Abstract
Background
Women with chest pain, ischemia, and no obstructive coronary artery disease often have coronary vascular dysfunction (CVaD). Peripheral vascular reactivity to mental stress may contribute mechanistic understanding of stress-induced ischemia in women with CVaD.
Methods
62 women (41 CVaD and 21 controls) underwent mental stress testing (MST) with anger recall, mental arithmetic, and forehead cold pressor (COP) challenge. Emotional arousal was measured (Likert scale). Reactive hyperemia index (RHI) was calculated before and after MST by peripheral arterial tonometry (PAT). Stress PAT ratio (SPR) of pulse amplitude during stress to rest was obtained to measure vasoconstriction. Wilcoxson rank sum test was used for analysis.
Results
Mean age of CVaD and control groups was 58±9 and 55±10 years (p=0.73). Baseline RHI correlated with coronary endothelial function (r=0.36, p=0.03) and inversely with RHI change post-MST (r=−0.51, p <0.001). During MST, 10% controls reported chest pain vs. 41% CVaD subjects (p=0.01). RHI did not change significantly after MST in either group. CVaD subjects had lower SPR vs. controls during mental arithmetic (0.54 [0.15, 1.46] vs. 0.67 [0.36, 1.8], p=0.039), not evident in the other tasks. Vasoconstriction inversely correlated with anxiety (r=−3.4, p=0.03), frustration (r=−0.37, p=0.02), and feeling challenged (r=−0.37, p=0.02) in CVaD but not controls.
Conclusions
Mental stress peripheral vascular reactivity is elevated in women with CVaD compared to controls. Elevated vascular reactivity may be one contributor to stress-induced chest pain in CVaD. Interventions that modulate vasoconstrictive responses may be of benefit and should be tested in clinical trials in women with CVaD.
Keywords: vascular reactivity, mental stress, microvascular dysfunction, women heart disease
Background
Chest pain with evidence of myocardial ischemia and no obstructive coronary arteries occurs more commonly in women and is associated with an adverse prognosis.1–3 Approximately 50% of these women have coronary vascular dysfunction (CVaD). 2,4 CVaD encompasses endothelial and non-endothelial dependent macro- and microvascular dysfunction, and data from the Women’s Ischemia Syndrome Evaluation (WISE) and other studies indicate that CVaD is associated with adverse cardiovascular prognosis, including myocardial infarction, stroke, congestive heart failure, and sudden cardiac death.3,5–7 Invasive coronary reactivity testing (CRT) can be performed to clarify the diagnosis when CVaD is suspected.8
Women with CVaD also often present with non-exertional, emotional stress-induced chest pain; while anxiety/pain disorders and abnormal cardiac nociception are also relatively more common in women with chest pain,9,10 the mechanistic pathways of emotional stress-induced chest pain are not well understood. Acute mental stress has been associated with endothelial dysfunction and impaired vasoreactivity in patients with obstructive CAD.11,12 This may result in mental stress-induced myocardial ischemia (MSIMI), which is more common in women with CAD. Patients with MSIMI are also more likely to have exaggerated peripheral vasoconstriction during mental stress.12,13 Further evidence suggests that mental stress induces peripheral vasoreactivity, which may be useful in the detection of MSIMI in patients with CAD.14–18 However, it is not known whether women with CVaD and no obstructive CAD have abnormal peripheral vasoreactivity during acute mental stress. Hence, this study evaluated peripheral vascular reactivity to acute mental stress in women with CVaD as compared to reference control subjects.
Methods
CVaD Subjects
44 CVaD women enrolled in the NHLBI-sponsored Women’s Ischemia Syndrome Evaluation Coronary Vascular Dysfunction (WISE-CVD) study (PI: Bairey Merz) at a single site (Cedars-Sinai Medical Center) were recruited and enrolled in the NHLBI-sponsored Cardiac Autonomic Nervous System (CANS) substudy between October 2010 - June 2015. These women had persistent chest pain, evidence of myocardial ischemia by routine stress testing, and no obstructive CAD on invasive coronary angiography. Inclusion and exclusion criteria were as previously published for WISE-CVD19. Three subjects who did not have coronary reactivity testing (CRT) to diagnose CVaD were excluded due to inadequate data. The study was IRB approved, and all subjects were provided informed consent.
Coronary Reactivity Testing (CRT)
Clinically-indicated invasive CRT was performed to diagnose CVaD as previously described8. Briefly, after confirmation of no obstructive CAD by angiography, a doppler Flowire (Volcano®) was placed in the proximal left anterior descending artery and vasoactive agents (adenosine 18 and 36 mcg, acetylcholine 36.4 mcg, and nitroglycerin 200 mcg) were used to assess endothelial and non-endothelial dependent, macro- and micro vascular function, using previously published methods.7,20 The peak coronary flow reserve (CFR) response to either dose of adenosine was used for analysis. Abnormal CRT responses were defined as the following four pathways: (a) an abnormal non-endothelial microvascular response was CFR <2.5 to intracoronary adenosine; (b) an abnormal endothelial microvascular response was change in coronary blood flow (CBF) ≤ 50% to high dose acetylcholine; (c) an abnormal endothelial macro-vascular response was <5% change in coronary artery diameter to high dose acetylcholine; and (d) an abnormal non-endothelial macrovascular response was change in coronary artery diameter <20% to nitroglycerin.8,21 Coronary endothelial dysfunction was defined as abnormal coronary diameter response or abnormal coronary blood flow to acetylcholine. Patients with at least one abnormal pathway were included in the study.
Mental Stress Testing (MST)
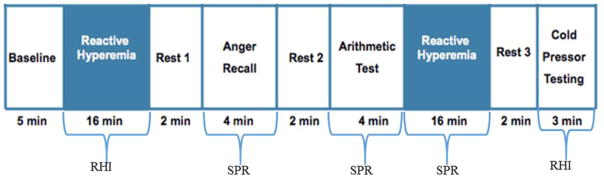
MST was performed in the morning after an overnight fast; caffeine was withheld for 24 hours and smoking was prohibited for at least 6 hours. Subjects were asked to withhold caffeine and medications for 24 hours (beta blocker, short acting calcium channel blocker, angiotensin-converting enzyme inhibitors and angiotensin receptor blockers, renin blockers, and ranolazine) or for 48 hours (long acting calcium channel blockers and nitrates) when medically able. After IV placement, subjects rested for 30 minutes in a quiet, dimly lit, temperature-controlled room. A mood survey with Likert scale22–25 questions was administered to assess subjective levels of psychological stress, including anxiety, frustration, anger, and irritation. Baseline blood pressure, heart rate, and reactive hyperemia index (RHI) by peripheral arterial tonometry (PAT) were measured. All subjects underwent MST in supine position via a standardized protocol (Appendix Figure 1).
Appendix Figure 1.
Mental Stress Testing Protocol. All subjects underwent standardized mental stress testing with anger recall and mental arithmetic, with blood pressure, heart rate, and peripheral arterial tonometric response. RHI: reactive hyperemia index; SPR: stress PAT ratio.
Two mental stress tests were administered in counterbalanced order with a two minute rest period between each stress test task: (a) 4 minute anger recall speech task modified from Ironson et al26 requires the subject to recall a situation of extreme anger or frustration and (b) 4-minute mental arithmetic task, which requires the subject to count backwards by 7’s from a randomly chosen number. These are standard MST tasks that have been used successfully by our group and others.22,24,27 During the two minute rest period in between tasks, blood pressure and heart rate were assessed to ensure a return to baseline levels, prior to proceeding with the next task. During the MST tasks, BP was monitored every minute by automated cuff, and heart rate and rhythm continuously monitored. For the first 26 subjects, one-lead ECG monitoring was used, but for better detection of ST segment changes, we changed to 12-lead ECG monitoring (Mortara Instruments®) for the later subjects. Likert scale was re-administered after each MST task. PAT pulse amplitude was continuously monitored during MST.
At the end of MST, 3 minutes of modified forehead cold pressor (COP) test was performed. A 1.5 liter bag filled with 800ml crushed ice and 200 ml of water (Temp 4°C) was placed on subject’s forehead for 3 minutes. Hand PAT probes precluded traditional COP testing with hand in ice bucket, thus forehead COP was used. HR, BP, and PAT pulse amplitude response were also measured during COP test.
Peripheral Arterial Tonometry
Peripheral endothelial function and vascular reactivity were measured by peripheral arterial tonometric (PAT) plethysmographic device (EndoPAT 2000, Itamar Medical®) as previously described and during supine position, under conditions for MST described above..28,29 The deviceconsists of a finger probe with a transducer to assess digital volume changes accompanying pulse-waves. A pressure of 40–70mmHg is applied by the probe to the index fingers for venous occlusion of both hands and arterial pulse amplitude is recorded. A blood pressure cuff is placed on one arm (test arm) while amplitudes are recorded from the test arm and the contralateral arm. After 10 minutes of equilibration period, reactive hyperemia index (RHI) is obtained by the following: 5 minutes of baseline amplitude signal detection, 5-minutes of arterial occlusion by suprasystolic blood pressure cuff inflation, and then 6-minutes of post-occlusion amplitude signal detection. RHIis a unit-less measure and a marker of endothelial function, and was determined at baseline and post MST by EndoPAT® automated software which takes into account the baseline signal and adjusts it to the changes in the contralateral arm15,16,28. RHI of less than 2.0 has been reported to correlate with coronary endothelial dysfunction and coronary artery disease, and is prognostic (sensitivity and specificity of ~80%).30–32 Peripheral vascular reactivity was determined by stress PAT ratio (SPR) which is a ratio of stress to rest pulse amplitude as previously described.15 The lowest pulse amplitude for 30 second segment during mental stress test task was used to compare to an average 3 minute rest amplitude to obtain SPR. SPR was obtained from the arm not wearing a BP cuff and determined for each MST task, as a measure of vasoconstriction, where less than 0.8 is considered abnormal.28,33
Reference Control Subjects
22 women were recruited from the Reference Control Study in Women (PI: Bairey Merz) at Cedars-Sinai and enrolled in the CANS study. One subject was a screen failure and excluded. Women in this group were age- and BMI-matched to the CVaD group, had no cardiac risk factors, were not on cardiac medications, and had a normal maximal exercise treadmill testing (Bruce Protocol) to serve as reference controls.
Statistical Analysis
Summary data are expressed as means, standard deviations, medians, and ranges for continuous variables and frequencies (%) for categorical ones. Comparison of categorical variables between groups was done using Fisher’s Exact test due to low counts in the control group. Wilcoxon Rank Sum tests were used to compare continuous outcomes between groups due to the presence of outliers. Spearman correlation coefficients are reported due to outliers. A p-value < 0.05 was considered to indicate statistical significance. Multiple linear regression was performed with RHI as the outcome and adjusted for various cardiac medications. All statistical analysis was performed using SAS (The SAS Institute, Cary, NC; ver. 9.3).
Results
Baseline characteristics are shown in Table 1 and demonstrate no difference between mean age and BMI between the two groups. Mean time between CRT and MST was 2.0 ± 1.6 years. There were only 7 subjects with MST within 6 months of CRT, precluding further analysis given small numbers. CRT results demonstrated that 34% had abnormal CFR (< 2.5), 63% had abnormal acetylcholine diameter response, 44% had abnormal coronary blood flow change to acetylcholine, and 61% had an abnormal smooth muscle vasodilation to nitroglycerin. Only one subject had CVaD diagnosis because of abnormal nitroglycerin response with the other 3 pathways being normal. Excluding this subject did not change the overall results.
Table 1.
Baseline Characteristics
| CVaD Subjects (n = 41) | Reference Control Subjects (n=21) | p-Value | |
|---|---|---|---|
| Age (years) | 58±9 | 55±10 | 0.73 |
| BMI (kg/m2) | 26.8±5 | 26.6±4 | 0.81 |
| Hypertension | 14 (32%) | 0 | 0.0064 |
| Diabetes | 4 (9%) | 0 | 0.5686 |
| Hyperlipidemia | 5 (11%) | 0 | 0.3093 |
| Previous history of smoking | 16 (36%) | 0 | 0.0028 |
| Currently smoking | 0 (0%) | 0 | - |
| Aspirin | 32 (73%) | 0 | <0.0001 |
| Hormone Therapy | 11 (25%) | 3 (18%) | 0.74 |
| Beta-Blockers | 22 (50%) | 0 | <0.0001 |
| Calcium Channel Blockers | 9 (20%) | 0 | 0.05 |
| Nitrates | 23 (52%) | 0 | <0.0001 |
| ACE-Inhibitors/ARBs | 21 (48%) | 0 | <0.0001 |
| Statins | 29 (67%) | 0 | <0.0001 |
| Peak CFR to IC adenosine (36 mcg) | 2.83 ±0.74 | - | - |
| CBF to IC acetylcholine (36.4 mcg) | 84.07 ±107.05 | - | - |
| Coronary Diameter Change to IC acetylcholine (36.4 mcg) | −1.39 ± 17.36 | - | - |
| Diameter change to IC nitroglycerin (200 mcg) | 16.47 ± 12.94 | - | - |
CBF: coronary blood flow; CFR: coronary flow reserve; CVaD: coronary vascular dysfunction; IC: intracoronary; LAD: left anterior descending coronary artery; QCA: quantitative coronary angiography
Cardiovascular Reactivity and Likert Scale
Hemodynamic changes to anger, mental arithmetic, and COP were similar in both groups (Appendix Table 1). There were no stress-induced arrhythmias detected on continuous rhythm monitoring during MST. During MST, 2/21 (10%) reference controls reported transient, self-limited chest pain, compared to 17/41 (41%) of CVaD subjects (p=0.010). Chest pain was not associated with rhythm or ST segment changes, and no one required treatment with SL NTG for chest pain resolution during MST.
Appendix Table 1.
Hemodynamic Changes with Mental Stress Testing
| CVaD Subjects Median [min, max] |
Reference Control Subjects Median [min, max] |
p-value | ||
|---|---|---|---|---|
| Baseline | HR | 67 [47, 90] | 61 [50, 83] | 0.27 |
| SBP | 115 [90, 165] | 116 [94, 145] | 0.70 | |
| DBP | 64 [44, 80] | 67 [31, 85] | 0.27 | |
| RPP | 7725 [4794, 12330] | 7192 [4700, 9570] | 0.56 | |
| Anger | Δ HR | 12 [2, 42] | 11 [6, 52] | 0.49 |
| Δ SBP | 18 [2, 50] | 22 [−4, 54] | 0.88 | |
| Δ DBP | 12 [−2, 27] | 12 [1, 69] | 0.49 | |
| Δ RPP | 2994 [724, 10020] | 2702 [715, 13514] | 0.56 | |
| Arithmetic | Δ HR | 10 [−1, 53] | 17 [−6, 41] | 0.08 |
| Δ SBP | 17 [−5, 62] | 23 [−6, 39] | 0.23 | |
| Δ DBP | 8 [−8, 29] | 11 [0, 16] | 0.27 | |
| Δ RPP | 2682 [−217, 13472] | 3267 [−212, 8349] | 0.17 | |
| Cold Pressor | Δ HR | 1 [−21, 30] | −1 [−14, 17] | 0.90 |
| Δ SBP | 19 [−15, 60] | 21 [−4, 40] | 0.60 | |
| Δ DBP | 11 [−8, 29] | 11 [−7, 27] | 0.90 | |
| Δ RPP | 1206 [−1594, 8700] | 1525 [−1480, 3900] | 0.87 |
DBP= diastolic blood pressure, HR= heart rate, RPP= rate pressure product, SBP= systolic blood pressure
At baseline, there were no significant differences in emotional arousal measured by Likert scale subjective measures of emotion between the two groups. While both MST tasks resulted in significant changes in emotion compared to baseline in both groups (Table 2), CVaD subjects were more emotionally aroused compared to reference controls. During recovery at the end of both MST tasks, CVaD subjects remained significantly more emotionally aroused compared to controls (Table 2).
Table 2.
Emotion During Mental Stress Testing and Recovery in CVaD vs. Controls
| CVaD n=41 (mean ±SD) | p-Value (Compared to Baseline) | Reference Controls n=21 (mean ± SD) | p-Value (Compared to Baseline) | p-Value (CVaD vs. Reference Controls) | |
|---|---|---|---|---|---|
| Δ Anger Recall | |||||
| Δ Anxious | 3.24 ± 2.21 | <0.0001 | 2.48 ± 2.16 | <0.0001 | 0.16 |
| Δ Tense | 3.39 ± 1.93 | <0.0001 | 3.10 ± 1.84 | <0.0001 | 0.55 |
| Δ Frustrated | 4.80 ± 1.55 | <0.0001 | 3.62 ± 2.18 | <0.0001 | 0.02 |
| Δ Irritated | 4.39 ± 1.93 | <0.0001 | 3.29 ± 1.79 | <0.0001 | 0.008 |
| Δ Depressed | 1.66 ± 2.23 | <0.0001 | 1.24 ± 1.81 | 0.005 | 0.42 |
| Δ Angry | 4.05 ± 1.95 | <0.0001 | 3.71 ± 2.28 | <0.0001 | 0.72 |
| Δ Stressed | 4.29 ± 1.66 | <0.0001 | 3.62 ± 1.77 | <0.0001 | 0.14 |
| Δ Challenged | 3.05±2.6 | <0.0001 | 2.29 ±2 | <0.0001 | 0.42 |
| Δ Mental Arithmetic | |||||
| Δ Anxious | 4.30 ± 1.68 | <0.0001 | 3.30 ± 1.89 | <0.0001 | 0.04 |
| Δ Tense | 4.13 ± 1.68 | <0.0001 | 3.95 ± 2.01 | <0.0001 | 0.94 |
| Δ Frustrated | 4.83 ± 1.57 | <0.0001 | 4.30 ± 1.95 | <0.0001 | 0.34 |
| Δ Irritated | 3.85 ± 2.21 | <0.0001 | 3.90 ± 1.86 | <0.0001 | 0.92 |
| Δ Depressed | 1.48± 2.33 | 0.0003 | 1.00 ± 1.62 | 0.012 | 0.61 |
| Δ Angry | 3.60 ± 2.37 | <0.0001 | 2.40 ± 2.58 | 0.0005 | 0.07 |
| Δ Stressed | 4.43 ± 1.81 | <0.0001 | 4.00 ± 2.08 | <0.0001 | 0.45 |
| Δ Challenged | 4.16 ±2.01 | <0.0001 | 4.81 ± 1.64 | <0.0001 | 0.25 |
Peripheral Endothelial Function (PAT-RHI)
Baseline peripheral endothelial function, measured by RHI, was normal in both CVaD and reference controls (2.49 [1.25, 3.74] vs 2.31 [1.49, 4.67], p=0.54, respectively). RHI within CVaD patients with an abnormal coronary acetylcholine response was lower compared to those with normal acetylcholine response (RHI: (2.15 [1.25, 3.74]) vs. 3.04 [1.81, 3.59], p=0.049). Likewise, median RHI was lower in those subjects with hypertension (n = 9) compared to those without hypertension (n=23) (RHI: 2.05 [1.61, 2.74] vs. 2.68 [1.25, 3.74], p = 0.016). Median RHI remained normal after acute MST in both CVaD and controls (2.57 [1.39, 4.21] vs. 2.40 [1.52, 5.03], p=0.76, respectively). Baseline RHI correlated with coronary endothelial function (r=0.36, p=0.03) and inversely with RHI change post-MST (r = −0.51, p = <0.001). In those with abnormal coronary endothelial function (abnormal acetylcholine diameter response or abnormal CBF), there was no significant difference in RHI between pre- and post-MST (p = 0.20). There were no differences in RHI in the CVaD group based on cardiac medications.
Peripheral Vascular Reactivity (stress PAT ratio)
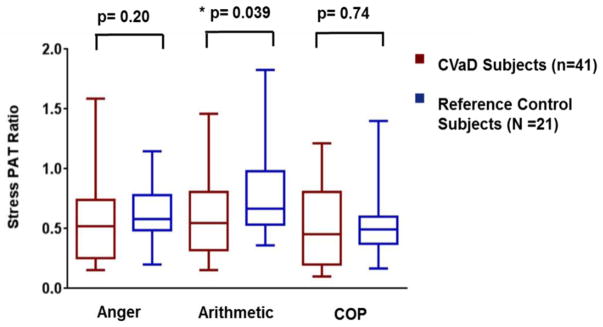
A comparison of individual tasks showed that CVaD subjects had a significantly lower SPR (consistent with more vasoconstriction) to arithmetic compared to control subjects (median [range]: 0.54 [0.15, 1.46] vs. 0.67 [0.36, 1.8], p=0.039) (Figure 1). Similar results were obtained when outliers were excluded. Women with coronary endothelial dysfunction demonstrated more vasoconstriction with mental arithmetic compared to controls (median [range]: 0.57 [0.32, 0.71] vs. 0.67 [0.54, 0.92] (p = 0.04). Subgroup analysis of those who had an abnormal CFR (n=13) (<2.5, i.e. non-endothelial microvascular dysfunction) compared to reference controls demonstrated no difference in peripheral vasoreactivity with the stressors. There was no difference in proportion of CVaD subjects who had a low SPR (defined as <0.8) during arithmetic vs. anger compared to controls. There was also no difference in the proportion of CVaD subjects who had an SPR <0.8 to at least one of the stressors compared to control subjects. There were no differences in SPR on the basis of medications. During anger recall, some measures of emotional arousal [anxiety (r=−3.4, p=0.03), frustration (r=−0.37, p=0.02), and feeling challenged (r=−0.37, p=0.02)], inversely correlated with vasoconstriction (SPR) in CVaD subjects, but not in controls. There were no significant correlations between emotional arousal and SPR during mental arithmetic in either group.
Figure 1.
Stress PAT Ratio (SPR) to Mental Stress in CVaD vs. Reference Control Subjects. CVaD subjects had abnormally low peripheral vasoreactivity to mental arithmetic compared to reference controls.
Invasive coronary reactivity and PAT measures
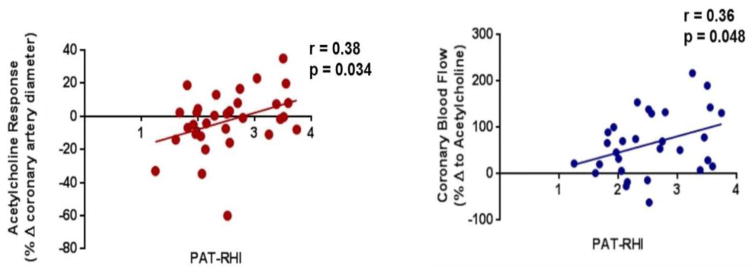
There was a moderate correlation between coronary endothelial function and coronary blood flow by acetylcholine testing to RHI (Figure 2). There were no significant correlations between peripheral RHI and non-endothelial dependent pathways (CFR to adenosine, r = −0.05, p = 0.77; NTG response, r = 0.20, p = 0.26). SPR to anger, mental arithmetic, or cold pressor did not correlate with any of the CRT measures.
Figure 2.
Correlation of coronary diameter response to acetylcholine and peripheral endothelial function. PAT determined RHI mildly correlated with invasive coronary endothelial function by acetylcholine and coronary blood flow determine by acetylcholine. There was no correlation between RHI and invasive adenosine CFR or coronary artery diameter response to nitroglycerin.
Discussion
The novel finding of this investigation is that women with CVaD demonstrate more peripheral vasoconstriction to mental stress compared to reference control subjects. Women with CVaD also reported more emotional arousal during MST and recovery compared to reference controls, with peripheral vasoconstriction correlating with emotional arousal in women with CVaD compared to controls. Other vascular measures, including baseline RHI, were not different between the two groups. To our knowledge, this is the first study that compares women with CVaD diagnosed by invasive CRT to reference controls in a systematic mental stress protocol to evaluate digital peripheral arterial tonometric response. Taken together, these data add novel mechanistic insight into emotionally triggered symptoms in CVaD.
Autonomic dysregulation may contribute to this elevated vasoconstrictor response. Our study indicates that while peripheral vasoconstriction with MST occurs in both CVaD subjects and controls, it occurs significantly more in CVaD compared to controls during mental arithmetic, suggesting that mental arithmetic is a more potent stress test to detect group differences to understand mechanistic pathways of CVaD in women. A prior study comparing CAD subjects to those with normal coronary angiograms demonstrated during acute mental stress (by 10-min video game) greater coronary microvascular dysfunction in a non-obstructed coronary among the CAD subjects compared to those with normal angiograms.34 “Demand ischemia” due to mismatch from increased myocardial oxygen demand and failure of the microvasculature to dilate is often used to explain angina and ischemia in those with no obstructive CAD, however our data suggest that abnormal vascular constriction may be a mechanistic pathway for emotionally triggered angina in CVaD. In a report from the WISE study among women who underwent flow-mediated brachial artery reactivity testing, brachial artery constriction post-hyperemia was a marker associated with adverse cardiovascular outcomes compared to those without constriction, and the risk was not related to CAD or traditional risk factors.35 There were some differences in risk factors among the two groups. While both groups had similar ages, BMI, and no current smokers, a small percentage had diabetes and hyperlipidemia, while a third had hypertension. Thus, it cannot be established from this study whether the observed differences in peripheral vasoreactivity is typical of subjects with CVaD compared to those with a large presence of cardiovascular risk factors.
The mean baseline PAT-RHI in our study was similar between CVaD and reference control subjects. RHI is determined by hyperemic blood flow in the index fingers (skin microcirculation), which is a different than coronary reactivity testing which uses vasoactive agents to assess coronary circulation. We report an RHI of 2.5 [1.25, 3.74] in CVaD, which is consistent with prior literature. Median RHI in the iPOWER study from Denmark in women with microvascular dysfunction (diagnosed by dipyridamole stress echo coronary flow velocity reserve) was similar to ours (mean 2.1 [range 1.6, 2.6]). They also reported a lack of correlation between CVaD and resting RHI.36 In a study by Martin et al, RHI scores in post-menopausal controls were 2.35 ± 0.49 (similar to our reference controls), and there was no difference between controls and those with history of Takotsubo syndrome, which has been linked to CVaD. However, patients with a history of stress cardiomyopathy with apical ballooning demonstrated increased peripheral vasoconstriction to mental stress compared to those post-menopausal controls or patients with myocardial infarction.15,37 In contrast, a study in women from Japan 38 reported an abnormal low RHI of 1.58 [1.4, 1.78] in the no obstructive CAD group compared to 2.15 [1.85, 2.48] in those with no ischemia. These differences could be explained by ethnic variation; however, a more likely explanation would be the older age in the Japanese study compared to ours (age: 64±10 vs. 58±9), and higher burden of risk factors of hypertension, hyperlipidemia, and diabetes in the Japanese study, which would impact RHI.
As expected, we found that peripheral endothelial function in those with a history of hypertension was lower compared to non-hypertensive women. We also found that those women who had an abnormal response to acetylcholine did have a lower RHI and lower SPR compared to those with normal acetylcholine response. We therefore interpret our RHI data to reflect the vast heterogeneity of CVaD mechanistic pathways in women. The non-endothelial pathways of CVaD did not correlate with RHI, possibly due to small numbers. However, the endothelial pathways of CVaD did moderately correlate with RHI in this study, as would be expected, since previous reports have published that RHI correlates with coronary endothelial function in those with no obstructive CAD. 31,39 Studies have demonstrated that RHI has a sensitivity of 85% and specificity of 80% for detection of endothelial dysfunction in CAD patients.40,41
Ramadan et al. reported that in patients with CAD, SPR is not related to the angiographic severity of CAD.12 In another study in patients with stable CAD, those who had mental stress induced myocardial ischemia on nuclear imaging had greater peripheral vasoconstriction to mental stress compared to those who did not have mental stress myocardial ischemia.42 We find that SPR does not correlate with severity of coronary flow reserve abnormalities. However, SPR correlates with some measures of emotional arousal in women with CVaD during MST. During anger recall, emotional arousal (higher anxiety, frustration, and feeling challenged) inversely correlated with SPR in CVaD subjects, but not in controls. There were no significant correlations between emotions and SPR during mental arithmetic in either group. While more detailed measures of anxiety, depression, and stress were not collected in this study, these findings are thought-provoking and serve as pilot data for a larger study which we are planning to connect psychological measures to various CVaD presentations and mechanisms.
Women often have emotional stress/low heart rate-related angina that is not always exertional; ischemia from mental stress typically occurs at low cardiac workloads 25,43, and likely involves the autonomic nervous system.44–46 A large body of evidence links mental stress to endothelial dysfunction, atheroma progression, ischemia, and CAD morbidity and mortality.23,27,43,47,48 During mental stress, the normal 26–44% dilation response of coronary microvasculature is blunted in patients with atherosclerosis compared to patients with normal angiograms34. An exaggerated mental stress-induced rate pressure product may trigger acute ischemia in the setting of chronic coronary endothelial dysfunction. A high rate-pressure product is also predictive of outcomes in CAD patients, particularly in post-menopausal women 49. In this study, however, the CVaD group did not demonstrate an exaggerated hemodynamic response compared to reference control women when exposed to MST or COP testing. The increase in HR and BP that we achieved is consistent with hemodynamic change related to mental stress induction as reported in the literature22.
Limitations
It must be noted that diagnoses of CVaD by CRT was determined within 2 years of PAT, and not concomitantly. It is possible that if PAT RHI was performed around the same time as CRT, our results may have been different as symptoms and potential underlying mechanisms can fluctuate over time. A true control group for the CVaD group would be women who have a normal CRT, however, our reference control group was asymptomatic and invasive testing is not appropriate. It is possible that we missed eliciting a stronger autonomic response to pain because forehead cold pressor testing may not be as strong a stimulus as traditional hand in ice bucket cold pressor testing. While PAT provides insight into sympathetic vasoconstriction, direct measures of muscle and skin sympathetic nerve activity were not assessed and warrant further investigation. Menstrual cycle may affect endothelial function and in this study, menstrual phase was not assessed. There were relatively low numbers of women with CFR less than 2.5 to adenosine in this study. The doses of vasoactive substances used in CRT may impact the diagnosis (e.g. the highest dose of intracoronary adenosine used was 36 mcg per WISE-CVD study protocol, which may differ from CFR by intravenous adenosine). By design, there were no men enrolled, so conclusions about sex differences in peripheral vasoconstrictive response cannot be made.
Conclusions
Peripheral vasoreactivity to mental stress is higher in patients with coronary vascular dysfunction compared to reference controls. Increased vasoconstriction may be one of the mechanisms that may explain stress induced chest pain and ischemia in women with CVaD. Interventions that modulate autonomic vasoconstrictive responses may be of benefit and should be tested in women with CVaD.
Acknowledgments
Support: This work was supported by contracts from the National Heart, Lung and Blood Institute K23HL105787, K23HL127251, R01 HL090957, T32HL69751, N01-HV-68161, N01-HV-68162, N01-HV-68163, N01-HV-68164, U0164829, U01 HL649141, U01 HL649241, 1R03AG032631 from the National Institute on Aging, GCRC grant MO1-RR00425 from the National Center for Research Resources, the National Center for Advancing Translational Sciences (NCATS) grant UL1TR000124, and grants from the Gustavus and Louis Pfeiffer Research Foundation, Danville, NJ, The Women’s Guild of Cedars-Sinai Medical Center, Los Angeles, CA, The Ladies Hospital Aid Society of Western Pennsylvania, Pittsburgh, PA, and QMED, Inc., Laurence Harbor, NJ, Society for Women’s Health Research (SWHR), Washington, D.C., the Edythe L. Broad and the Constance Austin Women’s Heart Research Fellowships, the Barbra Streisand Women’s Cardiovascular Research and Education Program, the Linda Joy Pollin Women’s Heart Health Program, and the Erika Glazer Women’s Heart Research Initiative and the Adelson Family Foundation, Cedars-Sinai Medical Center, Los Angeles, CA.
Footnotes
nih.gov identifier: NCT01568177
Conflicts of interest: Mehta reports research support from Gilead and General Electric; Dr. Bairey Merz reports consulting revenue paid to Cedars-Sinai from Gilead, Medscape, Research Triangle Institute, research money from Gilead, Erika Glazer, payments for lectures from Beaumont Hospital, European Horizon, Florida Hospital, INOVA, Korean Cardiology Society, Practice Point Communications, Pri-Med, University of Chicago, VBWG, University of Colorado, University of Utah, WomenHeart, Harold Buchwald Heart-Health, and Tufts; Lerman: Advisory board Itamar Medical; All others: none.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bugiardini R, Bairey Merz CN. Angina with “normal” coronary arteries: a changing philosophy. JAMA : the journal of the American Medical Association. 2005;293(4):477–484. doi: 10.1001/jama.293.4.477. [DOI] [PubMed] [Google Scholar]
- 2.Gulati M, Shaw LJ, Bairey Merz CN. Myocardial ischemia in women: lessons from the NHLBI WISE study. Clinical cardiology. 2012;35(3):141–148. doi: 10.1002/clc.21966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pepine CJ, Ferdinand KC, Shaw LJ, et al. Emergence of Nonobstructive Coronary Artery Disease: A Woman’s Problem and Need for Change in Definition on Angiography. J Am Coll Cardiol. 2015;66(17):1918–1933. doi: 10.1016/j.jacc.2015.08.876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reis SE, Holubkov R, Conrad Smith AJ, et al. Coronary microvascular dysfunction is highly prevalent in women with chest pain in the absence of coronary artery disease: results from the NHLBI WISE study. Am Heart J. 2001;141(5):735–741. doi: 10.1067/mhj.2001.114198. [DOI] [PubMed] [Google Scholar]
- 5.Camici PG, Crea F. Coronary microvascular dysfunction. N Engl J Med. 2007;356(8):830–840. doi: 10.1056/NEJMra061889. [DOI] [PubMed] [Google Scholar]
- 6.Pepine CJ, Anderson RD, Sharaf BL, et al. Coronary microvascular reactivity to adenosine predicts adverse outcome in women evaluated for suspected ischemia results from the National Heart, Lung and Blood Institute WISE (Women’s Ischemia Syndrome Evaluation) study. J Am Coll Cardiol. 2010;55(25):2825–2832. doi: 10.1016/j.jacc.2010.01.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.von Mering GO, Arant CB, Wessel TR, et al. Abnormal coronary vasomotion as a prognostic indicator of cardiovascular events in women: results from the National Heart, Lung, and Blood Institute-Sponsored Women’s Ischemia Syndrome Evaluation (WISE) Circulation. 2004;109(6):722–725. doi: 10.1161/01.CIR.0000115525.92645.16. [DOI] [PubMed] [Google Scholar]
- 8.Wei J, Mehta PK, Johnson BD, et al. Safety of coronary reactivity testing in women with no obstructive coronary artery disease: results from the NHLBI-sponsored WISE (Women’s Ischemia Syndrome Evaluation) study. JACC. Cardiovascular interventions. 2012;5(6):646–653. doi: 10.1016/j.jcin.2012.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rutledge T, Kenkre TS, Bittner V, et al. Anxiety associations with cardiac symptoms, angiographic disease severity, and healthcare utilization: the NHLBI-sponsored Women’s Ischemia Syndrome Evaluation. International journal of cardiology. 2013;168(3):2335–2340. doi: 10.1016/j.ijcard.2013.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rutledge T, Linke SE, Krantz DS, et al. Comorbid depression and anxiety symptoms as predictors of cardiovascular events: results from the NHLBI-sponsored Women’s Ischemia Syndrome Evaluation (WISE) study. Psychosomatic medicine. 2009;71(9):958–964. doi: 10.1097/PSY.0b013e3181bd6062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yeung AC, Vekshtein VI, Krantz DS, et al. The effect of atherosclerosis on the vasomotor response of coronary arteries to mental stress. N Engl J Med. 1991;325(22):1551–1556. doi: 10.1056/NEJM199111283252205. [DOI] [PubMed] [Google Scholar]
- 12.Ramadan R, Sheps D, Esteves F, et al. Myocardial ischemia during mental stress: role of coronary artery disease burden and vasomotion. Journal of the American Heart Association. 2013;2(5):e000321. doi: 10.1161/JAHA.113.000321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vaccarino V, Shah AJ, Rooks C, et al. Sex differences in mental stress-induced myocardial ischemia in young survivors of an acute myocardial infarction. Psychosomatic medicine. 2014;76(3):171–180. doi: 10.1097/PSY.0000000000000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burg MM, Graeber B, Vashist A, et al. Noninvasive detection of risk for emotion-provoked myocardial ischemia. Psychosomatic medicine. 2009;71(1):14–20. doi: 10.1097/PSY.0b013e318187c035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martin EA, Prasad A, Rihal CS, Lerman LO, Lerman A. Endothelial function and vascular response to mental stress are impaired in patients with apical ballooning syndrome. J Am Coll Cardiol. 2010;56(22):1840–1846. doi: 10.1016/j.jacc.2010.03.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martin EA, Tan SL, MacBride LR, Lavi S, Lerman LO, Lerman A. Sex differences in vascular and endothelial responses to acute mental stress. Clin Auton Res. 2008;18(6):339–345. doi: 10.1007/s10286-008-0497-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hassan M, Quyyumi AA, Sheps DS. A noninvasive clinically useful predictor for mental stress-induced ischemia. Psychosomatic medicine. 2009;71(1):21–22. doi: 10.1097/psy.0b013e3181967c3d. [DOI] [PubMed] [Google Scholar]
- 18.Hassan M, York KM, Li H, et al. Usefulness of peripheral arterial tonometry in the detection of mental stress-induced myocardial ischemia. Clinical cardiology. 2009;32(9):E1–6. doi: 10.1002/clc.20515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thomson LE, Wei J, Agarwal M, et al. Cardiac magnetic resonance myocardial perfusion reserve index is reduced in women with coronary microvascular dysfunction. A National Heart, Lung, and Blood Institute-sponsored study from the Women’s Ischemia Syndrome Evaluation. Circulation. Cardiovascular imaging. 2015;8(4) doi: 10.1161/CIRCIMAGING.114.002481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hasdai D, Gibbons RJ, Holmes DR, Jr, Higano ST, Lerman A. Coronary endothelial dysfunction in humans is associated with myocardial perfusion defects. Circulation. 1997;96(10):3390–3395. doi: 10.1161/01.cir.96.10.3390. [DOI] [PubMed] [Google Scholar]
- 21.Bairey Merz CN, Handberg EM, Shufelt CL, et al. A randomized, placebo-controlled trial of late Na current inhibition (ranolazine) in coronary microvascular dysfunction (CMD): impact on angina and myocardial perfusion reserve. Eur Heart J. 2016;37(19):1504–1513. doi: 10.1093/eurheartj/ehv647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gottdiener JS, Kop WJ, Hausner E, McCeney MK, Herrington D, Krantz DS. Effects of mental stress on flow-mediated brachial arterial dilation and influence of behavioral factors and hypercholesterolemia in subjects without cardiovascular disease. Am J Cardiol. 2003;92(6):687–691. doi: 10.1016/s0002-9149(03)00823-3. [DOI] [PubMed] [Google Scholar]
- 23.Gottdiener JS, Krantz DS, Howell RH, et al. Induction of silent myocardial ischemia with mental stress testing: relation to the triggers of ischemia during daily life activities and to ischemic functional severity. J Am Coll Cardiol. 1994;24(7):1645–1651. doi: 10.1016/0735-1097(94)90169-4. [DOI] [PubMed] [Google Scholar]
- 24.Kop WJ, Krantz DS, Nearing BD, et al. Effects of acute mental stress and exercise on T-wave alternans in patients with implantable cardioverter defibrillators and controls. Circulation. 2004;109(15):1864–1869. doi: 10.1161/01.CIR.0000124726.72615.60. [DOI] [PubMed] [Google Scholar]
- 25.Krantz DS, Kop WJ, Santiago HT, Gottdiener JS. Mental stress as a trigger of myocardial ischemia and infarction. Cardiol Clin. 1996;14(2):271–287. [PubMed] [Google Scholar]
- 26.Ironson G, Taylor CB, Boltwood M, et al. Effects of anger on left ventricular ejection fraction in coronary artery disease. Am J Cardiol. 1992;70(3):281–285. doi: 10.1016/0002-9149(92)90605-x. [DOI] [PubMed] [Google Scholar]
- 27.AD, Becker LC, Bonsall R, et al. Ischemic, hemodynamic, and neurohormonal responses to mental and exercise stress. Experience from the Psychophysiological Investigations of Myocardial Ischemia Study (PIMI) Circulation. 1996;94(10):2402–2409. doi: 10.1161/01.cir.94.10.2402. [DOI] [PubMed] [Google Scholar]
- 28.Martin EA, Nelson RE, Felmlee-Devine MD, Brown TE, Lerman A. Comparing EndoPAT and BIOPAC measurement of vascular responses to mental stress. Cell Biochem Funct. 2011;29(4):272–278. doi: 10.1002/cbf.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martin EA, Prasad A, Rihal CS, Lerman LO, Lerman A. Endothelial function and vascular response to mental stress are impaired in patients with apical ballooning syndrome. J Am Coll Cardiol. 2010;56(22):1840–1846. doi: 10.1016/j.jacc.2010.03.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matsuzawa Y, Li J, Aoki T, et al. Predictive value of endothelial function by noninvasive peripheral arterial tonometry for coronary artery disease. Coron Artery Dis. 2015;26(3):231–238. doi: 10.1097/MCA.0000000000000208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bonetti PO, Pumper GM, Higano ST, Holmes DR, Jr, Kuvin JT, Lerman A. Noninvasive identification of patients with early coronary atherosclerosis by assessment of digital reactive hyperemia. Journal of the American College of Cardiology. 2004;44(11):2137–2141. doi: 10.1016/j.jacc.2004.08.062. [DOI] [PubMed] [Google Scholar]
- 32.Matsuzawa Y, Kwon TG, Lennon RJ, Lerman LO, Lerman A. Prognostic Value of Flow-Mediated Vasodilation in Brachial Artery and Fingertip Artery for Cardiovascular Events: A Systematic Review and Meta-Analysis. Journal of the American Heart Association. 2015;4(11) doi: 10.1161/JAHA.115.002270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goor DA, Sheffy J, Schnall RP, et al. Peripheral arterial tonometry: a diagnostic method for detection of myocardial ischemia induced during mental stress tests: a pilot study. Clinical cardiology. 2004;27(3):137–141. doi: 10.1002/clc.4960270307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dakak N, Quyyumi AA, Eisenhofer G, Goldstein DS, Cannon RO., 3rd Sympathetically mediated effects of mental stress on the cardiac microcirculation of patients with coronary artery disease. Am J Cardiol. 1995;76(3):125–130. doi: 10.1016/s0002-9149(99)80043-5. [DOI] [PubMed] [Google Scholar]
- 35.Sedlak TL, Johnson BD, Pepine CJ, Reis SE, Bairey Merz CN. Brachial artery constriction during brachial artery reactivity testing predicts major adverse clinical outcomes in women with suspected myocardial ischemia: results from the NHLBI-sponsored women’s ischemia Syndrome Evaluation (WISE) Study. PLoS One. 2013;8(9):e74585. doi: 10.1371/journal.pone.0074585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Michelsen MM, Mygind ND, Pena A, et al. Peripheral Reactive Hyperemia Index and Coronary Microvascular Function in Women With no Obstructive CAD: The iPOWER Study. JACC Cardiovasc Imaging. 2016;9(4):411–417. doi: 10.1016/j.jcmg.2016.02.005. [DOI] [PubMed] [Google Scholar]
- 37.Patel SM, Lerman A, Lennon RJ, Prasad A. Impaired coronary microvascular reactivity in women with apical ballooning syndrome (Takotsubo/stress cardiomyopathy) Eur Heart J Acute Cardiovasc Care. 2013;2(2):147–152. doi: 10.1177/2048872613475891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Matsuzawa Y, Sugiyama S, Sugamura K, et al. Digital assessment of endothelial function and ischemic heart disease in women. J Am Coll Cardiol. 2010;55(16):1688–1696. doi: 10.1016/j.jacc.2009.10.073. [DOI] [PubMed] [Google Scholar]
- 39.Sondergaard E, Moller JE, Egstrup K. Relationship between vascular dysfunction in peripheral arteries and ischemic episodes during daily life in patients with ischemic heart disease and hypercholesterolemia. Am Heart J. 2002;144(1):108–114. doi: 10.1067/mhj.2002.123147. [DOI] [PubMed] [Google Scholar]
- 40.Bonetti PO, Lerman LO, Lerman A. Endothelial dysfunction: a marker of atherosclerotic risk. Arterioscler Thromb Vasc Biol. 2003;23(2):168–175. doi: 10.1161/01.atv.0000051384.43104.fc. [DOI] [PubMed] [Google Scholar]
- 41.Bonetti PO, Pumper GM, Higano ST, Holmes DR, Jr, Kuvin JT, Lerman A. Noninvasive identification of patients with early coronary atherosclerosis by assessment of digital reactive hyperemia. J Am Coll Cardiol. 2004;44(11):2137–2141. doi: 10.1016/j.jacc.2004.08.062. [DOI] [PubMed] [Google Scholar]
- 42.Hammadah M, Alkhoder A, Al Mheid I, Wilmot K, et al. Hemodynamic, catecholamine, vasomotor and vascular responses: Determinants of myocardial ischemia during mental stress. International journal of cardiology. 2017;(243) doi: 10.1016/j.ijcard.2017.05.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rozanski A, Bairey CN, Krantz DS, et al. Mental stress and the induction of silent myocardial ischemia in patients with coronary artery disease. N Engl J Med. 1988;318(16):1005–1012. doi: 10.1056/NEJM198804213181601. [DOI] [PubMed] [Google Scholar]
- 44.McCraty R, Atkinson M, Tiller WA, Rein G, Watkins AD. The effects of emotions on short-term power spectrum analysis of heart rate variability. Am J Cardiol. 1995;76(14):1089–1093. doi: 10.1016/s0002-9149(99)80309-9. [DOI] [PubMed] [Google Scholar]
- 45.Pagani M, Mazzuero G, Ferrari A, et al. Sympathovagal interaction during mental stress. A study using spectral analysis of heart rate variability in healthy control subjects and patients with a prior myocardial infarction. Circulation. 1991;83(4 Suppl):II43–51. [PubMed] [Google Scholar]
- 46.Tuininga YS, Crijns HJ, Brouwer J, et al. Evaluation of importance of central effects of atenolol and metoprolol measured by heart rate variability during mental performance tasks, physical exercise, and daily life in stable postinfarct patients. Circulation. 1995;92(12):3415–3423. doi: 10.1161/01.cir.92.12.3415. [DOI] [PubMed] [Google Scholar]
- 47.Burg MM, Jain D, Soufer R, Kerns RD, Zaret BL. Role of behavioral and psychological factors in mental stress-induced silent left ventricular dysfunction in coronary artery disease. J Am Coll Cardiol. 1993;22(2):440–448. doi: 10.1016/0735-1097(93)90048-6. [DOI] [PubMed] [Google Scholar]
- 48.Sheps DS, McMahon RP, Becker L, et al. Mental stress-induced ischemia and all-cause mortality in patients with coronary artery disease: Results from the Psychophysiological Investigations of Myocardial Ischemia study. Circulation. 2002;105(15):1780–1784. doi: 10.1161/01.cir.0000014491.90666.06. [DOI] [PubMed] [Google Scholar]
- 49.Bairey Merz CN, Kop W, Krantz DS, Helmers KF, Berman DS, Rozanski A. Cardiovascular stress response and coronary artery disease: evidence of an adverse postmenopausal effect in women. Am Heart J. 1998;135(5 Pt 1):881–887. doi: 10.1016/s0002-8703(98)70050-x. [DOI] [PubMed] [Google Scholar]