Abstract
Petroleum ether, chloroform, ethyl acetate, and n-butanol fractions of Greek juniper (Juniperus excelsa M.Bieb. from the family Cupressaceae) were evaluated for antileishmanial activities against Leishmania major promastigotes compared to meglumine antimoniate (Glucantime). In vitro toxicity assay was performed using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide and microplate ELISA reader. Extracts were prepared in ethanol/dimethyl sulfoxide (80/20) at 10 to 0.62 mg/mL. The standard was prepared in phosphate-buffered saline at 500 to 15.62 mg/mL. Both leaf and fruit extracts and related fractions showed strong inhibitory effects against promastigotes, significantly different from that of the standard. The leaf extract and the respective petroleum ether fraction showed maximum effectiveness compared to other fractions and also fruit extract and fractions (IC90 = 1.89 ± 0.03 and 0.90 ± 0.03 mg/mL, respectively). Regarding the potent activities of nonpolar fractions of Greek juniper leaf extract, these fractions can be suggested for further investigation.
Keywords: antileishmanial activity, Juniperus excelsa, Leishmania major
Leishmaniasis is a vector-borne disease caused by a protozoan parasite Leishmania and is transmitted by the bite of certain types of sandflies.1 This disorder can be presented in 3 ways: cutaneous, mucocutaneous, and visceral leishmaniasis.2 The cutaneous type is associated with skin ulcers, whereas the mucocutaneous form presents with skin, mouth, and nose ulcers. The visceral form, which starts with skin ulcers, presents with fever later, decline in red blood cells, and causes splenomegaly and hepatomegaly. Leishmaniasis is reported as an endemic disease in about 90 countries where 350 million people are at risk. It is reported that more than 12 million people are currently infected by the parasite.3,4 Among those prevalent types of leishmaniasis, the cutaneous type is common in some parts of Asia, Africa, and central and south America.5,6 In Iran, cutaneous leishmaniasis has been increasing during the past 10 years and more than 70% of this disease is distributed in the rural parts of the country.7
Treatment lines for leishmaniasis are generally application of pentavalent antimony compounds, pentamidine, and numerous antifungal formulations, such as amphotericin B, and currently, alkylphosphocholine compounds such as miltefosine.8 Additionally, nonpharmacological interventions such as cryotherapy, localized controlled heat, and photodynamic therapy are also used to treat the cutaneous type.9–11 In spite of various chemical medicaments applied for the treatment of this disease, results have not yet been satisfactory. Thus, seeking for new and safe medication is considered crucial.12
Other than the conventional remedies, natural medicaments were also investigated. With reference to herbal and complementary medicine, antileishmanial activities of plants, such as feverfew,13 onion,14 and Zhumeria majdae Rech.f. & Wendelbo15 were studied.
Traditional Persian medicine is a summation of development of ideas and knowledge from ancient civilizations and Iranian scientists (Hakim) during the medieval era (Islamic golden ages of medical knowledge). This medical system is founded on 4 humor concept, namely, phlegm (Balgham), blood (Dam), yellow bile (Ṣafrā), and black bile (Saudā). Balance in the proportion of these humors sustains health preservation, whereas lack of this balance results in the incidence of various diseases.16 This school of medicine, which is not only a collection of previous integrative medical information but also a summation of its own scholars and practitioners,17 contains numerous medical suggestions for these various types of diseases. In this regard, the present study aimed to assess the antileishmanial activities of Greek juniper or Juniperus excelsa M.Bieb. (Family Cupressaceae) extracts yielded from its leaves and fruits on Leishmania major in comparison with meglumine antimoniate. The herb is native to Iran and has been recently evaluated for some activities such as antimicrobial, antioxidant, and antitumor.18,19
Materials and Methods
Extraction and Fractionation of Fruits and Leaves of the Herb
Fruits and leaves of Greek juniper were collected from Genu Mountain, northern parts of Bandar-Abbas (Hormozgan Province, Iran). Specifying a voucher number (PM-947), the sample was identified by S. Khademian, Herbalist of the Department of Traditional Pharmacy, School of Pharmacy, Shiraz University of Medical Sciences. Dried leaves (9000 g) and fruits (500 g) were separately subjected to alcoholic extraction via the percolation procedure. Both extracts were subsequently concentrated using a rotary evaporator under reduced pressure. The time consumed for the extraction of fruits and leaves was 48 hours and 10 days, respectively.
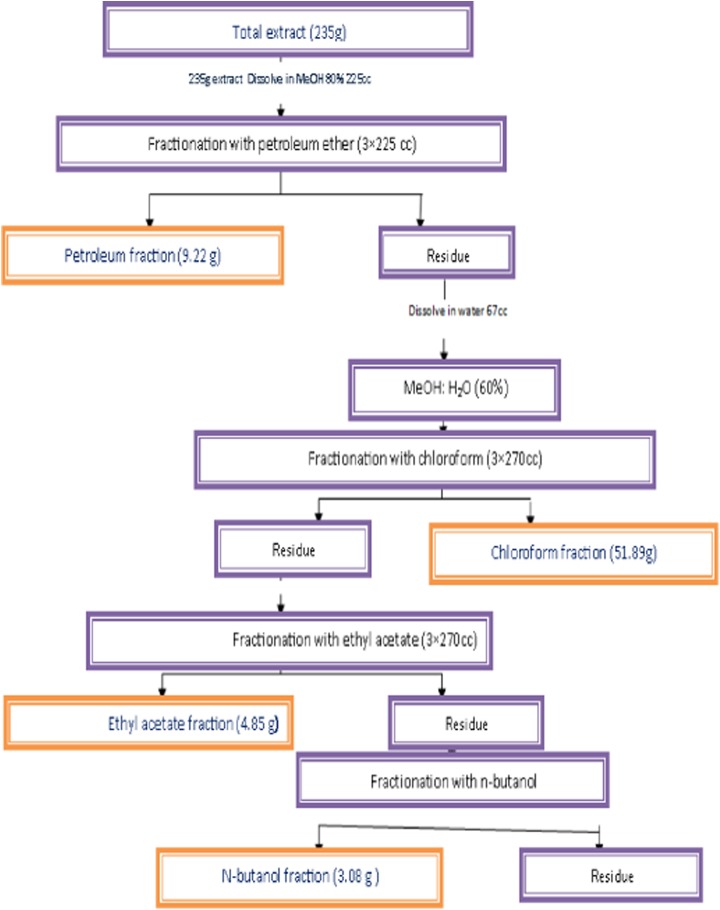
To fractionate the fruit extract, 88 g of crude extract was dissolved in 225 mL of methanol (80%) and extracted with petroleum ether (3 × 225 mL) using a separating funnel. The petroleum ether fraction was separated and the residue was dissolved in 67 mL distilled water. Subsequently, this phase was mixed and extracted with chloroform (3 × 270 mL) using the same method. The chloroform fraction was collected and the residue was later dissolved in distilled water up to 270 mL. This phase was then mixed and extracted with ethyl acetate (3 × 270 mL). The aqueous phase that separated from the ethyl acetate fraction was mixed eventually with n-butanol and the n-butanol fractions were separated. The same procedure was performed for leaves extract, and all 4 prepared fractions related to both leaf and fruit extracts were subjected to antileishmanial activity. Figure 1 schematically represents the fractionation procedure for the fruit extract.
Figure 1.
Fractionation of Greek juniper fruits extract via liquid-liquid extraction.
Growth Media and Parasite Culture
Blood agar–based biphasic Novy-MacNeal-Nicolle (NNN) as well as brain-heart infusion (BHI) media were employed for the recovery and mass cultivation of the parasite, respectively. Reference strain of Leishmania major (MRHO/IR/75/ER) was purchased from Pasteur Institute in Iran and was cultivated on NNN medium having BHI as the liquid phase. The promastigotes produced were then transferred to the fresh culture for further processing.20 The number of parasites was adjusted to 1 × 106/mL for inoculation.
Evaluation of the In Vitro Leishmanicidal of Fractions and Meglumine Antimoniate
Fractions were dissolved in an appropriate solvent involving dimethyl sulfoxide (DMSO)-ethanol (20:80) with proper dilutions. The employed concentrations for the fractions and meglumine antimoniate were at different ranges of 0.625 to 10 and 15.625 to 500 mg/mL, respectively. Briefly, 10 mg of each fraction was dissolved in 1000 μL of the solvent. In parallel, 500 mg of meglumine antimoniate was dissolved in phosphate-buffered saline (PBS) to the concentration of 500 mg/mL. Each fraction was then treated with promastigotes in 5 different concentrations.
Counting the Parasites
Approximately, 100 λ of the parasites was transferred to a neobar lam. The WBC chamber of the lam was used to count the parasites.
MTT Method for Assessment of Antileishmanial Activity
MTT, or 3-(4,5-dimethylthiazol-2yl)-2,5-diphenyltetrazoliumbromide, was applied for in vitro promastigotes cell toxicity assay of all fractions and extracts of leaf and fruit of Greek juniper as well as that of the meglumine antimoniate. This substance is used as a marker to determine the cell viability.21 Briefly, 100 μL of BHI culture (2.5 × 106 promastigotes) was transferred to each well of a 96-well standard microplate. Subsequently, 10 μL of different concentrations (10-0.625 mg/mL) of fruits and leaves of the herb as well as related fractions were added to the respective well. The plate was then incubated at 25°C for 48 hours. Blank and control wells contained medium and promastigotes plus solvent, respectively. Following incubation, the wells containing fluid were centrifuged and, subsequently, the supernatant was removed. Afterwards, 50 μL of MTT (2 mg/mL) in PBS was added to the wells. The plate was then incubated in darkness at 25°C for 3 hours. At the end, 100 μL of DMSO, as stopper, was added to the wells to stop the enzymatic reaction. The plate was left standing for 5 minutes. Finally, the optical density was determined at 570 nm using a microplate ELISA reader to read continuously. Lysis of the parasites (%) by extracts and fractions as well was meglumine antimoniate was determined using the following formula:
Lysis % = 100 − [(test − blank)/(control − blank)] × 100
The values of IC90 were determined by plotting the amounts of promastigotes lysis (%) with respect to that of the control against treatment concentrations.
All tests were performed in triplicate, and the average data were reported as the final results. GraphPad InStat software was used to compare the resulting data. Statistical analysis was done by using one-way ANOVA and Tukey post hoc tests. In this study, P < .05 was considered statistically significant.
Results
Results of the lethal effects and leishmanicidal activities of Greek juniper leaves and fruits were determined in 5 different concentrations. As the parasite had shown sensitivity to the extracts, the employed concentrations were 50 times diluted compared to those of the standard (meglumine antimoniate). The standard was assessed for the same activity in 6 different concentrations. Data related to the evaluation of leaves and fruits extract and related fractions are shown in Table 1. Data related to the antileishmanial activity of meglumine antimoniate are presented in Table 2. Lethal effect values which were calculated fewer than 20% were considered inactive. To have a better outline, Figures 2 and 3 present profiles related to the lethal effects of extracts and respective fractions of Greek juniper fruits and leaves compared to those of the standard, respectively. Furthermore, values related to IC90 of leaf and fruit extracts and also respective fractions are presented in Table 3.
Table 1.
Data of Lethal Effects of Leaves and Fruits Total Extracts and Respective Fractions.a,b
| Concentration (mg/mL) | |||||
|---|---|---|---|---|---|
| 10 | 5 | 2.5 | 1.25 | 0.625 | |
| Fruits extract | |||||
| Total extract | 100 | 87.11 ± 1.98 | 76.84 ± 1.04 | 62.84 ± 1.75 | 46.88 ± 2.88 |
| Petroleum ether | 100 | 100 | 100 | 99.64 ± 0.25 | 92.41 ± 1.62 |
| Chloroform | 89.98 ± 2.76 | 76 ± 3.48 | 53.32 ± 0.91 | 42.36 ± 1.12 | 31.1 ± 2.04 |
| Ethyl acetate | 66.47 ± 9.74 | 57.17 ± 1.71 | 42.34 ± 6.00 | 35.44 ± 1.68 | 27.66 ± 2.22 |
| n-Butanol | Not active | Not active | Not active | Not active | Not active |
| Leaves extract | |||||
| Total extract | 100 | 100 | 98.43 ± 0.01 | 84.51 ± 2.07 | 65.59 ± 1.02 |
| Petroleum ether | 100 | 100 | 98.08 ± 0.40 | 98.01 ± 0.19 | 82.90 ± 0.90 |
| Chloroform | 100 | 100 | 99.77 ± 0.07 | 92.72 ± 1.81 | 73.10 ± 2.28 |
| Ethyl acetate | 100 | 100 | 99.22 ± 0.98 | 86.14 ± 1.62 | 72.26 ± 0.36 |
| n-Butanol | Not active | Not active | Not active | Not active | Not active |
aResults are presented as lethal effects (%) ± standard deviation.
bThe values which were calculated as less than 20% were considered as “not active.”
Table 2.
Data of Lethal Effects of Meglumine Antimoniate as Standard.
| Concentration (mg/mL) | ||||||
|---|---|---|---|---|---|---|
| 500 | 250 | 125 | 62.5 | 31.25 | 15.625 | |
| Lethal effects (%) | 98.14 ± 1.21 | 96.55 ± 0.77 | 68.08 ± 3.19 | 47.34 ± 0.78 | 23.24 ± 8.51 | 3.10 ± 3.83 |
Figure 2.
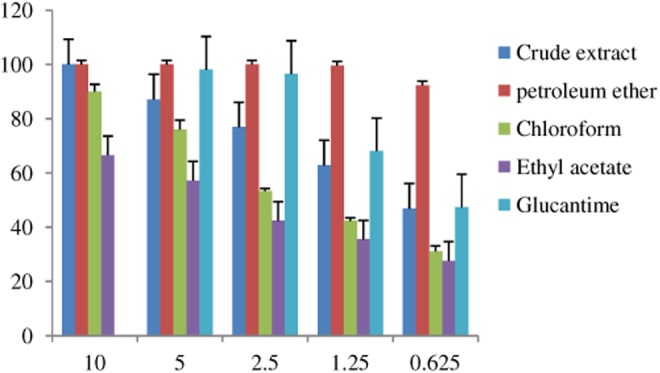
Lethal effects of Greek juniper fruits extract and respective fractions on promastigotes, compared to meglumine antimoniate.
Figure 3.
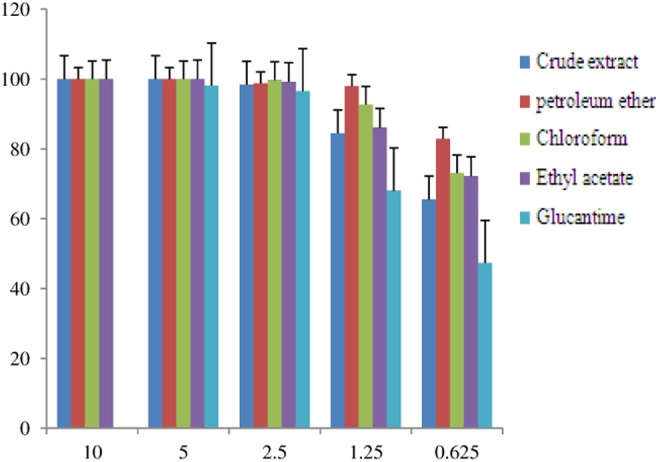
Lethal effects of Greek juniper leaves extract and respective fractions on promastigotes, compared to meglumine antimoniate.
Table 3.
Data Related to IC90 of Leaves and Fruits Extracts and Respective Fractions.
| IC90 ± SD (mg/mL) | ||
|---|---|---|
| Leaves Extract | Fruits Extract | |
| Total extract | 1.89 ± 0.03 | 6.96 ± 0.11 |
| Petroleum ether | 0.90 ± 0.03 | 0.83 ± 0.01 |
| Chloroform | 1.14 ± 0.05 | 9.08 ± 0.13 |
| Ethyl acetate | 1.76 ± 0.07 | 15.57 ± 3.71 |
Discussion and Conclusion
In the current study, a potent extract and respective fractions of a medicinal plant has been applied for the efficacy assessment on leishmaniasis. According to the medical and pharmaceutical manuscripts of Persian medicine, this disorder appears as a wound, either dry or wet, and could be contagious as well. The nearest term for this disorder is defined as Rish-e-balkhi (Balkh wound). The mentioned signs and symptoms are in line with cutaneous leishmaniasis in current medicine.22 With reference to the documents of Persian medical and pharmaceutical manuscripts and prior experimental findings, Greek juniper was selected for the current research. In Makhzan al-adviyah (The Storehouse of Medicaments by Alavī Shīrāzī, 18th century), a main pharmacopeia of Persian medicine, fruits and leaves of this plant have been strongly recommended for the treatment of such wounds.23
This medicinal plant has shown to exert various pharmacological activities. With regard to skin diseases, investigations have demonstrated considerable cytotoxic activities for Greek juniper. A study revealed the cytotoxic activity of J sabina berries against tumor cell lines.24 Another investigation showed that the cytotoxic activity of a hydroalcoholic extract of fruits of this plant was similar to that of Taxus baccata, a popular cytotoxic plant.25 Greek juniper, on the other hand, has repeatedly exerted antimicrobial activities. A potent fraction of the plant’s essential oil, terpenes, was effective against microbial strains.26 Antimicrobial investigation of the herb essential oil showed considerable effectiveness (minimum inhibitory concentration = 0.625-2.5 μg/mL) on both gram-positive and gram-negative strains.18 On the other hand, the essential oil of a Juniperus species was assessed for antileishmanial activities and the result was satisfactory.27 With regard to the role of antioxidants in wounds and skin ulcers, Greek juniper has exhibited antioxidant and radical scavenging activities, which could be effective in wound healing. A study showed that essential oil of leaves and fruits of this plant could effectively inhibit lipid peroxidation in the employed cells.28 Using 2,2′-azobis(2-amidinopropane)dihydrochloride in another assessment, the essential oil exerted antioxidant activity as compared to vitamin E.29
To get more active constituents extracted, percolation was selected as the extraction procedure. Subsequently, the MTT method was employed for the determination of antileishmanial activities. In this method, living promastigotes can convert MTT to formazan via an enzymatic reaction. Later, DMSO can produce a uniform purple solution. The absorption of this solution can be determined by a microplate ELISA reader. The amount of produced formazan corresponds with number of living promastigotes.30,31
In this assessment, the promastigotes form of the parasite was employed due to the simplicity of medium and preservation as well as the usefulness of this form for chemotherapeutic screening.3,31
According to the findings of this study, the petroleum ether fraction of the fruit extract showed the highest activity among fruit total extract and respective fractions at the concentration of 0.312 mg/mL. Similar results were achieved for the leaf extract. It is also considerable that the potency of the aforementioned fraction from the leaf extract was found higher than that of the fruit (P < .001). On the other hand, these extracts and fractions were used at 1/50 concentration of meglumine antimoniate. These findings, thus, show that these extracts and fractions can be introduced as good candidates for clinical research, in combination or as an alternative to meglumine antimoniate.
In summary, the current study assessed the antileishmanial activity of fruits and leaves of Greek juniper at the concentration range of 0.625 to 10 mg/mL. The results revealed that activities of leaves and respective fractions were higher than those of the fruits. However, in the aforementioned concentration range both extracts were highly active. Since the lethal effects of both extracts were found to be higher than 50%, determination of IC90 was considered instead of IC50.
Previously, other medicinal plants were evaluated for antileishmanial activity. Plumbagin isolated from bark of Pera benensis Rusby showed considerable effects against some Leishmania species (IC90 = 5 μg/mL).32 Berberine, with a concentration of 10 μg/mL, was found effective on Leishmania-infected golden hamsters.33 Other compounds such as senegalene, squamocine, and asimicine (25-100 μg/mL) isolated from Annona senegalensis Pers. showed antileishmanial effects against related promastigotes.34 Alkaloids of Galipea longiflora K. Krause leaves has shown antileishmanial effects (IC90 = 25 μg/mL) against Leishmania braziliensis.35 In another investigation, Peganum harmala L. extract was checked for activity. The value of IC50 for the extract (concentration range of 5-20 mg/mL) was calculated as 1832.65 μg/mL compared to that of the potassium antimonyl tartrate (62.5-500 μg/mL) as the standard (IC50 = 17.87 μg/mL).3 In our study, the IC90 values of Greek juniper leaf extract and respective petroleum ether fractions were calculated as 1.89 and 0.90 mg/mL, respectively. Therefore, it can be concluded that the antileishmanial activity of Greek juniper from the current study is higher than that of the Peganum harmala L. It is also notable that fractions with antileishmanial activity may be parallel with reduction in polarity. This fact may be due to the increase in terpenoids in the petroleum fraction.
Regarding the potent activities of nonpolar fractions of Greek juniper leaf extract, these fractions can be suggested for further investigation. In addition, concerned fractions can be purified and subjected to in vivo and also human comprehensive studies.
Acknowledgement
This work was derived from the PharmD thesis of Dr Razieh Taghavi-Moghadam. The authors express their appreciation to Shiraz University of Medical Sciences, Shiraz, Iran (Project numbers: 5493).
Footnotes
Author Contributions: MM and GH defined the research theme and designed methods and experiments. TMR carried out the laboratory experiments under the supervision of the other authors. MM, MMZ, and GH analyzed the data and interpreted the results. MMZ wrote the draft of the article. All authors have reviewed and confirmed the final draft.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Ethical Approval: As this work was an experimental assessment, no ethical approval was required.
References
- 1. Ahua KM, Ioset JR, Ioset KN, Diallo D, Mauel J, Hostettmann K. Antileishmanial activities associated with plants used in the Malian traditional medicine. J Ethnopharmacol. 2007;110:99–104. [DOI] [PubMed] [Google Scholar]
- 2. Desjeux P. Leishmaniasis: current situation and new perspectives. Comp Immunol Microbiol Infect Dis. 2004;27:305–318. [DOI] [PubMed] [Google Scholar]
- 3. Mirzaie M, Nosratabadi SJ, Derakhshanfar A, Sharif I. Antileishmanial activity of Peganum harmala extract on the in vitro growth of Leishmania major promastigotes in comparison to a trivalent antimony drug. Veterinarski Arhiv. 2007;77:365–375. [Google Scholar]
- 4. Barrett MP, Croft SL. Management of trypanosomiasis and leishmaniasis. Br Med Bull. 2012;104:175–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ejazi SA, Ali N. Developments in diagnosis and treatment of visceral leishmaniasis during the last decade and future prospects. Expert Rev Anti Infect Ther. 2013;11:79–98. [DOI] [PubMed] [Google Scholar]
- 6. Reithinger R, Dujardin JC, Louzir H, Pirmez C, Alexander B, Brooker S. Cutaneous leishmaniasis. Lancet Infect Dis. 2007;7:581–596. [DOI] [PubMed] [Google Scholar]
- 7. Ahmadizadeh E, Soleimani K, Hosseinpour M, Zakeri A, Nejhad MN, Ahmadizadeh A. Epidemiology of cutaneous leishmaniasis in Hormozgan province (2007-2011). Life Sci J. 2013;10(3). http://www.lifesciencesite.com/lsj/life1003/220_20307life1003_1473_1475.pdf. Accessed December 12, 2015. [Google Scholar]
- 8. Santos DO, Coutinho CE, Madeira MF, et al. Leishmaniasis treatment—a challenge that remains: a review. Parasitol Res. 2008;103:1–10. [DOI] [PubMed] [Google Scholar]
- 9. Akilov OE, Kosaka S, O’Riordan K, Hasan T. Photodynamic therapy for cutaneous leishmaniasis: the effectiveness of topical phenothiaziniums in parasite eradication and Th1 immune response stimulation. Photochem Photobiol Sci. 2007;6:1067–1075. [DOI] [PubMed] [Google Scholar]
- 10. González U, Pinart M, Reveiz L, et al. Designing and reporting clinical trials on treatments for cutaneous leishmaniasis. Clin Infect Dis. 2010;51:409–419. [DOI] [PubMed] [Google Scholar]
- 11. Mosleh IM, Geith E, Natsheh L, Schönian G, Abotteen N, Kharabsheh SA. Efficacy of a weekly cryotherapy regimen to treat Leishmania major cutaneous leishmaniasis. J Am Acad Dermatol. 2008;58:617–624. [DOI] [PubMed] [Google Scholar]
- 12. Rocha LG, Almeida JRGS, Macêdo RO, Barbosa-Filho JM. A review of natural products with antileishmanial activity. Phytomedicine. 2005;12:514–535. [DOI] [PubMed] [Google Scholar]
- 13. Tiuman TS, Ueda-Nakamura T, Garcia Cortez DA, et al. Antileishmanial activity of parthenolide, a sesquiterpene lactone isolated from Tanacetum parthenium. Antimicrob Agents Chemother. 2005;49:176–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Saleheen D, Ali SA, Yasinzai MM. Antileishmanial activity of aqueous onion extract in vitro. Fitoterapia. 2004;75:9–13. [DOI] [PubMed] [Google Scholar]
- 15. Moein MR, Pawar RS, Khan SI, Tekwani BL, Khan IA. Antileishmanial, antiplasmodial and cytotoxic activities of 12,16-dideoxy aegyptinone B from Zhumeria majdae Rech.f. & Wendelbo. Phytother Res. 2008;22:283–285. [DOI] [PubMed] [Google Scholar]
- 16. Zarshenas MM, Petramfar P, Firoozabadi A, Moein MR, Mohagheghzadeh A. Types of headache and those remedies in traditional Persian medicine. Pharmacogn Rev. 2013;7:17–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hamedi A, Zarshenas MM, Sohrabpour M, Zargaran A. Herbal medicinal oils in traditional Persian medicine. Pharm Biol. 2013;59:1208–1218. [DOI] [PubMed] [Google Scholar]
- 18. Moein MR, Ghasemi Y, Moein S, Nejati M. Analysis of antimicrobial, antifungal and antioxidant activities of Juniperus excelsa M. B subsp. Polycarpos (K. Koch) Takhtajan essential oil. Pharmacognosy Res. 2010;2:128–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Unlu M, Vardar-Unlu G, Vural N, Donmez E, Cakmak O. Composition and antimicrobial activity of Juniperus excelsa essential oil. Chem Nat Compd. 2008;44:129–131. [Google Scholar]
- 20. Habibi P, Sadjjadi S, Owji M, et al. Characterization of in vitro cultivated amastigote like of Leishmania major: a substitution for in vivo studies. Iran J Parasitol. 2008;3:6–15. [Google Scholar]
- 21. Bhattacharya S, Biswas M, Haldar PK. The triterpenoid fraction from Trichosanthes dioica root exhibits in vitro antileishmanial effect against Leishmania donovani promastigotes. Pharmacognosy Res. 2013;5:109–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mosaddegh M, Naghibi F, Sadr M. Oriental sore in traditional Persian medicine. J Islamic Iran Tradit Med. 2010;1:59–62. [Google Scholar]
- 23. Shirazi A. Makhzan al-adviyah (The Storehouse of Medicaments). Tehran, Iran: Tehran University of Medical Sciences; 2009. [Google Scholar]
- 24. Asili J, Emami SA, Rahimizadeh M, Fazly-Bazzaz BS, Hassanzadeh MK. Chemical and antimicrobial studies of Juniperus sabina L. and Juniperus foetidissima Willd. essential oils. J Essential Oil Bearing Plants. 2010;13:25–36. [Google Scholar]
- 25. Shokrzadeh M, Azadbakht M, Ahangar N, Naderi H, Saeedi Saravi SS. Comparison of the cytotoxic effects of Juniperus sabina and Zataria multiflora extracts with Taxus baccata extract and cisplatin on normal and cancer cell lines. Pharmacogn Mag. 2010;6:102–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Akimov Iu A, Kharchenko GI, Krylova AP, Belova NN. Antimicrobial effect of terpenes from the Cossack juniper, Juniperus sabina L. Prikl Biokhim Mikrobiol. 1977;13:185–188. [PubMed] [Google Scholar]
- 27. Machado M, Santoro G, Sousa MC, Salgueiro L, Cavaleiro C. Activity of essential oils on the growth of Leishmania infantum promastigotes. Flavour Fragrance J. 2010;25:156–160. [Google Scholar]
- 28. Asgary S, Sahebkar A, Naderi GA, et al. Essential oils from the fruits and leaves of Juniperus sabina possess inhibitory activity against protein glycation and oxidative stress: an in vitro phytochemical investigation. J Essential Oil Res. 2013;25:70–77. [Google Scholar]
- 29. Emami S, Khayyat MH, Doosti F, Bagherpasand N, Zolfaghar F. Investigation of antioxidant activity of the essential oils of different parts of Juniperus sabina (Cupressaceae) by TBARS method in comparison with vitamin E. Res Pharm Sci. 2012;7:798. [Google Scholar]
- 30. Dutta A, Bandyopadhyay S, Mandal C, Chatterjee M. Development of a modified MTT assay for screening antimonial resistant field isolates of Indian visceral leishmaniasis. Parasitol Int. 2005;54:119–122. [DOI] [PubMed] [Google Scholar]
- 31. Dutta A, Mandal G, Mandal C, Chatterjee M. In vitro antileishmanial activity of Aloe vera leaf exudate: a potential herbal therapy in leishmaniasis. Glycoconj J. 2007;24:81–86. [DOI] [PubMed] [Google Scholar]
- 32. Fournet A, Angelo A, Muñoz V, Roblot F, Hocquemiller R, Cavé A. Biological and chemical studies of Pera benensis, a Bolivian plant used in folk medicine as a treatment of cutaneous leishmaniasis. J Ethnopharmacol. 1992;37:159–164. [DOI] [PubMed] [Google Scholar]
- 33. Vennerstrom J, Lovelace J, Waits V, Hanson W, Klayman D. Berberine derivatives as antileishmanial drugs. Antimicrob Agents Chemother. 1990;34:918–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chan-Bacab MJ, Pena-Rodriguez LM. Plant natural products with leishmanicidal activity. Nat Prod Rep. 2001;18:674–688. [DOI] [PubMed] [Google Scholar]
- 35. Calla-Magariños J, Quispe T, Giménez A, Freysdottir J, Troye-Blomberg M, Fernández C. Quinolinic alkaloids from Galipea longiflora Krause suppress production of proinflammatory cytokines in vitro and control inflammation in vivo upon Leishmania infection in mice. Scand J Immunol. 2013;77:30–38. [DOI] [PubMed] [Google Scholar]