Abstract
Objective
To evaluate the diagnostic accuracy of T2*-mapping for detecting acetabular cartilage damage in patients with symptomatic femoroacetabular impingement (FAI).
Design
A total of 29 patients (17 females, 12 males, mean age 35.6 ± 12.8 years, mean body mass index 25.1 ± 4.1 kg/m2, 16 right hips) with symptomatic FAI underwent T2* MRI and subsequent hip arthroscopy. T2* values were obtained by region of interest analysis in seven radially reformatted planes around the femoral neck (anterior, anterior-superior, superior-anterior, superior, superior-posterior, posterior-superior, posterior). Intraoperatively, a modified Outerbridge classification was used for assessment of the cartilage status in each region. T2* values and intraoperative data were compared, and sensitivity, specificity, negative predictive values (NPV) and positive predictive values (PPV) as well as the correlation between T2*-mapping and intraoperative findings, were determined. The mean time interval between MRI and arthroscopy was 65.7 ± 48.0 days.
Results
Significantly higher T2* values were noted in arthroscopically normal evaluated cartilage than in regions with cartilage degeneration (mean T2* 25.6 ± 4.7 ms vs. 19.9 ± 4.5 ms; P < 0.001). With the intraoperative findings as a reference, sensitivity, specificity, NPV and PPV were 83.5%, 67.7%, 78.4% and 74.4%, respectively. The correlation between T2*-mapping and intraoperative cartilage status was moderate (ρ = −0.557; P < 0.001).
Conclusions
T2*-mapping enabled analysis of acetabular cartilage with appropriate correlation with intraoperative findings and promising results for sensitivity, specificity, PPV, and NPV in this cohort. Our results emphasize the value of T2*-mapping for the diagnosis of hip joint cartilage pathologies in symptomatic FAI.
Keywords: hip, MRI, FAI, T2*-mapping, arthroscopy
Introduction
In symptomatic femoroacetabular impingement (FAI), the ongoing pathological abutment between the acetabular rim and the femoral head-neck junction leads to early cartilage degeneration, damage to the acetabular labrum and synovitis. Although it remains unclear which specific FAI-related morphology and corresponding damage to the hip really causes symptoms, if left untreated in symptomatic cases, early osteoarthritis (OA) is known to occur.1,2 As early intervention including different joint preserving approaches can potentially alter the course of disease progression in a prearthritic condition, a reliable and reproducible cartilage assessment at various stages of damage is critical.3,4
Magnetic resonance imaging (MRI) remains the modality of choice for morphological hip joint cartilage assessment. However, the ability to detect cartilage lesions using standard MRI is limited particularly in early stages of cartilage degeneration. For an evaluation of subtle changes that occur early in the course of degeneration, different biochemically sensitive MRI techniques have been proven as robust biomarkers that can pick up changes in cartilage composition before macroscopic alterations are notable. While some of these techniques are susceptible to cartilage glycosaminoglycan (GAG) content (delayed gadolinium-enhanced magnetic resonance imaging of cartilage, dGEMRIC5; T1rho6), others are sensitive to the cartilage water content and interactions between water molecules and the collagen fiber network (T2/T2*-mapping7,8). T2*-mapping is advantageous in many ways. For example, it does not require the application of contrast media, and it allows for a 3-dimensional (3D), high-resolution, isotropic cartilage evaluation with short acquisition time.9
In this study, we sought to determine the accuracy of isotropic 3D T2*-mapping for hip joint cartilage analysis in patients with FAI at 3 T. Using intraoperative data as a reference, we calculated the sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) in different regions of the hip joint and determined the correlation between MRI and intraoperative findings.
Method
Study Population
The procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation and with the Helsinki Declaration of 1975, as revised in 2000.
We included 29 patients (17 females, 12 males, mean age 35.6 ± 12.8 years, mean body mass index 25.1 ± 4.1 kg/m2, 16 right hips) who underwent MRI, including T2*-mapping, prior to hip arthroscopy in the clinical setting of symptomatic FAI at our institution between 2010 and 2016. To minimize the risk of bias from possible progressive cartilage degeneration that could occur between MRI and surgery, we included only those patients in whom surgery was performed within 6 months of the MRI (mean time interval between MRI and arthroscopy 65.7 ± 48.0 days, range 5-182 days). All patients were clinically examined by 1 of 2 orthopedic surgeons (B.B., C.Z.). Both are fellowship-trained in Hip Preservation specialty, are currently practicing at a national referral center for hip conditions, and have more than 10 years of experience in clinical management of FAI patients. Regarding surgical experience in hip arthroscopy, both surgeons have, respectively, 9 and 8 years of experience, while coauthors of this study (R.K., H.H.) are national and international hip specialists with more than 2 decades of experience in treating hip conditions individually. The flow chart of inclusion and exclusion criteria is provided in Figure 1 . Before participation, each patient signed written informed consent and ethical approval was obtained from the local ethics committee.
Figure 1.
Flowchart for study inclusion and exclusion.
Magnetic Resonance Imaging
MRI was conducted on a 3-T scanner (Magnetom Trio, Siemens Medical Solutions, Erlangen, Germany) in the supine position with a 4-channel phased-array, flex surface coil around the hip being investigated. To increase comfort on the examination table and to reduce motion artifacts, the leg was stabilized with blankets and pillows.
Along with localizer images and standard pulse sequences, the MRI protocol included (1) a high-resolution 3D double-echo steady-state (DESS) sequence for morphological cartilage assessment (TR 14.75 ms, TE 5.03 ms, flip angle 25°, NEX 1, FOV 192 mm2, slice thickness 0.6 mm, in-plane resolution 0.6 × 0.6 mm, slice gap 0.2 mm, bandwidth 260 Hz/pixel, acquisition time 13.17 minutes) and (2) A gradient-echo high-resolution 3D multi-echo data image combination (MEDIC) sequence with 6 consecutive echoes for the T2* measurements (TR 38 ms, TE 4.62 ms, 9.41 ms, 15.28 ms, 21.15 ms, 27.02 ms, 32.89 ms, flip angle 25°, NEX 1, FOV 192 mm2, slice thickness 0.6 mm, in-plane resolution 0.6 × 0.6 mm, slice gap 0.2 mm, bandwidth 260 Hz/pixel, acquisition time 13.29 minutes).
Image Postprocessing and T2* Relaxometry
Postprocessing and analyses of images were conducted by one reader (C.S.) with several years of experience in biochemical cartilage imaging who was blinded to patients’ clinical information. Following image acquisition, all MR data sets of the DESS and MEDIC, which included the inline T2* maps, were transferred to a Leonardo workstation (Siemens Medical Solutions, Erlangen, Germany). Before analysis, multiplanar reconstruction software was used to create 7 radial reformats with a slice thickness of 2 mm around the femoral neck and with the femoral head as the center of rotation (anterior, anterior-superior, superior-anterior, superior, superior-posterior, posterior-superior, posterior). In each reformat, acetabular cartilage was divided into a peripheral and central zone, separated by a line that bisects the acetabular cartilage between the acetabular fossa and the cartilage-labral junction. T2* relaxometry was conducted by region of interest (ROI) analysis in central and peripheral acetabular cartilage in each reformat. Corresponding DESS reformats served as a guide to ensure ROI placement within cartilage boundaries ( Fig. 2 ).
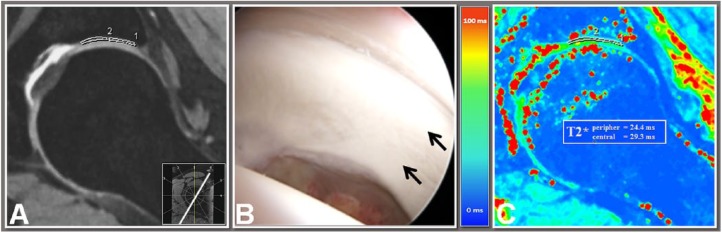
Figure 2.
A 10 o’clock double-echo steady-state (DESS) reformat (A), an intraoperative view (B) of the corresponding region (arrows), and a 10 o’clock T2* map reformat (C) of a 15-year-old male patient with anterosuperior femoroacetabular impingement (FAI). T2* values were obtained using a region of interest (ROI) analysis in central and peripheral acetabular cartilage. The corresponding DESS reformat served as a guide to ensure ROI placement within cartilage boundaries. Note no cartilage degeneration was observed in this region, either with DESS MRI, intraoperative correlation or T2* relaxometry.
Analysis of Arthroscopic Data
In all 29 patients, hip arthroscopy was carried out using a similar technique by 1 of 2 experienced orthopedic arthroscopy surgeons (B.B., C.Z.). All patients were placed in supine position on the extension table, an anterior and anterolateral portal were established and, in cases that needed labral refixation, a distal anterolateral portal was established. Additional portals were established, if needed, on a case to case basis. Retrospectively, all intraoperative data (intraoperative images, videos and operative notes) were reviewed by 1 reader (B.B.) for cartilage assessment. For localization purposes, a clock-face scheme was implemented, which is consistent with MR interpretation, with 3 o’clock being anterior, 12 o’clock superior, and 9 o’clock posterior ( Fig. 3 ). For each region central and peripheral acetabular cartilage was graded according to a modified Outerbridge classification system as follows: grade 0 = normal cartilage; grade 1 = cartilage softening; grade 2 = cartilage abrasion; grade 3 = cartilage loss; grade 4 = no evaluation of cartilage possible.10 In cases of multiple cartilage defects within one region the highest grade of defect was chosen.
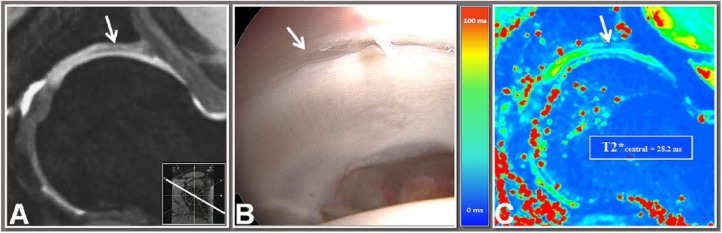
Figure 3.
Double-echo steady-state (DESS) reformat (A), arthroscopic view (B) and T2* map reformat (C) of the same 15-year-old male patient with femoroacetabular impingement (FAI) revealing acetabular rim cartilage delamination (arrow; hypointense zone in the DESS image; low T2* values) in the 1 o’clock region. The peripheral region was excluded from this analysis because T2* assessment cannot be performed in areas with severe cartilage loss.
Statistical Analysis
Statistical analysis in this study was conducted with the support of a biostatistician who used SPSS software, version 22 (IBM Corp, Armonk, NY, USA). All P values <0.05 were considered statistically significant. Exploring the intra- and interreader reliability regarding the retrospective cartilage grading, which was based on intraoperative images, videos, and operative notes, all cases were reviewed again by the first (time interval between both measurements >12 weeks) and a second observer (C.Z.). Then, an intraclass correlation coefficient (ICC) analysis was performed with pairwise comparison and absolute agreement definition. For analysis of correlation between T2*-mapping and intraoperative data Spearman’s correlation coefficient (ρ) along with the 95% confidence interval (CI) was calculated. In each investigated region of the hip, cartilage was classified as disease positive or disease negative according to intraoperative data. For MRI evaluation, in each investigated ROI T2* values were classified as either “normal” or “decreased.” The ROIs were classified as “decreased” if T2* values were noted below the 95% CI of mean T2* values that were previously published in a population of asymptomatic controls at 3 T.11 In each case, the mean values of the corresponding hip region were used as baseline values to compensate for regional differences in the T2* relaxation times possibly due to the magic angle effect,12 which promotes an increase in T2/T2* relaxation when collagen fibers are orientated 54.7° to the main magnetic field, and potential regional differences in collagen density, fiber orientation, and water content. Sensitivity, specificity, PPV, and NPV were obtained via cross-table calculations. For evaluation of variances across the hip joint, correlation, sensitivity, specificity, PPV, and NPV were also evaluated in different subregions (1, anterior, anterosuperior; 2, superoanterior to superoposterior; 3, posterosuperior, posterior).
Results
We investigated 406 ROI (29 patients, 7 regions, central and peripheral acetabular cartilage). A total of 238 ROIs that had complete intraoperative grading and no apparent cartilage loss on MRI underwent statistical analysis. The intra- and interreader variabilities regarding retrospective cartilage grading were low (ICC = 0.89; P < 0.001 for intrareader and ICC = 0.92; P < 0.001 for interreader agreement).
With superior cartilage degeneration, according to the modified Outerbridge classification, we observed lower T2* values (90 ROIs graded “normal cartilage”: mean T2* 25.6 ± 4.7 ms; 11 ROIs graded “cartilage softening”: mean T2* 24.2 ± 3.3 ms; 132 ROIs graded “cartilage abrasion”: mean T2* 19.9 ± 4.5 ms). The correlation between intraoperative cartilage status and T2* values was moderate (ρ = −0.557; P < 0.001). However, subregion analysis revealed good correlation in subregion 1 (anterior, anterosuperior; ρ = −0.750; P < 0.001) compared with subregion 2 (superoanterior to superoposterior; ρ = −0.486; P < 0.001) and subregion 3 (posterosuperior, posterior; ρ = −0.331; P = 0.004; Table 1 ). The correlation between T2*-mapping and intraoperative grading in central (ρ = −0.573; P < 0.001) and peripheral (ρ = −0.513; P < 0.001) acetabular cartilage was similar.
Table 1.
Spearman’s ρ Correlation Analysis Assessing Agreement Between Cartilage Grading with Either T2* Relaxometry or Intraoperative Assessment in Various Regions of the Hip Joint.
| Region | Correlation | 95% Confidence Interval | P |
|---|---|---|---|
| Anterior Anterosuperior |
−0.750 | −0.841 to −0.616 | <0.001 |
| Superoanterior Superior Superoposterior |
−0.486 | −0.622 to −0.322 | <0.001 |
| Posterosuperior Posterior |
−0.331 | −0.522 to −0.110 | 0.004 |
| Total | −0.557 | −0.639 to −0.463 | <0.001 |
Sensitivity, specificity, PPV, and NPV were 83.5%, 67.7%, 78.4%, and 74.4%, respectively, in total. Subregion analysis revealed values of 85.3%, 89.7%, 90.6%, and 83.9% for subregion 1 (anterior, anterosuperior), 85.5%, 57.6%, 80.8%, and 65.5% for subregion 2 (superoanterior to superoposterior), and 77.8%, 59.5%, 65,1%, and 73.3% for subregion 3 (posterosuperior, posterior; Table 2 ), respectively. For central acetabular cartilage the respective equivalent values were 85.1%, 68.3%, 80.8%, and 74.5%. In peripheral acetabular cartilage, the respective values were 80.0%, 66.7%, 73.5%, and 74.3%.
Table 2.
Sensitivity, Specificity, Positive Predictive Value, and Negative Predictive Value for Cartilage Status Assessment by Means of T2*-Mapping Using Intraoperative Data as a Reference.
| Region | Arthroscopy |
||||
|---|---|---|---|---|---|
| Healthy | Damaged | ||||
| Anterior Anterosuperior |
T2* | Healthy | n | 26 | 3 |
| Negative predictive value | 83.9% | ||||
| Specificity | 89.7% | ||||
| Damaged | n | 5 | 29 | ||
| Positive predictive value | 90.6% | ||||
| Sensitivity | 85.3% | ||||
| Superoanterior Superior Superoposterior |
T2* | Healthy | n | 19 | 14 |
| Negative predictive value | 65.5% | ||||
| Specificity | 57.6% | ||||
| Damaged | n | 10 | 59 | ||
| Positive predictive value | 80.8% | ||||
| Sensitivity | 85.5% | ||||
| Posterosuperior Posterior |
T2* | Healthy | n | 22 | 15 |
| Negative predictive value | 73.3% | ||||
| Specificity | 59.9% | ||||
| Damaged | n | 8 | 28 | ||
| Positive predictive value | 65.1% | ||||
| Sensitivity | 77.8% | ||||
| Total | T2* | Healthy | n | 67 | 32 |
| Negative predictive value | 74.4% | ||||
| Specificity | 67.7% | ||||
| Damaged | n | 23 | 116 | ||
| Positive predictive value | 78.4% | ||||
| Sensitivity | 83.5% | ||||
Discussion
We have continued to expand our understanding of the changing mechanics of the hip joint and nonphysiological abutment motion that occurs in FAI and causes progressive damage of cartilage and early OA in untreated cases of symptomatic FAI. Surgical management via both open and arthroscopic approaches has advanced rapidly to address the pathomorphology, repair the labral tear and damage, treat cartilage defects, and try and restore morphology for an attempted restoration of physiology and function of the hip, thereby altering the course of rapid degeneration to early OA. However, clinical outcomes following these procedures may be unpredictable and sometimes unsatisfactory if higher grades of joint degeneration are already present at the time of surgery.13-15 Biochemical cartilage evaluation to accurately and reliably assess the cartilage status before cartilage alterations occur will likely provide critical information for decision making related to joint preservation or joint replacement and also for treatment monitoring and postintervention follow-up studies.
In this retrospective study, we sought to determine the accuracy of isotropic high-resolution 3D T2* mapping for hip joint cartilage analysis in patients with symptomatic FAI. Compared with arthroscopic data, T2*-mapping revealed promising results for sensitivity, specificity, PPV, and NPV in this cohort. The ICC measures indicate a high similarity between the surgeon grading of intraoperative cartilage damage in which all intraoperative data, including intraoperative images, videos and operative notes were reviewed. The correlation between MRI and intraoperative cartilage status was moderate. Notably, subregion analysis revealed good correlation in the anterior and anterosuperior aspect of the joint, a region more prone to damage in most FAI patients thereby implying that T2*-mapping is effective in this target group. However, our study raises concerns regarding the relative lack of diagnostic agreement between the T2* mapping values and corresponding intraoperative findings in the posterior aspect of the acetabulum. Although it remains hypothetical, some confirmation and selection bias with the tendency to find more cartilage damage in the anterior aspect while potentially overlooking existent (mild) cartilage degeneration in the posterior portion of the hip may have played a role. Also, T2*-mapping and arthroscopic cartilage evaluation are differently targeted diagnostic modalities where some cartilage degeneration or cartilage alteration picked up with T2*-mapping may not have been seen during hip arthroscopy. The clinical relevance of this finding discrepancy is still unclear, and T2*-imaging artifacts such as magic angle and susceptibility effects9 may have a further influence. A further critical aspect is the appropriate use of portals for cartilage status assessment in which the anterior or a mid-anterior portal may be advantageous to observe the posterior part of the acetabulum, whereas it might be difficult to observe the superoposterior acetabulum from the anterolateral portal. In the retrospective review of articular surface, it is sometimes difficult to evaluate whole parts of a hip joint and in the retrospective, although viewing portals did not differ between surgeons, it is not absolutely clear which portal was used for the assessment of a specific cartilage region.
Only a few studies on the correlation between biochemical cartilage imaging and intraoperative data in FAI cohorts have been published previously ( Table 3 ). In 16 FAI patients (mean age 31.0 ± 11.3 years) who underwent MR arthrography, including dGEMRIC analysis within three months prior to safe surgical hip dislocation, Bittersohl et al.16 noted a moderate correlation between standard MRI and intraoperative data (r = 0.535; P < 0.001) but a weak correlation between dGEMRIC analysis and such data (r = 0.114; P < 0.126). However, regions with intraoperative cartilage damage had significantly lower T1Gd values compared with normal cartilage (453.1 ± 113.6 ms vs. 510.1 ± 141.2 ms; P = 0.003). In contrast to our study, using a 1.5-T scanner, acetabular and (in FAI patients) presumably healthy femoral head cartilage were analyzed as one ROI, which may have biased the dGEMRIC analysis and correlation with the intraoperative acetabular cartilage status in that study.
Table 3.
Synopsis of Studies Performed as a Comparative Analysis of Magnetic Resonance Imaging (MRI) and Intraoperative Data Assessing the Reliability of Biochemically Sensitive MRI in the Evaluation of Hip Joint Cartilage.
| Study | Cohort | Methodology | Fiel -Strength (Tesla) | Selected Sequences /Resolution (mm) | Results | Further Key Findings and Limitations | |||
|---|---|---|---|---|---|---|---|---|---|
| Bittersohl et al. (2011)16
Correlation dGEMRIC, open hip surgery Interval MRA/OP: >3 months |
16 FAI patients Mean age: 31 years (range 17-57 years) |
ROI: Acetabulofemoral cartilage ant., ant.-sup., sup.-ant., sup., sup.-post., post.-sup., post. peripheral, central T1Gd threshold 500 ms |
1.5 | Dual FA 3D GRE VIBE (coronal oblique) 0.78 × 0.78 × 0.78 |
Correlation dGEMRIC/Surgery k = 0.114 (P = 0.126) Correlation MRI / Surgery k = 0.535 (P < 0.001) SE (peripheral/central): 50% / 37% SP (peripheral/central): 60% / 76% |
T1Gd evident damage: 453 ms T1Gd no evident damage: 510 ms P = 0.003 Small study group Femoral and acetabular ROI combined |
|||
| Lattanzi et al. (2012)17
Correlation dGEMRIC, arthroscopy Interval MRI OP: <4 months |
10 FAI patients Mean age: 19.9 years (range 14-32 years) |
ROI: acetabulum ant.-sup., sup., post.-sup. central femoral head (=control) Cartilage damage: Mod. Outerbridge classification + grade 5 = delaminated cartilage dGEMRIC: absolute T1Gd values & T1Gd threshold 500 ms dGEMRIC: standardized values (z) z threshold < −1, < −2, < −3 |
1.5 | T1 spin echo fat suppression 0.4 × 0.4 × 3 dGEMRIC 0.3 × 0.3 × 4 |
dGEMRIC | dGEMRIC | Outerbridge | Small study group Retrospective study Femoral and acetabular ROI combined Selected group of patients Central femoral head cartilage may not be healthy in other pathologies |
|
| z < −2 | T1Gd < 500 ms | ||||||||
| SE (%) | 88 | 47 | 47 | ||||||
| SP (%) | 51 | 58 | 79 | ||||||
| ACC (%) 62 55 | 62 | 55 | 70 | ||||||
| PPV (%) | 42 | 31 | 47 | ||||||
| NPV (%) | 92 | 74 | 79 | ||||||
| Ellermann et al. (2014)18
Correlation T2*-mapping, arthroscopy Interval MRA / OP: 3 months |
26 FAI patients (28 hips) Mean age: 28.2 years (range 12-53 years) |
ROI: acetabulum ant.-sup.: 5 ROIs (superficial and deep zone) acetabulum post.-med. (=control): 2 ROIs (superficial and deep zone) Cartilage damage: Mod. Beck scale Cartilage segmentation and generation of T2* surface map |
3 | T2* GRE fat saturated (sagittal) 0.52 × 0.52 × 3 interpolated: 0.26 × 0.26 × 3 |
Beck Grade | T2* values | Interobserver reliability: 0.88 Differentiation between 1 and higher grades No differentiation between higher grades T2* variation due to orientation to B0 (magic angle) max. 2% Retrospective study |
||
| Bulk | Superficial Zone | Deep Zone | |||||||
| 1 | 35 | 40 | 31 | ||||||
| 2 | 21 | 24 | 17 | ||||||
| 3 + 4 | 20 | 23 | 17 | ||||||
| 5 + 6 | 17 | 18 | 16 | ||||||
| Bulat et al. (2015)19
Correlation dGEMRIC, hip surgery (surgery n/s) Interval MRA / OP: <6 months |
45 FAI patients (47 hips) intraoperative evaluation in 44 hips Mean age: 29 years (range: n/s) |
ROI: acetabulum ant.-post., ant.-cor., sup.-post., sup.-cor., post.-post., post.-cor. Cartilage damage (MRI): Mod. Outerbridge classification Cartilage damage (surgery): Mod. Beck scale dGEMRIC: Radial and planar T1Gd-maps |
1.5 | 3-dimensional VFA VIBE (dGEMRIC) 0.83 × 0.83 × 2 TrueFISP 0.63 × 0.63 × 0.63 sagittal, oblique = source 18 radial reformats (10° interval) |
Planar T1Gd correlation (range) with Beck scale specific to subregions | Faster cartilage evaluation with planar map (av. 55 s = 27%) Intraobserver agreement: Beck: κ = 0.80 Outerbridge: κ = 0.64 dGEMRIC: κ = 0.89 Retrospective study SE, SP not reported |
|||
| Outerbridge | dGEMRIC | ||||||||
| 0.16-0.52 | 0.14 to −0.57 | ||||||||
| No planar map-dependent effect on correlation strength | |||||||||
ACC = accuracy; dGEMRIC = delayed gadolinium-enhanced magnetic resonance imaging of cartilage; FA = flip angle; FISP = fast imaging with steady-state precession; GRE = gradient echo; MRA = magnetic resonance arthrography; NPV = negative predictive value; PPV = positive predictive value; ROI = region of interest; SE = sensitivity; SP = specificity; VFA = variable flip angle; VIBE = volumetric interpolated breathhold examination.
Bulat et al.19 noted a correlation of between 0.14 and -0.63 for dGEMRIC analysis and intraoperative cartilage condition. Their retrospective study on 45 FAI patients (47 hips, mean age 29 ± 11 years) included dGEMRIC analysis in six different regions in central and peripheral acetabular cartilage from anterior to posterior that were obtained in radial reformats and in planar T1Gd maps. Although planar T1Gd maps revealed slightly faster cartilage analysis (average of 55 seconds = 27% faster cartilage evaluation compared with radial image analysis), no map-dependent effect on correlation strengths was noted in that study. With a moderate correlation in superior cartilage regions (r = −0.31 to r = −0.63), cartilage analysis in the anterior aspect of the hip joint was poor to moderate (r = −0.03 to r = −0.35). This is in contrast to our study, in which we observed a good correlation to the surgical findings in the anterior region, and, on the other hand, a not so good correlation in the superior and the posterior region. Regional differences in the GAG distribution among central weightbearing and peripheral cartilage regions with an increased GAG content toward the superior and central regions (correlates with higher T1Gd values) might account for some of the region-dependent variation in this context. However, detailed information on the surgical procedure (open vs. arthroscopically), sensitivity and specificity were not provided in this study.
To overcome limitations in the dGEMRIC interpretation due to technical alterations and inter- and intra-subject variances in GAG distribution and gadolinium uptake, Lattanzi et al.17 proposed a technique in which standard scores (z) were calculated from T1Gd values that were obtained from the mean and the standard deviation of T1Gd of presumably healthy femoral head cartilage. Compared with arthroscopic data, this approach showed better diagnostic ability (sensitivity, specificity, and accuracy were 88%, 51%, and 62%, respectively, when assuming z = −2 as the threshold between normal and degenerated cartilage) to identify cartilage damage than applying threshold values (500 ms) or morphological cartilage grading. This study included only 10 patients and (following femoral head cartilage assessment for z-score calculation) acetabular and femoral head cartilage were evaluated as one entity. In our study, sensitivity and specificity were 83.5% and 67.7%, respectively, in total (85.3% and 89.7% for subregion anterior, anterosuperior, 85.5% and 57.6% for subregion superoanterior to superoposterior, and 77.8% and 59.5% for subregion posterosuperior to posterior, respectively). Accuracy was not measured in our study. These results are roughly similar although considerable differences (example radial vs. coronar planes, 1,5 T vs. 3 T MR scanners, analyzing acetabular and femoral head cartilage as one ROI in the study of Lattanzi et al.17) in the methodology need to be considered.
With recent concerns and debates related to the safety of gadolinium involving MR methodologies,20,21 noncontrast MRI for biochemical cartilage assessment has received increasing attention. Including 28 hips in FAI patients (mean age 28.2 years, range 12-53 years), Ellermann et al.18 correlated T2*-mapping with arthroscopic cartilage assessment (according to a modified Beck score). Their group reported significantly lower T2* values in areas with intraoperative cartilage damage compared with areas with normal appearing cartilage (T2* = 20.7 ± 6.0 ms vs. 35.3 ± 7.0 ms; P < 0.001). Although this study group included only patients with Tönnis grade 0 or Tönnis grade 1 on plain radiographs, 68% of the intraoperatively documented cartilage areas had apparent cartilage damage. This again highlights the limitations of plain radiography in identifying and picking up cartilage degeneration in early stages. Using receiver operating characteristic curve analysis, the authors proposed a T2* threshold value of 28 ms to differentiate healthy from damaged cartilage (91% true-positive and 13% false-positive rate to differentiate Beck score 1 from all other cartilages). However, given the previously published heterogeneity of T2* values in different cohorts of asymptomatic volunteers owing to differences in cartilage composition, cartilage thickness, and the magic angle effect, we believe it is crucial to thoroughly scrutinize T2* in each case, especially when a distinctive cartilage disease pattern is present. Our T2* values in normal appearing cartilage were lower (mean T2* = 25.6 ± 4.7 ms vs. 35.3 ± 7.0 ms) while the mean T2* values in damaged cartilage were similar (mean T2* = 19.9 ± 4.5 ms vs. 20.7 ± 6.0 ms). A potential explanation for this discrepancy is the different average age of both study cohorts. We included patients with a mean age of 35.6 ± 12.8 years while the average patient age in the study by Ellermann et al.18 was 28.2 years. Therefore, age-dependent differences of T2* relaxation times despite morphologically normal appearing cartilage have to be considered in these patient groups with a prearthritic hip condition.
Our study has a few limitations. This study cohort of 29 patients is small, and the retrospective study design, including possible errors of documentation for pre-, intra-, and postoperative assessment have to be considered. There may also have been some individual variations in reading the intra-operative damage due to human error. Also, the study-period was long in which changes to the background information and the documentation may have some effect on its scientific value. On the other hand, hip arthroscopy was performed by orthopedic surgeons specialized in hip arthroscopy with similar technique, and the MR imaging protocol, including hardware and the T2* assessment was not changed at all within this study period. Therefore, we believe this time-window bias is likely slight and not contradicting our conclusion. Furthermore, the possible progressive cartilage degeneration between the time points of MRI and surgery that might have biased our results should be considered, although it remains entirely unclear how and when the changes in cartilage damage become apparent in a hip joint with symptomatic FAI. For this reason, we believe that keeping this interval within 6 months is reasonable. One limitation was the lack of a diagnostic gold standard in the form of specimens and histological analysis. Therefore, knowing the sensitivity of T2* mapping to changes in water content and collagen fiber network, which may occur without macroscopically visible change, it is not surprising that the correlation between T2*-mapping and arthroscopic findings was only moderate. Finally, the T2* measurement using ROI analysis obtains mean values that represent the entire encircled area. Consequently, minor but remarkable changes may have been underestimated.
In summary, T2*-mapping reliably assisted in the assessment of acetabular cartilage in patients with symptomatic FAI in which the correlation with intraoperative findings revealed promising results for sensitivity, specificity, PPV, and NPV. Our results certainly emphasize the value of T2*-mapping for the diagnosis of hip joint cartilage pathologies, including FAI and other prearthritic hip conditions.
Footnotes
Acknowledgments and Funding: We would like to thank Mrs. Erika Rädisch for helping to perform the MRI, and Mr. Sebastian Ullrich, who helped with conducting the statistical analysis in this study.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Approval: This study was approved by the local ethics committee (Study-ID: 5574R).
Informed Consent: Written informed consent was obtained from all subjects before the study.
Trial Registration: Not applicable.
References
- 1. Leunig M, Ganz R. Femoroacetabular impingement. A common cause of hip complaints leading to arthrosis [in German]. Unfallchirurg. 2005;108:9-10, 12-7. [DOI] [PubMed] [Google Scholar]
- 2. Ganz R, Leunig M, Leunig-Ganz K, Harris WH. The etiology of osteoarthritis of the hip: an integrated mechanical concept. Clin Orthop Relat Res. 2008;466:264-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bittersohl B, Hosalkar HS, Hesper T, Tiderius CJ, Zilkens C, Krauspe R. Advanced imaging in femoroacetabular impingement: current state and future prospects. Front Surg. 2015;2:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ho CP, Ommen ND, Bhatia S, Saroki AJ, Goljan P, Briggs KK, et al. Predictive value of 3-T magnetic resonance imaging in diagnosing grade 3 and 4 chondral lesions in the hip. Arthroscopy. 2016;32:1808-13. [DOI] [PubMed] [Google Scholar]
- 5. Zilkens C, Miese F, Kim YJ, Jager M, Mamisch TC, Hosalkar H, et al. Direct comparison of intra-articular versus intravenous delayed gadolinium-enhanced MRI of hip joint cartilage. J Magn Reson Imaging. 2014;39:94-102. [DOI] [PubMed] [Google Scholar]
- 6. Borthakur A, Mellon E, Niyogi S, Witschey W, Kneeland JB, Reddy R. Sodium and T1rho MRI for molecular and diagnostic imaging of articular cartilage. NMR Biomed. 2006;19:781-821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mosher TJ, Dardzinski BJ. Cartilage MRI T2 relaxation time mapping: overview and applications. Semin Musculoskelet Radiol. 2004;8:355-68. [DOI] [PubMed] [Google Scholar]
- 8. Hesper T, Miese FR, Hosalkar HS, Behringer M, Zilkens C, Antoch G, et al. Quantitative T2* assessment of knee joint cartilage after running a marathon. Eur J Radiol. 2015;84:284-9. [DOI] [PubMed] [Google Scholar]
- 9. Hesper T, Hosalkar HS, Bittersohl D, Welsch GH, Krauspe R, Zilkens Cet al. T2* mapping for articular cartilage assessment: principles, current applications, and future prospects. Skeletal Radiol. 2014;43:1429-45. [DOI] [PubMed] [Google Scholar]
- 10. Holstein A, Zilkens C, Bittersohl B, Jäger M, Haamberg T, Mamisch TC, et al. Delayed gadolinium-enhanced MRI of cartilage (dGEMRIC) and morphologic MRI of cartilage in the long-term follow-up after Legg-Calvé-Perthes disease (LCPD). J Med Imaging Radiat Oncol. 2011;55:259-65. [DOI] [PubMed] [Google Scholar]
- 11. Hesper T, Schleich C, Buchwald A, Hosalkar HS, Antoch G, Krauspe R, et al. T2* mapping of the hip in asymptomatic volunteers with normal cartilage morphology: an analysis of regional and age-dependent distribution. Cartilage. Epub 2016. December 30. doi: 10.1177/1947603516684591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Xia Y. Magic-angle effect in magnetic resonance imaging of articular cartilage: a review. Invest Radiol. 2000;35:602-21. [DOI] [PubMed] [Google Scholar]
- 13. Nepple JJ, Byrd JW, Siebenrock KA, Prather H, Clohisy JC. Overview of treatment options, clinical results, and controversies in the management of femoroacetabular impingement. J Am Acad Orthop Surg. 2013;21(Suppl 1):S53-58. [DOI] [PubMed] [Google Scholar]
- 14. Philippon MJ, Briggs KK, Carlisle JC, Patterson DC. Joint space predicts THA after hip arthroscopy in patients 50 years and older. Clin Orthop Relat Res. 2013;471:2492-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Skendzel JG, Philippon MJ, Briggs KK, Goljan P. The effect of joint space on midterm outcomes after arthroscopic hip surgery for femoroacetabular impingement. Am J Sports Med. 2014;42:1127-33. [DOI] [PubMed] [Google Scholar]
- 16. Bittersohl B, Hosalkar HS, Apprich S, Werlen SA, Siebenrock KA, Mamisch TC. Comparison of pre-operative dGEMRIC imaging with intra-operative findings in femoroacetabular impingement: preliminary findings. Skeletal Radiol. 2011;40:553-61. [DOI] [PubMed] [Google Scholar]
- 17. Lattanzi R, Petchprapa C, Glaser C, Dunham K, Mikheev AV, Krigel A, et al. A new method to analyze dGEMRIC measurements in femoroacetabular impingement: preliminary validation against arthroscopic findings. Osteoarthritis Cartilage. 2012;20:1127-33. [DOI] [PubMed] [Google Scholar]
- 18. Ellermann J, Ziegler C, Nissi MJ, Goebel R, Hughes J, Benson M, et al. Acetabular cartilage assessment in patients with femoroacetabular impingement by using T2* mapping with arthroscopic verification. Radiology. 2014;271:512-23. [DOI] [PubMed] [Google Scholar]
- 19. Bulat E, Bixby SD, Siversson C, Kalish LA, Warfield SK, Kim YJ. Planar dGEMRIC maps may aid imaging assessment of cartilage damage in femoroacetabular impingement. Clin Orthop Relat Res. 2016;474:467-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Heverhagen JT, Krombach GA, Gizewski E. Application of extracellular gadolinium-based MRI contrast agents and the risk of nephrogenic systemic fibrosis. Rofo. 2014;186:661-9. [DOI] [PubMed] [Google Scholar]
- 21. Robert P, Violas X, Grand S, Lehericy S, Idee JM, Ballet S, et al. Linear gadolinium-based contrast agents are associated with brain gadolinium retention in healthy rats. Invest Radiol. 2016;51:73-82. [DOI] [PMC free article] [PubMed] [Google Scholar]