Abstract
Background.
Cyclophosphamide (CP) is a nitrogen mustard alkylating drug used for the treatment of chronic and acute malignant lymphomas, myeloma, leukemia, neuroblastoma, adenocarcinoma, retinoblastoma, breast carcinoma, and immunosuppressive therapy. Despite its vast therapeutic uses, it is known to cause severe cardiac toxicity. Kolaviron (KV), a Garcinia kola seed extract containing a mixture of flavonoids, is reputed for its antioxidant and membrane stabilizing properties.
Objective.
This study investigated the protective effect of KV on CP-induced cardiotoxicity in rats.
Methods.
Thirty rats were used, and they were divided into 6 groups of 5 rats each. Group I received 2 mL/kg propylene glycol orally for 14 days; group II received CP (50 mg/kg/d, intraperitoneally [i.p.]) for 3 days; groups III and IV received 200 and 400 mg/kg/d KV, respectively, orally for 14 days and groups V and VI were pretreated with 200 and 400 mg/kg/d KV, respectively, orally for 14 days followed by CP (50 mg/kg/d, i.p.) for 3 days.
Results.
CP treatment resulted in a significantly lower food consumption and body weight in rats. The lactate dehydrogenase and creatine kinase enzymes in cardiac tissues of rats treated with CP were significantly higher. In cardiac tissues, 3-day doses of CP resulted in significantly higher heart weight, cardiac troponin I, myeloperoxidase, malondialdehyde, hydrogen peroxide and lower superoxide dismutase, catalase, glutathione peroxidase activities, and reduced glutathione levels. Histological examination of cardiac tissues showed sign of necrosis of myocardium after CP treatment. However, administration of KV at 200 and 400 mg/kg for 14 days prior to CP treatment, increase food consumption, body weight, and attenuates the biochemical and histological changes induced by CP.
Conclusions.
These results revealed that KV attenuates CP-induced cardiotoxicity by inhibiting oxidative stress and preserving the activity of antioxidant enzymes.
Keywords: kolaviron, cyclophosphamide, oxidative stress, cardiotoxicity, cardiac troponin I, antioxidant
Cyclophosphamide (CP) is a nitrogen mustard alkylating agent with potent antineoplastic, immunosuppressive and immunomodulatory properties.1,2 It is widely used as an antineoplastic agent for the treatment of human hematological malignancies and a variety of solid tumors such as breast cancer, carcinoma of the lung, acute leukemia, and ovarian cancer.3–5 Despite its wide spectrum of clinical uses, CP is known to cause multiple dose-dependent organ toxicity.6
The cellular mechanism of CP toxicity is due to the production of highly reactive oxygen free radicals by the metabolites; phosphoramide and acrolein.7 High therapeutic effect of CP is attributed to phosphoramide, while the other CP metabolite, acrolein is associated with toxic side effect which interferes with the tissue antioxidant defense system and produces highly reactive oxygen free radicals which are mutagenic to mammalian cells.8–10 High therapeutic doses of CP caused a lethal cardiotoxicity that presents a combination of symptoms and signs of myopericarditis, which could lead to fatal complication such as congestive heart failure, arrhythmias, and cardiac tamponade.11 Long-term administration of CP has been associated with increased lipid peroxidation and significant depletion of antioxidant molecules, such as reduced glutathione (GSH), catalase, and superoxide dismutase.12–14 Multiple clinical studies have suggested that the use of antioxidants in combination with chemotherapy and irradiation prolong the survival time of patients compared with expected outcome without antioxidant supplements.15–17 Therefore, antioxidant agents such as dl-α-lipoid acid and melatonin have protective actions against CP-induced toxicity.18,19 Thus, the combination of drug delivery together with antioxidant agent may be a potential therapeutic approach to reverse or arrest the progress of CP adverse effects.
Kolaviron (KV) is a mixture of flavonoids extracted from the seeds of Garcinia kola, which has numerous therapeutic benefits against cancer, genotoxicity, and hepatotoxicity.20–22 It protects against oxidative stress induced by toxins in experimental animal models.23,24 Previous studies have identified antioxidant capacity of KV.25,26 KV has favorable pharmacological effect in suppressing oxidative stress in various experimental models,25,26 nevertheless, its protective capacity against cardiotoxicity has not been explored previously. Hence, its effects on CP-induced cardiotoxicity remain to be explicated. This study is therefore designed to investigate the protective effects of KV on CP-induced cardiac toxicity in rats.
Materials and Methods
Drugs and Chemicals
CP (200 mg/10 mL) injection was purchased in Celon Laboratory, Hyderabad, India; ketamine hydrochloride (50 mg/10 mL) injection was purchased from Popular Pharmaceuticals Ltd, Gazipur, Bangladesh; propylene glycol was purchased from Biovision, Milpitas, CA, USA; petroleum ether, acetone, and ethyl acetate were of analytical grade and purchased from Sigma (St Louis, MO, USA). Assay kits for biochemical parameters that were carried out were purchased from Randox Laboratories Limited, Crumlin, UK.
Extraction of Kolaviron
Garcinia kola seeds were purchased from Oja Oba, in Ikere Ekiti, Nigeria and certified by a taxonomist at the herbarium of the Department of Botany, Obafemi Awolowo University, with a voucher number (IFE 17540).
Fresh seeds of Garcinia kola were peeled, air dried, and crushed into powder using an electric pulverizer (DIK-2910, Daiki Rika Kogyo Co Ltd, Tokyo, Japan). Powdered seeds of Garcinia kola weighing 2.1 kg were defatted with 3.5 L of petroleum ether (boiling point 40°C to 60°C) in a Soxhlet extractor for 24 hours. The defatted dry product was further extracted with 3.5 L of 80% acetone (1:2 w/v) in a Soxhlet extractor for 24 hours. The extract was concentrated at 40°C using a rotary evaporator, diluted to twice its volume with distilled water and partitioned with 2 L of ethyl acetate. The concentrated ethyl acetate fraction yielded KV as a golden brown solid and was freeze-dried in a lyophilizer (Ilshin Lab Co Ltd, Seoul, Republic of Korea) to a solid form. The sample obtained as a product of freeze drying was weighed to calculate for the percentage yield of the plant extract.
Scheme illustration of extraction process of kolaviron.
Stock Solutions of Kolaviron
KV was prepared at doses of 200 and 400 mg/kg. Two grams of KV were dissolved in 20 mL of propylene glycol to obtain a sample preparation (stock solution) for 200 mg/kg of KV. From the stock solution, the rats received 0.2 mL/100 g/d of the extract orally (p.o.). Stock solutions for 400 mg/kg of KV was prepared by dissolving 4 g of KV in 20 mL of propylene glycol. The control rats received 0.2 mL/100 g/d of propylene glycol. However, 200 mg anhydrous CP was dissolved in 10 mL sterile water for injection immediately before injected to the animals in order to attain the required dose.
Animals
The male experimental rats of Wistar strain weighing 120 to 150 g used in the present studies were procured from the Animal Holding of the College of Health Sciences, Obafemi Awolowo University, Ile-Ife. All the animals were kept under the standard environment condition at 12-hour light/12-hour dark cycle at room temperature and were allowed free access to a standard diet and clean drinking water. The rats were kept in animal house for 2 weeks before starting the experiments. All the animals received humane care according to the criteria outlined in the Guide for the Care and Use of Laboratory Animals prepared by the National Academy of Science and approved by Institutional Research Committee.
Experimental Design
In this experiment, 30 rats were used. The animals were divided into 6 groups of 5 rats in each group. Group I: normal control rats were treated with 2 mL/kg propylene glycol orally once daily for 14 consecutive days. Group II (CP): rats were maintained on normal rat chow for the first 14 days before injected with CP (50 mg/kg/d, intraperitoneally [i.p.]) for the last 3 days of the study. Groups III and IV: rats were treated with KV (200 and 400 mg/kg/d, p.o., respectively) for 14 consecutive days. Groups V and VI: rats were pretreated with KV (200 and 400 mg/kg/d, p.o., respectively) for 14 consecutive days, 24 hours after the last dose; rats were injected with CP (50 mg/kg/d, i.p.) for 3 consecutive days. Twenty-four hours after the last treatment, all the animals were sacrificed under ketamine anesthesia. The right and left ventricles of each rat was separated. The right ventricle of each rat was carefully excised and immediately washed in ice cold normal saline, blotted dry, and weighed. A 20% w/v of the homogenate was prepared in 50 mM Tris-HCl buffer (pH 7.4). The homogenates were centrifuged at 10 000 revolutions per minute for 15 minutes at −4°C to separate the nuclear debris. The supernatant was obtained for the estimation of cardiac marker enzymes and antioxidant status. The left ventricle of each rat was also excised, weighed, and fixed in 10% formal saline for histopathological studies using hematoxylin-eosin stain.
Measurement of Body Weight, Heart Weight, and Food Consumption
The food consumption (g) and body weight change (%) of each experimental rat were measured by digital weighing balance (Hanson, China) to assess weekly feed consumption and weight gain or loss while the weight of the dissected hearts (g) was measured with the aid of a Camry weighing balance.
Determination of Creatine Kinase and Lactate Dehydrogenase
The activity of creatine kinase (CK) and lactate dehydrogenase (LDH) were assayed in right heart tissues homogenate using commercial kits purchased from Randox Laboratories (Antrim, Crumlin, UK) following the methods of Swanson and Wilkinson,27 and Weisshaar et al,28 respectively.
Estimation of Cardiac Antioxidant Status Activities and Lipid Peroxidation Products
Superoxide dismutase (SOD) activity in cardiac tissue homogenate was determined following the methods of Misra and Fridovich.29 Estimation of catalase (CAT) activity was done by using hydrogen peroxide as substrate according to the method of Clairborne.30 GSH was determined using the method described by Beutler et al.31 Glutathione peroxidase (GPx) activity was determined by the method of Rotruck et al.32 Hydrogen peroxide (H2O2) generation was assessed by the method of Wolff,33 while lipid peroxidation was measured as malondialdehyde (MDA) and according to the method described by Ohkawa et al.34 The absorbance was read at 532 nm against the reagent blank. The results were expressed as nmol/mg protein.
Determination of Cardiac Troponin I (cTn l) Levels and Myeloperoxidase Activities
Troponin I as a marker of cardiac damage was measured using commercially available enzyme-linked immunosorbent assay (ELISA) kits (R & D Systems) according to the manufacturer’s instructions.
Myeloperoxidase (MPO) as marker of inflammation and cardiac injury was measured according to the method of Xia and Zweier.35 To 2 mL of O-dianisidine mixture (16.7 mg of O-dianisidine, 100 mL of 0.05 M potassium phosphate buffer, and 50 μL of diluted H2O2) in a cuvette, 70 μL of Proton Motive Force (PMF) was added. The increase in absorbance was monitored every 30 seconds for 1 minute. The absorbance was read at 450 nm. One unit of MPO activity can be defined as the quantity of enzyme able to convert/degrade 1 μmol of H2O2 to water in 1 minute at room temperature.
Histological Processes
The heart biopsies of the rats were fixed in 10% formalin, dehydrated in graded alcohol, cleared in xylene, and embedded in paraffin wax. The tissues were then cut into 2- to 3-μm thick sections by a microtome, fixed on the slides, and stained with hematoxylin-eosin. The slides were examined under a light microscope (Olympus CH; Olympus, Tokyo, Japan) and photomicrographs were taken with a Leica DM 750 camera at 100× and 400× magnifications.
Statistical Analysis
Data are expressed as means ± standard errors of the mean (SEM), n = 5. Statistical comparison between different groups was performed using 1-way analysis of variance (ANOVA) followed by Student-Newman-Keuls multiple comparisons test. A P value of .05 or less was taken as a criterion for a statistically significant difference. The statistical analysis was performed with the aid of Graph Pad Prism 5.03.
Results
Body Weight Change, Heart Weight, and Food Consumption
A significantly lower percentage weight change was observed in group II (CP) (−11.93% ± 1.06%) when compared with group I (control) (4.64% ± 1.49%) and groups III, IV, V, and VI (13.50% ± 0.91%; 13.94% ± 1.35%; 4.30% ± 1.12%; and 3.40% ± 0.96%, respectively) (P < .0001). There was a significantly higher percentage weight change in groups III and IV (6.46% ± 1.70% and 6.77% ± 1.47%, respectively) when compared with the control (4.64% ± 1.49%) (P = .00917). However, groups V and VI (4.30% ± 1.12% and 3.40% ± 0.96%, respectively) showed no significant difference in percentage weight change when compared with group I (control) (4.64% ± 1.49%) (P = .5917) (Table 1).
Table 1.
Effects of Kolaviron on Body Weight Change, Relative Heart Weight, and Food Consumption in Rats Treated With Cyclophosphamide.a
| Groups | Body Weight Change (%) | Relative Heart Weight (%) | Food Consumption (g) |
|---|---|---|---|
| I (control) | 4.64 ± 1.49 | 0.34 ± 0.01 | 18.20 ± 0.57 |
| II (CP) | −11.93 ± 1.06b | 0.56 ± 0.02b | 10.20 ± 0.49b |
| III (200 mg/kg KV) | 9.46 ± 1.70b | 0.40 ± 0.01c | 24.20 ± 0.27b,c |
| IV (400 mg/kg KV) | 8.77 ± 1.47b | 0.38 ± 0.02c | 23.50 ± 0.76b,c |
| V (200 mg/kg KV + CP) | 4.30 ± 1.12c | 0.37 ± 0.02c | 17.00 ± 0.43c |
| VI (400 mg/kg KV + CP) | 3.40 ± 0.96c | 0.42 ± 0.02b,c | 16.80 ± 0.53c |
Abbreviations: CP, cyclophosphamide; KV, kolaviron.
aValues are given as mean ± standard error of the mean (SEM) (n = 5).
b P < .05 compared with control.
c P < .05 compared with CP.
There was a significantly higher relative heart weight in group II (CP) (0.56% ± 0.02%) when compared with that of the group I (control) (0.34% ± 0.01%) and groups III, IV, V, and VI (0.40% ± 0.01%; 0.38% ± 0.02%; 0.37% ± 0.02%; and 0.42% ± 0.02%, respectively) (P < .0001). However, groups III, IV, V and VI (0.40% ± 0.01%; 0.38% ± 0.02%; 4.30% ± 1.12%; and 3.40% ± 0.96%, respectively) showed no significant difference in relative heart weight when compared with group I (control) (0.34% ± 0.01%) (P = .9498) (Table 1).
Group II (CP) had a significantly lower food intake (10.20 ± 0.49 g) when compared with the group I (control) (18.20 ± 0.57 g) and groups III, IV, V, and VI (24.20 ± 0.27; 23.50 ± 0.76; 17.00 ± 0.43; and 16.80 ± 0.53 g, respectively) (P < .0001). However, groups III and IV had a significantly higher food intake (24.20 ± 0.27 and 23.50 ± 0.76 g, respectively) when compared with group I (control) (18.20 ± 0.57 g) (P < .0001), but groups V and VI showed no significant difference in food intake when compared with group I (control) (17.00 ± 0.43 and 16.80 ± 0.53 g, respectively) (P = .16758) (Table 1).
Creatine Kinase and Lactate Dehydrogenase
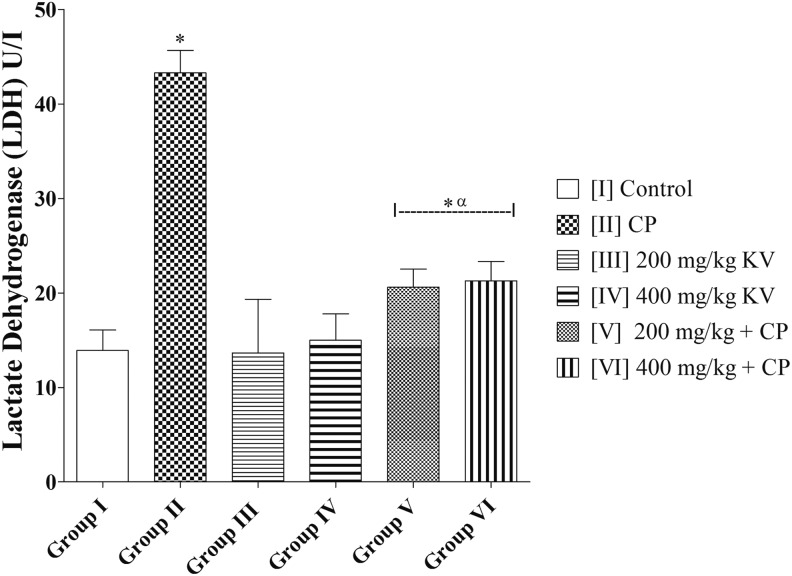
LDH activity in group II (CP) was significantly higher (43.28 ± 2.38 U/L) when compared with group I (control) (13.90 ± 2.17 U/L) and groups III, IV, V, and VI (13.65 ± 5.67; 14.99 ± 2.79; 20.62 ± 1.90; and 21.29 ± 2.03 U/L, respectively) (P = .0002). There was no significant difference in LDH of groups III and IV (13.65 ± 5.67 and 14.99 ± 2.79 U/L, respectively) when compared with group I (control) (13.90 ± 2.17 U/L) (P = .9663). However, groups V and VI had a significantly higher LDH activity (20.62 ± 1.90 and 21.29 ± 2.03 U/L, respectively) when compared with the group I (control) (13.90 ± 2.17 U/L) (P = .0460) (Figure 1).
Figure 1.
Effects of kolaviron on lactate dehydrogenase (LDH) activities in rats treated with cyclophosphamide (CP). Values are given as mean ± standard error of the mean (SEM) (n = 5). *P < .05 compared with control and α P < .05 compared with CP.
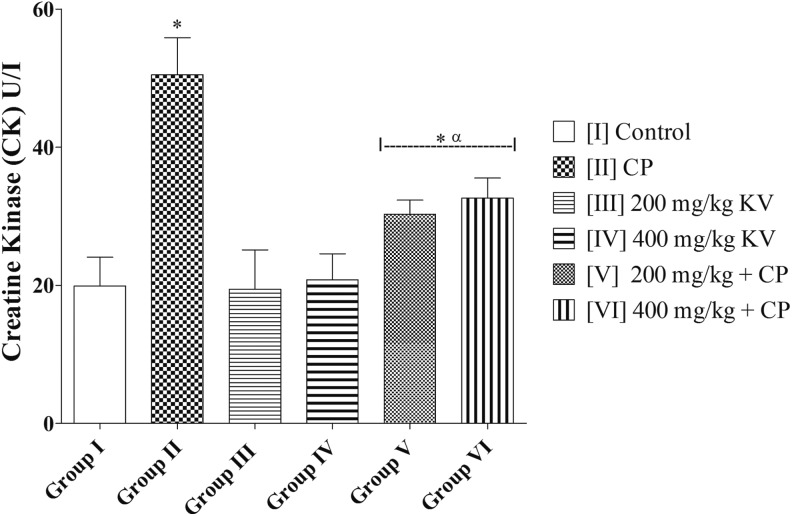
CK activity in group II (CP) was significantly higher (50.48 ± 5.38 U/L) when compared with group I (control) (19.90 ± 4.17 U/L) and groups III, IV, V, and VI (19.45 ± 5.67; 20.79 ± 3.79; 30.29 ± 2.03; and 32.62 ± 2.90 U/L, respectively) (P = .0003). There was no significant difference in CK of groups III and IV (19.45 ± 5.67 and 20.79 ± 3.79 U/L, respectively) when compared with group I (control) (19.90 ± 4.17 U/L) (P = .9784). However, groups V and VI had a significantly higher CK activity (30.29 ± 2.03 and 32.62 ± 2.90 U/L, respectively) when compared with the group I (control) (19.90 ± 4.17 U/L) (P = .0329) (Figure 2).
Figure 2.
Effects of kolaviron on creatine kinase (CK) activities in rats treated with cyclophosphamide (CP). Values are given as mean ± standard error of the mean (SEM) (n = 5). *P < .05 compared with control and α P < .05 compared with CP.
Antioxidant Status and Lipid Peroxidation
SOD activity of group II (CP) (0.59 ± 0.06) was significantly lower when compared with the group I (control) (1.27 ± 0.16) and groups III, IV, V, and VI (1.33 ± 0.17; 1.24 ± 0.13; 1.31 ± 0.16; and 1.23 ± 0.13, respectively) (P = .0053). Groups III and IV (1.33 ± 0.17 and 1.24 ± 0.13, respectively) showed no significant difference in SOD activity when compared with group I (control) (1.27 ± 0.16) (P = .9199). Similarly, groups V and VI (1.31 ± 0.16 and 1.23 ± 0.13, respectively) showed no significant difference in SOD activity when compared with group I (control) (1.27 ± 0.16) (P = .9271) (Table 2).
Table 2.
Effects of Kolaviron on SOD, CAT, GPx Activities, GSH, and MDA Levels in Rats Treated With Cyclophosphamide.a
| Groups | SOD (µM/mg Protein) | CAT (µM/mg Protein) | GPx (µM/mg Protein) | GSH (µg/mg Protein) | MDA (nM/mg Protein) | H2O2 (nM/g Protein) |
|---|---|---|---|---|---|---|
| I (Control) | 1.27 ± 0.16 | 121.2 ± 5.22 | 8.29 ± 0.48 | 1.06 ± 0.11 | 11.15 ± 0.57 | 17.91 ± 1.28 |
| II (CP) | 0.59 ± 0.06b | 56.59 ± 5.54b | 4.66 ± 0.33b | 0.40 ± 0.03b | 21.15 ± 1.01b | 32.42 ± 1.63b |
| III (200 mg/kg KV) | 1.33 ± 0.17c | 119.0 ± 5.44c | 7.98 ± 0.53c | 1.16 ± 0.19c | 12.21 ± 1.08c | 18.12 ± 1.51c |
| IV (400 mg/kg KV) | 1.24 ± 0.13c | 118.1 ± 6.56c | 7.67 ± 0.58c | 1.08 ± 0.16c | 13.55 ± 0.84c | 18.89 ± 1.24c |
| V (200 mg/kg KV + CP) | 1.31 ± 0.16c | 117.8 ± 5.53c | 7.76 ± 0.41c | 0.69 ± 0.04b,c | 12.67 ± 1.23c | 19.67 ± 1.07c |
| VI (400 mg/kg KV + CP) | 1.23 ± 0.13c | 105.8 ± 3.86b,c | 6.65 ± 0.35b,c | 0.55 ± 0.03b,c | 17.29 ± 1.32b,c | 22.68 ± 1.53b,c |
Abbreviations: CAT, catalase; CP, cyclophosphamide; GPx, glutathione peroxidase; GSH, reduced glutathione; MDA, malondialdehyde; SOD, superoxide dismutase.
aValues are given as mean ± standard error of the mean (SEM) (n = 5).
b P < .05 compared with control.
c P < .05 compared with CP.
There was a significantly lower GSH level in group II (CP) (0.40 ± 0.03) when compared with the control group (1.06 ± 0.11) and groups III, IV, V, and VI (1.16 ± 0.19; 1.08 ± 0.16; 0.69 ± 0.04; and 0.55 ± 0.03, respectively) (P = .0048). Groups III and IV (1.16 ± 0.19 and 1.08 ± 0.16, respectively) showed no significant difference in GSH level when compared with group I (control) (1.06 ± 0.11) (P = .8972). However, groups V and VI had a significantly lower level of GSH (0.69 ± 0.04 and 0.55 ± 0.03, respectively) when compared with the control (1.06 ± 0.11) (P = .0012) (Table 2).
There was a significantly lower CAT activity in group II (CP) (56.59 ± 5.54) when compared with the control group (121.2 ± 5.22) and groups III, IV, V, and VI (119.0 ± 5.44; 118.1 ± 6.56; 117.8 ± 5.53; and 105.8 ± 3.86, respectively) (P = .0001). Groups III, IV, and V (7.98 ± 0.53; 7.67 ± 0.58; and 7.76 ± 0.41, respectively) showed no significant difference in CAT level when compared with group I (control) (121.2 ± 5.22) (P = .9739). However, group VI had a significantly lower level of CAT (6.65 ± 0.35) when compared with the control (121.2 ± 5.22) (P = .0456) (Table 2).
There was a significantly lower GPx level in group II (CP) (4.66 ± 0.33) when compared with the control group (8.29 ± 0.48) and groups III, IV, V, and VI (7.98 ± 0.53; 7.67 ± 0.58; 7.76 ± 0.41; and 6.65 ± 0.35, respectively) (P = .0001). Groups III, IV, and V (7.98 ± 0.53; 7.67 ± 0.58; and 7.76 ± 0.41, respectively) showed no significant difference in GPx level when compared with group I (control) (8.29 ± 0.48) (P = .8742). However, group VI had a significantly lower level of GPx (6.65 ± 0.35) when compared with the control (8.29 ± 0.48) (P = .0249) (Table 2).
The MDA level was significantly higher in group II (CP) (21.15 ± 1.01) when compared with the control group (11.15 ± 0.57) and groups III, IV, V, and VI (12.21 ± 1.08; 13.55 ± 0.84; 12.67 ± 1.23; and 17.29 ± 1.32, respectively) (P < .0001). Groups III and IV (12.21 ± 1.08 and 13.55 ± 0.84, respectively) showed no significant difference in MDA level when compared with the control (11.15 ± 0.57) (P = .1829). Group V (12.67 ± 1.23) showed no significant difference in MDA level when compared with the control (11.15 ± 0.57) (P = .2942). There was a significantly higher MDA level in group VI (17.29 ± 1.32) when compared with the control (11.15 ± 0.57) (P = .4030) (Table 2).
H2O2 level of group II (CP) (32.42 ± 1.63) was significantly higher when compared with the group I (control) (17.91 ± 1.28) and groups III, IV, V, and VI (18.12 ± 1.51; 18.89 ± 1.24; 19.67 ± 1.07; and 22.68 ± 1.53, respectively) (P = .0001). Groups III and IV (1.33 ± 0.17 and 1.24 ± 0.13, respectively) showed no significant difference in H2O2 level when compared with group I (control) (17.91 ± 1.28) (P = .8659). Similarly, group V (19.67 ± 1.07) showed no significant difference in H2O2 level when compared with group I (control) (17.91 ± 1.28) (P = .3227). There was a significantly higher H2O2 level in group VI (22.68 ± 1.53) when compared with group I (control) (17.91 ± 1.28) (P = .0439) (Table 2).
Cardiac Troponin I (cTn l) Levels and Myeloperoxidase (MPO) Activities
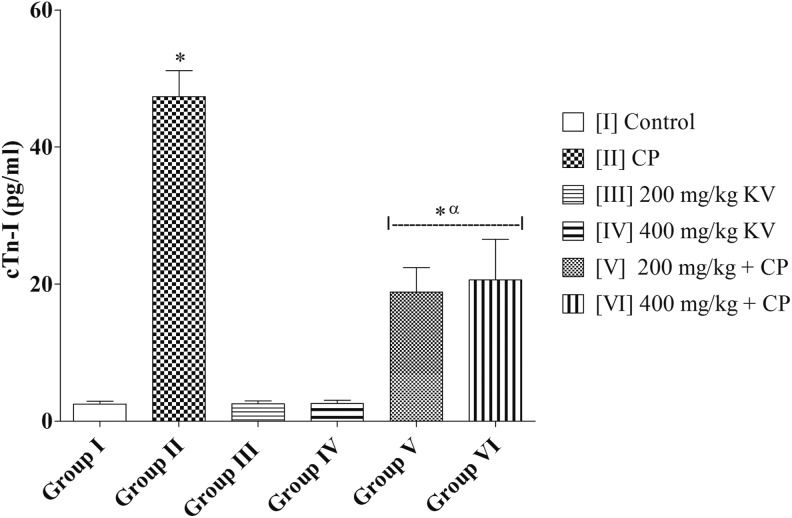
Cardiac troponin I (cTn l) levels of group II (CP) (47.36 ± 3.78 pg/mL) was significantly higher when compared with the group I (control) (2.48 ± 0.44 pg/mL) and groups III, IV, V, and VI (2.54 ± 0.45; 2.59 ± 0.48; 18.83 ± 3.58; and 20.62 ± 5.90 pg/mL, respectively) (P = .0001). Groups III and IV (2.54 ± 0.45 and 2.59 ± 0.48 pg/mL, respectively) showed no significant difference in cTn l levels when compared with group I (control) (2.48 ± 0.44 pg/mL) (P = .9850). However, groups V and VI (18.83 ± 3.58 and 20.62 ± 5.90 pg/mL, respectively) showed a significantly higher cTn l levels when compared with group I (control) (2.48 ± 0.44 pg/mL) (P = .0137) (Figure 3).
Figure 3.
Effects of kolaviron on myeloperoxidase (MPO) activities in rats treated with cyclophosphamide (CP). Values are given as mean ± standard error of the mean (SEM) (n = 5). *P < .05 compared with control and α P < .05 compared with CP.
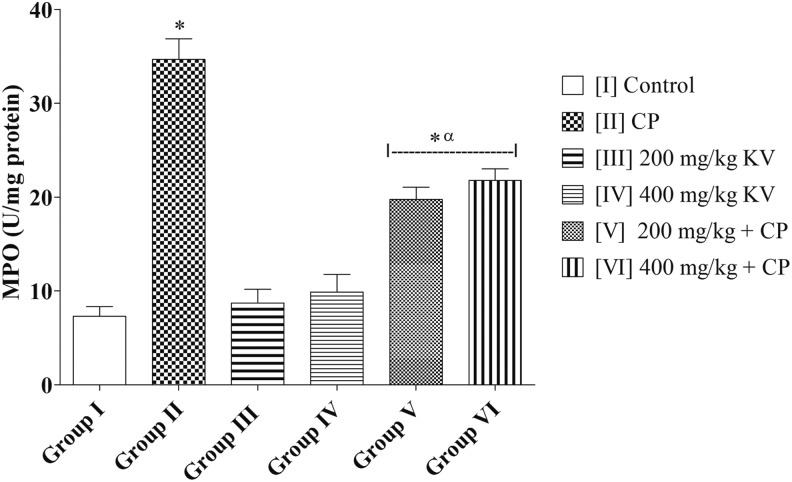
There was a significantly higher MPO activity in group II (CP) (34.66 ± 2.21) when compared with the control group (7.31 ± 1.01) and groups III, IV, V, and VI (8.72 ± 1.46; 9.89 ± 1.86; 19.74 ± 1.33; and 21.78 ± 1.23, respectively) (P = .0001). Groups III and IV (8.72 ± 1.46 and 9.89 ± 1.86, respectively) showed no significant difference in MPO activities when compared with group I (control) (7.31 ± 1.01) (P = .4917). However, groups V and VI had a significantly higher MPO activities (19.74 ± 1.33 and 21.78 ± 1.23, respectively) when compared with the control (7.31 ± 1.01) (P = .0001) (Figure 4).
Figure 4.
Effects of kolaviron on cardiac troponin I (cTn I) in rats treated with cyclophosphamide (CP). Values are given as mean ± standard error of the mean (SEM) (n = 5). *P < .05 compared with control and α P < .05 compared with CP.
Histopathological Results
Figures 5 and 6 show the histological results of heart tissues of rat treated with KV and CP.
Figure 5.
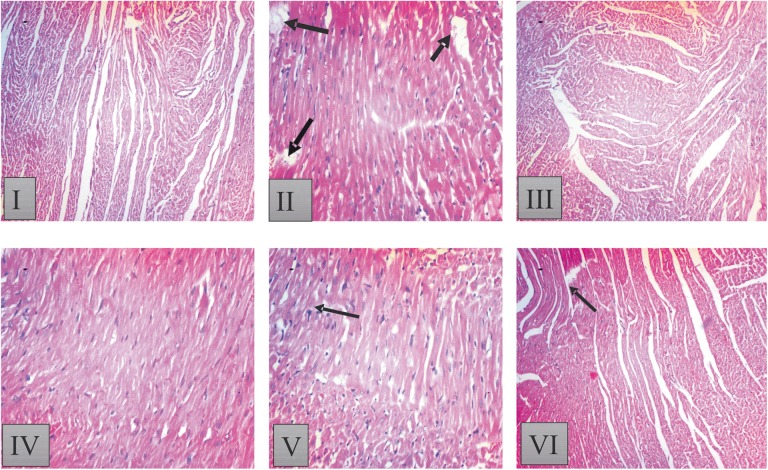
Histopathological result of heart tissue in [I] control: showed normal histoarchitecture of the heart with no visible lesion; [II] Cyclophosphamide (CP) treated. CP-treated group [II] shows distorted and wavy myocardial fibers with focal fatty change in some area of myocardial fibers. Loss of cellular constituents of the myocardial cells (black arrow), myofibrillar loss and hypertrophic myocardial fiber with inflammation; [III] 200 mg/kg kolaviron (KV) showed intact myocardial fibers, but focal areas of hyalinization and some wavy myocardial fibers; [IV] 400 mg/kg KV showed a few wavy myocardial fibers as well as few losses of cellular constituents; [V] 200 mg/kg KV + CP treated: showed improvement in histoarchitecture with mild waviness of myofibers as well as separation of some myocardial fibers and [VI] 400 mg/kg KV + CP treated rats; showed mild fatty change in some muscle cells and separation of some myocardial fibers. Sections of heart tissues of rats were stained with hematoxylin-eosin (magnification 100×).
Figure 6.
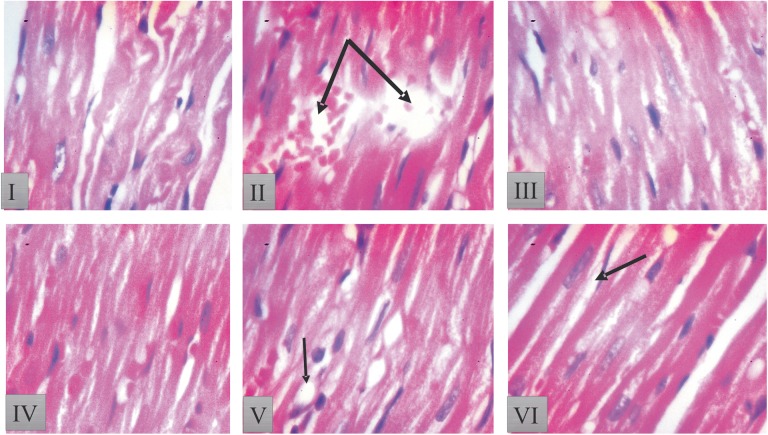
Histopathological result of heart tissue in [I] control: rats heart tissue showed intact myocardial fibers and pericardium; [II] cyclophosphamide (CP) alone: rats heart tissue produced massive change in the myocardium showing a varying degree of vacuolar changes in the cardiac muscle fibers mainly in the form of degeneration of myocardial fibers, vacuolization of the cardiomyocytes, infiltration of inflammatory cells, myofibrillar loss, and hypertrophic myocardial fiber with inflammation (double arrow); [III] 200 mg/kg kolaviron (KV) showed intact myocardial fibers, but focal areas of hyalinization and some wavy myocardial fibers; [IV] 400 mg/kg KV showed a few wavy myocardial fibers as well as few losses of cellular constituents; [V] 200 mg/kg KV + CP treated showed improvement in histoarchitecture with mild waviness of myofibers as well as separation of some myocardial fibers; and [VI] 400 mg/kg KV + CP treated rats showed mild fatty change in some muscle cells and separation of some myocardial fibers. Sections of heart tissues of rats were stained with hematoxylin-eosin (magnification 400×).
Group I (control) rats showed intact myocardial fibers and pericardium. Group II (CP alone) produced massive change in the myocardium showing a varying degree of vacuolar changes in the cardiac muscle fibers mainly in the form of degeneration of myocardial fibers, vacuolization of the cardiomyocytes, infiltration of inflammatory cells, myofibrillar loss, and hypertrophic myocardial fiber with inflammation (Figures 5 and 6). Group III and IV (200 and 400 mg/kg KV) showed intact myocardial fibers, but focal areas of hyalinization and some wavy myocardial fibers were seen compared with group I (control) (Figures 5 and 6). Heart tissue of rats in groups V and VI (200 mg/kg KV + CP and 400 mg/kg KV + CP) showed improvement in histoarchitecture when compared with that of rats treated with group II (CP alone) (Figures 5 and 6).
Discussion
This study aimed to study the effect of KV on CP-induced cardiotoxicity in Wistar rats. To our best knowledge, this is the first report on the effects of KV on drug-induced cardiotoxicity in an experimental animal model.
In the present study, there was a significant decrease in the body weight in the CP-treated group when compared with the control group. The decrease in body weight observed in this study may be attributed to the anorexia that was observed in the group treated with CP. It was observed that the CP-treated group had a significant decreased in food intake when compared with the control group. The significant decrease in food intake in the CP-treated group that was observed in this study when compared with the control may suggests that CP has deleterious effects on the gastrointestinal tract or appetite center in the hypothalamus. Compared with the control group, rats treated with KV at 200 and 400 mg/kg showed an accelerated weight gain. But, KV groups treated with CP had no significant difference in body weight when compared with the control. The increased body weight gain that was observed in KV-treated rats could be as a result of polyphagia caused by the stimulation of the feeding center in the hypothalamus.36 Thus, KV was able to prevent the deleterious effect of CP on feeding pattern of rats in this study.
It was observed in this study that CP administration produced a significant increase in the relative heart weight of the rats when compared with the control group. CP-induced cardiotoxicity, consists of—in order of increasing severity—swelling of sarcoplasmic reticulum, cytoplasmic vacuolization, myofibrillar degeneration, myocyte disruption, and fibrosis.37 The observed increased in heart weight is corroborated by photomicrograph in this study which showed the sign of hypertrophy of the myocyte fiber, disruption of myocyte structure, including damage to microtubules, cytoplasmic vacuolization, loss of myofibrils, marked congestion, edema and extravasation in the cardiac tissues, as well as a marked leucocytes infiltration.38,39 Thus, the decrease in body weight and increase in relative heart weight that was observed in this study as a result of CP treatment are in accordance with the previous studies.40 KV at 200 and 400 mg/kg reversed this increase in the relative heart weight (cardiac tissue mass) of the rats toward control group. Hammon41 reported that flavonoids have a favorable effect on hearts by dilating the blood vessels resulting in reduced peripheral resistance and increased coronary circulation. This study indicates that regular intake of KV might probably protect cardiac tissues mass against CP-induced cardiac tissues enlargement and failure.
In the present study, CP administration significantly increased the activities of heart tissues LDH and CK when compared with the control group. Similarly, cTn I levels of rats significantly increased following treatment with CP. LDH, CK, and cTn I are one of the specific and sensitive marker of heart muscle damage. The observed increased in LDH, CK activities, and cTn I levels in CP-treated group is in accordance with the previous studies.42–44 CP is a cardiotoxic agent inducing a direct myocardial endothelial damage and destruction of myocardial cells. As a result, LDH, CK, and cTn I are increased in heart tissues and in the blood stream.40,44 Elevation of LDH, CK, and cTn I in heart tissues of CP-treated rats that was observed in this study might be due to the overproduction of reactive oxygen species during CP treatment, which cause membrane injury by triggering the production of lipid peroxidation that result in loss of function and integrity of myocardial membranes.45 Thus, this effect might be due to the CP toxic metabolite; acrolein.46
Treatment with KV at 200 and 400 mg/kg ameliorates CP-induced alteration in the heart tissue activities of LDH, CK, and cTn I toward control level. These suggested that KV preserved the structural integrity of the cardiac cells membranes and subsequently prevented the overproduction of LDH, CK, and cTn I in the heart tissue. This result agrees with the work of Nwaneri et al,47 who reported cardioprotective effect of KV in cholesterol-fed rats. Interestingly, the effect of KV on LDH, CK, and cTn I, might be probably due to its antioxidant and membrane stabilizing properties.25,48
MPO is found primarily in the azurophilic granules of neutrophils,49 however, to a much lesser extent in monocytes and some macrophages.49 The assay of this enzyme has been widely used as an index of neutrophil infiltration (inflammation) and cardiac damage in various experimental studies.49,50 Relative to the normal control group, there was marked elevation in the activities of MPO in the CP-treated group.
Excessive production of MPO causes oxidative damage.51,52 This could be as a result of the deficiency in the endogenous antioxidant system, which functions to scavenge deleterious free radicals. The elevated in MPO activities in CP-treated rats was as result of increase in the infiltration of the cardiac tissue with inflammatory cells and subsequently lead to myocardial damage. However, the results of the rats pretreated with KV showed marked reduction in the activities of MPO and histopathological changes induced by CP injection only showed less myocardial injury with less infiltration of monocyte and cellular changes in KV-treated rats.
The present study shows a significant decrease in SOD, CAT, GPx activities, and reduced GSH levels as well as increased MDA and H2O2 hydrogen peroxide levels in rats treated with CP when compared with the control group. It has been reported that free radicals generated during treatment with CP causes membrane injury, which resulted to the loss of function and integrity of myocardial membrane.41 GSH is a major low-molecular-weight scavenger of free radicals and an important inhibitor of free radical generation mediated by lipid peroxidation. It is known to result in enhanced lipid peroxidation, which causes increased glutathione consumption. CP-induced significant decrease in SOD, CAT, GPx activities and GSH level promotes the formation of OH radicals, and initiation and propagation of lipid peroxidation. However, it is suggested that the decrease in the activities of antioxidant enzymes is a consequence of increased oxidative stress in the cardiac tissues due to the overproduction of active reactive oxygen species.53,54 Conversely, the increase in MDA and H2O2 levels along with the decreased GSH levels, might be due to the ability of acrolein to deprive the cell of its natural defense (GSH) against the reactive oxygen species55 or to the direct conjugation of CP and its metabolites with free or protein-bound SH groups,56 stimulating the formation of free radicals thereby interfering with the antioxidant functions.55,57 Free radical scavenging enzymes are the first line of cellular defense against oxidative injury. SOD detoxifies the superoxide radicals to hydrogen peroxide and CAT dismutates H2O2 to water (H2O) and O2, while GPx converts glutathione to oxidize glutathione, and at the same time reduces H2O2 to H2O, and lipid hydroperoxides (ROOH) to the corresponding stable alcohols.53
Treatment of CP-treated animals with KV restored the GSH level and the antioxidant enzyme (SOD, CAT, and GPx) activities and decreased MDA and H2O2 levels in the cardiac tissues toward control level, indicating a protective effect of KV against reactive oxygen species. This effect might be due to its ability to decrease oxidative stress and preserve the activity of antioxidant enzymes as well as its ability to inhibit lipid peroxidation hydroxyl radical.23,24 Another possible mechanism of kolaviron protection against CP-induced cardiotoxicity, may be due to its ability to correct deficient thiol status of the cardiac cells by increasing the synthesis of GSH.58 In this study, KV has a strong cardioprotective effect, decreases lipid peroxidation in the heart tissue and prevents free radicals–induced damage to the myocardium by its free radical scavenging effect.59,60 In addition, protective effect of KV against insults from various xenobiotics has been reported to be attributed to its antioxidant properties.61 KV act as precursor of glutathione molecule.61 It was demonstrated that GSH has a potential effect to reduce CP-induced lipid peroxidation and also increase therapeutic index of the drug by way of reducing its toxicity that may be mediated through free radical mechanisms.62
Conclusion
In conclusion, the results from the present investigation indicate that KV at 200 and 400 mg/kg prevents deleterious effect of CP on food consumption of rats and protects the cardiac tissues against CP-induced cardiotoxicity by decreasing oxidative stress and preserve the activity of antioxidant enzymes of the cardiac tissues.
Footnotes
Author Contributions: JGM, OBA and QKA made significant contribution to conception, design, experimentation, acquisition and interpretation of the data and writing of manuscript.. MAA, MAA, BOO, and BAF made substantial contribution in interpretation of data and revising the manuscript for intellectual content. All authors read and approved the final manuscript.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Quadri Kunle Alabi  http://orcid.org/0000-0002-9984-0565
http://orcid.org/0000-0002-9984-0565
Ethical Approval: All the animals received humane care according to the criteria outlined in the “Guide for the Care and Use of Laboratory Animals” prepared by the National Academy of Science (NAS) and approved by Institutional Research Committee.
References
- 1. Demirer T, Buckner CD, Appelbaum FR, et al. Busulfan, cyclophosphamide and fractionated total body irradiation for autologous or syngeneic marrow transplantation for acute and chronic myelogenous leukemia: phase I dose escalation of busulfan based on targeted plasma levels. Bone Marrow Transplant. 1996;17:491–495. [PubMed] [Google Scholar]
- 2. Itescu SE, Burke K, Lietz R, et al. Intravenous pulse administration of cyclophosphamide is an effective and safe treatment for sensitized cardiac allograft recipients. Circulation. 2002;105:1214–1219. [DOI] [PubMed] [Google Scholar]
- 3. Pagel JM, Appelbaunm FR, Earyis IF, et al. 131I-anti-CD45 antibody plus busulfan and cyclophosphamide before allogeneic hematopoietic cell transplantation for treatment of acute myeloid leukemia in first remission. Blood. 2006;107:2184–2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhang J, Tian Q, Zhou SF. Clinical pharmacology of cyclophosphamide and ifosfamide. Curr Drug Ther. 2006;1:55-84(30). [Google Scholar]
- 5. Hirano A, Shimizu T, Watanabe O, et al. Epirubicin and cyclophosphamide followed by docetaxel as primary systemic chemotherapy in locally advanced breast cancer. Anticancer Res. 2008;28:4137–4142. [PubMed] [Google Scholar]
- 6. De Souza CA, Santini G, Mario S, et al. Amifostine (WR-2721), a cytoprotective agent during high-dose cyclophosphamide treatment of non-Hodgkin’s lymphomas: a phase II study. Braz J Med Biol Res. 2000;33:791–798. [DOI] [PubMed] [Google Scholar]
- 7. Lee CK, Harman GS, Hohl RJ, Gingrich RD. Fatal cyclophosphamide cardiomyopathy: its clinical course and treatment. Bone Marrow Transplant. 1996;18:573–577. [PubMed] [Google Scholar]
- 8. Colvin OM. An overview of cyclophosphamide development and clinical applications. Curr Pharm Des. 1999;5:555–560. [PubMed] [Google Scholar]
- 9. Kern JC, Kehrer JP. Acrolein-induced cell death: a caspase-influenced decision between apoptosis and oncosis/necrosis. Chem Biol Interact. 2002;139:79–95. [DOI] [PubMed] [Google Scholar]
- 10. Pass GJ, Carrie D, Boylan M, et al. Role of hepatic cytochrome P450s in the pharmacokinetics and toxicity of cyclophosphamide: studies with the hepatic cytochrome P450 reductase null mouse. Cancer Res. 2005;65:4211–4217. [DOI] [PubMed] [Google Scholar]
- 11. Gharib MI, Burnett AK. Chemotherapy-induced cardiotoxicity: current practice and prospects of prophylaxis. Eur J Heart Fail. 2002;4:235–242. [DOI] [PubMed] [Google Scholar]
- 12. Patel JM, Block ER. Cyclophosphamide-induced depression of the antioxidant defense mechanisms of the lung. Exp Lung Res. 1985;8:153–165. [DOI] [PubMed] [Google Scholar]
- 13. Patel JM. Stimulation of cyclophosphamide-induced pulmonary microsomal lipid peroxidation by oxygen. Toxicology. 1987;45:79–91. [DOI] [PubMed] [Google Scholar]
- 14. Dorr RT, Lagel K. Effect of sulfhydryl compounds and glutathione depletion on rat heart myocyte toxicity induced by 4-hydroperoxycyclophosphamide and acrolein in vitro. Chem Biol Interact. 1994;93:117–28. [DOI] [PubMed] [Google Scholar]
- 15. Singh DK, Lippman SM. Cancer chemoprevention. Part 1: retinoids and carotenoids and other classic antioxidants. Oncology (Williston Park). 1988;12:1643–1660. [PubMed] [Google Scholar]
- 16. Manda K, Bhatia AL. Prophylactic action of melatonin against cyclophosphamide-induced oxidative stress in mice. Cell Biol Toxicol. 2003;19:367–372. [DOI] [PubMed] [Google Scholar]
- 17. Sudharsan PT, Mythili Y, Selvakumar E, Varalakshmi P. Lupeol and its ester ameliorate the cyclophosphamide provoked cardiac lysosomal damage studied in rat. Mol Cell Biochem. 2006;282:23–29. [DOI] [PubMed] [Google Scholar]
- 18. Libey YO, Ozbek E, Simsek A, Otunctemur A, Cekmen M, Somey A. Potential chemoprotective effect of melatonin in cyclophosphamide- and cisplatin-induced testicular damage in rats. Fertile Steril. 2009;92:1124–1132. [DOI] [PubMed] [Google Scholar]
- 19. Selvakumar E, Prahalathan C, Mythili Y, Varalakshmi P. Beneficial effects of dl-α-lipoid acid on cyclophosphamide-induced oxidative stress in mitochondria fraction of rat testis. Chem Biol Interact. 2005;152;59–66. [DOI] [PubMed] [Google Scholar]
- 20. Iwu MM, Igboko OA, Elekwa OK. Prevention of thioacetamide-induced hepatoxicity by biflavanones of Garcinia kola . Phytother Res. 1990;4:157–159. [Google Scholar]
- 21. Nwankwo JO, Tanhnteng JG, Emerole GO. Inhibition of aflatoxin B1 genotoxicity in human liver-derived HepG2 cells by kolaviron biflavonoids and molecular mechanisms of action. Eur J Cancer Prev. 2000;9:351–361. [DOI] [PubMed] [Google Scholar]
- 22. Alabi QA, Akomolafe RO, Olukiran OS, et al. The Garcinia kola biflavonoid kolaviron attenuates experimental hepatotoxicity induced by diclofenac. Pathophysiology. 2017;24:281–290. [DOI] [PubMed] [Google Scholar]
- 23. Akintonwa A, Essien AR. Protective effects of Garcinia kola seed extract against paracetamol induced hepatotoxicity in rats. J Ethnopharmacol. 1990;29:207–211. [DOI] [PubMed] [Google Scholar]
- 24. Farombi EO. Mechanisms for the hepatoprotective action of kolaviron: studies on hepatic enzymes, microsomal lipids and lipid peroxidation in carbon tetrachloride-treated rats. Pharmacol Res. 2000;42:75–80. [DOI] [PubMed] [Google Scholar]
- 25. Farombi EO, Akanni OO, Emerole GO. Antioxidant and scavenging activities of flavonoid extract (kolaviron) of Garcinia kola seeds. Pharm Biol. 2002;40:107–116. [Google Scholar]
- 26. Adedara IA, Farombi EO. Influence of kolaviron and vitamin E on ethylene glycol monoethyl ether-induced haematotoxicity and renal apoptosis in rats. Cell Biochemical Function. 2013;32:32–38. [DOI] [PubMed] [Google Scholar]
- 27. Swanson JR, Wilkinson JH, Conn RB, Hess JW, Natho GJW. Measurement of creatine kinase in serum In: Cooper GR, ed. Standard Method of Clinical Chemistry. Vol 7 New York, NY: Academic Press; 1972:33–42. [Google Scholar]
- 28. Weisshaar HD. The photometric determination of LDH. Med Welt. 1975;26:387–390.1121268 [Google Scholar]
- 29. Misra HP, Fridovich I. The role of superoxide anion in the autooxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem. 1972;247:3170–3175. [PubMed] [Google Scholar]
- 30. Clairborne A. Catalase activity In: Greewald AR, ed. Handbook of Methods for Oxygen Radical Research. Boca Raton, FL: CRC Press; 1995:237–242. [Google Scholar]
- 31. Beutler E, Durgun O, Kelly BM. Improved method for the determination of blood glutathione. J Lab Clin Med. 1963;51:882–888. [PubMed] [Google Scholar]
- 32. Rotruck JT, Pope AL, Ganther HE, Swanson AB, Hafeman DG, Hoekstra WG. Selenium: biochemical role as a component of glutathione peroxidase. Science. 1973;179:588–590. [DOI] [PubMed] [Google Scholar]
- 33. Wolff SP. Ferrous ion oxidation in the presence of ferric ion indicator xylenol orange for measurement of hydroperoxides. Methods Enzymol. 1994;233:182–189. [Google Scholar]
- 34. Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–358. [DOI] [PubMed] [Google Scholar]
- 35. Xia Y, Zweier JL. Measurement of myeloperoxidase in leukocyte-containing tissues. Anal Biochem. 1997;245:93–96. [DOI] [PubMed] [Google Scholar]
- 36. Alabi QK, Akomolafe RO, Olukiran OS, et al. Assessment of haematological and biochemical effects of kolaviron in male Wistar rats. Br J Pharm Res. 2017:16;1–14. [Google Scholar]
- 37. Baky NA, Al-Rasheed NM, Al-Rasheed NM, Zaghloul IY, Radwan MA. α-Lipoic acid and amlodipine ameliorate myocardial infarction induced by isoproterenol in rats. Int J Acad Res. 2009;1:68–77. [Google Scholar]
- 38. Lieber IH, Stoneburner SD, Floyd M, McGuffin WL. Potassium wasting nephropathy secondary to chemotherapy simulating Bartter’s syndrome. Cancer. 1984;54:808–810. [DOI] [PubMed] [Google Scholar]
- 39. Malley SE, Vizzard MA. Changes in urinary bladder cytokine mRNA and protein after cyclophosphamide-induced cystitis. Physiol Genomics. 2002;9:5–13. [DOI] [PubMed] [Google Scholar]
- 40. Viswanatha SAH, Patel UM, Koti BC, Gadad PC, Patel NL, Thippeswamy AH. Cardioprotective effect of Saraca indica against cyclophosphamide-induced cardiotoxicity in rats: a biochemical, electrocardiographic and histopathological study. Indian J Pharmacol. 2013;45:44–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hamon NW. Hawthorns: the genus crataegus. Can Pharm J. 1996;121:708–709. [Google Scholar]
- 42. Asiri YA. Probucol attenuates cyclophosphamide induced oxidative apoptosis, p53 and Bax signal expression in rat cardiac tissues. Oxid Med Cell Longev. 2010;3:308–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Nagi MN, Al-Shabanah OA, Hafez MM, Sayed-Ahmed MM. Thymoquinone supplementation attenuates cyclophosphamide-induced cardiotoxicity in rats. J Biochem Mol Toxicol. 2011;25:135–142. [DOI] [PubMed] [Google Scholar]
- 44. Bertinchant JP, Polge A, Juan JM, et al. Evaluation of cardiac troponin I and T levels as markers of myocardial damage in doxorubicin-induced cardiomyopathy rats, and their relationship with echocardiographic and histological findings. Clin Chim Acta. 2003;329:39–51. [DOI] [PubMed] [Google Scholar]
- 45. Chakraborty P, Hossain Sk U, Murmu N, Das JK, Pal S, Bhattacharya S. Modulation of cyclophosphamide-induced cellular toxicity by diphenyl-methyl selenocyanate. In vivo, an enzymatic study. J Cancer Mol. 2009;4:183–189. [Google Scholar]
- 46. Senthilkumar S, Devaki T, Manohar BM, Babu M.S. Effect of squalene on cyclophosphamide induced toxicity. Clin Chim Acta. 2006;364:335–342. [DOI] [PubMed] [Google Scholar]
- 47. Chidozie NVO, Anyanwu KC, Adaramoye OA, Emerole GO. Cardioprotective effect of kolaviron (a Garcinia kola seed extract) in cholesterol-fed rats. Biores Bull. 2014;4:1–6. [Google Scholar]
- 48. Farombi EO, Nwaokeafor IA. Anti-oxidant mechanisms of kolaviron: studies on serum lipoprotein oxidation, metal chelation and oxidative membrane damage in rats. Clin Exp Pharmacol Physiol. 2005;32:667–674. [DOI] [PubMed] [Google Scholar]
- 49. Klebanoff SJ. Myeloperoxidase: friend and foe. J Leukoc Biol. 2005;77:598–625. [DOI] [PubMed] [Google Scholar]
- 50. Nicholls SJ, Hazen SL. Myeloperoxidase and cardiovascular disease. Arterioscler Thromb Vasc Biol. 2005;25:1102–1111. [DOI] [PubMed] [Google Scholar]
- 51. Hiraishi H, Terano A, Ota S, et al. Protection of cultured rat gastric cells against oxidant-induced damage by exogenous glutathione. Gastroenterology. 1994;106:1199–1207. [DOI] [PubMed] [Google Scholar]
- 52. Suzuki M, Mori M, Miura S, et al. Omeprazole attenuates oxygen-derived free radical production from human neutrophils. Free Radic Biol Med. 1996;21:727–731. [DOI] [PubMed] [Google Scholar]
- 53. Jnaneshwari S, Hemshekhar M, Santhosh MS, et al. Crocin, a dietary colorant mitigates cyclophosphamide-induced organ toxicity by modulating antioxidant status and inflammatory cytokines. J Pharm Pharmacol. 2013;65:604–614. [DOI] [PubMed] [Google Scholar]
- 54. Merwid-Lad A, Trocha M, Chlebda-Sieragowska E, et al. Effect of cyclophosphamide and morin-5-sulfonic acid sodium salt, alone or in combination, on ADMA/DDAH pathway in rats. Pharmacol Rep. 2013;65:201–207. [DOI] [PubMed] [Google Scholar]
- 55. Al-Yahya AA, Al-Majed AA, Gado AM, et al. Acacia Senegal gum exudate offers protection against cyclophosphamide-induced urinary bladder cytotoxicity. Oxid Med Cell Longev. 2009;2:207–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Yousefipour Z, Ranganna K, Newaz MA, Milton SG. Mechanism of acrolein-induced vascular toxicity. J Physiol Pharmacol. 2005;56:337–353. [PubMed] [Google Scholar]
- 57. Tsakadze NL, Srivastava S, Awe SO, Adeagbo AS, Bhatnagar A, D’Souza SE. Acrolein induced vasomotor responses of rat aorta. Am J Physiol. 2003;285:H727–H734. [DOI] [PubMed] [Google Scholar]
- 58. Livingstone C, Davis J. Review. Targeting therapeutics against glutathione depletion in diabetes and its complications. Br J Diabetes Vasc Dis. 2007;7:258–265. [Google Scholar]
- 59. Arica V, Demir IH, Tutanc M, et al. N-Acetylcysteine prevents doxorubucin-induced cardiotoxicity in rats. Hum Exp Toxicol. 2013;32:655–661. [DOI] [PubMed] [Google Scholar]
- 60. Owoeye O, Adedara IA, Adeyemo OA, Bakare OS, Egun C, Farombi EO. Modulatory role of kolaviron in phenytoin-induced hepatic and testicular dysfunctions in Wistar rats. J Diet Suppl. 2015;12:105–117. [DOI] [PubMed] [Google Scholar]
- 61. Farombi EO, Møller P, Dragsted LO. Ex-vivo and in vitro protective effects of kolaviron against oxygen-derived radical-induced DNA damage and oxidative stress in human lymphocytes and rat liver cells. Cell Biol Toxicol. 2004;20:71–82. [DOI] [PubMed] [Google Scholar]
- 62. Ray S, Pandit B, Ray SD, Das S, Chakraborty S. Cyclophosphamide induced lipid peroxidation and changes in cholesterol content: protective role of reduced glutathione. Int J Pharmtech Res. 2010;2:704–718. [Google Scholar]