Abstract
Background:
In this paper, our aim was to systematically evaluate published evidence of bone fracture risk associated with tamoxifen and aromatase inhibitors in women aged 65 and under, and diagnosed with nonmetastatic breast cancer.
Methods:
We comprehensively searched MEDLINE, EMBASE and CINAHL databases from January 1997 through May 2015, and reference lists of the selected articles to identify English-language randomized controlled trials and cohort studies of fracture risk. Two independent reviewers screened articles and assessed methodological quality using Risk of Bias assessment for randomized controlled trials and the Newcastle–Ottawa Scale for cohort studies. Fracture risk was estimated as pooled risk ratios using a random-effects model and inverse variance method.
Results:
Of 1926 identified articles, 21 independent studies fulfilled our selection criteria. Similar fracture risk was observed in women treated and not treated with tamoxifen [pooled risk ratio (RR) 0.95; 95% confidence interval (CI) 0.84–1.07]. A 35% (95% CI 1.21–1.51) higher fracture risk was observed in the aromatase inhibitor group compared with the tamoxifen group. A 17% (95% CI 1.07–1.28) higher fracture risk was observed in the aromatase inhibitor group than the no aromatase inhibitor group. Compared with the tamoxifen group, aromatase inhibitor-associated fracture risk increased by 33% (pooled RR 1.33; 95% CI 1.21–1.47) during the tamoxifen/aromatase inhibitor treatment period, but did not increase (pooled RR 0.99; 95% CI 0.72–1.37) during the post-tamoxifen/aromatase inhibitor treatment period.
Conclusions:
Fracture risk is significantly higher in women treated with aromatase inhibitors, especially during the treatment period. Tamoxifen is not associated with lower fracture risk while tamoxifen could potentially preserve bone mass. Better osteoporosis management programs, especially during the treatment period, are needed for this group of women.
Keywords: aromatase inhibitors, breast cancer, fracture risk, hormonal treatment, tamoxifen, women
Introduction
Adjuvant systemic treatments, such as chemotherapy and hormonal treatment, have been used widely to treat breast cancer.1 Hormonal treatment is recommended for women with hormone receptor-positive breast cancer, accounting for at least two-thirds of all breast cancer cases.2,3 The two most common hormonal treatments are tamoxifen and aromatase inhibitors (AIs).
Tamoxifen, a selective estrogen receptor modulator (SERM), was introduced in the 1970s. Tamoxifen is currently recommended to treat early and advanced-stage breast cancer in premenopausal and postmenopausal women.4 Tamoxifen is also an optional treatment in women with stage 0 (in situ) breast cancer.5 Tamoxifen reduces the available estrogen to cancer cells by competitively inhibiting the binding of estrogen to the estrogen receptors on breast tissues. The effect of tamoxifen on bone tissues is inconsistent across studies and seems to differ by menopausal status. Tamoxifen caused a bone mineral density (BMD) decrease in healthy premenopausal women but a BMD increase in healthy postmenopausal women.6 In women diagnosed with breast cancer, tamoxifen preserves bone mass in premenopausal women, and either slightly increases or decreases BMD in postmenopausal women.7–12 Tamoxifen may have a beneficial effect on bone health in women diagnosed with breast cancer. However, tamoxifen has not been approved for the treatment or prevention of osteoporosis in any populations by the US Food and Drug Administration.
AIs were introduced in the early 2000s. AIs are currently recommended to treat early and advanced-stage breast cancer in postmenopausal women, especially women unable to tolerate tamoxifen or at higher risk of cancer relapse. AIs reduce the circulating estrogen levels by inhibiting the aromatase enzyme from converting androgen into estrogen in nonovarian tissues. AIs significantly increase bone loss10,13 and are associated with higher fracture risks in several major trials.14,15 However, AI-associated fracture risk has not been reviewed systematically.
The initial goal of this study was to determine the effects of adjuvant systemic breast cancer treatments on BMD changes and fracture risk, compared with locoregional treatments (i.e. surgery and radiation therapy) or no breast cancer treatment in women aged 65 and under. In women diagnosed with breast cancer, younger women (aged 65 and under) are less likely than older women to be assessed for fracture risk before fractures occur using 10-year fracture risk assessment tools or BMD testing. This is because cancer treatment-associated fracture risk is not universally recognized as an indicator in the 10-year fracture risk assessment tools and BMD testing.16 Fractures, however, have a higher clinical impact on healthcare systems than BMD changes. Tamoxifen and AIs are used to treat breast cancer more often than other adjuvant systemic treatments. Hence, we focussed our research questions on the differential fracture risks associated with tamoxifen and AIs in younger women aged 65 years and under, and diagnosed with nonmetastatic breast cancer. This study is targeting younger women, as it is more challenging to identify high-risk young women before fractures occur.
Method
This was a systematic review with meta-analysis study using aggregate data from randomized controlled trials (RCTs) and cohort studies on fracture risks associated with tamoxifen and AIs in younger women aged 65 years and under, and diagnosed with nonmetastatic breast cancer. We registered the review protocol at PROSPERO (registration number CRD42015015604, available at: https://www.crd.york.ac.uk/PROSPERO/). We reported study results using criteria from the Preferred Reporting Items for Systematic Review and Meta-analysis Protocols (PRISMA).17 Article search was conducted by the first author. Study selection (NR/OT for title/abstract screening; WH/OT for full-text article review), study quality evaluation (WH/OT), and data extraction (WH/OT) were performed independently by two reviewers using Excel spreadsheets. Disagreements between reviewers were resolved by discussion. Persistent disagreements between reviewers were arbitrated by another designated team member (MD).
Search strategy
We searched PubMed, MEDLINE, CINAHL, EMBASE, and Cancerlit databases for article published from 1 January 1970 to 1 May 2015, on 3 May 2016. We included search terms “breast” and “wom*n OR female” and “tumor OR cancer OR neoplasm OR malignanc?” and “fracture OR BMD OR densit? OR densitometr? OR absorptiometry?”. Studies were then limited to human studies and English language articles. Review articles were then excluded. The reference lists of the included articles were hand searched. Approximately 20% of included and excluded articles at each step of the article search were randomly reviewed to ensure proper article search strategies.
Study selection
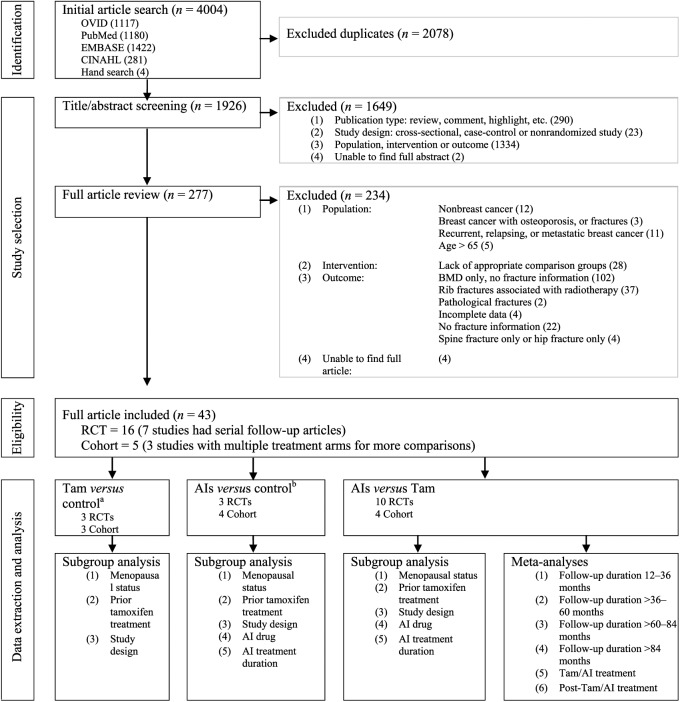
Articles were initially screened by title and abstract, followed by full article reviews (Figure 1). Articles fulfilling the inclusion criteria: (a) RCTs or cohort studies;18 (b) women diagnosed with nonmetastatic breast cancer; (c) at least one participant aged 65 years and under at baseline; (d) breast cancer treatments of tamoxifen, AIs or both; and (e) fracture outcomes, were selected. We defined the outcomes in this study as count of fracture events or participants with fractures. Articles reporting pathological fractures or any specific fracture type (e.g. spine fracture only) were excluded.
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) flow diagram for systematic review of the fracture risks associated with breast cancer treatments.
aNo tamoxifen;
bNo AIs.
AIs, aromatase inhibitors; BMD, bone mineral density; RCT, randomized controlled trial; Tam, tamoxifen.
Study quality assessment
We evaluated the methodological quality of the selected articles using two separate assessment tools suggested by the Cochrane Collaboration Review Group. RCTs were evaluated using the Cochrane Risk of Bias assessment tool. Each RCT was assessed and rated as ‘low risk of bias’, ‘high risk of bias’ or ‘unknown risk of bias’ in the seven domains of potential bias.19,20 Cohort studies were evaluated in three categories using the Newcastle–Ottawa Scale with a range of zero to nine stars. Each cohort study was awarded a maximum of one star per item within the selection category with four items and outcome category with three items, and a maximum of two stars for the single item within the comparability category.21,22
Data extraction
Articles reporting data with the same follow-up times from the same independent study were collated (ID 5, 16, 18, 21, 30). We extracted data from each included study on method, participant, treatment, fracture outcome, and factors controlled for multivariate regression models. Fracture outcome information included definition of fractures, count of fracture events (allowing more than one fracture event per participant), count of participants who developed fractures, and relative measures consisting of odds ratios (ORs), risk ratios (RRs), incidence rate ratios (IRRs), or hazard ratios (HRs) using Cox regression models.
There were two articles (ID 12, 34) each reporting combined data from two independent studies.23,24 Extracted data from each independent study were inadequate for meta-analysis. The authors of both articles were contacted by email but we were unable to obtain additional information on these four studies.
Data synthesis
Meta-analyses were undertaken to estimate the differential fracture risks of tamoxifen and AIs, and risks between tamoxifen and AI. Each fracture risk was stratified by three to five factors of menopausal status (prespecified), prior tamoxifen treatment, study design, AI treatment duration and AI drugs, using subgroup analysis. Menopausal status was determined using age in the two cohort studies with missing menopausal status information (ID 4, 35).
The time effect on differential fracture risk between tamoxifen and AI was evaluated by ranges of follow-up durations (12–36, >36–60, >60–84, >84 months) and treatment period (on- and post-Tam/AI treatment). Meta-analyses were conducted independently for each range of follow-up duration and treatment period. The Tam/AI-treatment period was defined as the time period when women were receiving tamoxifen or AIs during the study period.
For each independent study with serial follow-up data, the article with the longest follow-up duration was included for each individual meta-analysis to avoid double counting of study participants. For studies with multiple treatment arms, the arms were either grouped as a single pair-wise comparison (ID 13, 14) or a three-group comparison with each other (ID 35, 36, 37) of tamoxifen, AIs, and control group (no tamoxifen alone, no AIs alone, and no combination of tamoxifen and AIs). Articles with double-zero events (zero-cell counts in both intervention arms) were excluded from meta-analysis.25
Statistical analysis
Meta-analyses were restricted to studies reporting counts of participants with fractures and not fracture events. For RCTs included in meta-analysis, RRs with 95% confidence intervals (CIs) were calculated. For cohort studies included in meta-analysis, published adjusted hazard ratios (aHRs) with 95% CIs were used first. RRs were calculated for cohort studies without available aHRs. aHRs were treated as adjusted RRs due to the low incidence of fracture outcomes. Overall differential fracture risk was pooled as weighted RRs using a generic inverse variance method with random effects models. The weight of each study was based on the inverse of that study’s variance. Statistical significance of the pooled RRs was evaluated using chi-square tests. Statistical heterogeneity was evaluated using Cochrane’s Q statistic and quantified as I2 measures. Sensitivity tests were conducted when combining RRs and aHRs. Funnel plots were not used to evaluate publication bias, as each analysis included less than ten studies.26 All statistical tests were performed using RevMan 5.2 analysis software (The Cochrane Collaboration, Copenhagen, Denmark).27
Results
There were 4004 articles identified, of which 2078 were duplicate articles (Figure 1). This left 1926 unique articles for title/abstract screening. Of them, 1649 were excluded, leaving 277 articles for full article review. A total of 43 articles from 21 independent studies fulfilled our selection criteria and proceeded to methodological quality assessment.
Characteristics of included studies
Sixteen RCTs, four retrospective cohort studies, and one prospective cohort study were included (Table 1). All RCTs were designed to evaluate primary outcome of efficacy and secondary outcome of safety, including fractures, using intent-to-treat analysis with the exception of one study (ID 7). All cohort studies were designed to evaluate fracture outcomes. Seven of the 16 RCTs reported serial follow-up data. Eight of the 16 RCTs involved postmenopausal women only.
Table 1.
Summary of studies.
| Study Information | Study participants (safety population) | Treatment | Published fracture outcomes - fracture | Meta-analysis | Factors | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ID | Study name Author, year (ref) | Design | Country | Data source | Median follow-up duration | Age (Years)a | Post-menopausal (%) | Prior tamoxifen b (duration) | No. | Arms | Duration | Type | Participants with fractures No. | Fracture events per 1000 PY | Risk measure (95% CI) | Risk Measure used in meta-analysis (95% CI) | Adjusted |
| Tamoxifen vs. Control / placebo (reference) | |||||||||||||||||
| 1 | Kristense, 199428 | RCT | Denmark | Self-report | 44 M | 57 (NR, 65) | 100 | N | 20 23 |
Tam Control |
24 M | Osteo-porotic | 0 / – 0 / – |
– | – | – | – |
| 2 | Love, 199429 | RCT | USA | Self-report | Mean 60.5 M |
58 ± 4 | 100 | N | 70 70 |
Tam Placebo | 24 M | Any | 6 / 7 8 / 10 |
– | – | Calculated RR 0.75 (0.27, 2.05) |
– |
| 3 | Sacco, 200330 | RCT | Italy | Self-report | 52 M | 61 ± 6 | 95 | Y (24 M) | 943 958 |
Tam Control | 36 M | Any | 8 / – 10 / – |
– | – | Calculated RR 0.81 (0.32, 2.05) |
- |
| Aromatase inhibitors (AIs) vs. Control / placebo (reference) | |||||||||||||||||
| 4 | Mincey, 200631 | Cohort | US | Data-linkage | Range 1998- 2005 |
66 ± 11 64 ± 13 |
<100c | N | 1354 11,014 |
AIs Control |
– | Any | 183 / – 1132 / – |
86 / – 63.6 / – |
aHR 1.21 (1.03, 1.43) IRR 1.35 (1.16, 1.58) |
Published aHR 1.21 (1.03, 1.43) |
Age, 1, 2, 3, 4 |
| 5 |
MA 17
Goss, 200332; DeGrendele, 200333 |
RCT | Multiple, 9 | Self-report | 2.4 Y | 62 (NR) | 100 | Y (60 M) | 2154 2145 | AIs (Let) Placebo |
60 Months | Any | 77 / – 63 / – |
– | – | – | – |
| 6 |
MA 17
Goss, 200534 |
30 M | 2572 2577 | AIs (Let) Placebo | 137 / – 119 / – |
– | – | Calculated RR 1.15 (0.94, 1.41) | – | ||||||||
| 7 |
MA 17
Goss, 200835 |
1.1 Yd (after unblinding) | 1579e 804 | AIs (Let) Placebo |
82 / – 25 / – |
– | – | – | – | ||||||||
| 8 |
Norwegian
Lonning, 200536 |
RCT | Norway | Self-report | 24 M | 59 (46–73) | 100 | N | 73 74 |
AIs (Exe) Placebo |
24 months | Any | 4 / – 5 / – |
– | – | – | – |
| 9 |
Norwegian
Geisler, 200637 |
36 M | 73 74 |
AIs (Exe) Placebo |
4 / – 5 / – |
– | – | Calculated RR 0.81 (0.23, 2.90) |
– | ||||||||
| 10 |
NSABP (B-33)
Mamounas, 2008 38 |
RCT | USA / Canada | Self-report | Till April 2004 |
60 (NR) | 100 | Y (57–66 M) | 783 779 |
AIs (Exe) Placebo |
60 months | Any | 28 / – 20 / – |
– | – | Calculated RR 1.39 (0.79, 2.45) |
– |
| Aromatase inhibitors (AIs) vs. Tamoxifen (reference) | |||||||||||||||||
| 11 | Koopal, 201539 |
Cohort | Netherland | Charts + X-ray |
Post-Tam/AI (3.1 Y) |
52 ± 7 (pre-m) 71 ± 10 (post-m) |
0 | N | 39 92 |
AIs Tam |
5.7 – 6 years |
Any | 4 / – 24 / – |
– | – | Calculated RR 0.39 (0.15, 1.06) |
– |
| 12 |
ABCSG – 8 /
ARNO 95 Jakesz, 200523 |
RCT | Germany / Austria, | Self-report | 28 M | 62 (41–80) | 100 | Y (24 M) | 1602 1597 | AIs (Ana) Tam |
36 months | Any | 34 / – 16 / – |
– | OR 2.14 (1.14, 4.17) |
– | |
| 13 |
ABCSG-12
Grant, 200940 |
RCT | Austria | Self-report | 47.8 M | 45 (26–57) | 0 | N | 903 900 | AIs (Ana) Tam |
36 months | Any | 12 / – 12 / – |
– | – | Calculated RR 1.00 (0.45, 2.21) |
– |
| 14 |
ABCSG-12
Grant, 201141 |
62 M | 903 900 |
AIs (Ana) Tam |
13 / – 12 / – |
– | – | Calculated RR 1.08 (0.50-2.35) |
– | ||||||||
| Aromatase inhibitors (AIs) vs. Tamoxifen (reference) | |||||||||||||||||
| 15 |
ARNO 95
Kaufmann, 200742 |
RCT | Germany | Self-report | 30.1 M | 61 (46–74) | 100 | Y (24 M) | 445 452 | AIs (Ana) Tam |
36 months | Any | 10 / – 10 / – |
– | – | Calculated RR 1.02 (0.43, 2.42) |
– |
| 16 |
ATAC
Buzdar, 200243; Fisher, 200244; Baum, 200245 |
RCT | Multiple, 21 | Self-report | 33.3 M | 64 ± 9 | 100 | N | 3092 3094 |
AIs (Ana) Tam |
60 months | Any | 183 / – 115 / – |
– | – | Calculated RR 1.59 (1.27,2.00) |
– |
| 17 |
ATAC
Baum, 200346 |
42 M | 3092 3093 | AIs (Ana) Tam |
219 / – 137 / – |
– | – | Calculated RR 1.60 (1.30, 1.97) |
– | ||||||||
| 18 |
ATAC
Howell, 200547; Cuzick, 200748 |
68 M | 3092 3094 | AIs (Ana) Tam |
340 / – 237 / – |
22.6 / – 15.6 / – |
OR 1.49 (1.25, 1.77) HR 1.44 (1.21, 1.68) |
Calculated RR 1.44 (1.23, 1.68) |
– | ||||||||
| 19 |
ATAC
Arimidex, 200849 |
100 M | 3092 3094 | AIs (Ana) Tam |
– | – | – | – | – | ||||||||
| On Tam/AI | 3092 3094 |
AIs (Ana) Tam |
– / 375 – / 234 |
– / 29.3 – / 19 |
IRR 1.55 (1.31-1.83) |
– | – | ||||||||||
| Post Tam/AI | 2496 2419 |
AIs (Ana) Tam |
– / 146 – / 143 |
– / 15.6 – / 15.1 |
IRR 1.03 (0.81, 1.31) |
– | – | ||||||||||
| 20 |
ATAC
Cuzick, 201014 |
120 M | 3092 3094 | AIs (Ana) Tam |
– | – | – | – | – | ||||||||
| On Tam/AI | 3092 3094 |
451 / – 351 / – |
– | – | Calculated RR 1.29 (1.13, 1.46) |
– | |||||||||||
| Post-Tam/AI | 2223 2246 |
110 / – 112 / – |
– | – | Calculated RR 0.99 (0.77, 1.28) |
– | |||||||||||
| 21 |
BIG 1-98
Thurlimann, 200550; Monnier, 200551 |
RCT | Multiple, 27 | Self-report | 25.8 M | 61 (38–90) | 100 | N | 3975 3988 |
AIs (Let) Tam |
60 M | Any | 225 / – 159 / – |
22 / – 15 / – |
OR 1.44 |
Calculated RR 1.42 (1.16, 1.73) |
– |
| 22 |
BIG 1-98
Crivellari, 200852 |
40.4 M | 2448 2447 |
AIs (Let) Tam |
196 / – 132 / – |
– | – | – | – | ||||||||
| 23 |
BIG 1-98
Coates, 200753 |
51 M | 2448 2447 |
AIs (Let) Tam |
211 / – 141 / – |
– | – | Calculated RR 1.50 (1.22, 1.84) |
– | ||||||||
| 24 |
BIG 1-98
Rabaglio, 2009,15 |
On Tam/AI 60.3 Mf | 2448 2447 |
AIs (Let) Tam |
228 / – 160 / – |
25.2 / 27.1 18.1 / 18.7 |
HR 1.38 (1.13, 1.69) aHR 1.40 (1.14, 1.71) |
Calculated RR 1.42 (1.17, 1.73) |
Age, 5, 6, 7, 8, 9, 10 | ||||||||
| 25 |
BIG 1-98
Mouridsen, 200954 |
71 M | 1540 1534 | AIs (Let) Tam |
– | – | – | – | – | ||||||||
| on Tam/AI (Y1–2) | 1540 1534 |
AIs (Let) Tam |
65 / – 50 / – |
– | – | – | – | ||||||||||
| on Tam/AI (Y 3–5)g | 1540 1534 | AIs (Let) Tam |
90 / – 67 / – |
– | – | – | – | ||||||||||
| on Tam/AI (Y 1–5) |
1540 1534 | AIs (Let) Tam |
150 / – 112 / – |
– | – | – | – | ||||||||||
| 26 |
BIG 1-98
Colleoni, 201155 |
74 M | 2448 2447 | AIs (Let) Tam |
244 / – 165 / – |
– | – | Calculated RR 1.48 (1.22, 1.79) |
– | ||||||||
| 27 |
HOBOE
Nuzzo, 201256 |
RCT | Italy | Self-report | 12 M | 50 (29–80) | 46 | N | 148 152 |
AIs (Let) Tam |
60 M | Any | 0 / 0 0 / 0 |
– | – | – | – |
| 28 |
IES
Coombes, 200457 |
RCT | Multiple, 37 | Self-report | 30.6 M | 64 ± 8 | 100 | Y ( 2.4 Y) | 2305 2329 |
AIs (Exe) Tam |
2-3 Y | Any | 72 / – 53 / – |
– | – | Calculated RR 1.37 (0.97, 1.95) |
– |
| 29 |
IES
Coleman, 200758 |
58 M | 2320 2338 |
AIs (Exe) Tam |
162 / 188 115 / 143 |
17.6 / 20.1 13.2 / 16.0 |
OR 1.45 (1.13-1.87) |
Calculated RR 1.42 (1.13, 1.79) |
– | ||||||||
| 30 |
IES
Bliss, 201259; Clomean, 20109 |
91 M | 2319 2338 | AIs (Exe) Tam |
249 / 280 190 / 214 |
– | ORh
1.36 (1.04, 1.76) |
Calculated RR 1.32 (1.10, 1.58) |
– | ||||||||
| On Tam/AI | 2319 2338 |
AIs (Exe) Tam |
113 / 117 86 / 83 |
– / 21 – / 12.3 |
ORh
1.39 (0.94, 2.06) HRh 1.39 (0.96, 2.01) |
Calculated RR 1.37 (1.04, 1.81) |
– | ||||||||||
| Post Tam/AI | 2105 2036 |
AIs (Exe) Tam |
144 / 163 117 / 128 |
– / 20.3 – / 20.6 |
ORh
1.20 (0.86, 1.69) HRh 1.20 (0.89, 1.63) |
Calculated RR 1.19 (0.94, 1.51) | – | ||||||||||
| 31 |
ITA
Boccardo. 200560 |
RCT | Italy | Self-report | 36 M | 63 (38–77) | 100 | Y (28 M) | 223 225 |
AIs (Ana) Tam |
2-3 Y | Any | 2 / – 2 / – |
– | – | Calculated RR 1.01 (0.14, 7.10) |
– |
| 32 |
ITA
Boccardo, 201361 |
128 M | 223 225 |
AIs (Ana) Tam |
Hospital events, |
4 / – 4 / – |
– | – | Calculated RR 1.01 (0.26, 3.98) |
– | |||||||
| 33 |
N-SAS BC03
Aihara, 201062 |
RCT | Japan | Self-report | 42 M | 60 ± 7 | 100 | Y (1-4 Y) | 347 349 |
AIs (Ana) Tam |
1-4 Y | Any | 5 / – 9 / – |
– | – | Calculated RR 0.56 (0.19, 1.65) |
– |
| 34 |
TEXT / SOFT (IBCSG)
Pagani, 201424 |
RCT | Multiple | Self-report | 68 M | 43 ± NR | 0 | N | 2318 2325 |
AIs (Exe) Tam |
60 M | Any | 158 / – 120 / – |
– | – | – | – |
| Multiple treatment arms | |||||||||||||||||
| 35 | Ligibel, 201263 | Cohort | US | Data linkage | 30 M | 67 ± NR | <100 c | N | Total 44,026 | Tam Control |
– | Any | – | 26.8 / – 38.1 / – |
aHR 0.93 (0.82-1.06) |
Published aHR 0.93 (0.82-1.06) |
Age, 1, 2, 3, 11, 12, 13, 14, 15 |
| Total 44,026 | AIs Control |
33.3 / – 38.1 / – |
aHR 1.13 (1.02-1.25) |
Published aHR 1.13 (1.02-1.25) |
|||||||||||||
| Total 44,026 | AIs Tam |
33.3 / – 26.8 / – |
– | ||||||||||||||
| 36 | Robinson, 201464 | Cohort | Australia | Self-report | Mean 5.7 Y |
57 (27–87) | 35 | N | 393 252 |
Tam Control |
– | Minimal trauma | 56 / – 30 / – |
– | – | Calculated RR 1.20 (0.79-1.81) |
– |
| 306 252 |
AIs Control |
46 / – 30 / – |
OR 1.31 (0.80, 2.14) |
Calculated RR 1.26 (0.82-1.94) |
– | ||||||||||||
| 306 393 |
AIs Tam |
46 / – 56 / – |
Calculated RR 1.05 (0.74, 1.51) |
– | |||||||||||||
| 37 | Xu, 201465 | Cohort | China | Self-report | 32.5 M | 56 ± 8 61 ± 9 |
76–88 | N | 52 89 |
Tam Control |
– | Any | 1 / – 1 / – |
– | aHR 2.64 (0.14, 48.73) |
Published aHR 2.64 (0.14, 48.73) |
10, 16, 17 |
| 61 ± 7 61 ± 9 |
70 89 |
AIs Control |
9 / – 1 / – |
aHR 20.08 (1.7, 234.1) |
Published aHR 20.08 (1.7, 234.1) |
||||||||||||
| 61 ± 7 56 ± 8 |
70 52 |
AIs Tam |
9 / – 1 / – |
– | Calculated RR 6.69 (0.87, 51.14) |
||||||||||||
Mean ± SD or median (range)
Tamoxifen treatment prior to the study
Menopausal status was determined based on age range
Information of fracture outcome was collected for 1.1 years after unblinding on October 2003
1579 participants crossed over from placebo group after unblinding
Fracture data obtained from participants on medications only
25.2% crossover
99% confidence intervals
Abbreviations: aHR adjusted hazard ratio, AIs Aromatase inhibitors, Ana anatrozole, CI confidence interval, Exe Exemestane, HR hazard ratio, IRR incidence rate ratio, Let letrozole, M month, No number, NR not recorded, OR odds ratio, pre-m pre-menopausal, post-m post-menopausal, ref reference, RCT randomized controlled trail, RR risk ratio, SD standardized deviation, Tam Tamoxifen, Y year,
Study abbreviations: ABCSG Austrian Breast & Colorectal Cancer Study Group, ARNO Arimidex-Nolvadex, ATAC Arimidex, Tamoxifen, Alone or in Combination, BIG Breast International Group, HOBOE Hormonal Bone Effects, IES Intergroup Exemestane Study, ITA Italian Tamoxifen Anastrozole, NSABP National Surgical Adjuvant Breast and Bowel Project, SOFT Suppression of Ovarian Function Trial, TEXT Tamoxifen and Exemestane
Adjusted factor: 1 Charlson Comorbidity Index, 2 residential regions, 3 health plan, 4 income, 5 body mass index, 6 smoking, 7 osteoporosis, 8 fracture history, 9 hormonal replacement therapy, 10 bisphosphonates, 11 index year, 12 urban/rural status, 13 drug class, 14 education, 15 % of black, 16 age of diagnosis, 17 age of menopause
Mean or median age ranged from 43 to 67 years. Treatment dose was unknown in four cohort studies (ID 4, 11, 35, 36). Doses of tamoxifen were 20 mg/day in almost all studies, with one (ID 1) of 30 mg/day and two of 20–30 mg/day (ID 12, 15). Doses of AIs were consistent across all studies as follows: anastrozole (1 mg/day), letrozole (2.5 mg/day), and exemestane (25 mg/day). Treatment duration ranged from 12 to 72 months while follow-up duration ranged from 12 to 128 months. About 17–25% crossover was reported in a few studies (ID 25, 26). Fracture outcomes were measured as any self-reported fracture (15 studies), self-reported osteoporotic/minimal-trauma fracture (ID 1, 36), self-reported hospitalized fracture (ID 32), any fracture event in medical records (ID 11), or any fracture using data linkage (ID 4, 35).
Study quality assessment
High risk of bias was observed primarily in domains of blinding of participants, blinding of outcome assessors, incomplete data, and other biases (e.g. funding) among RCTs (Appendix Figure A, online only). Unblinding of participants and their outcome assessment was observed in at least half of the RCTs that were either open RCTs or unblinded during their study periods.
Financial support from pharmaceutical companies was noted in at least 80% of the RCTs. The quality of all cohort studies was consistently high with either seven or nine out of a maximum of nine stars (Appendix Table B, online only).
Tamoxifen
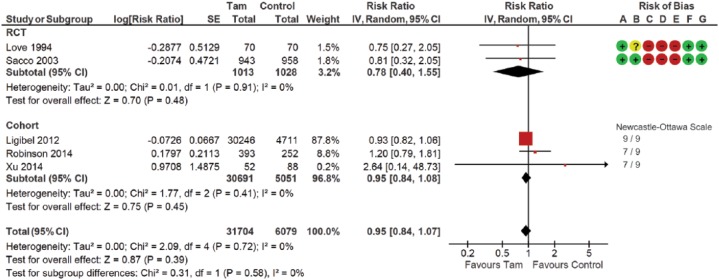
Three RCTs and three cohort studies compared fracture outcomes between women treated and not treated with tamoxifen (Table 2; Figure 2). One RCT with double-zero events was excluded from this meta-analysis. This analysis included 37,783 participants. Fracture risk did not differ between tamoxifen and no-tamoxifen groups (pooled RR 0.95; 95% CI 0.84–1.07). The statistical heterogeneity was low with an I2 measure of 0% (p = 0.72). No statistical significance was reported in subgroup analyses.
Table 2.
Meta-analysis including subgroup analysis of aromatase inhibitors, tamoxifen, and control groups on fractures.
| Treatment arms | Study (n) | Participant (n) | Pooled RR (95% CI) | p for effect | I2 (%)a | p for subgroup differences | ID of article included |
|---|---|---|---|---|---|---|---|
| Tam versus control (no-Tam)b | |||||||
| Total effect | 5 | 37,783 | 0.95 (0.84, 1.07) | 0.39 | 0 | 0 | 2, 3, 35, 36, 37 |
| Subgroup analysis | |||||||
| Menopausal status | 0.65 | ||||||
| Premenopausal | 0 | – | – | – | – | ||
| Pre-/postmenopausal | 4 | 0.95 (0.84, 1.08) | 0.42 | 0 | 3, 35, 36, 37 | ||
| Postmenopausal | 1 | 0.75 (0.27, 2.05) | 0.57 | – | 2 | ||
| Prior tamoxifen treatment | 0.74 | ||||||
| No | 4 | 0.95 (0.84, 1.07) | 0.41 | 0 | 2, 35, 36, 37 | ||
| Yes | 1 | 0.81 (0.32, 2.05) | 0.66 | – | 3 | ||
| Study design | 0.58 | ||||||
| RCT | 2 | 0.78 (0.40, 1.55) | 0.48 | 0 | 2, 3 | ||
| Cohort | 3 | 0.95 (0.84, 1.08) | 0.45 | 0 | 35, 36, 37 | ||
| AIs versus control (no-AIs)b | |||||||
| Total effect | 7 | 59,258 | 1.17 (1.07, 1.28) | <0.01 | 8 | 4, 6, 9, 10, 35, 36, 37 | |
| Subgroup analysis | |||||||
| Menopausal status | 0.88 | ||||||
| Premenopausal | 0 | – | – | – | – | ||
| Pre-/postmenopausal | 4 | 1.19 (1.01, 1.41) | 0.04 | 49 | 4, 35, 36, 37 | ||
| Postmenopausal | 3 | 1.17 (0.97, 1.41) | 0.10 | 0 | 6, 9, 10 | ||
| Prior tamoxifen treatment | 0.99 | ||||||
| No | 5 | 1.18 (1.02, 1.37) | 0.03 | 35 | 4, 9, 35, 36, 37 | ||
| Yes | 2 | 1.18 (0.97, 1.42) | 0.09 | 0 | 6, 10 | ||
| Study design | 0.88 | ||||||
| RCT | 3 | 1.17 (0.97, 1.41) | 0.10 | 0 | 6, 9, 10 | ||
| Cohort | 4 | 1.19 (1.01, 1.41) | 0.04 | 49 | 4, 35, 36, 37 | ||
| AI treatment duration | 0.57 | ||||||
| ⩽48 months | 2 | 1.18 (0.97, 1.42) | 0.09 | 0 | 6, 10 | ||
| 60 months | 1 | 0.81 (0.23, 2.90) | 0.75 | – | 9 | ||
| AI drug | 0.93 | ||||||
| Nonsteroidal (letrozole and anastrozole) | 1 | 1.15 (0.94, 1.41) | 0.16 | – | 6 | ||
| Steroidal (exemestane) | 2 | 1.27 (0.76, 2.14) | 0.36 | 0 | 9, 10 | ||
| Any AI | 4 | 1.19 (1.01, 1.41) | 0.04 | 49 | 35, 36, 37 | ||
| AIs versus Tamb | |||||||
| Total effect | 9 | 20,403 | 1.35 (1.21, 1.51) | <0.01 | 12 | 14, 15, 18, 26, 30, 32, 33, 36, 37 | |
| Subgroup analysis | |||||||
| Menopausal status | 0.75 | ||||||
| Premenopausal | 1 | 1.08 (0.5, 2.35) | 0.85 | – | 14 | ||
| Pre-/postmenopausal | 2 | 2.00 (0.36, 11.21) | 0.43 | 67 | 36, 37 | ||
| Postmenopausal | 6 | 1.39 (1.26, 1.54) | <0.01 | 0 | 15, 18, 26, 30, 32, 33 | ||
| Prior tamoxifen treatment | 0.5 | ||||||
| No | 5 | 1.38 (1.18, 1.62) | <0.01 | 27 | 14, 18, 26, 36, 37 | ||
| Yes | 4 | 1.27 (1.07, 1.51) | <0.01 | 0 | 15, 30, 32, 33 | ||
| Study design | 0.68 | ||||||
| RCT | 7 | 1.39 (1.26, 1.53) | <0.01 | 0 | 14, 15, 18, 26, 30, 32, 33 | ||
| Cohort | 2 | 2.00 (0.36, 11.21) | 0.43 | 67 | 36, 37 | ||
| AI treatment duration | 0.19 | ||||||
| ⩽48 months | 5 | 1.26 (1.07, 1.50) | <0.01 | 0 | 14, 15, 30, 32, 33 | ||
| 60 months | 2 | 1.45 (1.29, 1.64) | <0.01 | 0 | 18, 26 | ||
| AI drug | 0.76 | ||||||
| Nonsteroidal (letrozole and anastrozole) | 6 | 1.41 (1.26, 1.59) | <0.01 | 0 | 14, 15, 18, 26, 32, 33 | ||
| Steroidal (exemestane) | 1 | 1.32 (1.10, 1.58) | <0.01 | – | 30 | ||
| Any AI | 2 | 2.00 (0.36, 11.21) | 0.43 | 67 | 36, 37 | ||
Values in bold indicate statistical significance.
AI, aromatase inhibitor; CI, confidence interval; RCT, randomized controlled trial; RR, risk ratio; Tam, tamoxifen.
For heterogeneity.
Reference group.
Figure 2.
Forest plot of comparison for fracture risk between women treated with tamoxifen and not treated with tamoxifen (control) by study design subgroups.
The large diamond at the bottle of the table represents the pooled risk ratio of all studies. The width of the diamond represents with 95% CI. Results of study quality assessment were included.
Risk of bias: (A) random sequence generation (selection bias); (B) allocation concealment (selection bias); (C) blinding of participants and personnel (performance bias); (D) blinding of outcome asessment (detection bias); (E) incomplete outcome data (attrition bias); (F) selective reporting (reporting bias); (G) other bias.
 Low risk of bias
Low risk of bias  Unknown risk of bias
Unknown risk of bias  High risk of bias
High risk of bias
CI, confidence interval; d.f., degrees of freedom; IV, inverse variance; RCT, randomized controlled trial; SE, standard error; Tam, tamoxifen.
Aromatase inhibitors
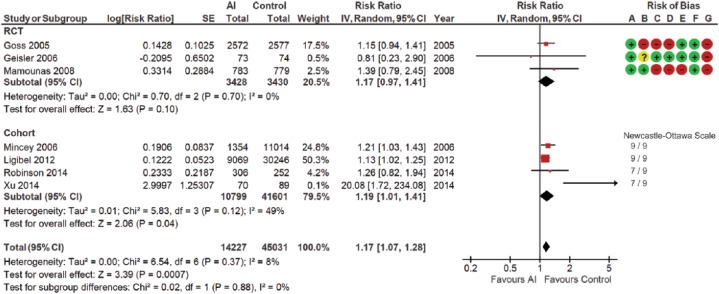
Three RCTs and four cohort studies compared fracture outcomes between women treated and not treated with AIs. All seven studies were included in this meta-analysis (Table 2; Figure 3). Data from the longest follow-up durations were selected for the two included studies (ID 6, 9). This analysis included 59,258 participants. A 17% (95% CI 1.07–1.28) higher fracture risk was observed in the AI group than the no-AI group. Statistical heterogeneity was low, with an I2 measure of 8% (p = 0.37). No statistical significance was noted in subgroup analyses. Sensitivity analyses, excluding the Xu et al. study65 (ID 37), resulted in a similar estimate of 16% RR increase with a zero I2 measure across all analyses.
Figure 3.
Forest plot of comparison for fracture risk between women treated with aromatase inhibitors and not treated with aromatase inhibitors (control) by study design subgroups.
The large diamond at the bottle of the table represents the pooled risk ratio of all studies. The width of the diamond represents with 95% CI. Results of study quality assessment were included.
Risk of bias: (A) random sequence generation (selection bias); (B) allocation concealment (selection bias); (C) blinding of participants and personnel (performance bias); (D) blinding of outcome asessment (detection bias); (E) incomplete outcome data (attrition bias); (F) selective reporting (reporting bias); (G) other bias.
 Low risk of bias
Low risk of bias  Unknown risk of bias
Unknown risk of bias  High risk of bias
High risk of bias
AI, aromatase inhibitor; CI, confidence interval; d.f., degrees of freedom; IV, inverse variance; RCT, randomized controlled trial; SE, standard error.
Comparison of aromatase inhibitors and tamoxifen
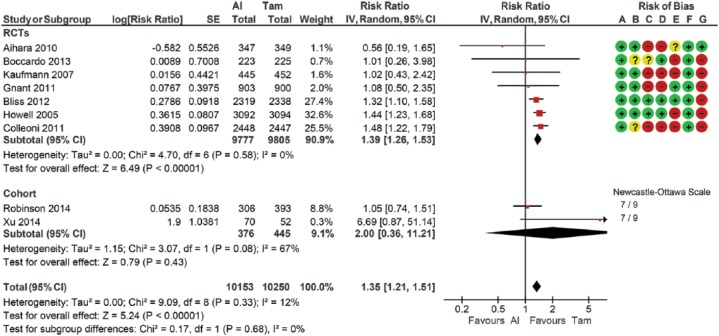
Ten RCTs and four cohort studies compared fracture outcomes between women treated with AIs and treated with tamoxifen (Table 2; Figure 4). Four studies (ID 12, 27, 34, 35) were excluded due to either missing data, double-zero events, or reporting combined data from more than one independent study. Data from the longest follow-up duration was selected for the five included studies (ID 14, 18, 26, 30, 32). This analysis included 20,403 participants. A 35% (95% CI 1.21–1.51) higher fracture risk was observed in the AI group compared with the tamoxifen group. The statistical heterogeneity was low with an I2 measure of 12% (p = 0.43). No statistical significance was observed in subgroup analysis. Sensitivity analyses excluding the Xu et al. study65 (ID 37) resulted in a similar estimate of 36% RR increase with a low I2 measure (range 0–7) across all analyses.
Figure 4.
Forest plot of comparison for fracture risk between women treated with aromatase inhibitors and treated with tamoxifen by study design subgroups.
The large diamond at the bottle of the table represents the pooled risk ratio of all studies. The width of the diamond represents with 95% CI. Results of study quality assessment were included.
Risk of bias: (A) random sequence generation (selection bias); (B) allocation concealment (selection bias); (C) blinding of participants and personnel (performance bias); (D) blinding of outcome asessment (detection bias); (E) incomplete outcome data (attrition bias); (F) selective reporting (reporting bias); (G) other bias.
 Low risk of bias
Low risk of bias  Unknown risk of bias
Unknown risk of bias  High risk of bias
High risk of bias
AI, aromatase inhibitor; CI, confidence interval; IV, inverse variance; RCT, randomized controlled trial; SE, standard error; Tam, tamoxifen.
Comparison of tamoxifen and aromatase inhibitors: time effect
Twenty articles from ten independent studies were included for these meta-analyses (Appendix Table C; Figure D, E, online only). Compared with the tamoxifen group, increased AI-associated fracture risk showed a downward trend when the range of follow-up duration increased. The pooled RRs were 1.47 (95% CI 1.28–1.68), 1.46 (1.27–1.68), 1.39 (1.23–1.57) and 1.32 (1.10–1.57) when the range of follow-up duration was 12–36, >36–60, >60–84 and >84 months, respectively. Compared with the tamoxifen group, AI-associated fracture risk increased by 33% (pooled RR 1.33; 95% CI 1.21–1.47) during the Tam/AI treatment period, but did not increase (pooled RR 0.99; 95% CI 0.72–1.37) during the post-Tam/AI treatment period. Sensitivity analysis excluding the Koopal et al. study39 (ID 11) resulted in a similar RR estimate (pooled RR 1.09; 95% CI 0.92–1.31) with a reduction of I2 measure by 56% for the post-Tam/AI treatment period.
Discussion
This study systematically summarized fracture risks associated with tamoxifen and AIs in women diagnosed with breast cancer. Results showed that fracture risk did not differ between women treated and not treated with tamoxifen. AI-associated fracture risk was 17 and 35% higher than the risks in the no-AI group and tamoxifen group, respectively. Compared with the tamoxifen group, increased AI-associated fracture risk trended down when the range of follow-up duration increased. AI-associated fracture risk increased by 30% during the Tam/AI treatment period but did not increase during the post-Tam/AI treatment period when compared with the tamoxifen group.
Our results showed that fracture risk did not differ between the tamoxifen and no-tamoxifen groups. This finding is consistent with the fact that tamoxifen has no effect on reducing vertebral or hip fractures in general populations.66,67 By contrast, tamoxifen treatment for 1 year increased the risk of trochanteric fractures (HR 2.12; 95% CI 1.12–4.01) among 1716 postmenopausal women with nonmetastatic breast cancer during the 12-year follow up in the Danish Breast Cancer Cooperative Group (DBCG) trial.68 While evidence shows that tamoxifen may preserve BMD, tamoxifen has not been approved for the treatment or prevention of osteoporosis in any population by the US Food and Drug Administration. Women who receive tamoxifen breast cancer treatment should not skip BMD testing recommended for women diagnosed with breast cancer.
Our analysis showed that AI-associated fracture risk increased by 17 and 35% when compared with the no-AI and tamoxifen groups respectively. The result in comparison with the tamoxifen group is consistent with the Early Breast Cancer Trialists Collaborative Group (EBCTCG) study (rate ratio 1.42; 95% CI 1.28–1.57),69 with methodological differences in data type (aggregate versus individual), type of study included (RCTs and cohort versus RCTs only), effect size (RR versus rate ratio), outcome measure (number of participants with fractures versus number of fracture events) and data synthesis.
When comparing the AI with tamoxifen groups, differential fracture risks were higher without a statistical difference in the prior tamoxifen treatment subgroup (pooled RR 1.38; 95% CI 1.18–1.62) than the no prior tamoxifen treatment subgroup (pooled RR 1.27; 95% CI 1.07–1.51). This might be because prior tamoxifen treatment may reduce AI-associated fracture risk. Or it may be because follow-up time was longer in the prior tamoxifen subgroup (30–128 months) than the no prior tamoxifen subgroup (32–74 months), and fracture risk decreased when follow-up duration increased.
AIs are given alone for 5 years or in sequence for 2–3 years before or after tamoxifen (sequential AI-Tam or sequential Tam-AI).70 Sequential treatments, compared with either tamoxifen or AIs alone, reduce the exposure times of both tamoxifen and AIs, which may reduce the long-term side effects associated with either tamoxifen or AIs, such as fracture risk. Differential fracture risk between sequential AI-Tam and sequential Tam-AI treatments were not included nor compared in this study due to limited available data. However, the BIG-98 trial showed sequential AI-Tam treatment reducing fracture risk by 22% (calculated RR 0.78; 95% CI 0.62–0.99) compared with the sequential Tam-AI treatment in approximately 3000 participants during the 45-month follow up.54
Longer AI treatment duration did not affect fracture risk in our study, but increased fracture risk by 47% in the Amir et al. study in 2011.71 This could be explained primary by different data synthesis methods. Our study evaluated the effect of AI treatment duration on differential fracture risk between AIs and tamoxifen. The Amir et al. study71 evaluated differential fracture risk of AI treatment duration.
A steroid AI (exemestane) with irreversible binding properties may affect bone health differently than nonsteroidal AIs (letrozole and anastrozole) with reversible binding properties.72 Our results showed no difference between steroidal and nonsteroidal AI subgroups when evaluating differential fracture risks of AIs, and between AIs and tamoxifen. This finding is consistent with findings from two other major trials; a bone substudy of the Tamoxifen Exemestane Adjuvant Multinational (TEAM) in Japan73 and MA.2774 comparing nonsteroidal anastrozole with steroidal exemestane.
While extracting and synthesizing data, we noted that fracture risk was not consistent over time. The RR decreased from 1.60 to 1.44 when the follow-up duration increased from 42 to 68 months in the Arimidex, Tamoxifen, Alone or in Combination (ATAC) trial.46,47 The IRRs decreased significantly from 1.55 during the Tam/AI treatment period to 1.03 during the post-Tam/AI treatment period in the ATAC trial.49 In response to this, we evaluated the time effect on fracture risk by conducting four individual meta-analyses with four ranges of follow-up durations and two individual meta-analyses for Tam/AI treatment and post-Tam/AI treatment periods.
Our results showed that AI-associated fracture risk, compared with the tamoxifen group, increased by 33% (95% CI 1.21–1.47) during the Tam/AI treatment period but did not increase during the post-Tam/AI treatment period. This is consistent with the EBCTCG study which shows the AI-associated fracture risk increased by 43% (95% CI 1.30–1.57) during the first 4 years from treatment allocation (treatment period), but did not increase during the 5–9 years (primarily post-treatment period, 95% CI 0.61–1.18). This is also consistent with our other result: AI-associated fracture risk, compared with the tamoxifen group, decreased when follow-up duration increased and more participants entered their post-Tam/AI treatment periods. This also can be explained by changes in BMD but not fracture risks upon discontinuation of Tam/AI treatment. The median BMD changes during the first 24 months of the post-treatment period are either stable (hip) or increased (1.5–3.8% in spine) in the AI group, but decrease (1–1.9% in spine, 2.3–2.6% in hip) in the tamoxifen group, compared with the BMD in the final treatment year.75 The fracture incidence rates (per 1000 person-years) in the AI group decreased significantly from 29.3 (95% CI 26.5–32.4) during the treatment period to 15.6 (95% CI 13.2–18.3) during the post-treatment period, while rates in the tamoxifen group were stable (treatment period: 19.0, 95% CI 16.7–21.5; post-treatment period: 15.1, 95% CI 12.8–17.8) in the ATAC trial (ID 19).49 Contrasting this, the fracture incidence rates (per 1000 person-years) in the AI group were stable during both the treatment period (21.0; 95% CI 14.5–27.5) and post-treatment period (20.3; 95% CI 13.7–26.9), while rates in the tamoxifen group increased from 12.3 (95% CI 7.3–17.3) during the treatment period to 20.6 (95% CI 13.8–27.4) during the post-treatment period in the Intergroup Exemestane Study (IES).9 The causes of differences in fracture risks between the treatment and post-treatment periods remain unclear. It may be due to the independent effect of AI on fracture risk, the independent effect of tamoxifen on fracture risk, or both effects combined.
Recommended osteoporosis management for women diagnosed with breast cancer is inconsistent across guidelines, which should include: (a) healthy lifestyles; (b) risk screening using predefined risk factors; (c) fracture risk assessment using BMD testing alone, Fracture Risk Assessment Tool (FRAX) alone or both; and (d) treatments.76 It remains challenging to identify women at high fracture risk for treatment initiation before fractures occur. BMD measurements using dual-energy X-ray absorptiometry fail to identify everyone who will develop fractures77,78 while promoting lifestyle modification79 and willingness to initiate treatment.80 Fracture risk assessment using FRAX in this population is limited by uncertain accuracy and potential underestimation. This is because FRAX was validated using population-based studies without considering the negative effects of breast cancer treatments on bones.81 More recently, the role of bisphosphonates (such as zoledronic acid or clodronate) has shifted from being a fracture prevention treatment to an adjuvant treatment for postmenopausal women who are diagnosed with nonmetastatic breast cancer and candidates for adjuvant systemic treatments82 due to their abilities to reduce bone recurrence and improve survival.
Similar estimates between RCTs and cohort subgroups were observed for fracture risk in our study and for treatment effects of other noncancer drugs in other studies.83,84 This is likely because both RCTs and cohort studies included in this study had large participant populations, sufficient follow-up time, and low risk of bias.85 Most included cohort studies reported relative measures adjusted for confounders, which further reduced selection bias. While at least 50% of included RCTs were unblinded to outcome assessment, it has a minimal effect on assessing objective outcomes including fractures.
Risk differences, differences in proportions of participants with fractures, between two treatments were not analyzed in this study due to significant variation in fracture rates (10 times), heterogeneous participant groups and baseline risk between studies. Number needed to treat, the average number of participants who need to be treated to prevent one fracture, was not estimated for the same reason.
All selected RCTs and cohort studies in this study reported relative measures as ORs, HRs or IRRs. RRs were selected to estimate effect sizes, as RRs are more appropriate measures and easier to interpret than ORs.86,87 RRs were favored over HRs and IRRs, as RRs can be recalculated for almost all included articles except one. A generic inverse variance method with random effects model was selected in this study to account for different risk measures and heterogeneity across the included studies. Although we chose random effects models in this study, statistical heterogeneity was low (<15%) in the majority of our analyses except the analysis for post-Tam/AI treatment period and some subgroup analyses. Effect sizes were almost identical using either random or fixed effects models based on our internal analysis.
Mild to moderate statistical heterogeneity (27–67%) was noted in our meta-analyses. This statistical heterogeneity decreased significantly to 0–7% after excluding the Xu et al. study65 (ID 37) or the Koopal et al. study39 (ID 11). This statistical heterogeneity associated with both these studies could be explained primarily by uncontrolled confounders due to a lack of reported adjusted relative measures. These two studies also differed from most of the included studies in this review in study setting (one center versus national/multinational) and sample size.
Limitation
This review was limited by the relative low numbers of available articles on certain subgroups, especially premenopausal groups. When comparing AIs with tamoxifen, fracture risks did not differ among subgroups of premenopausal, a mixture of pre- and postmenopausal, and postmenopausal women. Only two included studies (ID 13, 34) involved 100% premenopausal women. However, the Tamoxifen and Exemestane Trial (TEXT)/Suppression Ovarian Functions (SOFT) study (ID 34) was not included in our reported meta-analysis, as it reported combined data from two independent studies, TEXT and SOFT. An internal analysis including data from the TEXT/SOFT study was conducted. It resulted in a similar RR estimate, with a slightly narrower 95% CI of 1.24–1.48.
Conclusion
Fracture risk is significantly higher in women treated with AIs, especially during the treatment period. Tamoxifen is not associated with lower fracture risk while tamoxifen could potentially preserve bone mass. Women who receive tamoxifen or AI breast cancer treatment should be encouraged to have BMD testing as recommended for women diagnosed with breast cancer. Optimal osteoporosis management programs, especially during the treatment period, are needed for this group of women.
Supplemental Material
Supplemental material, Systematic Review - Appendix 2017_10_15 for Aromatase inhibitors are associated with a higher fracture risk than tamoxifen: a systematic review and meta-analysis by Olivia L. Tseng, John J. Spinelli, Carolyn C. Gotay, Wan Y. Ho, Mary L. McBride and Martin G. Dawes in Therapeutic Advances in Musculoskeletal Disease
Acknowledgments
The first author, Dr Olivia L Tseng was financially supported by the Clinician Scholar Program through the Department of Family Practice of the University of British Columbia and a Roman M Babicki Fellowship in Medical Research through the University of British Columbia. Special thanks go to Dr Nicole Redding, a second-year family practice resident at the University of British Columbia, for her help in study selection.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors declare that there is no conflict of interest.
ORCID iD: Olivia L Tseng  https://orcid.org/0000-0002-7987-4338
https://orcid.org/0000-0002-7987-4338
Contributor Information
Olivia L. Tseng, Department of Family Practice, University of British Columbia, 3rd floor David Strangway Building, 5950 University Boulevard Building, Vancouver, BC V6T 1Z3, Canada.
John J. Spinelli, Cancer Control Research Department, BC Cancer Research Centre, BC, Canada School of Population and Public Health, University of British Columbia, BC, Canada
Carolyn C. Gotay, Cancer Control Research Department, BC Cancer Research Centre, BC, Canada School of Population and Public Health, University of British Columbia, BC, Canada
Wan Y. Ho, Faculty of Pharmaceutical Science, University of British Columbia, Vancouver, BC, Canada
Mary L. McBride, Cancer Control Research Department, BC Cancer Research Centre, BC, Canada School of Population and Public Health, University of British Columbia, BC, Canada
Martin G. Dawes, Department of Family Practice, University of British Columbia, Vancouver, BC, Canada
References
- 1. Berry DA, Cronin KA, Plevritis SK, et al. Effect of screening and adjuvant therapy on mortality from breast cancer. N Engl J Med 2005; 353: 1784–1792. [DOI] [PubMed] [Google Scholar]
- 2. Howlader N, Altekruse SF, Li CI, et al. US Incidence of breast cancer subtypes defined by joint hormone receptor and HER2 status. JNCI J Natl Cancer Inst 2014; 106: dju055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Davidson A, Chia S, Olson R, et al. Stage, treatment and outcomes for patients with breast cancer in British Columbia in 2002: a population-based cohort study. CMAJ Open 2013; 1: E134–E141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gnant M, Thomssen C, Harbeck N. St. Gallen/Vienna 2015: a brief summary of the consensus discussion. Breast Care 2015; 10: 124–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Goldhirsch A, Wood WC, Gelber RD, et al. Meeting highlights: updated international expert consensus on the primary therapy of early breast cancer. J Clin Oncol 2003; 21: 3357–3365. [DOI] [PubMed] [Google Scholar]
- 6. Powles T, Hickish T, Kanis JA, et al. Effect of tamoxifen on bone mineral density measured by dual-energy X-ray absorptiometry in healthy premenopausal and postmenopausal women. J Clin Oncol 1996; 14: 78–84. [DOI] [PubMed] [Google Scholar]
- 7. Rodriguez-Rodriguez LM, Rodriguez-Rodriguez EM, Oramas-Rodriguez JM, et al. Changes on bone mineral density after adjuvant treatment in women with non-metastatic breast cancer. Breast Cancer Res Treat 2005; 93: 75–83. [DOI] [PubMed] [Google Scholar]
- 8. Zidan J, Keidar Z, Basher W, et al. Effects of tamoxifen on bone mineral density and metabolism in postmenopausal women with early-stage breast cancer. Med Oncol 2004; 21: 117–121. [DOI] [PubMed] [Google Scholar]
- 9. Coleman RE, Banks LM, Girgis SI, et al. Reversal of skeletal effects of endocrine treatments in the Intergroup Exemestane Study. Breast Cancer Res Treat 2010; 124: 153–161. [DOI] [PubMed] [Google Scholar]
- 10. Zaman K, Thurlimann B, Huober J, et al. Bone mineral density in breast cancer patients treated with adjuvant letrozole, tamoxifen, or sequences of letrozole and tamoxifen in the BIG 1-98 study (SAKK 21/07). Ann Oncol 2012; 23: 1474–1481. [DOI] [PubMed] [Google Scholar]
- 11. Kristensen B, Ejlertsen B, Dalgaard P, et al. Tamoxifen and bone metabolism in postmenopausal low-risk breast-cancer patients: a randomized study. J Clin Oncol 1994; 12: 992–997. [DOI] [PubMed] [Google Scholar]
- 12. Love RR, Mazess RB, Barden HS, et al. Effects of tamoxifen on bone mineral density in postmenopausal women with breast cancer. N Engl J Med 1992; 326: 852–856. [DOI] [PubMed] [Google Scholar]
- 13. Eastell R, Adams JE, Coleman RE, et al. Effect of anastrozole on bone mineral density: 5-year results from the anastrozole, tamoxifen, alone or in combination trial 18233230. J Clin Oncol 2008; 26: 1051–1058. [DOI] [PubMed] [Google Scholar]
- 14. Cuzick J, Sestak I, Baum M, et al. Effect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 10-year analysis of the ATAC trial. Lancet Oncol 2010; 11: 1135–1141. [DOI] [PubMed] [Google Scholar]
- 15. Rabaglio M, Sun Z, Price KN, et al. Bone fractures among postmenopausal patients with endocrine-responsive early breast cancer treated with 5 years of letrozole or tamoxifen in the BIG 1-98 trial. Ann Oncol 2009; 20: 1489–1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Papaioannou A, Morin S, Cheung AM, et al. 2010 clinical practice guidelines for the diagnosis and management of osteoporosis in Canada: summary. CMAJ 2010; 182: 1864–1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev 2015; 4: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Golder S, Loke YK, Bland M. Meta-analyses of adverse effects data derived from randomised controlled trials as compared to observational studies: methodological overview. PLoS Med 2011; 8: e1001026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Higgins JPT, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011; 343: d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Savović J, Weeks L, Sterne JAC, et al. Evaluation of the Cochrane Collaboration’s tool for assessing the risk of bias in randomized trials: focus groups, online survey, proposed recommendations and their implementation. Systematic Reviews 2014; 3: 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zeng X, Zhang Y, Kwong JSW, et al. The methodological quality assessment tools for preclinical and clinical studies, systematic review and meta-analysis, and clinical practice guideline: a systematic review. J Evid Based Med 2015; 8: 2–10. [DOI] [PubMed] [Google Scholar]
- 22. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analysis, http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (2011, accessed 19 February 2018).
- 23. Jakesz R, Jonat W, Gnant M, et al. Switching of postmenopausal women with endocrine-responsive early breast cancer to anastrozole after 2 years’ adjuvant tamoxifen: combined results of ABCSG trial 8 and ARNO 95 trial. Lancet 2005; 366: 455–462. [DOI] [PubMed] [Google Scholar]
- 24. Pagani O, Regan MM, Walley BA, et al. Adjuvant exemestane with ovarian suppression in premenopausal breast cancer. N Engl J Med 2014; 371: 107–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Higgins JPT, Green S. (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. http://handbook.cochrane.org (2011, accessed 19 February 2018).
- 26. Sterne JA, Sutton AJ, Ioannidis JP, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ 2011; 343: d4002. [DOI] [PubMed] [Google Scholar]
- 27. The Cochrane Collaboration. Review Manager (RevMan) [Computer Program]. Version 5.2. Copenhagen: The Nordic Cochrane Centre, 2014. [Google Scholar]
- 28. Kristensen B, Ejlertsen B, Dalgaard P, et al. Tamoxifen and bone metabolism in postmenopausal low-risk breast cancer patients: a randomized study. J Clin Oncol 1994; 12: 992–997. [DOI] [PubMed] [Google Scholar]
- 29. Love RR, Barden HS, Mazess RB, et al. Effect of tamoxifen on lumbar spine bone mineral density in postmenopausal women after 5 years. Arch Intern Med 1994; 154: 2585–2588. [PubMed] [Google Scholar]
- 30. Sacco M, Valentini M, Belfiglio M, et al. Randomized trial of 2 versus 5 years of adjuvant tamoxifen for women aged 50 years or older with early breast cancer: Italian Interdisciplinary Group Cancer Evaluation Study of Adjuvant Treatment in Breast Cancer 01. J Clin Oncol 2003; 21: 2276–2281. [DOI] [PubMed] [Google Scholar]
- 31. Mincey BA, Duh MS, Thomas SK, et al. Risk of cancer treatment-associated bone loss and fractures among women with breast cancer receiving aromatase inhibitors. Clin Breast Cancer 2006; 7: 127–132. [DOI] [PubMed] [Google Scholar]
- 32. Goss PE, Ingle JN, Martino S, et al. A randomized trial of letrozole in postmenopausal women after five years of tamoxifen therapy for early-stage breast cancer. N Engl J Med 2003; 349: 1793–1802. [DOI] [PubMed] [Google Scholar]
- 33. DeGrendele H, O’Shaughnessy JA. Benefit of letrozole in postmenopausal women after five years of tamoxifen therapy for early-stage breast cancer. Clin Breast Cancer 2003; 4: 311–312. [DOI] [PubMed] [Google Scholar]
- 34. Goss PE, Ingle JN, Martino S, et al. Randomized trial of letrozole following tamoxifen as extended adjuvant therapy in receptor-positive breast cancer: updated findings from NCIC CTG MA.17. J Natl Cancer Inst 2005; 97: 1262–1271. [DOI] [PubMed] [Google Scholar]
- 35. Goss PE, Ingle JN, Pater JL, et al. Late extended adjuvant treatment with letrozole improves outcome in women with early-stage breast cancer who complete 5 years of tamoxifen. J Clin Oncol 2008; 26: 1948–1955. [DOI] [PubMed] [Google Scholar]
- 36. Lonning PE, Geisler J, Krag LE, et al. Effects of exemestane administered for 2 years versus placebo on bone mineral density, bone biomarkers, and plasma lipids in patients with surgically resected early breast cancer. J Clin Oncol 2005; 23: 5126–5137. [DOI] [PubMed] [Google Scholar]
- 37. Geisler J, Lonning PE, Krag LE, et al. Changes in bone and lipid metabolism in postmenopausal women with early breast cancer after terminating 2-year treatment with exemestane: a randomised, placebo-controlled study. Eur J Cancer 2006; 42: 2968–2975. [DOI] [PubMed] [Google Scholar]
- 38. Mamounas EP, Jeong JH, Wickerham DL, et al. Benefit from exemestane as extended adjuvant therapy after 5 years of adjuvant tamoxifen: intention-to-treat analysis of the National Surgical Adjuvant Breast and Bowel Project B-33 trial. J Clin Oncol 2008; 26: 1965–1971. [DOI] [PubMed] [Google Scholar]
- 39. Koopal C, Janssen-Heijnen ML, Van de, Wouw AJ, et al. Fracture incidence in pre- and postmenopausal women after completion of adjuvant hormonal therapy for breast cancer. Breast 2015; 24: 153–158. [DOI] [PubMed] [Google Scholar]
- 40. Gnant M, Mlineritsch B, Schippinger W, et al. Endocrine therapy plus zoledronic acid in premenopausal breast cancer. N Engl J Med 2009; 360: 679–691. [DOI] [PubMed] [Google Scholar]
- 41. Gnant M, Mlineritsch B, Stoeger H, et al. Adjuvant endocrine therapy plus zoledronic acid in premenopausal women with early-stage breast cancer: 62-month follow-up from the ABCSG-12 randomised trial. Lancet Oncol 2011; 12: 631–641. [DOI] [PubMed] [Google Scholar]
- 42. Kaufmann M, Jonat W, Hilfrich J, et al. Improved overall survival in postmenopausal women with early breast cancer after anastrozole initiated after treatment with tamoxifen compared with continued tamoxifen: the ARNO 95 study. J Clin Oncol 2007; 25: 2664–2670. [DOI] [PubMed] [Google Scholar]
- 43. Buzdar AU. ‘Arimidex’ (anastrozole) versus tamoxifen as adjuvant therapy in postmenopausal women with early breast cancer-efficacy overview. J Steroid Biochem Mol Biol 2003; 86: 399–403. [DOI] [PubMed] [Google Scholar]
- 44. Fisher MD, O’Shaughnessy J, Sparano JA. Anastrozole may be superior to tamoxifen as adjuvant treatment for postmenopausal patients with breast cancer. Clin Breast Cancer 2002; 2: 269–271. [Google Scholar]
- 45. Baum M, Budzar AU, Cuzick J, et al. Anastrozole alone or in combination with tamoxifen versus tamoxifen alone for adjuvant treatment of postmenopausal women with early breast cancer: first results of the ATAC randomised trial. Lancet 2002; 359: 2131–2139. [DOI] [PubMed] [Google Scholar]
- 46. Baum M, Buzdar A, Cuzick J, et al. Anastrozole alone or in combination with tamoxifen versus tamoxifen alone for adjuvant treatment of postmenopausal women with early-stage breast cancer: results of the ATAC (Arimidex, Tamoxifen Alone or in Combination) trial efficacy and safety update analyses. Cancer 2003; 98: 1802–1810. [DOI] [PubMed] [Google Scholar]
- 47. Howell A, Cuzick J, Baum M, et al. Results of the ATAC (Arimidex, Tamoxifen, Alone or in Combination) trial after completion of 5 years’ adjuvant treatment for breast cancer. Lancet 2005; 365: 60–62. [DOI] [PubMed] [Google Scholar]
- 48. Cuzick J. The ATAC trial: the vanguard trial for use of aromatase inhibitors in early breast cancer. Expert Rev Anticancer Ther 2007; 7: 1089–1094. [DOI] [PubMed] [Google Scholar]
- 49. Forbes JF, Cuzick J, Buzdar A, et al. ; Arimidex, Tamoxifen, Alone or in Combination (ATAC) Trialists’ Group. Effect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 100-month analysis of the ATAC trial. Lancet Oncol 2008; 9: 45–53. [DOI] [PubMed] [Google Scholar]
- 50. Thurlimann B, Keshaviah A, Coates AS, et al. A comparison of letrozole and tamoxifen in postmenopausal women with early breast cancer. N Engl J Med 2005; 353: 2747–2757. [DOI] [PubMed] [Google Scholar]
- 51. Monnier A. The evolving role of letrozole in the adjuvant setting: first results from the large, phase III, randomized trial BIG 1-98. Breast 2006; 15(Suppl. 1): S21–S29. [DOI] [PubMed] [Google Scholar]
- 52. Crivellari D, Sun Z, Coates AS, et al. Letrozole compared with tamoxifen for elderly patients with endocrine-responsive early breast cancer: the BIG 1-98 trial. J Clin Oncol 2008; 26: 1972–1979. [DOI] [PubMed] [Google Scholar]
- 53. Coates AS, Keshaviah A, Thurlimann B, et al. Five years of letrozole compared with tamoxifen as initial adjuvant therapy for postmenopausal women with endocrine-responsive early breast cancer: update of study BIG 1-98. J Clin Oncol 2007; 25: 486–492. [DOI] [PubMed] [Google Scholar]
- 54. Mouridsen H, Giobbie-Hurder A, Goldhirsch A, et al. Letrozole therapy alone or in sequence with tamoxifen in women with breast cancer. N Engl J Med 2009; 361: 766–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Colleoni M, Giobbie-Hurder A, Regan MM, et al. Analyses adjusting for selective crossover show improved overall survival with adjuvant letrozole compared with tamoxifen in the BIG 1-98 study. J Clin Oncol 2011; 29: 1117–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Nuzzo F, Gallo C, Lastoria S, et al. Bone effect of adjuvant tamoxifen, letrozole or letrozole plus zoledronic acid in early-stage breast cancer: the randomized phase 3 HOBOE study. Ann Oncol 2012; 23: 2027–2033. [DOI] [PubMed] [Google Scholar]
- 57. Coombes RC, Hall E, Gibson LJ, et al. A randomized trial of exemestane after two to three years of tamoxifen therapy in postmenopausal women with primary breast cancer. Women’s Oncol Rev 2004; 4: 135–136. [DOI] [PubMed] [Google Scholar]
- 58. Coleman RE, Banks LM, Girgis SI, et al. Skeletal effects of exemestane on bone-mineral density, bone biomarkers, and fracture incidence in postmenopausal women with early breast cancer participating in the Intergroup Exemestane Study (IES): a randomised controlled study. Lancet Oncol 2007; 8: 119–127. [DOI] [PubMed] [Google Scholar]
- 59. Bliss JM, Kilburn LS, Coleman RE, et al. Disease-related outcomes with long-term follow-up: an updated analysis of the intergroup exemestane study. J Clin Oncol 2012; 30: 709–717. [DOI] [PubMed] [Google Scholar]
- 60. Boccardo F, Rubagotti A, Puntoni M, et al. Switching to anastrozole versus continued tamoxifen treatment of early breast cancer: preliminary results of the Italian Tamoxifen Anastrozole Trial. J Clin Oncol 2005; 23: 5138–5147. [DOI] [PubMed] [Google Scholar]
- 61. Boccardo F, Guglielmini P, Bordonaro R, et al. Switching to anastrozole versus continued tamoxifen treatment of early breast cancer: long term results of the Italian Tamoxifen Anastrozole trial. Eur J Cancer 2013; 49: 1546–1554. [DOI] [PubMed] [Google Scholar]
- 62. Aihara T, Takatsuka Y, Ohsumi S, et al. Phase III randomized adjuvant study of tamoxifen alone versus sequential tamoxifen and anastrozole in Japanese postmenopausal women with hormone-responsive breast cancer: N-SAS BC03 study. Breast Cancer Res Treat 2010; 121: 379–387. [DOI] [PubMed] [Google Scholar]
- 63. Ligibel JA, James O’Malley A, Fisher M, et al. Risk of myocardial infarction, stroke, and fracture in a cohort of community-based breast cancer patients. Breast Cancer Res Treat 2012; 131: 589–597. [DOI] [PubMed] [Google Scholar]
- 64. Robinson PJ, Bell RJ, Zecena Morales CS, et al. Minimal-trauma fracture in women with breast cancer surviving for at least 5 years from diagnosis. Osteoporos Int 2014; 26: 795–800. [DOI] [PubMed] [Google Scholar]
- 65. Xu L, Wang J, Xue DD, et al. Aromatase inhibitors associated musculoskeletal disorders and bone fractures in postmenopausal breast cancer patients: a result from Chinese population. Med Oncol 2014; 31: 128. [DOI] [PubMed] [Google Scholar]
- 66. MacLean C, Newberry S, Maglione M, et al. Systematic review: comparative effectiveness of treatments to prevent fractures in men and women with low bone density or osteoporosis. Ann Intern Med 2008; 148: 197–213. [DOI] [PubMed] [Google Scholar]
- 67. Qaseem A, Snow V, Shekelle P, et al. Pharmacologic treatment of low bone density or osteoporosis to prevent fractures: a clinical practice guideline from the American College of Physicians. Ann Intern Med 2008; 149: 404–415. [PubMed] [Google Scholar]
- 68. Kristensen B, Ejlertsen B, Mouridsen HT, et al. Femoral fractures in postmenopausal breast cancer patients treated with adjuvant tamoxifen. Breast Cancer Res Treat 1996; 39: 321–326. [DOI] [PubMed] [Google Scholar]
- 69. Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Aromatase inhibitors versus tamoxifen in early breast cancer: patient-level meta-analysis of the randomised trials. Lancet 2015; 386: 1341–1352. [DOI] [PubMed] [Google Scholar]
- 70. Harbeck N, Thomssen C, Gnant M. St. Gallen 2013: brief preliminary summary of the consensus discussion. Breast Care 2013; 8: 102–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Amir E, Seruga B, Niraula S, et al. Toxicity of adjuvant endocrine therapy in postmenopausal breast cancer patients: a systematic review and meta-analysis. JNCI J Natl Cancer Inst 2011; 103: 1299–1309. [DOI] [PubMed] [Google Scholar]
- 72. McCloskey EV. Aromatase inhibitors and bone health. IBMS BoneKEy 2006; 3: 5–13. [Google Scholar]
- 73. Aihara T, Suemasu K, Takei H, et al. Effects of exemestane, anastrozole and tamoxifen on bone mineral density and bone turnover markers in postmenopausal early breast cancer patients: results of N-SAS BC 04, the TEAM Japan substudy. Oncology 2010; 79: 376–381. [DOI] [PubMed] [Google Scholar]
- 74. Goss PE, Ingle JN, Pritchard KI, et al. Exemestane versus anastrozole in postmenopausal women with early breast cancer: NCIC CTG MA.27—a randomized controlled phase III trial. J Clin Oncol 2013; 31: 1398–1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Eastell R, Adams J, Clack G, et al. Long-term effects of anastrozole on bone mineral density: 7-year results from the ATAC trial. Ann Oncol 2011; 22: 857–862. [DOI] [PubMed] [Google Scholar]
- 76. Lustberg MB, Reinbolt RE, Shapiro CL. Bone health in adult cancer survivorship. J Clin Oncol 2012; 30: 3665–3674. [DOI] [PubMed] [Google Scholar]
- 77. Schuit SC, Van der Klift M, Weel AE, et al. Fracture incidence and association with bone mineral density in elderly men and women: the Rotterdam Study. Bone 2004; 34: 195–202. [DOI] [PubMed] [Google Scholar]
- 78. Wainwright SA, Marshall LM, Ensrud KE, et al. Hip fracture in women without osteoporosis. J Clin Endocr Metab 2005; 90: 2787–2793. [DOI] [PubMed] [Google Scholar]
- 79. Marci CD, Anderson WB, Viechnicki MB, et al. Bone mineral densitometry substantially influences health-related behaviors of postmenopausal women. Calcif Tissue Int 2000; 66: 113–118. [DOI] [PubMed] [Google Scholar]
- 80. Barr RJ, Stewart A, Torgerson DJ, et al. Population screening for osteoporosis risk: a randomised control trial of medication use and fracture risk. Osteoporos Int 2010; 21: 561–568. [DOI] [PubMed] [Google Scholar]
- 81. Body JJ. Increased fracture rate in women with breast cancer: a review of the hidden risk. BMC Cancer 2011; 11: 384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Dhesy-Thind S, Fletcher GG, Blanchette PS, et al. Use of adjuvant bisphosphonates and other bone-modifying agents in breast cancer: a cancer care Ontario and American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol 2017; 35: 2062–2081. [DOI] [PubMed] [Google Scholar]
- 83. Ioannidis JA, Haidich A, Pappa M, et al. Comparison of evidence of treatment effects in randomized and nonrandomized studies. JAMA 2001; 286: 821–830. [DOI] [PubMed] [Google Scholar]
- 84. Benson K, Hartz AJ. A comparison of observational studies and randomized, controlled trials. N Engl J Med 2000; 342: 1878–1886. [DOI] [PubMed] [Google Scholar]
- 85. Vandenbroucke JP. When are observational studies as credible as randomised trials? Lancet 2004; 363: 1728–1731. [DOI] [PubMed] [Google Scholar]
- 86. Balasubramanian H, Ananthan A, Rao S, et al. Odds ratio vs risk ratio in randomized controlled trials. Postgrad Med 2015; 127: 359–367. [DOI] [PubMed] [Google Scholar]
- 87. Knol MJ, Duijnhoven RG, Grobbee DE, et al. Potential misinterpretation of treatment effects due to use of odds ratios and logistic regression in randomized controlled trials. PLoS One 2011; 6: e21248. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Systematic Review - Appendix 2017_10_15 for Aromatase inhibitors are associated with a higher fracture risk than tamoxifen: a systematic review and meta-analysis by Olivia L. Tseng, John J. Spinelli, Carolyn C. Gotay, Wan Y. Ho, Mary L. McBride and Martin G. Dawes in Therapeutic Advances in Musculoskeletal Disease