Abstract
High-dose therapy followed by autologous stem cell transplantation (ASCT) can improve outcomes for mantle cell lymphoma (MCL) but is associated with a high incidence of relapse. A retrospective study of 191 MCL patients who underwent ASCT at City of Hope was performed to examine prognostic factors for outcomes following ASCT. For all patients, the 5-year overall survival (OS) was 71% (confidence interval [CI]: 63% – 77%) and progression-free survival (PFS) was 53% (CI: 45% – 60%). The 5-year cumulative incidence of relapse was 41% (CI: 34% – 48%) with a continuous pattern of relapse events occurring at a median of 2.1 years (range: 0.2 – 13.4) after ASCT. In multivariate analysis, post-transplant maintenance rituximab was the factor most significantly associated with both OS (relative risk [RR] 0.17, CI: 0.07 – 0.38) and PFS (RR 0.25, CI: 0.14 – 0.44). For the subset of patients who had positron emission tomography (PET) data available and were in a PET-negative first complete remission (CR1) at ASCT (n = 105), Maintenance rituximab was significantly associated with superior OS (RR 0.17, CI: 0.05 – 0.59) and PFS (RR 0.20, CI: 0.09 – 0.43). These results support a benefit with maintenance rituximab for all MCL patients treated with ASCT.
Keywords: Autologous hematopoietic cell transplantation, mantle cell lymphoma, rituximab
INTRODUCTION
Mantle cell lymphoma (MCL) comprises a biologically heterogeneous group of B-cell non-Hodgkin lymphoma (NHL) united by the near-canonical translocation t(11;14)(q13;q32) involving the cyclin D1 gene. Although a small subset of patients present with very indolent disease and can be simply observed(1), the majority of patients will require treatment due to aggressive disease. The management of MCL has historically represented a great challenge as frontline chemoimmunotherapy, although associated with a high response rate, is not curative, and relapses tend to be the rule rather than the exception(2). Risk stratification models such as the Mantle Cell Lymphoma International Prognostic Index (MIPI) clearly have a role in predicting the prognosis(3), and intrinsic biological factors and the diversity of treatment patterns in MCL largely account for the wide variation of clinical outcomes seen in MCL.
The overall outcome of MCL has improved over time due to refinements in induction therapy, the incorporation of early consolidative ASCT, and the introduction of novel targeted therapies such as lenalidomide, bortezomib, and ibrutinib(4–6). Intensive induction regimens such as R-hyper-CVAD (rituximab, hyper-fractionated cyclophosphamide, vincristine, adriamycin, and dexamethasone alternating with rituximab, high-dose methotrexate, and high-dose cytarabine), the Nordic regimen (rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone alternating with rituximab, dexamethasone, cytarabine, and cisplatin), and other high-dose cytarabine-containing regimens, with or without early ASCT, have been associated with superior outcomes as compared to historical controls(7–11). Maintenance rituximab post induction immunochemotherapy regimens such as rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP)(12), modified R-HyperCVAD(13), and rituximab, bortezomib, modified hyper-fractionated cyclophosphamide, doxorubicin, vincristine, dexamethasone (VcR-CVAD)(14) has been shown to reduce the risk of relapse and high rates of durable remission in older and transplant ineligible patients with MCL although a recent report by Rummel, et al. did not find any benefit from maintenance rituximab after frontline bendamustine and rituximab therapy(15). In the context of ASCT, data from the phase III LyMa trial were recently presented at ASH 2016 and showed an improvement in survival with post-ASCT maintenance rituximab after induction therapy with rituximab, dexamethasone, cytarabine, and cisplatin (RDHAP) in younger patients(16). Nonetheless, despite these advances, a troubling pattern of ongoing relapses over time without a clear plateau in progression-free survival remains the norm in MCL, highlighting the need for improved therapies as well as a better understanding of relevant baseline and treatment-related prognostic factors.
Here we present a single-center experience of 191 consecutive MCL patients treated with ASCT with an analysis of prognostic factors.
PATIENTS AND METHODS
Patients and eligibility
This is a single-center retrospective study of 191 consecutive MCL patients treated with ASCT at the City of Hope National Medical Center (COH) from January 1997 to November 2013. Medical records were retrospectively reviewed for comprehensive patient characteristics and clinical outcomes. The follow-up cut off was February 2016. Initially 202 cases during the study period were identified based on Center for International Blood and Marrow Transplant Research (CIBMTR) database. Excluded from this analysis were patients with non-MCL diagnosis based on medical record review (n=1), MCL patients who received preemptive post-transplant therapy with CD19-directed chimeric antigen receptor T-cell infusion (n = 1) or maintenance using rituximab and bortezomib (n = 9). The COH Institutional Review Board approved this study.
Treatment
ASCT conditioning regimens were selected by the individual transplant physician according to patient age, treatment history, and clinical trial availability at the time of transplant. There were two ongoing clinical trials with the CD20-directed radioimmunotherapy ibritumomab tiuxetan (Zevalin®) in combination with either high dose cyclophosphamide and etoposide (High-Z), or carmustine, etoposide, cytarabine, and melphalan (Zevalin-BEAM). Patients who were less than 55 years old and had not received prior radiotherapy were eligible to receive total body irradiation (TBI) followed by etoposide and cyclophosphamide. The remaining patients were conditioned with either cyclophosphamide, carmustine, and etoposide (CBV) or carmustine, etoposide, cytarabine, and melphalan (BEAM). Maintenance rituximab post-transplant was recommended but given on an individual basis at the discretion of the treating physician.
Statistical analysis
Patient demographics, disease, and treatment characteristics were compared between patients who received maintenance rituximab vs. patients who did not by the Wilcoxon rank sum test for continuous variables and Chi-square test or Fisher’s exact test for categorical variables. PFS was defined as the time from ASCT to disease relapse/progression or death due to any cause, whichever occurred first; patients who were free of these events at the last contact were censored. OS was defined as the time from ASCT to death due to any cause; patients alive at the last contact were censored. PFS and OS were estimated by the Kaplan-Meier product limit estimator with Greenwood estimator for standard error; 95% confidence interval was constructed based on log-log transformation. Cumulative incidence curves were computed for relapse/progression and non-relapse mortality (NRM) by competing risk methodology where relapse/progression and NRM were treated as competing risks.
In the Cox regression analysis on PFS and OS, all explanatory variables were treated as time-independent variables except maintenance rituximab, which was treated as a time-dependent binary variable taking a value of 0 for no-maintenance rituximab patients and switching from 0 to 1 in patients after the start of maintenance rituximab. The relative risk (RR) which referred to the estimated hazard ratio from these Cox models were reported. Since MIPI had been shown to be an important predictor of outcome, in multivariate Cox regression analysis it was always included in the model regardless of its statistical significance. For all other explanatory variables, a step-wise model selection algorithm (based on score test with variable entry criteria p<0.25 and stay criteria p<0.2) was used to select the variables in the final model.
For the landmark analysis of PFS and OS by maintenance rituximab status, PFS/OS was computed from day 180 post HCT; patients who experienced an event for PFS/OS prior to day 180 were excluded. Patients who started maintenance rituximab before day 180 were categorized as maintenance rituximab while patients who never received maintenance rituximab or those who started it after day 180 were categorized as nomaintenance rituximab in the landmark analysis. A log rank test was used to compare the PFS/OS curves in the landmark analysis.
All analyses were performed in SAS version 9.4, and all P values cited were 2-sided. A P value < 0.05 was considered statistically significant.
RESULTS
Patient and Treatment Characteristics
One-hundred and ninety-one patients treated with ASCT between January 1997 and November 2013 were evaluable for this study (Table 1). The median age at diagnosis was 59 years (range: 33 – 76), and 142 (74%) were male. Nearly all (98%) patients had stage III or IV disease. The MIPI score at diagnosis could be determined for 131 (69%) patients, of which 52% were intermediate or high risk. The majority of patients had received one frontline therapy (67%, n = 128) prior to ASCT and one hundred and six (56%) patients received a frontline regimen containing high-dose cytarabine, either Hyper-CVAD (n = 93), the Nordic regimen (n = 10), or both (n = 3). One hundred and forty-four patients (75%) underwent ASCT in CR1 while twenty eight (15%) patients were transplanted in PR1, and nineteen (10%) patients were transplanted in a second remission or later. One hundred and one patients (53%) received chemotherapy-only ASCT conditioning regimens, either CBV or BEAM, and the remainder (47%, n = 90) received a radiation-based conditioning regimen, either TBI (17%, n = 32), Zevalin-BEAM (26%, n = 49), or High-Z (5%, n = 9).
Table 1.
Patient and Transplant Characteristics
| Total number of patients | 191 |
| Median age at diagnosis, years (range) | 59 (33 – 76) |
| Year of Diagnosis (Median 2006) | |
| 1992–2005 | 89 (47%) |
| 2006 and later | 102 (53%) |
| Male gender, no. (%) | 142 (74%) |
| Stage, at diagnosis | |
| I/II | 3(2%) |
| III | 20 (10%) |
| IV | 168 (88%) |
| B Symptoms, at diagnosis | 36 (19%) |
| Bone marrow involvement, at diagnosis | 149 (78%) |
| Extranodal involvement, at diagnosis | 67 (35%) |
| Blastoid variant | 16 (8%) |
| MIPI (n=131)a | |
| Low risk | 63 (48%) |
| Intermediate risk | 42 (32%) |
| High risk | 26 (20%) |
| High-dose cytarabine pre-ASCT, no. (%)b | 106 (56%) |
| Rituximab pre-ASCT, no. (%) | 175 (92%) |
| No. or regimens pre-ASCT, no. (%) | |
| 1 | 128 (67%) |
| 2 | 48 (25%) |
| 3 | 15 (8%) |
| Median time from diagnosis to ASCT, months (range) | 8 (4 – 105) |
| Year of Transplant (Median 2007) | |
| 1997–2006 | 88 (46%) |
| 2007 and later | 103 (54%) |
| Disease status at ASCT, no. (%) | |
| CR1 | 144 (75%) |
| PR1 | 28 (15%) |
| CR2/PR2/PR3 | 19 (10%) |
| Conditioning, no. (%) | |
| Chemotherapy only | 101 (53%) |
| Radiation-based | 90 (47%) |
| Conditioning, no. (%) | |
| BEAM | 54 (28%) |
| CBV | 47 (25%) |
| TBI/VP/CY | 32 (17%) |
| Z-BEAM | 49 (26%) |
| High-Z | 9 (5%) |
| Rituximab maintenance, no. (%) | |
| 375mg/m2 weekly × 4 every 6 mos. for 2 yrs. | 46 (24%) |
| 375mg/m2 every 2 mos. for 2 yrs. | 16 (8%) |
| 375mg/m2 every 3 mos. for 2 yrs. | 13 (7%) |
| None | 116 (61%) |
| Median follow-up post-ASCT, yrs. (range)c | 6.3 (2.1 – 17.8) |
Abbreviations: CR, complete remission; ASCT, high-dose therapy; MIPI, mantle cell lymphoma International prognostic index; PR, partial remission; BEAM, carmustine, etoposide, cytarabine, melphalan; CBV, cyclophosphamide, carmustine, etoposide; TBI/VP/CY, total body irradiation, etoposide, cyclophosphamide; Z-BEAM, ibritumomab tiuxetan, carmustine, etoposide, cytarabine, melphalan; High-Z, ibritumomab tiuxetan, cyclophosphamide, etoposide
Data not available for all patients.
Patients treated with high-dose cytarabine received Hyper-CVAD (n=93), MaxiCHOP (n=10), or both (n=3).
Surviving patients (n=119).
One hundred and seventy-five patients (92%) received rituximab prior to ASCT. Seventy-five (39%) patients were given maintenance rituximab after ASCT. Rituximab at a dose of 375mg/m2 was administered using one of three dosing schedules: weekly dosing for 4 weeks every 6 months for four courses over 2 years (n = 46), every 2 month dosing for twelve doses over 2 years (n = 16), or every 3 month dosing for eight doses over 2 years (n = 13). Maintenance rituximab was started and completed, respectively, at a median of 130 days (range: 36 – 451) and 735 days (range: 70 – 1121) after ASCT. Among patients receiving maintenance rituximab, ten (13%) did not complete all scheduled rituximab infusions, most commonly due to disease progression (n = 9).
PET scan results prior to ASCT were available in 133 patients (70%), of which 105 patients (79%) were in a PET-negative CR1 prior to ASCT (Table 2). All but one of these patients (99%) received rituximab prior to ASCT. Eighteen patients had PET-positive disease prior to ASCT. PET-negative CR1 patients who received maintenance rituximab as opposed to those who did not were significantly more likely have received high-dose cytarabine prior to ASCT (74% vs 45%, p=0.002). The two groups were otherwise similar with respect to age, gender, stage, presence of B symptoms, extranodal disease, MIPI score, time from diagnosis to ASCT, ASCT regimen, and median follow-up time post ASCT (5.9 years vs 5.2 years, p=0.20).
Table 2.
Characteristics of patients undergoing ASCT in PET-negative CR1 a
| All (n = 105) | maintenance rituximab (n = 58) |
No-maintenance rituximab (n = 47) |
P-valueb | |
|---|---|---|---|---|
|
|
||||
| Median age at diagnosis, years (range) | 58 (35–74) | 56 (35–70) | 60 (43–74) | 0.054 |
| Male | 73 (70%) | 36 (62%) | 37 (79%) | 0.065 |
| Year of Diagnosis: 2006 and later | 81 (77%) | 48 (83%) | 33 (70%) | 0.13 |
| Stage, at diagnosis | ||||
| I/II | 3 (3%) | 1 (2%) | 2 (4%) | 0.51 |
| III | 7 (7%) | 5 (9%) | 2 (4%) | |
| IV | 95 (90%) | 52 (90%) | 43 (91%) | |
| B symptoms, at diagnosis | 16 (15%) | 6 (10%) | 10 (21%) | 0.12 |
| Bone marrow involvement, at diagnosis | 85 (81%) | 46 (79%) | 39 (83%) | 0.63 |
| Extranodal involvement, at diagnosis | 39 (37%) | 23 (40%) | 16 (34%) | 0.55 |
| Blastoid variant | 8 (8%) | 5 (9%) | 3 (6%) | 0.67 |
| MIPI | 0.36 | |||
| Low | 42 (40%) | 26 (45%) | 16 (34%) | |
| Intermediate/high | 33 (31%) | 15 (26%) | 18 (38%) | |
| Unknown | 30 (29%) | 17 (29%) | 13 (28%) | |
| High-dose cytarabine pre-ASCT | 64 (61%) | 43 (74%) | 21 (45%) | 0.002 |
| Rituximab pre-ASCT | 104 (99%) | 57 (98%) | 47 (100%) | 1.0 |
| Median time from dx to ASCT (months) | 7.4 (4.8–105.3) | 7.0 (4.8–105.3) | 8.0 (4.9–20.7) | 0.052 |
| Year of ASCT, 2007 and later | 81 (77%) | 48 (83%) | 33 (70%) | 0.13 |
| No of regimens pre-ASCT, 2 or 3 | 18 (17%) | 11 (19%) | 7 (15%) | 0.58 |
| Radiation-based conditioning | 46 (44%) | 24(41%) | 22 (47%) | 0.58 |
| Median followup post-ASCT, yrs. (range)c | 5.5 (2.1–13.0) | 5.9 (2.1–12.3) | 5.2 (2.1–13.0) | 0.20 |
| Median time from ASCT to maintenance rituximab (days) | n/a | 129 (36–451) | n/a | |
Abbreviations: CR, complete remission; ASCT, high-dose therapy; MIPI, mantle cell lymphoma International prognostic index; maintenance rituximab, maintenance rituximab.
PET scans were available for 133 patients, 105 of whom were in CR1 prior to transplant.
P-values comparing the maintenance rituximab and no-maintenance rituximab groups.
Treatment Outcome
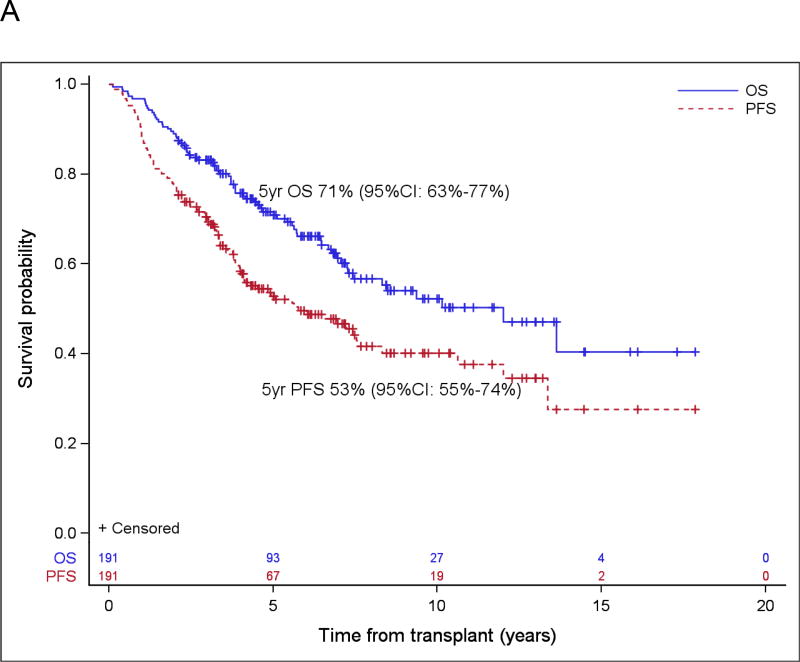
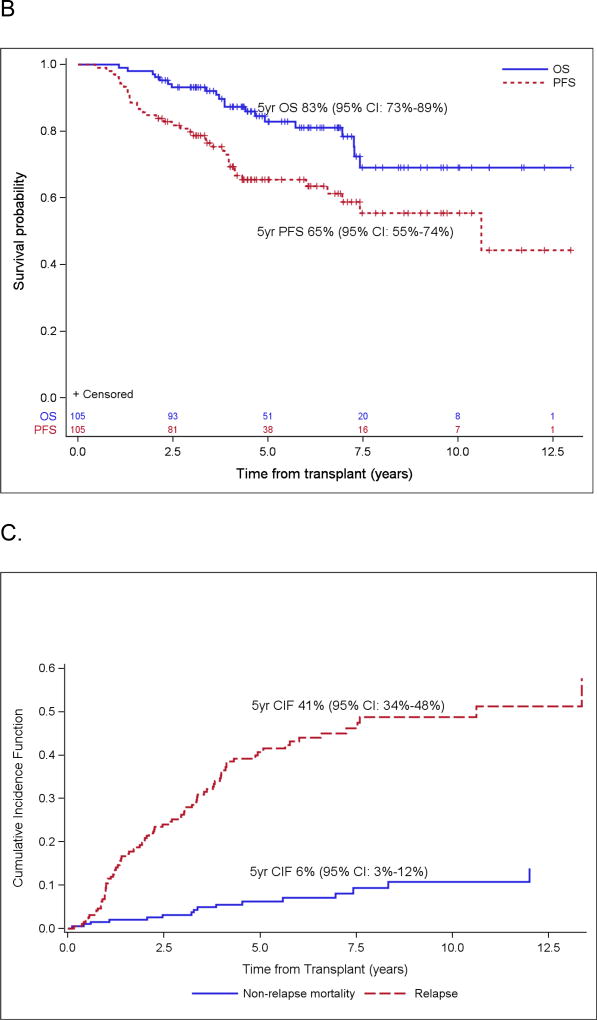
With a median follow-up for surviving patients of 6.3 years (range: 2.1 – 17.8), the 5-year estimate of OS and PFS were 71% (95% confidence interval [CI]: 63% – 77%) and 53% (95% CI: 45% – 60%), respectively (Figure 1A). For patients in PET-negative CR1 at ASCT, the 5-year estimates of OS and PFS were 83% (95% CI: 73% – 89%) and 65% (95% CI: 55% – 74%), respectively (Figure 1B). Forty-seven patients were transplanted with a disease status other than CR1; in this group, the 5-year OS and PFS were 49% (95% CI: 33%–63%) and 27% (95% CI: 15%–41%), respectively. There were 40 patients over the age of 65 at diagnosis included in our analysis, and 5-year OS and PFS were 68% (95% CI: 50%, 80%) and 55% (95% CI: 38%, 69%) in this subgroup.
Figure 1.
Probabilities of PFS/OS for the entire cohort (A) and for PET negative CR1 (B), and cumulative incidence function of relapse and non-relapse mortality (C).
The 5-year cumulative incidence of relapse was 41% (95% CI: 34% – 48%). A total of 83 relapses occurred at a median of 2.1 years (range: 0.2 – 13.4) post-transplant (Figure 1C). Fourteen of 83 patients who relapsed subsequently underwent allogeneic transplant. Secondary malignancies occurred in 14 patients (7%), of which 5 were therapy-related MDS or AML (3%) (Table 3). Non-relapse mortality, defined as any death without a prior relapse, occurred in 16 patients (8%) and was a result of a secondary malignancy in 10 patients (5%).
Table 3.
Secondary cancers
| N | Death due to SMN |
Conditioning Regimen |
|
|---|---|---|---|
| CBV:1 | |||
| AML/MDS | 5 | 2/5 | BEAM: 3 |
| TBI/eto/Cy: 1 | |||
| Adenocarcinoma | 1 | Y | Chemo only |
| Bladder cancer | 1 | Y | TBI/Eto/Cy |
| Breast cancer | 1 | N | Z-BEAM |
| Cholangiocarcinoma | 1 | Y | BEAM |
| Liposarcoma | 1 | Y | CBV |
| Lung cancer (squamous) | 1 | Y | Z-BEAM |
| Melanoma | 1 | Y | BEAM |
| Mesothelioma | 1 | Y | BEAM |
| Skin cancer | 1 | Y | TBI/Eto/Cy |
| Total | 14 | 10 | Chemo only: 9 |
| Radiation-based: 5 |
Prognostic Factors
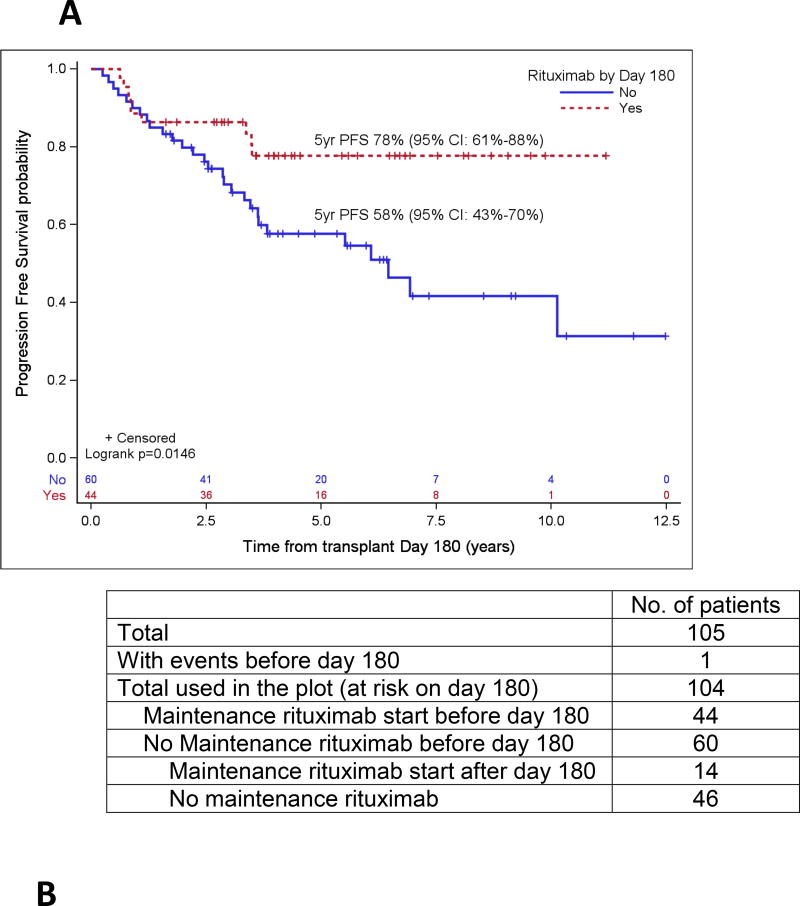
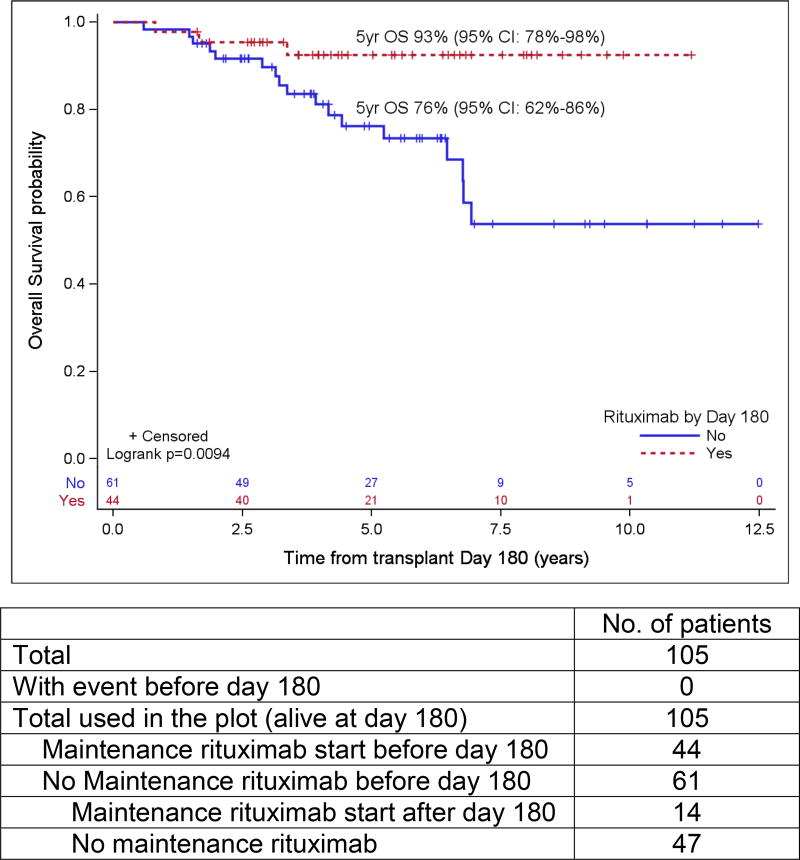
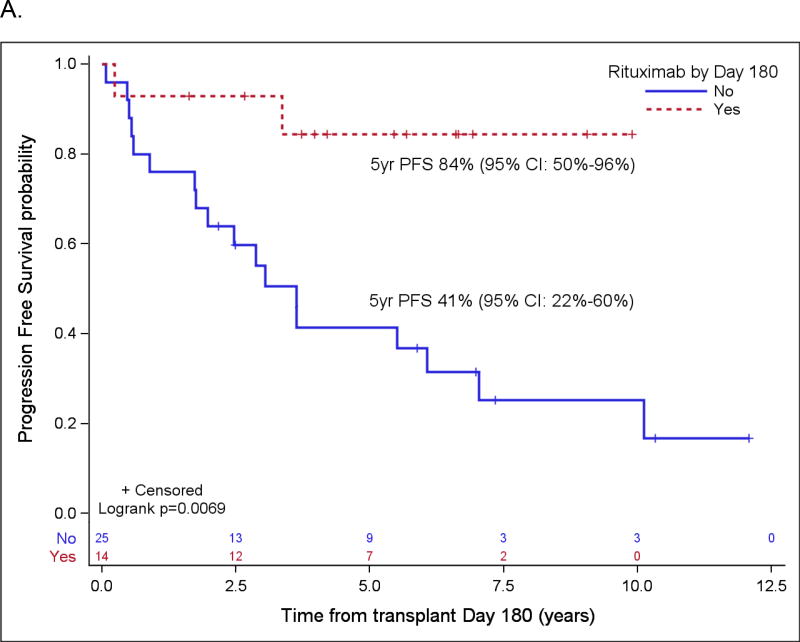
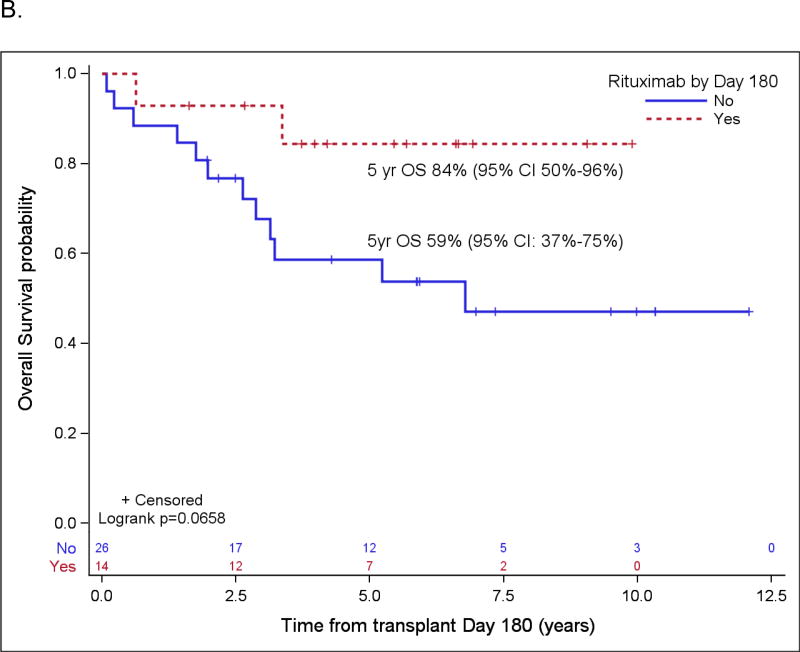
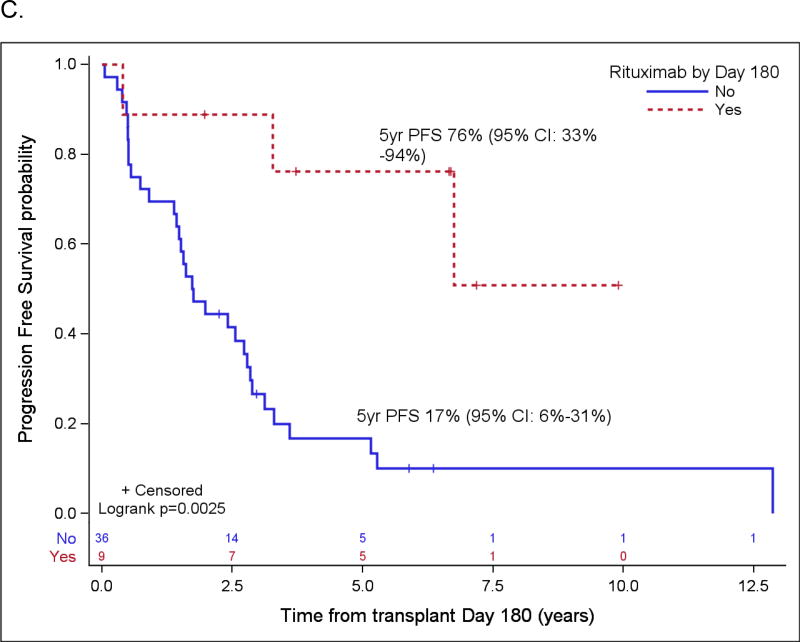
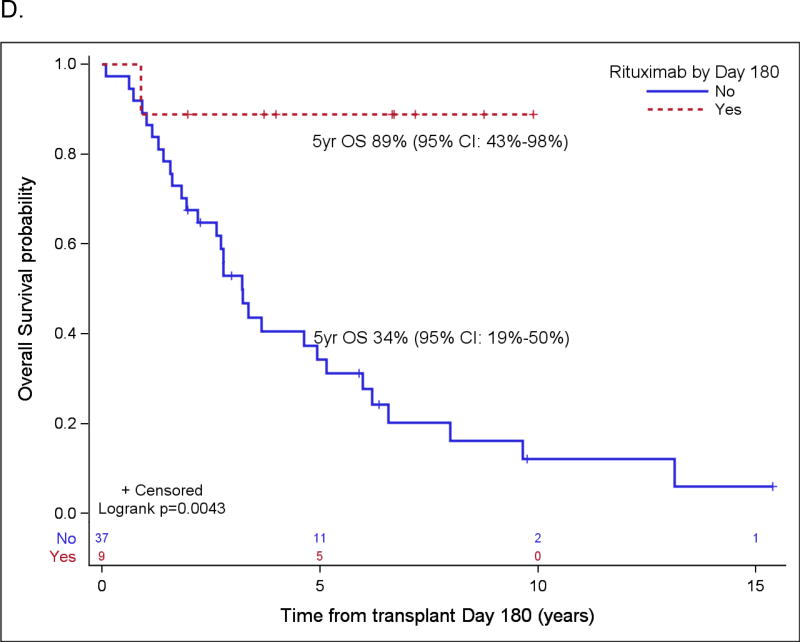
Patient variables were examined first in univariate analyses, and then in a multivariate analysis, and the results are summarized in Table 4 and Table 5, respectively. The strongest prognostic indicator for both OS and PFS was maintenance rituximab post-transplant. For all patients (Table 5A), maintenance rituximab conferred a relative risk for OS of 0.17 (95% CI: 0.07 – 0.38) and for PFS of 0.25 (95% CI: 0.14 – 0.44). Of the 75 patients who received maintenance rituximab, 66 (88%) were in CR1, compared to 78 CR1 among 116 patients (67%) who did not receive maintenance rituximab (p=0.001). When evaluated in the subset of patients in PET-negative CR1 at ASCT (Table 5B), maintenance rituximab remained significantly predictive for both OS and PFS with relative risks of 0.17 (95% CI: 0.05 – 0.59) and 0.20 (95% CI: 0.09 – 0.43), respectively. For all patients who started maintenance rituximab within 180 days post ASCT, the 5-year OS and PFS were 88% (95% CI: 75%–95%) and 75% (95% CI: 60%–85%), respectively, as compared to 63% (95% CI: 53%–71%) and 45% (95% CI: 35%–54%), respectively, in patients who did not receive maintenance rituximab by then (both p<0.001). For patients in a PET-negative CR1 who received maintenance rituximab within 180 days post ASCT, the 5-year estimates of OS and PFS were 93% (95% CI: 78% – 98%) and 78% (95% CI: 61% – 88%), respectively, as compared to 76% (95% CI: 62%–86%) and 58% (95% CI: 43%–70%) for patients in a PET-negative CR1 who did not receive maintenance rituximab by then (p=0.009 and 0.0146) (Figure 2). In the cohort of patients over 65 years, maintenance rituximab was associated with superior PFS and a trend towards improved OS, and in patients whose disease status at ASCT was > CR1, maintenance rituximab was associated with both improved PFS and OS (Figure 3).
Table 4.
Univariate Cox model for PFS/OS for (A) entire cohort and (B) subset of PET- negative CR1 patients (B), where maintenance rituximab was modeled as a time-dependent covariate and all other variables time-independent covariates.
| A. Entire Cohort | PFS | OS | ||
|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | |
|
|
||||
| Gender: Female vs. Male (Ref) | 0.46(0.27,0.78) | 0.004 | 0.44(0.23,0.84) | 0.013 |
| Age (year) | 1.02(1.00,1.05) | 0.074 | 1.03(1.00,1.06) | 0.054 |
| Diagnosis Year: 2006 and later vs. before 2006 (Ref) | 0.46(0.3,0.71) | <0.001 | 0.34(0.19,0.59) | <0.001 |
| B Symptoms: No vs. Yes (Ref) | 0.83(0.50,1.36) | 0.45 | 0.84(0.48,1.49) | 0.56 |
| Bone marrow involvement: No vs. Yes (Ref) | 1.07(0.66,1.73) | 0.79 | 0.94(0.53,1.69) | 0.84 |
| Extranodal involvement: No vs. Yes (Ref) | 1.26(0.82,1.94) | 0.28 | 1.43(0.85,2.42) | 0.18 |
| Blastoid variant: No vs. Yes (Ref) | 0.58(0.29,1.15) | 0.12 | 0.43(0.21,0.91) | 0.027 |
| MIPI | ||||
| Intermediate/high vs. low risk (Ref) | 1.62(0.98,2.70) | 0.061 | 1.49(0.84,2.62) | 0.17 |
| Unknown vs. low risk (Ref) | 1.47(0.87,2.47) | 0.15 | 1.02(0.56,1.88) | 0.94 |
| High-dose cytarabine pre-ASCT: No vs. Yes (Ref) | 1.56(1.05,2.32) | 0.027 | 1.59(1.00,2.54) | 0.052 |
| Rituximab pre-ASCT: No vs. Yes (Ref) | 2.16(1.22,3.82) | 0.008 | 1.69(0.87,3.27) | 0.12 |
| Regimens pre-ASCT: 2–3 vs. 1 (Ref) | 2.11(1.42,3.15) | <0.001 | 2.6(1.63,4.13) | <0.001 |
| Time from diagnosis to ASCT (month) | 1.01(1.00,1.02) | 0.069 | 1.01(1.00,1.02) | 0.17 |
| ASCT year: 2007 and later vs. before 2007 (Ref) | 0.41(0.27,0.63) | <0.001 | 0.29(0.17,0.52) | <0.001 |
| Disease status : PR1/CR2/PR2/PR3 vs. CR1 (Ref) | 2.74(1.82,4.14) | <0.001 | 3.15(1.97,5.02) | <0.001 |
| ASCT Conditioning: Radiation-based vs. Chemotherapy-only (Ref) | 0.96(0.64,1.44) | 0.85 | 0.74(0.46,1.19) | 0.21 |
| Maintenance rituximab: Yes vs. No (Ref) | 0.22 (0.13,0.37) | <0.001 | 0.14 (0.07, 0.31) | <0.001 |
| B. PET negative CR1a | PFS | OS | ||
|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | |
|
|
||||
| Gender: Female vs. Male (Ref) | 0.47(0.21,1.04) | 0.062 | 0.19(0.04,0.82) | 0.026 |
| Age (year) | 1.06(1.01,1.11) | 0.010 | 1.11(1.04,1.19) | 0.002 |
| Diagnosis Year: 2006 and later vs. before 2006 (Ref) | 0.70(0.35,1.40) | 0.31 | 0.44(0.18,1.09) | 0.076 |
| B Symptoms: No vs. Yes (Ref) | 0.45(0.20,1.00) | 0.049 | 0.58(0.19,1.75) | 0.33 |
| Bone marrow involvement: No vs. Yes (Ref) | 0.78(0.32,1.87) | 0.57 | 0.24(0.03,1.77) | 0.16 |
| Extranodal involvement: No vs. Yes (Ref) | 1.35(0.67,2.73) | 0.41 | 2.93(0.86,10.02) | 0.087 |
| Blastoid variant: No vs. Yes (Ref) | 0.41(0.14,1.16) | 0.093 | 0.28(0.08,0.98) | 0.046 |
| MIPI | ||||
| Intermediate/high vs. low risk (Ref) | 2.38(1.03,5.51) | 0.042 | 2.25(0.80,6.34) | 0.12 |
| Unknown vs. low risk (Ref) | 2.19(0.96,5.02) | 0.063 | 0.81(0.25,2.68) | 0.73 |
| High-dose cytarabine Pre-ASCT: No vs. Yes (Ref) | 2.38(1.25,4.54) | 0.009 | 2.68(1.07, 6.73) | 0.036 |
| Regimens pre-ASCT: 2–3 vs. 1 (Ref) | 1.44(0.66,3.14) | 0.36 | 2.19(0.79,6.07) | 0.13 |
| Time from diagnosis to ASCT (month) | 1.00(0.97,1.03) | 0.91 | 1.00(0.96,1.04) | 0.92 |
| ASCT year: 2007 and later vs. before 2007 (Ref) | 0.71(0.35,1.43) | 0.34 | 0.45(0.18,1.12) | 0.088 |
| ASCT Conditioning: Radiation-based vs. Chemotherapy-only (Ref) | 0.76 (0.39,1.47) | 0.41 | 0.35 (0.13,0.94) | 0.037 |
| Maintenance rituximab: Yes vs. No (Ref) | 0.22 (0.11,0.46) | <0.001 | 0.11 (0.03,0.38) | <0.001 |
Abbreviations: CR, complete remission; ASCT, high-dose therapy; MIPI, mantle cell lymphoma International prognostic index; PR, partial remission.
Variables excluded: disease status due to all patients being CR1; pre-HCT Rituxan due to only 1 patient being “No”.
Table 5.
Multivariate Cox model for PFS/OS for (A) entire cohort and (B) PET-negative CR1 patients, where maintenance rituximab was modeled as a time-dependent covariate and all other variables time-independent covariates. MIPI was always included in each final model. The other variables in each final model were chosen based on a model-selection procedure where all baseline characteristics were initially considered and only those who reached p<0.2 in the selection process were kept in the final model.
| A. Entire Cohort a | PFS | OS | ||
|---|---|---|---|---|
|
| ||||
| HR (95% CI) | P-value | HR (95% CI) | P-value | |
| Gender: Female vs. Male (Ref) | 0.56(0.32,0.98) | 0.044 | 0.63(0.31,1.27) | 0.19 |
| Blastoid variant: No vs. Yes (Ref) | 0.35(0.16,0.74) | 0.006 | 0.21(0.09,0.49) | <0.001 |
| Regimens pre-ASCT: 2–3 vs. 1 (Ref) | 1.49(0.95,2.32) | 0.083 | 1.82(1.07,3.09) | 0.026 |
| MIPI | ||||
| Intermediate/high vs. low risk (Ref) | 1.25(0.74,2.10) | 0.40 | 1.37(0.77,2.44) | 0.28 |
| Unknown vs. low risk (Ref) | 1.20(0.71,2.05) | 0.49 | 0.86(0.46,1.60) | 0.63 |
| ASCT year: 2007 and later vs. Before 2007 (Ref) | 0.55(0.34,0.89) | 0.014 | 0.44(0.24,0.81) | 0.008 |
| Disease status: PR1/CR2/PR2/PR3 vs. CR1 (Ref) | 1.48(0.93,2.37) | 0.10 | 1.43(0.84,2.45) | 0.19 |
| ASCT Conditioning: Radiation-based vs. Chemotherapy-only (Ref) | 0.69(0.45,1.06) | 0.087 | 0.50(0.31,0.83) | 0.007 |
| Maintenance rituximab: Yes vs. No (Ref) | 0.25(0.14,0.44) | <.001 | 0.17(0.07,0.38) | <.001 |
| B. PET negative CR1a,b | PFS | OSc | ||
|---|---|---|---|---|
|
| ||||
| HR (95% CI) | P-value | HR (95% CI) | P-value | |
| Age | - | - | 1.10(1.01,1.21) | 0.033 |
| Gender: Female vs. Male (Ref) | 0.53(0.23,1.22) | 0.14 | 0.20(0.04,1.05) | 0.057 |
| Blastoid variant: No vs. Yes (Ref) | 0.20(0.06,0.62) | 0.006 | - | - |
| B Symptoms: No vs. Yes (Ref) | - | - | 0.36(0.10,1.32) | 0.12 |
| Regimens pre-ASCT: 2–3 vs. 1 (Ref) | 2.27(0.95,5.43) | 0.067 | - | - |
| MIPI | ||||
| Intermediate/high vs. low risk (Ref) | 2.20(0.93,5.19) | 0.072 | 1.09(0.28,4.18) | 0.91 |
| Unknown vs. low risk (Ref) | 2.40(1.02,5.68) | 0.046 | 0.36(0.09,1.43) | 0.15 |
| Maintenance rituximab: Yes vs. No (Ref) | 0.20(0.09,0.43) | <.0001 | 0.17(0.05,0.59) | 0.005 |
Abbreviations: CR, complete remission; ASCT, high-dose therapy; MIPI, mantle cell lymphoma International prognostic index; PR, partial remission.
Diagnosis year was not included in the multivariate analysis due to its high correlation with ASCT year.
Variables excluded: disease status due to all patients being CR1; pre-ASCT rituximab due to only 1 patient being “No”.
Blastoid variant was excluded due to small number which led to extreme/unstable estimate if included in the model.
Figure 2.
Probabilities of PFS (A) and OS (B) according to rituximab maintenance for patients in PET-negative CR1 based on landmark analysis. Only patients who are still at risk for failure at day 180 after transplant were included in the plots. Patients who started maintenance rituximab prior to day 180 are counted as maintenance rituximab while patients who did not start maintenance rituximab by then are counted as no-maintenance rituximab.
Figure 3.
Probabilities of PFS and OS according to rituximab maintenance for patients over the age of 65 (A and B) and for patients not in CR1 (C and D) based on landmark analysis. Only patients who are still at risk for failure at day 180 after transplant were included in the plots. Patients who started maintenance rituximab prior to day 180 are counted as maintenance rituximab, while patients who did not start maintenance rituximab by then are counted as no-maintenance rituximab (n=3 in A & B, n=0 in C & D).
Other variables significantly associated with both increased PFS and OS in the multivariate analysis were lack of the blastoid variant histology and ASCT performed after 2007. Radiation-based conditioning was associated with a superior OS on the multivariate analysis and showed a trend towards improved PFS.
DISCUSSION
In this study, we have summarized the treatment outcomes of patients who underwent ASCT for MCL at our center with an analysis of both baseline clinical and treatment-related factors. This is one of the largest data sets for MCL for patients treated with ASCT at a single center (n = 191) and captures a wide range of patients treated with several different induction and conditioning regimens. Moreover, the long follow-up (median 6.3 years, range 2.1 –17.8) period also adds value to the data interpretation.
Our data identify maintenance rituximab after ASCT as the strongest factor resulting in significant improvement of PFS and OS in all patients. In patients who were already in a PET-negative CR1 prior to ASCT, maintenance rituximab remained strongly associated with superior PFS and OS, especially when initiated in the first 180 days post ASCT. In fact, patients who received maintenance rituximab had a 75% risk reduction in progression and 83% in death. Two other retrospective studies have also reported a benefit of rituximab maintenance in PFS and potentially OS when given post-ASCT (17, 18); nevertheless, our study has the largest number of patients. The only prospective randomized trial of post-ASCT maintenance rituximab is the phase 3 LyMa study, and the results were recently presented in ASH 2016. The treatment consisted of 4 cycles of R-DHAP, and patients in a PET-negative CR proceeded directly to ASCT conditioned with rituximab-BEAM, whereas patients with evidence of persistent disease received R-CHOP for four more cycles prior to ASCT. Post-ASCT patients were randomized 1:1 either to observation or maintenance rituximab (375 mg/m2) given every 2 months for 3 years. Ultimately 240 patients were randomized post ASCT, and 4-years PFS (82.2% vs. 64.6%, p=0.0005) and 4-year OS (88.7% vs. 81.4%) were superior in patients receiving rituximab maintenance.(16). However, unlike the LyMa study which only included younger patients and homogenous induction therapy, our analysis included patients who achieved CR after a variety of induction therapy. Furthermore, 40 patients who were over 65 years, of whom 17 received maintenance rituximab; a benefit from maintenance rituximab was also seen in this advanced age group. Nine of 10 patients discontinued rituximab due to disease progression, suggesting maintenance rituximab was well tolerated and the adverse events were manageable.
In our study, the incorporation of radiation into the conditioning regimen was associated with a statistically improved OS on the multivariate analysis as well as a trend towards increased PFS in the overall cohort. In an attempt to prevent relapse post-transplant, many patients were transplanted on clinical protocols that incorporated ibritumomab tiuxetan into the conditioning regimen. However, there was no survival benefit seen with radiation-based conditioning specifically for patients who were in a PET-negative CR1 at the time of transplant. Our results are in line with those obtained in a recent retrospective analysis from the University of Washington finding improved outcomes with RIT when adjusted for pre-transplant prognostic factors with the benefit from RIT primarily in patients with a worse pre-transplant disease status.(19) At the same time, the Nordic MCL3 trial did not find any improvement with the addition of RIT specifically for patients who were not in CR at the time of ASCT.(19, 20)
Strengths of our study include the large number of patients, long follow-up period, and a substantial proportion of patients who had received maintenance rituximab and/or were transplanted using a radiation-based conditioning regimen. Limitations of this study include the large heterogeneity of treatments used and its retrospective nature. Although the multivariate analysis attempted to control for many of these pre-treatment and treatment-related factors including the year of transplant, certainly there may have been other relevant factors that were not taken into consideration. Furthermore, treatment-related factors such as radiation were not administered in a uniform fashion due to patient age and clinical trial availability at the time of transplant. Multiple maintenance rituximab regimens were used which were adopted from ongoing practice changes with maintenance rituximab in indolent lymphoma. To examine each of these factors separately would have diluted the statistical power further. Finally, early diagnosis, improvements in supportive care, and the incorporation of novel agents such as bortezomib, lenalidomide, and ibrutinib, as well as novel multidrug combination in the frontline treatment for MCL in recent years have also altered the natural history of relapsed and refractory disease(4–6), and these would be expected to affect the survival results in a time-dependent fashion. In support of this notion, patients who underwent ASCT after 2007 in our center enjoyed significantly improved outcomes.
In summary, our study details the experience at COH with ASCT for MCL across more than 15 years and captures a variety of treatment patterns that have changed over time in response to therapeutic advances in the field. In spite of the heterogeneity of the data, the large benefit conferred by rituximab maintenance stands out and adds to the increasing body of evidence supporting this practice for all MCL patients after ASCT regardless of age and frontline induction regimens. This study also set the stage for prospective investigation aiming at optimization of maintenance therapy following ASCT.
Hightlights.
-
❖
The 5-year OS was 71% and PFS 53% for MCL patients receiving ASCT in our center
-
❖
Maintenance rituximab given post ASCT significantly associated with superior OS and PFS
-
❖
The benefit of maintenance rituximab was seen in all age groups
-
❖
Secondary cancer occurred in 7% of patients and was the most common cause of non-relapse mortality
Acknowledgments
We acknowledge our transplant coordinators and transplant nurses for their dedicated care of the patients.
FUNDING
This work was supported by research funding from NCI P50 CA107399. LEB is a member of Lymphoma Research Foundation MCL consortium and a recipient of Damon Runyon Cancer Research Foundation Clinical Investigator Award.
We thank all the patients who participated in this study and the support from Toni Stephenson Lymphoma Center at City of Hope.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
AUTHORSHIP
L.E.B., T.M.C., and A.P.N. designed the study.
M.G.M., L.E.B., T.M.C., and C.L. wrote the paper.
C.L. and J.P. performed the statistical analysis.
T.S., J.L.C, L.T.F, M.G.M, M.M.A., A.S. I.A., L.L.P., R.W.C, S.T.R., L.K., and S.J.F participated in data collection and contributed patients to the study.
All authors approved the final version of the manuscript.
CONFLICT-OF-INTEREST DISCLOSURE
The authors declare no competing financial interests.
References
- 1.Hsi ED, Martin P. Indolent mantle cell lymphoma. Leuk Lymphoma. 2014 Apr;55(4):761–7. doi: 10.3109/10428194.2013.815353. [DOI] [PubMed] [Google Scholar]
- 2.Campo E, Rule S. Mantle cell lymphoma: evolving management strategies. Blood. 2015;125(1):48–55. doi: 10.1182/blood-2014-05-521898. [DOI] [PubMed] [Google Scholar]
- 3.Geisler CH, Kolstad A, Laurell A, et al. The Mantle Cell Lymphoma International Prognostic Index (MIPI) is superior to the International Prognostic Index (IPI) in predicting survival following intensive first-line immunochemotherapy and autologous stem cell transplantation (ASCT) Blood. 2010;115(8):1530–3. doi: 10.1182/blood-2009-08-236570. [DOI] [PubMed] [Google Scholar]
- 4.Goy A, Sinha R, Williams ME, et al. Single-agent lenalidomide in patients with mantle-cell lymphoma who relapsed or progressed after or were refractory to bortezomib: phase II MCL-001 (EMERGE) study. J Clin Oncol. 2013 Oct 10;31(29):3688–95. doi: 10.1200/JCO.2013.49.2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goy A, Bernstein S, Kahl B, et al. Bortezomib in patients with relapsed or refractory mantle cell lymphoma (MCL): Preliminary results of the PINNACLE study. J Clin Oncol. 2005;23(90160):6563-. [Google Scholar]
- 6.Wang ML, Rule S, Martin P, et al. Targeting BTK with ibrutinib in relapsed or refractory mantle-cell lymphoma. N Engl J Med. 2013 Aug 08;369(6):507–16. doi: 10.1056/NEJMoa1306220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Romaguera JE, Fayad LE, Feng L, et al. Ten-year follow-up after intense chemoimmunotherapy with Rituximab-HyperCVAD alternating with Rituximab-high dose methotrexate/cytarabine (R-MA) and without stem cell transplantation in patients with untreated aggressive mantle cell lymphoma. Br J Haematol. 2010 Jul;150(2):200–8. doi: 10.1111/j.1365-2141.2010.08228.x. [DOI] [PubMed] [Google Scholar]
- 8.Hermine O, Hoster E, Walewski J, et al. Addition of high-dose cytarabine to immunochemotherapy before autologous stem-cell transplantation in patients aged 65 years or younger with mantle cell lymphoma (MCL Younger): a randomised, open-label, phase 3 trial of the European Mantle Cell Lymphoma Network. Lancet. 2016 Aug 06;388(10044):565–75. doi: 10.1016/S0140-6736(16)00739-X. [DOI] [PubMed] [Google Scholar]
- 9.Robak T, Huang H, Jin J, et al. Bortezomib-based therapy for newly diagnosed mantle-cell lymphoma. N Engl J Med. 2015 Mar 05;372(10):944–53. doi: 10.1056/NEJMoa1412096. [DOI] [PubMed] [Google Scholar]
- 10.Bernstein SH, Epner E, Unger JM, et al. A phase II multicenter trial of hyperCVAD MTX/Ara-C and rituximab in patients with previously untreated mantle cell lymphoma; SWOG 0213. Ann Oncol. 2013 Jun;24(6):1587–93. doi: 10.1093/annonc/mdt070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rule S. Frontline therapy and role of high-dose consolidation in mantle cell lymphoma. Hematology Am Soc Hematol Educ Program. 2016 Dec 02;2016(1):419–24. doi: 10.1182/asheducation-2016.1.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kluin-Nelemans JC, Hoster E, Walewski J, et al. R-CHOP Versus R-FC Followed by Maintenance with Rituximab Versus Interferon-Alfa: Outcome of the First Randomized Trial for Elderly Patients with Mantle Cell Lymphoma. Blood. 2011;118(21):439-. [Google Scholar]
- 13.Kenkre VP, Long WL, Eickhoff JC, et al. Maintenance rituximab following induction chemo-immunotherapy for mantle cell lymphoma: long-term follow-up of a pilot study from the Wisconsin Oncology Network. Leuk Lymphoma. 2011;52(9):1675–80. doi: 10.3109/10428194.2011.580404. 2011/09/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chang JE, Li H, Smith MR, et al. Phase 2 study of VcR-CVAD with maintenance rituximab for untreated mantle cell lymphoma: an Eastern Cooperative Oncology Group study (E1405) Blood. 2014;123(11):1665–73. doi: 10.1182/blood-2013-08-523845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mathias J, Rummel WK, Martin Goerner, Ulrike Soeling, Elisabeth Lange, Bernd Hertenstein, Jochen Eggert, Georg C Schliesser, Rudolf Weide, Klaus Blumenstengel, Ninia Detlefsen, Axel Hinke, Frank Kauff, Juergen Barth Universitaetsklinik, Giessen, Germany; Centre for Haematology & Oncology Bethanien, Berlin, Germany; Stadt Klinikum Bielefeld, Bielefeld, Germany; Onkologie Kassel, Kassel, Germany; Evangelisches Krankenhaus Hamm, Hamm, Germany; Department of Hematology and Oncology, Klinikum Bremen Mitte, Bremen, Germany; Onkologische Praxis Moers, Moers, Germany; Medical Office, Giessen, Germany; Hematology and Oncology Group Practice, Koblenz, Germany; Medical Practice for Oncology and Hematology, Eisenach, Germany; Universitaetsklinikum, Giessen, Germany; Wisp Gmbh, Langenfeld, Germany. Two years rituximab maintenance vs. observation after first-line treatment with bendamustine plus rituximab (B-R) in patients with mantle cell lymphoma: First results of a prospective, randomized, multicenter phase II study (a subgroup study of the StiL NHL7-2008 MAINTAIN trial) J Clin Oncol. 2016;34(suppl) abstr 7503. [Google Scholar]
- 16.Le Gouill S, Thieblemont C, Oberic L, et al. Rituximab Maintenance after Autologous Stem Cell Transplantation Prolongs Survival in Younger Patients with Mantle Cell Lymphoma: Final Results of the Randomized Phase 3 LyMa Trial of the Lysa/Goelams Group. Blood. 2016;128(22):145-. [Google Scholar]
- 17.Graf SA, Stevenson PA, Holmberg LA, et al. Maintenance rituximab after autologous stem cell transplantation in patients with mantle cell lymphoma. Ann Oncol. 2015;26(11):2323–8. doi: 10.1093/annonc/mdv364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dietrich S, Weidle J, Rieger M, et al. Rituximab maintenance therapy after autologous stem cell transplantation prolongs progression-free survival in patients with mantle cell lymphoma. Leukemia. 2014 Mar;28(3):708–9. doi: 10.1038/leu.2013.332. [DOI] [PubMed] [Google Scholar]
- 19.Cassaday RD, Stevenson PA, Gooley TA, et al. High-Dose CD20-Targeted Radioimmunotherapy-Based Autologous Transplantation Improves Outcomes for Persistent Mantle Cell Lymphoma. Br J Haematol. 2015;171(5):788–97. doi: 10.1111/bjh.13773. 10/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kolstad A, Laurell A, Jerkeman M, et al. Nordic MCL3 study: 90Y-ibritumomabtiuxetan added to BEAM/C in non-CR patients before transplant in mantle cell lymphoma. Blood. 2014 May 08;123(19):2953–9. doi: 10.1182/blood-2013-12-541953. [DOI] [PMC free article] [PubMed] [Google Scholar]