haplotypes.30,31 This method allowed for the detection of the Central African Republic (CAR, also known as Bantu), Benin (BEN), Senegal (SEN), Cameroon (CAM), and Arab-Indian (ARAB) haplotypes.
Statistical Methods
Categorical and continuous data were summarized by frequency (percent) and mean (standard deviation), respectively. Differences in categorical variables between sites were assessed by the Chi-squared test when appropriate. In cases were the expected number for any cell was less than 5, a Fisher exact test was used in place of the Chi-squared test. Differences in continuous variables between sites were tested using one way ANOVA. All calculations were done in R (version 3.2.4).
RESULTS
Enrollment
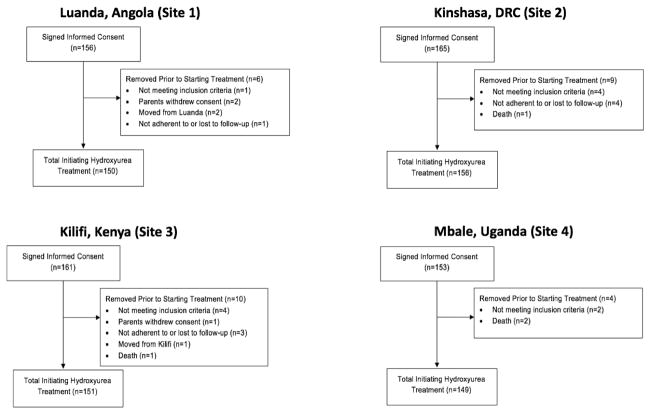
With the goal of enrolling and screening enough children to treat 600 with hydroxyurea, REACH enrollment was brisk due to the large volume of patients at each clinical site, coupled with great interest among the families and staff. Enrollment commenced at each site after all required approvals were obtained and the study teams were fully assembled and trained. The timing of enrollment initiation differed across sites, due to variations in timelines for Ethics Committees and regulatory approval at each site and within each country. Site personnel consented approximately 2–3 new patients per week, which gave ample time to complete the screening and enrollment forms, as well as collect appropriate and timely samples for all required laboratory studies. Sites 2 and 3 were the first to begin enrollment, while Sites 1 and 4 started approximately 8 months later, with a total of 635 participants enrolled between July 2014 and December 2016 (Supplementary Figure 1). There was an intentional pause in enrollment at each site after 60 participants were enrolled, as part of the two-staged study design, which is reflected in the stepped pattern of the enrollment curve. Almost all of the enrolled participants (n=606, 95.4%) completed screening and initiated hydroxyurea study treatment. A total of 29 enrolled participants did not complete screening including 10 screen failures, 3 elective withdrawals of consent by the family, 9 participants not adherent to visits during the screening period, 3 participants who moved out of the region or country, and 4 deaths (Figure 1). The reasons for the 10 screen failures included not meeting the age criteria for inclusion (n=4), hemoglobin electrophoresis confirming a diagnosis of HbAA (n=1) or HbAS (n=1), a positive HIV test (n=2), and low HAZ or WHZ <−3 documenting severe malnutrition (n=2). The 9 non-adherent participants were removed from the study prior to starting hydroxyurea at the discretion of the local site lead investigators, due to concerns that they would not be able to attend the protocol-direct clinic visits reliably during the treatment phase of the study.
Figure 1. REACH Enrollment Consort Diagram.
REACH aimed to enroll 150 children at each site with replacement of participants who did not complete three months of hydroxyurea treatment. This figure summarizes enrollment at each site, including those participants who did not complete the two-month screening period.
Demographics
Table 1 summarizes the demographics of the 606 study participants who completed screening and initiated hydroxyurea treatment. Their mean (SD) age at enrollment was 5.4 (2.4) years and 46.7% of participants were <5.0 years of age. There was an equal split between males (51.2%) and females (48.8%), with no significant differences in age or sex across sites. Mild to moderate malnutrition was common at all sites, with a mean (SD) WHZ = −0.7 (1.0), HAZ = −0.9 (1.1), and WAZ = −0.9 (1.0). However, significant differences in nutritional status were observed across the four clinical sites (Table 1); for example, there was poor growth at Site 1 and Site 3, but essentially normal growth among participants at Site 4. A majority of enrolled participants at Site 3 (107/151=71%) had WAZ and HAZ <−1, and 23% of participants at site 1 (35/150) met the clinical definition for wasting with WHZ <−2.
Table 1.
Baseline Characteristics
| Site 1 (Angola) | Site 2 (DRC) | Site 3 (Kenya) | Site 4 (Uganda) | Total | p-value | |
|---|---|---|---|---|---|---|
| N | 150 | 156 | 151 | 149 | 606 | |
| Age at Consent | 5.3 (2.4) | 5.4 (2.5) | 5.8 (2.3) | 5.1 (2.4) | 5.4 (2.4) | 0.80 |
| Male N (%) | 72 (48) | 83 (53) | 83 (55) | 72 (48) | 310 (51) | 0.57 |
| Height (cm) | 108.2 (14.9) | 109.3 (15.5) | 107.3 (12.9) | 105.7 (15.6) | 107.6 (14.8) | 0.09 |
| Weight (kg) | 17.2 (4.9) | 17.9 (4.8) | 16.9 (3.9) | 17.1 (4.7) | 17.3 (4.6) | 0.43 |
| Height-for-age z-score (HAZ) | −0.7 (1.1) | −0.6 (1.2) | −1.4 (1.0) | −0.9 (1.0) | −0.9 (1.1) | <0.01 |
| Weight-for-height z-score (WHZ) | −1.0 (1.1) | −0.6 (1.0) | −0.8 (0.9) | −0.4 (1.0) | −0.7 (1.0) | <0.01 |
| Weight-for-age z-score (WAZ) | −1.1 (0.9) | −0.7 (1.1) | −1.4 (0.9) | −0.5 (1.0) | −0.9 (1.0) | <0.01 |
Values are represented as mean (standard deviation).
Travel Distance to Clinic
Many enrolled study participants lived far from the clinical sites, yet agreed to travel long distances on a regular basis to enroll and complete screening. The median distance from home to clinic was 12.1km (IQR 6.2–20.1) but with significant differences across the four sites. In general, the urban settings of Site 1 and Site 2 (median distance 10.7 and 6.9km, respectively) recruited patients living closer to clinic than the rural settings of Site 3 and Site 4 (median distance 19.9 and 15.3km, respectively). Supplementary Figure 2 illustrates the home location for each study participant, in reference to the clinical site.
Past Medical History and Physical Examination
Almost all enrolled children reported previous vaso-occlusive pain (98%) and dactylitis (80%), while 6% of the patients had previous stroke (Supplementary Table 1). At the time of enrollment, >70% of study participants at each site reported a history of previous blood transfusion except at Site 3, where fewer children (46%) had a history of transfusion. Routine care at all sites included prophylactic penicillin for children <5 years of age and folic acid supplementation, though there were significant differences in malaria preventive measures. The reported use of bed nets was common (85%), but there were significant differences noted across sites with participants at Site 1 (69%) less likely to report use of bed nets compared to Site 2 (90%), Site 3 (80%), and Site 4 (99%). Routine malaria chemoprophylaxis was provided year round at two sites (proguanil once daily at Site 3 and chloroquine once weekly at Site 4). Overall immunization rates were high, although some families did not have full documentation of previous vaccinations. Routine vaccinations for most participants across sites included Streptococcus pneumoniae, Haemophilus influenzae type b, measles, diphtheria, tetanus, pertussis, hepatitis B, and BCG. On physical examination, many children (50%) had icterus while baseline palpable splenomegaly was recorded in 99 participants (16%). Six enrolled children reported a history of surgical splenectomy.
Laboratory Parameters
Table 2 summarizes the baseline laboratory characteristics for all 606 study participants who completed screening across the four clinical sites. The laboratory values were generally as expected for untreated children with SCA, but some significant between-site differences were noted. For example, the average hemoglobin concentration was significantly lower at Site 1 (7.2 g/dL) and Site 2 (7.0 g/dL), compared to Sites 3 (7.4 g/dL) and 4 (7.5 g/dL). Similarly, the baseline proportion of fetal hemoglobin was significantly higher at Site 4 than the other three clinical sites. There were additional small but significant differences in the baseline reticulocyte, leukocyte and neutrophil counts, and MCV (Table 2).
Table 2.
Baseline Laboratory Results
| Site 1 (Angola) | Site 2 (DRC) | Site 3 (Kenya) | Site 4 (Uganda) | Total | p-value | |
|---|---|---|---|---|---|---|
| N | 150 | 156 | 151 | 149 | 606 | |
| HbF (%) | 10.5 (6.8) | 10.2 (7.1) | 10.9 (6.5) | 11.8 (6.5) | 10.8 (6.7) | 0.08 |
| WBC (x109/L) | 14.9 (4.6) | 15.7 (5.1) | 17.6 (11.0) | 17.5 (9.3) | 16.4 (8.0) | <0.01 |
| Hb (g/dL) | 7.2 (1.0) | 7.0 (1.1) | 7.4 (1.1) | 7.5 (1.1) | 7.3 (1.1) | <0.01 |
| MCV (fL) | 75 (8) | 77 (8) | 74 (10) | 80 (9) | 77 (9) | <0.01 |
| ANC (x109/L) | 6.5 (2.7) | 6.6 (2.9) | 7.0 (3.0) | 7.1 (3.5) | 6.8 (3.0) | 0.06 |
| ARC (x109/L) | 298 (133) | 334 (121) | 308 (99) | 436 (184) | 344 (147) | <0.01 |
| Platelets (x109/L) | 398 (168) | 427 (190) | 387 (156) | 432 (167) | 411 (172) | 0.34 |
| Creatinine (mg/dL) | 0.5 (0.2) | 0.3 (0.1) | 0.5 (0.1) | 0.4 (0.1) | 0.4 (0.2) | 0.15 |
| AST (IU/L) | 69 (34) | 54 (80) | 62 (25) | 55 (37) | 59 (50) | 0.14 |
| ALT (IU/L) | 23 (27) | 28 (53) | 24 (10) | 23 (25) | 24 (33) | 0.66 |
| Bilirubin (mg/dL) | 2.8 (1.6) | 2.6 (1.9) | 2.1 (1.0) | 2.2 (1.6) | 2.4 (1.6) | <0.01 |
Values are represented as mean (standard deviation).
Genetic Modifiers
Adequate DNA was extracted from all FTA cards transported to CCHMC for genetic testing (Table 3). Laboratory analysis confirmed the diagnosis of homozygous HbSS on the vast majority of samples, but 11 children (1.8%) had an unexpected diagnosis of HbS/β0 thalassemia, including 1 from Site 1 and 10 from Site 3. Three null β-thalassemia mutations were identified by DNA sequencing. The most common mutation, found in 9 Kenyan children, was a G to T change at position 117 in exon 1 (rs33959855), which causes premature chain termination with Glu23Term. Two additional beta-null mutations included a 24 base pair deletion in Intron 2 that includes the donor acceptor site for Exon 2 (rs193922563) in one Kenyan child, and a C to T change at position 168 in Exon 2 (rs193922563) in one Angolan child that causes premature termination with Gln40Term. All eleven children with HbS/β0 thalassemia had a low MCV (59.7±5.0 fL) and elevated HbA2 (5.0±0.9%).
Table 3.
Genetic Modifiers of REACH participants
| Site 1 (Angola) | Site 2 (DRC) | Site 3 (Kenya) | Site 4 (Uganda) | Total | p-value | |
|---|---|---|---|---|---|---|
| N | 150 | 156 | 151 | 149 | 606 | |
| Sickle cell anemia | <0.0001 | |||||
| HbSS | 149 (0.99) | 156 (1.0) | 141 (0.93) | 149 (1.0) | 595 (0.98) | |
| Hb S/β0thalassemia | 1 (0.01) | 0 | 10 (0.07) | 0 | 11 (0.02) | |
| α –thalassemia | 0.08 | |||||
| None (αα/αα) | 55 (0.37) | 64 (0.41) | 58 (0.38) | 75 (0.50) | 252 (0.42) | |
| 1-gene deletion (−α3.7/αα) | 75 (0.50) | 79 (0.51) | 72 (0.48) | 64 (0.43) | 290 (0.48) | |
| 2-gene deletion (−α3.7/−α3.7) | 20 (0.13) | 13 (0.08) | 21 (0.14) | 10 (0.07) | 64 (0.11) | |
| β-globin haplotype | 0.28 | |||||
| CAR/CAR | 135 (0.90) | 143 (0.92) | 136 (0.90) | 142 (0.95) | 556 (0.92) | |
| CAR/Other | 14 (0.09) | 12 (0.08) | 12 (0.08) | 6 (0.04) | 44 (0.07) | |
| ARAB/Other | 0 | 1 (0.01) | 3 (0.02) | 1 (0.01) | 5 (0.01) | |
| SEN/SEN | 1 (0.01) | 0 | 0 | 0 | 1 (0.002) | |
| G6PD genotype | ||||||
| Males (N) | 72 | 83 | 83 | 72 | 310 | 0.73 |
| Deficient (A−) | 17 (0.24) | 17 (0.20) | 15 (0.18) | 12 (0.17) | 61 (0.20) | |
| Not Deficient | 55 (0.76) | 66 (0.80) | 68 (0.82) | 60 (0.83) | 249 (0.80) | |
| Females (N) | 78 | 73 | 68 | 77 | 296 | 0.71 |
| Deficient (A−/A−) | 2 (0.03) | 1 (0.01) | 2 (0.03) | 2 (0.03) | 7 (0.02) | |
| Carrier (A−/A or B) | 29 (0.37) | 29 (0.40) | 21 (0.31) | 21 (0.27) | 100 (0.33) | |
| Unaffected | 47 (0.60) | 43 (0.59) | 45 (0.66) | 54 (0.70) | 189 (0.64) |
Data are presented as number (proportion) unless otherwise indicated. P-values represent statistical comparisons between sites for each genetic modifier.
G6PD deficiency was common at all clinical sites, with the prevalence of affected hemizygous males ranging from 16–24% and heterozygous female carriers ranging from 27–39% (Table 3). The B isoform was more common than the A isoform, with an allele frequency of 0.62. The normal A isoform and the pathologic A− isoform were both present with allele frequencies of 0.18 and 0.19, respectively. There was also a very high frequency of deletional α-thalassemia across sites, as defined by the presence of the African 3.7kb α-globin gene deletion. Nearly half (290/606=48%) of enrolled participants had one α-globin gene deletion (−α3.7/αα), while 64 participants (11%) had two gene deletions (−α3.7/−α3.7) (Table 3). The presence of deletional α-thalassemia resulted in a significant incremental increase in hemoglobin concentration and absolute reticulocyte count (ARC), and a similar decrease in MCV for each gene deletion (Hb=7.1 g/dL, ARC=367 × 109/L, MCV=82 fL for αα/αα; Hb=7.3 g/dL, ARC=336 × 109/L, MCV=75 fL for −α3.7/αα; and Hb=7.7 g/dL, ARC=282 × 109/L, MCV=63 fL for (−α3.7/−α3.7, p<0.01). There was a trend toward lower HbF for participants without any α-globin gene deletions compared to those with one or two deletions, but this was not statistically significant (p=0.06). There were similar rates of α-globin gene deletions among the 34 participants with previous stroke. Participants with two α-globin gene deletions were significantly less likely (p<0.01) to have previous blood transfusion (48%) than those with zero (70%) or one α-globin deletion (70%). There were no differences in the reported history of malaria across the α-globin genotypes. The β-globin haplotype was almost exclusively Central African Republic (CAR), with 92% of children having the homozygous CAR/CAR genotype; however, five children were identified with an Arab-Indian haplotype. Neither G6PD deficiency nor β-globin haplotype was associated with any significant differences in baseline laboratory values or reported clinical history.
DISCUSSION
The design of the REACH trial was ambitious, aiming to enroll and treat 600 children with SCA across four clinical sites in four different countries, with the goal of providing an accurate assessment of the safety and benefits of hydroxyurea in each clinical setting. Site readiness was initiated by a careful selection process based on interest and resources, followed by personnel training using web-based tools and training in three languages, plus face-to-face interactions; these efforts culminated with formal site activation after all regulatory and logistical hurdles were cleared. Questions about the feasibility of enrolling a large number of children into a therapeutic trial in sub-Saharan Africa were voiced by several external review groups. However, the brisk enrollment rates quickly refuted those concerns, reflecting the careful training and dedication of the local clinical teams, as well as strong interest from families. Almost every family approached by the local team agreed to participate in the trial, reflecting the severity of SCA and the lack of available, affordable and effective treatment options for this life-threatening disease. Figure 1 illustrates that almost all enrolled study participants successfully completed screening and initiated hydroxyurea treatment, with <5% dropout across all four clinical sites. This degree of study retention is remarkable, especially when considering the effort required to attend the clinic for study visits, and the fact that many families have limited resources with challenging living conditions, multiple children, lack of reliable transportation, and long travel distances. Supplementary Figure 2 shows that 40% of participants traveled more than 15 km each way for study visits, often by various forms of connecting public transportation. Feasibility for the subsequent treatment phase will be assessed through recording the frequency of attended clinic visits within the protocol-specified study windows.
These pre-treatment REACH enrollment data allow comparisons among large cohorts of children with SCA across four countries in sub-Saharan Africa, with several important similarities and differences noted in their baseline laboratory values and clinical characteristics. Given the large population of patients at each site eligible to enroll in the REACH study, local investigators chose to approach patients with more clinically severe phenotypes. Each clinical site had many patients who might benefit from hydroxyurea, and local investigators had discretion to determine which patients were offered enrollment. Similar to the Phase I/II HUG-KIDS trial in the US, REACH offered treatment to those with the highest clinical severity to boost enrollment and adherence, while providing potential benefit to sicker children.32 The study purposefully provided relatively flexible inclusion and exclusion criteria, allowing local sites to determine which patients might benefit most from hydroxyurea therapy. This may have led to some selection bias that contributed to the differences in baseline characteristics among sites. Unsurprisingly, nearly all enrolled participants reported previous painful vaso-occlusive crises and malaria infection. The reported prevalence of each of these clinical diagnoses likely underestimates the actual value, however, given that this information was mostly obtained through parental self-report. Many diagnoses, such as acute chest syndrome, stroke, sepsis, and osteomyelitis require high clinical suspicion and advanced technology, which limits diagnostic accuracy in these resource-limited settings. Prophylactic treatment with penicillin were routinely prescribed at all sites and malaria chemoprophylaxis was prescribed at 2 of the 4 clinical sites, but adherence to these medications was not documented and may have been limited due to socioeconomic conditions. Most study participants also had received at least one blood transfusion, though Site 3 uses transfusions less frequently, due to decreased blood availability at this more rural site. As expected for untreated children with SCA living in low and middle-income countries, there was moderate malnutrition among most enrolled participants, with more wasting (lower WHZ) noted at Site 1 but more chronic malnutrition (lower HAZ) and underweight children (low WAZ) at Site 3. The causes of these differences are not clear, but they may be important factors that influence the dosing, response, and toxicity of hydroxyurea therapy. It will also be important to carefully investigate how baseline nutritional differences contribute to these hydroxyurea parameters, and to monitor the effects of hydroxyurea therapy on these growth parameters over time.
Laboratory values revealed expected degrees of anemia and reticulocytosis, along with increased neutrophil and platelet counts. On average, however, the baseline hematological parameters demonstrated more severe anemia than age-matched patients in the US33 and Jamaica34, with a mean baseline hemoglobin concentration of 7.3 g/dL across the entire trial. These baseline hematological parameters were consistent with those previously described for other children with SCA in sub-Saharan Africa.35–37 There were several significant differences in baseline laboratory characteristics across sites (Table 2) that are possibly due to differences in the unique genetic, nutritional, and environmental backgrounds at each of the four locations. There were statistically significant differences noted in both bilirubin and ARC that could reflect important differences in baseline levels of hemolysis, which will require prospective evaluation to determine if these participants require or tolerate higher doses of hydroxyurea. Reflecting the CAR β-globin haplotype, average fetal hemoglobin levels were only 10–12% in these young cohorts (Table 2). This relatively low HbF starting point will allow prospective evaluation of the therapeutic benefits of hydroxyurea, both at the 15–20 mg/kg/day starting dose and after escalation to maximum tolerated dose in the subsequent treatment phase of the study.
Genetic analyses demonstrated the feasibility and utility of using dried bloodspots to isolate genomic DNA and perform several genotyping assays. Despite variation in local collection practices and storage conditions, DNA was successfully extracted from nearly all dried blood spot samples with evaluable results for all molecular assays performed. Despite a given diagnosis of homozygous HbSS for all participants, genetic analyses identified an unexpected diagnosis of HbS/β0-thalassemia for 11 REACH participants (1.8%), which has been rarely described in sub-Saharan Africa.38,39 The finding that eight children at Site 3 carry the same null β-globin mutation suggests a founder effect, whose origin and migration patterns remain to be elucidated. This is a novel finding that deserves further investigation to determine the incidence of both HbS/β0-thalassemia and homozygous β-thalassemia major in East Africa. These findings are important and novel and demonstrate that β-thalassemia mutations indeed exist in both East and Central Africa, and that HbS/β0 or HbS/β+-thalassemia should be considered for patients with elevated %HbA2 and decreased MCV. As previously described,40–42 there was a high frequency of both α–thalassemia and G6PD deficiency in our cohort, which may ameliorate the risk of severe malaria43–45 and sickle phenotype,46–52 but may also affect the responses to hydroxyurea therapy.53 Study participants with two α-globin gene deletions had significantly higher hemoglobin concentrations and were less likely to have been transfused prior to study entry, in comparison to those with one or zero α-globin gene deletions.
These baseline data from the REACH trial demonstrate important differences in the clinical and laboratory characteristics of children with SCA across Central and East Africa. The observed variability provides an important and unprecedented opportunity to prospectively evaluate the benefits, potential toxicity, and appropriate dosing of hydroxyurea within this population. The current experience with hydroxyurea is limited mostly to high income countries where nutrition, antibiotics, and comprehensive care are the norm. Although the dosing, safety, and benefits of hydroxyurea have been clearly documented in high-income settings, it is not clear whether these clinical guidelines will be suitable or appropriate for lower income settings of sub-Saharan Africa. The REACH study has the opportunity to determine whether these differences in baseline nutritional, laboratory, genetic and clinical characteristics will influence the overall safety, benefits, or dosing of hydroxyurea therapy. However, while REACH includes a wide representation of patients across sub-Saharan Africa, our study population may not be representative of the entire African continent. Following the prospective evaluation of feasibility, safety, and dosing of hydroxyurea within REACH, it will be important to more widely evaluate the use of hydroxyurea in other clinical and geographical settings across sub-Saharan Africa, particularly in West Africa with additional variation in cultural, nutritional, socioeconomic, and genetic background.
Despite the overwhelming burden of disease, fewer than 0.1% of more than 250,000 registered studies on ClinicalTrials.gov are focused on SCA and listed as either active or recruiting; as of November 2017, only 12 such studies are being conducted in Africa.54 REACH is therefore a critically important research trial, representing the first and only multi-national study of hydroxyurea for patients with SCA living in sub-Saharan Africa. The successful enrollment and high-quality baseline data demonstrate an important step for SCA research within sub-Saharan Africa, and the study has the potential to provide robust, data-driven guidelines for the optimal dosing and monitoring of hydroxyurea therapy. In addition, the REACH collaborative research network, fully trained and highly energetic, is now poised to address many additional unanswered questions about the clinical course, outcomes, and treatment options for children with SCA across sub-Saharan Africa.
Supplementary Material
Acknowledgments
The authors would like to thank the extensive, energetic, and dedicated clinical teams from each of the REACH clinical sites in Luanda, Kinshasa, Kilifi, and Mbale. Special thanks go to Gideon Nyutu for assistance with the geospatial maps. The REACH study would not be possible without the generous donation of hydroxyurea by the Bristol-Myers Squibb Foundation, through an investigator-sponsored research agreement (REW). REACH is supported by U01 HL 133883 from the National Heart, Lung, and Blood Institute (REW). TNW is funded through a Senior Research Fellowships (202800 and 091758) from the Wellcome Trust. PTM is supported by a training grant from the National Heart, Lung, and Blood Institute (K23 HL 128885). This paper is published with the permission of the Director of KEMRI.
Footnotes
DISCLOSURES
Dr. Ware is a consultant for Global Blood Therapeutics and Nova Laboratories; is on an advisory board for Agios Pharmaceuticals; receives research support from Addmedica; and serves on a Data and Safety Monitoring Board for the US Food and Drug Administration. None of these disclosures is relevant to the results and conclusions of the REACH trial. The remaining authors have nothing to disclose.
References
- 1.Ware RE, de Montalembert M, Tshilolo L, Abboud MR. Sickle cell disease. Lancet. 2017;390(10091):311–323. doi: 10.1016/S0140-6736(17)30193-9. [DOI] [PubMed] [Google Scholar]
- 2.Piel FB, Patil AP, Howes RE, Nyangiri OA, Gething PW, Dewi M, et al. Global epidemiology of sickle haemoglobin in neonates: a contemporary geostatistical model-based map and population estimates. Lancet. 2013;381:142–151. doi: 10.1016/S0140-6736(12)61229-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McGann PT, Hernandez AG, Ware RE. Sickle cell anemia in sub-Saharan Africa: advancing the clinical paradigm through partnerships and research. Blood. 2017;192(2):155–161. doi: 10.1182/blood-2016-09-702324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grosse SD, Odame I, Atrash HK, Amendah DD, Piel FB, Williams TN. Sickle cell disease in Africa: a neglected cause of early childhood mortality. Am J Prev Med. 2010;41(6 Suppl 4):S398–405. doi: 10.1016/j.amepre.2011.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization Regional Office for Africa. Sickle-cell disease: A strategy for the WHO African Region. Jun, 2010. [Google Scholar]
- 6.Rahimy MC, Gangbo A, Ahouignan G, Alihonou E. Newborn screening for sickle cell disease in the Republic of Benin. J Clin Pathol. 2009;62:46–8. doi: 10.1136/jcp.2008.059113. [DOI] [PubMed] [Google Scholar]
- 7.Tshilolo L, Aissi LM, Lukusa D, Kinsiama C, Wembonyama S, Gulbis B, et al. Neonatal screening for sickle cell anaemia in the Democratic Republic of the Congo: experience from a pioneer project on 31 204 newborns. J Clin Pathol. 2009;62:35–8. doi: 10.1136/jcp.2008.058958. [DOI] [PubMed] [Google Scholar]
- 8.Ndeezi G, Kiyaga C, Hernandez AG, et al. Burden of sickle cell trait and disease in the Uganda Sickle Surveillance Study (US3): a cross-sectional study. Lancet Glob Health. 2016;4(3):e195–200. doi: 10.1016/S2214-109X(15)00288-0. [DOI] [PubMed] [Google Scholar]
- 9.McGann PT, Ferris MG, Ramamurthy U, Santos B, de Oliveira V, Bernardino L, et al. A prospective newborn screening and treatment program for sickle cell anemia in Luanda, Angola. Am J Hematol. 2013;88:984–9. doi: 10.1002/ajh.23578. [DOI] [PubMed] [Google Scholar]
- 10.McGann PT, Grosse SD, Santos B, et al. A Cost-Effectiveness Analysis of a Pilot Neonatal Screening Program for Sickle Cell Anemia in the Republic of Angola. J Pediatr. 2015;167(6):1314–1319. doi: 10.1016/j.jpeds.2015.08.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sins JW, Magar DJ, Davis SCAT, et al. Pharmacotherapeutical strategies in the prevention of acute, vaso-occlusive pain in sickle cell disease: a systematic review. Blood Advances. 2017;1(19):1598–1616. doi: 10.1182/bloodadvances.2017007211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.WHO. Model List of Essential Medications for Children. World Health Organization; Mar, 2017. 6th list. http://www.who.int/medicines/publications/essentialmedicines/6th_EMLc2017.pdf?ua=1. [Google Scholar]
- 13.WHO. Model List of Essential Medications. World Health Organization; Mar, 2017. 20th list. http://www.who.int/medicines/publications/essentialmedicines/20th_EML2017.pdf?ua=1. [Google Scholar]
- 14.Charache S, Terrin ML, Moore RD, et al. Effect of hydroxyurea on the frequency of painful crises in sickle cell anemia. N Engl J Med. 1995;18(330):1317–1322. doi: 10.1056/NEJM199505183322001. [DOI] [PubMed] [Google Scholar]
- 15.Zimmerman SA, Schultz WH, Davis JS, et al. Sustained long-term hematologic efficacy of hydroxyurea at maximum tolerated dose in children with sickle cell disease. Blood. 2004;103:2039–2045. doi: 10.1182/blood-2003-07-2475. [DOI] [PubMed] [Google Scholar]
- 16.Wang WC, Ware RE, Miller ST, et al. Hydroxycarbamide in very young children with sickle-cell anaemia: a multicentre, randomised, controlled trial (BABY HUG) Lancet. 2011;377:1663–1672. doi: 10.1016/S0140-6736(11)60355-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ware RE, Davis BR, Schultz WH, et al. Hydroxycarbamide versus chronic transfusion for maintenance of transcranial doppler flow velocities in children with sickle cell anaemia-TCD With Transfusions Changing to Hydroxyurea (TWiTCH): a multicentre, open-label, phase 3, non-inferiority trial. Lancet. 2016;387(10019):661–670. doi: 10.1016/S0140-6736(15)01041-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Steinberg MG, McCarthy WF, Castro O, et al. The risks and benefits of long-term use of hydroxyurea in sickle cell anemia: A 17. 5 year follow-up. Am J Hematol. 2010;85(6):403–408. doi: 10.1002/ajh.21699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Voskaridou E, Christoulas D, Bilalis A, et al. The effect of prolonged administration of hydroxyurea on morbidity and mortality in adult patients with sickle cell syndromes: results of a 17-year, single-center trial (LaSHS) Blood. 2010;115(12):2354–2363. doi: 10.1182/blood-2009-05-221333. [DOI] [PubMed] [Google Scholar]
- 20.Lobos CL, Pinto JF, Nascimento EM, et al. The effect of hydroxycarbamide therapy on survival of children with sickle cell disease. Br J Haematol. 2013;161(6):852–860. doi: 10.1111/bjh.12323. [DOI] [PubMed] [Google Scholar]
- 21.Lê PQ, Gulbis B, Dedeken L, et al. Survival among children and adults with sickle cell disease in Belgium: Benefit from hydroxyurea treatment. Pediatr Blood Cancer. 2015;61(11):1956–1961. doi: 10.1002/pbc.25608. [DOI] [PubMed] [Google Scholar]
- 22.Ware RE. How I use hydroxyurea to treat young patients with sickle cell anemia. Blood. 2010;115:5300–5311. doi: 10.1182/blood-2009-04-146852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Patel DK, Mashon RS, Patel S, Das BS, Purohit P, Bishwal SC. Low dose hydroxyurea is effective in reducing the incidence of painful crisis and frequency of blood transfusion in sickle cell anemia patients from eastern India. Hemoglobin. 2012;36(5):409–420. doi: 10.3109/03630269.2012.709897. [DOI] [PubMed] [Google Scholar]
- 24.Jain DL, Apte M, Colah R, et al. Efficacy of fixed low dose hydroxyurea in children with sickle cell anemia: a single centre experience. Indian Pediatr. 2013;50(10):929–933. doi: 10.1007/s13312-013-0264-0. [DOI] [PubMed] [Google Scholar]
- 25.Strouse JJ. Is low dose hydroxyurea the solution to the global epidemic of sickle cell disease? Pediatr Blood Cancer. 2015;62(6):929–930. doi: 10.1002/pbc.25471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McGann PT, Tshilolo L, Santos B, et al. Hydroxyurea therapy for children with sickle cell anemia in sub-Saharan Africa: Rationale and design of the REACH trial. Pediatr Blood Cancer. 2016;63(1):98–104. doi: 10.1002/pbc.25705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.World Health Organization. The WHO Child Growth Standards. http://www.who.int/childgrowth/standards/en/
- 28.McGann PT, Williams AM, McElhinney KE, et al. Genetic Causes of Anemia in Malawian Children Less than 5 Years of Age: Results from the Malawi Demographic and Health Survey. 2017 (Under Review) [Google Scholar]
- 29.Grimholt RM, Urdal P, Klingenberg O, Piehler AP. Rapid and reliable detection of α-globin copy number variations by quantitative real-time PCR. BMC Hematol. 2014;14:4. doi: 10.1186/2052-1839-14-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nagel RL, Fabry ME, Pagnier J, et al. Hematologically and Genetically Distinct Forms of Sickle Cell Anemia in Africa — The Senegal Type and the Benin Type. NEJM. 1985;312:880–884. doi: 10.1056/NEJM198504043121403. [DOI] [PubMed] [Google Scholar]
- 31.Sutton M, Bouhassira E, Nagel RL. Polymerase chain reaction amplification applied to the determination of β-like globin gene cluster haplotypes. Am J Hematol. 1989;32(1):66–69. doi: 10.1002/ajh.2830320113. [DOI] [PubMed] [Google Scholar]
- 32.Kinney TR, Helms RW, O’Branski EE, et al. Safety of hydroxyurea in children with sickle cell anemia: results of the HUG-KIDS study, a phase I/II trial. Pediatric Hydroxyurea Group. Blood. 1999;94(5):1550–54. [PubMed] [Google Scholar]
- 33.West MS, Wethers D, Smith J, et al. Laboratory profile of sickle cell disease: a cross-sectional analysis. The Cooperative Study of Sickle Cell Disease. J Clin Epidemiol. 1992;45(8):893–909. doi: 10.1016/0895-4356(92)90073-v. [DOI] [PubMed] [Google Scholar]
- 34.Serjeant GR, Grandison Y, Lowrie Y, et al. The development of haematological changes in homozygous sickle cell disease: a cohort study from birth to 6 years. Br J Haematol. 1981;48(4):533–543. doi: 10.1111/j.1365-2141.1981.tb02750.x. [DOI] [PubMed] [Google Scholar]
- 35.Tshilolo L, Wembonyama S, Summa V, Avvisati G. Hemogram findings in Congolese children with sickle cell disease in remission. Med Trop. 2010;70(5–6):459–463. [PubMed] [Google Scholar]
- 36.Heeney MH, Hoppe CC, Abboud MR, et al. A multinational trial of prasugrel for sickle cell vaso-occlusive events. New Eng J Med. 2016;374:625–635. doi: 10.1056/NEJMoa1512021. [DOI] [PubMed] [Google Scholar]
- 37.Macharia AW, Mochomah G, Uyoga S, et al. The clinical epidemiology of sickle cell anemia In Africa. A J Hematol. 2017 doi: 10.1002/ajh.24986. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kotilla TR. Beta thalassemia in Nigeria: myth of fact? Afr J Med Med Sci. 2013;42(4):355–358. [PubMed] [Google Scholar]
- 39.Tubman VN, Marshall R, Jallah W, et al. Newborn screening for sickle cell disease in Liberia: a pilot study. Pediatr Blood Cancer. 2016;63(4):671–676. doi: 10.1002/pbc.25875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Flint J, Hill AV, Bowden DK, Oppenheimer SJ, Sill PR, Serjeantson SW, Bana-Koiri J, Bhatia K, Alpers MP, Boyce AJ, Weatherall DJ, Clegg JB. High frequencies of alpha-thalassemia are the result of natural selection by malaria. Nature. 1986;321:744–750. doi: 10.1038/321744a0. [DOI] [PubMed] [Google Scholar]
- 41.Howes RE, Piel FB, Patil AP, et al. G6PD deficiency prevalence and estimates of affected populations in malaria endemic countries: a geostatistical model-based map. PLoS Med. 2012;9:e1001339. doi: 10.1371/journal.pmed.1001339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Olupot-Olupot P, Engoru C, Uyoga S, et al. High frequency of blackwater fever among children presenting to hospital with severe febrile illness in Eastern Uganda. Clin Infect Dis. 2017;64(7):939–946. doi: 10.1093/cid/cix003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Uyoga S, Ndila CM, Macharia AW, et al. Glucose-6-phosphate dehydrogenase deficiency and the risk of malaria and other diseases in children in Kenya: a case-control and a cohort study. Lancet Haematol. 2015;2(10):e437–e444. doi: 10.1016/S2352-3026(15)00152-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Enevold A, Lusingu JP, Mmbando B, et al. Reduced risk of uncomplicated malaria episodes in children with alpha+-thalassemia in northeastern Tanzania. Am J Top Med Hyg. 2008;78:714–20. [PubMed] [Google Scholar]
- 45.Lopez C, Saravia C, Gomez A, Joebeke J, Patarroyo MA. Mechanisms of genetically-based resistance to malaria. Gene. 2010;467:1–12. doi: 10.1016/j.gene.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 46.Embury SH, Dozy AM, Miller J, et al. Concurrent sickle-cell anemia and alpha-thalassemia: effect on severity of anemia. N Engl J Med. 1982;306(5):270–274. doi: 10.1056/NEJM198202043060504. [DOI] [PubMed] [Google Scholar]
- 47.Embury SH, Clark MR, Monroy G, Mohandas N. Concurrent sickle cell anemia and alpha-thalassemia. Effect on pathological properties of sickle erythrocytes. J Clin Invest. 1984;73(1):116–123. doi: 10.1172/JCI111181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bernaudin F, Verlhac S, Chevret S, et al. G6PD deficiency, absence of alpha-thalassemia, and hemolytic rate at baseline are significant independent risk factors for abnormally high cerebral velocities in patients with sickle cell anemia. Blood. 2008;112(10):4314–4317. doi: 10.1182/blood-2008-03-143891. [DOI] [PubMed] [Google Scholar]
- 49.Williams TN, Mwangi TW, Wambua S, et al. Negative epistasis between the malaria-protective effects of α+-thalassemia and the sickle cell trait. Nat Genet. 2005;37(11):1253–1257. doi: 10.1038/ng1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sheehan VA, Luo Z, Flanagan JM, et al. Genetic modifiers of sickle cell anemia in the BABY HUG cohort: influence on laboratory and clinical phenotypes. Am J Hematol. 2013;88:571–6. doi: 10.1002/ajh.23457. [DOI] [PubMed] [Google Scholar]
- 51.Wonkam A, Rumaney MB, Ngo Bitoungui VJ, Vorster AA, Ramesar R, Ngogang J. Coinheritance of sickle cell anemia and a-thalassemia delays disease onset and could improve survival in Cameroonian’s patients (sub-Saharan Africa) Am J Hematol. 2014;89:664–5. doi: 10.1002/ajh.23711. [DOI] [PubMed] [Google Scholar]
- 52.Cox SE, Makani J, Soka D, et al. Haptoglobin, alpha-thalassaemia and glucose-6-phosphate dehydrogenase polymorphisms and risk of abnormal transcranial Doppler among patients with sickle cell anaemia in Tanzania. Br J Haematol. 2014;165(5):699–706. doi: 10.1111/bjh.12791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Darbari DS, Nouraie M, Taylor JG, et al. Alpha thalassaemia and response to hydroxyurea in sickle cell anaemia. Eur J Haematol. 2014;92(4):341–345. doi: 10.1111/ejh.12245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.https://clinicaltrials.gov/ct2/results/map/click?map.x=6&map.y=224&recrs=adf&cond=Sickle+Cell&mapw=890
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.