Abstract
Background
Cystoisospora suis causes diarrhoeal disease and reduced weight gain in suckling piglets, and a toltrazuril-based oral suspension is available for treatment. Recently a combinatorial product with toltrazuril plus iron has been developed for parenteral application. In this study we compared the efficacy of the injectable product with the oral suspension against experimentally induced piglet cystoisosporosis.
Methods
In a randomised controlled study, three groups of piglets (n = 10–13) were treated either with a fixed dose of 45 mg toltrazuril + 200 mg gleptoferron i.m. per piglet (Forceris®) on the second day of life (study day 2; SD 2) or with 20 mg toltrazuril/kg body weight as an oral suspension (Baycox® 5%) on SD 4 or left untreated (Control group). The Baycox® and the Control group received 200 mg of iron dextran/piglet on SD 2. All piglets were infected with 1000 sporulated C. suis oocysts on SD 3. Faecal samples were taken daily from SD 7 to SD 20 to determine faecal consistency, oocyst shedding and other diarrhoeal pathogens. Body weight was recorded on SD 1 and then weekly until SD 29. Animals were observed daily for general health and after treatment for possible adverse events.
Results
In the Control group all animals shed oocysts for 3.1 days on average and all animals showed diarrhoea for an average of five days. Excretion peaked on SD 9 (max. 48,618 oocysts per gram of faeces). Treatment with Forceris® completely suppressed oocyst excretion. In the Baycox® group, low levels of excretion could be detected. Diarrhoea was reduced to single piglets in the treated groups. Body weight development was reduced in the Control group compared to the treated groups. Enteropathogenic bacteria (Escherichia coli, Clostridium perfringens) could be detected. All parameters related to oocyst excretion, faecal consistency and weight gain were significantly improved in the treated groups compared to the Control group without significant differences between the treated groups. Both products were safe to use.
Conclusions
Treatment with both the injectable (Forceris®) and the oral (Baycox®) formulation of toltrazuril in the prepatent period were safe and highly effective against experimental infection with C. suis in newborn piglets.
Keywords: Pig, Swine, Coccidiosis, Cystoisospora suis, Isospora suis, Diarrhoea, Experimental infection, Toltrazuril, Efficacy
Background
Porcine neonatal coccidiosis caused by Cystoisospora suis (syn. Isospora suis) is a major cause of diarrhoea and unthriftiness in piglets worldwide [1–6] and induces substantial economic losses in the pig breeding industry [7–10]. Currently, the only effective chemotherapeutic drug available for treatment is the triazinetrione toltrazuril [11]. It is available as a 5% suspension to be applied as a single oral treatment during the third to fifth day of life. Oral treatment with toltrazuril is highly effective in controlling oocyst excretion and diarrhoea both in experimental and natural infections of suckling piglets [12–17].
Recently an injectable combination product, toltrazuril + iron (30 mg toltrazuril/ml; 133.4 mg iron/ml as gleptoferron) has been developed for the prevention of piglet coccidiosis together with a prevention of iron deficiency (Forceris®, Ceva, Libourne, France). Treatment is scheduled from the first to the third day of life (i.e. between 24 and 96 h after birth) as a single intramuscular injection of 1.5 ml/piglet corresponding to 45 mg of toltrazuril and 200 mg of iron.
Forceris® is the first toltrazuril product to be marketed in an injectable formulation for the control of C. suis in piglets from the first day of life, and this study aimed to evaluate its efficacy against porcine coccidiosis in an experimental infection model [18] in comparison to an established reference product, an oral toltrazuril suspension (Baycox® 5%, Bayer Animal Health, Monheim, Germany).
Methods
Animals and husbandry
A total of 35 piglets from four litters were enrolled in the trial on study day (SD) 1 and finished it on SD 29. Sows (Landrace × Large White) were moved to the large animal facilities of the Institute of Parasitology of the Vetmeduni Vienna two weeks before farrowing to acclimatise to the housing conditions. Animals were kept on straw with a heat lamp for the piglets and fed with conventional feed free of coccidiostats. Water was provided ad libitum. On the first day of the study (SD 1; within 24 h after birth of the piglets) animals were marked individually and enrolled in the study if they were clinically healthy and weighed at least 900 g. They were randomly allocated to one of three groups according to body weight on SD 1 in the order of the birth of the litters. The general health of sows and their piglets was observed and recorded daily from SD 1 to SD 29.
Treatment
Animals were treated either with toltrazuril + gleptoferron (investigational product: Forceris®, Ceva, France) at a fixed dose of 1.5 ml/piglet corresponding to 45 mg of toltrazuril and 200 mg of iron, i.m. on SD 2 (Forceris® group, n =13 piglets) or with a toltrazuril suspension (reference product: Baycox® 5%, Bayer Animal Health, Monheim, Germany) at a dose of 20 mg/kg orally on SD 4 (Baycox® group, n = 12 piglets). A third group (Control group, n = 10 piglets) remained untreated. The Baycox® and the Control groups received iron dextran (Uniferon® 200, Virbac, Kolding, Denmark) at a fixed dose of 200 mg/piglet, i.m. on SD 2. Allocation to treatment groups and treatment were carried out under blinded conditions, i.e. only the dispenser was aware of the allocation of piglets to a treatment group during the course of the study. Post-treatment observations for any adverse events (swelling/bleeding of the injection site, inability to stand, walk, suckle or other abnormal behaviour, including dyspnoea, vomiting, limping, lateral recumbency, signs of pain, distress or neurological alterations, [19]) were conducted under blinded conditions by a veterinarian at 2, 6 and 24 h after treatment, and after that daily until SD 8.
Experimental infection
Each piglet was orally infected with a single dose of approximately 1000 sporulated oocysts of C. suis, strain Wien-I [15] on SD 3.
Determination of concomitant bacterial and viral infections
On the first day of faecal sampling (SD 7) pooled faecal samples from each litter (n = 4) were submitted for bacteriological and virological examination to the Institute of Microbiology and Institute of Virology, respectively, of the Vetmeduni Vienna. A general qualitative/semiquantitative bacteriological examination, followed by specific differentiation and virulence factor/toxin typing in positive cases, was conducted. Porcine rotavirus A antigen and nucleic acids of porcine coronaviruses TGEV and PRCoV were determined from the faecal material. In case of diarrhoea after treatment with toltrazuril, individual samples were taken on the first day of diarrhoea and examined for bacterial pathogens as well.
Efficacy parameters
Individual faecal samples were taken daily from SD 7 to SD 20. Efficacy of toltrazuril was evaluated by qualitative (autofluorescence; [20]) and quantitative (McMaster counting of oocysts per gram of faeces, OpG) determination of oocysts and faecal consistency as previously described [18]. Faecal consistency of each sample was scored immediately with faecal score (FS) 1 describing firm, FS 2 pasty, FS 3 semi-liquid and FS 4 liquid faeces, with FS 3 and FS 4 considered as diarrhoea. Individual body weight was recorded for each enrolled piglet on SD 1, 8, 15, 22 and 29.
To evaluate the efficacy of treatment, oocyst excretion, faecal consistency and body weight development were analysed statistically. This included the area under the curve (AUC) for quantitative oocyst excretion and FS, the number of days with oocyst excretion/diarrhoea, and the number of animals that showed excretion/diarrhoea in each group, as well as the body weight on each weighing day and body weight gain. Data were evaluated for homogeneity of the groups and analysed by means of the Kruskal-Wallis H-test (for continuous parameters) or by means of the Chi-square test (for discrete parameters). Pairwise comparisons were performed either using Fisher’s exact test (excretion/diarrhoea present or not) or Wilcoxon-Mann-Whitney U-test (other parameters). For all tests, P-value correction with Bonferroni was applied.
Results
Safety
No animal showed treatment-related adverse events that required veterinary intervention. Two animals from the Forceris® group showed a slight temporary swelling at the injection site within the first day of observation after treatment. Two animals from the Control group had to be treated with Ringer’s lactate due to dehydration.
Qualitative oocyst excretion determined by autofluorescence
All piglets of the Control group and 25% of the piglets in the Baycox® group excreted oocysts detectable by autofluorescence (AF), while none of the animals in the Forceris® group shed oocysts. The mean duration of oocyst excretion was 1.3 days in the Baycox® group and 3.1 days in the Control group (Table 1).The number of days with AF detectable excretion and the number of piglets that excreted AF detectable oocysts were significantly reduced in the Forceris® and Baycox® groups compared to the Control group without statistical difference between the treatment groups (Table 2).
Table 1.
McMaster countable oocyst excretion, autofluorescence detectable oocyst excretion and diarrhoea values per group
| Forceris® | Baycox® | Control | |
|---|---|---|---|
| No. of piglets | 13 | 12 | 10 |
| No. of samples over all sampling days | 181 | 168 | 140 |
| McMaster countable oocyst excretion | |||
| No. (%) positive piglets | 0 (0) | 2 (16.7) | 9 (90.0) |
| No. (%) excretion days | 0 (0) | 3 (1.8) | 21 (15.0) |
| Autofluorescence detectable oocyst excretion | |||
| No. (%) positive piglets | 0 (0) | 3 (25.0) | 10 (100) |
| No. (%) excretion days | 0 (0) | 4 (2.4) | 31 (22.1) |
| Diarrhoea | |||
| No. (%) positive piglets | 2 (15.4) | 4 (33.3) | 10 (100) |
| No. (%) diarrhoea days | 3 (1.7) | 6 (3.6) | 50 (35.7) |
| Liquid diarrhoea - faecal score (FS) 4 | |||
| No. (%) FS 4 piglets | 1 (7.7) | 1 (8.3) | 7 (70.0) |
| No. (%) FS 4 days | 1 (0.6) | 3 (1.8) | 22 (15.7) |
Table 2.
P-values (given as -log10) are given for the parameters of oocyst excretion and faecal score. Differences at P < 0.05 are indicated in bold
| Parameter | Forceris® vs Control | Baycox® vs Control | Forceris® vs Baycox | χ2, df |
|---|---|---|---|---|
| Area under the Curve for OpG | 3.89 | 3.19 | 0.35 | 24.87, 2 |
| No. of days with McMaster countable excretion | 3.89 | 2.70 | 0.35 | 23.17, 2 |
| McMaster countable excretion present or not | 4.43 | 2.24 | 0.18 | 23.09, 2 |
| No. of days with AF detectable excretion | 4.62 | 3.80 | 0.70 | 28.29, 2 |
| AF detectable excretion present or not | 5.52 | 2.85 | 0.54 | 25.36, 2 |
| Area under the Curve for FS | 3.72 | 4.72 | 0.00 | 20.64, 2 |
| No. of days with diarrhoea | 4.13 | 3.60 | 0.00 | 24.46, 2 |
| Diarrhoea present or not | 3.70 | 2.29 | 0.00 | 17.44, 2 |
| Daily body weight gain SD 1–29 | 1.56 | 2.59 | 0.88 | 14.59, 2 |
Abbreviations: OpG oocysts per gram of faeces, AF autofluorescence, FS faecal score, χ2 and df statistics and degrees of freedom according to Kruskal-Wallis rank sum test or Chi-square test
Quantitative oocyst excretion determined by McMaster counting
McMaster countable oocyst excretion was observed in nine out of ten piglets of the Control group, in two animals of the Baycox® group and in none of the piglets of the Forceris® group. When excretion days were evaluated, positive animals in the Control group excreted McMaster countable oocysts between one and four days (mean 2.3 days), whereas in the Baycox® group two positive animals excreted for one and two days, respectively (Table 1).
The maximum OpG value in the Control group was 48,618 OpG on SD 9 (Fig. 1); on that day, the prevalence of McMaster countable excretion also reached its peak at 50% (Fig. 2), while in the Baycox® group the OpGs did not exceed 333.
Fig. 1.
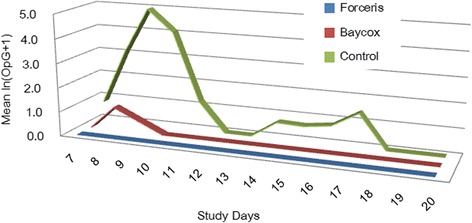
Mean oocyst excretion given as ln (OpG+1) per group during the whole study period
Fig. 2.
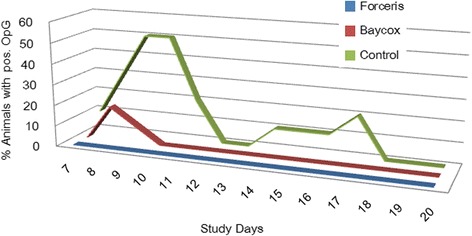
Prevalence of McMaster countable excretion
The area under the curve for OpG, the number of days with McMaster countable excretion and the number of piglets that excreted countable oocyst numbers were significantly reduced in the Forceris® and Baycox® groups compared to the Control group without statistical difference between the treatment groups (Table 2).
Faecal consistency and diarrhoea
The average faecal score increased above 2 in the Control group from SD 8 to SD 14, while in the treated groups the mean FS never exceeded 2. The maximum prevalence of diarrhoea was 100% in the Control group (SD 11 and SD 12) with an average duration of 5.0 days, while in the Baycox® group 33.3% of the animals showed diarrhoea for an average of 1.5 days, and in the Forceris® group two animals (15.2%) showed diarrhoea for one and two days, respectively. FS 4 (watery diarrhoea) was observed in 70% of the Control animals (average duration: 3.1 days) while in the Baycox® group one animal had watery diarrhoea for three days and in the Forceris® group one animal for one day (Table 1).
The peak of diarrhoea was observed on SD 11 to SD 12 when all animals in the Control group showed diarrhoea with a mean FS of 3.6 (Figs. 3 and 4). The area under the curve for the FS, the number of days with diarrhoea and the number of piglets that had diarrhoea were significantly reduced in the Forceris® and Baycox® groups compared to the Control group without statistical difference between the treatment groups (Table 2).
Fig. 3.
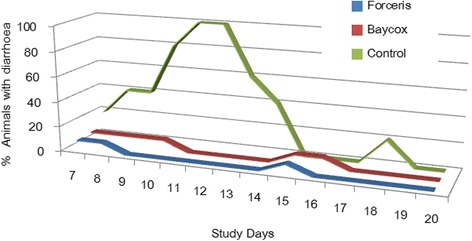
Course of diarrhoea (FS 3 and 4) in the different groups
Fig. 4.
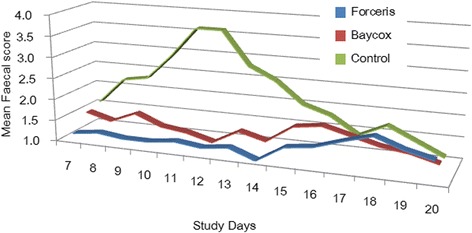
Course of the average faecal score (FS) in the different groups
Body weight development
Body weights were not significantly different between the groups on SD 1, the day of randomisation (Kruskal-Wallis H-test, χ2 = 95145, df = 2, P = 0.62). Daily body weight gain and total weight gain from SD 1 to SD 29 were lowest in the Control group due to a severe depression of weight gain in the acute phase of infection (from SD 8 to SD 15) during which the Control group only gained 975.8 g on average compared to 2008.3 g in the Baycox® group and 1976.9 g in the Forceris® group (Fig. 5).
Fig. 5.
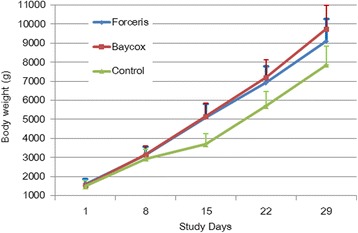
Body weight development during the study (study days 1–29). Vertical lines depict the upper standard deviations
The daily body weight gain from SD 1 to SD 29 was significantly higher in the Forceris® and Baycox® groups compared to the Control group without statistical difference between the treatment groups (Fig. 5).
Concomitant infections
All litters showed infections with a similar pattern of bacteria. Haemolytic Escherichia coli which were positive for virulence factors fimH, papC, iucD, and cnf1 and β-2 toxin positive Clostridium perfringens Type A were detected in high amounts at the beginning of sampling and were also diagnosed in those animals that showed diarrhoea after treatment. No viral infections were detected.
Discussion
Piglets experimentally infected with C. suis were treated with toltrazuril either parenterally or orally before the onset of oocyst excretion and diarrhoea. Both, Forceris® and Baycox® had a comparable effect on oocyst shedding, faecal consistency and body weight development.
Oocyst shedding was significantly suppressed in both treated groups compared to the Control group. Despite the intra-litter randomisation which entailed a high environmental contamination in all litters due to the presence of untreated control animals, no piglets of the Forceris® group excreted oocysts during the trial. This indicates that the treatment was highly effective and the level of false-positive samples due to coprophagy [21] in such an experimental setting is likely to be low. In the Baycox® group three samples were positive with one oocyst in McMaster counting each, resulting in OpG values of 333. It is difficult to unequivocally conclude that these findings are derived from true infections for the reason stated above; however, one of the two animals that excreted countable oocysts did so on two consecutive days indicating that at least this individual shed oocysts due to infection and not due to coprophagy, but data on the extent of false positive samples due to this in experimentally infected mixed litters are not available. Low levels of oocyst shedding can occur despite treatment with Baycox® [14] while in other experimentally induced infections suppression of oocyst development was complete [15]. Despite low levels of oocyst shedding the reduction of environmental recontamination by disruption of the parasite’s life-cycle was highly effective.
Diarrhoea is a hallmark of porcine cystoisosporosis [2, 3, 18, 22]; it can be induced by parasite infection in the absence of other enteropathogens [23, 24] but exacerbated by rotavirus [24] or Cl. perfringens [25, 26], and probably by other enteropathogens as well. Early studies on the use of toltrazuril in pigs indicated that C. suis has a forerunner role for bacterial infections as anticoccidial treatment reduced diarrhoea and simultaneously the amount of antibiotics required to control bacterial infections on affected farms, but a low level of diarrhoea was still seen after toltrazuril application [12]. In the light of these findings, it is conceivable that the E. coli and clostridia that were circulating in the litters (and also detected in the animals with diarrhoea after treatment) contributed to the clinical outcome in some individuals despite successful treatment of cystoisosporosis.
Body weight development and diarrhoea are inversely related [18] and consequently body weight gain was increased in treated animals (which showed less diarrhoea), with the result of higher weights around weaning on SD 29 compared to the Control group. Improved weight gain has also been shown upon treatment in C. suis-positive herds with no apparent clinical signs [27], indicating that the presence of the parasite may influence performance of piglets even at low infection levels.
Parasite shedding, diarrhoea and body weight development showed no significant differences between the two toltrazuril formulations; both were highly efficacious. Forceris® can be used to simultaneously treat metaphylactically against C. suis infection and supply the iron required to support the rapid growth and increase in blood volume during the first days of life of the piglets, thus reducing the required handling of the piglets. Time needed for oral treatment of one piglet was estimated at 10 seconds with a labour cost estimation of € 18/hour [27], significantly contributing to the costs of coccidiosis control. Forceris® can be applied from the first day of life (Baycox®: third to fifth day of life) and may thus also be more useful in cases where C. suis infections occur very early after birth and are often seriously exacerbated by bacterial infections, such as with Cl. perfringens [17]. Accurate dosing of piglets by injection may also be of advantage when vomiting occurs for a variety of reasons, including infection with C. suis [28].
Conclusions
Oocyst shedding was reduced to a minimum in the Baycox® group and suppressed completely in the Forceris® group. Diarrhoea was also reduced significantly in both treatment groups, resulting in significantly better weight gain. The latter was depressed during the acute stage of infection (in the second week of life) in animals that were clinically affected by pasty to watery diarrhoea. Both applications were safe to use and effective in a single application. Early (metaphylactic) treatment of piglet coccidiosis during the prepatent phase of infection can control infection and significantly improve piglet health when C. suis is present on a farm. Treatment with toltrazuril together with iron by injection was safe and effective. Handling of animals for medication was reduced by the combination product without interfering with the efficacy of toltrazuril treatment.
Acknowledgements
The authors sincerely thank the staff of the Institute of Parasitology, Vetmeduni Vienna, for competent sampling and sample processing.
Funding
This study was funded by Ceva, France. The funding body had no role in the collection, analysis, or interpretation of the data. DS and HK are employees of Ceva and were involved in the design of the study and the writing of the manuscript.
Availability of data and materials
Raw data will not be shared as study documentation is protected by confidentiality agreements.
Abbreviations
- AF
Autofluorescence
- AUC
Area under the curve
- FS
Faecal score
- OpG
Oocysts per gram of faeces
- SD
Study day
Authors’ contributions
AJ, HK and DS designed the study. AS and BF analysed the samples and supervised the animal study part. NP carried out the statistical analysis and BH carried out the dispensing, blinding and deblinding of the staff involved and the sponsor. All authors have read and approved the final manuscript.
Ethics approval
The procedures involving piglets for collection of oocysts were approved by the institutional ethics committee and the national authority according to § 26ff of Animal Experiments Act, Tierversuchsgesetz 2012-TVG 2012 (license number: BMWF-68.205/0034-WF/V/3b/2016; Austrian Federal Ministry of Science, Health and Economy).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests. DS and HK are employees of Ceva. No member of the staff of the Vetmeduni Vienna involved in the trial received allowances or other personal benefits from the Sponsor.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Anja Joachim, Email: Anja.Joachim@vetmeduni.ac.at.
Aruna Shrestha, Email: Aruna.Shrestha@vetmeduni.ac.at.
Barbara Freudenschuss, Email: Barbara.Freudenschuss@vetmeduni.ac.at.
Nicola Palmieri, Email: Nicola.Palmieri@vetmeduni.ac.at.
Barbara Hinney, Email: Barbara.Hinney@vetmeduni.ac.at.
Hamadi Karembe, Email: hamadi.karembe@ceva.com.
Daniel Sperling, Email: daniel.sperling@ceva.com.
References
- 1.Stuart B, Lindsay D, Ernest J, Gosser H. Isospora suis enteritis in piglets. Vet Pathol. 1980;17:84–93. doi: 10.1177/030098588001700109. [DOI] [PubMed] [Google Scholar]
- 2.Lindsay DS, Current WL, Taylor JR. Effects of experimentally induced Isospora suis infection on morbidity, mortality, and weight gains in nursing pigs. Am J Vet Res. 1985;46(7):1511–1512. [PubMed] [Google Scholar]
- 3.Lindsay DS, Current WL, Power TA. Enteric coccidial infections and coccidiosis in swine. Compend Contin Educ Vet. 1992;14:698–702. [Google Scholar]
- 4.Martineau GP, del Castillo J. Epidemiological, clinical and control investigations on field porcine coccidiosis: clinical, epidemiological and parasitological paradigms? Parasitol Res. 2000;86(10):834–837. doi: 10.1007/PL00008509. [DOI] [PubMed] [Google Scholar]
- 5.Niestrath M, Takla M, Joachim A, Daugschies A. The role of Isospora suis as a pathogen in conventional piglet production in Germany. J Vet Med B Infect Dis Vet Public Health. 2002;49(4):176–180. doi: 10.1046/j.1439-0450.2002.00459.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mundt HC, Cohnen A, Daugschies A, Joachim A, Prosl H, Schmäschke R, et al. Occurrence of Isospora suis in Germany, Switzerland and Austria. J Vet Med B Infect Dis Vet Public Health. 2005;52(2):93–97. doi: 10.1111/j.1439-0450.2005.00824.x. [DOI] [PubMed] [Google Scholar]
- 7.Chae C, Kwon D, Kim O, Min K, Cheon DS, Choi C, et al. Diarrhoea in nursing piglets associated with coccidiosis: prevalence, microscopic lesions and coexisting microorganisms. Vet Rec. 1998;143(15):417–420. doi: 10.1136/vr.143.15.417. [DOI] [PubMed] [Google Scholar]
- 8.Scala A, Demontis F, Varcasia A, Pipia AP, Poglayen G, Ferrari N, et al. Toltrazuril and sulphonamide treatment against naturally Isospora suis infected suckling piglets: is there an actual profit? Vet Parasitol. 2009;163(4):362–365. doi: 10.1016/j.vetpar.2009.04.028. [DOI] [PubMed] [Google Scholar]
- 9.Skampardonis V, Sotiraki S, Kostoulas P, Leontides L. Effect of toltrazuril treatment in nursing piglets naturally infected with Isospora suis. Vet Parasitol. 2010;172(1–2):46–52. doi: 10.1016/j.vetpar.2010.04.020. [DOI] [PubMed] [Google Scholar]
- 10.Kreiner T, Worliczek HL, Tichy A, Joachim A. Influence of toltrazuril treatment on parasitological parameters and health performance of piglets in the field - an Austrian experience. Vet Parasitol. 2011;183(1–2):14–20. doi: 10.1016/j.vetpar.2011.07.019. [DOI] [PubMed] [Google Scholar]
- 11.Mehlhorn H, Greif G. Baycox. In: Mehlhorn H, editor. Encyclopedia of Parasitology. Berlin: Springer Berlin; 2016. pp. 299–300. [Google Scholar]
- 12.Driesen SJ, Fahy VA, Carland PG. The use of toltrazuril for the prevention of coccidiosis in piglets before weaning. Aust Vet J. 1995;72(4):139–141. doi: 10.1111/j.1751-0813.1995.tb15034.x. [DOI] [PubMed] [Google Scholar]
- 13.Pommier P, Keita A, Wessel-Robert S, Dellac B, Mundt HC. Efficacy of toltrazuril in the prevention and the treatment of suckling piglets coccidiosis: results of two field trials. Rev Med Vet. 2003;1:41–46. [Google Scholar]
- 14.Mundt HC, Mundt-Wüstenberg S, Daugschies A, Joachim A. Efficacy of various anticoccidials against experimental porcine neonatal isosporosis. Parasitol Res. 2007;100(2):401–411. doi: 10.1007/s00436-006-0314-9. [DOI] [PubMed] [Google Scholar]
- 15.Joachim A, Mundt HC. Efficacy of sulfonamides and Baycox® against Isospora suis in experimental infections of suckling piglets. Parasitol Res. 2011;109(6):1653–1659. doi: 10.1007/s00436-011-2438-9. [DOI] [PubMed] [Google Scholar]
- 16.Rypula K, Porowski M, Kaba J, Gorczykowski M, Deniz A. Effect of isosporiasis prevention with toltrazuril on long-term pig performance. Sci World J. 2012;2012:486324. doi: 10.1100/2012/486324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mengel H, Krüger M, Krüger MU, Westphal B, Swidsinski A, Schwarz S, et al. Necrotic enteritis due to simultaneous infection with Isospora suis and clostridia in newborn piglets and its prevention by early treatment with toltrazuril. Parasitol Res. 2012;110(4):1347–1355. doi: 10.1007/s00436-011-2633-8. [DOI] [PubMed] [Google Scholar]
- 18.Mundt HC, Joachim A, Becka M, Daugschies A. Isospora suis: an experimental model for mammalian intestinal coccidiosis. Parasitol Res. 2006;98(2):167–175. doi: 10.1007/s00436-005-0030-x. [DOI] [PubMed] [Google Scholar]
- 19.Oldham JG. Clinical measurement of pain, distress and discomfort in pigs. In: Gibson TE, editor. The Detection and Relief of Pain in Animals. London: BVA Animal Welfare Foundation; 1985. pp. 88–90. [Google Scholar]
- 20.Daugschies A, Bialek R, Joachim A, Mundt HC. Autofluorescence microscopy for the detection of nematode eggs and protozoa, in particular Isospora suis, in swine faeces. Parasitol Res. 2001;87(5):409–412. doi: 10.1007/s004360100378. [DOI] [PubMed] [Google Scholar]
- 21.Joyner LP, Gregory MW, Norton CC, Done JT, Wells GWH. Coccidiosis and coprophagy in pigs. Vet Rec. 1981;108:264–265. doi: 10.1136/vr.108.12.264-a. [DOI] [PubMed] [Google Scholar]
- 22.Joachim A, Schwarz L, Hinney B, Ruttkowski B, Vogl C, Mundt HC. Which factors influence the outcome of experimental infection with Cystoisospora suis? Parasitol Res. 2014;113(5):1863–1873. doi: 10.1007/s00436-014-3834-8. [DOI] [PubMed] [Google Scholar]
- 23.Harleman JH, Meyer RC. Pathogenicity of Isospora suis in gnotobiotic and conventionalised piglets. Vet Rec. 1985;116(21):561–565. doi: 10.1136/vr.116.21.561. [DOI] [PubMed] [Google Scholar]
- 24.Vítovec J, Koudela B. Double alteration of the small intestine in conventional and gnotobiotic piglets experimentally infected with the coccidium Isospora suis (Apicomplexa, Eimeriidae) Folia Parasitol. 1990;37:21–33. [PubMed] [Google Scholar]
- 25.Vítovec J, Koudela B, Kudweis M, Stepanek J, Smid B, Dvorak R. Pathogenesis of experimental combined infections with Isospora suis and rotavirus in conventional and gnotobiotic piglets. J Vet Med B. 1991;38(3):215–226. doi: 10.1111/j.1439-0450.1991.tb00864.x. [DOI] [PubMed] [Google Scholar]
- 26.Ruiz VL, Bersano JG, Carvalho AF, Catroxo MH, Chiebao DP, Gregori F, et al. Case-control study of pathogens involved in piglet diarrhea. BMC Res Notes. 2016;9:22. doi: 10.1186/s13104-015-1751-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maes D, Vyt P, Rabaeys P, Gevaert D. Effects of toltrazuril on the growth of piglets in herds without clinical isosporosis. Vet J. 2007;173(1):197–199. doi: 10.1016/j.tvjl.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 28.Stuart BP, Lindsay DS. Coccidiosis of swine. Veterinary Clinics of North America: Food Anim Pract. 1986;2:455–468. doi: 10.1016/s0749-0720(15)31256-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Raw data will not be shared as study documentation is protected by confidentiality agreements.


