Abstract
Background:
Symptomatic articular cartilage lesions of the knee are common and are being treated surgically with increasing frequency. While many studies have reported outcomes following a variety of cartilage restoration procedures, few have investigated outcomes of revision surgery after a failed attempt at cartilage repair or reconstruction.
Purpose:
To investigate outcomes of revision cartilage restoration procedures for symptomatic articular cartilage lesions of the knee following a previously failed cartilage reconstructive procedure.
Study Design:
Systematic review; Level of evidence, 4.
Methods:
A literature search was performed by use of the PubMed, EMBASE, and MEDLINE/Ovid databases for relevant articles published between 1975 and 2017 that evaluated patients undergoing revision cartilage restoration procedure(s) and reported outcomes using validated outcome measures. For studies meeting inclusion criteria, relevant information was extracted.
Results:
Ten studies met the inclusion criteria. Lesions most commonly occurred in the medial femoral condyle (MFC) (52.8%), with marrow stimulation techniques (MST) the index procedure most frequently performed (70.7%). Three studies demonstrated inferior outcomes of autologous chondrocyte implantation (ACI) following a previous failed cartilage procedure compared with primary ACI. One study comparing osteochondral allograft (OCA) transplant following failed microfracture (MFX) with primary OCA transplant demonstrated similar clinical outcomes and graft survival at midterm follow-up. No studies reported outcomes following osteochondral autograft transfer (OAT) or newer techniques.
Conclusion:
This systematic review of the literature reporting outcomes following revision articular cartilage restoration procedures (most commonly involving the MFC) demonstrated a high proportion of patients who underwent prior MST. Evidence is sufficient to suggest that caution should be taken in performing ACI in the setting of prior MST, likely secondary to subchondral bone compromise. OCA appears to be a good revision treatment option even if the subchondral bone has been violated from prior surgery or fracture.
Keywords: revision cartilage, microfracture, osteochondral allograft, autologous chondrocyte implantation, marrow stimulation techniques, osteochondral autograft
Articular cartilage defects are common and can be found in 60% to 66% of patients undergoing knee arthroscopy,1,10,24 with full-thickness defects found in approximately 36% of knees in athletes.14 These lesions are being treated surgically with an increasing frequency; more than 200,000 procedures are performed in the United States annually, with an increase of approximately 5% per year.12,32 This growth has occurred, in large part, secondary to improving technologies and a well-established increased risk of osteoarthritis progression in the setting of these lesions.38,46 Articular cartilage defects commonly occur in young, active patients eager to return to a high level of activity. Arthroplasty options may be limited in these relatively young patients; therefore, cartilage restoration procedures must be considered.
Symptomatic full-thickness articular cartilage defects are currently managed with a variety of procedures depending on numerous factors, including lesion size and location, number of defects, surgeon preference, and insurance coverage. Currently used treatments include autologous chondrocyte implantation (ACI),3 osteochondral autograft transfer (OAT),30 osteochondral allograft (OCA),11 BioCartilage Extracellular Matrix (Arthrex Inc), minced juvenile articular cartilage allograft (DeNovo NT Graft; Zimmer-Biomet Inc),13 prefabricated OCA (Cartiform; Arthrex Inc), and marrow stimulation techniques (MST) including drilling, abrasion arthroplasty, and microfracture (MFX).27,40,48 Other techniques that are used outside of the United States have been described, such as Hyalgraft C (Fidia Advanced Biopolymers Laboratories), matrix-induced ACI (Sanofi Bioservices), and the Cartilage Autograft Implantation System (CAIS; DePuy Mitek).5
While many studies have reported clinical outcomes following primary cartilage restoration procedures, few studies have reported outcomes of revision cartilage procedures following a previously failed cartilage reconstructive procedure. As the number of primary reconstruction procedures continues to increase,12,32 the number of treatment failures warranting reoperation will also likely increase. Failure rates following primary cartilage reconstructive procedures are significant, ranging from 14% to 43%.‡
Given the methodological difficulties of conducting a prospective, randomized study comparing one or more procedures for revision cartilage restoration, a systematic review of the existing literature may help guide surgical decision making in the setting of previously treated, symptomatic articular cartilage lesions of the knee. Therefore, the purpose of this study was to investigate the outcomes of revision cartilage restoration procedures of the knee following a previously failed articular cartilage reconstructive procedure.
Methods
Study Identification
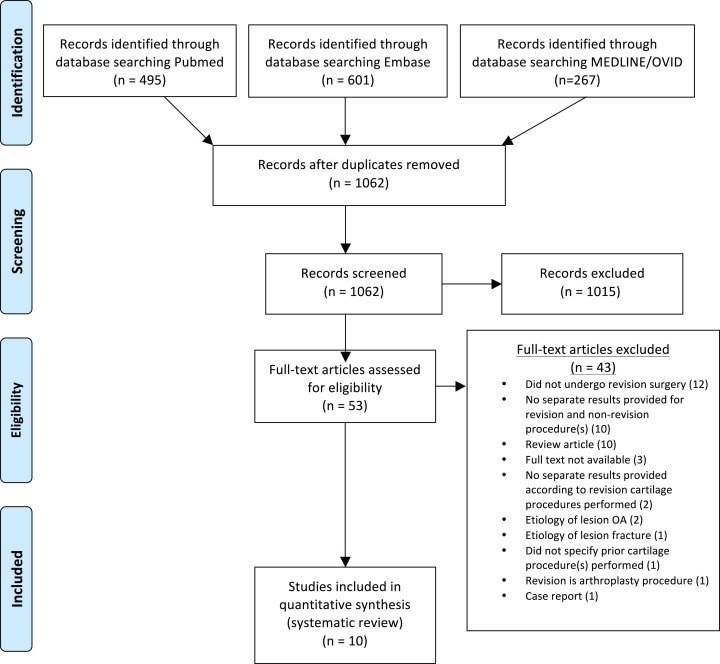
A systematic review was performed according to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analysis) guidelines. A medical librarian assisted with the creation and execution of the search strategy. The search identified all articles containing terms related to (“revision” or “failed”) and (“cartilage” or “chondral” or “osteochondral”) and “knee.” A literature search was independently performed by 2 authors (J.D.L. and K.A.S.) in December 2017. PubMed, EMBASE, and MEDLINE/Ovid were searched from their earliest entries through December 1, 2017. Full-length manuscripts of studies to potentially be included based on title and abstract were then independently reviewed by the same 2 authors to verify the meeting of inclusion criteria. Citations within all included studies were manually reviewed to identify any additional studies that may have been missed during the initial database searches (Figure 1).
Figure 1.
PRISMA flow diagram. OA, osteoarthritis.
Studies were considered for inclusion if they were primary research articles published in English; evaluated patients undergoing revision articular cartilage restoration procedures of any articular surface of the knee; and reported outcomes using validated outcome measures. Studies that included patients who underwent concurrent procedures such as osteotomy, ligamentous reconstruction, or meniscal surgery were also included because of the frequency of these associated procedures. Studies were excluded if they did not specify what types of prior cartilage procedures were performed; results were not reported separately for patients undergoing revision cartilage restoration procedures and those not undergoing a revision procedure; results were not reported separately according to the revision cartilage restoration procedure performed; the cause of the osteochondral lesion was fracture or osteoarthritis; revision treatment was arthroplasty or other artificial surface replacement; the full-text version was not available; the study was not published in English; or the study was either a case report or a review.
Data Extraction
A standardized data sheet was prepared, and all relevant information and outcome data were extracted from the included studies by 2 authors (J.D.L. and K.A.S.). independently. When necessary, means and measure of dispersion (standard deviation based on error bars from figures) were estimated. Collected data included title, author, publication year, publication journal, study type, level of evidence, patient population, location of the cartilage lesion, size of cartilage lesion, type of prior cartilage procedure, type of revision cartilage procedure, clinical outcome, reoperation rate, and graft survivorship. Quality assessment of the included studies was performed using the MINORS (Methodological Index for Non-randomized Studies) checklist.47
Two authors (J.D.L. and K.A.S.) independently reviewed each study and recorded data on these prespecified forms. Disagreements were resolved by consensus.
Results
Study Characteristics
Initial searches of PubMed, MEDLINE/Ovid, and EMBASE returned 495, 267, and 601 records, respectively. Fifty-three studies underwent full-text review. Following application of eligibility criteria, 43 of these studies were excluded, leaving 10 studies for final inclusion.
Individual study characteristics and patient demographics of all 10 included studies are summarized in Table 1. Clinical outcomes from these studies are summarized in Table 2. Two studies were characterized as level 2 evidence, two as level 3 evidence, and six as level 4 evidence.25 Quality assessment using the MINORS checklist can be seen in Appendix Table A1. The mean score using the MINORS checklist was 12.9 (SD, ±3.3; range, 10-20). The final analysis contained 608 knees. Mean age ranged from 24 to 37.4 years. Minimum follow-up ranged from 1 to 10 years.
TABLE 1.
Study Characteristicsa
| Lead Author (Year) | Site of Lesion (% Population) | Minimum Follow-up, y | No. of Knees | MST Knees, % | Age, y, mean | Prior Cartilage Procedures | Revision Cartilage Procedure | Defect Size, cm2, mean | Outcome Measures | LOE |
|---|---|---|---|---|---|---|---|---|---|---|
| Minas33 (2009) | Not reported | 2 | 111 | 100 | 35.4 (range, 14-55) | MST (drilling, abrasion chondroplasty, microfracture) (100%) | ACI | 5.2 (SD, ±3.1) | Treatment failure | 2 |
| Minas34 (2014) | Not reported | 10 | 89 | 100 | 35.8 (SD, ±9.6)b | Drilling (52%), abrasion arthroplasty (34%), microfracture (14%) | ACI | 8.4 (SD, ±5.5)b | Graft failure, WOMAC, KSS, SF-36 | 4 |
| Pestka39 (2012) | MFC (57%), LFC (7%), PF (36%) | 1 | 28 | 100 | 34.1 (range, 14.8-45.8) | Microfracture (100%) | ACI | 4.6 (SD, ±2.7; range, 1.5-7.5) | IKDC, KOOS, VAS pain, VAS knee function, ARS | 3 |
| Vijayan52 (2014) | MFC (50%), LFC (9%), PF (41%) | 1.3 | 22 | 0 | 37.4 (range, 18-48) | ACI (77%), MACI (23%) | ACI | 4.5 (range, 1.5-8.8) | Cincinnati, Stanmore Bentley, VAS | 4 |
| Zaslav53 (2009) | MFC (67%), LFC (18%), PF (16%) | 3.8 | 126 | 44 | 34.5 (SD, ±8.1) | Debridement (48%), microfracture (27%), drilling (10%), abrasion arthroplasty (6%), other (9%)c | ACI | 4.6 (SD, ±3.2) | Modified Cincinnati, KOOS, VAS, SF-36 | 2 |
| Gracitelli19 (2015) | MFC (61%), LFC (31%), MFC+LFC (7%), PF (4%) | 2 | 46d | 100e | 26.2 (SD, ±10.4) | MST (microfracture or drilling) (100%) | OCA | 8 (SD, ±3.2)e | Merle d’Aubigne-Postel, IKDC, KOOS, KSS-F | 3 |
| Gracitelli20 (2015) | MFC (45%), LFC (17%), PF (12%), multiple sites (25%) | 2 | 164 | 88 | 32.6 (range, 11-59) | MST (88%), OAT (2%), multiple procedures (7%) | OCA | 6.8 (SD, ±8) | Merle d’Aubigne-Postel, IKDC, KOOS, KSS-F | 4 |
| Horton25 (2013) | MFC (42%), LFC (27%), PF (27%), TP (15%)f | 2 | 33 | 0 | 33.0 (range, 16-64) | OCA (100%) | OCA | 9.5 (range, 2-30)b | IKDC, KSS-F, modified Merle d’Aubigne-Postel | 4 |
| Stone49 (2014) | MFC (71%), LFC (29%) | 2 | 7 | 29 | 24.0 (range, 15-39) | OCD repair (43% refixation, 29% drilling, 29% OAT) | OCG | 3.3 (SD, ±1.5) | IKDC, WOMAC, Raw Tegner, MRI analysis | 4 |
| Niethammer37 (2015) | MFC (37%), LFC (6%), PF (56%)b | 2 | 28 | 0 | 34.1 (range, 11-66)b | ACI | Retrograde drilling or microfractureg | 5 (SD, ±2.5) | IKDC, VAS | 4 |
aACI, autologous chondrocyte implantation; ARS, Activity Rating Scale; IKDC, International Knee Documentation Committee; KOOS, Knee injury and Osteoarthritis Outcome Score; KSS, Knee Society Score; KSS-F, Knee Society Score–Function; LFC, lateral femoral condyle; LOE, level of evidence; MACI, autologous cultured chondrocytes on porcine collagen membrane; MFC, medial femoral condyle; MRI, magnetic resonance imaging; MST, marrow stimulation technique; OAT, osteochondral autograft transfer; OCA, osteochondral allograft; OCD, osteochondritis dissecans; OCG, osteochondral grafting (notch plugs harvested, morselized, and then impacted); PF, patellofemoral; SF-36, Short Form Health Survey–36; TP, tibial plateau; VAS, Visual Analog Scale; WOMAC, Western Ontario and McMaster Universities Osteoarthritis Index.
bData for entire study population of index and revision procedures.
cOther: OAT (5%), chondroplasty (2%), MST unspecified (1%).
dIncluded in 164 patients in the Gracitelli et al20 study.
eGraft area (defect area not reported).
fTotal >100%, as some patients had multiple graft sites.
gRetrograde drilling or microfracture: (1) retrograde drilling + infusion therapy for bone marrow edema; (2) microfracture for partial graft cartilage deficiency.
TABLE 2.
Study Resultsa
| Lead Author (Year) | Revision Cartilage Procedure | Clinical Outcomes | Reoperation, % | Graft Survivorship, % |
|---|---|---|---|---|
| Minas33 (2009) | ACI |
Defects with prior treatment affecting subchondral bone failed at a rate 3 times higher than nontreated defects. Failure rates of ACI were 28% following drilling, 27% following abrasion arthroplasty, and 20% following microfracture. |
Not reported | 74% at minimum 2 yb |
| Minas34 (2014) | ACI |
Survivorship of ACI was lower after prior MST compared with no prior MST at 10 y (95% CI, 55%-75% vs 76%-90%) and 15 y (95% CI, 50%-72% vs 69%-87%). Significant difference in 15-y survivorship following prior microfracture compared with no prior microfracture (95% CI, 17%-68% vs 69%-87%). Among patients treated with concurrent HTO, 29% with prior MST experienced failure compared with none without prior MST (P < .001). |
68 | 71% at 10 y |
| Pestka39 (2012) | ACI | ACI following microfracture, when compared with primary ACI, had the following results, respectively: failure rate 25% vs 3.6% (P = .0241), IKDC 58.4 vs 69.0 (P = .0583), KOOSpain 69.2 vs 80.1 (P = .034), KOOSADL 78.5 vs 86.3 (P = .024), VASknee function 6.2 vs 6.9 (P = .032). | 25 | 75% at minimum 1 y |
| Vijayan52 (2014) | MACI (78%), ACI (22%) | Modified Cincinnati score from 40.5 to 64.9, VAS from 6.1 to 4.7, 64% “good” or “excellent” outcome. | 36 | 86% at minimum 1.3 y |
| Zaslav53 (2009) | ACI |
Significant improvement in all KOOS subscales, modified Cincinnati 3.3 to 6.3, VAS 28.8 to 69.9, SF-36 physical health 33.0 to 44.4. Duration of benefit 31 months longer following revision ACI than non-ACI index procedure. 49% had subsequent procedures, which was not predictive of failure. |
49 | 76%b at 4 y |
| Gracitelli19 (2015) | OCA |
Reoperation in 24% of primary OCA compared with 44% of OCA after prior MST (P = .04). OCA failure in 11% of primary OCA compared with 15% of OCA after prior MST (P = .53). 10-y survivorship 87.4% following primary OCA compared with 86% in OCA after prior MST. Satisfaction 87% in primary compared with 97% in OCA after prior MST. Significant improvements in pain and function (modified Merle d’Aubigne-Postel, IKDC, KOOS) with no significant between-group difference. |
44 | 86% at 10 y |
| Gracitelli20 (2015) | OCA |
Median time to failure 2.6 ± 6.8 y. 89% “extremely satisfied” or “satisfied.” Significant improvement in modified Merle d’Aubigne-Postel, IKDCpain, function, total, KSS-F, and KOOS postoperatively compared with preoperatively. |
42 | 82% at 10 y 74.9% at 15 y |
| Horton25 (2013) | OCA | Mean time to failure of 5.5 y. Among those with graft survival: 63% “excellent” or “good” based on Merle D’Aubigne-Postel score, 95% satisfaction rate with 68% “extremely satisfied.” | 67 | 79% at 5 y 61% at 10 y |
| Stone49 (2014) | OCG |
Significant improvement in IKDC, WOMAC, and Tegner postoperatively compared with preoperatively. Complete cartilage fill and adjacent tissue integration on MRI in 71.4%. |
71 | 57.1%c at minimum 2 y |
| Niethammer37 (2015) | Retrograde drilling or microfractured | Improvement in IKDCsubjective, VAS during activity, and VAS at rest postoperatively compared with preoperatively. | Not reported | Not reported |
aACI, autologous chondrocyte implantation; ADL, activities of daily living; HTO, high tibial osteotomy; IKDC, International Knee Documentation Committee; KOOS, Knee injury and Osteoarthritis Outcome Score; KSS-F, Knee Society Score–Function; MACI, autologous cultured chondrocytes on porcine collagen membrane; MRI, magnetic resonance imaging; MST, marrow stimulation technique; OCA, osteochondral allograft; OCG, osteochondral grafting (notch plugs harvested, morselized, and then impacted); SF-36, Short Form Health Survey–36; VAS, visual analog scale; WOMAC, Western Ontario and McMaster Universities Osteoarthritis Index.
bDid not meet a priori definition of treatment failure.
cOne patient had partial revision of graft and is included as failure.
dRetrograde drilling or microfracture: (1) retrograde drilling + infusion therapy for bone marrow edema; (2) microfracture for partial graft cartilage deficiency.
Heterogeneity in lesion location was noted, with 52.8% of the lesions occurring within the medial femoral condyle (MFC). A variety of failed index cartilage procedures were performed prior to the revision cartilage surgery. In 6 studies, no concomitant procedures were performed.19,20,25,37,39,52 In the 4 other studies, concomitant procedures were performed (Appendix Table A2).33,34,49,53 In 3 studies, a considerable number of patients underwent concomitant procedures.33,34,53 Due to marked heterogeneity in outcome measures, previous cartilage procedures performed, lesion location, and definition of treatment failure, a formal meta-analysis could not be reliably performed.
Autologous Chondrocyte Implantation
Five studies reported outcomes for ACI33,34,39,52,53 following a failed index cartilage procedure (Table 1). Lesion size was similar among 4 of these studies, ranging from a mean of 4.5 to 5.2 cm2; one study34 reported a larger mean size of 8.4 cm2. Only one study reported outcomes of revision following a failed primary ACI.52 Four studies reported outcomes of ACI following prior MST. Three comparative studies demonstrated inferior outcomes of ACI following prior MST compared with primary ACI.33,34,39 Two studies performed subanalysis of patients with prior MFX (excluding other MSTs33,39), and inferior outcomes were demonstrated in both studies among patients who underwent prior MFX compared with primary ACI. One study that analyzed patients who underwent concomitant high tibial osteotomy (HTO) and ACI with or without prior MFX33 demonstrated inferior outcomes among those with prior MFX. Together, the results of these studies suggest inferior outcomes of ACI following a previous failed cartilage procedure compared with primary ACI, particularly following prior MST including MFX.
Osteochondral Allograft
Three studies investigated outcomes of OCA transplant following a prior failed cartilage procedure.19,20,25 Mean lesion size in these 3 studies was higher than in those studies reporting outcomes of ACI following a prior failed cartilage procedure, with lesion size ranging from a mean of 6.8 to 9.5 cm2. While 2 case series lacked a comparative group,20,25 one study compared primary OCA transplant with OCA transplant following prior failed MST.19 While the reoperation rate was higher among patients undergoing OCA transplant following prior failed MST, failure rates between groups did not differ, and survivorship was nearly identical at 10 years (87.4% for primary OCA vs 86% for revision OCA, P = .841). Two other studies20,25 without comparative groups demonstrated varying results, which may be attributable to lesion size and prior cartilage procedure performed. In a cohort of patients undergoing revision OCA transplant following prior OCA transplant with a mean lesion size of 9.5 cm2, Horton et al25 reported a failure rate of 39%, with a mean graft survival of 10 years. Gracitelli et al20 reported a cohort of patients who underwent OCA transplant following a prior failed cartilage procedure (88.4% had prior MST), noting a 10-year and 15-year graft survivorship of 82% and 74.9%, respectively. Together, the findings of these studies suggest good midterm survivorship of OCA following prior cartilage procedures. OCA transplant following prior MST does not appear to affect graft survival as it does for ACI following prior MST.
Other Cartilage Repair Techniques
Two studies reported outcomes following cartilage repair techniques other than ACI or OCA transplant following a prior failed procedure. Stone et al49 reported on the use of osteochondral grafting following failed osteochondritis dissecans repair using morselized autologous osteochondral plugs harvested from the intercondylar notch. A relatively high reoperation rate was noted, with 71.4% undergoing revision surgery. Niethammer et al37 demonstrated improvements in clinical outcome measures using multiple treatments, including retrograde drilling and infusion therapy as well as MFX or drilling following third-generation ACI. Due to a limited number of studies using these techniques, further study is needed to determine their utility in the setting of revision cartilage restoration.
Discussion
In our review of the literature, we identified 10 studies reporting the clinical outcomes of revision articular cartilage procedures following failed cartilage restoration surgery. A high proportion of these patients (70.7%) underwent previous MST (MFX, drilling, abrasion chondroplasty/arthroplasty) likely because of the ease, familiarity, and low cost of these techniques. Although no study directly compared outcomes of ACI and OCA transplant following prior failed MST, several of the included studies did report mid- to long-term graft survival or failure rates. In a study of 164 knees, of which 88% had undergone prior MFX, Gracitelli et al20 reported 88% and 82% survivorship at 5 and 10 years, respectively, following OCA transplant. In a separate study, Gracitelli et al19 performed a matched-pairs analysis comparing a group of patients undergoing primary OCA transplant with a group undergoing OCA transplant after failed MFX. The investigators found a near-equivalent 10-year graft survivorship between these 2 groups (87.4% and 86%, respectively; P = .841). However, the group receiving an OCA after failed prior treatment had nearly double the reoperation rate compared with those undergoing primary treatment (24% vs 44%), with more than 50% of these reoperations being an arthroscopic debridement. Finally, in a recent study not meeting the inclusion criteria of this review, Frank et al16 demonstrated no significant difference in 10-year OCA graft survival when comparing patients with and without a history of prior MFX, with survival rates of 89.9% and 84.9%, respectively (P = .370). Furthermore, neither concomitant meniscal allograft transplant nor the compartment of OCA transplant (MFC, lateral femoral condyle, or multiple sites) was found to significantly affect graft survivorship. Together, these studies suggest that similar results can be achieved following primary OCA transplant or revision OCA transplant after previous failed cartilage procedures, most notably MST.
ACI following prior MST appears to have inferior results compared with primary ACI. Zaslav et al53 reported a 24% treatment failure at a minimum 4-year follow-up of patients who underwent prior cartilage surgery, although fewer than 50% of their patients underwent prior MST. Pestka et al39 reported a 25% graft failure at a minimum 15-month follow-up among patients with prior MFX, with only a 3.6% failure rate among those undergoing primary ACI (P = .024). Similarly, Minas et al33 found that patients undergoing ACI after prior MST had a significantly higher failure rate than those undergoing ACI without prior MST (26% vs 8%, P < .001). Failure rates among the different types of MST (eg, drilling, abrasion arthroplasty, MFX) were not significantly different. In that study, Minas et al33 defined “simple” defects as single lesions less than 4 cm2 on the femoral condyles. “Complex” lesions were either multifocal, single lesions larger than 4 cm2 or those involving the trochlea, tibia, or patella. “Salvage” lesions were defined as those occurring on articulating surfaces (bipolar) or lesions with early arthritic changes. Overall failure rates were 3 times higher in knees with prior marrow stimulation, but the numbers were too low to report outcomes of “simple” lesions alone. In a separate study, Minas et al34 demonstrated that survivorship of ACI was significantly lower following MST at 10- and 15-year follow-up, with failure rates of 34% and 38%, respectively, compared with 16% and 21%, respectively, among patients who did not undergo prior MST. Considering MFX, alone, the authors reported a 56% graft failure rate at 15-year follow-up among patients with prior failed MFX compared with 21% among patients who did not undergo prior MFX. Finally, among patients who underwent concurrent HTO with ACI, those who underwent prior failed MST had a significantly higher failure rate (29%) compared with those who did not undergo prior MST (0%). Collectively, these findings suggest that caution should be taken when ACI is considered as a treatment option after a failed prior MST.
Multiple studies have suggested that MST techniques induce changes to the subchondral architecture similar to those in osteoarthritis, resulting in a thickened and stiffer subchondral plate that may be less receptive to cell-based therapies such as ACI.33,41 In contrast, OCA techniques replace this altered subchondral bone in the setting of prior MST or subchondral injury, thereby addressing both the articular cartilage and osseous components of injury. Therefore, when ACI is considered in the setting of one or more previous failed cartilage procedures, prior operative notes should be thoroughly reviewed, if available, and any advanced imaging should be carefully scrutinized for signs of subchondral osseous changes. In addition to potentially limiting treatment options in the revision setting, index MFX has been shown to have inferior outcomes compared with primary ACI and OAT, especially for chronic lesions or those occurring in patients older than 30 years.2,8,9,22,44 Although cost-effective,45 MFX and other MSTs may need to be reconsidered as primary cartilage restoration procedures.
In the setting of prior subchondral injury or treatment involving violation of the subchondral bone such as MST, the senior author (M.J.M.) uses OAT for lesions less than approximately 2 cm2 and uses OCA for lesions larger than 2 cm2, for multiple lesions, and for lesions without stable surrounding bone architecture.30 Microfracture or other MSTs are used exclusively in younger patients (<30 years) with small, acute unipolar lesions or for defects located peripherally on either femoral condyle or central trochlea. While limited data exist regarding outcomes of treatment using relatively new technologies, these newer, unproven graft options may have a role in the revision setting.4,7,13,15,42,50,51 This is especially true for patellar or trochlear lesions, where it may be technically difficult to contour osteochondral autograft or allograft plugs. Unfortunately, no long-term follow-up is available for these newer technologies, and their feasibility may be limited by governmental regulatory agencies and/or third-party payers.
Evaluation of Treatment Failure
The management of symptomatic patients with a failed reconstructive articular cartilage procedure is challenging and requires a careful approach. Several factors must be considered, including the index procedure; the location, size, and number of lesions; the cause of failure; the symptom complex; physical examination and imaging results; and concurrent injury or disease. Surgical planning for symptomatic patients depends on an understanding of the multiple causes of treatment failure. Failure of primary cartilage reconstructive surgery can result from recurrent trauma, failure of graft incorporation, poor surgical technique, untreated concomitant injury or disease, or a combination of these factors. Risk factors for failure following various cartilage reconstruction procedures have been previously described.§ Obesity (defined as a body mass index >30), age older than 45 years, higher preoperative activity scores, and lesions larger than 2 to 4 cm2 have been found to be risk factors for failure after MFX.17,18,21,26,29,35 Risk factors for failure following ACI include obesity, higher preoperative activity levels, and female sex.6,7,18,23,28,36 First-generation ACI using a periosteal patch has led to a higher reoperation rate compared with newer ACI techniques.18 Advanced age has been found to be the sole risk factor for failure following OAT and OCA transplant.31,43
History and Physical Examination
A complete history should be obtained, including mechanism of injury (if any), symptom complex (swelling, giving-way, locking, catching, crepitus, gait alteration), symptom duration, previous injuries, and surgical interventions including ligamentous, meniscal, alignment, and articular cartilage procedures. Current symptoms should be compared with those preoperatively. If the patient describes recurrent symptoms and is unable to recall a causative traumatic episode, this may suggest technical or biological reasons for graft failure. The patient should be asked to describe the postoperative course following the previous cartilage procedure, detailing the time course and return to activity or sport. Failure to return to the same level of activity may suggest a technical error, inadequate rehabilitation, or failure of graft maturation or incorporation. Previous operative notes, clinic notes, therapy notes, imaging studies, and intraoperative arthroscopic images should be reviewed, if available. A complete physical examination should be performed, including an assessment of gait, limb alignment, location of preexisting scars, and ligamentous integrity.
Imaging
Plain radiographs, including weightbearing 40° posteroanterior (Rosenberg), 30° lateral, and Merchant patellofemoral views, should be obtained in all patients. Full-length lower extremity radiographs should be obtained to assess the mechanical axis, which may alter the operative tactic to include either a distal femoral osteotomy (DFO) or HTO for associated coronal malalignment. In the setting of patellofemoral instability or dysplasia, an assessment of patellar height and alignment is imperative prior to any concurrent patellar realignment procedure. A complete discussion of these conditions and their treatment is beyond the scope of this systematic review.
We routinely obtain 1.5-T or 3.0-T magnetic resonance imaging (MRI) to assess the cartilage lesion as well as any additional intra-articular abnormality. An MRI is useful to evaluate the lesion diameter, lesion depth, and health of the subchondral bone. Computed tomography (CT) can be useful to better evaluate any congenital or traumatic structural irregularities of the osseous architecture. MRI or CT may be used to calculate the tibial tubercle–trochlear groove distance in patients with symptomatic patellar instability. Skeletal scintigraphy (bone scan) is rarely indicated to evaluate discordant pain patterns in patients presenting with coexistent articular cartilage lesions.
If a complete understanding of the causes of treatment failure and the extent of the cartilage lesion cannot be discerned following a comprehensive history, physical examination, and review of imaging, then diagnostic arthroscopy may be indicated. However, in most cases, diagnostic arthroscopy alone, without a definitive treatment plan, is not indicated. Limb malalignment affecting the involved compartment in which the chondral lesion is located should be addressed. Untreated malalignment may either contribute to or, in some cases, be the sole cause of treatment failure. In 3 studies included in this systematic review,33,34,53 a relatively high proportion of patients underwent realignment osteotomies concurrently with revision cartilage restoration. In the setting of malalignment, which preferentially loads the compartment affected by the symptomatic chondral lesion, we advocate a realignment procedure (HTO, DFO, or tibial tubercle osteotomy) either concurrently or prior to the revision cartilage procedure. A staged procedure may also be considered if the surgeon does not feel comfortable performing both the realignment and articular cartilage procedures at the same setting. Similarly, any ligamentous insufficiency or meniscal abnormality should be addressed concurrently with, or prior to, any revision cartilage procedure.
Revision cartilage surgery may be categorized based on the index procedure and subcategorized based on lesion location (tibiofemoral or patellofemoral). Furthermore, the clinician must consider whether the subchondral bone has been affected by injury or prior treatment. In the setting of a prior MST, OAT, or OCA, the subchondral bone should be considered violated, and caution should be taken when ACI is contemplated in these patients.33,34,39,53
In the setting of a failed primary MST, an OAT or OCA transplant should be considered; OAT is generally recommended for lesions 2 cm2 or smaller, and OCA is used for larger lesions. This recommendation is based on an “average” sized knee, as a smaller knee may not provide enough osteochondral plugs to fill a 2-cm2 lesion. In this situation, one or more plugs may also be harvested from the contralateral knee. Although no data are available to support the use of prefabricated OCAs in the revision setting, products such as the Cartiform graft, BioCartilage Extracellular Matrix, and DeNovo NT Graft offer flexibility in shaping the graft to a variety of surface contours (ie, patellofemoral surfaces) while maintaining the natural cartilage-bone interface.
In the setting of a failed ACI procedure, the health of the subchondral bone must be considered. For lesions that entail compromised subchondral bone, the treatment options are similar to those following a failed MST. Regarding healthy subchondral bone, current evidence does not demonstrate superiority of OAT, OCA, or ACI. However, our preference is to use either OAT or OCA (depending on lesion size and graft availability) due to the high cost of ACI and required 2-stage procedure.45
In the setting of a failed OAT or OCA, revision options are limited, as the subchondral bone has been violated during the index procedure. Similar to revision following other failed index cartilage procedures, graft choice is largely based on lesion size. In the setting of a failed OCA, the indication for the index procedure, the health of the surrounding cartilage and other compartments, and patient age and activity demands should be considered.25 Horton et al25 demonstrated a trend toward increased failure rates in older patients and those whose index procedure was performed as a salvage operation for osteoarthritis. Caution should be taken with these patients, and arthroplasty options should be considered depending on age and activity level.
Limitations
Limitations of the current systematic review include only 10 studies meeting the inclusion criteria and only 608 knees constituting the entire study group. Given the number of articular cartilage procedures performed in the United States each year, this is a relatively small number of patients on which to base a treatment algorithm. Related to this is the fact that 6 of the 10 studies were level 4 evidence, only 2 studies were level 2, and none were level 1. Therefore, the majority of studies were case series, with the inherent limitations associated with nonrandomized, retrospectively collected data. Several studies33,34,49,53 included patients who underwent concurrent ligamentous or meniscal procedures, which likely contributed to clinical improvement among those patients undergoing multiple concomitant or staged procedures with revision cartilage restoration (Appendix Table A2). Minimum follow-up varied between studies, with a range from 1 to 10 years. Follow-up less than 5 years is a potential weakness of any study evaluating the outcome of an articular cartilage procedure. The studies entailed a relative lack of variety in terms of the primary cartilage repair, as the majority involved an MST and approximately 94% (573/608) of the revision procedures were either ACI or OCA. As well, lesion size was heterogeneous among the included studies. The years of inclusion of this systematic review, from 1975 to 2017, comprise a long time span, and technologies used in the 1970s through the 1990s may not be applicable in today’s practice. In fact, all 10 studies were published between 2009 and 2015. However, any comprehensive literature search must include a wide range of years. Finally, we found no studies evaluating the outcome of newer techniques for cartilage restoration in the revision setting. Therefore, our conclusions and recommendations must be interpreted within this context.
Conclusion
This systematic review of the literature reporting outcomes following revision articular cartilage restoration procedures (most commonly involving the MFC) demonstrated that a high proportion of patients underwent prior MST. Evidence is sufficient to suggest that caution should be taken in performing ACI in the setting of prior MST due to its negative effects on the subchondral bone. OCA transplant appears to be a good treatment option in the setting of a failed prior cartilage restoration surgery, even if the subchondral bone has been violated from prior surgery or fracture. Further investigation is needed to assess outcomes following osteochondral autografts in the revision setting as well as newer techniques.
Appendix
TABLE A1.
MINORS Quality Assessmenta
| Endpoints Appropriate to Aim of Study | Prospective Calculation of Study Size | For Comparative Studies Only | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lead Author (Year) | Clearly Stated Aim | Inclusion of Consecutive Patients | Prospective Data Collection | Unbiased Assessment of Study | Follow-up Period Appropriate | Loss of Follow-up <5% | Comparative Study | Adequate Control | Contemporary Groups | Baseline Equivalence Groups | Adequate Statistical Analysis | Total Score | ||
| Minas33 (2009) | 2 | 2 | 2 | 2 | 0 | 1 | 2 | 1 | Yes | 2 | 2 | 2 | 2 | 20 |
| Minas34 (2014) | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 0 | No | 12 | ||||
| Pestka39 (2012) | 2 | 2 | 0 | 2 | 0 | 1 | 2 | 0 | Yes | 2 | 2 | 2 | 2 | 17 |
| Vijayan52 (2014) | 2 | 2 | 2 | 1 | 0 | 1 | 2 | 0 | No | 10 | ||||
| Zaslav53 (2009) | 2 | 2 | 2 | 2 | 0 | 2 | 0 | 2 | No | 12 | ||||
| Gracitelli19 (2015) | 2 | 2 | 0 | 2 | 0 | 1 | 0 | 0 | Yes | 2 | 2 | 2 | 2 | 15 |
| Gracitelli20 (2015) | 2 | 2 | 2 | 2 | 0 | 1 | 2 | 0 | No | 11 | ||||
| Horton25 (2013) | 2 | 2 | 2 | 2 | 0 | 2 | 0 | 0 | No | 10 | ||||
| Stone49 (2014) | 2 | 2 | 2 | 2 | 0 | 1 | 2 | 0 | No | 11 | ||||
| Niethammer37 (2015) | 2 | 2 | 2 | 2 | 0 | 1 | 2 | 0 | No | 11 | ||||
aThe items are scored 0 (not reported), 1 (reported but inadequate), or 2 (reported and adequate). The global ideal score is 16 for noncomparative studies and 24 for comparative studies.
TABLE A2.
Concomitant Procedures Performed During Revision Cartilage Restoration Procedurea
| Lead Author (Year) | Concomitant Procedures |
|---|---|
| Minas33 (2009) | 23 (21%) varus/valgus osteotomy 30 (27%) TTO 9 (8%) ligament reconstruction |
| Minas34 (2014) | 33 (15.7%) HTO 3 (1.4%) DFO 49 (23.3%) TTO 15 (7.1%) HTO/TTO 12 (5.7%) ligament reconstruction 18 (8.6%) meniscal procedures |
| Pestka39 (2012) | None |
| Vijayan52 (2014) | None |
| Zaslav53 (2009) | 13 (8%) TTO 11 (7%) lateral release 9 (6%) other (see text) 5 (3%) HTO 1 (1%) loose body removal 1 (1%) partial lateral meniscectomy 1 (1%) synovectomy |
| Gracitelli19 (2015) | None |
| Gracitelli20 (2015) | None |
| Horton25 (2013) | None |
| Stone49 (2014) | 1 (14%) lateral meniscus allograft |
| Niethammer37 (2015) | None |
aIncludes all patients in study, not only revisions. DFO, distal femoral osteotomy; HTO, high tibial osteotomy; TTO, tibial tubercle osteotomy.
Footnotes
One or more of the authors has declared the following potential conflict of interest or source of funding: M.J.M. is a paid consultant for Arthrex, Schwartz Biomedical, and Pacira Pharmaceuticals; has received research support from Arthrex and Breg; and has received other financial/material support from Pacira Pharmaceuticals.
References
- 1. Aroen A, Loken S, Heir S, et al. Articular cartilage lesions in 993 consecutive knee arthroscopies. Am J Sports Med. 2004;32(1):211–215. [DOI] [PubMed] [Google Scholar]
- 2. Basad E, Ishaque B, Bachmann G, Sturz H, Steinmeyer J. Matrix-induced autologous chondrocyte implantation versus microfracture in the treatment of cartilage defects of the knee: a 2-year randomised study. Knee Surg Sports Traumatol Arthrosc. 2010;18(4):519–527. [DOI] [PubMed] [Google Scholar]
- 3. Brittberg M, Lindahl A, Nilsson A, Ohlsson C, Isaksson O, Peterson L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994;331(14):889–895. [DOI] [PubMed] [Google Scholar]
- 4. Brophy RH, Wojahn RD, Lamplot JD. Cartilage restoration techniques for the patellofemoral joint. J Am Acad Orthop Surg. 2017;25(5):321–329. [DOI] [PubMed] [Google Scholar]
- 5. Camp CL, Stuart MJ, Krych AJ. Current concepts of articular cartilage restoration techniques in the knee. Sports Health. 2014;6(3):265–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chahal J, Thiel GV, Hussey K, Cole BJ. Managing the patient with failed cartilage restoration. Sports Med Arthrosc. 2013;21(2):62–68. [DOI] [PubMed] [Google Scholar]
- 7. Cole BJ, DeBerardino T, Brewster R, et al. Outcomes of autologous chondrocyte implantation in Study of the Treatment of Articular Repair (STAR) patients with osteochondritis dissecans. Am J Sports Med. 2012;40(9):2015–2022. [DOI] [PubMed] [Google Scholar]
- 8. Cole BJ, Farr J, Winalski CS, et al. Outcomes after a single-stage procedure for cell-based cartilage repair: a prospective clinical safety trial with 2-year follow-up. Am J Sports Med. 2011;39(6):1170–1179. [DOI] [PubMed] [Google Scholar]
- 9. Crawford DC, DeBerardino TM, Williams RJ., III NeoCart, an autologous cartilage tissue implant, compared with microfracture for treatment of distal femoral cartilage lesions: an FDA phase-II prospective, randomized clinical trial after two years. J Bone Joint Surg Am. 2012;94(11):979–989. [DOI] [PubMed] [Google Scholar]
- 10. Curl WW, Krome J, Gordon ES, Rushing J, Smith BP, Poehling GG. Cartilage injuries: a review of 31,516 knee arthroscopies. Arthroscopy. 1997;13(4):456–460. [DOI] [PubMed] [Google Scholar]
- 11. Demange M, Gomoll AH. The use of osteochondral allografts in the management of cartilage defects. Curr Rev Musculoskelet Med. 2012;5(3):229–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Farr J, Cole B, Dhawan A, Kercher J, Sherman S. Clinical cartilage restoration: evolution and overview. Clin Orthop Relat Res. 2011;469(10):2696–2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Farr J, Tabet SK, Margerrison E, Cole BJ. Clinical, radiographic, and histological outcomes after cartilage repair with particulated juvenile articular cartilage: a 2-year prospective study. Am J Sports Med. 2014;42(6):1417–1425. [DOI] [PubMed] [Google Scholar]
- 14. Flanigan DC, Harris JD, Trinh TQ, Siston RA, Brophy RH. Prevalence of chondral defects in athletes’ knees: a systematic review. Med Sci Sports Exerc. 2010;42(10):1795–1801. [DOI] [PubMed] [Google Scholar]
- 15. Fortier LA, Chapman HS, Pownder SL, et al. BioCartilage improves cartilage repair compared with microfracture alone in an equine model of full-thickness cartilage loss. Am J Sports Med. 2016;44(9):2366–2374. [DOI] [PubMed] [Google Scholar]
- 16. Frank RM, Lee S, Levy D, et al. Osteochondral allograft transplantation of the knee: analysis of failures at 5 years. Am J Sports Med. 2017;45(4):864–874. [DOI] [PubMed] [Google Scholar]
- 17. Gobbi A, Karnatzikos G, Kumar A. Long-term results after microfracture treatment for full-thickness knee chondral lesions in athletes. Knee Surg Sports Traumatol Arthrosc. 2014;22(9):1986–1996. [DOI] [PubMed] [Google Scholar]
- 18. Gomoll AH, Probst C, Farr J, Cole BJ, Minas T. Use of a type I/III bilayer collagen membrane decreases reoperation rates for symptomatic hypertrophy after autologous chondrocyte implantation. Am J Sports Med. 2009;37(suppl 1):20S–23S. [DOI] [PubMed] [Google Scholar]
- 19. Gracitelli GC, Meric G, Briggs DT, et al. Fresh osteochondral allografts in the knee: comparison of primary transplantation versus transplantation after failure of previous subchondral marrow stimulation. Am J Sports Med. 2015;43(4):885–891. [DOI] [PubMed] [Google Scholar]
- 20. Gracitelli GC, Meric G, Pulido PA, McCauley JC, Bugbee WD. Osteochondral allograft transplantation for knee lesions after failure of cartilage repair surgery. Cartilage. 2015;6(2):98–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gudas R, Gudaite A, Pocius A, et al. Ten-year follow-up of a prospective, randomized clinical study of mosaic osteochondral autologous transplantation versus microfracture for the treatment of osteochondral defects in the knee joint of athletes. Am J Sports Med. 2012;40(11):2499–2508. [DOI] [PubMed] [Google Scholar]
- 22. Gudas R, Simonaityte R, Cekanauskas E, Tamosiunas R. A prospective, randomized clinical study of osteochondral autologous transplantation versus microfracture for the treatment of osteochondritis dissecans in the knee joint in children. J Pediatr Orthop. 2009;29(7):741–748. [DOI] [PubMed] [Google Scholar]
- 23. Harris JD, Siston RA, Pan X, Flanigan DC. Autologous chondrocyte implantation: a systematic review. J Bone Joint Surg Am. 2010;92(12):2220–2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hjelle K, Solheim E, Strand T, Muri R, Brittberg M. Articular cartilage defects in 1,000 knee arthroscopies. Arthroscopy. 2002;18(7):730–734. [DOI] [PubMed] [Google Scholar]
- 25. Horton MT, Pulido PA, McCauley JC, Bugbee WD. Revision osteochondral allograft transplantations: do they work? Am J Sports Med. 2013;41(11):2507–2511. [DOI] [PubMed] [Google Scholar]
- 26. Jamali AA, Emmerson BC, Chung C, Convery FR, Bugbee WD. Fresh osteochondral allografts: results in the patellofemoral joint. Clin Orthop Relat Res. 2005;(437):176–185. [PubMed] [Google Scholar]
- 27. Johnson LL. Arthroscopic abrasion arthroplasty historical and pathologic perspective: present status. Arthroscopy. 1986;2(1):54–69. [DOI] [PubMed] [Google Scholar]
- 28. Jungmann PM, Salzmann GM, Schmal H, Pestka JM, Sudkamp NP, Niemeyer P. Autologous chondrocyte implantation for treatment of cartilage defects of the knee: what predicts the need for reintervention? Am J Sports Med. 2012;40(1):58–67. [DOI] [PubMed] [Google Scholar]
- 29. Knutsen G, Drogset JO, Engebretsen L, et al. A randomized trial comparing autologous chondrocyte implantation with microfracture: findings at five years. J Bone Joint Surg Am. 2007;89(10):2105–2112. [DOI] [PubMed] [Google Scholar]
- 30. Krych AJ, Harnly HW, Rodeo SA, Williams RJ., III Activity levels are higher after osteochondral autograft transfer mosaicplasty than after microfracture for articular cartilage defects of the knee: a retrospective comparative study. J Bone Joint Surg Am. 2012;94(11):971–978. [DOI] [PubMed] [Google Scholar]
- 31. Levy YD, Gortz S, Pulido PA, McCauley JC, Bugbee WD. Do fresh osteochondral allografts successfully treat femoral condyle lesions? Clin Orthop Relat Res. 2013;471(1):231–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. McCormick F, Harris JD, Abrams GD, et al. Trends in the surgical treatment of articular cartilage lesions in the United States: an analysis of a large private-payer database over a period of 8 years. Arthroscopy. 2014;30(2):222–226. [DOI] [PubMed] [Google Scholar]
- 33. Minas T, Gomoll AH, Rosenberger R, Royce RO, Bryant T. Increased failure rate of autologous chondrocyte implantation after previous treatment with marrow stimulation techniques. Am J Sports Med. 2009;37(5):902–908. [DOI] [PubMed] [Google Scholar]
- 34. Minas T, Von Keudell A, Bryant T, Gomoll AH. The John Insall Award: a minimum 10-year outcome study of autologous chondrocyte implantation. Clin Orthop Relat Res. 2014;472(1):41–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mithoefer K, McAdams T, Williams RJ, Kreuz PC, Mandelbaum BR. Clinical efficacy of the microfracture technique for articular cartilage repair in the knee: an evidence-based systematic analysis. Am J Sports Med. 2009;37(10):2053–2063. [DOI] [PubMed] [Google Scholar]
- 36. Nawaz SZ, Bentley G, Briggs TW, et al. Autologous chondrocyte implantation in the knee: mid-term to long-term results. J Bone Joint Surg Am. 2014;96(10):824–830. [DOI] [PubMed] [Google Scholar]
- 37. Niethammer TR, Valentin S, Ficklscherer A, Gulecyuz MF, Pietschmann MF, Muller PE. Revision surgery after third generation autologous chondrocyte implantation in the knee. Int Orthop. 2015;39(8):1615–1622. [DOI] [PubMed] [Google Scholar]
- 38. Pearle AD, Warren RF, Rodeo SA. Basic science of articular cartilage and osteoarthritis. Clin Sports Med. 2005;24(1):1–12. [DOI] [PubMed] [Google Scholar]
- 39. Pestka JM, Bode G, Salzmann G, Sudkamp NP, Niemeyer P. Clinical outcome of autologous chondrocyte implantation for failed microfracture treatment of full-thickness cartilage defects of the knee joint. Am J Sports Med. 2012;40(2):325–331. [DOI] [PubMed] [Google Scholar]
- 40. Pridie KH. A method of resurfacing osteoarthritic joints. J Bone Joint Surg Br. 1959;41:618–619. [Google Scholar]
- 41. Radin EL, Rose RM. Role of subchondral bone in the initiation and progression of cartilage damage. Clin Orthop Relat Res. 1986;(213):34–40. [PubMed] [Google Scholar]
- 42. Riboh JC, Cvetanovich GL, Cole BJ, Yanke AB. Comparative efficacy of cartilage repair procedures in the knee: a network meta-analysis. Knee Surg Sports Traumatol Arthrosc. 2017;25(12):3786–3799. [DOI] [PubMed] [Google Scholar]
- 43. Robb CA, El-Sayed C, Matharu GS, Baloch K, Pynsent P. Survival of autologous osteochondral grafts in the knee and factors influencing outcome. Acta Orthop Belg. 2012;78(5):643–651. [PubMed] [Google Scholar]
- 44. Saris DB, Vanlauwe J, Victor J, et al. Treatment of symptomatic cartilage defects of the knee: characterized chondrocyte implantation results in better clinical outcome at 36 months in a randomized trial compared to microfracture. Am J Sports Med. 2009;37(suppl 1):10S–19S. [DOI] [PubMed] [Google Scholar]
- 45. Schrock JB, Kraeutler MJ, Houck DA, McQueen MB, McCarty EC. A cost-effectiveness analysis of surgical treatment modalities for chondral lesions of the knee: microfracture, osteochondral autograft transplantation, and autologous chondrocyte implantation. Orthop J Sports Med. 2017;5(5):23259 67117704634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Shelbourne KD, Jari S, Gray T. Outcome of untreated traumatic articular cartilage defects of the knee: a natural history study. J Bone Joint Surg Am. 2003;85-A(suppl 2):8–16. [DOI] [PubMed] [Google Scholar]
- 47. Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J. Methodological index for non-randomized studies (MINORS): development and validation of a new instrument. ANZ J Surg. 2003;73(9):712–716. [DOI] [PubMed] [Google Scholar]
- 48. Steadman JR, Rodkey WG, Briggs KK. Microfracture: its history and experience of the developing surgeon. Cartilage. 2010;1(2):78–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Stone KR, Pelsis JR, Crues JV, III, Walgenbach AW, Turek TJ. Osteochondral grafting for failed knee osteochondritis dissecans repairs. Knee. 2014;21(6):1145–1150. [DOI] [PubMed] [Google Scholar]
- 50. Vanlauwe J, Huylebroek J, Van Der Bauwhede J, et al. Clinical outcomes of characterized chondrocyte implantation. Cartilage. 2012;3(2):173–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Vanlauwe JJ, Claes T, Van Assche D, Bellemans J, Luyten FP. Characterized chondrocyte implantation in the patellofemoral joint: an up to 4-year follow-up of a prospective cohort of 38 patients. Am J Sports Med. 2012;40(8):1799–1807. [DOI] [PubMed] [Google Scholar]
- 52. Vijayan S, Bentley G, Rahman J, Briggs TW, Skinner JA, Carrington RW. Revision cartilage cell transplantation for failed autologous chondrocyte transplantation in chronic osteochondral defects of the knee. Bone Joint J. 2014;96-B(1):54–58. [DOI] [PubMed] [Google Scholar]
- 53. Zaslav K, Cole B, Brewster R, et al. A prospective study of autologous chondrocyte implantation in patients with failed prior treatment for articular cartilage defect of the knee: results of the Study of the Treatment of Articular Repair (STAR) clinical trial. Am J Sports Med. 2009;37(1):42–55. [DOI] [PubMed] [Google Scholar]