Abstract
Objective
To perform a systematic review of clinical outcomes following microfracture augmented with biological adjuvants (MFX+) compared with microfracture (MFX) alone.
Design
The MEDLINE, Scopus, and Cochrane databases were searched for clinical studies on MFX+ for chondral defects of the knee. Study characteristics and clinical outcome score data were collected. Subjective synthesis was performed using data from randomized controlled studies to determine effect size of MFX+ procedures performed with either injectable or scaffold-based augmentation compared with MFX alone.
Results
A total of 18 articles reporting on 625 patients (491 MFX+, 134 MFX) were identified. Six studies were level II evidence and 1 study was level I evidence. Mean patient age range was 26 to 51 years, and mean follow-up ranged from 2 to 5 years. All studies demonstrated significant improvement in reported clinical outcome scores at follow-up after MFX+ therapy, and 87% of patients reported satisfaction with treatment. The most commonly reported treatment complication was postoperative stiffness (3.9% of patients). Subjective synthesis on randomized controlled trials demonstrated that 2/2 injectable MFX+ interventions had significantly greater improvements in International Knee Documentation Committee Subjective Knee Form (IKDC; P = 0.004) and Knee injury and Osteoarthritis Outcome Score (KOOS; P = 0.012) scores compared with MFX alone, while 2/2 trials on scaffolding MFX+ adjuvants showed comparable postoperative improvements.
Conclusions
MFX+ biological adjuvants are safe supplements to marrow stimulation for treating cartilage defects in the adult knee. Early literature is heterogenous and extremely limited in quality. Individual trials report both equivalent and superior clinical outcomes compared with MFX alone, making definitive conclusions on the efficacy of MFX+ difficult without higher quality evidence.
Keywords: articular cartilage, microfracture, marrow stimulation, biomaterials
Introduction
Articular cartilage lesions of the knee remain a challenging clinical entity due to the limited intrinsic healing capacity of cartilage tissue and the potential progression of lesions to generalized osteoarthritis.1 The lack of a successful endogenous repair mechanism has been attributed to the poor recruitment of regenerative cells into the cartilage defect area.2 After the theory of marrow stimulation by subchondral drilling was proposed by Pridie in 1959, Steadman popularized the concept by introducing the microfracture (MFX) technique, whereby migration of mesenchymal stem cells and growth factors from subchondral bone facilitates restoration of hyaline-like fibrocartilage.3 In contrast to other cartilage restoration techniques, MFX is minimally invasive, cost effective, and relatively simple to perform in a single-stage procedure. Although MFX is still considered the “gold standard” first-line treatment for chondral defects of the knee with good short-term results,4 there are concerns surrounding suboptimal repair with fibrocartilage infill5 and long-term clinical outcomes compared with other available cartilage restoration procedures such as autologous chondrocyte implantation (ACI), mosaicplasty/osteoarticular transfer system (OATS), and osteochondral allograft transplantation.6-10 In response, some, including the UK National Institute for Health and Care Excellence in its recent assessment report, have advocated for the abandonment of MFX in favor of ACI or other options as first line treatment for articular cartilage lesions.11
Recent studies have investigated modifications of traditional marrow-stimulating techniques to enhance efficacy, including the use of synthetic and autologous biological adjuvants to improve repair tissue quality and durability.6,12 Some investigators hypothesize that suboptimal outcomes with traditional MFX may be due to insufficient concentration of mesenchymal stem cells (MSCs) and growth factors being released from subchondral marrow.13 To this end, recent studies have reported success by supplementing MFX with intra-articular injectable adjuvants, including MSCs,13,14 platelet-rich plasma (PRP),15,16 and hyaluronic acid.17,18 Others have proposed that augmentation of the microfractured defect with a scaffolding matrix19-23 and cell-free polymer-based implants24,25 may have a “bioreactor” like effect in which marrow elements are entrapped and concentrated to facilitate efficient cartilage restoration.26 While individual reports of these biological augmentations have shown successful histological and clinical outcomes, the literature on these “microfracture plus” (MFX+) techniques has been limited by significant heterogeneity among study cohorts in the form of case series and a paucity of prospective clinical outcomes data.
The purpose of this systematic review was 2-fold: (1) to summarize the available scientific evidence comparing the clinical outcomes of MFX+ with traditional MFX and (2) perform a subjective synthesis of functional and pain outcomes comparing these techniques. We hypothesized that MFX+ supplemented with biological augmentation would have clinical outcomes superior to MFX alone due to the potential for higher quality repair tissue infill of the chondral defect.
Materials and Methods
A systematic review was performed using the MEDLINE, Scopus, and Cochrane Library databases and subsequently registered on the PROSPERO database (Registration number: CRD42016037619, University of York, York, United Kingdom). Search terms were “microfracture” AND “knee” along with associated MeSH terms. Duplicate studies and published abstracts without an associated full-text manuscript were excluded.
Following the primary search, a manual title and abstract review was performed in accordance with the standard PRISMA checklist to identify articles that contained relevant information. If relevant information was identified, articles were then systematically assessed in order to determine compliance with the following inclusion criteria: (1) a minimum of 5 subjects (e.g., no case reports or small case series), (2) intervention of MFX plus biological adjuvant for symptomatic chondral defect of the knee, (3) minimum of 2 years clinical follow-up, and (4) study published in the English language. Articles were excluded if they were review articles, systematic reviews, or meta-analyses. Cadaveric, in vitro, and animal studies were also excluded. Full-text review of the remaining included entries was performed for further application of the above inclusion and exclusion criteria.
Studies that satisfied inclusion/exclusion criteria were used to extract cohorts of patients who had either scaffolding or injectable products as a MFX adjuvant for intervention. Relevant data on patient demographics (age, sex), lesion size, length of follow-up, incidence of postoperative complications, and all reported pre- and postoperative clinical outcome scores (International Knee Documentation Committee Subjective Knee Form [IKDC], visual analog scale–pain [VAS pain], Lysholm, Cincinnati, Western Ontario and McMaster Universities Osteoarthritis Index [WOMAC], Tegner, Knee injury and Osteoarthritis Outcome Score [KOOS]) were collected and reviewed. For outcome scores where complete descriptive data (e.g., mean/mean, variance/standard deviation, cohort size) was not available at baseline and/or follow-up, 3 separate attempts were made to contact the primary and/or corresponding author for primary data.
Statistics
Subjective synthesis was performed on clinical outcomes measures collected from randomized, controlled comparative studies with a MFX comparison cohort (level I and level II evidence). For each individual study where data were available, pre- to postoperative improvements in clinical outcome scores (IKDC, VAS pain, Cincinnati, and KOOS) from baseline to final follow-up were used to calculate effect size using standard difference in means (d) with 95% confidence intervals and associated P values for significance. When standard deviations were not reported and unobtainable from the original study author(s), they were calculated using the sample data range under the assumption the data were contained within 2 standard deviations of the mean (98% confidence).27 All statistical analysis was performed using Comprehensive Meta-Analysis version 2 software (Biostat, Inc, Englewood, NJ, USA).
Results
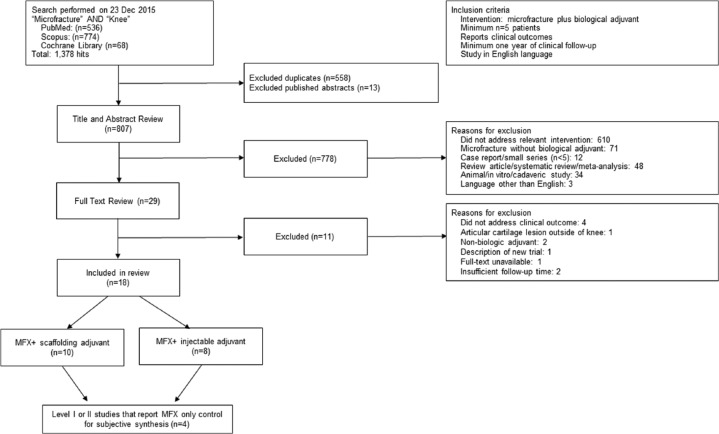
A PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) flowchart ( Fig. 1 ) outlines the application of the systematic review inclusion and exclusion criteria for the 807 unique articles that were identified in the initial search. A total of 18 articles met study inclusion criteria. Of these 18 articles, 1 study was level I evidence and 6 studies were level II evidence ( Table 1 ), while the remainder were level IV case series. Ten studies reported on a scaffolding adjuvant20-24,28-32 and 8 studies reported on an injectable adjuvant13-16,33-36 ( Table 2 ). No studies described a combined scaffolding and injectable MFX+ adjuvant. In total, there were 625 unique patients identified across all the studies; 491 underwent MFX+ therapy (78.6%) and 134 (21.4%) underwent MFX alone. Of the entire cohort, 328 (52.5%) of these patients were male. Mean patient age ranged from 26 to 51 years, and mean follow-up ranged from 2 to 5 years. The average lesion size ranged from 1.4 to 4.2 cm2 ( Table 1 ). Nearly half of all studies (8/18) neither excluded for nor specified the location (e.g., condylar, patellar), depth, or grade of lesions treated. Description of Kellgren-Lawrence x-ray grade (3/18) and concomitant meniscal or ligamentous pathology (14/18) was also inconsistently reported.
Figure 1.
PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) diagram outlining the application of the inclusion and exclusion criteria for the systematic review.
Table 1.
Summary of the 18 Articles Identified Through the Systematic Review.
| Study No. | First Author (Year of Publication) | N | Male | Female | Age in Years, Mean (Range) | Lesion Size (cm2) Mean (Range) | Level of Evidence | Level of Evidence, Study Methodology | Scaffolding Adjuvant | Injectable Adjuvant | Microfracture Only Cohort | IKDC | VAS Pain | Lysholm | Cincinnati | WOMAC | Tegner | KOOS |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Becher et al. (2015)33 | 5 | 3 | 2 | 27.6 (15-40) | 4.0 (3.0-5.0) | Level IV | Case series | ✓ | ✓ | ||||||||
| 2 | Enea et al. (2015)28 | 9 | 5 | 4 | 51.0 (28-70) | 3.5 (1.8-9.0) | Level IV | Case series | ✓ | ✓ | ✓ | ✓ | ✓ | |||||
| 3 | Koh et al. (2016)13 | 80 | 30 | 50 | 38.7 (18-50) | 4.7 | Level II | Randomized controlled trial | ✓ | ✓ | ✓ | ✓ | ✓ | |||||
| 4 | Shive et al. (2015)24 | 80 | 48 | 32 | 36.1 (18-55) | 2.1 | Level I | Randomized controlled trial | ✓ | ✓ | ✓ | |||||||
| 5a | Siclari et al. (2014)34 | 52 | 20 | 32 | 44.0 (31-65) | 2.7 (1.5-5.0) | Level IV | Case series | ✓ | ✓ | ||||||||
| 6 | Anders et al. (2013)22 | 38 | 31 | 7 | 37.1 (21-50) | 3.4 (2.1-6.6) | Level II | Randomized controlled trial | ✓ | ✓ | ✓ | |||||||
| 7 | Chung et al. (2013)29 | 36 | 16 | 20 | 46.3 | 1.4 | Level II | Randomized controlled trial | ✓ | ✓ | ✓ | ✓ | ||||||
| 8 | Enea et al. (2013)30 | 9 | 5 | 4 | 48 | 2.6 | Level IV | Case series | ✓ | ✓ | ✓ | ✓ | ✓ | |||||
| 9 | Gille et al. (2013)22 | 57 | 38 | 19 | 37.3 (17-61) | 3.4 (1.0-9.0) | Level IV | Case series | ✓ | ✓ | ✓ | ✓ | ||||||
| 10 | Saw et al. (2013)35 | 49 | 17 | 32 | 39.9 (22-50) | Not stated | Level II | Randomized controlled trialb | ✓ | ✓ | ✓ | |||||||
| 11 | Siclari et al. (2013)16 | 52 | 20 | 32 | 44.0 (31-65) | 2.7 (1.5-5.0) | Level IV | Case series | ✓ | |||||||||
| 12 | Lee et al. (2012)15 | 49 | 29 | 20 | 46 (41-48) | <4 | Level II | Randomized controlled trial | ✓ | ✓ | ✓ | ✓ | ✓ | |||||
| 13 | Lee et al. (2012)14 | 70 | 36 | 34 | <55 | N/A | Level II | Randomized controlled trialb | ✓ | ✓ | ✓ | ✓ | ||||||
| 14a | Siclari et al. (2012)36 | 52 | 20 | 32 | 44.0 (31-65) | 2.7 (1.5-5.0) | Level IV | Case series | ✓ | ✓ | ||||||||
| 15 | Dhollander et al. (2011)31 | 5 | 2 | 3 | 29.8 (16-39) | 2.5 (1.5-5.0) | Level IV | Case series | ✓ | ✓ | ✓ | |||||||
| 16 | Kusano et al. (2011)23 | 40 | 23 | 17 | 45.3 | 3.9 | Level IV | Case series | ✓ | ✓ | ✓ | ✓ | ||||||
| 17 | Gille et al. (2010)32 | 27 | 16 | 11 | 39 (16-50) | 4.2 (1.3-8.8) | Level IV | Case series | ✓ | ✓ | ✓ | |||||||
| 18 | Pascarella et al. (2009)20 | 19 | 12 | 7 | 26 (18-50) | 3.6 (2.8-3.9) | Level IV | Case series | ✓ | ✓ | ✓ |
IKDC = International Knee Documentation Committee Subjective Knee Form; KOOS = Knee injury and Osteoarthritis Outcome Score; N/A = not applicable; VAS = visual analog scale; WOMAC = Western Ontario and McMaster Universities Osteoarthritis Index.
Denotes case series following the same group of enrolled patients with outcomes described in three separate publications.
Randomized trial compares 2 different microfracture plus adjuvant interventions.
Table 2.
List of Injectable and Scaffolding Biological Adjuvants Identified in Systemic Review.
| Scaffolding Adjuvant (n = 10) | Injectable Adjuvant (n = 8) |
|---|---|
| Collagen I/III matrix (AMIC Chondro-Gide) (n = 6) | PRP (n = 1) |
| Chitosan polymer matrix (BST-CarGel) (n = 1) | PGA + PRP (n = 2) |
| Collagen II, GAGs from porcine decellularized biomembrane (Artifilm ECM) | Adipose-derived MSCs |
| PGA-HA cell-free matrix (BioTissue AG Chondrotissue) | Intra-articular autologous BMC, HA mixture |
| PGA-HA cell-free matrix(BioTissue AG Chondrotissue) with autologous BMC | PGA-HA + PRPPGA-HAPGA-HA with autologous BMC |
AMIC = autologous matrix-induced chondrogenesis; BMC = bone marrow concentrate; GAG = glycosaminoglycans; HA = hyaluronic acid; MSC = mesenchymal stem cell; PGA = polyglycolic acid; PRP = platelet-rich plasma.
The most commonly reported clinical outcome measures were the IKDC (8/18 studies), Lysholm (9/18 studies), and VAS pain (7/18 studies) scores. All studies demonstrated statistically significant improvement in each reported clinical outcome score at follow-up (mean 2-5 years) after the reported MFX+ intervention compared with baseline. Of those reported, 86.8% of patients were satisfied with the treatment outcome. The most commonly reported treatment complication for patients undergoing MFX+ was stiffness requiring manipulation under anesthesia (3.9% of patients). Other specifically reported adverse events were deep vein thrombosis (0.5%) and hematoma (0.3%). No adverse events attributable to the MFX+ adjuvant treatments were reported.
Among the 7 level I and level II studies, 2 were level II studies comparing 2 different MFX+ treatments with one another ( Table 3 ) and therefore were treated as a single cohort for the purpose of our subjective synthesis. Of the remaining 5 level I and level II studies, 4 provided sufficient data to perform post hoc statistical analysis to determine standard mean differences in improvement. Subjective synthesis was performed on these four comparative clinical outcomes studies with treatment arms randomized to either injectable or scaffolding MFX+ and MFX alone ( Table 4 ). Among scaffolding adjuvants, neither Chung et al.29 using Artifilm ECM (IKDC: d = 0.30, 95% CI −0.40 to 1.00, P = 0.401; VAS pain: d = 0.36, 95% CI −0.40 to 1.00, P = 0.398) nor Anders et al.22 using AMIC Chondro-Gide (Cincinnati: d = −0.33, 95% CI −1.24 to 0.58, P = 0.482) were found to have a significantly different postoperative improvement following treatment in either pain or functional outcome scores at 2-year follow-up. Among injectable adjuvants, Lee et al.15 found a statistically significantly greater improvement in postoperative IKDC scores (d = 0.87, 95% CI 0.29 to 1.46, P = 0.004), but no statistically significant difference with regard to VAS pain score improvement compared with MFX only controls (d = 0.29, 95% CI −0.05 to 1.09, P = 0.071). Using injectable adipose-derived MSCs as an adjuvant, Koh et al.13 reported statistically significantly greater improvements in KOOS scores at 2 years (d = 0.57, 95% CI 0.13 to 1.02, P = 0.012).
Table 3.
Level of Evidence Analysis of Randomized Controlled Trials.
| First Author (Year of Publication) | N | Level of Evidence | Category | Comparison | Follow-up | Outcome Measures | Included in Subjective Synthesis? |
|---|---|---|---|---|---|---|---|
| Koh et al. (2016)13 | 80 | Level II | Injectable | MFX+ injectable adipose-derived MSC injections vs. MFX alone | 2 years | KOOS | Yes |
| Shive et al. (2015)24 | 80 | Level I | Scaffolding | MFX+ BST-CarGel vs. MFX alone | 1 year, 5 years | WOMAC, SF-36 | No—insufficient statistics provided |
| Anders et al. (2013)22 | 38 | Level II | Scaffolding | MFX+ AMIC Chondro-Gide vs. MFX alone | 2 years | Cincinnati | Yes |
| Chung et al. (2013) | 36 | Level II | Scaffolding | MFX+ Artifilm ECM vs. MFX alone | 2 years | IKDC, VAS pain | Yes |
| Saw et al. (2013)35 | 49 | Level IIa | Injectable | MFX+ HA + peripheral blood stem cells vs. MFX+ HA alone | 2 years | IKDC, KOOS | No—no MFX only comparison cohort |
| Lee et al. (2012)15 | 49 | Level II | Injectable | MFX+ PRP injections vs. MFX alone | 2 years | IKDC, VAS pain | Yes |
| Lee et al. (2012)14 | 70 | Level IIa | Injectable | MFX+ Postoperative MSCs + HA vs. MFX+ intraoperative MSCs + HA | 2 years | IKDC, Lysholm | No—no MFX only comparison cohort |
AMIC = autologous matrix-induced chondrogenesis; HA = hyaluronic acid; IKDC = International Knee Documentation Committee Subjective Knee Form; KOOS = Knee injury and Osteoarthritis Outcome Score; MFX = microfracture; MSC = mesenchymal stem cell; PRP = platelet-rich plasma; VAS = visual analog scale; WOMAC = Western Ontario and McMaster Universities Osteoarthritis Index.
Randomized trial compares 2 different microfracture plus adjuvant interventions (effectively level IV for purpose of this review).
Table 4.
Subjective Synthesis of MFX+ Randomized Controlled Trials.
| First Author (Year of Publication) | Intervention | Follow-up | Standard Difference in Mean Improvement (95% CI) | P | Interpretation |
|---|---|---|---|---|---|
| IKDC | |||||
| Chung et al. (2013)29 | Scaffolding—Artifilm ECM | 2 years | 0.30 (−0.40 to 1.00) | 0.401 | No statistically significant difference |
| Lee et al. (2012)15 | Injectable—PRP | 2 years | 0.87 (0.29-1.46) | 0.004 | MFX+ significantly greater improvement than MFX alone |
| VAS pain | |||||
| Chung et al. (2013)29 | Scaffolding—Artifilm ECM | 2 years | 0.36 (−0.40 to 1.00) | 0.398 | No statistically significant difference |
| Lee et al. (2012)15 | Injectable—PRP | 2 years | 0.29 (−0.05 to 1.09) | 0.071 | No statistically significant difference |
| Cincinnati | |||||
| Anders et al. (2013)22 | Scaffolding—AMIC Chondro-Gide | 2 years | −0.33 (−1.24 to 0.58) | 0.482 | No statistically significant difference |
| KOOS | |||||
| Koh et al. (2016)13 | Injectable—Adipose-derived MSCs | 2 years | 0.57 (0.13-1.02) | 0.012 | MFX+ significantly greater improvement than MFX alone |
AMIC = autologous matrix-induced chondrogenesis; IKDC = International Knee Documentation Committee Subjective Knee Form; KOOS = Knee injury and Osteoarthritis Outcome Score; MFX, microfracture; MSC = mesenchymal stem cell; PRP = platelet-rich plasma; VAS = visual analog scale.
Discussion
Although MFX remains the current gold standard for articular cartilage repair, this notion is currently being challenged given an abundance of literature that demonstrates long-term clinical outcomes of varying success.6-10 This has been largely attributed to the questionable durability of fibrocartilage tissue, which lacks the nascent hyaline articular structure.5 In this systematic review, we identified 18 studies that investigated the use of injectable or polymer-based scaffolding as a biologic adjuvant to enhance cartilage repair. As hypothesized, MFX+ demonstrated significant improvements in clinical outcome scores compared with baseline, and the majority (86.9%) of patients were satisfied with treatment. Furthermore, our subjective synthesis of 4 individual randomized controlled trials demonstrated that patients treated with injectable MFX+ adjuvants (either PRP or adipose-derived MSCs) showed statistically significantly greater improvement in postoperative IKDC and KOOS scores while patients treated with scaffolding MFX+ adjuvants (either Artifilm ECM or AMIC Chondro-Gide) had equivalent postoperative improvements with respect to IKDC, VAS pain, and Cincinnati scores. Of note, patients undergoing MFX+ therapy did not have any complications solely attributable to the adjuvant treatment.
Previous animal and human studies have attributed the poor tissue quality and gradual decline in clinical outcomes following MFX to 1 of 2 mechanisms: (1) the instability of marrow-derived blood clots formed during the healing process, which shrink and detach in response to subchondral stimulation37,38 and (2) the insufficient concentration of marrow precursors required to facilitate cartilage repair.13,25,39 The proposed advantage of MFX+ scaffolding and injectable adjuvants is the theoretical ability to increase the concentration of mesenchymal stem cells in the formed clot as well as facilitate stabilization on clot formation.40 In studies dating back to the early to mid-2000s, small and large animal models of MFX supplemented with a wide variety of these adjuvant treatments have consistently demonstrated superior histologic integration and cartilage restoration, biomechanical properties, and repair tissue durability in vivo.18,41-44 In one study, Strauss et al.18 demonstrated that viscosupplementation with intra-articular hyaluronic acid injections promoted more tissue infill and more hyaline-like tissue quality compared with controls in a New Zealand White rabbit model. Among these marrow stimulation adjuncts, scaffolding supplements that have been investigated include matrices derived from synthetic copolymers, chitosan, and collagen.12 Animal studies on injectable products have been primarily hyaluronic acid, PRP, and autologous marrow-derived MSCs12, but have also recently included stimulant cytokines and growth factors.26
While the benefits of MFX+ techniques were initially demonstrated in preclinical models with histologic and biomechanical data, case series and clinical trials investigating these techniques began to surface in the literature in 2009.20 Using subjective synthesis to determine effect size of individual studies identified in this systematic review, we found that studies using injectable PRP and adipose-derived MSCs demonstrate statistically significantly greater postoperative improvements with MFX+, while others using scaffolding adjuvants show no difference with regard to postoperative improvements ( Table 4 ). We believe this may be partially due to insufficient statistical power in the constituent studies, which failed to demonstrate superiority.45 For instance, the 2 largest randomized controlled trials by Koh et al.13 using adipose-derived MSC/fibrin glue injections and Lee et al.15 using injectable PRP demonstrated greater improvement in KOOS and IKDC scores, respectively, in comparison with MFX only control groups. Conversely, the smaller trials describing scaffolding adjuvants showed slightly higher scores in their respective MFX+ cohorts but did not achieve statistical significance.21,29 These equivocal findings demonstrate the need for larger and higher quality studies to delineate the efficacy of specific MFX+ scaffolding and injectable adjuvants, which could potentially translate to improved long-term outcomes where MFX alone has been less successful.18,26,41 Outside of the design of this subjective synthesis, we also acknowledge the findings of Stanish and colleagues who in 2 separate publications reported sustained and significantly superior repair tissue quantity and quality at 1 year25 and 5 years24 using the BST-CarGel biopolymer scaffold as a MFX+ adjuvant in a level I study of 80 patients. The original study was not included in this systematic review as it reported only 1-year clinical follow-up, and the follow-up work, while included in the review, reported insufficient data for subjective synthesis of postoperative improvements in WOMAC scores.
The clinical implications of this study are significant as it pertains to treatment of articular cartilage lesions of the knee. As demonstrated in this review, the amount of high-quality methodological literature in cartilage surgery is relatively low, leading to ambiguous or contradictory conclusions regarding individual surgical options.46 Given recent data that demonstrates inferior long-term outcomes following MFX, many surgeons are giving stronger consideration to alternative cartilage restoration procedures such as ACI, mosaicplasty/OATS, and osteochondral allograft transplantation, despite the limitations of some of these techniques including the need for 2-stage procedures, donor site morbidity, and the increased cost and limited availability of allograft donor tissue. In a 2-year prospective randomized controlled trial of 144 patients, Saris et al.8 demonstrated superior clinical outcomes with matrix-applied characterized ACI for symptomatic chondral defects ≥3 cm relative to microfracture.2 Similarly, Krych et al.7 demonstrated that osteochondral autograft/mosaicplasty has superior activity levels as measured by the Marx Activity Rating Scale at 2-year follow-up and beyond when compared with microfracture.7 The findings of this particular study suggest that certain “microfracture plus” adjuvants may be superior to traditional marrow stimulation alone, and may be a viable single-stage alternative for cartilage repair. Interestingly, one published model using short-term pain, long-term clinical outcomes and complication data from the largest clinical trial referenced in this study projected that BST-CarGel, a chitosan-β glycerolphosphate based scaffold commercially available in Europe, would yield a 20-year cumulative cost savings of €6448 per patient relative to MFX alone.47 However, future high-quality randomized controlled trials are necessary to directly compare augmented MFX with other cartilage restoration procedures to determine differential efficacy and cost-effectiveness.
Despite the aforementioned findings of our study, there are several limitations. First, the majority of the 18 studies reported observational data (level of evidence 4) in the form of case series on a small group of patients without an internal control, and only 5 studies comparing MFX+ with a MFX control were available. Second, because there was a diverse number of scaffolding and injectable adjuvants in the constituent studies with primarily low-quality evidence, this systematic review and subjective synthesis cannot advocate for any single technique without higher quality evidence. This is reflective of both the relative novelty of MFX+ as a treatment option and the greater number of lower-quality observational studies rather than high-quality prospective comparative clinical data. Indeed, future clinical studies and conclusions drawn from them should be in the context of a specific MFX+ supplement in comparison to traditional MFX and other cartilage restoration techniques. Methodological heterogeneity is also evident in the wide variety of clinical outcome measures reported ( Tables 1 - 3 ) in the constituent studies. This precludes the ability to perform quantitative synthesis such as meta-analysis and to make more robust and definitive conclusions. This also underscores the principle of high reporting variability in the orthopedic literature, and emerging fields such as cartilage surgery in particular.48 Third, we acknowledge that the age and lesion size generalizability of these findings may be limited, as the constituent studies did not include youth patient populations and larger defect sizes. Furthermore, these studies inconsistently reported important baseline variables such as lesion depth, grade, and location, as well as concomitant pathologies and progression to osteoarthritis. Fourth, because the average lesion size in this study was small, it would be difficult to demonstrate superiority for MFX+ therapy given that MFX shows acceptable results for lesions <4 cm2 and that the majority of studies reported only short-term follow-up at 2 years. Fifth, the statistically significantly greater improvement achieved by certain MFX+ adjuvants compared with MFX alone may have limited clinical significance given the small magnitude of effect size differences ( Table 4 ). Further comparative trials with appropriately powered sample sizes and analysis with regard to minimal clinically important difference (MCID) criteria will be important in comparing MFX+ with traditional MFX and other cartilage repair strategies. Finally, although we limited our search criteria to English studies only, it has been reported that restricting systematic reviews to English-only does not introduce bias into systematic reviews and meta-analyses.49
In summary, the current study demonstrated that MFX+ is an emerging concept and biological adjuvants are safe supplements to marrow stimulation with mixed results among individual studies demonstrating both statistically superior and equivalent clinical outcomes compared to MFX alone. Existing literature on MFX+ is in its infancy with many new adjuvants emerging and the body of prospective high-quality data growing. Based on these observations, we are unable to make definitive conclusions regarding the benefit of the current forms of augmentation to date, but this collection of available clinical studies in conjunction with current basic science data does demonstrate the potential for MFX+ to be a viable option worthy of further investigation for clinically significant differences. When considering options for biologic augmentation and further research to justify their use, it is important to weigh the potential for improved clinical outcomes and more durable hyaline-like repair cartilage against the added cost of adjuvant and the technical simplicity of microfracture relative to other cartilage repair procedures.
Footnotes
Authors’ Note: This study was primarily performed at the Department of Orthopedic Surgery in the David Geffen School of Medicine at University of California, Los Angeles.
Acknowledgments and Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1. Widuchowski W, Widuchowski J, Trzaska T. Articular cartilage defects: study of 25,124 knee arthroscopies. Knee. 2007;14(3):177-82. [DOI] [PubMed] [Google Scholar]
- 2. Farr J, Cole B, Dhawan A, Kercher J, Sherman S. Clinical cartilage restoration: evolution and overview. Clin Orthop Relat Res. 2011;469(10):2696-705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Steadman JR, Rodkey WG, Rodrigo JJ. Microfracture: surgical technique and rehabilitation to treat chondral defects. Clin Orthop Relat Res. 2001;(391 Suppl):S362-9. [DOI] [PubMed] [Google Scholar]
- 4. Knutsen G, Engebretsen L, Ludvigsen TC, Drogset JO, Grøntvedt T, Solheim E, et al. Autologous chondrocyte implantation compared with microfracture in the knee. A randomized trial. J Bone Joint Surg Am. 2004;86-A(3):455-64. [DOI] [PubMed] [Google Scholar]
- 5. Hoemann CD, Tran-Khanh N, Chevrier A, Chen G, Lascau-Coman V, Mathieu C, et al. Chondroinduction is the main cartilage repair response to microfracture and microfracture with BST-CarGel results as shown by ICRS-II histological scoring and a novel zonal collagen type scoring method of human clinical biopsy specimens. Am J Sports Med. 2015;43(10):2469-80. [DOI] [PubMed] [Google Scholar]
- 6. Bedi A, Feeley BT, Williams RJ. Management of articular cartilage defects of the knee. J Bone Joint Surg Am. 2010;92(4):994-1009. [DOI] [PubMed] [Google Scholar]
- 7. Krych AJ, Harnly HW, Rodeo SA, Williams RJ. Activity levels are higher after osteochondral autograft transfer mosaicplasty than after microfracture for articular cartilage defects of the knee. J Bone Joint Surg Am. 2012;94(11):971-8. [DOI] [PubMed] [Google Scholar]
- 8. Saris DBF, Vanlauwe J, Victor J, Almqvist KF, Verdonk R, Bellemans J, et al. Treatment of symptomatic cartilage defects of the knee characterized chondrocyte implantation results in better clinical outcome at 36 months in a randomized trial compared to microfracture. Am J Sports Med. 2009;37(1 Suppl):10S-19S. [DOI] [PubMed] [Google Scholar]
- 9. Gobbi A, Karnatzikos G, Kumar A. Long-term results after microfracture treatment for full-thickness knee chondral lesions in athletes. Knee Surg Sports Traumatol Arthrosc. 2014;22(9):1986-96. [DOI] [PubMed] [Google Scholar]
- 10. Solheim E, Hegna J, Inderhaug E, Oyen J, Harlem T, Strand T. Results at 10-14 years after microfracture treatment of articular cartilage defects in the knee. Knee Surg Sports Traumatol Arthrosc. 2014;24(5):1587-93. [DOI] [PubMed] [Google Scholar]
- 11. Mistry H, Connock M, Pink J, Shyangdan D, Clar C, Royle P, et al. Autologous chondrocyte implantation in the knee: systematic review and economic evaluation. Health Technol Assess. 2017;21(6):1-294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Case JM, Scopp JM. Treatment of articular cartilage defects of the knee with microfracture and enhanced microfracture techniques. Sports Med Arthrosc. 2016;24(2):63-8. [DOI] [PubMed] [Google Scholar]
- 13. Koh Y-G, Kwon O-R, Kim Y-S, Choi Y-J, Tak D-H. Adipose-derived mesenchymal stem cells with microfracture versus microfracture alone: 2-year follow-up of a prospective randomized trial. Arthroscopy. 2016;32(1):97-109. [DOI] [PubMed] [Google Scholar]
- 14. Lee KBL, Wang VTZ, Chan YH, Hui JHP. A novel, minimally-invasive technique of cartilage repair in the human knee using arthroscopic microfracture and injections of mesenchymal stem cells and hyaluronic acid—a prospective comparative study on safety and short-term efficacy. Ann Acad Med Singapore. 2012;41(11):511-7. [PubMed] [Google Scholar]
- 15. Lee GW, Son J-H, Kim J-D, Jung G-H. Is platelet-rich plasma able to enhance the results of arthroscopic microfracture in early osteoarthritis and cartilage lesion over 40 years of age? Eur J Orthop Surg Traumatol. 2012;23(5):581-7. [DOI] [PubMed] [Google Scholar]
- 16. Siclari A, Mascaro G, Gentili C, Kaps C, Cancedda R, Boux E. Cartilage repair in the knee with subchondral drilling augmented with a platelet-rich plasma-immersed polymer-based implant. Knee Surg Sport Traumatol Arthrosc. 2013;22(6):1225-34. [DOI] [PubMed] [Google Scholar]
- 17. Saw K-Y, Hussin P, Loke S-C, Azam M, Chen HC, Tay YG, et al. Articular cartilage regeneration with autologous marrow aspirate and hyaluronic acid: an experimental study in a goat model. Arthroscopy. 2009;25(12):1391-400. [DOI] [PubMed] [Google Scholar]
- 18. Strauss E, Schachter A, Frenkel S, Rosen J. The efficacy of intra-articular hyaluronan injection after the microfracture technique for the treatment of articular cartilage lesions. Am J Sports Med. 2009;37(4):720-6. [DOI] [PubMed] [Google Scholar]
- 19. Schiavone Panni A, Cerciello S, Vasso M. The manangement of knee cartilage defects with modified amic technique: preliminary results. Int J Immunopathol Pharmacol. 2011;24(1 Suppl 2):149-52. [DOI] [PubMed] [Google Scholar]
- 20. Pascarella A, Ciatti R, Pascarella F, Latte C, Di Salvatore MG, Liguori L, et al. Treatment of articular cartilage lesions of the knee joint using a modified AMIC technique. Knee Surg Sport Traumatol Arthrosc. 2009;18(4):509-13. [DOI] [PubMed] [Google Scholar]
- 21. Anders S, Volz M, Frick H, Gellissen J. A randomized, controlled trial comparing autologous matrix-induced chondrogenesis (AMIC®) to microfracture: analysis of 1- and 2-year follow-up data of 2 centers. Open Orthop J. 2013;7:133-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gille J, Behrens P, Volpi P, de Girolamo L, Reiss E, Zoch W, et al. Outcome of autologous matrix induced chondrogenesis (AMIC) in cartilage knee surgery: data of the AMIC Registry. Arch Orthop Trauma Surg. 2013;133(1):87-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kusano T, Jakob RP, Gautier E, Magnussen RA, Hoogewoud H, Jacobi M. Treatment of isolated chondral and osteochondral defects in the knee by autologous matrix-induced chondrogenesis (AMIC). Knee Surg Sport Traumatol Arthrosc. 2011;20(10):2109-15. [DOI] [PubMed] [Google Scholar]
- 24. Shive MS, Stanish WD, McCormack R, Forriol F, Mohtadi N, Pelet S, et al. BST-CarGel® treatment maintains cartilage repair superiority over microfracture at 5 years in a multicenter randomized controlled trial. Cartilage. 2015;6(2):62-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Stanish WD, McCormack R, Forriol F, Mohtadi N, Pelet S, Desnoyers J, et al. Novel scaffold-based BST-CarGel treatment results in superior cartilage repair compared with microfracture in a randomized controlled trial. J Bone Joint Surg Am. 2013;95(18):1640-50. [DOI] [PubMed] [Google Scholar]
- 26. Strauss EJ, Barker JU, Kercher JS, Cole BJ, Mithoefer K. Augmentation strategies following the microfracture technique for repair of focal chondral defects. Cartilage. 2010;1(2):145-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. NCSS. Standard deviation estimator. PASS Sample Size Software. Kaysville, UT: NCSS. [Google Scholar]
- 28. Enea D, Cecconi S, Calcagno S, Busilacchi A, Manzotti S, Gigante A. One-step cartilage repair in the knee: collagen-covered microfracture and autologous bone marrow concentrate. A pilot study. Knee. 2015;22(1):30-5. [DOI] [PubMed] [Google Scholar]
- 29. Chung JY, Lee D, Kim TH, Kwack K-S, Yoon KH, Min B-H. Cartilage extra-cellular matrix biomembrane for the enhancement of microfractured defects. Knee Surg Sport Traumatol Arthrosc. 2013;22(6):1249-59. [DOI] [PubMed] [Google Scholar]
- 30. Enea D, Cecconi S, Calcagno S, Busilacchi A, Manzotti S, Kaps C, et al. Single-stage cartilage repair in the knee with microfracture covered with a resorbable polymer-based matrix and autologous bone marrow concentrate. Knee. 2013;20(6):562-9. [DOI] [PubMed] [Google Scholar]
- 31. Dhollander AA, Verdonk PC, Lambrecht S, Almqvist KF, Elewaut D, Verbruggen G, et al. The combination of microfracture and a cell-free polymer-based implant immersed with autologous serum for cartilage defect coverage. Knee Surg Sport Traumatol Arthrosc. 2011;20(9):1773-80. [DOI] [PubMed] [Google Scholar]
- 32. Gille J, Schuseil E, Wimmer J, Gellissen J, Schulz AP, Behrens P. Mid-term results of autologous matrix-induced chondrogenesis for treatment of focal cartilage defects in the knee. Knee Surg Sport Traumatol Arthrosc. 2010;18(11):1456-64. [DOI] [PubMed] [Google Scholar]
- 33. Becher C, Ettinger M, Ezechieli M, Kaps C, Ewig M, Smith T. Repair of retropatellar cartilage defects in the knee with microfracture and a cell-free polymer-based implant. Arch Orthop Trauma Surg. 2015;135(7):1003-10. [DOI] [PubMed] [Google Scholar]
- 34. Siclari A, Mascaro G, Kaps C, Boux E. A 5-year follow-up after cartilage repair in the knee using a platelet-rich plasma-immersed polymer-based implant. Open Orthop J. 2014;8:346-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Saw K-Y, Anz A, Siew-Yoke Jee C, Merican S, Ching-Soong Ng R, Roohi SA, et al. Articular cartilage regeneration with autologous peripheral blood stem cells versus hyaluronic acid: a randomized controlled trial. Arthroscopy. 2013;29(4):684-94. [DOI] [PubMed] [Google Scholar]
- 36. Siclari A, Mascaro G, Gentili C, Cancedda R, Boux E. A cell-free scaffold-based cartilage repair provides improved function hyaline-like repair at one year. Clin Orthop Relat Res. 2012;470(3):910-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hoemann CD, Hurtig M, Rossomacha E, Sun J, Chevrier A, Shive MS, et al. Chitosan-glycerol phosphate/blood implants improve hyaline cartilage repair in ovine microfracture defects. J Bone Joint Surg Am. 2005;87(12):2671-86. [DOI] [PubMed] [Google Scholar]
- 38. Chevrier A, Hoemann CD, Sun J, Buschmann MD. Chitosan-glycerol phosphate/blood implants increase cell recruitment, transient vascularization and subchondral bone remodeling in drilled cartilage defects. Osteoarthritis Cartilage. 2007;15(3):316-27. [DOI] [PubMed] [Google Scholar]
- 39. Fortier LA, Potter HG, Rickey EJ, Schnabel LV, Foo LF, Chong LR, et al. Concentrated bone marrow aspirate improves full-thickness cartilage repair compared with microfracture in the equine model. J Bone Joint Surg Am. 2010;92(10):1927-37. [DOI] [PubMed] [Google Scholar]
- 40. Bark S, Piontek T, Behrens P, Mkalaluh S, Varoga D, Gille J. Enhanced microfracture techniques in cartilage knee surgery: fact or fiction? World J Orthop. 2014;5(4):444-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Dorotka R, Windberger U, Macfelda K, Bindreiter U, Toma C, Nehrer S. Repair of articular cartilage defects treated by microfracture and a three-dimensional collagen matrix. Biomaterials. 2005;26(17):3617-29. [DOI] [PubMed] [Google Scholar]
- 42. Miller RE, Grodzinsky AJ, Barrett MF, Hung HH, Frank EH, Werpy NM, et al. Effects of the combination of microfracture and self-assembling peptide filling on the repair of a clinically relevant trochlear defect in an equine model. J Bone Joint Surg Am. 2014;96(19):1601-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Vaisman A, Figueroa D, Calvo R, Espinosa M, Melean P, Gallegos M, et al. Steroids and platelet-rich plasma as coadjuvants to microfracture for the treatment of chondral lesions in an animal model. Cartilage. 2012;3(2):118-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Milano G, Deriu L, Sanna Passino E, Masala G, Manunta A, Postacchini R, et al. Repeated platelet concentrate injections enhance reparative response of microfractures in the treatment of chondral defects of the knee: an experimental study in an animal model. Arthroscopy. 2012;28(5):688-701. [DOI] [PubMed] [Google Scholar]
- 45. Abdullah L, Davis DE, Fabricant PD, Baldwin K, Namdari S. Is there truly “no significant difference”? Underpowered randomized controlled trials in the orthopaedic literature. J Bone Joint Surg Am. 2015;97(24):2068-73. [DOI] [PubMed] [Google Scholar]
- 46. Arshi A, Siesener NJ, McAllister DR, Williams RJ, 3rd, Sherman SL, Jones KJ. The 50 most cited articles in orthopedic cartilage surgery. Cartilage. 2016;7(3):238-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Frappier J, Stanish W, Brittberg M, Steinwachs M, Crowe L, Castelo D, et al. Economic evaluation of BST-CarGel as an adjunct to microfracture vs microfracture alone in knee cartilage surgery. J Med Econ. 2014;17(4):266-78. [DOI] [PubMed] [Google Scholar]
- 48. Makhni EC, Padaki AS, Petridis PD, Steinhaus ME, Ahmad CS, Cole BJ, et al. High variability in outcome reporting patterns in high-impact ACL literature. J Bone Joint Surg Am. 2015;97(18):1529-42. [DOI] [PubMed] [Google Scholar]
- 49. Morrison A, Polisena J, Husereau D, Moulton K, Clark M, Fiander M, et al. The effect of English-language restriction on systematic review-based meta-analyses: a systematic review of empirical studies. Int J Technol Assess Health Care. 2012;28(2):138-44. [DOI] [PubMed] [Google Scholar]