SUMMARY
Restoring adult stem cell function provides an exciting approach for rejuvenating the aging brain. However, molecular mechanisms mediating neurogenic rejuvenation remain elusive. Here we report that the enzyme ten eleven translocation methylcytosine dioxygenase 2 (Tet2), which catalyzes the production of 5-hydroxymethylcytosine (5hmC), rescues age-related decline in adult neurogenesis and enhances cognition in mice. We detected a decrease in Tet2 expression and 5hmC levels in the aged hippocampus associated with adult neurogenesis. Mimicking an aged condition in young adults by abrogating Tet2 expression within the hippocampal neurogenic niche, or adult neural stem cells, decreased neurogenesis and impaired learning and memory. In a heterochronic parabiosis rejuvenation model, hippocampal Tet2 expression was restored. Overexpressing Tet2 in the hippocampal neurogenic niche of mature adults increased 5hmC associated with neurogenic processes, offset the precipitous age-related decline in neurogenesis, and enhanced learning and memory. Our data identify Tet2 as a key molecular mediator of neurogenic rejuvenation.
In Brief
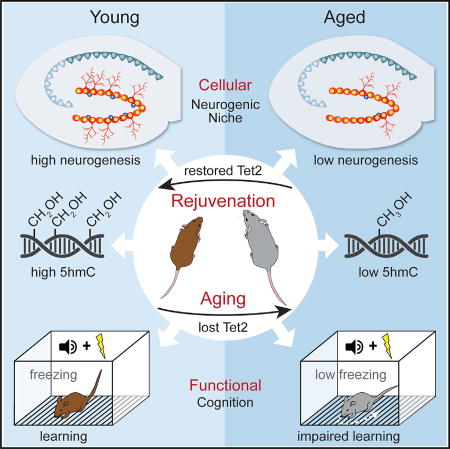
Gontier et al. find a link between Tet2 and neurogenic rejuvenation. Age-related loss of hippocampal Tet2 and 5hmC associates with decreased neurogenesis. Mimicking age-related loss of Tet2 in the young mouse hippocampus decreases neurogenesis and impairs cognition. Restoring Tet2 in the mature hippocampus rejuvenates regenerative capacity and enhances cognition.
INTRODUCTION
During aging, the number of neural stem/progenitor cells (NPCs), and subsequently neurogenesis, precipitously declines in the subgranular zone of the dentate gyrus (DG) in the hippocampus (Fan et al., 2017). Mounting evidence in animal models indicates the potential for rejuvenation of regenerative and cognitive functions in the aging brain through interventions, such as heterochronic parabiosis (which exposes aged animals to young blood) (Fan et al., 2017; Katsimpardi et al., 2014; Villeda et al., 2011). However, the ability to utilize this neurogenic potential is predicated on identifying molecular targets that reverse the effects of aging in the brain.
Recent studies have begun to link changes in the functions of epigenetic mediators, such as those necessary for covalent DNA modifications, to age-related regenerative decline (Beerman and Rossi, 2015; Brunet and Rando, 2017). Interestingly ten eleven translocation methylcytosine dioxygenase 2 (Tet2), which catalyzes the oxidation of 5-methylcytosine (5mC) to 5-hydroxymethylcytosine (5hmC), is emerging as a potential epigenetic regulator of aging (Burgess, 2015). Human genetic studies identified an increased frequency of somatic TET2 mutations with age that are associated with elevated risk for aging-associated disorders, such as cancer, cardiovascular disease, and stroke (Burgess, 2015; Jaiswal et al., 2014; Nadarajah et al., 2015). Notwithstanding, the involvement of Tet2 in mediating the aging process in the adult brain has yet to be investigated. While Tet proteins and 5hmC are highly expressed in the brain (Hahn et al., 2013; Kriaucionis and Heintz, 2009; Szulwach et al., 2011), with Tet1 and Tet3 implicated in proper brain function (Kaas et al., 2013; Li et al., 2014; Rudenko et al., 2013; Zhang et al., 2013), the role of Tet2 remains relatively unexplored.
Here we demonstrate that Tet2 offsets age-related neurogenic decline and enhances cognition in the hippocampus of adult mice. We detect an age-dependent decrease in the levels of Tet2 and 5hmC in the aging hippocampus coincident with decreased adult neurogenesis. Mimicking an age-related loss of Tet2 in the adult neurogenic niche of the hippocampus, or adult NPCs, impairs regenerative capacity and associated hippocampal-dependent learning and memory processes. Conversely, increasing Tet2 in the hippocampus of mature animals increases 5hmC associated with neurogenic processes, restores adult neurogenesis to youthful levels, and enhances cognitive function. These findings indicate that Tet2-mediated hydroxymethylation regulates age-related regenerative decline in the aging hippocampus, with functional implications for neurogenic rejuvenation.
RESULTS
Tet2 and 5hmC Levels Decrease in the Aged Hippocampus and Are Associated with Neurogenic Processes
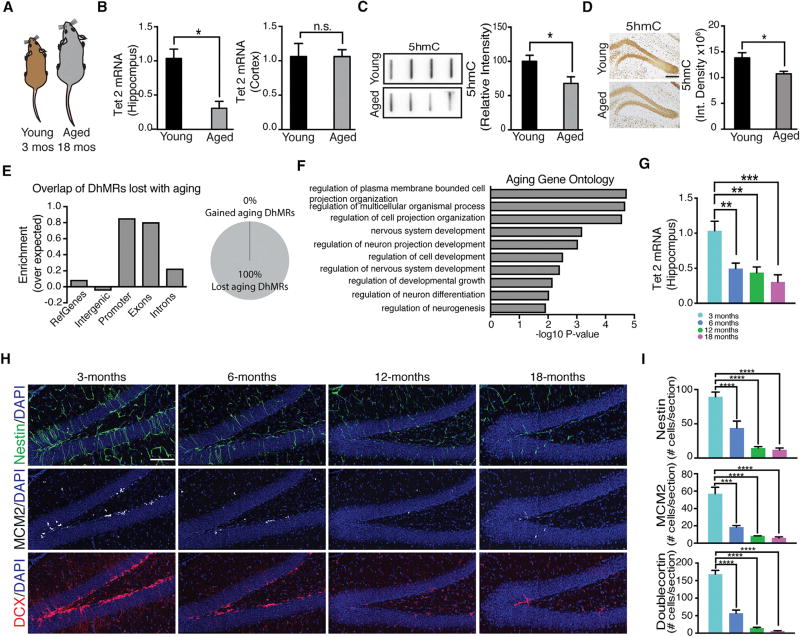
We characterized changes in the levels of Tet2 mRNA in the hippocampus and cortex of normal young and aged animals by qPCR, and we detected an age-related decrease in Tet2 expression within the aged hippocampus (Figures 1A and 1B). We examined changes in hippocampal 5hmC and 5mC levels by slot blot and immunohistochemical analysis, and we observed an age-related decrease in 5hmC (Figures 1C and 1D), but not 5mC (Figures S1A and S1B). To gain mechanistic insight into which genomic loci were affected by these changes, we performed antibody-based 5hmC immunoprecipitation combined with deep sequencing (hMeDIP-seq) in the young and aged hippocampus (Figures 1E and 1F; Figures S1C–S1H). We characterized differentially 5-hydroxymethylated regions (DhMRs) (Feng et al., 2012), and we identified 345 DhMRs lost, while detecting none gained, with age (Figure 1E; Table S1). Lost DhMRs were enriched in intragenic regions, in the aged compared to young hippocampus (Figure 1E), and we observed DhMR-associated genes were involved in neurogenic processes by gene ontology analysis (Figure 1F).
Figure 1. Tet2 Expression and 5hmC Levels Decline in the Aged Hippocampus and Are Associated with Neurogenic Processes.
(A) Schematic of young adult (3-month-old) versus aged (18-month-old) mice.
(B) Quantitative reverse-transcription PCR of Tet2 mRNA from hippocampal and cortical lysates of young and aged mice (n = 5 per group; t test, *p < 0.05; n.s., not significant).
(C) Representative slot blot and quantification of isolated hippocampal DNA probed with anti-5hmC antibodies from young and aged mice (n = 4 per group; t test, *p < 0.05).
(D) Representative field and quantification of 5hmC expression in the dentate gyrus (DG) of the young and aged hippocampus (n = 4 per group; scale bar, 100 mm; t test, *p < 0.05).
(E) Association of regions of 5hmC lost during age with genomic elements (differentially 5-hydroxymethylated regions [DhMRs] lost between 3 and 18 months; 345 DhMRs at q = 0.05). Pie chart depicts overall loss and gain of DhMRs during aging.
(F) Top gene ontology terms for genes overlapping with age-associated 5hmC loss (>2-fold enrichment; ordered by p value).
(G) Reverse-transcription qPCR of Tet2 mRNA from hippocampal lysates of aging mice (n = 5; ANOVA with Dunnett’s post hoc test, **p < 0.01).
(H and I) Neurogenesis was analyzed by immunostaining and confocal microscopy. Representative field (H) and quantification (I) of Nestin-positive, MCM2-positive, and Doublecortin (DCX)-positive cells in aging dentate gyrus (DG) at 3, 6, 12, and 18 months (n = 5; scale bar, 100 mm; ANOVA with Dunnett’s post hoc test, ***p < 0.001 and ****p < 0.0001).
Data are represented as mean ± SEM. See also Figure S1.
To further investigate the relationship between age-related changes in Tet2 expression and adult neurogenesis, we compared the temporal kinetics of decreased adult neurogenesis and Tet2 expression with age (Figures 1G–1I; Figure S1I). Adult neurogenesis and Tet2 expression were quantified in contralateral hippocampi of the same animals by immunohistochemical analysis and qPCR, respectively. We observed a precipitous decline in adult neurogenesis by 6 months of age in mature adults (Figures 1H and 1I; Figure S1I) that was paralleled by a sharp decrease in Tet2 expression (Figure 1G; Figure S1I). Altogether, these data raise the possibility that decreased Tet2 and 5hmC levels in the aging hippocampus are associated with age-related impairments in adult neurogenesis.
Reducing Tet2 in the Young Adult Hippocampus Impairs Neurogenesis
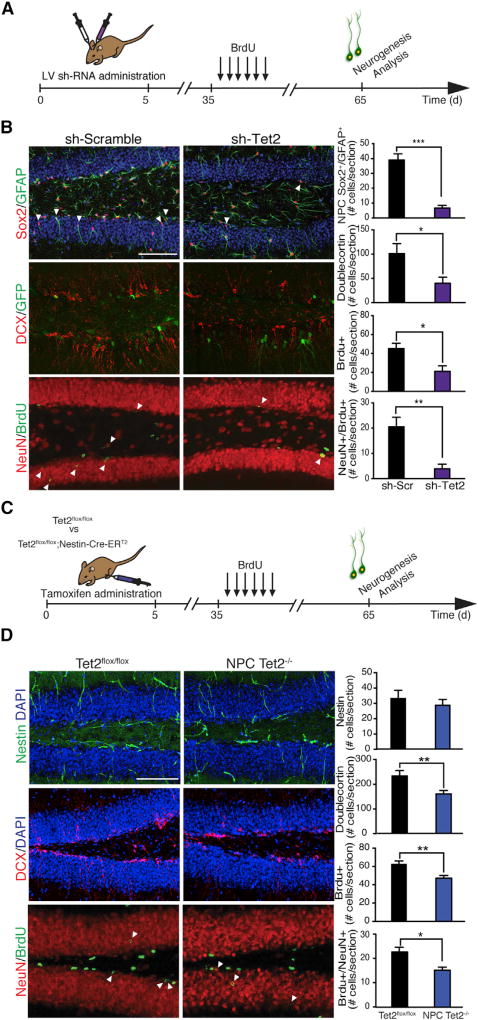
We next asked whether mimicking an age-related decline in Tet2 within the young adult hippocampus would impair neurogenesis. We first abrogated Tet2 expression in the young adult hippocampus utilizing an in vivo viral-mediated RNAi approach (Figure 2A). Young adult animals were stereotaxically injected with high-titer lentivirus (LV) encoding Tet2 or scramble control small hairpin RNA (shRNA) sequences into the DG of contralateral hippocampi, and Tet2 abrogation was confirmed in vivo (Figures S2A–S2C). No changes in Tet1 or Tet3 were detected (Figure S2D). Subsequently, we analyzed NPC function and maturation in an independent cohort of young adult animals by immunohistochemical analysis. Abrogation of Tet2 in the DG resulted in a significant decrease in the number of Sox2/GFAP-positive NPCs, Doublecortin (Dcx)-positive newly born neurons, Bromodeoxyuridne (BrdU)-positive cells, and BrdU/NeuN-positive mature differentiated neurons compared with the control contralateral DG (Figure 2B).
Figure 2. Abrogation of Tet2 in the Adult Dentate Gyrus or Loss of Tet2 in Adult NPCs Impairs Hippocampal Neurogenesis.
(A and B) Young adult (3-month-old) mice were given unilateral stereotaxic injections of lentivirus (LV) encoding either shRNA targeting Tet2 or scramble control sequences in tandem with a GFP reporter into contralateral dentate gyrus (DG). Mice were administered BrdU by intraperitoneal injections for 6 days and euthanized 30 days later. Neurogenesis was analyzed by immunostaining and confocal microscopy.
(A) Schematic illustrating stereotaxic injection paradigm and experimental timeline.
(B) Representative field and quantification of GFAP/Sox2-positive, Doublecortin (Dcx)-positive, BrdU-positive, and NeuN/BrdU-positive cells (n = 5–9 per group; scale bar, 100 mm; t test, *p < 0.05, **p < 0.01, and ***p < 0.001).
(C and D) A cell type-specific temporally controlled Tet2flox/flox/NestinCre-ERT2 genetic model was generated, in which Tet2 was excised selectively in adult neural stem/progenitor cells (NPCs) upon tamoxifen administration (Tet2−/−). Neurogenesis was analyzed in young adult (3-month-old) NPC Tet2−/− and littermate control (Tet2flox/flox) mice by immunostaining and confocal microscopy.
(C) Schematic illustrating tamoxifen injection paradigm and experimental timeline. Mice were administered BrdU by intraperitoneal injections for 6 days and euthanized 30 days later.
(D) Representative field and quantification of Nestin-positive, Dcx-positive, BrdU-positive, and NeuN/BrdU-positive cells (n = 7–8 per group; scale bar, 100 mm; t test, *p < 0.05, **p < 0.01, and ***p < 0.001).
Data are represented as mean ± SEM. See also Figure S2.
To gain mechanistic insight at a cellular level, we generated Tet2floxf/lox mice carrying an inducible NestinCre-ERT2 gene, in which Tet2 is excised specifically in adult NPCs (Tet2−/−) upon tamoxifen administration (Figure 2C; Figure S2E). We examined changes in neurogenesis in young adult Tet2−/− and littermate control (Tet2floxf/lox) mice by immunohistochemical analysis. The absence of Tet2 expression in adult NPCs resulted in a selective decrease in the number of Dcx-positive newly born neurons, BrdU-positive cells, and BrdU/NeuN-positive mature differentiated neurons in Tet2−/− mice compared to Tet2flox/flox controls, without altering the number of Nestin-positive NPCs (Figure 2D). This decrease in newly born and mature neurons is consistent with increased levels of 5hmC upon neuronal differentiation in vivo (Figure S2F). Collectively, our in vivo RNAi and genetic data suggest an involvement of Tet2 in regulating adult neurogenesis at both the levels of the neurogenic niche and adult NPCs.
Reducing Tet2 in the Young Adult Hippocampus Impairs Cognitive Function
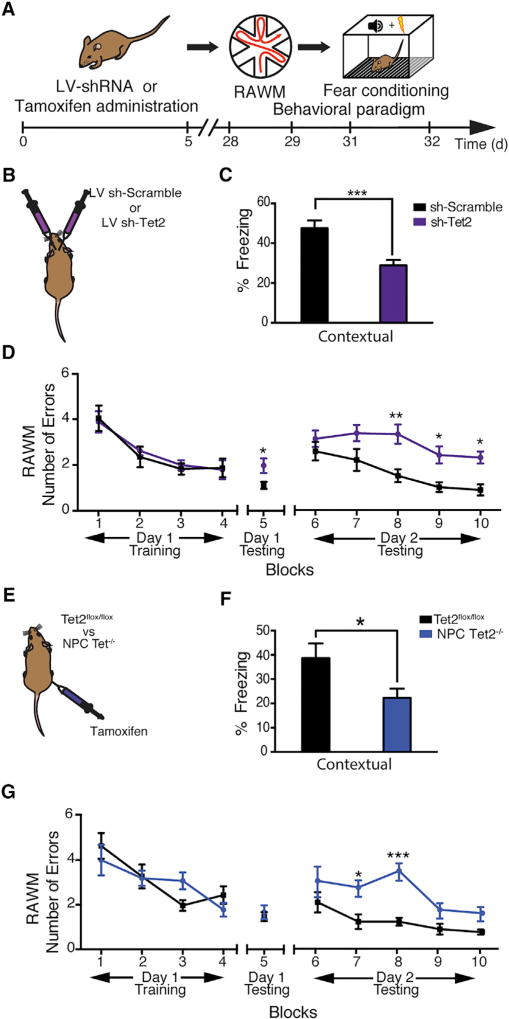
To investigate whether decreased Tet2 in the young adult DG, or loss of Tet2 in adult NPCs, impaired cognitive processes, hippocampal-dependent learning and memory were assessed using radial arm water maze (RAWM) and contextual fear-conditioning paradigms (Figure 3A). To test the effect of decreased Tet2 in the adult DG, young adult animals were given bilateral stereotaxic injections into the hippocampi of high-titer LV encoding Tet2 shRNA or scramble control sequences (Figure 3B). To test the effect of selective loss of Tet2 in adult NPCs, we utilized young adult NPC Tet2−/− and littermate control Tet2flox/flox mice (Figure 3E). All mice showed similar learning capacity during the training phase of the RAWM (Figures 3D and 3G). Abrogation of Tet2 in the adult DG resulted in significantly more errors in locating the target platform during both short-term(Figure 3D; Figure S3B) and long-term (Figure 3D; Figure S3C) learning and memory testing compared to control conditions. Interestingly, selective loss of Tet2 in adult NPCs resulted in impairments only during long-term learning and memory testing compared to control mice (Figure 3G; Figures S3E and S3F). During fear conditioning training, mice exhibited no differences in baseline freezing time (Figures S3A and S3D). However, both abrogation of Tet2 in the adult DG and loss of Tet2 in adult NPCs resulted in decreased freezing time during contextual (Figures 3C and 3F), but not cued (Figures S3A and S3D), memory testing. These data demonstrate that decreased Tet2 in the adult neurogenic niche, or adult NPCs, impairs long-term hippocampal-dependent spatial learning and memory and associative fear memory acquisition.
Figure 3. Abrogation of Tet2 in the Adult Dentate Gyrus or Loss of Tet2 in Adult NPCs Impairs Hippocampal-Dependent Cognitive Function.
(A) Schematic of experimental paradigm and cognitive testing timeline. Hippocampal-dependent learning and memory were assessed by radial arm water maze (RAWM) and contextual fear-conditioning paradigms.
(B) Young adult (3-month-old) wild-type mice were given bilateral stereotaxic injections of lentivirus (LV) encoding either shRNA targeting Tet2 (sh-Tet2) or scramble control sequences (sh-Scramble) into the dentate gyrus (DG).
(C) Associative fear memory was assessed in sh-Tet2- and sh-Scramble control-injected mice using contextual fear conditioning. Quantification of percentage freezing 24 hr after training is shown (n = 14 per group; t test, ***p < 0.001).
(D) Hippocampal-dependent spatial learning and memory were assessed in sh-Tet2- and sh-Scramble control-injected mice using RAWM. Quantification of the number of entry errors during RAWM training and testing is shown (n = 14 per group; repeated-measures ANOVA with Bonferroni post hoc correction, *p < 0.05 and **p < 0.01).
(E) Young adult Tet2flox/flox/NestinCre-ERT2 NPC-specific knockout (Tet2−/−) or littermate control (Tet2flox/flox) mice were administered tamoxifen. Data from 9–10 animals per group are shown.
(F) Associative fear memory was assessed in Tet2−/− and Tet2flox/flox control mice using contextual fear conditioning. Quantification of percentage freezing 24 hr after training is shown (n = 9–10 per group; t test, *p < 0.05).
(G) Hippocampal-dependent spatial learning and memory were assessed in in Tet2−/− and Tet2flox/flox control mice using RAWM. Quantification of the number of entry errors during RAWM training and testing is shown (n = 9–10 per group; repeated-measures ANOVA with Bonferroni post hoc correction, ***p < 0.001).
Data are represented as mean ± SEM. See also Figure S3.
Decreased Tet2 in the Aged Hippocampus Is Reversed in a Heterochronic Parabiosis Model of Brain Rejuvenation
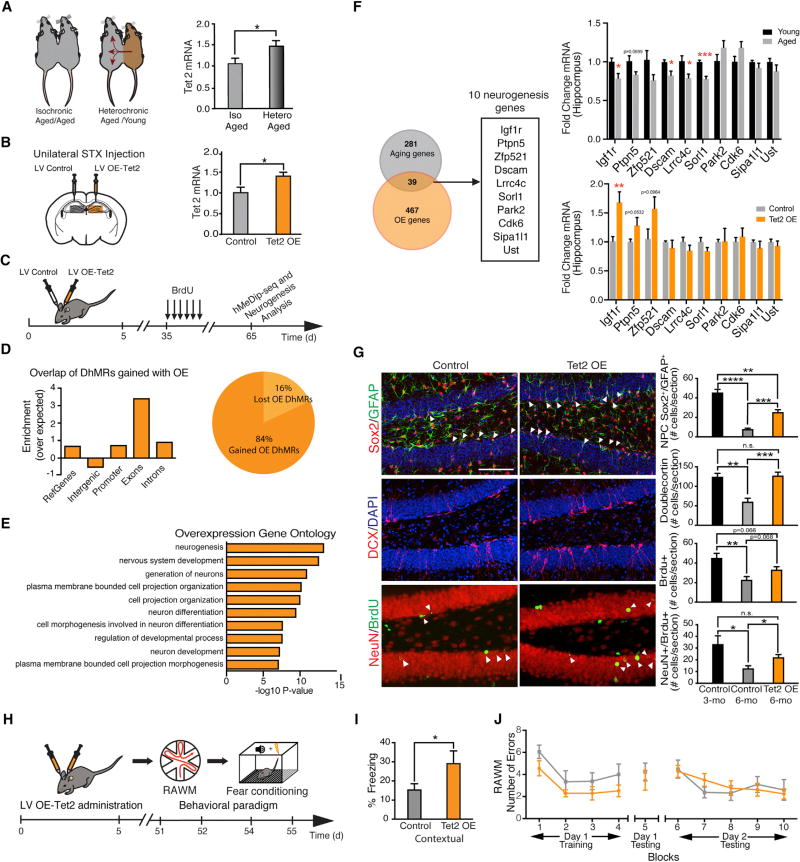
Thus far, at a cellular level, our findings indicate that mimicking an age-related decline in Tet2 impairs adult neurogenesis. However, it remains an open question whether restoring Tet2 in the adult brain can rescue age-related regenerative decline. Using a model of heterochronic parabiosis, we and others have demonstrated that rejuvenation of adult neurogenesis is possible in the aged brain (Katsimpardi et al., 2014; Villeda et al., 2011). To gain insight into the potential involvement of Tet2 in neurogenic rejuvenation, we measured mRNA levels of Tet2 in the aged hippocampus after heterochronic parabiosis. We detected an increase of Tet2 in heterochronic parabionts after exposure to young blood compared to age-matched isochronic parabionts exposed to old blood (Figure 4A). No changes in Tet1 or Tet3 were observed (Figure S7A). These data implicate Tet2 in conditions of brain rejuvenation.
Figure 4. Increasing Tet2 in the Mature Adult Dentate Gyrus Rescues Age-Related Regenerative Decline and Enhances Cognitive Function.
(A) Schematic and reverse-transcription qPCR of Tet2 mRNA from hippocampal lysates of aged (18-month-old) isochronic and heterochronic parabionts 5 weeks after parabiosis (n = 5 per group; t test, *p < 0.05).
(B) Mature adult (6-month-old) mice were given unilateral stereotaxic (STX) injections of lentivirus (LV) encoding Tet2 or control LV into contralateral dentate gyrus (DG). Schematic and qPCR of Tet2 mRNA from hippocampal lysates following STX injection are shown (n = 5 per group; t test, *p < 0.05).
(C) Schematic illustrating stereotaxic injection paradigm and experimental timeline.
(D) Association of regions of 5hmC gained after Tet2 overexpression with genomic elements (DhMRs gained; 558 DhMRs at q = 0.05). Pie chart depicts overall loss and gain of DhMRs after Tet2 overexpression (OE).
(E) Top gene ontology terms (2-fold enrichment, ordered by p value) for genes associated with gained DhMRs after Tet2 overexpression (OE).
(F) Venn diagram representing the overlap of genes paired with DhMRs from those lost during aging (3 months over 18 months) and those gained from Tet2 overexpression (OE over control). From the overlap, genes associated with neurogenesis using gene ontology are shown, and mRNA expression in hippocampal lysates was measured by qPCR (n = 5 per group; t test, *p < 0.05, **p < 0.01, and ***p < 0.001).
(G) Neurogenesis was analyzed by immunostaining and confocal microscopy in mature adult (6-month-old) animals after LV administration. As a reference, young adult (3-month-old) mice were given unilateral STX injections of control LV. All mice were administered BrdU by intraperitoneal injections for 6 days and euthanized 30 days later. Representative field and quantification of GFAP/Sox2-positive, Dcx-positive, BrdU-positive, and NeuN/BrdU-positive cells are shown (n = 5 per group; scale bar, 100 mm; t test, *p < 0.05, **p < 0.01, and ***p < 0.001).
(H) Schematic of experimental paradigm and cognitive testing timeline. Mature adult (6-month-old) wild-type mice were given bilateral stereotaxic (STX) injections of lentivirus (LV) encoding Tet2 or control LV into the dentate gyrus (DG).
(I) Associative fear memory was assessed using contextual fear conditioning. Quantification of percentage freezing 24 hr after training is shown (n = 12–15 per group; t test, ***p < 0.001).
(J) Hippocampal-dependent spatial learning and memory was assessed using RAWM. Quantification of the number of entry errors during RAWM training and testing is shown (n = 6–9 per group; repeated-measures ANOVA with Bonferroni post hoc correction).
Data are represented as mean ± SEM. See also Figure S4.
Restoring Tet2 in the Mature Adult Hippocampus Rescues Age-Related Regenerative Decline
To investigate whether restoring Tet2 in the adult hippocampal neurogenic niche could counteract age-related regenerative decline, we utilized an in vivo viral-mediated overexpression approach. Aging analysis demonstrated a concomitant age-related decrease in adult neurogenesis and Tet2 expression in mature adult animals at 6 months of age (Figures 1G–1I). Correspondingly, mature adult animals at this age were stereotaxically injected with high-titer LV encoding Tet2 or control into the DG of contralateral hippocampi, and Tet2 overexpression was confirmed in vivo (Figure 4B; Figure S4B). No changes in Tet1 or Tet3 were detected (Figure S4C).
We performed hMeDIP-seq in the mature adult hippocampi following viral-mediated Tet2 overexpression (Figures 4C–4E; Figures S4D–S4H), and we found 558 DhMRs gained and 110 lost after Tet2 overexpression (Figure 4D; Table S2). We observed gained DhMRs enriched in intragenic regions (Figure 4D), and we identified associated neurogenic processes by gene ontology analysis (Figure 4E). We compared genes whose DhMRs were lost during aging with those gained by Tet2 overexpression, and we detected 39 overlapping genes (Table S3), of which 10 are involved in neurogenesis (Figure 4F).
Next, we examined whether restoring Tet2 was sufficient to rescue age-related decline in adult hippocampal neurogenesis by immunohistochemical analysis. We observed that increasing Tet2 in the mature adult DG resulted in a significant enhancement in the number of Sox2/GFAP-positive NPCs, Dcx-positive newly born neurons, BrdU-positive cells, and BrdU/NeuN-positive mature differentiated neurons in the DG compared with the control contralateral DG (Figure 4G). Excitingly, levels of adult neurogenesis achieved in the mature adult hippocampus by increased Tet2 mirrored levels normally observed in the young adult hippocampus (Figure 4G). We overexpressed Tet2 in the DG of young adult animals at 3 months of age, and we detected no changes in adult neurogenesis (Figure S4J). Our data indicate an age-dependent role for Tet2 in regulating regenerative decline in the aging brain.
Restoring Tet2 in the Mature Adult Hippocampus Enhances Cognitive Function
We next investigated the functional consequence of increasing Tet2 in the mature adult DG on cognition. Hippocampal-dependent learning and memory were assessed using RAWM and contextual fear-conditioning paradigms. Mature adult animals were given bilateral stereotaxic injections of high-titer LV encoding Tet2 or control into the DG of the hippocampus (Figure 4H; Figures S4K and S4L). All mice showed similar learning capacity during the training phase of the RAWM (Figure 4J), and no differences were detected in locating the target platform during short-term (Figure 4J; Figure S4N) and long-term (Figure 4J; Figure S4N) learning and memory testing. During fear conditioning training, mice exhibited no differences in baseline freezing time (Figure S4M). However, increased Tet2 expression in the mature adult DG resulted in increased freezing time during contextual (Figure 4I), but not cued (Figure S4M), memory testing. These data indicate that restoring Tet2 in the mature adult neurogenic niche is sufficient to enhance associative fear memory acquisition.
DISCUSSION
Cumulatively, our data indicate that age-related loss of Tet2 leads to decreased adult neurogenesis, with functional implications for cognitive impairment. Our in vivo RNAi and genetic data dissect the involvement of Tet2 in regulating adult neurogenesis at the level of the neurogenic niche and adult NPCs, pointing to complementary cell-autonomous and non-autonomous roles for Tet2 in regulating NPC function versus neuronal differentiation processes. Moreover, we demonstrate that increasing Tet2 in the hippocampus is sufficient to rescue the precipitous age-related regenerative decline observed in the mature adult brain and enhance associated cognitive processes. Ultimately, our data suggest that restoring Tet2 in the aging brain can promote rejuvenation.
Recently, it has been demonstrated that constitutive whole-body loss of Tet2 yields opposing effects on neurogenic processes, resulting in increased adult NPC proliferation but decreased neuronal differentiation (Li et al., 2017). In contrast, our data indicate that decreasing Tet2 expression acutely in the adult neurogenic niche impairs all stages of hippocampal neurogenesis, while loss of Tet2 in adult NPCs impairs neuronal differentiation processes. These data point to differential regulation of distinct stages of neurogenesis by Tet2 that arise from the loss of Tet2 at the level of the whole organism, neurogenic niche, and adult NSC during development versus adult ages. In the context of aging, our data implicate decreased Tet2 in the aging hippocampus with age-related regenerative decline.
While a causal link between age-related decreased neurogenesis and cognitive decline remains obfuscated (Drapeau et al., 2003; Merrill et al., 2003; Seib et al., 2013; Smith et al., 2015), decreasing Tet2 within the young adult hippocampus, or loss of Tet2 in adult NPCs, resulted in cognitive impairments. Interestingly, impairments in short-term spatial learning and memory that are not typically associated with changes in adult neurogenesis were observed after Tet2 abrogation in the adult hippocampus, but not in adult NPCs, beginning to delineate complementary roles for Tet2 in regulating neurogenesis-dependent versus -independent cognitive functions. Collectively, our behavioral data identify a unique role for Tet2 in cognitive function distinct from other Tet family members, with previous studies implicating Tet1 and Tet3 in memory extinction but finding no role in long-term memory acquisition (Kaas et al., 2013; Li et al., 2014; Rudenko et al., 2013; Zhang et al., 2013).
Outside of the brain, aging-associated regenerative decline is also attributed to age-related dysfunction in tissue-specific adult stem cells throughout the body. Therefore, improving adult stem cell function provides an exciting approach for broadly rejuvenating regenerative capacity (Fan et al., 2017; Beerman and Rossi, 2015; Brunet and Rando, 2017). Mounting evidence indicates that interventions eliciting neurogenic rejuvenation (Katsimpardi et al., 2014; Villeda et al., 2011) concomitantly promote rejuvenation in peripheral tissues (Baht et al., 2015; Conboy et al., 2005; Sinha et al., 2014). Thus, our data raise the exciting possibility that rejuvenation of regenerative capacity could be promoted across tissues by targeting epigenetic regulators associated with the aging process. Moreover, the activities of several chromatin regulators are regulated by metabolites, notably Tet enzymes by vitamin C (Blaschke et al., 2013; Cimmino et al., 2017), raising the possibility that non-invasive metabolic interventions may further contribute to promoting epigenetic-mediated rejuvenation.
EXPERIMENTAL PROCEDURES
Further details and an outline of resources used in this work can be found in the Supplemental Experimental Procedures.
Animals
The following mouse lines were used: young (3-month-old), mature (6-monthold), and aged (18-month-old) male C57BL/6 mice (The Jackson Laboratory), Tet2flox/flox mice (The Jackson Laboratory), Gt(Rosa)26Sorflox-stop-floxCAG-TdTomato mice (The Jackson Laboratory), and NestinCreERT2 mice (The Jackson Laboratory). Animal use was in accordance with institutional guidelines approved by the University of California, San Francisco Institutional Animal Care and Use Committee (IACUC).
Data and Statistical Analyses
All experiments were randomized and blinded by an independent researcher. Groups were un-blinded at the end of each experiment upon statistical analysis. The distribution of data in each set of experiments was tested for normality using D’Agostino-Pearson omnibus test or Shapiro-Wilk test. Statistical analysis was performed with Prism 7.0 software (GraphPad). Means between two groups were compared with two-tailed, unpaired Student’s t test. Comparisons of means from multiple groups with each other or against one control group were analyzed with one-way ANOVA, followed by the appropriate post hoc tests (indicated in the figure legends).
Supplementary Material
Highlights.
Loss of Tet2 and 5hmC in the aged hippocampus associates with regenerative decline
Decreasing Tet2 in the young mouse hippocampus impairs neurogenesis and cognition
Heterochronic parabiosis restores Tet2 in the aged hippocampus
Restoring Tet2 in the mature hippocampus rescues neurogenesis and enhances cognition
Acknowledgments
We thank Dr. Barbara Panning for critical insight and technical assistance. Work was funded by the Irene Diamond Fund AFAR award, NIH Ruth L. Kirschstein NRSA fellowships (E.G.W., F31-AG050415 and J.M.S., F32-AG055292), a gift from Marc and Lynne Benioff (S.A.V.), the Glenn Foundation (S.A.V.), and the NIA (R01 AG053382 and R01 AG055797).
Footnotes
DATA AND SOFTWARE AVAILABILITY
The accession number for the hMeDIP-seq data reported in this paper is GEO: GSE102473.
Supplemental Information includes Supplemental Experimental Procedures, four figures, and three tables and can be found with this article online at https://doi.org/10.1016/j.celrep.2018.02.001.
AUTHOR CONTRIBUTIONS
G.G. and S.A.V. developed the concept and designed experiments. G.G. collected and analyzed data. G.G. and M.I. performed histological and cognitive studies. J.M.S. performed 5meDIP-seq studies. G.B. and E.G.W. generated viral constructs. E.G.W. and S.A.V. performed parabiosis studies. M.R.-S. provided scientific expertise. G.B. generated schematics. G.G., J.M.S., and S.A.V. wrote the manuscript. S.A.V. supervised all aspects of the project. All authors had the opportunity to discuss results and comment on the manuscript.
DECLARATION OF INTERESTS
The authors declare no competing interests.
References
- Baht GS, Silkstone D, Vi L, Nadesan P, Amani Y, Whetstone H, Wei Q, Alman BA. Erratum: Exposure to a youthful circulation rejuvenates bone repair through modulation of β-catenin. Nat. Commun. 2015;6:7761. doi: 10.1038/ncomms8761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beerman I, Rossi DJ. Epigenetic Control of Stem Cell Potential during Homeostasis, Aging, and Disease. Cell Stem Cell. 2015;16:613–625. doi: 10.1016/j.stem.2015.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaschke K, Ebata KT, Karimi MM, Zepeda-Martínez JA, Goyal P, Mahapatra S, Tam A, Laird DJ, Hirst M, Rao A, et al. Vitamin C induces Tet-dependent DNA demethylation and a blastocyst-like state in ES cells. Nature. 2013;500:222–226. doi: 10.1038/nature12362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunet A, Rando TA. Interaction between epigenetic and metabolism in aging stem cells. Curr. Opin. Cell Biol. 2017;45:1–7. doi: 10.1016/j.ceb.2016.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess DJ. Human genetics: somatic mutations linked to future disease risk. Nat. Rev. Genet. 2015;16:69. doi: 10.1038/nrg3889. [DOI] [PubMed] [Google Scholar]
- Cimmino L, Dolgalev I, Wang Y, Yoshimi A, Martin GH, Wang J, Ng V, Xia B, Witkowski MT, Mitchell-Flack M, et al. Restoration of TET2 function blocks aberrant self-renewal and leukemia progression. Cell. 2017;170:1079–1095.e20. doi: 10.1016/j.cell.2017.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conboy IM, Conboy MJ, Wagers AJ, Girma ER, Weissman IL, Rando TA. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature. 2005;433:760–764. doi: 10.1038/nature03260. [DOI] [PubMed] [Google Scholar]
- Drapeau E, Mayo W, Aurousseau C, Le Moal M, Piazza PV, Abrous DN. Spatial memory performances of aged rats in the water maze predict levels of hippocampal neurogenesis. Proc. Natl. Acad. Sci. USA. 2003;100:14385–14390. doi: 10.1073/pnas.2334169100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan X, Wheatley EG, Villeda SA. Mechanisms of hippocampal aging and the potential for rejuvenation. Annu. Rev. Neurosci. 2017;40:251–272. doi: 10.1146/annurev-neuro-072116-031357. [DOI] [PubMed] [Google Scholar]
- Feng J, Liu T, Qin B, Zhang Y, Liu XS. Identifying ChIP-seq enrichment using MACS. Nat. Protoc. 2012;7:1728–1740. doi: 10.1038/nprot.2012.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn MA, Qiu R, Wu X, Li AX, Zhang H, Wang J, Jui J, Jin SG, Jiang Y, Pfeifer GP, Lu O. Dynamics of 5-hydroxymethylcytosine and chromatin marks in Mammalian neurogenesis. Cell Rep. 2013;3:291–300. doi: 10.1016/j.celrep.2013.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaiswal S, Fontanillas P, Flannick J, Manning A, Grauman PV, Mar BG, Lindsley RC, Mermel CH, Burtt N, Chavez A, et al. Age-related clonal hematopoiesis associated with adverse outcomes. N. Engl. J. Med. 2014;371:2488–2498. doi: 10.1056/NEJMoa1408617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaas GA, Zhong C, Eason DE, Ross DL, Vachhani RV, Ming GL, King JR, Song H, Sweatt JD. TET1 controls CNS 5-methylcytosine hydroxylation, active DNA demethylation, gene transcription, and memory formation. Neuron. 2013;79:1086–1093. doi: 10.1016/j.neuron.2013.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsimpardi L, Litterman NK, Schein PA, Miller CM, Loffredo FS, Wojtkiewicz GR, Chen JW, Lee RT, Wagers AJ, Rubin LL. Vascular and neurogenic rejuvenation of the aging mouse brain by young systemic factors. Science. 2014;344:630–634. doi: 10.1126/science.1251141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriaucionis S, Heintz N. The nuclear DNA base 5-hydroxymethylcytosine is present in Purkinje neurons and the brain. Science. 2009;324:929–930. doi: 10.1126/science.1169786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Wei W, Zhao QY, Widagdo J, Baker-Andresen D, Flavell CR, D’Alessio A, Zhang Y, Bredy TW. Neocortical Tet3-mediated accumulation of 5-hydroxymethylcytosine promotes rapid behavioral adaptation. Proc. Natl. Acad. Sci. USA. 2014;111:7120–7125. doi: 10.1073/pnas.1318906111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Yao B, Chen L, Kang Y, Li Y, Cheng Y, Li L, Lin L, Wang Z, Wang M, et al. Ten-eleven translocation 2 interacts with forkhead box O3 and regulates adult neurogenesis. Nat. Commun. 2017;8:15903. doi: 10.1038/ncomms15903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrill DA, Karim R, Darraq M, Chiba AA, Tuszynski MH. Hippocampal cell genesis does not correlate with spatial learning ability in aged rats. J. Comp. Neurol. 2003;459:201–207. doi: 10.1002/cne.10616. [DOI] [PubMed] [Google Scholar]
- Nadarajah N, Meggendorfer M, Kern W, Haferlach C, Haferlach T. Accumulation of somatic mutations as a function of aging: A study on 4843 TET2 mutated patients in comparison to their respective SNP pattern. Blood. 2015;126:4111. [Google Scholar]
- Rudenko A, Dawlaty MM, Seo J, Cheng AW, Meng J, Le T, Faull KF, Jaenisch R, Tsai LH. Tet1 is critical for neuronal activity-regulated gene expression and memory extinction. Neuron. 2013;79:1109–1122. doi: 10.1016/j.neuron.2013.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seib DR, Corsini NS, Ellwanger K, Plaas C, Mateos A, Pitzer C, Niehrs C, Celikel T, Martin-Villalba A. Loss of Dickkopf-1 restores neurogenesis in old age and counteracts cognitive decline. Cell Stem Cell. 2013;12:204–214. doi: 10.1016/j.stem.2012.11.010. [DOI] [PubMed] [Google Scholar]
- Sinha M, Jang YC, Oh J, Khong D, Wu EY, Manohar R, Miller C, Regalado SG, Loffredo FS, Pancoast JR, et al. Restoring systemic GDF11 levels reverses age-related dysfunction in mouse skeletal muscle. Science. 2014;344:649–652. doi: 10.1126/science.1251152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith LK, He Y, Park JS, Bieri G, Snethlage CE, Lin K, Gontier G, Wabl R, Plambeck KE, Udeochu J, et al. β2-microglobulin is a systemic pro-aging factor that impairs cognitive function and neurogenesis. Nat. Med. 2015;21:932–937. doi: 10.1038/nm.3898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szulwach KE, Li X, Li Y, Song CX, Wu H, Dai Q, Irier H, Upadhyay AK, Gearing M, Levey AI, et al. 5-hmC-mediated epigenetic dynamics during postnatal neurodevelopment and aging. Nat. Neurosci. 2011;14:1607–1616. doi: 10.1038/nn.2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villeda SA, Luo J, Mosher KI, Zou B, Britschgi M, Bieri G, Stan TM, Fainberg N, Ding Z, Eggel A, et al. The ageing systemic milieu negatively regulates neurogenesis and cognitive function. Nature. 2011;477:90–94. doi: 10.1038/nature10357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang RR, Cui QY, Murai K, Lim YC, Smith ZD, Jin S, Ye P, Rosa L, Lee YK, Wu HP, et al. Tet1 regulates adult hippocampal neurogenesis and cognition. Cell Stem Cell. 2013;13:237–245. doi: 10.1016/j.stem.2013.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.