Abstract
Rasa Shastra is an exclusive branch of ayurveda that uses processed metals and minerals in various combinations. Though the formulations are time tested, safety and quality concerns are being raised since the past two decades. In view of this, it becomes mandatory to generate quality control profiles of such formulations by following available parameters. Considering this, we attempted to develop standard manufacturing procedures of Maha Yogaraja Guggulu and generate preliminary physicochemical profiles using inductively coupled plasma mass spectrometry, X-ray diffraction, and high-performance thin-layer chromatography. The results from high-performance thin-layer chromatography revealed presence of organic constituents from plant material. X-ray diffraction indicated that the prepared drug contained cinnabar (mercury sulfide; Rasa sindhura), cassiterite (tin oxide; Vanga bhasma), litharge (lead oxide; Naga bhasma), and iron dioxide and magnetite (di-iron oxide; Loha and Mandura bhasma). The observations of the present study are preliminary and first of its kind that may be considered as baseline data for future studies.
Keywords: bhasma, loha, maha yogaraja guggulu, mandura, naga, rasa sindhura, vanga
Maha yogaraja guggulu (MYG) is a well-known ayurvedic herbo-mineral formulation known for its Rasayana (rejuvenative), Shothahara (anti-inflammatory), Vedanahara (analgesic) properties. It is in practice mainly for Vatavyadhi (specific disorders occurring due to Vata dosha), Kustha (skin diseases), Arshas (hemorrhoids), Prameha (diabetes), Vatarakta (gout), Nabhi shula (pain around umbilicus), Bhagandara (fistula-in-ano), Gulma (abdominal tumors), Swasa (dyspnea), Kasa (cough), Aruchi (anorexia), Retasdosha (seminal abnormalities), and Rajodosha (menstrual disorders).1,2
Considering its importance in clinical use, the detailed standard operative procedures for the preparation, standardization, and chemical characterization of this formulation should be well documented with substantial evidences for worldwide acceptance. Hence, this study was conducted with the objectives to evolve standard operative procedures for the preparation as per classical ayurvedic texts and physicochemical profile of Maha Yogaraja Guggulu.
Materials and Methods
Pharmaceutical Processing
Maha yogaraja guggulu was prepared as per standard methods mentioned in the Ayurvedic Formulary of India.3 The details about the ingredients used are presented in Table 1. The whole process of preparation was divided into following steps.
Table 1.
Formulation Composition of Maha Yogaraja Guggulu a.
| Materials Used | Botanical/English Name | Part/Form Used | Proportion |
|---|---|---|---|
| Shunthi | Zingiber officinale Rose. | Dried rhizome | 1 part |
| Pippali | Piper longum Linn. | Dried fruit | 1 part |
| Chavya | Piper chaba Hunter. | Dried stem | 1 part |
| Pippali mula | Piper longum Linn. | Dried root | 1 part |
| Chitraka | Plumbago zeylanica Linn. | Dried root | 1 part |
| Hingu | Ferula foetida Regel. | Exudates | 1 part |
| Ajamoda | Apium graveolens Linn. | Dried fruit | 1 part |
| Sarshapa | Brasica compestris Linn. | Dried seed | 1 part |
| Shweta jiraka | Cuminum cyminum Linn. | Dried fruit | 1 part |
| Krishna jiraka | Carum carvi Linn. | Dried fruit | 1 part |
| Renuka | Vitex agnus-castus Linn. | Dried seed | 1 part |
| Indrayava | Holarrhena antidysentrica Wall. | Dried seed | 1 part |
| Patha | Cissampelos pareira Linn. | Dried root | 1 part |
| Vidanga | Embelia ribes Burn. f. | Dried fruit | 1 part |
| Gaja pippali | Sciendapsus officinalis Schott | Dried fruit | 1 part |
| Katuki | Picrorhiza kurroa Royle ex Benth. | Dried root/rhizome | 1 part |
| Ativisha | Aconitum heterophyllum Wall ex Royle. | Dried root | 1 part |
| Bharangi | Clerodendrum serratum Moon. | Dried root | 1 part |
| Vacha | Acorus calamus Linn. | Dried rhizome | 1 part |
| Murva | Marsdenia tenacissima Wight and Arn. | Dried root | 1 part |
| Triphala | 40 parts | ||
| Amalaki | Emblica officinalis Gaertn. | Dried pericarp | |
| Haritaki | Terminalia chebula Retz. | Dried pericarp | |
| Bibhitaka | Terminalia bellerica Roxb. | Dried pericarp | |
| Guggulu | Commiphora wightii (Arn.) Bhandari | Oleo-gum resin | 60 parts |
| Vanga | Tin | Bhasma (incinerated tin) | 16 parts(320 g) |
| Rajata | Silver | Bhasma (incinerated silver) | 16 parts |
| Naga | Lead | Bhasma (incinerated lead) | 16 parts |
| Loha | Iron | Bhasma (incinerated iron) | 16 parts |
| Abhraka | Biotite mica | Bhasma (incinerated mica) | 16 parts |
| Mandura | Bhasma (incinerated sludge iron) | 16 parts | |
| Rasa sindoor | Sulfide of mercury (HgS) | A type of kupipakwa rasayana of mercury | 16 parts |
aSource: The Ayurvedic Formulary of India, Government of India, Part 1, 5:6.
Processing of Guggulu
One part (1700 g) of Guggulu was made into small pieces carefully by removing physical impurities like stone, glass, and so on, bundled into a Pottali, suspended in a vessel containing Triphala kashaya (3400 mL), and boiled on moderate flame maintaining the temperature at around 80°C. On complete dissolution of Guggulu in the liquid, the contents were filtered through mesh No. 60 (to remove possible insoluble impurities, if any) and the filtrate was again heated till the liquid converts into a semisolid mass. This semisolid mass was shifted into steel trays and dried in a tray drier at 50°C. The dried mass was taken out from the tray and used in the preparation of maha yogaraja guggulu.4
Preparation of Bhasmas (Vanga/Rajata/Naga/Lauha/Abhraka/Mandura/Rasa Sindhura)
Preparation of vanga bhasma
Vanga was melted and poured 3 times each in Tila taila, takra, kanjika, gomutra, and decoction of the seeds of kulattha (Dolichus biflorus L.).5 It was further processed for vishesha sodhana, where molten vanga was poured in the leaf juice of Nirgundi (Vitex negundo Linn.) and powder of haridra (Curcuma longa Linn.) consecutively 3 times.6
Vanga collected at this stage was again molten in an iron pan, added with powder of ashvattha (bark of Ficus religiosa Linn.) and chincha tvak (bark of Tamarindus indica Linn.) in small quantities, and stirred continuously with loha darvi (iron spatula). This process was continued till reduction of vanga to powder (jarita vanga).7 Furthermore, equal quantity of orpiment powder was added to this jarita vanga and levigated with lemon juice; small chakrikas (flat cakes) were prepared, dried, placed in sarava samputa, and subjected to gaja puta. From the second puta onwards, one fourth part of the orpiment powder was added to vanga. The process of puta was repeated 10 times to obtain vanga bhasma.
Preparation of rajata bhasma
Rajata patras were heated to red hot and immersed consecutively in tila taila, takra, kanjika, gomutra, and decoction of the seeds of kulattha. The whole process was repeated 3 times. Rajata patra thus obtained was further processed to vishesha sodhana by processing in agastya swarasa (leaf juice of Sesbania grandiflora) 3 times.8 In the next step, this was added with suddha hingula (processed cinnabar in lemon juice), ground well, and heated in urdhva patana yantra for 6 hours. On cooling, the apparatus was opened to collect rajata bhasma from the lower vessel.9
Preparation of naga bhasma
Molten naga was poured consecutively in tila taila, takra, kanji, gomutra, and decoction of the seeds of kulattha 3 times each in all the liquids. This was further collected in an iron pan and heated. On melting, powders of chincha tvak and asvattha tvak were sprinkled in small quantities and stirred with loha darvi (iron spatula). This process was continued till the molten naga is reduced to powder form (jarita naga). Furthermore, equal quantity of manahsila was added to jarita naga and levigated with kanji; small chakrikas were prepared, dried, and placed in sarava samputa and ardha gaja puta is given. This process was repeated 60 times to obtain naga bhasma of desired quality.10
Preparation of abhraka bhasma
Abhraka was heated to red hot and immersed in decoction of Triphala (3 myrobalans) 7 times.11 The shodhita abhraka was bundled in a jute bag (into pottali form) with one fourth quantity of paddy and immersed in kanji 12 for 3 days. Thereafter, the pottali was rubbed thoroughly and squeezed in the liquid itself so that only fine abhraka particles can escape through the holes of the bag. The bag was removed from the kanji and the contents were allowed to settle down. The supernatant liquid layers were separated carefully to collect fine particles of abhraka that had settled down in the container. These particles (dhanyabhraka) were dried and stored for further use.13
Dhanyabhraka was levigated with required quantity of arka ksheera (latex of Calotropis procera (Ait) R.Br.) for a day; chakrikas were prepared and dried in the sun. These chakrikas were placed in a sharava samputa, the junctions were sealed properly, and subjected to gaja puta. 14 The material thus obtained at the end of this puta was processed in similar way 6 more times. At the end of the seventh puta, the contents were levigated with nyagrodha mula kwatha (decoction of Ficus bengalensis roots), dried, and gaja puta was given. The process was repeated 3 times followed by levigation with Rambha rasa (juice of rhizome of Musa paradisiaca) and 7 gaja putas were given. Finally, this was grounded in nyagrodha mula kwatha and 3 gaja putas were given. After completion of these putas, the finished product abhraka bhasma was obtained.15
Preparation of mandura bhasma
Shuddha mandura 16 was levigated in triphala kvatha and chakrikas were prepared and dried that are placed in sharava samputa and subjected to heat in sadharan puta. The process was repeated 30 times to obtain red-colored mandura bhasma.17
Preparation of lauha bhasma
Lauha was heated to red hot and immersed 3 times each consecutively in tila taila, takra, kanji, gomutra, and decoction of the seeds of kulattha. This was further processed in equal quantities of triphala kashaya and gomutra.18 Lauha churna thus obtained was further processed through bhanupaka (processed in sun rays), followed by sthalipaka (heated with decoction of triphala in stainless steel vessel).19,20 This was further levigated with triphala kwatha, Chakrikas were prepared, dried, and placed in sharava samputa, and subjected to gaja puta. The same procedure was repeated 60 times to get lauha bhasma of desired quality.21 The details of media used, nature, and number of putas and temperature used in the preparation of each bhasma are mentioned in Table 2.
Table 2.
Details of Media Used, Nature and Number of Puta, and Temperature Used for the Preparation of Each Bhasma.
| Name of Bhasma | Reference | Media Used | Nature of Puta | Number of Putas |
|---|---|---|---|---|
| Vanga | Ayurved Sara Sangrah | Nimbu rasa | Ardha gaja puta | 10 |
| Rajata | Rasa Tarangini, Taranga 16 | Nimbuk swarsa | Urdhav patan yantra | For 6 hours |
| Naga | Sarangdhar Samhita | Kanjika | Ardha gaja puta and gaja puta | 60 (where last 10 putas are gaja puta) |
| Abhraka | Rasa Tarangini, Taranga 10 | Arka ksheera | Gaja puta | Arka Ksheera—7 putas |
| Nyagrodha mula kwatha | Nyagrodha mula kwatha—3 putas | |||
| Kadali rasa | Kadali rasa—7 putas | |||
| Mandura | Rasa Tarangini, Taranga 20 | Triphala kashaya | Sadharana puta | 30 |
| Lauha | Rasa Tarangini, Taranga 16 | Triphala kashaya | Gaja puta | 60 |
Preparation of rasa sindhura
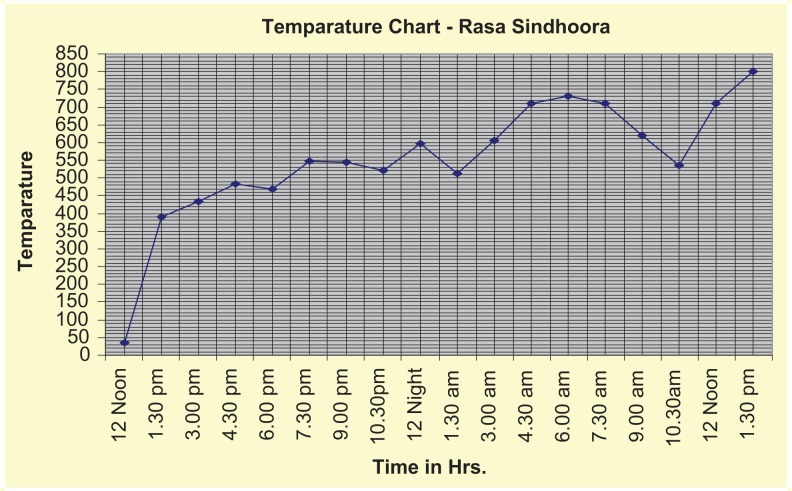
Preparation of rasa sindhura involves preparation of kajjali, bhavana (levigation) with vatankura jala (decoction of leaf buds of Ficus bengalenesis Linn.), and processing in valuka yantra. 22–24 Kajjali was prepared by triturating equal quantities of hingulottha parada (mercury obtained from cinnabar) and shuddha gandhaka (processed sulfur) in a khalva yantra (mortar pestle), till the formation of a black-colored, soft, lusterless fine powder like collyrium. This was further levigated with vatankura jala and then dried. This was filled in a strong amber-colored kachakupi (glass bottle) in valuka yantra (heating device) and subjected to heat by increasing the temperature gradually. Mild heat was applied for the first 6 hours, followed by moderate heat. The temperature pattern for the preparation of rasa sindhura is shown in Figure 1.
Figure 1.
The temperature pattern for the preparation of rasa sindhura.
When the bottom of the bottle appears red, the mouth of the bottle was blocked with cork and sealed with mud mixed with lime and jaggery smeared cloth. This was followed by application of strong heat for the next 6 hours. Thereafter, the valuka yantra was allowed to cool down on its own (Figure 1). The bottle was then removed from the valuka yantra and the mud-smeared cloth was scrapped with a knife; the bottle was broken down carefully to collect crystallized rasa sindhura from the neck of the bottle.
Preparation of Finished Product
Shuddha guggulu (1.2 kg) was dissolved in 3 L of water to prepare a thick paste, to which fine powders of other components were added in end runner followed by levigation for 3 days. At the end of this process, the material was removed from the end runner, shifted to clean stainless steel trays, and dried at 50°C.
The dried material was converted into granules by passing through No. 40 sieve and shifted to the tablet section. Two percent of talcum powder was added as excipient to the granules and were compressed into tablets of 125 mg in a rotary tablet machine.
Physicochemical Analysis
Physicochemical analysis, that is, description, estimation of loss on drying, ash content, acid insoluble ash, water/alcohol soluble extractive, pH, and so on; qualitative/quantitative elemental testing; residual pesticide; microbiological examination; and tablet parameters, that is, hardness, friability, average weight, dissolution time, and so on, were carried out by following standard methods as per Ayurvedic Pharmacopoeia of India25–27 guidelines. The quantitative estimation of heavy metals, that is, Pb, Cd, As, Hg, and Sn was carried out by atomic absorption spectrometer (Perkin Elmer Analyst 400), and the other elements that is, Ca, Mg, Cu, B, Mn, Al, Cr, Fe, and Ag were analyzed on ICP-AES (THERMO EECTRON Corporation’s model IRIS INTREPRID II XDL). However, sulfur and silica were quantified by using conventional methods.27
High-Performance Thin-Layer Chromatography Method
Sample preparation
Two grams of powders each of the 3 batches of maha yogaraja guggulu were soaked overnight separately in 20 mL of methanol. The solutions were continuously stirred for 6 hours and kept for the next 18 hours and then the samples were filtered, dried, and made into 10% solution.
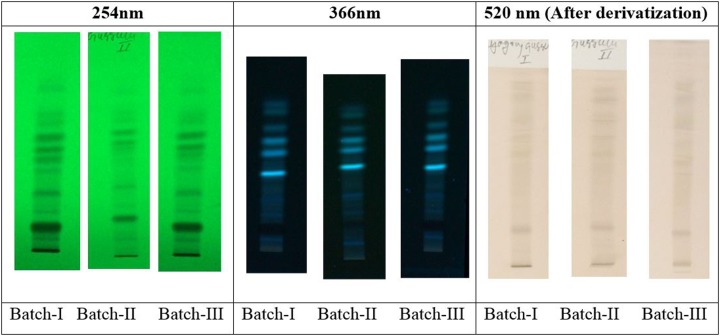
High-performance thin-layer chromatography was performed on thin-layer chromatography plates precoated with 0.25 μm thin layers of silica gel 60 F254 (E. Merck). Ten microliters of methanolic solution of formulation (3 batches) were applied on the plates as bands 8.0 mm wide by use of a Linomat-V applicator (CAMAG, Switzerland) fitted with a 100 μL syringe (Hamilton, Switzerland). The application positions X and Y were both 10 mm, to avoid edge effects. Linear ascending development to a distance of 80 mm with toluene–ethyl acetate–formic acid 10:3:1 (v/v) as mobile phase was performed in a twin-trough glass chamber previously saturated with vapors of the mobile phase for 20 minutes. The plates were dried in air and visualized under 254 nm and 366 nm for ultraviolet detection and taken the fingerprints as evident. The same thin-layer chromatography plate was also derivatized with anisaldehyde-sulfuric acid reagent and visualized in white light.
X-Ray Diffraction Study
Powder X-ray diffraction analysis was carried out using Rigaku Ultima-IV X-ray diffractometer with CuKα radiation (λ = 1.54 A) operating at 40 kV and 30 mA. The pattern was recorded for angle (2θ) ranging from 10° to 100° at a scanning rate of 1°/second and scan step of 0.1°. X-ray diffraction pattern of maha yogaraja guggulu (3 batches) is shown in the spectra. Sample identification was done by matching d-spacing with the standard database.
Results and Discussion
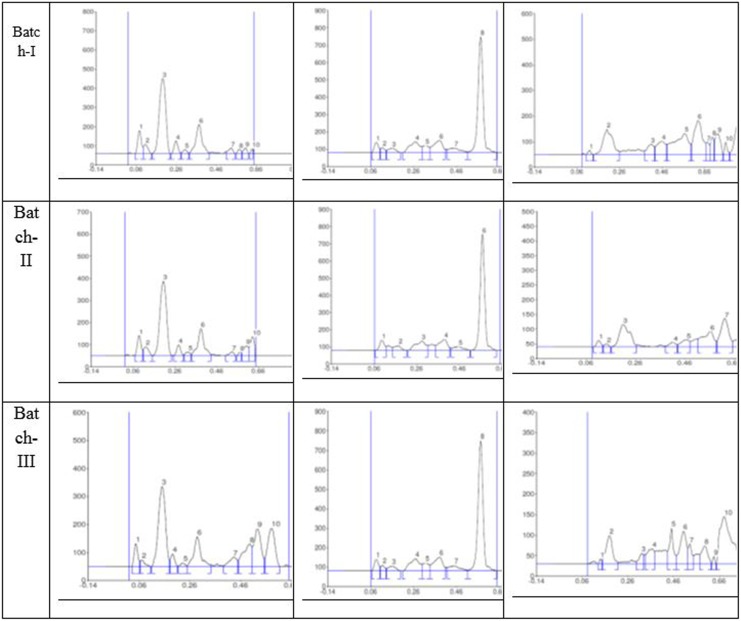
Organoleptic observations show that maha yogaraja guggulu is in the form of a brownish-colored tablet having characteristic pleasant odor and slightly acrid in taste. The qualitative analysis shows positivity for the presence of mercury, silver, sulfur, calcium, copper, iron, and lead. Chemical analysis revealed 2.39% of lead, 1.31% of mercury, 2.25% of sulfur, 4.36% of iron, 1.63% of silver, 2.73% of calcium, 1.08% of magnesium, and 1.18% of silica along with other trace elements like aluminum, manganese, arsenic, copper, tin, boron, chromium, and cadmium, which were found in <1.0% range. Moisture content 3.62% was found when determining loss on drying at 105°C. Total ash content (44.38%) is ash left after burning of organic and volatile matter (56.29%). As shown in the observations, water soluble (12.03%) and alcohol soluble (9.06%) matter were also present in this formulation. The drug also tested for residual pesticides and microbial contamination, which was found to be within permissible limits (Table 3). The results from high-performance thin-layer chromatography, as shown in Figures 2 and 3, revealed presences of organic constituents from plant material.
Table 3.
Preliminary Physicochemical Profile of Maha Yogaraja Guggulu.
| Parameter Tested | Observations |
|---|---|
| Organoleptic characters | |
| Color | Blackish |
| Taste | Acrid |
| Odor | Pleasant |
| Appearance | Tablet |
| Physicochemical | |
| Identification | Yields the reaction characteristics of silver, mercury, lead, tin, and iron |
| Loss on drying, % w/w | 2.0-5.0 |
| Total ash, % w/w | 43.0-46.0 |
| Acid-insoluble ash, % w/w | 12.0-14.50 |
| Water-soluble extractive, % w/w | 11.0-14.0 |
| Alcohol (90%) soluble extractive, % w/w | 8.0-10.0 |
| Resin content, % w/w | 12.50-14.0 |
| pH of aqueous extract | 5.0-6.0 |
| Organic and volatile matter, % w/w | 55.50-57.50 |
| Specific gravity | 0.9980-0.9990 |
| Particle size distribution | |
| 10% | 2.15-2.76 µm |
| 50% | 11.01-20.35 µm |
| 90% | 54.11-81.65 µm |
| Assay of elements | |
| Silver (% w/w) | 1.5-2.0 |
| Iron (% w/w) | 4.0-5.0 |
| Sulfur (% w/w) | 2.0-2.5 |
| Silica (% w/w) | 0.5-2.0 |
| Calcium (% w/w) | 2.5-3.0 |
| Copper(% w/w) | 0.1-0.5 |
| Boron (% w/w) | 0.01-0.30 |
| Manganese (% w/w) | 0.05-0.07 |
| Magnesium (% w/w) | 0.50 -1.50 |
| Chromium (% w/w) | 0.03-0.05 |
| Aluminum (% w/w) | 0.50 -1.0 |
| Mercury (% w/w) | 1.0 -1.5 |
| Lead (% w/w) | 2.0-2.5 |
| Arsenic (% w/w) | 0.3-0.4 |
| Cadmium (% w/w) | 0.1-0.2 |
| Tin (ppm) | 350-750 |
| Residual pesticide (μg/kg) | |
| Alpha and beta HCH | Not detected |
| Gamma HCH | Not detected |
| Delta HCH | Not detected |
| DDT and metabolites | Not detected |
| D.T. | 18-20 minutes |
| Hardness | 2.5 kg/cm2 |
| Friability | 0.30-0.60% |
| Average weight | 125-127 mg/tablet |
| Microbial contamination | |
| Total aerobic count | 20 000-40 000 |
| Coliform | Not detected |
| Escherichia coli | Not detected |
| Salmonella sp. | Not detected |
| Staphylococcus aureus | Not detected |
| Yeasts | Not detected |
| Molds | 70-100 |
| Pseudomonas aeruginosa | Absent |
| High-performance thin-layer chromatography | Incorporated |
Figure 2.
High-performance thin-layer chromatography profiles of maha yogaraja guggulu.
Figure 3.
High-performance thin-layer chromatography fingerprints of maha yogaraja guggulu.
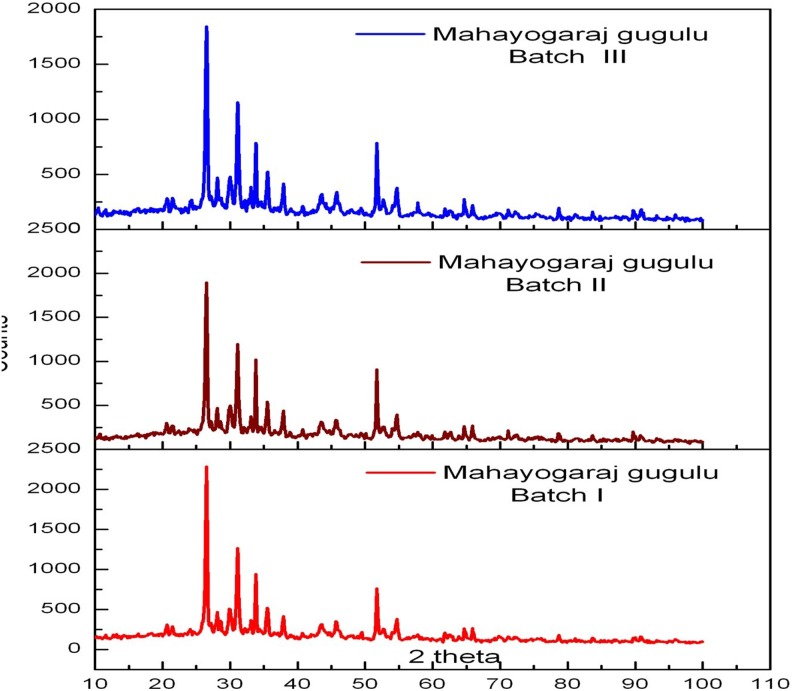
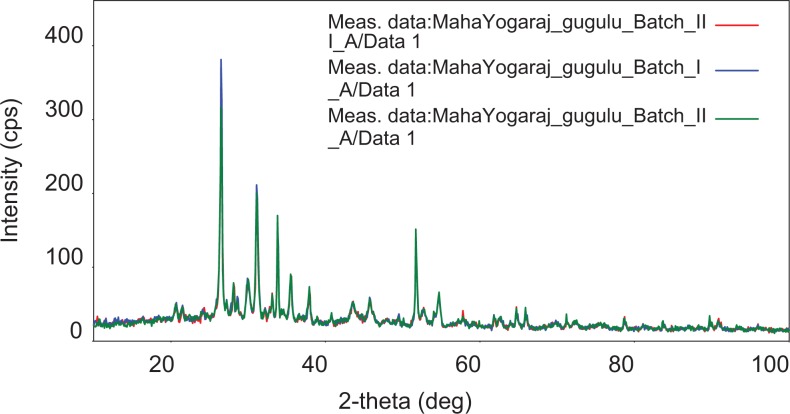
The X-ray diffraction patterns of the 3 batches, as shown in Figures 4 and 5 and Table 4, are nearly identical, showing clear crystalline phases of the inorganic constituents. The X-ray diffraction results have indicated that all the samples contained cinnabar (mercury sulfide added as rasa sindhura), cassiterite (tin oxide, vanga bhasma), litharge (lead oxide; naga bhasma), and iron dioxide and magnetite (di-iron oxide; loha and mandura bhasma). Some clear but weak signature of elemental tin is also seen. No signature of rajat bhasma could be identified in these spectra, indicating that the method used for its preparation did not yield any crystalline products or the signature is buried under the strong lines of other constituents. The X-ray diffraction lines at 21.5° and 49.5° could show the presence of elemental tin. The crystalline form of abhrak bhasma does not appear to be present. It can be seen that 3 X-ray diffraction patterns on the samples are qualitatively same in the relative intensities of the peaks, and the X-ray diffraction results indicate that the inorganic contents have remained intact over time.
Figure 4.
X-ray diffraction pattern of maha yogaraja guggulu.
Figure 5.
Overlay of X-ray diffraction pattern of maha yogaraja guggulu.
Table 4.
d-Spacing and 2-Theta (°) Values of X-Ray Diffraction Analysis.
| 2 theta | d (A) | Size (A) | Chemical Formula | Phase Data Name | cps |
|---|---|---|---|---|---|
| 21.5 | 4.1 | 214.2 | Sn | Tin (1,1,0) | 12.7 |
| 26.5 | 3.4 | 381.0 | HgS, SnO2 | Cinnabar (1,0,1), cassiterite, syn (1,1,0) | 271.0 |
| 28.0 | 3.2 | 106.0 | HgS | Cinnabar (0,0,3) | 17.2 |
| 28.7 | 3.1 | 217.2 | PbO | Litharge (1,0,1) | 16.1 |
| 29.9 | 3.0 | 191.0 | Fe3O4 | Iron di-iron (III) oxide, magnetite HP, syn (2,2,0) | 35.0 |
| 31.1 | 2.9 | 268.0 | HgS | Cinnabar (1,0,2) | 123.0 |
| 34.0 | 2.6 | 220.1 | SnO2 | Cassiterite, syn (1,0,1) | 122.3 |
| 35.4 | 2.5 | 336.0 | Fe3O4, PbO | Iron di-iron (III) oxide, magnetite HP, syn (3,1,1), litharge (0,0,2) | 47.0 |
| 37.9 | 2.4 | 297.0 | HgS, SnO2 | Cinnabar (1,0,3), cassiterite, syn (2,0,0) | 27.0 |
| 43.4 | 2.1 | 117.0 | HgS, Fe3O4, Sn | Cinnabar (1,1,0), iron di-iron (III) oxide, magnetite HP, syn (4,0,0), tin (2,2,0) | 17.8 |
| 45.7 | 2.0 | 228.0 | HgS, PbO | Cinnabar (1,0,4), litharge (2,0,0) | 21.5 |
| 49.5 | 1.8 | 231.7 | Sn | Tin (3,1,0) | 12.9 |
| 51.7 | 1.8 | 593.0 | HgS, SnO2 | Cinnabar (2,0,1), cassiterite, syn (2,1,1) | 95.0 |
| 52.8 | 1.7 | 235.0 | HgS | Cinnabar (1,1,3) | 17.4 |
| 54.7 | 1.7 | 224.0 | HgS, SnO2, PbO | Cinnabar (2,0,2), cassiterite, syn (2,2,0), litharge (2,1,1) | 26.0 |
| 61.8 | 1.5 | 245.3 | SnO2 | Cassiterite, syn (3,1,0) | 14.3 |
| 62.5 | 1.5 | 246.2 | Fe3O4, SnO2, Sn | Iron di-iron (III) oxide, magnetite HP, syn (4,4,0), cassiterite, syn (2,2,1), tin (1,1,2) | 6.8 |
| 64.8 | 1.4 | 249.2 | HgS, SnO2 | Cinnabar (2,0,4), cassiterite, syn (1,1,2) | 22.8 |
| 66.0 | 1.4 | 914.0 | Fe3O4, SnO2 | Iron di-iron (III) oxide, magnetite HP, syn (5,3,1), cassiterite, syn (3,0,1) | 32.0 |
| 69.7 | 1.3 | 29.0 | HgS, SnO2, Sn | Cinnabar (2,1,0), cassiterite, syn (3,1,1), tin (2,0,2) | 3.0 |
| 71.2 | 1.3 | 258.9 | Fe3O4, SnO2 | Iron di-iron (III) oxide, magnetite HP, syn (6,2,0), cassiterite, syn (2,0,2) | 4.9 |
| 78.7 | 1.2 | 272.2 | SnO2 | Cassiterite, syn (3,2,1) | 12.7 |
| 83.7 | 1.2 | 282.6 | SnO2, PbO | Cassiterite, syn (2,2,2), litharge (1,1,4) | 7.7 |
| 89.8 | 1.1 | 297.0 | Fe3O4, SnO2 | Iron di-iron (III) oxide, magnetite HP, syn (7,3,1), cassiterite, syn (3,1,2) | 5.9 |
| 90.9 | 1.1 | 212.0 | HgS, SnO2, PbO | Cinnabar (2,0,7), cassiterite, syn (4,1,1), litharge (3,2,1) | 6.0 |
Conclusion
This study reveals that yogaraja guggulu prepared following the classical guidelines seems to be very effective in converting the macro elements into therapeutically effective medicines in micro form. Well-prepared herbo-mineral drugs offer many advantages over plant medicines due to their longer shelf-life, lesser doses, easy storing facilities, better palatability, and so on. The inferences and the standards laid down in this study certainly can be utilized as baseline data of standardization and quality assurance of this herbo-mineral formulation. It will be helpful in laying down further pharmacopoeia standards of maha yogaraja guggulu.
Acknowledgment
The authors express their heartfelt thanks and would like to acknowledge Dr S. K. Sharma, Former Advisor (Ayurveda), Ministry of AYUSH, Government of India; Dr M. M. Padhi, Former Deputy Director General, CCRAS; and Dr Pramila Pant, Assistant Director (Chemistry) for valuable guidance; and Dr V. K. Singh, M/s Maharishi Ayurveda Pharmacy, Noida, India, for technical inputs. The authors are thankful to Dr J. Arunachalam, Former Director, National Centre for Compositional Characterization of Materials (BARC), Hyderabad, India, for helping in data analysis and interpretation of the results. Thanks are also conveyed to Bhavana Dwivedi, Dr Aarti Sheetal, Suman Singh, Divya Mishra, and Yadunandan Dey, senior research fellows of CCRAS, for technical assistance.
Footnotes
Author Contributions: Arjun Singh: Data generation, data interpretation, and drafted the chemical characterization part of the article.
Sarada Ota: Coordination of the project and drafted the ayurvedic part and standard operative procedures for the preparation of the formulation in the article.
Narayan Srikanth: Monitored the project work and revised the article critically.
Galib: Generated the data related to the standard operative procedures for the preparation of the formulation.
Sreedhar Bojja: Generated and analyzed the data related to chemical characterization.
Kartar Singh Dhiman: Supervision and final approval of the article for publication.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The study was funded by the Ministry of AYUSH, Government of India.
Ethical Approval: Not applicable for this research study.
References
- 1. Sharangadhara. Madhyam khanda In: Vidyasagar PS, ed. Sharangadhara Samhita. Varanasi, India: Chaukhamba Orientalia; 2002:202. [Google Scholar]
- 2. Govind D. Vatavyadi adhikara In: Haridutt S, Vaidya L, eds. Bhaisajaya Ratanavali. Varanasi, India: Motilal Banarasidass; 1998:331. [Google Scholar]
- 3. Government of India. The Ayurvedic Formulary of India—Part-I. 2nd ed New Delhi, India: Ministry of Health and Family Welfare, Government of India; 2003:69. [Google Scholar]
- 4. Government of India. The Ayurvedic Formulary of India—Part-I. 2nd ed New Delhi, India: Ministry of Health and Family Welfare, Government of India; 2003:65. [Google Scholar]
- 5. Sharangadhara. Madhyam khanda In: Vidyasagar PS, ed. Sharangadhara Samhita. Varanasi, India: Chaukhamba Orientalia; 2002:241. [Google Scholar]
- 6. Sharms S. Vangavigyaniya asthadas tarang In: Shastri K, ed. Rasa Taringini. Varanasi, India: Motilal Banarasidass; 1979:438. [Google Scholar]
- 7. Upadhyaya M. Chapter 3 In: Mishra GS, ed. Ayurvedaprakasha. Varanasi, India: Chowkhambha Vidya Bhawan; 2008:379–380. [Google Scholar]
- 8. Sharma S. Rajatavigyaniya shodash tarang In: Shastri K, ed. Rasa Taringini. Varanasi, India: Motilal Banarasidass; 1979:387. [Google Scholar]
- 9. Sharma S. Rajatavigyaniya shodash tarang In: Shastri K, ed. Rasa Taringini. Varanasi, India: Motilal Banarasidass; 1979:392–393. [Google Scholar]
- 10. Sharangadhara. Madhyam khanda In: Vidyasagar PS, ed. Sharangadhara Samhita. Varanasi, India: Chaukhamba Orientalia; 2002:245. [Google Scholar]
- 11. Sharma S. Abharakvigayniyo dasham tarang In: Shastri K, ed. Rasa Taringini. Varanasi, India: Motilal Banarasidass; 1979:225. [Google Scholar]
- 12. Government of India. The Ayurvedic Formulary of India—Part-I. 2nd ed New Delhi, India: Ministry of Health and Family Welfare, Government of India; 2003:348. [Google Scholar]
- 13. Upadhyaya M. Chapter 3 In: Mishra GS, ed. Ayurvedaprakasha. Varanasi, India: Chowkhambha Vidya Bhawan; 2008:289–290. [Google Scholar]
- 14. Anonymous. The Ayurvedic Formulary of India (AFI)—Part-I. 2nd ed New Delhi, India: Ministry of Health and Family Welfare, Government of India; 2003:354. [Google Scholar]
- 15. Sharma S. Abharakvigayniyo dasham tarang In: Shastri K, ed. Rasa Taringini. Varanasi, India: Motilal Banarasidass; 1979:228–229. [Google Scholar]
- 16. Sharma S. Lohadivigyaniyanaam vinsa tarang In: Shastri K, ed. Rasa Taringini. Varanasi, India: Motilal Banarasidass; 1979:516–517. [Google Scholar]
- 17. Sharma S. Lohadivigyaniyanaam vimsa tarang In: Shastri K, ed. Rasa Taringini. Varanasi, India: Motilal Banarasidass; 1979:517. [Google Scholar]
- 18. Sharma S. Lohadivigyaniyanaam vinsa tarang In: Shastri K, ed. Rasa Taringini. Varanasi, India: Motilal Banarasidass; 1979:495–496. [Google Scholar]
- 19. Sharma S. Rajatavigyaniya shodash tarang In: Shastri K, ed. Rasa Taringini. Varanasi, India: Motilal Banarasidass; 1979:496–497. [Google Scholar]
- 20. Sharma S. Rajatavigyaniya shodash tarang In: Shastri K, ed. Rasa Taringini. Varanasi, India: Motilal Banarasidass; 1979:497–498. [Google Scholar]
- 21. Sharma S. Rajatavigyaniya shodash tarang In: Shastri K, ed. Rasa Taringini. Varanasi, India: Motilal Banarasidass; 1979:498–500. [Google Scholar]
- 22. Sharma S. Murchhanavigyaniya sastha tarang In: Shastri K, ed. Rasa Taringini. Varanasi, India: Motilal Banarasidass; 1979:135–136. [Google Scholar]
- 23. Vaghbhatta A. Chapter 8 In: Shastri A, ed. Rasa Ratna Samuchchaya. Varanasi, India: Amarbharti Prakashan; 1995:135. [Google Scholar]
- 24. Sharma S. Yantravigyaniya chaturth tarang In: Shastri K, ed. Rasa Taringini. Varanasi, India: Motilal Banarasidass; 1979:52–53. [Google Scholar]
- 25. Government of India. The Ayurvedic Pharmacopoeia of India—Part-II. Vol I. 1st ed. New Delhi, India: Ministry of Health and Family Welfare, Government of India; 2007. [Google Scholar]
- 26. Government of India. The Ayurvedic Pharmacopoeia of India—Part-II. Vol II. 1st ed. New Delhi, India: Ministry of Health and Family Welfare, Government of India; 2008. [Google Scholar]
- 27. Government of India. Pharmacopoeial Standards for Ayurvedic Formulations (Revised Edition). New Delhi, India: CCRAS, Ministry of Health and Family Welfare, Government of India; 1987. [Google Scholar]