Abstract
Van Braeckel-Budimmir et al. (2017) reveal that the pathogenic response of mice to a Plasmodium berghei infection is dominated by a Vβ8.1 T cell response. Mice lacking Vβ8.1 T cells fail to mount a pathogenic response, thus showing that the TCR locus can be an Immune Response (Ir) gene.
One of the seminal discoveries in modern immunology was that the ability to mount an immune response to an antigen is genetically controlled. Baruj Benacerraf and colleagues in 1963 showed that two inbred strains of guinea pigs differed in their ability to mount a T cell response to synthetic amino acid polymers (Levine et al., 1963). Strain 2 responded to the antigen poly L-lysine, whereas strain 13 did not. Conversely, Strain 2 did not respond to glutamic acid/tyrosine, while strain 13 did. This Ir (immune response) gene was identified as being autosomal dominant, but further genetic analysis in guinea pigs proved difficult.
Subsequently, Hugh McDevitt at Mill Hill in London with John Humphrey and Ita Askonas mapped the Ir gene to the MHC locus. Using synthetic co-polymers developed by Michael Sela, McDevitt initially observed that rabbits differed in their ability to respond to the co-polymers, but like the guinea pig, genetic analysis of this response proved difficult. Turning to MHC congenic mouse strains, McDevitt showed that the Ir genes mapped to the MHC locus (McDevitt and Sela, 1965). Up until this time, MHC molecules were the prerogative of transplantation biologists, and their real physiological role was not appreciated. McDevitt’s discovery placed the MHC molecules into their appropriate framework-at the center of adaptive immune responses. Further mapping using intra-H-2 recombinant mouse strains mapped the Ir genes to a region between H-2K and H-2D, initially named the I region for Immune response, which we now call the MHC class II region (McDevitt, 2000).
The molecular mechanism of how Ir genes functioned was at the center of immunology research in the 1970s and many theories, entire meetings, and whole books were devoted to this subject (Katz and Benacerraf, 1976). Ir genes were identified for several small protein antigens such as cytochrome c, or hen egg-white lysozyme, but not when larger more complex antigens (such as KLH) were tested. A major breakthrough in the field of Ir genes was made by Unanue and colleagues, when they reported the first demonstration of a peptide binding to an MHC molecule (Babbitt et al., 1985). They showed that the HEL(46-61) peptide bound to I-Ak molecules from a responder strain, but not to I-Ad molecules from a non-responder strain. Thus, the basis for responder/non-responder “status” in mice was the ability (or inability) of a peptide to bind to an MHC molecule, and subsequently be recognized by a TCR. It now also made sense why, for large protein antigens, responder/non-responder mouse strains could not be found: there were always one or more peptides that could bind to any particular MHC molecule, and stimulate the T cells. Ir genes were mostly a class II restricted phenomenon, but a few class I Ir genes were reported. As more and more class I- and class II-restricted epitopes were identified, another unexplained phenomenon arose: that of immunodominant responses against viruses and bacteria. For example, a dominant epitope in the class I-restricted response against LCMV is the gp33/H-2Db epitope, comprising ~10 % of the responding CD8+ T cells (Wherry et al., 2003).
Infection of mice with P. berghei is an important model of severe malarial disease. Previous studies have reported that the development of experimental cerebral malaria (ECM) during P. berghei infection is absolutely dependent on pathogenic CD8+ T cells, in particular Vβ8+ cells recognizing the immunodominant H-2Db-restricted epitope GAP5040-48, but, It had not been established why these T cells are pathogenic in ECM. In this issue, Van Breackel-Budimir et al. established the basis for this pathogenic response (Van Braeckel-Budimmir et al., 2017).
The authors first compared specific responses against GAP5040-48 immunization to those of other P. berghei-derived epitopes. They found that, unlike responses to other epitopes, CD8+ T cells responding to GAP5040-48 have a uniform and absolute usage of Vβ8.1+. Using mutational analysis of the GAP5040-48 peptide, they identified two key C-terminal residues that constituted “hotspots” for TCR recognition. A crystal structure of a protypical TCR:peptide-MHC (pMHC) revealed that the TCR β chain overwhelmingly dictated interactions with the peptide, while the TCRα chain mostly interacted with the MHC molecule. Intriguingly, this suggested that the peptide itself, not the MHC in this case, was driving the preference for Vβ8.1 usage. Structurally speaking, Vβ8.1 and Vβ8.2 TCR have been the most extensively studied TCRs. Evidence for a germline affinity between the TCR and MHC has been found for both Vβ8.1 and Vβ8.2 TCRs, in the form of conserved interaction motifs in the CDR2β and framework TCRβ chain (Feng et al., 2007). Strikingly, the GAP5040-48-specific TCRs do not use the same conserved residues to bind H-2Db, thereby challenging the concept of a germline encoded affinity between the TCR and MHC.
Focusing then on the naive repertoire that serves as the source of the ECM response to P. berghei, the authors used tetramer enrichment to estimate that there exists a pool of ~3000 GAP5040-48-specific precursor cells per mouse; notably, this is the largest antigen-specific naive population ever described. This finding implied that thymic positive selection was a major contributor to the robust CD8+ T cell response to GAP5040-48. To further support this idea, the authors found that levels of the immunomodulatory molecule CD5 are especially high on this population, indicating strong selection of these cells during thymic development. By then expressing the GAP5040-48 epitope in Listeria monocytogenes, they showed that this rapidly expanding GAP5040-48-specific precursor population conferred enhanced protection in the context of a bacterial infection.
To demonstrate the in vivo significance of the GAP5040-48. immunodominant response, the authors examined responses to P. berghei infection in C57/L mice, which are H-2b-restricted, but lack several TCR Vβ genes, including Vβ8.1. Unlike their Vβ8.1-replete B6 counterparts, the C57/L mice produced no detectable GAP5040-48 responses. The C57/L mice did not succumb to ECM like B6 mice, but rather died from uncontrollable parasite burdens several weeks after infection. This remarkable finding shows that the absence of germline-encoded Vβ8.1 results in not only a lack of the pathogenic response necessary for ECM development, but also the ability to control P. berghei infections (Preview Figure, left panel). This finding by Van Braeckel-Budimir et al., revealed that the TCR locus can be an Ir gene, and can determine if a T cell response against a pathogen will occur or not (Van Braeckel-Budimmir et al., 2017). Thus, this novel demonstration shows that the T cell can also be responsible for an in vivo Ir gene response- a concept that had been previously proposed, but not supported by experiments (Preview Figure, right panel) (Katz and Benacerraf, 1976).
Figure. Mice must express Vβ8.1 to mount a pathogenic CD8+ T cell response against P. berghei infection.
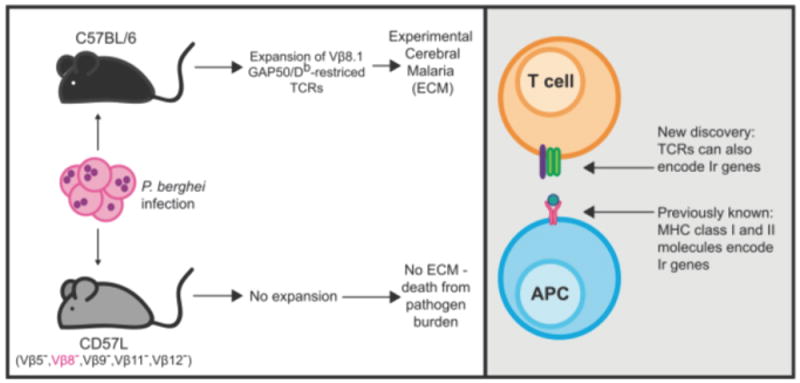
(Left) C57/BL6 mice mount a robust pathogenic CD8+ T cell response against P. berghei in a murine model of malaria, which ultimately leads to development of Experimental Cerebral Malaria (ECM). This response is overwhelmingly specific for an H-2Db-restricted epitope that is derived from GAP50. In contrast, CD57L mice, which express H-2Db but lack several TCR Vβ chains (including Vβ8), fail to control P. berghei infection and ultimately succumb to a large pathogen burden. (Right) Herein, the authors show that the capacity of C57/BL6 mice to mount this response lies in their germline expression of Vβ8.1. While it has been previously shown that MHC class I and II molecules can encode germline immune response (Ir) genes, Van Breackel-Budimir et al. for the first time show that TCR chains can also encodeIr genes.
One implication of these studies is that there must exist a single peptide involved in positive selection of the Vβ8.1 GAP5040-48 T cells. The identification of this positively selecting peptide (and the explanation for this incredibly large naive precursor population) would form the basis of fascinating future studies. What is the significance of the finding that the TCR can be an Ir gene, in the context of human immune responses? The authors’ findings raise the possibility that polymorphisms in the TCR loci could dictate whether an individual could respond (or not respond) to a vaccine challenge. Two recent reports describe a process of finding patterns in human T cell recognition of pMHC using tetramer enrichment, high-throughput TCR sequencing, and novel algorithms (Dash et al., 2017; Glanville et al., 2017). These studies could provide an approach to investigate the TCR Ir gene phenomenon in depth. Overall, these findings by Van Breackel-Budimir et al. show how the TCR can take some of the responsibility away from the pMHC in determining whether an individual can respond to a pathogen.
Contributor Information
Ashley A. Viehmann Milam, Department of Pathology and Immunology, Washington University
Paul M. Allen, Department of Pathology and Immunology, Washington University
References
- Babbitt BP, Allen PM, Matsueda G, Haber E, Unanue ER. Binding of immunogenic peptides to Ia histocompatibility molecules. Nature. 1985;317:359–361. doi: 10.1038/317359a0. [DOI] [PubMed] [Google Scholar]
- Dash P, Fiore-Gartland AJ, Hertz T, Wang GC, Sharma S, Souquette A, Crawford JC, Clemens EB, Nguyen THO, Kedzierska K, et al. Quantifiable predictive features define epitope-specific T cell receptor repertoires. Nature. 2017;547:89–93. doi: 10.1038/nature22383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng D, Bond CJ, Ely LK, Maynard J, Garcia KC. Structural evidence for a germline-encoded T cell receptor-major histocompatibility complex interaction ‘codon’. Nat Immunol. 2007;8:975–983. doi: 10.1038/ni1502. [DOI] [PubMed] [Google Scholar]
- Glanville J, Huang H, Nau A, Hatton O, Wagar LE, Rubelt F, Ji X, Han A, Krams SM, Pettus C, et al. Identifying specificity groups in the T cell receptor repertoire. Nature. 2017;547:94–98. doi: 10.1038/nature22976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz DH, Benacerraf B. The role of products of the histocompatibility gene complex in immune responses. New York: Academic Press, Inc.; 1976. [Google Scholar]
- Levine BB, Ojeda A, Benacerraf B. Studies on Artificial Antigens. Iii. The Genetic Control of the Immune Response to Hapten-Poly-L-Lysine Conjugates in Guinea Pigs. J Exp Med. 1963;118:953–957. doi: 10.1084/jem.118.6.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDevitt HO. Discovering the role of the major histocompatibility complex in the immune response. Annu Rev Immunol. 2000;18:1–17. doi: 10.1146/annurev.immunol.18.1.1. [DOI] [PubMed] [Google Scholar]
- McDevitt HO, Sela M. Genetic control of the antibody response. I. Demonstration of determinant-specific differences in response to synthetic polypeptide antigens in two strains of inbred mice. J Exp Med. 1965;122:517–531. doi: 10.1084/jem.122.3.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Braeckel-Budimmir N, Gras S, Ladell K, Josephs TM, Pewe L, Urban SL, Miners KL, Farenc C, Price DA, Rossjohn J, Harty JA. An immune response gene in a T cell receptor locus. Immunity. 2017;xx(this issue):xxxx–xxxx. doi: 10.1016/j.immuni.2017.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wherry EJ, Blattman JN, Murali-Krishna K, van der Most R, Ahmed R. Viral persistence alters CD8 T-cell immunodominance and tissue distribution and results in distinct stages of functional impairment. J Virol. 2003;77:4911–4927. doi: 10.1128/JVI.77.8.4911-4927.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]


