Abstract
Objective
To determine the impact of a cerebrospinal fluid (CSF) enteroviral polymerase chain reaction (PCR) test performance on hospital length of stay (LOS) in a large multicenter cohort of infants undergoing evaluation for central nervous system infection.
Study design
We performed a planned secondary analysis of a retrospective cohort of hospitalized infants ≤60 days of age who had a CSF culture obtained at one of 18 participating centers (2005–2013). After adjustment for patient age and study year as well as clustering by hospital center, we compared LOS for infants who had an EV PCR test performed vs. not performed and among those tested, for infants with a positive vs negative test result.
Results
Of 19,953 hospitalized infants, 4,444 (22.3%) had an EV PCR test performed and 945 (21.3% of tested infants) had positive test results. Hospital LOS was similar for infants who had an EV PCR test performed compared with infants who did not (Incident rate ratio [IRR] 0.98 hours; 95% CI: 0.89–1.06 hours). However, EV PCR-positive infants had a 38% shorter LOS than EV PCR-negative infants (IRR 0.62 hours; 95% CI: 0.57–0.68). No infant with a positive EV PCR test had bacterial meningitis (0%; 95% CI: 0–0.4%).
Conclusions
While EV PCR testing was not associated with a reduction in LOS, infants with a positive EV PCR test had a one-third shorter LOS compared with infants with a negative EV PCR test. Focused EV PCR test use could increase the impact on LOS for infants undergoing CSF evaluation.
Keywords: enterovirus, young infant, neonate, meningitis
Young infants brought to the emergency department (ED) for evaluation of fever are frequently hospitalized and treated with parenteral antibiotics while awaiting bacterial culture results.1,2 However, the majority of febrile infants have viral infections and require only supportive care.1,3 Distinguishing between viral and bacterial infections using clinical and laboratory findings available at the time of initial evaluation presents challenges.1,4 Enterovirus, a common cause of fever in young infants,5–7 can be detected in the cerebrospinal fluid (CSF) by a reverse transcriptase polymerase chain reaction (EV PCR),8,9 with results often available within a few hours.7,10
Previous studies have suggested that children with enteroviral infections are at low risk of bacterial co-infection.5,11 Rapid diagnosis of enteroviral infection may reduce the length of hospitalization for febrile infants by identifying infants at very low risk of invasive bacterial infections (eg, bacteremia and acute bacterial meningitis). A positive EV PCR test has been associated with shorter duration of hospital stay and of parenteral antibiotics,7,10,12,13 which may result in reduced healthcare costs,14 avoidance of iatrogenic complications (eg, nosocomial infections and medication errors), and reduced burden on families. However, previous investigations have been single-center studies and included few infants with bacterial meningitis, reducing generalizability and clinical applicability. Additionally, prior studies have not evaluated the impact of performing an EV PCR test on hospital length of stay (LOS).
To address these aforementioned limitations, we assembled a large multicenter retrospective cohort of hospitalized infants ≤60 days of age who had a CSF culture obtained as part of the ED evaluation. Our aims were to determine the association between performance of an EV PCR test and hospital LOS as well as between a positive EV PCR test result and hospital LOS.
METHODS
We performed a planned secondary analysis of a retrospective cohort study of infants ≤60 days of age who were brought to the ED and underwent evaluation for central nervous system infection. The parent study, designed to determine the prevalence of herpes simplex virus (HSV) infection, was endorsed by the Pediatric Emergency Medicine Collaborative Research Committee (PEM CRC) and included 23 hospitals. We limited this analysis to the 18 participating hospitals that contributed LOS and EV PCR data. The institutional review board at each participating institution approved the study protocol, with a waiver of informed consent and permission for data sharing.
We identified infants ≤ 60 days of age who were brought to the ED of a participating institution between January 1, 2005 and December 31, 2013, and in whom a CSF culture was obtained in the ED or within 24 hours of ED arrival. The exact study period varied somewhat between participating institutions based on available electronic data sources. Eligible infants were identified at each site using a site-specific electronic search strategy. Infants could be included more than once if they had multiple eligible ED encounters. For this study, we excluded infants who were discharged from the ED after initial evaluation or who were missing hospital LOS data.
The following data elements were extracted either electronically or manually from existing medical records at each participating site: date of visit, ED arrival time, demographics, disposition (discharge vs. admission and, for admitted infants, floor vs. intensive care unit), ED triage temperature, hospital discharge date and time, and laboratory data [urinalysis, complete blood count (CBC) with differential, and CSF cell count, glucose, protein, and Gram stain]. We also extracted results of blood, urine, and CSF bacterial cultures and the CSF EV PCR test. As the EV PCR test is currently only approved by the Federal Drug Administration (FDA) for testing CSF, we did not extract data related to the utilization of this PCR test on non-approved sample types.15
Infants with a positive EV PCR test result were classified as having enteroviral infection. We defined an invasive bacterial infection as growth of pathogenic bacteria from blood or CSF culture. Serious bacterial infection (SBI) was defined by the presence of any invasive bacterial infection or a urinary tract infection (UTI)16 defined as a catheterized urine culture with ≥50,000 colony-forming units (CFUs)/mL of a single pathogenic bacteria or 10,000–50,000 CFUs/mL of a single pathogenic bacteria with an abnormal urinalysis (i.e., positive nitrite or leukocyte esterase test on urine dipstick or >5 WBCs/hpf on urine microscopy).17,18 Cultures from which more than 1 bacterial species were isolated were considered contaminated unless 1 or more was a true pathogen. We defined a priori as contaminants normal skin or oral flora isolated from a bacterial culture of urine, blood, or CSF (Table I; available at www.jpeds.com).
Table I.
Organisms considered contaminants
| Organism | Bacterial species |
|---|---|
| Gram positive | Abiotrophia, Actinomyces, Aerococcus, Bacillus (non-anthracis, non-cereus), Bifidobacterium, Brevibacterium, Corynebacterium, Lactobacillus, Micrococcus, Propionibacterium acnes, Rothia, coagulase-negative staphylococci (S. epidermidis, S. haemolyticus, S. hominis, S. simulans, S. warneri, as well as staphylococci that do not produce coagulase, but that were unable to be speciated biochemically), Stomatococcus, Streptococcus bovis, Streptococcus gordonii, Streptococcus mitis, Streptococcus oralis, Streptococcus parasanguinis, Streptococcus salivarius group, alpha streptococci, gamma streptococci, viridans streptococci |
| Gram negative | Acinetobacter species, Comamonas testosteroni, Raoultella planticola |
The primary outcome measure was hospital LOS defined as the time from ED arrival to provider signature of the hospital discharge order.
Statistical Analyses
The unit of analysis was the ED encounter. We described categorical variables using counts and proportions with 95% confidence intervals (CI), and compared using the χ2 test. We described continuous variables using medians and interquartile ranges (IQR), and compared using the Mann-Whitney U test. We examined trends over time using the Mantel-Haenszel trend test.
We used quantile regression models to compare median LOS between groups. We also estimated negative binomial regression models to compare LOS counts between groups, adjusting for patient age and study year after clustering by center. These negative binomial models also utilized a robust variance estimator to accommodate the correlation resulting from the clustering of patients within hospitals. The output of the negative binomial model was an incident rate ratio (IRR), which is the ratio of LOS by hour between the comparison groups. We first compared infants with and without an EV PCR test performed. Next, for the subgroup of infants who had the EV PCR test performed, we compared LOS for infants with a positive vs. negative EV PCR test result. Due to differential clinical management,19,20 we also stratified each comparison by patient age (≤ 28 days vs 29–60 days) and by CSF pleocytosis (present vs. absent). We defined CSF pleocytosis using published age-based normal values: CSF WBC ≥20 cells/mm3 for infants ≤28 days of age and ≥10 cells/mm3 for infants 29 to 60 days of age.21
We also examined utilization and impact of EV PCR testing by participating hospital center and over the study period. We examined the relationship between the rate of EV PCR testing and percent positivity using a Spearman correlation coefficient.
We utilized both the Statistical Program for the Social Sciences (SPSS) version 22.0 (IBM Corporation; Armonk, New York) as well as Stata Data Analysis and Statistical Software version 13.0 (StataCorp, Inc; College Station, Texas) for statistical analyses.
RESULTS
We identified 22,397 infants with CSF cultures obtained at the 18 participating sites, of whom 19,994 (89.3%) were hospitalized. Of these 19,994 infants, 41 (0.2%) were missing LOS data and were excluded, resulting in an analytic sample of 19,953 infants (Figure 1). Of the 1,984 infants who were discharged from the ED, 186 (9.4% of discharged infants) had an EV PCR test performed and 38 (20.4% of tested discharged infants) were positive.
Figure 1.
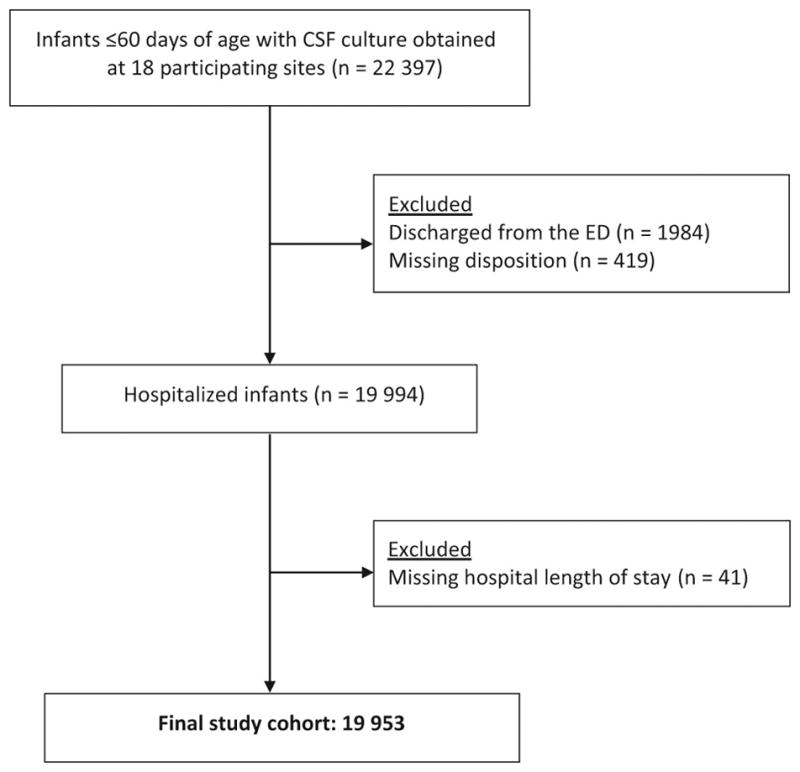
Study patients
Among the 19,953 admitted infants, 4,444 (22.3%; 95% CI: 21.7–22.9%) underwent EV PCR testing and 945 (21.3% of infants tested; 95% CI: 20.1–22.5%) had a positive EV PCR. EV PCR testing rates were higher in the peak enteroviral season between June and October [2,885/8,739 (33.0%) peak season vs. 1,559/11,214 (13.9%) non-peak season; difference 19.1%, 95% CI: 17.9–20.3%]. Of the 2,885 infants with an EV PCR test performed between June and October, 799 (27.7% of infants tested; 95% CI: 26.1–29.4%) had a positive test.
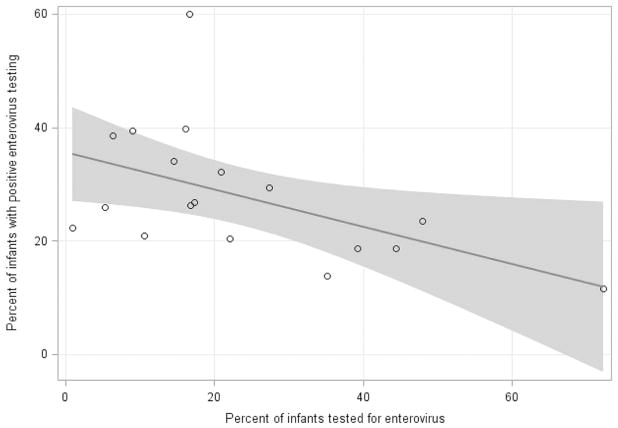
The proportion of infants who had an EV PCR test performed varied across the 18 participating hospitals (range 0.9%–72.3%; Figure 2). The EV PCR testing rate increased over the study period from 10.9% in 2005 to 24.6% in 2013 (p = 0.003). Centers with higher EV PCR testing rates had lower positive test rates (Spearman’s coefficient −0.5, 95% CI: −0.1 – −0.8; Figure 3; available at www.jpeds.com).
Figure 2.
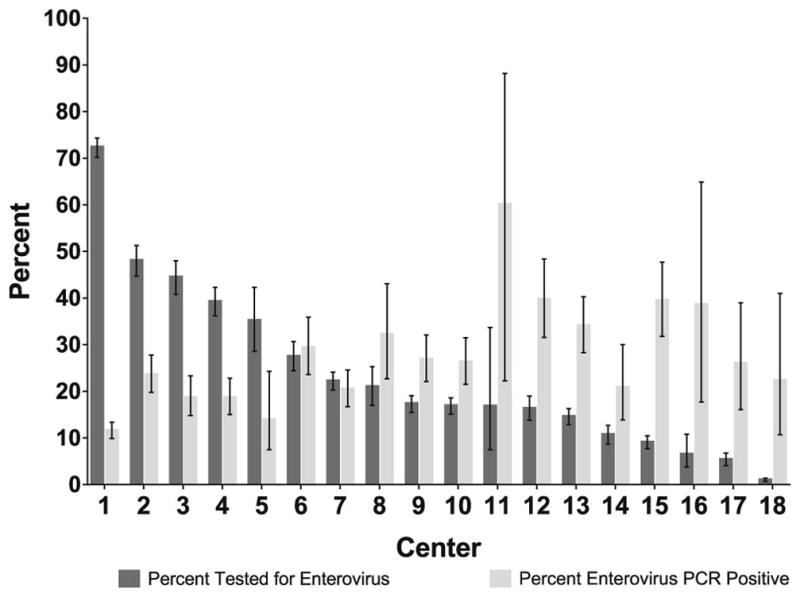
Distribution of EV PCR testing and rates of positive EV PCR test results by hospital center
Figure 3 Online.
Rate of EV PCR testing vs rate of test positivity rate by hospital center
Next, we compared infants who had an EV PCR test performed to those who did not have the test performed. Infants who were seen during peak enteroviral season or who had CSF pleocytosis were more likely to have an EV PCR test performed (Table II). Overall, 1,710 infants (8.6%) had any SBI, and 557 (2.8%) had an invasive bacterial infection, of which 182 (0.9%) had bacterial meningitis. Compared with infants with a positive EV PCR test result, infants with a negative test result had higher rates of both any SBI (6.2% vs. 0.8%; difference 5.4%, 95% CI: 4.3–6.3%) as well as invasive bacterial infection (2.5% vs. 0.4%; difference 2.1%, 95% CI: 1.3–2.7%). Of the 945 infants with a positive EV PCR, 8 had a concomitant SBI (0.8%; 95% CI 0.4–1.7%). Of these, 4 had an invasive bacterial infection, all of which were bacteremia (0.4%; 95% CI 0.2–1.1%) and none was bacterial meningitis (0%; 95% CI: 0–0.4%). The bacteremia pathogens isolated were: Staphylococcus aureus (2), Escherichia coli (1) and Klebsiella sp. (1).
Table II.
Patient characteristics by Enterovirus testing group
| Patient Characteristics | CSF EV PCR Not Tested N=15,509 n (%) |
CSF EV PCR Tested N=4,444 n (%) |
p-value |
|---|---|---|---|
|
| |||
| Demographics | |||
|
| |||
| Age 29–60 days | 6,909 (44.5) | 1,994 (44.9) | 0.86 |
|
| |||
| Male gender | 8,538 (55.1) | 2,523 (56.8) | 0.04 |
|
| |||
| Presentation during peak enteroviral seasona | 5,854 (37.7) | 2,885 (64.9) | < 0.001 |
|
| |||
| Laboratory | |||
|
| |||
| Abnormal urinalysisb | 2,774 (17.9) | 530 (11.9) | < 0.001 |
|
| |||
| WBC ≥ 15,000 cells/mm3 | 2,723 (17.6) | 673 (15.1) | < 0.001 |
|
| |||
| CSF cell count obtained | 12,897 (83.2) | 3,005 (67.6) | < 0.001 |
| CSF pleocytosis presentc | 2,924 (22.7) | 1,163 (38.7) | |
| CSF pleocytosis absentc | 9,973 (77.3) | 1,842 (61.3) | |
|
| |||
| Disposition | |||
|
| |||
| Intensive care unit admission | 2,624 (16.9) | 688 (15.5) | 0.02 |
|
| |||
| Bacterial infections | |||
|
| |||
| Invasive bacterial infectiond | 465 (3.0) | 92 (2.1) | 0.001 |
|
| |||
| Any SBIe | 1,483 (9.6) | 227 (5.1) | < 0.001 |
| UTI | 1,141 (7.4) | 151 (3.4) | |
| Bacteremia | 361 (2.3) | 76 (1.7) | |
| Bacterial meningitis | 152 (1.0) | 30 (0.7) | |
| No SBI | 14,026 (90.4) | 4,217 (94.9) | |
June to October
Positive nitrite, positive leukocyte esterase or > 5 WBCs/hpf
CSF WBC ≥ 20 cells/mm3 for infants 0 to 28 days of age and CSF WBC ≥ 10 cells/mm3 for infants 29 to 60 days of age
Bacteremia and/or bacterial meningitis
Infants may have had more than one type of SBI
CSF: cerebrospinal fluid; EV: Enterovirus; PCR: polymerase chain reaction; WBC: white blood cell; SBI: serious bacterial infection; UTI: urinary tract infection
Of the 4,087 infants with CSF pleocytosis, 1,163 (28.5%) had a CSF EV PCR test performed. Older infants 29 to 60 days of age with CSF pleocytosis had higher EV PCR testing rates [664/2149 (30.9%)] when compared with infants ≤28 days of age [499/1938 (25.7%); difference 5.2%, 95% CI: 2.4–7.9%]. Of the 1,163 infants with CSF pleocytosis who had an EV PCR test performed, 504 infants (43.3%) had a positive EV PCR test result. Among tested infants, the EV PCR was more frequently positive in those infants with CSF pleocytosis [504/1163 (43.3%) with CSF pleocytosis vs. 259/1842 (14.1%) without CSF pleocytosis; difference 29.3%, 95% CI: 26.0–32.5%]. During the June to October peak enteroviral season, 833/2,068 (40.3%) of infants with CSF pleocytosis had an EV PCR test performed, and 413 (49.6% of those tested) had a positive test.
Overall, the median LOS was 2.4 days [interquartile range (IQR) 1.9–3.3]. Infants who had an EV PCR test performed had only a slightly shorter LOS than those who did not have an EV PCR test performed (Table III). Of the 4,444 infants who had an EV PCR test performed, infants with a positive test result had a 0.4 day shorter LOS (95% CI: 0.3–0.5) compared with those with a negative test. We observed a similar impact of a positive EV PCR test in the older infants aged 29 to 60 days and in those with CSF pleocytosis. When the analysis was limited to infants without an SBI, infants with a positive EV PCR test had a 0.4 day shorter LOS (95% CI: 0.3–0.4) compared with infants with a negative test.
Table III.
Comparison of median LOS (in days) with interquartile range (IQR) for all infants and for infants who had an EV PCR test obtained
| All Infants | ||||
|---|---|---|---|---|
| CSF EV PCR Not Tested N=15,509 |
CSF EV PCR Tested N=4,444 |
Median Difference (95% CI) | Incident Rate Ratioa (95% CI) | |
| All infants (n=19,953) | 2.4 (2.0, 3.3) | 2.3 (1.9, 3.1) | 0.1 (0.1, 0.2) | 0.97 (0.89, 1.06) |
| 0–28 days of age (n=11,054) | 2.5 (2.0, 3.7) | 2.4 (2.0, 3.6) | 0.1 (0.0, 0.1) | 1.05 (0.93, 1.19) |
| 29–60 days of age (n=8,899) | 2.3 (1.9, 3.0) | 2.2 (1.8, 2.9) | 0.1 (0.1, 0.2) | 0.87 (0.80, 0.94) |
| CSF pleocytosisb (n=4,087) | 2.4 (2.0, 3.4) | 2.2 (1.8, 3.0) | 0.2 (0.1, 0.3) | 0.89 (0.78, 1.02) |
| Infants who had an EV PCR test obtained | ||||
| CSF EV PCR Negative N=3,499 |
CSF EV PCR Positive N=945 |
Median Difference (95% CI) | Incident Rate Ratioa (95% CI) | |
| All infants (n=4,444) | 2.4 (1.9, 3.6) | 2.0 (1.6, 2.6) | 0.4 (0.3, 0.5) | 0.62 (0.57, 0.68) |
| 0–28 days of age (n=2,450) | 2.5 (2.0, 3.9) | 2.1 (1.8, 2.8) | 0.4 (0.2, 0.5) | 0.65 (0.57, 0.74) |
| 29–60 days of age (n=1,994) | 2.3 (1.9, 3.1) | 1.9 (1.3, 2.4) | 0.4 (0.3, 0.5) | 0.59 (0.52, 0.68) |
| CSF pleocytosisb (n=1,163) | 2.5 (2.0, 3.8) | 2.0 (1.6, 2.6) | 0.5 (0.4, 0.7) | 0.56 (0.48, 0.64) |
Adjusted for patient age and study year, and clustered by hospital center
CSF WBC ≥ 20 cells/mm3 for infants 0 to 28 days of age and CSF WBC ≥ 10 cells/mm3 for infants 29 to 60 days of age
CSF: cerebrospinal fluid; EV: Enterovirus; LOS: length of stay; PCR: polymerase chain reaction
After adjusting for study year and patient age as well as clustering by center (Table III), hospital LOS was similar for infants who had an EV PCR test performed compared with those who did not undergo testing (IRR 0.98 hours; 95% CI: 0.89–1.06 hours). However, for infants who had an EV PCR test performed, infants with a positive test result had a 38% shorter LOS compared with those with a negative test result (IRR 0.62 hours; 95% CI: 0.57–0.68 hours).
DISCUSSION
In this multicenter cohort of nearly 20,000 hospitalized infants ≤ 60 days of age, EV PCR testing was obtained in almost one quarter of infants. Overall, the performance of the EV PCR test did not shorten hospital LOS. However, infants with a positive EV PCR test had an approximately one-third shorter length of hospital stay after adjustment for patient age and study year, and clustering by hospital center. Similar to studies of older children,11 no child with a positive EV PCR test had bacterial meningitis.
Our results are consistent with previous studies demonstrating an association between a positive EV PCR test result and a reduction in hospital LOS.7,10,12,13,22 Our study expands on previous findings in several important ways. First, prior investigations each were conducted at a single center, where clinical care could have been more determined by protocol.23 Our study demonstrates the impact of a positive EV PCR test result across clinical settings, which increases the generalizability of our findings. Second, our substantially larger sample size allowed stratification of results by patient age (infants 0 to 28 days vs 29 to 60 days) as well as by the presence of CSF pleocytosis. Third, we examined the overall impact of the decision to obtain an EV PCR test in infants undergoing evaluation for central nervous system infection. This assessment is critical as diagnostic tests have both high-value as well as low-value applications in practice.24 At the patient level, although we observed a statistically significant reduction in LOS for infants who had an EV PCR test performed compared with those who did not, the small reductions were of uncertain clinical significance and disappeared after adjustment for patient age and study year, and clustering by center. Our study highlights the importance of targeting EV PCR testing to infants most likely to have a positive test and who could be candidates for early discharge if the EV PCR test was positive. Otherwise, the costs savings from earlier hospital discharge may be outweighed by increased costs of additional viral testing.
Enterovirus PCR is the diagnostic gold standard to confirm enterovirus infection of the central nervous system. Enterovirus PCR testing technology has changed over the past two decades, with test results now available in a clinically relevant timeframe. Commercially available EV PCR testing platforms with fully automated processes have the potential for test turnaround in as little as one hour.25,26 However, in practice, test turnaround time typically ranges between 12 to 24 hours, reflecting limited testing hours and specimen batching.7,10,13 As clinical EV PCR testing evolves, earlier availability of test results may increase the impact on LOS.
The risk of bacterial meningitis in infants with a positive EV PCR test result is low. In a previous study of 735 children ≤18 years of age with meningitis and a positive EV PCR test, none had bacterial meningitis (0%, 95% CI 0–0.4%).11 Our study confirmed that the risk of bacterial meningitis is low in the youngest infants. None of the 945 study infants with a positive EV PCR test had bacterial meningitis. Given the low risk of co-infection, particularly bacterial meningitis, clinicians can safely consider outpatient management strategies for infants with a positive EV PCR test result. For those infants initially hospitalized, the majority of blood and CSF bacterial cultures that yield growth of a pathogenic organism are positive within 24 hours,27,28 although CSF cultures may be assessed for growth once daily at some microbiology laboratories. Therefore, an infant with a positive EV PCR test and negative bacterial cultures of the blood and CSF at 24 hours is at extremely low risk for co-infection, allowing for safe earlier hospital discharge. Treating clinicians must also consider the infant’s clinical status and results of other laboratory tests before making management decisions.
The optimal EV PCR testing strategy for febrile infants undergoing evaluation for CNS infection needs to be determined. In our study, centers with higher EV PCR testing rates had a lower proportion of positive tests. A prior cost-analysis found that EV PCR testing for infants with CSF pleocytosis would reduce healthcare costs if the prevalence of enteroviral infection was greater than 6%, assuming that infants with a positive test were discharged 24 hours after hospitalization.14 In our study, more than 20% of infants tested had a positive EV PCR, which exceeded this threshold. However, more than half of the infants with a positive EV PCR test result had a LOS of two or more days. The observed reductions in LOS were greatest for the infants with CSF pleocytosis, although almost three-quarters of these infants did not have an EV PCR test obtained. Even during the peak enteroviral season, 60% of infants with CSF pleocytosis did not have an EV PCR test performed even though half of the tested infants had a positive result. Targeted utilization of the EV PCR test (eg, for infants with CSF pleocytosis or during annual peak enteroviral season) and rapid turnaround time likely would increase the test’s impact on hospital LOS.
Our study has several limitations. First, our study was conducted at North American children’s hospitals, and may not be generalizable to other settings. Second, we were unable to determine the timing of EV PCR result availability to the clinical team, which may have varied between participating institutions and over the study period. Third, we relied on the time that the treating clinician signed the discharge order to calculate the hospital LOS. Although some infants may have lingered in the hospital while awaiting a caregiver’s arrival or for transportation home, the order was signed when the clinical team judged the infant appropriate for home management. Fourth, we do not have data on the infants’ clinical appearance and ill-appearing infants would have a longer hospital LOS, regardless of the EV PCR test result. Although we examined intensive care unit admission as a proxy measure, admission location for neonates varies across centers and, therefore, may not reflect illness severity. Fifth, we were unable to determine which infants had received antibiotics prior to performance of the diagnostic lumbar puncture, potentially rendering bacterial cultures falsely negative.29 However, we do know that the youngest infants with fever are infrequently pretreated with antibiotics.16,18 Sixth, EV PCR testing decisions were at the discretion of the clinical team. Although we adjusted for patient age and study year, and clustering by center, we cannot fully control for clinical differences between tested and untested infants, raising the potential for unadjusted confounding. Last, we excluded infants who were discharged from the ED. In the future, EV PCR testing platforms with very rapid test turnaround times (eg, as little as 1 hour) may provide results while the patient is still in the ED, which may reduce rates of hospitalization altogether in certain patients.
Acknowledgments
Supported by the Section of Emergency Medicine of the American Academy of Pediatrics (AAP) and Baylor College of Medicine. Paul L. Aronson received support for this work from CTSA grant number KL2 TR001862 from the National Center for Advancing Translational Science (NCATS), a component of the National Institutes of Health (NIH). Todd W. Lyons was supported by a training grant from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NIH) Childhood (5T32HD040128-12). Stephen Freedman is supported by the Alberta Children’s Hospital Foundation Professorship in Child Health and Wellness.
We would like to acknowledge the other site investigators in the Pediatric Emergency Medicine Clinical Research Network (PEM CRC) Herpes Simplex Virus (HSV) Study Group who contributed patient data for this study and/or the parent study. See online Appendix for the list of site investigators.
Abbreviations
- CSF
cerebrospinal fluid
- ED
emergency department
- EV
enterovirus
- LOS
length of stay
- PCR
polymerase chain reaction
- SBI
serious bacterial infection
- UTI
urinary tract infection
- WBC
white blood cell
Appendix. Pediatric Emergency Medicine Clinical Research Network (PEM CRC) Herpes Simplex Virus (HSV) Study Group
Elizabeth R. Alpern, MD, MSCE, Northwestern University Feinberg School of Medicine, Chicago, IL
Fran Balamuth, MD, PhD, MSCE, University of Pennsylvania Perelman School of Medicine, Philadelphia, PA
Stuart A. Bradin, DO, University of Michigan Medical School, Ann Arbor, MI
Sarah J. Curtis, MD, MSc, University of Alberta Faculty of Medicine and Dentistry, Edmonton, AB
Aris C. Garro, MD, MPH, Alpert Medical School of Brown University, Providence, RI
Kendra L. Grether-Jones, MD, University of California-Davis School of Medicine, Sacramento, CA
Paul T. Ishimine, MD, University of California-San Diego School of Medicine, San Diego, CA
Dina Kulik, MD, University of Toronto School of Medicine, Toronto, ON
Prashant Mahajan, MD, MPH, MBA, University of Michigan Medical School, Ann Arbor, MI
Aaron S. Miller, MD, MSPH, St. Louis University School of Medicine, St. Louis, MO
Rakesh D. Mistry, MD, MS, University of Colorado School of Medicine, Aurora, CO
Christopher M. Pruitt, MD, University of Alabama-Birmingham School of Medicine, Birmingham, AL
David Schnadower, MD, MPH, Washington University School of Medicine, St. Louis, MO
Samir S. Shah, MD, MSCE, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH
Joanna E. Thomson, MD, MPH, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH
Neil G. Uspal, MD, University of Washington School of Medicine, Seattle, WA
Footnotes
The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hui C, Neto G, Tsertsvadze A, Yazdi F, Tricco AC, Tsouros S, et al. Diagnosis and management of febrile infants (0–3 months) Evid Rep Technol Assess (Full Rep) 2012;(205):1–297. [PMC free article] [PubMed] [Google Scholar]
- 2.Aronson PL, Thurm C, Alpern ER, Alessandrini EA, Williams DJ, Shah SS, et al. Variation in Care of the Febrile Young Infant <90 Days in US Pediatric Emergency Departments. Pediatrics. 2014;134:667–77. doi: 10.1542/peds.2014-1382. [DOI] [PubMed] [Google Scholar]
- 3.Levine DA, Platt SL, Dayan PS, Macias CG, Zorc JJ, Krief W, et al. Risk of serious bacterial infection in young febrile infants with respiratory syncytial virus infections. Pediatrics. 2004;113:1728–34. doi: 10.1542/peds.113.6.1728. [DOI] [PubMed] [Google Scholar]
- 4.Baker MD, Avner JR, Bell LM. Failure of infant observation scales in detecting serious illness in febrile, 4- to 8-week-old infants. Pediatrics. 1990;85:1040–43. [PubMed] [Google Scholar]
- 5.Byington CL, Taggart EW, Carroll KC, Hillyard DR. A polymerase chain reaction-based epidemiologic investigation of the incidence of nonpolio enteroviral infections in febrile and afebrile infants 90 days and younger. Pediatrics. 1999;103:E27. doi: 10.1542/peds.103.3.e27. [DOI] [PubMed] [Google Scholar]
- 6.Rittichier KR, Bryan PA, Bassett KE, Taggart EW, Enriquez FR, Hillyard DR, et al. Diagnosis and outcomes of enterovirus infections in young infants. Pediatr Infect Dis J. 2005;24:546–50. doi: 10.1097/01.inf.0000164810.60080.ad. [DOI] [PubMed] [Google Scholar]
- 7.King RL, Lorch SA, Cohen DM, Hodinka RL, Cohn KA, Shah SS. Routine cerebrospinal fluid enterovirus polymerase chain reaction testing reduces hospitalization and antibiotic use for infants 90 days of age or younger. Pediatrics. 2007;120:489–96. doi: 10.1542/peds.2007-0252. [DOI] [PubMed] [Google Scholar]
- 8.Schlesinger Y, Sawyer MH, Storch GA. Enteroviral meningitis in infancy: potential role for polymerase chain reaction in patient management. Pediatrics. 1994;94:157–62. [PubMed] [Google Scholar]
- 9.Ahmed A, Brito F, Goto C, Hickey SM, Olsen KD, Trujillo M, et al. Clinical utility of the polymerase chain reaction for diagnosis of enteroviral meningitis in infancy. J Pediatr. 1997;131:393–97. doi: 10.1016/s0022-3476(97)80064-9. [DOI] [PubMed] [Google Scholar]
- 10.Lyons TW, McAdam AJ, Cohn KA, Monuteaux MC, Nigrovic LE. Impact of in-hospital enteroviral polymerase chain reaction testing on the clinical management of children with meningitis. J Hosp Med. 2012;7:517–20. doi: 10.1002/jhm.1947. [DOI] [PubMed] [Google Scholar]
- 11.Nigrovic LE, Malley R, Agrawal D, Kuppermann N Pediatric Emergency Medicine Collaborative Research Committee of the American Academy of P. Low risk of bacterial meningitis in children with a positive enteroviral polymerase chain reaction test result. Clin Infect Dis. 2010;51:1221–22. doi: 10.1086/656919. [DOI] [PubMed] [Google Scholar]
- 12.Ramers C, Billman G, Hartin M, Ho S, Sawyer MH. Impact of a diagnostic cerebrospinal fluid enterovirus polymerase chain reaction test on patient management. JAMA. 2000;283:2680–85. doi: 10.1001/jama.283.20.2680. [DOI] [PubMed] [Google Scholar]
- 13.Dewan M, Zorc JJ, Hodinka RL, Shah SS. Cerebrospinal fluid enterovirus testing in infants 56 days or younger. Arch Pediatr Adolesc Med. 2010;164:824–30. doi: 10.1001/archpediatrics.2010.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nigrovic LE, Chiang VW. Cost analysis of enteroviral polymerase chain reaction in infants with fever and cerebrospinal fluid pleocytosis. Arch Pediatr Adolesc Med. 2000;154:817–21. doi: 10.1001/archpedi.154.8.817. [DOI] [PubMed] [Google Scholar]
- 15.Nolte FS, Rogers BB, Tang YW, Oberste MS, Robinson CC, Kehl KS, et al. Evaluation of a rapid and completely automated real-time reverse transcriptase PCR assay for diagnosis of enteroviral meningitis. J Clin Microbiol. 2011;49:528–33. doi: 10.1128/JCM.01570-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Milcent K, Faesch S, Gras-Le Guen C, Dubos F, Poulalhon C, Badier I, et al. Use of Procalcitonin Assays to Predict Serious Bacterial Infection in Young Febrile Infants. JAMA Pediatr. 2016;170:62–9. doi: 10.1001/jamapediatrics.2015.3210. [DOI] [PubMed] [Google Scholar]
- 17.Hoberman A, Wald ER. Urinary tract infections in young febrile children. Pediatr Infect Dis J. 1997;16:11–7. doi: 10.1097/00006454-199701000-00004. [DOI] [PubMed] [Google Scholar]
- 18.Mahajan P, Kuppermann N, Mejias A, Suarez N, Chaussabel D, Casper TC, et al. Association of RNA Biosignatures With Bacterial Infections in Febrile Infants Aged 60 Days or Younger. JAMA. 2016;316:846–57. doi: 10.1001/jama.2016.9207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baskin MN, O’Rourke EJ, Fleisher GR. Outpatient treatment of febrile infants 28 to 89 days of age with intramuscular administration of ceftriaxone. J Pediatr. 1992;120:22–7. doi: 10.1016/s0022-3476(05)80591-8. [DOI] [PubMed] [Google Scholar]
- 20.Baker MD, Bell LM, Avner JR. Outpatient management without antibiotics of fever in selected infants. N Engl J Med. 1993;329:1437–41. doi: 10.1056/NEJM199311113292001. [DOI] [PubMed] [Google Scholar]
- 21.Kestenbaum LA, Ebberson J, Zorc JJ, Hodinka RL, Shah SS. Defining cerebrospinal fluid white blood cell count reference values in neonates and young infants. Pediatrics. 2010;125:257–64. doi: 10.1542/peds.2009-1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wallace SS, Lopez MA, Caviness AC. Impact of enterovirus testing on resource use in febrile young infants: a systematic review. Hosp Pediatr. 2017;7:96–102. doi: 10.1542/hpeds.2016-0060. [DOI] [PubMed] [Google Scholar]
- 23.Aronson PL, Thurm C, Williams DJ, Nigrovic LE, Alpern ER, Tieder JS, et al. Association of clinical practice guidelines with emergency department management of febrile infants ≤56 days of age. J Hosp Med. 2015;10:358–65. doi: 10.1002/jhm.2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Elshaug AG, McWilliams JM, Landon BE. The value of low-value lists. JAMA. 2013;309:775–76. doi: 10.1001/jama.2013.828. [DOI] [PubMed] [Google Scholar]
- 25.Ninove L, Nougairede A, Gazin C, Zandotti C, Drancourt M, de Lamballerie X, et al. Comparative detection of enterovirus RNA in cerebrospinal fluid: GeneXpert system vs. real-time RT-PCR assay. Clin Microbiol Infect. 2011;17:1890–94. doi: 10.1111/j.1469-0691.2011.03487.x. [DOI] [PubMed] [Google Scholar]
- 26.Leber AL, Everhart K, Balada-Llasat JM, Cullison J, Daly J, Holt S, et al. Multicenter Evaluation of BioFire FilmArray Meningitis/Encephalitis Panel for Detection of Bacteria, Viruses, and Yeast in Cerebrospinal Fluid Specimens. J Clin Microbiol. 2016;54:2251–61. doi: 10.1128/JCM.00730-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Biondi EA, Mischler M, Jerardi KE, Statile AM, French J, Evans R, et al. Blood culture time to positivity in febrile infants with bacteremia. JAMA Pediatr. 2014;168:844–49. doi: 10.1001/jamapediatrics.2014.895. [DOI] [PubMed] [Google Scholar]
- 28.Fielding-Singh V, Hong DK, Harris SJ, Hamilton JR, Schroeder AR. Ruling out bacteremia and bacterial meningitis in infants less than one month of age: is 48 hours of hospitalization necessary? Hosp Pediatr. 2013;3:355–61. doi: 10.1542/hpeds.2013-0009. [DOI] [PubMed] [Google Scholar]
- 29.Kanegaye JT, Soliemanzadeh P, Bradley JS. Lumbar puncture in pediatric bacterial meningitis: defining the time interval for recovery of cerebrospinal fluid pathogens after parenteral antibiotic pretreatment. Pediatrics. 2001;108:1169–74. [PubMed] [Google Scholar]