Abstract
Objective:
Several countries have developed Clinical Practice Guidelines regarding treatment of perinatal depressive symptoms and perinatal use of antidepressant. We aimed to compare guidelines to guide clinicians in best clinical practice.
Methods:
An extensive search in guideline databases, MEDLINE and PsycINFO was performed. When no guidelines were (publicly) available online, we contacted psychiatric-, obstetric-, perinatal- and mood disorder societies of all first world countries and the five largest second world countries. Only Clinical Practice Guidelines adhering to quality criteria of the Appraisal of Guidelines for Research and Evaluation instrument and including a systematic review of evidence were included. Data extraction focussed on recommendations regarding continuation or withdrawal of antidepressants and preferred treatment in newly depressed patients.
Results:
Our initial search resulted in 1094 articles. After first screening, 40 full-text articles were screened. Of these, 24 were excluded for not being an official Clinical Practice Guidelines. In total, 16 Clinical Practice Guidelines were included originating from 12 countries. Eight guidelines were perinatal specific and eight were general guidelines.
Conclusion:
During pregnancy, four guidelines advise to continue antidepressants, while there is a lack of evidence supporting this recommendation. Five guidelines do not specifically advise or discourage continuation. For new episodes, guidelines agree on psychotherapy (especially cognitive behavioural therapy) as initial treatment for mild to moderate depression and antidepressants for severe depression, with a preference for sertraline. Paroxetine is not preferred treatment for new episodes but switching antidepressants for ongoing treatment is discouraged (three guidelines). If mothers use antidepressants, observation of the neonate is generally recommended and breastfeeding encouraged.
Keywords: Clinical Practice Guideline, antidepressants, perinatal depression, pregnancy, depressive disorder
Introduction
Depression is a common mental disorder and the leading cause of disability worldwide (World Health Organization, 2017). In high-income countries, up to 15% of people experience at least one major depressive episode in their life (Kessler and Bromet, 2013; Kruijshaar et al., 2005). Women in the Western world are affected twice as often as men (Piccinelli and Wilkinson, 2000). Perinatal depression (considered here as depression arising in the period from conception to the end of the first postnatal year) affects up to 15% of women; a recent meta-analysis showed a pooled prevalence of 11.9% of all pregnancies, without significant differences between prevalence estimates for the prenatal and postnatal periods (Woody et al., 2017).
Several management options are available for depressive disorders (Malhi et al., 2015). Most patients, 65–80%, will be treated by a general practitioner (Alonso et al., 2004; Bijl and Ravelli, 2000; Bushnell et al., 2006; Kovess-Masfety et al., 2007; Verhaak et al., 2009, 2012; Wang et al., 2000), who are instructed to use a stepped care management approach (Van Weel-Baumgarten et al., 2012). Especially in mild to moderate depression, these approaches recommend psychotherapy as first-line treatment, before starting antidepressants. However, in current practice, around 70% of cases are primarily treated with antidepressants (Bushnell et al., 2006; Olfson et al., 2016; Sleath et al., 2001; Verhaak et al., 2012). Subsequently, many patients continue to take medication for a longer period; for example, over 60% of Americans continue medication for 2 years or more and 14% continues medication for 10 years or more (Pratt et al., 2011). Women in their reproductive ages are three times as likely to use antidepressants compared to men (Pratt et al., 2011). In case of a pregnancy, decisions regarding the use of antidepressants are complex.
Although antidepressants are generally considered safe to use during pregnancy, this remains controversial (Simoncelli et al., 2010). Antidepressant use has been associated with an increased risk for cardiovascular malformations (Grigoriadis et al., 2013a), persistent pulmonary hypertension of the neonate (Kieler et al., 2012), poor neonatal adaptation (Grigoriadis et al., 2013b), preterm delivery, lower birth weight (Ross et al., 2013) and psychiatric disorders in offspring (Liu et al., 2017).
Untreated perinatal depression is not risk free either. Children of women who suffered from depression during pregnancy have an increased risk of premature delivery, low birth weight, gestational hypertension (Grigoriadis et al., 2013c; Grote et al., 2010) and perinatal death (Howard et al., 2007). Perinatal depression can also lead to behavioural, emotional, cognitive and motor problems in early childhood (Field, 2011; Talge et al., 2007). Postnatal depression may influence the mother–infant relationship, which can lead to poor infant development and outcomes (Goodman et al., 2011; Tronick and Reck, 2009). Together, decisions regarding the prevention and treatment of perinatal depression (including the use of antidepressants) are complex.
To facilitate this decision-making, several countries have developed ‘Clinical Practice Guidelines’ (CPGs), to guide clinicians in choosing the most efficacious and least harmful intervention. According to the Institute of Medicine, CPGs are based on a systematic review of evidence and include recommendations to optimize patient care (Graham et al., 2011). The objective of this study was to review the content of the internationally available guidelines on the treatment of perinatal depression and the perinatal use of antidepressants.
Methods
Identification of guidelines
We initially performed an extensive search in databases for CPGs using the terms ‘pregnancy’, ‘mood disorders’, ‘depression’ and/or ‘antidepressants’. The following databases were searched: National Guideline Clearinghouse (US AHRQ), National Institute for Health and Care Excellence (UK): Evidence Services, Canadian Medical Association Infobase: CPG, Guidelines, National Health and Medical Research Council (NHMRC) (Australia): CPGs and the Guidelines International Network (G-I-N). Second, we searched MEDLINE (accessed via PubMed) using a combination of free text terms (antidepressant, pregnancy, antenatal period, depression, prenatal period, mental health), limiting the results with a filter to retrieve guidelines only. Third, we searched PsycINFO using a combination of title keywords (depression OR mental health OR mood disorder AND guideline), since PsycINFO does not have a search limit for guidelines and would otherwise retrieve too many hits.
Consecutively, we identified all professional societies of obstetricians and gynaecologists and all professional societies of psychiatrists for countries for which we did not yet retrieve a guideline. For feasibility reasons, we limited our search to societies of first world countries and the largest second and third world countries. First world refers to ‘so called developed, capitalist, industrial countries, roughly, a bloc of countries aligned with the United States after World War II, with more or less common political and economic interests’ (source: nationsonline.org). These include 25 countries: the United States, Canada, Australia, New Zealand, Japan, Korea, the United Kingdom, France, Germany, Belgium, the Netherlands, Spain, Italy, Portugal, Turkey, Greece, Luxembourg, Israel, Austria, Switzerland, Ireland, Sweden, Norway, Iceland and Denmark. In addition, we searched for guidelines in large second and third world countries including Brazil, China, India, Mexico and South Africa. When no guidelines were (publicly) available online, we contacted perinatal and mood disorder societies and send two reminders to non-responders.
Finally, we sent out an email to the members of the Marcé society (an international society for perinatal health) and to our international contacts asking for information on missing guidelines, independent on country of origin.
Selection of guidelines
Only CPGs, defined as statements that include recommendations, intended to optimize patient care, that are informed by a systematic review of evidence and an assessment of the benefits and harms of alternative care options, were selected. These should adhere to the quality criteria of the Appraisal of Guidelines for Research and Evaluation (AGREE) instrument (www.agreetrust.org). To avoid documents not meeting these quality criteria, consensus statements and guidance papers were excluded from this review. There were no limits for publication date or language. CPGs that did not comment on the perinatal management of mood disorders and/or on the perinatal use of antidepressants were excluded. Only the latest or more complete version of a guideline was selected when several versions of the same guideline were available.
Data extraction
Data extraction focussed on recommendations before, during and after pregnancy. Recommendations were investigated both for newly arising symptoms of depression and for pre-existent antidepressant use. We included recommendations regarding management of pre-existent antidepressant use, preferred treatment in newly depressed patients and breastfeeding with antidepressants. Recommendations were scored as follows: (blank) no mention of the measure in the guideline, (0) measure mentioned in the guideline but without a clear direction of the recommendation (no positive or negative advice), (+) measure advised by guideline or (–) measure discouraged by guideline.
Results
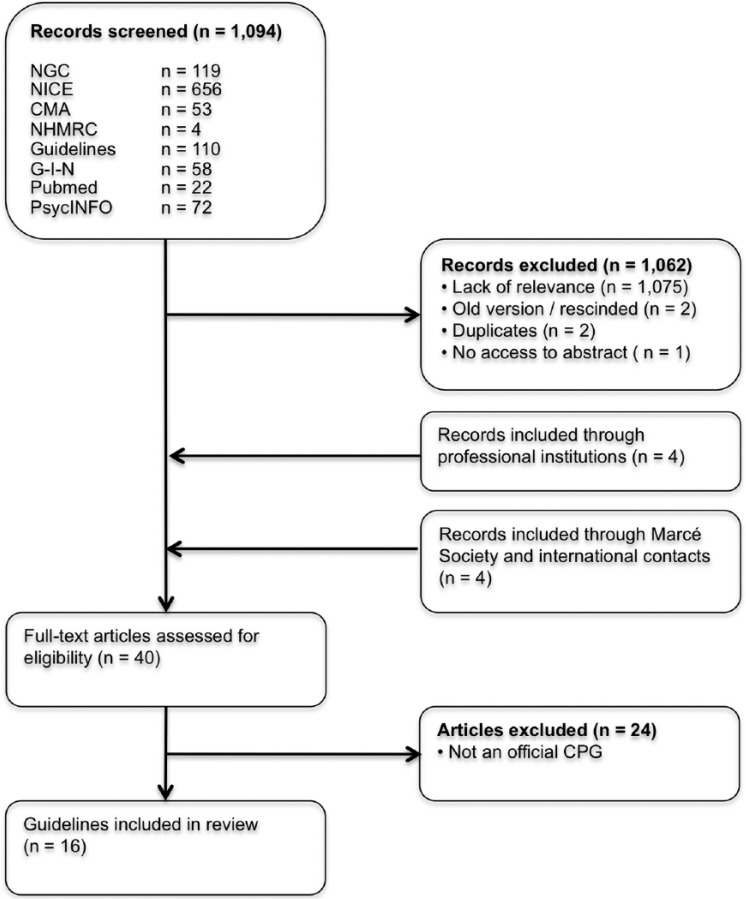
Our guideline database search strategy produced a set of 1000 articles. Our PubMed search added another 22 articles and our PsycINFO search 72. Of these 1094 articles, 1062 were excluded after screening on title and abstract (Figure 1). Our search strategy through the professional societies, the Marcé Society and international contacts resulted in an additional eight articles. After thorough assessment, 24 articles were excluded for not being an official CPG. This resulted in a total of 16 guidelines originating from 12 countries (ACOG Committee on Practice Bulletins-Obstetrics, 2008; American Psychiatric Association, 2010; Austin et al., 2017; BC Reproductive Mental Health Program, 2014; Dansk Psykiatrisk Selskab, et al., 2014; DGPPN et al., 2015; Li and Ma, 2015; MacQueen et al., 2016; Malhi et al., 2015; Management of Major Depressive Disorder Working Group, 2016; Ministry of Health, 2012; Ministry of Health, Social Services and Equality, 2014; National Collaborating Centre for Mental Health, 2014; Nederlandse Vereniging voor Obstetrie en Gynaecologie, 2012; Nordeng and Jettestad, 2015; Scottish Intercollegiate Guidelines Network (SIGN), 2012). In addition, we received information on the absence of a national guideline from the following countries: Austria, Belgium, France, Israel, Luxembourg, Mexico, Portugal, South Africa, Sweden, Switzerland and Turkey. Guidelines from India and Israel are in progress.
Figure 1.
Flowchart of the article selection process.
NGC: National Guideline Clearinghouse; NICE: National Institute for Health and Care Excellence; CMA: Canadian Medical Association Infobase; NHMRC: National Health and Medical Research Council; G-I-N: Guidelines International Network; CPG: Clinical Practice Guideline.
Table 1 shows the specifics and recommendations of these guidelines. Eight guidelines were exclusively on perinatal management; the remaining guidelines were general guidelines on treatment of depression but included a section on perinatal recommendations.
Table 1.
Summary of guideline recommendations pre-, during and post-pregnancy and perinatal medication recommendations.
| Pre-pregnancy |
Pregnancy |
Postpartum |
Medication recommendations |
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Country of origin | Year of publication | Perinatal specific | Pregnancy planning | Continue AD | Switch AD | Psychotherapy for new depression | Medication for new depression | Continue AD | Switch AD | Psychotherapy for new depression | Medication for new depression | Continue AD | Switch AD | Breastfeeding | Psychotherapy for new depression | Medication for new depression | Preferred medication | Non-preferred medication | |
| ACOG | USA | 2008 | √ | 0 | + | Paroxetine | |||||||||||||
| APA | USA | 2010 | + | + | 0 | 0 | + | + | + | 0 | 0 | Paroxetine | |||||||
| BC | Canada | 2014 | √ | + | – | 0 | – | + | + | + | + | Paroxetine | |||||||
| BMU | China | 2015 | + | + | |||||||||||||||
| CANMAT | Canada | 2016 | + | + | + | + | + | + | Sertraline, (es)citalopram | Paroxetine, fluoxetine | |||||||||
| COPE | Australia | 2017 | √ | + | + | + | + | + | + | + | |||||||||
| Danish | Denmark | 2014 | √ | + | + | + | 0 | Sertraline, citalopram | Paroxetine, fluoxetine | ||||||||||
| DGPPN | Germany | 2017 | + | 0 | 0 | Paroxetine, fluoxetine | |||||||||||||
| NFOG | Norway | 2015 | √ | – | + | – | + | + | + | Paroxetine | |||||||||
| NHS | Spain | 2014 | Fluoxetine | Paroxetine | |||||||||||||||
| NICE | UK | 2014 | √ | + | + | + | 0 | 0 | + | + | 0 | 0 | + | + | + | ||||
| NVOG | Netherlands | 2012 | √ | + | – | 0 | + | + | Paroxetine | ||||||||||
| MOH | Singapore | 2012 | 0 | + | + | + | + | ||||||||||||
| RANZCP | Australia and New Zealand | 2015 | + | + | + | + | Paroxetine, fluoxetine, venlafaxine | ||||||||||||
| SIGN | UK | 2012 | √ | + | – | + | + | + | + | + | Paroxetine | ||||||||
| VA/DoD | USA | 2016 | + | + | Sertraline | Paroxetine, fluoxetine | |||||||||||||
√: yes; +: advised by guideline; –: discouraged by guideline; 0: mentioned but no steering recommendation; ACOG: American College of Obstetricians and Gynaecologists; APA: American Psychiatric Association; VA/DoD: Department of Veterans Affairs/Department of Defense; BC: British Columbia Reproductive Mental Health Program & Perinatal Services British Columbia; BMU: Beijing Medical University; CANMAT: Canadian Network for Mood and Anxiety Treatments; COPE: Centre of Perinatal Excellence; RANZCP: Royal Australian and New Zealand College of Psychiatrists; NICE: National Institute for Health and Care Excellence; SIGN: Scottish Intercollegiate Guidelines Network; Danish: Danish Psychiatric Society, Danish Society for Obstetrics and Gynaecology, Danish Paediatric Society and Danish Company for Clinical Pharmacology; DGPPN: German Society for Psychiatry and Psychotherapy, Psychosomatics and Neurology; NVOG: Dutch Society of Obstetrics and Gynaecology; NFOG: Nordic Federation of Societies of Obstetrics and Gynaecology; NHS: Spanish ministry of health, social services and equality; MOH: Ministry of Health, Singapore.
Pre-pregnancy
Three guidelines discourage switching antidepressants. The Dutch Society of Obstetrics and Gynaecology (NVOG; the Netherlands) advises to continue antidepressants if the patient is psychiatrically stable.
With regard to initial therapy for new depressive symptomatology, the American Psychiatric Association (APA; USA), Centre of Perinatal Excellence (COPE; Australia) and National Institute for Health and Care Excellence (NICE; UK) guidelines give detailed recommendations. All three advise psychotherapy as initial treatment. In more severe cases of depression, the COPE and NICE guidelines advise antidepressants as initial therapy.
Pregnancy
During pregnancy, four guidelines advise to continue antidepressants. Five other guidelines mention the possibility of continuation but do not specifically advise or discourage continuation. Three guidelines discourage switching antidepressants during pregnancy. In contrast, the Danish guideline promotes switching when unfavourable antidepressants (paroxetine and fluoxetine) are used.
Most guidelines agree on psychotherapy as initial treatment for mild to moderate depression and antidepressants as initial therapy for severe depression. Only the American College of Obstetricians and Gynaecologists (ACOG; USA) guideline recommends antidepressants as preferred initial therapy instead of psychotherapy and independent of symptom severity.
There is general consensus that potential harms and benefits of antidepressants during pregnancy should be discussed by the clinician with the patient. This way, patients can make well-informed decisions on preferred treatment.
Management around delivery
Most guidelines recommend a hospital delivery, which is standard in most countries. In the Netherlands and Canada, home births are still common; therefore, these guidelines explicitly mention a hospital delivery with additional observation as preferred option.
Postpartum observation of the neonate is generally recommended but the length of observation is variable (ranging from 12 hours to 3 days). The BC guideline (Canada) recommends more intense monitoring of the neonate, including pulse oximetry for early detection of persistent pulmonary hypertension and on indication neonatal serum levels of antidepressants.
Postpartum
BC (Canada) and NVOG (the Netherlands) specifically recommend continuation of antidepressants to prevent relapse of depressive symptoms. For new episodes, most guidelines agree on psychotherapy as initial treatment for mild to moderate depression and consideration of antidepressants as initial therapy for severe depression. Most guidelines agree on encouraging breastfeeding, independent of the kind of antidepressant medication the patient is taking. The Nordic Federation of Societies of Obstetrics and Gynaecology (NFOG; Norway) advises switching medication when breastfeeding with unfavourable medication. Sertraline is named as favourable medication mainly due to its low level in breast milk and infants serum.
Medication preference
Recommended medication preferences are often not pregnancy stage specific. In general, guidelines agree on avoiding paroxetine during pregnancy, since the use of paroxetine is associated with increased risk of congenital cardiovascular malformations in the newborn (Grigoriadis et al., 2013a). In addition, the ACOG guideline (USA) recommends foetal examination by echocardiography if the foetus is exposed to paroxetine during early pregnancy.
Five guidelines marked fluoxetine as ‘unfavourable’, due to its long half-life and its presence in breast milk. Remarkably, the NHS (Spanish ministry of health, social services and equality; Spain) mentions fluoxetine as preferred medication.
There is general consensus on sertraline as preferred medication by the guidelines mentioning preferences for the postpartum period, mainly due to its favourable profile during lactation (Pinheiro et al., 2015). CANMAT (Canadian) and the Danish guideline also mention citalopram as preferred medication because of its minimized risk during lactation and available data on effectiveness during the postpartum period (Molyneaux et al., 2014).
Discussion
For new depressive episodes, there is general consensus within guidelines for what is considered ‘best clinical practice’. Guidelines recommend, independent of pregnancy stage, to discuss all potential treatment options available and their potential harms and benefits during and after pregnancy. Most guidelines agree that psychotherapy, especially cognitive behavioural therapy (CBT), should be considered as initial treatment for mild to moderate depression, both during pregnancy and the postpartum period. Psychotherapy, such as CBT or interpersonal therapy, has a robust treatment effect for depressive disorder during pregnancy (Van Ravesteyn et al., 2017) and research to other treatment options like bright light therapy is still ongoing (Bais et al., 2016). In more severe cases, antidepressants are preferred treatment options, although, until this date, there are no controlled studies on the effects of psychotropic medication for antepartum mental disorders (Van Ravesteyn et al., 2017); the consequence of ethical constraints of conducting clinical trials with pregnant participants. Paroxetine is not a first-choice treatment option, considering its possible increased risk for congenital heart malformations. Preferred medications during the perinatal period include sertraline and citalopram. Breastfeeding is encouraged with sertraline as preferred medication.
More complicated is the management of pre-pregnancy use of antidepressants and continuation during pregnancy. Unfortunately, evidence on the risks and benefits of tapering antidepressants during pregnancy is limited. One naturalistic study (n = 201) of women with long-standing depression (mean duration of illness 15.4 years) showed a significant increased risk of relapse in pregnant women who discontinued their medication, compared to those continuing medication (44 [68%] vs 21 [26%]) (Cohen et al., 2006), while another naturalistic study (n = 778) showed no clear difference in relapse rates of depression (16% in total) between women continuing and discontinuing antidepressants (Yonkers et al., 2011). Randomized controlled trials (RCTs) are currently lacking with only one RCT in progress (Molenaar et al., 2016). Four guidelines advise continuation of antidepressants during pregnancy, which is remarkable given the scarce evidence. Unfortunately, none of the guidelines discusses treatment options for patients with current depressive symptomatology despite antidepressant use.
Most guidelines acknowledge the importance of personalized medicine. For suitable decision-making, the following should be taken into consideration: psychiatric history and indication for antidepressant medication, current psychiatric symptoms, previous attempts of tapering medication, availability of alternative treatment options such as preventive psychotherapy and the presence of a social support network. Moreover, clinical algorithms need to be developed to improve decision-making. Currently, a pilot study is being executed to investigate if a patient decision aid (PDA) tool can reduce decision-making difficulty and lead to better treatment outcomes in pregnant women with antidepressant use (Vigod et al., 2016).
Overall, the guidelines have good quality (Santos et al., 2012), but most CPGs were not specifically developed for pregnant women and contained limited information on the measures of implementation and audit of the proposed measures. In our review, only eight guidelines were perinatal specific. Moreover, as pointed out by Santos et al. (2012), guidelines do not disclose recommendations on emerging clinical questions and on new available evidence.
For this review, we did not include Clinical Consensus Statements (CSSs) because they were not developed in accordance with CPGs. CSSs reflect the expert views of a panel of individuals who are well versed on the topic of interest while carefully examining and discussing the scientific data available. These consensus statements might give different recommendations than stated in the CPGs. For example, an Austrian CSS suggests tapering of antidepressants 2 weeks before the due date to reduce neonatal adaptation problems (Kasper et al., 2012). None of the CPGs mention this option, possibly because of available evidence suggesting reduction of exposure to selective serotonin reuptake inhibitors (SSRIs) at the end of pregnancy has no significant effect on improving neonatal health (Warburton et al., 2010). In clinical practice, CSSs and other guiding documents are frequently used instead of the formal guidelines and might contain a higher level of detail.
In summary, this overview of information might be helpful for the development of new CPGs. Clearly, there is a need for up-to-date and perinatal-specific CPGs and CSSs to help clinicians and patients in decision-making. It is challenging to develop these CPGs because evidence-based medicine, personalized medicine and legal liabilities need to be balanced.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship and/or publication of this article.
ORCID iD: Nina M Molenaar  https://orcid.org/0000-0003-2531-9467
https://orcid.org/0000-0003-2531-9467
References
- ACOG Committee on Practice Bulletins-Obstetrics (2008) Use of psychiatric medications during pregnancy and lactation. Obstetrics and Gynecology 111: 1001–1020. [DOI] [PubMed] [Google Scholar]
- Alonso J, Angermeyer MC, Bernert S, et al. (2004) Use of mental health services in Europe: Results from the European Study of the Epidemiology of Mental Disorders (ESEMeD) project. Acta Psychiatrica Scandinavica 420: 47–54. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association (2010) Practice Guideline for the Treatment of Patients with Major Depressive Disorder, 3rd Edition. Arlington, VA: American Psychiatric Association. [Google Scholar]
- Austin MP, Highet N. and the Expert Working Group (2017) Mental Health Care in the Perinatal Period: Australian Clinical Practice Guideline. Melbourne, VIC, Australia: Centre of Perinatal Excellence. [Google Scholar]
- Bais B, Kamperman AM, van der Zwaag MD, et al. (2016) Bright light therapy in pregnant women with major depressive disorder: Study protocol for a randomized, double-blind, controlled clinical trial. BMC Psychiatry 16: 381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BC Reproductive Mental Health Program (2014) Best Practice Guidelines for Mental Health Disorders in the Perinatal Period. Vancouver, BC, Canada: BC Reproductive Mental Health. [Google Scholar]
- Bijl RV, Ravelli A. (2000) Psychiatric morbidity, service use, and need for care in the general population: Results of the Netherlands Mental Health Survey and Incidence Study. American Journal of Public Health 90: 602–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushnell J, McLeod D, Dowell A, et al. (2006) The treatment of common mental health problems in general practice. Family Practice 23: 53–59. [DOI] [PubMed] [Google Scholar]
- Cohen LS, Altshuler LL, Harlow BL, et al. (2006) Relapse of major depression during pregnancy in women who maintain or discontinue antidepressant treatment. JAMA 295: 499–507. [DOI] [PubMed] [Google Scholar]
- Dansk Psykiatrisk Selskab, Dansk Selskab for Obstetrik og Gynaekologi, Dansk Paediatrisk Selskab, Dansk Selskab for Klinisk Farmakologi. (2014) Anvendelse af psykofarmaka ved graviditet og amning - kliniske reningslinjer. Edition 27October2014 [Google Scholar]
- DGPPN, BÄK, KBV, AWMF (Hrsg.) für die Leitliniengruppe Unipolare Depression (2015). S3-Leitlinie/Nationale Versor-gungsLeitlinie Unipolare Depression - Langfassung, 2. Auflage. Version 5. 2015. [cited: 2018-February-22]. DOI: 10.6101/AZQ/000364 www.depression.versorgungsleitlinien.de. [DOI]
- Field T. (2011) Prenatal depression effects on early development: A review. Infant Behavior & Development 34: 1–14. [DOI] [PubMed] [Google Scholar]
- Goodman SH, Rouse MH, Connell AM, et al. (2011) Maternal depression and child psychopathology: A meta-analytic review. Clinical Child and Family Psychology Review 14: 1–27. [DOI] [PubMed] [Google Scholar]
- Graham R, Mancher M, Wolman DM, et al. (2011) Clinical Practice Guidelines We Can Trust. Washington, DC: National Academies Press. [PubMed] [Google Scholar]
- Grigoriadis S, VonderPorten EH, Mamisashvili L, et al. (2013. a) Antidepressant exposure during pregnancy and congenital malformations: Is there an association? A systematic review and meta-analysis of the best evidence. Journal of Clinical Psychiatry 74: e293–e308. [DOI] [PubMed] [Google Scholar]
- Grigoriadis S, VonderPorten EH, Mamisashvili L, et al. (2013. b) The effect of prenatal antidepressant exposure on neonatal adaptation: A systematic review and meta-analysis. Journal of Clinical Psychiatry 74: e309–e320. [DOI] [PubMed] [Google Scholar]
- Grigoriadis S, VonderPorten EH, Mamisashvili L, et al. (2013. c) The impact of maternal depression during pregnancy on perinatal outcomes: A systematic review and meta-analysis. Journal of Clinical Psychiatry 74: e321–e341. [DOI] [PubMed] [Google Scholar]
- Grote NK, Bridge JA, Gavin AR, et al. (2010) A meta-analysis of depression during pregnancy and the risk of preterm birth, low birth weight, and intrauterine growth restriction. Archives of General Psychiatry 67: 1012–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard LM, Kirkwood G, Latinovic R. (2007) Sudden infant death syndrome and maternal depression. Journal of Clinical Psychiatry 68: 1279–1283. [DOI] [PubMed] [Google Scholar]
- Kasper S, Lehofer M, Doering S, et al. (2012) Depression - Medikametöse Therapie. CliniCum neuropsy. Sonderausgabe November 2012. [Google Scholar]
- Kessler RC, Bromet EJ. (2013) The epidemiology of depression across cultures. Annual Review of Public Health 34: 119–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieler H, Artama M, Engeland A, et al. (2012) Selective serotonin reuptake inhibitors during pregnancy and risk of persistent pulmonary hypertension in the newborn: Population based cohort study from the five Nordic countries. BMJ 344: d8012. [DOI] [PubMed] [Google Scholar]
- Kovess-Masfety V, Alonso J, Brugha TS, et al. (2007) Differences in lifetime use of services for mental health problems in six European countries. Psychiatric Services 58: 213–220. [DOI] [PubMed] [Google Scholar]
- Kruijshaar ME, Barendregt J, Vos T, et al. (2005) Lifetime prevalence estimates of major depression: An indirect estimation method and a quantification of recall bias. European Journal of Epidemiology 20: 103–111. [DOI] [PubMed] [Google Scholar]
- Li L, Ma X. (2015) Guideline on the Prevention and Treatment of Depressive Disorder in China, 2nd Edition. Beijing, China: Beijing Medical University Press. [Google Scholar]
- Liu X, Agerbo E, Ingstrup KG, et al. (2017) Antidepressant use during pregnancy and psychiatric disorders in offspring: Danish nationwide register based cohort study. BMJ 358: j3668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacQueen GM, Frey BN, Ismail Z, et al. (2016) Canadian Network for Mood and Anxiety Treatments (CANMAT) 2016 clinical guidelines for the management of adults with major depressive disorder: Section 6. Special populations – Youth, women, and the elderly. Canadian Journal of Psychiatry 61: 588–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhi GS, Bassett D, Boyce P, et al. (2015) Royal Australian and New Zealand College of Psychiatrists clinical practice guideline for mood disorders. Australian and New Zealand Journal of Psychiatry 49: 1087–1206. [DOI] [PubMed] [Google Scholar]
- Management of Major Depressive Disorder Working Group (2016) VA/DoD Clinical Practice Guideline for the Management of Major Depressive Disorder (version 3.0). Washington, DC: Department of Veterans Affairs and Department of Defense. [Google Scholar]
- Ministry of Health (2012) MOH Clinical Practice Guidelines 1/2012 – Depression. Singapore: Ministry of Health. [Google Scholar]
- Ministry of Health, Social Services and Equality (2014) Clinical Practice Guideline on the Management of Depression in Adults: SNS Clinical Practice Guidelines. Madrid: Ministry of Health, Social Services and Equality. [Google Scholar]
- Molenaar NM, Brouwer ME, Bockting CLH, et al. (2016) Stop or go? Preventive cognitive therapy with guided tapering of antidepressants during pregnancy: Study protocol of a pragmatic multicentre non-inferiority randomized controlled trial. BMC Psychiatry 16: 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molyneaux E, Howard LM, McGeown HR, et al. (2014) Antidepressant treatment for postnatal depression. Cochrane Database of Systematic Reviews 11: CD002018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Collaborating Centre for Mental Health (2014) Antenatal and Postnatal Mental Health: The NICE Guideline on Clinical Management and Service Guidance. London: British Psychological Society and Royal College of Psychiatrists. [PubMed] [Google Scholar]
- Nederlandse Vereniging voor Obstetrie en Gynaecologie (2012) Richtlijn: SSRI-gebruik in de zwangerschap en tijdens de lactatie. North Padre Island, TX: Nederlandse Vereniging voor Obstetrie en Gynaecologie. [Google Scholar]
- Nordeng H, Jettestad M. (2015) Depression during Pregnancy and Lactation. Oslo: Nordic Federation of Obstetrics and Gynaecology. [Google Scholar]
- Olfson M, Blanco C, Marcus SC. (2016) Treatment of adult depression in the United States. JAMA Internal Medicine 176: 1482–1491. [DOI] [PubMed] [Google Scholar]
- Piccinelli M, Wilkinson G. (2000) Gender differences in depression: Critical review. British Journal of Psychiatry 177: 486–492. [DOI] [PubMed] [Google Scholar]
- Pinheiro E, Bogen DL, Hoxha D, et al. (2015) Sertraline and breastfeeding: Review and meta-analysis. Archives of Women’s Mental Health 18: 139–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt LA, Brody DJ, Gu Q. (2011) Antidepressant use in persons aged 12 and over: United States, 2005–2008. NCHS Data Brief 76: 1–8. [PubMed] [Google Scholar]
- Ross LE, Grigoriadis S, Mamisashvili L, et al. (2013) Selected pregnancy and delivery outcomes after exposure to antidepressant medication: A systematic review and meta-analysis. JAMA Psychiatry 70: 436–443. [DOI] [PubMed] [Google Scholar]
- Santos F, Sola I, Rigau D, et al. (2012) Quality assessment of clinical practice guidelines for the prescription of antidepressant drugs during pregnancy. Current Clinical Pharmacology 7: 7–14. [DOI] [PubMed] [Google Scholar]
- Scottish Intercollegiate Guidelines Network (SIGN) (2012) Management of Perinatal Mood Disorders (Publication no. 127). Edinburgh: SIGN. [Google Scholar]
- Simoncelli M, Martin BZ, Berard A. (2010) Antidepressant use during pregnancy: A critical systematic review of the literature. Current Drug Safety 5: 153–170. [DOI] [PubMed] [Google Scholar]
- Sleath BL, Rubin RH, Huston SA. (2001) Antidepressant prescribing to Hispanic and non-Hispanic white patients in primary care. Annals of Pharmacotherapy 35: 419–423. [DOI] [PubMed] [Google Scholar]
- Talge NM, Neal C, Glover V, et al. (2007) Antenatal maternal stress and long-term effects on child neurodevelopment: How and why? J Child Psychol Psychiatry 48: 245–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tronick E, Reck C. (2009) Infants of depressed mothers. Harvard Review of Psychiatry 17: 147–156. [DOI] [PubMed] [Google Scholar]
- Van Ravesteyn LM, Lambregtse-van den, Berg MP, Hoogendijk WGJ, et al. (2017) Interventions to treat mental disorders during pregnancy: A systematic review and multiple treatment meta-analysis. PLoS ONE 12: e0173397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Weel-Baumgarten EM, Van Gelderen MG, Grundmeijer HGLM, et al. (2012) NHG-Standaard Depressie (tweede herziening). Huisarts Wet 55: 252–259. [Google Scholar]
- Verhaak PF, Prins MA, Spreeuwenberg P, et al. (2009) Receiving treatment for common mental disorders. General Hospital Psychiatry 31: 46–55. [DOI] [PubMed] [Google Scholar]
- Verhaak PF, van Dijk CE, Nuijen J, et al. (2012) Mental health care as delivered by Dutch general practitioners between 2004 and 2008. Scandinavian Journal of Primary Health Care 30: 156–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigod S, Hussain-Shamsy N, Grigoriadis S, et al. (2016) A patient decision aid for antidepressant use in pregnancy: Study protocol for a randomized controlled trial. Trials 29: 110–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang PS, Berglund P, Kessler RC. (2000) Recent care of common mental disorders in the United States: Prevalence and conformance with evidence-based recommendations. Journal of General Internal Medicine 15: 284–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warburton W, Hertzman C, Oberlander TF. (2010) A register study of the impact of stopping third trimester selective serotonin reuptake inhibitor exposure on neonatal health. Acta Psychiatrica Scandinavica 121: 471–479. [DOI] [PubMed] [Google Scholar]
- Woody CA, Ferrari AJ, Siskind DJ, et al. (2017) A systematic review and meta-regression of the prevalence and incidence of perinatal depression. Journal of Affective Disorders 219: 86–92. [DOI] [PubMed] [Google Scholar]
- World Health Organization (WHO) (2017) Depression and Other Common Mental Disorders: Global Health Estimates. Geneva: WHO. [Google Scholar]
- Yonkers KA, Gotman N, Smith MV, et al. (2011) Does antidepressant use attenuate the risk of a major depressive episode in pregnancy? Epidemiology 22: 848–854. [DOI] [PMC free article] [PubMed] [Google Scholar]