Abstract
Objectives
Research using very low nicotine content (VLNC) cigarettes has shown that participants underreport use of non-study cigarettes. Biomarkers of nicotine exposure could be used to verify compliance with VLNC cigarettes. This study aimed to characterize biomarkers of exposure when participants exclusively use VLNC cigarettes.
Methods
23 participants stayed in a hotel that permitted smoking for 5 days and 4 nights. They were provided 2 packs of VLNC cigarettes each day (0.4 mg of nicotine/g of tobacco; Spectrum cigarettes) and did not have access to other tobacco products. 24-hour urine samples were collected to assess exposure to nicotine and anatabine.
Results
After 4 days of exclusive use, the geometric means for urinary total cotinine, total nicotine equivalents (TNE), and anatabine were 1.13 nmol/ml (92% reduction), 3.17 nmol/ml (94% reduction) and 0.0031 nmol/ml (93% reduction). The population estimates of the 95th percentile of cotinine, TNE, and anatabine levels were 2.69, 6.41, and 0.0099 nmol/ml, respectively.
Conclusions
Study participants exclusively smoking 0.4 mg/g Spectrum cigarettes are unlikely to have biomarker values above these levels. The data presented here will be valuable to researchers conducting research on use of VLNC cigarettes.
Keywords: biomarkers, nicotine reduction, VLNC cigarettes, total nicotine equivalents, cotinine, anatabine
INTRODUCTION
The Family Smoking Prevention and Tobacco Control Act enables the United States Food and Drug Administration (FDA) to implement tobacco product standards, including reducing the nicotine content in cigarettes to non-addictive levels.1 Clinical trials have assessed the potential impact of a reduced nicotine product standard by providing very low nicotine content cigarettes to participants and instructing them to abstain from using their usual brand cigarettes and other tobacco products.2–5 Nicotine content in these products is reduced by as much as 98% compared to normal nicotine content cigarettes.5 However, researchers have reported that the reduction in biomarkers of nicotine exposure is less than expected given the reduction in nicotine content within the product.5,6
There are several possible explanations for why participants have higher than expected biomarkers of nicotine exposure in studies using very low nicotine content (VLNC) cigarettes. First, the content of nicotine in usual brand cigarettes varies across brands, so the relative reduction in nicotine content is not the same for all smokers. Second, smokers vary in the rate at which they metabolize nicotine, and individuals who metabolize nicotine more slowly may have higher levels of nicotine exposure than individuals who metabolize nicotine more quickly. Third, participants may be compensating for the reduction in nicotine content by increasing the number of cigarettes smoked per day and/or changing their smoking behavior to extract more nicotine per individual cigarette. Compensation is a potential negative outcome of nicotine reduction if it results in greater exposure to other tobacco smoke toxicants, and it is important that researchers accurately characterize any compensation that occurs. Finally, despite explicit instruction to use only the low-nicotine products provided to them, the most likely explanation is that participants may also be smoking normal nicotine content cigarettes in conjunction with VLNC cigarettes, or they may be using other tobacco or nicotine products such as e-cigarettes or nicotine replacement while smoking VLNC cigarettes. In this case, the higher than expected levels of exposure are attributable to the secondary source(s) of nicotine.
The use of other tobacco products, especially normal nicotine content cigarettes, in addition to VLNC cigarettes, is challenging for researchers interested in nicotine reduction for several reasons. First, non-compliance prevents researchers from characterizing the reduction in nicotine exposure that would occur if participants did not have access to other tobacco products. Second, non-compliance could minimize the potential benefits of reducing the nicotine content in cigarettes, including changes in smoking behavior and level of dependence. A recent study analyzed the variability in urinary cotinine levels in smokers randomized to smoke only VLNC cigarettes for 6 weeks prior to their quit date. Those smokers who had the lowest levels of total urinary cotinine after 6 weeks of VLNC use had the greatest abstinence rates. As nicotine exposure increased, likely due to use of other tobacco products, abstinence rates at the one-month follow up decreased.7 Third, use of conventional cigarettes or other nicotine-containing products may minimize potential negative outcomes associated with VLNC cigarettes, such as nicotine withdrawal symptoms or cognitive disruption, because smokers may use other tobacco products to alleviate their symptoms. While, it is not feasible to prevent non-compliance, researchers could obtain more precise estimates of the effect of nicotine reduction by providing incentives to participants for abstaining from non-study products and/or identifying participants who were not compliant following data collection. In both cases, researchers need to be able to verify a participant’s compliance.
Benowitz and colleagues recently proposed an analytical approach for establishing biochemical cutoffs for use of other nicotine/tobacco products besides VLNC cigarettes.6 They reasoned that non-compliance could be assessed by dividing the ratio of smokers’ baseline plasma cotinine to number of cigarettes per day (CPD) by the ratio of plasma cotinine to CPD after smoking VLNC cigarettes. The predicted ratio with no compensation based on a decrease in nicotine from 10 mg in conventional cigarettes to 0.5 mg in VLNC cigarettes would be 0.05. To conservatively account for extreme compensation and potentially other sources of variability, they also assumed a maximum 4-fold increase in bioavailability of nicotine, which resulted in a proposed cutoff of 0.2. Therefore, any ratio above 0.2 would indicate non-compliance. When applying this equation to previous data, they found that 60% of their sample had a ratio greater than 0.2; however, only 21% of the sample self-reported non-compliance during the study. This discrepancy highlights the limitation of relying solely on self-reported compliance. Although the approach described by Benowitz et al.,6 is useful for estimating non-compliance, it relies on assumptions about the bioavailability of nicotine in VLNC cigarettes, and needs to be validated before being used to verify compliance. The ideal approach would be to characterize biomarkers of nicotine exposure in a group of smokers known to be compliant with VLNC cigarettes.
METHODS
Overview
The primary aims of this study were to characterize the reduction in nicotine exposure in smokers known to be compliant with VLNC cigarettes and to develop biochemical cutoffs for compliance with VLNC cigarettes. This was done by assessing biomarkers of tobacco exposure in users of VLNC cigarettes under conditions of restricted access to other tobacco products. Participants were confined to a local hotel that permitted smoking, both in individual rooms and in several outdoor areas. A hotel was chosen as the location over an in-patient residential unit to mimic a more naturalistic environment. Participants stayed at the hotel for 5 days and 4 nights and were provided VLNC cigarettes to smoke. Research staff were onsite 24-hours per day.
Participants were instructed to collect all urine while at the hotel. Urine samples were collected in 4or 12 hour blocks of time (8AM-12PM, 12PM-4PM, 4PM-8PM, and 8PM-8AM). First void urine was collected separately (the protocol is described in Figure 1). All urine samples were analyzed for total cotinine; total nicotine, total trans-3’-hydroxycotinine (3-HCOT), and nicotine N-oxide. ("Total" refers to the sum of free and glucuronide-conjugated compounds). Total nicotine equivalents (TNE), are the sum of total nicotine, total cotinine, total 3-HCOT and nicotine N’-oxide. Urine samples from the first morning void on Day 1 and all samples collected on Days 4 and 5 were also analyzed for anatabine. The anatabine content of the tobacco in VLNC is reduced compared to conventional cigarettes,5 and the level of this minor alkaloid could also be used to verify compliance in VLNC cigarette smokers when using nicotine medications (ie, nicotine replacement therapy, NRT) which do not contain significant quantities of anatabine.
Figure 1.
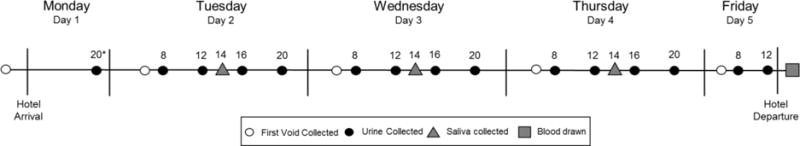
Protocol of sample collection
Spectrum cigarettes were provided upon hotel arrival. Participants did not have access to usual brand cigarettes after arriving at the hotel until after they completed the blood draw on Friday. Samples were collected at the indicated times. The total urine output was collected between time points and samples were labeled with the ending time of collection. The concentration of total nicotine equivalents was measured in each urine collection, and anatabine concentrations were determined for baseline first void and all samples on Days 4 and 5. Saliva and Plasma samples were analyzed for free cotinine. *Timepoints are listed in military time.
Design
Study enrollment was divided into 3 distinct weeks at the hotel, with each participant residing at the hotel for one week (Monday-Friday) in one of 2 study conditions. Participants in the first and third weeks were enrolled into one condition (n=24): these individuals consented to smoking only VLNC cigarettes (herein referred to as the ‘Compliant’ group). Participants in the second week were enrolled into the second study condition (n=7): they consented to smoking ~90% VLNC cigarettes and ~10% usual brand cigarettes (herein referred to as the 10% Usual Brand group). To avoid people self-selecting into the 10% Usual Brand group, individuals in both conditions were blind to the cigarettes smoked by the other smoking groups. Participants were told the research cigarettes may or may not have reduced nicotine levels.
Participants
Thirty-three daily smokers were recruited via Craigslist, newspaper advertisements, and flyers in Pittsburgh, PA. The study was described as collecting urine, saliva, and blood after smoking research cigarettes for 5 days. Inclusion criteria included: 1) age 18 or older; 2) smoking 5 or more cigarettes per day for the past year with no continuous periods of abstinence longer than 30 days; 3) expired carbon monoxide (CO) of >9 ppm (Smokerlyzer Bedfont Scientific, Covita™, Haddonfield, NJ) or urinary cotinine >2000 ng/ml (NicAlert® =6, Nymox Pharmaceutical Corporation, Hasbrouck Heights, NJ) 4) willing to have blood drawn; and 5) willing to spend 5 days/4 nights in a hotel. Exclusion criteria included: 1) intention to quit smoking in the next 30 days; 2) currently seeking treatment for smoking cessation; 3) a quit attempt in the past 30 days resulting in greater than 3 days of abstinence; 4) using non-cigarette tobacco products on more than 9 of the past 30 days; 5) significant unstable medical or psychiatric conditions as determined by the study physician after reviewing a brief medical history questionnaire; 6) positive toxicology screen for illicit drugs including cocaine, methamphetamines, PCP, and non-prescription opioids, benzodiazepines, barbiturates or amphetamines; 7) pregnant, trying to become pregnant or breastfeeding as determined by urinary hCG assays and self-report; 8) smoking ‘roll your own cigarettes’ exclusively; 9) currently taking medications that could alter nicotine metabolism including phenytoin, carbamazepine, oxcarbazenpine, primidone, phenobarbital, bendamustine, clopidogrel, clozapine, erlotinib, felcainide, fluvoxamine, irinotecan, olanzapine, ropinirole, tacrine, or theophylline; 10) suicidal ideation in the past month or suicide attempts in the past 10 years; and 11) participation in a previous study using the research cigarettes.
Hotel Information
Participants were sequestered in a small hotel located in a moderately isolated area of western Pennsylvania (ie limited businesses or residences within walking distance). Hotel rooms that allowed smoking were reserved during 3 separate weeks. Participants were provided their own room unless they preferred to share with another participant. Transportation to and from the hotel was provided by a shuttle service. All meals were provided either by the hotel or outside catering services. Participant meals sometimes included foods from the solanaceae family (eg, potatoes, tomatoes) which may have contained trivial amounts of nicotine.8
The research staff searched the participants’ personal belongings prior to arrival at the hotel. Usual brand cigarettes and alcohol were confiscated and returned upon study completion. Participants were required to remain on the hotel premises from Monday afternoon until Friday afternoon, except during supervised outings organized by the staff (eg kickball games, ice cream social). Outside visitors were prohibited and a curfew was enforced between 9PM-7AM. During the day, 3-6 staff members were at the hotel to complete the procedures as well as supervise the participants. The principal investigator, project manager, graduate student, and/or other staff members stayed overnight at the hotel to ensure at least 3 researchers were present each night. The study physician and clinical psychologist were available via phone 24-hours per day. Participants could earn $575 for completing all parts of the study.
Investigational Cigarettes
The investigational cigarettes were Spectrum brand cigarettes obtained from the National Institute on Drug Abuse (NOT-DA-14-004; NRC 102/103). The research cigarettes contained 0.4 mg/g of nicotine and 30 μg/g of anatabine.5 Nicotine and tar yields (ISO) were 0.03 mg nicotine and 9 mg, respectively.5 The nicotine content for the usual brand cigarettes of 78% of participants in the Compliant Group was obtained from the FDA through the Freedom of Information Act.9 For these participants the average nicotine content of their usual brand was 16.5 mg/g tobacco, so the reduction in the nicotine content of the research cigarettes was approximately 98%. The anatabine contents in usual brand cigarettes were not available from the FDA. However, the anatabine contents of the 50 top-selling cigarettes ranged from 92 to 1390 μg/g tobacco,10 so levels in the research cigarettes were likely reduced by ~97%. Participants were assigned to smoke menthol or non-menthol study cigarettes based on self-reported preference and were not permitted to switch flavors during the study. The protocol was reviewed by the FDA’s Center for Tobacco Products for the use of the described investigational tobacco products, and was approved by the University of Pittsburgh Institutional Review Board.
Procedures
Screening/baseline session
Participants deemed eligible during a preliminary phone screen were invited to complete an in-person screening visit. After providing written informed consent, participants provided CO and breath alcohol level (BAL) readings, and completed questionnaires about their smoking, medical, and psychiatric history. The standard operating procedure for CO measurement during the trial instructed participants to inhale a deep breath and hold it for 15 seconds while the Smokerlyzer CO monitor counted down to zero. Then they exhaled into a straw-like mouthpiece for at least 6 seconds or until their lungs were emptied.
Prior to entering the hotel, participants deemed eligible completed questionnaires about dependence (Fagerström Test for Cigarette Dependence, FTCD11; Wisconsin Inventory of Smoking Dependence Motives, WISDM-3612), withdrawal symptoms (Minnesota Nicotine Withdrawal Scale, MNWS13; Questionnaire on Smoking Urges, QSU-1014), and mood (Center for Epidemiological Studies-Depression, CESD15; Positive and Negative Affect Schedule, PANAS16). Data from these questionnaires are not reported in this manuscript. The study physician reviewed the medical and psychiatric history for each eligible subject and confirmed that it was appropriate for him/her to receive the study product.
Hotel procedures
While at the hotel, participants were provided with 2 packs of Spectrum cigarettes per day (40 cigarettes) with instructions to smoke at least 5 per day and not to share them with other participants or hotel patrons. They were also instructed to collect all of their urine in the provided receptacles. Used cigarettes butts were saved in individual collection tins and the times of each cigarette smoked were recorded on the tins. Discrepancies between cigarettes provided and cigarette butts returned were reconciled by participants and staff members.
Participants had ‘check-in’ times each day at 8AM, 12PM, 4PM, and 8PM. At each time point participants’ urine output was collected and a CO reading was taken. Heart rate, blood pressure, and BAL were measured twice per day to monitor safety and confirm abstinence from alcohol. Blood pressure readings above 160/100 or below 90/45 and heart rates above 105 bpm or below 45 bpm were reviewed by the study physician to determine if continued participation was appropriate. Breath alcohol levels above 0.01 g/l were grounds for study dismissal.
In the 10% Usual Brand group, all participants smoked one usual brand cigarette each day at 10AM. Participants in this group with a baseline smoking rate greater than 15 CPD smoked an additional usual brand cigarette at 2PM. Usual brand cigarettes were maintained by the research staff and were provided to the participants at the designated timepoint(s).
Saliva samples for cotinine measurement were collected once per day at 2PM on Tuesday-Thursday, and plasma samples for cotinine measurement were collected on Friday immediately upon returning to the laboratory at the University of Pittsburgh. The samples were stored at -20°C or cooler and were shipped on dry ice overnight to the University of Minnesota Masonic Cancer Center for processing and analysis.
Biomarker analysis
Urinary concentrations of total nicotine, total cotinine, total 3-HCOT, nicotine N-oxide and anatabine for all urine samples were determined by liquid chromatography tandem mass spectrometry (LC/MS/MS) analysis using previously described methods.17,18 The limits of quantitation (LOQ) for nicotine, cotinine and 3-HCOT were each 0.05 nmol/ml, for nicotine N-oxide the LOQ was 0.006 nmol/ml and for anatabine the LOQ was 0.0009 nmol/ml. TNE were calculated as the molar sum of total nicotine, total cotinine, total 3-HCOT and nicotine N’-oxide. Plasma and salivary cotinine concentrations was quantified by LC/MS/MS analysis as previously described and the LOQ was 0.3 ng/ml.19 Urinary anatabine concentrations were also quantified by LC/MS/MS analysis.18
Data Analysis
The primary objective was to characterize urinary cotinine, TNE, and anatabine across time. The geometric means of the biomarker concentrations (nmol/ml urine or nmol/mg creatinine) were calculated for each urine collection, and the change in the concentration over time was evaluated using repeated measures ANOVA on log-transformed values. The baseline first voided sample was collected at home on the day participants checked into the hotel, prior to receiving the Spectrum cigarettes (Monday). The final first voided sample was collected on the day participants checked out of the hotel (Friday). Biomarker levels on Days 4 and 5 were compared to assess whether levels had achieved steady state.
Population quantiles (80th, 90th and 95th percentile) were estimated for each biomarker in the Compliant group in the final first void sample. Population quantiles were estimated by maximum likelihood estimation assuming a gamma distribution for each biomarker. Maximum likelihood estimation was completed using the ‘fitdistr’ function in the MASS package20 for the R statistical programming language.21 95% confidence intervals for population quantiles were estimated using the parametric bootstrap.22
A ratio of the biomarker concentration per Cigarette Per Day (CPD) for the final first void sample compared to the biomarker concentration per CPD at baseline was calculated to estimate the reduction in nicotine and anatabine exposure per VLNC cigarette compared to the exposure per usual brand cigarette [(Hotel Final First Void Sample Biomarker/(Hotel CPD))/(Baseline First Void Sample Biomarker/Baseline CPD)]. For the baseline sample (Day 1, 1st morning void) CPD is defined as the average CPD during the 14 days prior to entering the hotel as assessed by a timeline follow back interview (Appendix Table 1). For urine samples collected at the hotel, the CPD is defined as the total number of cigarettes smoked while at the hotel divided by 4 (participants were at the hotel for approximately 4 24-hr periods).
One participant was excluded from all data analyses due to use of contraband non-study tobacco products while at the hotel. This participant’s baseline, final first void, and final sample biomarker data are included in Appendix Table A2 and A3 (Participant 24).
RESULTS
Of the 33 participants who met study criteria, one subject was withdrawn prior to entering the hotel due to elevated blood pressure. Another subject was excluded due to participation in a previous laboratory study. The 31 participants that completed the study ranged from 24 to 69 years of age, 52% were female, and 55% identified as African American. Mean baseline CPD was 18.2 and average FTND score was 6.5. Seventy-one percent of the sample smoked menthol cigarettes. Demographic information is provided for each participant in Appendix Table A1
Figure 2 depicts urinary total cotinine, TNE, and anatabine concentrations plotted across time. The data are expressed as nmol/ml (left figure panels) and nmol/mg creatinine (right figure panels). The concentrations of both total cotinine and TNE decreased markedly over the first 2 days. The 10% Usual Brand group is shown on the graphs for descriptive purposes only; the data from this group have not been included in the statistical analyses because there were only 7 participants. A repeated-measures ANOVA confirmed there was a statistically significant reduction in all biomarkers across time points (results presented in Appendix). This was true whether the biomarker was expressed per ml urine or per mg creatinine. We observed a significant reduction in total cotinine and TNEs with the first void on Day 2 (cotinine (nmol/ml), p = .011; cotinine (nmol/mg creatinine), p < .001; TNE (nmol/ml and nmol/mg creatinine), p < .001), and this difference persisted for the remainder of the study (p < .012 at all timepoints). Urine anatabine concentrations (nmol/ml and nmol/mg creatinine) were significantly reduced in the first sample analyzed (First Void on Day 4) compared to the baseline (p < 0.001). The relative reduction in the geometric means of cotinine, TNE, and anatabine from the first to final sample was 92% (95%CI: 89-94%), 94% (95%CI: 92%-96%), and 93% (95% CI: 89-96%). This was despite an observed increase in CPD while at the hotel (Compliant Group CPD at Baseline: Mean=20.09, Standard deviation=8.34, CPD at Hotel: Mean=28.30, Standard Deviation=7.90; 10% UB Group CPD at Baseline: M= 12.14, SD=4.88, CPD at Hotel: M=26.26, SD=6.95).
Figure 2.
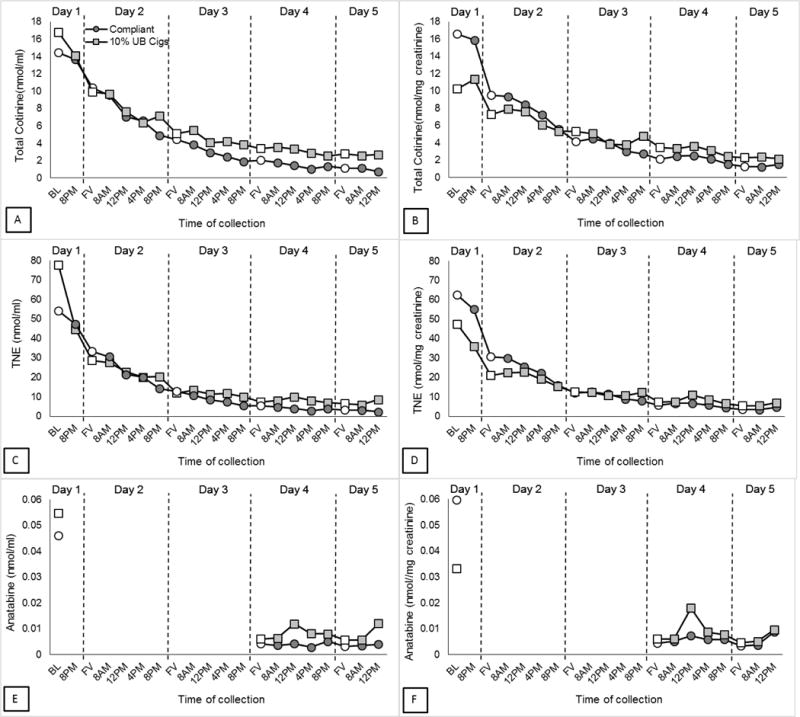
Geometric means for urinary biomarker concentrations of VLNC cigarette use by day
Geometric means of total cotinine (A), TNE (C), and anatabine (E) concentration (nmol/ml) were plotted for each urine collection for Compliant (n=23) and 10% Usual Brand (n=7) groups. Data in panels B, D, and F are expressed per mg of creatinine. First voids are represented with open symbols. Baseline sample is first void from Day 1. Anatabine levels were only analyzed in samples collected at baseline, Day 4, and Day 5.
A paired samples t-test was used to assess the extent to which biomarker concentrations at the end of the study had reached steady state by comparing the first void samples on Days 4 and 5. Total cotinine and TNE (both nmol/ml and nmol/mg creatinine) significantly decreased from Day 4 to Day 5 (p < 0.001), reduction in geometric means from First Void Day 4 to First Void Day 5 represents 4-7% (depending on biomarker) of total reduction from Baseline to Day 5) Anatabine (nmol/ml and nmol/mg creatinine) did not significantly change from Day 4 to Day 5. Data for each participant from the baseline and final first void samples are provided in Appendix Table A2, along with values for total nicotine, the minor alkaloid anabasine, salivary cotinine, total cotinine, and total number of cigarettes smoked at the hotel. Data from final urine sample (Day 5, 12pm collection) are provided in Appendix Table A3.
Individual subject biomarker concentrations from the first void urine samples at baseline (Day 1) through Day 5are plotted in Figure 3. The distribution of all 3 biomarker concentrations for the Compliant group overlapped with that of the 10% Usual Brand. However, distributions of total cotinine and TNE concentrations (nmol/ml) for the Compliant group on Day 5 were distinct from their baseline distribution, except for one participant with a low baseline TNE value of 6.75 nmol/ml. When TNE were expressed per mg creatinine there was no overlap between baseline and Day 5 concentrations.
Figure 3.
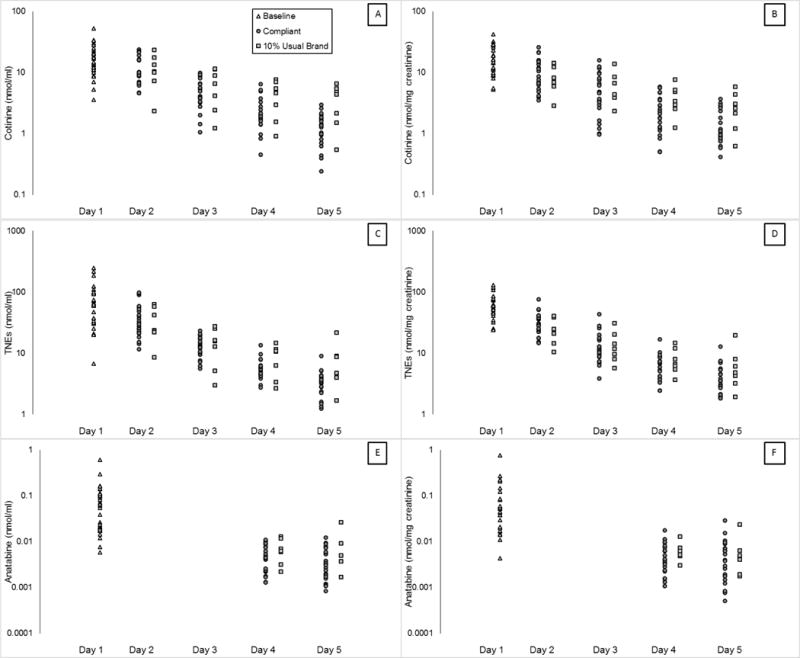
Biomarker concentrations in the first morning void samples collected on Days 1 through 5 for each participant.
The urinary concentration of total cotinine, TNE, and anatabine from Day 1 (baseline collection prior to hotel entry) through Day 5 first void samples are plotted on a log scale for each participant in the Compliant and 10% Usual Brand groups. Both groups are included in the Day 1 (baseline) distribution. Data in panels B, D, and F are expressed per mg of creatinine. Anatabine levels were not analyzed from the first void samples collected on Days 2 and 3.
Estimates and 95% confidence intervals for the 80th, 90th, and 95th percentile of TNE, total cotinine, and anatabine in a compliant population are presented in Table 1. These analyses estimate that in a fully compliant population smoking 0.4 mg/g Spectrum Cigarettes, 95% of smokers will have total cotinine, TNE, and anatabine levels (nmol/ml) at or below 2.69, 6.41, and 0.0099, and total cotinine, TNE, and anatabine levels (nmol/mg creatinine) at or below 3.13, 8.21, and 0.016. The percentage of the participants in the Compliant group (n=23) who met each of these criteria for each day of the study (first void of each day) are presented in Table 2. These data confirm that the cutoffs are appropriate for this sample (approximately 95% of participants would meet these criteria on Day 5). Very few (anatabine) or no (cotinine and TNE) individuals would meet these criteria with exclusive use of conventional cigarettes (Baseline). The one participant who did not meet the 95th percentile criterion on Day 5 for all 3 biomarkers also had the highest cotinine, TNE, and anatabine levels at baseline, 52.9, 248, and 0.612 nmol/ml, respectively. Therefore, it is likely that a significant amount of the biomarker present at Day 5 is due to residual nicotine/anatabine exposure from baseline smoking of usual brand cigarettes.
Table 1.
80th, 90th, and 95th percentile population estimates for cotinine, TNE, and anatabine in the Compliant group (n = 23) using biomarkers collected in the final first void sample. See data analysis section for description of analyses.
| Biomarker | 80th | 95% CI | 90th | 95% CI | 95th | 95% CI | |
|---|---|---|---|---|---|---|---|
| nmol/ml | Total Cotinine | 1.86 | (1.44, 2.3) | 2.29 | (1.74, 2.95) | 2.69 | (1.96, 3.44) |
| TNE | 4.72 | (3.82, 5.59) | 5.6 | (4.45, 6.87) | 6.41 | (4.9, 7.94) | |
| Anatabine | 0.006 | (0.004, 0.008) | 0.008 | (0.006, 0.011) | 0.010 | (0.007, 0.013) | |
| nmol/mg creatinine | Total Cotinine | 2.11 | (1.60, 2.58) | 2.64 | (1.88, 3.42) | 3.13 | (2.24, 4.06) |
| TNE | 5.68 | (4.37, 7) | 6.99 | (5.27, 8.89) | 8.21 | (6.02, 10.63) | |
| Anatabine | 0.009 | (0.006, 0.013) | 0.013 | (0.008, 0.018) | 0.016 | (0.010, 0.023) |
Table 2.
Percent of individuals in Compliant group (n=23) within the estimated population 80th, 90th, and 95th percentiles for the first void sample each day. “-“ indicates the sample was not analyzed.
| 80th | 90th | 95th | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hotel Day | Hotel Day | Hotel Day | ||||||||||||||
| Biomarker | BL | 2 | 3 | 4 | 5 | BL | 2 | 3 | 4 | 5 | BL | 2 | 3 | 4 | 5 | |
| nmol/ml | Total Cotinine | 0 | 0 | 9 | 48 | 83 | 0 | 0 | 13 | 74 | 91 | 0 | 0 | 13 | 74 | 96 |
| TNE | 0 | 0 | 0 | 43 | 87 | 0 | 0 | 0 | 57 | 96 | 0 | 0 | 9 | 83 | 96 | |
| Anatabine | 4 | – | – | 65 | 78 | 9 | – | – | 78 | 87 | 9 | – | – | 91 | 96 | |
| nmol/mg creatinine | Total Cotinine | 0 | 0 | 22 | 43 | 74 | 0 | 0 | 26 | 57 | 78 | 0 | 0 | 30 | 65 | 91 |
| TNE | 0 | 0 | 4 | 39 | 83 | 0 | 0 | 13 | 61 | 83 | 0 | 0 | 22 | 74 | 96 | |
| Anatabine | 4 | – | – | 78 | 78 | 4 | – | – | 96 | 91 | 13 | – | – | 96 | 96 | |
In some cases, researchers may have information to adjust for baseline cigarettes per day and biomarkers of exposure, as recommended by Benowitz et al.6 Figure 4 shows the distribution of the ratios of the biomarker concentrations per CPD at Day 5 to the concentration per CPD at baseline for each participant. This value is referred to as the proportion of the biomarker at baseline. For all participants in the Compliant group except one, ratios were less than 0.13 for both total cotinine and TNE when expressed as nmol/ml (median 0.05 for cotinine, 0.04 for TNE). The one exception is a participant who had low biomarker values at baseline (6.75 nmol/ml TNE, but within a normal range when expressed as nmol/mg creatinine, 61.3). When the proportion of baseline is calculated using nmol/mg creatinine all participants have values below 0.24 for cotinine and 0.14 for TNE (median 0.05 for cotinine, 0.04 for TNE). The proportion of baseline for anatabine was below 0.5 when data are expressed as nmol/ml or 0.24 when data are expressed as nmol/mg creatinine (median 0.04 and 0.03 respectively).
Figure 4.
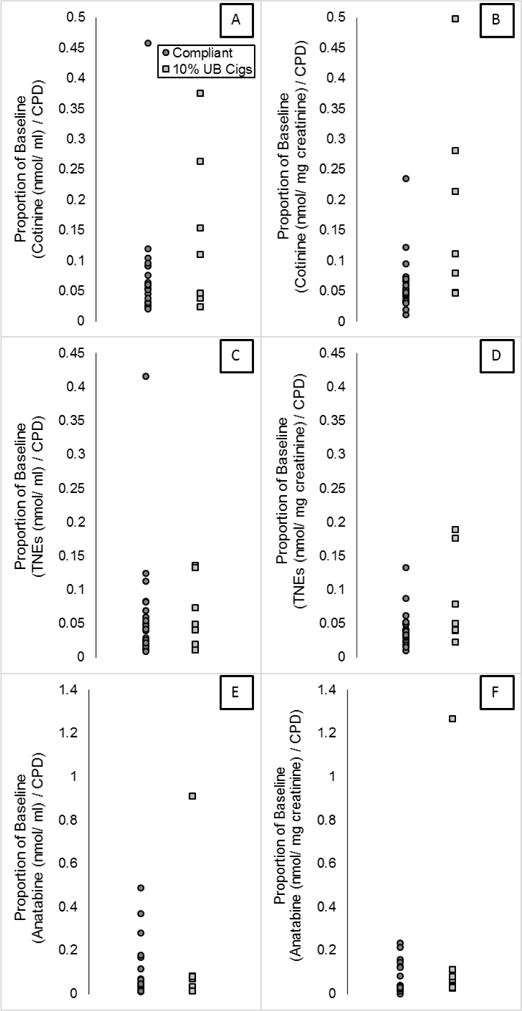
Biomarker concentration expressed per CPD and as a proportion of baseline concentrations per CPD.
The ratio of the urinary concentrations of each biomarker per CPD was calculated for the Day 1 (baseline collection prior to hotel entry) and Day 5 first void samples and the ratio of these ratios (referred to as proportion of baseline) was plotted for each participant.
DISCUSSION
The results of the present study are the first to characterize biomarkers of nicotine and other alkaloid exposure in smokers who are known to be exclusively using VLNC cigarettes. Urinary concentrations of total cotinine, TNE, and anatabine decreased sharply over the first 2 days of VLNC use, and remained low with continued use (Figure 2). The average within-subject reduction for each biomarker was very high (92%, 94%, and 93% for total cotinine, TNE, and anatabine, respectively) and approached the relative reduction of nicotine and anatabine contents in the Spectrum product (98% and 95%, respectively), suggesting that smokers who exclusively use VLNC cigarettes are likely to experience a reduction in nicotine exposure similar to the reduction in nicotine content from their usual brand cigarettes.
Data from the final sample were used to generate population estimates of the 80th, 90th, and 95th percentile for each of the 3 biomarkers (Table 1). These estimates can be used in future projects to assess (and potentially incentivize) compliance with instructions to avoid use of other (non-VLNC) cigarettes by verifying that participants displayed the expected reduction in one or more biomarkers of nicotine exposure. These estimates may also be useful for interpreting previously collected data from projects where participants were instructed to exclusively use VLNC cigarettes and biomarker data were collected.
The population estimates presented in Table 1 are highly sensitive to exclusive use of VLNC cigarettes in that almost all participants in the Compliant group would have met those criteria by the end of the study. However, any criteria used by researchers for identifying compliance is likely to lack specificity for excluding participants who are partially non-compliant. Indeed, there is substantial overlap in biomarkers between the Compliant group and the 10% Usual Brand group (Figure 3). However, Figure 3 shows there is little overlap in biomarkers between baseline and Day 5 levels in the Compliant group, suggesting that researchers will be able to determine when participants have a high level of non-compliance (Figure 3). No participants smoking 5 or more cigarettes per day would meet the 80th, 90th, or 95th percentile criteria for total cotinine or TNE at baseline (100% usual brand use) (Table 2). In practice, the population of non-compliant participants will be a mixture of fully non-compliant participants and partially non-compliant participants, which will include intermediate levels of partial non-compliance not evaluated in this study. Furthermore, this mixture may be study-dependent. Fully characterizing biomarker values in partially non-compliant participants is the subject of ongoing research.
There are at least 3 sources that may contribute to the slightly smaller reduction in biomarkers (92-94%) as compared to the reduction in nicotine (98%) and anatabine (95%) within the product. First, participants used the VLNC cigarettes for approximately 96 hours, but some metabolites from usual brand cigarette use prior to entering the hotel may not have been entirely metabolized and excreted. For example, cotinine’s half-life is between 16-19 hours,23 so after 96 hours, a smoker who metabolizes cotinine more slowly may still excrete about 3% of their baseline cotinine left in their system. For those participants who were heavy smokers, the cotinine derived from nicotine in their usual brand cigarettes could account for a substantial proportion of the cotinine excreted at the end of the study. Consistent with this interpretation, cotinine and TNE (nmol/ml) on Day 5 were significantly lower than on Day 4. Also consistent with this interpretation, the 2 individuals with the highest anatabine levels at Day 5 had TNE at baseline of 248 and 185 nmol/ml, and had the highest levels of anatabine at baseline (Appendix Table A2). If participants had used VLNC cigarettes exclusively for a longer period of time (ie greater than 96 hours), their cotinine, TNE, and anatabine levels would likely have continued to decrease. Second, participants increased the number of cigarettes they smoked per day while in the hotel, likely as a result of the lack of smoking restrictions in the hotel and having cigarettes provided for free. The increase in number of cigarettes smoked emphasizes the importance of maintaining or strengthening current tobacco control policies, such as smoke-free air policies and cigarette taxation. Third, it is possible that participants smoked the VLNC cigarettes differently than they smoked their usual brand cigarettes, increasing the bioavailability of nicotine and anatabine. However, the median biomarker per cigarette decreased by 95-98% while smoking the Spectrum cigarettes (Figure 4), and any discrepancy between this reduction and the reduction in nicotine or anatabine content could easily be accounted for by remaining biomarker from usual brand prior to entering the hotel (see above).
The present study reported anatabine levels so that studies focusing on combined use of VLNC cigarettes and non-combustible nicotine products (eg, nicotine replacement therapy or e-cigarettes that have very little or no anatabine24) could also assess compliance. Anatabine is a minor alkaloid found in tobacco products. Anatabine levels in Spectrum cigarettes are also markedly reduced compared to conventional cigarettes.5,25 Therefore, if a participant is compliant with smoking VLNC cigarettes while wearing a nicotine patch, levels of anatabine are expected to decrease while urinary cotinine and nicotine metabolites may not.26 However, the baseline and final sample distributions for anatabine appeared to overlap more than for other biomarkers. Relatively little is known about the half-life or metabolism of anatabine, especially at these low levels, and a long half-life might contribute to greater overlap between baseline and the final first void sample for anatabine. Regardless of the cause, criteria for assessing and incentivizing compliance using anatabine are likely to include smokers who are using a higher percentage of usual brand cigarettes than assessing and incentivizing compliance using cotinine or TNE. For example, a small percentage of participants in the Compliant group would have met criteria for anatabine at baseline when the data were expressed both with and without creatinine output (Table 2).
An alternative approach for assessing compliance is to express biomarkers as a ratio of biomarker concentrations per usual brand CPD and to the biomarker concentrations per VLNC CPD (Figure 4). Expressing the data in this manner did not appear to increase discrimination between compliance and non-compliance. The degree to which the biomarker distributions of the Compliant group and 10% Usual Brand group biomarkers distributions overlap appears similar whether or not the data are expressed per cigarette per day (Figure 3 vs. Figure 4). However, in individuals who smoke a very high number of VLNC cigarettes per day, an adjustment for CPD will improve sensitivity of any cutoff. This adjustment is the same approach taken by Benowitz and colleagues,6 in which a cutoff for cotinine and TNE below 0.2 is conservatively suggested for estimating compliance. This cutoff is overly conservative in that it allows for additional sources of variance including variability in usual brand nicotine content, variability in biomarker metabolism, and up to a 4-fold increase in bioavailability of nicotine. The results shown in Figure 4 confirm that a cutoff of 0.2 is conservative.
The primary strength of the paper is the hotel-based protocol in which access to non-study tobacco products was restricted. Some limitations should be noted. First, the 10% Usual Brand group only had 7 participants, so data analyses were limited and results from this group should be interpreted with caution. Second, the results of the present study are only applicable to Spectrum Cigarettes with 0.4 mg/g nicotine. Other VLNC cigarettes will differ in the content of nicotine and other alkaloids. Additionally, other product design characteristics (eg, flavor, tar yield, ventilation) could possibly affect smoking behavior and nicotine bioavailability. Such differences will affect biomarkers of exposure. Finally, over half of participants identified as African American, which could influence the results of our study. African Americans have a higher prevalence of variant CYP2A6 alleles than do whites. CYP2A6 codes for the enzyme that metabolizes nicotine to cotinine and cotinine to 3HC, and reduced CYP2A6 activity results in higher cotinine concentrations for any given level of daily nicotine intake.27 African Americans also have low cotinine glucuronidation due to the prevalence of a splice variant in UGT2B10 that catalyzes this reacation,17 and smokers who carry UGT2B10 alleles that code for non-functional enzyme have higher levels of circulating cotinine.28 Thus, the absolute concentrations of cotinine while smoking VLNC in our participants would be higher than those seen for a population with a more typical percentage of African American smokers. This likely makes our cotinine estimates conservative. This limitation does not apply when TNE is used or when within-subject changes in cotinine normalized for CPD are used as cut points.
IMPLICATIONS FOR TOBACCO REGULATION
The present paper is the first to characterize biomarkers of nicotine exposure in participants known to be exclusively using VLNC cigarettes. Participants’ TNE levels were approximately 92% lower than their baseline TNE levels after 96 hours of smoking only VLNC cigarettes. Previous literature has suggested that large decreases in nicotine exposure could lead to changes in smoking behavior, such as increases in smoking cessation attempts, which may ultimately lead to improved public health outcomes.29–31 Prior studies assessing VLNC cigarette use have reported smaller percentage decreases in cotinine or TNE levels indicating that participants in those studies were likely using other nicotine or tobacco products in part because access to alternative tobacco products was widely available to them.2,5,6 These data are the first to characterize the large reduction in nicotine exposure that might result from a regulation of the nicotine content in cigarettes when smokers do not have access to normal nicotine content cigarettes.
Additionally, the data presented here will be valuable to investigators conducting research on the use of reduced nicotine cigarettes. The criteria developed in Table 1 can be used to incentivize compliance or to retroactively exclude participants from trials who are unlikely to have been compliant. The ability to assess whether or not participants are compliant to smoking VLNC cigarettes during research studies is critical for tobacco regulatory scientists. Non-compliance within a study may lead researchers to underestimate the potential public health benefits of a nicotine reduction policy while simultaneously underestimating any potential unintended consequences of reduced nicotine exposure. By increasing product compliance during a study and/or analyzing data based on product compliance, researchers and regulators may gain a better understanding of how a potential nicotine reduction policy would impact smoking behavior with the ultimate goal of improving public health outcomes.
Acknowledgments
Thank you to all staff that was involved in conducting this study including: Erin Goldstein, Cathy Scott, Lee Bennett, Kristin Yahner, John Mahalchak, Jamie Pearson, and Ravi Choudhuri. Thank you to Kwangsoo Choi and Nicole Thomson for performing all of the LC/MS/MS analyses of biomarkers in the Analytical Biochemistry Core of the University of Minnesota Cancer Center. Research reported in this publication was supported by the National Institute on Drug Abuse and FDA Center for Tobacco Products (CTP) (U54 DA031659). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or the Food and Drug Administration. LC/MS/MS analyses were supported in part by the NIH CA77598. Tracy Smith was supported by the National Cancer Institute (T32 CA186783).
Table A1.
Demographic characteristics for all participants.
| Compliant Group | |||||
|---|---|---|---|---|---|
| Participant | Gender | Race | CPD | Usual Brand | Menthol Assignment |
| 1 | M | White | 20 | Marlboro Lights Gold | Non-Menthol |
| 2 | F | White | 18 | Newport 100s | Non-Menthol |
| 3 | M | Hispanic | 21 | Pall Mall Green Box | Menthol |
| 4 | F | Black | 12 | Salem Menthol Green | Menthol |
| 5 | F | Black | 14 | Newport 100s | Menthol |
| 6 | F | White | 28 | Pall Mall Black | Menthol |
| 7 | M | White | 15 | Pall Mall Red 100s | Non-Menthol |
| 8 | F | Black | 12 | Newport 100s | Menthol |
| 9 | F | White | 21 | Camel Crush | Menthol |
| 10 | M | White | 23 | Pall Mall Red 100s | Non-Menthol |
| 11 | F | White | 32 | Basic light Kings Gold Pack | Menthol |
| 12 | M | Multi/Unspecified | 24 | Winston Full Flavor 100s | Non-Menthol |
| 13 | F | American Native | 40 | Newport 100s | Menthol |
| 14 | F | American Indian/Alaska Native | 35 | Maverick 100s | Non-Menthol |
| 15 | F | Black | 11 | Newport Kings | Menthol |
| 16 | F | Black | 21 | Newport Kings | Menthol |
| 17 | M | Black | 21 | Newport Kings | Menthol |
| 18 | F | Black | 17 | Newport 100s | Menthol |
| 19 | M | Multi/Unspecified | 9 | Maverick 100s | Menthol |
| 20 | M | Black | 18 | Newport Kings | Menthol |
| 21 | F | Black | 11 | Waves 100s Light | Non-Menthol |
| 22 | M | Black | 10 | Waves Regular 100s | Menthol |
| 23 | F | White | 29 | Winston Kings | Non-Menthol |
| Average | 20.01 | ||||
| StDev | 8.34 | ||||
| *24 | F | Black | 14 | Newport 100s | Menthol |
| 10% Usual Brand Group | |||||
| 25 | F | Black | 7 | Newport 100s | Menthol |
| 26 | M | Black | 10 | Newport 100s | Menthol |
| 27 | M | White | 17 | Marlboro Reds Kings | Non-Menthol |
| 28 | M | Black | 6 | Newport 100s Smooth Select | Menthol |
| 29 | M | Black | 18 | Newport 100s | Menthol |
| 30 | M | Black | 11 | Salem 100s Green Box | Menthol |
| 31 | M | Black | 16 | Newport 100s | Menthol |
| Average | 12.14 | ||||
| StDev | 4.88 | ||||
Participant was excluded due to non-study tobacco use.
Table A2.
Biomarker data for each participant from the first and final first urine void samples*, total cigarettes smoked while at the hotel, and cotinine concentrations in saliva and plasma.
| Pt | Creatinine | NIC | COT | TNE | ANT | ANB | Creatinine | NIC | COT | TNE | ANT | ANB | Total Hotel Cigarettes |
Cotinine (Saliva) |
Cotinine (Plasma) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline First Void Sample (nmol/ml) | Final First Void Sample (nmol/ml) | (ng/ml) | |||||||||||||
| Compliant Group | |||||||||||||||
| 1 | 2.29 | 50.9 | 52.9 | 248.3 | 0.612 | 0.581 | 1.77 | 0.83 | 1.77 | 5.12 | 0.009 | 0.016 | 84 | 18.9 | 11.2 |
| 2 | 0.11 | 0.94 | 3.56 | 6.75 | 0.006 | 0.007 | 1.10 | 0.41 | 2.00 | 3.43 | 0.002 | 0.006 | 88 | 18.3 | 11.6 |
| 3 | 0.33 | 6.03 | 13.7 | 25.3 | 0.014 | 0.021 | 0.76 | 0.81 | 2.13 | 4.25 | 0.008 | 0.011 | 125 | 35.1 | 26.3 |
| 4 | 0.27 | 12.3 | 5.27 | 32.5 | 0.061 | 0.020 | 0.58 | 0.38 | 0.24 | 1.25 | 0.005 | 0.005 | 89 | 6.0 | 5.3 |
| 5 | 1.49 | 6.20 | 13.3 | 37.3 | 0.022 | 0.027 | 1.47 | 0.39 | 1.28 | 2.81 | 0.001 | 0.005 | 84 | 15.8 | 12.7 |
| 6 | 1.25 | 4.12 | 14.6 | 59.6 | 0.021 | 0.035 | 1.67 | 0.20 | 1.03 | 3.21 | 0.001 | 0.003 | 87 | 11.6 | 6.1 |
| 7 | 1.64 | 12.4 | 18.6 | 99.2 | 0.141 | 0.125 | 0.77 | 0.15 | 0.63 | 1.61 | 0.002 | 0.005 | 92 | 17.2 | MSSING |
| 8 | 1.26 | 5.59 | 11.2 | 31.6 | 0.018 | 0.022 | 1.47 | 0.43 | 1.35 | 2.85 | 0.001 | 0.005 | 108 | 68.4 | 74.0 |
| 9 | 1.23 | 8.72 | 23.0 | 72.0 | 0.071 | 0.087 | 1.28 | 0.26 | 0.96 | 2.35 | 0.001 | 0.004 | 65 | 8.6 | MISSING |
| 10 | 0.46 | 3.77 | 11.9 | 33.6 | 0.027 | 0.029 | 0.94 | 0.51 | 1.42 | 3.64 | 0.006 | 0.012 | 120 | 25.0 | 17.3 |
| 11 | 0.49 | 8.02 | 13.6 | 37.2 | 0.019 | 0.016 | 0.38 | 0.45 | 0.43 | 1.34 | 0.003 | 0.003 | 159 | 13.8 | 9.0 |
| 12 | 0.12 | 47.7 | 29.8 | 217 | 0.091 | 0.049 | 1.27 | 0.41 | 0.96 | 3.72 | 0.002 | 0.004 | 127 | 9.8 | 8.6 |
| 13 | 0.70 | 24.6 | 20.0 | 59.8 | 0.103 | 0.070 | 0.94 | 0.30 | 0.80 | 4.34 | 0.004 | 0.008 | 141 | 26.4 | 14.7 |
| 14 | 0.57 | 30.6 | 16.8 | 74.3 | 0.116 | 0.091 | 0.31 | 0.86 | 0.98 | 3.97 | 0.009 | 0.011 | 158 | 48.0 | 36.7 |
| 15 | 2.39 | 32.2 | 34.1 | 185 | 0.296 | 0.244 | 1.27 | 1.10 | 2.96 | 9.02 | 0.012 | 0.017 | 135 | 50.3 | 29.6 |
| 16 | 1.18 | 2.11 | 3.26 | 8.63 | 0.020 | 0.020 | 0.34 | 0.22 | 0.40 | 1.50 | 0.002 | 0.004 | 146 | 24.8 | 23.4 |
| 17 | 0.39 | 1.88 | 8.90 | 21.0 | 0.008 | 0.021 | 1.55 | 0.77 | 1.70 | 4.19 | 0.006 | 0.013 | 134 | 14.4 | 10.4 |
| 18 | 0.73 | 14.0 | 21.1 | 63.0 | 0.163 | 0.078 | 0.47 | 0.77 | 1.38 | 3.32 | 0.007 | 0.007 | 144 | 37.5 | 25.3 |
| 19 | 0.43 | 6.13 | 7.00 | 19.8 | 0.023 | 0.025 | 1.66 | 0.51 | 1.57 | 3.47 | 0.003 | 0.005 | 106 | 30.3 | 13.9 |
| 20 | 2.78 | 1.42 | 14.7 | 66.9 | 0.012 | 0.030 | 2.32 | 0.46 | 1.35 | 4.29 | 0.003 | 0.008 | 112 | 17.6 | 11.0 |
| 21 | 0.99 | 24.6 | 9.98 | 47.1 | 0.082 | 0.046 | 0.50 | 0.57 | 1.84 | 3.70 | 0.003 | 0.006 | 133 | 67.1 | 46.8 |
| 22 | 1.00 | 8.02 | 11.0 | 63.2 | 0.055 | 0.047 | 0.63 | 0.17 | 0.70 | 2.24 | 0.002 | 0.003 | 67 | 35.2 | 16.5 |
| 23 | 2.39 | 31.4 | 27.9 | 99.0 | 0.143 | 0.167 | 0.98 | 0.44 | 2.60 | 5.21 | 0.002 | 0.009 | 112 | 39.7 | 23.0 |
| GM | 0.77 | 8.83 | 13.7 | 48.8 | 0.046 | 0.045 | 0.92 | 0.43 | 1.13 | 3.17 | 0.003 | 0.007 | 113.74 | 22.9 | 16.5 |
| *24 | 2.23 | 22.1 | 14.0 | 132 | 0.155 | 0.093 | 0.37 | 8.02 | 1.21 | 11.7 | 0.025 | 0.012 | 136 | 57.3 | 169 |
| 10% Usual Brand Group | |||||||||||||||
| 25 | 2.25 | 15.0 | 33.2 | 124 | 0.101 | 0.162 | 1.08 | 0.45 | 4.72 | 8.72 | 0.005 | 0.018 | 104 | 117 | 76.1 |
| 26 | 2.25 | 17.0 | 12.4 | 72.3 | 0.066 | 0.060 | 2.11 | 0.76 | 5.48 | 8.96 | 0.004 | 0.009 | 67 | 172 | 114 |
| 27 | 1.55 | 6.51 | 25.1 | 95.4 | 0.017 | 0.022 | 1.12 | 7.45 | 6.53 | 21.9 | 0.026 | 0.021 | 115 | 75.2 | 68.2 |
| 28 | 2.48 | 30.9 | 20.0 | 111 | 0.111 | 0.066 | 1.24 | 0.68 | 1.49 | 3.96 | 0.005 | 0.007 | 75 | 48.1 | 36.7 |
| 29 | 1.90 | 62.3 | 17.5 | 97.7 | 0.163 | MISSING | 1.43 | 2.30 | 4.38 | 8.65 | 0.009 | 0.015 | 48 | 49.3 | 53.2 |
| 30 | 0.87 | 3.38 | 8.59 | 30.5 | 0.018 | 0.026 | 0.88 | 0.26 | 0.55 | 1.68 | 0.002 | 0.002 | 60 | 15.7 | 13.2 |
| 31 | 1.01 | 7.66 | 12.06 | 60.18 | 0.039 | 0.036 | 1.00 | 0.91 | 2.13 | 4.77 | 0.005 | 0.009 | 103 | 45.9 | 63.9 |
| GM | 1.64 | 13.5 | 16.8 | 77.6 | 0.055 | 0.049 | 1.22 | 0.99 | 2.78 | 6.46 | 0.006 | 0.010 | 81.71 | 59.8 | 51.6 |
NIC: total nicotine, COT: total cotinine, ANT: anatabine, ANB: anabasine, GM=Mean for total hotel cigarettes, Geometric Mean for all other values
Participant was excluded due to non-study tobacco use.
Table A3.
Biomarker data for each participant from the final sample, collected between 8am and 12pm on Day 5 at the hotel.
| Pt | Creatinine | Nicotine | Cotinine | TNE | Anatabine | Anabasine |
|---|---|---|---|---|---|---|
| Final Sample (nmol/ml) | ||||||
| Compliant Group | ||||||
| 1 | 0.47 | 0.80 | 0.87 | 2.70 | 0.008 | 0.011 |
| 2 | 0.04 | 0.23 | 0.33 | 0.73 | 0.001 | 0.002 |
| 3 | 0.50 | 0.64 | 1.42 | 2.85 | 0.009 | 0.010 |
| 4 | 0.22 | 0.16 | 0.11 | 0.55 | 0.001 | 0.001 |
| 5 | 0.71 | 0.83 | 0.78 | 2.28 | 0.007 | 0.008 |
| 6 | 0.16 | 0.17 | 0.08 | 0.41 | ND | ND |
| 7 | 0.42 | 0.29 | 0.44 | 1.34 | 0.003 | 0.004 |
| 8 | 0.56 | 0.33 | 2.06 | 4.23 | 0.001 | 0.005 |
| 9 | 0.66 | 0.64 | 0.98 | 2.98 | 0.008 | 0.012 |
| 10 | 0.25 | 0.38 | 1.37 | 3.46 | 0.003 | 0.006 |
| 11 | 0.09 | 0.28 | 0.16 | 0.62 | 0.001 | 0.001 |
| 12 | 1.18 | 0.80 | 0.67 | 3.03 | 0.003 | 0.003 |
| 13 | 2.29 | 2.40 | 3.68 | 9.18 | 0.022 | 0.020 |
| 14 | 0.44 | 0.90 | 1.11 | 4.42 | 0.010 | 0.012 |
| 15 | 0.23 | 0.42 | 0.75 | 2.05 | 0.004 | 0.004 |
| 16 | 1.14 | 1.02 | 0.64 | 4.62 | 0.012 | 0.017 |
| 17 | 1.22 | 0.60 | 1.15 | 2.94 | 0.006 | 0.009 |
| 18 | 0.58 | 0.74 | 1.49 | 3.75 | 0.008 | 0.009 |
| 19 | 0.71 | 0.56 | 0.70 | 1.86 | 0.005 | 0.005 |
| 20 | 1.33 | 0.31 | 0.80 | 2.51 | 0.003 | 0.004 |
| 21 | 0.22 | 1.34 | 1.81 | 4.52 | 0.010 | 0.011 |
| 22 | 1.15 | 0.25 | 0.69 | 3.09 | 0.003 | 0.004 |
| 23 | 0.24 | 0.22 | 0.88 | 1.66 | 0.002 | 0.004 |
| GM | 0.45 | 0.49 | 0.73 | 2.26 | 0.004 | 0.005 |
| *24 | 0.39 | 5.83 | 3.15 | 14.44 | 0.026 | 0.017 |
| 10% Usual Brand Group | ||||||
| 25 | 2.25 | 0.74 | 6.00 | 14.4 | 0.010 | 0.028 |
| 26 | 2.07 | 2.29 | 5.49 | 10.6 | 0.013 | 0.015 |
| 27 | 0.63 | 4.40 | 3.56 | 11.8 | 0.022 | 0.015 |
| 28 | 1.01 | 2.68 | 1.44 | 5.78 | 0.010 | 0.006 |
| 29 | 1.29 | 3.03 | 4.02 | 10.2 | 0.012 | 0.010 |
| 30 | 1.31 | 3.92 | 0.70 | 6.18 | 0.011 | 0.004 |
| 31 | 0.95 | 1.35 | 2.25 | 5.44 | 0.009 | 0.011 |
| GM | 1.25 | 2.28 | 2.71 | 8.64 | 0.012 | 0.011 |
NIC: total nicotine, COT: total cotinine, ANT: anatabine, ANB: anabasine, ND= Not detected below limit of quantitation (0.0009 for anatabine and 0.001 for anabasine), GM= Mean for total hotel cigarettes, Geometric Mean for all other values
Participant was excluded due to non-study tobacco use.
Table A4.
Table of p values for biomarker comparisons.
| Cotinine (nmol/ml) | Cotinine
(nmol/mg creatinine) |
TNE (nmol/ml) | TNE (nmol/mg creatinine) |
Anatabine (nmol/ml) |
Anatabine (nmol/mg creatinine) |
|
|---|---|---|---|---|---|---|
| Omnibus test (p < 0.001) | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| Baseline vs. Day 1 8pm | 0.665 | 0.757 | 0.404 | 0.304 | ||
| Baseline vs. Day 2 FV | 0.011 | <0.001 | 0.001 | <0.001 | ||
| Baseline vs. Day 2 8am | 0.012 | 0.001 | 0.006 | <0.001 | ||
| Baseline vs. Day 2 12pm | <0.001 | <0.001 | <0.001 | <0.001 | ||
| Baseline vs. Day 2 4 pm | <0.001 | <0.001 | <0.001 | <0.001 | ||
| Baseline vs. Day 2 8 pm | <0.001 | <0.001 | <0.001 | <0.001 | ||
| Baseline vs. Day 3 FV | <0.001 | <0.001 | <0.001 | <0.001 | ||
| Baseline vs. Day 3 8am | <0.001 | <0.001 | <0.001 | <0.001 | ||
| Baseline vs. Day 3 12pm | <0.001 | <0.001 | <0.001 | <0.001 | ||
| Baseline vs. Day 3 4 pm | <0.001 | <0.001 | <0.001 | <0.001 | ||
| Baseline vs. Day 3 8pm | <0.001 | <0.001 | <0.001 | <0.001 | ||
| Baseline vs. Day 4 FV | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| Baseline vs. Day 4 8am | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| Baseline vs. Day 4 12pm | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| Baseline vs. Day 4 4pm | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| Baseline vs. Day 4 8pm | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| Baseline vs. Day 5 FV | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| Baseline vs. Day 5 8am | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| Baseline vs. Day 5 12pm | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| Day 4 FV vs. Day 5 FV | <0.001 | <0.001 | <0.001 | <0.001 | 0.109 | 0.307 |
Footnotes
Portions of these data were also presented as part of a poster at the annual meeting for the Society for Nicotine and Tobacco Research in Philadelphia, PA on February 26th, 2015.
Human Subjects Statement
The protocol was reviewed by the FDA’s Center for Tobacco Products for the use of the described investigational tobacco products, and was approved by the University of Pittsburgh Institutional Review Board (PRO14040384).
Conflict of Interest Statement
Dr. Neal Benowitz serves as a consultant to several pharmaceutical companies that market smoking cessation medications and has served as a paid expert witness in litigation against tobacco companies. The other authors do not have any conflicts to report.
Contributor Information
Rachel L. Denlinger, Project Manager, Department of Psychology, University of Pittsburgh, Pittsburgh, PA.
Tracy T. Smith, Graduate Student, Department of Psychology, University of Pittsburgh, Pittsburgh, PA.
Sharon E. Murphy, Professor, Department of Biochemistry, Molecular Biology, and Biophysics, University of Minnesota, Minneapolis, MN.
Joseph S. Koopmeiners, Assistant Professor, Division of Biostatistics, School of Public Health, University of Minnesota, Minneapolis, MN.
Neal L. Benowitz, Professor, Departments of Medicine and Bioengineering & Therapeutic Sciences, University of California San Francisco, San Francisco, CA.
Dorothy K. Hatsukami, Professor, Department of Psychiatry, University of Minnesota, Minneapolis, MN.
Lauren R. Pacek, Lauren R. Pacek, Postdoctoral Fellow, Department of Psychiatry and Behavioral Sciences, Johns Hopkins University School of Medicine, Baltimore, MD.
Cirielle Colino, Cirielle Colino, Study Coordinator, Department of Psychiatry and Behavioral Sciences, Johns Hopkins University School of Medicine, Baltimore, MD.
Samantha N. Cwalina, Undergraduate, Department of Psychology, University of Pittsburgh, Pittsburgh, PA.
Eric C. Donny, Professor, Department of Psychology, University of Pittsburgh, Pittsburgh, PA.
References
- 1.Family Smoking Prevention and Tobacco Control Act (H.R. 1256) Available at https://www.govtrack.us/congress/bills/111/hr1256/text. Accessed 23 February 2016.
- 2.Benowitz NL, Dains KM, Hall SM, et al. Smoking behavior and exposure to tobacco toxicants during 6 months of smoking progressively reduced nicotine content cigarettes. Cancer Epidemiol Biomarkers Prev. 2012;21(5):761–769. doi: 10.1158/1055-9965.EPI-11-0644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hatsukami DK, Kotlyar M, Hertsgaard LA, et al. Reduced nicotine content cigarettes: effects on toxicant exposure, dependence and cessation. Addiction. 2010;105(2):343–355. doi: 10.1111/j.1360-0443.2009.02780.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hatsukami DK, Heishman SJ, Vogel RI, et al. Dose-response effects of spectrum research cigarettes. Nicotine Tob Res. 2013;15(6):1113–1121. doi: 10.1093/ntr/nts247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Donny EC, Denlinger RL, Tidey JW, et al. Randomized Trial of Reduced-Nicotine Standards for Cigarettes. N Engl J Med. 2015;373(14):1340–1349. doi: 10.1056/NEJMsa1502403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benowitz NL, Nardone N, Hatsukami DK, Donny EC. Biochemical estimation of noncompliance with smoking of very low nicotine content cigarettes. Cancer Epidemiol Biomarkers Prev. 2015;24(2):331–335. doi: 10.1158/1055-9965.EPI-14-1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dermody SS, Donny EC, Hertsgaard LA, Hatsukami DK. Greater reductions in nicotine exposure while smoking very low nicotine content cigarettes predict smoking cessation. Tob Control. 2015;24(6):536–539. doi: 10.1136/tobaccocontrol-2014-051797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Henningfield JE. More on the nicotine content of vegetables. N Engl J Med. 1993;329(21):1581–1582. doi: 10.1056/NEJM199311183292118. [DOI] [PubMed] [Google Scholar]
- 9.The Freedom of Information Act 5 U.S.C §552. Available at http://www.justice.gov/oip/blog/foia-update-freedom-information-act-5-usc-sect-552-amended-public-law-no-104-231-110-stat. Accessed 23 February 2016.
- 10.Lisko JG, Stanfill SB, Duncan BW, Watson CH. Application of GC-MS/MS for the analysis of tobacco alkaloids in cigarette filler and various tobacco species. Anal Chem. 2013;85(6):3380–3384. doi: 10.1021/ac400077e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addict. 1991;86(9):1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- 12.Smith SS, Piper ME, Bolt DM, et al. Development of the Brief Wisconsin Inventory of Smoking Dependence Motives. Nicotine Tob Res. 2010;12(5):489–499. doi: 10.1093/ntr/ntq032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hughes JR, Hatsukami D. Signs and symptoms of tobacco withdrawal. Arch Gen Psychiatry. 1986;43(3):289–294. doi: 10.1001/archpsyc.1986.01800030107013. [DOI] [PubMed] [Google Scholar]
- 14.Cox LS, Tiffany ST, Christen AG. Evaluation of the brief questionnaire of smoking urges (QSU-brief) in laboratory and clinical settings. Nicotine Tob Res. 2001;3(1):7–16. doi: 10.1080/14622200020032051. [DOI] [PubMed] [Google Scholar]
- 15.Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1(3):385–401. [Google Scholar]
- 16.Crawford JR, Henry JD. The positive and negative affect schedule (PANAS): construct validity, measurement properties and normative data in a large non-clinical sample. Br J Clin Psychol. 2004;43(Pt 3):245–265. doi: 10.1348/0144665031752934. [DOI] [PubMed] [Google Scholar]
- 17.Murphy SE, Park SS, Thompson EF, et al. Nicotine N-glucuronidation relative to N-oxidation and C-oxidation and UGT2B10 genotype in five ethnic/racial groups. Carcinogenesis. 2014;35(11):2526–2533. doi: 10.1093/carcin/bgu191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.von Weymarn L, Thomson NM, Donny EC, et al. Quantitation of the minor tobacco alkaloids nornicotine, anatabine, and anabasine in smokers’ urine by high throughput liquid chromatography mass spectrometry. Chem Res Toxicol. 2016 doi: 10.1021/acs.chemrestox.5b00521. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murphy SE, Wickham KM, Lindgren BR, et al. Cotinine and trans 3’-hydroxycotinine in dried blood spots as biomarkers of tobacco exposure and nicotine metabolism. J Expo Sci Environ Epidemiol. 2013;23(5):513–518. doi: 10.1038/jes.2013.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Venables WN, Ripley BD. Modern Applied Statistics with S. 4th. New York: Springer; 2002. [Google Scholar]
- 21.Team RC. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2013. [Google Scholar]
- 22.Efron B, Tibshirani RJ. Introduction to Bootstrap. Boca Raton, FL: Chapman and Hall/CRC Press; 1993. [Google Scholar]
- 23.Jarvis MJ, Russell MA, Benowitz NL, Feyerabend C. Elimination of cotinine from body fluids: implications for noninvasive measurement of tobacco smoke exposure. Am J Public Health. 1988;78(6):696–698. doi: 10.2105/ajph.78.6.696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cheng T. Chemical evaluation of electronic cigarettes. Tob Control. 2014;23(Suppl 2):ii11–17. doi: 10.1136/tobaccocontrol-2013-051482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu W, Ashley DL, Watson CH. Determination of nicotine and other minor alkaloids in international cigarettes by solid-phase microextraction and gas chromatography/mass spectrometry. Anal Chem. 2002;74(19):4878–4884. doi: 10.1021/ac020291p. [DOI] [PubMed] [Google Scholar]
- 26.Jacob P, 3rd, Hatsukami D, Severson H, Hall S, Yu L, Benowitz NL. Anabasine and anatabine as biomarkers for tobacco use during nicotine replacement therapy. Cancer Epidemiol Biomarkers Prev. 2002;11(12):1668–1673. [PubMed] [Google Scholar]
- 27.Zhu AZ, Renner CC, Hatsukami DK, et al. The ability of plasma cotinine to predict nicotine and carcinogen exposure is altered by differences in CYP2A6: the influence of genetics, race, and sex. Cancer Epidemiol Biomarkers Prev. 2013;22(4):708–718. doi: 10.1158/1055-9965.EPI-12-1234-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Berg JZ, von Weymarn LB, Thompson EA, et al. UGT2B10 genotype influences nicotine glucuronidation, oxidation, and consumption. Cancer Epidemiol Biomarkers Prev. 2010;19(6):1423–1431. doi: 10.1158/1055-9965.EPI-09-0959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Benowitz NL, Henningfield JE. Establishing a nicotine threshold for addiction. The implications for tobacco regulation. N Engl J Med. 1994;331(2):123–125. doi: 10.1056/NEJM199407143310212. [DOI] [PubMed] [Google Scholar]
- 30.Benowitz NL, Henningfield JE. Reducing the nicotine content to make cigarettes less addictive. Tob Control. 2013;22(Suppl 1):i14–17. doi: 10.1136/tobaccocontrol-2012-050860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Donny EC, Hatsukami DK, Benowitz NL, et al. Reduced nicotine product standards for combustible tobacco: building an empirical basis for effective regulation. Prev Med. 2014;68:17–22. doi: 10.1016/j.ypmed.2014.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]


