Abstract
Nowadays, increases in resistance of tumors to the current therapeutic agents have become a problematic issue. Therefore, efforts to discover new anticancer compounds with high sensitivity of cancer cells are extending. Animal and laboratory researches have shown that exogenous antioxidants are able to help prevent the free radical damage associated with the development of cancer. However, researches in human beings have not demonstrated convincingly that taking antioxidants can reduce the risk of developing cancer. Angiogenesis is also a natural condition that controls the formation of new blood vessels from the available vessels. Today, it is believed that most of the cancers have angiogenesis potential and their growth, metastasis, and invasion depend on angiogenesis. Several compounds with plant origin and with anti-angiogenic properties have been identified. The aim of this study is to review recently published articles about anticancer drugs obtained from plants with antioxidant and anti-angiogenesis properties.
Keywords: angiogenesis, antioxidants, cancer, medicinal plants, tumor
Plants are considered as important resources for researchers to prove and develop new drugs. Generally, using plants in the treatment of cancer has a long history and thus plants have been primary resources for producing traditional drugs effective in the treatment of cancer.1
After identifying new proteins that had important regulatory effects on the development of the tumors cell cycle, research in isolating molecules from plants and other natural organisms approved that plants are important resources for synthesis inhibitors that have potential to develop production of anticancer drugs.2
Moreover, researchers have reported a large number of plant species that have been used in the treatment of cancer since ancient times, and today, the tendency toward the use and evaluation of therapeutic effects of plants and their compounds as potential anticancer drugs are increasing. So at the present time, more than half of the used anticancer drugs are derived from natural resources like plants, microorganisms, and sea creatures.3
The mechanisms by which these drugs act on cancer cells are mostly unclear. However, the role of oxidative stress in the induction of cancer and antioxidants in the prevention and treatment of cancer is obvious, and most plants are good sources of antioxidants.4–8 Numerous studies have suggested that most cancers are diet related. Furthermore, the risks of most kinds of cancers can be reduced by dietary changes. In this regard, the studies in different countries have suggested that the prevalence of cancer is less in people who eat higher amounts of fruits or vegetables that have antioxidant activity.9,10
There are more than 25 000 phytochemicals in different plants that often have biological effects.11 Diets rich in herbal resources provide necessary essential vitamins and minerals to the body. The ability of the molecules present in medicinal plants to bond therapeutic sites holds promise for achievement of natural products and compounds from plants that are effective on cancer with low toxicity on healthy tissues.12
Angiogenesis is also a natural condition that controls the formation of new blood vessels from the available vessels and has a crucial role in cancer development. This process, unlike cancer, is the basis of several physiologic processes like embryonic development, reproduction cycle, and wound healing.13
In fact, it is believed that most of the cancers have angiogenesis potential and their growth, metastasis, and invasion depend on angiogenesis. Furthermore, the agents with anti-angiogenesis activities have the potential to control cancer development.14
In this study, we aim to review present data on recently published articles about the role of natural antioxidants and anti-angiogenesis agents on cancer development.
The Role of Plant Antioxidants on Cancer
Free radicals, and in particular reactive oxygen species, are formed naturally in the body when an atom or a molecule either loses or gains an electron. Free radicals play an important role in many normal cellular processes.15 However, at high concentrations, they can damage all major components of cells, including proteins, DNA, and cell membranes. These damages to cells, especially damage to DNA, play a crucial role in the development of cancer.16 Moreover, some environmental toxins, such as cigarette smoke, may contain larger amounts of free radicals or stimulate the body’s cells to produce more free radicals.16 Free radicals, other than cancer, are involved in a wide variety of disorders, especially in degenerative diseases such as neurological disorders,4,17 chronic inflammation, and the generation of damage, particularly during ischemia/reperfusion, diabetes,18,19 atherosclerosis,20,21 cardiovascular diseases,22,23 and wound complications.24,25 These conditions involve many changes, including alterations in the redox state.26,27 Antioxidants, especially medicinal plants with antioxidant activity, have the ability to counteract these conditions.28,29 Various clinical and experimental studies have demonstrated promising results for various conditions, especially for the treatment and prevention of life-threatening diseases.30–33 These agents are also effective in inhibition of toxic agents induced complications.8,34
The body makes some of the antioxidants that are called endogenous antioxidants. However, the body relies on exogenous sources of antioxidants for the rest of the antioxidants it needs.35 A lot of medicinal plants have antioxidant activity24,32,33,36 and have shown promising results in cancer therapy. Here we try to present the role of plants having antioxidant activity in cancer therapy.
In laboratory and preclinical studies in cancer prevention37 or immune system stimulation,38 promising results have been achieved. However, whether or not taking dietary antioxidants can prevent or reduce the risk of developing cancer in humans is not clear. The cohort and case-control studies that have investigated the use of dietary antioxidants in the risk of cancer in humans have achieved mixed results. Due to inadequate control for biases that might influence the results, observational studies must be viewed with caution. Randomized controlled clinical trials are considered to provide more reliable evidence of the harm or benefit of a health-related intervention. However, the randomized controlled trials of antioxidant supplementation for cancer prevention are inadequate to be concluded. The results of these trials are summarized below.
The first trial was a large-scale randomized trial investigating the effect of antioxidants on cancer risk of healthy Chinese people at increased risk of developing gastric and esophageal cancers. The participants were randomly assigned to take a combination of 50 μg selenium, 15 mg β-carotene, and 30 mg α-tocopherol per day for 5 years or no supplement. The results of the study demonstrated that the risk of developing gastric cancer and/or esophageal cancer was not affected by antioxidant supplementation. However, people who took antioxidants had lower rates of death due to gastric cancer, but not due to esophageal cancer.39 In this study, 10 years after antioxidant supplementation ended, reduced risk of gastric cancer death was no longer found for those who took antioxidants, in comparison to those who did not.40
In a second trial, which was conducted in Finland, 5 to 8 years of consumption of α-tocopherol and/or β-carotene could reduce the incidence of different cancers in middle-aged male smokers. The initial results of the study showed an increase in the incidence of lung cancer in the participants who took 20 mg per day β-carotene supplementation.41 There were no effects of α-tocopherol or β-carotene supplementation on the incidence of renal pelvis, bladder, ureter, pancreas, colorectal, kidney, pharyngeal, laryngeal, or esophageal cancers.42
In a trial in the United States, daily supplementation with β-carotene and vitamin A on people who were at high risk of lung cancer due to having a history of smoking or exposure to asbestos showed that daily supplementation with both 25 000 IU retinol and 15 mg β-carotene was associated with increased lung cancer and increased death from all-cause mortality.43 A later report in 2004 showed that the adverse effects persisted up to 6 years after the consumption of these supplements ended. However, the enhanced risks of all-cause mortality and lung cancer were no longer statistically significant.44
In another study in 1996, 50 mg β-carotene administration every other day for 12 years had no effect on cancer mortality, cancer incidence, and all-cause mortality among US male subjects.45
Every other day administration of 50 mg β-carotene, 600 IU vitamin E, and 100 mg aspirin has also had no benefit or the incidence of cancer and cardiovascular diseases in US women of over 45 years.46 In 2005, similar results were published for vitamin E consumption.47
In a trial published in 2004, daily supplementation with 100 μg selenium, 20 mg zinc, 6 mg β-carotene, 30 mg vitamin E, and 120 mg vitamin C, for a median of 7.5 years, had no effect on the incidence of cardiovascular or cancer diseases or all-cause mortality.48
In an international trial reported in 2005, no effect of daily supplementation with 400 IU α-tocopherol, which was administered for 7 years was seen in the incidence of major cardiovascular events—such as stroke, heart attack or death from heart disease, cancer incidence or death from cancer in people diagnosed with cardiovascular disease or diabetes.49
The next trial was conducted in the United States, began in 2001 and was stopped in 2008. The trial investigated whether daily supplementation with 200 μg selenium, 400 IU vitamin E, or both would reduce the incidence of prostate cancer in men over 50 years. The results showed that the use of these supplements for a period of about 5.5 years did not affect the incidence of prostate or other cancers.4 Updated findings, reported in 2011, demonstrated that, following 1.5 years of supplements, the cases of prostate cancer among men taking vitamin E alone were 17% more compared to the placebo group.50 No increase in prostate risk was observed for men assigned to take vitamin E plus selenium or selenium alone compared with men assigned to take a placebo.49
In a trial that was conducted in 2009, the use of 500 mg vitamin C, 400 IU vitamin E, or a combination of the two, every other day for a median of 7.6 years, did not reduce the incidence of prostate cancer or other cancers, including leukemia, melanoma, lymphoma, and cancers of the bladder, pancreas, lung, colon, and rectum.52
The aforementioned randomized controlled clinical trials did not show that dietary antioxidant supplements are beneficial in primary cancer prevention. However, it is possible that the lack of benefit in clinical studies can be related to the effects of the tested antioxidants. They all were consumed as purified chemicals and they might be opposed when they are consumed in foods, which contain complex mixtures of minerals, vitamins, and various antioxidants. This acquire a more complete understanding of the antioxidant content of individual foods, how the various antioxidants interact with each other, and the factors that influence the uptake and distribution of food-derived antioxidants in the body. These are all active areas of ongoing cancer prevention research.
Another question that might be raised is that whether or not people already diagnosed with cancer should take antioxidant supplements? A randomized controlled trial reported that antioxidant supplements during cancer treatment might alter the effectiveness or reduce the toxicity of specific therapies.53 Other trials have reported mixed results; some of them found that people who took antioxidants during cancer therapy had worse outcomes, especially if they were smokers.
Additional large randomized controlled studies are necessary to provide clear evidence about the benefits or harms of taking antioxidant supplements during cancer treatment. The role of natural compounds with angiogenesis inhibitory activity in cancer therapy.
Angiogenesis is a natural condition that controls the formation of new blood vessels from the available vessels. This process is the basis of several physiologic processes like embryonic development, reproduction cycle, and wound healing.13,54
In the natural formation of the new blood vessels, which is a completely controlled process, endothelial cells receive stimulator massage and secret special enzymes like matrix metalloproteinase and heparin that cause digestion of the extracellular matrix and basal lamina and as a result strong connections between endothelial cells are disjointed. When this process is continued, endothelial cells can be created and as a result immigration and reproduction are organized for forming new capillary tubes. In many crucial states of diseases, the control of angiogenesis is disturbed. When the cells of patients unnaturally produce large amounts of angiogenesis factors like endothelial cell growth factor (VEGF), fibroblastic growth factor (FGF-2), and hepatocyte growth factor, these factors overpass effects of the natural inhibitors like angiostatin, endostatin, and thrombospondin and thus excessive angiogenesis occurs.
Generally, there are more than 70 other diseases like obesity, rheumatoid arthritis, and asthma that are related to the unnatural angiogenesis.55 Today, it is believed that most of the cancers have angiogenesis potential and their growth, metastasis, and invasion depend on angiogenesis.14
One of the first identified and isolated anti-angiogenic agents was a natural compound, namely, Fumagillin, which is an antibiotic secreted from the Aspergillus fumigatus fungi.33 Gradually, other different compounds with plant origin and with anti-angiogenic properties were identified and even effective compounds of some of them were isolated. Now, different research models are used to screen plants that have anti-angiogenic activities.56 Results of studies show that foods, especially those with plant origin, have the potential to prevent one third of cancers.14 So consumption of a plant diet might prevent development and improvement of chronic diseases like malignant tumors whose development is associated with angiogenesis.57 However, it has been suggested that single anti-angiogenic factors have limited efficacy. Useful natural products contain a range of complex organic chemicals that may have synergic activity. Possibly, other than interacting with multiple angiogenesis pathways, they also act on other pathways like cell signaling and the apoptosis pathways or influence collision between cancer cells and immunity system and so inhibit angiogenesis.58
Some of the anti-angiogenic factors also have anticoagulant activity that may decrease metastasis of tumor cells.59 In addition, other anti-angiogenic compounds have been identified and isolated during the past years by conducting extensive researches. Examples are isolation of the inhibitory compound, camptothecin from Camptotheca acuminate in 1966, identification and isolation of the angiogenesis-inhibiting compound taxol from Taxus baccata plant in 1971, isolation of combretastin from Combretum caffrum in 1987, and other studies in the field of identification of anti-angiogenic properties of different plants and their effective compounds.
It should be noted that different plants contain very active compounds that some of them have properties effective on angiogenesis. In fact, plants are complex chemical cocktails with different properties. It means that not just one mechanism of a plant acts on cancer in angiogenesis but may include several mechanisms.
Some of reliable anticancer drugs obtained from plants with anti-angiogenic properties are introduced below.
Taxol
Researchers identified an extract made from the bark of the Pacific yew tree with the scientific name Taxus brevifolia in 1962 that has anticancer properties. The active compound in this extract was then identified in 1971and named taxol, which is a complex diterpene (Figure 1).46 Thereafter, taxol was produced synthetically and used.
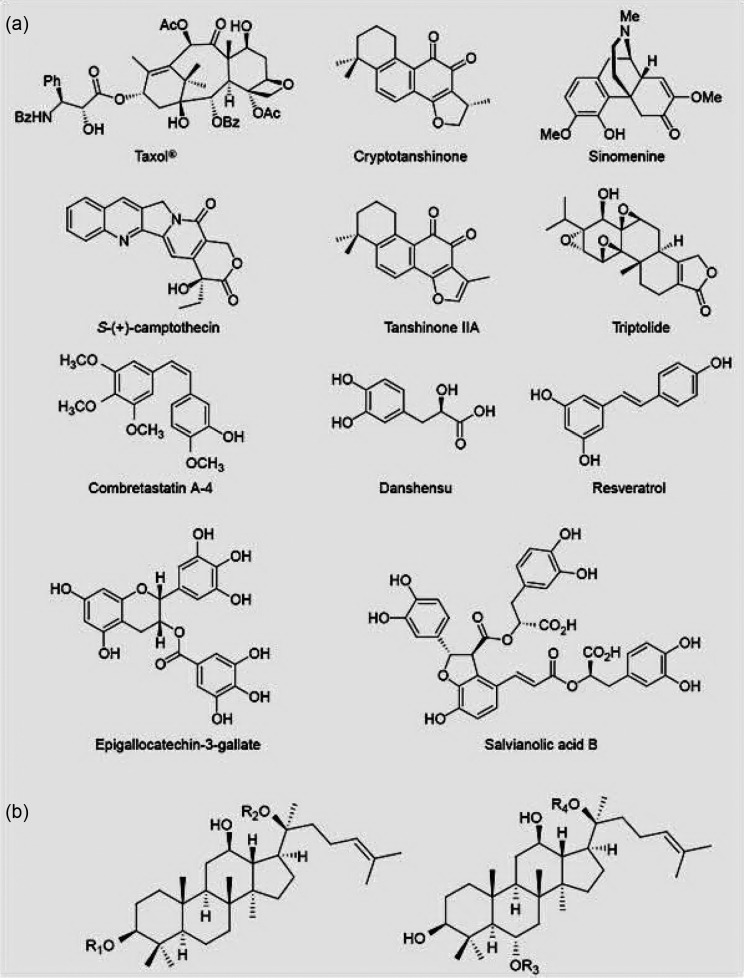
Figure 1.
The chemical structure of factors affecting angiogenesis that have been obtained from medicinal drugs.
Taxol combats cancer cells that are multiplying by disrupting their microtubule skeleton. Results of studies done by researchers have shown that taxol at low picomolar concentrations inhibits angiogenesis by inhibiting VEGF production and controlling the protein expression of the hypoxia-inducible factors. With regard to anti-angiogenic properties represented by taxol, its antitumor properties are further confirmed. It has been many years since the clinical application of taxol in the treatment of cancer has been approved and it is yet used.59
Camptothecin
Camptothecin was first isolated from the Camptotheca acuminate tree, a tree native to China, using the bioactivity-directed fractionation technique (Figure 1). Camptothecin, as an anticancer agent, was studied for 15 years and finally its exclusive procedure to kill tumor cells was identified. This compound traps topoisomerase I-DNA complexes, inhibits DNA replication, and leads to killing of cancer cells. The discovery of this issue encouraged researchers to produce compounds similar to camptothecin that have solubility in water and maintain their anticancer activity. Finally, 2 compounds similar to camptothecin, namely topotecan and trinotecan, were approved by the US Food and Drug Administration in 1990 to be used to treat colon, lung, and ovarian cancers.60 In 1999, it was shown that camptothecin and topotecan inhibit the growth of the human endothelial cells in vitro in a nontoxic manner and this inhibition continues up to 96 hours after the drug is stopped. They also suggested that these 2 compounds are effective in angiogenesis unlike the unspecific toxic drug, cisplatin or TNP-470, in inhibition of angiogenesis. For this reason, in addition to activities like killing tumors, camptothecin possibly possesses an indirect in vivo antitumor effect mediated through the inhibition of angiogenesis.45
Combretastatin
Combretastatin is a microtubule-targeting agent found in the bark of the African bush willow tree (Combretum caffrum), identified in 1987 as an anticancer agent. More studies led to the production of phosphate derivatives in aqueous solution with more bioavailability. Vincent et al61 also suggested that combretastatin A4 phosphate (CA4P; Figure 1) selectively targets endothelial cells and destroys new tumor vessels in mice by disrupting the function of the junctional molecule of cadherin in endothelial cells. CA4P also increases permeability of endothelial cells and inhibits immigration and formation of capillary tube mainly through disrupting the signaling pathway of E-cadherin/β-catenin/Akt that leads to the rapid collapse of vessels and necrosis. This compound has a synergistic effect with monoclonal anti–VE-cadherin antibody (when this antibody is administered in low doses), which stops aggregation of new vessels and so inhibits tumor growth. These findings show that CA4P selectively induces regression of unstable tumor neovessels and through disruption in the mentioned signaling pathway.44,61
Some other natural compounds derived from plants with anti-angiogenic activity are presented in Table 1.11
Table 1.
Natural Compounds That Have Direct or Indirect Anti-Angiogenic Potential.
| No. | Plant Name | Compound | Possible Mechanism |
|---|---|---|---|
| 1 | Camellia sinensis (green tea) | EGCG | Abrogates VEGF signaling by interfering with formation of VEGF receptor-2 complex |
| 2 | Camptotheca acuminate | Camptothecin | Blocks topoisomerase I, inhibits EC proliferation and tube formation, decreases HIF1α and VEGF expression |
| 3 | Combretum caffrum | Combretastatin | Inhibits tubulin assembly |
| 4 | Cordyceps militaris | Unknown | Inhibits FGF-2 expression in EC and MMP-expression in tumor cells |
| 5 | Ganoderma lucidum | Polysaccharide, peptide | Causes EC apoptosis by reducing Bcl-2 expression and increasing Bax expression, decreases VEGF secretion from tumor cells |
| 6 | Glycine max (soybean) | Genistein | Suppresses VEGF and FGF-2 expression, inhibits receptor tyrosine kinase, inhibits activation of NF-κB and Akt signaling pathways |
| 7 | Glycyrrhiza uralensis (liquorice) | Isoliquiritin | Inhibits tube formation |
| 8 | Panax ginseng | Ginsenosides Rb1 | Inhibits VEGF production by tumor cells |
| 9 | Sinomenium acutum | Sinomenine | G1-G0 arrest of ECs |
| 10 | Salvia miltiorrhiza (danshen) | Cryptotanshinone | G1-G0 arrest of ECs, apoptosis of ECs |
| 11 | Taxus brevifolia | Taxol | Disrupts microtubule cytoskeleton inhibits VEGF production, inhibits HIF-1α protein |
| 12 | Tripterygium wilfordii Hook.f | Triptolide | Inhibits VEGF expression and secretion from ECs, inhibits COX-1, COX2, and 5-lipoxygenase, decreases transcription of the gene encoding inducible nitric oxides synthase |
| 13 | Vinca rosea | Vincristine | Disrupts microtubule cytoskeleton, inhibits VEGF production |
| 14 | Vitis spp (grape) | Resveratrol | Disrupts Src-dependent VE cadherin tyrosine phosphorylation |
Some other plants and their derivatives that specifically inhibit the vessels’ endothelia growth factor (VEGF) and have antiangiogenic activity include the following: Artemisia annua or Chinese wormwood (containing 95% artemisinin), Vsicum album or European mistletoe (containing mistletoe lectin III (ML3A), Curcuma longa or turmeric (containing 95% curcumin), Camellia sinensis or green tea (containing 95% phenols; 50% epigallocatechin), Vitis vinifera or grape seed extract (containing 95% proanthocyanidins), Angelica sinensis or Dong quai (containing 4-hydroxyderricin), Scutellaria baicalensis or Chinese skullcap (containing 95% baicalin and flavonoids), Polygonum cuspidatum or Japanese knotweed (containing 20% resveratrol), Silybum marianum or milk thistle (containing 80% silymarin), and magnolia seed cones (containing 90% honokiol).
Some plant-derived compounds such as curcumin, gingiber, bromelain, epigallocatechin-3 gallate, resveratrol, proanthocyanidin, and antioxidants present in plants (vitamins A, C, E, selenium, zinc, carotenoids, flavonoids) have inhibitory activity on the cyclooxygenase enzyme in the angiogenesis pathway.11
Soybean Grains and Its Effective Compounds
The prevalence rate of breast and prostate cancer is very low in Asian countries like Japan and China compared to European countries and the United States of America. Studies show that this important difference in the prevalence rate of cancer in different ethnic groups depends to some extent on eating habits. In fact, one of the main differences in nutrition of these 3 types of population is that Japanese and Chinese use food in their diet that usually contain plant products including soybean. Moreover, Fotsis et al51 by studying the urine of persons who have used large amounts of plant foods observed that urine of these people contain isoflavones of soya and its metabolites, specially genistein, which inhibits proliferation of endothelial cells of brain capillaries. Other studies also have suggested that pure genistein has a strong dose-dependent inhibiting effect on the proliferation of the endothelial cells. Genistein also inhibits proliferation of other vessels’ endothelial cells like cells derived from the bovine adrenal cortex and the aorta.52
Isoflavonoids are present mainly in vegetables. Soybean grains and its products contain large amounts of isoflavonoids. These observations and epidemiological and laboratory evidence have shown that consumption of diets rich of soybean products is associated with lower risk of getting cancer. Flavonoids like 3,4-dihydroxyflavone, 1,3-dihydroxyflavone, fisetin, apigenin, and lutlin can inhibit in vitro angiogenesis in the micromolar concentration range.62 Studies of researchers have approved that genistein inhibits proliferation of VEGF-induced endothelial cells and signaling molecules of tyrosine phosphorylation in the micromolar range. Moreover, genistein inhibits effectively the mast cell chemotaxis as facilitator of new vessels’ formation in response to some incentive factors of angiogenesis. This compound can inhibit angiogenesis through different methods like adjusting the activity of proteolytic enzymes.62 Moreover, genistein suppress expression of VEGF and FGF-2 and inhibits phosphorylation of the receptor tyrosine kinase and activation of Akt. The mentioned compound prevents activation of NF-κB that leads to apoptosis even in cancer cells resistant to apoptosis.
Genistein as a phytostrogen also targets estrogen and androgen signaling pathways in carcinogenesis and has antioxidant properties. Generally, in vivo and in vitro studies have shown that this isoflavone is a promising factor to treat or inhibit cancer. This peptide is called Kunitz trypsin inhibitor and its anti-angiogenic property has been approved.63
Green Tea
Green tea, especially one of its compounds named epigallocatechin gallate (EGCG; Figure 1), inhibits angiogenesis significantly. EGCG inhibits production of cytokine-induced IL-8, inhibits AKt activation (induced by VEGF), and also prevents phosphorylation of V-E cadherin in physiologic concentrations. Findings also show that drinking green tea can be effective in inhibiting and treatment of angiogenesis-dependent diseases like cancer and diabetic retinopathy.64
Red Grape
Red grape is a delicious fruit that contains a compound named resveratrol (Figure 1). This compound can be found in peanut, morus, and some other medicinal plants like Polygonum cuspidatum. Studies have suggested that this compound inhibits angiogenesis when it is administered orally without important side effects. This compound, as one of the most promising chemical factors that inhibits cancer, has attracted excessive attention in recent years. For this reason, there is possibility to discover and identify more polyphenols in natural products as compounds that lead to discovery of more strong synthetic inhibitors of angiogenesis. Resveratrol may treat heart muscle damage through inducing angiogenesis by VEGF and the receptor tyrosine kinase FLK-1 (angiogenesis induction). However, recent inconsistent findings should be evaluated.65
Allium hirtifolium
This plant is one of the important species in Allium genus that has been used in many countries including Iran as condiment since the ancient times and has been used also in the traditional treatment of cancer. This plant has known properties like effect on hematologic indices, antioxidant potential, and antifungal and antibacterial properties. Moreover, the study of its chemical components shows that it has many compounds like organosoulfurs and polyphenols that are very similar to compounds available in other species in the same family. Aqueous extract of Allium hirtifolium has a dose-dependent inhibitory effect on the angiogenesis.66
Artemisia annua (Chinese Wormwood)
One of the compounds extracted from the Artemisia annua plant is the active compound of artemisin that has anti-angiogenic effects. This compound has been used in traditional medicine as an antimalarial medication. Recently, researchers have shown that this compound applies the toxic effect on cancer cells via induction of apoptosis. Artesunate also is a semisynthetic derivative of artemisinin that has anti-angiogenic activity in a dose-dependent manner. Moreover, the in vivo anti-angiogenic effect of artemisinin using transplantation of cancer cells of human ovary in nude mice has been evaluated and approved.67
Viscum album (European Mistletoe)
Viscum album, also known as iscador, often is used as an anticancer agent. Laboratory studies show that compounds of this plant via VEGF expression inhibit the angiogenesis and also induce apoptosis in cancer cells. Using extract of this plant in a mouse model leads to decreased lung metastasis and increase in survival. Also, a clinical study performed on individuals with different cancers indicates increase in their survival and longevity. Of course, more studies are required to specify different aspects of therapeutic effects of this plant.67
Curcuma longa (Curcumin)
It is a compound that interacts with cancer cells in different levels. Its antimetastatic effects are applied to some extent via a decrease in expression of the matrix metalloproteinase enzyme (MMP-2) and increase in expression of the tissue inhibitor of metalloproteinase 1 (TIMP1). It should be recalled that the mentioned enzymes have an important role in regulating angiogenesis and tumor cell invasion. Studies have also suggested that this compound inhibits transcription of angiogenesis factors VEGF and bFGF and also decreases production of nitric oxide in endothelial cells that have an important role in the development of angiogenesis and tumor growth. Other activities of this compound include binding to CD13 antibody expressed by blood vessel components and inhibiting its activity, decreasing expression of VEGF and MMP-9, and inhibiting EGF and VEGF receptors and also resisting intracellular signaling pathway of tyrosine kinases.68,69
Scutellaria baicalensis (Chinese Skullcap)
Baicalin and baicalein are main derivations of the Chinese skullcap plant. These 2 compounds are strong compounds that decrease activity of VEGF, bFGF, 12-lipooxigenase, and also matrix metalloproteinase. This plant is one of the known Chinese plants that have shown activity against the development of prostate cancer.70
Magnolia officinalis (Chinese Magnolia Tree)
Grains present in the fruit of Chinese magnolia contain substances that inhibit new blood vessels formation. The active component of this compound is honokiol. This component can decrease angiogenesis to some extent through regulating expression of the endothelial cell growth factor derived from placket and also transforming growth factor-β. Moreover, this compound inhibits nitric oxide synthesis and tumor necrosis factor expression. Studies using laboratory animals also have shown that honokiol inhibits proliferation of blood vessels’ endothelial cells and so decreases tumor growth.71
Silybum marianum (Milk Thistle)
Silymarin and silibinin are polyphenolic flavonoids isolated from fruits and seeds of Silybum marianum. Results obtained from studies of researchers indicate that silymarin has strong antitumor activity against types of tumors like ovary cancer via decreasing expression of VEGF and EGFR.72,73
Ginkgo Biloba
An extract of this plant has anticancer effect having some properties like gene regulation and anti-angiogenic activities. An extract of this plant contains about 25% flavonoids (ginkgo, flavone glycosides) and about 5% terpenoids (ginkgolides and bilobalides). Its most strong flavonoid is ginkgolide B. An extract of this plant inhibits angiogenesis via decreasing the VEGF expression.74
Plants Rich of Quercetin
Quercetin is a flavonoid compound that is found in apple, onion, red grapes, black-berry, citrus, cherry, broccoli, and also some of the species in the Allium family. This flavone inhibits angiogenesis through different mechanisms like interacting with cyclooxygenase-2 and 5-lipooxigenase, VEGFR enzymes, HER-2 intracellular signaling pathways, and also nuclear transcription protein NF-κB. Quercetin may enhance anticancer effects of tamoxifen drug via its anti- angiogenic activity.75
Poria cocos
Studies have suggested that this extract inhibits placket accumulation and apparently it has anti-angiogenic effect via decreasing expression of the NF-κB.76
Panax ginseng
Lipophilic compounds of ginseng namely saponins and ginsenosids have anticancer activities including inhibiting angiogenesis and inducing apoptosis in tumor cells.77
Rabdosia rubescens Hara (Rabdosia)
Rabdosia has been used to treat cancer since ancient time. This plant is one of the Chinese plants known as pc-spes and has activity against the prostate cancer. This plant includes 2 diterpenoids named ponicidin and oridonin that have significant anti-angiogenic activity.78
Conclusions
Nowadays, increases in resistance of tumors to the current therapeutic agents have become a problematic issue. Therefore, the efforts of researchers to discover new anticancer compounds with high sensitivity of cancer cells are extending. Some of cytotoxic drugs inhibit angiogenesis and have minimum toxicity. Studies on new plants will lead to discovery of new anticancer drugs from plant sources and this success will be very important.
Animal and laboratory researches have shown that exogenous antioxidants are able to help prevent the free radical damage associated with the development of cancer. However, researches in humans have not demonstrated convincingly that taking antioxidants can reduce the risk of developing cancer. Furthermore, a wide variety of medicinal plants, including Allium sativum, Stachys lavandulifolia, Artemisia abrotanum, and Salvia officinalis have been shown to have antioxidant activity.29,33,35,79–98 More important, it has been shown that there is a correlation between antioxidant activity and anticancer effects of some medicinal plants such as garlic.38 If antioxidants are one of the main components of anticancer therapy, then those plants that have antioxidant activity should all have anticancer property. These may be worth examining.
Acknowledgments
The authors would like to acknowledge the Razi Herbal Medicines Research Center, Lorestan University of Medical Sciences, Khorramabad, Iran, for supporting this study.
Footnotes
Author Contributions: All the authors contributed equally to the writing of the first draft of the article.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This article was prepared with support from the Razi Herbal Medicines Research Center, Lorestan University of Medical Sciences, Khorramabad, Iran.
Ethical Approval: As no human or animal subjects were involved in this study, ethical approval is not required.
References
- 1. Sewell RDE, Rafieian-Kopaei M. The history and ups and downs of herbal medicine usage. J HerbMed Pharmacol. 2014;3:1–3. [Google Scholar]
- 2. Fan TP, Yeh JC, Leung KW, Yue PYK, Wong RNS. Angiogenesis: from plants to blood vessels. Trends Pharmacol Sci. 2006;27:297–309. [DOI] [PubMed] [Google Scholar]
- 3. Cragg GM, Newman DJ. Plants as source of anticancer agents. J Ethnopharmacol. 2005;100:72–79. [DOI] [PubMed] [Google Scholar]
- 4. Roohafza H, Sarrafzadegan N, Sadeghi M, Rafieian-Kopaei M, Sajjadi F, Khosravi-Boroujeni H. The association between stress levels and food consumption among Iranian population. Arch Iran Med. 2013;16:145–148. [PubMed] [Google Scholar]
- 5. Rabiei Z, Rafieian-Kopaei M, Heidarian E, Saghaei E, Mokhtari S. Effects of Zizyphus jujube extract on memory and learning impairment induced by bilateral electric lesions of the nucleus Basalis of Meynert in rat. Neurochem Res. 2014;39:353–360. [DOI] [PubMed] [Google Scholar]
- 6. Hosseini-asl K, Rafieian-kopaei M. Can patients with active duodenal ulcer fast Ramadan? Am J Gastroenterol. 2002;97:2471–2472. [DOI] [PubMed] [Google Scholar]
- 7. Sedighi M, Rafieian-Kopaei M, Noori-Ahmadabadi M. Kelussia odoratissima Mozaffarian inhibits ileum contractions through voltage dependent and beta adrenergic receptors. Life Sci J. 2012;9:1033–1038. [Google Scholar]
- 8. Heidarian E, Rafieian-Kopaei M. Protective effect of artichoke (Cynara scolymus) leaf extract against lead toxicity in rat. Pharm Biol. 2013;51:1104–1109. [DOI] [PubMed] [Google Scholar]
- 9. Nasri H, Rafieian-Kopaei M. Medicinal plants and antioxidants: why they are not always beneficial? Iran J Public Health. 2014;43:255–257. [PMC free article] [PubMed] [Google Scholar]
- 10. Rafieian-Kopaei M. Medicinal plants and the human needs. J HerbMed Plarmacol. 2012;1:1–2. [Google Scholar]
- 11. Gordaliza M. Natural products as leads to anticancer drugs. Clin Transl Oncol. 2007;9:767–776. [DOI] [PubMed] [Google Scholar]
- 12. Nasri H, Shirzad H. Toxicity and safety of medicinal plants. J HerbMed Plarmacol. 2013;2:21–22. [Google Scholar]
- 13. Folkman J. Fundamental concepts of the angiogenic process. Curr Mol Med. 2003;3:643–651. [DOI] [PubMed] [Google Scholar]
- 14. Griggs J, Metcalfe JC, Hesketh R. Targeting tumor vasculature: the development of combretastatin A4. Lancet Oncol. 2001;2:82–87. [DOI] [PubMed] [Google Scholar]
- 15. Rafieian-Kopaei M, Baradaran A, Rafieian M. Oxidative stress and the paradoxical effects of antioxidants. J Res Med Sci. 2013;18:629. [PMC free article] [PubMed] [Google Scholar]
- 16. Bouayed J, Bohn T. Exogenous antioxidants—double-edged swords in cellular redox state: health beneficial effects at physiologic doses versus deleterious effects at high doses. Oxid Med Cell Longev. 2010;3:228–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rafieian-Kopaei M, Heidarian E, Saghaei E, Mokhtari S. Effects of Zizyphus jujube extract on memory and learning impairment induced by bilateral electric lesions of the nucleus Basalis of Meynert in rat. Neurochem Res. 2014;39:353–360. [DOI] [PubMed] [Google Scholar]
- 18. Kiani MA, Khodadad A, Mohammadi S, Mobarhan MG, Saeidi M, Jafari SA. Effect of peppermint on pediatrics’ pain under endoscopic examination of the large bowel. J HerbMed Pharmacol. 2013;2:41–44. [Google Scholar]
- 19. Bagheri N, Taghikhani A, Rahimian G, et al. Association between virulence factors of helicobacter pylori and gastric mucosal interleukin-18 mRNA expression in dyspeptic patients. Microb Pathog. 2013;65:7–13. [DOI] [PubMed] [Google Scholar]
- 20. Baradaran A, Madihi Y, Merrikhi A, Rafieian-Kopaei M, Nasri H. Serum lipoprotein(a) in diabetic patients with various renal function not yet on dialysis. Pak J Med Sci. 2013;29(suppl):354–357. [Google Scholar]
- 21. Behradmanesh S, Horestani MK, Baradaran A, Nasri H. Association of serum uric acid with proteinuria in type 2 diabetic patients. J Res Med Sci. 2013;18:44–46. [PMC free article] [PubMed] [Google Scholar]
- 22. Madihi Y, Merrikhi A, Baradaran A, et al. Bioactive components and the effect of hydroalcoholic extract of Vaccinium myrtillus on postprandial atherosclerosis risk factors in rabbits. Pak J Med Sci. 2013;29(suppl):384–389. [Google Scholar]
- 23. Setorki M, Nazari B, Asgary S, Azadbakht L, Rafieian-Kopaei Antiatherosclerotic effects of verjuice on hypocholesterolemic rabbits. Afr J Pharm Pharmacol. 2011;5:1038–1045. [Google Scholar]
- 24. Khosravi-Boroujeni H, Sarrafzadegan N, Mohammadifard N, et al. White rice consumption and CVD risk factors among Iranian population. J Health Popul Nutr. 2013;31:252–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sadeghi M, Khosravi-Boroujeni H, Sarrafzadegan N, et al. Cheese consumption in relation to cardiovascular risk factors among Iranian adults—IHHP Study. Nutr Res Pract. 2014;8:336–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Asadi SY, Parsaei P, Karimi M, et al. Effect of green tea (Camellia sinensis) extract on healing process of surgical wounds in rat. Int J Surg. 2013;11:332–337. doi:10.1016/j.ijsu.2013.02.014. [DOI] [PubMed] [Google Scholar]
- 27. Parsaei P, Karimi M, Asadi SY, Rafieian-Kopaei M. Bioactive components and preventive effect of green tea (Camellia sinensis) extract on postlaparotomy intra-abdominal adhesion in rats. Int J Surg. 2013;11:811–815. doi:10.1016/j.ijsu.2013.08.014. [DOI] [PubMed] [Google Scholar]
- 28. Nasri H, Rafieian-Kopaei M. Tubular kidney protection by antioxidants. Iran J Public Health. 2013;42:1194–1196. [PMC free article] [PubMed] [Google Scholar]
- 29. Baradaran A, Nasri H, Nematbakhsh M, Rafieian-Kopaei M. Antioxidant activity and preventive effect of aqueous leaf extract of aloe vera on gentamicin-induced nephrotoxicity in male Wistar rats. Clin Ter. 2014;165:7–11. [DOI] [PubMed] [Google Scholar]
- 30. Nasri H, Tavakoli M, Ahmadi A, Baradaran A, Nematbakhsh M, Rafieian-Kopaei M. Ameliorative effect of melatonin against contrast media induced renal tubular cell injury. Pak J Med Sci. 2014;30:261–265. [PMC free article] [PubMed] [Google Scholar]
- 31. Rafieian-Kopaei M, Nasri H. The ameliorative effect of Zingiber officinale in diabetic nephropathy. Iran Red Crescent Med J. 2014;16:e11324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nasri H, Rafieian-Kopaei M. Protective effects of herbal antioxidants on diabetic kidney disease. J Res Med Sci. 2014;19:82–83. [PMC free article] [PubMed] [Google Scholar]
- 33. Asgary S, Sahebkar A, Afshani M, Keshvari M, Haghjooyjavanmard S, Rafieian-Kopaei M. Clinical evaluation of blood pressure lowering, endothelial function improving, hypolipidemic and anti-inflammatory effects of pomegranate juice in hypertensive subjects. Phytother Res. 2013;28:193–199. doi:10.1002/ptr.4977. [DOI] [PubMed] [Google Scholar]
- 34. Taghikhani A, Afrough H, Ansari-Samani R, Shahinfard N, Rafieian-Kopaei M. Assessing the toxic effects of hydroalcoholic extract of Stachys lavandulifolia Vahl on rat’s liver. Bratisl Lek Listy. 2014;115:121–124. [DOI] [PubMed] [Google Scholar]
- 35. Rafieian-Kopaie M, Baradaran A. Plants antioxidants: from laboratory to clinic. J Nephropathol. 2013;2:152–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Baradaran A, Nasri H, Rafieian-Kopaei M. Oxidative stress and hypertension: possibility of hypertension therapy with antioxidants. J Res Med Sci. 2014;19:358–367. [PMC free article] [PubMed] [Google Scholar]
- 37. Shirzad H, Taji F, Rafieian-Kopaei M. Correlation between antioxidant activity of garlic extracts and WEHI-164 fibrosarcoma tumor growth in BALB/c mice. J Med Food. 2011;14:969–974. [DOI] [PubMed] [Google Scholar]
- 38. Shirzad H, Shahrani M, Rafieian-Kopaei M. Comparison of morphine and tramadol effects on phagocytic activity of mice peritoneal phagocytes in vivo. Int Immunopharmacol. 2009;9:968–970. [DOI] [PubMed] [Google Scholar]
- 39. Blot WJ, Li JY, Taylor PR, et al. Nutrition intervention trials in Linxian, China: supplementation with specific vitamin/mineral combinations, cancer incidence, and disease-specific mortality in the general population. J Natl Cancer Inst. 1993;85:1483–1491. [DOI] [PubMed] [Google Scholar]
- 40. Qiao YL, Dawsey SM, Kamangar F, et al. Total and cancer mortality after supplementation with vitamins and minerals: follow-up of the Linxian General Population Nutrition Intervention Trial. J Natl Cancer Inst. 2009;101:507–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. The Alpha-Tocopherol, Beta Carotene Cancer Prevention Study Group. The effects of vitamin E and beta carotene on the incidence of lung cancer and other cancers in male smokers. New Engl J Med. 1994;330:1029–1035. [DOI] [PubMed] [Google Scholar]
- 42. Wright ME, Virtamo J, Hartman AM, et al. Effects of alpha-tocopherol and beta-carotene supplementation on upper aerodigestive tract cancers in a large, randomized controlled trial. Cancer. 2007;109:891–898. [DOI] [PubMed] [Google Scholar]
- 43. Omenn GS, Goodman GE, Thornquist MD, et al. Effects of a combination of beta carotene and vitamin A on lung cancer and cardiovascular disease. N Engl J Med. 1996;334:1150–1155. [DOI] [PubMed] [Google Scholar]
- 44. Goodman GE, Thornquist MD, Balmes J, et al. The Beta-Carotene and Retinol Efficacy Trial: incidence of lung cancer and cardiovascular disease mortality during 6-year follow-up after stopping beta-carotene and retinol supplements. J Natl Cancer Inst. 2004;96:1743–1750. [DOI] [PubMed] [Google Scholar]
- 45. Hennekens CH, Buring JE, Manson JE, et al. Lack of effect of long-term supplementation with beta carotene on the incidence of malignant neoplasms and cardiovascular disease. N Engl J Med. 1996;334:1145–1149. [DOI] [PubMed] [Google Scholar]
- 46. Lee IM, Cook NR, Manson JE. Beta-carotene supplementation and incidence of cancer and cardiovascular disease: women’s health study. J Natl Cancer Inst. 1999;91:2102–2106. [DOI] [PubMed] [Google Scholar]
- 47. Lee IM, Cook NR, Gaziano JM, et al. Vitamin E in the primary prevention of cardiovascular disease and cancer: the Women’s Health Study: a randomized controlled trial. JAMA. 2005;294:56–65. [DOI] [PubMed] [Google Scholar]
- 48. Hercberg S, Galan P, Preziosi P, et al. The SU.VI.MAX study: a randomized, placebo-controlled trial of the health effects of antioxidant vitamins and minerals. Arch Intern Med. 2004;164:2335–2342. [DOI] [PubMed] [Google Scholar]
- 49. Lippman SM, Klein EA, Goodman PJ, et al. Effect of selenium and vitamin E on risk of prostate cancer and other cancers: the Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA. 2009;301:39–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Klein EA, Thompson IM, Tangen CM, et al. Vitamin E and the risk of prostate cancer: the Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA. 2011;306:1549–1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Fotsis Th, Pepper MS, Aktas E, et al. Flavonoids, Dietary-derived Inhibitors of Cell Proliferation and in Vitro Angiogenesis. Cancer Res. 1997;57:2916–2921. [PubMed] [Google Scholar]
- 52. Gaziano JM, Glynn RJ, Christen WG, et al. Vitamins E and C in the prevention of prostate and total cancer in men: the Physicians’ Health Study II randomized controlled trial. JAMA. 2009;301:52–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Sullivan R, Smith JE, Rowan NJ. Medicinal mushrooms and cancer therapy: translating a traditional practice in to western medicine. Perspect Biol Med. 2006;49:159–170. [DOI] [PubMed] [Google Scholar]
- 54. Ribatti D, Nico B, Crivellato E, Roccaro AM, Vacca A. The history of angiogenic switch concept. J Leukemia. 2007;21:44–52. [DOI] [PubMed] [Google Scholar]
- 55. Noonan DM, Benelli R, Albini A. Angiogenesis and cancer prevention: a vision. Recent Results Cancer Res. 2007;174:219–224. [DOI] [PubMed] [Google Scholar]
- 56. Mostafaie A, Mohammadi Motlagh HR, Mansouri K. Angiogenesis and the models to study angiogenesis. Yakhteh Med J. 2010;11:374–381. [Google Scholar]
- 57. Miller AB. Diet and cancer—a review. Rev Oncol. 1990;3:87–95. [DOI] [PubMed] [Google Scholar]
- 58. Neal CP, Berry DP, Doucas H, Manson MM, Steward W, Garcea G. Clinical aspects of natural anti-angiogenic drugs. Curr Drug Targets. 2006;7:371–383. [DOI] [PubMed] [Google Scholar]
- 59. Oberlies NH, Kroll DJ. Camptothecin and taxol: historic achievements in natural products research. J Nat Prod. 2004;67:129–135. [DOI] [PubMed] [Google Scholar]
- 60. Kingston DG, Newman DJ. Taxoids: cancer-fighting compounds from nature. Curr Opin Drug Discov Devel. 2007;10:130–144. [PubMed] [Google Scholar]
- 61. Vincent L, Kermani P, Young LM. Combretastatin A4 phosphate induces rapid regression of tumor neovessels and growth through interference with vascular endothelial-cadherin signaling. J Clin Invest. 2005;115:2992–3006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Sarkar FH, Li Y. Soy isoflavones and cancer prevention. Cancer Invest. 2003;21:744–757. [DOI] [PubMed] [Google Scholar]
- 63. Shakiba Y, Mansouri K, Mostafaie A. Anti-angiogenic effect of soybean kunitz trypsin inhibitor on human umbilical vein endothelial cells. Fitoterapia. 2007;78:587–589. [DOI] [PubMed] [Google Scholar]
- 64. Lamy S, Gingras D, Béliveau R. Green tea catechins inhibit vascular endothelial growth factor receptor phosphorylation. Cancer Res. 2002;62:381–385. [PubMed] [Google Scholar]
- 65. Cao Y, Cao R, Bråkenhielm E. Antiangiogenic mechanisms of diet-derived polyphenols. J Nutr Biochem. 2002;13:380–383. [DOI] [PubMed] [Google Scholar]
- 66. Keshavarz M, Mostafaie A, Mansouri K, Shakiba Y, Mohammadi Motlagh HR. Inhibition of corneal neovascularization with propolis extract. Arch Med Res. 2009;40:59–61. [DOI] [PubMed] [Google Scholar]
- 67. Harmsma M, Gromme M, Ummelen M, Dignef W, Tusenius KJ, Ramaekers FC. Differential effects of Viscum album extract, IscadorQu on cell cycle progression and apoptosis in cancer cells. Int J Oncol. 2004;25:1521–1529. [PubMed] [Google Scholar]
- 68. Khafif A, Hurst R, Kyker K, Fliss DM, Gil Z, Medina JE. Curcumin: a new radio-sensitizer of squamous cell carcinoma cells. Otolaryngol Head Neck Surg. 2005;132:317–321. [DOI] [PubMed] [Google Scholar]
- 69. Nasri H, Sahinfard N, Rafieian M, Rafieian S, Shirzad M, Rafieian-Kopaei M. Turmeric: a spice with multifunctional medicinal properties. J HerbMed Plarmacol. 2014;3:5–8. [Google Scholar]
- 70. Miocinovic R, McCabe NP, Keck RW, Jankun J, Hampton JA, Selman SH. In vivo and in vitro effect of baicalein on human prostate cancer cells. Int J Oncol. 2005;26:241–246. [PubMed] [Google Scholar]
- 71. Chen F, Wang T, Wu YF, Xu XL, Zheng S, Hu X. Honokiol: a potent chemotherapy candidate for human colorectal carcinoma. World J Gastroenterol. 2004;10:3459–3463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Gallo D, Giacomelli S, Ferlini C. Antitumor activity of the silybin-phosphatidylcholine complex, IdB1016, against human ovarian cancer. Eur J Cancer. 2003;39:2403–2410. [DOI] [PubMed] [Google Scholar]
- 73. Kabiri N, Ahangar-Darabi M, Setorki M, Rafieian-Kopaei M. The effect of silymarin on liver injury induced by Thioacetamide in rats. J HerbMed Pharmacol. 2013;2:29–33. [Google Scholar]
- 74. Zhang L, Rui YC, Yang PY, Qiu Y, Li TJ, Liu HC. Inhibitory effects of Ginkgo biloba extract on vascular endothelial growth factor in rat aortic endothelial cells. Acta Pharmacol Sin. 2002;23:919–923. [PubMed] [Google Scholar]
- 75. Igura K, Ohta T, Kuroda Y, Kaji K. Resveratrol and quercetin inhibit angiogenesis in vitro. Cancer Lett. 2001;171:11–16. [DOI] [PubMed] [Google Scholar]
- 76. Jin Y, Zhang L, Zhang M, et al. Antitumor activities of heteropolysaccharides of Poria cocos mycelia from different strains and culture media. Carbohydr Res. 2003;338:1517–1521. [DOI] [PubMed] [Google Scholar]
- 77. Sato K, Mochizuki M, Saiki I, Yoo YC, Samukawa K, Azuma I. Inhibition of tumor angiogenesis and metastasis by a saponin of panax ginseng, ginsenoside-Rb2. Biol Pharm Bull. 1994;17:635–639. [DOI] [PubMed] [Google Scholar]
- 78. Meade-Tollin LC, Wijeratne EM, Cooper D. Ponicidin and oridonin are responsible for the antiangiogenic activity of Rabdosia rubescens, a constituent of the herbal supplement PC SPES. J Nat Prod. 2004;67:2–4. [DOI] [PubMed] [Google Scholar]
- 79. Nasri H, Nematbakhsh M, Rafieian-Kopaei M. Ethanolic extract of garlic for attenuation of gentamicin-induced nephrotoxicity in Wistar rats. Iran J Kidney Dis. 2013;7:376–382. [PubMed] [Google Scholar]
- 80. Bahmani M, Rafieian-Kopaei M. Medicinal plants and secondary metabolites for leech control. Asian Pac J Trop Dis. 2014;4:315–316. [Google Scholar]
- 81. Amirmohammadi M, Khajoenia S, Bahmani M, Rafieian-Kopaei M, Eftekhari Z, Qorbani M. In vivo evaluation of antiparasitic effects of Artemisia abrotanum and Salvia officinalis extracts on Syphacia obvelata, Aspiculoris tetrapetra and Hymenolepis nana parasites. Asian Pac J Trop Dis. 2014;4(suppl 1):S250–S254. [Google Scholar]
- 82. Nasri H, Rafieian-Kopaei M. Medicinal plants and new concerns in statin consumption. Iran J Public Health. 2013;42:1071–1072. [PMC free article] [PubMed] [Google Scholar]
- 83. Rafieian-Kopaei M, Nasri H. Re: Erythropoietin ameliorates oxidative stress and tissue injury following renal ischemia/reperfusion in rat kidney and lung. Med Princ Pract. 2014;23(1):95 doi:10.1159/000350842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Baradaran A, Nasri H, Rafieian-Kopaei M. Comment on: anti-oxidative stress activity of Stachys lavandulifolia aqueous extract in humans. Cell J. 2013;15:272–273. [PMC free article] [PubMed] [Google Scholar]
- 85. Baradaran A, Madihi Y, Merrikhi A, et al. Nephrotoxicity of hydroalcoholic extract of Teucrium polium in Wistar rats. Pak J Med Sci. 2013;29(suppl):329–333. [Google Scholar]
- 86. Bahmani M, Zargaran A, Rafieian-Kopaei M, Saki M. Ethnobotanical study of medicinal plants used in the management of diabetes mellitus in the Urmia, Northwest Iran. Asian Pac J Trop Med. 2014;7(suppl 1):S348–S354. [DOI] [PubMed] [Google Scholar]
- 87. Delfan B, Bahmani M, Hassanzadazar H, Saki K, Rafieian-Kopaei M. Identification of medicinal plants affecting on headaches and migraines in Lorestan Province, West of Iran. Asian Pac J Trop Med. 2014;7(suppl 1):S376–S379. [DOI] [PubMed] [Google Scholar]
- 88. Asadi-Samani M, Bahmani M, Rafieian-Kopaei M. The chemical composition, botanical characteristic and biological activities of Borago officinalis: a review. Asian Pac J Trop Med. 2014;7(suppl 1):S22–S28. [DOI] [PubMed] [Google Scholar]
- 89. Saki K, Bahmani M, Rafieian-Kopaei M. The effect of most important medicinal plants on two important psychiatric disorders (anxiety and depression)—a review. Asian Pac J Trop Med. 2014;7(suppl 1):S34–S42. [DOI] [PubMed] [Google Scholar]
- 90. Saki K, Bahmani M, Rafieian-Kopaei M, et al. The most common native medicinal plants used for psychiatric and neurological disorders in Urmia city, northwest of Iran. Asian Pac J Trop Dis. 2014;4(suppl 2):895–901. [Google Scholar]
- 91. Sarrafchi A, Bahmani M, Shirzad H, Rafieian-Kopaei M. Oxidative stress and Parkinson’s disease: new hopes in treatment with herbal antioxidants. Curr Pharm Des. 2015;22:238–246. [DOI] [PubMed] [Google Scholar]
- 92. Bahmani M, Eftekhari Z, Saki K, Fazeli-Moghadam E, Jelodari M, Rafieian-Kopaei M. Obesity phytotherapy: review of native herbs used in traditional medicine for obesity [published online August 12, 2015]. J Evid Based Complementary Altern Med. doi:10.1177/2156587215599105. [DOI] [PubMed] [Google Scholar]
- 93. Shaygannia E, Bahmani M, Zamanzad B, Rafieian-Kopaei M. A review study on Punica granatum L [published online July 30, 2015]. J Evid Based Complementary Altern Med. doi:10.1177/2156587215598039. [DOI] [PubMed] [Google Scholar]
- 94. Bahmani M, Shirzad H, Mirhosseini M, Mesripour A, Rafieian-Kopaei M. A review on ethnobotanical and therapeutic uses of fenugreek (Trigonella foenum-graceum L). J Evid Based Complementary Altern Med. 2016;21:53–62. [DOI] [PubMed] [Google Scholar]
- 95. Ebrahimie M, Bahmani M, Shirzad H, Rafieian-Kopaei M, Saki K. A review study on the effect of Iranian herbal medicines on opioid withdrawal syndrome. J Evid Based Complementary Altern Med. 2015;20:302–309. doi:10.1177/2156587215577896. [DOI] [PubMed] [Google Scholar]
- 96. Bahmani M, Mirhoseini M, Shirzad H, Sedighi M, Shahinfard N, Rafieian-Kopaei M. A review on promising natural agents effective on hyperlipidemia. J Evid Based Complementary Altern Med. 2015;20:228–238. doi:10.1177/2156587214568457. [DOI] [PubMed] [Google Scholar]
- 97. Delfan B, Kazemeini H, Bahmani M. Identifying effective medicinal plants for cold in Lorestan province, West of Iran. J Evid Based Complementary Altern Med. 2015;20:173–179. doi:10.1177/2156587214568458. [DOI] [PubMed] [Google Scholar]
- 98. Bahmani M, Sarrafchi A, Shirzad H, Rafieian-Kopaei M. Autism: pathophysiology and promising herbal remedies. Curr Pharm Des. 2015;22:277–285. [DOI] [PubMed] [Google Scholar]