Abstract
Chikungunya viral fever results in extreme morbidity and arthralgia in affected individuals. Currently, modern medicines providing symptomatic relief for the acute febrile phase and the chronic arthritic phase are only options available. Traditional Indian medical system, however, uses specific formulations for treatment of this infection; one such polyherbal formulation used to treat the postpyretic phase of chikungunya is amukkara choornam. The current study was undertaken to study the efficacy of amukkara choornam in the treatment of chikungunya in C57BL/6J mice. The formulation when administered to chikungunya-infected mice relieved morbidity and joint swelling. Analysis of virus clearance in brain and joint tissues on formulation treatment revealed a direct correlation of viral load in brain to morbidity during infection; likewise, joint swelling receded prior to complete viral clearance explaining possible immunomodulatory effect of amukkara choornam. This study provides insight into the possible mode of action of amukkara choornam during chikungunya.
Keywords: polyherbal, siddha formulations, chikungunya, postpyretic phase, in vivo evaluation
Chikungunya, transmitted by Aedes mosquitoes, is a debilitating disease currently affecting people worldwide. Chikungunya usually starts with sudden onset of fever, chills, headache, nausea, joint pain with joint swelling, and rashes and exists in 2 phases, namely pyretic and postpyretic. Pyretic phase is the acute phase of chikungunya and last up to 2 to 7 days, leading to very high fever (104°F in some cases), nausea, and rashes accompanied with severe arthralgia.1–3 In the postpyretic phase, the arthralgia persists and results in severe arthritic pain that may last for months to several years.4,5 When present with other comorbid conditions, chikungunya has been reported to cause fatalities.6 Till now, there is no specific management for chikungunya virus (CHIKV) infection except for the symptomatic treatment which is recommended by most physicians worldwide. The line of management includes rest, intravenous fluids, antipyretic, anti-inflammatory, and analgesic agents. Chloroquine phosphate (250 mg) once daily for 5 days was tried in patients suffering fever with arthritis that demonstrated promising results,7 but in recent clinical trials in CHIKV-infected patients, no difference was observed between the treated and untreated groups.8 It was also reported that in mice model chloroquine enhances alphavirus replication and aggravates disease pathogenesis.9
Indian traditional medicine system has been used for centuries to find cure to human diseases based on the concept of tridosha theory (three-humor theory).10 For this purpose, traditional Indian medical practitioners have utilized herbs, plants, and minerals to develop formulations that could be used to build the host immunity to fight against infectious agents. One such important herb used extensively in Indian and Chinese medicines, especially in musculoskeletal conditions like arthritis and rheumatism, is Withania somnifera.11 This herb is the principal component of an important formulation called amukkara choornam that is used in the treatment of chikungunya, especially the postpyretic phase.
The present study was undertaken to evaluate this formulation for its efficacy in chikungunya, especially during the post pyretic phase, using an in vivo mice model, C57BL/6J. Our study shows that this formulation was quite effective in alleviating arthralgia in a significant manner and improved overall health in diseased condition.
Materials and Methods
Phytochemical Analysis for Amukkara Choornam
For preliminary phytochemical analysis, 100 g powdered formulation was extracted with water successively. The extracts were dried and weighed. The presence or absence of different phytoconstituents were detected by previously prescribed methods.10,12–14
Physiochemical Analysis for Amukkara Choornam
Percentage of total ash, acid-insoluble ash, water soluble ash, and sulfated ash were calculated as per the Indian pharmacopoeia. The different extracts of the formulation were prepared for the study of extractive value.15,16 Loss on drying was carried out. Behavior of powder with different chemical reagents was studied.
Virus, Cells, and Cell Viability Assay
The CHIKV isolate used in this experiment was a clinical isolate from an outbreak in Delhi in 2010, belonging to the ECSA genotype.17 The CHIKV strain was propagated in Vero cells and harvested after full cytopathic effect (CPE) was observed. Virus stock was purified using polyethylene glycol (PEG) concentration methods18 and stored at –80°C. MTT 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide) assay (Sigma, Cat No. D4540) was performed to evaluate the cytotoxicity of tested formulation against Vero cells using previously standardized protocols.19
Animal Experiments
Animal studies were carried out in animal biosafety level (ABSL)-2 facilities under an approved ICGEB Institutional Animal Ethics Committee (IAEC) protocol ICGEB/IAEC/08/2016. C57BL/6J mice (age matched, 20-24 g body weight) were challenged with 106 plaque-forming units (PFUs; 50 μL/mice, footpad injections) of virus described above. Mice were divided into 4 groups: G1 male and G1 female groups were the control groups that were placebo injected with phosphate-buffered saline (PBS) and was orally treated with 0.2 mL vehicle alone (2% methyl cellulose) twice a day, G2 male and female groups were challenged with 106 PFU of CHIKV treated orally with vehicle alone twice a day, G3 male and female groups were placebo infected with PBS and received 0.2 mL of 30 mg/kg/dose of amukkara choornam polyherbal formulation twice a day 48 hours postinfection (h.p.i.)20,21 G4 male and female groups were challenged with 106 PFU of CHIKV followed by the treatment with 0.2 mL 30 mg/kg/dose of amukkara choornam polyherbal formulation twice a day 48 h.p.i. At the end of the experiment, the survival data were collected and analyzed. Also, groupwise data were analyzed for parameters such as morbidity using Morton and Griffiths scale (1985)22 and limb swelling. Blood/sera was collected from animals every third day starting with 0 days postinfection (d.p.i.) and was used to perform plaque assay using standardized protocols.23 All animals were photographed every alternative day for the duration of this study to document changes in animals. Size of hind limbs (both left and right) was measured using digital Vernier caliper with a least count of 0.01 cm. At the end of the experiment at day 15 (or as and when animals died in case of G2) all animals were sacrificed, organs were recovered, pooled (group-wise, gender-wise) and viral nucleic acid isolation was done followed by viral load determination using uantitative real-time polymerase chain reaction (qRT-PCR) using standard protocols.23
Additionally, animals from the virus only group (G2), and virus challenged and drug treated (G4), were sacrificed every third d.p.i. starting day 0 postinfection and brain and joint tissues were dissected. The samples were pooled (organ-, group-, and gender-wise) and plaque assay was performed.
Statistical Studies
Analyses of various physical and biological parameters, group-wise, gender-wise analyses were conducted using 1-way analysis of variance (ANOVA) with regard to group G2. Kaplan-Meier survival curves were plotted and analyzed by the log rank test (Mantel-Cox) test for statistical significance. All statistical analysis was performed using GraphPad Prism 5 software.
Results and Discussion
Amukkara choornam is a polyherbal siddha formulation and a mix of various Indian herbs with Withania somnifera constituting for almost 60% of the composition (Supplementary Table 1). Basic physicochemical studies, fluorescence analysis, and phytochemical screening of extract of the formulation were performed before proceeding with further studies (Supplementary Tables 2a and 2b). Cytotoxicity assay of the formulation showed that concentration of amukkara choornam of 30 μg/mL and below was nontoxic (Supplementary Figure 1).
Amukkara choornam is traditionally administered to control arthritic pain in management of chikungunya.24 However, its mode of action is presently unclear and efforts were taken in this present study to evaluate its antiarthritic properties using an animal model. Mouse strain C57BL/6 J has been widely used as a model to evaluate CHIKV vaccine and drug candidates25,26 and for testing potential CHIKV inhibitors.27,28 Studies have established that this mouse strain is capable of hosting replication of CHIKV, when administered in the footpad of the hind limbs results in extreme morbidity in infected mice25,29 with 10% to 20% of the mice succumbing to it at high challenge doses.30,31 In the present study, 106 PFU was found to be suitable in establishing CHIK infection in the mice with 100% mortality observed 11 d.p.i. (data not shown).
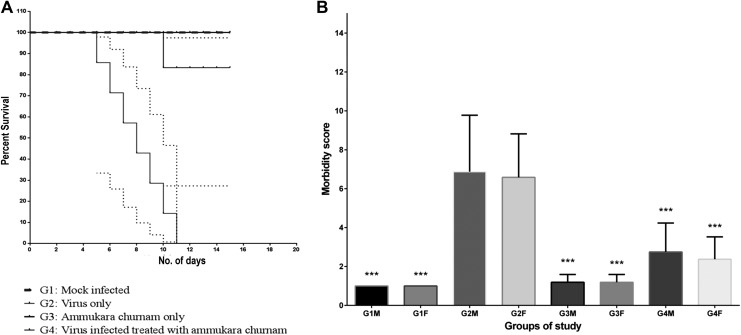
Mice in all groups were observed for differences in their survival and morbidity (Figure 1a and b, respectively). Mice started showing signs of morbidity within 48 hours of virus infection and reached maximum morbidity score of 10. Mice with >9 morbidity died within 24 hours. Illness that preceded death was characterized by rise in self-mutilation, immobilization due to swelling and pain, depression, and moderately abnormal behavior and lack of motor function coordination. It was observed that mortality in group 2 started to occur 7 d.p.i. and by 11 d.p.i., all the animals died. G1 (placebo-treated group) revealed no morbidity throughout the course of the study. G3 (formulation only) also behaved similar to G1, showing no signs of morbidity revealing that the formulation has no adverse side effects on the animals per se. Compared with the virus-only group, G4, exhibited maximum of 6 morbidity score from day 0 to day 6 postinfection, thereafter on treatment with the formulation this score was reduced to 1 on day 15 when all the animals were sacrificed, clearly emphasizing the protective efficacy of formulation. It is noteworthy that all but one mice died in G4 hence, the level of protection afforded by the formulation was statistically significant (P < .05).
Figure 1.
Survival and morbidity analysis of animals used to evaluate the therapeutic potential of amukkara choornam in animal studies. (a) Kaplan-Meier survival plot to evaluate the therapeutic potential of amukkara choornam in animal studies: In group G2 all mice died by 11 days postinfection whereas all mice in group G1, G3, and all but one mice in group G4 survived at the end of the study. Dotted lines represent standard deviations. (b) Mean morbidity score for animal for G1-G4: Morbidity in animals was performed on basis of Morton and Griffith scale. None of the mice in group G1 and G3 exhibited morbidity. Mice in group G2 exhibited high morbidity and mice died once morbidity score reached more than 9. Morbidity in mice in group G4 was like group G2 till day 3 postinfection but it decreased and became comparable to group G1 after treatment with the formulation. *Denotes the level of significance of reduction of morbidity score in comparison with group G2 (all male groups were compared with G2M and all female groups was compared with G2F).
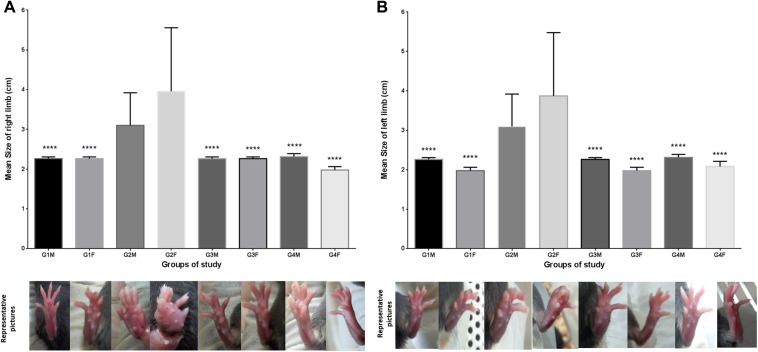
Previous studies have shown that chronic arthralgia is a common complication during postpyretic phase of chikungunya infection.4 In the present study, it was observed that all virus infected mice (group 2 and group 4) exhibited arthralgia 2 d.p.i. The limb size of both hind limbs was measured every third day postinfection for all groups (Figure 2a and b). In depth analysis of limb size in mice groups revealed that the swelling of both limbs of G4 was comparable with the virus only group (G2) till day 3 postinfection but it reduced significantly after the administration of this formulation and by 8 d.p.i., it reverted to the size comparable to placebo-treated control group (G1). Analyses of variance revealed that P values were <.0001 in case of G2 versus G4 male and P = .0001 in case of G2 versus G4 female (Supplementary Tables 3 and 4).
Figure 2.
Arthritic conditions/limb swelling of the animal groups used in the study at day 8. (a) Mean size of right limb for animal groups: Mean increase in the size of the right limb in groups G1, G3 and G4 in comparison with group G2. (b) Mean size of left limb for animal groups: Mean increase in the size of the left limb in groups G1, G3, and G4 in comparison with group G2. G1M and G1F. The swelling in group G2 infected with virus alone reached its maxima at day 10 before succumbing to death. The swelling of limbs in group G4 increased till day 3 postinfection but reduced and became comparable with group G1 after the treatment with the polyherbal formulation.
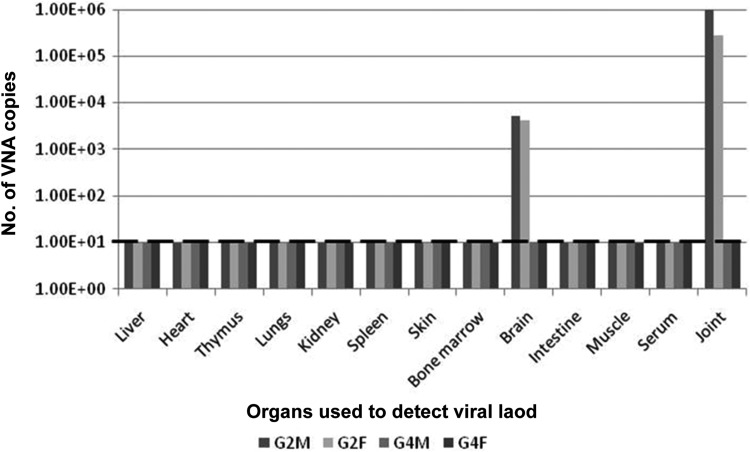
Virus tropism was determined by evaluating viral load in various tissues at different d.p.i. (Supplementary Information 1). Based on the results, it was established that maximum viral load was seen in joints during day 3 to day 15 postinfection, in muscles the viral load was similar to that of joint till day 5 postinfection but later reduced at day 7 postinfection and by day 15 postinfection, it was below the threshold range. Virus was present in the spleen and liver as well during days 3 to 7 postinfection albeit in lesser quantities. Tissues like bone marrow, intestine, and skin showed the presence of virus only during the initial days of infections (days 3-5 ppostinfection). It was interesting to note that CHIKV was present in the sera only in day 3 ppostinfection and was absent during the later days of screening. Brain showed presence of the virus at later stages of infection (days 7-15 postinfection). Presence of CHIKV was not detected in tissues like heart, thymus, lungs, and kidneys during the days tested (days 3-15 postinfection) (Supplementary Figure 2). Based on these observations, further analysis of viral load was performed in the brain and joint tissues once arthritis was established in the mice foot pads 3 d.p.i. to evaluate the mode of action of this formulation.
We sought to study the status of virus clearance in animals after formulation administration. For this purpose, animals were sacrificed on day 15 in case of G1, G3, and G4 and after animal mortality in case of G2, the animals were dissected, and various organs as mentioned in Supplementary Study 1 were collected. For groups G2 and G4, every organ was pooled separately (organ-wise, gender-wise) and processed for viral load detection using qRT-PCR. It was observed that in case of G2, at the time of death, CHIKV was present in joint and brain tissues in both male and female pooled samples. Viral load was below detectable levels in all other organs (Figure 3). In case of G4, viral load was below detectable range in both brain and joint tissues at the time of sacrifice, i.e. 15 d.p.i. (Figure 3).
Figure 3.
Organ-wise viral load for groups G2 and G4. Number of viral nucleic acid (VNA) copies were detected for pooled male and female mice of groups G2 and G4. 101 was the detection threshold for real-time polymerase chain reaction and samples showing VNA copy number more than that was considered positive for the presence of chikungunya virus. For group G2 mice, the animals were sacrificed when they died, and organs of male and female mice were separately collected and pooled before VNA isolation. In case of group G4, all mice were sacrificed at the end of the study, and organs of male and female mice were pooled separately before VNA isolation.
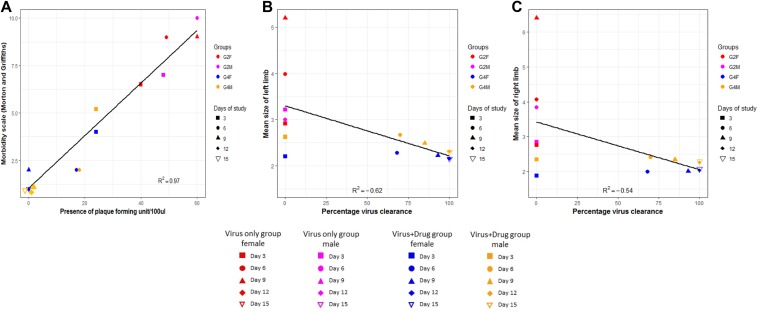
Furthermore, to evaluate the implications of formulation treatment on virus clearance in brain and joint, we sought to evaluate daywise virus clearance pattern on administration of the formulation and correlate morbidity and joint swelling in the virus-only group (G2) and group that was challenged with virus and subsequently treated with amukkara choornam (G4). In brain, it was observed that number of PFUs in G4 started to reduce after day 3 posttreatment in comparison with G2 where number PFUs increased was found to be >50 after day 3 posttreatment (data not shown). It was ascertained that amukkara choornam reduced the number of PFUs in the brain tissues. Additionally, evaluating viral titer with morbidity revealed that in the virus-only group (G2), the virus was seen in the brain tissues from day 3 onward, which coincided with increase in morbidity (Figure 4a). As the days progressed, viral load maximized in direct proportion with morbidity ultimately resulting in death of the animals on day 7 postinfection in case of males and on day 9 postinfection in case of females with comparable viral load among the 2 genders. In case of the formulation treated group (G4), it was seen that the brain showed presence of viruses on day 3 postinfection similar to that of the control group, albeit with lesser viral load. (It should be noted that formulation treatment was started from day 2 onward.) As the days progressed, the viral load was found to decrease along with morbidity and by day 9, the viral load in both males and females was found to be below detectable levels (Figure 4a). In both the groups the viral load was found to be highly correlated with morbidity (R 2 = 0.97 and P < .05).
Figure 4.
Correlation of animal parameters with viral load. (a) Correlation of morbidity score and plaque forming units (PFU)/100 μL of the brain homogenate of male and female mice in group G2F, G2M, G4F, and G4M at days 3, 6, 9, 12, and 15. (b) Correlation of left limb size and percentage virus clearance in male and female mice in group G2F, G2M, G4F, and G4M at days 3, 6, 9, 12, and 15. (c) Correlation of right limb size and percentage virus clearance in male and female mice in group G2F, G2M, G4F, and G4M at days 3, 6, 9, 12, and 15.
In our study, morbidity was scored based on distinct biological features, hence, we hypothesized that in G2, morbidity was linked with central nervous system involvement, which is also exhibited by the presence of viral load and was directly regulated by response to external stimuli, that is, CHIKV infection, leading to behavioral responses such as depression/exaggeration, unprovoked behavior such as self-mutilation and lack of motor activity. Previous reports on other alphaviruses that studied morbidity as an indication of brain infection also used it as a predictor of disease progression, especially for encephalitic alphaviruses.32,33 Even though chikungunya is not encephalitic alphavirus but is known to affect the central nervous system, our study indicates a correlation of morbidity and central nervous system involvement.
With regard to joint swelling and virus infection, we observed that viral clearance in formulation-treated group directly correlated to lessening of joint swelling from day 2 posttreatment and, by day 10 posttreatment, there was 100% viral clearance with no measurable swelling seen in the animals. It was also observed there were only slight differences between the left and right limbs (Figure 4b and c). When analyzed based on limb size, it was found to be negatively correlated with viral clearance (R2 values of −0.62 [left] and −0.54 [right], P < .05 in both cases). Alphavirus-induced arthritis has been well studied with regard to disease severity and tissue specific viral replication25 as well as status of immune system, virus strain virulence and host age31,34 reviewed in Assuncao-Miranda et al.35 In our study, day-wise observation and correlation of viral clearance with joint swelling revealed that swelling totally diminished (day 8 postinfection) even before the complete clearance of CHIKV in the joints that could be attributed to the immunomodulatory characteristics of amukkara choornam.
Even though the exact mode of action of this formulation is unknown, recent studies have reported that W somnifera, the core component in amukkara choornam exhibits both neuroprotective effect as well as anti-inflammatory effects in addition to immunomodulatory activity due to its primary active component, withanamides, known to cross the blood-brain barrier and provide therapeutic effect against stress-induced neurological disorders.36 Furthermore, another study demonstrated the effectiveness of W somnifera in reducing paw swelling and degenerative changes caused by Freund’s adjuvant-induced arthritis in rats, even though the study failed to provide evidence on the mode of action.37
Although, Indian traditional medical practice is effective it is in want of systematic evaluation of the underlying mechanism of their action through well-described experimental analyses. Thus, the present study was undertaken to assess efficacy of this siddha formulation amukkara choornam and has provided preliminary information about its anti-inflammatory and neuroprotective features and its possible immunomodulatory activity during the postpyretic phase of CHIKV in an animal model.
Supplementary Material
Acknowledgments
We duly thank Mr Munna and Mr Patra, Animal House, International Centre for Genetic Engineering and Biotechnology for their assistance in carrying out animal studies.
Footnotes
Author Contributions: SS and JJ designed the research. JJ and VN performed the research and analyzed the data. JJ, SS, and VN wrote the article. SC supervised the animal studies. SS and SP supervised the overall research work.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Financial support for the study was received from the Ministry of AYUSH, (Grant No. ND/AYU/15/019), Government of India and their support is duly acknowledged.
Ethical Approval: Animal studies were carried out in animal biosafety level (ABSL)-2 facilities under an approved ICGEB Institutional Animal Ethics Committee (IAEC) protocol ICGEB/IAEC/08/2016.
Supplemental Material: Supplementary material for this article is available online.
References
- 1. Jain M, Rai S, Chakravarti A. Chikungunya: a review. Trop Doct. 2008;38:70–72. [DOI] [PubMed] [Google Scholar]
- 2. Staples JE, Breiman RF, Powers AM. Chikungunya fever: an epidemiological review of a re-emerging infectious disease. Clin Infect Dis. 2009;49:942–948. [DOI] [PubMed] [Google Scholar]
- 3. Thiboutot MM, Kannan S, Kawalekar OU, et al. Chikungunya: a potentially emerging epidemic? PLoS Negl Trop Dis. 2010;4:e623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Schilte C, Staikovsky F, Couderc T, et al. Chikungunya virus-associated long-term arthralgia: a 36-month prospective longitudinal study. PLoS Negl Trop Dis. 2013;7:e2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Soumahoro MK, Gérardin P, Boëlle PY, et al. Impact of chikungunya virus infection on health status and quality of life: a retrospective cohort study. PLoS One. 2009;4:e7800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tandale BV, Sathe PS, Arankalle VA, et al. Systemic involvements and fatalities during chikungunya epidemic in India, 2006. J Clin Virol. 2009;46:145–149. [DOI] [PubMed] [Google Scholar]
- 7. Brighton SW. Chloroquine phosphate treatment of chronic Chikungunya arthritis. An open pilot study. S Afr Med J. 1984;66:217–218. [PubMed] [Google Scholar]
- 8. De Lamballerie X, Boisson V, Reynier JC, et al. On chikungunya acute infection and chloroquine treatment. Vector Borne Zoonotic Dis. 2008;8:837–839. [DOI] [PubMed] [Google Scholar]
- 9. Maheshwari RK, Srikantan V, Bhartiya D. Chloroquine enhances replication of Semliki Forest virus and encephalomyocarditis virus in mice. J Virol. 1991;65:992–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Thirunarayanan T. An Introduction to Siddha Medicine. Thiruchendur, India: Thirukumaran; 1981. [Google Scholar]
- 11. Mishra LC, Singh BB, Dagenais S. Scientific basis for the therapeutic use of Withania somnifera (ashwagandha): a review. Altern Med Rev. 2000;5:334–346. [PubMed] [Google Scholar]
- 12. Jeganathan N, Kannan K, Manavalan R, Vasanthi HR. Standardization of a siddha formulation amukkara curanam by HPTLC. Afr J Tradit Complement Altern Med. 2008;5:131–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mukherjee PK. Exploring botanicals in Indian system of medicine—regulatory perspectives. Clin Res Regul Aff. 2003;20:249–264. [Google Scholar]
- 14. Mukherjee PK, Wahile A. Integrated approaches towards drug development from Ayurveda and other Indian system of medicines. J Ethnopharmacol. 2006;103:25–35. [DOI] [PubMed] [Google Scholar]
- 15. Brain KR, Turner TD. The Practical Evaluation of Phytopharmaceuticals. Bristol, England: Wright-Scientechnica; 1975. [Google Scholar]
- 16. Evans WC. Trease and Evans’ Pharmacognosy. 14th ed London, England: WB Saunders; 1996:475. [Google Scholar]
- 17. Shrinet J, Jain S, Sharma A, et al. Genetic characterization of Chikungunya virus from New Delhi reveal emergence of a new molecular signature in Indian isolates. Virol J. 2012;9:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Athmaram TN, Saraswat S, Misra P, Das TK, Srinivasan A. A two-step purification strategy for chikungunya virions purification using sucrose buoyant density gradient separation. J Virol Res. 2013;2:18. [Google Scholar]
- 19. Sourisseau M, Schilte C, Casartelli N, et al. Characterization of reemerging chikungunya virus. PLoS Pathog. 2007;3:e89 doi:10.1371/journal.ppat.0030089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Erhirhie EO, Ekene NE, Ajaghaku DL. Guidelines on dosage calculation and stock solution preparation in experimental animals’ studies. J Nat Sci Res. 2014;4:100–106. [Google Scholar]
- 21. Patra KC, Kumar KJ. Establishing correlation of therapeutic activity of a siddha formulation with its antioxidant activity. A comparative study. Int J Pharma Bio Sci. 2010;1:1–8. [Google Scholar]
- 22. Morton DB, Griffiths PH. Guidelines on the recognition of pain, distress and discomfort in experimental animals and a hypothesis for assessment. Vet Rec. 1985;116:431–436. [DOI] [PubMed] [Google Scholar]
- 23. Jain J, Nayak K, Tanwar N, et al. Clinical, serological and virological analysis of 572 chikungunya patients from 2010-2013 in India. Clin Infect Dis. 2017;65:133–140. doi:10.1093/cid/cix283. [DOI] [PubMed] [Google Scholar]
- 24. Wilson E, Rajamanickam GV, Vyas N, Agarwal A, Dubey GP. Herbs used in siddha medicine for arthritis–a review. Indian J Tradit Knowledge. 2007;7:678–686. [Google Scholar]
- 25. Morrison TE, Oko L, Montgomery SA, et al. A mouse model of chikungunya virus-induced musculoskeletal inflammatory disease: evidence of arthritis, tenosynovitis, myositis, and persistence. Am J Pathol. 2011;178:32–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yeo LS, Chu JJH. Recent developments and challenges in mouse models of chikungunya virus infection. Future Virol. 2013;8:423–426. [Google Scholar]
- 27. Kuo SC, Wang YM, Ho YJ, et al. Suramin treatment reduces chikungunya pathogenesis in mice. Antiviral Res. 2016;134:89–96. [DOI] [PubMed] [Google Scholar]
- 28. Varghese FS, Thaa B, Amrun SN, et al. The antiviral alkaloid berberine reduces chikungunya virus-induced mitogen-activated protein kinase signaling. J Virol. 2016;90:9743–9757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wang E, Volkova E, Adams AP, et al. Chimeric alphavirus vaccine candidates for chikungunya. Vaccine. 2008;26:5030–5039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Couderc T, Chrétien F, Schilte C, et al. A mouse model for chikungunya: young age and inefficient type-I interferon signaling are risk factors for severe disease. PLoS Pathog. 2008;4:e29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gardner J, Anraku I, Le TT, et al. Chikungunya virus arthritis in adult wild-type mice. J Virol. 2010;84:8021–8032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cook SH, Griffin DE. Luciferase imaging of a neurotropic viral infection in intact animals. J Virol. 2003;77:5333–5338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Patterson M, Poussard A, Taylor K, et al. Rapid, non-invasive imaging of alphaviral brain infection: reducing animal numbers and morbidity to identify efficacy of potential vaccines and antivirals. Vaccine. 2011;29:9345–9351. doi:10.1016/j.vaccine.2011.09.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Suhrbier A, Jaffar-Bandjee MC, Gasque P. Arthritogenic alphaviruses—an overview. Nat Rev Rheumatol. 2012;8:420–429. doi:10.1038/nrrheum.2012.64. [DOI] [PubMed] [Google Scholar]
- 35. Assuncão-Miranda I, Cruz-Oliveira C, Da Poian AT. Molecular mechanisms involved in the pathogenesis of alphavirus-induced arthritis. Biomed Res Int. 2013;2013:973516 doi:10.1155/2013/973516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Vareed SK, Bauer AK, Nair KM, Liu Y, Jayaprakasam B, Nair MG. Blood-brain barrier permeability of bioactive withanamides present in Withania somnifera fruit extract. Phytother Res. 2014;28:1260–1264. doi:10.1002/ptr.5118. [DOI] [PubMed] [Google Scholar]
- 37. Begum VH, Sadique J. Long term effect of herbal drug Withania somnifera on adjuvant induced arthritis in rats. Indian J Exp Biol. 1988;26:877–882. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.