Abstract
Ocular tuberculosis (TB) commonly causes severe inflammation and vision loss in TB-endemic countries. The mechanism by which tuberculous infection becomes established in the eye is poorly understood. We have developed the zebrafish larva infected with Mycobacterium marinum as a model to study the early pathogenesis of ocular TB. We find that hematogenous bacterial seeding of the eye occurs despite a functional blood retinal barrier. Prototypical early granulomas form in response to bacteria in the eye. These granulomas involve the retinal vasculature and retinal pigment epithelium-choroid complex which are characteristic locations for human ocular TB. We find that peripheral blood monocytes are recruited to the nascent ocular granuloma further suggesting that the immune privileged nature of the eye is breached by this inflammatory focus.
Introduction
Ocular tuberculosis (TB) causes significant visual impairment in TB-endemic countries [1]. Several clinical presentations involving nearly every ocular tissue have been attributed to ocular TB [2]. The pathogenesis of ocular TB is poorly understood prompting several attempts to create animal models for investigation. These include rabbit models infected with Mycobacterium tuberculosis by intra-ocular or intra-carotid injections [3], and a recent model in aerosol-infected guinea pigs [4], both of which have demonstrated caseating as well as non-caseating granulomas in the eye, with acid-fast organisms. While these models have demonstrated mycobacterial seeding in the eye and reproduced the histopathological changes of human ocular TB, the factors influencing mycobacterial invasion of the eye in the presence of the blood-retinal barrier remain unknown.
The zebrafish (Danio rerio) model of Mycobacterium marinum infection has been established as a natural, relevant and genetically tractable host-pathogen conjugate, for dissecting mycobacterial interactions with the host in TB [5–6]. Zebrafish larvae are optically transparent, which allows real-time, intravital visualization of early host-pathogen interactions in the eye [5]. M. marinum forms early granulomas comprising of aggregates of infected and uninfected macrophages, inside the larvae. We reasoned that the zebrafish larva could be a useful model for studying the earliest host-pathogen interactions leading to ocular TB for the following reasons: 1) the larval eye completes substantial anatomical development even by 2 days post-fertilization (dpf) [7], 2) the inner (endothelial) and outer (retinal pigment epithelium, RPE) blood retinal barriers (BRB) are well established by 3 dpf, rendering it a distinct anatomical compartment structurally analogous to the human eye [8] and 3) the larva is highly amenable to intravital microscopy up to 3 weeks as well as to genetic manipulation [5]. Our results suggest that this model will be useful to understand how mycobacteria seed the eye and how the early granuloma develops in this immune privileged site.
Materials and methods
Fish husbandry: All zebrafish husbandry and experiments were performed in compliance with the UK Home Office and the Animal Ethics Committee of the University of Cambridge. Wild-type AB and various transgenic(Tg) zebrafish (Tg(mpeg::YFP), Tg(mpeg:DsRed2) Tg(kdrl:DsRed2) and Tg(mfap4:tdTomato) were maintained as described [6]. Eggs were collected following natural spawning and treated with N-Phenylthiourea 12 hours post-fertilization (hpf) onwards to prevent pigmentation.
Bacterial strains: We used M. marinum strain M (ATCC BAA-535) constitutively expressing the green- or red-fluorescent proteins, Wasabi or tdTomato (pTEC15 or pTEC27 deposited with Addgene). M. marinum single-cell preparations for infections were made as described [6], and stored in 5 μL aliquots at a concentration of 100 CFU per nL. For each experiment, a single vial was thawed and mixed with 0.25 μL of 20% phenol red before injection.
Caudal vein injections: Larvae at specified developmental stages (2 or 3 dpf) were anesthetized with tricaine and injected with M. marinum, or the specified reagent, as described [6].
Microscopy: Screening of eyes for infection with fluorescent M. marinum was performed on a Nikon E600 equipped with a CoolLED pE-300 light source. Larvae were anaesthetised in tricaine and placed laterally on a flat slide to image one eye at a time. Each eye was imaged along the z-axis, from superficial to deep layers and the presence of fluorescent bacteria was scored manually. For confocal microscopy, larvae were embedded in low-melting point agarose (1%), and imaged on a Nikon A1 confocal microscope with a 20x Plan Apo 0.75 NA objective. Z-stack images were generated using a 2 μm step size spanning a total depth of 140 μm. Images were analysed using NIS-Elements AR 4.4.
Peripheral monocyte recruitment assay: For these experiments, Tg(mfap4:tdTomato) fish with red-fluorescent macrophages were injected with a lower dose (25 CFU) of Wasabi-expressing green-fluorescent M. marinum. Fish were screened for granuloma formation at 6 days post infection (dpi) (corresponding to 9 dpf) and then injected with 200 μg/mL Hoecsht 33342 dye (Invitrogen). Recruitment of Hoechst-positive peripheral blood monocytes was evaluated by confocal microscopy 24 hours later.
Results
Hematogenous dissemination of M. marinum infection to the eye occurs in the presence of the blood retinal barrier
Most forms of human extra-pulmonary TB are thought to develop through hematogenous dissemination of M. tuberculosis [9]. In the case of ocular TB, this would require the bacteria to have dissemination into the eye crossing the BRB. Because the BRB is established in zebrafish larvae at between 2 and 3 days post fertilization (dpf) [8], we were able to use 3 dpf larvae to evaluate if mycobacterial dissemination occurs in the presence of the BRB. For this, we injected red-fluorescent M. marinum into the caudal vein of transgenic mpeg:YFP larvae with yellow fluorescent macrophages. We observed ocular infections in approximately 20% of the animals in multiple experiments, with the majority of infections already apparent 1 day post-injection (dpi) (Fig 1A). Since an appreciable frequency of seeding to the eye occurred in the presence of the BRB, it appeared that it was not a significant deterrent to bacterial traversal into the eye. To test this directly, we compared the frequency of infected eyes after 1 day of infection of 3 dpf larvae to 2 dpf larvae in which the BRB is not yet present [8]. If the BRB deters seeding of the eye, we should see an increased frequency of ocular infection in the animals infected at 2 dpf over 3 dpf. We found, however, that the presence of the BRB did not inhibit bacterial seeding of the eye. If anything, the frequency of ocular infection was slightly higher in animals infected at 3 dpf than in those infected at 2 dpf (19.2% for 3 dpf and 9.8% for 2 dpf) (Fig 1B), possibly due to an increased number of bacteria traversing through the eye due to the increased retinal vasculature at this stage of development [8]. In any case, these findings showed that the BRB is not a significant deterrent to hematogenous seeding of mycobacteria into the eye.
Fig 1. The blood retinal barrier (BRB) does not prevent seeding of infection within eye.
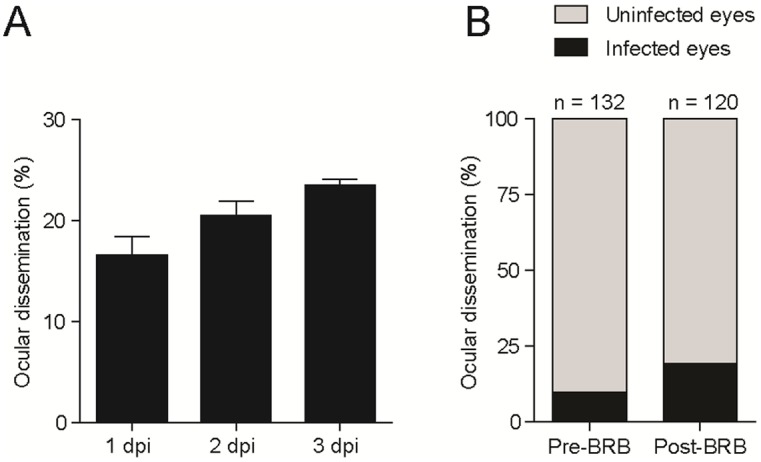
(A, B) Frequency of ocular dissemination (as % of infected eyes) after caudal vein injection of 100 CFU of Mycobacterium marinum. (A) Larvae infected at 3 days-post-fertilization, in three separate experiments (n = 108, 92, and 120). (B) Larvae infected at 2 dpf, before formation of the BRB (pre-BRB), or at 3 dpf, following formation of the BRB (post-BRB). Each eye was scored for the presence of infection at 24 hours post-infection.
Early granuloma formation in infected eyes
Sequential daily monitoring of the infection revealed the development of organized infected macrophage aggregates by 5 dpi (Fig 2). The steps leading to early granuloma formation in the retina, and granuloma morphology were similar to that seen in other parts of the body [6,10]. In a few instances (~5% eyes), we observed seeding of the eye at 1 dpi, with the infections not visible on repeat imaging, suggesting that they had resolved or that the bacteria were only transiently in the eye and had left. In support of the former possibility, we found that infection foci elsewhere in the body can also resolve, a phenomenon that has been observed in humans and nonhuman primates [11–12]. In support of the latter possibility, we noted occasional bacteria entering and leaving the eye through the hyaloid vasculature, suggesting that bacteria may transit through the eye without ultimately settling there. Overall, our findings suggest that mycobacterial infection of the eye results in the formation of macrophage aggregates similar to those found elsewhere in the body.
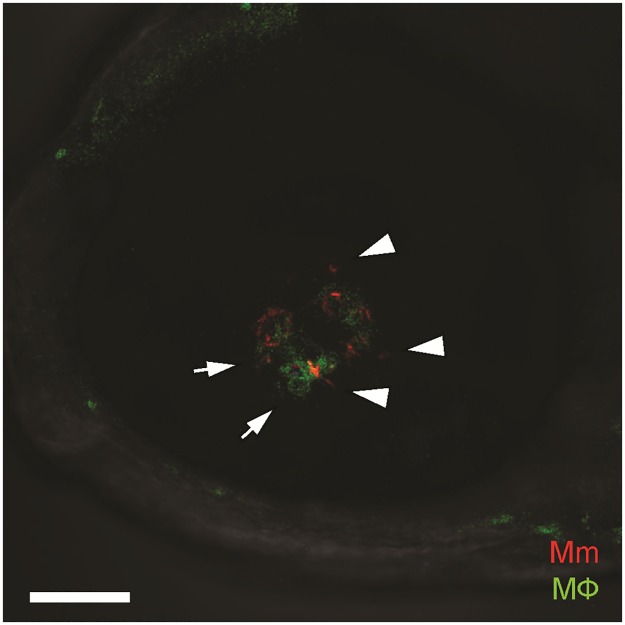
Fig 2. Confocal image of green-fluorescent macrophage aggregate surrounding red-fluorescent Mycobacterium marinum, located in the retinal parenchyma.
The mycobacteria are intracellular within macrophages (arrows) as well as extracellular (arrowheads). Scale bar, 50 μm.
The anatomical localization of ocular granulomas in the zebrafish larva resembles that in humans
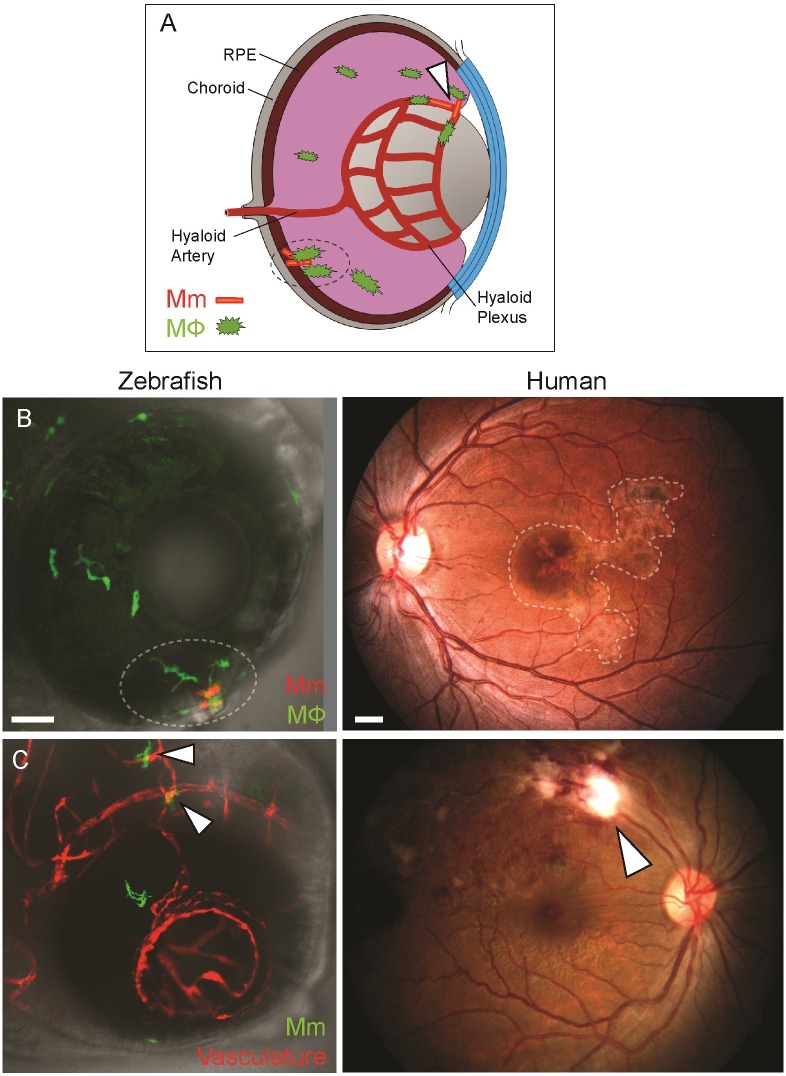
In humans, ocular TB is commonly localized to the retinal vasculature (retinal periphlebitis) or the retinal pigment epithelium—choroid complex (serpiginous-like choroiditis) (Fig 3A–3C). We found that mycobacterial infection was similarly localized in the zebrafish eye. Fig 3B showing the eye of a zebrafish with green-fluorescent macrophages infected with red-fluorescent bacteria, provides an example of an infected macrophage aggregate localized to the outer part of the eye, corresponding to the choroiditis lesion seen in the human eye (right panel). Fig 3C shows a Tg(kdrl:DsRed2) zebrafish eye with red-fluorescent retinal vasculature and green-fluorescent bacteria with bacteria abutting the blood vessels similar to retinal periphlebitis seen in human ocular TB. Since the macrophages in this animal are not labeled, we cannot tell if the bacteria are intracellular. It is likely that they are, given our observations in animals with labeled macrophages (Fig 3B). In sum, the zebrafish eye lesions resemble those seen in human ocular TB.
Fig 3. Anatomical localization of intraocular granuloma after M. marinum infection.
(A) Schematic representation showing localization of granulomas near the retinal vasculature (arrowhead), and in retinal pigment epithelium-choroid complex (dotted circle). (B) Localization of intraocular granulomas (within dotted regions); in the outer eye of zebrafish larvae corresponding to the retinal pigment epithelium-choroid complex, and in the choroid in human ocular TB. (C) Localization of perivascular infection (arrowheads); as seen as bacterial aggregates in close association of blood vessels in zebrafish, and retinal periphlebitis associated with focal chorioretinitis overlying the blood vessel in human ocular TB. (B-C) Confocal images of ocular infection in Tg(mpeg:YFP) and Tg(kdrl:dsRed2) zebrafish, and fundus photographs of human ocular TB. Scale bars, 25 μm and 750 μm, respectively.
Ocular granulomas recruit peripheral monocytes
The eye has a population of retinal microglia (tissue resident macrophages of the retina) that are already present even by this stage of development [13]. Their proximity to the site of infection would suggest that they participate in the formation of intraocular granulomas. We wondered if peripheral blood monocytes were also recruited to the forming granuloma in the eye, as they are in the context of granulomas forming in other parts of the body [10]. To determine this, we took advantage of the nuclear dye Hoechst 33342 that on intravenous injection labels peripheral blood monocytes but not retinal microglia owing to its inability to cross the BRB [10]. Next, we assessed zebrafish infected through the caudal vein at 3 dpf for granuloma formation in the eye at 6 dpi and injected the Hoechst dye in fish that had granulomas. Twenty-four hours after the injection, we found no dye leakage through the BRB, suggesting that there was not a gross breakdown of the BRB at this stage of the infection. When we imaged the developing granulomas, we found Hoechst-positive cells in 8 of 17 granulomas (47.1%) showing that they recruited peripheral blood monocytes (Fig 4A–4C). Altogether, this experiment confirms that the BRB does not suffer gross breakdown even when sizable aggregates have formed. Whether there is localized breakdown of the BRB close to the lesions remains to be assessed as our imaging was not of sufficient resolution. Further, the finding that peripheral blood monocytes do not circulate into the uninfected eye is consistent with it normally being an immune privileged site. The relatively high frequency with which peripheral blood monocytes are attracted to the forming granuloma suggests that its chemotactic influence overrides the mechanisms that prevent monocyte ingress under baseline conditions.
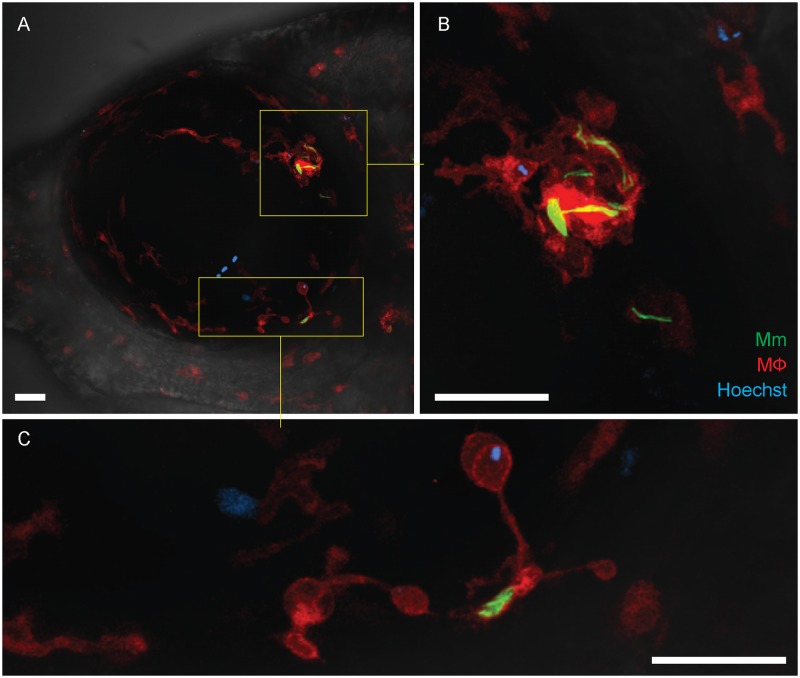
Fig 4. Recruitment of peripheral blood monocytes in granuloma formation.
(A-C) Granuloma formation in Tg(mfap4:tdTomato) fish with red-fluorescent macrophages and infected with green-fluorescent M. marinum. Hoechst-positive (blue-fluorescent) peripheral blood monocytes which have been recruited into the infected eye are seen within the granuloma (inset and panel B) and in contact with a single infected macrophage (inset and panel C). The retinal microglia are incorporated into the granuloma and also dispersed uniformly within the ocular tissues. Scale bars, 30 μm.
Discussion
We show that mycobacterial infection of zebrafish larvae can seed the eye hematogenously, consistent with the proposed model for extrapulmonary dissemination of TB in humans [9]. The similar spatial localization of human and zebrafish larval TB further validates the investigational utility of this model. A large study of 10,524 cases of active pulmonary TB in a sanatorium, showed that 1.4% had concurrent ocular TB [14]. The higher frequency of ocular dissemination (~20%) in our experiments likely reflects the relatively high inoculum introduced directly into the blood stream. Alternatively, we may be looking at very early events all of which do not necessarily progress to clinically apparent lesions, or the lack of adaptive immunity in fish larvae could reduce the host’s ability to control infection. In either case, the ability to study the first seeding event to the eye and the varying consequences thereof may shed light on these early events that have remained elusive so far.
Two insights into the pathogenesis of ocular TB emerge from this pilot study. First, mycobacteria can enter the eye from the systemic circulation and establish infection despite the presence of an intact BRB. Such hematogenous dissemination may also explain the clinical finding that ~ 50% of individuals presenting with ocular TB do not have evidence of TB elsewhere [2], suggesting that hematogenous dissemination from an initial infection focus that resolved may have seeded the eye [9]. Second, once the infection is established in the eye, peripheral monocytes can be recruited from the circulation, despite limited or no breakdown of the BRB. We previously demonstrated the role of mycobacteria in promoting macrophage recruitment to expand the forming granuloma and thereby their intracellular niche [10]. This work suggests that the monocyte-recruiting chemotactic signaling pathway induced by mycobacteria is operant in the face of the BRB, as it is in the context of the blood brain barrier [10].
Resident macrophages, including brain microglia, are more microbicidal to mycobacteria than blood monocytes [15], and it will be informative to see if this holds true in the context of retinal microglia and peripheral blood monocytes in the eye. It is conceivable that a preferential early involvement of the more proximate microglia in the forming ocular granuloma may tip the balance in the host’s favor to clear the infection, accounting for the resolution of several of the early lesions in this study.
We hope that this work will provide the foundation for future studies of the role of host and bacterial factors that influence dissemination to the eye and fate of the bacteria therein.
Data Availability
All relevant data are within the paper.
Funding Statement
The work was partially funded by Short-term fellowship to Soumyava Basu by Department of Health Research, Indian Council of Medical Research (Reference number: DHR/HRD/Short Term Foreign/ Type-II/1/2015).
References
- 1.Basu S, Monira S, Modi RR, Choudhury N, Mohan N, Padhi TR, et al. Degree, duration, and causes of visual impairment in eyes affected with ocular tuberculosis. J Ophthalmic Inflamm Infect 2014; 4:3 doi: 10.1186/1869-5760-4-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wroblewski KJ, Hidayat AA, Neafie RC, Rao NA, Zapor M. Ocular tuberculosis: a clinicopathologic and molecular study. Ophthalmology. 2011;118:772–777. doi: 10.1016/j.ophtha.2010.08.011 [DOI] [PubMed] [Google Scholar]
- 3.Finnoff WC. Changes found in eyes of rabbits following injection of living tubercle bacilli into the common carotid artery. Am J Ophthalmol, 1924;7:81–9. [PMC free article] [PubMed] [Google Scholar]
- 4.Rao NA, Albini TA, Kumaradas M, Pinn ML, Fraig MM, Karakousis PC., Experimental ocular tuberculosis in guinea pigs. Arch Ophthalmol. 2009;127:1162–6 doi: 10.1001/archophthalmol.2009.220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davis JM, Clay H, Lewis JL, Ghori N, Herbomel P, Ramakrishnan, L. Real-time visualization of mycobacterium-macrophage interactions leading to initiation of granuloma formation in zebrafish embryos. Immunity. 2002;17:693–702. [DOI] [PubMed] [Google Scholar]
- 6.Takaki K, Davis JM, Winglee K, Ramakrishnan L. Evaluation of the pathogenesis and treatment of Mycobacterium marinum infection in zebrafish. Nature Protocols. 2013;8:1114–24. doi: 10.1038/nprot.2013.068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schmitt EA, Dowling JE. Early-eye morphogenesis in the zebrafish, Brachydanio rerio. J Comparative Neurol, 1994;344:532–42. [DOI] [PubMed] [Google Scholar]
- 8.Xie J, Farage E, Sugimoto M Anand-Apte B. A novel transgenic zebrafish model for blood-brain and blood-retinal barrier development. BMC Dev Biol, 2010;10:76 doi: 10.1186/1471-213X-10-76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krishnan N, Robertson BD, Thwaites G. The mechanisms and consequences of the extra-pulmonary dissemination of Mycobacterium tuberculosis. Tuberculosis. 2010;90:361–6. doi: 10.1016/j.tube.2010.08.005 [DOI] [PubMed] [Google Scholar]
- 10.Davis JM, Ramakrishnan L. The role of the granuloma in expansion and dissemination of early tuberculous infection. Cell. 2009;136:37–49. doi: 10.1016/j.cell.2008.11.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Adams KN, Takaki K, Connolly LE, Wiedenhoft H, Winglee K, Humbert O, et al. Drug tolerance in replicating mycobacteria mediated by a macrophage-induced efflux mechanism. Cell. 2011;145:39–53. doi: 10.1016/j.cell.2011.02.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin PL, Ford CB, Coleman MT, Myers AJ, Gawande R, Ioerger T, et al. Sterilization of granulomas is common in active and latent tuberculosis despite within-host variability in bacterial killing. Nature Medicine. 2014;20:75–9. doi: 10.1038/nm.3412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Herbomel P, Thisse B, Thisse C. Zebrafish early macrophages colonize cephalic mesenchyme and developing brain, retina, and epidermis through a M-CSF receptor-dependent invasive process. Dev Biol. 2001;238:274–288. doi: 10.1006/dbio.2001.0393 [DOI] [PubMed] [Google Scholar]
- 14.Donahue HC. Ophthalmologic experience in a tuberculosis sanatorium. American journal of ophthalmology. 1967;64:742–8. [DOI] [PubMed] [Google Scholar]
- 15.Cambier CJ, O’Leary MO, O’Sullivan MP, Keane J, Ramakrishnan L. Phenolic glycolipid facilitates mycobacterial escape from a microbicidal population of tissue resident macrophages. Immunity, 2017;47:552–65.e4 doi: 10.1016/j.immuni.2017.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.