Abstract
Aim.
To evaluate the efficacy of topical use of Matricaria recutita L oil in the treatment of enuresis in children.
Methods.
Eighty patients diagnosed as monosymptomatic nocturnal or daytime enuresis were allocated to receive Matricaria recutita L (chamomile) oil or placebo topically for 6 weeks in a double-blind randomized placebo-controlled trial with a parallel design. Patients were evaluated prior to and following 8 weeks of the intervention in terms of frequency of enuresis and any observed adverse events.
Results.
The mean frequency of enuresis at the first, second, and third 2 weeks was lower in the intervention group compared with the placebo group, and the differences were statistically significant (P < .001, P = .03, and P < .001, respectively). There was no report of any adverse event in the study groups.
Conclusion.
The findings of this study showed that the topical use of (chamomile) oil can decrease the frequency of nocturia in children with monosymptomatic nocturnal or daytime enuresis.
Keywords: urinary incontinence, enuresis, Matricaria recutita, herbal medicine, traditional medicine
Urinary incontinence is the inability to control urination in a child who is expected to have reached a level of development and is able to control urine.1 Monosymptomatic enuresis is defined as enuresis in children without any other lower urinary tract symptoms and without a history of bladder dysfunction. Nocturnal enuresis (as the most prevalent type of enuresis) is the involuntary loss of urine at night, in the absence of physical illness, after age 5 when a child is reasonably expected to be dry.2 Although nocturnal enuresis is pathologically benign and has a high rate of spontaneous remission, it is a socially disruptive and stressful condition that affects around 15% to 20% of 5-year-old children.2,3
Various pharmacological, psychological/behavioral, and a variety of unconventional interventions are used to treat monosymptomatic enuresis. Less common interventions include surgery, fluid deprivation, and complementary therapies.2,3 Drugs include tricyclic antidepressants, anticholinergics, and desmopressin. Imipramine is the most commonly used tricyclic antidepressants with a success rate of 50% to 60% and a 60% relapse rate.4 It reduces the frequency and intensity of bladder contractions and number of enuresis. Although imipramine has many side effects, it is one of the most widely used drugs in the treatment of nocturia. Its side effects include anxiety, insomnia, and dry mouth.5 Desmopressin as an antidiuretic is also used for treatment of children’s nocturia with dilutional hyponatremia as its most important side effect.6 According to insufficient efficacy and significant side effect profile of the current interventions, many efforts are focused on the potential of complementary and alternative medicine for the treatment of enuresis in children.3,4
Matricaria recutita L (chamomile) has been used as a treatment for enuresis of children in traditional Persian medicine.7 Matricaria recutita L, commonly known as chamomile, is one of the most popular species from the genus Matricaria and the family Asteraceae.8 It is investigated for different pharmacologic effects such as anti-inflammatory, antioxidative, hepatoprotective, antibacterial, and antifungal properties.9–11 Its efficacy is also evaluated in different animal and human studies for a wide variety of diseases including premenstrual syndrome, polycystic ovary syndrome, wound healing, osteoarthritis, irritable bowel syndrome, peptic ulcer, oral aphthae, and carpal tunnel syndrome.12–18
Chamomile also has showed spasmolytic activity on smooth muscle tissues.19,20 This pharmacologic effect can reduce detrusor muscle overactivity, which is considered as one of the important etiological factors in children with enuresis.21 It has also shown anticholinergic activity, with beneficial effects in children with enuresis.22,23 According to mentioned traditional use of chamomile oil, besides its potential pharmacologic effects on bladder function, this study aimed to evaluate its efficacy and safety in children with monosymptomatic nocturnal or daytime enuresis.
Materials and Methods
Trial Design
The study was designed as a 2-arm, double-blind randomized placebo-controlled clinical trial using a parallel design with a 1:1 allocation ratio. There was no change in methods after trial commencement.
Participants
One hundred and twenty-five patients attending the Pediatric Clinic of Qom Azad University (Golpaygani Hospital) between March 2014 and August 2014 with a clinical diagnosis of monosymptomatic nocturnal or daytime enuresis were evaluated for inclusion in the study (more than 7 years old for nocturnal and more than 4 years old for daytime enuresis). Children with other lower urinary tract symptoms including consistently increased (≥8 times/day) or decreased (≤3 times/day) voiding frequency, urgency, hesitancy, straining, a weak stream, intermittency, holding maneuvers, a feeling of incomplete emptying, postmicturition dribble, and genital or lower urinary tract pain were excluded from the study based on the International Children’s Continence Society criteria.24
Sample Size
The sample size was calculated by a statistician considering a one-sided significance level of .05, a power of .90, and an α of .05 based on a previous study on complementary medicine intervention on enuresis.25 The minimum required sample size was calculated to be 32 participants per group.
Interventions
Preparation of the formulation was done in the School of Pharmacy according to traditional product instructions as described earlier.26 No change was made in production method because it was important to evaluate the efficacy of the same product available in the local market and used by patients. The chamomile flower was purchased from a local herbal shop in Iran and was verified by a botanist at the Herbarium Center of the School of Pharmacy, with voucher number PM407. Chamomile dry flowers were grinded and macerated in water for 24 hours and then decocted for 30 minutes. The resulting extract was then boiled in an oily vehicle (sweet almond oil) to evaporate the water portion (boiling and evaporating method). Sweet almond oil, as the vehicle of our drug, was considered to be a placebo. The parents were instructed to use 6 drops of the chamomile oil topically on the perineal and suprapubic area of children one time per night (based on traditional instruction). The control group received 6 drops of sweet almond oil one time per night in the mentioned area as in the intervention group.
Outcomes
Patients were evaluated prior to and following 2, 4, and 6 weeks of the intervention in terms of the frequency of enuresis. The number of participants with any observed or reported adverse events were also registered and compared between groups after the study.
Randomization, Blinding, and Allocation Concealment
Eighty eligible patients were randomly allocated to 2 parallel groups (the drug and placebo groups) by the clinic secretary, who had been instructed to use a randomized list. The randomized list was generated using Microsoft Excel with a block randomization method, as previously described.27 The physicians, researchers, and statisticians were blind to the allocation of patients. Based on the same shape and size of the drug and placebo containers and a similarity in oils color, the patients were blind to the drug allocation.
Statistical Methods
The descriptive data were presented by means and standard error of means in case of quantitative data and as percentage in case of qualitative data. Student t test, paired t test, and χ2 test were used for statistical comparison of primary characteristics and outcomes in each group and between the drug and placebo groups. Pearson correlation test was used to evaluate the significance of correlation between age and outcomes. Repeated measures ANOVA test was used to evaluate the significance of changes in outcomes during the study. A P value of less than .05 was considered significant.
Ethical Considerations
The study protocol was in compliance with the Declaration of Helsinki (1989 revision) and approved by the Local Medical Ethics Committee of Tehran University of Medical Sciences and was provided with the following reference number: 2181/130/92/ . The trial was registered in the Iranian Clinical Trials Registry (Registration ID: IRCT2014052617870N1).
. The trial was registered in the Iranian Clinical Trials Registry (Registration ID: IRCT2014052617870N1).
Results
From March 2014 to August 2014, a total of 125 patients were assessed for eligibility, and finally, 80 of them were randomized to receive the trial drug or placebo. All patients in the intervention and placebo groups were followed-up to end of the study. One patient in the intervention group and 3 patients in the placebo group did not have full adherence to the study protocol (taking other drugs out of the study protocol). Their data were analyzed in their allocated group according to intention to treat analysis protocol.28 Figure 1 is a flow chart that reveals detailed descriptions of patients’ enrolment, randomization, and outcomes.
Figure 1.
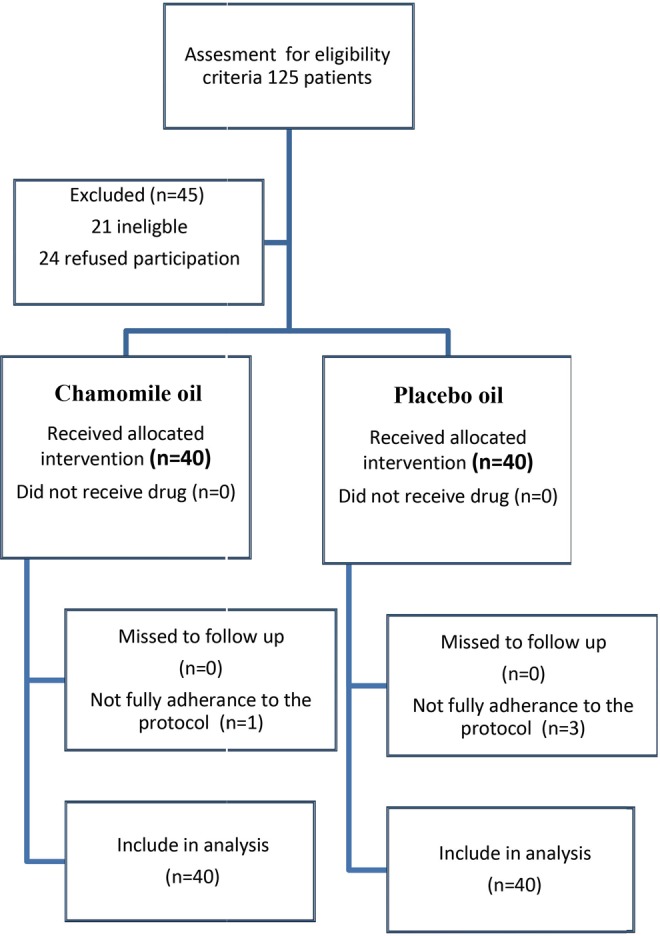
Flowchart of study inclusion, allocation, and follow-up.
In this study, 53 boys (66.2%) and 27 girls (33.8%) participated, in which 24 (60%) boys and 16 (40%) girls were in the case group and 29 (72.5%) boys and 11 (27.5%) girls were in the control group (P = .208). The mean age of the case group was 9.52 ± 1.88 while it was 9.90 ± 2.51 in the control group (P = .453). The youngest children in the case and control groups were, respectively, 7 and 6 years, and the oldest ones in the case and control groups were, respectively, 14 and 18 years. There was no significant difference between the 2 arms in terms of demographic characteristics.
The mean frequency of enuresis has decreased significantly in the intervention group in the first, second, and third 2 weeks when compared with the base scores (Table 1). The mean frequency of enuresis at the first, second, and third 2 weeks was lower in the intervention group and the differences were statistically significant (P < .001, P = .03, and P < .001, respectively; see Table 2 and Figure 2). Using ANOVA with repeated measures with a Greenhouse-Geisser correction, the mean frequency of enuresis in the chamomile group was statistically significantly different, F(2.44, 95.170) = 54.41, P < .001.
Table 1.
The Mean Frequency of Enuresis Before the Intervention Compared With the First, Second, and Third 2 Weeks After the Intervention.
| Groups | Paired Differences | |||||
|---|---|---|---|---|---|---|
| Mean Differences | SEM | 95% Confidence Interval of the Difference | ||||
| Lower | Upper | P Valuea | ||||
| Chamomile | Base—First 2 weeks | 2.575 | 0.373 | 1.819 | 3.330 | <.001 |
| Base—Second 2 weeks | 2.500 | 0.469 | 1.550 | 3.449 | <.001 | |
| Base—Third t weeks | 5.350 | 0.381 | 4.578 | 6.121 | <.001 | |
| Placebo | Base—First 2 weeks | 0.225 | 0.431 | −0.648 | 1.09856 | .605 |
| Base—Second 2 weeks | 1.425 | 0.480 | 0.453 | 2.396 | .005 | |
| Base—Third 2 weeks | 2.200 | 0.479 | 1.230 | 3.169 | <.001 | |
Abbreviation: SEM, standard error of mean.
aPaired t test.
Table 2.
Comparison of the Mean Frequency of Enuresis in the Chamomile and Placebo Groups.
| Frequency of Enuresis | Groups (Mean ± SEM) | Difference | 95% Confidence Interval of the Difference | P Valuea | ||
|---|---|---|---|---|---|---|
| Chamomile | Placebo | Lower | Upper | |||
| Baseline | 8.20 ± 0.31 | 8.22 ± 0.31 | −0.02 | −0.91 | 0.86 | .956 |
| After 2 weeks | 5.62 ± 0.31 | 8.00 ± 0.25 | −2.37 | −3.17 | −1.57 | <.001 |
| After 4 weeks | 5.70 ± 0.35 | 6.80 ± 0.34 | −1.10 | −2.09 | −0.10 | .03 |
| After 6 weeks | 2.85 ± 0.31 | 6.02 ± 0.36 | −3.17 | −4.13 | −2.21 | <.001 |
Abbreviation: SEM, standard error of mean.
aStudent’s t test.
Figure 2.
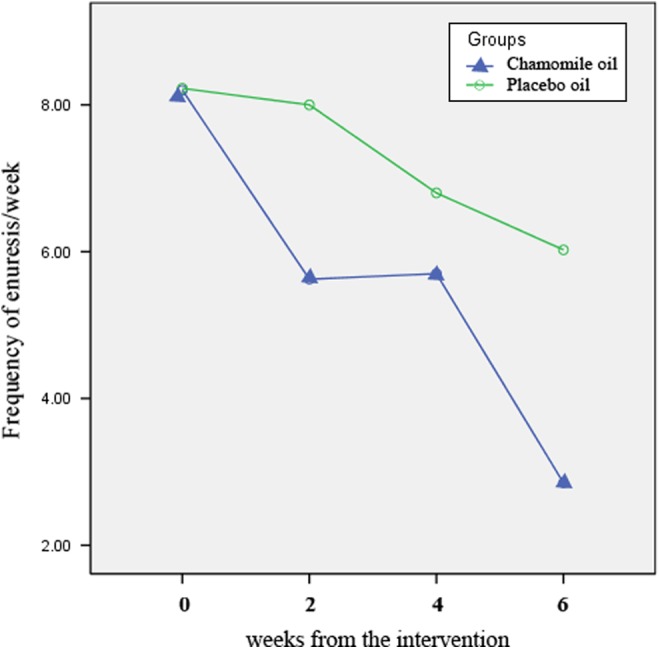
The mean frequency of enuresis in the chamomile and placebo groups at the first, second, and third 2 weeks of the intervention.
There was no significant correlation between age and frequency of nocturia at the end of the intervention (P = .926). There was also no significant difference between this outcome in boys and girls (P = .848). There was no report of any adverse event in the study groups. However, 5 parents complained about the greasy nature of the drug.
Discussion
This study aimed to evaluate the effectiveness of topical application of chamomile oil in the treatment of urinary incontinence in children. The results of this study showed the higher effectiveness of this traditional formulation compared with placebo so that the mean frequency of nocturia at the first, second, and third 2 weeks in the chamomile oil group was significantly lower than that in the placebo group.
Few clinical trials have studied complementary medicine for nocturia, and most of them compared herbal and chemical drugs.3 In Huang et al’s small clinical trial, which compared Chinese medicinal herbs with desmopressin, after the end of treatment, failure or relapse rate of enuresis in children taking herbal treatment was lower than children treated with desmopressin.3 In other trials, medicinal herbs appeared to be better than imipramine both during and after the treatment.3 In 3 clinical trials by Cheng et al, Feng et al, and Zhu et al, 3 different preparations of medicinal herbs were compared with imipramine. Although the outcome measures varied, during and after the treatment, medicinal herbs were better than imipramine. However, all these trials had small sample size and most of them did not compare medicinal herbs with placebo or control group. Thus, the evidence on the effectiveness of medicinal herbs for the treatment of children with enuresis is not strong and should be confirmed in further trials.3
In traditional Persian medicine, the reason of urinary incontinence is the inability of the bladder sphincter to maintain needed contraction due to its wet and cold distemperament.29 Since a medicinal herb with the opposite temperament of the disease is considered in traditional Persian medicine, warm and astringent herbs including chamomile is suggested for cold and wet temperament disease such as urinary incontinence. The topical use of oil-based formulations for drug delivery was popular in traditional Persian medicine.30–32 In the case of the treatment of enuresis, topical use of the oil-based formulation of chamomile is suggested in traditional Persian medicine for warming up the bladder.7
Spasmolytic and anticholinergic activity of Matricaria recutita L active constituents can highlight the potential mechanism of observed effect of its oil on enuresis in children. Detrusor muscle overactivity has an important role in children with enuresis.22 Defects in the circadian rhythm of detrusor inhibition are observed in children with monosymptomatic enuresis.34 Increased rate of bladder contractions occurring in association with the enuretic episodes have been observed in urodynamic studies performed during sleep in children with nocturia.33 Drugs with spasmolytic activity such as oxybutynin are well known in the treatment of enuresis.35 Chamomile also has shown spasmolytic activity on smooth muscle tissues.20,21 Inhibition of cAMP and cGMP phosphodiesterases by flavonoid components is considered as its mechanisms of spasmolytic activity.20 Topical or transdermal drug delivery for treatment of detresur muscle overactivity has been recently introduced in the medical literature.36 However, this type of drug delivery to visceral organs has old use in traditional Persian medicine. The mentioned evidences can lighten the mechanism of efficacy of topical use of chamomile oil in the treatment of enuresis of children.
The most important limitation of our study was the short duration of follow-up and not considering the recurrence rate after discontinuing the treatment. The absence of a standard treatment arm in the study is another limitation. Future research with higher methodological quality is suggested, using systemic formulation of chamomile and in comparison with most commonly used interventions like desmopressin and imipramin in appropriate settings and including follow-up periods of longer duration. Use of sweet almond oil as a placebo in the control group should be also considered in interpreting the results. Sweet almond oil cannot be mentioned as completely ineffective placebo, but because of the use of this oil as a vehicle of main drug, the observed results of the study can be considered as the separate effect of chamomile.
The findings of this study showed that the topical use of chamomile oil can decrease the nocturia episodes in the first, second, and third 2 weeks compared with the placebo. Therefore, it can be considered as a potential complementary option in the treatment of monosymptomatic enuresis in children.
Acknowledgment
The authors thank all the participants who cooperated during the study and Mrs Fatemeh Hosseinzadeh (Staff in the Clinical Research Development Center of Qom University of Medical Science) for English language editing in this article.
Footnotes
Author Contributions: The work presented in this article was carried out through collaboration between all authors. HS and MBM made the initial hypothesis. All authors participate in defining the research theme and providing the proposal. MJQ, HS, MG, and NA visited the patients, enrolled them, and followed-up on their progress. MH interpreted the data and wrote the article. MBM supervised the work. All authors have contributed to, edited, and approved the article.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This trial is funded by the Tehran University of Medical Sciences (Grant Number 2181/130/92).
Ethical Approval: The study protocol was approved by the Local Medical Ethics Committee of Tehran University of Medical Sciences and was provided with the following reference number: 2181/130/92/ .
.
References
- 1. World Health Organization. Nonorganic Enuresis. The ICD10 Classification of Mental and Behavioural Disorders: Clinical Descriptions and Diagnostic Guidelines. Geneva, Switzerland: World Health Organization; 1992. [Google Scholar]
- 2. Glazener CMA, Peto RE, Evans JHC. Effects of interventions for the treatment of nocturnal enuresis in children. Qual Saf Health Care. 2003;12:390–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Huang T, Shu X, Huang YS, Cheuk DK. Complementary and miscellaneous interventions for nocturnal enuresis in children. Cochrane Database Syst Rev. 2011;(12):CD005230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Vinchurkar SA, Arankalle DV. Integrating yoga therapy in the management of urinary incontinence: a case report. J Evid Based Complementary Altern Med. 2015;20:154–156. [DOI] [PubMed] [Google Scholar]
- 5. Ilyas M. Management of nocturnal childhood enuresis: a new challenge. Pediatr Ann. 1996;25:258–264. [DOI] [PubMed] [Google Scholar]
- 6. Monda JM, Husmann DA. Primary nocturnal enuresis: a comparison among observation, imipramine, desmopressin acetate and bed-wetting alarm systems. J Urol. 1995;154(2 pt 2):745–748. [PubMed] [Google Scholar]
- 7. Aghili Khorasani MH. Makhzan al Advieh. Tehran, Iran: Bavardaran Press; Research Institute for Islamic and Complementary Medicine, Iran University of Medical Sciences; 2001. [Google Scholar]
- 8. Zargaran A, Borhani-Haghighi A, Faridi P, Daneshamouz S, Kordafshari G, Mohagheghzadeh A. Potential effect and mechanism of action of topical chamomile (Matricaria chammomila L.) oil on migraine headache: a medical hypothesis. Med Hypotheses. 2014;83:566–569. [DOI] [PubMed] [Google Scholar]
- 9. Singh O, Khanam Z, Misra N, Srivastava MK. Chamomile (Matricaria chamomilla L.): an overview. Pharmacogn Rev. 2011;5:82–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Srivastava JK, Pandey M, Gupta S. Chamomile, a novel and selective COX-2 inhibitor with anti-inflammatory activity. Life Sci. 2009;85:663–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jamalian A, Shams-Ghahfarokhi M, Jaimand K, Pashootan N, Amani A, Razzaghi-Abyaneh M. Chemical composition and antifungal activity of Matricaria recutita L. flower essential oil against medically important dermatophytes and soil-borne pathogens. J Mycol Med. 2012;22:308–315. [DOI] [PubMed] [Google Scholar]
- 12. Tavakol HS, Farzad K, Fariba M, et al. Hepatoprotective effect of Matricaria chamomilla L in paraquat induced rat liver injury. Drug Res (Stuttg). 2015;65:61–64. [DOI] [PubMed] [Google Scholar]
- 13. Seyyedi SA, Sanatkhani M, Pakfetrat A, Olyaee P. The therapeutic effects of chamomilla tincture mouthwash on oral aphthae: a randomized clinical trial. J Clin Exp Dent. 2014;6:e535–e538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sharifi F, Simbar M, Mojab F, Majd HA. Comparison of the effects of Matricaria chamomila (Chamomile) extract and mefenamic acid on the intensity of premenstrual syndrome. Complement Ther Clin Pract. 2014;20:81–88. [DOI] [PubMed] [Google Scholar]
- 15. Asadi-Shahmirzadi A, Mozaffari S, Sanei Y, et al. Benefit of Aloe vera and Matricaria recutita L. mixture in rat irritable bowel syndrome: combination of antioxidant and spasmolytic effects [published online December 21, 2012]. Chin J Integr Med. doi:10.1007/s11655-012-1027-9. [DOI] [PubMed] [Google Scholar]
- 16. Shoara R, Hashempur MH, Ashraf A, Salehi A, Dehshahri S, Habibagahi Z. Efficacy and safety of topical Matricaria chamomilla L. (chamomile) oil for knee osteoarthritis: a randomized controlled clinical trial. Complement Ther Clin Pract. 2015; 21:181–187. [DOI] [PubMed] [Google Scholar]
- 17. Farideh ZZ, Bagher M, Ashraf A, Akram A, Kazem M. Effects of chamomile extract on biochemical and clinical parameters in a rat model of polycystic ovary syndrome. J Reprod Infertil. 2010;11:169–174. [PMC free article] [PubMed] [Google Scholar]
- 18. Hashempur MH, Lari ZN, Ghoreishi PS, et al. A pilot randomized double-blind placebo-controlled trial on topical chamomile (Matricaria chamomilla L.) oil for severe carpal tunnel syndrome. Complement Ther Clin Pract. 2015;21:223–228. [DOI] [PubMed] [Google Scholar]
- 19. Jarrahi M, Vafaei AA, Taherian AA, Miladi H, Rashidi Pour A. Evaluation of topical Matricaria chamomilla extract activity on linear incisional wound healing in albino rats. Nat Prod Res. 2010;24:697–702. [DOI] [PubMed] [Google Scholar]
- 20. Maschi O, Cero ED, Galli GV, Caruso D, Bosisio E, Dell’Agli M. Inhibition of human cAMP-phosphodiesterase as a mechanism of the spasmolytic effect of Matricaria recutita L. J Agric Food Chem. 2008;56:5015–5020. [DOI] [PubMed] [Google Scholar]
- 21. Achterrath-Tuckermann U, Kunde R, Flaskamp E, Isaac O, Thiemer K. Pharmacological investigations with compounds of chamomile. V. Investigations on the spasmolytic effect of compounds of chamomile and Kamillosan on the isolated guinea pig ileum [in German]. Planta Med. 1980;39:38–50. [DOI] [PubMed] [Google Scholar]
- 22. Yeung CK, Chiu HN, Sit FK. Bladder dysfunction in children with refractory monosymptomatic primary nocturnal enuresis. J Urol. 1999;162:1049–1054. [DOI] [PubMed] [Google Scholar]
- 23. Austin PF, Ferguson G, Yan Y, Campigotto MJ, Royer ME, Coplen DE. Combination therapy with desmopressin and an anticholinergic medication for nonresponders to desmopressin for monosymptomatic nocturnal enuresis: a randomized, double-blind, placebo-controlled trial. Pediatrics. 2008;122:1027–1032. [DOI] [PubMed] [Google Scholar]
- 24. Nevéus T, von Gontard A, Hoebeke P, et al. The standardization of terminology of lower urinary tract function in children and adolescents: report from the Standardisation Committee of the International Children’s Continence Society. J Urol. 2006;176:314–324. [DOI] [PubMed] [Google Scholar]
- 25. Reed WR, Beavers S, Reddy SK, Kern G. Chiropractic management of primary nocturnal enuresis. J Manipulative Physiol Ther. 1994;17:596–600. [PubMed] [Google Scholar]
- 26. Hamedi A, Zarshenas MM, Sohrabpour M, Zargaran A. Herbal medicinal oils in traditional Persian medicine. Pharm Biol. 2013;51:1208–1218. [DOI] [PubMed] [Google Scholar]
- 27. Kim J, Shin W. How to do random allocation (randomization). Clin Orthop Surg. 2014;6:103–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. White IR, Horton NJ, Carpenter J, Pocock SJ. Strategy for intention to treat analysis in randomised trials with missing outcome data. BMJ. 2011;342:d40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nojavan F, Sharifi H, Ghanbari Z, Kamalinejad M, Mokaberinejad R, Emami M. Causes and risk factors of urinary incontinence: Avicenna’s point of view vs. contemporary findings. Urol J. 2015;12:1995–1998. [PubMed] [Google Scholar]
- 30. Hashempur MH, Homayouni K, Ashraf A, Salehi A, Taghizadeh M, Heydari M. Effect of Linum usitatissimum L. (linseed) oil on mild and moderate carpal tunnel syndrome: a randomized, double-blind, placebo-controlled clinical trial. Daru. 2014;22:22–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Heydari M, Homayouni K, Hashempur MH, Shams M. Topical Citrullus colocynthis in painful diabetic neuropathy: a double-blind randomized placebo-controlled clinical trial. J Diabetes. 2016;8(2):246–252. doi:10.1111/1753-0407.12287. [DOI] [PubMed] [Google Scholar]
- 32. Heydari M, Shams M, Hashempur MH, Zargaran A, Dalfardi B, Borhani Haghighi A. The origin of the concept of neuropathic pain in early medieval Persia (9th-12th century CE). Acta Med Hist Adriat. 2015;(suppl 1):9–22. [PubMed] [Google Scholar]
- 33. Van Hoeck K, Bael A, Lax H, et al. Urine output rate and maximum volume voided in school-age children with and without nocturnal enuresis. J Pediatr. 2007;151:575–580. [DOI] [PubMed] [Google Scholar]
- 34. Nørgaard JP, Hansen JH, Nielsen JB, Rittig S, Djurhuus JC. Nocturnal studies in enuretics. A polygraphic study of sleep-EEG and bladder activity. Scand J Urol Nephrol Suppl. 1989;125:73–78. [PubMed] [Google Scholar]
- 35. Nevéus T. Oxybutynin, desmopressin and enuresis. J Urol. 2001;166:2459–2462. [DOI] [PubMed] [Google Scholar]
- 36. MacDiarmid SA. The evolution of transdermal/topical overactive bladder therapy and its benefits over oral therapy. Rev Urol. 2009;11:1–6. [PMC free article] [PubMed] [Google Scholar]


