Abstract
This research was aimed at investigating the antioxidant and antibacterial activity of Bunium persicum, Eucalyptus globulus, and rose water on multidrug-resistant Listeria species. The antibiotic resistance of Listeria spp obtained from seafood samples were determined by the Kirby-Bauer method. The antioxidant and antibacterial activity of the essential oils and extracts were evaluated using ferric reducing antioxidant power and microdilution methods, respectively. A total 2 samples (1.88%) were positive for Listeria spp. L monocytogenes was found to be resistant to ampicillin, amoxicillin/clavulanic acid, penicillin, vancomycin, and kanamycin. B persicum essential oil showed the greatest antioxidant activity (248.56 ± 1.09 µM Fe2+/g). The E globulus essential oil showed consistently strong antimicrobial activity against L monocytogenes and L grayi, while rose water showed no antimicrobial activity against any of the tested bacterial strains. The results showed that after adding the B persicum and E globulus essential oils to bacteria, the cell components’ release increased significantly.
Keywords: antibacterial activity, multidrug resistance, seafood, Listeria
Food-borne pathogenic bacteria have been considered as the main causes of food-borne infections in worldwide.1,2 Among food pathogens, Listeria monocytogenes is the only species of the genus Listeria that has been involved in known food-borne outbreaks of listeriosis and causing 10% to 40% mortality in patients.1,3,4 L monocytogenes is ubiquitous and can grow in high salt concentrations (10%), at a wide range of temperatures (1°C-45°C), at pH 4.6 to 9.6 and can be found in raw products of animal origin and fresh vegetables.5 Also, the contamination could happen in processed food through contaminated waters and environments as well as during transportation.
The Centers for Disease Control and Prevention6 has reported human listeriosis ascribing to consumption of contaminated cantaloupe in the United States. Also, numerous studies in Iran have detected L monocytogenes and its other species in various types of foods, including seafood,7 open-air fish markets,8 raw milk,9 meat and meat products, and ready-to-eat foods.10,11
Nowadays, the development of antibiotic resistance both in animal and human zoonotic bacterial pathogens has been related to the administration of antimicrobial agents in food animal production as growth promoters and their extensive therapeutic use. The antimicrobial agents can be passed through the food chain and contribute to the emergence of resistant bacteria that can be transferred directly to humans after consumption.12,13 Previous studies have reported the multidrug-resistant Listeria spp isolated from raw milk, milking equipment, dairy workers,14 ready-to-eat products of animal origin,15 and fresh and smoked fish.16
Therefore, there is an urgent need for control the spread of multidrug-resistant pathogens to new antimicrobial agents as a strategy. Presently, extracts and essential oils of medicinal plants are used as a novel source of effective antimicrobial agents.17 Previous works have demonstrated the antimicrobial properties of many plant essential oils and extracts as natural substances.1,2,17,18 The presence of bioactive compounds such as phenolic substances in extracts and essential oils may react with microorganisms and restrain microbial growth.19 Therefore, the aim of this study was to investigate the chemical composition, antioxidant and antibacterial activity of Bunium persicum, Eucalyptus globulus, and rose water on multidrug-resistant Listeria species.
Materials and Methods
Sample Collection
The minimum sample size required was estimated based on the single proportion formula:
where n is the minimum sample size required, Z is the reliability coefficient at 95% confidence interval (1.96), p is the prevalence estimate, q is equal to 1 − p, and d is the acceptable error (0.05). According to a previously published prevalence estimate of 7% reported from a study on Listeria species in fresh and frozen fish and shrimp in Iran.20
Accordingly, a total of 106 raw samples of various seafood including shrimp (n = 40) and fresh fish (n = 66) were purchased from randomly selected retail markets located in Kashan, Iran from March 2016 to June 2017. All samples were immediately transferred to the food microbiology laboratory, Kashan University of Medical Sciences, in portable insulated cold-boxes. The samples were analyzed on the day they were collected.
Isolation and Identification of Listeria spp
A 25-g portion of each food sample was added to 225 mL of Listeria enrichment broth (HiMedia Laboratories Limited, Mumbai, India) and stomached for 3 minutes and incubated at 37°C for 48 hours. Then, a loopful of each enrichment culture was spread plated onto Listeria Selective Agar (HiMedia Laboratories Pvt Ltd, India) and was incubated for 24 hours, at 37°C. Following incubation, the suspected colonies with a dark brown color or black halo were selected and transferred onto tryptic soy agar (Merck Co, Darmstadt, Germany) and incubated for 24 hours, at 37°C. The isolates were identified using conventional methods: Gram staining, catalase, typical umbrella motility and fermentation of mannitol, rhamnose, and xylose.21
Antimicrobial Susceptibility
Antimicrobial susceptibility testing of the Listeria isolates was performed by the Kirby-Bauer disc diffusion method using Mueller-Hinton agar (Merck Co, Darmstadt, Germany) according to the Clinical and Laboratory Standards Institute.22 A bacterial suspension with equivalent turbidity to 0.5 McFarland standard (1.5 × 108 cfu/mL) was prepared in sterile phosphate buffered saline (137 mM NaCl, 10 mM phosphate, 2.7 mM KCl, pH 7.4). The sterile swab stick was dipped into bacterial suspension and then on the surface of agar was uniformly inoculated. Five antibiotic disks were placed for each plate and incubated at 35°C for 24 hours. Inhibition zones on agar plate were measured after 24 hours and the results were recorded in accordance with interpretive criteria provided by the Clinical and Laboratory Standards Institute. The antibiotics discs (HiMedia Laboratories Pvt Ltd, Mumbai, India) are reported as follows: ampicillin (10 µg), amoxicillin/clavulanic acid, clindamycin (2 µg), erythromycin (15 µg), gentamycin (10 µg), ciprofloxacin (5 µg), penicillin (10 µg), tetracycline (30 µg), trimethoprim/sulfamethoxazole (10 µg), vancomycin (30 µg), nitrofurantoin (300 µg), norfloxacin (30 µg), kanamycin (30 µg), ceftriaxone (30 µg), and chloramphenicol (30 µg).
Plant Material and Preparation of the Essential Oils and Extracts
Samples of B persicum, E globulus, and rose water were purchased from a reputable grocery in Kashan, Iran. The collected samples were identified and stored at the herbarium of the Research Center for Biochemistry and Nutrition in Metabolic Diseases of Kashan University of Medical Sciences, Iran (Nos. 1b, 2e, and 3r).
To prepare essential oils, the 100 g dried plants were hydrodistilled for 4 hours using a Clevenger type apparatus. The essential oils were then dehydrated over anhydrous sodium sulfate and kept in sealed vials at 4°C.
To prepare ethanolic extracts, 100 g of the dried powder of the plants were mixed with 500 cm3 of 80% ethanol and kept at room temperature (22°C) for 24 hours. The obtained extracts were filtered by filter paper and entered into rotary device (to remove solvent). The obtained alcoholic extracts were dried at the temperature of 40°C in an incubator.18 For the antibacterial properties, several dilutions of the essential oils and extracts were done using 5% (v/v) aqueous dimethyl sulfoxide (DMSO; Merck Co, Darmstadt, Germany) and sterilized by filtration through a 0.45-μm membrane filter.
Essential Oils and Extracts Analysis
The gas chromatographic analyses of essential oils were carried out by an Agilent 6890 GC system with a HP-5MS (60 m × 0.25 mm, film thickness 0.25 μm). The carrier gas (helium) was used at a flow rate of 1.0 mL/min. The gas chromatograph oven temperature was kept at 40°C for 1 minute and programmed to 230°C at the rate of 3°C/min. The injector and detector temperatures were 230°C and 250°C, respectively. Quantitative data were obtained electronically from flam ionization detector area percent data. The main chemical constituents of essential oils were compared with analytical standard and tested in triplicate.
The amount of total phenolic content was measured in ethanolic extracts by colorimetric method using Folin-Ciocalteu. Standard solutions with concentration of 12.5, 25, 50, 62.5,100, and 125 ppm were prepared from gallic acid in 60% solution. A total of 0.1 mL of each solution was transferred to a test tube and 0.5 mL of reagent Folin-Ciocalteu solution 10% was added and after 3 to 8 minutes, 0.4 mL 7.5% sodium carbonate solution was added to it, then the tubes were kept at laboratory temperature for 30 minutes. The optical absorption was measured at a wavelength of 765 nm by a spectrophotometer (Unico UV-2100, Dayton, NJ, USA) and a standard curve was prepared. Then 0.01 to 0.02 g of the dried extract was dissolved in 60% methanol up to the volume of 10 mL. Total phenol content was determined based on Folin-Ciocalteu method with the difference that instead of the standard solution, 0.1 mL of the extract solution was added. The obtained absorbance rate was posed at the standard curve and thus the total phenol content of the extract in mg/g gallic acid equivalent was estimated.23
Ferric Reducing Antioxidant Power Assay
The determination of the total antioxidant activity of plant extracts and essential oils was done by iron reduction (ferric reducing antioxidant power assay).24 The stock solutions included 300 µM acetate buffer pH 3.6 (3.1 g C2H3NaO2ċ3H2O and 16 mL C2H4O2), 10 µM 2,4,6- tripyridyl-s-triazine (TPTZ) solution in 40 µM HCl, and 20 µM FeCl3ċ6H2O solution. FRAP reagent was prepared by mixing 25 mL acetate buffer, 2.5 mL TPTZ, and 2.5 mL FeCl3ċ6H2O solution. The temperature of the solution was raised to 37°C before use. A volume of 200 μL of the plant extracts (1 mg/mL) and essential oils was mixed with 2800 μL of the ferric reducing antioxidant power reagent. The absorbance was measured after 30-minute incubation at 37°C in dark condition at 593 nm. The values were calculated from a calibration curve obtained with FeSO4ċ7H2O (100-1000 μM). Final results were expressed as µM Fe2+/g extract or essential oil.
Minimum Inhibitory Concentration and Minimum Bactericidal Concentration Assays
The broth microdilution method was used to determine the minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC). The 96-well plastic microdilution tray were prepared by dispensing into each well 95 µL of Mueller-Hinton broth (MHB; Merck Co, Darmstadt, Germany) and 5 µL of each isolate suspension equivalent to 0.5 McFarland. Finally, 100 µL of consecutive dilution of (serial 2-fold dilutions) each extract or essential oil were added to each well. Positive and negative controls were considered as follows: positive control—195 µL MHB containing DMSO and 5 µL of bacterial suspension without extracts and essential oils and negative control—200 µL of MHB containing DMSO without bacterial inoculum. After mixing the samples by shaker (with the rate of 300 rpm per 20 S), they were placed in an incubator for 18 to 24 hours at 37°C. The wells were examined according to the presence or lack of turbidity. Dilution plate of the well containing the lowest concentration of plant extracts or essential oils that inhibited growth of bacteria (lack of turbidity) was determined as the MIC. Furthermore, the lowest concentration that showed no visible growth on Mueller-Hinton agar was determined as the MBC.25
Integrity of Cell Membrane
The cell integrity of Listeria strains is tested by determining the release of cell components into supernatant according to the method described by Xu et al26 with some modifications. Bacteria from the working culture of Listeria strains were collected by centrifuged for 15 minutes at 5000 rpm, washed 3 times, and resuspended in 0.1 M phosphate buffered solution. Then, cell suspension obtained were incubated at 37°C under agitation for 4 hours in the presence of essential oils and extracts at MIC concentration. After that, 10 mL of samples were collected and centrifuged at 11 000 rpm for 3 minutes. The concentration of the released components including especially of nucleic acids, 1 mL supernatant was used to measure ultraviolet absorption at 260 nm.
Statistical Analysis
Data were analyzed using SPSS version 18.0 (IBM Corp, Armonk, NY, USA) and descriptive statistic was used. The results of all experiments were expressed as the mean ± standard deviation of triplicates. Analysis of variance was carried out to determine significant differences (P < .05) between means.
Results
In the present study, using cultural techniques, 2 of 106 samples (1.88%) were positive for Listeria strains. No Listeria was isolated from fresh fish samples. In contrast, 2 shrimp (5%) samples were contaminated with L monocytogenes (2.5%) and L grayi (2.5%). The resistance pattern of Listeria spp isolates to 16 antibiotics tested in this study is shown in Table 1. The 2 isolates (100%) were multidrug resistant. In addition, 2 isolates were susceptible to trimethoprim/sulfamethoxazole and ciprofloxacin. The L monocytogenes was more sensitive to antibiotics than L grayi.
Table 1.
Antimicrobial Resistance of Listeria spp Isolated From Seafood in Kashan, Iran.
| Antimicrobials | Listeria monocytogenes | Listeria grayi |
|---|---|---|
| Ampicillin (10 µg) | R | R |
| Amoxicillin/clavulanic acid | R | R |
| Clindamycin (2 µg) | I | R |
| Erythromycin (15 µg) | I | R |
| Methicillin (5 µg) | R | R |
| Gentamycin (10 µg) | S | R |
| Ciprofloxacin (5 µg) | S | S |
| Penicillin (10 µg) | R | R |
| Tetracycline (30 µg) | I | R |
| Trimethoprim/Sulfamethoxazole (10 µg) | S | S |
| Vancomycin (30 µg) | R | R |
| Nitrofurantoin (300 µg) | I | R |
| Norfloxacin (30 µg) | S | R |
| Kanamycin (30 µg) | R | R |
| Ceftriaxone (30 µg) | I | R |
| Chloramphenicol (30 µg) | S | R |
Abbreviations: R, resistant; I, intermediate; S, susceptible.
The main chemical composition of the essential oils is presented in Table 2. The total phenolic content of B persicum and E globulus extracts were 4.32 ± 0.9 and 23.06 ± 1.4 mg/g gallic acid equivalent, respectively.
Table 2.
The Chemical Components of Bunium persicum, Eucalyptus globulus Essential Oils, and Rose Water.
| Plants | Compounds |
|---|---|
| Bunium persicum essential oil | β-pinene (11.72%), p-cymene (15.47%), γ-terpinene (18.32%), cumin aldehyde (38.4%), p-mentha-1,3-dien-7-al (5.37%), and p-mentha-1,4-dien-7-al (2.86%) |
| Eucalyptus globulus essential oil | Limonene (9.4%) and 1,8-cineole (70.3%) |
| Rose water | Linalool (6.6%), terpineol (3.3%), carvone (0.31%), citronellol (6.85%), trans-geraniol (7.11%), phenylethanol (66.84%), eugenol (4.53%), cytronellol, hydroxyl (1.15%), and geranic acid (1.2%) |
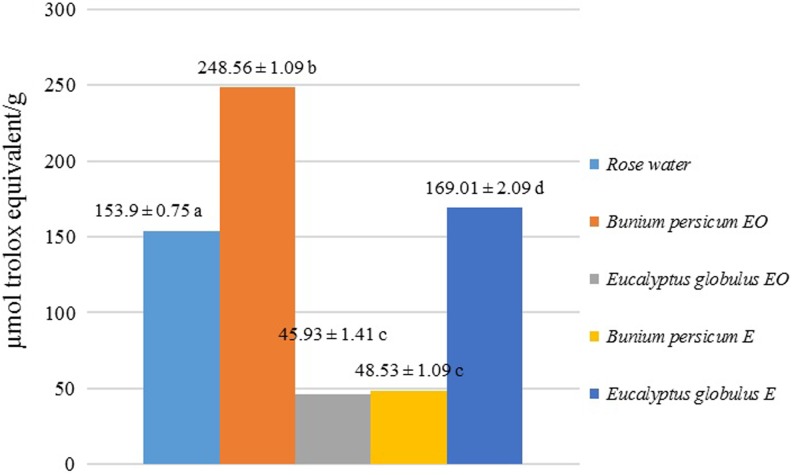
The total antioxidant activity of the 2 essential oils, 2 extracts, and rose water was measured by FRAP assay (Figure 1). B persicum essential oil showed the greatest antioxidant activity (248.56 ± 1.09 µM Fe2+/g). B persicum extract and E globulus essential oil were the least antioxidant activity. Overall, the total antioxidant activity ranked as follows: Bunium persicum essential oil > Eucalyptus globulus extract > rose water > Bunium persicum extract ≥ Eucalyptus globulus essential oil.
Figure 1.
Antioxidant activity determined by the ferric reducing antioxidant power assay.
The results related to the MIC and MBC of the essential oils and extracts by microdilution method are given in Table 3. The essential oil of E globulus showed consistently strong antimicrobial activity against L monocytogenes and L grayi, while rose water showed no antimicrobial activity against any of the tested bacterial strains. The essential oils generally exhibited a higher antimicrobial spectrum than extracts. Interestingly, L monocytogenes was more sensitive to essential oils and extracts than L grayi.
Table 3.
Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC) of the Bunium persicum, Eucalyptus globulus, and Rose Water Against Listeria spp Isolated From Seafood.
| Name of Medicinal Plants | Listeria monocytogenes | Listeria grayi | |
|---|---|---|---|
| MIC (mg/mL) | Bunium persicum EO | 0.351 | 2.812 |
| Eucalyptus globulus EO | 0.351 | 1.406 | |
| Rose water | NE | NE | |
| Bunium persicum E | 247 | 495 | |
| Eucalyptus globulus E | 45 | 361 | |
| MBC (mg/mL) | Bunium persicum EO | 0.703 | 5.625 |
| Eucalyptus globulus EO | 0.703 | 2.812 | |
| Rose water | NE | NE | |
| Bunium persicum E | 495 | 990 | |
| Eucalyptus globulus E | 90 | 723 | |
Abbreviations: E, ethanolic extract; EO, essential oil; NE, no effect.
Table 4 shows the results when Listeria strains were treated with MIC concentration of essential oils and extracts for 4 hours, respectively. The results showed that after adding the B persicum and E globulus essential oils to bacteria, the cell components’ release increased significantly. Compared with control and the cell components in suspensions treated with MIC concentration of B persicum and E globulus essential oils for L monocytogenes increased by 2.52 and 2.86 times, respectively and they increased by 1.52 and 1.67 times, respectively, for L grayi.
Table 4.
Effects of Bunium persicum, Eucalyptus globulus, and Rose Water in MIC Concentrations on Cell Constituents’ Release of Listeria spp Isolated From Seafood.*
| Medicinal Plants | Bunium persicum EO | Eucalyptus globulus EO | Rose water | Bunium persicum E | Eucalyptus globulus E | |
|---|---|---|---|---|---|---|
| Cell constituents (OD260 nm) | Listeria monocytogenes | 0.136 ± 0.001a | 0.675 ± 0.002a | — | ND | ND |
| Control | 0.054 ± 0.000b | 0.236 ± 0.000b | ||||
| Listeria grayi | 0.149 ± 0.001c | 0.797 ± 0.001c | — | ND | ND | |
| Control | 0.098 ± 0.000d | 0.476 ± 0.000d | ||||
E, ethanolic extract; EO: essential oil; ND, not detected; OD260 nm, optical density at 260 nm.
*Different superscript letters a, b, c, and d in the column indicate significant differences (P = .00).
Discussion
This study showed that prevalence of L monocytogenes in shrimp and fresh fish samples taken in the Kashan region, was low (0.94%). Previous studies in other areas in Iran have been demonstrated as follows; 7.8% of open-air fish market samples in Mazandaran,8 0% and 1.9% of fresh fish, frozen fish, and shrimp samples in Isfahan and Shahrekord, respectively.10,20 Also, our findings are similar to Momtaz and Yadollahi27 who revealed 2.5% (1 sample) L monocytogenes contamination in fresh shrimp in Iran. Zarei et al28 reported the low prevalence of L monocytogenes in Iranian seafood samples (1.4%). Latorre et al29 reported that L monocytogenes was not found in 154 samples of fresh seafood products in Italy.
The results of this work indicate resistance of Listeria spp to antibiotics (Table 1). L monocytogenes was resistant to ampicillin and penicillin as the first-choice antibiotics used for treatment of human listeriosis. Erythromycin and trimethoprim/sulfamethoxazole are generally used for treatment of listeriosis in pregnant women and patients having allergy to penicillin, respectively. In this study, L monocytogenes was susceptible to trimethoprim/sulfamethoxazole that is in agreement with the previous reports.30,31 In addition, L monocytogenes was resistant to vancomycin as one of the last therapeutic options for the treatment of human listeriosis.32 Lakhanpal et al33 showed that 3 L monocytogenes isolated from milk products were sensitive to ampicillin, gentamicin, vancomycin, and erythromycin. However, the isolates were resistant to nalidixic acid and ciprofloxacin. Rahimi et al34 showed that 3 L monocytogenes isolated from smoked and salted fish samples were resistant to nalidixic acid, penicillin, and tetracycline. Also, the isolates were sensitive to ciprofloxacin, vancomycin, gentamycin, and chloramphenicol.
Recently, due to the increasing problem of bacterial resistance, extracts and essential oils are regarded as good candidates for replacing chemical antimicrobial agents. Previous works have demonstrated the antioxidant activity and antibacterial properties of essential oils and extracts.2,23,25,26
Our results showed that B persicum, E globulus, and rose water had antioxidant activities (Figure 1), which was supported by previous studies.35–37 Rose water is called “Golab” in Iran. It has been used to prepare drinks and as a traditional medicine for several centuries in Iran.35 Ganesan et al38 reported that the phenolic compounds isolated from E globulus demonstrated antioxidant activity and antioxidative property against glucose- and oxalate-induced oxidative stress in NRK-49F cells. El-Sayed et al39 demonstrated that the Taif rose water byproduct had free radical scavenging activity toward artificial 1,1-diphenyl picrylhydrazyl radical with SC50 = 23.72 ± 0.36 µg/mL. The results in this work indicate that these plants could be a potential sources of natural antioxidant foods.
The 4 essential oils and extracts inhibited the growth of L monocytogenes and L grayi. In the present work, 2 essential oils studied had a lower MIC and MBC when used against the Gram-positive L monocytogenes and L grayi. Our results are in agreement with the previous studies.40,41 Patra and Beak41 have reported the Enteromorpha linza, Undaria pinnatifida, Laminaria japonica, and Porphyra tenera essential oils exhibited strong antilisterial activity against multiple strains of L monocytogenes. A previous study has demonstrated antibacterial activity of cumin and eucalyptus essential oils against L monocytogenes, Salmonella typhi, Streptococcus pyogenes, and Shigella dysenteriae. L monocytogenes has the same sensitivity to cumin and eucalyptus essential oils. The MIC and MBC values were 5.625 and 11.25 mg/mL, respectively.42
The difference antimicrobial properties of extracts and essential oils against Listeria spp may be linked to inoculation amount of bacteria, incubation time as experimental conditions, and the sources of extracts and essential oils and their chemical composition.40
The results of this study showed, increase in the optical density values at 260 nm of L monocytogenes and L grayi treated with B persicum and E globulus essential oils over time, which can result in changes of cell membrane structure. Also, the cellular leakage can result from the effect of penetration of the hydrophobic essential oils. Therefore, some reducing sugars, K+, Ca2+, and Na+ as small molecules or macromolecules, including proteins and nucleic acids released from the bacteria to the outside. Thus, we concluded that one of the modes of antibacterial action on Listeria spp was that the essential oils from B persicum and E globulus first destroyed the cell membranes, next causing the leakage of cytosolic materials, which finally resulted in the cell death.
Similar observations regarding the release of cytosolic materials outside of bacterial cells treated with essential oils from Enteromorpha linza, Undaria pinnatifida, Laminaria japonica, Porphyra tenera, Laurus nobilis, Mentha pulegium, Satureja calamintha, Lavandula stoechas, and Myrtus communis and other essential oils have been reported.26,40,41
Conclusion
Considering the low prevalence of L monocytogenes in this study, consumption of raw and undercooked seafoods, may pose a health risk particularly for susceptible populations.
The present work confirmed the antioxidant activity of the B persicum, E globulus, and rose water. Also, B persicum and E globulus essential oils and extracts had relatively high antimicrobial activity against Listeria spp. In addition, the essential oils can penetrate the cytoplasmic membrane, which causes a loss of the membrane permeability of the cellular membrane and leakage of the cytosolic materials. Therefore, it is suggested that the above extracts and essential oils used as a potential source of natural antibacterial agents against multidrug-resistant Listeria.
Acknowledgments
The authors are thankful to the Research Deputy of Kashan University of Medical Sciences, Kashan, Iran, for financial support.
Footnotes
Author Contributions: FSC contributed to the design of the study, supervised the work scientifically, and edited the English manuscript. MS contributed to the data collection and laboratory testing. RSC developed the original idea, analyzed and abstracted the data, and prepared the manuscript.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study received financial support from the Deputy of Research of Kashan University of Medical Sciences, Kashan, Iran (Grant No.: 95009).
Ethical Approval: The study protocol was approved by Ethical Committee of Kashan University of Medical Sciences, Kashan, Iran.
References
- 1. Abdollahzadeh E, Rezaei M, Hosseini H. Antibacterial activity of plant essential oils and extracts: the role of thyme essential oil, nisin, and their combination to control Listeria monocytogenes inoculated in minced fish meat. Food Control. 2014;35:177–183. [Google Scholar]
- 2. Mith H, Duré R, Delcenserie V, Zhiri A, Daube G, Clinquart A. Antimicrobial activities of commercial essential oils and their components against food-borne pathogens and food spoilage bacteria. Food Sci Nutr. 2014;2:403–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cocolin L, Rantsiou K, Iacumin L, Cantoni C, Comi G. Direct identification in food samples of Listeria spp. and Listeria monocytogenes by molecular methods. Appl Environ Microbiol. 2002;68:6273–6282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Morvan A, Moubareck C, Leclercq A, et al. Antimicrobial resistance of Listeria monocytogenes strains isolated from humans in France. Antimicrob Agents Chemother. 2010;54:2728–2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jamali H, Chai LC, Thong KL. Detection and isolation of Listeria spp. and Listeria monocytogenes in ready-to-eat foods with various selective culture media. Food Control. 2013;32:19–24. [Google Scholar]
- 6. Centers for Disease Control and Prevention. Multistate outbreak of listeriosis associated with Jensen Farms cantaloupe—United States, August-September 2011. MMWR Morb Mortal Wkly Rep. 2011;60:1357–1358. [PubMed] [Google Scholar]
- 7. Abdollahzadeh E, Ojagh SM, Hosseini H, Ghaemi EA, Irajian G, Naghizadeh Heidarlo M. Antimicrobial resistance of Listeria monocytogenes isolated from seafood and humans in Iran. Microb Pathog. 2016;100:70–74. [DOI] [PubMed] [Google Scholar]
- 8. Jamali H, Paydar M, Ismail S, et al. Prevalence, antimicrobial susceptibility and virulotyping of Listeria species and Listeria monocytogenes isolated from open-air fish markets. BMC Microbiol. 2015;15:144 doi:10.1186/s12866-015-0476-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mansouri-Najand L, Kianpour M, Sami M, Jajarmi M. Prevalence of Listeria monocytogenes in raw milk in Kerman, Iran. Vet Res Forum. 2015;6:223–226. [PMC free article] [PubMed] [Google Scholar]
- 10. Jalali M, Abedi D. Prevalence of Listeria species in food products in Isfahan, Iran. Int J Food Microbiol. 2008;122:336–340. [DOI] [PubMed] [Google Scholar]
- 11. Mashak Z, Zabihi A, Sodagari H, Noori N, Akhondzadeh Basti A. Prevalence of Listeria monocytogenes in different kinds of meat in Tehran province, Iran. Brit Food J. 2015;117:109–116. [Google Scholar]
- 12. Tan SL, Lee HY, Mahyudin NA. Antimicrobial resistance of Escherichia coli and Staphylococcus aureus isolated from food handler’s hands. Food Control. 2014;44:203–207. [Google Scholar]
- 13. Sharafati-Chaleshtori R, Sharafati-Chaleshtori F, Karimi A. Antibiotic resistance pattern of Staphylococcus strains isolated from orange and apple juices in Shahre-kord, Iran. Pak J Med Sci. 2010;26:615–618. [Google Scholar]
- 14. Tahoun ABMB, Abou Elez RMM, Abdelfatah EN, Elsohaby I, El-Gedawy AA, Elmoslemany AM. Listeria monocytogenes in raw milk, milking equipment and dairy workers: molecular characterization and antimicrobial resistance patterns. J Glob Antimicrob Resist. 2017;10:264–270. [DOI] [PubMed] [Google Scholar]
- 15. Escolar C, Gómez D, Del Carmen Rota García M, Conchello P, Herrera A. Antimicrobial resistance profiles of Listeria monocytogenes and Listeria innocua isolated from ready-to-eat products of animal origin in Spain. Foodborne Pathog Dis. 2017;14:357–363. [DOI] [PubMed] [Google Scholar]
- 16. Wieczorek K, Osek J. Prevalence, genetic diversity and antimicrobial resistance of Listeria monocytogenes isolated from fresh and smoked fish in Poland. Food Microbiol. 2017;64:164–171. [DOI] [PubMed] [Google Scholar]
- 17. Mulyaningsih S, Sporer F, Zimmermann S, Reichling J, Wink M. Synergistic properties of the terpenoids aromadendrene and 1,8-cineole from the essential oil of Eucalyptus globulus against antibiotic-susceptible and antibiotic-resistant pathogens. Phytomedicine. 2010;17:1061–1066. [DOI] [PubMed] [Google Scholar]
- 18. Sharafati-chaleshtori A, Sharafati-Chaleshtori R, Sharafati-Chaleshtori F, Rafieian M. Antibacterial effects of ethanolic extract of walnut leaves (Juglans regia) on propionibacterium acnes. J Zanjan Univ Med Sci. 2010;18:42–49. [Google Scholar]
- 19. Muthaiyan A, Martin EM, Natesan S, Crandall PG, Wilkinson BJ, Ricke SC. Antimicrobial effect and mode of action of terpeneless cold-pressed Valencia orange essential oil on methicillin-resistant Staphylococcus aureus . J Appl Microbiol. 2012;112:1020–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rahimi E, Shakerian A, Raissy M. Prevalence of Listeria species in fresh and frozen fish and shrimp in Iran. Ann Microbiol. 2012;62:37–40. [Google Scholar]
- 21. Ozbey G, Ertas HB, Kok F. Prevalence of Listeria species in camel sausages from retail markets in Aydin province in Turkey and RAPD analysis of Listeria monocytogenes isolates. Ir Vet J. 2006;59:342–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Basti AA, Misaghi A, Salehi TZ, Kamkar A. Bacterial pathogens in fresh, smoked and salted Iranian fish. Food Control. 2006;17:183–188. [Google Scholar]
- 23. Sharafati-Chaleshtor F, Sharafati-Chaleshtori R. In vitro antibacterial and antioxidant properties of Elettaria cardamomum Maton extract and its effects, incorporated with chitosan, on storage time of lamb meat. Vet Arh. 2017;87:301–315. [Google Scholar]
- 24. Benzie IF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem. 1996;239:70–76. [DOI] [PubMed] [Google Scholar]
- 25. Sharafati-Chaleshtori F, Sharafati-Chaleshtori R. Antimicrobial activity of chitosan incorporated with lemon and oregano essential oils on broiler breast meat during refrigerated storage. Nutr Food Sci. 2017;47:306–317. [Google Scholar]
- 26. Xu JG, Liu T, Hu QP, Cao XM. Chemical composition, antibacterial properties and mechanism of action of essential oil from clove buds against Staphylococcus aureus . Molecules. 2016;21:E1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Momtaz H, Yadollahi S. Molecular characterization of Listeria monocytogenes isolated from fresh seafood samples in Iran. Diagn Pathol. 2013;8:149–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zarei M, Maktabi S, Ghorbanpour M. Prevalence of Listeria monocytogenes, Vibrio parahaemolyticus, Staphylococcus aureus, and Salmonella spp. in seafood products using multiplex polymerase chain reaction. Foodborne Pathog Dis. 2012;9:108–112. [DOI] [PubMed] [Google Scholar]
- 29. Latorre L, Parisi A, Fraccalvieri R, et al. Low prevalence of Listeria monocytogenes in foods from Italy. J Food Prot. 2007;70:1507–1512. [DOI] [PubMed] [Google Scholar]
- 30. Fallah AA, Saei-Dehkordi SS, Mahzounieh M. Occurrence and antibiotic resistance profiles of Listeria monocytogenes isolated from seafood products and market and processing environments in Iran. Food Control. 2013;34:630–636. [Google Scholar]
- 31. Altuntas EG, Kocan D, Cosansu S, Ayhan K, Juneja VK, Materon L. Antibiotic and bacteriocin sensitivity of Listeria monocytogenes strains isolated from different foods. Food Nutr Sci. 2012;3:363–368. [Google Scholar]
- 32. Alonso-Hernando A, Prieto M, García-Fernández C, Alonso-Calleja C, Capita R. Increase over time in the prevalence of multiple antibiotic resistance among isolates of Listeria monocytogenes from poultry in Spain. Food Control. 2012;23:37–41. [Google Scholar]
- 33. Lakhanpal P, Panda AK, Thakur SD. Low prevalence of Listeria monocytogenes in ready-to-eat foods of animal origin from various tourist destinations of Himachal Pradesh, India. J Commun Dis. 2016;48:29–32. [Google Scholar]
- 34. Rahimi E, Safarpoor Dehkordi F, Yahaghi E, Khodaverdi Darian E. Prevalence and antimicrobial resistance of Listeria species isolated from smoked and salted fish. Iran J Med Microbiol. 2014;8:31–37. [Google Scholar]
- 35. Mahboubi M. Rosa damascena as holy ancient herb with novel applications. J Tradit Complement Med. 2015;6:10–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chizzola R, Saeidnejad AH, Azizi M, Oroojalian F, Mardani H. Bunium persicum: variability in essential oil and antioxidants activity of fruits from different Iranian wild populations. Genet Resour Crop Evol. 2014;61:1621–1631. [Google Scholar]
- 37. Vázquez G, Santos J, Freire MS, Antorrena G, González-Álvarez J. Extraction of antioxidants from eucalyptus (Eucalyptus globulus) bark. Wood Sci Technol. 2012;46:443–457. [Google Scholar]
- 38. Ganesan D, Al-Sayed E, Albert A, et al. Antioxidant activity of phenolic compounds from extracts of Eucalyptus globulus and Melaleuca styphelioides and their protective role on D-glucose-induced hyperglycemic stress and oxalate stress in NRK-49Fcells [published online June 21, 2017]. Nat Prod Res. doi:10.1080/14786419.2017.1343324. [DOI] [PubMed] [Google Scholar]
- 39. Abdel-Hameed ESS, Bazaid SA, Sabra ANA. Total phenolic, in vitro antioxidant activity and safety assessment (acute, sub-chronic and chronic toxicity) of industrial Taif rose water by-product in mice. Pharm Lett. 2015;7:251–259. [Google Scholar]
- 40. Cherrat L, Dumas E, Bakkali M, Degraeve P, Laglaoui A, Oulahal N. Effect of essential oils on cell viability, membrane integrity and membrane fluidity of Listeria innocua and Escherichia coli . J Essent Oil Bear Plants. 2016;19:155–166. [Google Scholar]
- 41. Patra JK, Baek KH. Anti-Listerial activity of four seaweed essential oils against Listeria monocytogenes . Jundishapur J Microbiol. 2016;9:e31784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sharafati-Chaleshtori F, Taghizadeh M, Rafieian-Kopaei M, Sharafati-Chaleshtori R. Effect of chitosan incorporated with cumin and eucalyptus essential oils as antimicrobial agents on fresh chicken meat. J Food Process Preserv. 2016;40:396–404. [Google Scholar]