Abstract
This research was aimed to evaluate anti–herpes simplex virus type-1 (anti-HSV-1) activity of crude ethanol extract and 4 corresponding fractions of Quercus brantii acorn in vitro. Crude ethanol extract was prepared and subjected to fractionation with different polarity. Anti-HSV-1 activity was evaluated on baby hamster kidney cell line using MTT assay. The inhibitory effect of the plant materials on adsorption and/or post-adsorption stages of HSV-1 replication cycle were determined. Regression analysis was used to determine 50% inhibitory concentration and 50% cytotoxicity concentration, from which selective index was calculated. Based on our results, the chloroform fraction and the crude extract had the highest effect against HSV-1 with selectivity indices of 53.8 and 48.4, respectively. The n-hexane, n-butanol, and chloroform fractions inhibited HSV-1 replication in postadsorption stage (P < .001). The results obtained indicated that the chloroform fraction of Q brantii acorn with high inhibitory effect against HSV-1 replication could be a new promising anti-HSV-1 agent.
Keywords: antiviral, Quercus brantii, herpes simplex virus
Human populations experience viral diseases frequently around the world and many of these viral infections remained to be treated or pose resistance to antiviral drugs.1–3 Infections caused by herpes simplex virus (HSV) are public health concern worldwide. These infections range from inapparent to severe life-threatening infections such as encephalitis.4 During the past two decades, the mechanisms of replication and pathogenesis of HSV-1 and thus the potential antiviral targets in this virus have been widely understood and leads to development of antiviral compounds that target this virus.4 A category of nucleoside analogues, acyclovir, is widely used to treat HSV-1 infections as a drug of choice.5 However, a major problem associated with use of acyclovir, is the development of drug-resistant HSV strains, particularly in patients with acquired immune deficiency syndrome.6 Therefore, antiviral agents from medicinal plants with new effective compounds exhibiting different modes of action against viral infections are urgently needed.
Medicinal plants have been used for many years for the treatment of human diseases7–10 and a number of herbal medicines have been developed into therapeutic agents or have had promising results.11–15
Belonging to the family of Fagaceae, genus Quercus contains 500 species, some of which such as Quercus brantii L are predominant in central and northern regions of Iran.16 The fruit of oak tree is called acorn and is placed within a cup called gland. Vitamins, nutrients, and carbohydrates have been reported to comprise a large portion (48%-85%) of acorn components. Acorn also contains considerable amounts of phenolic, tannin, catechin, epicathechin, and gallocatechin components.16–19
There are some reports indicating different biological activities of some species of genus Oak.20,21 Different species of Quercus have been reported to have antibacterial activity,17,22,23 antiviral activity,24,25 antioxidant activity,26,27 and gastroprotective effect.28
To the best of our knowledge, to date, there has been no report on the antiviral activity of different fractions of Q brantii acorn. Therefore, this research was aimed to prepare crude ethanol extract and 4 corresponding fractions of Q brantii acorn and to evaluate anti-HSV-1 activity of these plant materials in vitro.
Materials and Methods
Plant Collection
The fruits of oak (Q brantii) were gathered from the mountains around the Lordegan city southwest region of Iran. The genus and species of the plant were identified and confirmed by Professor M. Rafieian, in the herbarium of Medical Plants Research Center of Shahrekord University of Medical Sciences, Iran (herbarium number 325).
Extraction and Fractionation of Plant Material
Extraction and fractionation were according to Moradi et al26 with some modification. The acorn powder was dissolved in 70% ethyl alcohol and kept at room temperature for 96 hours. After that, the mixture was filtered and concentrated under nearly vacuum pressure and at 40°C using rotary evaporator. The process was repeated 3 times with intervals of 4 days. The crude extract was dissolved in 70% ethyl alcohol, and partitioned respectively with hexane, chloroform, ethyl acetate, and butanol. The hexane, chloroform, ethyl acetate, butanol, and last remaining aqueous fractions were evaporated to obtain fractions.26 The extracts were kept in sterile bottles, under refrigerated conditions, until further use. The extracts were suspended at 37°C in dimethyl sulfoxide (DMSO) to give a stock solution of 25 mg/mL, dissolved in culture medium, filtered (Millipore 0.22 μm) and stored at 4°C until use. The small percentage of DMSO present in the wells (maximal 0.2%) was found not to affect the experiment.29
Determination of Total Phenolic Content
The total phenolic content of the crude extract and 4 corresponding fractions of Q brantii fruits was determined using Folin-Ciocalteu method.30 Briefly, 0.1 mL of each of the diluted samples was added to 0.5 mL of 10% (v/v) Folin-Ciocalteu reagent and kept at room temperature for 3 to 8 minutes. Subsequently, 0.4 mL of 7.5% (w/v) sodium carbonate solution was added to the mixture. After being kept in total darkness for 30 minutes, the absorbance of the reaction mixture was measured at 765 nm using a ultraviolet-visible spectrophotometer (UNICO 2100). The total phenolic content were calculated using a gallic acid calibration curve. The results were expressed as milligrams gallic acid equivalents (GAE) per gram of dry plant matter.
Determination of Total Flavonoid and Flavonol Content
The total flavonoid and flavonol content of the extracts were measured as previously reported method.31 Briefly, 0.5 mL of each diluted plant material was independently mixed with 1.5 mL of methanol, 0.1 mL of 10% (w/v) aluminum chloride, 0.1 mL of 1 M potassium acetate, and 2.8 mL of distilled water. Following incubation at room temperature for 40 minutes (for total flavonoid) and for 2.5 hours (for total flavonol), the absorbance of the reaction mixture was read at 415 nm for total flavonoid and 440 nm for total flavonol) using an ultraviolet-visible spectrophotometer (UNICO 2100). The results were expressed in milligrams of rutin equivalents per gram of dry plant matter (mg RUT/g) by comparison with the standard curve, which was made in the same condition.
All measurements were carried out in triplicate and statistical analysis was done by statistical software using 1-way analysis of variance and the post hoc Tukey’s test.
Cell and Virus
Baby hamster kidney (BHK) was kindly provided by Pasteur Institute of Iran. The cells were grown in Dulbecco’s modified Eagle’s medium (DMEM; Sigma) supplemented with 10% of fetal bovine serum (FBS; Gibco), 100 µg/mL of streptomycin, 100 UI/mL of penicillin, and 0.25 µg/mL amphotericin B (Gibco), at 37°C and 5% CO2. The same medium containing 2% phosphate-buffered saline (PBS) was used for cytotoxicity and antiviral assays. HSV-1 (HSV-1, KOS strain) was kindly provided by University of Tarbiat Modares, Tehran, Iran. Virus stock was prepared by infection of confluent monolayer BHK cells in 75 cm2 culture flasks using DMEM medium with 2% FBS, at 37°C in 5% CO2. Virus titer was determined by cytopathic effect (CPE) of HSV-1 in BHK cells and was expressed as the 50% tissue culture infective dose (TCID50) per milliliter.
Cytotoxicity Assay
Prior to the investigation of anti-HSV-1 activity, the cytotoxic effect of the test compounds was determined. Briefly, BHK cells were seeded onto 96-well plates with a concentration of 10 000 cells/well with final volume of 100 µL per well. After incubation at 37°C for 24 hours, when the cell monolayer was confluent, the cell culture medium of cells aspirated and washed with PBS. Cells were incubated with 100 µL/well of various concentrations of ethanolic extract or each fraction (in triplicates) and incubated for further 3 days. The number of living cells was determined by the MTT [3-(4, 5-dimethylthiazol-2ol) 2,5 diphenyltetrazolium bromide] assay.32 Briefly, the supernatants were removed from the wells and 50 μL of an MTT (Sigma) solution (1 mg/mL in PBS) was added to each well. The plates were incubated for 4 hours at 37°C, and 100 μL of DMSO (Samchun Korea) was added to the wells to dissolve the MTT crystals. The plates were placed on a shaker for 15 minutes and the absorbance were read on an enzyme-linked immunosorbent assay reader (STATA FAX 2100) at 492 nm. Data were calculated as the percentage of toxicity using the following formula: toxicity (%) = [100 – (At/As) ×100]%, where At and As refer to the absorbance of the test substance and the solvent control, respectively.29 The 50% cytotoxic concentration (CC50) was defined as the cytotoxic concentration of the crude extract and the fractions by regression analysis.
Antiviral Assay
Antiviral activity of the fractions was evaluated by inhibitory activity assay using MTT method, as described previously.29 Briefly, 100 µL (100TCID50) virus suspension was added to confluent BHK cell monolayer in a 96-well plate, and incubated at 37°C for about 1 hour to allow virus adsorption. Thereafter, serial 2-fold dilutions prepared from nontoxic dose of the crude extract and the fractions (below the CC50 value) were added and tested in triplicate. As virus control, cells were infected with the same concentration of virus but without addition of extract. As a cell control, only 1% DMEM was added to the cells. The plates were incubated at 37°C for 3 days. DMSO with 0.1% concentration and a solution of acyclovir (Sigma) were used as negative and positive controls, respectively. Cell viability was also determined using previously described MTT assay.29 Data were calculated as the percentage of inhibition using the following formula: Antiviral activity (%) = (Atv – Acv)/(Acd – Acv) × 100%, where Atv, Acv, and Acd are the absorbance of the test compounds on virus infected cells, the absorbance of the virus control, and the absorbance of the cell control, respectively.29 The experiment was performed in triplicate. The 50% inhibitory concentration (IC50) was determined from a curve relating inhibition to the concentration of each extract/fractions. Selectivity index, as a marker of antiviral activity, was determined as the ratio of CC50 to IC50.
Time-of-Addition Assay
The time-of-addition effect of the crud extract and the corresponding fractions of Q brantii was evaluated with minor modifications in previously described method.33 To assess the effect of both the extract and the fractions both in the adsorption and postadsorption events of HSV-1 replication, the virus was treated with one time IC90 of these plant materials (crud extract = 10, n-butanol fraction = 6, chloroform fraction = 7, n-hexane fraction = 274, and remaining aqueous fraction = 127μg/mL) in 3 different manners, as both the extract and the fractions were present; (1) only during the adsorption period (adsorption), (2) after adsorption and until the end of the experiment (postadsorption), and (3) during and after the adsorption (throughout) (Figure 1). To carry out these experiments, 90% confluent cells were chilled at 4°C for 1 hour followed by infection with100 µL/well of HSV-1 (100TCID50) in the presence or absence of extract/fractions and further incubated at 4°C for 1 hour allowing only the adsorption step of the viral particles to the cells (adsorption). Subsequently, the supernatant was removed, the cells were washed twice with ice-cold PBS, and the medium with or without the extract/fractions was replaced with an equal volume of DMEM and 1% PBS, and incubated for 3 days. Using MTT assay, cell viability and the percentage of viral inhibition was evaluated compared with the control. Data represented as the percentage of virus inhibition compare with untreated control as mean ± standard deviation (n = 3). Statistical analysis was carried out by statistical software using 1-way analysis of variance and the post hoc Tukey’s test.
Figure 1.
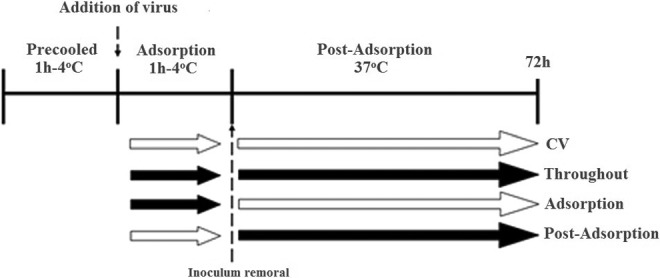
Scheme of addition of extract/fractions in the adsorption and post-adsorption stages of herpes simplex virus type-1 (HSV-1).33 Open and black arrows indicate the absence and presence of extract, respectively.
Statistical Analysis
All experiences were carried out in triplicate. The IC50 and CC50 values were calculated using dose-response analyses and related models with probit procedure using SPSS program. A P value of less than .05 was considered statistically significant.
Results
Total Phenolic, Flavonoid, and Flavonol Compounds
To standardize the crude extract and the corresponding fractions, total phenolic, flavonoid, and flavonol compounds were measured. Total amount of phenolic compounds showed that among the 4 fractions, the n-butanol (376.2 ± 7.1 mg GAE/g) and the n-hexane (24.4 ± 3.5 mg GAE/g) had the highest and the lowest amount of total phenolic compounds, respectively. The total phenolic of the crude extract, the chloroform fraction, the n-butanol fraction, and the aqueous fraction were significantly different from that of the n-hexane fraction (P < .05; Table 1). The highest amount of flavonoid and flavonol (91.6 and 105 mg RUT/g, respectively) were in cholorophorm fraction and the lease amount (5.8 and 1.1 mg RUT/g, respectively) was in aqueous fraction (P < .05; Table 1).
Table 1.
Total Phenolics, Flavonoid, and Flavonol Values of the Different Fractions of Quercus brantii Fruita
| Sample | Total Phenolicsb | Flavonoid Contentc | Flavonol Contentc |
|---|---|---|---|
| Crude extract | 201.6 ± 4.0 | 14.7 ± 2.2 | 33.6 ± 2.0 |
| n-Hexane fraction | 24.4 ± 3.5 | 16.8 ± 2 | 2.4 ± 0.5 |
| Choloroform fraction | 222.5 ± 2.5 | 91.6 ± 1.5 | 105 ± 4.5 |
| n-Butanol fraction | 376.2 ± 7.1 | 22 ± 2 | 70.9 ± 55 |
| Remaining aqueous fraction | 120 ± 3.5 | 5.8 ± 0.5 | 1.1 ± 0.4 |
| P | <.01 | <.01 | <.01 |
aAll results are presented as mean ± standard error of the mean of 3 assays.
bMilligrams gallic acid equivalent per gram of extract powder.
cMilligrams rutin equivalent per gram of extract powder.
Cytotoxicity and Anti-HSV-1 Activity
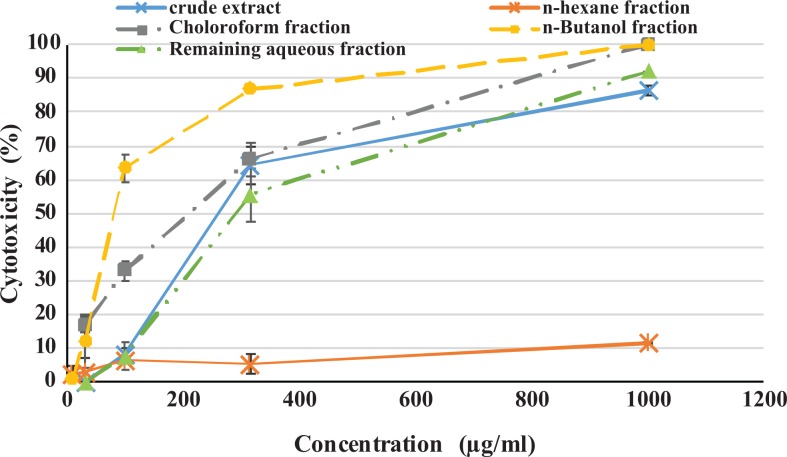
Based on MTT results and probit analysis the CC50 value of crude extract, n-butanol fraction, and chloroform fraction was 208, 112.5, and 156.1 µg/mL, respectively (Table 2). The analysis showed that there was significant relationship between the concentration of the crude extract, n-butanol, chloroform, and remaining aqueous fractions and cell death, with more extract concentration, more cell death (P < .01; Figure 2).
Table 2.
Cell Cytotoxicity, Anti–Herpes Simplex Virus Type-1 Activity, and Selectivity Index of the Crude Extract and 4 Corresponding Fractions of Quercus brantii Fruit.a
| Extract | Cell Cytotoxicityb (CC50, μg/mL) | Antiviral Activityc (IC50, μg/mL) | Selectivity Indexd |
|---|---|---|---|
| Crude extract | 208 | 4.3 | 48.4 |
| n-Hexane fraction | >1000 | 120.5 | >8.3 |
| Chloroform fraction | 156.1 | 2.9 | 53.8 |
| n-Butanol fraction | 112.5 | 3.8 | 29.6 |
| Remaining aqueous fraction | 330 | 59 | 5.6 |
| Acyclovire | 177.5 | 1.3 | 136.5 |
aCell cytotoxicity effect and antiviral activity was determined by MTT assay.
bCC50 was the concentration that showed 50% cellular cytotoxic effect.
cIC50 was the concentration that inhibited 50% of HSV-1.
dSelectivity index is the ratio of CC50 to IC50.
eAcyclovir was included as positive control for the antiviral activity of HSV-1.
Figure 2.
Cytotoxicity of crude ethanol extract and four corresponding fractions of Quercus brantii L acorn on baby hamster kidney (BHK) cells. Confluent BHK cells were exposed to different concentrations of crude ethyl alcohol extract and 4 fractions for 48 hours. Cytotoxicity was measured in MTT assay. Experiences were carried out in triplicate.
Our results showed that more the extract concentration, more the CPE inhibition in the crude extract, chloroform fraction, and n-butanol fraction (P < .05). Based on probit analysis, IC50 of crude extract, n-butanol fraction, and chloroform fraction, was 4.3, 3.8, and 2.9 µg/mL, respectively. The chloroform fraction, n-butanol fraction, and crude extract showed highest effect against HSV-1 with selectivity indicess of 53.8, 29.6, and 48.4, respectively (Table 2). There was good relation between the total flavonoid contents and the selectivity indices in the 4 fractions in this study (R = 0.984, P = .002).
Characterization of Antiviral Activity
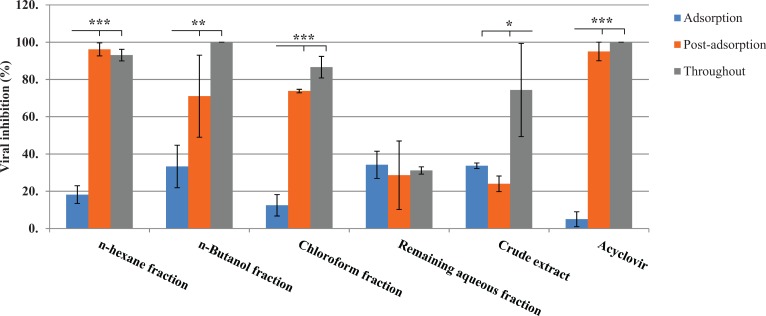
To assess the effect of the extract and the fractions both in the adsorption and postadsorption events of HSV-1 replication, different experiments were carried out with one time IC90 of these plant materials (Figure 1). Our results showed that n-hexane and chloroform fractions and acyclovir inhibit HSV-1 replication in the postadsorption stage (P < .001). There was no significance difference in the percentage of postadsorption viral inhibition when n-hexane and chloroform fractions and acyclovir were present during all the experimental time (throughout). In contrast, crude extract and n-butanol fraction increased the percentage of viral inhibition when it was added in the throughout step of HSV-1 to BHK cells (P < .05). The increase of viral inhibition observed in the postadsorption stage could be due to the inhibitory effect of the n-hexane and chloroform fractions on the replication steps of HSV-1 in BHK cells (Figure 3).
Figure 3.
The effect of crud extract and the corresponding fractions of Quercus brantii fruit on the adsorption and postadsorption of herpes simplex virus type-1 (HSV-1) to the baby hamster kidney (BHK) cell. Data represent the percentage of virus inhibition compared with untreated control as mean ± SD (n = 3). One time 90% inhibitory concentration (IC90) was used for this experiment. Statistical analysis was done using statistical software with 1-way analysis of variance and the post hoc Tukey’s test. ***P < .001 adsorption versus postadsorption and throughout. **P < .05 adsorption versus postadsorption and throughout. *P < .05 throughout versus postadsorption and adsorption.
Discussion
Initial screening of plants for exploring their possible active natural products typically begins by using crude aqueous or alcohol extraction and can be followed by various organic extraction methods. These naturally occurring products are often obtained through initial ethanol or methanol extraction.34 In this study, using ethanol as crude extraction solvent, we have shown that the ethanol crude extract and four corresponding fractions of Q brantii fruits have high antiviral effect against HSV-1. Although the antiviral activity of different species of Quercus has been previously reported,24,25 this is the first report on the antiviral activity of corresponding fractions of Q brantii fruit. In this report, crude ethanol extract of Q brantii fruits subjected to bioactivity guided fractionation using solubilization sequential partition with different solvents and with increasing polarity include n-hexane, chloroform, n-butanol, and remaining fractions.
Based on our results, the chloroform fraction showed highest effect against HSV-1 with selectivity index of 156.1. Our findings also indicated that the chloroform fraction had the highest flavonoid and flavonol content among the other fractions studied with significant relationship between flavonoid content and selectivity index (P = .002).
Chemical composition screening has shown that flavonoids, alkaloids, glycosides, tannins, phenolic compounds, resins, saponins, terpenes, and steroids are the main components of Quercus species acorn with no alkaloids and saponins compound detection.17,35,36 This potential has been attributed to phenolic compounds of Quercus species37 such as flavonoids and tannins.38–40
Flavonoids have been reported to have antiviral effect against a number of viruses, including dengue virus, hepatitis B virus, human cytomegalovirus herpes simplex virus, respiratory syncytial virus, parainfluenza virus, and adenovirus.41–45 The interaction of these plant materials with intracellular stages of viral replication cycle in some viruses was previously described.46 Glycone form of flavonoids has been shown to be more effective on rotavirus than their aglycone form.47 The flavonoid fraction was shown to be effective against both HSV-1 and HSV-2 with inhibitory effect on replication cycle of HSV-1.48,49 Therefore, in consistent with these findings, our results might indicate that promising anti-HSV-1 activity of chloroform fraction could be attributed to its flavonoid content.
Quercus species are a rich source of polyphenols and tannins,17,35,36 and tannins are contained in bark and fruits of various oak types. Tannins are subclassified into 2 kinds of condensed tannins (proanthocyanidins and gallotannins) and hydrolyzable tannins (ellagitannins). Condensed tannins have antiviral effects against both influenza A virus and HSV-1.50–52 The effect of condensed tannins on HSV-1 infection seems to be due to preventing the entry of the virus into the host cell, which is the first critical step in HSV-1 replication.53 Hydrolysable tannins also possess antiviral activities, particularly against HIV infection54,55 and manifest inhibitory effect on HSV-1 and/or HSV-2 replication including acyclovir-resistant strains, as well as Epstein-Barr virus.56 Thus, consistent with these results, the high anti-HSV-1 activity of the chloroform fraction revealed in this study most probably is due to its hydrolysable tannins.
On characterizing the antiviral activity, our results showed that n-hexane, n-butanol, and chloroform fractions inhibited HSV-1 replication during the postadsorption stage. This finding is in accordance with the reported results of Visintini Jaime et al,33 which demonstrated that Baccharis gaudichaudiana organic extract (30 μg/mL) reduced the formation of PV-2 plaques when it was added after the adsorption period.33
Conclusion
Based on our results, the chloroform fraction of Q brantii fruit with high inhibitory effect against HSV-1 replication could be a new promising anti-HSV-1 agent. More understanding of the mechanism of action and the natural components of this fraction seems to be valuable. The results of this study also showed high level of flavonoids in the extract. Hence, the antiviral activity of this plant might, in part, be attributed to flavonoid contents.
Acknowledgments
Authors are thankful to the Director of Medical Plants Research Center and to the Deputy of Research and Technology of Shahrekord University of Medical Sciences, Shahrekord, Iran, for financial support.
Footnotes
Author Contributions: AK contributed to the design of the study, supervised the work scientifically, and edited the English manuscript. MR-K contributed to the design and protocol and the scientific supervision. M-TM developed the original idea, analyzed and abstracted the data, and prepared the manuscript. SA contributed to the data collection and laboratory testing.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study received financial support from the Director of Medical Plants Research Center and the Deputy of Research and Technology of Shahrekord University of Medical Sciences, Shahrekord, Iran.
Ethical Approval: The study protocol was confirmed by Ethical Committee of Shahrekord University of Medical Science, Shahrekord, Iran.
References
- 1. Moezzi M, Imani R, Khosravi N, Pourheidar B, Ganji F, Karimi A. Hepatitis B seroprevalence and risk factors in adult population of Chaharmahal and Bakhtiari Province in 2013. Hepat Mon. 2014;14:e17398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Karimi A, Hoseini SM. Seroprevalence of hepatitis B and C virus and HIV markers among blood donors from Shahre-Kord, Iran (2004-2006). Kuwait Med J. 2008;40:285–287. [Google Scholar]
- 3. Karimi A, Imani-Rastabi R, Moezzi M, Moradi MT. Hepatitis A seroprevalence and associated risk factors: a community based cross-sectional study in Shahrekord, Iran. Arch Clin Infect Dis. 2016;11:e32288. [Google Scholar]
- 4. Koelle DM, Corey L. Herpes simplex: insights on pathogenesis and possible vaccines. Annu Rev Med. 2008;59:381–395. [DOI] [PubMed] [Google Scholar]
- 5. Villarreal EC. Current and potential therapies for the treatment of herpes-virus infections. Prog Drug Res. 2003;60:263–307. [DOI] [PubMed] [Google Scholar]
- 6. Elion GB. Acyclovir: discovery, mechanism of action, and selectivity. J Med Virol. 1993;41:2–6. [DOI] [PubMed] [Google Scholar]
- 7. Sarrafchi A, Bahmani M, Shirzad H, Rafieian-Kopaei M. Oxidative stress and Parkinson’s disease: new hopes in treatment with herbal antioxidants. Curr Pharm Des. 2016; 22: 238–246. [DOI] [PubMed] [Google Scholar]
- 8. Rafieian-Kopaei M, Sewell RD. The history and ups and downs of herbal medicines usage. J HerbMed Pharmacol. 2014;3:1–3. [Google Scholar]
- 9. Asadi-Samani M, Moradi MT, Bahmani M, Shahrani M. Antiviral medicinal plants of Iran: A review of ethnobotanical evidence. Int J Pharm Tech Res. 2016;9:427–434. [Google Scholar]
- 10. Bahmani M, Sarrafchi A, Shirzad H, Rafieian-Kopaei M. Autism: pathophysiology and promising herbal remedies. Curr Pharm Des. 2016;22:277–285. [DOI] [PubMed] [Google Scholar]
- 11. Moradi MT, Rafieian-Kopaei M, Karimi A. A review study on the effect of Iranian herbal medicines against in vitro replication of herpes simplex virus. Avicenna J Phytomed. 2016;6:506–515. [PMC free article] [PubMed] [Google Scholar]
- 12. Shahrani M, Rafieian M, Shirzad H, et al. Effect of Allium sativum L. extract on acid and pepsin secretion in basal condition and stimulated with vag stimulate in rat. J Med Plants. 2007;6:28–37. [Google Scholar]
- 13. Baradaran A, Rabiei Z, Rafieian M, Shirzad H. A review study on medicinal plants affecting amnesia through cholinergic system. J Herbmed Plarmacol. 2012;1:3–9. [Google Scholar]
- 14. Moradi MT, Karimi A, Alidadi S. In vitro antiproliferative and apoptosis-inducing activities of crude ethyle alcohole extract of Quercus brantii L. acorn and subsequent fractions. Chin J Nat Med. 2016;14:196–202. [DOI] [PubMed] [Google Scholar]
- 15. Karimi A, Moradi MT, Alidadi S, Hashemi L. Anti-adenovirus activity, antioxidant potential, and phenolic content of black tea (Camellia sinensis Kuntze) extract [published online August 30, 2016]. J Complement Integr Med. doi:10.1515/jcim-2016-0050. [DOI] [PubMed] [Google Scholar]
- 16. Saffarzadeh A, Vincze L, Csapo J. Determination of the chemical composition of acorn (Quercus branti), Pistacia atlantica and Pistacia Khinjk seeds as non-conventional feedstuffs. Acta Agr Kapos. 1999;3:59–69. [Google Scholar]
- 17. Andrensek S, Simonovska B, Vovk I, Fyhrquist P, Vuorela H, Vuorela P. Antimicrobial and antioxidative enrichment of oak (Quercus robur) bark by rotation planar extraction using ExtraChrom. Int J Food Microbiol. 2004;92:181–187. [DOI] [PubMed] [Google Scholar]
- 18. Cadahia E, Munoz L, Fernandez de Simon B, Garcia-Vallejo MC. Changes in low molecular weight phenolic compounds in Spanish, French, and American oak woods during natural seasoning and toasting. J Agric Food Chem. 2001;49:1790–1798. [DOI] [PubMed] [Google Scholar]
- 19. Popovic BM, Stajner D, Zdero R, Orlovic S, Galic Z. Antioxidant characterization of oak extracts combining spectrophotometric assays and chemometrics. ScientificWorldJournal. 2013;2013:134656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Aslani A, Emami S, Ghannadi A, Ajdari M. Formulation and physicochemical evaluation of an herbal antihemorrohid ointment from quercus, black cumin and fenugreek for the treatment of internal anal hemorrhoids. J Pharm Sci Tabriz Univ Med Sci. 2009;14:247–257. [Google Scholar]
- 21. Kaur G, Hamid H, Ali A, Alam MS, Athar M. Antiinflammatory evaluation of alcoholic extract of galls of Quercus infectoria. J Ethnopharmacol. 2004;90:285–292. [DOI] [PubMed] [Google Scholar]
- 22. Jamil M, ul Haq I, Mirza B, Qayyum M. Isolation of antibacterial compounds from Quercus dilatata L. through bioassay guided fractionation. Ann Clin Microbiol Antimicrob. 2012;11:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gulluce M, Adiguzel A, Ogutcu H, Sengul M, Karaman I, Sahin F. Antimicrobial effects of Quercus ilex L. extract. Phytother Res. 2004;18:208–211. [DOI] [PubMed] [Google Scholar]
- 24. Karimi A, Moradi MT, Saeedi M, Asgari S, Rafieian-Kopaei M. Antiviral activity of Quercus persica L.: High efficacy and low toxicity. Adv Biomed Res. 2013;2:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Muliawan SY, Kit LS, Devi S, Hashim O, Yusof R. Inhibitory potential of Quercus lusitanica extract on dengue virus type 2 replication. Southeast Asian J Trop Med Public Health. 2006;37:132–135. [PubMed] [Google Scholar]
- 26. Moradi MT, Karimi A, Alidadi S, Ghasemi-Dehkordi P, Ghaffari-Goosheh MS. Cytotoxicity and in vitro antioxidant potential of Quercus brantii acorn extract and the corresponding fractions. Int J Pharm Phytochem Res. 2016;8:558–562. [Google Scholar]
- 27. Chevolleau S, Mallet JF, Debal A, Ucciani E. Antioxidant activity of mediterranean plant leaves: occurrence and antioxidative importance of α-tocopherol. J Am Oil Chem Soc. 1993;70:807–809. [Google Scholar]
- 28. Gharzouli K, Khennouf S, Amira S, Gharzouli A. Effects of aqueous extracts from Quercus ilex L. root bark, Punica granatum L. fruit peel and Artemisia herba-alba Asso leaves on ethanol-induced gastric damage in rats. Phytother Res. 1999;13:42–45. [DOI] [PubMed] [Google Scholar]
- 29. Jadhav P, Kapoor N, Thomas B, Lal H, Kshirsagar N. Antiviral potential of selected Indian medicinal (ayurvedic) plants against herpes simplex virus 1 and 2. N Am J Med Sci. 2012;4:641–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Folin O, Ciocalteu V. On tyrosine and tryptophane determinations in proteins. J Biol Chem. 1927;73:627–650. [Google Scholar]
- 31. Asgari S, Setorki M, Rafieian-Kopaei M, et al. Postprandial hypolipidemic and hypoglycemic effects of Allium hertifolium and Sesamum indicum on hypercholesterolemic rabbits. Afr J Pharm Pharmacol. 2012;6:1131–1135. [Google Scholar]
- 32. Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. [DOI] [PubMed] [Google Scholar]
- 33. Visintini Jaime MF, Redko F, Muschietti LV, Campos RH, Martino VS, Cavallaro LV. In vitro antiviral activity of plant extracts from Asteraceae medicinal plants. Virol J. 2013;10:245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Vilegas JHY, Marchi E, Lancas FM. Extraction of low-polarity compounds (with emphasis on coumarin and kaurenoic acid) from Mikania glomerata (‘guaco’) leaves. Phytochem Anal. 1997;8:266–270. [Google Scholar]
- 35. Rakić S, Maletić RO, Perunović MN, Svrzić G. Influence of thermal treatment on tannin content and antioxidation effect of oak acorn Quercus cerris extract. J Agric Sci. 2004;49:97–107. [Google Scholar]
- 36. Kamalak A, Canbolat O, Ozay O, Aktas S. Nutritive value of oak (Quercus spp.) leaves. Small Ruminant Res. 2004;53:161–165. [Google Scholar]
- 37. Ohemeng KA, Schwender CF, Fu KP, Barrett JF. DNA gyrase inhibitory and antibacterial activity of some flavones(1). Bioorg Med Chem Lett. 1993;3:225–230. [Google Scholar]
- 38. Meng Z, Zhou Y, Lu J, Sugahara K, Xu S, Kodama H. Effect of five flavonoid compounds isolated from Quercus dentata Thunb on superoxide generation in human neutrophils and phosphorylation of neutrophil proteins. Clin Chim Acta. 2001;306:97–102. [DOI] [PubMed] [Google Scholar]
- 39. Ito H, Yamaguchi K, Kim TH, Khennouf S, Gharzouli K, Yoshida T. Dimeric and trimeric hydrolyzable tannins from Quercus coccifera and Quercus suber. J Nat Prod. 2002;65:339–345. [DOI] [PubMed] [Google Scholar]
- 40. Zhentian L, Jervis J, Helm RF. C-Glycosidic ellagitannins from white oak heartwood and callus tissues. Phytochemistry. 1999;51:751–756. [Google Scholar]
- 41. Chiang LC, Chiang W, Liu MC, Lin CC. In vitro antiviral activities of Caesalpinia pulcherrima and its related flavonoids. J Antimicrob Chemother. 2003;52:194–198. [DOI] [PubMed] [Google Scholar]
- 42. Evers DL, Chao CF, Wang X, Zhang Z, Huong SM, Huang ES. Human cytomegalovirus-inhibitory flavonoids: studies on antiviral activity and mechanism of action. Antiviral Res. 2005;68:124–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lyu SY, Rhim JY, Park WB. Antiherpetic activities of flavonoids against herpes simplex virus type 1 (HSV-1) and type 2 (HSV-2) in vitro. Arch Pharm Res. 2005;28:1293–1301. [DOI] [PubMed] [Google Scholar]
- 44. Xu G, Dou J, Zhang L, Guo Q, Zhou C. Inhibitory effects of baicalein on the influenza virus in vivo is determined by baicalin in the serum. Biol Pharm Bull. 2010;33:238–243. [DOI] [PubMed] [Google Scholar]
- 45. Zandi K, Teoh BT, Sam SS, Wong PF, Mustafa MR, Abubakar S. Antiviral activity of four types of bioflavonoid against dengue virus type-2. Virol J. 2011;8:560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kaul TN, Middleton E, Jr, Ogra PL. Antiviral effect of flavonoids on human viruses. J Med Virol. 1985;15:71–79. [DOI] [PubMed] [Google Scholar]
- 47. Bae EA, Han MJ, Lee M, Kim DH. In vitro inhibitory effect of some flavonoids on rotavirus infectivity. Biol Pharm Bull 2000;23:1122–1124. [DOI] [PubMed] [Google Scholar]
- 48. Garrett R, Romanos MTV, Borges RM, Santos MG, Rocha L, da Silva AJR. Antiherpetic activity of a flavonoid fraction from Ocotea notata leaves. Rev Bras Farmacogn. 2012;22:306–313. [Google Scholar]
- 49. Kemertelidze EP, Shalashvili KG, Korsantiya BM, Nizharadze NO, Chipashvili NS. Therapeutic effect of phenolic compounds isolated from Rhododendron ungernii leaves. Pharm Chem J. 2007;41:10–13. [Google Scholar]
- 50. Serrano J, Puupponen-Pimia R, Dauer A, Aura AM, Saura-Calixto F. Tannins: current knowledge of food sources, intake, bioavailability and biological effects. Mol Nutr Food Res. 2009;53(suppl 2):S310–S329. [DOI] [PubMed] [Google Scholar]
- 51. Isaacs CE, Wen GY, Xu W, et al. Epigallocatechin gallate inactivates clinical isolates of herpes simplex virus. Antimicrob Agents Chemother. 2008;52:962–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Song JM, Lee KH, Seong BL. Antiviral effect of catechins in green tea on influenza virus. Antiviral Res. 2005;68:66–74. [DOI] [PubMed] [Google Scholar]
- 53. Gescher K, Hensel A, Hafezi W, Derksen A, Kuhn J. Oligomeric proanthocyanidins from Rumex acetosa L. inhibit the attachment of herpes simplex virus type-1. Antiviral Res. 2011;89:9–18. [DOI] [PubMed] [Google Scholar]
- 54. Martino V, Morales J, Martinez-Irujo JJ, Font M, Monge A, Coussio J. Two ellagitannins from the leaves of Terminalia triflora with inhibitory activity on HIV-1 reverse transcriptase. Phytother Res. 2004;18:667–669. [DOI] [PubMed] [Google Scholar]
- 55. Notka F, Meier G, Wagner R. Concerted inhibitory activities of Phyllanthus amarus on HIV replication in vitro and ex vivo. Antiviral Res. 2004;64:93–102. [DOI] [PubMed] [Google Scholar]
- 56. Ito H, Miyake M, Nishitani E, et al. Cowaniin, a C-glucosidic ellagitannin dimer linked through catechin from Cowania mexicana. Chem Pharm Bull (Tokyo). 2007;55:492–494. [DOI] [PubMed] [Google Scholar]