Abstract
Myeloid cell production within the bone marrow is accelerated in the setting of cancer, and the numbers of circulating and infiltrating neutrophils and granulocytic myeloid derived suppressor cells (MDSCs) correlate with tumor progression and patient survival. Cancer is therefore able to hijack the normally host-protective immune system and use it to further fuel growth and metastasis. Myeloid cells secrete neutrophil elastase and neutrophil extracellular traps (NETs) in response to cues within the tumor microenvironment, thereby leading to enhanced activity in a variety of cancer types. Neutrophil elastase may indeed be a driver of tumorigenesis, since genetic deletion and pharmacological inhibition markedly reduces tumor burden and metastatic potential in numerous preclinical studies. In this review, we examine the current evidence for neutrophil elastase as a stimulatory factor in cancer, focusing on precise mechanisms by which it facilitates primary tumor growth and secondary organ metastasis. We conclude with a brief overview of neutrophil elastase inhibitors and discuss their potential use in cancer therapy.
Keywords: neutrophil elastase, myeloid derived suppressor cells (MDSC), tumor microenvironment, prostate cancer
Introduction
Cancer related inflammation is associated with poor prognosis and reduced survival in numerous human malignancies. Expansion of myeloid derived immune cells in response to tumor-secreted factors largely contributes to the heightened systemic and intra-tumoral inflammatory milieu observed in cancer patients. While the pattern of infiltrating immune cells is heterogeneous between different cancers, granulocytic myeloid cells are amongst the most predominant immune cell types that confer adverse clinical outcomes. This was most strikingly demonstrated by a recent transcriptomic analysis of 18,000 tumors, encompassing 39 distinct human cancers. The authors reported that a cancer-wide granulocytic gene signature was most significantly correlated with poor prognosis, thereby implicating infiltrating granulocytes and neutrophils as central and potentially targetable regulators of tumor growth [1]. In accordance with this major finding, a large number of preclinical animal studies confirm myeloid skewing, particularly towards the granulocytic lineage, as a characteristic phenomenon in the setting of most cancers [2–4]. Moreover, high neutrophil to lymphocyte ratios (NLR), which are likely peripheral manifestations of myeloid skewing, have prognostic value for human cancer patients [5, 6].
We recently reported that infiltrating myeloid cells exert pro-tumorigenic actions via neutrophil elastase in prostate cancer [7]. The contribution of granulocytic myeloid cells and neutrophils to cancer progression is multifaceted and has been extensively reviewed elsewhere [8, 9]; however, accumulating literature on the role of neutrophil elastase in cancer warrants further consideration. In this review, we examine the mechanisms by which neutrophil elastase may facilitate primary tumor growth and secondary organ metastasis. Importantly, we conclude with a brief discussion of neutrophil elastase as a novel therapeutic target in cancer.
Neutrophil elastase in health and malignancy
Neutrophil elastase was first described as a serine protease stored in azurophilic granules of neutrophils. While the enzyme is abundantly present within mature neutrophils, mRNA transcripts encoded by the ELANE gene are synthesized early during myelopoiesis, primarily during the pro-myelocyte and late pro-myelocyte stages [10]. Neutrophil elastase enzyme is released into the extracellular space through degranulation or during neutrophil extracellular trap formation, also known as NETosis [11]. There it carries out its chief physiological function of pathogen clearance during infection. Neutrophil elastase may also be a critical regulator of emergency myelopoiesis [12], leukocyte transmigration and homing [13], and general mounting of an inflammatory response [14, 15]. As a result of its nature as a protease with broad-spectrum specificity, neutrophil elastase is implicated in matrix remodeling in a variety of pathological processes, including but not limited to emphysema, chronic obstructive pulmonary disease (COPD) [16], pulmonary fibrosis [17], atherosclerosis [18], and cancer [19].
Indeed, neutrophil elastase expression and activity is up regulated in numerous cancer types. In lung cancer patients, elevated serological neutrophil elastase positively correlates with not only disease state but also disease progression. Interestingly, activity is three and five-fold greater in the bronchoalveolar lavage fluid and serum, respectively, of individuals with lung cancer compared to those with COPD [20]. Enhanced neutrophil elastase activity in lung cancer patients can also be detected indirectly through accumulation of a neutrophil elastase specific elastin degradation product, termed EL-NE [21]. Neutrophil elastase-degraded elastin levels in the serum of individuals with lung squamous cell, non-small-cell, and adenocarcinoma are significantly greater than in those with idiopathic pulmonary fibrosis. Similarly, a strong neutrophil elastase proteolytic fingerprint distinguishes the colon adenocarcinoma proteome from that of ulcerative colitis [22]. Taken together, these findings suggest that cancer is a unique and potent inducer of neutrophil elastase, since its levels are significantly elevated in the setting of cancer, even when compared to non-malignant inflammatory diseases. In breast cancer, high neutrophil elastase immunoreactivity is an independent indicator of poor prognosis, demonstrated by diminished metastasis-free survival, relapse-free survival, and overall survival even after correcting for traditional prognostic factors such as age and tumor grade [23–25]. Moreover, increased presence of neutrophil elastase predicts poor response to tamoxifen and trastuzumab therapy [24, 26].
Neutrophil elastase expression is also enhanced in several mouse models of cancer. A novel imaging modality utilizing a protease specific optical probe localizes activity to growing tumors in living animals. We show enhanced neutrophil elastase activity in xenografts of two human prostate cancer cell lines, as well as within tumors of probasin driven Pten-null mice compared to wild type prostates. Not surprisingly, tumors have abundant granulocytic infiltrates [7]. Human colorectal cancer xenografts are also reported to harbor abundant neutrophil elastase activity [27]. Neutrophil elastase expression and activity are similarly up regulated in uteri of Tsc2-null mice compared to healthy controls, especially at sites of leiomyoma development [28]. Furthermore, Zdhhc13 mutant mice are more susceptible to squamous cell carcinogenesis and exhibit elevated neutrophil elastase activity in the skin, concomitant with increased granulocytic infiltration [29].
Despite its name, neutrophil elastase is reportedly produced by a variety of immune cells, including neutrophils, myeloid derived suppressor cells or MDSCs, macrophages [30], and lymphocytes [31]. It is interesting to postulate that cancer-induced accumulation of MDSCs may explain the resultant enhanced intra-tumoral neutrophil elastase, particularly because both are correlated with disease progression in human patients. Indeed, we demonstrate that CD33 positive MDSCs produce neutrophil elastase in human prostate cancer [7]. Our findings are supported by multiple transcriptomic analyses of MDSCs in tumor bearing mice, revealing neutrophil elastase to be abundantly present in cells of both granulocytic and monocytic subtypes [32, 33]. Notably, studies have described neutrophil elastase expression in epithelial breast cancer cells, suggesting that, in some cases, its source may be from tumors themselves rather than surrounding stroma [34, 35]
As mentioned, neutrophil elastase is an integral component of neutrophil extracellular traps (NETs). In fact, not only is it present in NETs, but its activity is required for NET formation [11]. NETosis is a process whereby immune cells extrude DNA fibers decorated with histones, proteases, and other proteins – similar to casting a tangled net into the extracellular space. While NETosis was initially discovered in models of host-pathogen defense, it is a relevant process in many other pathological situations where neutrophil elastase is similarly implicated. A pro-tumorigenic role for NETs has only recently become appreciated, as they appear to facilitate both primary tumor growth and metastasis [36–38]. Intriguingly, enhanced NETosis occurs in response to tumor-derived factors such as G-CSF and IL8, identical to those that are required for systemic MDSC expansion and local recruitment [39–41].
Neutrophil elastase in primary tumor initiation and growth
The tumor burden of several genetic mouse models of cancer is significantly reduced in the setting of global neutrophil elastase deletion, suggesting an important role in cancer development and progression. Using the LSL-K-ras model of lung adenocarcinoma, mice lacking neutrophil elastase (Elane −/−) have a profound survival advantage over Elane +/+ controls [42]. While the number of lesions in both groups is the same, the grade, size, and proliferative indices are diminished, suggesting that neutrophil elastase mediates tumor growth rather than initiation [42]. Using the C3(1)Tag mouse model of breast cancer, neutrophil elastase knockout mice similarly have slower tumor growth kinetics and lower markers of proliferation [43]. In another model of tumor formation and growth, neutrophil elastase knockout SKH1 hairless mice are remarkably resistant to ultraviolet B-induced squamous cell tumorigenesis, suggesting that neutrophil elastase may contribute to tumor initiation in inflammation-induced cancers [44]. Indeed, neutrophil elastase expression and activity is enhanced in chronic colitis-associated carcinogenesis; moreover, neutrophil depletion reduces intra-tumoral neutrophil elastase as well as the number and size of tumors [45]. Likewise, in the POET3+ Pten+/− mouse model of inflammation-induced prostate carcinogenesis, the number of lesions is most strongly associated with neutrophil infiltration [46].
The role of neutrophil elastase in primary tumor growth is also demonstrated in numerous syngeneic and xenograft mouse models. Lewis lung carcinoma tumors subcutaneously injected into Elane −/− mice grow significantly slower [42]. Neutrophil elastase inhibition via curcumin similarly shrinks Lewis lung carcinoma tumors, though off target effects likely contribute [47, 48]. Remarkably, pharmacologically targeting neutrophil elastase with a specific inhibitor sivelestat (also called ONO-5046) reduces tumor progression in mice bearing human colorectal [27], lung [49], gastric [50], and prostate cancer xenografts [7]. Treatment of LSL-K-ras mice with ONO-5046 recapitulates the Elane −/− phenotype and results in smaller tumors [42], further supporting neutrophil elastase inhibition as an efficacious treatment strategy in cancer.
Several mechanisms have been proposed to account for the pro-tumorigenic activity of neutrophil elastase. First, neutrophil elastase can directly stimulate proliferative pathways by extracellular transactivation of membrane receptors such as epidermal growth factor receptor (EGFR) and toll-like receptor 4 (TLR4), inducing mitogen activated protein kinase (MAPK) signaling and downstream effects. In both breast and prostate cancer cells, neutrophil elastase acts through MAPK to induce ERK phosphorylation and transcription of ERK-dependent genes like FOS. Consequently, pre-treatment with MEK inhibitors abrogates neutrophil elastase induced proliferation [7, 43]. Neutrophil elastase may transactivate EGFR through cleavage and release of a variety of membrane bound ligands, including TGFα, EGF-like ligands, and PDGF [50–52]. In fact, pre-treatment with TGFα neutralizing antibodies abolishes downstream proliferative effects of neutrophil elastase in keratinocytes [51, 53]. Neutrophil elastase can additionally activate the phosphoinositide 3-kinase (PI3K)-Akt proliferative pathway through internalization and degradation of insulin receptor substrate 1 (IRS-1) in lung cancer cells [42, 47, 48, 54, 55]. Recent studies have identified possible methods of endosomal internalization of neutrophil elastase; specifically, clathrin and neuropilin-1 appear to mediate the process [54, 56]. Furthermore, co-culturing of human neutrophils with lung cancer cells in vitro reveals that direct cell-cell interactions are required for neutrophil elastase mediated release and induction of proliferation through a COX-2 dependent pathway [57]. Intriguingly, MDA-MB-231 breast cancer cells express neutrophil elastase endogenously, and shRNA mediated down-regulation results in decreased proliferation, migration, and invasion in vitro, as well as a drastic reduction in tumorigenic potential in vivo [34]. Nonetheless, the reason why different cancer cell types utilize preferential mechanisms for activation in response to neutrophil elastase is unclear at this time.
While activation of oncogenic signaling is an important way of promoting tumor growth, another possibility is disinhibition of proliferation through inactivation of tumor suppressors. In fact, the loss of tumor suppressor function is a critical step in initiation of many cancers, and neutrophil elastase may contribute to tumor progression in such a manner. For instance, neutrophil elastase-dependent cleavage compromises the tumor suppressor role of EMILIN1 [58, 59]. While extracellular matrix associated EMILIN1 normally inhibits proliferation of leiomyosarcoma cells through engagement of α4/β1 integrins, neutrophil elastase-cleaved EMILIN1 is unable to exert this effect [58, 59]. Furthermore, neutrophil elastase inactivates the anti-tumorigenic factor thrombospondin-1 (Tsp-1), enhancing growth of lung tumors and metastatic melanoma foci in the lung [60].
Within the tumor microenvironment, neutrophil elastase may also modulate additional important processes that contribute to growth but cannot be ascertained in a culture dish. Development of a supportive vasculature is crucial for continuous expansion of cancer cells and eventual hematologic dissemination. Therefore, targeting angiogenesis is a promising cancer therapy, particularly when combined with other drugs. Indeed, NETosis, possibly via neutrophil elastase, promotes endothelial cell proliferation and motility in vitro and vascularization in vivo [61]. Neutrophil elastase directly stimulates the release of vascular endothelial growth factor or VEGF from the surface of tumor cells, which may subsequently activate proliferation of tumor-associated endothelium in a paracrine fashion [47, 52]. Accordingly, markers of angiogenesis such as VEGF and CD31 are reduced in lung tumors of LSL-K-ras neutrophil elastase knockout mice [62]. Other neutrophil derived proteases, particularly matrix metalloproteinase 9 (MMP-9), are critical mediators of angiogenesis and likely act cooperatively in vivo [63, 64]. Neutrophil elastase can regulate the activity of several members of the matrix metalloproteinase family, including MMP-9 and MMP-2 [65–67]. This is achieved through direct action on the pro-enzymes as well as inactivation of regulators of their activity, such as tissue inhibitor of metalloproteinase-1 (TIMP-1) [68]. Interestingly, resistance to anti-angiogenic therapy is mediated by refractory accumulation of granulocytic myeloid cells, and the combination of antibodies against VEGF and Gr-1 significantly improves therapeutic effect [69]. It is therefore intriguing to postulate that inhibiting neutrophil elastase may similarly improve anti-angiogenic therapy, though this hypothesis remains to be tested.
Neutrophil elastase in the metastatic cascade
Much of the morbidity and mortality associated with cancer is due to development of secondary organ metastases. The metastatic cascade requires successful completion of several steps, including cancer cell acquisition of migratory and invasive phenotypes, intravasation into adjacent vasculature, survival within the circulation, homing and subsequent extravasation into a distant site, evasion of host immunity, and finally proliferation and colonization [70]. Accumulation of granulocytic myeloid cells in the periphery and at secondary organs is thought to facilitate metastasis in numerous mouse models of cancer; in fact, depletion using Gr-1 or Ly6G antibodies or treatment with inhibitors that target chemokines and chemokine receptors to prevent their recruitment consistently results in reduced metastatic burden [4, 71, 72]. The mechanism for this phenomenon is complex and still not well understood, but myeloid derived neutrophil elastase may be one important and targetable mediator that acts at multiple key steps within the metastatic cascade.
Mice subcutaneously inoculated with Lewis lung carcinoma cells develop spontaneous lung metastases; however, the number and size of metastatic foci are significantly reduced when injected into mice with a global deletion of neutrophil elastase [42]. While this strongly suggests a critical role in the metastatic cascade, it is difficult to interpret where exactly neutrophil elastase is exerting its effect. Using the tail vein injection model of Lewis lung carcinoma cells, it is apparent that immune cell derived neutrophil elastase is crucial, since wild type mice that receive bone marrow transplants from neutrophil elastase and cathepsin G knockouts are similarly resilient to metastasis. The mechanism by which neutrophil derived proteases facilitate metastatic outgrowth is dependent on their ability to inactivate the tumor suppressor Tsp-1, thereby retaining cancer cells within the lung [60]. Importantly, these results suggest that neutrophil elastase can act on the second half of the metastatic cascade (implantation), since cancer cells are introduced directly into the circulation and do not need to first leave the primary tumor. Interestingly, proteinase 3 (PR3), a neutrophil derived protease analogous to neutrophil elastase and cathepsin G, was recently demonstrated to facilitate homing to the bone marrow through interaction with receptor for advanced glycation end products (RAGE) on prostate cancer cells [73]. The role of neutrophil elastase in tissue specific homing of cancer cells, however, remains to be further investigated.
Pharmacologic inhibition of neutrophil elastase also reduces metastatic potential of cancer cells in vivo, recapitulating the findings in the neutrophil elastase knockout animals [36, 48]. However, the apparent reduction in metastasis may partially be a result of reduced neutrophil extracellular trap (NET) formation or NETosis. Indeed, numerous studies have implicated NETs in the development of metastases [74]. Inhibition of neutrophil elastase with sivelestat reduces the extension of cancer cell induced NETs and NET-mediated cancer cell invasion, suggesting that the effects of NETs and neutrophil elastase are difficult to distinguish [38]. Moreover, targeting NETs by other means such as treatment with DNAse I or inhibitors of peptidylarginine deiminase type 4 (PAD4) similarly reduces lung and hepatic metastases, particularly in the setting of enhanced inflammatory stress [36, 75].
One explanation for how neutrophil elastase and NETs facilitate metastatic spread is the induction of epithelial to mesenchymal transition (EMT), whereby cancer cells become more migratory and invasive. Indeed, treatment of a variety of cancer cell types with neutrophil elastase enhances migration and invasion in vitro. In ovarian cancer cells, neutrophil elastase down regulates the epithelial marker E-cadherin and activates β-catenin signaling [76]. In pancreatic cancer cells, neutrophil elastase similarly reduces E-cadherin and keratin expression and induces β-catenin mediated expression of mesenchymal markers ZEB1 and TWIST1 [77, 78]. Moreover, we find that neutrophil elastase induced migration is MAPK-independent in prostate cancer cells, since pre-treatment with a MEK inhibitor has minimal effect on migration [7]. Neutrophil elastase not surprisingly also induces cancer cell invasion in vitro, a process that requires overcoming an extracellular matrix barrier. Immunoreactive neutrophil elastase is associated with direct extension of non-small lung cancer into the aorta, suggesting human clinical relevance [79]. In vivo, it is likely that the combined effort of numerous proteases results in stromal remodeling, leading to a tumor microenvironment that is favorable for cancer cell dissemination.
Once cancer cells enter the circulation, they must survive until they reach their destination. The sequestration of circulating tumor cells within NETs is one possible mechanism for protection from host elimination. This is supported by the fact that localized NET deposits increase development of transient micro-metastases and subsequent gross metastatic disease [36, 38, 74, 75]. Neutrophil elastase also directly enhances vascular adherence of cancer cells, in part through up regulation of E-selectin on the endothelium [80]. In agreement with animal studies, the metastatic burden of advanced cancer patients consistently correlates with the number of circulating MDSCs, which are a source of neutrophil elastase and NETs [9].
Lastly, it should be noted that neutrophil elastase is a driver of several non-malignant lung pathologies, including pulmonary fibrosis and emphysema. Neutrophil elastase knockout mice are protected from the development of air space enlargement in response to chronic cigarette smoke exposure [16]. Neutrophil elastase knockout mice are also resistant to bleomycin and asbestos induced pulmonary fibrosis [17, 81]. Neutrophil elastase appears to directly promote myofibroblast differentiation and proliferation in an IRS-1/PI3K/Akt dependent manner, similar to what is seen in lung cancer [17]. Moreover, neutrophil elastase enhances inflammation in numerous disease models [12, 14, 15]; hence, targeting its activity may be a practical anti-inflammatory strategy in the setting of cancer.
Neutrophil elastase as a therapeutic target in cancer
The importance of MDSCs and their derived factors in promoting primary tumor growth and metastasis is evidenced by numerous preclinical studies, some of which are described above. Novel treatment strategies that target the tumor microenvironment in addition to proliferating tumor cells may lead to more favorable outcomes in patients. Indeed, several inhibitors of MDSCs are already showing promise in human clinical trials [82]. For instance, tasquinimod, a small molecule inhibitor that prevents accumulation of MDSCs and blocks angiogenesis by binding to S100A9 on myeloid cells, was recently tested in castration-resistant prostate cancer patients. Even as a monotherapy, it resulted in prolonged progression free survival, although overall survival was unchanged in this patient population [83]. Selective targeting of MDSCs in advanced cancer patients was also achieved using DS-8273a, an agonistic TRAIL-R2 antibody; similarly, a decrease MDSCs inversely correlated with the length of progression free survival [84]. Unfortunately, cancer patients that receive such experimental drugs are often in late stages of metastatic disease resistant to traditional chemotherapeutics. If, as mentioned, neutrophil elastase and MDSC are acting early in cancer to promote growth and metastasis, treating late-stage cancer with drugs directed against these species may be too late. Interestingly, response to chemotherapy is linked to a successful reduction in NLR and MDSCs in prostate and pancreatic cancer [85, 86], suggesting that perhaps a combinatorial treatment approach would be most favorable [87].
Inhibition or deletion of neutrophil elastase recapitulates the therapeutic effect of MDSC depletion on cancer progression, and thus could be considered a novel drug target. Several inhibitors are utilized safely in in vivo mouse models of cancer, including sivelestat or ONO-5046 and curcumin derivatives, though the latter lack specificity. While sivelestat has decent specificity and selectivity for neutrophil elastase and is well characterized in the literature, its oral bioavailability in humans is poor. Despite this, use in patients with acute lung injury and acute respiratory distress syndrome demonstrates modest benefit in limited clinical trials in Japan [88, 89]. In recent years, at least two new orally available neutrophil elastase inhibitors have been designed: AZD9668 [90], manufactured by AstraZeneca, and BAY-85-8501 [91], manufactured by Bayer, have already entered phase II clinical trials for a variety of pulmonary diseases and thus far appear to be well tolerated. However, since neutrophil elastase is required for the normal immune response to bacterial pathogens, heightened risk for infections is a potential significant side effect if the drug is given long term. Clinical trials for cancer have yet to be established, but in doing so, administration design (i.e. monotherapy or in combination with chemotherapy or immunotherapy), as well as the patient population, must carefully be considered in order to attain the greatest benefit.
Conclusions
Infiltrating and circulating myeloid cells exert important actions at both the primary tumor and metastatic sites. The pro-tumorigenic actions of these cells may be mediated in part by enhanced production and activity of neutrophil elastase. In one potential model, MDSCs may be drawn from the blood vessels to tumor sites, where they then secrete neutrophil elastase. This enzyme in turn promotes tumor proliferation, EMT, migration, and invasion, which ultimately leads to metastasis (Figure 1). As evidenced by numerous preclinical studies highlighted in this review, neutrophil elastase may therefore serve as a novel cancer biomarker or therapeutic target. That said, the recent development and safe utilization of more potent and bioavailable neutrophil elastase inhibitors in human patients is promising and warrants further investigation in the cancer field.
Figure 1.
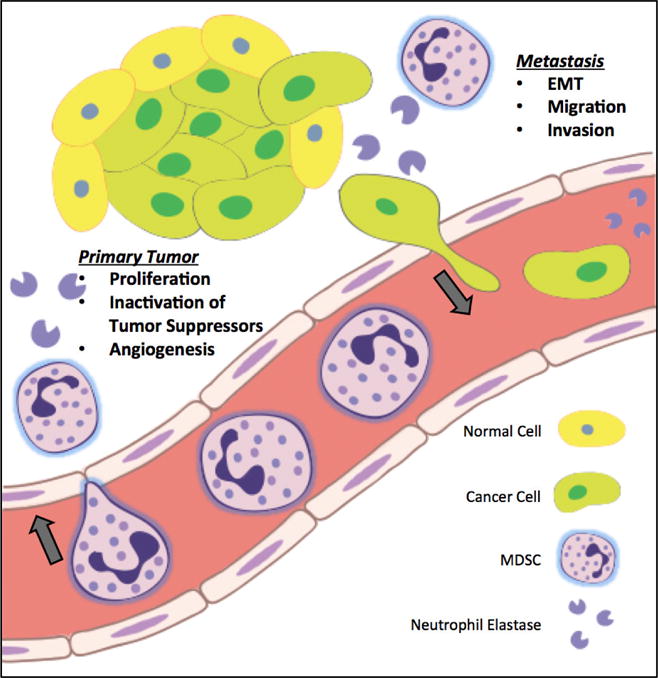
Infiltrating immune cells, including neutrophils and myeloid derived suppressor cells (MDSC), secrete neutrophil elastase into the tumor microenvironment. Neutrophil elastase then mediates important pathways involved in primary tumor growth, such as direct induction of proliferation, inactivation of tumor suppressors, and stimulation of angiogenesis. Neutrophil elastase also facilitates key steps in the metastatic cascade, including epithelial to mesenchymal transition (EMT), migration, invasion, and eventual homing to distant sites of metastasis.
Acknowledgments
This work was supported by NIH grants R01GM101709 (S.R.H.) and F30CA203517 (I.L.).
Abbreviations
- EMT
Epithelial to mesenchymal transition
- MAPK
Mitogen activated protein kinase
- MDSC
Myeloid derived suppressor cells
- NETs
Neutrophil extracellular traps
- VEGF
Vascular endothelial growth factor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gentles AJ, et al. The prognostic landscape of genes and infiltrating immune cells across human cancers. Nat Med. 2015;21(8):938–945. doi: 10.1038/nm.3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Casbon AJ, et al. Invasive breast cancer reprograms early myeloid differentiation in the bone marrow to generate immunosuppressive neutrophils. Proc Natl Acad Sci U S A. 2015;112(6):E566–75. doi: 10.1073/pnas.1424927112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Youn JI, et al. Characterization of the nature of granulocytic myeloid-derived suppressor cells in tumor-bearing mice. J Leukoc Biol. 2012;91(1):167–81. doi: 10.1189/jlb.0311177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ouzounova M, et al. Monocytic and granulocytic myeloid derived suppressor cells differentially regulate spatiotemporal tumour plasticity during metastatic cascade. Nat Commun. 2017;8:14979. doi: 10.1038/ncomms14979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tang L, et al. Prognostic Value of Neutrophil-to-Lymphocyte Ratio in Localized and Advanced Prostate Cancer: A Systematic Review and Meta-Analysis. PLoS One. 2016;11(4):e0153981. doi: 10.1371/journal.pone.0153981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ethier JL, et al. Prognostic role of neutrophil-to-lymphocyte ratio in breast cancer: a systematic review and meta-analysis. Breast Cancer Res. 2017;19(1):2. doi: 10.1186/s13058-016-0794-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lerman I, et al. Infiltrating Myeloid Cells Exert Protumorigenic Actions via Neutrophil Elastase. Mol Cancer Res. 2017;15(9):1138–1152. doi: 10.1158/1541-7786.MCR-17-0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coffelt SB, Wellenstein MD, de Visser KE. Neutrophils in cancer: neutral no more. Nat Rev Cancer. 2016;16(7):431–46. doi: 10.1038/nrc.2016.52. [DOI] [PubMed] [Google Scholar]
- 9.Marvel D, Gabrilovich DI. Myeloid-derived suppressor cells in the tumor microenvironment: expect the unexpected. J Clin Invest. 2015;125(9):3356–64. doi: 10.1172/JCI80005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fouret P, et al. Expression of the neutrophil elastase gene during human bone marrow cell differentiation. J Exp Med. 1989;169(3):833–45. doi: 10.1084/jem.169.3.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Papayannopoulos V, et al. Neutrophil elastase and myeloperoxidase regulate the formation of neutrophil extracellular traps. J Cell Biol. 2010;191(3):677–91. doi: 10.1083/jcb.201006052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang JY, et al. Neutrophil Elastase Regulates Emergency Myelopoiesis Preceding Systemic Inflammation in Diet-induced Obesity. J Biol Chem. 2017;292(12):4770–4776. doi: 10.1074/jbc.C116.758748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Young RE, et al. Neutrophil Elastase (NE)-Deficient Mice Demonstrate a Nonredundant Role for NE in Neutrophil Migration, Generation of Proinflammatory Mediators, and Phagocytosis in Response to Zymosan Particles In Vivo. The Journal of Immunology. 2004;172(7):4493–4502. doi: 10.4049/jimmunol.172.7.4493. [DOI] [PubMed] [Google Scholar]
- 14.Kessenbrock K, et al. Proteinase 3 and neutrophil elastase enhance inflammation in mice by inactivating antiinflammatory progranulin. J Clin Invest. 2008;118(7):2438–47. doi: 10.1172/JCI34694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Henry CM, et al. Neutrophil-Derived Proteases Escalate Inflammation through Activation of IL-36 Family Cytokines. Cell Rep. 2016;14(4):708–22. doi: 10.1016/j.celrep.2015.12.072. [DOI] [PubMed] [Google Scholar]
- 16.Shapiro SD, et al. Neutrophil elastase contributes to cigarette smoke-induced emphysema in mice. Am J Pathol. 2003;163(6):2329–35. doi: 10.1016/S0002-9440(10)63589-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gregory AD, et al. Neutrophil elastase promotes myofibroblast differentiation in lung fibrosis. J Leukoc Biol. 2015;98(2):143–52. doi: 10.1189/jlb.3HI1014-493R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alfaidi M, et al. Neutrophil elastase promotes interleukin-1beta secretion from human coronary endothelium. J Biol Chem. 2015;290(40):24067–78. doi: 10.1074/jbc.M115.659029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sato T, et al. Neutrophil elastase and cancer. Surg Oncol. 2006;15(4):217–22. doi: 10.1016/j.suronc.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 20.Vaguliene N, et al. Local and systemic neutrophilic inflammation in patients with lung cancer and chronic obstructive pulmonary disease. BMC Immunol. 2013;14:36. doi: 10.1186/1471-2172-14-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kristensen JH, et al. Serological assessment of neutrophil elastase activity on elastin during lung ECM remodeling. BMC Pulm Med. 2015;15:53. doi: 10.1186/s12890-015-0048-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kistowski M, et al. A Strong Neutrophil Elastase Proteolytic Fingerprint Marks the Carcinoma Tumor Proteome. Mol Cell Proteomics. 2017;16(2):213–227. doi: 10.1074/mcp.M116.058818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Akizuki M, et al. Prognostic Significance of Immunoreactive Neutrophil Elastase in Human Breast Cancer: Long-Term Follow-Up Results in 313 Patients. Neoplasia. 2007;9(3):260–264. doi: 10.1593/neo.06808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Foekens JA, et al. Elevated expression of polymorphonuclear leukocyte elastase in breast cancer tissue is associated with tamoxifen failure in patients with advanced disease. Br J Cancer. 2003;88(7):1084–90. doi: 10.1038/sj.bjc.6600813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Foekens JA, et al. The prognostic value of polymorphonuclear leukocyte elastase in patients with primary breast cancer. Cancer Res. 2003;63(2):337–41. [PubMed] [Google Scholar]
- 26.Yamashita J, et al. Neutrophil elastase predicts trastuzumab responsiveness in metastatic breast cancer. Breast J. 2006;12(3):288. doi: 10.1111/j.1075-122X.2006.00265.x. [DOI] [PubMed] [Google Scholar]
- 27.Ho AS, et al. Neutrophil elastase as a diagnostic marker and therapeutic target in colorectal cancers. Oncotarget. 2014;5(2):473–80. doi: 10.18632/oncotarget.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Prizant H, et al. Estrogen maintains myometrial tumors in a lymphangioleiomyomatosis model. Endocr Relat Cancer. 2016;23(4):265–80. doi: 10.1530/ERC-15-0505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Perez CJ, et al. Increased Susceptibility to Skin Carcinogenesis Associated with a Spontaneous Mouse Mutation in the Palmitoyl Transferase Zdhhc13 Gene. J Invest Dermatol. 2015;135(12):3133–3143. doi: 10.1038/jid.2015.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dollery CM, et al. Neutrophil elastase in human atherosclerotic plaques: production by macrophages. Circulation. 2003;107(22):2829–36. doi: 10.1161/01.CIR.0000072792.65250.4A. [DOI] [PubMed] [Google Scholar]
- 31.Vicuna L, et al. The serine protease inhibitor SerpinA3N attenuates neuropathic pain by inhibiting T cell-derived leukocyte elastase. Nat Med. 2015;21(5):518–23. doi: 10.1038/nm.3852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fridlender ZG, et al. Transcriptomic analysis comparing tumor-associated neutrophils with granulocytic myeloid-derived suppressor cells and normal neutrophils. PLoS One. 2012;7(2):e31524. doi: 10.1371/journal.pone.0031524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aliper AM, et al. Interactome analysis of myeloid-derived suppressor cells in murine models of colon and breast cancer. Oncotarget. 2014;5(22):11345–53. doi: 10.18632/oncotarget.2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hunt KK, et al. Elafin, an inhibitor of elastase, is a prognostic indicator in breast cancer. Breast Cancer Res. 2013;15(1):R3. doi: 10.1186/bcr3374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yamashita JI, et al. Production of immunoreactive polymorphonuclear leucocyte elastase in human breast cancer cells: possible role of polymorphonuclear leucocyte elastase in the progression of human breast cancer. Br J Cancer. 1994;69(1):72–6. doi: 10.1038/bjc.1994.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cools-Lartigue J, et al. Neutrophil extracellular traps sequester circulating tumor cells and promote metastasis. J Clin Invest. 2013 doi: 10.1172/JCI67484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Demers M, et al. Priming of neutrophils toward NETosis promotes tumor growth. Oncoimmunology. 2016;5(5):e1134073. doi: 10.1080/2162402X.2015.1134073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Park J, et al. Cancer cells induce metastasis-supporting neutrophil extracellular DNA traps. Sci Transl Med. 2016;8(361):361ra138. doi: 10.1126/scitranslmed.aag1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Alfaro C, et al. Tumor-Produced Interleukin-8 Attracts Human Myeloid-Derived Suppressor Cells and Elicits Extrusion of Neutrophil Extracellular Traps (NETs) Clin Cancer Res. 2016;22(15):3924–36. doi: 10.1158/1078-0432.CCR-15-2463. [DOI] [PubMed] [Google Scholar]
- 40.Leal AC, et al. Tumor-Derived Exosomes Induce the Formation of Neutrophil Extracellular Traps: Implications For The Establishment of Cancer-Associated Thrombosis. Sci Rep. 2017;7(1):6438. doi: 10.1038/s41598-017-06893-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Demers M, et al. Cancers predispose neutrophils to release extracellular DNA traps that contribute to cancer-associated thrombosis. Proc Natl Acad Sci U S A. 2012;109(32):13076–81. doi: 10.1073/pnas.1200419109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Houghton AM, et al. Neutrophil elastase-mediated degradation of IRS-1 accelerates lung tumor growth. Nat Med. 2010;16(2):219–23. doi: 10.1038/nm.2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Caruso JA, et al. The serine protease inhibitor elafin maintains normal growth control by opposing the mitogenic effects of neutrophil elastase. Oncogene. 2015;34(27):3556–67. doi: 10.1038/onc.2014.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Starcher B, et al. Inhibition of Neutrophil Elastase Suppresses the Development of Skin Tumors in Hairless Mice. Journal of Investigative Dermatology. 1996;107(2):159–163. doi: 10.1111/1523-1747.ep12329559. [DOI] [PubMed] [Google Scholar]
- 45.Shang K, et al. Crucial involvement of tumor-associated neutrophils in the regulation of chronic colitis-associated carcinogenesis in mice. PLoS One. 2012;7(12):e51848. doi: 10.1371/journal.pone.0051848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Burcham GN, et al. Impact of prostate inflammation on lesion development in the POET3 (+)Pten(+/−) mouse model of prostate carcinogenesis. Am J Pathol. 2014;184(12):3176–91. doi: 10.1016/j.ajpath.2014.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fan S, et al. Opposite angiogenic outcome of curcumin against ischemia and Lewis lung cancer models: in silico, in vitro and in vivo studies. Biochim Biophys Acta. 2014;1842(9):1742–54. doi: 10.1016/j.bbadis.2014.06.019. [DOI] [PubMed] [Google Scholar]
- 48.Xu Y, et al. Curcumin inhibits tumor proliferation induced by neutrophil elastase through the upregulation of alpha1-antitrypsin in lung cancer. Mol Oncol. 2012;6(4):405–17. doi: 10.1016/j.molonc.2012.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Inada M, Yamashita J, Ogawa M. Neutrophil elastase inhibitor (ONO-5046-Na) inhibits the growth of human lung cancer cell lines transplanted into severe combined immunodeficiency (scid) mice. Res Commun Mol Pathol Pharmacol. 1997;97(2):229–32. [PubMed] [Google Scholar]
- 50.Wada Y, et al. Sivelestat, a specific neutrophil elastase inhibitor, suppresses the growth of gastric carcinoma cells by preventing the release of transforming growth factor-alpha. Cancer Sci. 2006;97(10):1037–43. doi: 10.1111/j.1349-7006.2006.00278.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Meyer-Hoffert U, Wingertszahn J, Wiedow O. Human leukocyte elastase induces keratinocyte proliferation by epidermal growth factor receptor activation. J Invest Dermatol. 2004;123(2):338–45. doi: 10.1111/j.0022-202X.2004.23202.x. [DOI] [PubMed] [Google Scholar]
- 52.Wada Y, et al. Neutrophil elastase induces cell proliferation and migration by the release of TGF-alpha, PDGF and VEGF in esophageal cell lines. Oncol Rep. 2007;17(1):161–7. [PubMed] [Google Scholar]
- 53.Rogalski C, et al. Human leukocyte elastase induces keratinocyte proliferation in vitro and in vivo. J Invest Dermatol. 2002;118(1):49–54. doi: 10.1046/j.0022-202x.2001.01650.x. [DOI] [PubMed] [Google Scholar]
- 54.Gregory AD, et al. Clathrin pit-mediated endocytosis of neutrophil elastase and cathepsin G by cancer cells. J Biol Chem. 2012;287(42):35341–50. doi: 10.1074/jbc.M112.385617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xiaokaiti Y, et al. EGCG reverses human neutrophil elastase-induced migration in A549 cells by directly binding to HNE and by regulating alpha1-AT. Sci Rep. 2015;5:11494. doi: 10.1038/srep11494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kerros C, et al. Neuropilin-1 mediates neutrophil elastase uptake and cross-presentation in breast cancer cells. J Biol Chem. 2017;292(24):10295–10305. doi: 10.1074/jbc.M116.773051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hattar K, et al. Interactions between neutrophils and non-small cell lung cancer cells: enhancement of tumor proliferation and inflammatory mediator synthesis. Cancer Immunol Immunother. 2014;63(12):1297–306. doi: 10.1007/s00262-014-1606-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pivetta E, et al. Neutrophil elastase-dependent cleavage compromises the tumor suppressor role of EMILIN1. Matrix Biol. 2014;34:22–32. doi: 10.1016/j.matbio.2014.01.018. [DOI] [PubMed] [Google Scholar]
- 59.Maiorani O, et al. Neutrophil elastase cleavage of the gC1q domain impairs the EMILIN1-alpha4beta1 integrin interaction, cell adhesion and anti-proliferative activity. Sci Rep. 2017;7:39974. doi: 10.1038/srep39974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.El Rayes T, et al. Lung inflammation promotes metastasis through neutrophil protease-mediated degradation of Tsp-1. Proc Natl Acad Sci U S A. 2015;112(52):16000–5. doi: 10.1073/pnas.1507294112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Aldabbous L, et al. Neutrophil Extracellular Traps Promote Angiogenesis: Evidence From Vascular Pathology in Pulmonary Hypertension. Arterioscler Thromb Vasc Biol. 2016;36(10):2078–87. doi: 10.1161/ATVBAHA.116.307634. [DOI] [PubMed] [Google Scholar]
- 62.Gong L, et al. Promoting effect of neutrophils on lung tumorigenesis is mediated by CXCR2 and neutrophil elastase. Mol Cancer. 2013;12(1):154. doi: 10.1186/1476-4598-12-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bekes EM, et al. Tumor-recruited neutrophils and neutrophil TIMP-free MMP-9 regulate coordinately the levels of tumor angiogenesis and efficiency of malignant cell intravasation. Am J Pathol. 2011;179(3):1455–70. doi: 10.1016/j.ajpath.2011.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Deryugina EI, et al. Tissue-infiltrating neutrophils constitute the major in vivo source of angiogenesis-inducing MMP-9 in the tumor microenvironment. Neoplasia. 2014;16(10):771–88. doi: 10.1016/j.neo.2014.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Geraghty P, et al. Neutrophil Elastase Up-Regulates Cathepsin B and Matrix Metalloprotease-2 Expression. The Journal of Immunology. 2007;178(9):5871–5878. doi: 10.4049/jimmunol.178.9.5871. [DOI] [PubMed] [Google Scholar]
- 66.Jackson PL, et al. Human neutrophil elastase-mediated cleavage sites of MMP-9 and TIMP-1: implications to cystic fibrosis proteolytic dysfunction. Mol Med. 2010;16(5-6):159–66. doi: 10.2119/molmed.2009.00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shamamian P, et al. Activation of progelatinase A (MMP-2) by neutrophil elastase, cathepsin G, and proteinase-3: a role for inflammatory cells in tumor invasion and angiogenesis. J Cell Physiol. 2001;189(2):197–206. doi: 10.1002/jcp.10014. [DOI] [PubMed] [Google Scholar]
- 68.Itoh Y, Nagase H. Preferential inactivation of tissue inhibitor of metalloproteinases-1 that is bound to the precursor of matrix metalloproteinase 9 (progelatinase B) by human neutrophil elastase. J Biol Chem. 1995;270(28):16518–21. doi: 10.1074/jbc.270.28.16518. [DOI] [PubMed] [Google Scholar]
- 69.Shojaei F, et al. Tumor refractoriness to anti-VEGF treatment is mediated by CD11b+Gr1+ myeloid cells. Nat Biotechnol. 2007;25(8):911–20. doi: 10.1038/nbt1323. [DOI] [PubMed] [Google Scholar]
- 70.Valastyan S, Weinberg RA. Tumor metastasis: molecular insights and evolving paradigms. Cell. 2011;147(2):275–92. doi: 10.1016/j.cell.2011.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Steele CW, et al. CXCR2 Inhibition Profoundly Suppresses Metastases and Augments Immunotherapy in Pancreatic Ductal Adenocarcinoma. Cancer Cell. 2016;29(6):832–845. doi: 10.1016/j.ccell.2016.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Toh B, et al. Mesenchymal transition and dissemination of cancer cells is driven by myeloid-derived suppressor cells infiltrating the primary tumor. PLoS Biol. 2011;9(9):e1001162. doi: 10.1371/journal.pbio.1001162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kolonin MG, et al. Interaction between Tumor Cell Surface Receptor RAGE and Proteinase 3 Mediates Prostate Cancer Metastasis to Bone. Cancer Res. 2017;77(12):3144–3150. doi: 10.1158/0008-5472.CAN-16-0708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Erpenbeck L, Schon MP. Neutrophil extracellular traps: protagonists of cancer progression? Oncogene. 2017;36(18):2483–2490. doi: 10.1038/onc.2016.406. [DOI] [PubMed] [Google Scholar]
- 75.Tohme S, et al. Neutrophil Extracellular Traps Promote the Development and Progression of Liver Metastases after Surgical Stress. Cancer Res. 2016;76(6):1367–80. doi: 10.1158/0008-5472.CAN-15-1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mayer C, et al. Neutrophil Granulocytes in Ovarian Cancer - Induction of Epithelial-To-Mesenchymal-Transition and Tumor Cell Migration. J Cancer. 2016;7(5):546–54. doi: 10.7150/jca.14169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gaida MM, et al. Polymorphonuclear neutrophils promote dyshesion of tumor cells and elastase-mediated degradation of E-cadherin in pancreatic tumors. Eur J Immunol. 2012;42(12):3369–80. doi: 10.1002/eji.201242628. [DOI] [PubMed] [Google Scholar]
- 78.Grosse-Steffen T, et al. Epithelial-to-mesenchymal transition in pancreatic ductal adenocarcinoma and pancreatic tumor cell lines: the role of neutrophils and neutrophil-derived elastase. Clin Dev Immunol. 2012;2012:720768. doi: 10.1155/2012/720768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yamashita J-I, et al. Tumor Neutrophil Elastase Is Closely Associated With the Direct Extension of Non-small Cell Lung Cancer Into the Aorta. Chest. 1997;111(4):885–890. doi: 10.1378/chest.111.4.885. [DOI] [PubMed] [Google Scholar]
- 80.Nozawa F, et al. Elastase activity enhances the adhesion of neutrophil and cancer cells to vascular endothelial cells. J Surg Res. 2000;94(2):153–8. doi: 10.1006/jsre.2000.6002. [DOI] [PubMed] [Google Scholar]
- 81.Chua F, et al. Mice lacking neutrophil elastase are resistant to bleomycin-induced pulmonary fibrosis. Am J Pathol. 2007;170(1):65–74. doi: 10.2353/ajpath.2007.060352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tobin RP, et al. The clinical evidence for targeting human myeloid-derived suppressor cells in cancer patients. J Leukoc Biol. 2017;102(2):381–391. doi: 10.1189/jlb.5VMR1016-449R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sternberg C, et al. Randomized, Double-Blind, Placebo-Controlled Phase III Study of Tasquinimod in Men With Metastatic Castration-Resistant Prostate Cancer. J Clin Oncol. 2016;34(22):2636–43. doi: 10.1200/JCO.2016.66.9697. [DOI] [PubMed] [Google Scholar]
- 84.Dominguez GA, et al. Selective Targeting of Myeloid-Derived Suppressor Cells in Cancer Patients Using DS-8273a, an Agonistic TRAIL-R2 Antibody. Clin Cancer Res. 2017;23(12):2942–2950. doi: 10.1158/1078-0432.CCR-16-1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Markowitz J, et al. Patients with pancreatic adenocarcinoma exhibit elevated levels of myeloid-derived suppressor cells upon progression of disease. Cancer Immunol Immunother. 2015;64(2):149–59. doi: 10.1007/s00262-014-1618-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lorente D, et al. Baseline neutrophil-lymphocyte ratio (NLR) is associated with survival and response to treatment with second-line chemotherapy for advanced prostate cancer independent of baseline steroid use. Ann Oncol. 2015;26(4):750–5. doi: 10.1093/annonc/mdu587. [DOI] [PubMed] [Google Scholar]
- 87.Lu X, et al. Effective combinatorial immunotherapy for castration-resistant prostate cancer. Nature. 2017;543(7647):728–732. doi: 10.1038/nature21676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Aikawa N, et al. Reevaluation of the efficacy and safety of the neutrophil elastase inhibitor, Sivelestat, for the treatment of acute lung injury associated with systemic inflammatory response syndrome; a phase IV study. Pulm Pharmacol Ther. 2011;24(5):549–54. doi: 10.1016/j.pupt.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 89.Aikawa N, Kawasaki Y. Clinical utility of the neutrophil elastase inhibitor sivelestat for the treatment of acute respiratory distress syndrome. Ther Clin Risk Manag. 2014;10:621–9. doi: 10.2147/TCRM.S65066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Stevens T, et al. AZD9668: pharmacological characterization of a novel oral inhibitor of neutrophil elastase. J Pharmacol Exp Ther. 2011;339(1):313–20. doi: 10.1124/jpet.111.182139. [DOI] [PubMed] [Google Scholar]
- 91.von Nussbaum F, et al. Freezing the Bioactive Conformation to Boost Potency: The Identification of BAY 85-8501, a Selective and Potent Inhibitor of Human Neutrophil Elastase for Pulmonary Diseases. ChemMedChem. 2015;10(7):1163–73. doi: 10.1002/cmdc.201500131. [DOI] [PMC free article] [PubMed] [Google Scholar]


