Abstract
Background
Hashimoto’s thyroiditis is associated with serious alterations in serum lipids and glucose homeostasis. The aims of the current study were to evaluate the effect of powdered Nigella sativa on serum lipids, glucose homeostasis and anthropometric variables in patients with Hashimoto’s thyroiditis.
Methods
Forty patients with Hashimoto’s thyroiditis, aged between 22 and 50 years old, participated in the trial and were randomly allocated into two groups of intervention and control receiving powdered Nigella sativa or placebo daily for 8 weeks. Serum lipids, glucose homeostasis, and anthropometric variables were evaluated at baseline and after intervention.
Results
Treatment with Nigella sativa significantly reduced body weight and body mass index (BMI). Serum concentrations of low density lipoprotein cholesterol (LDL) and triglyceride (TG) also decreased in Nigella sativa-treated group after 8 weeks; while serum high density lipoprotein cholesterol (HDL) significantly increased after treatment with Nigella sativa (P < 0.05). None of these changes had been observed in placebo treated group. Serum Nesfatin-1 concentrations was in inverse relationship with serum triglyceride (TG) (r = − 0.31, P = 0.04).
Conclusions
Giving attention to the potent beneficial effects of powdered black cumin seeds in improving serum lipid profile and anthropometric features in patients with Hashimoto’s thyroiditis, this medicinal plant could be considered as a beneficial herbal supplement alongside with the disease- specific medications including Levothyroxine in management of Hashimoto’s thyroiditis- related metabolic abnormalities.
Trial registration
Iranian registry of clinical trials (registration number IRCT2014090819082N2- Registered 2014-09-29).
Keywords: Hashimoto’s thyroiditis, Nigella sativa, Black cumin, Lipid profile, Glucose homeostasis
Background
Hashimoto’s thyroiditis (HT) is one of the most common human autoimmune diseases and an organ-specific T-cell mediated disease that affects the thyroid glands [1, 2]. The disease is ten times more prevalent in women than in men and affects 2% of general population [3, 4]. A significant proportion of patients have asymptomatic chronic autoimmune thyroiditis and 8% of woman (10% of woman over 55 years of age) and 3% of men have subclinical hypothyroidism [5]. Hashimoto’s thyroiditis is associated with serious alterations in composition and the transport of lipoproteins; Hypothyroidism is characterized by hyper-cholesterolaemia and a marked increase in low-density lipoproteins (LDL) and apo-lipoprotein B (apo B) because of reduced fractional clearance of LDL by a reduced number of LDL receptors in the liver [6, 7]. Dyslipidemia occurred in thyroid abnormalities is a potent risk factor of cardiovascular events and myocardial infarction among patients with abnormal thyroid function. Numerous studies revealed that hypothyroidism is an independent risk factor of mortality from cardiovascular disease and all-cause mortality [8, 9]. Moreover, Hashimoto’s thyroiditis is a risk factor of non-insulin dependent diabetes mellitus (NIDDM) and more often these two diseases are in co-existence with each other [10]. Up to 38% of patients with NIDDM have also Hashimoto’s thyroiditis [11].
Considering these metabolic abnormalities in Hashimoto’s thyroiditis, therapeutic approaches in treatment of the disease will be important. Levothyroxine sodium is the treatment of choice for Hashimoto’s thyroiditis however its chronic use is related with cardiac dysfunction, left ventricular hypertrophy [12, 13] and rapid bone loss [14].There are limited data evaluating the effects of vitamins or herbal medications in treatment of thyroid abnormalities [15] and no study was available evaluating the effects of herbal medications in treatment of Hashimoto’s thyroiditis in human. Nigella sativais an amazing herb with a rich historical and religious background; it is one of the medicinal plants and belongs to the Ranunculaceae family [16]. The seeds of the Nigella sativa are the source of the active ingredients of this plants; it has considerable health promoting effects including its antioxidant, anti-inflammatory and immune-modulatory properties [16]. Numerous studies have extensively studied therapeutic actions of Nigella sativa in treating the disease especially in animal models; while human studies in this filed are scarce [17–19]. Lipid- lowering effects of Nigella sativa had been studied in several human diseases including hypercholesterolemia [20], type two diabetes mellitus [21] and coronary artery disease [22]. However, to our review of literature, the effect of this herbal medicine on dyslipidemia or glycemic status and thyroid function among patients with Hashimoto’s thyroiditis has not been evaluated before; therefore in the current study we aimed to test these hypotheses.
Methods
Patients
In the current double-blinded placebo-controlled trial, forty patients with Hashimoto’s thyroiditis were enrolled (Fig. 1). Subjects were recruited from outpatient endocrinology and metabolism clinics of Isfahan University of Medical Sciences. Inclusion criteria were as follows: age between 20 and 50 years, having Hashimoto’s thyroiditis according to physician diagnosis based on laboratory analysis of thyroid stimulating hormone (TSH), T3, T4 and anti-thyroid peroxidase concentrations. Exclusion criteria were as follows: taking any nutritional supplements for at least 3 months prior participation or during the trial, any history of autoimmune disease, cardiovascular events, other thyroid abnormalities including Grave’s disease, being pregnant or lactating, any history of thyroid surgeries and being on any dietary regimens during and 3 months before recruitment in the trial.
Fig. 1.
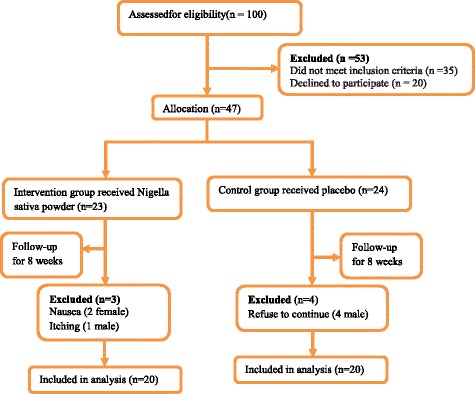
Flow diagram of subject recruitment
Study design
From one hundred recruited subjects, fifty three participants were excluded because of not meeting the inclusion criteria or decline to participate. Among forty seven patients random permuted block procedure was performed and participants were randomly allocated into Nigella sativa-treated (n = 24) or placebo-treated (n = 23) groups.
Patients in the intervention group received a daily dose of 2 g Nigella sativa powder per day and placebo group received 2 g starches per day for 8 weeks. The mature Nigella sativa seeds were obtained from a local market and were milled in a grinder. Both Nigella sativa powder and placebo were identically packaged to have similar appearance. Subjects were advised to receive the supplement or placebo packages in two divided dosages with lunch and dinner. Randomization procedure was performed by a third investigator with no clinical involvement in the trial for ensure in blinding. A follow-up procedure was done with weekly telephone contacts to ensure that subjects consumed the supplements regularly.
Written informed consent was obtained from all of the participants before participation in the trial and the study protocol was approved by the ethics committee of Tabriz University of Medical Sciences (Project number: 93173). The current trial was also registered in the Iranian Registry of Clinical Trials (Identifier: IRCT2014090819082N2) [23, 24].
Anthropometric and nutritional assessments
Body weight and height were measured with a calibrated digital scale and stadiometer respectively. BMI was calculated as weight (kg) divided by height (m) squared [25]. Waist circumference (WC) was measured in horizontal plane, midway between the lowest rib and the iliac crest with a measuring tape in centimeter. Waist to hip ratio (WHR) was calculated by WC divided by hip circumference (HC) [26]. The dietary assessments were performed using a 3-day food record, covering two weekdays and one weekend day, to estimate total energy, carbohydrate, protein, fat and vitamins consumption. Nutrient analysis of the 3-day food record was performed using the Nutritionist IV software (N-squared Computing, Salem, OR, USA).
Physical activity level
Physical activity was obtained by the questionnaire with nine different metabolic equivalent (MET) scales ranging from sleep/rest (0.9 METs) to high-intensity physical activities (> 6 METs). For each activity level, the MET value was multiplied by the time spent at that particular level. The MET-time at each level was added to obtain a total over 24 h MET-time, representing the physical activity level on an average weekday. Physical activities of different intensities were categorized to sedentary (< 3 METs), moderate (3–6 METs) and vigorous (> 6 METs) respectively [27].
Biochemical assays
Fasting blood samples were obtained from all of the participants at the beginning and end of the trail. The serum and plasma samples were separated by centrifugation at 2500 rpm for 10 min (Beckman Avanti J-25; Beckman Coulter, Brea, CA, USA) at room temperature. The serum samples were stored at − 70 °C immediately after centrifugation until their assays.Serum total cholesterol (TC), fasting serum glucose (FSG), triglyceride (TG), high density lipoprotein cholesterol (HDL-C) and low density lipoprotein cholesterol (LDL-C) were analyzed by enzymatic colorimetric method (Pars – Azmoon, Tehran – Iran). Serum insulin was analyzed with enzyme linked immunosorbent assay method (ELISA- Monobind Insulin AccuBind, CA 92630, USA). The Sensitivity of this assay was 0.75 μIU/ml and mean inter and intra assay coefficient of variations (CV) were < 9.8% and < 8% respectively. Atherogenic index of plasma (AIP) was calculated as log TG divided by HDL-C [28]. Assessment of insulin sensitivity was performed by the homeostasis model assessment of insulin resistance (HOMA-IR) based on fasting glucose and insulin measurements as follows: HOMA-IR: (glucose (mg/dl) × insulin (mU/l)) / 405. High HOMA-IR scores denote high insulin resistance [29]. Serum Nesfatin-1 concentration was also assessed by ELISA method (Hangzhou Eastbiopharm Co, USA). This assay had a sensitivity of 0.15 ng/ml.
Statistical assays
Statistical analysis was performed by SPSS™ statistical software (SPSS Inc., Chicago, IL, USA). Quantitative data were presented as mean ± standard deviation (SD), and qualitative data were demonstrated as frequency and percent. Kolmogorov-Smirnov test was used to assess the normality of data. Between groups comparisons of continuous variables were performed by independent sample t-test. Paired t-test was used for before and after intervention comparisons. Analysis of covariance (ANCOVA) was used to identify any differences between two treatment groups after intervention adjusting for the confounding effects of baseline concentrations of parameter, age and gender.
Results
In the current study 40 patients with Hashimoto’s thyroiditis were enrolled. The demographic and biochemical characteristics of study population are shown in Table 1. Participants in two Nigella-sativa and placebo treated group were similar in their mean age and gender distribution. Baseline values of anthropometric variables were also similar between groups. Whereas, weight and BMI of Nigella-sativa group significantly reduced after 8 weeks intervention period while no changes observed in placebo-treated group. Dietary energy and nutrient intakes before and after intervention are presented in Table 2. Energy and nutrient intakes were similar between groups before intervention and no significant change observed after intervention. Nigella sativa significantly reduced serum LDL, TG and AIP values (P < 0.05). Serum HDL increased after Nigella sativa supplementation. None of these changes were observed in placebo treated group. Serum Nesfatin-1 also did not change after intervention; moreover, serum TSH and anti-TPO concentrations reduced while serum T3 increased in Nigella sativa treated group (P < 0.05). (Table 3). A significant negative relationship was observed between baseline values of Nesfatin-1 and serum triglyceride concentrations (Fig. 2).
Table 1.
Demographic characteristics and anthropometric variables in treatment groups before and after intervention
| N | Nigella Sativa treated Group | Control group | P† |
|---|---|---|---|
| N = 20 | N = 20 | ||
| Age (years) | 35.70 ± 8.18 | 33.95 ± 8.72 | 0.52 |
| Female [n(%)] | 17 (85) | 17 (85) | 0.89 |
| Weight (kg) | |||
| Before | 70.52 ± 12.27 | 69.63 ± 11.75 | 0.81 |
| After | 69.39 11.84 | 69.62 11.80 | 0.95 |
| P‡ | 0.004 | 0.91 | |
| BMI (kg/m2) | |||
| Before | 27.10 ± 4.63 | 25.93 ± 4.07 | 0.40 |
| After | 26.63 ± 4.42 | 25.95 ± 4.11 | 0.61 |
| P‡ | 0.002 | 0.65 | |
| WHR | |||
| Before | 0.86 ± 0.052 | 0.87 ± 0.053 | 0.53 |
| After | 0.86 ± 0.05 | 0.87 ± 4.11 | 0.61 |
| P ‡ | 0.38 | 0.53 | |
| Physical activity (Met-min/day) | |||
| Before | 5.25 ± 0.40 | 5.29 ± 0.56 | 0.81 |
| After | 5.26 ± 0.43 | 5.55 ± 0.89 | 0.20 |
| P ‡ | 0.89 | 0.22 | |
Data are presented as mean ± SD or number (percent). BMI body mass index, WC waist circumference, HC hip circumference, WHR waist to hip ratio; †P values for ANCOVA after adjustment for age, duration of the disease and variable’s baseline value; ‡ P values for paired t-test
The bold data present statistically significant values
Table 2.
Dietary intakes of energy and nutrients in treatment groups before and after intervention
| N | Powdered Black Cumin Treated | Placebo treated | P |
|---|---|---|---|
| N = 20 | N = 20 | ||
| Energy (kcal/d) | |||
| Before | 2251.90 ± 349.58 | 2208.95 ± 327.80 | 0.69 |
| After | 2236.40 ± 248.27 | 2265.45 ± 270.73 | 0.72 |
| P‡ | 0.77 | 0.32 | |
| Carbohydrate (%) | |||
| Before | 57.11 ± 2.90 | 57.07 ± 3.78 | 0.97 |
| After | 57.24 ± 2.71 | 57.58 ± 3.32 | 0.72 |
| P‡ | 0.26 | 0.67 | |
| Protein (%) | |||
| Before | 15.73 ± 1.68 | 15.07 ± 1.29 | 0.17 |
| After | 15.77 ± 1.36 | 15.10 ± 1.81 | 0.19 |
| P‡ | 0.94 | 0.94 | |
| Fat (%) | |||
| Before | 27.34 ± 2.15 | 26.57 ± 2.32 | 0.28 |
| After | 26.56 ± 1.96 | 26.13 ± 2.48 | 0.55 |
| P‡ | 0.51 | 0.51 | |
| Vitamin E (mg/d) | |||
| Before | 2.98 ± 1.08 | 3.43 ± 1.48 | 0.36 |
| After | 2.89 ± 1.64 | 3.40 ± 1.19 | 0.28 |
| P‡ | 0.80 | 0.96 | |
| Vitamin C (mg/d) | |||
| Before | 79.75 ± 22.69 | 75.67 ± 17.24 | 0.52 |
| After | 78.37 ± 11.84 | 75.45 ± 15.36 | 0.59 |
| P‡ | 0.77 | 0.91 | |
Data are presented as mean ± SD. †P values for ANCOVA after adjustment for age, duration of the disease and baseline concentration of parameter; ‡P values for paired t-test
Table 3.
Metabolic parameters and thyroid hormones in treatment groups before and after intervention
| N | Powdered Black Cumin Treated | Placebo treated | P |
|---|---|---|---|
| N = 20 | N = 20 | ||
| FSG (mg/dl) | |||
| Before | 86.60 ± 8.46 | 88.10 ± 8.56 | 0.58 |
| After | 84.90 ± 7.01 | 87.80 ± 6.03 | 0.16 |
| P‡ | 0.31 | 0.85 | |
| Insulin (μIU/ml) | |||
| Before | 10.62 ± 7.51 | 7.71 ± 4.12 | 0.14 |
| After | 29.18 ± 19.93 | 17.30 ± 9.16 | 0.023 |
| P‡ | < 0.001 | < 0.001 | |
| HOMA-IR | |||
| Before | 2.32 ± 1.71 | 1.67 ± 0.92 | 0.14 |
| After | 6.09 ± 4.06 | 3.76 ± 2.08 | 0.03 |
| P‡ | < 0.001 | < 0.001 | |
| HDL (mg/dl) | |||
| Before | 41.55 ± 4.67 | 41.70 ± 6.50 | 0.93 |
| After | 43.75 ± 3.72 | 40.57 ± 4.87 | 0.027 |
| P‡ | 0.046 | 0.26 | |
| LDL (mg/dl) | |||
| Before | 130.65 ± 30.68 | 105.00 ± 34.48 | 0.018 |
| After | 107.85 ± 36.99 | 108.90 ± 32.88 | 0.92 |
| P‡ | 0.002 | 0.06 | |
| TG (mg/dl) | |||
| Before | 177.10 ± 34.50 | 186.00 ± 66.63 | 0.59 |
| After | 156.00 ± 21.92 | 185.55 ± 74.98 | 0.11 |
| P‡ | 0.02 | 0.93 | |
| TC (mg/dl) | |||
| Before | 183.70 ± 45.72 | 179.10 ± 43.66 | 0.74 |
| After | 175.10 ± 29.06 | 180.70 ± 44.95 | 0.64 |
| P‡ | 0.22 | 0.55 | |
| AIP | |||
| Before | 0.62 ± 0.11 | 0.63 ± 0.15 | 0.86 |
| After | 0.54 ± 0.08 | 0.63 ± 0.16 | 0.04 |
| P‡ | < 0.001 | 0.68 | |
| Nesfatin-1 (ng/ml) | |||
| Before | 41.80 ± 28.33 | 25.86 ± 20.91 | 0.049 |
| After | 37.63 ± 5.91 | 26.75 ± 23.95 | 0.175 |
| P‡ | 0.34 | 0.69 | |
| TSH (mIU/l) | |||
| Before | 6.42 ± 3.86 | 8.14 ± 7.28 | 0.35 |
| After | 4.13 ± 2.35 | 8.27 ± 7.21 | 0.02 |
| P‡ | 0.03 | 0.40 | |
| T3 (mmol/l) | |||
| Before | 0.92 ± 0.27 | 1.18 ± 0.36 | 0.017 |
| After | 1.06 ± 0.34 | 1.16 ± 0.35 | 0.39 |
| P‡ | 0.008 | 0.15 | |
| T4 (mmol/l) | |||
| Before | 8.07 ± 2.56 | 7.97 ± 3.11 | 0.91 |
| After | 8.89 ± 1.43 | 7.63 ± 2.23 | 0.04 |
| P‡ | 0.21 | 0.32 | |
| Anti-TPO (IU/ml) | |||
| Before | 294.55 ± 210.05 | 278.10 ± 170.77 | 0.78 |
| After | 147.99 ± 158.33 | 274.30 ± 167.20 | 0.01 |
| P‡ | 0.019 | 0.28 | |
Data are presented as mean ± SD. †P values for ANCOVA after adjustment for age, duration of the disease and baseline concentration of parameter; ‡P values for paired t-test. FSG fasting serum glucose, TC total cholesterol, TG triglycerides, HDL high-density lipoprotein cholesterol, AIP atherogenic index of plasma, TSH thyroid-stimulating hormone, T3 triiodothyronine T4 thyroxine, TPO thyroid peroxidase
The bold data present the statistically significant values
Fig. 2.
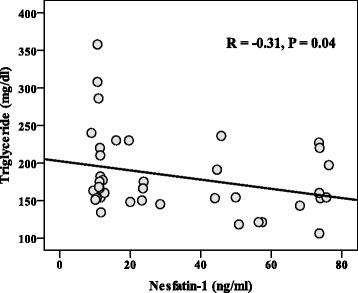
Relationship between serum Nesfatin-1 and triglyceride concentrations in total participants
Discussion
Hashimoto’s thyroiditis is accompanied with disturbances in serum lipids and glucose homeostasis. The disease is a known risk factor for hyperlipidemia and diabetes mellitus [6, 10]. The results of the current study provide insight into lipid-lowering effects of Nigella sativa in patients with Hashimoto’s thyroiditis. Moreover Nigella sativa reduced body weight and BMI in these patients. It is the first trial evaluating the effects of Nigella sativa on serum lipids and BMI in patients with Hashimoto’s thyroiditis.
Weight reducing effects of Nigella sativa has been observed in previous studies; Zaoui A [30] reported a significant reduction in body weight in rats after 6 weeks treatment with Nigella sativa fixed oil (P < 0.001). In other study 3 month supplementation with 1.5 g daily powdered Nigella sativa in central obese men significantly reduced body weight [31]. In other study in menopausal women also slight and non-significant reduction in body weight was observed after 2 month treatment with 1 g/ day Nigella sativa [32]. The anti-obesity effects of Nigella might be explained by increasing mean rates of satiety and fullness [33]. Other possible mechanisms includes reduction in lipid absorption, reduced energy intake, increased energy expenditure, decreased pre-adipocyte differentiation and proliferation, or decreased lipogenesis and increased lipolysis [34].
In fact, other health promising effects of Nigella sativa like its hypolipidemic or hypoglycemic effects observed in our study and also previous reports [31, 35] could be explained by these mechanisms; we observed a strong reduction in serum LDL and TG and AIP and increase in serum HDL concentrations (P < 0.001 and P < 0.05 respectively). A mild non-significant reduction in serum FSG was also observed in Nigella sativa treated group. Serum insulin was increased in both groups and comparison of mean difference in serum insulin between groups showed no statistically significant difference.
Although not clear explanation can be attributed to this phenomenon, however, the possible underlying reason can be the direct effects of pro-inflammatory cytokines in the Hashimoto’s thyroiditis against insulin resistance and deteriorating the pancreas’ β-cell function; TSH is a potent stimulator of interleukin (IL)-6, IL-2, C-reactive protein (CRP) and tumor necrosis factor (TNF)-α secretion from adipose tissue in patients with Hashimoto’s thyroiditis [36]. On the other hand, previous studies reporting the beneficial effects of Nigella sativa on glycemic status and insulin resistance used higher doses of Nigella sativa compared with our study [37, 38] or even more prolonged study duration time [39, 40]. Therefore, the beneficial effects of Nigella sativa on glycemic status and insulin resistance in the current dose and the study’s duration have not been observed.
The underlying mechanisms previously suggested for hypolipidemic effects of Nigella sativa included an up-regulation of LDL-C molecules through receptor mediated endocytosis [35], decreased dietary cholesterol absorption, stimulation of primary bile acid synthesis and its fecal losses probably contributed to its dietary soluble fibers and sterols [32] and non-enzymatic lipid peroxidation by antioxidant properties of Nigella sativa making liver cells more efficient to remove LDL-C from blood by increasing LDL-C receptor densities in the liver and binding to apolipoprotein, apo-B [41–44].
In the current study serum Nesfatin-1 concentrations did not change after Nigella sativa supplementation. However, its serum concentrations was in inverse relationship with serum triglyceride concentrations (r = − 0.31, P < 0.05). Nesfatin-1 is a new anorexigenic hormone which is expressed from several regions of hypothalamus and peripheral tissues, including the adipose tissue, gastric mucosa, pancreatic beta-cells. Recent studies have demonstrated that Nesfatin-1 is negatively related with obesity and insulin resistance [32, 45]. Our finding also confirmed these results. However no change after Nigella sativa supplementation was observed in serum Nesfatin-1 concentrations. Probably because of the study’s low sample size.
Conclusions
We have demonstrated beneficial effects of powdered black cumin in improving serum lipids and reducing body weight in patients with Hashimoto’s thyroiditis. Considering these health-promoting effects of Nigella sativa, it can be considered as a beneficial herbal supplement alongside with the disease- specific medications including Levothyroxine in management of Hashimoto’s thyroiditis- related metabolic abnormalities.
Acknowledgements
The current research was financially supported by a grant from Tabriz University of Medical Sciences.
Funding
This research has been performed by a grant from Tabriz University of Medical Sciences (Project number: 93173).
Availability of data and materials
The data are available for any scientific use with kind permission.
Abbreviations
- BMI
Body mass index
- HC
Hip circumference
- T3
Triiodotyronine
- T4, Thyroxine
TG-Ab, thyroglobulin antibody
- TPO-Ab
Thyroid peroxidase antibody
- TRH
Thyrotropin releasing hormone
- TSH
Thyroid stimulating hormone, VEGF, vascular endothelial growth factor
- WC
Waist circumference; WHR, waist to hip ratio
Authors’ contributions
MAF conceived and designed the project and wrote the manuscript and performed the statistical analysis, PD was invloved in study design and revision of the manuscritp, ST performed the sampling and data collection. All authors read and approved the final manuscript.
Ethics approval and consent to participate
All participants signed a written informed consent approved by the Institutional Review Board of Tabriz University of Medical Sciences. The study design and protocol was approved by the ethical committee of Tabriz University of Medical Sciences (Project number: 93173). The study has also been registered in Iranian registry of clinical trials (registration number IRCT2014090819082N2- Registered 2014-09-29).
Consent for publication
Not applicable.
Competing interests
The authors declare that there is no competing of interest.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Chistiakov DA. Immunogenetics of Hashimoto's thyroiditis. J Autoimmune Dis. 2005;2(1):1–21. doi: 10.1186/1740-2557-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jalilzadeh S, Bahrami A, Eftekhar-Sadat B, Pezeshki Z, Salek Zamani Y, Mobasseri M. Electrodiagnostic changes in patient with subclinical hypothyroidism. Med J Tabriz University of Med Sci Health Serv. 2007;29(1):11–20. [Google Scholar]
- 3.Wang C, Crapo LM. The epidemiology of thyroid disease and implications for screening. Endocrinol Metab Clin N Am. 1997;26:189–218. doi: 10.1016/s0889-8529(05)70240-1. [DOI] [PubMed] [Google Scholar]
- 4.Tunbridge WM, Vanderpump MP. Population screening for autoimmune thyroid disease. Endocrinol Metab Clin N Am. 2000;29:239–253. doi: 10.1016/s0889-8529(05)70129-8. [DOI] [PubMed] [Google Scholar]
- 5.Vanderpump MP, Tunbridge WM, French JM, Appleton D, Bates D, Clark F, et al. The incidence of thyroid disorders in the community: a twenty-year follow-up of the Whickham survey. Clin Endocrinol. 1995;43:55–69. doi: 10.1111/j.1365-2265.1995.tb01894.x. [DOI] [PubMed] [Google Scholar]
- 6.Duntas LH. Thyroid disease and lipids. Thyroid. 2002;12(4):287–293. doi: 10.1089/10507250252949405. [DOI] [PubMed] [Google Scholar]
- 7.Farhangi MA, Eshraghian M, Keshavarz SA, Saboor-Yaraghi AA. Thyroid-stimulating hormone, triiodotyronine and thyroxine concentrations and their relationship with metabolic parameters, anthropometric variables and body composition in premenopausal euthyroid obese women. Turk J Endocrinol Metab. 2015;19(1):1–6. [Google Scholar]
- 8.Singh S, Duggal J, Molnar J, Maldonado F, Barsano CP, Arora R. Impact of subclinical thyroid disorders on coronary heart disease, cardiovascular and all-cause mortality: a meta-analysis. Int J Cardiol. 2008;125(1):41–48. doi: 10.1016/j.ijcard.2007.02.027. [DOI] [PubMed] [Google Scholar]
- 9.Klein I, Danzi S. Thyroid disease and the heart. Circulation. 2007;116(15):1725–1735. doi: 10.1161/CIRCULATIONAHA.106.678326. [DOI] [PubMed] [Google Scholar]
- 10.McCanlies E, O’Leary LA, Foley TP, Kramer MK, Burke JP, Libman A, et al. Hashimoto’s thyroiditis and insulin-dependent diabetes mellitus: differences among individuals with and without abnormal thyroid function. J Clin Endocrinol Metab. 1998;83(5):1548–1551. doi: 10.1210/jcem.83.5.4769. [DOI] [PubMed] [Google Scholar]
- 11.Kontiainen S, Schlenzka A, Koskimies S, Rilva A, Maenpaa J. Autoantibodies and autoimmune diseases in young diabetics. Diabetes Res. 1990;13:151–156. [PubMed] [Google Scholar]
- 12.Monzani F, Di Bello V, Caraccio N, Bertini A, Giorgi D, Giusti C, et al. Effect of levothyroxine on cardiac function and structure in subclinical hypothyroidism: a double blind, placebo-controlled study. J Endocrinol Metab. 2001;86(3):1110–1115. doi: 10.1210/jcem.86.3.7291. [DOI] [PubMed] [Google Scholar]
- 13.Dai DZ, Hu HJ, Yang DM, Hao XM, Zhang GQ, Zhou PA, et al. Chronic levothyroxin treatment is associated with ion channel abnormalities in cardiac and neuronal cells. Clin Exp Pharmacol Physiol. 1999;26(10):819–821. doi: 10.1046/j.1440-1681.1999.03135.x. [DOI] [PubMed] [Google Scholar]
- 14.Panebianco P, Rosso D, Destro G, Scarpinato RA, Tropea S, Rizzo A, et al. Use of disphosphonates in the treatment of osteoporosis in thyroidectomized patients on levothyroxin replacement therapy. Arch Gerontol Geriatr. 1997;25(2):219–225. doi: 10.1016/s0167-4943(97)00013-7. [DOI] [PubMed] [Google Scholar]
- 15.Farhangi MA, Keshavarz SA, Eshraghian M, Ostadrahimi A, Saboor-Yaraghi AA. The effect of vitamin a supplementation on thyroid function in premenopausal women. J Am Coll Nutr. 2012;31(4):268–274. doi: 10.1080/07315724.2012.10720431. [DOI] [PubMed] [Google Scholar]
- 16.Salem ML. Immunomodulatory and therapeutic properties of the Nigella sativa L. seed. Int Immunopharmacol. 2005;5(13):1749–1770. doi: 10.1016/j.intimp.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 17.Kanter M, Coskun O, Korkmaz A, Oter S. Effects of Nigella sativa on oxidative stress and β-cell damage in streptozotocin-induced diabetic rats. Anat Rec A Discov Mol Cell Evol Biol. 2004;279(1):685–691. doi: 10.1002/ar.a.20056. [DOI] [PubMed] [Google Scholar]
- 18.Kanter M, Coskun O, Budancamanak M. Hepatoprotective effects of Nigella sativa and Urtica dioica on lipid peroxidation, antioxidant enzyme systems and liver enzymes in carbon tetrachloride-treated rats. WJG. 2005;11(42):6684–6688. doi: 10.3748/wjg.v11.i42.6684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abdel-Sater KA. Gastroprotective effects of Nigella sativa oil on the formation of stress gastritis in hypothyroidal rats. Int J Physiol Pathophysiol Pharmacol. 2009;1(2):143–149. [PMC free article] [PubMed] [Google Scholar]
- 20.Bhatti IU, Ur Rehman F, Khan MA, Marwat SK. Effect of prophetic medicine kalonji (Nigella sativa L.) on lipid profile of human beings. An in vivo approach. World Appl Sci J. 2009;6(8):1053–1057. [Google Scholar]
- 21.Akash MSH, Rehman K, Rasool F, Sethi A, Abrar MA, Irshad A, et al. Alternate therapy of type 2 diabetes mellitus (T2DM) with Nigella (Ranunculaceae) J Med Plants Res. 2011;5(31):6885–6889. [Google Scholar]
- 22.Tasawar Z, Siraj Z, Ahmad N, Lashari MH. The effects of Nigella sativa (Kalonji) on lipid profile in patients with stable coronary artery disease in Multan. Pakistan Pak J Nutr. 2011;10(2):162–167. [Google Scholar]
- 23.Farhangi MA, Dehghan P, Tajmiri S, Abbasi MM. The effects of Nigella sativa on thyroid function, serum vascular endothelial growth factor (VEGF) – 1, Nesfatin-1 and anthropometric features in patients with Hashimoto’s thyroiditis: a randomized controlled trial. BMC Complement Alternat Med. 2016;16:471–480. doi: 10.1186/s12906-016-1432-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tajmiri S, Farhangi MA, Dehghan P. Nigella sativa treatment and serum concentrations of thyroid hormones, transforming growth factor β (TGF-b) and interleukin 23 (IL-23) in patients with Hashimoto’s thyroiditis. Eur J Integrat Med. 2016;8:576–580. [Google Scholar]
- 25.Farhangi MA, Keshavarz SA, Eshraghian MR, Ostadrahimi A, Saboor-Yaraghi AA. Vitamin a supplementation, serum lipids, liver enzymes and C-reactive protein concentrations in obese women of reproductive age. Ann Clin Biochem. 2013;50:25–30. doi: 10.1258/acb.2012.012096. [DOI] [PubMed] [Google Scholar]
- 26.Sharifi N, Mahdavi R, Ebrahimi-Mameghani M. Perceived barriers to weight loss programs for overweight or obese women. Health Prom Perspect. 2013;3(1):11–22. doi: 10.5681/hpp.2013.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kelishadi R, Ardalan G, Gheiratmand R, Gouya MM, Razaghi EM, Delavari A. Association of physical activity and dietary behaviours in relation to the body mass index in a national sample of Iranian children and adolescents: CASPIAN study. Bull World Health Organ. 2007;85:19–26. doi: 10.2471/BLT.06.030783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Farhangi MA, Keshavarz SA, Eshraghian M, Ostadrahimi A, Saboor-Yaraghi AA. White blood cell count in women: relation to inflammatory biomarkers, haematological profiles, visceral adiposity, and other cardiovascular risk factors. J Health Popul Nutr. 2013;31(1):58–64. doi: 10.3329/jhpn.v31i1.14749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Farhangi MA, Jahangiry L, Mirinazhad MM, Shojaeezade D, Montazeri A, Yaghoubi A. A web-based interactive lifestyle modification program improves lipid profile and serum adiponectin concentrations in patients with metabolic syndrome: the “red ruby” study. Int J Diabetes Dev Ctries. 2017;37(1):21-30.
- 30.Zaoui A, Cherrah Y, Alaoui K, Mahassine N, Amarouch H, Hassar M. Effects of Nigella sativa fixed oil on blood homeostasis in rat. J Ethnopharmacol. 2002;79(1):23–26. doi: 10.1016/s0378-8741(01)00342-7. [DOI] [PubMed] [Google Scholar]
- 31.Datau EA, Surachmanto EE, Pandelaki K, Langi JA. Efficacy of Nigella sativa on serum free testosterone and metabolic disturbances in central obese male. Acta Med Indones. 2010;42(3):130–134. [PubMed] [Google Scholar]
- 32.Ibrahim RM, Hamdan NS, Ismail M, Saini SM, Abd Rashid SN, Abd Latiff L, et al. Protective effects of Nigella sativa on metabolic syndrome in menopausal women. Adv Pharm Bull. 2014;4(1):29–33. doi: 10.5681/apb.2014.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qidwai W, Hamza HB, Qureshi R, Gilani A. Effectiveness, safety, and tolerability of powdered Nigella sativa [kalonji] seed in capsules on serum lipid levels, blood sugar, blood pressure, and body weight in adults: results of a randomized, double-blind controlled trial. J Altern Complement Med. 2009;15:639–644. doi: 10.1089/acm.2008.0367. [DOI] [PubMed] [Google Scholar]
- 34.Hasani-Ranjbar S, Jouyandeh Z, Abdollahi M. A systematic review of anti-obesity medicinal plants-an update. J Diabetes Metab Disord. 2013;12(1):28–33. doi: 10.1186/2251-6581-12-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ibrahim RM, Hamdan NS, Mahmud R, Imam MU, Saini SM, SNA R, et al. A randomised controlled trial on hypolipidemic effects of Nigella sativa seeds powder in menopausal women. J Transl Med. 2014;12:82. doi: 10.1186/1479-5876-12-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Varim C, Kaya T, Varim P, Nalbant A, Vatan MB, Yaylaci S, Gokosmanoglu F, Tamer A. Insulin resistance in the patients with euthyroid Hashimoto thyroiditis. Biomed Res. 2017;28(4):1543–1547. [Google Scholar]
- 37.Najmi A, Haque SF, Naseeruddin M, Khan RA. Effect of Nigella sativa oil on various clinical and biochemical parameters of insulin resistance syndrome. Int J Diab Metab. 2008;16:85–87. doi: 10.4103/0973-3930.41980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Najmi A, Nasiruddin M, Ali Khan R, Haque SF. Therapeutic effect of Nigella Sativa oil On different clinical and biochemical parameters In metabolic syndrome. Int J Diabetes Dev Ctries. 2008;28(1):11–14. doi: 10.4103/0973-3930.41980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kaatabi H, Bamosa AO, Badar A, Al-Elq A, Abou-Hozaifa B, Lebda F, et al. Nigella sativa improves glycemic control and ameliorates oxidative stress in patients with type 2 diabetes mellitus: placebo controlled participant blinded clinical trial. PLoS One. 2015;10(2):e0113486. doi: 10.1371/journal.pone.0113486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bamosa AO, Kaatabi H, Lebdaa FM, Elq AM, Al-Sultanb A. Effect of Nigella sativa seeds on the glycemic control of patients with type 2 diabetes mellitus. Indian J Physiol Pharmacol. 2010;54(4):344–354. [PubMed] [Google Scholar]
- 41.Al-Naqeeb G, Ismail M, Al-Zubairi AS. Fatty acid profile, α- tocopherol content and total antioxidant activity of oil extracted from N sativa seeds. Int J Pharmacol. 2009;5(244–50):4. [Google Scholar]
- 42.Sokhanvar S, Mazaki R, Mousavinasab N, Golmohammadi Z. The association between serum lipoprotein (a) and other cardiac risk factors with the severity of coronary artery disease. J Cardiovasc Thorac Res. 2011;3(1):35–39. [Google Scholar]
- 43.Sokhanvar S, Khoshi A, Hajiaghaei S, Mousavinasab SN, Golmohammadi Z. Association between Apo lipoprotein B levels at admission of patients and short-term morbidity and mortality after myocardial infarction. J Cardiovas Thoracic Res. 2012;4(3):61–64. doi: 10.5681/jcvtr.2012.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Su Y, Zhang J, Tang Y, Bi F, Liu JN. The novel function of nesfatin-1: anti-hyperglycemia. Biochem Biophys Res Commun. 2010;391:1039–1042. doi: 10.1016/j.bbrc.2009.12.014. [DOI] [PubMed] [Google Scholar]
- 45.Bashir J, Gholami F, Rahbaran A, Tarmahi V. Effects of single bout of aerobic exercise on serum Nesfatin-1 levels in non-athlete elderly men. Med J Tabriz Uni Med Sci. 2012;34(4):25–30. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data are available for any scientific use with kind permission.


