Abstract
Leishmaniasis is caused by an obligate intracellular protozoa belonging to Leishmania genus. The current drugs for treatment of leishmaniasis possess many disadvantages; therefore, researchers are continuously looking for the more effective and safer drugs. The aim of this study is to review the effectiveness, toxicities, and possible mechanisms of pharmaceutical actions of different garlic extracts and organosulfur compounds isolated from garlic against Leishmania spp. in a variety of in vitro, in vivo and clinical trials reports. All relevant databases were searched using the terms “Allium sativum,” “Garlic,” “Allicin,” “Ajoene,” “Leishmania,” “in vitro,” “in vivo,” and “clinical trial,” alone or in combination from 5 English databases (Web of Science, PubMed, Science Direct, Scopus, Google Scholar) and 3 Persian databases (Scientific Information Database, Iran Medex, and Magiran) from 1990 to 2014. In summary, garlic with immunomodulatory effects and apoptosis induction contributes to the treatment of leishmaniasis.
Keywords: Allium sativum, garlic, Leishmania, organosulfur compounds, allicin, ajoene
Leishmaniasis is a vector-borne disease that is caused by an obligate intracellular protozoa belonging to Leishmania genus. In the Old World and New World it is transmitted by sandfly bites of Phlebotomus genus and Lutzomyia, respectively. Leishmaniasis is more prevalent in tropical and subtropical regions and based on clinical manifestations, it is responsible for at least 3 types of disease that range from self-healing cutaneous lesions to severe and life-threatening mucocutaneous and visceral forms. According to the World Health Organization reports, leishmaniasis as a major public health concern with 2.4 million disability-adjusted life years is found in 5 continents and about 100 countries. Also, about 12 million people are infected currently and 350 million individuals are at risk for acquiring disease as well. Annually, approximately 0.2 to 0.4 million new cases of visceral leishmaniasis and 0.7 to 1.2 million new cases of cutaneous leishmaniasis occur throughout the world.1 Pentavalent antimonial compounds like pentostam and glucantime have been used as the most important antileishmanial drugs from the 1940s till now, while unfortunately there is no effective and efficient vaccine against leishmaniasis yet. Also, amphotericin B, pentamidine, paromomycin, and miltefosine are being prescribed as second-choice drugs. Because of some drawbacks even after adjustment of course remedy and dose proportion of these drugs, including painful injection, prolonged treatment course, high cost, drug resistance, toxicity, and different side effects, there is an urgent need for investigators to find cheaper, affordable, safer, and more effective antileishmanial drugs.2
According to a report published by the World Health Organization, about 80% of people have a tendency to use traditional medicine to alleviate their pain and as a treatment for ailments.3 Recently, use of plant-derived and plant extracts has had a rising trend worldwide that is justified by low cost, fewer side effects, easy availability, and wealth of valuable sources of the plants.4–7 In recent years, several researches have been performed by the current authors about efficacy of different plant extracts and other compounds against Leishmania spp. viability, including Allium sativum,8 Cordia myxa,9 Crocus sativus,10 Camellia sinensis,11,12 Satureja khuzestanica,13 and some biurets compounds.14
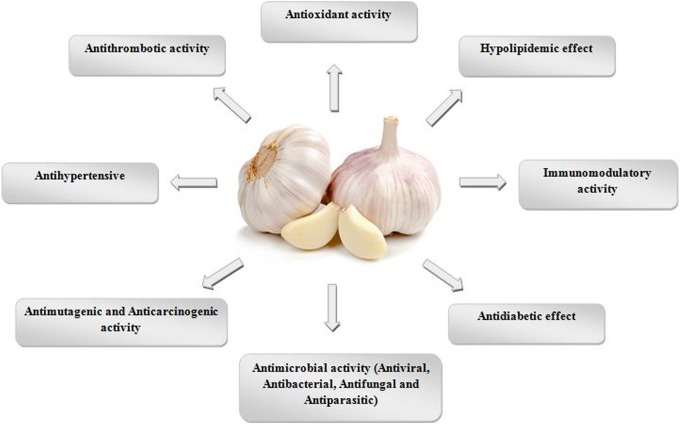
Allium sativum (garlic) belongs to Plantae kingdom, Asparagales order, Amaryllidaceae family, Allioideae subfamily and Allium genus (http://www.ncbi.nlm.nih.gov/Taxonomy/Browser/wwwtax.cgi?id=4682). Medicinal benefits and therapeutic properties of garlic are ascribed to presence of organosulfur compounds (OSCs).7,15 Historically, garlic has possessed medicinal and dietary importance in different cultures for more than 4000 years. In addition, there is evidence that proves garlic has been consumed for various purposes over the centuries. For instance, during the Greek Olympics for stamina enhancement in athletes, during the Second World War as an antibacterial agent for prevention of gangrene in soldiers, in India as an antiseptic lotion in order to wash ulcers, and in China as a remedy of headache, fever, dysentery, and cholera.16 Numerous therapeutic properties for A sativum have been mentioned and are summarized in Figure 1. Interestingly, garlic acts as a natural antibiotic but has no damaging effect on friendly bacterial flora.15 Antiparasitic effects of A sativum have previously been confirmed against Entamoeba,17 Cryptosporidium,18 Giardia,19,20 Eimeria,21 Spironucleus,22 Plasmodium,23 Leishmania,8,24 Trypanosoma,25 Trichomonas,26 Schistosoma,27 Hymenolepis,19 Angiostrongylus,28 and Trichuris.28
Figure 1.
Summary of some therapeutic properties of Allium sativum (garlic) bulbs and cloves.
It has previously been reported that garlic components possess immunomodulatory effects both in vitro and in vivo such as shifting the secretion pattern of cytokines from TH2 to TH1 (T helper cell type 2 and 1, respectively), interferon-gamma (IFN-γ), and nitric oxide (NO) production,29,30 although under certain conditions garlic extract may act as immunosuppressive for downregulation of pro-inflammatory responses.31
Immunity Against Leishmaniasis
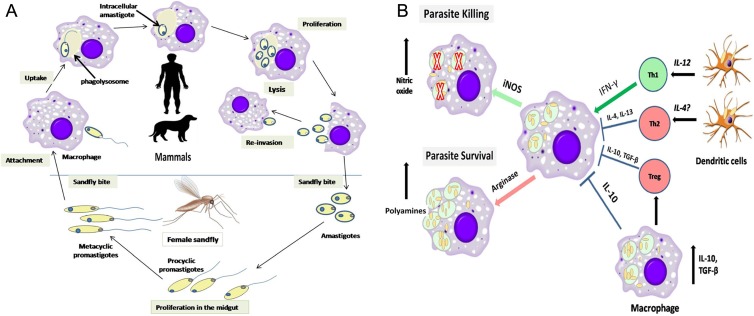
Leishmania life cycle in human body begins after inoculation of metacyclic promastigote forms by female sandflies during blood-feeding process. Shortly after injection, promastigotes are taken up by neutrophils and macrophages (MQs). Promastigotes after entrance to MQs, are quickly converted to a nonmotile forms known as amastigote inside the phagolysosome. Then these immobile forms are divided repeatedly using binary fission within MQs and finally lead to bursting and infecting other cells. Leishmania spp. are obligatory intracellular parasites; hence, they need MQs for differentiation, survival and multiplication (Figure 2A). When the infection starts, MQs and neutrophils are deployed to the site of infection in order to interact with parasite. MQs are considered as the main effector cells to eliminate intracellular protozoa; accordingly, their proper activation seems critical in order to destroy the Leishmania parasites. Overall, MQs activation is classified into 2 categories: alternative and classical activation. The first is promoted by TH2 cytokines like interleukin (IL-4), IL-10, and IL13, while the latter is induced using TH1 cytokines such as IFN-γ. IL-10 restrict secretion of some pro-inflammatory cytokines such as tumor necrosis factor alpha (TNF-α), IL-1, IL-12, and IFN-γ. IL-12 is an important cytokine produced by antigen-presenting cells such as dendritic cells (DCs) and MQs that are necessary for expansion of TH1 and IFN-γ production. It is worth noting that IFN-γ is indispensable for upregulation of inducible nitric oxide synthase (iNOS) mRNA expression and eventually leads to NO production, which plays a crucial role in destroying Leishmania inside MQs, although some inflammatory cytokines like TNF-α, IL-1, IFN-α, and IFN-β also act alike IFN-γ in iNOS gene expression; while TH2 cytokines suppress the TH1 responses and help Leishmania survive within MQs.32 Hence if each compound promoted the TH1-type responses, it would aid in the treatment of leishmaniasis (Figure 2B).29
Figure 2.
(A) Schematic diagram of the Leishmania life cycle. (B) Role of dendritic cells and macrophages in leishmaniasis outcome. Abbreviations: IFN-γ, interferon-gamma; IL, interleukin; iNOS, inducible nitric oxide synthase; TGF-β, transforming growth factor beta; TH1 and TH2, T helper cell type 1 and 2, respectively; Treg, T regulatory cell.
Phytochemistry of Allium sativum
Allium sativum contains many chemical ingredients, including 17 amino acids, more than 33 OSCs, 8 minerals (calcium, potassium, magnesium, germanium, selenium, copper, zinc, and iron), vitamins (A, B1, B2, B3 B6, B12, C, D, E), and some enzymes (allinase).33 Allicin as the major biologically active component of fresh garlic is a candidate in antitumor survey. Several studies have been conducted to determine the chemopreventive and anticarcinogenic role of garlic’s main component since the in vitro and in vivo studies of Weisberger and Pensky,34 which showed garlic thiosulfinate inhibit the tumor cell growth. Allicin (diallyl thiosulfinate) which is quickly created from alliin using alliinase enzyme in freshly crushed garlic, is considered a very reactive thiosulfinate and the main ingredient responsible for biological functions of garlic. Allicin is metabolized and converted into other OSCs like ajoene, diallyl disulfide (DADS), diallyl trisulfide (DATS), and vinyl dithiines (Figure 3). So allicin is not found in garlic unless it is crushed or damaged. In addition, the pungent flavor of garlic is due to the presence of allicin.15 Molecular structure of allicin and other OSCs is shown in Table 1. Allicin is a potential anticancer and antimicrobial agent, the activity of which has been the focus of many researchers in past decades.7,35,36 Numerous publications have introduced allicin as an effective molecule against infectious agents and this fact has been repeatedly confirmed.35,37 Based on reports, bactericidal activity of allicin has been verified against important pathogens, including Enterococcus spp, Bacillus spp, Helicobacter pylori, Escherichia coli, Staphylococcus aureus, Salmonella typhimurium, and Vibrio cholera,35 as well as methicillin-resistant Staphylococcus aureus strains.38 Also, antifungal effects of allicin against Epidermophyton spp, Trichophyton spp, Cryptococcus spp, and Candida spp. are clear.35 Furthermore, it has been reported that allicin possesses antiparasitic activity against Schistosoma,39 Plasmodium,23,40 Babesia,41 Theileria,41 Trypanosoma,42 Leishmania,43 and Entamoeba.44 In addition, allicin by inhibiting the cysteine proteases of protozoa plays an important role as antitrypanosomal and antiplasmodial agent.42 Antimicrobial activity of allicin is justified by its reaction with SH groups of different enzymes such as thioredoxin reductase, alcohol dehydrogenase, cysteine proteinase, and so on.37
Figure 3.
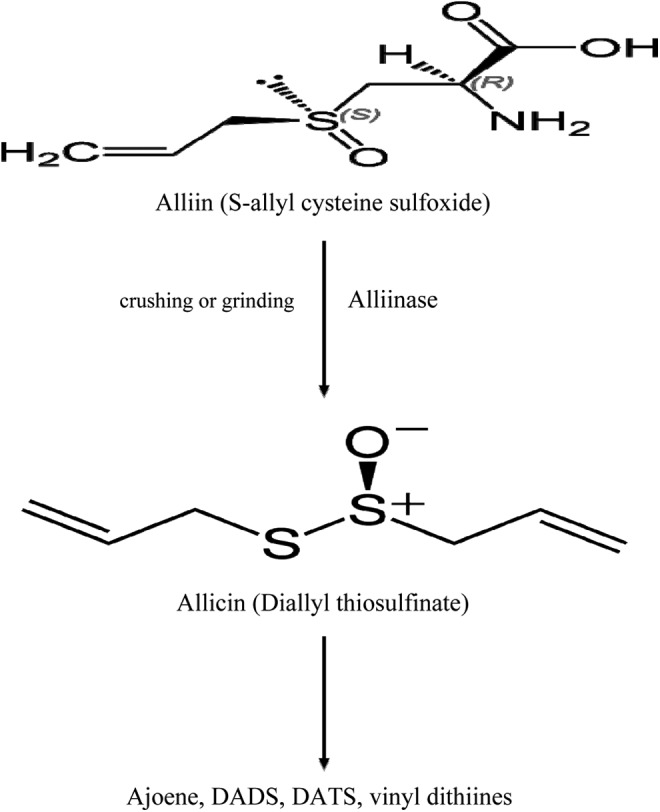
Formation of allicin from alliin, which is catalyzed using alliinase enzyme.
Table 1.
Chemical Structure and Function of Some Organosulfur Compounds (OSCs) Present in Garlic.
| Sulfur Components | Molecular Structure | Function | References |
|---|---|---|---|
| Allicin (diallylthiosulfinate) |
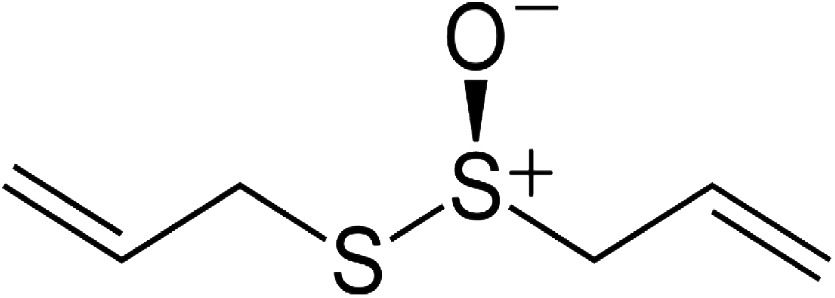
|
Antileishmanial effect on Leishmania infantum and Leishmania donovani promastigotes and amastigotes Antimalarial activity Antiparasitic effect versus Schistosoma mansoni, Trypanosoma brucei, Entamoeba histolytica, and Giardia lamblia Anticancer activity Apoptosis induction in cancer cells Antibacterial, Antifungal and Antiviral activity | 43 23, 40 37, 39, 42 45 46, 47 37 |
| Alliin (S-allylcysteine sulfoxide) |
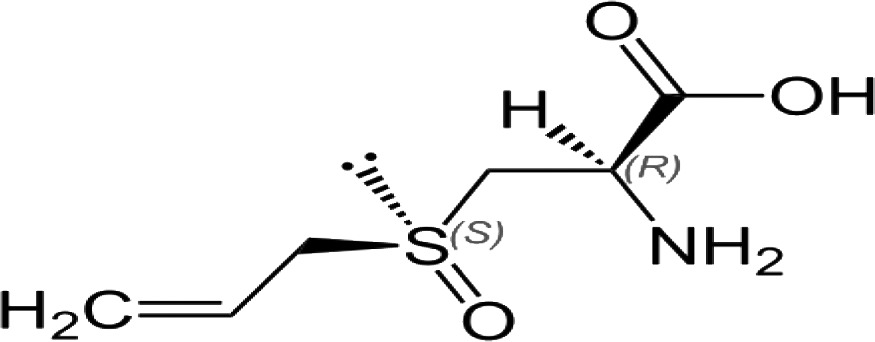
|
The most OSC present in whole garlic | 36 |
| Ajoene |
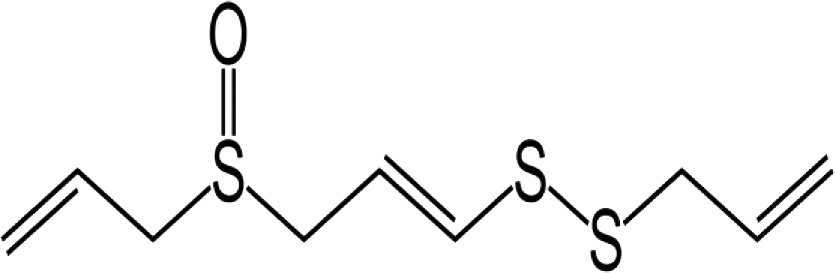
|
Antileishmanial effects on various Leishmania spp. Antitrypanosomal activity The most effective virucidal compound found in garlic Antifungal activity Reduce tumor size using apoptosis induction | 48 49 50 51 52 |
| DADS (diallyl disulfide) |
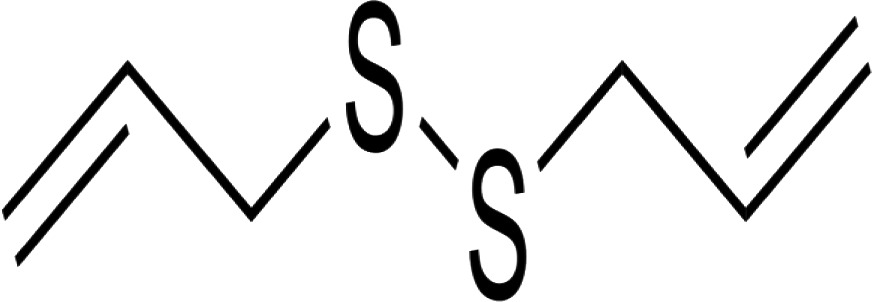
|
Inhibit in vitro proliferation of human A549 lung tumor cells Inhibit the growth of human colon tumor cells Apoptosis induction in human leukemia HL-60 cells Antifungal activity | 53 54 55 56, 57 |
| DATS (diallyl trisulfide) |
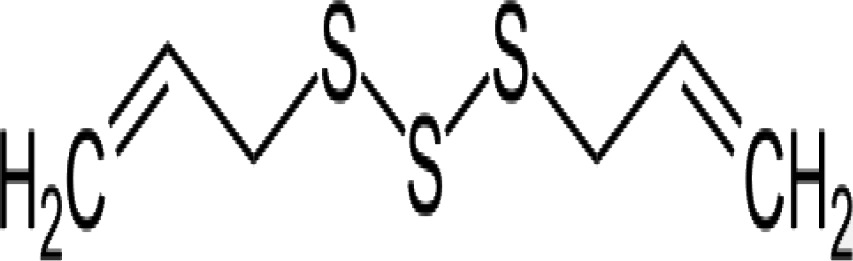
|
Antiparasitic activity against Entamoeba histolytica, Giardia lamblia, and Trypanosoma spp. in vitro Inhibit in vitro proliferation of human A549 lung tumor cells Apoptosis induction in human prostate cancer cells Antifungal activity | 58 53 59 57 |
| SAC (S-allyl cysteine) |
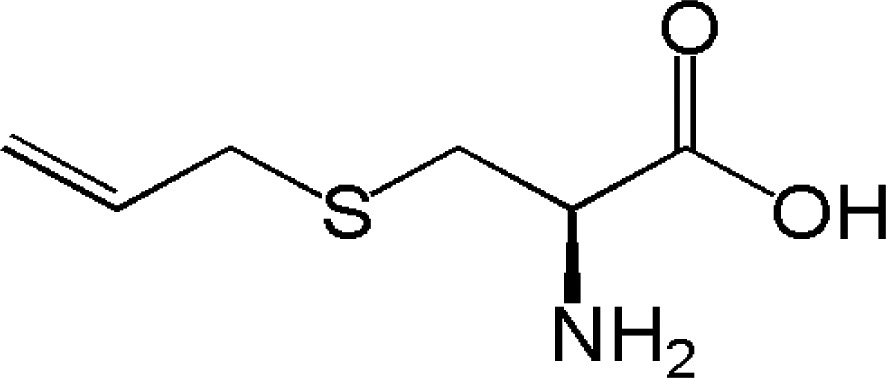
|
Anticancer activity | 36 |
| SAMC (S-allylmercaptocysteine) |
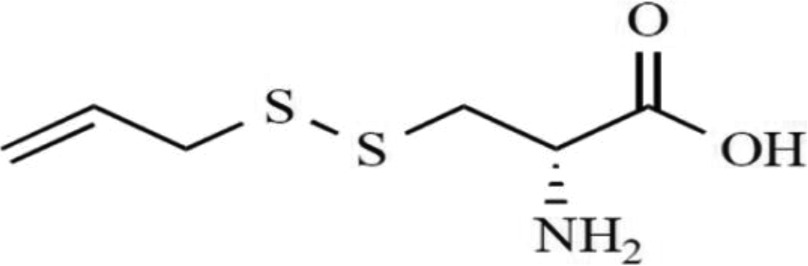
|
Antiproliferative effect on colon cancer cells, apoptosis induction by enhancement in caspase3-like activity Apoptosis induction in erythroleukemia cell lines Anticancer activity | 60 61 36 |
Alliin in the amount of 5.3 to 30 mg/g is the highest OSC present in whole garlic and is considered as a derivative of cysteine amino acid. Content of OSCs present in garlic is different worldwide. For example, alliin in German fresh garlic was found approximately 5.3 to 9.4 mg/g, while in Korean garlic cloves it ranged from 25 to 30 mg/g. The content of OSCs of garlic is summarized in Table 2.36 Ajoene similar to allicin is a potential antimicrobial agent with wide spectrum effects against both Gram-negative and Gram-positive bacteria, protozoa, and fungi35 while in the study by Weber et al,50 ajoene was introduced as the most effective virucidal compound compared with other OSCs in garlic. Ajoene is considered as a Trypanosoma trypanothione reductase inhibitor.49 Also, it is inhibited from phosphatidylcholine biosynthesis in Trypanosoma cruzi.62 The antiparasitic and antiproliferative activity of this OSC is attributed to several functional effects on key enzymes of the antioxidant thiol metabolism.49 Several studies suggest that garlic because of containing the sulfur compounds like allicin, ajoene, and DATS can delay and prevent growth of parasites such as Leishmania major, Crithidia fasciculata, Leptomonas colosoma, Giardia lamblia, Tetratrichomonas gallinarum, Cryptosporidium baileyi, Plasmodium berghei, Histomonas meleagridis, and Trypanosoma spp.4
Table 2.
The Content of Organosulfur Compounds Present in Garlic.a
| Sulfur Components | Contents Range (mg/g) |
|---|---|
| Allicin | 2.3-7.7 |
| Alliin | 5.3-30 |
| Ajoene | 0.12-0.47 |
| Diallyl disulfide | 0.06-0.89 |
| Diallyl trisulfide | 0.01-0.39 |
| S-allyl cysteine | 0.36-0.60 |
a Taken from Yun et al.36
Phytochemical ingredients and OSCs originated from garlic such as allicin, ajoene, DADS, DATS, S-allyl cysteine (SAC) and S-allylmercaptocysteine (SAMC) induce apoptosis via mitochondrial pathway in numerous cancer cells both in vitro and in vivo.36 For instance, DADS induce apoptosis via caspase-3 activation, which led to cleavage of poly-ADP-ribose polymerase (PARP) in human leukemia HL-60 cells.55 Also, ajoene using apoptosis induction led to reduction in basal cell carcinoma tumor size.52 In recent years, a new OSC has been isolated from garlic and is known as thiacremonone (2,4-dihydroxy-2,5-dimethyl-thiophene-3-one).63 Since the considerarable attention given by the World Health Organization to discover the antileishmanial agents from natural products, many studies have been carried out in order to introduce effective drug candidates for leishmaniasis treatment worldwide3; thus, the aim of this study is to review the effectiveness, toxicities, and possible mechanisms of pharmaceutical actions of different garlic extracts and organosulfur compounds isolated from garlic against Leishmania spp. in a variety of in vitro, in vivo, and clinical trials reports.
Search Strategy and Study Selection
To evaluate the antileishmanial effects of garlic, all relevant databases were searched using the terms “Allium sativum,” “Garlic,” “Allicin,” “Ajoene,” “Leishmania,” “in vitro,” “in vivo,” and “clinical trial” alone or in combination from January 1990 up to December 2014. Information of A sativum was collected from 5 English databases (Web of Science, PubMed, Science Direct, Scopus, and Google Scholar) and 3 Persian databases (Scientific Information Database, Iran Medex, and Magiran). In order to evaluate the eligibility and inclusion criteria, articles were reviewed by 2 reviewers (MF-R and SK) and discrepancies among studies were unraveled by consensus. Afterward the desired data were extracted carefully using a data extraction form on the basis of title, kind of preparation, type of study (in vitro, in vivo, and clinical trial), parasite strain, and main findings.
Antiproliferative Activity of Garlic Against Leishmania spp In Vitro
Antileishmanial activity of drugs and other compounds is usually assessed by 3-(4, 5-methylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) colorimetric method in vitro, which evaluates the mitochondrial succinate dehydrogenase enzyme activity of parasite. MTT assay compared with direct counting method has some advantages, including ease of use, reproducibility and reliability features, excellent precision, high sensitivity, no need for use of radioisotope, and radioactive materials; accordingly, it is extensively utilized for drug responses and has become popular throughout the globe.64 Natural compounds of garlic such as allicin and ajoene play a key role against growth inhibition of several species of Leishmania in vitro.43,48 Furthermore, aqueous garlic extract (AGE) was reported to be effective against L mexicana (MNYC/BZ/62/M379 strain).30 Based on the findings of Khademvatan et al,8 AGE illustrated a dose-dependent cytotoxic effect against L major (MRHO/IR/76/ER) promastigotes and observation by optical microscopic revealed some changes in parasites including: cell shrinkage, cytoplasmic condensation, blebbing of cell membrane, nuclear morphology changes and immobilization approximately 48 hours after treatment.
In the study of Mahmoudvand et al,65 antileishmanial effect of A sativum extracts on L tropica (MHOM/IR/2002/Mash2) was investigated. IC50 (50% inhibitory concentration) value of aqueous and methanolic extracts was obtained as 19.2 ± 2.51 μg/mL and 12.3 ± 1.15 μg/mL against promastigotes, respectively, while in control group IC50 was 15.4 ± 2.51 μg/mL. Also, findings indicated aqueous and methanolic extracts of A sativum possess very low cytotoxicity against murine peritoneal MQ cells with 50% cytotoxic concentration (CC50) 348.2 ± 4.6 μg/mL and 291.4 ± 3.08 μg/mL, respectively, while CC50 in control group was 283.6 ± 4.6 μg/mL. However, methanolic extract against promastigotes demonstrated better efficacy than glucantime, which is currently being used as the first remedy of choice for leishmaniasis. Hence methanolic extract with greater effectiveness compared with aqueous extract and glucantime can be considered as candidate for L tropica drug.65 Recently, in similar studies, the effectiveness of methanolic extract of garlic against L major and L donovani promastigotes and amastigotes has been reported. These findings suggest that A sativum extract has high potential to control and combat both cutaneous and visceral leishmaniasis agents.66,67 The majority of investigations have shown that antileishmanial effects of methanolic extract are more effective than aqueous extract65 and usage from methanol solvent is more common than others.65–67 This fact may be due to the greater ability of methanol in isolation and extraction of a wide range of chemical ingredients compared with aqueous extraction. However, in the studies by Kinuthia et al,68,69 the results were in contrast, which could be justified by the type of extraction, methodology, and strain of parasite as well as contents of OSC of garlic. Antileishmanial activity of garlic extracts and garlic-derived compounds that have been performed in vitro are summarized in Table 3.
Table 3.
Antileishmanial Activity of Garlic Extracts and Garlic-Derived Compounds as Examined In Vitro.
| Preparation | Leishmania Strain | Findings | Reference |
|---|---|---|---|
| AGE | L major (MRHO/IR/76/ER) | IC50: 37 mg/mL Inhibit the proliferation and growth of promastigotes Cytotoxic effect against parasite Cell cycle arrest Cell shrinkage Nuclear morphology changes Apoptosis induction by overexpression from PARP and metacaspase genes PS externalization Internucleosomal cleavage of DNA (DNA fragmentation) | 8 |
| AGE | L major (MRHO/IR/76/ER) | IC50: 37 mg/mL Overexpression from IFN-γ and iNOS genes in J774 cells infected by parasite | 70 |
| AGE | L major (MRHO/IR/76/ER) | IC50: 37 mg/mL ↑ IL-12 production by infected MQs | 71 |
| MGE | L major | IC50: 4.94 μg/mL Inhibit the proliferation and growth of promastigotes | 72 |
| AGE MGE | L tropica (MHOM/IR/2002/Mash2) | IC50: 19.2 ± 2.51 μg/mL and CC50: 348.2 ± 4.6 µg/mL for aqueous extract IC50: 12.3 ± 1.15 µg/mL and CC50: 291.4 ± 3.08 µg/mL for methanolic extract Acceptable cytoxicity against promastigotes specially for methanolic extract Low cytotoxicity against murine MQs for both extracts | 65 |
| MGE | L donovani (MHOM/IN/1998/KE16) | IC50: 89 ± 7 µg/mL and 67 ± 5 µg/mL for promastigotes and amastigotes, respectively Show appreciable activity against both promastigotes and amastigotes forms | 67 |
| CSA | L. donovani (MHOM/IN/80/DD8) and 5 other local strains | ↓ Promastigotes proliferation | 73 |
| AGE Ethanolic extract | L mexicana and L chagasi | Inhibition of L mexicana and L chagasi growth | 74 |
| Allicin | L mexicana and L chagasi | Inhibition of L mexicana and L chagasi growth | 75 |
| SAC | L major (MRHO/IR/76/ER) | ↑ Survival and promastigotes growth in culture medium | 76 |
| Ajoene | L mexicana (Lm: MHOM/VE/80/NR) L mexicana venezuelensis (Lmv: MHOM/VE/80/H16) L mexicana amazonensis (Lma: M112, IFLA/BR/67/PH8) L donovani chagasi (Ldch: MHOM/BR/74/PP75) | IC50: 1.83 µM Leishmanicidal effect against all species Ultrastructural survey showed: morphological changes, nuclear and mitochondrial membrane alterations | 48 |
| AGE | L major (IDUB/KD/94=NLB-144) | IC50: 575.75 µg/mL | 69 |
| MGE | L major (IDUB/KD/94=NLB-144) | IC50: 863.12 µg/mL | 68 |
| Allicin | L infantum and L donovani | Inhibit the proliferation of L infantum and L donovani promastigotes Transmission electron microscope showed: decrease in cytoplasmic electron dense bodies, chromatin condensation of nucleus and kDNA, deteriorate the mitochondria and kinetoplast membranes integrity and cell swelling | 43 |
| AGE | L. major (MRHO/IR/76/ER) | Killing amastigotes inside MQs | 77 |
| MGE | L. major and L. donovani | IC50: 34.22 μg/mL and 37.41 μg/mL for L major and L donovani promastigotes, respectively Cytotoxic effect against parasite | 66 |
| AGE R10 | L. mexicana (MNYC/BZ/62/M379) | ↓ Proportion of infected MQs and number of parasites per cell | 30 |
| Active ingredients derived from garlic | Not mentioned | Cytotoxic effects against parasites in vitro | 78 |
Abbreviations: AGE, aqueous garlic extract; CC50, 50% cytotoxic concentration; CSA, crude soluble antigen; IC50, 50% inhibitory concentration; IFN-γ, interferon-gamma; IL, interleukin; iNOS, inducible nitric oxide synthase; MGE, methanolic garlic extract; MQs, macrophages; PARP, poly ADP-ribose polymerase; PS, phospholipid phosphatidylserine; SAC, S-allyl cysteine.
Various properties of allicin such as anticancer activity and its antimicrobial effects, which are apparent, as well as numerous articles have verified antiproliferative activity of allicin against a broad spectrum of infectious agents.35,37 It is noteworthy that mechanism of action of allicin is correlated by interaction with thiol (SH) groups of some enzymes.37 In the study by Jesus Corral-Caridad et al,43 allicin, an OSC isolated from garlic, inhibited the proliferation of L infantum and L donovani promastigotes effectively in a time- and dose-dependent manner. The dose of 120 µM allicin completely destroyed the promastigotes after 72 hours. In addition, allicin was able to restrict the multiplication of L. infantum and L. donovani intracellular amastigotes in both J774 cell line and murine peritoneal MQs after 24 hours.43 Allicin with interference in thiol-redox proteins like glutathione and trypanothione/trypanothione reductase led to damaging the Leishmania parasites. In addition, microtubule disruption is another target for allicin.43 In other investigations, aqueous and ethanolic extracts of garlic as well as allicin inhibited the growth of L mexicana and L chagasi in vitro.74,75 It is known that allicin by disrupting the activity of cysteine proteases enzyme, is considered as an antiplasmodial and antitrypanosomal agent; therefore, a similar mechanism might be responsible for antileishmanial effect of allicin.42
Ajoene, an OSC isolated from garlic, is a known antimicrobial and anticancer agent. The cytostatic and antiprotozoal activity of ajoene may be partially due to several effects on key enzymes which contribute to antioxidant thiol metabolism.49,51,52 In the study by Ledezma et al,48 antiproliferative effects of ajoene were evaluated on Leishmania spp. such as L mexicana (Lm: MHOM/VE/80/NR), L mexicana venezuelensis (Lmv: MHOM/VE/80/H16), L mexicana amazonensis (Lma: M112, IFLA/BR/67/PH8), and L donovani chagasi (Ldch: MHOM/BR/74/PP75). Interestingly, a dose-dependent antiproliferative activity from 0.1 to 10 µM ajoene was seen in all Leishmania spp. Concentrations greater than 1 µM exhibited cytotoxic activity (IC50: 1.83 µM), while higher than 10 µM induced complete destruction and lysis of all parasite species after 96 hours of incubation.48 It has been shown that ajoene, by inhibition of phosphatidylcholine biosynthesis and trypanothione reductase enzyme, prevented multiplication of Trypanosoma cruzi.49,62 Hence antileishmanial activity of ajoene can be justified using the mentioned mechanism in Trypanosoma parasites.
There is evidence which suggests that some molecules that act anticancer agents could be effective against Leishmania spp.79,80 SAC has anticancer activity, although in the study by Eslami et al,76 SAC (a water-soluble OSC isolated from garlic) increased the survival and L major (MRHO/IR/76/ER) promastigotes growth in culture medium, which found the concentration of 10 mM had the best effect on growth. It seems that SAC protects the parasites from destruction and oxidative damage.76 The finding of Eslami et al76 is inconsistent with previous studies that were performed by other OSCs isolated from garlic like allicin and ajoene on Leishmania spp.43,48 To clarify the reason for this issue, further investigation is required.
Antileishmanial Effects of Garlic In Vivo and Clinical Trials Studies
Investigations which have exhibited acceptable and effective results in vitro must be followed as in vivo and clinical trials studies. Based on previous findings, BALB/c mice are considered as a vulnerable experimental animal to L major infection and are afflicted with chronic and non-healing ulcers. These murine strains secreted TH2-related cytokines, including IL-4, IL-5 and IL-10 during leishmaniasis infection while cellular immune responses are weak.81 Garlic extract stimulate the TH1-type responses and augment the engulfment activity of peritoneal MQs in L major–infected vulnerable mice that consequently help to cure the disease.29,77 Treatment with AGE simultaneously leads to dramatic reduction in footpad swelling and parasitic—L mexicana (MNYC/BZ/62/M379 strain)—burden in the lesions in mice model while these changes in control group that were treated with glucantime were not statistically significant.30 Also, combination of Tridax procumbens and A sativum extracts is more effective against L mexicana (Hd18-(MHET/MX/ 97/Hd18) in mice than when used alone and leads to increase in IgG2a/IgG1 (immunoglobulin G) ratio.24 It seems a combined therapy of A sativum via other compounds by synergistic effects shows better effectiveness in leishmaniasis treatment, which has been repeatedly confirmed in numerous studies.24,29,82 Methanolic garlic extract (MGE) after oral and peritoneal administration led to a dramatic reduction in hamster L donovani and L major burden.66 Also, in an in vivo survey conducted by Ghaffarifar et al,83 AGE and R10 fraction were used as ointment in order to evaluate diameter of ulcers. R10 showed better efficacy than AGE in BALB/c, C57BL/6 and outbred Swiss Webster (SW) mice in ulcer size.83 In a few reports, after treatment with garlic extract no effective response was observed although the lesion size in mice models was increased.84,85
Garlic extracts and OSCs isolated from garlic like allicin by intensifying the host pro-inflammatory responses, DCs activation, provoking the expansion of MQs and T cells, lead to decrease in parasitemia and protection against acute murine malaria infection, but it has no effect on IL-10 production,40 which is similar to our previous study on L major–infected MQs.71 This issue is in accordance with the fact that allicin in some circumstances is able to downregulate the inflammatory responses.31 There is a similar strategy about usage of garlic in Leishmania spp.70,77,82 In an investigation performed by Ahmadi-Renani et al,82 AGE showed acceptable response in reduction of ulcer size in mice but combination of vitamin A and garlic had better efficacy compared with either garlic or vitamin A alone. Also, a direct correlation was observed between amount of NO production and healing process.82 Some surveys that have been conducted on animal models are summarized in Table 4.
Table 4.
Antileishmanial Activity of Garlic Extracts and Garlic-Derived Compounds as Examined In Vivo.
| Preparation | Parasite strain | Findings | Reference |
|---|---|---|---|
| AGE | L major (IDUB/KD/94=NLB-144) | ↓ Lesion size, footpad swelling and parasitic burden | 69 |
| MGE | L major (IDUB/KD/94=NLB-144) | ↓ Lesion size, footpad swelling and parasitic burden | 68 |
| Allicin | L infantum and L donovani | Inhibit the proliferation of L infantum and L donovani intracellular amastigotes in both J774 cell line and murine peritoneal MQs | 43 |
| AGE | L major (MRHO/IR/76/ER) | ↑ Peritoneal MQs phagocytic activity and killing amastigotes inside MQs | 77 |
| MGE | L major and L donovani | Cytotoxic effect against parasite ↑ NO production Healing effect on ulcer size ↓ Parasite burden | 66 |
| AGE R10 | L mexicana (MNYC/BZ/62/M379) | ↓ Lesion size, footpad swelling and parasitic burden ↓ Proportion of infected MQs and number of parasites per cell ↑ IFN-γ production by T cells ↑ NO production and killing power of MQs Shifting toward TH1 immune responses | 30 |
| AGE | L mexicana Hd18-(MHET/MX/ 97/Hd18) | ↓ Lesion size, footpad swelling and parasitic burden ↑ Provoke production of antibodies (IgG) Combination of Tridax procumbens and Allium sativum extracts lead to increase the IgG2a/IgG1 ratio Tendency to TH1 immune responses | 24 |
| AGE | Leishmania spp. | ↓ Diameter of ulcers in mice ↑ NO production Combination of vitamin A and garlic showed better efficacy compared with when used alone | 82 |
| AGE | L major (MRHO/IR/76/ER) | ↓ Lesions growth in mice and course of disease ↑ DTH responses ↑ Destruction of Leishmania amastigotes in peritoneal MQs ↑ Level of IFN-γ and IL-2 as indicator of TH1-type response ↓ Level of IL-4 and IL-10 as indicator of TH2-type response | 29 |
| AGE R10 fraction (use as ontiment) | L major (MRHO/IR/76/ER) | R10 showed better efficacy than AGE in BALB/c, C57BL/6, and outbred SW mice in diameter of ulcers AGE illustrated dramatically differences with control group in ulcer size of BALB/c mice (P < .05) R10 exhibited significantly differences with control group in ulcers size of BALB/c and outbred SW mice (P < .05) | 83 |
| Hydroalcoholic extract | L major (MRHO/IR/76/ER) | No effective response was observed although the lesion size in mice models was increased | 84 |
| Ethanolic extract | L major (MRHO/IR/76/ER) | No effective response was observed although the lesion size in mice models was increased | 85 |
| Active ingredients derived from garlic as gel form (use as topical gel) | Not mentioned | Curing the ulcers in all experimental mice during 6 weeks | 78 |
Abbreviations: AGE, aqueous garlic extract; DTH, delayed type hypersensitivity; IFN-γ, interferon-gamma; IgG, immunoglobulin G; IL, interleukin; MGE, methanolic garlic extract; MQs, macrophages; NO, nitric oxide; SW mice, Swiss Webster mice; TH1 and TH2, T helper cell type 1 and 2, respectively.
Few reports are available about the efficacy of garlic extracts and garlic-derived compounds that were tested as clinical trials studies in patients with leishmaniasis. Although some results may be in conflict with each other, but overall garlic is reported to be an antileishmanial agent in all kinds of studies. In the following we will discuss, in summary, the performed studies.
Recently, a comprehensive investigation was performed by Samdani and Iqbal Choudhary78 based on laboratory studies and clinical trials about novel formulation from garlic extract on cutaneous leishmaniasis remedy. In this survey, a topical gel was prepared containing active compounds of garlic in order of next tests. Antileishmanial effects in vitro followed in vivo, in which this topical gel was administered once daily on mice. During six weeks their ulcers healed perfectly. It was also followed in humans as clinical trial. Seventy patients with cutaneous leishmaniasis whose disease was confirmed by smear examination were included in the study. After applying the gel, 57 patients (81.42%) responded to treatment whereas in 13 patients (18.57%) no response was seen. Also, 10 patients and 40 patients perfectly recovered after 6 and 8 weeks of therapy, respectively (Table 5).78 In another clinical trial study, MGE was used topically twice a day with concentration of 0.1 g/mL in patients with cutaneous leishmaniasis. All (100%) the patients with cutaneous leishmaniasis responded against MGE treatment,86 which is similar to the study by Samdani and Iqbal Choudhary,78 whereas in a randomized, double blind survey done on 197 patients with cutaneous leishmaniasis, after the usage of placebo or 5% garlic cream, no significant differences were seen between the 2 groups. The authors recommended concentration higher than 5% and different combinations of garlic should be used in other studies.87 The discrepancy between different findings might be due to study population, immune system condition of patients, severity of illness, and kind of Leishmania spp. agents. The studies by Samdani and Iqbal Choudhary78 and Khalid et al86 confirmed that garlic is an excellent source for treatment of cutaneous leishmaniasis and for better understanding of its therapeutic applications, further studies are required. Since the amount of OSCs found in garlic varies in different regions (Table 2)36 the results obtained by authors might also differ from one another.
Table 5.
Antileishmanial Activity of Garlic Extracts and Garlic-Derived Compounds as Examined in Clinical Trials
| Preparation | Findings | Reference |
|---|---|---|
| Active ingredients derived from garlic as gel form (use as topical gel) | 57 (81.42%) out of 70 CL patients responded to treatment | 78 |
| MGE (used topically) | 100% of CL patients responded against MGE treatment 91.6% and 8.4% of CL patients cured during 15-22 and 30 days, respectively | 86 |
| 5% garlic cream | No significant differences were seen between 2 groups (P = .9865) | 87 |
Abbreviations: CL, cutaneous leishmaniasis; MGE, methanolic garlic extract.
Garlic Extract and Garlic-Derived Compounds Induce Apoptosis
Programmed cell death (PCD), also called apoptosis, is characterized using several features including: activation of caspases cascade, change of nucleus morphology, chromatin condensation, cell shrinkage, blebbing of plasma membrane, formation of apoptotic bodies, loss of mitochondrial functions, phospholipid phosphatidylserine (PS) externalization and DNA fragmentation.88 There are numerous evidences that confirmed apoptosis induction by different garlic-derived and garlic extracts occurring in various cells, especially cancerous cells.46,47,52,89 Initially, it was believed that apoptosis happens exclusively in multicellular animals; however, recently it has been revealed that this phenomenon also occurs in unicellular eukaryotes such as Plasmodium, Leishmania, Trypanosoma, Toxoplasma, Trichomonas, Entamoeba, Blastocystis, and Giardia.79,88,90 So, any compound that is able to induce PCD process in Leishmania spp, could be considered as an appropriate candidate drug for the treatment.
In our recent study, PS externalization was measured after AGE treatment using annexin-V FLUOS staining kit. PS exposure is a hallmark of early stage PCD that translocated from inner cell membrane to outer membrane during this process. Also, annexin is a dye that binds with high affinity to PS. Percentage of annexin-positive L major promastigotes 36 and 48 hours after AGE treatment (IC50: 37 mg/mL) was 65.55% and 86.11%, respectively; while in control group it was 3.9% in both time points. DNA fragmentation is another technique used in order to differentiate between necrosis and apoptosis and is considered a hallmark of late-stage PCD in which during this phenomenon internucleosomal cleavage of DNA (approximately between 180-200 bp) happens, whereas in necrosis DNA cleavage is random. In this survey, after 24 hours fragmented DNA in agarose gel electrophoresis confirmed the PCD process, which indicates that A sativum extract is able to induce apoptosis. In addition, overexpression of metacaspase and PARP genes was detected 4 hours after A sativum extract treatment using reverse transcriptase–polymerase chain reaction (RT-PCR); therefore, aqueous A sativum extract is able to induce apoptosis by several mechanisms and this fact was verified using flow cytometry (PS externalization), DNA fragmentation assay (DNA cleavage) and RT-PCR (overexpression of PARP and metacaspase gene). PCD induction by AGE in L major promastigotes might be due to the presence of water-soluble OSCs such as SAC and SAMC8 that in other researches have shown similar activity in cancer cells.15,36,60,61 AGE-induced apoptosis is caspase-independent in L major promastigotes,8 while apoptosis induction using SAMC in SW-480 and HT-29 cell lines, correlated with enhancement in caspase3-like activity.60 Apoptosis induction by garlic extract has been shown in cultured human nonneoplastic (MRC-5) and neoplastic (A549) lung cancer cells,53 in human colon HT-29 and SW-480 cancer cells,60 on human colon cancer cells,91 on breast cancer,92 and on murine transitional cell carcinoma.93 A proteomic study showed a strong correlation between organosulfur-sensitive proteins and apoptotic pathways that supports previous surveys.94
Allicin initiates and induces the apoptosis in cancerous cells whose mechanisms contribute to mitochondrial pathway of Bcl-2/Bax and antioxidant enzyme systems.47 Since L major, due to possessing the mitochondria has similarities with the multicellular animals, it can be assumed that a similar mechanism is involved in apoptosis induction of Leishmania spp. In the investigation of Jesus Corral-Caridad et al,43 allicin was treated with L infantum and L. donovani promastigotes and then examined by transmission electron microscope. Resulting changes included: decrease in cytoplasmic electron-dense bodies, vacuolization, cell swelling, deterioration of the mitochondria and kinetoplast membranes integrity, chromatin condensation of nucleus and kDNA.43 In another ultrastructural study, time- and dose-dependent morphological changes such as nuclear and mitochondrial membrane alterations were seen in L mexicana amazonensis (Lma: M112, IFLA/BR/67/PH8) amastigotes treated with ajoene.48
Leishmanicidal Activities of Garlic by Immunomodulatory Effects
In the literature, it has been reported that garlic shows immunomodulatory activity such as alteration of cytokine production, antibody secretion, lymphocyte proliferation, MQs and DCs activation and phagocytosis promotion.29,30,77 It is assumed that garlic derivatives and garlic extracts augment cellular immune response and concurrently reduce the course of Leishmania spp infection and burden of parasites in mice.24,30,66 NO acts as a key component in eradication and growth inhibition of Leishmania, killing the parasites within MQs, its production is stimulated by IFN-γ cytokine.32 Furthermore, it has been reported some cytokines like IFN-γ and GM-CSF (granulocyte macrophage colony-stimulating factor) alone or in combination with chemotherapy have been effective in limiting the leishmaniasis. Treatment with AGE enhances the IFN-γ production by T-cell in mice, indicating tendency toward TH1 immune response in order to improve a strong cell mediated response for better control against leishmaniasis, while in control groups, glucantime effect is not associated with IFN-γ and NO production, thus antileishmanial effect of garlic extract due to immunomodulatory feature is significantly higher than glucantime both in vitro and in vivo.30 In garlic-treated groups, a regression in lesion development was reported with shifting from TH2 to TH1 immune response and cytokine pattern, which finally reduced the course of infection in BALB/c mice.29 In addition, a single dose of A sativum extract in mouse model could lead to control of infection as a result of increasing phagocytosis and killing power of L major using MQs.77 Garlic extracts and garlic-derived compounds may change lipid trafficking and protein in the surface of cell membrane.95 According to these findings, increased phagocytosis using garlic extract could be explained by changing the protein content and probably enhancing the receptor expression in MQs surface cell membrane.77
Garlic extract may enhance lectin-induced lymphocyte proliferation and natural killer cells activity.96 Furthermore, garlic extract at dose of 20 mg/kg enhance the delayed type hypersensitivity response in mice and histologically lead to hypertrophy and hyperplasia both of the paracortical zone in lymph nodes and periarteriolar sheaths of spleen. The immunopotentiation and immunomodulatory properties of garlic in enhancement of delayed type hypersensitivity and natural killer cells activity can be an excellent mechanism for cell-mediated immunotherapy.97 Some studies have demonstrated garlic extract and its OSCs might contribute to the decrease of NO production in MQ cell lines,98–100 whereas in other researches, enhancement of NO production by MQs was seen.30,70,82,101 Gained results from the survey by Gharavi et al70 illustrated that AGE increases the overexpression of iNOS gene in J774 cell lines that finally lead to NO production. Discrepancy between authors may be associated with type of study (in vitro and in vivo), methodology, kind of extracts, and its concentrations. Antileishmanial activity of garlic extracts and garlic-derived compounds by immunomodulatory effects are summarized in Tables 3 and 4.
Cytokines play a crucial role in adopting the appropriate immune response. TH1-associated cytokines such as IL-2 and IFN-γ stimulate tightly cellular immune response while TH2-associated cytokines such as IL-4, IL-5, and IL-10 play a role in humoral immunity. High level of IL-10 suppresses the TH1 immune responses and cause long-term persistence of Leishmania infection in both experimental animals and human while increased production of IL-12 and IFN-γ cytokines, that is essential for healing process leads to a decrease in both duration of infection and burden of protozoa concurrently (Figure 2B).32 Garlic extract could provoke indirectly or directly IFN-γ production by TH1 cells in L mexicana–infected mice, and consequently stimulate the NO production inside MQs. Also, the effect of R10 fraction of garlic on NO production was evaluated and R10 was shown to increase IFN-γ and subsequently NO value30; hence, immunomodulatory activity of garlic was ascribed to a low molecular weight 14 kDa (kilodalton) glycoprotein known as R1097 and saponin content.35 In fact, saponin is identified as vaccine adjuvant, especially in antileishmanial vaccines.102 According to results from the study by Gamboa-León et al,30 antileishmanial effects of garlic extract both in vitro and in vivo was mediated by several mechanisms associated with TH1 and MQ activation by which immunomodulatory feature of R10 fraction and other ingredients of garlic lead to a shift toward TH1 response. Then, IFN-γ secreted from TH1 affects MQs and enhances their activities like killing power and over production of NO. As was mentioned before, increase in NO level directly correlated with leishmanicidal activity inside MQs.30
In the study by Gharavi et al,71 the effect of AGE on IL-12 and IL-10 levels in L major infected MQs were assessed. Results illustrated IC50 dose (37 mg/mL) of AGE lead to increased secretion of IL-12 from infected MQs while no changes were observed in IL-10 production.71 Also, AGE over 48-hour incubation destroyed L major amastigotes using over expression of IFN-γ and iNOS genes in J774 MQ cell lines.70 Thus IL-12, IFN-γ, and subsequent NO production were identified as a crucial factor against Leishmania amastigotes defense inside MQs; whereas IL-10 delay improving the Leishmania infection.70,71 Also, Miltefosine (an anticancer drug which is used as an alternative drug in leishmaniasis) demonstrated similar effect with AGE on IFN-γ and iNOS mRNA expression, IL-12 and IL-10 production in L major–infected J774 cell lines.80
In L. major–infected BALB/c mice that were treated with AGE, ulcer growth was significantly inhibited, whereas in glucantime treated mice a retardation in ulcer expansion was seen in comparison with control groups, but was unable to suppress the lesions growth. A combined therapy with both glucantime and garlic extract illustrated more effectiveness against lesions growth in mice models than when used alone. In AGE-treated mice, high level of IFN-γ and IL-2 and low level of IL-4 and IL-10 were seen that was indicative of a cell-mediated immune response while in control groups results were in contrast. Also, in glucantime-treated mice, TH2-type response was observed with low level of IFN-γ and IL-2 and high level of IL-4 and IL-10.29 These findings are supported by other reports, which suggest that treatment with glucantime possesses insufficient efficacy in human and experimental animal models.103 Combined therapy with both garlic and glucantime induce a TH1 immune response similar to that stimulated by garlic alone.29 Also, AGE induces production of IgG antibodies in treated mice.24 Finally, in order to achieve successful treatment in leishmaniasis, and additionally to decrease parasite burden, development of strong cell-mediated immune response by IL-2, IL-12, and IFN-γ is required.29,71 In recent years, some researchers succeeded in treating the chronic non healing cutaneous leishmaniasis by combined therapy of IFN-γ and IL-2 or anti IL-4 plus pentostam drug.104,105 Recently, many studies for leishmaniasis treatment have been performed based on immunotherapy methods that have acceptable perspectives for future.106,107
Conclusion and Future Perspective
Garlic as an important traditional medicine in many studies has been more effective than glucantime. In summary, different garlic extracts and garlic-derived compounds, especially OSCs showed cytotoxic activity against several Leishmania species in a variety of in vitro, in vivo and clinical trials reports. A sativum contains potential antimicrobial agents like OSCs such as allicin, ajoene, DADS, and DATS. In addition, apoptosis phenomenon by these ingredients occurs in different cells and pathogen agents. Also, garlic, due to possessing the immunomodulatory activity such as antibody secretion, increase in lymphocytes proliferation, MQs and DCs activation, phagocytosis promotion and by creating a shift in cytokine production pattern from TH2 to TH1, leads to expansion of a strong cell-mediated immunity and reduces the course of treatment. It seems combined therapy of garlic via other extracts, vitamins, and so on leads to increasing their efficacy using synergistic effects. In order to improve the efficiency, initially garlic compounds should be purified and then used as complementary medicine in leishmaniasis treatment. It seems that further bioinformatic and in silico studies are needed to predict major biologically active components as potential antileishmanial derivations.
Acknowledgments
The authors would like to thank all staff of Department of Medical Parasitology of Urmia University of Medical Sciences. The authors would like to thank Dr Alborz Eskandari for his help in preparation of figures.
Footnotes
Author Contributions: MF-R and SK contributed to the concept, design, searching the literature, and writing of the manuscript. KHT contributed to manuscript review and manuscript editing.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Ethical Approval: As this review did not involve any human or animal subjects, ethical approval was not required.
References
- 1. Alvar J, Vélez ID, Bern C, et al. Leishmaniasis worldwide and global estimates of its incidence. PLoS One. 2012;7(5):e35671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Croft SL, Seifert K, Yardley V. Current scenario of drug development for leishmaniasis. Indian J Med Res. 2006;123:399–410. [PubMed] [Google Scholar]
- 3. World Health Organization. A report of the consultation meeting on traditional and modern medicine: harmonizing two approaches, 22-26 November 1999 Beijing, China, West Pacific Region 2000. [Google Scholar]
- 4. Anthony JP, Fyfe L, Smith H. Plant active components—a resource for antiparasitic agents? Trends Parasitol. 2005;21:462–468. [DOI] [PubMed] [Google Scholar]
- 5. Kooti W, Moradi M, Akbari SA, et al. Therapeutic and pharmacological potential of Foeniculum vulgare Mill: a review. J HerbMed Pharmacol. 2015;4:1–9. [Google Scholar]
- 6. Kooti W, Ali-Akbari S, Asadi-Samani M, et al. A review on medicinal plant of Apium graveolens. Adv Herbal Med. 2015;1:48–59. [Google Scholar]
- 7. Asadi-Samani M, Kooti W, Aslani E, Shirzad H. A systematic review of Iran’s medicinal plants with anticancer effects. J Evid Based Complementary Altern Med. 2016;21(2):143–153. doi:10.1177/2156587215600873. [DOI] [PubMed] [Google Scholar]
- 8. Khademvatan S, Saki J, Gharavi MJ, Rahim F. Allium sativum extract induces apoptosis in Leishmania major (MRHO/IR/75/ER) promastigotes. J Med Plant Res. 2011;5:3725–3732. [Google Scholar]
- 9. Saki J, Khademvatan S, Pazyar N, et al. In vitro activity of Cordia myxa mucilage extract against Leishmania major and L. infantum promastigotes. Jundishapur J Microbiol. 2015;8(3):e19640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yousefi E, Eskandari A, Gharavi MJ, Khademvatan S. In vitro activity and cytotoxicity of Crocus sativus extract against Leihmania major (MRHO/IR/75/ER). Infect Disord Drug Targets. 2014;14:56–60. [DOI] [PubMed] [Google Scholar]
- 11. Feily A, Saki J, Maraghi S, et al. In vitro activity of green tea extract against Leishmania major promastigotes. Int J Clin Pharmacol Ther. 2012;50:233–236. [DOI] [PubMed] [Google Scholar]
- 12. Allahdin S, Khademvatan S, Hashemitabar M, Eskandari A. In vitro activity of Camellia sinensis extracts against L. major and L. infantum promastigotes using the colorometric MTT assay. Urmia Med J. 2014;25:893–900. [Google Scholar]
- 13. Sadeghi-Nejad B, Saki J, Khademvatan S, Nanaei S. In vitro antileishmanial activity of the medicinal plant—Satureja khuzestanica Jamzad. J Med Plant Res. 2011;5:5912–5915. [Google Scholar]
- 14. Khademvatan S, Adibpour N, Eskandari A, et al. In silico and in vitro comparative activity of novel experimental derivatives against Leishmania major and Leishmania infantum promastigotes. Exp Parasitol. 2013;135:208–216. [DOI] [PubMed] [Google Scholar]
- 15. Iciek M, Kwiecien I, Wlodek L. Biological properties of garlic and garlic-derived organosulfur compounds. Environ Mol Mutagen. 2009;50:247–265. [DOI] [PubMed] [Google Scholar]
- 16. Corzo-Martínez M, Corzo N, Villamiel M. Biological properties of onions and garlic. Trends Food Sci Technol. 2007;18:609–625. [Google Scholar]
- 17. Behnia M, Haghighi A, Komeilizadeh H, et al. In vitro antiamoebic activity of Iranian Allium sativum in comparison with metronidazole against Entamoeba histolytica. Iran J Parasitol. 2008;3(4):32–38. [Google Scholar]
- 18. Gaafar MR. Efficacy of Allium sativum (garlic) against experimental cryptosporidiosis. Alexandria J Med. 2012;48:59–66. [Google Scholar]
- 19. Soffar SA, Mokhtar GM. Evaluation of the antiparasitic effect of aqueous garlic (Allium sativum) extract in hymenolepiasis nana and giardiasis. J Egypt Soc Parasitol. 1991;21:497–502. [PubMed] [Google Scholar]
- 20. Harris JC, Plummer S, Turner MP, Lloyd D. The microaerophilic flagellate Giardia intestinalis: Allium sativum (garlic) is an effective antigiardial. Microbiol-Uk. 2000;146:3119–3127. [DOI] [PubMed] [Google Scholar]
- 21. Dkhil MA, Abdel-Baki A, Wunderlich F, Sies H, Al-Quraishy S. Anticoccidial and anti-inflammatory activity of garlic in murine Eimeria papillata infections. Vet Parasitol. 2011;175:66–72. [DOI] [PubMed] [Google Scholar]
- 22. Millet CO, Lloyd D, Williams C, et al. Effect of garlic and allium-derived products on the growth and metabolism of Spironucleus vortens. Exp Parasitol. 2011;127:490–499. [DOI] [PubMed] [Google Scholar]
- 23. Coppi A, Cabinian M, Mirelman D, Sinnis P. Antimalarial activity of allicin, a biologically active compound from garlic cloves. Antimicrob Agents Chemother. 2006;50:1731–1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gamboa-Leon R, Vera-Ku M, Peraza-Sanchez SR, Ku-Chulim C, Horta-Baas A, Rosado-Vallado M. Antileishmanial activity of a mixture of Tridax procumbens and Allium sativum in mice. Parasite. 2014;21:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nok AJ, Williams S, Onyenekwe PC. Allium sativum-induced death of African trypanosomes. Parasitol Res. 1996;82:634–637. [DOI] [PubMed] [Google Scholar]
- 26. Ahmed SA. In vitro effects of aqueous extracts of garlic (Allium sativum) and onion (Allium cepa) on Trichomonas vaginalis. Parasitol Un J. 2010;3:45–54. [Google Scholar]
- 27. Mantawy MM, Aly HF, Zayed N, Fahmy ZH. Antioxidant and schistosomicidal effect of Allium sativum and Allium cepa against Schistosoma mansoni different stages. Eur Rev Med Pharmacol Sci. 2012;16(suppl 3):69–80. [PubMed] [Google Scholar]
- 28. Klimpel S, Abdel-Ghaffar F, Al-Rasheid KA, et al. The effects of different plant extracts on nematodes. Parasitol Res. 2011;108:1047–1054. [DOI] [PubMed] [Google Scholar]
- 29. Ghazanfari T, Hassan ZM, Ebtekar M, et al. Garlic induces a shift in cytokine pattern in Leishmania major–infected BALB/c mice. Scand J Immunol. 2000;52:491–495. [DOI] [PubMed] [Google Scholar]
- 30. Gamboa-León M, Aranda-González I, Mut-Martín M, et al. In vivo and in vitro control of Leishmania mexicana due to garlic-induced NO production. Scand J Immunol. 2007;66:508–514. [DOI] [PubMed] [Google Scholar]
- 31. Hodge G, Hodge S, Han P. Allium sativum (garlic) suppresses leukocyte inflammatory cytokine production in vitro: potential therapeutic use in the treatment of inflammatory bowel disease. Cytometry. 2002;48:209–215. [DOI] [PubMed] [Google Scholar]
- 32. Liu D, Uzonna JE. The early interaction of Leishmania with macrophages and dendritic cells and its influence on the host immune response. Front Cell Infect Microbiol. 2012;2:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rizwani G, Shareef H. Genus Allium: the potential nutritive and therapeutic source. J Pharmacy Nutr Sci. 2011;1:158–165. [Google Scholar]
- 34. Weisberger AS, Pensky J. Tumor inhibition by a sulfhydryl-blocking agent related to an active principle of garlic (Allium sativum). Cancer Res. 1958;18:1301–1308. [PubMed] [Google Scholar]
- 35. Lanzotti V, Scala F, Bonanomi G. Compounds from Allium species with cytotoxic and antimicrobial activity. Phytochem Rev. 2014;13:769–791. [Google Scholar]
- 36. Yun HM, Ban JO, Park KR, et al. Potential therapeutic effects of functionally active compounds isolated from garlic. Pharmacol Ther. 2014;142:183–195. [DOI] [PubMed] [Google Scholar]
- 37. Ankri S, Mirelman D. Antimicrobial properties of allicin from garlic. Microbes Infect. 1999;1:125–129. [DOI] [PubMed] [Google Scholar]
- 38. Cutler RR, Wilson P. Antibacterial activity of a new, stable, aqueous extract of allicin against methicillin-resistant Staphylococcus aureus. Br J Biomed Sci. 2004;61:71–74. [DOI] [PubMed] [Google Scholar]
- 39. Lima CM, Freitas FI, Morais LC, et al. Ultrastructural study on the morphological changes to male worms of Schistosoma mansoni after in vitro exposure to allicin. Rev Soc Bras Med Trop. 2011;44:327–330. [DOI] [PubMed] [Google Scholar]
- 40. Feng Y, Zhu X, Wang Q, et al. Allicin enhances host pro-inflammatory immune responses and protects against acute murine malaria infection. Malar J. 2012;11:268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Salama AA, AbouLaila M, Terkawi MA, et al. Inhibitory effect of allicin on the growth of Babesia and Theileria equi parasites. Parasitol Res. 2014;113:275–283. [DOI] [PubMed] [Google Scholar]
- 42. Waag T, Gelhaus C, Rath J, et al. Allicin and derivates are cysteine protease inhibitors with antiparasitic activity. Bioorg Med Chem Lett. 2010;20:5541–5543. [DOI] [PubMed] [Google Scholar]
- 43. Jesus Corral-Caridad M, Moreno I, Torano A, et al. Effect of allicin on promastigotes and intracellular amastigotes of Leishmania donovani and L. infantum. Exp Parasitol. 2012;132:475–482. [DOI] [PubMed] [Google Scholar]
- 44. Ankri S, Miron T, Rabinkov A, et al. Allicin from garlic strongly inhibits cysteine proteinases and cytopathic effects of Entamoeba histolytica. Antimicrob Agents Chemother. 1997;41:2286–2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lee J, Gupta S, Huang JS, et al. HPLC-MTT assay: anticancer activity of aqueous garlic extract is from allicin. Anal Biochem. 2013;436:187–189. [DOI] [PubMed] [Google Scholar]
- 46. Miron T, Wilchek M, Sharp A, et al. Allicin inhibits cell growth and induces apoptosis through the mitochondrial pathway in HL60 and U937 cells. J Nutr Biochem. 2008;19:524–535. [DOI] [PubMed] [Google Scholar]
- 47. Cha JH, Choi YJ, Cha SH, et al. Allicin inhibits cell growth and induces apoptosis in U87MG human glioblastoma cells through an ERK-dependent pathway. Oncol Rep. 2012;28:41–48. [DOI] [PubMed] [Google Scholar]
- 48. Ledezma E, Jorquera A, Bendezu H, et al. Antiproliferative and leishmanicidal effect of ajoene on various Leishmania species: ultrastructural study. Parasitol Res. 2002;88:748–753. [DOI] [PubMed] [Google Scholar]
- 49. Gallwitz H, Bonse S, Martinez-Cruz A, et al. Ajoene is an inhibitor and subversive substrate of human glutathione reductase and Trypanosoma cruzi trypanothione reductase: crystallographic, kinetic, and spectroscopic studies. J Med Chem. 1999;42:364–372. [DOI] [PubMed] [Google Scholar]
- 50. Weber ND, Andersen DO, North JA, et al. In vitro virucidal effects of Allium sativum (garlic) extract and compounds. Planta Med. 1992;58:417–423. [DOI] [PubMed] [Google Scholar]
- 51. Yoshida S, Kasuga S, Hayashi N, et al. Antifungal activity of ajoene derived from garlic. Appl Environ Microbiol. 1987;53:615–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Tilli CM, Stavast-Kooy AJ, Vuerstaek JD, et al. The garlic-derived organosulfur component ajoene decreases basal cell carcinoma tumor size by inducing apoptosis. Arch Dermatol Res. 2003;295:117–123. [DOI] [PubMed] [Google Scholar]
- 53. Sakamoto K, Lawson LD, Milner JA. Allyl sulfides from garlic suppress the in vitro proliferation of human A549 lung tumor cells. Nutr Cancer. 1997;29:152–156. [DOI] [PubMed] [Google Scholar]
- 54. Sundaram SG, Milner JA. Diallyl disulfide suppresses the growth of human colon tumor cell xenografts in athymic nude mice. J Nutr. 1996;126:1355–1361. [DOI] [PubMed] [Google Scholar]
- 55. Kwon KB, Yoo SJ, Ryu DG, et al. Induction of apoptosis by diallyl disulfide through activation of caspase-3 in human leukemia HL-60 cells. Biochem Pharmacol. 2002;63:41–47. [DOI] [PubMed] [Google Scholar]
- 56. Yousuf S, Ahmad A, Khan A, et al. Effect of diallyldisulphide on an antioxidant enzyme system in Candida species. Can J Microbiol. 2010;56:816–821. [DOI] [PubMed] [Google Scholar]
- 57. Avato P, Tursi F, Vitali C, et al. Allylsulfide constituents of garlic volatile oil as antimicrobial agents. Phytomedicine. 2000;7:239–243. [DOI] [PubMed] [Google Scholar]
- 58. Lun ZR, Burri C, Menzinger M, Kaminsky R. Antiparasitic activity of diallyl trisulfide (Dasuansu) on human and animal pathogenic protozoa (Trypanosoma sp., Entamoeba histolytica and Giardia lamblia) in vitro. Ann Soc Belg Med Trop. 1994;74:51–59. [PubMed] [Google Scholar]
- 59. Xiao D, Choi S, Johnson DE, et al. Diallyl trisulfide-induced apoptosis in human prostate cancer cells involves c-Jun N-terminal kinase and extracellular-signal regulated kinase-mediated phosphorylation of Bcl-2. Oncogene. 2004;23:5594–5606. [DOI] [PubMed] [Google Scholar]
- 60. Shirin H, Pinto JT, Kawabata Y, et al. Antiproliferative effects of S-allylmercaptocysteine on colon cancer cells when tested alone or in combination with sulindac sulfide. Cancer Res. 2001;61:725–731. [PubMed] [Google Scholar]
- 61. Sigounas G, Hooker JL, Li W, et al. S-allylmercaptocysteine, a stable thioallyl compound, induces apoptosis in erythroleukemia cell lines. Nutr Cancer. 1997;28:153–159. [DOI] [PubMed] [Google Scholar]
- 62. Urbina JA, Marchan E, Lazardi K, et al. Inhibition of phosphatidylcholine biosynthesis and cell proliferation in Trypanosoma cruzi by ajoene, an antiplatelet compound isolated from garlic. Biochem Pharmacol. 1993;45:2381–2387. [DOI] [PubMed] [Google Scholar]
- 63. Ban JO, Yuk DY, Woo KS, et al. Inhibition of cell growth and induction of apoptosis via inactivation of NF-κB by a sulfurcompound isolated from garlic in human colon cancer cells. J Pharmacol Sci. 2007;104:374–383. [DOI] [PubMed] [Google Scholar]
- 64. Fumarola L, Spinelli R, Brandonisio O. In vitro assays for evaluation of drug activity against Leishmania spp. Res Microbiol. 2004;155:224–230. [DOI] [PubMed] [Google Scholar]
- 65. Mahmoudvand H, Sepahvand P, Jahanbakhsh S, Azadpour M. Evaluation of the antileishmanial and cytotoxic effects of various extracts of garlic (Allium sativum) on Leishmania tropica. J Parasit Dis. 2016;40(2):423–426. doi:10.1007/s12639-014-0520-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Wabwoba BW, Anjili CO, Ngeiywa MM, et al. Experimental chemotherapy with Allium sativum (Liliaceae) methanolic extract in rodents infected with Leishmania major and Leishmania donovani. J Vector Borne Dis. 2010;47:160–167. [PubMed] [Google Scholar]
- 67. Sharma U, Velpandian T, Sharma P, Singh S. Evaluation of anti-leishmanial activity of selected Indian plants known to have antimicrobial properties. Parasitol Res. 2009;105:1287–1293. [DOI] [PubMed] [Google Scholar]
- 68. Kinuthia G, Kabiru E, Anjili C, et al. Efficacy of crude methanolic extracts of Allium sativum L. and Moringa stenopetala (Baker f.) Cufod. against Leishmania major. Int J Med Arom Plants. 2014;4:16–25. [Google Scholar]
- 69. Kinuthia G, Anjili C, Gikonyo N, et al. In vitro and in vivo activities of blends of crude aqueous extracts from Allium sativum L, Callistemon citrinus (Curtis) Skeels and Moringa stenopetala (Baker F) Cufodontis against Leishmania major. Int J Med Arom Plants. 2013;3:234–246. [Google Scholar]
- 70. Gharavi M, Nobakht M, Khademvatan S, et al. The effect of garlic extract on expression of IFN-γ and iNOS genes in macrophages infected with Leishmania major. Iran J Parasitol. 2011;6:74–81. [PMC free article] [PubMed] [Google Scholar]
- 71. Gharavi M, Nobakht M, Khademvatan S, et al. The effect of aqueous garlic extract on interleukin-12 and 10 levels in Leishmania major (MRHO/IR/75/ER) infected macrophages. Iran J Public Health. 2011;40:105–111. [PMC free article] [PubMed] [Google Scholar]
- 72. Fatima F, Khalid A, Nazar N, et al. In vitro assessment of anti-cutaneous leishmaniasis activity of some Sudanese plants. Turkiye Parazitol Derg. 2005;29:3–6. [PubMed] [Google Scholar]
- 73. Singh SK, Bimal S, Narayan S, et al. Leishmania donovani: assessment of leishmanicidal effects of herbal extracts obtained from plants in the visceral leishmaniasis endemic area of Bihar, India. Exp Parasitol. 2011;127:552–558. [DOI] [PubMed] [Google Scholar]
- 74. McClure CD, Nolan LL. Herb extracts as potential antiprotozoal agents Paper presented at: International Symposium on Medicinal and Aromatic Plants; August 27, 1995; Amherst, MA. [Google Scholar]
- 75. McClure CD, Nolan LL, Zatyrka SA. Antileishmanial properties of Allium sativum extracts and derivatives Paper presented at: International Symposium on Medicinal and Aromatic Plants; August 27, 1995; Amherst, MA. [Google Scholar]
- 76. Eslami G, Shams A, Fasahat M, et al. Effects of S-allyl-cysteine on survival, apoptosis, and proliferation of Leishmania major in vitro. J Isfahan Med Sch. 2013;31(225):121–130. [Google Scholar]
- 77. Ghazanfari T, Hassan ZM, Khamesipour A. Enhancement of peritoneal macrophage phagocytic activity against Leishmania major by garlic (Allium sativum) treatment. J Ethnopharmacol. 2006;103:333–337. [DOI] [PubMed] [Google Scholar]
- 78. J Samdani A, Iqbal Choudhary M. Laboratory studies and clinical trials on new formulations from garlic extract against cutaneous leishmaniasis. Anti-Infective Agents. 2012;10:111–116. [Google Scholar]
- 79. Khademvatan S, Gharavi MJ, Rahim F, Saki J. Miltefosine-induced apoptotic cell death on Leishmania major and L. tropica strains. Korean J Parasitol. 2011;49:17–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Khademvatan S, Gharavi MJ, Yousefi E, Saki J. INOS and IFNγ gene expression in Leishmania major–infected J774 cells treated with miltefosine. Int J Pharmacol. 2011;7:843–849. [Google Scholar]
- 81. Loria-Cervera EN, Andrade-Narvaez FJ. Animal models for the study of leishmaniasis immunology. Rev Inst Med Trop Sao Paulo. 2014;56:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Ahmadi-Renani K, Mahmoodzadeh A, Cheraghali A, Esfahani A. Effect of garlic extract on cutaneous leishmaniasis and the role of nitric oxide. Iran J Med Sci. 2002;27:97–100. [Google Scholar]
- 83. Ghaffarifar F, Mahmmoudzadeh Poornaki A, Ghazanfari T, et al. Evaluation treatment of cutaneous leishmaniasis with garlic extract and R10 fraction in BALB/c, C57BL/6 and outbred SW mice. Modares J Med Sci: Pathobiol. 2011;14(3):25–34. [Google Scholar]
- 84. Hejazi S, Shirani-Bidabadi L, Zolfaghari-Baghbaderani A, et al. Comparision effectiveness of extracts of thyme, yarrow, henna and garlic on cutaneous leishmaniasis caused by L. major in animal model (Balb/c). J Med Plant. 2009;2:129–136. [Google Scholar]
- 85. Babaee-Khou L, Mohebali M, Niakan-Lahiji M, Mehrabi-Tavana A. The therapeutic effects of eucalyptus, myrtus, ferula, aretmisia, allium and urtica extracts against cutaneous leishmaniasis caused by Leishmanaia major in small white mice (out-bred). Hakim Res J. 2007;10(2):21–27. [Google Scholar]
- 86. Khalid N, Mohomed H, Toum A, et al. Treatment of cutaneous leishmaniasis with some local Sudanese plants (neem, garad & garlic). Turkiye Parazitol Derg. 2004;28:129–132. [Google Scholar]
- 87. Gholami A, Khamesipour A, Momeni A, et al. Treatment of cutaneous leishmaniasis with 5% garlic cream: a randomized, double-blind study. Iran J Dermatol. 2000;3:2–6. [Google Scholar]
- 88. Jimenez-Ruiz A, Alzate JF, Macleod ET, et al. Apoptotic markers in protozoan parasites. Parasit Vectors. 2010;3:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Jakubikova J, Sedlak J. Garlic-derived organosulfides induce cytotoxicity, apoptosis, cell cycle arrest and oxidative stress in human colon carcinoma cell lines. Neoplasma. 2005;53:191–199. [PubMed] [Google Scholar]
- 90. Khademvatan S, Gharavi MJ, Saki J. Miltefosine induces metacaspase and PARP genes expression in Leishmania infantum. Braz J Infect Dis. 2011;15:442–448. [DOI] [PubMed] [Google Scholar]
- 91. Su CC, Chen GW, Tan TW, et al. Crude extract of garlic induced caspase-3 gene expression leading to apoptosis in human colon cancer cells. In Vivo. 2006;20:85–90. [PubMed] [Google Scholar]
- 92. Tsubura A, Lai YC, Kuwata M, et al. Anticancer effects of garlic and garlic-derived compounds for breast cancer control. Anticancer Agents Med Chem. 2011;11:249–253. [DOI] [PubMed] [Google Scholar]
- 93. Riggs DR, DeHaven JI, Lamm DL. Allium sativum (garlic) treatment for murine transitional cell carcinoma. Cancer. 1997;79:1987–1994. [DOI] [PubMed] [Google Scholar]
- 94. Zhang YW, Wen J, Xiao JB, et al. Induction of apoptosis and transient increase of phosphorylated MAPKs by diallyl disulfide treatment in human nasopharyngeal carcinoma CNE2 cells. Arch Pharm Res. 2006;29:1125–1131. [DOI] [PubMed] [Google Scholar]
- 95. Rendu F, Daveloose D, Debouzy JC, et al. Ajoene, the antiplatelet compound derived from garlic, specifically inhibits platelet release reaction by affecting the plasma membrane internal microviscosity. Biochem Pharmacol. 1989;38:1321–1328. [DOI] [PubMed] [Google Scholar]
- 96. Hassan ZM, Yaraee R, Zare N, et al. Immunomodulatory affect of R10 fraction of garlic extract on natural killer activity. Int Immunopharmacol. 2003;3:1483–1489. [DOI] [PubMed] [Google Scholar]
- 97. Ghazanfari T, Hassan ZM, Ebrahimi M. Immunomodulatory activity of a protein isolated from garlic extract on delayed type hypersensitivity. Int Immunopharmacol. 2002;2:1541–1549. [DOI] [PubMed] [Google Scholar]
- 98. Ippoushi K, Itou H, Azuma K, Higashio H. Effect of naturally occurring organosulfur compounds on nitric oxide production in lipopolysaccharide-activated macrophages. Life Sci. 2002;71:411–419. [DOI] [PubMed] [Google Scholar]
- 99. Kim KM, Chun SB, Koo MS, et al. Differential regulation of NO availability from macrophages and endothelial cells by the garlic component S-allyl cysteine. Free Radic Biol Med. 2001;30:747–756. [DOI] [PubMed] [Google Scholar]
- 100. Dirsch VM, Kiemer AK, Wagner H, Vollmar AM. Effect of allicin and ajoene, two compounds of garlic, on inducible nitric oxide synthase. Atherosclerosis. 1998;139:333–339. [DOI] [PubMed] [Google Scholar]
- 101. Morihara N, Sumioka I, Moriguchi T, et al. Aged garlic extract enhances production of nitric oxide. Life Sci. 2002;71:509–517. [DOI] [PubMed] [Google Scholar]
- 102. Santos WR, de Lima VM, de Souza EP, et al. Saponins, IL12 and BCG adjuvant in the FML-vaccine formulation against murine visceral leishmaniasis. Vaccine. 2002;21:30–43. [DOI] [PubMed] [Google Scholar]
- 103. Haldar AK, Sen P, Roy S. Use of antimony in the treatment of leishmaniasis: current status and future directions. Mol Biol Int. 2011;2011:571242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Li J, Sutterwala S, Farrell JP. Successful therapy of chronic, nonhealing murine cutaneous leishmaniasis with sodium stibogluconate and gamma interferon depends on continued interleukin-12 production. Infect Immun. 1997;65:3225–3230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Li J, Scott P, Farrell JP. In vivo alterations in cytokine production following interleukin-12 (IL-12) and anti-IL-4 antibody treatment of CB6F1 mice with chronic cutaneous leishmaniasis. Infect Immun. 1996;64:5248–5254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Singh OP, Sundar S. Immunotherapy and targeted therapies in treatment of visceral leishmaniasis: current status and future prospects. Front Immunol. 2014;5:296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Dalton JE, Kaye PM. Immunomodulators: use in combined therapy against leishmaniasis. Expert Rev Anti Infect Ther. 2010;8:739–742. [DOI] [PubMed] [Google Scholar]