Abstract
Objective.
The object of present study was to investigate the effects of direct addition of Tribulus terrestris extract on human sperm parameters.
Design.
Semen specimens from 40 healthy men volunteers were divided into 4 groups: one group received no treatment (control group) while the others were incubated with 20, 40, and 50 µg/mL of T terrestris extract (experimental groups). Motility, viability, and DNA fragmentation were assessed in all groups.
Results.
The incubation of human semen with 40 and 50 μg/mL of T terrestris extract significantly enhanced total sperm motility, number of progressive motile spermatozoa, and curvilinear velocity over 60 to 120 minutes’ holding time (P < .05 or P < < .01). Furthermore, viability was significantly enhanced by using T terrestris extract (P < .01).
Conclusions.
In vitro addition of the T terrestris extract to human sperm could affect male fertility capacity.
Keywords: Tribulus terrestris, human sperm, motility, viability, DNA fragmentation
Infertility is described as failure to conceive after one year of intercourse without any prevention,1 approximately 8% to 12% of couples worldwide are infertile.1 Many factors are involved in the process of conception that affects both men and women,2 whereas 40% to 50% of infertility cases are the results of male infertility.2 The initial step in assessment of male factor infertility is the semen quality, which is commonly evaluated by sperm concentration, morphology, and motility via semen analysis.3
The ability of sperm to move properly toward an oocyte is described as sperm motility.4 It is known that sperm motility is an important factor in evaluation of semen quality.4 Insufficient sperm motility is considered as a one of the most important causes of subfertility or infertility.4 The fertilization capacity of sperm is not only dependent on motility but also on other parameters such as viability,5 and sperm DNA fragmentation is increasingly being recognized as important factors of this.6
In recent years, in view of beneficial effects of botanical preparations as medicine, the use of spices and herbs has been gradually increasing in developing countries.7 Although for male infertility improvement, numerous medicinal plants have been investigated, but only a few plants were traditionally used to treat this problem. Use of herbal antioxidants, has been gaining attention in several earlier reports.8–10 Many medicinal plants have high antioxidant potential.11 For instance, the plant species such as Tribulus terrestris have been tested for development of the natural antioxidant formulations in the areas of medicine and nutrition.2,12
Tribulus terrestris L belonging to the family of Zygophyllaceae has been successfully used in Europe and Asia to treat sexual dysfunctions.2 This plant is composed of several biologically active compounds such as steroids, saponins, flavonoids, alkaloids, unsaturated fatty acids, vitamins, tannins, and so on.13 The main active components of T terrestris are saponins from furostanol type that are termed protodioscin14,15 These compounds have been extensively used for treatment of various diseases, such as urinary16 and cardiovascular disorder.17 Several studies have been reported that, administration of T terrestris extract in human and animals improves libido and spermatogenesis.18,19
Previous reports indicated that this plant has a positive effect on the fertility potential of oliguzoospermia patients and reproductive parameters and sperm quality in human, sexual activity, spermatogenesis, and erection of experimental animals such as rat, ram, and rabbit.12,13,20–24 Zheleva-Dimitrova et al10 demonstrated that regard to high antioxidant and inhibited lipid peroxidation activity of T terrestris, this plant could be benefit in infertility therapy.10 Adaay and Mattar25 reported the extract of T terrestris increased sperm concentration and motility and decreased abnormal morphology in mice. There are no data concerning the effect of T terrestris extract on human sperm parameters. Therefore, this study aimed at investigating the effect of T terrestris extract on human sperm parameters in vitro.
Material and Methods
Plant material
Tribulus terrestris was collected from the local vegetable markets in Kermanshah in April-May, 2013. The plant material was identified with the help of experts in Department of Agriculture, Razi University, Kermanshah, Iran.
Fresh plant material was cleaned and shed dried at 25°C. Then, the dried material was ground with a blender. The powder was kept in nylon bags in a freezer (−20°C) before starting the experiments.
Solvent Extraction
The plant powder was thoroughly mixed with distilled water using a stirrer for 24 hours. Then, the mixture was filtered and centrifuged for 15 minutes at 5000 × g. The supernatant was collected and the solvent was evaporated under reduced pressure at 37°C. In the experimental setup using human spermatozoa, the stock solution was mixed in Ham’s-F10 (Sigma), resulting in final concentrations of 20, 40, and 50 µg/mL. Ham’s-F10 without T terrestris extract served as a control agent.
Semen Samples Collection
This study was carried out at Infertility Treatment Research Center, Motazedi Hospital, Kermanshah, Iran. Healthy fertile men (n = 40) aged 20 to 35 years (average 27.8 years) were enrolled. Written informed consent was obtained from the participants before recruitment. Consent forms and protocols were approved by Ethics Committee of Kermanshah University of Medical Sciences.
Semen samples were collected by masturbation into sterile container after 3 days of abstinence and kept at 37°C immediately prior to the examination for liquefying. The nonliquefied samples were checked at 20-minute intervals until they were liquefied. Microscopic analysis was conducted according to World Health Organization (WHO) manual. All the routine semen parameters were consistent with the normal ranges according to WHO guidelines.26–28 Exclusion criteria included abnormal semen analysis according to the WHO criteria,29 sexual transmitted disease (HIV, syphilis, and hepatitis B), or genital tract infection diseases.
Sperm Processing
Semen specimens were preprocessed by swim-up optimization technique to obtain highly motile spermatozoa. They were then diluted with the Ham’s F-10 medium (Sigma) to obtain a sperm suspension with a concentration of 50 × 106/mL. The percentage of progressively motile spermatozoa (level a + b) and sperm motility should be higher than 45% and 80%, respectively.
In Vitro Incubation of Spermatozoa With Tribulus terrestris Extract
The sperms of each sample were divided into 4 groups. For group 1 (control), Ham’s F-10 medium (0 drug concentration) was added. For groups 2, 3, and 4, Ham’s F-10 medium with T terrestris extract at doses of 20, 40, and 50 µg/mL were added, respectively. The parameters recorded for each sample were measured after 15, 30, 60, and 120 minutes of incubation at 37°C, 5% CO2, and humidity 90%.
Sperm Concentration and Motility Analysis
Semen quality analysis was performed using the computer-assisted semen analysis (CASA) version 12 IVOS (Hamilton Thorne Biosciences). For automatic analysis, 5 μL semen samples were dropped on the sperm analysis chamber. Using CASA, at least 10 fields were evaluated regarding sperm concentration, sperm motility, and different sperm motion variables, including percentage of progressive motility (percentage of A + B level of spermatozoa) and movement characteristics such as curvilinear velocity (VCL) and straight line velocity (VSL).
After 0 seconds and once in every 15, 30, 60, and 120 minutes of incubation, the sperm motility parameters were estimated in 10 randomly chosen fields using the CASA system.
Sperm Viability Analysis
Eosin B staining30 was carried out to assess sperm viability. Four drops of semen were mixed thoroughly with one drop of 1% eosin B and mixed well. Immediately, a drop of the mixture was placed on a clean glass slide and allowed to be air dried. The prepared slide was examined that 100 cells per sample were determined. Pink-stained dead sperms were differentiated from unstained live sperm, and their numbers were recorded. The percentage of the live spermatozoa was calculated triplicately.
Determination of DNA Fragmentation
For all the experimental and control groups 30 µL of samples were mixed with70 µL of 1% low-melting point aqueous agarose (to obtain a 0.7% final agarose concentration) at 37°C. Aliquots of 50 mL of the mixture were pipetted onto a precoated glass slide with 0.65% standard agarose dried at 80°C and covered with a coverslip. Then, slides were left to be solidified at 4°C for 4 minutes. The coverslips were removed slowly, then the slides were immediately immersed horizontally in fresh acid denaturation solution (0.08 N HCl) at 22°C for 7 minutes in a dark space. The slides were transferred to a tray with neutralizing and lysing solution No. 1 (0.4 M Tris, 0.8 M dithiothretol [DTT], 1% sodium dodecyl sulfate [SDS], and 50 mM ethylenediamine tetra-acetic acid [EDTA] with pH of 7.5) at room temperature for 10 minutes. All the slides were incubated in neutralizing and lysing solution No. 2 (0.4 M Tris, 2 M NaCl, and 1% SDS with pH of 7.5) at room temperature for 5 minutes. This stage was followed by washing in Tris-borate-EDTA buffer (0.09 M Tris-borate and 0.002 M EDTA with pH of 7.5) for 2 minutes, dehydrating in sequential 70%, 90%, and 100% ethanol baths (each for 2 minutes), and air drying. The cells were stained with Wright stain (1:1 in phosphate buffered saline) (Merck) for 10 minutes. Then the slides were studied with light microscopy under 100× magnification.31
Statistical Analysis
The results of experiments are expressed using mean ± standard error of mean. Differences between two groups were compared by using Student’s t test, and statistical comparisons between experimental groups were made by analysis of variance and Tukey’s post test, as offered by GraphPad InStat version 3.0 (GraphPad Software Inc, La Jolla, CA). Statistical significance was determined as P < .05.
Results
All semen samples were examined using light microscopy and classified as normal according to WHO guidelines.32 Aliquots of all the experimental groups were incubated for 120 minutes with 20, 40, and 50 μg/mL concentration of T terrestris extract and untreated semen under the same conditions was used as a control agent.
Effects of Tribulus terrestris Extract on Sperm Motion Parameters
Sperm motility was assessed in all the treated and untreated groups. The sperm motility was evaluated during counting all motile and immotile spermatozoa in 10 randomly chosen fields using a CASA system. The effect of T terrestris extract on the total motility of sperm in all the experimental and controlling groups are reported in Figure 1. This figure demonstrated that the incubation of human semen with 20 μg/mL of T terrestris extract (group 2) for all holding times (0, 15, 30, 60, and 120 minutes) had no effect on total sperm motility compared with the control. When the concentration of T terrestris extract was elevated to 40 and 50 μg/mL (groups 3 and 4, respectively), the total sperm motility had no increase at 0 to 30-minute holding times, but was significantly was enhanced over 60- to 120-minute holding times (P < .01) compared with the control group (Figure 1).
Figure 1.
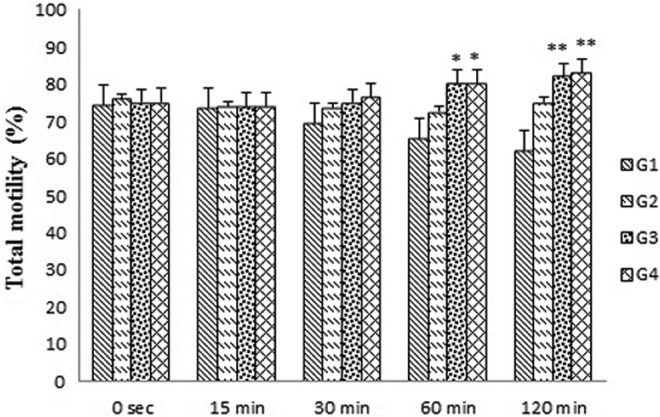
Effects of Tribulus terrestris extract on total motile spermatozoa in human sperm at different concentrations in vitro. Values = mean ± standard error of the mean (SEM), N = 10. *P < .05, **P < .01.
Tribulus terrestris extract could consistently increase the number of progressive motile spermatozoa over 30- to 120-minute holding times in groups 3 and 4 compared with the control group (P < .05 and P < .01, respectively) (Figure 2). In groups 3 and 4, the VCL was significantly enhanced by this extracts for 60 minutes and remained higher until 120 minutes in groups 3 and 4 compared with the control group (P < .05) (Figure 3). The VSL was not significantly changed in all experimental groups after exposure to the drug for 0 to 120-minute holding times (Figure 4).
Figure 2.
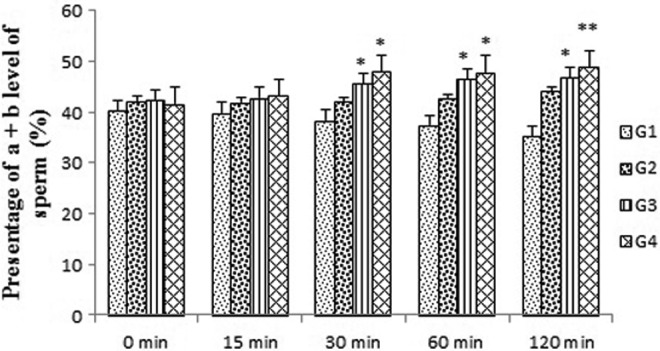
Effects of Tribulus terrestris extract on number of progressive motile spermatozoa in human sperm at different concentrations in vitro. Values = mean ± standard error of the mean (SEM), N = 10. *P < .05, **P < .01.
Figure 3.
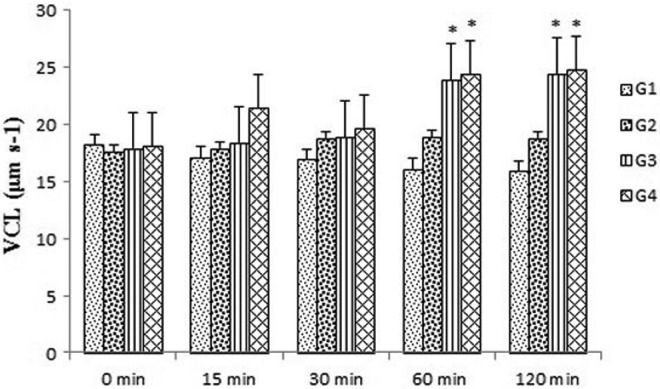
Effects of Tribulus terrestris extract on curvilinear velocity (VCL) in human sperm at different concentrations in vitro. Values = mean ± standard error of the mean (SEM), N = 10. *P < .05.
Figure 4.
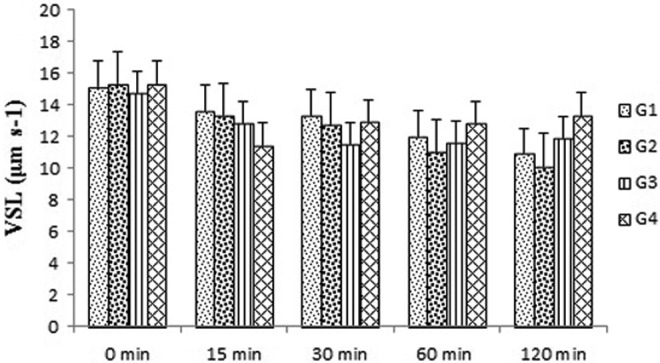
Effects of Tribulus terrestris extracts on straight line velocity (VSL) in human sperm at different concentrations in vitro. Values = mean ± standard error of the mean (SEM), N = 10.
Effect of Tribulus terrestris Extract on Spermatozoa Viability (Live/Dead Ratio)
The effect of T terrestris extract on the viability of sperm are reported in Figure 5. This figure demonstrated that the incubation of human semen with 20 μg/mL (group 2) of T terrestris extract for all the holding times (0, 15, 30, 60, and 120 minutes) had no effect on the viability of sperm versus the controlling group. Effect of T terrestris extract on the viability of sperm was insignificantly changed from 0 to 60 minutes’ exposure time to the drug at dose of 40 and 50 μg/mL (groups 3 and 4), whereas the viability of sperm was considerably increased at doses of 40 and 50 μg/mL of T terrestris extract versus the controlling (P < .05) after 120 minutes’ holding time (Figure 5).
Figure 5.
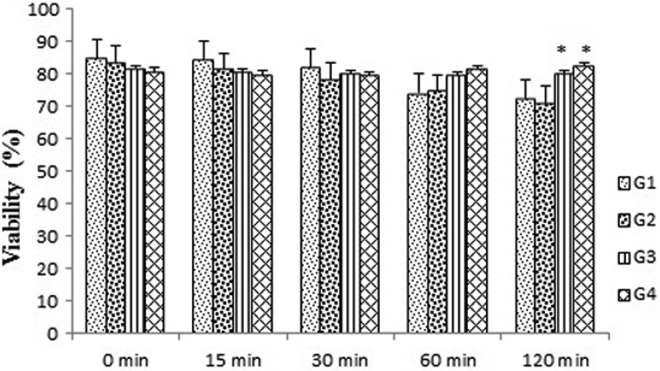
Effects of Tribulus terrestris extract on viability of human sperm at different concentrations in vitro. Values = mean ± standard error of the mean (SEM), N = 10. *P < .05.
Sperm Chromatin Dispersion Test Values
In order to determine the DNA fragmentation levels in semen samples the sperm chromatin dispersion test was performed. The results of sperm chromatin dispersion tests in all experimental groups indicated that T terrestris extract had no significant effect on DNA fragmentation versus the control group (Table 1).
Table 1.
Effect of Tribulus terrestris Extracts on DNA Fragmentation in Human Sperm at Different Concentrations In Vitro.a
| Group | n | Concentration (×106/mL) | DNA Fragmentation | P |
|---|---|---|---|---|
| Group 1 | 10 | 108.66 ± 3.8 | 11.43 ± 1.5 | Nonsignificant |
| Group 2 | 10 | 102.80 ± 2.7 | 12.25 ± 1.3 | Nonsignificant |
| Group 3 | 10 | 105.30 ± 3.3 | 12.00 ± 0.7 | Nonsignificant |
| Group 4 | 10 | 104.40 ± 4.1 | 12.30 ± 0.6 | Nonsignificant |
aValues are presented as mean ± standard error of the mean.
Discussion
The results of present study indicated that T terrestris extract has a considerable effect on the motility and viability of human sperm. However, the extract of T terrestris as a supplementation had no effect on DNA fragmentation of human sperm in vitro.
In procedures such as intrauterine insemination, in vitro fertilization, and gamete intra fallopian transfer, washed spermatozoa is used.33 In many cases of infertility, the problem encountered is poor sperm motility.33 Advanced techniques for optimizing sperm function in these procedures are of evident value.33
Many herbal medicines have been reported to enhance sperm motility. Apparently, the studies about the effect of T terrestris extract on human sperm motility in vitro have not been reported to the best of our knowledge. Previous studies on the in vivo effect of T terrestris extract on sperm motility showed that this extract could significantly increase the sperm motility in mice.25,33
The results of the present work demonstrated that T terrestris extract had considerable effect on improvement of total motile spermatozoa and enhancing the progressive motility due to significantly increase in VCL motility significantly after 60 to 120 minutes of treatment. The results also showed that this extract had no effect on the VSL in all the experiments. The velocity, one of the important sperm motility parameters, had a significant predictive value in male fertility.33 The threshold value for evaluation of sperm motility was known as VCL > 25 μm and was used as special and independent parameter in estimating male fertility by CASA system.34 Recent reports showed that improved VCL, rather than VSL, can be correlated with advanced rates of in vitro fertilization.35 Because mean linearity VSL/VCL, LIN had no increase with a little increase in VCL.
Although the mechanisms of T terrestris extract for improving sperm motility are unknown, Nassar et al36 suggested that a significant stimulatory effect on human sperm motility might have a relation with the trace elements, especially Ca2+ that is known in T terrestris extract. Ca2+ could inhibit the enzyme phosphate diesterase, which prevents cyclic adenosine monophosphate degradation and also enhances the sperm motility.37 Zinc, another trace elements in this extract, leads to improve the sperm motility because of its involvement in protein synthesis and nuclear chromatin stabilization.16 Furthermore, free radicals have an important role on male infertility as the antioxidants could prevent their harmful effects on the sperm.38 It has been reported that antioxidants have the greatest effect on sperm motility.38 T terrestris extract contains total polyphenols, which have a wide class of components such as phenolic acids and flavonols. These components are highly correlated with antioxidant activity.39,40 So this extract with antioxidant compounds has an antioxidant activity, which could be another effective mechanism on human sperm motility improvement.
A useful analysis of sperm is viability test, which is an important factor for in vivo and in vitro male fertilization capacity. There is no previous report on the effect of T terrestris extract for evaluation of the human sperm viability.
The results of this investigation demonstrated that T terrestris extract significantly increased the human sperm viability after 120 minutes of incubation at doses of 40 and 50 μg/mL in vitro. This activity might be through the same effective mechanism of this extract on the sperm motility. Oxidative stress is harmful to sperm function and impairs male fertility by affecting the sperm viability.41 High concentrations of NO could affect the sperm viability by cytotoxic effect that is probably mediated by oxidative stress and lipid peroxidation of sperm membranes.
Another important factor for male infertility is the negative effect of sperm DNA fragmentation. The effect of T terrestris extract on sperm DNA fragmentation was investigated and no change in sperm DNA fragmentation was shown during exposure to the T terrestris extract after 120-minute holding time. This result was similar to a previous investigation about the effect of crude extract of Polygala tenuifolia Willd on sperm DNA fragmentation.42 Lower DNA fragmentation levels in processed semen samples have to be regarded as an effect of the sperm preparation process.43,44
Collectively, the present results showed that the effect of T terrestris extract on human sperm parameters at the dependent doses significantly increased the motility and viability, while it had no positive effect on DNA fragmentation. Nevertheless, further studies are necessary to investigate fertilization capacity of sperms and quality of embryos after exposure to T terrestris extract.
Acknowledgments
Our sincere appreciation goes to Infertility Center in Motazedi Hospital of Kermanshah, Iran.
Footnotes
Author Contributions: All authors contributed to the design, execution, analysis, and write up of this article.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This project was funded by a grant from the Medical Biology Research Center, Kermanshah University of Medical Sciences, Kermanshah, Iran.
Ethical Approval: The project received approval from the Department of Agriculture, Razi University, Kermanshah, Iran.
References
- 1. Kumar N, Singh AK. Trends of male factor infertility, an important cause of infertility: a review of literature. J Hum Reprod Sci. 2015;8:191–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Elahi RK, Asl S, Shahian F. Study on the effects of various doses of Tribulus terrestris extract on epididymal sperm morphology and count in rat. Glob Veterin. 2013;10:13–17. [Google Scholar]
- 3. Fekrazad E, Keyhan H, Fekrazad R, Tajik A. Effect of diode lasers on human sperm motility. Acad Res Int. 2014;5:21–25. [Google Scholar]
- 4. Remya M, Sharma RC, Shoaib H, et al. In vitro effect of Aegle marmelos on human sperm motility. J Med Plants Res. 2009;3:1137–1139. [Google Scholar]
- 5. Mukhopadhyay AK, Kumar A. Follicular Growth Ovulation and Fertilization: Molecular and Clinical Basis. Boca Raton, FL: CRC Press; 2002. [Google Scholar]
- 6. López G, Lafuente R, Checa MA, Carreras R, Brassesco M. Diagnostic value of sperm DNA fragmentation and sperm high-magnification for predicting outcome of assisted reproduction treatment. Asian J Androl. 2013;15:790–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ganguly NK, Medappa N, Srivastava VR. Ginger: its role in xenobiotic metabolism. ICMR Bull. 2003;33:57–58. [Google Scholar]
- 8. Gvozdjakova A, Kucharska J, Lipkova J, et al. Importance of the assessment of coenzyme Q10, α-tocopherol and oxidative stress for the diagnosis and therapy of infertility in men. Bratisl Lekarske Listy. 2012;114:607–609. [DOI] [PubMed] [Google Scholar]
- 9. Liu JH, Li HY, Cao ZG, et al. Influence of several uropathogenic microorganisms on human sperm motility parameters in vitro. Asian J Androl. 2002;4:179–182. [PubMed] [Google Scholar]
- 10. Zheleva-Dimitrova D, Obreshkova D, Nedialkov P. Antioxidant activity of Tribulus terrestris—natural product in infertility therapy. Int J Pharm Pharm Sci. 2012;4:508–511. [Google Scholar]
- 11. Brunetti C, Di Ferdinando M, Fini A, et al. Flavonoids as antioxidants and developmental regulators: relative significance in plants and humans. Int J Mol Sci. 2013;14:3540–3555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sellandi TM, Thakar AB, Baghel MS. Clinical study of Tribulus terrestris Linn. in oligozoospermia: a double blind study. Ayu. 2012;33:356–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Adaikan PG, Gauthaman K, Prasad RN, Ng SC. Proerectile pharmacological effects of Tribulus terrestris extract on the rabbit corpus cavernosum. Ann Acad Med Singapore. 2000;29:22–26. [PubMed] [Google Scholar]
- 14. Kostova I, Dinchev D. Saponins in Tribulus terrestris—chemistry and bioactivity. Phytochem Rev. 2005;4:111–137. [Google Scholar]
- 15. Tomova M, Gjulemetova R, Zarkova S, et al. Steroidal saponins from Tribulus terrestris L. with a stimulating action on the sexual functions. In: Proc 1st Int Conf Chem Biotechnol Biol Active Nat Products Varna. 1981;298–302. [Google Scholar]
- 16. Wang B, Ma L, Liu T. 406 cases of angina pectoris in coronary heart disease treated with saponin of Tribulus terrestris [in Chinese]. Zhong xi yi jie he za zhi. 1990;10:85–87. [PubMed] [Google Scholar]
- 17. Joshi VS, Parekh BB, Joshi MJ, Vaidya AD. Inhibition of the growth of urinary calcium hydrogen phosphate dihydrate crystals with aqueous extracts of Tribulus terrestris and Bergenia ligulata. Urol Res. 2005;33:80–86. [DOI] [PubMed] [Google Scholar]
- 18. Gauthaman K, Ganesan AP, Prasad RNV. Sexual effects of puncturevine (Tribulus terrestris) extract (protodioscin): an evaluation using a rat model. J Altern Complement Med. 2003;9:257–265. [DOI] [PubMed] [Google Scholar]
- 19. Martino-Andrade AJ, Morais RN, Spercoski KM, et al. Effects of Tribulus terrestris on endocrine sensitive organs in male and female Wistar rats. J Ethnopharmacol. 2010;127:165–170. [DOI] [PubMed] [Google Scholar]
- 20. Kistanova E, Zlatev H, Karcheva V, Kolev A. Effect of plant Tribulus terrestris extract on reproductive performances of rams. Biotechnol Anim Husband. 2005;21:55–63. [Google Scholar]
- 21. Roaiah MF, Elkhayat YI, Saleh SF, Abd El Salam MA. Prospective analysis on the effect of botanical medicine (Tribulus terrestris) on Serum testosterone level and semen parameters in males with unexplained infertility. J Diet Suppl. 2016;20:1–7. [DOI] [PubMed] [Google Scholar]
- 22. Shalaby MA, Hammouda AA. Assessment of protective and anti-oxidant properties of Tribulus terrestris fruits against testicular toxicity in rats. J Intercul Ethnopharmacol. 2014;3:113–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sharma P, Huq AU, Singh R. Cypermethrin induced reproductive toxicity in male Wistar rats: protective role of Tribulus terrestris. J Environ Biol. 2013;34:857–862. [PubMed] [Google Scholar]
- 24. Singh S, Nair V, Gupta YK. Evaluation of the aphrodisiac activity of Tribulus terrestris Linn. in sexually sluggish male albino rats. J Pharmacol Pharmacother. 2012;3:43–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Adaay MH, Mattar AG. Effect of aqueous and ethanolic extracts of Tribulus terrestris, Phoenix dactylifera and Nasturtium officinale mixture on some reproductive parameters in male mice. J Baghdad Sci. 2012;9:640–650. [Google Scholar]
- 26. Kistanova E. Improvement of the reproductive performances of rams by the biological active substances—plant extract and probiotic. Biotechnol Anim Husband. 2005;21:69–72. [Google Scholar]
- 27. Kotdawala AP, Kumar S, Salian SR, et al. Addition of zinc to human ejaculate prior to cryopreservation prevents freeze-thaw-induced DNA damage and preserves sperm function. J Assist Reprod Gen. 2012;29:1447–1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Petyim S, Neungton C, Thanaboonyawat I, et al. Sperm preparation before freezing improves sperm motility and reduces apoptosis in post-freezing-thawing sperm compared with post-thawing sperm preparation. J Assist Reprod Gen. 2014;31:1673–1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lu J, Huang Y, Lu N. WHO Laboratory Manual for the Examination and Processing of Human Semen: its applicability to andrology laboratories in China [in Chines]. Zhonghua nan ke xue. 2010;16:867–871. [PubMed] [Google Scholar]
- 30. Aalseth EP, Saacke RG. Vital staining and acrosomal evaluation of bovine sperm. Gamete Res. 1986;15:73–81. [Google Scholar]
- 31. Fernandes JL, Muriel L, Rivero MT, et al. The sperm chromatin dispersion test: a simple method for the determination of sperm DNA fragmentation. J Androl. 2003;24:59–66. [PubMed] [Google Scholar]
- 32. World Health Organization. WHO Laboratory Manual for the Examination of Human Semen and Sperm-Cervical Mucus Interaction. Cambridge, England: Cambridge University Press; 1999. [Google Scholar]
- 33. Liu J, Liang P, Yin C, et al. Effects of several Chinese herbal aqueous extracts on human sperm motility in vitro. Andrologia. 2004;36:78–83. [DOI] [PubMed] [Google Scholar]
- 34. Larsen L, Scheike T, Jensen TK, et al. Computer-assisted semen analysis parameters as predictors for fertility of men from the general population. Hum Reprod. 2000;15:1562–1567. [DOI] [PubMed] [Google Scholar]
- 35. Barlow P, Delvigne A, Van Dromme J, et al. Predictive value of classical and automated sperm analysis for in-vitro fertilization. Hum Reprod. 1991;6:1119–1124. [DOI] [PubMed] [Google Scholar]
- 36. Nassar A, Mahony M, Blackmore P, et al. Increase of intracellular calcium is not a cause of pentoxifylline-induced hyperactivated motility or acrosome reaction in human sperm. Fertil Steril. 1998;69:748–754. [DOI] [PubMed] [Google Scholar]
- 37. Keshtmand Z, Oryan S, Ghanbari A, et al. Protective effect of Tribulus terrestris hydroalcoholic extract against cisplatin-induced cytotoxicity on sperm parameters in male mice. Int J Morphol. 2014;32:551–557. [Google Scholar]
- 38. Alizadeh H, Khaki A, Farzadi L, et al. The therapeutic effects of a medicinal plant mixture in capsule form on catalase levels in the semen of men with oligospermia. Crescent J Med Biol Sci. 2015;34:9–16. [Google Scholar]
- 39. Giovanelli G, Buratti S. Comparison of polyphenolic composition and antioxidant activity of wild Italian blueberries and some cultivated varieties. Food Chem. 2009;112:903–908. [Google Scholar]
- 40. Manach C, Williamson G, Morand C, Scalbert A, Rémésy C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am J Clin Nutr. 2005;81(1 suppl):230S–242S. [DOI] [PubMed] [Google Scholar]
- 41. Sikka SC. Relative impact of oxidative stress on male reproductive function. Curr Med Chem. 2001;8:851–862. [DOI] [PubMed] [Google Scholar]
- 42. Qiu Y, Wang LG, Jia YF, et al. Effects of the crude extract of Polygala tenuifolia Willd on human sperm in vitro. J Zhejiang Univ-Sc B. 2011;12:448–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Jackson RE, Bormann CL, Hassun PA, et al. Effects of semen storage and separation techniques on sperm DNA fragmentation. Fertil Steril. 2010;94:2626–2630. [DOI] [PubMed] [Google Scholar]
- 44. Younglai EV, Holt D, Brown P, et al. Sperm swim-up techniques and DNA fragmentation. Hum Reprod. 2001;16:1950–1953. [DOI] [PubMed] [Google Scholar]


