Abstract
Chronic hepatitis C virus (HCV) infection is a leading cause of chronic liver diseases and hepatocellular carcinoma (HCC) worldwide. In the past few years, anti-HCV therapies have undergone a revolution with the approval of multiple direct-acting antivirals (DAAs), which enable interferon-free treatments with considerable improvement of sustained virologic response in patients. Today, DAAs have become the standard of care for HCV therapy. However, several limitations remain, which include access to therapy, treatment failure in a subset of patients and persistent risk of HCC development following cure in patients with advanced fibrosis. By targeting conserved host proteins involved in the HCV life cycle, host-targeting agents (HTAs) offer opportunities for pan-genotypic antiviral approaches with a high barrier to drug resistance. Moreover, when applied in combination with DAAs, HTAs could improve the management of difficult-to-treat patients by acting through a complementary mechanism of action. In this review, we summarize the different HTAs evaluated in preclinical and clinical development and discuss their potential role for anti-HCV therapies.
Keywords: clinical trial, direct-acting antivirals, hepatitis C virus, host-targeting agents, treatment
Introduction
Hepatitis C virus (HCV) is a hepatotropic RNA virus, which belongs to the Flaviviridae family.1 Chronic HCV infection is a major public health problem, affecting more than 150 million individuals worldwide. Chronically infected patients are at high risk for developing liver fibrosis, cirrhosis and hepatocellular carcinoma (HCC). In many parts of the world, HCV infection is the major cause of HCC and the leading indication for liver transplantation (LT).2 There is no vaccine to prevent HCV infection. In the past, interferon (IFN)-based regimens were the standard of care for HCV infection, but only led to a sustained virologic response (SVR) in 50% of patients with serious adverse effects.3 Recent approval of novel antivirals directly targeting the virus, named direct-acting antivirals (DAAs), have enabled IFN-free treatments with considerable SVR improvement (SVR rates over 90%). Although the development of DAAs has revolutionized HCV therapy, several limitations remain: these include limited access to therapy in the majority of infected patients, treatment failure in a subset of patients, potential adverse effects in patients with comorbidity and persistent HCC risk following SVR in patients with advanced fibrosis.4 Targeting host factors required for virus infection is an attractive complementary strategy to address these challenges. An improved understanding of the viral life cycle based on the development of advanced HCV model systems has enabled the design of new molecules that target key factors of the HCV life cycle, named host-targeting agents (HTAs).5 HTAs provide a broad antiviral activity with very high genetic barrier to drug resistance due to the extremely low mutational rate occurring within host cells.5,6 Several HTAs are now being evaluated in phase II and III clinical trials. Here, we review the different classes of HTAs in preclinical or clinical development and highlight their future role in anti-HCV therapy.
Treatment of HCV infection in the era of DAAs
In recent years, the treatment of chronic HCV infection has dramatically improved with the development of IFN-free regimens based on DAAs. Indeed, a better understanding of the HCV life cycle has led to the development of multiple DAAs, with highly improved SVR rates, shortened treatment duration and reduced side effects.7 DAAs are molecules that specifically target defined nonstructural (NS) viral proteins playing a crucial role in the HCV life cycle. At least four classes of DAAs are available in the US and Europe: NS3/NS4A protease inhibitors (e.g. simeprevir, grazoprevir, paritaprevir), NS5B nucleoside and non-nucleoside polymerase inhibitors (e.g. sofosbuvir and dasabuvir, respectively) as well as NS5A inhibitors (e.g. daclatasvir, ledipasvir, ombitasvir).3 Combinations of DAAs are currently the standard of care for patients with HCV infection. In 2014, the combination of sofosbuvir and ledipasvir (Harvoni, Gilead, Foster City, CA, USA) was approved for the treatment of HCV genotype 1 infection, with a SVR of more than 95%.8–10 Moreover, in the same year, the US Food and Drug Administration (FDA) also approved the combination of three DAAs, namely ombitasvir, paritaprevir and dasabuvir (Viekira Pak, Abbvie, North Chicago, IL, USA) for the treatment of HCV genotype 1 infection.11–13 In 2016, additional DAA-based regimens including sofosbuvir/velpatasvir (Epclusa, Gilead) and grazoprevir/elbasvir (Zepatier, Merck, Kenilworth, NJ, USA) were approved for the treatment of pan-genotypic HCV infection with a SVR rate of about 95%.14,15
Limitations of DAA-based therapies
Today, it is estimated that more than 90% of patients with chronic hepatitis C can be cured with DAA-based regimens. Clinical studies involving large numbers of patients confirmed excellent efficacy, safety and tolerability of the new DAA combinations. However, several challenges remain unsolved.
The most important limitation is probably the accessibility of DAA regimens. Indeed, access to DAAs is limited to less than 10% of patients with HCV infection, especially in low-resource countries.16 Moreover, the management of special populations, or ‘difficult-to-treat’ patients still requires special attention.17 One challenge remains the treatment of patients with advanced cirrhosis and decompensated liver disease. Recent studies revealed that patients with or without cirrhosis respond equally well to DAAs, whereas patients with advanced cirrhosis appear to have a reduced ability to clear the virus, leading to lower SVR rates in this population.18,19 For these patients, treatment regimens should be adapted to higher doses or longer treatment duration. In this regard, it is important to note that patients with advanced cirrhosis (Child-Pugh classes B and C) were excluded in many phase II and phase III clinical trials and fewer studies were conducted in patients with decompensated liver disease in the past.8,10–12,20–22 Consequently, the dosage of DAAs, either alone or in combination, their efficacy and their safety have only been partially addressed in these special populations. Furthermore, several DAA-based regimens (e.g. the NS34A protease inhibitors simeprevir and asunaprevir) cannot easily be used in patients with decompensated cirrhosis because of their impaired drug-metabolizing capacity, leading to severe adverse effects.19,23,24 DAA-based regimens can also be challenging for patients with advanced renal insufficiency who often need DAA dose adjustments.19 Furthermore, few treatment options are available for patients with end-stage renal disease. Indeed, current recommendations only allow the use of grazoprevir (NS3/NS4A inhibitor) and elbasvir (NS5A inhibitor) in combination in these patients.25,26 Despite overall promising results, the optimal DAA combination, the treatment duration and treatment dosages in these special populations require further investigations.
Limitations in the application of DAA regimens also include certain viral genotypes. HCV genotype 3 is considered to be the most difficult-to-cure genotype with DAA-based therapy.27,28 Currently available DAAs are less efficient against this genotype. Two regimens are recommended for the management of these patients: the combinations of daclatasvir and sofosbuvir or sofosbuvir and velpatasvir. The SVR rates are lower compared with other genotypes, ranging from 50% to 90% depending on the treatment history and the disease stage.27–31 Interestingly, HCV genotype 3 is the second most prevalent genotype worldwide with high morbidity and mortality rates compared with other genotypes.32 Therefore, additional studies are needed to improve new therapeutic options for patients with HCV genotype 3 infection.
Another aspect that requires further studies is the persistent risk of HCC post cure in patients with advanced fibrosis or comorbidities. Although the risk of de novo HCC development is reduced after SVR, HCC can occur even more than 10 years following successful HCV clearance.4,33,34 Evidence from several cohorts appears to suggest that post-SVR HCC development and recurrence may be more frequent after DAA treatment compared with IFN-based therapy, potentially due to a difference in host immune modulation between IFN- and DAA-treated patients.4,35 Given the increasing incidence of HCC and the urgent unmet needs for prevention and detection of HCC, alternative strategies for these populations should be explored.7,34
Although not an issue in the majority of treated patients, HCV resistance to DAAs can occur in a small minority of patients. HCV has a quasispecies distribution. The high replication rate of HCV (1010−1012 virions per day in patients with chronic infection) and the low fidelity of its RNA polymerase (1.5–2.0 × 10−3 base substitutions per genome site per year) result in a high degree of genetic variants. Therefore, patients are infected by a mix of distinct but closely related viral populations.36 Viral polymorphisms can naturally appear in regions targeted by DAA and may confer DAA resistance. When a DAA treatment is administered, sensitive wild-type populations are completely inhibited while drug-resistant variants are rapidly selected, leading to treatment failure (breakthrough) or relapse after therapy.17,37 Moreover, DAAs from the same class share cross resistance, meaning that specific mutations can confer reduced susceptibility to all molecules from the same class, thereby limiting retreatment options.17,38 Consequently, more studies and clinical trials are ongoing to define the best retreatment strategies for patients with viral resistance or treatment failure.
Targeting of viral host-dependency factors to prevent and cure HCV infection
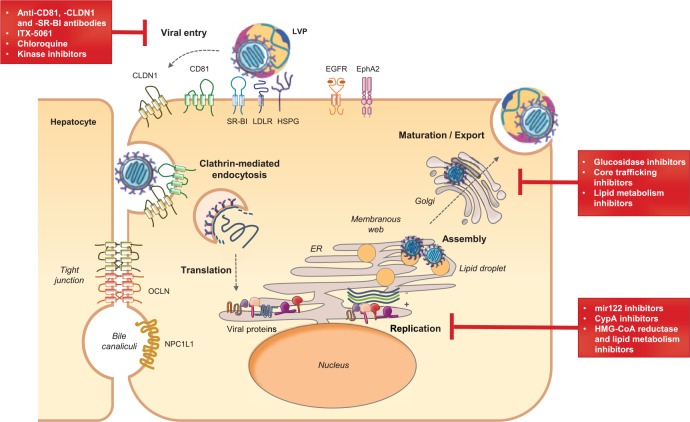
The HCV life cycle can be divided into three major steps: viral attachment and cell entry; viral translation and replication; and assembly and release of the new infectious virions (Figure 1).1 Numerous host cell factors are involved in each step of the HCV life cycle. A hallmark of HCV is the association of circulating viruses with very-low-density and low-density lipoproteins (VLDL and LDL) forming infectious lipo-viro particles (LVPs).39 HCV infection of hepatocytes begins with a complex interaction between these LVPs and several cellular attachment or entry factors. After attachment, viral particles are then internalized through clathrin-mediated endocytosis.40 The viral RNA is released into the cytosol, and translated into a polyprotein that is targeted to the endoplasmic reticulum (ER) where it is subsequently processed by host and viral proteases to generate three structural proteins (Core, E1 and E2) and seven NS proteins (p7, NS2, NS3, NS4A, NS4B, NS5A and NS5B).41 The NS proteins form the replication complex at the surface of ER-derived membranes, the so-called membranous web. Viral replication is catalyzed by the viral polymerase NS5B.42 Viral RNA and proteins accumulate and new virions are assembled in an ER-related compartment in close vicinity to hepatic lipid droplets (LDs). Finally, HCV uses the VLDL production and secretory pathways to generate and export infectious LVPs.43 As reviewed below, many of these host-dependency factors are targets for antiviral drugs. This review will focus on host factors that have been explored as targets of HTAs in antiviral therapies.
Figure 1.
The HCV life cycle and host targets for antiviral therapy.
The major steps of the hepatitis C virus (HCV) life cycle are shown schematically. Lipo-viro particle (LVP) interaction with cellular surface receptors initiates the viral entry process. After clathrin-mediated endocytosis and uncoating, the HCV genome is translated into a polyprotein that is processed into 10 viral proteins anchored in the endoplasmic reticulum (ER). Viral replication takes place in ER-derived membranes named the ‘membranous web’. The assembly process is triggered by core protein trafficking to lipid droplets (LDs). Virion morphogenesis is coupled to very-low-density lipoprotein (VLDL) synthesis. New virions are transported and maturated through the Golgi before being released as LVPs. The main inhibitors of viral entry, replication and assembly or release are highlighted in red squares. Examples of host-targeting agents (HTAs) in preclinical or early clinical development are indicated. These include miR-122 inhibitors: miravirsen/SPC3649, RG-101; cytochrome P450 A (CypA) inhibitors: alisporivir/Debio 025, NIM811, SCY-635 and CPI-431-32; lipid metabolism inhibitors: statins [3-hydroxyl-3-methylglutaryl coenzyme A (HMG-CoA) inhibitors], PF-429242 (SKI-1/S1P inhibitor); α-glucosidase inhibitors: celgosivir; Core protein trafficking inhibitors: LCQ908/pradigastat, quercetin (diglyceride acyltransferase I inhibitors); lipid metabolism inhibitors: naringenin and amiodarone (microsomal triglyceride transfer protein inhibitors), mipovirsen [apolipoprotein B (ApoB) inhibitor], resveratrol, pterostilbene; bezafibrate and torimefene (peroxisome proliferator-activated receptor α agonists). CLDN1, Claudin 1; EGFR, epidermal growth factor receptor; EphA2, ephrin receptor A2; HSPG, heparan sulfate proteoglycan; LDLR, low-density lipoprotein receptor; miR-122, microRNA 122; NPC1L1, Niemann-Pick C1 Like 1 protein; OCLN, Occludin; SR-BI, scavenger receptor BI.
Inhibitors of HCV entry: prevention of HCV infection during transplantation
The HCV entry process has been particularly well characterized in the past few years. First, heparan sulfate proteoglycans (HSPGs) are involved in the attachment of viral particles to hepatocytes. Viral entry is then mediated by several entry factors. The main entry factors are the scavenger receptor BI (SR-BI), the tetraspanin CD81 and the tight-junction proteins Claudin 1 (CLDN1) and Occludin (OCLN).44 This process also involves numerous cofactors, notably the two receptor tyrosine kinases epidermal growth factor receptor (EGFR) and ephrin receptor A2 (EphA2) that promote CD81–CLDN1 interaction, as well as other tetraspanin-associated proteins.45,46 Finally, other regulators of the host lipid metabolism and innate immune responses such as the Niemann-Pick C1 Like 1 protein (NPC1L1) and the sodium taurocholate cotransporting polypeptide (NTCP) are required for HCV entry.44–48 Furthermore, transferrin receptor 1 (TfR1) has recently been proposed as an HCV entry factor.44
Targeting host proteins involved in virus entry is a particularly attractive strategy for the prevention of organ infection in LT or during transplantation of HCV-positive organs, as reviewed recently.49,50 Chronic HCV infection is a leading cause of LT. Infection of the engrafted organ is universal and patients exhibit accelerated progression to advanced liver disease following LT.49,50 A very appealing option to prevent HCV infection and viral-induced disease in the graft is blocking viral cell entry using entry inhibitors alone or in combination with other antiviral treatments.
The first HTA having entered clinical development is the small molecule ITX-5061 that blocks the interaction of HCV with SR-BI. In vitro studies have shown efficient reduction of virus entry by ITX-5061 for HCV genotypes 1–6.51 Although SR-BI is a key component of the host lipid metabolism, ITX-5061 was well tolerated in clinical phase I studies with the only major adverse effect being elevated serum levels of high-density lipoproteins (HDLs).52 While ITX-5061 showed only limited efficacy in clinical phase I studies enrolling patients with chronic HCV infection, it significantly limited viral evolution in patients undergoing LT.52,53 Of note, long-term treatment with ITX-5061 in cell culture resulted in escape mutations in HCV envelope glycoprotein E2, but these findings might be limited to in vitro settings, since the mutants also showed increased sensitivity to antibody neutralization and thus might not arise in vivo.54 Limited potency of the compound combined with viral escape may have been the reasons for incomplete protection.
Another attractive strategy to inhibit virus entry is to target host factors using specific monoclonal antibodies. These antibodies bind to host proteins and block their engagement by HCV, thereby blocking virus entry into hepatocytes. Antibodies targeting CD81, CLDN1 and SR-BI have been shown to elicit strong antiviral effects in preclinical mouse studies.55–60 Of note, anti-CLDN1 antibodies are also able to cure chronic HCV infection in the albumin enhancer-promoter-driven urokinase-type plasminogen activator/severe combined immunodeficiency (uPA/SCID) liver chimeric mouse model, suggesting that entry inhibitors also hold potential to treat chronic hepatitis C.59,60 Completion of preclinical development is the next step for clinical translation of these compounds. Further host factors involved in HCV entry that are subject to (pre-)clinical drug development include NPC1L1 and EGFR. Ezetimibe, a molecule targeting NPC1L1, elicited only minor effects on HCV viral loads in a phase I clinical trial that enrolled two patients who underwent organ transplantation.61 Additionally, a phase I/II clinical trial has been initiated to evaluate the potential of the clinically approved EGFR inhibitor erlotinib to treat chronic HCV infections [ClinicalTrials.gov identifier: NCT01835938].
Silymarin/silibinin is a clinically approved natural product isolated from milk thistle that is regularly used to treat liver damage after intoxications and chronic liver disease. In vitro studies have shown an inhibitory effect of this compound on clathrin-dependent viral trafficking.62 Clinical phase I and phase II studies of patients with chronic HCV, including those who have had LT, revealed conflicting results. While some studies reported a significant reduction in viral loads and alanine transaminase (ALT) levels, other studies reported viral rebound and treatment failure.63–66 Further studies are thus needed to clarify the potential of silymarin/silibinin for the management of chronic hepatitis C.
Another drug targeting virus entry that has been evaluated in a phase IV clinical study is the malaria drug chloroquine that affects clathrin-mediated endocytosis and virus-mediated autophagy.67,68 Monotherapy with chloroquine of patients with chronic HCV infection not responding to IFN-based regimens with chloroquine resulted in a significant reduction of viral RNA and liver ALT levels. However, viral titers quickly relapsed after cessation of treatment, indicating that monotherapy with chloroquine might not be sufficient to cure HCV infection.69
Interestingly, combinations of host-targeting HCV entry inhibitors and DAAs are characterized by synergistic antiviral effects.58,70 The prophylactic properties of HTAs targeting virus entry could make them a valuable asset in the prevention of graft infection during LT. Additional clinical trials are required to establish the place of entry inhibitors in the management of HCV graft infection.
Inhibitors of HCV RNA replication
Cyclophilin A inhibitors
To facilitate its replication, HCV uses a plethora of host factors, including cyclophilins, that interact with the viral protein NS5A.42,71–73 It was demonstrated that cyclosporine A (CsA), a common immunosuppressive drug targeting cytochrome P450 A (CypA), efficiently suppresses viral replication in vitro and in LT recipients.74,75 Based on these observations, CsA derivatives lacking immunosuppressive activity but retaining antiviral activity have been developed.76–78 Three molecules have so far exhibited clinical efficacy in patients with HCV infection treated with both IFN-based and IFN-free regimens: alisporivir/Debio 025, NIM811 and SCY-635.79,80 Safety limitations have delayed the clinical development and further studies are needed to investigate the role of these compounds in the management of patients with HCV infection. These compounds act through two distinct mechanisms. On one hand, they inhibit HCV replication by disrupting and preventing Cyp–NS5A interaction. On the other hand, CypA inhibitors can restore the host innate immune responses against HCV.79,81,82 Interestingly, it was demonstrated that alisporivir also restricts human immunodeficiency virus (HIV) replication in vitro and in vivo by inhibiting CypA–HIV capsid protein interaction.83–85 Based on this knowledge, Gallay and collaborators described a novel CypA inhibitor, named CPI-431-32, that simultaneously blocks HCV and HIV replication with a higher efficiency than alisporivir.86 CypA inhibitors may thus be of interest to treat patients with HIV/HCV coinfection.
MicroRNA-122 inhibitors
After viral entry, the stability of HCV RNA and its propagation in hepatocytes depend on the interactions between the HCV genome and microRNA-122 (miR-122), a miRNA highly expressed in the liver.87–89 It was demonstrated that sequestering miR-122 using miravirsen/SPC3649, a miR-122 antisense locked nucleic acid, strongly reduces HCV replication in vitro.87,90 Moreover, as the miR-122 binding sites are highly conserved across HCV genotypes, miravirsen exhibits a pan-genotypic antiviral effect.91 It also appears safe and well tolerated and elicits a prolonged dose-dependent reduction in HCV RNA levels in patients without serious adverse effects.92,93 Although both in vitro and clinical data indicated that miravirsen provides a high barrier to viral resistance, some patients experienced viral rebound within 14 weeks after treatment cessation, suggesting that miravirsen may not be used as a monotherapy but rather in combination with other HTAs or DAAs.91,92 In this respect, promising results have already shown that miravirsen and DAAs exert an additive effect in vitro and that miravirsen is fully active against DAA-resistant HCV variants.74,94 Finally, a recent phase I clinical study assessed the safety and the antiviral effect of RG-101, a hepatocyte-targeted N-acetylgalactosamine-conjugated oligonucleotide that antagonizes miR-122. A single dose of this compound resulted in a significant decrease in HCV load and was well tolerated in patients with chronic HCV infection with various genotypes. However, viral rebound was observed in two patients, likely due to substitutions in miR-122 binding sites in the HCV genome, which led to viral resistance.95 More studies are therefore needed to evaluate the potential of these antiviral strategies. A phase II study is now underway (EudraCT 2015-001535-21) to establish the efficacy of RG-101 in combination with DAAs. Since HTAs target host factor functions and not the virus itself, these compounds may have adverse effects linked to their mode of action.3,83,96 Thus, although no side effects have been observed so far after administration of miR-122 inhibitors, the effect of a long-term use of such compounds must definitely be investigated because low hepatic miR-122 levels have been associated with liver disease progression and HCC development.97–99
Inhibitors of lipid biosynthesis pathways
Statins are drugs widely used for the treatment of hypercholesterolemia. They act through the inhibition of the 3-hydroxyl-3-methylglutaryl coenzyme A (HMG-CoA) reductase, the rate-limiting enzyme in cholesterol biosynthesis in the liver. HCV replication can be disrupted in vitro by several statins such as lovastatin, atorvastatin, fluvastatin and simvastatin.100–102 Thus, statins have been proposed as candidates for the treatment of HCV infection. Of note, all HMG-CoA reductase inhibitors do not affect HCV replication, such as apravastatin, which does not exhibit an anti-HCV activity.103 The precise mechanism of action is not yet fully understood. Some studies suggested that the anti-HCV activity of statins could be due to the inhibition of geranylgeranylation of cellular proteins rather than to the inhibition of cholesterol biosynthesis.100,104 Geranylgeranylation is a post-transcriptional modification that attaches geranylgeranyl groups produced through the cholesterol biosynthesis pathway to host proteins to facilitate their association with the host-cell membrane, which is essential for viral replication.105 Initial clinical studies indicated that statin monotherapies do not significantly modulate viremia in patients with chronic infection.106–108 Broad retrospective studies subsequently demonstrated that statins constitute interesting adjuvants for anti-HCV therapies. Fluvastatin and pitavastatin have been reported to increase SVR in patients with HCV infection treated with pegylated IFNα and ribavirin.109–113 Moreover, statins have been suggested to offer chemoprevention against HCV-induced HCC by inhibiting cell growth and tumor spread, and by exerting immunomodulatory effects.114–116 The use of statins as adjunctive therapy in HCV treatment has been limited to IFN-based therapies. Although statins in combination with DAAs have been shown to increase antiviral efficacy against HCV infection in vitro, the clinical benefit of statins in the era of DAA is still uncertain. Moreover, concerns have been raised about possible statin–DAA interactions.102,117–119 Therefore, future studies are needed to better characterize the benefits of statins in anti-HCV therapies and for the prevention of HCC development.
Another example of a lipid biosynthesis pathway inhibitor is PF-429242, which inhibits the human subtilase SKI-1/S1P. SKI-1/S1P regulates a master lipogenic pathway upstream of HMG-CoA reductase through the activation of the sterol-regulating element binding protein. It was shown that the antiviral activity of PF-429242 was higher than that of statins in cell culture and that this compound decreased virion production in vitro.120,121 However, their suitability for antiviral treatment still needs to be investigated.
Inhibitors of HCV assembly and release
α-Glucosidase inhibitors
The HCV particle consists of a nucleocapsid containing the viral RNA, surrounded by an ER-derived membrane where E1 and E2 glycoproteins are anchored as heterodimers.43 In the ER, E1 and E2 proteins are highly glycosylated by α-glucosidases I and II, which ensure proper folding of these proteins. Celgosivir, a glucosidase I inhibitor, was shown to reduce virion production and viral infectivity in vitro.122,123 It also exhibited antiviral activity in preclinical trials and was investigated in a phase II clinical trial. Although this compound is not efficient as a monotherapy, it demonstrated a synergistic effect in combination with IFN-based therapies [ClinicalTrials.gov identifier: NCT00332176].122 The clinical trials for celgosivir were stopped as stated in the Migenix financial report for 2010.
Inhibitors of HCV core protein trafficking
Since HCV circulates as a LVP in the blood of patients with infection, viral assembly and egress of infectious LVP rely on host factors, which are required for lipid metabolism and VLDL production. Therefore, they represent potential targets for anti-HCV therapies.39,124,125 Virus assembly is triggered by core protein recruitment on LD by the diglyceride acyltransferase I (DGAT-I).126,127 It was demonstrated that DGAT-I inhibitors efficiently suppress production of infectious viral particles in vitro while LD formation is not affected. Interestingly, quercetin, a natural flavonoid that inhibits DGAT-I, was reported to have anti-HCV properties.128 In a phase I dose-escalation study, quercetin exhibited both high safety (up to 5 g/day) and high antiviral efficacy in patients with chronic infection.129 Quercetin is widely available and cheap, and could thus be developed as an inexpensive adjuvant for HCV treatment. Recently, the antiviral efficacy of the DGAT-I inhibitor LCQ908/pradigastat was assessed in phase II clinical trials in patients with HCV infection. Pradigastat was also safe and well tolerated. However, the clinical study was prematurely interrupted for lack of efficacy: no significant change in serum viral RNA levels was observed in patients after pradigastat treatment compared with the placebo group.130 More studies are now needed to determine whether the DGAT-I inhibitor could be beneficial for patients in combination with DAAs.
During HCV assembly, HCV core protein is recruited from LD to the viral assembly site. This process involves several host factors, including clathrin assembly protein complex 2 medium chain μ1 (AP2M1), which directly interacts with core protein. Furthermore, the adaptor-associated kinase 1 (AAK1) and the cyclin-associated kinase (GAK) are known to regulate core-AP2M1 interaction and are essential for HCV assembly.131–133 Accordingly, Neveu and colleagues discovered that AAK1 and GAK inhibitors, including the approved anticancer drugs sunitinib and erlotinib, can block HCV assembly.131,132 However, these compounds were initially developed to target other kinases and could have adverse effects due to their lack of specificity. To overcome this problem, a more specific GAK inhibitor, isothiazolo[5,4-b]pyridine, was recently developed. This new drug efficiently inhibits HCV entry and assembly in vitro with limited off-target effects and has been proposed as an antiviral strategy.133
Inhibitors of host lipid metabolism
During the later stages of assembly, HCV coopts the VLDL pathway.124,134 Formation of VLDL as well as LVP requires the microsomal triglyceride transfer protein (MTP). This host enzyme mediates triglyceride incorporation into nascent LD and allows lipid loading of ApoB in the ER. Compounds that inhibit the VLDL assembly pathway, such as MTP inhibitors or ApoB inhibitors, are therefore interesting candidates to block HCV assembly and release.124,134 In line with these observations, the MTP inhibitor naringenin, a grapefruit flavonoid, has been shown to inhibit VLDL secretion in vitro and in vivo as well as HCV secretion in cell culture.135 More recently, a study demonstrated that the MTP inhibitor amiodarone also downregulates HCV assembly and release.136 However, a recent report indicated that amiodarone could induce bradycardia in patients when administered in combination with DAAs.96 Several other MTP inhibitors are currently being evaluated in clinical trials for the treatment of dyslipidemia but their in vivo efficacy against HCV remains to be demonstrated.137–139 Interestingly, mipomersen, an antisense inhibitor of ApoB synthesis used for the treatment of hypercholesterolaemia was also shown to efficiently block HCV morphogenesis in vitro.140 Mipomersen now needs to be tested in vivo.
In 2011, Goldwasser and colleagues showed that naringenin blocks HCV assembly not only by inhibiting MTP but also by activating the peroxisome proliferator-activated receptor α (PPARα) in HCV-infected cells.141,142 PPARα is a nuclear transcription factor regulating several aspects of the lipid metabolism in the liver. Notably, its activation leads to increase of fatty acid oxidation and impairment of HCV assembly and release.143,144 During chronic HCV infection, the viral core protein inhibits PPARα activity to promote HCV replication. Thus, restoring PPARα activity constitutes an interesting strategy to inhibit HCV infection.145,146 Accordingly, several approved drugs targeting PPARα were shown to display antiviral activity against HCV. This holds true for resveratrol and its methylated form pterostilbene (two natural compounds extracted from grapes and blueberries) as well as for torimefene (a tamoxifene derivative).146,147 In addition, Fujita and collaborators demonstrated the efficacy of bezafibrate, another PPARα agonist commonly used against hyperlipidemia in patients with chronic HCV infection.148 However, in 2013, Knop and colleagues observed that the beneficial effect of bezafibrate in patients is not due to a decrease in HCV RNA levels but rather to a significant reduction of liver enzymes and improved liver function in patients.149 Finally, more recent studies corroborated the efficacy of bezafibrate in the regulation of lipid metabolism and in the reduction of viral loads in cell culture, indicating that further studies are needed to ascertain its potential for anti-HCV therapies.150,151 Clinical trials are needed to investigate the clinical antiviral efficacy of assembly inhibitors.
Future role of HTAs in HCV therapy
DAA-based treatment is standard of care for the management of patients with chronic HCV infection. Next-generation DAAs with an even higher barrier to resistance and pan-genotypic activity are currently under clinical development.3 In this context, one can wonder about the positioning of HTAs in anti-HCV therapy. A key advantage of HTAs could be linked to the question of whether next-generation DAAs can address the limitations of currently licensed combination therapies (patients who are difficult to treat, resistance, access and HCC risk). While it is expected that next-generation DAAs will indeed address several of these issues, it is likely that DAAs will not be able to address all of these remaining challenges.
Multiresistance, especially in patients with reinfection such as those who are drug abusers with limited compliance and have had multiple treatment courses, may require complementary compounds. This is already blatant for nosocomial bacterial infections in patients who are critically ill, in whom current antibiotics are no longer effective. HTAs may also prove useful to ameliorate current treatment approaches when combined with DAAs. Combinations of HTAs and DAAs may even further reduce treatment duration, increase efficacy and thus improve adherence and access to therapy. HTAs may also be used for the treatment of patients with advanced disease, to lower HCC risk since this is a limitation of current DAA regimens, which have been proven unable to prevent HCC, especially in patients with advanced fibrosis or comorbidity. Finally, host-targeting entry inhibitors are good candidates to prevent HCV infection during LT or transplantation of HCV-positive organs such as kidneys. It is clear that infection prevention is by far conceptually a better option than DAA treatment post transplantation, since if the infection develops, it entails the risk of acute or chronic HCV-induced liver disease (with fibrosing cholestatic hepatitis or HCC as the most severe forms). If a short-term preventive approach can effectively prevent HCV infection during transplantation, this concept may also improve patient care as it will be administered perioperatively during hospitalization.
Taken together, further clinical trials are needed to define the place of HTAs in the management of patients with HCV infection and to determine their role in comparison to or in combination with DAAs.
Acknowledgments
The authors acknowledge Dr Marie Meister for critical reading of the manuscript.
Footnotes
Funding: The authors acknowledge funding by ARC, Paris and IHU Strasbourg (TheraHCC IHUARC IHU201301187), the European Union (ERC-AdG-2014-671231-HEPCIR, EU H2020-667273-HEPCAR, FP7 HEPAMAB GAN 305600), ANR (LABEX ANR-10-LABX-0028_HEPSYS), the National Institutes of Health (1U19AI123862-01) and the DoD (CA150281)
Conflict of interest statement: C.S., M.B.Z. and T.F.B are inventors of patents or patent applications on anti-receptor antibodies for prevention and treatment of HCV infection.
Contributor Information
Emilie Crouchet, Institut National de la Santé et de la Recherche Médicale (Inserm), U1110, Institut de Recherche sur les Maladies Virales et Hépatiques, Strasbourg, France Université de Strasbourg, Strasbourg, France.
Florian Wrensch, Institut National de la Santé et de la Recherche Médicale (Inserm), U1110, Institut de Recherche sur les Maladies Virales et Hépatiques, Strasbourg, France Université de Strasbourg, Strasbourg, France.
Catherine Schuster, Institut National de la Santé et de la Recherche Médicale (Inserm), U1110, Institut de Recherche sur les Maladies Virales et Hépatiques, Strasbourg, France Université de Strasbourg, Strasbourg, France.
Mirjam B. Zeisel, Institut National de la Santé et de la Recherche Médicale (Inserm), U1110, Institut de Recherche sur les Maladies Virales et Hépatiques, Strasbourg, France Université de Strasbourg, Strasbourg, France Inserm U1052, CNRS UMR 5286, Cancer Research Center of Lyon (CRCL), Université de Lyon (UCBL), Lyon, France
Thomas F. Baumert, Inserm U1110, Institut de Recherches sur les Maladies Virales et Hépatiques, Université de Strasbourg, 3 Rue Koeberlé, F-67000 Strasbourg, France.
References
- 1. Manns MP, Buti M, Gane E, et al. Hepatitis C virus infection. Nat Rev Dis Primer 2017; 3: 17006. [DOI] [PubMed] [Google Scholar]
- 2. Hoshida Y, Fuchs BC, Bardeesy N, et al. Pathogenesis and prevention of hepatitis C virus-induced hepatocellular carcinoma. J Hepatol 2014; 61(Suppl. 1): S79–S90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pawlotsky JM. New Hepatitis C therapies: the toolbox, strategies, and challenges. Gastroenterology 2014; 146: 1176–1192. [DOI] [PubMed] [Google Scholar]
- 4. Baumert TF, Jühling F, Ono A, et al. Hepatitis C-related hepatocellular carcinoma in the era of new generation antivirals. BMC Med 2017; 15: 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zeisel MB, Crouchet E, Baumert TF, et al. Host-targeting agents to prevent and cure hepatitis C virus infection. Viruses 2015; 7: 5659–5685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Baugh JM, Garcia-Rivera JA, Gallay PA. Host-targeting agents in the treatment of hepatitis C: a beginning and an end? Antiviral Res 2013; 100: 555–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chung RT, Baumert TF. Curing chronic hepatitis C: the arc of a medical triumph. N Engl J Med 2014; 370: 1576–1578. [DOI] [PubMed] [Google Scholar]
- 8. Afdhal N, Zeuzem S, Kwo P, et al. Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection. N Engl J Med 2014; 370: 1889–1898. [DOI] [PubMed] [Google Scholar]
- 9. Afdhal N, Reddy KR, Nelson DR, et al. Ledipasvir and sofosbuvir for previously treated HCV genotype 1 infection. N Engl J Med 2014; 370: 1483–1493. [DOI] [PubMed] [Google Scholar]
- 10. Kowdley KV, Gordon SC, Reddy KR, et al. Ledipasvir and sofosbuvir for 8 or 12 weeks for chronic HCV without cirrhosis. N Engl J Med 2014; 370: 1879–1888. [DOI] [PubMed] [Google Scholar]
- 11. Feld JJ, Kowdley KV, Coakley E, et al. Treatment of HCV with ABT-450/r-ombitasvir and dasabuvir with ribavirin. N Engl J Med 2014; 370: 1594–1603. [DOI] [PubMed] [Google Scholar]
- 12. Poordad F, Hezode C, Trinh R, et al. ABT-450/r-ombitasvir and dasabuvir with ribavirin for hepatitis C with cirrhosis. N Engl J Med 2014; 370: 1973–1982. [DOI] [PubMed] [Google Scholar]
- 13. Zeuzem S, Jacobson IM, Baykal T, et al. Retreatment of HCV with ABT-450/r-ombitasvir and dasabuvir with ribavirin. N Engl J Med 2014; 370: 1604–1614. [DOI] [PubMed] [Google Scholar]
- 14. Asselah T, Boyer N, Saadoun D, et al. Direct-acting antivirals for the treatment of hepatitis C virus infection: optimizing current IFN-free treatment and future perspectives. Liver Int 2016; 36(Suppl. 1): 47–57. [DOI] [PubMed] [Google Scholar]
- 15. Li G, De Clercq E. Current therapy for chronic hepatitis C: the role of direct-acting antivirals. Antiviral Res 2017; 142: 83–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Edlin BR. Access to treatment for hepatitis C virus infection: time to put patients first. Lancet Infect Dis 2016; 16: e196–e201. [DOI] [PubMed] [Google Scholar]
- 17. Pawlotsky JM. Hepatitis C virus resistance to direct-acting antiviral drugs in interferon-free regimens. Gastroenterology 2016; 151: 70–86. [DOI] [PubMed] [Google Scholar]
- 18. Hézode C, Fontaine H, Dorival C, et al. Triple therapy in treatment-experienced patients with HCV-cirrhosis in a multicentre cohort of the French Early Access Programme (ANRS CO20-CUPIC)—NCT01514890. J Hepatol 2013; 59: 434–441. [DOI] [PubMed] [Google Scholar]
- 19. Ferenci P. Treatment of hepatitis C in difficult-to-treat patients. Nat Rev Gastroenterol Hepatol 2015; 12: 284–292. [DOI] [PubMed] [Google Scholar]
- 20. Andreone P, Colombo MG, Enejosa JV, et al. ABT-450, ritonavir, ombitasvir, and dasabuvir achieves 97% and 100% sustained virologic response with or without ribavirin in treatment-experienced patients with HCV genotype 1b infection. Gastroenterology 2014; 147: 359–365.e1. [DOI] [PubMed] [Google Scholar]
- 21. Chayama K, Notsumata K, Kurosaki M, et al. Randomized trial of interferon- and ribavirin-free ombitasvir/paritaprevir/ritonavir in treatment-experienced hepatitis C virus-infected patients. Hepatology 2015; 61: 1523–1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Younossi ZM, Stepanova M, Charlton M, et al. Patient-reported outcomes with sofosbuvir and velpatasvir with or without ribavirin for hepatitis C virus-related decompensated cirrhosis: an exploratory analysis from the randomised, open-label ASTRAL-4 phase 3 trial. Lancet Gastroenterol Hepatol 2016; 1: 122–132. [DOI] [PubMed] [Google Scholar]
- 23. Saxena V, Nyberg L, Pauly M, et al. Safety and efficacy of simeprevir/sofosbuvir in hepatitis C-infected patients with compensated and decompensated cirrhosis. Hepatology 2015; 62: 715–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jacobson IM, Lawitz E, Kwo PY, et al. Safety and efficacy of elbasvir/grazoprevir in patients with hepatitis C virus infection and compensated cirrhosis: an integrated analysis. Gastroenterology 2017; 152: 1372–1382.e2. [DOI] [PubMed] [Google Scholar]
- 25. Roth D, Nelson DR, Bruchfeld A, et al. Grazoprevir plus elbasvir in treatment-naive and treatment-experienced patients with hepatitis C virus genotype 1 infection and stage 4-5 chronic kidney disease (the C-SURFER study): a combination phase 3 study. Lancet 2015; 386: 1537–1545. [DOI] [PubMed] [Google Scholar]
- 26. Cacoub P, Desbois AC, Isnard-Bagnis C, et al. Hepatitis C virus infection and chronic kidney disease: time for reappraisal. J Hepatol 2016; 65(Suppl. 1): S82–S94. [DOI] [PubMed] [Google Scholar]
- 27. Zeuzem S, Dusheiko GM, Salupere R, et al. Sofosbuvir and ribavirin in HCV genotypes 2 and 3. N Engl J Med 2014; 370: 1993–2001. [DOI] [PubMed] [Google Scholar]
- 28. Nelson DR, Cooper JN, Lalezari JP, et al. All-oral 12-week treatment with daclatasvir plus sofosbuvir in patients with hepatitis C virus genotype 3 infection: ALLY-3 phase III study. Hepatology 2015; 61: 1127–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Foster GR, Afdhal N, Roberts SK, et al. Sofosbuvir and velpatasvir for HCV genotype 2 and 3 infection. N Engl J Med 2015; 373: 2608–2617. [DOI] [PubMed] [Google Scholar]
- 30. Hull MW, Yoshida EM, Montaner JSG. Update on current evidence for hepatitis C therapeutic options in HCV mono-infected patients. Curr Infect Dis Rep 2016; 18: 22. [DOI] [PubMed] [Google Scholar]
- 31. Leroy V, Angus P, Bronowicki JP, et al. Daclatasvir, sofosbuvir, and ribavirin for hepatitis C virus genotype 3 and advanced liver disease: a randomized phase III study (ALLY-3+). Hepatology 2016; 63: 1430–1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Messina JP, Humphreys I, Flaxman A, et al. Global distribution and prevalence of hepatitis C virus genotypes. Hepatology 2015; 61: 77–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. van der Meer AJ, Veldt BJ, Feld JJ, et al. Association between sustained virological response and all-cause mortality among patients with chronic hepatitis C and advanced hepatic fibrosis. JAMA 2012; 308: 2584. [DOI] [PubMed] [Google Scholar]
- 34. El-Serag HB, Kanwal F, Richardson P, et al. Risk of hepatocellular carcinoma after sustained virological response in Veterans with hepatitis C virus infection. Hepatology 2016; 64: 130–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kozbial K, Moser S, Schwarzer R, et al. Unexpected high incidence of hepatocellular carcinoma in cirrhotic patients with sustained virologic response following interferon-free direct-acting antiviral treatment. J Hepatol 2016; 65: 856–858. [DOI] [PubMed] [Google Scholar]
- 36. Di Lorenzo C, Angus AGN, Patel AH. Hepatitis C virus evasion mechanisms from neutralizing antibodies. Viruses 2011; 3: 2280–2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Esposito I, Trinks J, Soriano V. Hepatitis C virus resistance to the new direct-acting antivirals. Expert Opin Drug Metab Toxicol 2016; 12: 1197–1209. [DOI] [PubMed] [Google Scholar]
- 38. Benítez-Gutiérrez L, Barreiro P, Labarga P, et al. Prevention and management of treatment failure to new oral hepatitis C drugs. Expert Opin Pharmacother 2016; 17: 1215–1223. [DOI] [PubMed] [Google Scholar]
- 39. André P, Komurian-Pradel F, Deforges S, et al. Characterization of low- and very-low-density hepatitis C virus RNA-containing particles. J Virol 2002; 76: 6919–6928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zeisel MB, Felmlee DJ, Baumert TF. Hepatitis C virus entry. Curr Top Microbiol Immunol 2013; 369: 87–112. [DOI] [PubMed] [Google Scholar]
- 41. Niepmann M. Hepatitis C virus RNA translation. Curr Top Microbiol Immunol 2013; 369: 143–166. [DOI] [PubMed] [Google Scholar]
- 42. Paul D, Madan V, Bartenschlager R. Hepatitis C virus RNA replication and assembly: living on the fat of the land. Cell Host Microbe 2014; 16: 569–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lindenbach BD. Virion assembly and release. Curr Top Microbiol Immunol 2013; 369: 199–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Douam F, Lavillette D, Cosset FL. The mechanism of HCV entry into host cells. Prog Mol Biol Transl Sci 2015; 129: 63–107. [DOI] [PubMed] [Google Scholar]
- 45. Lupberger J, Zeisel MB, Xiao F, et al. EGFR and EphA2 are host factors for hepatitis C virus entry and possible targets for antiviral therapy. Nat Med 2011; 17: 589–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zona L, Lupberger J, Sidahmed-Adrar N, et al. HRas signal transduction promotes hepatitis C virus cell entry by triggering assembly of the host tetraspanin receptor complex. Cell Host Microbe 2013; 13: 302–313. [DOI] [PubMed] [Google Scholar]
- 47. Sainz B, Barretto N, Martin DN, et al. Identification of the Niemann-Pick C1-like 1 cholesterol absorption receptor as a new hepatitis C virus entry factor. Nat Med 2012; 18: 281–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Verrier ER, Colpitts CC, Bach C, et al. Solute carrier NTCP regulates innate antiviral immune responses targeting hepatitis C virus infection of hepatocytes. Cell Rep 2016; 17: 1357–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Colpitts CC, Verrier ER, Baumert TF. Targeting viral entry for treatment of hepatitis B and C virus infections. ACS Infect Dis 2015; 1: 420–427. [DOI] [PubMed] [Google Scholar]
- 50. Felmlee DJ, Coilly A, Chung RT, et al. New perspectives for preventing hepatitis C virus liver graft infection. Lancet Infect Dis 2016; 16: 735–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Syder AJ, Lee H, Zeisel MB, et al. Small molecule scavenger receptor BI antagonists are potent HCV entry inhibitors. J Hepatol 2011; 54: 48–55. [DOI] [PubMed] [Google Scholar]
- 52. Sulkowski MS, Kang M, Matining R, et al. Safety and antiviral activity of the HCV entry inhibitor ITX5061 in treatment-naive HCV-infected adults: a randomized, double-blind, phase 1b study. J Infect Dis 2014; 209: 658–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Rowe IA, Tully DC, Armstrong MJ, et al. Effect of scavenger receptor class B type I antagonist ITX5061 in patients with hepatitis C virus infection undergoing liver transplantation. Liver Transplant 2016; 22: 287–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Zhu H, Wong-Staal F, Lee H, et al. Evaluation of ITX 5061, a scavenger receptor B1 antagonist: resistance selection and activity in combination with other hepatitis C virus antivirals. J Infect Dis 2012; 205: 656–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Meuleman P, Hesselgesser J, Paulson M, et al. Anti-CD81 antibodies can prevent a hepatitis C virus infection in vivo. Hepatology 2008; 48: 1761–1768. [DOI] [PubMed] [Google Scholar]
- 56. Lacek K, Vercauteren K, Grzyb K, et al. Novel human SR-BI antibodies prevent infection and dissemination of HCV in vitro and in humanized mice. J Hepatol 2012; 57: 17–23. [DOI] [PubMed] [Google Scholar]
- 57. Vercauteren K, Van Den Eede N, Mesalam AA, et al. Successful anti-scavenger receptor class B type I (SR-BI) monoclonal antibody therapy in humanized mice after challenge with HCV variants with in vitro resistance to SR-BI-targeting agents. Hepatology 2014; 60: 1508–1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Xiao F, Fofana I, Thumann C, et al. Synergy of entry inhibitors with direct-acting antivirals uncovers novel combinations for prevention and treatment of hepatitis C. Gut 2015; 64: 483–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Mailly L, Xiao F, Lupberger J, et al. Clearance of persistent hepatitis C virus infection in humanized mice using a claudin-1-targeting monoclonal antibody. Nat Biotechnol 2015; 33: 549–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Colpitts CC, Tawar RG, Mailly L, et al. Humanisation of a claudin-1-specific monoclonal antibody for clinical prevention and cure of HCV infection without escape. Gut. Epub ahead of print 30 March 2017. DOI: 10.1136/gutjnl-2016-312577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Monrroy-Bravo H, Angulo J, Pino K, et al. Effect of ezetimibe in HCV viral load after liver transplantation. Ann Hepatol 2016; 15: 803–805. [DOI] [PubMed] [Google Scholar]
- 62. Blaising J, Lévy PL, Gondeau C, et al. Silibinin inhibits hepatitis C virus entry into hepatocytes by hindering clathrin-dependent trafficking. Cell Microbiol 2013; 15: 1866–1882. [DOI] [PubMed] [Google Scholar]
- 63. Mariño Z, Crespo G, D’Amato M, et al. Intravenous silibinin monotherapy shows significant antiviral activity in HCV-infected patients in the peri-transplantation period. J Hepatol 2013; 58: 415–420. [DOI] [PubMed] [Google Scholar]
- 64. Neumann UP, Biermer M, Eurich D, et al. Successful prevention of hepatitis C virus (HCV) liver graft reinfection by silibinin mono-therapy. J Hepatol 2010; 52: 951–952. [DOI] [PubMed] [Google Scholar]
- 65. Bárcena R, Moreno A, Rodríguez-Gandía MA, et al. Safety and anti-HCV effect of prolonged intravenous silibinin in HCV genotype 1 subjects in the immediate liver transplant period. J Hepatol 2013; 58: 421–426. [DOI] [PubMed] [Google Scholar]
- 66. Aghemo A, Bhoori S, De Nicola S, et al. Failure of intravenous silibinin monotherapy to prevent hepatitis C genotype 2A liver graft reinfection. Hepat Mon 2012; 12: 411–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Blanchard E, Belouzard S, Goueslain L, et al. Hepatitis C virus entry depends on clathrin-mediated endocytosis. J Virol 2006; 80: 6964–6972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Mizui T, Yamashina S, Tanida I, et al. Inhibition of hepatitis C virus replication by chloroquine targeting virus-associated autophagy. J Gastroenterol 2010; 45: 195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Peymani P, Yeganeh B, Sabour S, et al. New use of an old drug: chloroquine reduces viral and ALT levels in HCV non-responders (a randomized, triple-blind, placebo-controlled pilot trial). Can J Physiol Pharmacol 2016; 94: 613–619. [DOI] [PubMed] [Google Scholar]
- 70. Paciello R, Urbanowicz RA, Riccio G, et al. Novel human anti-claudin 1 mAbs inhibit hepatitis C virus infection and may synergize with anti-SRB1 mAb. J Gen Virol 2016; 97: 82–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Hanoulle X, Badillo A, Wieruszeski JM, et al. Hepatitis C virus NS5A protein is a substrate for the peptidyl-prolyl cis/trans isomerase activity of cyclophilins A and B. J Biol Chem 2009; 284: 13589–13601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Kaul A, Stauffer S, Berger C, et al. Essential role of cyclophilin A for hepatitis C virus replication and virus production and possible link to polyprotein cleavage kinetics. PLoS Pathog 2009; 5: e1000546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Liu Z, Yang F, Robotham JM, et al. Critical role of cyclophilin A and its prolyl-peptidyl isomerase activity in the structure and function of the hepatitis C virus replication complex. J Virol 2009; 83: 6554–6565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Liu F, Shimakami T, Murai K, et al. Efficient suppression of hepatitis C virus replication by combination treatment with miR-122 antagonism and direct-acting antivirals in cell culture systems. Sci Rep. Epub ahead of print 3 August 2016. DOI: 10.1038/srep30939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Watashi K, Hijikata M, Hosaka M, et al. Cyclosporin A suppresses replication of hepatitis C virus genome in cultured hepatocytes. Hepatology 2003; 38: 1282–1288. [DOI] [PubMed] [Google Scholar]
- 76. Paeshuyse J, Kaul A, De Clercq E, et al. The non-immunosuppressive cyclosporin DEBIO-025 is a potent inhibitor of hepatitis C virus replication in vitro. Hepatology 2006; 43: 761–770. [DOI] [PubMed] [Google Scholar]
- 77. Hopkins S, Scorneaux B, Huang Z, et al. SCY-635, a novel nonimmunosuppressive analog of cyclosporine that exhibits potent inhibition of hepatitis C virus RNA replication in vitro. Antimicrob Agents Chemother 2010; 54: 660–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Gallay PA, Lin K. Profile of alisporivir and its potential in the treatment of hepatitis C. Drug Des Devel Ther 2013; 7: 105–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Naoumov NV. Cyclophilin inhibition as potential therapy for liver diseases. J Hepatol 2014; 61: 1166–1174. [DOI] [PubMed] [Google Scholar]
- 80. Pawlotsky JM, Flisiak R, Sarin SK, et al. Alisporivir plus ribavirin, interferon free or in combination with pegylated interferon, for hepatitis C virus genotype 2 or 3 infection. Hepatology 2015; 62: 1013–1023. [DOI] [PubMed] [Google Scholar]
- 81. Hopkins S, Bobardt M, Chatterji U, et al. The cyclophilin inhibitor SCY-635 disrupts hepatitis C virus NS5A-cyclophilin A complexes. Antimicrob Agents Chemother 2012; 56: 3888–3897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Daito T, Watashi K, Sluder A, et al. Cyclophilin inhibitors reduce phosphorylation of RNA-dependent protein kinase to restore expression of IFN-stimulated genes in HCV-infected cells. Gastroenterology 2014; 147: 463–472. [DOI] [PubMed] [Google Scholar]
- 83. Flisiak R, Horban A, Gallay P, et al. The cyclophilin inhibitor Debio-025 shows potent anti-hepatitis C effect in patients coinfected with hepatitis C and human immunodeficiency virus. Hepatology 2008; 47: 817–826. [DOI] [PubMed] [Google Scholar]
- 84. Ptak RG, Gallay PA, Jochmans D, et al. Inhibition of human immunodeficiency virus type 1 replication in human cells by Debio-025, a novel cyclophilin binding agent. Antimicrob Agents Chemother 2008; 52: 1302–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Daelemans D, Dumont JM, Rosenwirth B, et al. Debio-025 inhibits HIV-1 by interfering with an early event in the replication cycle. Antiviral Res 2010; 85: 418–421. [DOI] [PubMed] [Google Scholar]
- 86. Gallay PA, Bobardt MD, Chatterji U, et al. The novel cyclophilin inhibitor CPI-431-32 concurrently blocks HCV and HIV-1 infections via a similar mechanism of action. PLoS One 2015; 10: e0134707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Jopling CL, Yi M, Lancaster AM, et al. Modulation of hepatitis C virus RNA abundance by a liver-specific MicroRNA. Science 2005; 309: 1577–1581. [DOI] [PubMed] [Google Scholar]
- 88. Henke JI, Goergen D, Zheng J, et al. microRNA-122 stimulates translation of hepatitis C virus RNA. EMBO J 2008; 27: 3300–3310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Jangra RK, Yi M, Lemon SM. Regulation of hepatitis C virus translation and infectious virus production by the microRNA miR-122. J Virol 2010; 84: 6615–6625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Jopling CL, Schütz S, Sarnow P. Position-dependent function for a tandem microRNA miR-122-binding site located in the hepatitis C virus RNA genome. Cell Host Microbe 2008; 4: 77–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Li YP, Gottwein JM, Scheel TK, et al. MicroRNA-122 antagonism against hepatitis C virus genotypes 1-6 and reduced efficacy by host RNA insertion or mutations in the HCV 5’ UTR. Proc Natl Acad Sci USA 2011; 108: 4991–4996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Janssen HLA, Reesink HW, Lawitz EJ, et al. Treatment of HCV infection by targeting microRNA. N Engl J Med 2013; 368: 1685–1694. [DOI] [PubMed] [Google Scholar]
- 93. van der Ree MH, van der Meer AJ, de Bruijne J, et al. Long-term safety and efficacy of microRNA-targeted therapy in chronic hepatitis C patients. Antiviral Res 2014; 111: 53–59. [DOI] [PubMed] [Google Scholar]
- 94. Ottosen S, Parsley TB, Yang L, et al. In vitro antiviral activity and preclinical and clinical resistance profile of miravirsen, a novel anti-hepatitis C virus therapeutic targeting the human factor miR-122. Antimicrob Agents Chemother 2015; 59: 599–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Van Renne N, Roca Suarez AA, Duong FHT, et al. miR-135a-5p-mediated downregulation of protein tyrosine phosphatase receptor delta is a candidate driver of HCV-associated hepatocarcinogenesis. Gut. Epub ahead of print 3 February 2017. DOI: 10.1136/gutjnl-2016-312270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Renet S, Chaumais MC, Antonini T, et al. Extreme bradycardia after first doses of sofosbuvir and daclatasvir in patients receiving amiodarone: 2 cases including a rechallenge. Gastroenterology 2015; 149: 1378–1380.e1. [DOI] [PubMed] [Google Scholar]
- 97. Hsu S, Wang B, Kota J, et al. Essential metabolic, anti-inflammatory, and anti-tumorigenic functions of miR-122 in liver. J Clin Invest 2012; 122: 2871–2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Tsai WC, Hsu SD, Hsu CS, et al. MicroRNA-122 plays a critical role in liver homeostasis and hepatocarcinogenesis. J Clin Invest 2012; 122: 2884–2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Janssen HLA, Reesink HW, Lawitz EJ, et al. Treatment of HCV infection by targeting microRNA. N Engl J Med 2013; 368: 1685–1694. [DOI] [PubMed] [Google Scholar]
- 100. Ye J, Wang C, Sumpter R, et al. Disruption of hepatitis C virus RNA replication through inhibition of host protein geranylgeranylation. Proc Natl Acad Sci USA 2003; 100: 15865–15870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Kim SS, Peng LF, Lin W, et al. A cell-based, high-throughput screen for small molecule regulators of hepatitis C virus replication. Gastroenterology 2007; 132: 311–320. [DOI] [PubMed] [Google Scholar]
- 102. Delang L, Paeshuyse J, Vliegen I, et al. Statins potentiate the in vitro anti-hepatitis C virus activity of selective hepatitis C virus inhibitors and delay or prevent resistance development. Hepatology 2009; 50: 6–16. [DOI] [PubMed] [Google Scholar]
- 103. Ikeda M, Abe K, Yamada M, et al. Different anti-HCV profiles of statins and their potential for combination therapy with interferon. Hepatology 2006; 44: 117–125. [DOI] [PubMed] [Google Scholar]
- 104. Kapadia SB, Chisari FV. Hepatitis C virus RNA replication is regulated by host geranylgeranylation and fatty acids. Proc Natl Acad Sci USA 2005; 102: 2561–2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Wang C, Gale M, Keller BC, et al. Identification of FBL2 as a geranylgeranylated cellular protein required for hepatitis C virus RNA replication. Mol Cell 2005; 18: 425–434. [DOI] [PubMed] [Google Scholar]
- 106. O’Leary JG, Chan JL, McMahon CM, et al. Atorvastatin does not exhibit antiviral activity against HCV at conventional doses: a pilot clinical trial. Hepatology 2007; 45: 895–898. [DOI] [PubMed] [Google Scholar]
- 107. Bader T, Fazili J, Madhoun M, et al. Fluvastatin inhibits hepatitis C replication in humans. Am J Gastroenterol 2008; 103: 1383–1389. [DOI] [PubMed] [Google Scholar]
- 108. Patel K, Jhaveri R, George J, et al. Open-label, ascending dose, prospective cohort study evaluating the antiviral efficacy of Rosuvastatin therapy in serum and lipid fractions in patients with chronic hepatitis C. J Viral Hepat. Epub ahead of print May 2011. DOI: 10.1111/j.1365-2893.2010.01310.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Sezaki H, Suzuki F, Akuta N, et al. An open pilot study exploring the efficacy of fluvastatin, pegylated interferon and ribavirin in patients with hepatitis C virus genotype 1b in high viral loads. Intervirology 2009; 52: 43–48. [DOI] [PubMed] [Google Scholar]
- 110. Harrison SA, Rossaro L, Hu KQ, et al. Serum cholesterol and statin use predict virological response to peginterferon and ribavirin therapy. Hepatology 2010; 52: 864–874. [DOI] [PubMed] [Google Scholar]
- 111. Rao GA, Pandya PK. Statin therapy improves sustained virologic response among diabetic patients with chronic hepatitis C. Gastroenterology 2011; 140: 144–152. [DOI] [PubMed] [Google Scholar]
- 112. Shimada M, Yoshida S, Masuzaki R, et al. Pitavastatin enhances antiviral efficacy of standard pegylated interferon plus ribavirin in patients with chronic hepatitis C: a prospective randomized pilot study. J Hepatol 2012; 56: 299–300. [DOI] [PubMed] [Google Scholar]
- 113. Grammatikos G, Farnik H, Bon D, et al. The impact of antihyperlipidemic drugs on the viral load of patients with chronic hepatitis C infection: a meta-analysis. J Viral Hepat 2014; 21: 533–541. [DOI] [PubMed] [Google Scholar]
- 114. Demierre MF, Higgins PDR, Gruber SB, et al. Statins and cancer prevention. Nat Rev Cancer 2005; 5: 930–942. [DOI] [PubMed] [Google Scholar]
- 115. Singh S, Singh PP, Singh AG, et al. Statins are associated with a reduced risk of hepatocellular cancer: a systematic review and meta-analysis. Gastroenterology 2013; 144: 323–332. [DOI] [PubMed] [Google Scholar]
- 116. Mansourian PG, Yoneda M, Krishna Rao M, et al. Effects of statins on the risk of hepatocellular carcinoma. Gastroenterol Hepatol 2014; 10: 417–426. [PMC free article] [PubMed] [Google Scholar]
- 117. Lee JE, van Heeswijk R, Alves K, et al. Effect of the hepatitis C virus protease inhibitor telaprevir on the pharmacokinetics of amlodipine and atorvastatin. Antimicrob Agents Chemother 2011; 55: 4569–4574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Kiser JJ, Burton JR, Anderson PL, et al. Review and management of drug interactions with boceprevir and telaprevir. Hepatology 2012; 55: 1620–1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Simon TG, Butt AA. Lipid dysregulation in hepatitis C virus, and impact of statin therapy upon clinical outcomes. World J Gastroenterol 2015; 21: 8293–8303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Blanchet M, Seidah NG, Labonté P. SKI-1/S1P inhibition: a promising surrogate to statins to block hepatitis C virus replication. Antiviral Res 2012; 95: 159–166. [DOI] [PubMed] [Google Scholar]
- 121. Blanchet M, Sureau C, Guévin C, et al. SKI-1/S1P inhibitor PF-429242 impairs the onset of HCV infection. Antiviral Res 2015; 115: 94–104. [DOI] [PubMed] [Google Scholar]
- 122. Durantel D. Celgosivir, an alpha-glucosidase I inhibitor for the potential treatment of HCV infection. Curr Opin Invest Drugs 2009; 10: 860–870. [PubMed] [Google Scholar]
- 123. Qu X, Pan X, Weidner J, et al. Inhibitors of endoplasmic reticulum α-glucosidases potently suppress hepatitis C virus virion assembly and release. Antimicrob Agents Chemother 2011; 55: 1036–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Gastaminza P, Cheng G, Wieland S, et al. Cellular determinants of hepatitis C virus assembly, maturation, degradation, and secretion. J Virol 2008; 82: 2120–2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Popescu CI, Riva L, Vlaicu O, et al. Hepatitis C virus life cycle and lipid metabolism. Biology 2014; 3: 892–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Miyanari Y, Atsuzawa K, Usuda N, et al. The lipid droplet is an important organelle for hepatitis C virus production. Nat Cell Biol 2007; 9: 1089–1097. [DOI] [PubMed] [Google Scholar]
- 127. Herker E, Harris C, Hernandez C, et al. Efficient hepatitis C virus particle formation requires diacylglycerol acyltransferase-1. Nat Med 2010; 16: 1295–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Rojas Á, Campo JAD, Clement S, et al. Effect of quercetin on hepatitis C virus life cycle: from viral to host targets. Sci Rep 2016; 6: 31777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Lu NT, Crespi CM, Liu NM, et al. A phase I dose escalation study demonstrates quercetin safety and explores potential for bioflavonoid antivirals in patients with chronic hepatitis C. Phytother Res 2016; 30: 160–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Gane E, Stedman C, Dole K, et al. A diacylglycerol transferase 1 inhibitor is a potent hepatitis C antiviral in vitro but not in patients in a randomized clinical trial. ACS Infect Dis 2017; 3: 144–151. [DOI] [PubMed] [Google Scholar]
- 131. Neveu G, Barouch-Bentov R, Ziv-Av A, et al. Identification and targeting of an interaction between a tyrosine motif within hepatitis C virus core protein and AP2M1 essential for viral assembly. PLoS Pathog 2012; 8: e1002845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Neveu G, Ziv-Av A, Barouch-Bentov R, et al. AP-2-associated protein kinase 1 and cyclin G-associated kinase regulate hepatitis C virus entry and are potential drug targets. J Virol 2015; 89: 4387–4404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Kovackova S, Chang L, Bekerman E, et al. Selective inhibitors of cyclin G associated kinase (GAK) as anti-hepatitis C agents. J Med Chem 2015; 58: 3393–3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Huang H, Sun F, Owen DM, et al. Hepatitis C virus production by human hepatocytes dependent on assembly and secretion of very low-density lipoproteins. Proc Natl Acad Sci USA 2007; 104: 5848–5853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Nahmias Y, Goldwasser J, Casali M, et al. Apolipoprotein B-dependent hepatitis C virus secretion is inhibited by the grapefruit flavonoid naringenin. Hepatology 2008; 47: 1437–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Cheng YL, Lan KH, Lee WP, et al. Amiodarone inhibits the entry and assembly steps of hepatitis C virus life cycle. Clin Sci 2013; 125: 439–448. [DOI] [PubMed] [Google Scholar]
- 137. Cuchel M, Bloedon LT, Szapary PO, et al. Inhibition of microsomal triglyceride transfer protein in familial hypercholesterolemia. N Engl J Med 2007; 356: 148–156. [DOI] [PubMed] [Google Scholar]
- 138. Samaha FF, McKenney J, Bloedon LT, et al. Inhibition of microsomal triglyceride transfer protein alone or with ezetimibe in patients with moderate hypercholesterolemia. Nat Clin Pract Cardiovasc Med 2008; 5: 497–505. [DOI] [PubMed] [Google Scholar]
- 139. Ahn CH, Choi SH. New drugs for treating dyslipidemia: beyond statins. Diabetes Metab J 2015; 39: 87–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Schaefer EAK, Meixiong J, Mark C, et al. Apolipoprotein B100 is required for hepatitis C infectivity and Mipomersen inhibits hepatitis C. World J Gastroenterol 2016; 22: 9954–9965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Goldwasser J, Cohen PY, Lin W, et al. Naringenin inhibits the assembly and long-term production of infectious hepatitis C virus particles through a PPAR-mediated mechanism. J Hepatol 2011; 55: 963–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Goldwasser J, Cohen PY, Yang E, et al. Transcriptional regulation of human and rat hepatic lipid metabolism by the grapefruit flavonoid naringenin: role of PPARalpha, PPARgamma and LXRalpha. PLoS One 2010; 5: e12399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Yamaguchi A, Tazuma S, Nishioka T, et al. Hepatitis C virus core protein modulates fatty acid metabolism and thereby causes lipid accumulation in the liver. Dig Dis Sci 2005; 50: 1361–1371. [DOI] [PubMed] [Google Scholar]
- 144. Dharancy S, Malapel M, Perlemuter G, et al. Impaired expression of the peroxisome proliferator-activated receptor alpha during hepatitis C virus infection. Gastroenterology 2005; 128: 334–342. [DOI] [PubMed] [Google Scholar]
- 145. Tanaka N, Moriya K, Kiyosawa K, et al. PPARalpha activation is essential for HCV core protein-induced hepatic steatosis and hepatocellular carcinoma in mice. J Clin Invest 2008; 118: 683–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Jiang L, Gu Y, Ye J, et al. Resveratrol prevents hepatic steatosis induced by hepatitis C virus core protein. Biotechnol Lett 2012; 34: 2205–2212. [DOI] [PubMed] [Google Scholar]
- 147. Gastaminza P, Whitten-Bauer C, Chisari FV. Unbiased probing of the entire hepatitis C virus life cycle identifies clinical compounds that target multiple aspects of the infection. Proc Natl Acad Sci USA 2010; 107: 291–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Fujita N, Kaito M, Kai M, et al. Effects of bezafibrate in patients with chronic hepatitis C virus infection: combination with interferon and ribavirin. J Viral Hepat 2006; 13: 441–448. [DOI] [PubMed] [Google Scholar]
- 149. Knop V, Bergk A, Schlosser B, et al. Bezafibrate maintenance therapy in patients with advanced chronic hepatitis C. Eur J Gastroenterol Hepatol 2013; 25: 594–600. [DOI] [PubMed] [Google Scholar]
- 150. Singaravelu R, Chen R, Lyn RK, et al. Hepatitis C virus induced up-regulation of microRNA-27: a novel mechanism for hepatic steatosis. Hepatology 2014; 59: 98–108. [DOI] [PubMed] [Google Scholar]
- 151. Li X, Jiang H, Qu L, et al. Hepatocyte nuclear factor 4α and downstream secreted phospholipase A2 GXIIB regulate production of infectious hepatitis C virus. J Virol 2014; 88: 612–627. [DOI] [PMC free article] [PubMed] [Google Scholar]